ABSTRACT
Background and Objectives:
Gastric varices (GV) with spontaneous portosystemic shunt (SPSS) are associated with ectopic embolism in endoscopic cyanoacrylate. This study targeted to assess the efficacy and safety of EUS-guided coil embolization combined with endoscopic cyanoacrylate injection versus balloon-occluded retrograde transvenous obliteration (BRTO) for GV with high-risk ectopic embolism.
Materials and Methods:
We retrospectively analyzed six tertiary hospitals’ 104 patients with GV at high-risk ectopic embolism (the narrowest diameter of SPSS was greater than or equal to 5 mm and the maximum diameter usually >8 mm) who underwent EUS-guided coil embolization combined with endoscopic cyanoacrylate injection or BRTO from January 2014 to December 2020. The outcomes included rebleeding, survival, and complications.
Results:
The EUS group and BRTO group contained 59 and 45 patients, respectively. The technical success rate between the two groups was similar (96.6% vs. 95.6%, P = 1.000). During the follow-up, both groups’ 5-day rebleeding rate and 6-week mortality rate were 0%. One-year all-cause rebleeding rate (20.0% vs. 18.9%, P = 0.900) and 1-year mortality rate (2.0% vs. 0%, P = 1.000) in the EUS group were similar to the BRTO group. One patient experienced ectopic embolism in the EUS group, while the BRTO group did not. Both groups had similar mean days (16.0 [interquartile range (IQR), 12.0–19.0] vs. 16.5 [IQR, 11.8–26.0], P = 0.165) and cost of hospitalization (¥ 45950.6 [IQR, 39330.2–55768.2] vs. ¥ 51205.8 [IQR, 31628.8–74251.5], P = 0.680). Multivariate analysis showed that the narrowest diameter of the shunt (odds ratio [OR] = 1.86; 95% confidence interval [CI]: 1.062–3.258; P = 0.03) and content of hemoglobin (OR = 0.941; 95% CI: 0.892–0.992; P = 0.025) were the prognostic factors for survival.
Conclusions:
The efficacy and safety of EUS-guided coil embolization combined with endoscopic cyanoacrylate injection for GV with high-risk ectopic embolism are comparable to BRTO.
Keywords: Balloon-occluded retrograde transvenous obliteration, coil, EUS, gastric varices, spontaneous portosystemic shunt
INTRODUCTION
With the progress of portal hypertension and the establishment of collateral circulation, approximately 60% of patients with liver cirrhosis develop spontaneous portosystemic shunt (SPSS).[1] Gastrorenal shunt (GRS) and splenorenal shunt (SRS) are the most common types and they are compensatory pathophysiological changes formed under the condition of portal hypertension. SPSS also prominently decreases hepatic portal venous perfusion when it reduces the portal pressure. At the same time, it is prone to cause portal vein thrombosis[2,3,4,5] and increase the risk of hepatic encephalopathy (HE). When the SPSS’s narrowest diameter is greater than or equal to 5 mm and its maximum diameter usually >8 mm, it was at high-risk ectopic embolism in traditional endoscopic cyanoacrylate injection.
The current treatments for GV with GRS or SRS mainly include endoscopic cyanoacrylate injection, transjugular intrahepatic portosystemic shunt (TIPS), balloon-occluded retrograde transvenous obliteration (BRTO) and EUS-guided coil embolization and/or cyanoacrylate injection. However, there are no criteria or guidelines. Endoscopic cyanoacrylate injection is one of the first-line therapy recommended by the guidelines and expert consensus for primary and secondary prophylaxis treatment of GV bleeding and control of acute bleeding.[6,7] However, the existence of GRS and SRS will greatly increase the risk of ectopic embolism, among the serious adverse events of endoscopic cyanoacrylate injection.[8,9,10,11] When GV merges with GRS or SRS, it is advisable to cautiously use traditional endoscopic cyanoacrylate injection. TIPS is a safe and effective alternative of portal hypertension. It can significantly reduce the portal pressure in patients with liver cirrhosis and portal hypertension and basically alleviate the complications caused by portal hypertension, such as ascites and variceal bleeding. However, the hepatic portal venous perfusion decreases after TIPS, which makes it more vulnerable to HE and deterioration of liver function.[12,13]
Conventional BRTO is a proven safe and effective method of treating GV with SPSS, which GV is approached with an occlusive balloon from the systemic veins via GRS or SRS. American Association for the Study of Liver Diseases (AASLD) recommends BRTO for secondary prophylaxis of gastric variceal bleeding, especially in patients with SPSS.[14]
EUS-guided coil and cyanoacrylate injection is an effective approach for GV which notably reduces the risk of ectopic embolism and increase the rate of complete vascular embolization. While these potential advantages of EUS-guided treatment of GV are proven, more studies are needed to confirm the efficacy and safety of EUS-guided coil embolization and cyanoacrylate injection for patients with SPSS.
Until the present time, studies comparing EUS-guided coil embolization combined with endoscopic cyanoacrylate injection with BRTO in the treatment of GV with SPSS were few. This study is a multicenter retrospective cohort study to compare and evaluate the efficacy and safety of EUS-guided coil embolization combined with endoscopic cyanoacrylate injection versus BRTO for GV with high-risk ectopic embolism, defined as the SPSS’s narrowest diameter is greater than or equal to 5 mm and its maximum diameter usually >8 mm.
MATERIALS AND METHODS
Study design and patients
This was a multicenter retrospective cohort study. We reviewed cirrhotic patients with GV at high-risk ectopic embolism who underwent EUS-guided coil embolization combined with endoscopic cyanoacrylate injection or BRTO at six tertiary hospitals in China from January 2014 to December 2020. Patients were considered eligible when they met the following criteria: (1) Age ≥18 years; (2) endoscopy confirmed the presence of GV; (3) computed tomography angiography (CTA) of portal vein and EUS revealed the presence of a single and dominant shunt that the narrowest diameter was greater than or equal to 5 mm; (4) treated by EUS-guided coil embolization combined with endoscopic cyanoacrylate injection or BRTO. The exclusion criteria were as follows: (1) combined with malignant tumors; (2) combined with HE, hepatorenal syndrome or multiple organ failure; (3) previously received esophagus or stomach surgery; and (4) pregnant. Depending on patient’s preference and clinician discretion, either EUS-guided coil embolization combined with endoscopic cyanoacrylate injection or BRTO as treatment was selected. According to the treatments received, the eligible patients were divided into the EUS group and BRTO group.
We collected the data on the baseline characteristics, treatment and follow-up of the patients. Events of rebleeding, survival and complications were recorded during the follow-up period. This study was approved by the Ethics Committee of the Renmin Hospital of Wuhan University (WDRY2021-K033) and applied for the exemption of informed consent. The study was conducted according to the provisions of the Declaration of Helsinki (2013).
EUS-guided coil embolization combined with endoscopic cyanoacrylate injection procedure
Varices were assessed using the classification of Sarin’s by endoscopy firstly.[15] After endoscopy examination, EUS was used to assess varices and shunt, mainly focusing on the anatomical structure and blood flow. Then, to puncture the gastric fundal variceal vein at the lower esophagus and place coil at the entrance of the shunt that near the gastric wall under the guidance of EUS followed by injected with cyanoacrylate. If there was residual blood flow signal in GV, endoscopic cyanoacrylate injection was performed. After the injection, color Doppler was used to observe the blood flow in the variceal veins to evaluate the embolization effect [Supplementary Figures 1 (231.6KB, tif) and 2 (462.5KB, tif) ].
Balloon-occluded retrograde transvenous obliteration procedure
Varices were assessed using the classification of Sarin’s by endoscopy firstly.[15] After endoscopy examination, a balloon catheter was inserted through femoral vein to the GRS or SRS. Immediately, retrograde venography was performed through the inflated balloon catheter. If contrast agent showed visualization in GV, coils were placed and/or foam sclerosant was injected. Moreover, the inflated balloon was kept for 6–24 h. After 6–24 h, the left renal vein and inferior vena cava were absence on venography indicating the embolization was successful. The balloon was deflated and the balloon catheter was removed [Supplementary Figure 3 (460.2KB, tif) ].
Outcomes
The primary outcome was rebleeding. Rebleeding was defined as upper gastrointestinal hemorrhage after treatment, such as obvious hematemesis, melena and was evaluated through endoscopy if possible. The secondary outcomes were mortality, technical success, incidence of ectopic embolism, mean days, and cost of hospitalization. Mortality included death of all causes.
Data analysis
IBM SPSS Statistics version 26.0 for Windows (IBMCorp., Armonk, N.Y., USA) was used for all statistical analyses and GraphPad Prism version 8.0.2 for Windows (GraphPad Software, San Diego, California USA, www.graphpad.com) was used for drawing the survival curve. The results were presented as mean ± standard deviation or interquartile range (IQR) for the continuous data and proportions for the categorical data. The comparisons between the two groups were performed using the Student’s t-test or Mann–Whitney U-test for the continuous data and the Chi-squared test or Fisher’s exact test for the categorical data. Kaplan–Meier curve was used to analyze the cumulative occurrence of free rebleeding and survival and Log rank test was used to compare the outcomes between the two groups. Logistic regression was used for univariate analysis, and variables with P ≤ 0.1 would be included in the multivariate analysis. The results were presented as odds ratio (OR) with 95% confidence interval (95% CI). All the tests were two-sided, and P < 0.05 was considered statistically significant.
RESULTS
Baseline characteristics
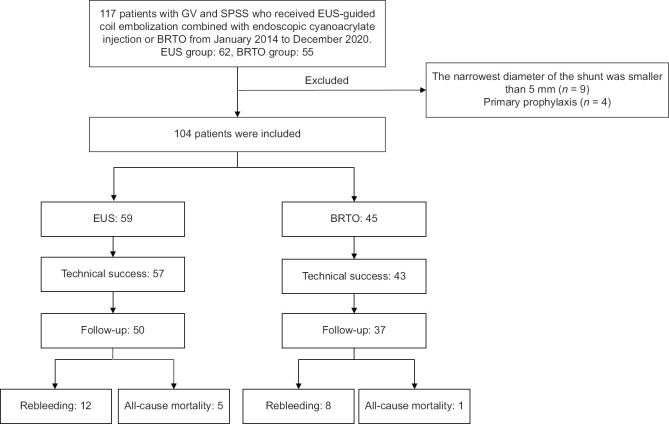
A total of 117 patients received EUS-guided coil embolization combined with endoscopic cyanoacrylate injection therapy or BRTO. Thirteen patients were excluded. Fifty-nine patients in the EUS group and 45 patients in the BRTO group were assessed for eligibility [Figure 1]. The baseline characteristics of patients are shown in Table 1. The BRTO group had a higher proportion of patients previously received endoscopic treatments (26.7% vs. 5.1%, P = 0.002) and the diameters of GV were larger (2.0 [IQR, 1.0, 3.0] vs. 3.0 [IQR, 3.0,4.8], P < 0.001).
Figure 1.
Flow chart of study profile
Table 1.
Baseline clinical characteristics of patients
| EUS (n=59), n (%) | BRTO (n=45), n (%) | P | |
|---|---|---|---|
| Male | 32 (54.2) | 28 (62.2) | 0.414* |
| Age (years) | 58.5±9.5 | 57.0±9.4 | 0.403† |
| Etiology of cirrhosis | |||
| HBV | 26 (44.1) | 26 (57.8) | 0.162‡ |
| HCV | 2 (3.4) | 2 (4.4) | |
| Alcoholic | 5 (8.5) | 4 (8.9) | |
| Schistosomiasis | 3 (5.1) | 3 (6.7) | |
| ALD | 3 (5.1) | 5 (11.1) | |
| Cryptogenic | 16 (27.1) | 3 (6.7) | |
| Others | 4 (6.8) | 2 (4.4) | |
| Previous treatment | |||
| Endoscopic treatment | 3 (5.1) | 12 (26.7) | 0.002* |
| Vascular intervention | 1 (1.7) | 2 (4.4) | 0.577‡ |
| Surgery | 1 (1.7) | 0 | 1.000‡ |
| WBC (×109/L) | 3.4 (2.6–4.7) | 2.8 (2.2–4.0) | 0.119§ |
| Hb (g/L) | 93.0 (74.0–114.0) | 84.0 (76.0–96.5) | 0.181§ |
| PLT (×109/L) | 76.0 (55.0–116.0) | 73.0 (52.0–101.0) | 0.618§ |
| ALT (U/L) | 23.0 (16.0–35.0) | 18.0 (13.0–25.5) | 0.033§ |
| TBIL (μmol/L) | 19.0 (13.3–24.0) | 17.0 (12.3–21.7) | 0.499§ |
| ALB (g/L) | 33.8 (29.5–38.1) | 34.0 (31.6–37.0) | 0.854§ |
| PT (s) | 14.2 (13.2-15.1) | 13.8 (12.7-14.7) | 0.181§ |
| Ascites | 19 (32.2) | 7 (15.6) | 0.052* |
| Child-Pugh grade | |||
| A | 36 (61.0) | 30 (66.7) | 0.773‡ |
| B | 20 (33.9) | 14 (31.1) | |
| C | 3 (5.1) | 1 (2.2) | |
| Child-Pugh score | 6.0 (5.0–7.0) | 6.0 (5.0–7.5) | 0.830§ |
| MELD score | 9.0 (8.0–11.0) | 9.0 (8.0–10.0) | 0.448§ |
| Types of varices | |||
| GOV1 | 3 (5.1) | 4 (8.9) | 0.567‡ |
| GOV2 | 25 (42.4) | 22 (48.9) | |
| IGV1 | 29 (49.2) | 19 (42.2) | |
| IGV2 | 2 (3.4) | 0 | |
| Diameter of GV (cm) | 2.0 (1.0–3.0) | 3.0 (3.0–4.8) | <0.001§ |
| Type of shunt | |||
| SRS | 29 (49.2) | 7 (15.6) | <0.001‡ |
| GRS | 30 (50.8) | 38 (84.4) | |
| The narrowest diameter of the shunt (mm) | 6.0 (5.5–8.0) | 6.0 (5.0–8.0) | 0.296§ |
| Purposes of treatment | |||
| Secondary prophylaxis | 56 (94.4) | 44 (97.8) | 0.632‡ |
| Management of acute bleeding episode | 3 (5.1) | 1 (2.2) |
*Chi-square test; †Independent-sample t-test; ‡Fisher’s exact test; §Mann–Whitney U-test. Other etiology of cirrhosis: Drug-induced, inherited metabolic diseases and cholestasis. HBV: Hepatitis B virus; HCV: Hepatitis C virus; ALD: Autoimmune liver diseases; WBC: White blood cell; Hb: Hemoglobin; PLT: Platelet; ALT: Alanine aminotransferases; TBIL: Total bilirubin; ALB: Albumin; PT: Prothrombin time; GOV1: Type 1 gastroesophageal varices; GOV2: Type 2 gastroesophageal varices; IGV1: Type 1 isolate gastric varices; IGV2: Type 2 isolate gastric varices; BRTO: Balloon-occluded retrograde transvenous obliteration; SRS: Splenorenal shunt; GRS: Gastrorenal shunt
Treatment
Table 2 summarizes the results of treatment. There was no statistically significant difference in technical success, amount of cyanoacrylate and sclerosant, days, and cost of hospitalization. The number of coils ranged from 1 to 5. Two patients failed to place the coil. One was because the puncture pathway was too far and the angle was too large, the other one was mainly because the perigastric varices were so abundant that puncture could not avoid them and this may cause severe bleeding. Two patients failed to performed BRTO. One was due to portal vein system vascular angulation deformity under X-ray and the balloon could not reach the GRS, the other one’s diameter of shunt was 9 to18 mm, the largest balloon (14 mm) still failed to completely occlude the shunt. Finally, BRTO combined with EUS-guided coil embolization and endoscopic cyanoacrylate injection was applied.
Table 2.
Results about treatment
| EUS | BRTO | P | |
|---|---|---|---|
| Technical success, n (%) | 57 (96.6) | 43 (95.6) | 1.000* |
| Amount of cyanoacrylate (mL)|| | 3.0 (1.5–5) | 5.0 (1.9–7.1) | 0.418§ |
| Days of hospitalization|| | 16.0 (12.0–19.0) | 16.5 (11.8–26.0) | 0.165§ |
| Cost of hospitalization (CNY)¶ | 45950.6 (39330.2–55768.2) | 51205.8 (31628.8–74251.5) | 0.680§ |
*Chi-square test; †Independent-sample t-test; ‡Fisher’s exact test; §Mann– Whitney U-test; ||EUS group (n=57), BRTO group (n=43); ¶EUS group (n=43), BRTO group (n=42). BRTO: Balloon-occluded retrograde transvenous obliteration; CNY: Chinese Yuan
Rebleeding, survival, and complications
Fifty patients in the EUS group had a follow-up with the median follow-up time of 11.5 (IQR, 2.9–19.2) months, whereas 37 patients in the BRTO group with the median follow-up time of 18.0 (IQR, 6.7–30.0) months. The rate of 5-day rebleeding and 6-week mortality was 0%. The 1-year all-cause rebleeding rate (20.0% vs. 18.9%, P = 0.900), 1-year mortality rate (2.0% vs. 0%, P = 1.000), and all-cause mortality rate (10.0% vs. 2.7%, P = 0.234) were similar between the both groups [Table 3]. The cumulative occurrence of free-rebleeding and survival was no significant difference [Figures 2 and 3].
Table 3.
Comparison of event of rebleeding, survival and complications
| EUS (n=50), n (%) | BRTO (n=37), n (%) | P | |
|---|---|---|---|
| Five-day rebleeding | 0 | 0 | 1.000‡ |
| Six-week mortality | 0 | 0 | 1.000‡ |
| All-cause Rebleeding | 12 (24.0) | 8 (21.6) | 0.794* |
| One-year all-cause rebleeding | 10 (20.0) | 7 (18.9) | 0.900* |
| All-cause mortality | 5 (10.0) | 1 (2.7) | |
| Rebleeding | 1 (2.0) | 0 | 0.234‡ |
| Ectopic embolism | 0 | 0 | |
| Others | 4 (8.0) | 1 (2.7) | |
| One-year mortality | 1 (2.0) | 0 | 1.000‡ |
| Ectopic embolism | 1 (2.0) | 0 | 1.000‡ |
*Chi-square test; †Independent-sample t-test; ‡Fisher’s exact test; §Mann–Whitney U-test. BRTO: Balloon-occluded retrograde transvenous obliteration
Figure 2.

Cumulative incidence of free from rebleeding using Kaplan–Meier methods
Figure 3.

Cumulative incidence of survival using Kaplan–Meier methods
As for the incidence of ectopic embolism, one patient’s CTA of portal vein in the EUS group showed partial embolism of the splenic vein trunk in the 2nd day after treatment, but he did not present with any clinical manifestations and was successfully treated with conservative therapy. During the follow-up, no embolism reappeared. No patient occurred ectopic embolism in the BRTO group. Nine patients (18.0%) in the EUS group and seven in the BRTO group (18.9%) required retreatment. In the EUS group, two patients underwent splenectomy after treatment due to splenomegaly, hypersplenism and repeated rebleeding, and one patient received TIPS 6 months after treatment due to refractory ascites. Two patients in the BRTO group received TIPS at 9 days and 26 months after treatment due to poor efficacy of BRTO and rebleeding after failed in the conservative treatment and endoscopic treatment.
Multivariate analysis and subgroup analysis
In the logistic regression analysis, multivariate analysis showed that count of white blood cell (OR = 0.438; 95% CI: 0.419–0.973; P = 0.037) was a prognostic factor for rebleeding, while narrowest diameter of the shunt (OR = 1.86; 95% CI: 1.062–3.258; P = 0.030) and content of hemoglobin (OR = 0.941; 95% CI: 0.892–0.992; P = 0.025) were the prognostic factors for the survival [Tables 4 and 5].
Table 4.
Univariate and multivariate analysis of rebleeding
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
|
|
|
|||
| OR (95%CI) | P | OR (95%CI) | P | |
| Male | 0.492 (0.175–1.388) | 0.180 | ||
| Age (years) | 0.976 (0.928–1.027) | 0.348 | ||
| BRTO | 0.874 (0.316–2.415) | 0.794 | ||
| WBC (×109/L) | 0.626 (0.416–0.940) | 0.024 | 0.438 (0.419–0.973) | 0.037 |
| PLT (×109/L) | 0.995 (0.983–1.006) | 0.367 | ||
| Hb (g/L) | 0.991 (0.971–1.012) | 0.408 | ||
| ALT (U/L) | 0.973 (0.935–1.013) | 0.189 | ||
| ALB (g/L) | 0.992 (0.926–1.063) | 0.820 | ||
| PT (s) | 0.949 (0.801–1.123) | 0.542 | ||
| Ascites | 1.466 (0.505–4.256) | 0.482 | ||
| Child-Pugh score | 0.976 (0.668–1.425) | 0.900 | ||
| MELD score | 0.785 (0.577–1.068) | 0.123 | ||
| Diameter of GV (cm) | 1.352 (0.958–1.907) | 0.086 | 1.236 (0.861–1.774) | 0.251 |
| The narrowest diameter of the shunt (mm) | 1.278 (0.914–1.787) | 0.152 | ||
BRTO: Balloon-occluded retrograde transvenous obliteration; CI: Confidence interval; WBC: White blood cell; Hb: Hemoglobin; PLT: Platelet; ALT: Alanine aminotransferases; ALB: Albumin; PT: Prothrombin time; OR: Odds ratio; GV: Gastric varices; MELD: Model for end-stage liver disease
Table 5.
Univariate and multivariate analysis of survival
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
|
|
|
|||
| OR (95%CI) | P | OR (95%CI) | P | |
| Male | 1.077 (0.205–5.656) | 0.930 | ||
| Age (years) | 1.097 (0.996–1.208) | 0.060 | 1.095 (0.976–1.228) | 0.121 |
| BRTO | 0.250 (0.028–2.237) | 0.215 | ||
| WBC (×109/L) | 0.913 (0.619–1.346) | 0.646 | ||
| PLT (×109/L) | 1.002 (0.986–1.019) | 0.774 | ||
| Hb (g/L) | 0.950 (0.907–0.997) | 0.035 | 0.941 (0.892–0.992) | 0.025 |
| ALT (U/L) | 0.937 (0.849–1.034) | 0.195 | ||
| ALB (g/L) | 1.001 (0.899–1.115) | 0.986 | ||
| PT (s) | 0.715 (0.370–1.384) | 0.320 | ||
| Ascites | 2.682 (0.503–14.297) | 0.248 | ||
| Child-Pugh score | 0.806 (0.398–1.632) | 0.549 | ||
| MELD score | 0.760 (0.441–1.308) | 0.321 | ||
| Diameter of GV (cm) | 1.431 (0.814–2.516) | 0.213 | ||
| The narrowest diameter of the shunt (mm) | 1.723 (1.044–2.843) | 0.033 | 1.86 (1.062–3.258) | 0.030 |
BRTO: Balloon-occluded retrograde transvenous obliteration; CI: Confidence interval; WBC: White blood cell; Hb: Hemoglobin; PLT: Platelet; ALT: Alanine aminotransferases; ALB: Albumin; PT: Prothrombin time; OR: Odds ratio; GV: Gastric varices; MELD: Model for End-stage Liver Disease
DISCUSSION
Our study was the first to compare the safety and efficacy of EUS-guided coil embolization combined with endoscopic cyanoacrylate injection and BRTO. According to Simón et al., SPSS was classified in large or small size according to its maximum diameter, with a cut-off at 8 mm.[1] In the clinical practice, we found that its maximum diameter is usually >8 mm when the narrowest diameter of the shunt is ≥5 mm. Moreover, the success of the treatment or the occurrence of ectopic embolism often depends on the narrowest diameter of the shunt. Therefore, in this study, we enrolled patients with GV accompanied by SPSS with the narrowest diameter of the shunt ≥5 mm who should be paid more attention to and had a higher risk of ectopic embolism during endoscopic cyanoacrylate injection and HE. We found that 5-day rebleeding rate and 6-week mortality were 0% in both groups. Both groups had similar technical success rate, incidence of ectopic embolism, 1-year rebleeding rate, and 1-year mortality rate. It can be seen that EUS-guided coil embolization combined with endoscopic cyanoacrylate injection and BRTO have comparable and pretty good efficacy and safety in the treatment of GV with high-risk ectopic embolism.
In the research conducted by the Baveno VI co-operative group, it is noted that the existence of SPSS was independently related to the mortality of patients with liver cirrhosis and the need for liver transplantation.[1] Traditional endoscopic cyanoacrylate injection is at a higher risk of ectopic embolism in patients with GV and SPSS and TIPS is at a higher risk of HE. To date, there is a paucity of data to guide the management. Therefore, the treatment of GV with GRS or SRS is riskier and more challenging.
BRTO is a traditional vascular interventional therapy and widely practiced for GV with SPSS, with high technical success rate and low rebleeding rate.[16,17] For cirrhotic patients with portal hypertension and GRS or SRS, BRTO can increase the blood flow of portal vein and promote liver metabolism of toxic substances, which leads to the prevention of HE and improve survival rate. AASLD recommends that BRTO be used for secondary prophylaxis of gastric variceal bleeding, especially in patients with SPSS.[14] Moreover, American Gastroenterological Association (AGA) also recommends that when a GRS is present, local expertise is available, and severe comorbid complications of portal hypertension are absent, BRTO is the optimal endovascular therapy for the management of GV bleeding.[18] Many studies and meta-analysis results showed that BRTO was superior to traditional endoscopic therapy and TIPS in terms of rate of rebleeding, mortality and incidence of adverse events and was often used as a treatment for patients with HE.[19,20,21,22] A meta-analysis of 1016 patients showed that technical success rate ranged from 79% to 100%. The 1-year survival rate ranged from 83.1% to 100% (mean 92.6% ± 4.3%) and the 3-year survival rate ranged from 75% to 100% (mean 84.5% ± 10.1%).[21] In a comparative study of BRTO and endoscopic cyanoacrylate injection in the treatment of GV, the 6-week mortality of BRTO was 13.1% lower than endoscopic cyanoacrylate injection (14.4%, P = 0.85). Moreover, rebleeding occurred in 1 year after treatment in 22.0% of patients treated with endoscopic cyanoacrylate injection and 3.5% of patients treated with BRTO (P < 0.01).[22]
Most studies considered that the rebleeding rate of GV was <10% after BRTO.[17] Compared with previous study, the 1-year all-cause rebleeding rate of BRTO (21.6%) in our study was no significant difference. Moreover, the rate of technical success, survival, and adverse events was similar with the previous studies. It proved BRTO was a safe, feasible, and effective modality for GV patients with SPSS.
The occlusion of GV and shunt and the redistribution of blood flow after treatment may lead to the increase of portal pressure and the aggravation of esophageal varices (EV), which perhaps increase the risk of EV bleeding and may also be one of the reasons for the high rate of rebleeding. Studies have reported that the incidence of EV aggravation after BRTO was about 33.3% (9.8%−72.2%).[21,23,24] Therefore, the increase of portal vein pressure is also one of the concerns, and better prevention methods should be studied. Unfortunately, because it was a retrospective study and lack of endoscopic follow-up data of some patients, we were unable to obtain the whole data of postoperative EV, portal hypertensive gastropathy (PHG) and the shunts, and also unable to compare the influence of EV and PHG between two methods. Besides, the time of follow-up in our study was still not long enough. It is possible that the impact of portal hypertension on the shunt is a long-term effect, and it is difficult to observe obvious changes in the short term. Therefore, the impact of the two treatments on the shunt and varices needs to be compared in cohort studies with longer follow-up time.
EUS is an important and emerging technology in the development of digestive endoscopy, and it shows a good application prospect in the field of portal hypertension. A number of studies have confirmed that EUS-guided cyanoacrylate injection and EUS-guided coil placement can significantly reduce the risk of rebleeding, incidence of ectopic embolism, and amount of cyanoacrylate in the treatment of GV.[25,26,27,28,29,30,31] Mohan et al. conducted a meta-analysis on the efficacy and safety of EUS-guided therapy versus endoscopic cyanoacrylate injection in the treatment of GV.[30] The results revealed that the early rebleeding rate was 7.7%, the late rebleeding rate was 9.2%, the incidence of ectopic embolism was 4.3%, and the all-cause mortality rate was 9% in EUS-guided coil embolization and cyanoacrylate injection. Among them, the mortality due to GV rebleeding was 4.5%, which showed better efficacy and safety than EUS-guided coil embolization, EUS-guided cyanoacrylate injection, and endoscopic cyanoacrylate injection.
In the procedure of therapy, what we concerned was ectopic embolism. Romero-Castro et al. noted that pulmonary embolism developed in 9 of 19 patients (47.4%) who underwent EUS-guided cyanoacrylate injection. These were all asymptomatic and were detected on routine imaging performed as the part of the study protocol.[32] In other studies, the published rate of ectopic embolism for EUS-guided coil embolization or cyanoacrylate injection ranged from 0% to 6.5%.[26,28,33,34,35] In our study, asymptomatic ectopic embolism was only occurred in one patient and successfully managed by the conservative treatment. No other serious adverse events were found. It revealed EUS-guided coil embolization combined with endoscopic cyanoacrylate injection was safe and effective for patients with GV and SPSS.
The multivariate analysis showed that the narrowest diameter of the shunt (OR = 1.86; 95% CI: 1.062–3.258; P = 0.030) and content of hemoglobin (OR = 0.941; 95% CI: 0.892–0.992; P = 0.025) were the prognostic factors for the survival. However, in this study, the narrowest diameters of shunt were 5, 6, 8, 8, 8 and 9 mm in 6 deaths, and the Child-Pugh grade was A or B. Only one death was associated with rebleeding. The relationship between shunt diameter and mortality remained questionable. Simón et al. conducted a study of about 1729 patients with liver cirrhosis, which found that the existence of SPSS was related to the deterioration of liver function and death, but failed to find the relationship between mortality and diameter or types of SPSS.[1] Considering a relatively small number of patients and the unbalanced proportion of the types of shunts in this study, the results may not be steady enough. The reliability of these results needs further research to confirm.
As a retrospective study, in addition to the limitations mentioned above, there still are some limitations. First, patients with all types of esophagogastric varices were included in this study. Whether the efficacy and safety of the two treatments are consistent for different types of varices remains to be studied. Second, most of the patients were for secondary prophylaxis and there were a few patients of Child-Pugh C. For patients with poor liver function, Child-Pugh C, and high Model for End-Stage Liver Disease scores or patients for primary prophylaxis and management of acute bleeding episode, whether the two methods still have satisfying efficacy and safety needs to be confirmed. Third, we only analyzed the data of 85 patients’ cost of hospitalization due to missing some data, which may cause bias to the results. Moreover, we only took into account the procedure costs based on days of hospitalization and had not taken into account events of retreatment and many other factors during the follow-up that will finally influence the cost-effectiveness. Finally, there was no comparison with TIPS or endoscopic cyanoacrylate injection. Large randomized controlled trials comparing the safety and efficacy of these two methods are still absent. Although their advantages have been addressed in numerous recent studies, the quality of the evidence remains inadequate.
CONCLUSIONS
EUS-guided coil embolization combined with endoscopic cyanoacrylate injection and BRTO have comparable and pretty efficacy and safety for patients with GV at high-risk ectopic embolism. The efficacy and safety of the two methods still need to be further explored in large sample prospective randomized controlled trials. Especially, the deterioration of EV and PHG after treatment should be noted. Hence, better prevention and follow-up strategies should be studied. The relationship between the diameter or types of the shunt and mortality, and the best treatment for different types of varices and shunts also need to be confirmed by large randomized controlled trials.
Supplementary materials
Supplementary information is linked to the online version of the paper on the Endoscopic Ultrasound website.
Financial support and sponsorship
The work in this paper is supported by The Key Research and Development Program of Hubei Province (NO.2020BCB007).
Conflicts of interest
There are no conflicts of interest.
CTA of portal vein showed GV (a) and three-dimensional reconstruction computed tomography image showed GRS (b, c). CTA: Computed tomography angiography, GV: Gastric varices, GRS: Gastrorenal shunt
The procedure of EUS-guided coil embolization combined with endoscopic cyanoacrylate injection. EUS showed multiple anechoic lesions (a) and color Doppler revealed abundant blood flow signals (b). Punctured the gastric fundal variceal vein at the lower esophagus near the cardia and placed coil under the guidance of EUS followed by injecting with cyanoacrylate (c). The blood flow signal was almost disappeared (d). Endoscopic cyanoacrylate injection of GV was performed (e, f). GV: Gastric varices
The procedure of BRTO. The preoperative radiography showed the GRS (a). Contrast agent showed visualization in GV (b). Placed coils and/or injected foam sclerosant and kept the balloon inflated for 6–24 h (c). After 6–24 h, the left renal vein and inferior vena cava were absence on venography and the contrast agent did not reflux (d). Gastric fundal varices became tough under the endoscopy (e). The balloon was removed and the contrast agent did not reflux (f). BRTO: Balloon-occluded retrograde transvenous obliteration
Acknowledgement
Thanks to Haowen Gu and Yuanbin Liu for writing assistance and proofreading. Thanks to Zhuo Cao, Jiao Li and Shuzhong Liu for providing guidance in the process of collecting data.
REFERENCES
- 1.Simón-Talero M, Roccarina D, Martínez J, et al. Association between portosystemic shunts and increased complications and mortality in patients with cirrhosis. Gastroenterology. 2018;154:1694–705. doi: 10.1053/j.gastro.2018.01.028. e4. [DOI] [PubMed] [Google Scholar]
- 2.Plessier A, Darwish-Murad S, Hernandez-Guerra M, et al. Acute portal vein thrombosis unrelated to cirrhosis:A prospective multicenter follow-up study. Hepatology. 2010;51:210–8. doi: 10.1002/hep.23259. [DOI] [PubMed] [Google Scholar]
- 3.Ponziani FR, Zocco MA, Campanale C, et al. Portal vein thrombosis:Insight into physiopathology, diagnosis, and treatment. World J Gastroenterol. 2010;16:143–55. doi: 10.3748/wjg.v16.i2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rathi S, Brocco S, Formentin C, et al. Spontaneous portosystemic shunts in cirrhosis:Detection, implications, and clinical associations. Dig Liver Dis. 2021;53:1468–75. doi: 10.1016/j.dld.2020.11.020. [DOI] [PubMed] [Google Scholar]
- 5.Zocco MA, Di Stasio E, De Cristofaro R, et al. Thrombotic risk factors in patients with liver cirrhosis:Correlation with MELD scoring system and portal vein thrombosis development. J Hepatol. 2009;51:682–9. doi: 10.1016/j.jhep.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 6.European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406–60. doi: 10.1016/j.jhep.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 7.de Franchis R, Baveno VI Faculty. Expanding consensus in portal hypertension:Report of the Baveno VI Consensus Workshop:Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743–52. doi: 10.1016/j.jhep.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 8.Lo GH, Lai KH, Cheng JS, et al. A prospective, randomized trial of butyl cyanoacrylate injection versus band ligation in the management of bleeding gastric varices. Hepatology. 2001;33:1060–4. doi: 10.1053/jhep.2001.24116. [DOI] [PubMed] [Google Scholar]
- 9.Mahadeva S, Bellamy MC, Kessel D, et al. Cost-effectiveness of N-butyl-2-cyanoacrylate (histoacryl) glue injections versus transjugular intrahepatic portosystemic shunt in the management of acute gastric variceal bleeding. Am J Gastroenterol. 2003;98:2688–93. doi: 10.1111/j.1572-0241.2003.08769.x. [DOI] [PubMed] [Google Scholar]
- 10.Rengstorff DS, Binmoeller KF. A pilot study of 2-octyl cyanoacrylate injection for treatment of gastric fundal varices in humans. Gastrointest Endosc. 2004;59:553–8. doi: 10.1016/s0016-5107(03)02865-7. [DOI] [PubMed] [Google Scholar]
- 11.Tan PC, Hou MC, Lin HC, et al. A randomized trial of endoscopic treatment of acute gastric variceal hemorrhage:N-butyl-2-cyanoacrylate injection versus band ligation. Hepatology. 2006;43:690–7. doi: 10.1002/hep.21145. [DOI] [PubMed] [Google Scholar]
- 12.Zhang H, Xiao J, Tu J, et al. Prevention of variceal rebleeding in cirrhotic patients with spontaneous portosystemic shunts:Transjugular intrahepatic portosystemic shunt versus endoscopic treatment. Eur J Gastroenterol Hepatol. 2021;33:752–61. doi: 10.1097/MEG.0000000000002079. [DOI] [PubMed] [Google Scholar]
- 13.Zheng M, Chen Y, Bai J, et al. Transjugular intrahepatic portosystemic shunt versus endoscopic therapy in the secondary prophylaxis of variceal rebleeding in cirrhotic patients:Meta-analysis update. J Clin Gastroenterol. 2008;42:507–16. doi: 10.1097/MCG.0b013e31815576e6. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Tsao G, Abraldes JG, Berzigotti A, et al. Portal hypertensive bleeding in cirrhosis:Risk stratification, diagnosis, and management:2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65:310–35. doi: 10.1002/hep.28906. [DOI] [PubMed] [Google Scholar]
- 15.Sarin SK, Kumar A. Gastric varices:Profile, classification, and management. Am J Gastroenterol. 1989;84:1244–9. [PubMed] [Google Scholar]
- 16.Garcia-Pagán JC, Barrufet M, Cardenas A, et al. Management of gastric varices. Clin Gastroenterol Hepatol. 2014;12:919–28. doi: 10.1016/j.cgh.2013.07.015. e1. [DOI] [PubMed] [Google Scholar]
- 17.Lee EW, Shahrouki P, Alanis L, et al. Management options for gastric variceal hemorrhage. JAMA Surg. 2019;154:540–8. doi: 10.1001/jamasurg.2019.0407. [DOI] [PubMed] [Google Scholar]
- 18.Henry Z, Patel K, Patton H, et al. AGA clinical practice update on management of bleeding gastric varices:Expert review. Clin Gastroenterol Hepatol. 2021;19:1098–107. doi: 10.1016/j.cgh.2021.01.027. e1. [DOI] [PubMed] [Google Scholar]
- 19.Luo X, Xiang T, Wu J, et al. Endoscopic cyanoacrylate injection vs. BRTO for prevention of gastric variceal bleeding:A randomized controlled trial. Hepatology. 2021;74:2074–84. doi: 10.1002/hep.31718. [DOI] [PubMed] [Google Scholar]
- 20.Paleti S, Nutalapati V, Fathallah J, et al. Balloon-occluded retrograde transvenous obliteration (BRTO) versus transjugular intrahepatic portosystemic shunt (TIPS) for treatment of gastric varices because of portal hypertension:A systematic review and meta-analysis. J Clin Gastroenterol. 2020;54:655–60. doi: 10.1097/MCG.0000000000001275. [DOI] [PubMed] [Google Scholar]
- 21.Park JK, Saab S, Kee ST, et al. Balloon-occluded retrograde transvenous obliteration (BRTO) for treatment of gastric varices:Review and meta-analysis. Dig Dis Sci. 2015;60:1543–53. doi: 10.1007/s10620-014-3485-8. [DOI] [PubMed] [Google Scholar]
- 22.Stein DJ, Salinas C, Sabri S, et al. Balloon retrograde transvenous obliteration versus endoscopic cyanoacrylate in bleeding gastric varices:Comparison of rebleeding and mortality with extended follow-up. J Vasc Interv Radiol. 2019;30:187–94. doi: 10.1016/j.jvir.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 23.Imai Y, Nakazawa M, Ando S, et al. Long-term outcome of 154 patients receiving balloon-occluded retrograde transvenous obliteration for gastric fundal varices. J Gastroenterol Hepatol. 2016;31:1844–50. doi: 10.1111/jgh.13382. [DOI] [PubMed] [Google Scholar]
- 24.Ninoi T, Nishida N, Kaminou T, et al. Balloon-occluded retrograde transvenous obliteration of gastric varices with gastrorenal shunt:Long-term follow-up in 78 patients. AJR Am J Roentgenol. 2005;184:1340–6. doi: 10.2214/ajr.184.4.01841340. [DOI] [PubMed] [Google Scholar]
- 25.Bazarbashi AN, Wang TJ, Jirapinyo P, et al. Endoscopic ultrasound-guided coil embolization with absorbable gelatin sponge appears superior to traditional cyanoacrylate injection for the treatment of gastric varices. Clin Transl Gastroenterol. 2020;11:e00175. doi: 10.14309/ctg.0000000000000175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhat YM, Weilert F, Fredrick RT, et al. EUS-guided treatment of gastric fundal varices with combined injection of coils and cyanoacrylate glue:A large U. S. experience over 6 years (with video) Gastrointest Endosc. 2016;83:1164–72. doi: 10.1016/j.gie.2015.09.040. [DOI] [PubMed] [Google Scholar]
- 27.Binmoeller KF, Weilert F, Shah JN, et al. EUS-guided transesophageal treatment of gastric fundal varices with combined coiling and cyanoacrylate glue injection (with videos) Gastrointest Endosc. 2011;74:1019–25. doi: 10.1016/j.gie.2011.06.030. [DOI] [PubMed] [Google Scholar]
- 28.Kozieł S, Pawlak K, Błaszczyk Ł, et al. Endoscopic ultrasound-guided treatment of gastric varices using coils and cyanoacrylate glue injections:Results after 1 year of experience. J Clin Med. 2019;8:1786. doi: 10.3390/jcm8111786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lôbo MR, Chaves DM, DE Moura DT, et al. Safety and efficacy of EUS-guided coil plus cyanoacrylate versus conventional cyanoacrylate technique in the treatment of gastric varices:A randomized controlled trial. Arq Gastroenterol. 2019;56:99–105. doi: 10.1590/S0004-2803.201900000-08. [DOI] [PubMed] [Google Scholar]
- 30.Mohan BP, Chandan S, Khan SR, et al. Efficacy and safety of endoscopic ultrasound-guided therapy versus direct endoscopic glue injection therapy for gastric varices:Systematic review and meta-analysis. Endoscopy. 2020;52:259–67. doi: 10.1055/a-1098-1817. [DOI] [PubMed] [Google Scholar]
- 31.Robles-Medranda C, Oleas R, Valero M, et al. Endoscopic ultrasonography-guided deployment of embolization coils and cyanoacrylate injection in gastric varices versus coiling alone:A randomized trial. Endoscopy. 2020;52:268–75. doi: 10.1055/a-1123-9054. [DOI] [PubMed] [Google Scholar]
- 32.Romero-Castro R, Ellrichmann M, Ortiz-Moyano C, et al. EUS-guided coil versus cyanoacrylate therapy for the treatment of gastric varices:A multicenter study (with videos) Gastrointest Endosc. 2013;78:711–21. doi: 10.1016/j.gie.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 33.Bang JY, Al-Haddad MA, Chiorean MV, et al. Mo1485 comparison of direct endoscopic injection (DEI) and EUS-guided fine needle injection (EUS-FNI) of 2-Octyl-cyanoacrylate for treatment of gastric varices. Gastrointest Endosc. 2015;81:AB437. [Google Scholar]
- 34.Bick BL, Al-Haddad M, Liangpunsakul S, et al. EUS-guided fine needle injection is superior to direct endoscopic injection of 2-octyl cyanoacrylate for the treatment of gastric variceal bleeding. Surg Endosc. 2019;33:1837–45. doi: 10.1007/s00464-018-6462-z. [DOI] [PubMed] [Google Scholar]
- 35.Thiruvengadam SS, Sedarat A. The role of endoscopic ultrasound (EUS) in the management of gastric varices. Curr Gastroenterol Rep. 2021;23:1. doi: 10.1007/s11894-020-00801-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CTA of portal vein showed GV (a) and three-dimensional reconstruction computed tomography image showed GRS (b, c). CTA: Computed tomography angiography, GV: Gastric varices, GRS: Gastrorenal shunt
The procedure of EUS-guided coil embolization combined with endoscopic cyanoacrylate injection. EUS showed multiple anechoic lesions (a) and color Doppler revealed abundant blood flow signals (b). Punctured the gastric fundal variceal vein at the lower esophagus near the cardia and placed coil under the guidance of EUS followed by injecting with cyanoacrylate (c). The blood flow signal was almost disappeared (d). Endoscopic cyanoacrylate injection of GV was performed (e, f). GV: Gastric varices
The procedure of BRTO. The preoperative radiography showed the GRS (a). Contrast agent showed visualization in GV (b). Placed coils and/or injected foam sclerosant and kept the balloon inflated for 6–24 h (c). After 6–24 h, the left renal vein and inferior vena cava were absence on venography and the contrast agent did not reflux (d). Gastric fundal varices became tough under the endoscopy (e). The balloon was removed and the contrast agent did not reflux (f). BRTO: Balloon-occluded retrograde transvenous obliteration