ABSTRACT
Background and Objectives:
EUS-guided biliary drainage (EUS-BD) is a promising alternative to ERCP in malignant distal biliary obstruction (MDBO). Despite accumulating data, however, its application in clinical practice has been impeded by undefined barriers. This study aims to evaluate the practice of EUS-BD and its barriers.
Methods:
An online survey was generated using Google Forms. Six gastroenterology/endoscopy associations were contacted between July 2019 and November 2019. Survey questions measured participant characteristics, EUS-BD in different clinical scenarios, and potential barriers. The primary outcome was the uptake of EUS-BD as a first-line modality, without previous ERCP attempts, in patients with MDBO.
Results:
Overall, 115 respondents completed the survey (2.9% response rate). Respondents were from North America (39.2%), Asia (28.6%), Europe (20%), and other jurisdictions (12.2%). Regarding the uptake of EUS-BD as first-line treatment for MDBO, only 10.5% of respondents would consider EUS-BD as a first-line modality regularly. The main concerns were the lack of high-quality data, fear of adverse events, and limited access to EUS-BD dedicated devices. On multivariable analysis, lack of access to EUS-BD expertise was an independent predictor against the use of EUS-BD, odds ratio 0.16 (95% confidence interval, 0.04–0.65). In salvage situations following failed ERCP, most favored EUS-BD (40.9%) over percutaneous drainage (21.7%) in unresectable cancer. In borderline resectable or locally advanced disease, however, most favored the percutaneous approach due to fear of EUS-BD complicating future surgery.
Conclusions:
EUS-BD has not reached widespread clinical adoption. Identified barriers include lack of high-quality data, fear of adverse events, and lack of access to EUS-BD dedicated devices. Fear of complicating future surgery was also identified as a barrier in potentially resectable disease.
Keywords: EUS, EUS-guided biliary drainage, stent, malignant biliary obstruction, pancreatic cancer, therapeutic EUS
INTRODUCTION
The endoscopic decompression of malignant distal biliary obstruction (MDBO) has historically been achieved through ERCP. Due in part to the requirement for transpapillary access, the performance of ERCP is associated with a significant risk of adverse events. These include post-ERCP pancreatitis (5%–15%)[1,2,3] and stent dysfunction due to tumor tissue ingrowth or overgrowth (20%–40%).[3,4,5,6] Stent dysfunction is a particular concern for this typically frail patient population, as it has the potential to lead to cholangitis, delays in chemotherapy, risks associated with repeat interventions, and prolonged recurrent hospitalizations, all associated with significant cost burdens to the health-care system.[7]
EUS-guided biliary drainage (EUS-BD) is an emerging technique that was first described as a rescue modality in cases where ERCP failed to achieve decompression of MDBO.[8] This endoscopic transluminal approach is especially attractive in cases of MDBO, since a biliary bypass is created with a stent through a choledochoduodenostomy from the duodenal bulb or through a hepatogastrostomy from the stomach, thereby limiting the risk for stent dysfunction secondary to tumor tissue ingrowth or overgrowth. As such, this technique has gained considerable traction not only as a rescue modality but also as a method of first intent.
Early randomized controlled trials (RCTs) comparing EUS-BD with ERCP as a method of first intent in patients with MDBO using non-EUS dedicated devices reported no significant differences in technical success, clinical response, and adverse events.[9,10] Recently, more robust data from a larger RCT demonstrated excellent technical and clinical success rates of >90% with both EUS-BD and ERCP.[3] Furthermore, EUS-BD was associated with lower rates of stent dysfunction needing re-intervention.[3] A meta-analysis of available evidence has largely confirmed these findings.[11]
Despite the accumulating evidence in support of EUS-BD, clinical adoption has lagged in both the setting of first-intent BD or as a salvage modality following ERCP failure. EUS-BD remains in the development phase (phase 2a) of a technology’s lifecycle,[12] where the procedure is performed in only a few select patients by a few select experts. The American Society for Gastrointestinal Endoscopy (ASGE) has identified EUS-BD as a priority research area for over 10 years without considerable progress being made in clinical practice.[13] Therefore, we performed an international survey to identify the barriers to the implementation of EUS-BD in clinical practice. This step is essential in identifying potential areas to target for successful knowledge translation of EUS-BD.
METHODS
The Checklist for Reporting Results of Internet E-Surveys criteria were used to present the results of our survey.[14]
Study design
A cross-sectional international survey study was conducted. The target population consisted of gastroenterologists and surgeons with an active practice, regardless of subspecialty, or experience. The survey was developed through discussion and consensus between three gastroenterologists (Y. C., A. B., N. F.). The study protocol and survey were approved by the McGill Institutional Research Ethics Board. No identifiable information was collected or stored during the survey.
Recruitment process
Six gastroenterology, endoscopy, and surgical associations were contacted between July 2019 and November 2019. These associations were asked to distribute our online survey to their members using any combination of their mailing list (s), newsletter (s), and/or social media platforms (including Twitter and Facebook). Survey participation was voluntarily. The survey was described as taking a total of 5 min to complete. No monetary or nonmonetary incentives were provided for survey completion.
Survey content
We used Google Forms to administer an online questionnaire comprising 22 questions [Appendix 1]. The questions were listed in the same order for all participants. Participants were able to return to previous pages to modify their answers. The survey was only accessible through the link provided to the contacted associations. Survey response was anonymous. The primary outcome of interest was the response regarding the practice of performing EUS-BD of first intent (without initially attempting ERCP) in patients with MDBO. Secondary outcomes included the response regarding the practice of performing EUS-BD in MDBO of different tumor stages in the salvage setting following failed ERCP as well as the response regarding both the perceived feasibility of, and barriers to, EUS-BD of first intent.
The survey collected information on participant demographics and practice setting data. The latter included the following: country of practice; medical specialty; years in practice; private, academic, or mixed practice setting; training specialty; number of yearly ERCP performed; whether the participants performed or had access to EUS-BD at their institution; and whether participants had formal training in EUS-BD.
Statistical analysis
We used descriptive analyses with frequency and percentages to describe the participants’ characteristics and their answers. To examine the association between participant characteristics and the likelihood of that participant having adopted EUS-BD as a first-line modality at the time of survey response, we used a standard stepwise multivariate analysis. Variable included in the univariate analysis included age, gender, location of practice, specialty, years in practice, type of practice, access to EUS-BD expertise, whether the respondent performs ERCP, whether the respondent performs EUS-BD, and whether the respondent had formal EUS-BD training. A P ≤ 0.05 was considered statistically significant. For all statistical analyses, SPSS 25 was used.
RESULTS
Overall, 115 respondents completed the survey, corresponding to a 2.9% response rate (number of responses/total number of members from each society). With respect to respondent demographics, 54.8% were 45 years of age and younger and 15.7% were female [Table 1]. The geographical distribution of respondents was as follows 39.2% from North America, 28.6% from Asia, 20% from Europe, 11.3% from Australia/New Zealand, and 0.9% from other locations. The majority of respondents were gastroenterologists with therapeutic endoscopy training (67.8%); the others included gastroenterologists without therapeutic endoscopy expertise (25.2%) as well as surgeons, internists, or radiologists (7%). Survey results revealed that 56.5% of participants had <10 years of independent practice, with most working in an academic center (53%). In terms of participants’ ERCP experience, 22.6% performed more than 250 ERCPs per year, 27% performed between 100 and 250 ERCPs per year, 18.3% performed fewer than 100 ERCPs per year, and 32.2% did not perform any ERCPs. The majority (67.8%) of respondents had EUS-BD expertise at their institution. A total of 47.8% of the participants performed EUS-BD in clinical practice. Most respondents performing EUS-BD had low-modest case volumes, with 58.2% performing <10 cases per year and 32.8% performing <20 cases per year. Among those performing EUS-BD, only 27.8% had received formal training in the procedure.
Table 1.
Respondents’ characteristics
| Characteristics | n (%) (n=115) |
|---|---|
| Gender | |
| Female | 18 (15.7) |
| Male | 97 (84.3) |
| Geography | |
| North America | 45 (39.2) |
| Asia | 33 (28.6) |
| Europe | 23 (20) |
| Australia/New Zealand | 13 (11.3) |
| Other | 1 (0.9) |
| Specialty | |
| Gastroenterology with therapeutic endoscopy training | 78 (67.8) |
| Gastroenterology | 29 (25.2) |
| Surgery | 6 (5.2) |
| Other | 2 (1.8) |
| Practice setting | |
| Academic | 61 (53) |
| Community | 20 (17.4) |
| Private | 5 (4.3) |
| Mixed | 29 (25.2) |
| Respondents’ ERCP volume (cases/year) | |
| >250 | 26 (22.6) |
| <250 | 52 (45.3) |
| None | 37 (32.2) |
| Respondents’ EUS-BD volume (cases/year) | |
| >20 | 5 (4.3) |
| 10–20 | 18 (15.7) |
| <10 | 32 (27.8) |
| None | 60 (52.2) |
EUS-BD: EUS guided biliary drainage
In terms of the participants’ impressions of the technical and clinical outcomes of EUS-BD, 81.7% considered EUS-BD to have either equivalent or better technical and clinical success than ERCP, with 38.2% considering EUS-BD to be associated with a shorter procedure time. Most respondents (85.2%) also felt that EUS-BD was either equivalent or superior to ERCP regarding their rates of stent occlusion and re-intervention. Finally, the majority of participants (86.1%) felt that EUS-BD was associated with lower risks for postprocedural pancreatitis when compared to ERCP [Figure 1].
Figure 1.
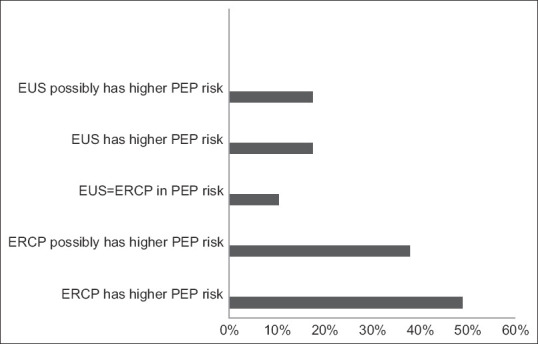
Perceived risk of pancreatitis with EUS-biliary drainage vsersus ERCP
For the primary endpoint of responses regarding the uptake of EUS-BD as first-line treatment for MDBO (without prior ERCP attempts), only 10.5% of respondents would consider EUS-BD as a first-line modality regularly, 83.5% of respondents answered that they would either never perform EUS-BD as a primary modality for MDBO or would do so only in very select cases, while 6.1% had no opinion on the matter [Figure 2]. The three major concerns regarding EUS-BD as a first-line modality were the lack of high-quality data to support such practice (40.9%), fear of adverse events (36.5%), and limited access to EUS or dedicated EUS-BD devices (29.6%) [Figure 3]. On multivariable analysis, lack of local access to EUS-BD expertise was an independent predictor against the use of EUS-BD as a first-line modality in MDBO, with an adjusted odds ratio of 0.16 (95% confidence interval, confidence interval, 0.04–0.65) [Tables 2 and 3].
Figure 2.
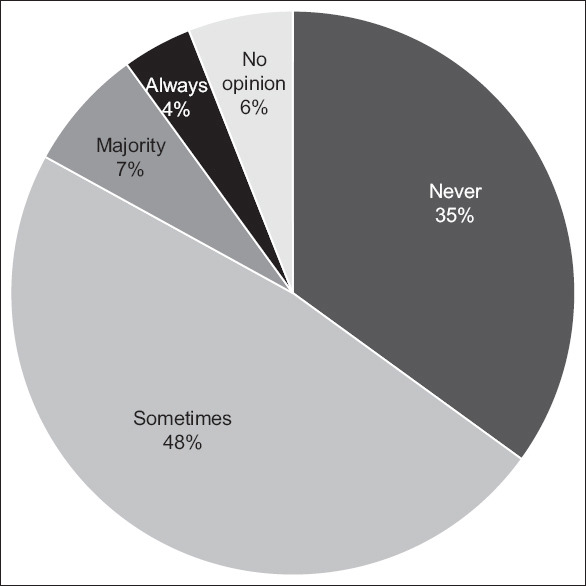
EUS-guided biliary drainage as a first-line modality without previous ERCP attempts in malignant distal biliary obstruction
Figure 3.
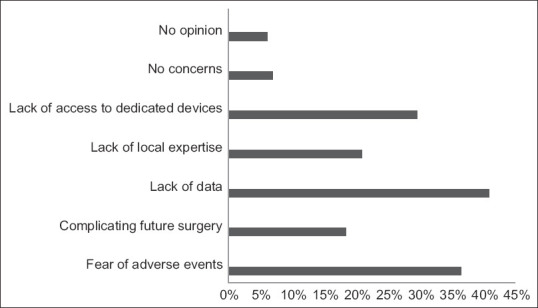
Concerns regarding EUS-guided biliary drainage as a first-line modality
Table 2.
Characteristics of respondents who consider EUS-biliary drainage as a first-line modality vs. respondents who generally does not consider EUS-biliary drainage as a first-line treatment
| Variable | Considers EUS-BD as a first-line treatment, n (%) | Does not consider EUS-BD as a first-line treatment, n (%) | P |
|---|---|---|---|
| Age ≤55 | 10 (83.3) | 38 (86.5) | 0.77 |
| Female | 3 (25.0) | 11 (11.5) | 0.19 |
| Geography | |||
| North Americac | 1 (8.3) | 41 (42.7) | <0.01 |
| Europe | 1 (8.3) | 20 (20.8) | |
| Asia | 9 (75.0) | 23 (24.0) | |
| Other | 1 (8.3) | 12 (12.5) | |
| Formal training in therapeutic endoscopy | 7 (58.3) | 68 (70.8) | 0.38 |
| Years in practice <11 | 9 (75.0) | 52 (54.2) | 0.17 |
| Academic center | 3 (25.0) | 56 (58.3) | 0.03 |
| Performs ERCP | 6 (50.0) | 71 (74.0) | 0.10 |
| Access to EUS-BD expertise | 3 (25.0) | 72 (75.0) | <0.01 |
| Performs EUS-BD | 3 (25.0) | 51 (53.1) | 0.07 |
| Formal EUS-BD training | 4 (33.3) | 27 (28.1) | 0.74 |
EUS-BD: EUS-guided biliary drainage
Table 3.
Multivariable analysis of independent predictors of EUS-biliary drainage as a first-line modality in malignant distal biliary obstruction
| Effect | OR estimates | |
|---|---|---|
|
| ||
| Point estimate | 95% Wald confidence limits | |
| North America vs. other | 0.309 | 0.028–3.375 |
| Academic center vs. nonacademic center | 0.510 | 0.102–2.539 |
| Lack of access to EUS-BD expertise | 0.155 | 0.037–0.648 |
OR: Odds ratio; EUS-BD: EUS-guided biliary drainage
When considering EUS-BD as a salvage modality following failed ERCP in unresectable MDBO, many participants favored EUS-BD (40.9%) over percutaneous drainage (21.7%), while 35.6% favored a rendezvous approach. In patients with borderline or locally advanced MDBO undergoing neoadjuvant chemotherapy of curative intent, fewer respondents indicated they would opt for EUS-BD following ERCP failure (22.6%), with the majority favoring either a percutaneous or rendezvous approach. The perceived potential for complicating future curative surgeries (36.5%) and potential adverse events (32.2%) were identified by participants as the main concerns limiting their choice of EUS-BD in patients with borderline resectable or locally advanced disease.
DISCUSSION
In this cross-sectional international survey, we aimed to identify barriers to the clinical adoption of EUS-BD. Overall, there were 115 respondents, most of whom were gastroenterologists with training in advanced therapeutic endoscopy. Consistent with recently published data,[3,9,10,11] the majority of participants felt that EUS-BD could achieve similar technical and clinical success as ERCP while being associated with lower risks for stent dysfunction and postprocedural pancreatitis. Nevertheless, the adoption of EUS-BD in clinical practice is limited. Overall, 67.8% of participants had access to EUS-BD expertise which, when compared to the availability of interventional radiology or ERCP, is far from being universally available. Furthermore, among respondents who performed EUS-BD, the vast majority (91%) performed fewer than 20 cases per year. In terms of EUS-BD as a potential first-line treatment for MDBO, only 10.5% of the respondents would consider its use in the majority of patients. On multivariable analysis, lack of EUS-BD expertise is identified as an independent predictor against the use of EUS-BD as a first-line modality. Even in salvage situations following ERCP failure, only 40.9% and 22.6% would consider EUS-BD as the next step in the management in unresectable and borderline resectable/locally advanced disease, respectively. Therefore, despite 20 years since its first description and having been identified as a research priority by the ASGE for over 10 years with accumulating data, EUS-BD remains far from “primetime” and is stuck in the exploration phase (2b) of a technology’s lifecycle where the procedure is performed by a few experts in a few select patients. The three major barriers of clinical adoption for EUS-BD as a primary modality identified in our study are the lack of high-quality data, fear of adverse events, and lack of access to EUS or EUS-BD dedicated devices. EUS biliary stents such as the lumen apposing metal stents are also very expensive likely further limiting access to dedicated devices. An additional concern that was identified for patients undergoing neoadjuvant chemotherapy of curative intent was the fear of EUS-BD complicating future potential surgeries.
Novel technologies typically follow a life cycle of development: stage 1 (innovation), stage 2a (development), stage 2b (exploration), stage 3 (assessment), and stage 4 (long term).[12] Widespread clinical adoption does not occur until stages 3 and 4 of this cycle. One of the major barriers potentially preventing EUS-BD from progressing past stage 2b is the arguably suboptimal technology development achieved in stage 1. Specifically, our results identified the lack of dedicated devices for EUS-BD as a major barrier. Currently, EUS-BD is technically demanding and taxing; in fact, they are mostly performed using devices designed for ERCP. Although dedicated stents have been developed in Asia,[3] none have gained widespread availability outside of select centers. A cautery-assisted lumen apposing metal stent dedicated for EUS-guide BD (Axios, Boston Scientific Inc., Marlborough, MA, USA) has recently been introduced. These stents measure 6 or 8 mm in diameter and can be inserted directly from the duodenum to the mid common bile duct using EUS guidance and cautery assistance without the need for wire guidance or tract dilation. While retrospective data suggest the great ease, safety, and clinical efficacy of these stents, its role as a potentially disruptive technology remains to be confirmed through an ongoing RCT by our group.[15]
Lack of high-quality data and fear of adverse events were also identified as major barriers to EUS-BD implementation. Most studies on EUS-BD have been retrospective in design from expert centers. There are currently only three RCTs comparing EUS-BD to ERCP as a method of first intent in the decompression of MDBO, with only one of them being sufficiently powered to detect a major clinical primary endpoint.[3,9,10] Importantly, none of the studies used a readily available dedicated device for EUS-BD (most used ERCP stents or specialized stents only available in Asia). In addition, all three trials were designed and executed by world leaders in this technique, limiting their generalizability. No studies were adequately powered to detect differences in adverse events between EUS-BD and ERCP, and there are no published prospective registries for EUS-BD. Another major concern identified in our survey is the lack of data on EUS-choledochoduodenostomy or EUS-hepatogastrostomy and their potential effects on subsequent potentially curative surgeries. Theoretically, EUS-guided choledochoduodenostomy should not affect subsequent pancreaticoduodenectomy, and this statement is supported by data from a handful of patients in a small RCT[9] as well as anecdotal experience from expert centers. However, there are no data on the potential effects of EUS-hepatogastrostomy on subsequent surgery.
The major limitation to our study is the low response rate of 2.9%. This low response rate is likely due to the subspecialized nature of pancreaticobiliary therapeutic endoscopy. Unlike other gastroenterology subspecialties such as inflammatory bowel disease or functional disease, most general gastroenterologists or surgeons are not involved with pancreaticobiliary therapeutic endoscopy and are thus unlikely to answer a survey on this topic. This low response rate creates a risk for significant selection bias. Any bias, however, likely favors the use of EUS-BD given that the majority of respondents are therapeutic endoscopists with EUS-BD experience. As such, the availability and adoption of EUS-BD in clinical practice are likely less than those reported in our survey. Even in this “best case scenario,” our study plainly demonstrates that EUS-BD is currently not considered a first-line modality. In addition, EUS-BD is also not the clear frontrunner as a salvage modality with many still preferring the percutaneous approach even in a survey of largely therapeutic endoscopists.
CONCLUSIONS
Despite being a research priority with accumulating evidence on efficacy and safety, the adoption of EUS-BD in clinical practice remains limited. Our survey has identified three major barriers to the use of EUS-BD as a first-line modality including lack of high-quality data, fear of adverse events, and lack of access to EUS-BD dedicated devices. In potentially resectable diseases, fear of complicating future surgery was also identified as a barrier. High-quality RCTs with dedicated EUS-BD stents, data on surgical outcomes, further technology development of EUS-BD dedicated devices, and cost-effectiveness analysis are therefore needed to clarify the role of EUS-BD in MDBO.
Financial support and sponsorship
Yen-I Chen received salary support from the Fonds de la Recherche en Santé du Québec for his technology assessment program in pancreaticobiliary endoscopy, which includes this survey study.
Conflicts of interest
Yen-I Chen is a consultant for Boston Scientific. Alan Barkun is a consultant for Pendopharm Inc., Boston Scientific Inc., Olympus Inc., Cook Inc., and ATGen Inc. Gurpal Sandha is a consultant for Boston Scientific. Anand Sahai is a consultant for Boston Scientific and Pentax. Sarto Paquin is a consultant for Boston Scientific. Anand Sahai is a Honorary Editor-in-Chief of the journal. This article was subject to the journal’s standard procedures, with peer review handled independently of the editor and his research group.
APPENDIX
Appendix 1: Survey Questionnaire
Role of EUS biliary drainage in malignant distal biliary obstruction: an internationally survey involving gastroenterologists and surgeons.
1. Age
< 36
36-45
46-55
56-65
>65
2. Gender
Female
Male
3. Where do you practice?
United States
Canada
Central/South America
Western Europe
Eastern Europe
Asia
Africa
Australia/New Zealand
4. What is your specialty:
General surgery
Gastroenterology
Hepatobiliary surgery
Radiology
5. Years in practice:
< 5 years
5-10 years
11-15 years
16-20 years
>20 years
6. Type of practice:
Academic
Community practice
Private
Mixed
7. Do you perform ERCP?
No
Yes, < 100 cases/year
Yes, 100-250 cases/year
Yes, > 250 cases/year
8. Do you perform EUS biliary drainage for malignant biliary obstruction
No
Yes <10 cases/year
Yes between 10 AND 20 cases/year
Yes >20 cases/year
9. If no to answer 2 do you have access to EUS BD services at your institutions
Yes
No
10. In patients with unresectable malignant distal biliary obstruction without concomitant gastric outlet obstruction, what is your next step for biliary drainage if failure with ERCP?
Percutaneous biliary drainage
Rendezvous procedure via the percutaneous route
Rendezvous via EUS
EUS guided biliary drainage via a choledochoduodenostomy (stent created track between the common bile duct and duodenal bulb) or hepatogastrostomy (stent created tract between the left intrahepatic bile duct BII/III and the stomach)
11. In patients with borderline resectable pancreatic cancer undergoing neoadjuvant chemotherapy with curative intent with distal biliary obstruction, what is your next step for biliary drainage if failure with ERCP?
EUS biliary drainage with a choledochoduodenostomy (stent created track between the common bile duct and duodenal bulb)
Rendezvous through the percutaneous route
Rendezvous through the EUS route
Percutaneous biliary drainage
12. Overall, do you consider EUS biliary drainage (BD) to be a good alternative in case of ERCP failure in unresectable distal malignant biliary obstruction?
Never
Sometimes
In the majority of cases
Always
No opinion
13. What is your main concern if any regarding EUS BD in unresectable distal malignant biliary obstruction
Lack of local expertise
Fear for adverse events
Limited access to EUS or devices for EUS BD
Lack of high quality data to support this practice
No concerns
14. Overall, do you consider EUS biliary drainage with a choledochoduodenostomy (stent created track between common bile duct and duodenal bulb) as a good alternative in case of ERCP failure in borderline resectable distal malignant biliary obstruction?
Never
Sometimes
In the majority of cases
Always
No opinion
15. What is your main concern if any regarding EUS BD through a choledochoduodenostomy in borderline resectable disease?
Fear of complicating eventual Whipple surgery with the choledochoduodenostomy approach
Fear for adverse events
Limited access to EUS or devices for EUS BD
Lack of high quality data to support this practice
Lack of local expertise
16. Overall, would you consider EUS BD as a first line drainage approach without previous ERCP attempts in malignant distal biliary obstruction
Never
Sometimes
In the majority of cases
Always
No opinion
17. What is your main concern if any regarding EUS BD as a first line modality
Lack of local expertise
Limited data to support this practice
Fear of adverse events
Limited access to EUS or EUS BD devices
18. For those who perform EUS BD what patient population do you include
Unresectable patients only
Both unresectable and borderline resectable patient undergoing neoadjuvant therapy with curative intent
No comment as I do not perform EUS BD
19. For those who perform EUS BD what is your preferred approach in unresectable distal malignant biliary obstruction (what do you attempt first?)
EUS guided hepatogastrostomy
EUS guided choledochoduodenostomy
Anterograde stenting through the papilla
Rendezvous
No comment as I do not perform EUS BD
20. For those who perform EUS BD what is your preferred stent for hepatogastrostomy
PCSEMS (ERCP stent)
FCSEMS (ERCP stent)
Specialized tubular dedicated EUS BD stent
No comment as I do not perform EUS BD
21. For those who perform EUS BD what is your preferred stent for choledochoduodenostomy
PCSEMS (ERCP stent)
FCSEMS (ERCP stent)
Lumen apposing metal stent (LAMS) 6 x 8 mm
Lumen apposing metal stent (LAMS) 8 x 8mm
Specialized tubular dedicated EUS BD stent (Non LAMS)
No comment as I do not perform EUS BD
22. For those who perform EUS BD, what is your main challenge in disseminating this technique
Limited access to EUS or devices due to cost
Discomfort towards the technical aspects of the procedure
Lack of EUS BD dedicated devices
Lack of surgeon buy in and support
Lack of high quality data support this practice
Lack of adequate training for trainees
REFERENCES
- 1.Freeman ML, Nelson DB, Sherman S, et al. Complications of endoscopic biliary sphincterotomy. N Engl J Med. 1996;335:909–18. doi: 10.1056/NEJM199609263351301. [DOI] [PubMed] [Google Scholar]
- 2.Kochar B, Akshintala VS, Afghani E, et al. Incidence, severity, and mortality of post-ERCP pancreatitis:A systematic review by using randomized, controlled trials. Gastrointest Endosc. 2015;81:143–9. doi: 10.1016/j.gie.2014.06.045. e9. [DOI] [PubMed] [Google Scholar]
- 3.Paik WH, Lee TH, Park DH, et al. EUS-guided biliary drainage versus ERCP for the primary palliation of malignant biliary obstruction:A multicenter randomized clinical trial. Am J Gastroenterol. 2018;113:987–97. doi: 10.1038/s41395-018-0122-8. [DOI] [PubMed] [Google Scholar]
- 4.Almadi MA, Barkun A, Martel M. Self-expandable metal stents versus plastic stents for malignant biliary obstruction. Gastrointest Endosc. 2016;83:852–3. doi: 10.1016/j.gie.2015.10.035. [DOI] [PubMed] [Google Scholar]
- 5.Almadi MA, Barkun JS, Barkun AN. Stenting in malignant biliary obstruction. Gastrointest Endosc Clin N Am. 2015;25:691–711. doi: 10.1016/j.giec.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Gardner TB, Spangler CC, Byanova KL, et al. Cost-effectiveness and clinical efficacy of biliary stents in patients undergoing neoadjuvant therapy for pancreatic adenocarcinoma in a randomized controlled trial. Gastrointest Endosc. 2016;84:460–6. doi: 10.1016/j.gie.2016.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barkun AN, Adam V, Martel M, et al. Partially covered self-expandable metal stents versus polyethylene stents for malignant biliary obstruction:A cost-effectiveness analysis. Can J Gastroenterol Hepatol. 2015;29:377–83. doi: 10.1155/2015/743417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giovannini M, Moutardier V, Pesenti C, et al. Endoscopic ultrasound-guided bilioduodenal anastomosis:A new technique for biliary drainage. Endoscopy. 2001;33:898–900. doi: 10.1055/s-2001-17324. [DOI] [PubMed] [Google Scholar]
- 9.Bang JY, Navaneethan U, Hasan M, et al. Stent placement by EUS or ERCP for primary biliary decompression in pancreatic cancer:A randomized trial (with videos) Gastrointest Endosc. 2018;88:9–17. doi: 10.1016/j.gie.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 10.Park JK, Woo YS, Noh DH, et al. Efficacy of EUS-guided and ERCP-guided biliary drainage for malignant biliary obstruction:Prospective randomized controlled study. Gastrointest Endosc. 2018;88:277–82. doi: 10.1016/j.gie.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 11.Miller CS, Barkun AN, Martel M, et al. Endoscopic ultrasound-guided biliary drainage for distal malignant obstruction:A systematic review and meta-analysis of randomized trials. Endosc Int Open. 2019;7:E1563–73. doi: 10.1055/a-0998-8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barkun JS, Aronson JK, Feldman LS, et al. Evaluation and stages of surgical innovations. Lancet. 2009;374:1089–96. doi: 10.1016/S0140-6736(09)61083-7. [DOI] [PubMed] [Google Scholar]
- 13.Holt BA, Hawes R, Hasan M, et al. Biliary drainage:Role of EUS guidance. Gastrointest Endosc. 2016;83:160–5. doi: 10.1016/j.gie.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 14.Eysenbach G. Improving the quality of Web surveys:The Checklist for Reporting Results of Internet E-Surveys (CHERRIES) J Med Internet Res. 2004;6:e34. doi: 10.2196/jmir.6.3.e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen YI, Callichurn K, Chatterjee A, et al. ELEMENT TRIAL:Study protocol for a randomized controlled trial on endoscopic ultrasound-guided biliary drainage of first intent with a lumen-apposing metal stent vs. endoscopic retrograde cholangio-pancreatography in the management of malignant distal biliary obstruction. Trials. 2019;20:696. doi: 10.1186/s13063-019-3918-y. [DOI] [PMC free article] [PubMed] [Google Scholar]


