ABSTRACT
Background and Objectives:
EUS-guided biliary drainage (BD) through hepaticogastrostomy (HGS) is an option in case of ERCP failure. Available data suggest that this procedure may be challenging with possible severe adverse events (AEs) mainly due to stent migration. The aim of our pilot study was to prospectively assess the technical and clinical outcomes of EUS-HGS using a new dedicated partially covered self-expandable metal stent with anti-migratory systems.
Methods:
This is a single-center prospective study enrolling patients with malignant biliary obstruction undergoing EUS-HGS after failed ERCP, between June 2020 and March 2021. The primary endpoint was the technical success rate. Evaluation of specific stent-related technical features as compared with commonly used self-expandable metal stent, clinical success rate, and procedure-related AEs was also assessed.
Results:
Twenty-two patients (15%–68.2%, female; mean age, 66.0 ± 10.0) were enrolled in the study analysis. Different causes of ERCP failure were infiltration of papilla by neoplastic tissue (4, 18.2%), unreachable papilla for duodenal stricture (9, 40.9%), surgically altered anatomy with Roux-en-Y reconstruction (4, 18.2%), and incomplete BD after transpapillary stent placement (5, 22.7%). Technical success was achieved in all patients, in a mean procedural time of 43.3 ± 26.8 min. Technical features were graded as high or medium in all cases. The clinical success rate was 91% (20/22, mean follow-up: 10.8 ± 3.1 months). There were no cases of stent misplacement or stent migration. Three (13.6%) cases of a hepatic abscess requiring percutaneous drainage and systemic antibiotics were reported, with no impact on clinical success and following oncologic treatments. No deaths occurred.
Conclusion:
EUS-HGS with a new dedicated stent with anti-migratory systems is feasible and effective, preventing stent migration, and misplacement. Although the persistent procedural challenges, dedicated devices may contribute to outcomes improvement and procedure diffusion.
Keywords: Biliary tract, intervention EUS, pancreatobiliary
BACKGROUND
In recent years, interventional EUS has been showing an increasing role in biliary drainage (BD) as an alternative to ERCP biliary decompression. As a matter of fact, even when performed by expert endoscopists, ERCP fails up to 12.9% of cases.[1,2] In these cases, standard alternative approaches include surgical bypass and percutaneous transhepatic biliary drainage (PTBD).[3] However, these procedures are associated with patient discomfort and prolonged hospital stay.[4,5]
The most diffuse approaches for EUS-BD are choledochoduodenostomy (CDS) or hepaticogastrostomy (HGS); however, there is still no consensus on the technique of choice in EUS-BD. The advent of a dedicated stent, namely, a lumen-apposing metal stent (LAMS), permitted the spread of CDS as the most diffuse approach, although the EUS-HGS possesses the advantage of being a viable option in case of inaccessible papilla for surgically altered anatomy or gastric outlet obstruction.[6,7] However, the diffusion of EUS-HGS is still limited due to the complexity of the procedure with possible severe adverse events (AEs) mainly due to the risk of stent migration.[8]
After the first reports of EUS-HGS with plastic stents, over the years, a self-expandable metal stent (SEMS) has taken over due to the higher stability and longer patency.[9,10] The use of asymmetrical partially covered SEMS (PCSEMS) has further improved the potential of EUS-HGS, due to the transgastric covered portion preventing bile leakage and the intrahepatic uncovered portion aiming to prevent both stent migration and peripherical bile duct obstruction.
The aim of our pilot study was to prospectively assess the technical and clinical outcomes of EUS-HGS using a new dedicated PCSEMS with anti-migratory systems.
METHODS
This is a single-center, prospective, single-arm study enrolling patients with malignant biliary obstruction undergoing EUS-HGS after ERCP. The study protocol was approved by the institutional review board for human research at Humanitas Research and Clinical Center (FIT_29042020; n° Hum_2753) and was registered on clinicaltrial.gov (NCT 04403893). Written informed consent was obtained from all patients before they underwent the endoscopic procedure.[11] The study did not receive any funding.
Inclusion criteria
Patient with malignant biliary obstruction who failed ERCP due to:
- Unreachable papilla for altered anatomy
- Unreachable papilla for duodenal stricture
- Failed biliary cannulation for infiltration of the papilla by neoplastic tissue incomplete BD after ERCP in proximal biliary obstruction.
Exclusion criteria
- Age <18 years old
- Contraindication for an endoscopic procedure or radiologic exposure (i.e. patients on antithrombotic therapy, precluding interventional procedures, and pregnancy)
- Unsigned informed consent form.
Procedure and devices
The procedures were performed by endoscopists with extensive experience in ERCP and both diagnostic and interventional EUS. All procedures were carried out under deep sedation and administered by a dedicated anesthesiologist using an Olympus linear echoendoscope, with CO2 insufflation. Patients were in the left lateral decubitus or in a supine position depending on the anesthesiologist’s preference. Access to the appropriate intrahepatic ductal system was achieved, under combined EUS and fluoroscopic guidance, by puncturing the distal part of the left hepatic duct (segment II or III) with a 19-gauge needle. The access was confirmed by injection of contrast medium. A flexible guidewire measuring 0.025” in diameter and 450 cm in length (VisiGlide II, Olympus) was deployed into the duct of the proposed stent location over which a 6 Fr Cystotome was used to dilate the tract, followed by placement of the dedicated PCSEMS (Hanarostent BPE, M. I. Tech, Korea) [Figure 1]. The stent is either 6, 8, or 10 cm in length, with a diameter of 10 mm. The stent length to be used was selected at the discretion of the endoscopist. The distal (gastric) portion has a flanged covered end with a diameter of 20 mm for anti-migration toward the peritoneum and the liver. The proximal (hepatic) end has a fixed 2-cm uncovered portion with multiple anti-migratory flaps for ensuring the stent anchor reduces the risk of migration toward the stomach. Twelve radiopaque markers on both ends visible on fluoroscopy help to release the stent [Video 1].
Figure 1.
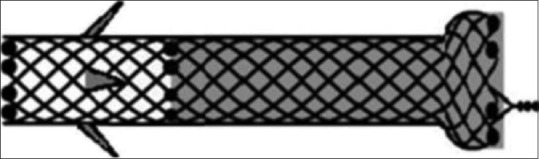
PCSEMS (Hanarostent BPE, M.I. Tech, Korea). PCSEMS: Partially covered self-expandable metal stent
Outcomes
The primary endpoint was the technical success rate defined as the success in deploying the stent confirming the flow of contrast and/or bile into the stomach [Figure 2].
Figure 2.

PCSEMS. Endoscopic view. PCSEMS: Partially covered self-expandable metal stent
Different stent-related technical features, namely, stent loadability, pushability, trackability, deployment accuracy, fluoroscopy visualization, and repositioning capability, were evaluated by the endoscopists at the time of stent positioning on a 3-level scale (high quality, medium quality, and low quality) as compared to currently used fully covered tubular SEMS for EUS-HGS.[12] Clinical success rate, defined as the drop of bilirubin to half the baseline level within 2 weeks from the procedure, procedural time, and procedure-related AEs, defined and graded as per the American Society of Gastrointestinal Endoscopy (ASGE) lexicon,[13] was assessed as secondary outcomes.
Descriptive statistics were used to summarize the baseline characteristics of patients. Continuous variables were expressed as the mean ± standard deviation, whereas qualitative variables were expressed as frequencies and percentages.
RESULTS
From June 2020 to March 2021, 20 patients (15%–68.2% – females; mean age, 66.0 ± 10.0) were enrolled in the study analysis [Figure 3]. The baseline characteristics are provided in Table 1.
Figure 3.

Study flowchart. *Unreachable papilla: 9; incomplete biliary drainage: 5; infiltrated papilla: 4; altered anatomy: 4
Table 1.
Baseline features
| Features | n/total(%) - mean±SD |
|---|---|
| Age | 66.0±10.0 |
| Gender | |
| Female | 15 (68.2) |
| Male | 7 (31.8) |
| Antithrombotics | |
| Anticoagulants | 0 (0) |
| Antiplatelets | 5 (22.7) |
| Etiology | |
| Pancreatic adenocarcinoma | 18 (81.8) |
| Cholangiocarcinoma | 2 (9.1) |
| Gallbladder adenocarcinoma | 1 (4.5) |
| Duodenal adenocarcinoma | 1 (4.5) |
| Reason for ERCP failure | |
| Infiltrated papilla | 4 (18.2) |
| Unreachable papilla | 9 (40.9) |
| Altered anatomy | 4 (18.2) |
| Incomplete biliary drainage | 5 (22.7) |
| Mean diameter of the intrahepatic bile duct punctured (range) | 6.3±1.9 mm (4.3–8.2) |
SD: Standard deviation
Different causes of ERCP failure were infiltration of papilla by neoplastic tissue (4, 18.2%), unreachable papilla for duodenal stricture (9, 40.9%), surgically altered anatomy with Roux-en-Y reconstruction (4, 18.2%), and incomplete BD after transpapillary stent placement (5, 22.7%).
Technical outcomes
Technical success was achieved in all patients, in a mean procedural time of 43.3 ± 26.8 min. Procedural information is provided in Supplementary Table 1. Technical features (stent loadability, pushability, trackability, deployment accuracy, fluoroscopy visualization, and repositioning capability) were graded as high or medium in all cases, and are detailed in Supplementary Table 1.
Supplementary Table 1.
Study results
| Outcomes | n/total(%) - mean±SD |
|---|---|
| Technical outcomes | |
| Technical success | 22/22 (100) |
| Procedural time (min) | 43.3±26.8 |
| Clinical outcomes | |
| Clinical success | 20/22 (91) |
| AEs | 3/22 (13.6) |
| Migration | 0 |
| Misplacement | 0 |
| Bleeding | 0 |
| Hepatic abscess | 3 |
| Death | 0 |
AEs: Adverse events, SD: Standard deviation
Clinical outcomes
The clinical success rate was 91.0% (20/22) in a mean follow-up time of 10.8 ± 3.1 months. The two patients who failed to achieve the expected drop in bilirubin level were referred for right hepatic biliary duct drainage through PTBD, due to the incomplete communication between the two hepatic systems due to hilar neoplastic invasion. The normalization of bilirubin levels was achieved in both patients permitting to begin the oncologic therapy as planned.
There were no cases of stent misplacement or stent migration. Three (13.6%) cases of hepatic abscess graded as moderate for the ASGE lexicon requiring hospital readmission with percutaneous drainage and systemic antibiotics were reported after a mean time of 18.7 ± 2.5 days from the EUS-HGS, with no impact on stent patency and following oncologic treatments. No fatal AEs occurred.
DISCUSSION
According to our pilot study, the benefit/risk ratio of using a new dedicated stent for EUS-guided HGS, is favorable, as shown by the 100% rate of technical success coupled with the absence of intra or postprocedural stent migration.
Our findings are relevant for the following reasons: First of all, although the first description of such technique was proposed in 2001, both the technical challenges and the risk of severe AEs have prevented the diffusion of the procedure, with most of such AE being strongly related to the risk of stent migration.[8,14] As the recent diffusion of EUS-guided BD through CDS was clearly related to the development of dedicated stents, namely, LAMS, the absence of any device specifically thought for EUS-guided HGS has probably represented the main factor preventing to appropriately face the most challenging procedural tasks. In our series, we achieve a successful stent placement in all cases, without any misdeployment, and this may be possibly related to the stent design. Further, once the stents were in place we had no cases of stent migration. Comparing this result with previous studies of EUS-HGS performed with standard tubular biliary stents, the anti-migratory systems of this dedicated stent seem to be effective in preventing the most feared AE, such as perforation, bile leaks, and peritonitis, all related to misplacement or postprocedural stent migration.[8] As a matter of fact, the anti-migratory system works in both directions, with the uncovered extremity and the four flaps preventing the migration of the intrahepatic extremity, and the flanged covered end preventing the migration of the intragastric extremity. It may be argued that three cases of postprocedural hepatic abscesses may not completely reassure the safety profile of the procedure even when performed with a dedicated device. As a matter of fact, the abscesses location (right hepatic lobe, far from the stent intrahepatic end) and the reports of such complication in previous series, suggest it may be not related to the stent itself. However, given most of these patients who failed the standard endoscopic drainage would have been treated through PTBD, we must consider the high risk of infective AEs burdening such approach[15] and often preventing an adequate oncological treatment. In this regard, our patients could undergo the oncologic therapy as planned after being successfully treated with systemic antibiotic and percutaneous drainage.
The second main result of our study is a clinical success rate as high as 90%. This may be even more relevant considering we included a difficult-to-treat population. The two patients with altered anatomy who failed in achieving an adequate drop in bilirubin level were treated with a PTBD approach aimed to drain the right hepatic lobe. On the other hand, the adequate drainage of the left biliary system reached even in these patients put the accent on selecting the patients who can better fit the indication for HGS [Figure 4]. In all cases, an imaging technique aiming to evaluate the biliary tree should be always suggested before the procedure to avoid partial drainage due to not communicating biliary ducts.
Figure 4.
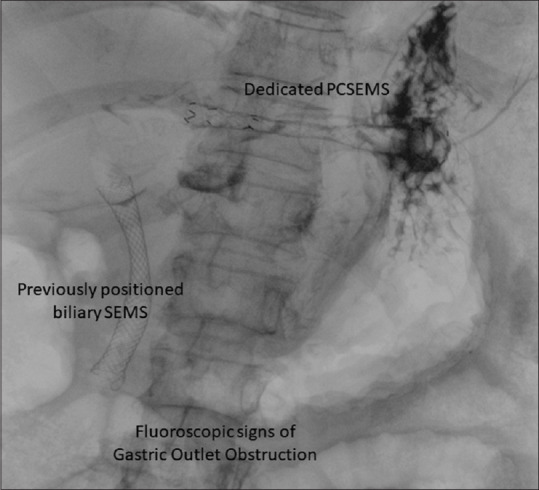
PCSEMS. Fluoroscopic view. PCSEMS: Partially covered self-expandable metal stent
Although its strength, several limitations of this study need to be discussed. The lack of a control group prevents any conclusive statement on using a dedicated stent for EUS-HGS compared to traditional SEMS. Further, the limited sample size was precluded to rule out the risk of rarer AEs. However, the promising outcomes in terms of both technical feasibility and safety may reassure in designing future comparative well-powered studies.
CONCLUSION
EUS-HGS with the new dedicated stent is feasible and effective and prevents stent migration and misplacement. Although the procedural challenges and the persistent risk of AEs unrelated to stent migration, dedicated devices may contribute to outcomes improvement and procedure diffusion.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Supplementary materials
Supplementary information is linked to the online version of the paper on the Endoscopic Ultrasound website.
Video available on: www.eusjournal.com
REFERENCES
- 1.Fugazza A, Troncone E, Amato A, et al. Difficult biliary cannulation in patients with distal malignant biliary obstruction:An underestimated problem? Dig Liver Dis. 2022;54:529–36. doi: 10.1016/j.dld.2021.07.010. [DOI] [PubMed] [Google Scholar]
- 2.Testoni PA, Mariani A, Aabakken L, et al. Papillary cannulation and sphincterotomy techniques at ERCP:European Society of Gastrointestinal Endoscopy (ESGE) clinical guideline. Endoscopy. 2016;48:657–83. doi: 10.1055/s-0042-108641. [DOI] [PubMed] [Google Scholar]
- 3.Anderloni A, Troncone E, Fugazza A, et al. Lumen-apposing metal stents for malignant biliary obstruction:Is this the ultimate horizon of our experience? World J Gastroenterol. 2019;25:3857–69. doi: 10.3748/wjg.v25.i29.3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee TH, Choi JH, Park do H, et al. Similar efficacies of endoscopic ultrasound-guided transmural and percutaneous drainage for malignant distal biliary obstruction. Clin Gastroenterol Hepatol. 2016;14:1011. doi: 10.1016/j.cgh.2015.12.032. [DOI] [PubMed] [Google Scholar]
- 5.Sportes A, Camus M, Greget M, et al. Endoscopic ultrasound-guided hepaticogastrostomy versus percutaneous transhepatic drainage for malignant biliary obstruction after failed endoscopic retrograde cholangiopancreatography:A retrospective expertise-based study from two centers. Therap Adv Gastroenterol. 2017;10:483–93. doi: 10.1177/1756283X17702096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amato A, Sinagra E, Celsa C, et al. Efficacy of lumen-apposing metal stents or self-expandable metal stents for endoscopic ultrasound-guided choledochoduodenostomy:A systematic review and meta-analysis. Endoscopy. 2021;53:1037–47. doi: 10.1055/a-1324-7919. [DOI] [PubMed] [Google Scholar]
- 7.Anderloni A, Fugazza A, Troncone E, et al. Single-stage EUS-guided choledochoduodenostomy using a lumen-apposing metal stent for malignant distal biliary obstruction. Gastrointest Endosc. 2019;89:69–76. doi: 10.1016/j.gie.2018.08.047. [DOI] [PubMed] [Google Scholar]
- 8.Hedjoudje A, Sportes A, Grabar S, et al. Outcomes of endoscopic ultrasound-guided biliary drainage:A systematic review and meta-analysis. United European Gastroenterol J. 2019;7:60–8. doi: 10.1177/2050640618808147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ogura T, Yamada M, Nishioka N, et al. One-step stent deployment of EUS-guided hepaticogastrostomy using a novel covered metal stent with a fine-gauge stent delivery system (with video) Endosc Ultrasound. 2020;9:267–9. doi: 10.4103/eus.eus_38_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakai Y, Sato T, Hakuta R, et al. Long-term outcomes of a long, partially covered metal stent for EUS-guided hepaticogastrostomy in patients with malignant biliary obstruction (with video) Gastrointest Endosc. 2020;92:623–31. doi: 10.1016/j.gie.2020.03.3856. e1. [DOI] [PubMed] [Google Scholar]
- 11.Spadaccini M, Binda C, Fugazza A, et al. Informed consent for endoscopic biliary drainage:Time for a New Paradigm. Medicina (Kaunas) 2022;58:331. doi: 10.3390/medicina58030331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cotton PB, Eisen GM, Aabakken L, et al. Alexicon for endoscopic adverse events:Report of an ASGE workshop. Gastrointest Endosc. 2010;71:446–54. doi: 10.1016/j.gie.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 13.Hathorn KE, Canakis A, Baron TH. EUS-guided transhepatic biliary drainage:A large single-center U. S. experience. Gastrointest Endosc. 2022;95:443–51. doi: 10.1016/j.gie.2021.10.013. [DOI] [PubMed] [Google Scholar]
- 14.Ochiai K, Fujisawa T, Ishii S, et al. Risk factors for stent migration into the abdominal cavity after endoscopic ultrasound-guided hepaticogastrostomy. J Clin Med. 2021;10:3111. doi: 10.3390/jcm10143111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta P, Maralakunte M, Rathee S, et al. Percutaneous transhepatic biliary drainage in patients at higher risk for adverse events:Experience from a tertiary care referral center. Abdom Radiol (NY) 2020;45:2547–53. doi: 10.1007/s00261-019-02344-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


