Abstract
There is increasing interest in the involvement of antioxidative systems in protecting from depression. Among these, Nrf2 occupies a central place. We aimed to review the role of Nrf2 in depression. For this reason, we conducted a PubMed search using as search strategy (psychiatr*[ti] OR schizo*[ti] OR psychot*[ti] OR psychos*[ti] OR depress*[ti] OR MDD[ti] OR BD[ti] OR bipolar[ti] OR Anxiety[ti] OR antidepress*[ti] OR panic[ti] OR obsess*[ti] OR compulsio*[ti] OR “mood disord*”[ti] OR phobi*[ti] OR agoraphob*[ti] OR anorex*[ti] OR anorect*[ti] OR bulimi*[ti] OR “eating disorder*”[ti] OR neurodevelopm*[ti] OR retardation[ti] OR autism[ti] OR autistic[ti] OR ASM[ti] OR adhd[ti] OR “attention-deficit”[ti]) AND nrf2, which on the 9th of March produced 208 results of which 89 were eligible for our purposes. Eligible articles were studies reporting data of Nrf2 manipulations or content by any treatment in human patients or animals with any animal model of depression. Most studies were on mice only (N = 58), 20 on rats only, and three on both rats and mice. There were two studies on cell lines (in vitro) and one each on nematodes and fish. Only four studies were conducted in humans, one of which was post mortem. Most studies were conducted on male animals; however, human studies were carried out on both men and women. The results indicate that Nrf2 is lower in depression and that antidepressant methods (drugs or other methods) increase it. Antioxidant systems and plasticity-promoting molecules, such as those in the Nrf2–HO-1, BDNF–TrkB, and cyclic AMP–CREB pathways, could protect from depression, while glycogen synthase kinase-3β and nuclear factor κB oppose these actions, thus increasing depressive-like behaviours. Since Nrf2 is also endowed with tumorigenic and atherogenic potential, the balance between benefits and harms must be taken into account in designing novel drugs aiming at increasing the intracellular content of Nrf2.
Keywords: depression, Nuclear factor erythroid-2 (Nrf2), pathophysiology, antioxidant pathways, Haemoxygenase (HO-1), Nuclear factor kappa B (NF-κB)
1. Introduction
Recently, much research has been devoted to the study of inflammation and its role in the context of a wide variety of pathological conditions. Inflammation and oxidative stress were found to enhance each other, thus establishing a pathological state [1], which may be found in various psychiatric disorders. The variety of external stimuli to which organisms are subjected triggers adaptive responses, which are designed to restore homoeostasis through a fine balance between oxidation and antioxidant activity [2].
An imbalance between the generation of reactive oxygen species (ROS) and antioxidant defences results in increased oxidative stress [3], with a consequent increase in neuroinflammation, mitochondrial dysfunction, and cell degeneration processes such as apoptosis and ferroptosis, which proved to be crucial in many psychiatric disorders. This is not surprising if we consider that the brain consumes massive doses of oxygen and contains high concentrations of oxidative lipids, thus being extremely vulnerable to oxidative stress-induced damage [2].
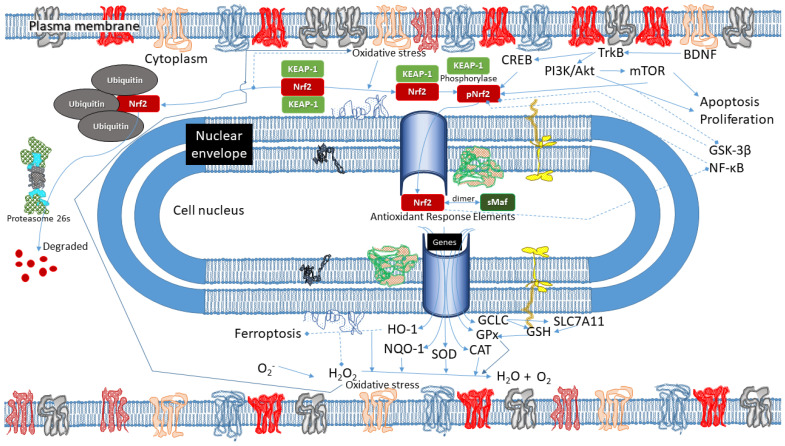
In this framework, it is important to draw attention to nuclear factor erythroid-2 (Nrf2), the main endogenous negative regulator of oxidation [4]. Its activation determines the expression of numerous antioxidants and cytoprotective genes capable of modulating oxidative stress. Nrf2 also presides over the regulation of genes involved in the oxidative stress-related pathological processes mentioned above. Consequently, dysregulation in Nrf2 expression with reduced cortical levels may contribute to the aetiopathogenesis of numerous pathological conditions, including psychiatric disorders and neurodegenerative diseases.
Nrf2 is a protein transcription factor composed of 605 amino acids, encoded by the NFE2L2 gene and belonging to the Cap’n’collar (CNC) family of transcription factors [5]. It contains seven highly conserved functional domains called Nrf2-ECH homology 1 (Neh1-Neh7). Neh1 and Neh3 interact with specific DNA sequences called antioxidant response elements (ARE) [6], thus promoting the transcription of enzymes with antioxidant activity. Neh2 interacts with Kelch-like ECH-associated protein 1 (Keap1), the main negative regulator of Nrf2. Neh4 and Neh5 interact with the cyclic adenosine monophosphate (cAMP)-response element-binding protein (CREB), which also promotes transcriptional activation [7]. In contrast, Neh6 can link to β-transducin repeat-containing protein (β-TrCP) and is involved in Keap1-independent degradation of Nrf2. Finally, Neh7 inhibits the Nrf2-ARE signalling pathway by binding to retinoic X receptor alpha (RXRα) [8]. Therefore, the activity of Nrf2 is subjected to both Keap1-dependent and Keap1-independent regulation.
Keap 1 represents the main Nrf2 suppressor; it forms a homodimer capable of binding ETGE and DLG motifs (stronger and weaker binding sites of Keap-1, respectively; the former is located in the loop region of the antiparallel β-sheet, while the latter is N-terminal to the α-helix [9]) included in the Neh2 domain of Nrf2. Under physiological conditions, the Keap1-Nrf2 complex combines with the E3-ubiquitin ligase Cullin 3 (Cul3) complex, leading to ubiquitination and proteasomal degradation of Nrf2 [10]. Negative regulation is also mediated by the phosphorylation of Nrf2 by glycogen synthase kinase-3 beta (GSK-3β) and mitogen-activating protein kinase (MAPK) [11].
When oxidative stress increases and ROS accumulation occurs, there is a dissociation of the Keap1-Nrf2 complex induced by conformational changes of a Keap1 domain, inhibiting ubiquitination and subsequent degradation of Nrf2. Dissociated from Keap1, Nrf2 is free to translocate into the nucleus and bind specific genomic sequences in order to promote antioxidant enzyme transcription. At the same time, there is a positive Keap1-independent regulation mediated by other kinases. The kinases involved in the phosphorylation and subsequent activation of Nrf2 include protein kinase C (PKC), casein kinase II (CK2), protein kinase R (PKR), c-Jun N-terminal kinase (JNK), and extracellular regulated kinases (ERKs) [12,13,14,15]. Finally, brain-derived neurotrophic factor (BDNF) can also promote the activation and subsequent migration of Nrf2 into the nucleus [16].
In addition to its antioxidant activity, Nrf2 is also directly involved in oxidative stress-related pathological processes by regulating their activation. In particular, there is a direct cross-talk between Nrf2 and p62, an autophagy key protein. Nrf2 can promote the expression of genes involved in autophagy, while p62 can compete with Nrf2 in binding to Keap1 in a positive feedback that is associated with cytoprotection [6,17]. In addition, Nrf2 is also involved in a special form of autophagy called mitophagy, the alteration of which allegedly plays an important role in psychiatric disorders. While it preserves mitochondrial integrity, mitophagy entails the elimination of damaged or redundant mitochondria through autophagy [18].
Furthermore, recent studies of Nrf2 found that its inducers promote the suppression of the pro-inflammatory phenotype of microglia through regulating BDNF, the reduction of which is found in many psychiatric disorders characterised by neuroinflammation [16,19,20,21]. The existence of reciprocal regulation has emerged between BDNF and Nrf2; the latter, in connecting with the exon I promoter of bdnf, can activate BDNF; on the other side, BDNF can increase the nuclear translocation of Nrf2, thus promoting its antioxidant activity [16].
Finally, several studies also cast light on the involvement of Nrf2 in ferroptosis, an iron-dependent form of programmed cell death characterised by the accumulation of lipid peroxides (lipids damaged by oxidation). Notably, Nrf2 does not only regulate numerous genes involved in iron metabolism and homoeostasis but also promotes the basal expression of the lipid hydroperoxidase, glutathione peroxidase-4 (GPX4), which converts toxic lipid peroxides to nontoxic lipid alcohols [22].
Impaired response to oxidative stress has been shown in animal models for stress disorders, such as post-traumatic stress disorder (PTSD) [23,24,25,26], but only a few studies have focused on Nrf2 [27,28,29]. PTSD and depression are long considered to represent stress disorders and share common neurobiological patterns [30]. Recently, the Nrf2-depression connection has received attention [2]. We decided to search the literature for studies investigating the ties between depression paradigms in the animal and/or major depressive disorder in humans and Nrf2 as a proxy of a mechanism that counters oxidative stress. Establishing such a relationship would allow us to concentrate on the production of drugs that would promote the search for drugs interfering with intracellular oxidative processes.
2. Methods
To systematically review the ties between depression and Nrf2, we first conducted a PubMed search involving all possible mental and psychiatric disorders and then focused on depression. The inclusion of other than depressive disorders/states was to make sure that depression during the course of other mental disorders was not dealt with. We employed the following search strategy: (psychiatr*[ti] OR schizo*[ti] OR psychot*[ti] OR psychos*[ti] OR depress*[ti] OR MDD[ti] OR BD[ti] OR bipolar[ti] OR Anxiety[ti] OR antidepress*[ti] OR panic[ti] OR obsess*[ti] OR compulsio*[ti] OR “mood disord*”[ti] OR phobi*[ti] OR agoraphob*[ti] OR anorex*[ti] OR anorect*[ti] OR bulimi*[ti] OR “eating disorder*”[ti] OR neurodevelopm*[ti] OR retardation[ti] OR autism[ti] OR autistic[ti] OR ASM[ti] OR adhd[ti] OR “attention-deficit”[ti]) AND nrf2. The choice of the search strategy was based on consultations among all authors.
Following search performance, we characterised the nature of all ensuing records and labelled them accordingly. This resulted in their being either included or excluded. The inclusion/exclusion labelling with the reasons for exclusion is shown in the Online Supplemental material and in Figure 1, where the PRISMA flow diagram is displayed. In carrying out our review, we followed the 2020 Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement [31]. The 2020 PRISMA Checklist may be found in the Online Supplement. We assessed the Risk of bias (RoB) of the included studies with the Cochrane RoB 2.0 tool [32]. We performed an evaluation of the RoB for each included study. The results are shown in the Online Supplement.
Figure 1.
PRISMA 2020 flow diagramme (From [31]: Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. https://doi.org/10.1136/bmj.n71. For more information, visit: http://www.prisma-statement.org/ accessed on 12 February 2023) of the review.
Eligibility was based on being an original study on any animal or tissue, including humans, on investigating depression or depression models and providing data on Nrf2 levels. All other studies were excluded. Excluded were case reports, opinion articles, such as editorials, letters to the editor, comments of other work, reviews, and meta-analyses (however, we hand-searched their reference lists to identify other possibly eligible studies), and studies not providing data. Eligibility for each paper was established with the consensus of all authors obtained through Delphi rounds, in which all authors participated, either in-person or online. The same applied to the compilation of the RoB.
3. Results
Our search, eventually conducted on the 10th of March 2023, yielded 208 results on PubMed, of which 89 studies were eligible, as summarised in Table 1 (human studies, of which two used cell lines in vitro) and Table 2 (animal studies). In particular, there were 78 articles labelled Depression and 11 Depression and Anxiety; these amounted to 89 articles. The remaining 116 studies were excluded. Depression-free articles focused on autism (N = 17), schizophrenia (N = 10), anxiety (N = 9), attention-deficit/hyperactivity disorder (N = 3), insomnia (N = 2), and bipolar disorder alone (N = 1). Other articles that did not meet the inclusion criteria were Opinions (N = 1), Case reports (N = 1), and Reviews (N = 23), while many articles were off-target (N = 49) in that they did not focus any psychiatric disorder and were unfocused in their designs or they were unrelated to the subject of our inquiry. Furthermore, there were two duplicates and a retracted paper, but another paper from the same group had not been retracted and dealt with the same issue as the retracted one. Publication dates spanned from 23-March-2006 to 1-March-2023 for the searched papers and 23-April-2013 to 1-March-2023 for the eligible ones. The included and excluded studies with their reasons for exclusion are shown in the Supplement. The selection process and reasons for exclusion are depicted in the PRISMA flowchart (Figure 1).
Of the 89 studies included in this review, most were conducted on mice only (N = 58; 65.17%), 20 were conducted on rats only (22.47%), and three on both rats and mice (3.37%), four on humans (4.49%), two on human cell lines in vitro (2.25%), one on fish and worms each (1.12%). Of the 58 studies carried out on mice only, 50 used only male animals, seven used only female, and one both used animals of both sexes; 31 used C57BL/6 strains; one reported unspecified C57 mice (which were presumably C57BL/6, based on other articles by the same group of authors), 10 used unspecified Swiss strains, five used CD-1, eight used Balb/c, five ICR, four Kunming, one BXD Recombinant Inbred, and one Murphy Roths Large lymphoproliferative Mouse (MRL/lpr); in eight of these studies, investigators used more than one strain. All 20 studies conducted on rats used only male animals, 14 Sprague Dawley and six Wistar, of which two were Hannover and two albino Wistar, while all three studies that employed both mice and rats were conducted using male-only animals, all three used Sprague Dawley rats (1 also Groningen, a strain characterised by high aggression levels [33]) and C57BL/6 mice. The four human studies included patients and matched controls of both sexes, while the only post mortem study did not report the sex or the age of included patients. The study that used fish employed the Japanese rice fish, medaka (Oryzias latipes), both male and female, and the one that used nematodes used Caenorhabditis elegans, while of the two conducted on cell lines, one used the macrophage RAW26.7 line (primary CD14+ monocytes from human donors of both sexes transformed in macrophages through 1-week Colony Stimulation Factor-1 stimulation) and the other used human neuroblastoma SH-SY5Y cells. Of the 85 studies that specified the sex of the animals they used in their experiments, 79 used males (92.94%) and only 12 used females (14.12%). Limiting the sex of animals to the 81 rodent studies, which constituted the bulk of eligible studies included in this review, it results that 73 studies employed male-only animals (90.12%), while only seven studies (9.59%) used female-only animals. This shows a strong bias toward the use of male animals in depression studies of rodent models that cannot be easily translated to humans, given that the majority of people with depression are women [34,35] or female adolescents [36].
Of the eligible studies, most were conducted in China (N = 49, 55.06%; only three were located in Beijing, China’s capital, while six studies were conducted in the Guangdong Province, i.e., three in Shenzhen, two in Guangzhou, and one in Zhanjiang), eight in Brazil (8.99%) and seven in Spain, of which one was a multinational study shared with other four countries (6.97%), five in Japan (5.62%), four in Egypt (4.49%), three in the US (3.37%), two in South Korea and France (2.25% each), two in Germany and Poland, with one multinational shared (1.35% each), one each in India, Iran, Italy, Nigeria, Pakistan, Serbia, and Turkey (1.12% each), and one in both Romania and Sweden, sharing the same multinational study as the other three (0.22% each). Of the 89 included studies, 15 did not use any specific drug to identify its effects on Nrf2 but rather focused on the effects of specific animal depression models on the entire antioxidant system. These 15 studies were conducted in China (N = 8), Spain (N = 3), Serbia, the USA, Brazil, and South Korea (N = 1 each). Plant extracts or animal tissue extracts were tested in 29 Chinese studies, three Japanese studies, one Pakistani, one Nigerian, and one South Korean study, for a total of 35 studies, representing 39.33% of all included studies, with China accounting for 82.86% of these studies and plant extract using studies for 59.18% of all Chinese studies.
Table 1.
Summary of human studies investigating the role of Nrf2 in depression (in vivo, post mortem, in vitro on cell lines).
| Study | Animal | Paradigm/Model | Location | Design | Results Relating to Nrf2 | Conclusions/Observations |
|---|---|---|---|---|---|---|
| Lukic et al., 2014 [37] | Man; 30 patients with MDD (17 ♁; 13 ♂; Ham-D > 14; age 44.77 ± 7.58 yr) vs. 35 HC (19 ♁; 16 ♂; age 39.49 ± 9.64 yr, younger, p = 0.018) | OS | Belgrade, Serbia | PBMC investigated with WB for Nrf2, Keap1, NF-κB, AOEs (MnSOD, CuZnSOD, GPx, GLR) in MDD patients and HC | ↑ Nrf2, Keap1, NF-κB, MnSOD, CuZnSOD, and CAT in MDD vs. HC in PBMC cytoplasm; ≈GPx and GLR between MDD and HC, but ↓GLR/GPx in MDD; MnSOD, CuZnSOD, and CAT levels correlated directly with Nrf2 levels, while MnSOD and CuZnSOD correlated with NF-κB levels | Impaired oxidative detoxification capacity in MDD, ↓ capacity of GPx to defend from OS in PBMC of MDD patients; the up-regulation of Nrf2 and NF-κB and their down-stream targets MnSOD, CuZnSOD, and CAT indicate OS status in PBMCs of MDD patients |
| Mellon et al., 2016 [38] | Man; 20 unmedicated MDD patients (♂, ♁) + 20 HC | MDD human model | San Francisco, CA, USA | I group = 20 unmedicated MDD subjects; II group = 20 age-, sex- and ethnicity-matched HC, before initiation of AD treatment, and in 17/20 of the unmedicated MDD subjects after 8 wk of sertraline treatment→ transcriptome-driven bioinformatic strategy to evaluate the activity of several transcriptional Ctrl pathways | In leukocytes from unmedicated MDD subjects ↑ transcriptional activity of cAMP response element-binding/activating TF (CREB/ATF) and Nrf2. 8 wk sertraline treatment was associated with ↓ in Ham-D scores and ↓ activity of Nrf2, but not in CREB/ATF activity. Several other transcriptional regulation pathways, including the glucocorticoid receptor, NF-κB and (EGR1–4) and interferon-responsive TFs, showed either no significant differences as a function of disease or treatment | CREB/ATF and Nrf2 signalling may contribute to MDD by activating immune cell transcriptome dynamics that ultimately influence central nervous system (CNS) motivational and affective processes via circulating mediators |
| Martín-Hernández et al., 2018 [39] | Post-mortem dlPFC samples of 30 Caucasian pts with MDD, ethnic origin-, gender-, and age-matched to 30 HC (sex not specified, although it was said that men were more, age of death not declared) | NI and OS in MDD post mortem | Bilbo, Bizcaia, Euskal Herria, Spain | 2 MDD groups: AD-free (N = 15) and AD-treated (N = 15). WB for levels of TLR-4, Hsp60, Hsp70, p-ERK 1/2, p-JNK, p-p38, p38 α/β, I3K, Keap-1, p11,DUSP-2, Nrf-2, NF-κB p65 subunit in cytosol and nucleus of dlPFC neurones | ↓ Nrf2 pathway in pts with MDD. AD treatments do not reverse the trend | ↑ ERK 1/2 (+22%, t = 2.293, p = 0.03) and JNK (+56%, t = 2.468, p = 0.02) expression in MDD pts, but not p38-MAPK, compared to HC. p-JNK/total JNK and p-p38/total p38 ↑ in MDD > HC. AD-free and AD-treated showed no significant ↑ in Keap-1 expression compared to HC. 21% ↓ of nuclear expression of Nrf2 in MDD pts |
| Kubick et al., 2020 [40] | In vitro cells (macrophage cell line RAW26.7 from 3 ♁ and 4 ♂ human donors, treated with LPS) | Drug repurposing, LPS-induced OS | Hamburg, Germany; Madrid, Spain; Bucharest, Romania; Stockholm, Sweden; Garbatka, Poland | RNA-seq Data Analysis, AI workflow (which drugs activate NRF2?), in vitro cells treated with ZT, Protein Assay (anti-NRF2), Chemiluminescence | RAW264.7 cells treated with ZT (10 μM, 16 h) showed ↑NRF2 levels compared to plac-treated Ctrl cells | Nrf2 pathway is a putative regulator of M1 function in depression; Nrf2 is a potential drug target; ZT activates Nrf2 and its downstream targets |
| Goetzl et al., 2021 [41] | Man; 10 MDD Resp patients (6 ♁; 4 ♂; Ham-D > 14; age 39.0 ± 9.4 yr) 10 MDD NResp patients (6 ♁; 4 ♂; Ham-D > 23; age 41.3 ± 11.6 yr) vs. 10 HC (5 ♁; 5 ♂; age 37.5 ± 10.5 yr) | OS | San Francisco, CA; New Haven, CT, USA | Two groups: MDD Resp → sertraline or escitalopram or fluoxetine × 8 wk at sertraline-equivalent doses; MDD NResp → sertaline or escitalopram, fluoxetine or citalopram at sertraline-equivalent doses; ELISA for Nrf2 and MCh proteins | NDEV levels of NRF2 were statistically ↓ in the NResp at BL and Resp at BL groups than in their Ctrl groups; levels ↑ in the NResp and Resp groups after treatment | NDEV levels of MPs of all functional classes, except complex I-6, NRF2 and PGC-1α, were normalised in MDD participants who responded to SSRI treatment but not in those who failed to respond, as assessed by the psychiatrist; the sample was small |
| X. Li et al., 2022 [42] | Human neuroblastoma SH-SY5Y cells | H2O2 induced SH-SY5Y cell damage | Beijing, China | Human neuroblastoma SH-SY5Y cells used to mimic OS damage in vitro. Four groups: untreated Ctrl, H2O2-induced injury model, kaempferol treatment, and ginsenoside rh2 treatment. WB to detect Nrf2, Trx, and Akt1. TrxR activity was Measur with the Solarbio thioredoxin reductase activity Kit | Kaempferol and ginsenoside rh2 ↑ the expression of Akt1 and Nrf2, which boosted the targets in the Akt1/Nrf2/Trx pathways cascade working conjointly. Kaempferol works better than ginsenoside rh2 in the Akt1/Nrf2/Trx pathways | Kaempferol and Rh2 could enhance the activity of the Trx system by up-regulating Akt1 to activate Nrf2 in vitro |
For abbreviations, see note to Table 2.
Table 2.
Summary of animal studies investigating the role of Nrf2 in depression.
| Study | Animal | Paradigm/Model | Location | Design | Results Relating to Nrf2 | Conclusions/Observations |
|---|---|---|---|---|---|---|
| Martín-de-Saavedra et al., 2013 [43] | ♂ WT C517BL/6 mice (Nrf2+/+) and Nrf2 KO (Nrf2−/−) and ♂ Swiss mice (3–4-month-old) | LPS-induced DLB | Madrid, Spain | 1. WT (Nrf2++) and KO (Nrf2--) mice were subjected to behav tests (TST, OFT, SPlT)+ biochemical analysis; WT mice received LPS (0.1 mg/kg via IP)+ SFN (1 mg/kg/day via ip for 7 days); 3. KO mice + Rofecoxib (2 mg/kg/day for 7 days) | Nrf2 deletion resulted in DLB (↑ in the immobility time in the TST and by a ↓ in the grooming time in the SPlT); ↓ of Dopa and Ser and ↑ Glu in the PFC;↑ of VEGF and synaptophysin;↑microgliosis. Nrf2 KO mice treatment with rofecoxib reversed their DLB; SFN in LPS-induced depression of WT mice afforded AD-like effects | Inflammation due to a deletion of Nrf2 can lead to a depressive-like phenotype, while the induction of Nrf2 could become a new and interesting target for developing novel AD drugs |
| Mendez-David et al., 2015 [44] | Adult ♂ C57BL/6Ntac mice, Nrf2 WT (+/+) and knock-out Nrf2(−/−) | Mouse CORT model of DLB | Paris, France | Chronic corticosteroids, chronic fluoxetine 4 wk later; OFT, E + M, NSFT, and SPIT testing during the wk following chronic fluoxetine. Immunoblotting for BDNF, Nrf2, and its downstream targets GCLC, NQO1, and HO-1, in cortical and Hippoc membranes | Chronic fluoxetine restored Nrf2 levels in mouse cortex, as well as GCLC, HO-1, and NQO1 levels that were ↓ by chronic CORT. In the Hippoc, Nrf2 was not ↓, but GCLC, HO-1, and NQO1 levels were ↓; chronic fluoxetine restored GCLC and NQO1 levels, but not HO-1. Chronic fluoxetine ↑ cortical BDNF levels and reversed CORT-induced ↓ in Hippoc BDNF; ↓ cortical and Hippoc BDNF levels in Nrf2(−/−) mice; these were ↑ by chronic fluoxetine | Chronic fluoxetine reverts CORT-induced Nrf2 pathway changes in cortex and Hippoc in a mouse model of depression; Nrf2 enhances BDNF, but fluoxetine enhances cortical and Hippoc BDNF through both Nrf2-dependent and independent pathways |
| Cunha et al., 2016 [45] | Adult ♂ Swiss mice (30–40 g) | Stress-induced DLB | Florianópolis, SC, Brazil | 1. I group: creatine or plac + LY294002 icv (PI3K inhibitor), wortmannin icv (PI3K inhibitor), or plac→TST or OFT 2. II group: lithium chloride (nonselective GSK3 inhibitor) or ARA01441 (selective GSK3 inhibitor)→TST or OFT 3. III group: subeffective doses of creatine + subeffective doses of GSK3 inhibitors (ARA01441 or lithium chloride)→TST or OFT 4.1 IV group: CoPP icv (HO-1 activator)→ TST or OFT; creatine or plac + HO-1 inhibitor ZnPP icv or plac→TST and OFT; creatine or plac + CoPP icv or plac→TST or OFT 5. V group: creatine or plac + rapamycin (mTOR inhibitor) or plac→TST and OFT |
Treatment with creatine↑ Akt and P70S6K phosphorylation, HO-1/Nrf2, GPx and PSD95 immunocontents. The pretreatment with LY294002 wortmannin, ZnPP (HO-1 inhibitor), or rapamycin (mTOR inhibitor) prevented the AD-like effect of creatine in the TST. Subbeffective dose of either the ARA014418, lithium chloride, or the HO-1 inductor CoPP + subeffective dose of creatine ↓ the immobility time in the TST | The AD-like effect of creatine in the TST depends on the activation of Akt, Nrf2/HO-1, GPx, mTOR, and GSK3 inhibition |
| Freitas et al., 2016 [46] | ♁, Swiss mice, WT C57BL/6 mice (Nrf2+/+) and Nrf2 KO (Nrf2−/−) (3–months-old, BW 40–45 g) | Mouse CORT model of DLB | Madrid, Spain | 1. Mice assigned to 6 groups (8 mice each): (a) plac, (b) IMI/plac, (c) agmatine/plac as the Ctrl groups, (d) plac/CORST, (e) IMI/CORST, and (f) agmatine/CORST. 2. Mice were assigned to 3 groups (6 mice each): (a) Nrf2+/+/plac, (b) Nrf2+/+/agmatine, as the Ctrl groups, (c) Nrf2−/−/plac, and (d) Nrf2−/−/agmatine |
Agmatine ↓ CORST-induced DLB, ↑BDNF, synaptotagmin I, Ser and Glut levels; ↓the CORST-induced changes in the morphology of astrocytes and microglia in CA1 subregion of Hippoc; ↑ Nora, Ser, and Dopa levels, CREB phosphorylation, mature BDNF and synaptotagmin I immunocontents, in the Hippoc of Ctrl group. Agmatine’s ability to produce an AD-like effect was abolished in Nrf2(−/−) mice | Chronic administration of a low dose of agmatine is able to abolish the behavioural responses in the TST and splash test elicited by the CORST-induced model of depression by a mechanism dependent on the activation of Nrf2 and neuroplasticity-related signalling in mice |
| Martín-Hernández et al., 2016 [47] | ♂ outbred Wistar Hannover rats initial BW 200–225 g | CMS-induced DLB | Madrid, Spain | The following groups (n 8 each) were used: (1) Ctrl; (2) Ctrl group +ip injection of sterile plac for 7 days (CT þ Veh), (3) CMS group; (4) a CMS group+ ip plac (CMS þ Veh) group. For experiments requiring the ip injection of AD, 3 other experimental groups were used: (5) CMS group + desipramine; (6) CMS group + escitalopram and (7) CMS group +duloxetine→behav tests + biochemical analysis | In the PFC, CMS ↓Akt and PI3K mRNA expression. Desipramine and duloxetine ↑ CMS-induced Akt levels, but only desipramine restored PI3K levels. CMS ↓Nrf2 mRNA and protein expression levels. Nrf2 inhibitors Keap-1 and p-GSK-3β/GSK-3β ratio ↑ after CMS. Desipramine and duloxetine to CMS rats restored the expression of Nrf2, returned Keap-1 to Ctrl levels and showed a trend towards returning the p-GSK-3b/GSK-3b ratio to its Ctrl levels. CMS ↓ NQO-1, GPx1.AD treatment restored GPx1 levels. Desipramine ↑HO-1. PAR g is modulated by the AD treatments in the PFC | Nrf2 pathway is differentially regulated by AD in the PFC and Hippoc. The Nrf2 pathway is involved in the oxidative/nitrosative damage detected in the PFC, and AD has a therapeutic action through this pathway. It seems that Nrf2 is not involved in the effects caused by CMS in the Hippoc |
| Martín-Hernández et al., 2016 [48] | ♂ outbred Wistar Hannover rats; initial BW 200–225 g | CMS-induced DLB | Madrid, Spain | Mice divided into 3 groups (n = 8 each): (1) a Ctrl group; (2) CMS group; (3) CMS group treated with antibiotics (CMS þ ATB)→behav test+ biochemical analysis | CMS protocol ↑intestinal permeability and bacterial translocation. CMS also ↑the expression of the activated form of the MAPK p38 while ↓the expression of Nrf2. The actions of antibiotic administration to prevent bacterial translocation↓MAPK and ↑ Nrf2 pathways | Translocated bacteria could play a role in the pathophysiology of depression through the p38 MAPK pathway, which could aggravate the neuroinflammation and the oxidative/nitrosative damage present in this pathology. Moreover, Nrf2 and its activators may be involved in the consequences of the CMS on the brain |
| Wojnicz et al., 2016 [49] | Adult ♂ Sprague Dawley rats (2.5–3 months old and BW 250–300 g); Nrf2 KO mice | Nrf2 KO mouse model of depression | Madrid, Spain | Seven adult WT rat brain samples→ LC–MS/MS to detect concentrations of neurotransmitters (Adre, Nora, Glu, GABA, DA, 5-HT) and their metabolites (MHPG and 5-HIAA)c. Same procedure in the Hippoc samples of Nrf2 KO mice | LC–MS/MS in adult WT and Nrf2 KO rats showed no significant differences in neurotransmitter values except for GABA, which was strongly ↓ in KO rats | LC–MS/MS method enables rapid quantification of neurotransmitters and their metabolites. It was precise, accurate, sensitive and reproducible. Its application to the mouse model of depression (Nrf2 KO) recorded a↓of Hippoc GABA |
| Yao et al., 2016 [50] | Adult ♂ C57BL/6 mice, aged 8 wk (BW 20–25 g) and 5 wk; CD-1 mice, aged 14 wk (BW 40–45 g) and ♂adult Nrf2 KO (Nrf2−/−) mice | SDS model of depression | Chiba, Japan | WT and KO mice subjected to SDS to induced DLB (exposed to a different CD1 aggressor mouse each day for 10 min for 10 days)→I group: DLB mice + SFN (10 mg/kg), II group: DLB mice + 7,8-DHF (10 mg/kg); III group: DLB mice + ANA-12 (0.5 mg/kg); IV group: Ctrl→behav tests+ biochemical analysis | ↓ Keap1 and Nrf2 in the PFC, CA3 and DG of Hippoc in mice with DLB compared to Ctrl; ↑ serum levels of pro-inflammatory cytokines in Nrf2 KO mice compared to WT mice; ↓ BDNF and TrkB in PFC, CA3 and DG play a role in DLB of Nrf2 KO mice. TrkB agonist, 7,8-DHF, but not antagonist ANA-12, produced AD effects in Nrf2 KO mice. Pretreatment with Nrf2 activator sulforaphane (SFN) prevented the DLB induced after repeated SDS | Keap1-Nrf2 system plays a key role in depression and dietary intake of SFN-rich food during juvenile stages and adolescence can confer stress resilience in adulthood (dietary intake of 0.1% glucoraphanin (a precursor of SFN) containing food during juvenile and adolescent stages also prevented the depression-like phenotype evoked in adulthood, after repeated social defeat stress) |
| Yao et al., 2016 [51] | Adult ♂ C57BL/6N mice BW 20–26 g | LPS-induced DLB | Chiba, Japan | Mice received ip injection of LPS (0.5 mg/Kg)+ Nrf2 activators TBE-31 or MCE-1→behav tests + ELISA | TBE-31 and MCE-1 ↑ nerve growth factor (NGF)-induced neurite outgrowth in PC12 cells in a concentration-dependent manner. TBE-31 or MCE-1↓ an increase in serum levels of TNF-α after LPS administration. In the TST and FST, TBE-31 or MCE-1 ↑ the mobility time after LPS administration | The Nrf2 activators have AD effects in animal models of depression. The novel Nrf2 activators such as TBE-31 and MCE-1 might be potential therapeutic drugs for inflammation-related depression |
| Bouvier et al., 2017 [52] | ♂ Sprague Dawley rats BW 290–310 g (intruder rats); ♂ WT Groningen rats (WTG, resident rats); C57BL/6J background WT (Nrf2+/+) and Nrf2-KO (Nrf2−/−) 6-wk-old mice | SDS and CMS-induced DLB | Paris, France | Rats received intense stress first hit produced (SD) + second stressful hit (CMS)→ behav test and biochemical analysis; antioxidants were Tempol, 4-hydroxy-2,2,6,6-tetramethylpiperidine 1-oxyl; 288 μmol/kg−1·day−1, 7,8-DHF on days 5, 7 and 9 after the end of the social defeat protocol, and t-BHQ (continuous infusion during 6–7 days) | Only vulnerable animals developed a DLB after CMS derived from a persistent state of OS and reversed by treatment with antioxidants. This persistent state of OS was due to ↓ BDNF levels. ↓BDNF→↓ nuclear translocation of Nrf2. In Nrf2+/+ mice, the activation of Nrf2 translocation restored redox homoeostasis and reversed vulnerability to depression. This mechanism was absent in Nrf2−/−mice | Low BDNF levels in vulnerable animals prevented Nrf2 translocation and consequently prevented the activation of detoxifying/antioxidant enzymes, resulting in the generation of sustained OS |
| Zhang et al., 2017 [53] | ♂ adult C57BL/6 mice, aged 8 wk (BW 20–25 g) | LPS-induced DLB | Chiba, Japan | Mice received an injection of LPS and SFN→behav test and biochemical analysis. One subgroup of mice received a dietary amount of 0.1% glucoraphanin (a glucosinolate precursor of SFN) at 5 wk→behav test in adulthood (9 wk) | Pretreatment with SFN blocked an ↑ in the serum TNF-α level and an ↑ in microglial activation after LPS administration (0.5 mg/kg); SFN ↑serum IL-10 after LPS administration. In the TST and FST, SFN ↓immobility time after LPS administration, SFN significantly recovered to Ctrl levels for LPS-induced alterations in the proteins such as BDNF, postsynaptic density protein 95 and AMPA receptor 1 (GluA1) and dendritic spine density. Dietary intake of 0.1% glucoraphanin (SFN precursor) food during the juvenile stage and adolescence could prevent the onset of LPS-induced DBS | Dietary intake of SFN-rich broccoli sprouts has prophylactic effects on inflammation-related depressive symptoms→supplementation of SFN-rich broccoli sprouts could be a prophylactic vegetable to prevent or ↓ the relapse by inflammation in the remission state of depressed patients |
| Zhao et al., 2017 [54] | C57BL/6J ♂ mice (adult, 8-wk-old) BW 20–25 g | CRS and ARS-induced depression | Nanjing, Jiangsu, China | Mice subjected to 2 stress paradigms: 8 wk of CRS and 2 h ARS; mice divided into 2 groups: prolonged (4 wk) and short-term (a single inj) Ipt treatment (i.p. 10 mg/kg)→behav tests+ biochemical analysis (ELISA, RT-PCR, WB, IF) | HPA axis was altered after stress, with different responses to CRS (↓r ACTH and CORT, ↑ AVP, but normal CRH) and ARS (↑ CRH, ACTH and CORT, but normal AVP). Prolonged and short-term Ipt treatment normalised stress-induced HPA axis disorders and abnormal behav in mice. CRS and ARS ↑mRNA levels of TNFα, IL-1_, IL-6 and TLR4 and OS molecules (gp91phox, iNOS and Nrf2) in the hypothalamus. IF showed CRS and ARS ↑ microglia activation (CD11b and TNF_) and OS in neurons (NeuN and gp91phox), which were ↓ by Ipt | Activation of ATP-sensitive potassium channel by ipt normalises stress-induced HPA axis disorder and depressive behav by alleviating inflammation and OS in mouse hypothalamus |
| López-Granero et al., 2017 [55] | BXD RI strains and C57BL/6 WT mice (5–6 wk), ♂, ♁ | BXD recombinant inbred mice depression and anxiety model | New York, NY, USA | Two BXD RI mouse strains, BXD21/TyJ RI, BXD84/RwwJ RI and C57BL/6 WT mice were used with 12 animals per strain and 6 animals per sex→behav tests + biochemical analysis | BXD84/RwwJ RI exhibits social avoidance behav and ↓time in elevated open spaces during the EMT. BXD21/TyJ RI ↓immobility time in the and ♂-specific sensitivity is noted; they also ↑Nrf2mRNA levels (no changes in Keap-1). Same cerebral cortex Gpx1 mRNA in BXD21/TyJ RI, BXD84/RwwJ RI and C57BL/6 WT mice. ↑pro-inflammatory response in ♂ BXD21/TyJ RI compared to BXD84/RwwJ RI and C57BL/6 WT (↑ IL-6 and TNF mRNA) | BXD84/RwwJ RI strain exhibits anxiety disorders, emotional disorders, anxiety-like behav, and social avoidance-like behavior (2) BXD21/TyJ RI strain shows resistance to depression illness |
| Abuelezz and Hendawy, 2018 [56] | Adult ♂ Wistar rats BW 150–200 g | CRS-induced DLB | Cairo, Egypt | Animals were allocated randomly to one of the following 5 groups (n 12 each): non-restrained Ctrl group, CRS group, and 3 other CRS-groups treated with cilostazol, a phosphodiesterase-3 and ROS inhibitor (7.5, 15, 30 mg/kg/day for 4 wk)→SPT, OFT, FST+ Biochemical, RT-PCR, WB analysis | Hippoc cytoplasmic and nuclear Nrf2 expressions were ↓ in CRS-rats, as well as HO-1 and NQO-1mRNA, compared with the Ctrl group. Cilostazol (15 mg/kg/day) prevented ↓nuclear Nrf2, whereas cilostazol (30 mg/kg/day) prevented ↓ in cytoplasmic and nuclear Nrf2 expression. Cilostazol (15 mg/kg/day) prevented ↓ in HO-1, whereas cilostazol (30 mg/kg/day) prevented the decrease in both HO-1 and NQO-1 mRNA | Cilostazol prevented CRS-induced DBL, improving behav tests and hypothalamus–pituitary–adrenal axis hyperactivity. Cilostazol prevented CRS-induced ↑ in Hippoc lipid peroxidation and 8-hydroxy-2′-deoxyguanosine, and a ↓ in antioxidant activities |
| Omar and Tash, 2017 [57] | 50 Adult ♂ Sprague Dawley rats BW 180–220 g | Chronic mild stress model of depression | Cairo, Egypt | Rats divided into the Ctrl (n = 10) and stress (n = 40) groups. Ctrl rats received distilled water. The stress group, subjected to the CMS procedure, was further subdivided into 4 subgroups (n 10 each): I group = distilled water; II group = fluoxetine (10 mg/kg/day); III group = zinc (15 mg/kg/day); IV group = fluoxetine + zinc (treatment for 28 days)→behav tests + biochemical investigations (ELISA, WB, RT-PCR) | Hippoc mRNA and protein levels of Nrf2, HO-1, MTs, GPR39 and BDNF ↑ in response to a combined therapy of fluoxetine and zinc than to either monotherapy. HO-1 and MTs gene expression was correlated with that of Nrf2 in the fluoxetine-only group | Fluoxetine therapy activated the expression of MTs and HO-1 through an Nrf2-dependent pathway. When fluoxetine was escorted by zinc, activated MTs had a positive impact on BDNF through the zinc signalling receptor GPR39, resulting in ↑in neuronal plasticity as well as ↓ of neuronal atrophy and neuronal cell loss |
| Li et al., 2017 [58] | 8- to 10-wk-old ♂ ICR mice | LPS-induced DLB | Ningbo, Zhejiang, China | Mice treated with IL-1β shRNA lentivirus or NS shRNA (Ctrl) lentivirus by DG regions inj + LPS (1 mg/kg, i.p.) or plac→ behav tests (memory deficits with NORT; anxiety-like behaviors with EZM; DLB with SPTand FST). Furthermore, the levels of MDA, SOD, Nrf2, HO-1, TNFα, VGF and BDNF were assayed | IL-1β KO in the Hippoc ↓ the memory deficits, anxiety- and DLB induced by LPS in mice; it also ameliorated the oxidative and neuroinflammatory responses and abolished the ↓ of VGF and BDNF induced by LPS. Finally, the ↑MDA and ↓SOD, Nrf2 and HO1 induced by LPS were completely prevented with IL-1β shRNA | IL-1β is necessary for the oxidative and neuroinflammatory responses produced by LPS and offers a novel drug target in the IL-1β/oxidative/neuroinflammatory/neurotrophic pathway for treating neuropsychiatric disorders that are closely associated with neuroinflammation, OS and ↓ of VGF and BDNF |
| Yang et al., 2018 [59] | 24 ♂ Sprague Dawley rats, 8-wk-old | LPS-induced DLB | Jining, China | Three groups of 8 rats each: Ctrl, LPS, and LPS + NPB. 24 h after last injection, behav tests + brain tissue analysis | Nrf2 ↓ in LPS group, Nrf, HO1 and NQO-1 levels ↓ in NBP group | Prolonged NBP treatment ameliorated LPS-induced DLB, attenuating LPS-induced NI, and OS |
| Gao et al., 2019 [60] | ♂ CD-1 mice BW 23–25 g and 8-wk-old ♂ C57BL/6 J mice | Effects of allicin on DLB | Yichang, China | Five groups 10 mice each: Ctrl, CSDS, CSDS + allicin (2, 10, or 50 mg/kg). SPT, SIT, and FST → Hippoc tissue collected. Inflam mediator levels assayed through ELISA. Iron concentration and iron-related protein expression Measur by WB. OS and apoptosis markers detected by WB | Allicin ↓ production of ROS, MDA NOX4, and ↑ activities of SOD and Nrf2/HO-1 pathway; CSDS mice performed worse than Ctrl on SPT, SIT, and FST; allicin reversed these impairments, with the highest dose being more effective | Microglia activation and ↑ cytokine in Hippoc of CSDS were ↓ by allicin. Content of iron and protein expression of iron metabolism were aberrant in CSDS mouse Hippoc; allicin improved this phenomenon. It also attenuated enhanced neuronal apoptosis and promoted NLRP3 inflammasome suppression (↓ Hippoc ASC, caspase-1, and IL-1β) |
| Fan et al., 2018 [61] | 72 ♂ 220–240 g Wistar rats | CUMS-induced DLB | Jinan, China | Four groups with N = 18/group: (a) Ctrl (non-CUMS), (b) CUMS, (c) ginsenoside-Rg1 pretreatment (40 mg/kg), (d) ginsenoside-Rg1 pretreatment (40 mg/kg) followed by CUMS. Behav tests + brain removed for immunofluorescence assay, immunohistochemistry and TUNEL staining | Ginsenoside-Rg1 ↑ Nrf2 expression and inhibits p-p38 MAPK and p65 NFκB subunit activation within the vmPFC | Ginsenoside-Rg1 prevented depression-like effects in a rat CUMS model. Chronic ginsenoside-Rg1 pretreatment prior to stress exposure suppressed inflam pathway activity via ↓ proinflam cytokine overexpression and microglial/astrocytic activation; ↓ dendritic spine and synaptic deficits parallel to ↑ synaptic-related proteins in vmPFC. ↓ apoptosis induced by CUMS exposure, ↑ Bcl-2 expression and ↓ cleaved caspase-3 and caspase-9 expression within the vmPFC region |
| Chu et al., 2019 [62] | 24 ♂ pathogen-free Sprague Dawley, 6-wk-old rats + 30 WT and 30 Nrf2−/− KO ♂, 6-wk-old mice | Pollution-induced DLB; tested the Nrf2/NLRP3 pathway in DLB | Shijiazhuang, Hebei Province, China | Twenty-four rats randomised into 3 groups: exposed to FiA, UnA, and CA × 12 wk. 30 WT and 30 Nrf2−/− KO mice randomised into clean air exposure and to UnA × 9 wk. Mice and rats had behav testing. Toxic elements in PFC of rats after PM2.5 exposure were Measur by ICP-MS; neurotransmitter and their metabolites’ determination (NA, 5-HIAA, 5-HT, DA, L-Dopa, DOPAC), GSH and GSSG levels in PFC were Measur by HPLC; histopathological changes, neurotrophic factor levels, cytokines, and NLRP3 inflammasome-related protein expression in PFC of rats detected with IHC and WB | CA rats and KO-UA mice displayed depressive-like responses. The NLRP3 signalling pathway was more activated in Nrf2−/− KO than WT mice after PM2.5 exposure × 9 wk | Li, Be, Al, Cr, Co, Ni, Se, Cd, Ba, Ti and Pb were deposited in rat PFC after PM2.5 exposure. Neurotransmitters were significantly altered in PFC of CA rats. The NLRP3 signalling pathway was more activated in Nrf2−/− than WT mice after PM2.5 exposure × 9 wk. The Nrf2/NLRP3 signalling pathway, by modulating inflammation, might play an important role in ambient PM2.5-induced depression |
| Dang et al., 2019 [63] | Adult, 8-wk-old C57BL/6 ♂ mice | PCMS in LPS-induced DLB | Xi’an, 710032, Shaanxi, China | Mice with PCMS (5 min with no mobility × 4 wk) and stress-naïve mice. LPS or plac administered via ip injection → Behav tests (FST, OFT, E + M) → brain removal, analysis through IF to detect IBA-1, IL-1β, Nrf2; WB for NLRP3, ASC, caspase-1, Nrf2, HO-1, NQO-1,TXNIP, Trx and β-actin; Real-time PCR to assess the amount and integrity of total Hippoc RNA | mRNA and protein levels of Nrf2 in stress naïve mice ↓ 26 h post-LPS administration compared with plac-treated mice. Though a significant difference in Nrf2 protein levels was not observed, PCMS mice showed increased gene expression of Nrf2 compared with stress-naïve mice; stress-naïve mice performed worse than PCMS mice on FST, OFT, and E + M | PCMS promotes recovery from LPS-induced behav deficits. Stress naïve mice showed nuclear condensation and acidophilic degeneration after LPS treatment; these neuronal injuries were alleviated in PCMS mice. IF for IBA-1 was used to analyse microglial activation, which was attenuated in PCMS mice. PCMS ameliorated LPS-induced OS, with decreased MDA level, enhanced SOD activities and reduced 8-OHdG. Gene expression of pro-apoptotic Bax was largely ↑ in the Hippoc of stress-naïve mice 26 h post-LPS and ↓ in PCMS mice. PCMS mice showed partially inhibited NLRP3 inflammasome activation (↓ in NLRP3 inflammasome component levels and attenuated IL-1β and TNF-α expression) |
| Gao et al., 2019 [64] | 50 5-wk-old ♂ C57 mice | HFD-induced DLB | Yichang, China | To study OS, MCh function, autophagy, insulin resistance, and NOX/Nrf2 imbalance, mice were randomised into 5 groups of 10 each: Ctrl, HFD, HFD + allicin (50, 100, or 200 mg/kg). After HFD and allicin × 15 wk → behav testing. Blood samples were collected after 12 h fasting periods. All hippocampi were removed for subsequent detection | ↑mRNA and protein expressions of NOX2 and NOX4, ↓Nrf2/HO-1 signalling in Hippoc of obese mice. Allicin ↓ NOX2/NOX4 expression and ↑ Nrf2/HO-1 levels | Allicin ↓ weight of obese mice, metabolic indicators, CORST, IR, and corrected HFD-triggered aberrant insulin signalling. HFD induced DLB, which was ameliorated by allicin.↑ ROS, MDA, protein carbonylation triggered by HFD were inhibited by allicin. HFD caused ↑ protein expression of autophagy in the Hippoc, which was reverted by allicin. Allicin ameliorated OS-induced damage through ↑ antioxidant SOD, CAT, GSH, and GPx activity |
| Arioz et al., 2019 [65] | ♁ Balb/c, 12–14-wk-old mice | OS, LPS-DLB | Izmir, Turkey | Effect of MT on NLRP3 inflammasome activation and SIRT1/Nrf2 pathway. Mice randomised into 3 groups: Ctrl, LPS, MT (30 mg/kg × 4) + LPS (5 mg/kg ip). 24 h later, animals performed behav experiments TST, FST → sacrificed. Hippocampi were isolated and used for further analyses; glial cell culture | MT ↑ Nrf2 translocation to nucleus (WB) and Nrf2 target genes HO-1, NQO1, GSTP1, GCLM (qPCR). Cross-talk between Nrf2 and SIRT1 protective pathways: siRNA-mediated Nrf2 knockdown inhibited basal SIRT1 expression; siRNA-mediated SIRT1 knockdown ↓Nrf2 translocation. The beneficial effects of MT on NLRP inflammasome activation were associated with Nrf2 and SIRT1 | MT ameliorated LPS-induced behav abnormalities in a mouse model of acute systemic inflammation and depression and decreased NLRP3 inflammasome activation in mice hippocampi (qPCR, WB and IF staining). Beneficial actions of MT are partly and significantly dependent on Nrf2 and SIRT1 activation in LPS and ATP-challenged murine microglia |
| Cigliano et al., 2019 [66] | 24 ♂ MRL/lpr mice brain samples (8-, 22- or 17-wk-old) | MRL/MpJ-Faslpr lupus-prone depression murine model | Napoli, Italy | To test CLA and FO modulation of the Nrf2 pathway in a mouse depression model, brain samples from 2 groups (n = 8 each), composed of 8- (Young) or 22-wk old (Old) mice were examined to evaluate the age-dependent occurrence of depressive disorder markers (BDNF, TrkB, Synaptophysin, Synapsin I; Synaptotagmin I, PPAR-α, PPAR-γ and the modification of DHA, C18:1, C16:0, and C18:0 content) with rtPCR and WB. 2 additional groups composed of 17-wk-old mice (n = 8 each) were supplemented with FO or CLA × 5 wk, when they reached old age, and were compared with untreated Old mice | FO or CLA ability in modulating Nrf2 pathway was investigated in brain cortex of all experimental groups. Old animals exhibited higher G6PD and GSR activities. Compensatory hyperactivation of GSR and G6PD, as well as ↑GCL and GSRmRNA levels exhibited by Old mice (p < 0.05), were ↓by FO and CLA. ↑Nrf2 involvement in the antioxidant activity elicited by FO or CLA (↓ Nrf2 content in nuclear extracts of FO + Old and CLA + Old animals) | Old mice exhibit disrupted Redox homoeostasis, compensatory Nrf2 hyperactivation, ↓ DHA, ↓ BDNF and ↓ of synaptic function proteins (Synaptophysin, Synaptotagmin I, Synapsin I) compared to Young mice. FO and CLA relieve almost all depression markers at a level comparable to Young mice, improving Nrf2-mediated antioxidant defences, ↓ auto-antibody titre and TNF-α concentration, ↑ BDNF and synaptic function proteins (FO > CLA) |
| Liu et al., 2019 [67] | ♂ WT C57BL/6 mice (adult, PRMT1+/+) BW 22–25 g; PRMT1 KO (PRMT1−/−) mice with C57BL/6 background | LPS-induced DLB | Liaocheng, China | PRMT1+/+ and PRMT1−/−mice received plac (10 mL/kg) or LPS (0.5 mg/kg) (ip) → behav testing → sacrifice; Hippoc analysed for total RNA with rt-qPCR. pNF-κB p65, NF-κB, Nrf-2, GFAP, PRMT1 and IBA-1, GAPDH were detected with WB. ROS levels in AST were determined using a specific probe | LPS ↓Nrf-2 expression; PRMT1 deficiency countered this effect. Nrf-2 expression in AST ↓ by ML385, an Nrf-2 inhibitor. PRMT1 KO ↓ expression of IL-1 β and TNF-α in LPS-exposed AST; this was prevented by ML385 pretreatment. PRMT1−/− ↓ ROS generation in LPS-exposed cells; levels were restored by ML385 pretreatment | PRMT1−/− mice ameliorated LPS-induced DLB and ↑BDNF and PSD-95 expression. PRMT1 deletion alleviates LPS-induced brain injury; down-regulating LPS-promoted expression levels of GFAP and IBA-1 compared with PRMT1+/+ mice. PRMT1 deficiency ↓IL-1β and TNF-α in Hippoc and PFC of LPS-challenged mice, ↓ pNF-κB, ↑ SOD and GSH-pX activities in Hippoc and ↑ Nrf-2 |
| Rosa et al., 2019 [68] | Adult ♁ Swiss mice (3 months, 30–40 g) | Guanosine AD-like effect via GSK-3β inhibition and MAPK/ERK and Nrf2/HO-1 activation | Florianópolis, Santa Catarina, Brazil | -Effective dose of guanosine (0.05 mg/kg, p.o.)/plac was administered to mice → TST -Sub-effective dose of guanosine (0.01 mg/kg, p.o.)/plac + sub-effective dose of lithium chloride (a non-selective GSK-3β inhibitor, 10 mg/kg, p.o.)/plac → TST, OFT -Sub-effective dose of guanosine/distilled H2O + sub-effective dose of the selective GSK-3β inhibitor, ARA014418 (0.01 μg/site, icv)/plac → TST and OFT -Effective dose of guanosine→ Hippoc and PFC WB for β-catenin and Nrf2 immunocontent -Effective dose of guanosine/plac + MEK1/2 inhibitor (5 μg/site, icv)/plac → TST -Effective dose of guanosine/+ ZnPP (HO-1 inhibitor, 10 μg/site, icv)/plac→ TST, OFT and WB Hippoc and PFC analysis for HO-1 detection |
Guanosine ↓ immobility time on the TST but did not alter OFT parameters. Guanosine ↑ Nrf2 cytosolic fraction immunocontent in Hippoc and PFC, compared to Ctrl. Nrf2 Hippoc nuclear fraction was not altered with guanosine | The combined treatment with sub-effective doses of guanosine (0.01 mg/kg, p.o.) and selective/non-selective GSK-3β inhibitors produced a synergistic AD-like effect in the TST. The AD-like effect of guanosine (0.05 mg/kg, p.o.) was completely prevented by the treatment with MEK1/2 inhibitors, or ZnPP. Guanosine administration (0.05 mg/kg, p.o.) ↑ the immunocontent of β-catenin in the nuclear fraction and Nrf2 in the cytosolic fraction in the Hippoc and PFC. HO-1 immunocontent was also ↑ in the Hippoc and PFC treated with guanosine. Guanosine ↓ depression by ↓ GSK-3β and ↑ MAPK/ERK and Nrf2/HO-1 pathways |
| Huang et al., 2020 [69] | 18 ♂ C57BL/6 mice (7–8-wk-old) | CMS-induced DBL | Shanghai, China | ADSCs were isolated from mouse fat pads and intravenously administered to CMS-exposed C57BL/6 mice at the dose of 1 × 106/wk × 3 wk. Behav test (SPT, FST, TST) + rt-qPCR analysis of brain RNA, ELISA microglia analysis to detect MCP-1, IL-6, TNF-α, and IL-1β; WB with anti-Nrf2, anti-HO-1, anti-NF-κB1, anti-CD29, anti-CD90, anti-CD44, anti-CD105, anti-CD34, anti-vWF, anti-BDNF, anti-TrkB, and anti-GAPDH Abs | CMS promoted DLB, TLR4/NFκB but suppressed Nrf2/HO-1; ADSC treatment had the opposite effect, ↑SPT and ↓ immobility on TST and FST. ADSC and BV2 microglia cell cocultures showed that the ↑ of TLR4 and NFκB induced by LPS was ↓ by treatment with Nrf2-overexpressing vector ADSCs, while the ↓ of Nrf2 decreased the inhibitory effect of ADSCs on LPS-induced TLR4 and NFκB expression. The ↑ of Nrf2 in ADSCs ↓ BV2 LPS-induced inflammatory factor secretion (MCP-1, TNF-α, IL-1β, and IL-6) | ADSC treatment reversed CMS-induced DLB. The BW of the mice in the CMS group slowly ↓ compared to Ctrl, and ADSC treatment restored the CMS-induced BW reduction. ADSC reversed CMS-induced DBS, CMS-induced inflammatory factor expression, and Hippoc microglial polarisation. CMS ↑ MCP-1, TNF-α, IL-1β, and IL-6 expression in serum, but ADSC reversed CMS-induced inflammatory factor production. Immunohisto-chemical detection also showed that the number of apoptotic neuronal cells ↓ with ADSC treatment. BDNF and TrkB ↓ with but ↑with ADSC. CMS induction promoted TLR4/NFκB signalling but suppressed Nrf2/HO-1 signalling, while ADSC treatment had the opposite effect |
| Zborowski et al., 2020 [70] | 24 ♂ adult Swiss mice, 60 days old, 25–35 g | DLB in STZ-induced DM mice | Santa Maria, Rio Grande do Sul, Brazil | Animals separated into 4 groups (n = 6 each): Ctrl; STZ-induced DM; (p-lPhSe); DM + (p-ClPhSe)2. Groups II and IV received STZ at a single dose of 200 mg/kg. After 14 days, DM+ mice (blood glucose ≥ 200 mg/dL) were enrolled. At day 21, mice performed behav tests (LP, TST, FST). For ex vivo assays, brains were removed, and the samples of the whole cerebral cortex were subjected to WB and OS assays | ↓ in Keap1, Nrf2 and HO-1 levels in the cerebral cortex of DM mice compared to Ctrl. (p-ClPhSe)2 ↑ Keap1, Nrf2, and HO-1 levels. A negative correlation was found between glycaemia on the one hand and Keap1, Nrf2 and HO-1 levels on the other | (p-ClPhSe)2 reversed DM+ mice DLB but did not alter mouse spontaneous behaviour; hyperglycaemia; counteracted DM-induced cortical oxidative damage. It did not reverse the ↑ in adrenal gland weight and the DM-induced decrease in GR content. It modulated the Keap1/Nrf2/HO-1 signalling pathway in DM mice and ↓ FJC+ cells (a measure of neurodegeneration) in the cerebral cortex of diabetic mice |
| Casaril et al., 2020 [71] | ♁ BALB/c 5–6-wk-old mice | Tumour-induced DLB | Pelotas, Rio Grande do Sul, Brazil | Mice were injected with 50 μL of tumour cell suspension sc; Ctrl mice were injected with PBS. Once tumours became palpable (day 7), tumour size was monitored wkly, and BW and body temperature were recorded. Treatment with CMI (10 mg/kg, i.g.) or canola oil was initiated at day 14 and continued until day 20. 24 h later, mice were submitted to behav tests followed by killing. PFC and Hippoc samples were analysed | 4T1 tumour-bearing mice had ↑ of NFκB, IL-1β, TNF-α, IDO, COX-2, and iNOS and ↓ of IL-10, Nrf2, and BDNF. CMI treatment ↓ the expression of inflammatory markers and ↑ the expression of IL-10, Nrf2, and BDNF | CMI abolished tumour-induced DLB and cognitive impairment; ↓ tumour-induced NI (↓NFκB, IL-1β, TNF-α, IL-10, IDO, and COX-2) and OS (altered expression of iNOS and Nrf2, ROS, NO, lipid peroxidation, and SOD activity) in mouse PFC and Hippoc |
| Tian et al., 2020 [72] | Adult ♂ Sprague Dawley rats (8–12-wk-old), BW 180–220 g | CUMS-induced DLB | Xi’an, Shaanxi, China | CUMS was used to establish depression and anxiety-like behaviour in rats. The rTMS was performed with a commercially available stimulator for 7 days, and then depression and anxiety-like behav were Measur. Nrf2 expression was Measur by WB and TNF-α, iNOS, IL-1b, IL-6 Measur with ELISA. A small interfering RNA was employed to knockdown Nrf2, after which the neurobehav assessment, Nrf2 nuclear expression, and the amount of inflammation factors were evaluated | CUMS-exposed rats had ↓ Nrf2 expression compared to Ctrl (F1,8 = 2.97, p < 0.05). One-wk rTMS treatment ↑ nuclear Nrf2 protein expression compared to CUMS (F1,18 = 3.48, p < 0.05) | Application of rTMS exhibited significant AD and anxiolytic-like effects associated with ↑ Nrf2 nuclear translocation and ↓ level of TNF-α, iNOS, IL-1β, and IL-6 in the Hippoc. Following Nrf2 silencing, AD and anxiolytic-like effects produced by rTMS were abolished. Moreover, the ↑ of Nrf2 nuclear translocation, and the ↓of TNF-α, iNOS, IL-1β, and IL-6 in Hippoc mediated by rTMS, were reversed by Nrf2 knockdown |
| Li et al., 2020 [73] | 6-wk-old ♂ ICR mice, 20−22 g | LPS-induced DLB | Liaocheng, ShanDong, China | For the acute inflammation experiment, mice received ip plac or ip Fen (10, 20 and 40 mg/kg) × 7 days prior to LPS injection. After behav tests, all mice were sacrificed. Blood was collected. Brain tissues were isolated for further analysis (siRNA, WB) | Fen dose-dependently ↑ Nrf2 expression from mRNA and Nrf2 protein levels and ↓ Nrf2 ubiquitination. Fen treatment ↑ Nrf2 expression and nuclear translocation in mouse bEnd.3 cells, promoting Nrf2-ARE transcription activity. Nrf2, HO-1, NQO1, and GCLM mRNA; Fen-induced protein expression levels were abolished by Nrf2 knockdown | Fen ↑ antioxidant capacity in bEnd.3 cells after LPS exposure: ↑ SOD, ↑ GPx, ↑ CAT, ↓ ROS, ↓ MDA; ↓ apoptotic rate promoted by LPS; ↓ IL-1β, IL-18, IL-6, TNF-α, and NO; ↓ TNF-κB nuclear expression; ↓ phosphorylation of IKKβ, IκBα and NF-κB. Fen had minimal impact on mouse histological changes and could alleviate symptoms of LPS-induced DLB |
| Nakayama et al., 2020 [74] | Adult ♂ and ♁ WT Japanese rice fish (medaka, Oryzias latipes) | Seasonal changes-induced DLB | Nagoya, Japan | Medaka fish under winter conditions (SC) were divided into 2 groups: one remained in SC with the other transferred to summer-like conditions (LW). 2 wk later, behav tests. Metabolomic and transcriptomic whole brain analyses (microarray analyses, qPCR). Drug screening was conducted to treat winter-induced depression (celastrol) | Inactivation of Nrf2-mediated antioxidant response under winter-like conditions. Celastrol induced Nrf2 expression and NRF2 target genes (GSTω1, GSH, GPx, PG reductase 1, proteasome subunit α type-6,and β type-7 and c-x-c chemokine receptor type 2) | SC in medaka fish: ↓ sociability; ↑ anxiety-like behav; ↓ in circadian clock genes (PER2, PER3, BMAL1, CLOCK, NPAS2, CRY2); ↓ GSH, tryptophan, and tyrosine;↑ inflammation markers (IL6, IL10, and BAFF, IL1R2); ↑serotonin levels but ↓ serotonin turnover, ↑glutamate and ↓ taurine; inactivation of RAR and glucocorticoid receptor signalling, with HPA dysregulation. Celastrol activated Nrf2 pathway |
| Ali et al., 2020 [75] | 8–10 wk ♂ C57BL/6J mice, divided in 5 groups of 6: normal, plac, LPS (1 mg/kg/day), LPS + MT (10 mg/kg/day), LPS + Fluoxetine (10 mg/kg/day), MT (10 mg/kg/day) | OS; LPS-induced DLB | Shenzhen, Guangdong, China | Open field test, Sucrose preference test, FST, TST, ROS-Measur; ELISA, IF, WB for ATG gene products and FOX03a | ↑ NF-κB signalling in LPS-treated mice, associated with alterations of redox molecules (Akt, Nrf2, HO-1), which were reversed by MT treatment | MT ↓LPS-induced DLB and autophagy impairment in the brain (via FOX03a signalling), ↓LPS-induced OS and NInfl |
| Wang et al., 2020 [76] | 6 wk ♂ C57BL/6J mice, divided in 5 groups of 8: Ctrl; (10 mg/kg) PB; CUMS; CUMS + (10 mg/kg) PB; CUMS + (10 mg/kg) IMI | CUMS-induced DLB mouse model; OS | Nanchang, Jiangxi, China | SPT, OFT, FST, TST, commercial kit for ROS, WB, TUNEL assay | PB treatment → Nrf2 and HO-1 expression, indicating that PB alleviated DLB in mice via activating Nrf2/HO-1 signal pathway | PB alleviated the ↓of sucrose preference and BW, ↓CUMS-induced DLB, ↓ ROS concentrations and inhibited cell apoptosis in Hippoc of CUMS-induced mice. PB ↓ DLB via inhibiting OS and NI, resulting in ↓cell apoptosis in CUMS-induced mice |
| Liao et al., 2020 [77] | 24 ♂ Sprague Dawley rats, 3 groups of 8: Ctrl; CMS group (×4 wk); CMS + SalB (30 mg/kg/day) | DLB in CMS-treated rats | Changsha, Hunan, China | WB, PCR (biomarkers of NI); SPT, FST, NSFT | SalB reversed CMS-induced up-regulation of the gene expression of IL-6, IL-1β, and TNF-α in the Hippoc; SalB → anti-inflammatory effect by activating the Nrf2 signal | SalB could alleviate CMS-induced damage to Hippoc neurones. SalB normalised behav changes in CMS rats |
| Severo et al., 2020 [78] | 46 ♂ 32-day-old Swiss mice in 4 groups: Ctrl; receiving each 4 cncs, 7 cncs; 10 cncs | Protocol of recurrent cncs (4, 7, or 10) | Santa Maria, Rio Grande do Sul, Brazil | TST, HRR, MCh respiration assays, estimation of ROS production and SOD activity, WB | Recurrent cncs did not alter SOD activity, but ↑ expression of NRF2 and SOD2 | Cncs ↓MCh oxygen flux vs. Ctrl; cncs do not induce significant changes in TST |
| Ali et al., 2020 [79] | 8 wk C57BL/6J ♂mice in 7groups of 6): normal plac, LPS (1 mg/kg/day), LPS + MT (10 mg/kg/day), LPS + Fluoxetine (10 mg/kg/day), MT (10 mg/kg/day), LPS + MT + luzindole (5 mg/kg/day), LPS + luzindole | LPS induced-DLB | Shenzhen, Guangdong, China | OFT, SPT, FST, TST, Serum ROS Measur, TBARs assay, ELISA (IL-6, IL-1B, TNFa), Immunofluorescence, WB (Nrf2, p-NFkB, NFkB, p-GSK-3β, GSK-3β, Sirt, Ho-1, GAPDH) | MT treatment significantly ↑ Nrf2 and anti-inflammatory protein HO-1 expression which was down-regulated in the presence of MT receptor (MT1/MT2) inhibitor, suggesting that MT regulates NF-kB/Nrf2/HO-1 expression in a receptor-dependent manner | MT suppressed LPS-induced DLB, ↓cytokines level, ↓oxidative stress, and normalised LPS-altered Sirt1, Nrf2, and HO-1 expression |
| Camargo et al., 2020 [80] | ♂ Swiss mice (30–40 g, 45–60 days of age) divided into 8 groups: plac + plac; plac + ketamine (0.1 mg/kg); plac + guanosine (0.01 mg/kg); (4) plac + ketamine + guanosine; CORT + plac; CORT + ketamine; ketamine+ guanosine; CORT + ketamine + guanosine | CORT-induced animal model of depression | Florianópolis, Santa Catarina, Brazil | Behav tests (TST, OFT, SPlT); WB (GR, NF-κB, IDO-1, GLT-1, Nrf2, HO-1); biochemical analysis (Glutamine synthetase activity, determination of antioxidant enzyme activities and OS markers) | CORT administration ↓ Nrf2 (cytosolic fraction) and HO-1 immunocontent in the Hippoc; a single coadministration with KT + GN could not restore CORT-induced down-regulation on Hippoc Nrf2 and HO-1 | Single administration of ketamine (0.1 mg/kg, i.p.) + guanosine (0.01 mg/kg, p.o.) ↓ DLB and Hippoc slice impairments induced by CORT. The behav response obtained by Ketamine + Guanosine was paralleled by the re-establishment of the CORT-induced molecular alterations on Hippoc GR, NF-κB, IDO-1, and GLT-1 immunocontent |
| Park et al., 2020 [81] | 7-wk-old ♂ C57BL/6 mice | Reserpine-induced depression and in vitro LPS-stimulated BV2 microglia | Daejeon, South Korea | Behav tests (OFT, TST, FST); Electrospray Ionisation Mass Spectrometry; IF (for BDNF, cAMP, CREB); ELISA (IL-6, IL-1b, TNF-a, and IL-10); PCR (Il1b, Il6, TNFα, NOs2, Cox2, Hmox1); WB (iNOS, NF-kB p65, HO-1, Nrf2, p-CREB, CREB, p-p38, p38, p-Erk, Erk, p-JNK, JNK, p-Akt, Akt, and BDNF) | BTS ↑ nuclear translocation of Nrf2 and p-CREB, which act as upstream modulators of HO-1 expression in BV2 microglia | BTS has considerable potential as an anti-NI and AD agent, as it has clear effects on depressive behaviours and associated factors caused by reserpine-induced depression. BDNF and pCREB in the Hippoc ↑ in BTS-treated mice vs. reserpine-treated mice. Il1β, Il6, and TNFα mRNA levels in BTS mice were ↓ vs. reserpine-treated mice. |
| Zhu et al., 2020 [82] | Adult ♂ Sprague Dawley rats BW 200–220 g, 3 groups: Ctrl, low-dose Hsd (50 mg/kg, Hsd-L), high-dose Hsd (50 mg/kg, Hsd-L) | STZ model of type 1 diabetes | Xuzhou, northwestern Jiangsu province, China | Behav test (OFT, TST); ELISA (CORST); Immunohistochemistry (Nrf2) | Hsd caused significant ↑ in Nrf2 levels and up-regulated g-glutamylcysteine synthetase, target gene of Nrf2/ARE signalling | Hsd ameliorate DLB and anxiety-like behaviours of diabetic rats, which are mediated by the enhancement of Glo-1, possibly due to the activation of the Nrf2/ARE pathway |
| Liao et al., 2020 [83] | ♂ Sprague Dawley rats (BW 180–220 g) | CUMS-induced depression model in rats | Changsha, Hunan, China | Rats randomised into 3 groups of 8: Ctrl, CUMS, CUMS + CUR. After 4 wk: behav tests (SPT, FST, OFT, NSFT); Determination of serum CORST; Hippoc: WB (NOX2, 4-HNE, Nrf2, pCREB, CREB, PSD-95, synaptophysin, PCNA), PCR (Nrf2, NQO-1, HO-1); Immunohistochemical staining | Nrf2 signal pathway was inhibited under CUMS, and chronic administration of CUR enhanced Nrf2 translocation from cytoplasm to nucleus and ↑ expression of antioxidant enzymes through Nrf2 signal pathway, thereby protecting the brain against CUMS-induced depression | CUR relieves depressive-like state through the mitigation of OS and the activation of Nrf2-ARE signalling pathway. DLB in CUMS-treated rats successfully corrected after CUR; CUR could effectively ↓ protein expression of OS markers (NOX2, 4-HNE, and MDA) and ↑ the activity of CAT; CUR also reversed CUMS-induced inhibition of Nrf2-ARE signalling pathway along with ↑ the mRNA expression of NQO-1 and HO-1; CUR also ↑ the ratio of pCREB/CREB and synaptic-related protein (BDNF, PSD-95, and synaptophysin); CUR could effectively reverse CUMS-induced reduction in spine density and total dendritic length. |
| Qu et al., 2021 [84] | ♂ adult C57BL/6 mice and ♂ adult Nrf2 KO mice (Nrf2−/−) mice | Nrf2 KO mice depression-like phenotypes | Chiba, Japan | Behav tests (LMT, TST, FST, SPT); brain mPFC homogenates: WB for GluA1 and PSD-95 | (R)-ketamine(10 mg/kg) could produce rapid-acting and long-lasting AD-like effects in Nrf2 KO mice via the BDNF-TrkB signalling pathway | (R)-KT can produce rapid and long-lasting AD-like actions in Nrf2 KO mice via TrkB signalling: (R)-KT significantly attenuated TST and FST ↑ immobility in Nrf2 KO mice; on the SPT, (R)-KT significantly ameliorated ↓ SPT preference. ↓ expression of GluA1 and PSD-95 in the mPFC of Nrf2 KO mice was significantly improved after a single (R)-KT injection and pretreatment with the TrkB antagonist ANA-12 (0.5 mg/kg) blocked the rapid and long-lasting AD-like effects of (R)-KT. ANA-12 significantly antagonised the beneficial effects of (R)-KT on ↓ expression of synaptic proteins in the mPFC |
| Li et al., 2021 [85] | Adult C57BL/6J ♂ mice BW 25–30 g | LPS-induced DLB | Shenzhen, Guangdong, China | Animals were divided into four groups of 10: Ctrl, LPS (2 mg/kg/day), LPS + Ibrutinib (50 mg/kg/day), and Ibrutinib (50 mg/kg/day). Behav tests (OFT, SPT, FST); ROS, NO, H2O2; TBAR Assay, ELISA, IF, Golgi staining, WB (BDNF, Nrf-2, NF-κB, HO-1) | Ibrutinib alleviated redox signalling changes, including altered LPS-induced Nrf2, HO-1, and SOD2 expression; ibrutinib, in the presence of LPS, ↑ the expression of Nrf2 and its target proteins, including HO-1 and SOD2 | Ibrutinib ↓ LPS-induced DLB and NI by inhibiting NF-κB activation, ↓ pro-inflammatory cytokine levels, normalising redox signalling and its downstream components, including Nrf2, HO-1, and SOD2, and glial cell activation markers, such as IBA-1 and GFAP; ibrutinib ↓ LPS-activated inflammasome activation by targeting NLRP3/P38/Caspase-1 signalling. LPS ↓ the number of dendritic spines and expression of BDNF, and synaptic-related markers, including PSD95, SNAP25, and synaptophysin, were ↑ by ibrutinib in mouse Hippoc |
| Yan et al., 2021 [86] | ♂ Kunming mice BW 18–22 g | D-GalN-induced animal model | Shenyang, Lioning, China | Four groups: Ctrl, D-GalN, NKT (5 mg/kg), NKT (10 mg/kg). Behav tests (SPT, FST, TST, NFT), WB (Ho-1, Nrf-2) | NKT can effectively ↓ OS in the model group, which may be caused by activating the Nrf2/HO-1/NQO1 signalling pathway, promoting the nuclear translocation of Nrf2, and ↑ the expression of downstream antioxidant protein HO-1 and NQO1 to weaken OS | NKT (5 mg/kg) co-treatment remarkably ameliorates D-GalN-induced anxiety- and depression-like behaviours. NKT could ↑ serum alanine transaminase and aspartate transaminase levels, alleviate hyperammonaemia-induced OS by activating Keap1/Nrf2/HO-1 antioxidant pathways, ↓ the expression of inducible NOs and NOX2 in Hippoc and prefrontal cortex, ↑ the vitality of SOD, ↑ catalase and GSH levels in serum, liver, and brain, and significantly ↓ the generation of MDA. NKT also ↓ the level of ammonia in serum and brain and ↑ the activity of glutamine synthase in the Hippoc and prefrontal cortex |
| Herbet et al., 2021 [87] | ♂ adult Albino Swiss mice BW 25–35 g | Mouse CORT model of DLB | Lublin, Poland | Five groups of 8: (1) Ctrl; (2) stress Ctrl or positive Ctrl of depression: CORST (20 mg/kg) for 21 days; (3) fluoxetine (10 mg/kg) and CORST for 21 days; (4) Mito-TEMPO (1 mg/kg) and CORST; (5) fluoxetine, Mito-TEMPO and CORST. Behav tests (FST, TST). Evaluation of the level of mRNA expression of Adora1, Ogg1, Msra, Nrf2 and Tfam in mouse Hippoc | ↑ of Ogg1, Adora1 and Nrf2 in the Hippoc of mice receiving CORST and Mito-TEMPO as compared to the CORST Ctrl group | Behavioural research data showed the AD effect of fluoxetine and Mito-TEMPO administered to mice alone and in combination. Molecular findings indicate a significant impact of chronic stress on the oxidation-reduction balance and an antioxidant effect of Mito-TEMPO. The results obtained in the study suggest that Mito-TEMPO protects DNA against oxidative damage and may be beneficial in the way of cellular function improvement under the conditions of chronic stress. Adora1, Msra, Nrf2 and Tfam genes may be involved in mediating the antioxidant effect of the combined fluoxetine–Mito-TEMPO treatment |
| Yao et al., 2021 [88] | ♂ adult C57BL/6 mice (8-wk-old, 20–25 g BW), CD-1 mice (14-wk-old, 40–45 g BW), and ♂ adult Nrf2 homozygous KO mice (Nrf2−/−) | LPS-induced and CSDS models of depression; Nrf2 KO mice | Guangzhou, Guangdong, China | PCR for Nrf2, Bdnf, Gapdh genes, and BDNF; WB; Behav tests (locomotion, TST, FST) | Nrf2 activator SFN showed AD-like effects in the LPS-induced and CSDS models of depression by ↑ the expression of BDNF | Activation of Nrf2 by SFN showed fast-acting AD-like effects in mice by activating BDNF, ↓ expression of its transcriptional co-repressors (HDAC2, mSin3A, and MeCP2), and restoring normal synaptic transmission; in contrast, SFN did not affect the protein expression of BDNF and its transcriptional repressor proteins in mPFC and Hippoc, nor did it ↓ DLB and abnormal synaptic transmission in Nrf2 KO mice. In the CSDS mouse model, Nrf2 and BDNF protein levels in mPFC and Hippoc were ↓ compared to Ctrl and CSDS-resilient mice; in contrast, protein levels of BDNF transcriptional repressors in the CSDS-susceptible mice were ↑ than those of Ctrl and CSDS-resilient mice |
| Salama et al., 2021 [89] | Adult ♂ Wistar albino rats, BW 150 ± 20 g | Ciprofloxacin-induced depression | Cairo, Egypt | Tested camphor as AD. 5 groups. I (normal Ctrl): normal plac. II: camphor (10 mg/kg; i.p.) × 21 days. Group III (depression Ctrl): ciprofloxacin only. Groups IV and V: ciprofloxacin + camphor (5 and 10 mg/kg; i.p.) × 21 days. Behav tests (FST, activity cage, and Rotarod). Measur of OS and antioxidant biomarkers (MDA, NO, Nrf2), inflammatory biomarkers (TLR4, TNF-α), neurotransmitters; histopathology | Camphor ↑ catalase and Nrf-2 activities, ↓ NO, MDA, TNF-α, TLR4 serum levels, and ↑ brain contents of 5-HT, DA, GABA, and P190-RHO GTP protein, normalising fronto-cortical neuronal cell structure and function | The beneficial effect of camphor as AD could be mainly attributed to its antioxidant and anti-inflammatory abilities that ↑ catalase, Nrf-2 expression, and ↓ NO, MDA, TNF-α, and TLR4 production. In addition, it up-regulated P190-RHO GTP protein, an actin reorganiser, thus improving locomotor activity and restoring neurotransmitter function and structure, countering histopathological changes; hence, it may be beneficial in ↓ ciprofloxacin-induced depression |
| Naß et al., 2021 [90] | C. elegans strains (N2 WT, QV225 skn-1 deficient, and VC289 prdx2 deficient) | Skn-1 (which corresponds to the human Nrf2) and prdx2-deficient mutants of C. elegans | Mainz, Germany | Examination of the antioxidant activity of UA compared to fluoxetine in C. elegans WT and skn-1- and prdx2-deficient strains through H2DCF-DA and jugl., and osmotic and heat-stress assays. Analysis of the binding of UA to human PRDX2 and Skn-1 proteins by molecular docking and microscale thermophoresis | UA exerted stronger antioxidant activities than fluoxetine. Additionally, induction of stress resistance towards osmotic and heat stress was observed. qRT-PCR showed UA to up-regulate skn-1 and prdx2 expression | UA exerted antioxidant effects and induced stress resistance through Prdx2 and Skn-1. Additionally, it ↑ the expression of antioxidant genes and prolonged lifespan. In many of these experiments, UA outperformed fluoxetine |
| Yang et al., 2021 [91] | Adult ♁ BALB/c (10-wk) mice, BW 20–22 g | Cancer-related fatigue model of depression | Changsha, Hunan, China | Test effect of Chinese herb couple Fuzi and Ganjiang (Aconitum carmichaelii Debx and Zingiber officinale Rosc) on NI (tested on cultured BV2 microglial cells; tested viability, LPS-induction), which in turn induces cancer-related fatigue (tumour inoculation). NO detected through NO2-, ROS, ELISA, IF, Nrf2 siRNA transfection of BV2 cells, WB, immunohistochemistry, Hippoc and cortex; 7 days post-inoculation, mice randomised into Ctrl, tumour-model, minocycline, low-, intermediate- and high-dose Fuzi and Ganjiang → FST, OFT, TST, and E + M → sacrifice | Fuzi and Ganjiang did not affect BV2 viability, ↓ TNF-α, ↓ IL-6, and ↓ ROS production, abolished iNOS-mediated NO, ↓ COX2-mediated prostaglandin E2 and ↓ NF-κB in LPS-induced BV2 microglia; in the same cells, Fuzi and Ganjiang ↑ Nrf2/HO-1 signalling pathway; low and high doses ↓ immobility in the TST, high dose ↑ open arm time in the E + M and ↓ immobility in the FST in tumour-model mice, ↓ iNOS and COX2 in PFC and Hippoc of tumour model mice with cancer-related fatigue–induced depression | Fuzi and Ganjiang counteracted NI and related depression by activating the Nrf2 pathway |
| Zhu et al., 2021 [92] | ♂ Sprague Dawley rats (BW 200–220 g) | DM-associated DLB | Xuzhou, northwestern Jiangsu province, China | Rats received 60 mg/kg, ip STZ injection and were divided into 3 groups of 10 each: DM model group; low dose hesperetin-treated DM group (50 mg/kg), high-dose hesperetin-treated DM group (150 mg/kg) + normal Ctrl → behav tests, biochemical analysis | Hesperetin ↑ Nrf2 and its related genes and proteins (Glo-1 and γ-GCS); ↓ high glucose-induced neuronal damage through the activation of the Nrf2/ARE pathway in SH-SY5Y cells | Hesperetin ameliorated DM-associated anxiety and DLB in rats (↑OFT, SPT, and FST performance) and ↑ Nrf2/ARE pathway activation |
| Wang et al., 2021 [93] | ♂ Sprague Dawley rats (BW 160–180 g) | CUMS-induced depression | Zhengzhou, Henan, China | Rats divided into 5 groups (10 rats each): Ctrl, catalpol; CUMS model; CUMS + catalpol; fluoxetine +CUMS→ behav tests (OFT, SPT, and FST) before and after stress/drug; hippoc for histological and biochemical analysis | CUMS caused ↓ mRNA and protein expression of Nrf2 and HO-1 in rat hippoc, whereas separate administration of both catalpol and fluoxetine reversed CUMS-induced Nrf2 and HO-1 abnormalities | Catalpol improved OFT, FST CUMS-induced abnormalities; ↑ Hippoc PI3K, Akt, Nrf2, HO-1, TrkB, and BDNF (↓ in CUMS-rats); ↑ the Hippoc SOD, catalase, GPX, GSTs; ↑ glutathione levels, ↓ in thiobarbituric acid reactive substances level in CUMS-induced depression |
| Wu et al., 2021 [94] | Adult ♂ Kunming mice (BW 18–22 g, 3–4-wk-old) | Hyperglycaemia-induced DLB | Zhengzhou, Henan, China | Ten mice selected as the Ctrl group. 80 mice received STZ (150 mg/kg once, ip) injection; 50 mice selected (blood glucose >200 mg/dL) and divided into 5 groups (10 mice each):the plac group; the catalpol (5–10–20 mg/kg)) group; the fluoxetine (20 mg/kg)+ metformin (100 mg/kg) group→ behav tests+ biochemical analysis on brain tissues | The levels of Nrf2 and HO-1 in hippoc and frontal cortex of STZ-induced hyperglycaemic mice significantly ↓, while 20 mg/kg catalpol reversed the abnormal Nrf2 and HO-1 protein levels | Catalpol reversed TST, FST, and OFT abnormalities and abnormal PI3K and Akt phosphorylation; ↑Nrf2-HO1, SOD, GPX and GSTs; ↓ GSH and MDA in hippoc and frontal cortex of STZ-induced hyperglycaemic mice with DLB |
| Rahman et al., 2021 [95] | Adult BALB/c ♂ mice BW 25–30 g (7–8-wk-old) | LPS-induced DLB | Zhengzhou, Henan, China | Mice divided into 5 groups of 10: (1) plac Ctrl; (2) Plac-LPS (LPS); (3) Xn-LPS (Xn = 10 mg/kg BW, i.g.) (4) Xn-LPS (Xn = 20 mg/kg BW, i.g.); (5) Fluoxetine-LPS→ behav tests; blood collected brain tissues collected for biochemical analysis | Xn significantly (p < 0.001) ↑ Nrf2 and HO-1 expression in the Hippoc, ↓ OS | Pretreatment with Xn (10 and 20 mg/kg, i.g.) reversed the behav impairments (FST and TST) with no effect on Locomotion; 20 mg improved anhedonic behavior (SPT). Xn dose-dependently prevented the LPS-induced NI, OS and nitrosative stress; ↓ activated gliosis via ↓ of Iba-1 and GFAP in hippoc; ↓ the expression of p-NF-κB and cleaved caspase-3 |
| Tao et al., 2021 [96] | 60 ♁ C57BL/6J mice BW 18–22 g | CUMS induced DLB | Nanjing 210023, China | Four groups: Ctrl, CUMS, CUMS + Magnolol (50 mg/kg, i.g. × 3 wk), and CUMS + Magnolol (100 mg/kg, MA-H i.g. × 3 wk). → behav tests (SPT,OFT,SFT,TST) → sacrificed → Hippoc tissue collected; ELISA for TNF-α, IL-1β, IL-6 IL-4, IL-10; PCR for Arg1, Ym1, Fizz1 and Klf4; Flow cytometry for ROS. Immunofluorescence for Iba-1 + CD16/32+ and Iba-1 + CD206+; WB for Nrf2, HO-1, NLRP3, caspase-1 p20 and IL-1β | Magnolol ↑ Nrf2, HO-1; ↓ NLRP3, caspase-1 p20, IL-1β both in vivo and in vitro. Magnolol ↓ ROS concentration, promoted Nrf2 nucleus translocation, and prevented Nrf2 ubiquitination. Nrf2 knockdown abolished the Magnolol-mediated microglial polarisation | Magnolol attenuated CUMS-stimulated depression by inhibiting M1 polarization and inducing M2 polarisation via Nrf2/HO-1/NLRP3 signalling |
| Huang et al., 2021 [97] | ♂ Sprague Dawley rats BW 180–220 g | CUMS induced depression model | Wenzhou, Zhejiang, China | Rats divided into 4 groups of 8: Ctrl, CUMS, CUMS+ NC (Hippoc injection LV-pCDH-Nrf2-NC) CUMS+ Nrf2 (Hippoc injection LV-pCDH-Nrf2) → behave tests (SPT, EMZ, OFT, FST, MWM) → sacrifice → Hippoc tissue collected →ELISA for TNF-α, IL-1β, IL-10; WB for Nrf2/β-actin/Wfs1; PCR for miR-17-5P/Nrf2/Wfs1 | Nrf2 weakly expressed in CUMS-treated rats. Nrf2 ↓ cognitive dysfunction and inflammatory brain injury. Nrf2 ↓ in CUMS treated rats. Nrf2 up-regulation reversed the trends in behav tests and the changes inflammation-related cytokine levels in CUMS-treated rats. Nrf2 inhibited miR-17-5p → limit Wfs1 transcription. miR- 17–5p ↑ or Wfs1 ↓ reversed the role of Nrf2 in reliving inflammatory injury of murine Hippoc neurones | CUMS group ↓ performance in behave tests. CUMS treated group ↑ TNF-α, IL-1β and ↓ IL-10 |
| Song et al., 2021 [98] | Adult ♂ Kunming mice BW 16–18 g | CORST-induced depression model | Zhengzhou, Henan, China | Four groups of 11: Ctrl group, CORST group, the CORST + catalpol (20 mg/kg) and CORST + fluoxetine (20 mg/kg i-gastr) 21 days → behav test (FST,OFT,TST) → sacrifice → Hippoc tissue, cortex and serum collected → WB for NF-κB/Nrf2/HO-1; ELISA for IL-1β, TNF-α, iNOS, and NO. Cortical tissue ELISA for IL-1β, TNF-α, iNOS, and NO. Detected in the serum level of CORST, ACH and CRH | Catalpol ↑ Nrf2 and antioxidant defence (GSH/GST) and ↓ oxidative damage | CORST → DLB in mice in behav tests, ↑ serum CORT/CRH/ACTH, ↑ NF-κB in the Hippoc and frontal cortex, and ↓ Nrf2. CORST ↑ IL-1β, TNF-α, iNOS NO and MDA while ↓ GSH and GST in Hippoc and frontal cortex. Catalpol administration suppressed the abnormalities of the above indicators |
| Guan et al., 2021 [99] | Adult ♂ Kunming mice BW 18–22 g | CUMS- induced depression model | Zhengzhou, Henan, China | Six group of 10: Ctrl group, CUMS group (CUMS + double distilled water i-gastr), CUMS + quercetin (10 mg/kg i-gastr), CUMS + quercetin (20 mg/kg i-gastr), CUMS + quercetin (40 mg/kg i-gastr), CUMS + FH (20 mg/kg i-gastr) for 21 days → behav test (SPT,OFT,FST) → sacrifice → Hippoc tissue collected → WB for PI3K/Akt/Nrf2/HO-1 detection kits for iNOS/NO/SOD/GST/GPx/GSH/MD | CUMS ↓ PI3K/Akt, Nrf2/HO-1in the Hippoc of mice. (all p < 0.01); Quercetin (40 mg/Kg) ↑ PI3K/Akt, Nrf2/HO-1 | CUMS for 21 days ↓ performance in behav tests (SPT,OFT,FST) quercetin(40 mg/kg) ↑ (SPT,OFT,FST) performance; CUMS ↓ SOD GST (both p < 0.01),quercetin at 40 mg/kg ↑ SOD,GST. CUMS ↑MDA, NO, iNOS while quercetin 40 mg/kg ↓ them; quercetin (20 mg/kg) ↑ performance (SPT,OFT,FST) and Akt, SOD, HO-1 and ↓ iNOS/MDA; quercetin (10 mg/kg) no reversal effect on the above indicators |
| D.-c. Sun et al., 2022 [100] | 5–6-wk-old ♂ ICR mice BW 18–22 g | CUMS-induced depression; literature-based prediction | Tianjin, China | Animals were divided into 6 groups (n = 10) including the “normal” (non-stressed mice), the CUMS (CUMS mice administered with distilled water), the venlafaxine (CUMS + venlafaxine), SE high-, medium-, and low-dose groups (CUMS + SE 1.8, 1.35 and 0.9 g/kg). → FST, TST, and OFT. DA in Hippoc and cerebral cortex, IL-2 and CORST in blood, Nrf2, Keap1, NADP, NQO1 and HO-1 in mice were Measur by ELISA and WB | In the SE group: up-regulation of Nrf2, Keap1, NQO1 and HO-1 vs. Ctrl. SE (1.8, 1.35 g/kg) ↓ immobility in FST and TST, while SE exhibited no significant effect in OFT. Compared with the CUMS group, the SE group (0.9 g/kg) showed significant differences in Hippoc and cortical DA levels. The SE (1.8 g/kg) group significantly ↓ the activities of CORST (p < 0.05) but not serum IL-2 (p > 0.05). WB showed ↑ Nrf2, Keap1, NQO1, and HO-1 in the SE group (1.8 g/kg) | Compared with the CUMS group, the protein contents of Nrf2, Keap1, NQO1, HO-1 ↑ in the SE group; SE may enhance antioxidant effects through regulating Nrf2-ARE signalling pathways. SE ↑ Nrf2 and downstream anti-OS targets |
| Cheng et al., 2022 [101] | ♂ Sprague Dawley rats; BW 130–150 g | CUMS-induced depression model in rats | Xiamen, China | Rats divided into 4 groups of 10: CUMS group, acupuncture group (acupoints in the skull) fluoxetine group (2.1 mg/kg, i-gastr for 28 days) and Ctrl group. Behav test (OFT, SPT, FST) → sacrificed → Hippoc tissue collected → ELISA for ROS, WB for Nrf2/HO-1, Immunohistochemical for Bcl-2/caspase-3 | CUMS↓ Nrf2/HO-1. Acupuncture pretreatment ↑ antioxidant enzymes in the Nrf2 pathway | Acupuncture/fluoxetine ↑ sugar preference SPT and ↓ immobility time in Behav test. Acupuncture ↓ Bax2/caspase-3 and ↑ Bcl-2. Acupuncture improved DLB of CUMS rats. Acupuncture showed AD effects in ↓oxidative stress products regulating the Nrf2/HO-1 pathway |
| Dang et al., 2021 [102] | ♂ C57BL/6J mice (aged 7–8 wk) and retired ♂ CD-1 mice (aged 16–20 wk) | CSDS depression model | Yuzhong District, Chongqing, 400016, China | Behav tests (SI, SPT, OFT, EPM, NOR, TST, FST). Hippoc and mPFC tissues for Nissl staining, immunofluorescence, targeted energy metabolomics analysis, ELISA, Measur of MDA, SOD, GSH, GSH-PX, T-AOC. WB and PCR for Sirt1/Nrf2/HO-1/Gpx4 signalling pathway. EX527, a Sirt1 inhibitor and ML385, an Nrf2 inhibitor 30 min before EDA injection daily | EDA (in CSDS model) ↑ expressions of Sirt1, Nrf2, HO-1 and Gpx4 in the Hip. EX527 and ML385 reversed the effect of EDA | EDA ameliorated CSDS-induced depressive and anxiety-like behaviours (↓ of neuronal loss, microglial activation, astrocyte dysfunction, OS damage, energy metabolism and pro-inflammatory cytokines activation in the Hip and mPFC) |
| Xia et al., 2022 [103] | ♂ C57BL/6 mice (8-wk-old) | DSS-induced IBF-associated depression and anxiety | Yangling, China | Test the effect of 6-wk 100 mg/kg BW/day sesamol for DSS-induced mice who developed IBF after DSS induction; Behav E + M (time spent and % entries in open arms), MBT (total and % travelled distance); TST (immobility time); Tissue Claudin 1, TNF-α, IL-1β, Iba1, and GFAP; Cortical mRNA expression of TLR-4, iNOS, COX-2, TNF-α, IL-1β, and IL-6; MDA, SOD, and GSH content in serum and cortex; Nrf2–HO-1, NQO1, HO-1 and their mRNA expression in cortex; BDNF in Hippoc CA3, mRNA expression of BDNF in cortex and Hippoc, p-TrkB/TrkB, p-CREB/CREB, PSD length and width, PSD-95 in cortex and Hippoc CA3; cortical NA and 5-HT content and mRNA expression of Htr1a and Htr2a, and PSD-95 mRNA expression in Hippoc and cortex; WB for 5-HT1AR F and 5-HT2AR F, Hippoc PSD-95, TLR-4, p-NF-κB/NF-κB, iNOS, COX-2, and p-IKBα/IKBα | Sesamol ↓ TLR-4/NF-κB, ↓ OS, ↑ Nrf2-HO-1 pathway, ↑ BDNF, ↑ BDNF/TrkB/CREB signalling pathway, and ↑ NA and 5-HT levels; ↑ Claudin 1 levels, thus reducing epithelial junction dysfunction, and improved behav test performance. It ↓ TNF-α, IL-1β, ↑ NQO1, ↑ Hippoc, cortical BDNF | Sesamol ↓ inflam, epithelial barrier dysfunction, and DLB and anxiety-like behaviours via the gut–brain axis; it restored synaptic impairment in a DSS-induced IBF mouse model of depression and anxiety and ↓ DLB and anxiety |
| Li J. et al., 2022 [104] | ♂ Sprague Dawley rats; BW 200–250 g | CRS-induced DLB | Beijing, China | Experiment 1: rats divided into 4 groups: CRS, Ctrl, CRS + Escitalopram 1 mg/kg/day i.g. × 28 days, CRS+ Rg1 20 mg/kg/day i.g. × 28 days. → behav tests (SPT, OFT, FST) → sacrifice → Hippoc tissue and serum collected. Experiment 2: rats divided into 4 groups: Ctrl, CRS, CRS + lenti + shNC (injection in vivo with lenti-shRNA into lateral ventricle), CRS + lenti + shGAS5 (injection in vivo with lenti-shGAS5 into lateral ventricle) → 48h CRS × 28 days → behav tests (SPT, OFT, FST) → sacrifice → Hippoc tissue and serum collected → qPCR for GAS5. Experiment 3: rats divided into 4 group: Ctrl, CRS, CRS + Rg1 (20 mg/Kg/day i.g. 1 h after CRS stimulation) +lenti vector (in vivo injection with lenti-shRNA into lateral ventricle), CRS+ Rg1(20/mg/Kg/day i-gastr 1 h after CRS stimulation) + lenti-GAS5 (in vivo injection with lenti-shGAS5 into lateral ventricle) → 48 h CRS × 28 days → behav tests (SPT, OFT, FST). → sacrificed → Hippoc tissue collected → qPCR for GAS-5; IF for IBA-1. Experiment 4: Rat microglia cell line HAPI → with LPS, (1μM) or solution × 2 h → treated with Rg1 (5 μM, 10 μM, 20 μM) for 24 h → HAPI WB for COX-2/iNOS/SOCS3. PC-12cell → with CORST (400 μM) or solution for 2 h → treated with Rg1 (5 μM/10 μM/20 μM) for 24 h. Experiment 5: HAPI → GAS5 shRNA after 48 h → LPS (1 μM) × 24 h. HAPI qPCR GAS5, ELISA for TNF-α, IL-1β, IL-6, WB for COX-2/iNOS/SOCS3. PC-12 → GAS5 shRNA after 48 h → CORST (400 μM) × 24 h. PC-12 qPCR for GAS5. Experiment 6: GAS5 KO Nrf2/SOCS3. HAPI/PC-12 → GAS5 shRNA. 48 after → HAPI qPCR, WB for SOCS3. PC-12 qPCR, WB for Nrf2. RIP and RNA pull-down assay → validate binding between GAS5/EZH2. ChIP-qPCR for EZH2/H3K27me3 in the promoter region of SOCS3 in HAPI. Experiment 7: SOCS3/NRF2 KO ↓ protective effects of Rg1 in HAPI7 PC-12 in vitro model. HAPI → SOCS3 shRNA. 48 h after → LPS (1 μM) for 2 h, → Rg1 (5 μM, 10 μM, 20 μM) × 24 h. ELISA for TNF-α, IL-1β, IL-6 WB for COX2/iNOS/SOCS3. PC-12 → NRF2 shRNA 48 h after → CORST (400 μM) × 2 h →Rg1 (5 μM, 10 μM, 20 μM) × 24 h. IF for IBA-1, WB for Nrf2/SOCS3/EZH2; ELISA for TNF-α/IL-1β/IL-6, TEM for MCh morphology, mitoSOX kit for MCh ROS detection, JC-1 assay to assess MCh membrane potential; ATP assay kit for ATP; q PCR for GAS5. ChIP-qPCR for EZH2/H3K27me3 in Nrf2 promoter region in PC-12 | SOCS3/Nrf2 ↓ in CRS group compared to CRS+ Rg1, CRS + Escitalopram and Ctrl. CRS rats showed ↑ EZH2 in Hippoc, ↓ SOCS3/Nrf2, ↑ EZH2 than other groups. GAS5 → ↑ EZH2, ↑ GAS5 in CRS rats compared to other groups. CRS + lenti + shGAS5 → ↑ Nrf2 compared to CRS + lenti + shNC and CRS; Rg1 ↑ ATP, SOCS3/Nrf2 ↓ GAS5 overexpression-related ATP, SOCS3, and Nrf2 reduction. CORST ↓ Nrf2/HO-1; Rg1 treatment ↑ Nrf2/HO-1. CORST stimulation ↓ Nrf2 and HO-1, GAS5 knockdown-related Nrf2 and HO-1 increase. GAS5 knockdown ↓ EZH2 and H3K27me3 enrichment in Nrf2 promoter region in PC-12 cells. GAS5 knockdown ↑ Nrf2 in PC12 and HAPI. In PC-12, Nrf2 knockdown reversed the protective effect of Rg1 on ROS production and on MCh membrane potential | CRS ↓ behav test performance, ↑ Hippoc IBA-1, ↑ Hippoc TNF-α/IL-1β/IL-6, ↓ Hippoc ATP and ↑ MCh injury compared to CRS + Rg1, CRS + Escitalopram and Ctrl. CRS, CRS + lenti + shNC ↑ Hippoc GAS5 compared to CRS + lenti + shGAS5 and Ctrl. ↑ performance on OFT and FST in CRS + lenti + shGAS5 and Ctrl compared to other groups. SFT ↑ performance in CRS + lenti + shGAS5 and Ctrl > than in other groups. GAS5 knockdown ↓ CRS-induced DLB. CRS + lenti + shGAS5 ↓ Hippoc IBA-1 compared to other groups. ↓ serum and Hippoc TNF-α, IL-1β, and IL-6 levels in Ctrl and CRS + lenti + shGAS5 groups compared to other groups. ↓ Hippoc ATP in CRS than in CRS + lenti + shNC, CRS + lenti + shGAS5, and Ctrl. ↑ MCh injury in CRS compared to CRS + lenti + shGAS5; GAS5 knockdown did not affect EZH2 expression. ↑ Hippoc GAS5 5 in CRS than CRS + Rg1 + lenti; Rg1 ↓ DLB, ↓ IBA-1, ↓ microglial activation, and ↓ MCh dysfunction; ↑ GAS5 overexpression → partly reversed the effect of Rg1 on DLB, on microglial activation, on IBA-1, and on MCh dysfunction. GAS5 overexpression → ↑ IBA-1, LPS ↑ TNF-α, IL-1β, and IL-6; Rg1 treatment ↓ TNF-α, IL-1β, no significant effect on IL-6. LPS ↑ COX-2/iNOS and ↓ SOCS3 in HAPI. Rg1 treatment ↓ MCh dysfunction. GAS5 inhibition exhibited a protective effect, similar to that of Rg1 treatment |
| Muhammad et al., 2022 [105] | ♂ Sprague Dawley rats; BW 180–200 g | LPS-induced DLB | Islamabad, Pakistan | Rats divided into 2 groups of 10, one treated with fluoxetine (5 mg/kg) + LPS, CAR20 or CAR50 + LPS → single dose of each × 5 days, ip + LPS → or single dose or after CAR20/50 (3rd, 4th day 1 mg/kg), and another, divided into 3 subgroupings (10 animals/group): ATRA + LPS, ATRA + LPS + CAR20/50, ATRA + LPS+ fluoxetine. ATRA ip injection 30 min before LPS. At Day 2, → behav tests (FST, LDB, E + M, sucrose SPIT) → sacrificed → Hippoc and cortical tissue collected and processed to RT-PCR and ELISA for Nrf2/HO-1. Immunohistochemical analysis for OS-related molecules | LPS ↓ Nrf2/HO-1 expression in cortex and Hippoc compared to Ctrl (p < 0.05). CAR20/50 + LPS ↑ Nrf2 and HO-1 compared to the LPS group (p < 0.01) | LPS ↑ ROS and DLB. CAR20/50 ↓ DLB and↑ Nrf2/HO-1; no differences between CAR20/50 + ATRA + LPS and ATRA + LPS |
| Shen et al., 2022 [106] | ♂ C57BL/6J mice (2 months old, BW 20–25 g) | LPS-induced CUMS and DLB | Hefei, Anhui, China | Experiment 1, mice randomised into 5 groups of 6: Ctrl, 2 h post-LPS, 6 h post-LPS, 12 h post-LPS, and 24 h post-LPS. After LPS injection → mice sacrificed → Hippoc tissue collected. Experiment 2, mice randomised into 6 groups of 6: Ctrl, LPS, LPS + PSP, LPS + fluoxetine, (30 mg/kg ip), LPS + calpeptin (2 mg/kg calpeptin ip), and LPS+ MCC (50 mg/kg ip). 24 h after LPS injection → sacrificed → Hippoc tissue collected. Experiment 3, mice randomised into 3 groups of 6: Ctrl, LPS, and LPS + NAC. 24 h after LPS injection → sacrificed → Hippoc tissue collected. Mice further randomised into 4 groups: Ctrl, CUMS, CUMS + PSP (i-gastr × 21 days 400 mg/kg) and CUMS + MCC (5 mg/kg ip × 7 days) After 21 day → behav tests (TST, SPT, FST, OFT) → sacrificed → Hippoc tissue collected. Hippoc tissue WB for OS-related molecules and IF for Nrf2 |
LPS ↓ Nrf2 expression compared to Ctrl in Hippoc CA1 region (p < 0.05). Treatment with PSP improved LPS-mediated ↓ in Nrf2 expression in CA1 (p < 0.05). LPS+ PSP ↑ Nrf2 compared to Ctrl (p < 0.05) | LPS treatment ↑ calpain-1 expression and substrate degradation, activated the NLRP3 inflammasome signalling pathway and glial cells, and Nrf2 and calpastatin expression. Treatment with PSP prevented these LPS-induced changes. Nrf2 expression was ↓ in the hippocampi of animals treated with LPS or subjected to CUMS, and this decrease was prevented by PSP |
| Jiang et al., 2022 [107] | 6–8–wk-old ♂ C57BL/6J mice (n = 60, BW 20–22 g); and 12-month-old retired breeders ♂ CD-1 mice (n = 70, BW 20–22 g) | CSDS-induced DLB | Beijing, Changsha, Luzhou, China | After a 1-wk adaptation, mice randomised into Ctrl, model, and treatment groups IMI (15 mg/kg ip), and Rb1 (35 and 70 mg/kg ip). Except for Ctrl, all animals subjected to CSDS treatment → behav testing (SIT, OFT, TST, FST) over the next 4 wk. IMI p.o. × 32 days. Hippoc protein concentration, CAT, SOD, and LPO Measur with specific kits. Pro-inflammatory mediator (TNF-α, IL-18, IL-1β) serum levels Measur with ELISA. WB for SIRT1, NLRP3, cleaved Caspase-1, ASC, IL-1β, HO-1, Nrf2, and β-actin | Nrf2 and HO-1 expression substantially ↓ in rat CSDS Hippoc compared to Ctrl. 70 mg/kg Rb1 ↑ Nrf2 expression | Rb1 treatment can rescue DLB-like social avoidance and behav despair of CSDS-induced mice. Rb1 attenuates pro-inflammatory cytokine production and inhibits the activation of NLRP3 inflammasome. Furthermore, Rb1 normalised OS following Nrf2/HO-1 and SIRT1 activation. Findings show that Rb1 attenuates NI |
| de Souza et al., 2022 [108] | ♂ adult Swiss mice BW 25–30 g | CUMS-induced depression | Fortaleza, Ceará, Brazil | Mice were exposed to a variety of stressful events (restraint, tilted cage, intermittent circle between lights on and off, constant light, water deprivation, wet cage, electric shock) × 28 days; from the 14th day they received DMF 50 and 100 mg/kg or fluoxetine 10 mg/kg or plac. On the 29th day → behav tests (OFT, FST, SPT, NOR). Mice divided into two groups, Ctrl and CUMS, which were subdivided into plac (carboxymethyl cellulose 0.5%), DMF50, DMF100 or fluoxetine 10 mg/kg (FLU) groups. 14 days after the beginning of the procedure, treatments were started in all groups, 30 min before daily stress application in CUMS groups. Simultaneously, Ctrl received the same treatments. Immunoenzymatic assay for TNF-α and IL-1β concentrations in Hippoc. IF to analyse subfields CA1, CA3, and DG | MMF (active metabolite of DMF) showed an efficient binding interaction with the Keap1 protein, leading to Nrf2 activation | DMF is a promising prodrug for the treatment of MDD and for comorbidities such as memory deficit. DMF modulated the neuroinflammatory pathway, ↑ astrocyte expression, ↓ microglia expression and the production of the pro-inflammatory cytokines IL-1β and TNF-α. MMF bound the Keap1 protein → activated Nrf2; it also bound HCAR2 protein, leading to a complex signalling cascade cross-talking with the Nrf2 pathway. It is suggested that one of the mechanisms involved in the AD effect of DMF is NI suppression, triggered by the binding of its metabolite MMF to the HCAR2 protein |
| Fasakin et al., 2022 [109] | Adult ♂ Wistar Albino rats, BW 181 ± 13 g | Depression and anxiety linked to NI and OS. | Akure, Nigeria | Six rats randomly housed in each cage: Group 1 = normal Ctrl. Groups 2 to 5 = CSAE once daily (doses of 5, 50, 500, and 2000 mg/kg BW, respectively). Groups 6 to 9 = DSAE once daily (doses of 5, 50, 500, and 2000 mg/kg BW, respectively). Groups 10 to 13 = NTAE once daily (doses of 5, 50, 500, and 2000 mg/kg BW, respectively). Groups 14 to 17 = CMAE once daily (doses 5, 50, 500, and 2000 mg/kg BW, respectively). Behav Tests: E + M, FST, TST. 91 days after alkaloid administration, rt-qPCR on Hippoc tissue. Biochemical analyses were performed to assess MAO activity, dopamine concentration, ACE activity, AChE activity, GDH activity, ROS concentrations. Concentrations of TNF-α, IL-1β, and IL-10 were evaluated using ELISA. Gas chromatograph–mass spectrometry analysis of alkaloid-rich extracts was performed | CSAE, NTAE, and CMAE ↑ 5-HT, BDNF, CREB and Nrf2 while ↓ NF-ĸB, GSK3β JNK3 and nesfatin-1 at 5, 50 and 500 mg/kg BW sub-chronic exposure. However, DSAE sub-chronic administration ↓ serotonin, BDNF, CREB and Nrf2 while ↑ NF-ĸB, GSK3β and JNK3 | This study established the AD and anxiolytic potentials of CSAE, CMAE, and NTAE via improving monoaminergic bioavailability, activation of neurotrophic signalling cascades and de-hyper-activation of hypothalamic–pituitary–adrenal axis. The difference between the effects of CSAE, CMAE, and NTAE at low and high doses may be the underlying factor in the discrepancies between the results of studies that have evaluated depression and psychoactive substance use |
| Tan et al., 2022 [110] | Adult ♂ C57BL/6 mice (BW 20 ± 2 g) | Behav despair mouse model in vivo and H2O2-induced PC12 cell model in vitro | Shenyang, 110016, China | Mice randomised into 4 groups of 10: plac, Fluoxetine 15 mg/kg, i.g.), SEO 250 group (250 mg/kg, i.g.) and SEO 750 group (750 mg/kg, i.g.). Flu and SEO suspended in 0.5% CMC-Na, plac 0.5% CMC-Na. Treatment once daily × 9 consecutive days. Behav tests (FST, TST). Serum, liver, brain, and cell supernatant MDA, SOD, CAT, and GSH levels detected through ELISA. Hippoc, cortex, and cell supernatant processed through WB (Nrf2, HO-1,PI3K,p-PI3K,p-Akt, GSK3β, p-GSK3β, LaminB, β-actin). Hippoc and cortical histopathology. PC12 cell culture. Groups: Ctrl, plac (300 μmol/L H2O2), SEO (0.78–25 μg/mL + 300 μmol/L H2O2). IF for PC12 cells with anti-Nrf2. 18 batches of SEO analysed by GC-MS. DPPH and ABTS assay | After FST and TST, expressions of nuclear Nrf2 in Hippoc and cortex significantly ↓. SEO treatment could reverse these changes, which indicates that SEO might promote Nrf2 translocation to reduce OS | SEO showed a promising AD-like effect in mice; the mechanisms of this effect might contribute to the antioxidant activities of SEO. SEO could ↑ Nrf2/HO-1 pathway to improve OS status and exert AD-like effects. PI3K/Akt/GSK3β signalling pathway might also be involved |
| Wu et al., 2022 [111] | Adult ♂ Sprague Dawley rats (BW 230–250 g, 6–7-wk-old) | CUMS-induced depression | Zhengzhou, Henan, China | After 7 days of adaptive life, all rats were evaluated by behav tests (OFT, FST and SPT. On the next day, rats were randomly divided into five groups: (1) Ctrl group (n = 10); (2) CUMS group (n = 10); (3) CUMS plus Catalpol group (n = 10); (4) CUMS plus ZnPP group (n = 6); (5) CUMS plus ZnPP + Catalpol group (n = 6). CUMS lasted for 3 wk. Catalpol was administered orally (dose of 10 mg/kg and volume of 20 mL/kg) for 3 wk. ZnPP injected into the intracerebroventricular (dose of 10 μg/rat and volume of 5 μL/rat) for 3 wk. 24 h after the last drug intervention, behav. tests were performed. Biochemical analyses were performed to determine Hippoc total protein and the levels of iNOS, GPX and GST in the Hippoc (using catalytic reaction plus chemical colourimetry method and the levels of NO, MDA, SOD, and GSH (nitrate reductase method, barbituric acid sulfate method, hydroxylamine method, and dithiodinitrobenzoic acid method, respectively) | CUMS exposure ↓ the phosphorylation level of ERK1/2, the nuclear expression level of Nrf2, and the total intracellular levels of HO-1, SOD, GPX, GST, and GSH and ↑ the level of peroxide MDA. Before intervention by HO-1′s antagonist ZnPP, catalpol significantly reversed these abnormalities. However, after intervention by HO-1′s antagonist ZnPP, the reversal effects of oral administration catalpol on the phosphorylation level of ERK1/2, the nuclear expression level of Nrf2, and the total intracellular levels of HO-1, SOD, GPX, GST, GSH, and MDA were completely abolished, which implied that HO-1′s antagonist ZnPP may abolish the regulation of catalpol on antioxidant and peroxide related factors in the Hippoc of CUMS-induced DLB rats | The AD-like effects of catalpol might be due to HO-1 that up-regulates the ERK1/2/Nrf2/HO-1 pathway-related factors to enhance the antioxidant defence, triggering the down-regulation of the COX-2/iNOS/NO pathway-related factors to inhibit NI, and the up-regulation of the BDNF/TrkB pathway to enhance neurotrophic effects |
| Zhao et al., 2022 [112] | ♂ C57BL/6 mice (8-wk-old) | CP-induced anxiety and DBL | Zhanjiang, Guangdong, China | Mice received CP ip × 2 wk and TSP × 4 wk → behav, biochemical, and molecular tests conducted | Protein expression of Keap1 ↑, Nrf2 and HO-1 ↓ in CP compared with Ctrl. TSP ↑ Nrf2 and HO-1 | TSP ↓ behav test abnormalities, ↓ SOD, MDA, and GSP abnormalities; ↓ expression of IBA-1, TNF-α and IL-1β, ↓ Hippoc apoptosis in CA1 and CA3 subfields; ↑ Nrf2/HO-1 signalling pathway; ↑ BDNF/TrkB/CREB signalling pathway, and ↓ the Bcl-2/BAX/caspase-3 apoptosis pathway |
| Bansal et al., 2022 [113] | 3-month-old ♂ BALB/c mice (BW 25–30 g) | OBX-induced depression | Chandigarh, Panjab, India | Thirty mice randomised in 3 groups of 10. I group = sham; II = OBX + plac plac; III and IV groups = OBX + Ro61-8048 (0.2 and 0.4 mg/kg) × 14 days → behav tests (OFT, SFT, SPT), biochemical analysis, ELISA (Nrf2, BDNF, NF-κB, TNF-α and IFN-γ), HPLC (5-HT, 5-HIAA, TRP, and KYN levels), SF(QA) and qPCR (Hippoc and cortex based gene expression analysis) were performed | KMO inhibition with higher dose of Ro61-8048 (0.4 mg/kg) in OBX mice showed: ↓ CORST levels; ↑ Nrf2 and GCLC with no significant effect on HO-1, ↑ PI3K and Akt, ↓ GSK3β gene expression and ↑ GFAP expression, ↓ MDA and nitrite, ↓ NF-κB and IFN-γ levels with no effect on TNF-α, ↑ BDNF, ↑ TRP, 5-HT and 5-HIAA, ↓ QA. The lower dose of Ro61-8048 (0.2 mg/kg) showed no significant effect | OBX ↑ OS in the brain; Ro61-8048 ↓ the effect of KMO and ↓ OS. KMO inhibition and QA-associated down-regulation ↓ Keap-1 and ↑ Nrf2 expression |
| Yu et al., 2022 [114] | ♂ C57BL/6 mice (6–8 wk, BW 20 ± 2 g) | LPS-induced DLB | Fuzhou, Fujian, China | Mice were divided into 4 groups (9 each): CON, the Ctrl group; CON + RA (80 mg/kg); LPS (0.1 mg/kg); LPS + RA (80 mg/kg). Behav tests were carried out 24 h later → mice sacrificed, and brains used for IF, histopathological analysis, WB, and rt-qPCR | Nrf2 ↓ after LPS injection compared to Ctrl, RA administration attenuated the ↓ of Nrf2 | RA improved behav test performances and BW; ↓ histologic brain damage in LPS-exposed mice; blocked the ↓ of BDNF (rt-qPCR and WB) and of Nrf2 (WB). LPS ↓ LC3 (IF), ↓ p21 and p62, the levels of which were restored by RA. ME1, IDH1, and 6-PGDH mRNA were ↓ in the LPS group and ↑ after RA. RA inhibited the LPS-induced ↑of CD44, iNOS, TNFα, and IL-1β mRNA expression |
| Kang et al., 2022 [115] | ♂ C57BL/6J mice (8-wk-old, BW 21–23 g, n = 36) | CRS-induced DLB | Daejeon, South Korea | Mice divided into 4 groups of 9: normal; CRS (stress and distilled water), PSE (stress and 100 mg/kg PSE), and positive Ctrl (stress and 100 mg/kg NAC). Behav tests (OFT, FST, TST); murine microglial cells (BV2) and Hippoc neuronal cells (HT22) were cultured for cytotoxicity, cytokines, and phagocytosis assays. IF and WB were also performed | p-Nrf2 ↑ with PSE (p < 0.05 for 40 μg/mL). PSE pretreatment increased the level of HO-1 protein expression (p < 0.05 for the 10 and 20 μg/mL doses). NAC exerted similar effects as PSE | PSE (10, 20, and 40 μg/mL) attenuated the ↑ in inflammatory factors (NO and TNF-α), translocation of NF-κB, and phenotypic transformations in a dose-dependent manner. These inhibitory effects of PSE on microglia were supported by its regulatory effects on the CX3CR1/Nrf2 pathway. PSE (100 mg/kg) ↓ sickness, anxiety, and DLB in mice subjected to CRS and improved behav test performance |
| Wang et al., 2022 [116] | 40 ♂ ICR 7-wk-old mice | PTZ-induced seizures and depression | Liaocheng, western Shandong, China | Mice divided into 4 groups of 10: Ctrl, PTZ, PTZ + EPA, and PTZ + DHA groups. Behav tests (OFT; TST; FST). Hippoc DHA, EPA and neurotransmitter levels were detected by GC. Histopathology of Hippoc was conducted through H&E-staining, FJB-staining and IF. Hippoc proteins were analysed by WB | The protein expressions of p-Nrf2 and HO-1 were significantly ↓ in the PTZ group compared with Ctrl. EPA and DHA significantly ↑ p-Nrf2 and HO-1 (higher levels in EPA) group | ↓ GSH, ↓ SOD activity, ↑ MDA, ↓ GPx4, xCT, HO-1, and p-Nrf2 in the PTZ group, reversed by EPA and DHA. EPA and DHA improved behav test results; ↑ GABA and GABARA1A levels without changing Glu levels; improved the morphology of cell damage (EPA repaired > than DHA); ↓ Hippoc total Fe (which was ↑ in the PTZ group) and ameliorated iron-homoeostasis-related proteins; ↓ pro-IL-1β, IL-1𝛽, and TNF𝛼 (which were ↑ in the PTZ group); EPA inhibited NLRP3 and ↓ Caspase-1 levels; DHA ↓ ASC, pro-Caspase-1 and Caspase-1 |
| Nasehi et al., 2022 [117] | Adult ♂ mice, sons of pregnant mothers from Pasteur Institute, Tehran, Iran (Balb/c?), fed ad libitum and kept in 12 h/12 h dark/light cycles | Maternal separation-induced depression associated with CVD | Zanjan, Iran | Depression was induced with 3 h daily separation from mothers while weaning; mice with maternal separation-induced depression and CV comorbidities treated with fluoxetine or SAHA + fluoxetine ip →behav tests (OFT [horizontal and vertical locomotion], E + M (Y-maze), TST, FST, SPlT [grooming]) + gene analysis with RT- qPCR; content and gene expression of PPAR-𝛼, NOX-2, -4, PGC-1α, and Nrf2 | SAHA ↓the NOX-4 gene expression level in mice treated with SAHA + fluoxetine without significantly changing NOX-2 expression. SAHA ↑ PGC-1α and Nrf2 in heart tissues of maternally separated mice, but had no effect on PPAR-𝛼 gene expression. Maternal separation induced depressive behaviour on all behav tests. SAHA and fluoxetine had no effect on OFT locomotion, but ↑ time spent in open arm of the Y-maze, and ↓ immobility time on FST and TST | SAHA reversed DLB and restored mitochondrial function in the heart via an effect on NOX-4 and mitochondrial biogenesis gene (PGC-1α and Nrf-2) expression levels. The authors speculated that when fluoxetine lacks effectiveness on cardiac mitochondrial biogenesis and cardiac NOX-4 gene expression, patients with depression may develop a recurrence of their depression |
| Pei et al., 2022 [118] | 50 ♂ Balb/c mice (BW = 25 g) | LPS-induced depression | Changchun, Jilin, China | Mice were divided into 5 groups (n = 10 each): Ctrl, LPS-induced, palmatine (50 and 100 mg/kg) group, and fluoxetine groups. Each group except Ctrl received LPS (5 g/L, 3 μL/mice). OFT and EPM were performed; SOD, TNFα, IL1β, and IL6 detected with ELISA; apoptosis and ROS with flow cytometry; total Hippoc proteins analysed with WB (BAX, Bcl-2, Nrf2, HO-1, β-actin) | Nrf2 and HO-1 in the LPS-induced group were ↓ compared with the Ctrl group. After palmatine treatment, their protein expression levels were significantly ↑ | Palmitine ameliorated mitochondrial damage, improved behav tests, ↓ levels of SOD, TNF-α, IL-1β, and IL-6. It also ↓ neuronal apoptosis in the Hippoc, and depression through BAX/Bcl-2 and Nrf2/HO-1 signalling pathways |
| J.Y. Sun et al., 2022 [119] | 60 ICR ♂ mice (6–8-wk, BW 18–22 g) | CORT-induced depression | Chengdu, Sichuan, China | All mice were randomly divided into 6 groups (n = 6): Ctrl group, CORT group, Fluoxetine (3 mg/kg), EOP low-dose group (1 mg/kg), EOP medium-dose group (2 mg/kg) and EOP high-dose group (3 mg/kg). Except for the Ctrl group, mice in other groups were injected with CORT 20 mg/kg ip daily for 4 consecutive wk→behav tests (TST, FST, SPT, OFT)+ blood samples and brain tissues for analysis | EOP ↓ CORT-induced DLB in mice (by improving behav tests), ↓ CRH, ACTH and cortisol in the brain tissues, ↑the phosphorylation of PI3K and Akt in Hippoc neurons and PC12 cells, ↑ Nrf2 and ↓ the CORT-induced OS. It also ↓ CORT-induced Hippoc neuron injury and apoptosis and ↑ the proliferation ability and cell viability of PC12 cells | EOP had a significant AD effect on the symptoms of CORT-induced depression in mice. EOP exerted anti-apoptotic effects on Hippoc neurons through PI3K/Akt/Nrf2 signalling pathway |
| He et al., 2022 [120] | ♂ adult C57BL/6 mice (8-wk-old, BW 20–25 g), CD-1 mice (14-wk-old, BW 40–45 g), ♂ adult YFP-Nrf2 WT mice, and YFP-Nrf2 KO mice | CSDS-induced DLB | Guangzhou, Guangdong, China | For the CSDS procedure, C57BL/6 mice or YFP mice were defeated by CD1 mice (10 min × 10 days). SIT performed to select susceptible mice (70%) → locomotion test, FST, and SPT. TREM2-HDO (icv) was injected on day 0, SFN (ip) 30 min before the CSDS and LPS. CSDS-susceptible mice were used for luciferase assay (lateral habenular BV2 cultured microglia cells treated with SFN or siRNA-Nrf2), ChIP assay (with Nrf2 antibody), qPCR (levels of TREM2 promoter mRNA and Trem2 mRNA); ImBl (TREM2, Nrf2, BDNF, arginase, phospho-TrkB, PSD-95, TrkB, β-actin antibody, β-tubulin antibody, GAPDH), IF (anti-TREM2, anti-IBA-1, anti-arginase1 [microglial anti-inflammatory phenotype marker]), dendritic spine analysis | SFN → ↑ Nrf2 → ↑ microglial arginase 1+ phenotype by initiating TREM2 transcription in mPFC, improving DLB in CSDS mice. Nrf2 KO ↓ TREM2 and microglial arginase 1+; both → DBL. Nrf2 KO and ↓ TREM2 expression associated with ↓ BDNF/TrkB signalling pathway | Luciferase assay showed that Nrf2 regulates TREM2 transcription; qPCR showed that ↑ SFN and ↑ Nrf2 → ↑ TREM2 promoter activity and ↑ TREM2 mRNA (which were ↓ in CSDS-mouse mPFC). ImBl and qPCR → TREM2-HDO dose-dependently decreased mRNA and protein expression of both TREM2 and Nrf2. IF showed TREM2 to colocalise with IBA1 and arginase 1+ in microglial cells. LPS ↓ TREM2 and arginase 1 and ↑ IBA1; these effects were reversed by SFN. qPCR → reversal by SFN of the reduction in the anti-inflammatory cytokines IL-4 and IL-10 in the mPFC of CSDS mice. ImBl → reversal by SFN of the reduction in Nrf2, TREM2, and arginase 1 expression in the mPFC of CSDS mice. Nrf2 KO ↓ TREM2 and arginase 1+ microglial phenotypes in mPFC |
| Samy et al., 2022 [121] | 10 wk-old ♁ Balb/c mice (BW = 25 g) | Post-OVX depression | Alexandria, Egypt | Eighty mice assigned to 5 groups (n = 16 each) receiving treatments or plac × 3 wk: Ctrl (sham-operated + plac); OVX mice + CarA (20 mg/kg/day, p.o.); OVX+ SnPP-IX (50 μmol/kg/day i.p); combined-treatment OVX + CarA + SnPP-IX received CarA; Behav tests. HO-1 activity and brain oxidative and antioxidant markers were determined. Isolation of Hippoc and cortex RNA for RT-qPCR; protein extraction for WB; determination of brain 5-HT levels through HPLC | ↓ Nrf2, Trx-1, and BDNF in OVX mouse brains compared with Ctrl. ↑ Nrf2, Trx-1, and BDNF with CarA either solely or combined with SnPP-IX (except for BDNF, which ↓ in the treatment combination group). Mice treated with SnPP-IX alone did not show any significant impact on Hippoc or cortical Nrf2, Trx-1, or BDNF | Improvement of FST, TST, SPT with CarA and partial improvement with CarA + SnPP-IX. ↓ Nrf2, Trx-1, and BDNF in OVX mouse brain, ↑ by CarA. ↑ in MDA and ↓ in GSH and SOD in OVX mice, ↑ GSH, SOD activity and ↓ MDA in CarA group (antioxidant effect attenuated in CarA + SnPP-IX). ↑ OS, ↑ MDA, ↓ GSH and SOD with SnPPIX alone. CarA ↑ 5-HT level in mouse Hippoc and PFC. Significant thinning of the pyramidal and granule cell layers of the HippP and DG of OVX mice, which was restored to the original thickness by CarA |
| Si et al., 2023 [122] | ♂ 6–8-wk-old Sprague Dawley rats (BW 160–180 g, n = 70) | CUMS-induced DLB | Wuhan, Hubei, China | Rats sorted into 2 groups of 35, Ctrl and CUMS. Behav tests performed to select CUMS-susceptible rats (SUS). SUS randomly divided into 2 groups: CUMS-SS (1 SUS + 3 Ctrl) and CUMS-SI (1 SUS). After 4 wk of SI or SS, the rats in different groups underwent behav tests. Total RNA was isolated for RT-PCR and total protein for WB. IF performed to observe Hippoc astrocyte changes | mRNA expression of Nrf2, HO-1 and NQO1 ↓in CUMS-SI and significantly lower in the CUMS-SI group compared to CUMS-SS | SUS exhibited DLB, memory deficits and social withdrawal. qRT-PCR and WB analysis showed ↓ Nrf2, HO-1, NQO1 and p-ERK in SUS and ↑ Keap1. IF showed ↓GFAP+ astrocytes in SUS. SI after CUMS perpetuated DLB, memory deficits and social withdrawal (overall worsening in behav tests and no BW ↑); ERK/Keap1/Nrf2 signalling was suppressed in CUMS-SI (↓ Nrf2, ERK, and ↑ Keap1 compared to CUMS-SS) |
| Wang et al., 2023 [123] | ♂ 8–10-wk-old adult C57BL6 mice | CUMS-induced DLB | Cangzhou, Hebei, China | 13 mice per group: Ctrl, exposed to CUMS + plac, CUMS + PD 100 mg/kg/day delivered by oral gavage, CUMS + PD 200 mg/kg/day oral gavage. → SPT, FST, TST, SIT for depression and MBI, E + M for anxiety → killing → Hippoc neuronal culture and staining, IF for Iba-1 and GFAP in Hippoc CA1, CA3, and DG, rt-qPCR for Iba-1, Gfap, Gapdh, Hmox1, Nqo1, and Nrf2 forward and reverse primers, WB for synaptophysin and PSD-95, ELISA for DA, 5-HT, IL-1β, IL-6, and TNF-α | CUMS → ↓ BW, in SPT ↓ sucrose preference, in FST and TST ↑ immobility time, in SIT ↓ affiliation, sociability, memory, and novelty; all these effects were counteracted by oral PD 200 mg/kg/day; CUMS ↑ marble burying in the MBI and time on closed arms in E + M, both counteracted by PD 200 mg/kg/day. PD ↑ PSD-95 and synaptophysin content in Hippoc, dendrite length and number. CUMS ↑ NI markers Iba-1 and GFAP, IL-1β, IL-6, and TNFα in Hippoc, ↑ release and nuclear translocation of NFκB, ↑ ROS, SOD, GPx, ↓ Hmox1, Nqo1, and Nrf2 levels; all these CUMS effects were counteracted by PD | PD reverts changes induced by CUMS on depressive and anxiety-related behaviour; PD restored all changes induced by CUMS and hippocampal neuronal function |
| Smaniotto et al., 2023 [124] | Swiss ♂ mice (BW 25–35 g) | CUMS-induced DLB | Pelotas, Rio Grande do Sul, Brazil | CUMS × 28 days → intranasal IL-4 vs. plac; 8 mice per group, 4 groups (Ctrl, Ctrl–IL-4, CUMS, CUMS–IL-4) subjected to water/food/movement restriction, tail pressure, and inclined box with wet bedding; → OFT, TST, SPlT → killing, tissue extraction and analyses (PFC and Hippoc, adrenals, lymph nodes, thymus, spleen [organs], blood to determine ROS, IDO, CAT, SOD, LPO, MDA, NO metabolites, gene expression, CORST levels) | In OFT no differences in crossings and rearings among groups; in TST, CUMS ↑ immobility and IL-4 reversed it; in the SPlT, CUMS ↓ grooming and IL-4 reversed it. CUMS → ↑ CORST and lymph organs, ↑ NF-κB expression, IL-4 → ↓ CORST and organ weight; ↓ NF-κB expression; in both PFC and Hippoc, CUMS → ↑ IDO, IL-1β, NF-κB, Nrf2, ROS, SOD, and CAT and ↓ BDNF (no effect on LPO in Hippoc and ↑ in PFC; similar effects for MDA, but no effect on NO metabolites in both PFC and Hippoc); all CUMS effects were reversed by IL-4 | IL-4 counteracts CUMS-induced DLB; the effects of IL-4 are most evident in the PFC. IL-4 protects against chronic stress-induced depressive behaviour through ↓ of NI and OS |
| Zhang et al., 2023 [125] | ♂ 7–8-wk-old adult C57BL6 mice | LPS-induced depression and anxiety | Guiyang, Guizhou, China | Mice randomised into Plac (Ctrl), to the phenolic glycoside gastrodin (major component of Gastrodia elata) 25, 50, or 100 mg/kg/day) or minocycline (microglial inhibitor) 50 mg/kg/day i.p. × 3 days → Plac (Ctrl), gastrodin 25, 50, or 100 mg/kg/day + Plac or LPS 0.25 mg/day, or minocycline 50 mg/kg/day + Plac or LPS 0.25 mg/day i.p. × 10 days; gastrodin-receiving animals were also treated with Nrf2 antagonist ML385 30 mg/kg/day i.p. × 10 days. Network pharmacology analysis, molecular docking on PPAR-γ, STAT6, and Nrf2; → OFT, SPT, E + M, FST → killing, cultured neonatal microglia, Hippoc microglia, rt-qPCR for Iba-1, β-actin, Nlrp3, iNOS, IL-1β, IL-10, TNF-α, Cd11b, Cd68, and Arg-1+; ELISA for IL-1β, IL-6, IL-10, TNF-α, and NO; WB for Nrf2, pNrf2, and GAPDH; IF for Hippoc Iba-1, iNOS, Arg-1, Nrf2 | LPS ↓ sucrose preference in SPT, ↑ immobility time in FST, ↓ number of entries or time of stay in open arms in E + M, ↓ time of stay in central area in OFT; all DLB and anxiety behav reversed by higher doses of gastrodin and minocycline; LPS ↑ Iba-1 staining in Hippoc microglia, thickened cell-body and shortened cell processes; LPS ↑ CD11b, CD86, NLRP3, iNOS; gastrodin 100 mg/kg/day and minocycline reversed NI-induced changes. Gastrodin 100 mg/kg/day or minocycline ↑ the microglial anti-inflammatory marker Arg-1 and Hippoc Arg-1+ microglia in LPS-treated, while they ↓ IL-1β and IL-6 and ↑ IL-10. ML385 blocked the effect of gastrodin on ↑ Arg-1+ cell proportion in Hippoc microglia and the subsequent ↑ of Nrf2, pNrf2 and nuclear Nrf2 translocation | Gastrodin through Nrf2 prevents depressive and anxiety behaviours through counteracting NI actions. Gastrodin could represent a useful addition to the treatment of depressive and anxiety disorders, as it promptly crosses the BBB |
Note. Abbreviations: Ab(s), antibody(ies); ABTS, 2,2′-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid; ACE Angiotensin-converting enzyme; AChE, Acetylcholinesterase; ACMS, adipose-derived mesenchymal stem cell; AD, antidepressant; ADSC, adipose-derived mesenchymal stem cells; AI, Artificial Intelligence, Akt, Protein kinase B; AOEs, antioxidant enzymes; ARE, antioxidant response elements; ARS, acute restraint stress ASC, apoptosis-associated speck-like; AST, astrocytes; ATP, adenosine triphosphate; ATRA, all-trans retinoic acids; BAFF, B-cell-activating factor of the tumour-necrosis-factor family, B-cell activating factor; BAX, Bcl-2-associated X protein, bcl-2-like protein 4; BBB, blood–brain barrier; Bcl-2, Bcl-2, B-cell lymphoma 2; BDNF, brain-derived neurotrophic factor; behav, behavioural; bEnd.3, brain endothelial cell line 3; Bilat, bilateral;BL, baseline; BTS, Bangpungtongsung-san; BW, body weight; C. elegans, Caenorhabditis elegans, a common worm (nematode); CA, concentrated PM2.5 air; cAMP, cyclic adenosine monophosphate; CarA, carnosic acid; CAR20, carveol 20 mg/kg; CAR50, carveol 50 mg/kg; CAT, catalase; ChIP, Chromatin immunoprecipitation; CLA, conjugated linoleic acid; CMAE, Carica papaya; CMC-Na, Sodium carboxymethylcellulose; CMI, 3-[(4-chlorophenyl) selanyl]-1-methyl-1H-indole; CMS, Chronic mild stress; cncs, concussions; CORT model, elevated corticosteroid anxiety/depression mouse model; CORST, corticosterone; CP, cyclophosphamide; CRS, chronic restraint stress; CREB, cyclic AMP responsive element binding protein; CRS, chronic restraint stress; CSAE, Cannabis sativa; CSDS, chronic social defeat stress; Ctrl, control(s); CUMS, chronic unpredictable mild stress; CUR, Curcumine; CuZnSOD, copper zinc superoxide dismutase; CVD, cardiovascular disease; CX3CR1, CX3C chemokine receptor 1; DA, dopamine; DG, dentate gyrus; D-GalN, D-galactosamine; DHA, docosaheaxaenoic acid; 7,8-DHF, 7,8-Dihydroxyflavone; DLB, depressive-like behaviour; DM, diabetes mellitus; DMF, Dimethyl fumarate; DOPAC, 3,4-dihydroxyphenylacetic acid; DPPH, 2,2-diphenyl-1-picrylhydrazyl; DSAE, Datura stramonium; DSS, dextran sulfate sodium; EGR1–4, early growth response proteins 1–4; ELISA, enzyme-linked immune sorbent test; EPA, eicosapentaenoic acid; ERK, extracellular signal-regulated protein kinase; EZH2, Enhancer of zeste homolog 2; E + M, elevated plus (or X or Y) maze; EZM, elevated zero maze; Fen, fenretinide; FiA, filtered air; FJB, Fluoro-Jade B; FJC+, Fluoro-Jade C-positive cells; FO, fish oil; FST, forced swimming test (Porsolt); GABA, γ-hydroxybutyric acid; GABARA1A, the 𝛼1 subunit of the GABAA receptor; GAPDH, glyceraldehyde-3-dehydrogenase; GAS5, long non-coding RNA growth arresting-specifc 5; GC, gas chromatography; GC-MS, gas chromatography–mass spectrometry; GCLC, glutamate-cysteine ligase catalytic subunit; GCLM, glutamate-cysteine ligase modifier subunit; GDH, Glutamate dehydrogenase; GFAP, glial fibrillary acid protein; Glo-1, glyoxalase 1; GLR, glutathione reductase; Glu, glutamate; GPx, glutathione peroxidase; GR, glucocorticoid receptor; GSH, glutathione; GSK-3β, glycogen synthase kinase 3-beta; GST, Glutathione S transferase; GSTP1, Glutathione S-transferase Pi; GSTω1, Glutathione S-transferase omega-1; GSSG, glutathione disulphide; G6PD, glucose-6-phosphodehydrogenase; h, hours; Ham-D, Hamilton Depression Rating Scale; HAPI, microglial cell line Highly Aggressively Proliferating Immortalised; HCAR2, Hydroxycarboxylic Acid Receptor 2; HC, healthy control(s); HDAC2, Histone Deacetylase 2 gene; HDOs, heteroduplex oligonucleotides; HFD, high fat diet; Hippoc, hippocampus, hippocampal; HippP, hippocampus proper; HO-1, haemoxygenase; HPLC, high-performance liquid chromatography; HRR, High resolution respirometry; Hsd, hesperidin; hsp-60, -70, 60 and 70 kDa heat shock proteins; Hst, hesperetin, i.e., the aglycone of the flavonoid hesperidin; H&E, Haematoxylin-Eosin; H2DCF-DA, 2′,7′ -dichlorodihydrofluorescein diacetate; H2O, water; H3K27me3, Methylation of histone 3 on lysine 27; IBA-1, ionised calcium-binding adapter molecular 1; IBF, inflammatory bowel disease; ICP-MS, inductively coupled plasma mass spectrometry; ICR, imprinting control region (mice); icv, intracerebroventricular; IDO-1, indoleamine-2,3-dioxygenase 1; IF, immunofluorescence; i.g., intragastric gavage; IKK-β, inhibitor of nuclear factor kappa-B kinase subunit beta; IL-1…10, Interleukin 1, 2, 3, …-10; IL-1β, Interleukin 1-beta; inflam, inflammation, inflammatory; ImBl, immunoblotting; IMI, Imipramine Hydrocloride; iNOS, inducible nitric oxide synthase; ip, intraperitoneal; Ipt, iptakalim IκBα, nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha; i-gastr, intragastrically; JNK, c-Jun N-terminal kinase; jugl., juglone, a naphthoquinone from walnut; Keap1, kelch-like ECH-associated protein 1; KMO, kynurenine monooxygenase; KO, knock-out; KT, ketamine; L-dopa, levodopa, L-3,4-dihydroxyphenylalanine; LDB, light-dark box; lenti, lentivirus; Lenti-GAS5, lentivirus-packaged GAS5 overexpressing plasmid; LPO, lipid peroxidase; LPS, lipopolysaccharide; LW, long day and warm temperature; M1, Macrophage M1 phenotype, MAO, Monoamine oxidase; MAPK, mitogen-activated protein kinase; MBT, marble burying test; MCE-1, (±)-3-ethynyl-3-methyl-6-oxocyclohexa-1,4-dienecarbonitrile, Nrf2 activator; MCC, MCC950; MCh, mitochondrium, mitochondrial; MDA, malondialdehyde; MDD, major depressive disorder; Measur, measurement, measured; MeCP2, methyl CpG binding protein 2; MEK1/2, mitogen-activated protein kinase kinases 1 and 2; Mito–TEMPO, 2-(2,2,6,6-Tetramethylpiperidin-1-oxyl-4-ylamino)-2-oxoethyl) triphenylphosphonium chloride; MMF, Monomethyl fumarate; MnSOD, manganese superoxide dismutase; MT, melatonin; NA, noradrenaline; NAC, N-acetyl-Lcysteine; NADP, Nicotinamide adenine dinucleotide phosphate; NBP, Dl-3-n-Butylphthalide; NBT, nesting building test; NDEVs, Enrichment of plasma neuron-derived extracellular vesicles; NF-κB, nuclear factor kappa B; NI, neuroinflammation; NKT, Nootkatone; NLRP3, Nucleotide-binding oligomerization domain containing 3 inflammasome; NO, nitric oxide; NORT, Novel Object Recognition Test; NOs, nitric oxide synthase; NOX, NADP oxidase; NQO-1, NAD(P)H Quinone Dehydrogenase (oxidoreductase) 1; NResp, non-responder(s); Nrf2, redox-sensing nuclear factor (erythroid-derived 2)-like 2; NS, non-silencing; NSFT, Novelty-suppressed feeding test; NTAE, Nicotiana tabacum; OBX, olfactory bulbectomy; OFT, open-field test; OS, oxidative stress; OVX, ovariectomised, ovariectomy; p, phospho, phosphorylated; p, statistical significance probability; PB, Pinocembrin; PBMC, peripheral blood mononuclear cells; PBS, phosphate-buffered saline; (p-ClPhSe)2, p-chlorodiphenyl diselenide; PCMS, predictable, chronic mild stress; PD, Polydatin, a resveratrol derivative of the herbal medicine Polygonum cuspidatum; PFC, prefrontal cortex, dl, dorsolateral, vm, ventromedial; PI3K, phosphatidylinositol 3-kinase; plac, placebo or vehicle or saline; p.o., per os, orally; PG, prostaglandin; PGC-1α, peroxisome proliferator-activated receptor-gamma coactivator; PPAR-α, -γ, Peroxisome Proliferator Receptor-alpha, -gamma; PRC2, Polycomb repressive complex 2; PRMT1, protein arginine methyltransferase; PSD-95, 95 kD post-synaptic density protein; pts, patients; PSE, pinus spp. succinum extract;. PSP, Polysaccharides from Polygonatum cyrtonema; PTZ, pentylenetetrazol; QA quinolinic acid; qPCR, quantitative polymerase chain reaction; RA, rosmarinic acid; Rb1, Retinoblastoma protein-1; Resp, responder(s); Rg1, Ginsenoside Rg1, the main active compound of ginseng; RI, recombinant inbred; RIP, RNA-binding protein immunoprecipitation; ROS, reactive oxygen species; Ro61-8048, 3,4-dimethoxy-N-[4-(3-nitrophenyl)thiazol-2-yl]benzenesulfonamide, high-affinity kynurenine 3-hydroxylase (KMO) inhibitor; rt-qPCR, real-time quantitative polymerase chain reaction; SAHA, suberoyanilide hydroxamic acid, vorinostat, a histone deacetylase inhibitor; SalB, Salvianolic Acid; SC, short day and cool temperature; SDS, social defeat stress; SE, Salicornia europaea; SEO, Schisandra Chinensis oil; SF spectrofluorometer; SFN, sulforaphane; shGAS5, lentivirus-packaged GAS5 shRNA; shNC, shRNA-containing lentivirus, negative-control; shRNA, short (small) hairpin RNA (to silence specific genes, e.g., lentivirus-packaged GAS5 shRNA); SI, social isolation; siRNA, small interfering ribonucleic acid; SIRT1, sirtuin-1, silent information regulator 2 homolog 1; SIT, social interaction test; SNAP25, 25kD synaptosomal associated protein; SnPP-IX, tin protoporphyrin IX; SOCS3, Suppressor of cytokine signalling 3; SODs, superoxide dismutases; SPlT, splash test; SPT, sucrose preference test; SS, social support; STAT6, signal transducer and activator of transcription 6; STZ, streptozotocin; SUS, CUMS-susceptible; TBAR, Thiobarbituric acid reactive substance; TBE-31, (±)-(4bS,8aR,10aS)-10a-ethynyl-4b,8,8-trimethyl-3,7-dioxo-3,4b,7,8,8a,9,10,10a-octahydrophenanthrene-2,6-dicarbonitrile, Nrf2 activator; t-BHQ, tert-Butylhydroquinone, Nrf2 nuclear translocator; TEM, transmission electron microscopy; Tempol, 4-hydroxy-2,2,6,6-tetramethylpiperidine 1-oxyl; 288 μmol/kg-1‧day-1, an antioxidant; TLR4, Toll-like receptor 4; TMS, transcranial magnetic stimulation, d, deep, r, repetitive; TNF-α, tumour necrosis factor-alpha; TREM2, Triggering receptor expressed on myeloid cell-2; TrkB, tropomyosin receptor kinase B; Trx, thioredoxin; TrxR, thioredoxin reductase; TSP, Tilapia Skin Peptides; TST, tail suspension test; TUNEL, Terminal-deoxynucleoitidyl Transferase mediated Nick end labelling; TXNIP, thioredoxin-interacting protein; UA, ursolic acid; UnA, unfiltered air; WB, Western blot(ting); Wfs1, Wolfram syndrome 1; wk, week(s); WT, wild type; , mean; xCT, solute carrier family 7 member 11, SLC7A11; YFP, Thy1-yellow fluorescent protein; Xn, Xanthohumol; yr(s), year(s); ZnPP, zinc protoporphyrin IX; ZT, Zileuton; γ-GCS, γ-glutamylcysteine synthetase; 4-HNE, 4-hydroxy-2-nonenal; 5-HIAA, 5-hydroxyindoleacetic acid; 5-HT, 5-hydroxytryptamine, serotonin; 8-OHdG, 8-hydroxy-2′-deoxyguanosine; ±, SD, standard deviation; ×, for, per; ≈, about equal, not different; ♁, females; ♂, males; ↓, decreased, lower; ↑, increased, higher, →, induced, followed.
4. Discussion
In this review, we collected data on animal and human studies on depression and the role of Nrf2 as a probe of the antioxidant system function and found an antidepressant effect to be the consequence of the activation of the Nrf2-HO-1 pathway, with no studies pointing to the opposite direction. In this context, the role of neuroinflammation in being associated with depressive-like states clearly emerges, as well as the role of antioxidant systems in countering neuroinflammation and its markers, with Nrf2 standing at its crossroad. We may thus suppose that depression involves multiple systems and the antioxidant system, and Nrf2, in particular, counteract many actions of the failing multi-system state associated with depressive behaviour. Many of the substances that help overcome the alterations caused in the organism by depression are of natural origin and constitute long-used remedies for many other conditions. The antioxidant research line in depression that focuses on the actions of Nrf2 is relatively recent, dating back to 2013 [43], although the substance was isolated in 1994 [126], almost three decades ago.
The rate of publications on this issue has considerably increased across the years, with papers focusing on psychiatric disorders increasing almost exponentially from 26 in 2020 to 37 in 2021 and to 58 in 2022, witnessing increased interest and awareness of its importance in the pathophysiology of psychiatric disorders. Studies on depression reflect a similar trend. From eight studies in 2019, which increased to 16 in 2020, 18 in 2021, and 23 in 2022 to the beginning of 2023.
Most of the studies were conducted on rodents, mainly mice and, most importantly, male animals. This limits the extension of results to female animals, but it should be noted that findings in females were not dissimilar from those in males.
The studies included here were based on different animal depression paradigms, with many studies using stress-induced DLB, mostly elicited by LPS [40,43,51,53,58,59,63,65,67,73,75,79,85,88,95,105,106,125], or through corticosterone or cortisol [44,46,80,87,98], while other studies used chronic unpredictable mild stress [61,72,76,83,93,96,97,99,100,101,106,108,111,122,124] or social defeat stress [50,52,88,102,107,120]. Two studies used tumour-related depression models [71,91], while just one study each used post-ovariectomised depression [121], pentylenetetrazol-induced seizures [116], maternal separation [117], and olfactory bulbectomy [113] models (Table 1). Various studies used Nrf2 knock-out animals [49,62,84,88,120] or knock-out of the inflammation and histone methylation modulator protein arginine methyltransferase [67]. Nrf2 knock-out or knock-down apparently impairs neurochemical indexes and behavioural test performance, while protein arginine methyltransferase knock-out decreases depressive indexes [67]. Despite heterogeneity, findings all pointed to the Nrf2-antioxidant pathway restoring impaired neurochemistry and behavioural test performance.
Anti-inflammatory or anti-neuroinflammatory effects of employed antidepressants and putative antidepressant substances, such as the many plant extracts used in the studies we considered here, were shown to be related to various improvements in neurochemical indexes and behavioural tests [43,48,59,71,76,81,85,91,95,108,111,123,125].
Studies were consistent in measuring cyclic AMP responsive element binding protein (CREB) activity, in that increased phospho-CREB or CREB levels were associated with an antidepressant response [38,46,81,83,103,109,112]; remarkably, studies on peripheral tissues in man [38] and hippocampal content in mice [81] pointed to the same direction. Additionally, studies of BDNF showed that increased BDNF activity was associated with antidepressant effects and that animals displaying depressive features have low BDNF contents in the periphery and the brain [44,52,57,57,58,66,67,69,81,83,85,88,93,111,112,114,121]. It has recently been shown that most, if not all, antidepressant drugs bring about their antidepressant effects by binding the tyrosine kinase receptor 2 (TrkB), a molecule tied to the action of BDNF [127,128]. The findings of the studies reviewed here quite match this concept; increased TrkB/BDNF signalling was related to antidepressant effects, and low levels were associated with depressive behaviour [11,50,66,69,84,93,103,111,120]. Increased activity also of protein kinase B (Akt-1), which is an antioxidant in the wingless-GSK pathway and carries on some of the biological [128,129,130] and clinical [131] actions of lithium, which also inhibits GSK-3β [132,133,134], was found to be associated with antidepressant effects in the studies included in this review [42,45,47,74,75,93,94,110,119]. On the opposite side are found nuclear factor kappa B (NF-κB) and glycogen synthase kinase (GSK) pathways; the higher the NF-κB [38,39,67,69,73,75,80,85,91,98,100,103,113,115] and GSK3β levels [45,79,109], the worse the depressive indexes. Therefore, it appears that on one side, there are antioxidant systems and growth factors promoting plasticity, such as Nrf2–HO-1, BDNF, Akt, TrkB, and CREB, which are protective from depression and, on the other side, GSK-3β and NF-κB, which promote depressive behaviour (Figure 2). Therefore, we may suppose that new antidepressant drug discovery could involve promoting drugs that increase Nrf2–HO-1, BDNF, Akt, TrkB, and CREB activity, while downplaying GSK-3β and NF-κB. However, it is not so simple to identify the characteristics of drugs that act only where they will wishfully carry on the desired effect because drugs acting within cells must cross plasma membranes selectively, reach their intracellular target in those cells needing their actions, and not everywhere. Depression is a multi-system and multi-organ condition but not an all-system derangement, so the presence of an extraordinarily penetrating drug in some cells could be related to undesirable or adverse effects. Furthermore, not all actions of Nrf2 are good for the body. Nrf2 may promote tumours [135,136] and atherogenesis [137,138], so any drug acting on Nrf2 must avoid these two and maybe other unknown potentially harmful actions.
Figure 2.
Simplified depiction of the mechanism of action of Nrf2 and the pathways it interacts with. Arrows, activation; broken lines with rhomboid ending, inhibition. Abbreviations. Akt, Protein kinase B; BDNF, brain-derived neurotrophic factor; CREB, cyclic AMP responsive element binding protein; GCLC, Glutamate-cysteine ligase; GPx, glutathione peroxidase; GSH, glutathione; GSK-3β, glycogen synthase kinase 3-beta; HO-1, haem oxygenase 1; mTOR, mammalian target of rapamycin; NF-κB, nuclear factor kappa B; NQO-1, NAD(P)H quinone dehydrogenase 1; PI3K, phosphatidylinositol-3-kinase; SLC7A11, Solute Carrier Family 7 Member 11 (cystine/glutamate transporter); sMAF, small musculoaponeurotic fibrosarcoma proteins; SOD, superoxide dismutase; TrkB, tropomyosin receptor kinase B.
5. Limitations
This review has several limitations in that the animal studies focused mostly on rodents (mice and rats), and few focused on other animals (fish and nematodes) or cell lines; therefore, the results cannot extend to other animal species. Human studies were only four (one post mortem), and their methodologies were too different from animal protocols; hence the results from the latter are not translatable to the former. Furthermore, a sex bias exists in animal studies (mostly males) and not in the few human studies. Depression is overrepresented in women in humans, so it might be that testing it in male animals is not a good idea, but female rodents pose significant problems in conducting scientific experiments using the paradigms of the studies included in this review. The animal models of depression are not easy to translate to humans and into clinical practice.
6. Conclusions
Nrf2 is at the crossroad of many cellular actions. It directs antioxidant pathways and receives many intracellular signals to which it attempts to respond in a balanced way. Its function appears to be impaired in depression, and it is also possible that its manipulation could prove to be beneficial in human depression; however, there is much to discover. There is hope to discover drugs that cross the blood–brain barrier, or use some phytopharmaca or their derivatives, such as gastrodin, or use drugs stimulating anti-inflammatory cytokines that could counteract neuroinflammation. From the studies included in this review, depression appears to be strictly tied to inflammatory mechanisms. Provided that new drugs acting on the Nrf2 antioxidant pathway can avoid tumorigenesis and atherogenesis, Nrf2 can be a useful target for novel drug development.
Acknowledgments
The authors are grateful to the Scientific Administration of the Bibliographic and Bibliometric Support Service, Fondazione Policlinico A. Gemelli IRCCS, in particular, Maria Pattuglia, and Mimma Ariano, Ales Casciaro, Teresa Prioreschi, and Susanna Rospo, Librarians of the Sant’Andrea Hospital, Faculty of Medicine and Psychology, Sapienza University of Rome, for rendering precious bibliographical material accessible. They all have consented to the acknowledgement.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antiox12040817/s1, Online Supplement.
Author Contributions
G.S., G.D.K., M.M., D.P.R.C. and L.M. (Laura Monti) conceived the review; S.M., A.B. and G.D.K. wrote the introduction; S.M., A.B., O.M.F., L.M. (Lorenzo Moccia), E.B., A.S., D.P.R.C. and G.D.K. wrote the methods, decided eligibility along all others and wrote results and discussion; L.M. (Laura Monti), L.M. (Lorenzo Moccia), M.M., D.J., G.D.K., L.J., S.M., A.B., D.P.R.C. and G.D.K. performed literature searches; S.M., A.B., L.M. (Laura Monti), L.M. (Lorenzo Moccia), M.M. and G.D.K. provided the first draft; A.S., D.J., L.J., L.M. (Lorenzo Moccia), D.P.R.C., G.D.K. and G.S. supervised the writing of the manuscript, wrote the conclusions, and edited the final form. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Cao H., Zuo C., Gu Z., Huang Y., Yang Y., Zhu L., Jiang Y., Wang F. High frequency repetitive transcranial magnetic stimulation alleviates cognitive deficits in 3xTg-AD mice by modulating the PI3K/Akt/GLT-1 axis. Redox Biol. 2022;54:102354. doi: 10.1016/j.redox.2022.102354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zuo C., Cao H., Song Y., Gu Z., Huang Y., Yang Y., Miao J., Zhu L., Chen J., Jiang Y., et al. Nrf2: An all-rounder in depression. Redox Biol. 2022;58:102522. doi: 10.1016/j.redox.2022.102522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Betteridge D.J. What is oxidative stress? Metabolism. 2000;49((Suppl. 1)):3–8. doi: 10.1016/S0026-0495(00)80077-3. [DOI] [PubMed] [Google Scholar]
- 4.Francisqueti-Ferron F.V., Ferron A.J.T., Garcia J.L., Silva C.C.V.A., Costa M.R., Gregolin C.S., Moreto F., Ferreira A.L.A., Minatel I.O., Correa C.R. Basic concepts on the role of nuclear factor erythroid-derived 2-like 2 (Nrf2) in age-related diseases. Int. J. Mol. Sci. 2019;20:3208. doi: 10.3390/ijms20133208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saha S., Buttari B., Panieri E., Profumo E., Saso L. An overview of Nrf2 signaling pathway and its role in inflammation. Molecules. 2020;25:5474. doi: 10.3390/molecules25225474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang W., Feng C., Jiang H. Novel target for treating Alzheimer’s Diseases: Crosstalk between the Nrf2 pathway and autophagy. Ageing Res. Rev. 2021;65:101207. doi: 10.1016/j.arr.2020.101207. [DOI] [PubMed] [Google Scholar]
- 7.Sivandzade F., Prasad S., Bhalerao A., Cucullo L. NRF2 and NF-κB interplay in cerebrovascular and neurodegenerative disorders: Molecular mechanisms and possible therapeutic approaches. Redox Biol. 2019;21:101059. doi: 10.1016/j.redox.2018.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Osama A., Zhang J., Yao J., Yao X., Fang J. Nrf2: A dark horse in Alzheimer’s disease treatment. Ageing Res. Rev. 2020;64:101206. doi: 10.1016/j.arr.2020.101206. [DOI] [PubMed] [Google Scholar]
- 9.Tong K.I., Padmanabhan B., Kobayashi A., Shang C., Hirotsu Y., Yokoyama S., Yamamoto M. Different electrostatic potentials define ETGE and DLG motifs as hinge and latch in oxidative stress response. Mol. Cell. Biol. 2007;27:7511–7521. doi: 10.1128/MCB.00753-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohan S., Gupta D. Crosstalk of toll-like receptors signaling and Nrf2 pathway for regulation of inflammation. Biomed. Pharmacother. 2018;108:1866–1878. doi: 10.1016/j.biopha.2018.10.019. [DOI] [PubMed] [Google Scholar]
- 11.Chowdhry S., Zhang Y., McMahon M., Sutherland C., Cuadrado A., Hayes J.D. Nrf2 is controlled by two distinct β-TrCP recognition motifs in its Neh6 domain, one of which can be modulated by GSK-3 activity. Oncogene. 2013;32:3765–3781. doi: 10.1038/onc.2012.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang H.-C., Nguyen T., Pickett C.B. Phosphorylation of Nrf2 at Ser-40 by protein kinase C regulates antioxidant response element-mediated transcription. J. Biol. Chem. 2002;277:42769–42774. doi: 10.1074/jbc.M206911200. [DOI] [PubMed] [Google Scholar]
- 13.Cullinan S.B., Diehl J.A. PERK-dependent activation of Nrf2 contributes to redox homeostasis and cell survival following endoplasmic reticulum stress. J. Biol. Chem. 2004;279:20108–20117. doi: 10.1074/jbc.M314219200. [DOI] [PubMed] [Google Scholar]
- 14.Keum Y.-S., Yu S., Chang P.P.-J., Yuan X., Kim J.-H., Xu C., Han J., Agarwal A., Kong A.-N.T. Mechanism of action of sulforaphane: Inhibition of p38 mitogen-activated protein kinase isoforms contributing to the induction of antioxidant response element-mediated heme oxygenase-1 in human hepatoma HepG2 cells. Cancer Res. 2006;66:8804–8813. doi: 10.1158/0008-5472.CAN-05-3513. [DOI] [PubMed] [Google Scholar]
- 15.Pi J., Bai Y., Reece J.M., Williams J., Liu D., Freeman M.L., Fahl W.E., Shugar D., Liu J., Qu W., et al. Molecular mechanism of human Nrf2 activation and degradation: Role of sequential phosphorylation by protein kinase CK2. Free Radic. Biol. Med. 2007;42:1797–1806. doi: 10.1016/j.freeradbiomed.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen C.D., Yoo J., Hwang S.Y., Cho S.Y., Kim M., Jang H., No K.O., Shin J.C., Kim J.H., Lee G. Bee venom activates the Nrf2/HO-1 and TrkB/CREB/BDNF pathways in neuronal cell responses against oxidative stress induced by Aβ1-42. Int. J. Mol. Sci. 2022;23:1193. doi: 10.3390/ijms23031193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lau A., Wang X.-J., Zhao F., Villeneuve N.F., Wu T., Jiang T., Sun Z., White E., Zhang D.D. A noncanonical mechanism of Nrf2 activation by autophagy deficiency: Direct interaction between Keap1 and p62. Mol. Cell. Biol. 2010;30:3275–3285. doi: 10.1128/MCB.00248-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo L., Jiang Z.-M., Sun R.-X., Pang W., Zhou X., Du M.-L., Chen M.-X., Lv X., Wang J.-T. Repeated social defeat stress inhibits development of hippocampus neurons through mitophagy and autophagy. Brain Res. Bull. 2022;182:111–117. doi: 10.1016/j.brainresbull.2022.01.009. [DOI] [PubMed] [Google Scholar]
- 19.Hashimoto K. Essential role of Keap1-Nrf2 signaling in mood disorders: Overview and future perspective. Front. Pharmacol. 2018;9:1182. doi: 10.3389/fphar.2018.01182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Björkholm C., Monteggia L.M. BDNF a key transducer of antidepressant effects. Neuropharmacology. 2016;102:72–79. doi: 10.1016/j.neuropharm.2015.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang R., Cao Q.-Q., Hu S.-W., He L.-J., Du P.-F., Chen G., Fu R., Xiao F., Sun Y.-R., Zhang J.-C., et al. Sulforaphane activates anti-inflammatory microglia, modulating stress resilience associated with BDNF transcription. Acta Pharmacol. Sin. 2022;43:829–839. doi: 10.1038/s41401-021-00727-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abdalkader M., Lampinen R., Kanninen K.M., Malm T.M., Liddell J.R. Targeting Nrf2 to suppress ferroptosis and mitochondrial dysfunction in neurodegeneration. Front. Neurosci. 2018;12:466. doi: 10.3389/fnins.2018.00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson C.B., McLaughlin L.D., Nair A., Ebenezer P.J., Dange R., Francis J. Inflammation and oxidative stress are elevated in the brain, blood, and adrenal glands during the progression of post-traumatic stress disorder in a predator exposure animal model. PLoS ONE. 2013;8:e76146. doi: 10.1371/journal.pone.0076146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun X.R., Zhang H., Zhao H.T., Ji M.H., Li H.H., Wu J., Li K.Y., Yang J.J. Amelioration of oxidative stress-induced phenotype loss of parvalbumin interneurons might contribute to the beneficial effects of environmental enrichment in a rat model of post-traumatic stress disorder. Behav. Brain Res. 2016;312:84–92. doi: 10.1016/j.bbr.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 25.Alzoubi K.H., Al Subeh Z.Y., Khabour O.F. Molecular targets for the interactive effect of etazolate during post-traumatic stress disorder: Role of oxidative stress, BDNF and histones. Behav. Brain Res. 2019;369:111930. doi: 10.1016/j.bbr.2019.111930. [DOI] [PubMed] [Google Scholar]
- 26.Li H., Tofigh A.M., Amirfakhraei A., Chen X., Tajik M., Xu D., Motevalli S. Modulation of astrocyte activity and improvement of oxidative stress through blockage of NO/NMDAR pathway improve posttraumatic stress disorder (PTSD)-like behavior induced by social isolation stress. Brain Behav. 2022;12:e2620. doi: 10.1002/brb3.2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hong C., Cao J., Wu C.F., Kadioglu O., Schüffler A., Kauhl U., Klauck S.M., Opatz T., Thines E., Paul N.W., et al. The Chinese herbal formula Free and Easy Wanderer ameliorates oxidative stress through KEAP1-NRF2/HO-1 pathway. Sci. Rep. 2017;7:11551. doi: 10.1038/s41598-017-10443-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou C.H., Xue F., Xue S.S., Sang H.F., Liu L., Wang Y., Cai M., Zhang Z.J., Tan Q.R., Wang H.N., et al. Electroacupuncture pretreatment ameliorates PTSD-like behaviors in rats by enhancing hippocampal neurogenesis via the Keap1/Nrf2 antioxidant signaling pathway. Front. Cell. Neurosci. 2019;13:275. doi: 10.3389/fncel.2019.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Recinella L., Chiavaroli A., Orlando G., Ferrante C., Veschi S., Cama A., Marconi G.D., Diomede F., Gesmundo I., Granata R., et al. Effects of growth hormone-releasing hormone receptor antagonist MIA-602 in mice with emotional disorders: A potential treatment for PTSD. Mol. Psychiatry. 2021;26:7465–7474. doi: 10.1038/s41380-021-01228-5. [DOI] [PubMed] [Google Scholar]
- 30.McEwen B.S. Mood disorders and allostatic load. Biol. Psychiatry. 2003;54:200–207. doi: 10.1016/S0006-3223(03)00177-X. [DOI] [PubMed] [Google Scholar]
- 31.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Higgins J.P.T., Savović J., Page M.J., Elbers R.G., Sterne J.A.C. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins J.P.T., Thomas J., Chandler J., Cumpston M., Li T., Page M.J., Welch V.A., editors. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane; London, UK: 2022. [(accessed on 12 February 2023)]. Version 6.3, updated February 2022. Available online: www.training.cochrane.org/handbook. [Google Scholar]
- 33.de Boer S.F., van der Vegt B.J., Koolhaas J.M. Individual variation in aggression of feral rodent strains: A standard for the genetics of aggression and violence? Behav. Genet. 2003;33:485–501. doi: 10.1023/A:1025766415159. [DOI] [PubMed] [Google Scholar]
- 34.Albert P.R. Why is depression more prevalent in women? J. Psychiatry Neurosci. 2015;40:219–221. doi: 10.1503/jpn.150205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuehner C. Why is depression more common among women than among men? Lancet Psychiatry. 2017;4:146–158. doi: 10.1016/S2215-0366(16)30263-2. [DOI] [PubMed] [Google Scholar]
- 36.Tang J., Zhang T. Causes of the male-female ratio of depression based on the psychosocial factors. Front. Psychol. 2022;13:1052702. doi: 10.3389/fpsyg.2022.1052702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lukic I., Mitic M., Djordjevic J., Tatalovic N., Bozovic N., Soldatovic I., Mihaljevic M., Pavlovic Z., Radojcic M.B., Maric N.P., et al. Lymphocyte levels of redox-sensitive transcription factors and antioxidative enzymes as indicators of pro-oxidative state in depressive patients. Neuropsychobiology. 2014;70:1–9. doi: 10.1159/000362841. [DOI] [PubMed] [Google Scholar]
- 38.Mellon S.H., Wolkowitz O.M., Schonemann M.D., Epel E.S., Rosser R., Burke H.B., Mahan L., Reus V.I., Stamatiou D., Liew C.C., et al. Alterations in leukocyte transcriptional control pathway activity associated with major depressive disorder and antidepressant treatment. Transl. Psychiatry. 2016;6:e821. doi: 10.1038/tp.2016.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martín-Hernández D., Caso J.R., Javier M.J., Callado L.F., Madrigal J.L.M., García-Bueno B., Leza J.C. Intracellular inflammatory and antioxidant pathways in postmortem frontal cortex of subjects with major depression: Effect of antidepressants. J. Neuroinflamm. 2018;15:251. doi: 10.1186/s12974-018-1294-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kubick N., Pajares M., Enache I., Manda G., Mickael M.E. Repurposing zileuton as a depression drug using an AI and in vitro approach. Molecules. 2020;25:2155. doi: 10.3390/molecules25092155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goetzl E.J., Wolkowitz O.M., Srihari V.H., Reus V.I., Goetzl L., Kapogiannis D., Heninger G.R., Mellon S.H. Abnormal levels of mitochondrial proteins in plasma neuronal extracellular vesicles in major depressive disorder. Mol. Psychiatry. 2021;26:7355–7362. doi: 10.1038/s41380-021-01268-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li X., Wang Y.B., Wang C.C., Jing R., Mu L.H., Liu P., Hu Y. Antidepressant mechanism of kaixinsan and its active compounds based on upregulation of antioxidant thioredoxin. Evid.-Based Complement. Altern. Med. 2022;2022:7302442. doi: 10.1155/2022/7302442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martín-de-Saavedra M.D., Budni J., Cunha M.P., Gómez-Rangel V., Lorrio S., Del Barrio L., Lastres-Becker I., Parada E., Tordera R.M., Rodrigues A.L.S., et al. Nrf2 participates in depressive disorders through an anti-inflammatory mechanism. Psychoneuroendocrinology. 2013;38:2010–2022. doi: 10.1016/j.psyneuen.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 44.Mendez-David I., Tritschler L., Ali Z.E., Damiens M.H., Pallardy M., David D.J., Kerdine-Römer S., Gardier A.M. Nrf2-signaling and BDNF: A new target for the antidepressant-like activity of chronic fluoxetine treatment in a mouse model of anxiety/depression. Neurosci. Lett. 2015;597:121–126. doi: 10.1016/j.neulet.2015.04.036. [DOI] [PubMed] [Google Scholar]
- 45.Cunha M.P., Budni J., Ludka F.K., Pazini F.L., Rosa J.M., Oliveira Á., Lopes M.W., Tasca C.I., Leal R.B., Rodrigues A.L.S. Involvement of PI3K/Akt signaling pathway and its downstream intracellular targets in the antidepressant-like effect of creatine. Mol. Neurobiol. 2016;53:2954–2968. doi: 10.1007/s12035-015-9192-4. [DOI] [PubMed] [Google Scholar]
- 46.Freitas A.E., Egea J., Buendia I., Gómez-Rangel V., Parada E., Navarro E., Casas A.I., Wojnicz A., Ortiz J.A., Cuadrado A., et al. Agmatine, by improving neuroplasticity markers and inducing Nrf2, prevents corticosterone-induced depressive-like behavior in mice. Mol. Neurobiol. 2016;53:3030–3045. doi: 10.1007/s12035-015-9182-6. [DOI] [PubMed] [Google Scholar]
- 47.Martín-Hernández D., Bris Á.G., MacDowell K.S., García-Bueno B., Madrigal J.L., Leza J.C., Caso J.R. Modulation of the antioxidant nuclear factor (erythroid 2-derived)-like 2 pathway by antidepressants in rats. Neuropharmacology. 2016;103:79–91. doi: 10.1016/j.neuropharm.2015.11.029. [DOI] [PubMed] [Google Scholar]
- 48.Martín-Hernández D., Caso J.R., Bris Á.G., Maus S.R., Madrigal J.L., García-Bueno B., MacDowell K.S., Alou L., Gómez-Lus M.L., Leza J.C. Bacterial translocation affects intracellular neuroinflammatory pathways in a depression-like model in rats. Neuropharmacology. 2016;103:122–133. doi: 10.1016/j.neuropharm.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 49.Wojnicz A., Avendaño Ortiz J., Casas A.I., Freitas A.E., López M.G., Ruiz-Nuño A. Simultaneous determination of 8 neurotransmitters and their metabolite levels in rat brain using liquid chromatography in tandem with mass spectrometry: Application to the murine Nrf2 model of depression. Clin. Chim. Acta. 2016;453:174–181. doi: 10.1016/j.cca.2015.12.023. [DOI] [PubMed] [Google Scholar]
- 50.Yao W., Zhang J.-c., Ishima T., Dong C., Yang C., Ren Q., Ma M., Han M., Wu J., Suganuma H., et al. Role of Keap1-Nrf2 signaling in depression and dietary intake of glucoraphanin confers stress resilience in mice. Sci. Rep. 2016;6:30659. doi: 10.1038/srep30659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yao W., Zhang J.-C., Ishima T., Ren Q., Yang C., Dong C., Ma M., Saito A., Honda T., Hashimoto K. Antidepressant effects of TBE-31 and MCE-1, the novel Nrf2 activators, in an inflammation model of depression. Eur. J. Pharmacol. 2016;793:21–27. doi: 10.1016/j.ejphar.2016.10.037. [DOI] [PubMed] [Google Scholar]
- 52.Bouvier E., Brouillard F., Molet J., Claverie D., Cabungcal J.H., Cresto N., Doligez N., Rivat C., Do K.Q., Bernard C., et al. Nrf2-dependent persistent oxidative stress results in stress-induced vulnerability to depression. Mol. Psychiatry. 2017;22:1701–1713. doi: 10.1038/mp.2016.144. Erratum in Mol. Psychiatry 2017, 22, 1795. [DOI] [PubMed] [Google Scholar]
- 53.Zhang J.-c., Yao W., Dong C., Yang C., Ren Q., Ma M., Han M., Wu J., Ushida Y., Suganuma H., et al. Prophylactic effects of sulforaphane on depression-like behavior and dendritic changes in mice after inflammation. J. Nutr. Biochem. 2017;39:134–144. doi: 10.1016/j.jnutbio.2016.10.004. Erratum in J. Nutr. Biochem. 2021, 88, 108550; J. Nutr. Biochem. 2021, 89, 108562. [DOI] [PubMed] [Google Scholar]
- 54.Zhao X.-J., Zhao Z., Yang D.-D., Cao L.-L., Zhang L., Ji J., Gu J., Huang J.-Y., Sun X.-L. Activation of ATP-sensitive potassium channel by iptakalim normalizes stress- induced HPA axis disorder and depressive behaviour by alleviating inflammation and oxidative stress in mouse hypothalamus. Brain Res. Bull. 2017;130:146–155. doi: 10.1016/j.brainresbull.2017.01.026. [DOI] [PubMed] [Google Scholar]
- 55.López-Granero C., Antunes Dos Santos A., Ferrer B., Culbreth M., Chakraborty S., Barrasa A., Gulinello M., Bowman A.B., Aschner M. BXD recombinant inbred strains participate in social preference, anxiety and depression behaviors along sex- differences in cytokines and tactile allodynia. Psychoneuroendocrinology. 2017;80:92–98. doi: 10.1016/j.psyneuen.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abuelezz S.A., Hendawy N. Insights into the potential antidepressant mechanisms of cilostazol in chronically restraint rats: Impact on the Nrf2 pathway. Behav. Pharmacol. 2018;29:28–40. doi: 10.1097/FBP.0000000000000335. [DOI] [PubMed] [Google Scholar]
- 57.Omar N.N., Tash R.F. Fluoxetine coupled with zinc in a chronic mild stress model of depression: Providing a reservoir for optimum zinc signaling and neuronal remodeling. Pharmacol. Biochem. Behav. 2017;160:30–38. doi: 10.1016/j.pbb.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 58.Li M., Li C., Yu H., Cai X., Shen X., Sun X., Wang J., Zhang Y., Wang C. Lentivirus-mediated interleukin-1β (IL-1β) knock-down in the hippocampus alleviates lipopolysaccharide (LPS)-induced memory deficits and anxiety- and depression-like behaviors in mice. J. Neuroinflamm. 2017;14:190. doi: 10.1186/s12974-017-0964-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang M., Dang R., Xu P., Guo Y., Han W., Liao D., Jiang P. Dl-3-n-Butylphthalide improves lipopolysaccharide-induced depressive-like behavior in rats: Involvement of Nrf2 and NF-κB pathways. Psychopharmacology. 2018;235:2573–2585. doi: 10.1007/s00213-018-4949-x. [DOI] [PubMed] [Google Scholar]
- 60.Gao W., Wang W., Liu G., Zhang J., Yang J., Deng Z. Allicin attenuated chronic social defeat stress induced depressive-like behaviors through suppression of NLRP3 inflammasome. Metab. Brain Dis. 2019;34:319–329. doi: 10.1007/s11011-018-0342-z. [DOI] [PubMed] [Google Scholar]
- 61.Fan C., Song Q., Wang P., Li Y., Yang M., Yu S.Y. Neuroprotective effects of ginsenoside-Rg1 against depression-like behaviors via suppressing glial activation, synaptic deficits, and neuronal apoptosis in rats. Front. Immunol. 2018;9:2889. doi: 10.3389/fimmu.2018.02889. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 62.Chu C., Zhang H., Cui S., Han B., Zhou L., Zhang N., Su X., Niu Y., Chen W., Chen R., et al. Ambient PM2.5 caused depressive-like responses through Nrf2/NLRP3 signaling pathway modulating inflammation. J. Hazard. Mater. 2019;369:180–190. doi: 10.1016/j.jhazmat.2019.02.026. [DOI] [PubMed] [Google Scholar]
- 63.Dang R., Guo Y.-Y., Zhang K., Jiang P., Zhao M.-G. Predictable chronic mild stress promotes recovery from LPS-induced depression. Mol. Brain. 2019;12:42. doi: 10.1186/s13041-019-0463-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gao W., Wang W., Zhang J., Deng P., Hu J., Yang J., Deng Z. Allicin ameliorates obesity comorbid depressive-like behaviors: Involvement of the oxidative stress, mitochondrial function, autophagy, insulin resistance and NOX/Nrf2 imbalance in mice. Metab. Brain Dis. 2019;34:1267–1280. doi: 10.1007/s11011-019-00443-y. [DOI] [PubMed] [Google Scholar]
- 65.Arioz B.I., Tastan B., Tarakcioglu E., Tufekci K.U., Olcum M., Ersoy N., Bagriyanik A., Genc K., Genc S. Melatonin attenuates LPS-induced acute depressive-like behaviors and microglial NLRP3 inflammasome activation through the SIRT1/Nrf2 pathway. Front. Immunol. 2019;10:1511. doi: 10.3389/fimmu.2019.01511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cigliano L., Spagnuolo M.S., Boscaino F., Ferrandino I., Monaco A., Capriello T., Cocca E., Iannotta L., Treppiccione L., Luongo D., et al. Dietary supplementation with fish oil or conjugated linoleic acid relieves depression markers in mice by modulation of the Nrf2 pathway. Mol. Nutr. Food Res. 2019;63:e1900243. doi: 10.1002/mnfr.201900243. [DOI] [PubMed] [Google Scholar]
- 67.Liu H., Jiang J., Zhao L. Protein arginine methyltransferase-1 deficiency restrains depression-like behavior of mice by inhibiting inflammation and oxidative stress via Nrf-2. Biochem. Biophys. Res. Commun. 2019;518:430–437. doi: 10.1016/j.bbrc.2019.08.032. [DOI] [PubMed] [Google Scholar]
- 68.Rosa P.B., Bettio L.E.B., Neis V.B., Moretti M., Werle I., Leal R.B., Rodrigues A.L.S. The antidepressant-like effect of guanosine is dependent on GSK-3β inhibition and activation of MAPK/ERK and Nrf2/heme oxygenase-1 signaling pathways. Purinergic Signal. 2019;15:491–504. doi: 10.1007/s11302-019-09681-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huang X., Fei G.-Q., Liu W.-J., Ding J., Wang Y., Wang H., Ji J.-L., Wang X. Adipose-derived mesenchymal stem cells protect against CMS-induced depression-like behaviors in mice via regulating the Nrf2/HO-1 and TLR4/NF-κB signaling pathways. Acta Pharmacol. Sin. 2020;41:612–619. doi: 10.1038/s41401-019-0317-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zborowski V.A., Heck S.O., Vencato M., Pinton S., Marques L.S., Nogueira C.W. Keap1/Nrf2/HO-1 signaling pathway contributes to p-chlorodiphenyl diselenide antidepressant-like action in diabetic mice. Psychopharmacology. 2020;237:363–374. doi: 10.1007/s00213-019-05372-3. [DOI] [PubMed] [Google Scholar]
- 71.Casaril A.M., Domingues M., Bampi S.R., Lourenço D.A., Smaniotto T.Â., Segatto N., Vieira B., Seixas F.K., Collares T., Lenardão E.J., et al. The antioxidant and immunomodulatory compound 3-[(4-chlorophenyl)selanyl]-1-methyl-1H-indole attenuates depression-like behavior and cognitive impairment developed in a mouse model of breast tumor. Brain Behav. Immun. 2020;84:229–241. doi: 10.1016/j.bbi.2019.12.005. [DOI] [PubMed] [Google Scholar]
- 72.Tian L., Sun S.S., Cui L.B., Wang S.Q., Peng Z.W., Tan Q.R., Hou W.G., Cai M. Repetitive transcranial magnetic stimulation elicits antidepressant- and anxiolytic-like effect via nuclear factor-E2-related factor 2-mediated anti-inflammation mechanism in rats. Neuroscience. 2020;429:119–133. doi: 10.1016/j.neuroscience.2019.12.025. [DOI] [PubMed] [Google Scholar]
- 73.Li T., Zheng L.N., Han X.H. Fenretinide attenuates lipopolysaccharide (LPS)-induced blood-brain barrier (BBB) and depressive-like behavior in mice by targeting Nrf-2 signaling. Biomed. Pharmacother. 2020;125:109680. doi: 10.1016/j.biopha.2019.109680. [DOI] [PubMed] [Google Scholar]
- 74.Nakayama T., Okimura K., Shen J., Guh Y.J., Tamai T.K., Shimada A., Minou S., Okushi Y., Shimmura T., Furukawa Y., et al. Seasonal changes in NRF2 antioxidant pathway regulates winter depression-like behavior. Proc. Natl. Acad. Sci. USA. 2020;117:9594–9603. doi: 10.1073/pnas.2000278117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ali T., Rahman S.U., Hao Q., Li W., Liu Z., Ali Shah F., Murtaza I., Zhang Z., Yang X., Liu G., et al. Melatonin prevents neuroinflammation and relieves depression by attenuating autophagy impairment through FOXO3a regulation. J. Pineal Res. 2020;69:e12667. doi: 10.1111/jpi.12667. [DOI] [PubMed] [Google Scholar]
- 76.Wang W., Zheng L., Xu L., Tu J., Gu X. Pinocembrin mitigates depressive-like behaviors induced by chronic unpredictable mild stress through ameliorating neuroinflammation and apoptosis. Mol. Med. 2020;26:53. doi: 10.1186/s10020-020-00179-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liao D., Chen Y., Guo Y., Wang C., Liu N., Gong Q., Fu Y., Fu Y., Cao L., Yao D., et al. Salvianolic acid B improves chronic mild stress-induced depressive behaviors in rats: Involvement of AMPK/SIRT1 signaling pathway. J. Inflamm. Res. 2020;13:195–206. doi: 10.2147/JIR.S249363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Severo L., Godinho D., Machado F., Hartmann D., Fighera M.R., Soares F.A., Furian A.F., Oliveira M.S., Royes L.F. The role of mitochondrial bioenergetics and oxidative stress in depressive behavior in recurrent concussion model in mice. Life Sci. 2020;257:117991. doi: 10.1016/j.lfs.2020.117991. [DOI] [PubMed] [Google Scholar]
- 79.Ali T., Hao Q., Ullah N., Rahman S.U., Shah F.A., He K., Zheng C., Li W., Murtaza I., Li Y., et al. Melatonin act as an antidepressant via attenuation of neuroinflammation by targeting Sirt1/Nrf2/HO-1 signaling. Front. Mol. Neurosci. 2020;13:96. doi: 10.3389/fnmol.2020.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Camargo A., Dalmagro A.P., Rosa J.M., Zeni A.L.B., Kaster M.P., Tasca C.I., Rodrigues A.L.S. Subthreshold doses of guanosine plus ketamine elicit antidepressant-like effect in a mouse model of depression induced by corticosterone: Role of GR/NF-κB/IDO-1 signaling. Neurochem. Int. 2020;139:104797. doi: 10.1016/j.neuint.2020.104797. [DOI] [PubMed] [Google Scholar]
- 81.Park B.-K., Kim N.-S., Kim Y.-R., Yang C., Jung I.-C., Jang I.-S., Seo C.-S., Choi J.-J., Lee M.-Y. Antidepressant and anti-neuroinflammatory effects of bangpungtongsung-san. Front. Pharmacol. 2020;11:958. doi: 10.3389/fphar.2020.00958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhu X., Liu H., Liu Y., Chen Y., Liu Y., Yin X. The antidepressant-like effects of hesperidin in streptozotocin-induced diabetic rats by activating Nrf2/ARE/Glyoxalase 1 pathway. Front. Pharmacol. 2020;11:1325. doi: 10.3389/fphar.2020.01325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liao D., Lv C., Cao L., Yao D., Wu Y., Long M., Liu N., Jiang P. Curcumin attenuates chronic unpredictable mild stress-induced depressive-like behaviors via restoring changes in oxidative stress and the activation of Nrf2 signaling pathway in rats. Oxid. Med. Cell. Longev. 2020;2020:9268083. doi: 10.1155/2020/9268083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Qu Y., Shan J., Wang S., Chang L., Pu Y., Wang X., Tan Y., Yamamoto M., Hashimoto K. Rapid-acting and long-lasting antidepressant-like action of (R)-ketamine in Nrf2 knock-out mice: A role of TrkB signaling. Eur. Arch. Psychiatry Clin. Neurosci. 2021;271:439–446. doi: 10.1007/s00406-020-01208-w. [DOI] [PubMed] [Google Scholar]
- 85.Li W., Ali T., He K., Liu Z., Shah F.A., Ren Q., Liu Y., Jiang A., Li S. Ibrutinib alleviates LPS-induced neuroinflammation and synaptic defects in a mouse model of depression. Brain Behav. Immun. 2021;92:10–24. doi: 10.1016/j.bbi.2020.11.008. [DOI] [PubMed] [Google Scholar]
- 86.Yan T., Li F., Xiong W., Wu B., Xiao F., He B., Jia Y. Nootkatone improves anxiety- and depression-like behavior by targeting hyperammonemia-induced oxidative stress in D-galactosamine model of liver injury. Environ. Toxicol. 2021;36:694–706. doi: 10.1002/tox.23073. [DOI] [PubMed] [Google Scholar]
- 87.Herbet M., Szumełda I., Piątkowska-Chmiel I., Gawrońska-Grzywacz M., Dudka J. Beneficial effects of combined administration of fluoxetine and mitochondria- targeted antioxidant at in behavioural and molecular studies in mice model of depression. Behav. Brain Res. 2021;405:113185. doi: 10.1016/j.bbr.2021.113185. [DOI] [PubMed] [Google Scholar]
- 88.Yao W., Lin S., Su J., Cao Q., Chen Y., Chen J., Zhang Z., Hashimoto K., Qi Q., Zhang J.-C. Activation of BDNF by transcription factor Nrf2 contributes to antidepressant-like actions in rodents. Transl. Psychiatry. 2021;11:140. doi: 10.1038/s41398-021-01261-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Salama A., Mahmoud H.A., Kandeil M.A., Khalaf M.M. Neuroprotective role of camphor against ciprofloxacin induced depression in rats: Modulation of Nrf-2 and TLR4. Immunopharmacol. Immunotoxicol. 2021;43:309–318. doi: 10.1080/08923973.2021.1905658. [DOI] [PubMed] [Google Scholar]
- 90.Naß J., Abdelfatah S., Efferth T. The triterpenoid ursolic acid ameliorates stress in Caenorhabditis elegans by affecting the depression-associated genes skn-1 and prdx2. Phytomedicine. 2021;88:153598. doi: 10.1016/j.phymed.2021.153598. [DOI] [PubMed] [Google Scholar]
- 91.Yang S., Yang Y., Chen C., Wang H., Ai Q., Lin M., Zeng Q., Zhang Y., Gao Y., Li X., et al. The anti-neuroinflammatory effect of fuzi and ganjiang extraction on LPS-induced BV2 microglia and its intervention function on depression-like behavior of cancer-related fatigue model mice. Front. Pharmacol. 2021;12:670586. doi: 10.3389/fphar.2021.670586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhu X., Zhang Y.-M., Zhang M.-Y., Chen Y.-J., Liu Y.-W. Hesperetin ameliorates diabetes- associated anxiety and depression-like behaviors in rats via activating Nrf2/ARE pathway. Metab. Brain Dis. 2021;36:1969–1983. doi: 10.1007/s11011-021-00785-6. [DOI] [PubMed] [Google Scholar]
- 93.Wang J., Chen R., Liu C., Wu X., Zhang Y. Antidepressant mechanism of catalpol: Involvement of the PI3K/Akt/Nrf2/HO-1 signaling pathway in rat hippocampus. Eur. J. Pharmacol. 2021;909:174396. doi: 10.1016/j.ejphar.2021.174396. [DOI] [PubMed] [Google Scholar]
- 94.Wu X., Wang J., Song L., Guan Y., Cao C., Cui Y., Zhang Y., Liu C. Catalpol weakens depressive-like behavior in mice with streptozotocin-induced hyperglycemia via PI3K/AKT/Nrf2/HO-1 signaling pathway. Neuroscience. 2021;473:102–118. doi: 10.1016/j.neuroscience.2021.07.029. [DOI] [PubMed] [Google Scholar]
- 95.Rahman S.U., Ali T., Hao Q., He K., Li W., Ullah N., Zhang Z., Jiang Y., Li S. Xanthohumol attenuates lipopolysaccharide-induced depressive like behavior in mice: Involvement of NF-κB/Nrf2 signaling pathways. Neurochem. Res. 2021;46:3135–3148. doi: 10.1007/s11064-021-03396-w. [DOI] [PubMed] [Google Scholar]
- 96.Tao W., Hu Y., Chen Z., Dai Y., Hu Y., Qi M. Magnolol attenuates depressive-like behaviors by polarizing microglia towards the M2 phenotype through the regulation of Nrf2/HO-1/NLRP3 signaling pathway. Phytomedicine. 2021;91:153692. doi: 10.1016/j.phymed.2021.153692. [DOI] [PubMed] [Google Scholar]
- 97.Huang X., Qu Q., Ren D. Nrf2 alleviates cognitive dysfunction and brain inflammatory injury via mediating Wfs1 in rats with depression-like behaviors. Inflammation. 2022;45:399–413. doi: 10.1007/s10753-021-01554-4. [DOI] [PubMed] [Google Scholar]
- 98.Song L., Wu X., Wang J., Guan Y., Zhang Y., Gong M., Wang Y., Li B. Antidepressant effect of catalpol on corticosterone-induced depressive-like behavior involves the inhibition of HPA axis hyperactivity, central inflammation and oxidative damage probably via dual regulation of NF-κB and Nrf2. Brain Res. Bull. 2021;177:81–91. doi: 10.1016/j.brainresbull.2021.09.002. [DOI] [PubMed] [Google Scholar]
- 99.Guan Y., Wang J., Wu X., Song L., Wang Y., Gong M., Li B. Quercetin reverses chronic unpredictable mild stress-induced depression-like behavior in vivo by involving nuclear factor-E2-related factor 2. Brain Res. 2021;1772:147661. doi: 10.1016/j.brainres.2021.147661. [DOI] [PubMed] [Google Scholar]
- 100.Sun D.-C., Wang R.-R., Xu H., Zhu X.-H., Sun Y., Qiao S.-H., Qiao W. A network pharmacology-based study on antidepressant effect of Salicornia europaea L. extract with experimental support in chronic unpredictable mild stress model mice. Chin. J. Integr. Med. 2022;28:339–348. doi: 10.1007/s11655-022-2879-2. [DOI] [PubMed] [Google Scholar]
- 101.Cheng W.-J., Li P., Huang W.-Y., Huang Y., Chen W.-J., Chen Y.-P., Shen J.-L., Chen J.-K., Long N.-S., Meng X.-J. Acupuncture relieves stress-induced depressive behavior by reducing oxidative stress and neuroapoptosis in rats. Front. Behav. Neurosci. 2022;15:783056. doi: 10.3389/fnbeh.2021.783056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dang R., Wang M., Li X., Wang H., Liu L., Wu Q., Zhao J., Ji P., Zhong L., Licinio J., et al. Edaravone ameliorates depressive and anxiety-like behaviors via Sirt1/Nrf2/HO-1/Gpx4 pathway. J. Neuroinflamm. 2022;19:41. doi: 10.1186/s12974-022-02400-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xia B., Liu X., Li X., Wang Y., Wang D., Kou R., Zhang L., Shi R., Ye J., Bo X., et al. Sesamol ameliorates dextran sulfate sodium-induced depression- like and anxiety-like behaviors in colitis mice: The potential involvement of the gut-brain axis. Food Funct. 2022;13:2865–2883. doi: 10.1039/D1FO03888E. [DOI] [PubMed] [Google Scholar]
- 104.Li J., Gao W., Zhao Z., Li Y., Yang L., Wei W., Ren F., Li Y., Yu Y., Duan W., et al. Ginsenoside Rg1 reduced microglial activation and mitochondrial dysfunction to alleviate depression-like behaviour via the GAS5/EZH2/SOCS3/NRF2 axis. Mol. Neurobiol. 2022;59:2855–2873. doi: 10.1007/s12035-022-02740-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Muhammad A.J., Hao L., Al Kury L.T., Rehman N.U., Alvi A.M., Badshah H., Ullah I., Shah F.A., Li S. Carveol promotes Nrf2 contribution in depressive disorders through an anti-inflammatory mechanism. Oxid. Med. Cell. Longev. 2022;2022:4509204. doi: 10.1155/2022/4509204. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 106.Shen F., Xie P., Li C., Bian Z., Wang X., Peng D., Zhu G. Polysaccharides from Polygonatum cyrtonema Hua reduce depression-like behavior in mice by inhibiting oxidative stress-calpain-1-NLRP3 signaling axis. Oxid. Med. Cell. Longev. 2022;2022:2566917. doi: 10.1155/2022/2566917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jiang N., Zhang Y., Yao C., Huang H., Wang Q., Huang S., He Q., Liu X. Ginsenosides Rb1 attenuates chronic social defeat stress-induced depressive behavior via regulation of SIRT1-NLRP3/Nrf2 pathways. Front. Nutr. 2022;9:868833. doi: 10.3389/fnut.2022.868833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.de Souza A.G., Lopes I.S., Filho A.J.M.C., Cavalcante T.M.B., Oliveira J.V.S., de Carvalho M.A.J., de Lima K.A., Jucá P.M., Mendonça S.S., Mottin M., et al. Neuroprotective effects of dimethyl fumarate against depression-like behaviors via astrocytes and microglia modulation in mice: Possible involvement of the HCAR2/Nrf2 signaling pathway. Naunyn Schmiedebergs Arch. Pharmacol. 2022;395:1029–1045. doi: 10.1007/s00210-022-02247-x. [DOI] [PubMed] [Google Scholar]
- 109.Fasakin O.W., Oboh G., Ademosun A.O., Lawal A.O. The modulatory effects of alkaloid extracts of Cannabis sativa, Datura stramonium, Nicotiana tabacum and male Carica papaya on neurotransmitter, neurotrophic and neuroinflammatory systems linked to anxiety and depression. Inflammopharmacology. 2022;30:2447–2476. doi: 10.1007/s10787-022-01006-x. [DOI] [PubMed] [Google Scholar]
- 110.Tan L., Yang Y., Peng J., Zhang Y., Wu B., He B., Jia Y., Yan T. Schisandra chinensis (Turcz.) Baill. essential oil exhibits antidepressant-like effects and against brain oxidative stress through Nrf2/HO-1 pathway activation. Metab. Brain Dis. 2022;37:2261–2275. doi: 10.1007/s11011-022-01019-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wu X., Liu C., Wang J., Guan Y., Song L., Chen R., Gong M. Catalpol exerts antidepressant-like effects by enhancing anti-oxidation and neurotrophy and inhibiting neuroinflammation via activation of HO-1. Neurochem. Res. 2022;47:2975–2991. doi: 10.1007/s11064-022-03641-w. [DOI] [PubMed] [Google Scholar]
- 112.Zhao Y.-T., Yin H., Hu C., Zeng J., Zhang S., Chen S., Zheng W., Li M., Jin L., Liu Y., et al. Tilapia skin peptides ameliorate cyclophosphamide-induced anxiety- and depression-like behavior via improving oxidative stress, neuroinflammation, neuron apoptosis, and neurogenesis in mice. Front. Nutr. 2022;9:882175. doi: 10.3389/fnut.2022.882175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bansal Y., Singh R., Sodhi R.K., Khare P., Dhingra R., Dhingra N., Bishnoi M., Kondepudi K.K., Kuhad A. Kynurenine monooxygenase inhibition and associated reduced quinolinic acid reverses depression-like behaviour by upregulating Nrf2/ARE pathway in mouse model of depression: In-vivo and In-silico studies. Neuropharmacology. 2022;215:109169. doi: 10.1016/j.neuropharm.2022.109169. [DOI] [PubMed] [Google Scholar]
- 114.Yu Y., Li Y., Qi K., Xu W., Wei Y. Rosmarinic acid relieves LPS-induced sickness and depressive-like behaviors in mice by activating the BDNF/Nrf2 signaling and autophagy pathway. Behav. Brain Res. 2022;433:114006. doi: 10.1016/j.bbr.2022.114006. [DOI] [PubMed] [Google Scholar]
- 115.Kang J.-Y., Baek D.-C., Son C.-G., Lee J.-S. Succinum extracts inhibit microglial- derived neuroinflammation and depressive-like behaviors. Front. Pharmacol. 2022;13:991243. doi: 10.3389/fphar.2022.991243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang X., Xiao A., Yang Y., Zhao Y., Wang C.C., Wang Y., Han J., Wang Z., Wen M. DHA and EPA prevent seizure and depression-like behavior by inhibiting ferroptosis and neuroinflammation via different mode-of-actions in a pentylenetetrazole-induced kindling model in mice. Mol. Nutr. Food Res. 2022;66:e2200275. doi: 10.1002/mnfr.202200275. [DOI] [PubMed] [Google Scholar]
- 117.Nasehi L., Morassaei B., Ghaffari M., Sharafi A., Dehpour A.R., Hosseini M.-J. The impacts of vorinostat on NADPH oxidase and mitochondrial biogenesis gene expression in the heart of mice model of depression. Can. J. Physiol. Pharmacol. 2022;100:1077–1085. doi: 10.1139/cjpp-2022-0098. [DOI] [PubMed] [Google Scholar]
- 118.Pei H., Zeng J., He Z., Zong Y., Zhao Y., Li J., Chen W., Du R. Palmatine ameliorates LPS-induced HT-22 cells and mouse models of depression by regulating apoptosis and oxidative stress. J. Biochem. Mol. Toxicol. 2022;37:e23225. doi: 10.1002/jbt.23225. [DOI] [PubMed] [Google Scholar]
- 119.Sun J.Y., Liu Y.T., Jiang S.N., Guo P.M., Wu X.Y., Yu J. Essential oil from the roots of Paeonia lactiflora pall. has protective effect against corticosterone-induced depression in mice via modulation of PI3K/Akt signaling pathway. Front. Pharmacol. 2022;13:999712. doi: 10.3389/fphar.2022.999712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.He L., Zheng Y., Huang L., Ye J., Ye Y., Luo H., Chen X., Yao W., Chen J., Zhang J.C. Nrf2 regulates the arginase 1+ microglia phenotype through the initiation of TREM2 transcription, ameliorating depression-like behavior in mice. Transl. Psychiatry. 2022;12:459. doi: 10.1038/s41398-022-02227-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Samy D.M., Mostafa D.K., Saleh S.R., Hassaan P.S., Zeitoun T.M., Ammar G.A.G., Elsokkary N.H. Carnosic acid mitigates depression-like behavior in ovariectomized mice via activation of Nrf2/HO-1 pathway. Mol. Neurobiol. 2022;60:610–628. doi: 10.1007/s12035-022-03093-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Si L., Xiao L., Xie Y., Xu H., Yuan G., Xu W., Wang G. Social isolation after chronic unpredictable mild stress perpetuates depressive-like behaviors, memory deficits and social withdrawal via inhibiting ERK/KEAP1/NRF2 signaling. J. Affect. Disord. 2023;324:576–588. doi: 10.1016/j.jad.2022.12.092. [DOI] [PubMed] [Google Scholar]
- 123.Wang J., Men Y., Wang Z. Polydatin alleviates chronic stress-induced depressive and anxiety-like behaviors in a mouse model. ACS Chem. Neurosci. 2023;14:977–987. doi: 10.1021/acschemneuro.2c00758. [DOI] [PubMed] [Google Scholar]
- 124.Smaniotto T.Â., Casaril A.M., de Andrade Lourenço D., Sousa F.S., Seixas F.K., Collares T., Woloski R., da Silva Pinto L., Alves D., Savegnago L. Intranasal administration of interleukin-4 ameliorates depression-like behavior and biochemical alterations in mouse submitted to the chronic unpredictable mild stress: Modulation of neuroinflammation and oxidative stress. Psychopharmacology. 2023;240:935–950. doi: 10.1007/s00213-023-06336-4. [DOI] [PubMed] [Google Scholar]
- 125.Zhang J., Li L., Liu Q., Zhao Z., Su D., Xiao C., Jin T., Chen L., Xu C., You Z., et al. Gastrodin programs an Arg-1+ microglial phenotype in hippocampus to ameliorate depression- and anxiety-like behaviors via the Nrf2 pathway in mice. Phytomedicine. 2023;113:154725. doi: 10.1016/j.phymed.2023.154725. [DOI] [PubMed] [Google Scholar]
- 126.Moi P., Chan K., Asunis I., Cao A., Kan Y.W. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc. Natl. Acad. Sci. USA. 1994;91:9926–9930. doi: 10.1073/pnas.91.21.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Casarotto P.C., Girych M., Fred S.M., Kovaleva V., Moliner R., Enkavi G., Biojone C., Cannarozzo C., Sahu M.P., Kaurinkoski K., et al. Antidepressant drugs act by directly binding to TRKB neurotrophin receptors. Cell. 2021;184:1299–1313.e19. doi: 10.1016/j.cell.2021.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Casarotto P., Umemori J., Castrén E. BDNF receptor TrkB as the mediator of the antidepressant drug action. Front. Mol. Neurosci. 2022;15:1032224. doi: 10.3389/fnmol.2022.1032224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Meffre D., Massaad C., Grenier J. Lithium chloride stimulates PLP and MBP expression in oligodendrocytes via Wnt/β-catenin and Akt/CREB pathways. Neuroscience. 2015;284:962–971. doi: 10.1016/j.neuroscience.2014.10.064. [DOI] [PubMed] [Google Scholar]
- 130.Mehrafza S., Kermanshahi S., Mostafidi S., Motaghinejad M., Motevalian M., Fatima S. Pharmacological evidence for lithium-induced neuroprotection against methamphetamine-induced neurodegeneration via Akt-1/GSK3 and CREB-BDNF signaling pathways. Iran. J. Basic Med. Sci. 2019;22:856–865. doi: 10.22038/ijbms.2019.30855.7442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sani G., Perugi G., Tondo L. Treatment of Bipolar Disorder in a Lifetime Perspective: Is Lithium Still the Best Choice? Clin. Drug Investig. 2017;37:713–727. doi: 10.1007/s40261-017-0531-2. [DOI] [PubMed] [Google Scholar]
- 132.Rahimi H.R., Ghahremani M.H., Dehpour A.R., Sharifzadeh M., Ejtemaei-Mehr S., Razmi A., Ostad S.N. The neuroprotective effect of lithium in cannabinoid dependence is mediated through modulation of cyclic AMP, ERK1/2 and GSK-3β phosphorylation in cerebellar granular neurons of rat. Iran. J. Pharm. Res. 2015;14:1123–1135. [PMC free article] [PubMed] [Google Scholar]
- 133.Li B., Ren J., Yang L., Li X., Sun G., Xia M. Lithium Inhibits GSK3β Activity via Two Different Signaling Pathways in Neurons After Spinal Cord Injury. Neurochem. Res. 2018;43:848–856. doi: 10.1007/s11064-018-2488-9. [DOI] [PubMed] [Google Scholar]
- 134.Monaco S.A., Ferguson B.R., Gao W.J. Lithium inhibits GSK3β and augments GluN2A receptor expression in the prefrontal cortex. Front. Cell. Neurosci. 2018;12:16. doi: 10.3389/fncel.2018.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.De Nicola G.M., Karreth F.A., Humpton T.J., Gopinathan A., Wei C., Frese K., Mangal D., Yu K.H., Yeo C.J., Calhoun E.S., et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475:106–109. doi: 10.1038/nature10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cirone M., D’Orazi G. NRF2 in cancer: Cross-talk with oncogenic pathways and involvement in gammaherpesvirus-driven carcinogenesis. Int. J. Mol. Sci. 2022;24:595. doi: 10.3390/ijms24010595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Barajas B., Che N., Yin F., Rowshanrad A., Orozco L.D., Gong K.W., Wang X., Castellani L.W., Reue K., Lusis A.J., et al. NF-E2-related factor 2 promotes atherosclerosis by effects on plasma lipoproteins and cholesterol transport that overshadow antioxidant protection. Arterioscler. Thromb. Vasc. Biol. 2011;31:58–66. doi: 10.1161/ATVBAHA.110.210906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wu W., Hendrix A., Nair S., Cui T. Nrf2-mediated dichotomy in the vascular system: Mechanistic and therapeutic perspective. Cells. 2022;11:3042. doi: 10.3390/cells11193042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available in the article and Supplementary Materials.