Abstract
Purpose:
This retrospective cohort study examined patterns of endocrine therapy initiation over time and by demographic, tumor, and treatment characteristics.
Methods:
We included 7,777 women from three U.S. integrated healthcare systems diagnosed with incident stage I-III hormone receptor-positive breast cancer between 2001-2016. We extracted endocrine therapy from pharmacy dispensings, defining initiation as dispensings within 12 months of diagnosis. Demographic, tumor, and treatment characteristics were collected from electronic health records. Using generalized linear models with a log link and Poisson distribution, we estimated initiation of any endocrine therapy, tamoxifen, and aromatase inhibitors (AI) over time with relative risks (RR) and 95% confidence intervals (CI) adjusted for age, tumor characteristics, diagnosis year, other treatment, and study site.
Results:
Among women aged 20+ (mean 62 years), 6,329 (81.4%) initiated any endocrine therapy, and 1,448 (18.6%) did not initiate endocrine therapy. Tamoxifen initiation declined from 67% to 15% between 2001-2016. AI initiation increased from 6% to 69% between 2001-2016 in women aged ≥55 years. The proportion of women who did not initiate endocrine therapy decreased from 19% to 12% between 2002-2014 then increased to 17% by 2016. After adjustment, women least likely to initiate endocrine therapy were older (RR=0.81, 95%CI=0.77-0.85 for age 75+ versus 55-64), Black (RR=0.93, 95%CI=0.87-1.00 versus white), and had stage I disease (RR=0.88, 95%CI=0.85-0.91 versus stage III).
Conclusions:
Despite an increase in AI use over time, at least one in six eligible women did not initiate endocrine therapy, highlighting opportunities for improving endocrine therapy uptake in breast cancer survivors.
Keywords: breast cancer, endocrine therapy, initiation, trend
Introduction
Clinical trials have demonstrated that using endocrine therapy (tamoxifen or aromatase inhibitors [AI]) is associated with reduced breast cancer recurrence and mortality risk.[1-6] Several studies have demonstrated that for most women, endocrine therapy benefits outweigh the risks.[3,4,7-9] Potential risks include side effects (hot flashes and night sweats for tamoxifen and AIs),[10-12] increased risk of osteoporosis and bone fracture for AIs,[13,14] and increased risk of uterine cancer, deep vein thrombosis, stroke, and pulmonary embolism for tamoxifen.[15-18] For women with hormone receptor (HR) positive breast cancer (approximately 75-80% of all women with breast cancer), American Society of Clinical Oncology[19] and National Comprehensive Cancer Network (NCCN)[20] guidelines recommend daily use of endocrine therapy for five years in postmenopausal women and daily use of tamoxifen for five years in premenopausal women with consideration of up to 10 years total treatment.[20-22] NCCN guidelines recommend sequential administration of endocrine therapy following surgery and chemotherapy (if indicated); endocrine therapy may be administered concurrently with radiotherapy and/or trastuzumab treatment.[20]
While numerous studies have evaluated adherence to endocrine therapy,[23-33] fewer have evaluated initial uptake of these drugs. Studies of women diagnosed with invasive HR-positive breast cancer have shown that approximately 60-90% of eligible women initiate endocrine therapy within 12 months of diagnosis.[34-40] Previous research has shown lower endocrine therapy initiation among women who were Black (versus white),[34,35,41,42] diagnosed at older ages (versus younger),[34,35,40-42] had multiple comorbidities (versus fewer),[34,35,40] or diagnosed with less (versus more) aggressive breast cancer tumors.[34,37,39,40,42] Multiple studies have also shown an increase in endocrine therapy use over time; however, few studies extended beyond women diagnosed in the late 2000’s.[42,43]
We evaluated endocrine therapy initiation using electronic prescription records in a cohort of women diagnosed with stage I-III HR-positive breast cancer between 2001-2016 from three U.S. integrated health systems. We previously evaluated endocrine therapy initiation in a cohort of 1,501 women with stage I-II breast cancer diagnosed between 2001-2008.[11] This analysis updates that cohort to include additional geographic sites, women with stage III breast cancer, and more contemporary years of diagnosis. Our objective was to evaluate patterns of endocrine therapy initiation within 12 months of diagnosis over time and by demographic, tumor, and treatment characteristics.
Methods
Study population
This was a retrospective cohort study of breast cancer survivors diagnosed between 2001-2016 with follow-up through 2017 and enrolled in Kaiser Permanente (KP) Colorado (years of diagnosis 2001-2014), KP Northwest (years of diagnosis 2001-2008), or KP Washington (years of diagnosis 2001-2016).[44] The cohort included women aged 20 and older with a first primary unilateral breast cancer diagnosis who did not have a second cancer diagnosis within 12 months of their initial diagnosis (n=10,994) (Figure 1). Women had to be alive and continuously enrolled in their health plan during those 12 months. We excluded women with stage 0, stage IV, or unknown stage (n=1,182); women without definitive surgery (n=91); and women with both estrogen receptor (ER) negative and progesterone receptor (PR) negative disease (n=1,534) or unknown/borderline ER or PR status (n=227). We further excluded women whose first use of endocrine therapy did not include tamoxifen, letrozole, anastrozole or exemestane (n=15). Finally, we excluded women missing information on breast cancer grade (n=133) or radiotherapy (n=35) because these variables had missing values in our adjusted models. Our final analytic population included 7,777 women. Each site obtained Institutional Review Board permission with a waiver of consent to access data.
Fig.1.
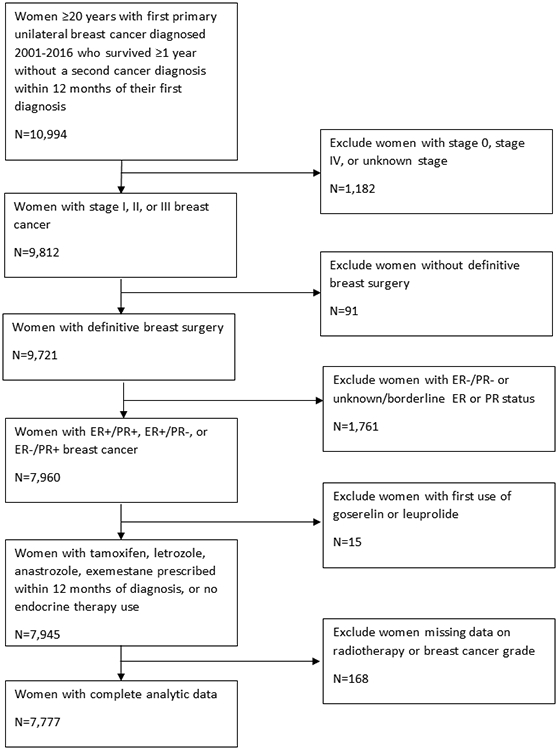
Inclusion and exclusion criteria used to create analytic cohort from Kaiser Permanente Breast Cancer Survivors Cohort. This figure shows the number of women that were included in the analytic cohort after each exclusion criterion was applied.
Data collection
We extracted data on demographic characteristics from the Virtual Data Warehouse (VDW), a distributed data system located behind individual site firewalls.[45] The VDW consists of standardized data tables with information from clinical and administrative data sources, including electronic health records, tumor registries, and pharmacy databases. Age, diagnosis year, race, breast cancer stage, tumor characteristics, and initial treatment were extracted from Surveillance Epidemiology and End Results (SEER) (KP Washington) or local (KP Colorado and Northwest) tumor registries. SEER is a population-based registry that collects data on cancer diagnoses, treatment, and outcomes for people who reside in a specific geographic area (in this case, the Seattle-Puget Sound region) whereas the local tumor registries collect cancer data on people enrolled their health systems. We used American Joint Committee on Cancer stage where possible, combining versions 5, 6, and 7 depending on what was available from tumor registries, and filled in missing values with SEER summary stage.
We collected data on any endocrine therapy dispensings (tamoxifen, letrozole, anastrozole, and exemestane) from electronic pharmacy records within 12 months of the initial breast cancer diagnosis, which we refer to as “use”. We separately evaluated first use of tamoxifen therapy and aromatase inhibitor (AI) therapy (letrozole, anastrozole, and exemestane). If a woman used both tamoxifen and AI therapy within 12 months, we only included the therapy she used first because we were interested in initial uptake (N=326: 150 used tamoxifen first and 176 AIs first). Women were considered non-users if they did not use any endocrine therapy at any time after diagnosis (not limited to the first 12 months).
Statistical analyses
We examined descriptive characteristics by endocrine therapy use categories: any endocrine therapy, tamoxifen first, AI first, and no endocrine therapy. All subsequent analyses for AIs were limited to postmenopausal women using age 55 years and older as a proxy,[46,47] because AIs are recommended for postmenopausal women only. Using generalized linear models with a log link and Poisson distribution, we estimated rates of any endocrine therapy, tamoxifen, and AI use for each diagnosis year adjusting for age at diagnosis, stage, grade, hormone receptor status, and study site. We calculated relative risks of any endocrine therapy use (versus no endocrine therapy use), first tamoxifen use (versus no endocrine therapy use), and first AI use (versus no endocrine therapy use and versus first tamoxifen use). We ran two adjusted models: 1) adjusting for age at diagnosis, diagnosis year, stage, grade, HR status, and study site; 2) adjusting for all variables in model 1 plus initial surgery/radiotherapy, and chemotherapy recorded in tumor registries or electronic pharmacy databases. In a sensitivity analysis, we restricted to women diagnosed between 2001-2008 to minimize any difference in results by study site (each site contributed women with different years of diagnosis).
Results
A total of 7,777 women ages 20-103 (mean and median age=62 years, standard deviation=12 years, Table 1) were included in the analyses for any initial endocrine therapy and tamoxifen use. Among all women, 6,329 (81.4%) initiated endocrine therapy and 1,448 (18.6%) did not initiate endocrine therapy. Women who did not initiate endocrine therapy were older (30.7% ≥75 years), had less aggressive tumor characteristics (79.4% with stage I disease and 40.0% with tumor sizes <1cm), and less aggressive treatment (15.7% with BCS alone without radiotherapy and 85.9% with no chemotherapy) compared with women who initiated tamoxifen or AIs.
Table 1.
Descriptive characteristics of women with hormone receptor positive breast cancer diagnosed between 2001-2016 by endocrine therapy use within 12 months of diagnosis, Kaiser Permanente Breast Cancer Survivors’ Cohort
| All ages (N=7777) | ||||
|---|---|---|---|---|
| Characteristic | Any endocrine therapya N (%) |
Tamoxifen first N (%) |
AIs first N (%) |
No endocrine therapyb N (%) |
| Total | 6329 (81.4) | 3105 (39.9) | 3224 (41.5) | 1448 (18.6) |
| Age at diagnosis, mean (SD) | 61.1 (11.7) | 57.7 (12.9) | 64.3 (9.4) | 67.3 (13.4) |
| Age at diagnosis | ||||
| <45 | 517 (8.2) | 470 (15.1) | 47 (1.5) | 86 (5.9) |
| 45-<55 | 1474 (23.3) | 1043 (33.6) | 431 (13.4) | 198 (13.7) |
| 55-<65 | 1974 (31.2) | 671 (21.6) | 1303 (40.4) | 319 (22.0) |
| 65-<75 | 1567 (24.8) | 557 (17.9) | 1010 (31.3) | 400 (27.6) |
| ≥75 | 797 (12.6) | 364 (11.7) | 433 (13.4) | 445 (30.7) |
| Race | ||||
| White | 5769 (91.2) | 2798 (90.1) | 2971 (92.2) | 1312 (90.6) |
| Black | 165 (2.6) | 83 (2.7) | 82 (2.5) | 48 (3.3) |
| American Indian/ Alaska Native | 42 (0.7) | 25 (0.8) | 17 (0.5) | 8 (0.6) |
| Asian/ Pacific Islander | 270 (4.3) | 154 (5.0) | 116 (3.6) | 47 (3.2) |
| Other | 28 (0.4) | 14 (0.5) | 14 (0.4) | 6 (0.4) |
| Unknown | 55 (0.9) | 31 (1.0) | 24 (0.7) | 27 (1.9) |
| Stage | ||||
| I | 3548 (56.1) | 1762 (56.7) | 1786 (55.4) | 1150 (79.4) |
| II | 2220 (35.1) | 1100 (35.4) | 1120 (34.7) | 254 (17.5) |
| III | 561 (8.9) | 243 (7.8) | 318 (9.9) | 44 (3.0) |
| Histology | ||||
| Ductal | 4796 (75.8) | 2359 (76.0) | 2437 (75.6) | 1099 (75.9) |
| Lobular | 691 (10.9) | 321 (10.3) | 370 (11.5) | 119 (8.2) |
| Mixed | 559 (8.8) | 275 (8.9) | 284 (8.8) | 89 (6.1) |
| Other | 283 (4.5) | 150 (4.8) | 133 (4.1) | 141 (9.7) |
| HR Status | ||||
| ER+/PR+ | 5469 (86.4) | 2731 (88.0) | 2738 (84.9) | 1190 (82.2) |
| ER+/PR− | 828 (13.1) | 354 (11.4) | 474 (14.7) | 213 (14.7) |
| ER−/PR+ | 32 (0.5) | 20 (0.6) | 12 (0.4) | 45 (3.1) |
| HER2 status | ||||
| Positive | 507 (8.0) | 230 (7.4) | 277 (8.6) | 79 (5.5) |
| Negative | 3560 (56.2) | 1605 (51.7) | 1955 (60.6) | 845 (58.4) |
| Missing | 2262 (35.7) | 1270 (40.9) | 992 (30.8) | 524 (36.2) |
| Tumor size, cm | ||||
| <1 | 1236 (19.5) | 611 (19.7) | 625 (19.4) | 579 (40.0) |
| 1-<2 | 2801 (44.3) | 1377 (44.4) | 1424 (44.2) | 569 (39.3) |
| 2-<5 | 1972 (31.2) | 971 (31.3) | 1001 (31.1) | 262 (18.1) |
| ≥5 | 269 (4.3) | 118 (3.8) | 151 (4.7) | 25 (1.7) |
| Missing | 51 (0.8) | 28 (0.9) | 23 (0.7) | 13 (0.9) |
| Grade | ||||
| Well differentiated | 2119 (33.5) | 1102 (35.5) | 1017 (31.5) | 684 (47.2) |
| Moderately differentiated | 2925 (46.2) | 1422 (45.8) | 1503 (46.6) | 534 (36.9) |
| Poorly differentiated/undifferentiated | 1285 (20.3) | 581 (18.7) | 704 (21.8) | 230 (15.9) |
| Surgery | ||||
| BCS | 310 (4.9) | 133 (4.3) | 177 (5.5) | 228 (15.7) |
| BCS + radiotherapy | 3623 (57.2) | 1746 (56.2) | 1877 (58.2) | 780 (53.9) |
| Bilateral mastectomy | 2396 (37.9) | 1226 (39.5) | 1170 (36.3) | 440 (30.4) |
| Chemotherapy | ||||
| No | 3868 (61.1) | 1792 (57.7) | 2076 (64.4) | 1244 (85.9) |
| Yes | 2461 (38.9) | 1313 (42.3) | 1148 (35.6) | 204 (14.1) |
Abbreviations: HR - Hormone receptor; ER - Estrogen receptor; PR – Progesterone receptor; HER2 - Human epidermal growth factor receptor 2; BCS - Breast conserving surgery (lumpectomy/partial mastectomy)
Includes any use of tamoxifen, letrozole, anastrozole, exemestane within 12 months of breast cancer diagnosis
Defined as no use of endocrine therapy at any time.
Among 5,502 women aged 55 and older (Table 2), 4,338 (78.8%) initiated endocrine therapy and 1,164 (21.2%) did not use endocrine therapy. Patterns of endocrine therapy use by demographic and tumor characteristics were similar to the overall population.
Table 2.
Descriptive characteristics of women age ≥55 years at diagnosis of hormone receptor positive breast cancer (2001-2016) by endocrine therapy use within 12 months of diagnosis
| Age ≥55 years (N=5502) | ||||
|---|---|---|---|---|
| Characteristic | Any endocrine therapya N (%) |
Tamoxifen first N (%) |
AIs first N (%) |
No endocrine therapyb N (%) |
| Total | 4338 (78.8) | 1592 (28.9) | 2746 (49.9) | 1164 (21.2) |
| Age at diagnosis, mean (SD) | 67.2 (8.2) | 68.0 (8.6) | 66.7 (7.9) | 72.2 (9.8) |
| Age at diagnosis | ||||
| 55-<65 | 1974 (45.5) | 671 (42.1) | 1303 (47.5) | 319 (27.4) |
| 65-<75 | 1567 (36.1) | 557 (35.0) | 1010 (36.8) | 400 (34.4) |
| ≥75 | 797 (18.4) | 364 (22.9) | 433 (15.8) | 445 (38.2) |
| Year of diagnosis | ||||
| 2001-2004 | 1138 (26.2) | 846 (53.1) | 292 (10.6) | 393 (33.8) |
| 2005-2008 | 1350 (31.1) | 442 (27.8) | 908 (33.1) | 350 (30.1) |
| 2009-2012 | 949 (21.9) | 166 (10.4) | 783 (28.5) | 228 (19.6) |
| 2013-2016 | 901 (20.8) | 138 (8.7) | 763 (27.8) | 193 (16.6) |
| Race | ||||
| White | 4021 (92.7) | 1482 (93.1) | 2539 (92.5) | 1071 (92.0) |
| Black | 95 (2.2) | 29 (1.8) | 66 (2.4) | 37 (3.2) |
| American Indian/Alaska Native | 21 (0.5) | 8 (0.5) | 13 (0.5) | 5 (0.4) |
| Asian/Pacific Islander | 142 (3.3) | 47 (3.0) | 95 (3.5) | 24 (2.1) |
| Other | 21 (0.5) | 7 (0.4) | 14 (0.5) | 4 (0.3) |
| Missing | 38 (0.9) | 19 (1.2) | 19 (0.7) | 23 (2.0) |
| Stage | ||||
| I | 2591 (59.7) | 1012 (63.6) | 1579 (57.5) | 959 (82.4) |
| II | 1420 (32.7) | 508 (31.9) | 912 (33.2) | 181 (15.5) |
| III | 327 (7.5) | 72 (4.5) | 255 (9.3) | 24 (2.1) |
| Histology | ||||
| Ductal | 3242 (74.7) | 1185 (74.4) | 2057 (74.9) | 870 (74.7) |
| Lobular | 509 (11.7) | 178 (11.2) | 331 (12.1) | 102 (8.8) |
| Mixed | 390 (9.0) | 141 (8.9) | 249 (9.1) | 71 (6.1) |
| Other | 197 (4.5) | 88 (5.5) | 109 (4.0) | 121 (10.4) |
| HR Status | ||||
| ER+/PR+ | 3690 (85.1) | 1365 (85.7) | 2325 (84.7) | 969 (83.2) |
| ER+/PR− | 631 (14.5) | 219 (13.8) | 412 (15.0) | 172 (14.8) |
| ER−/PR+ | 17 (0.4) | 8 (0.5) | 9 (0.3) | 23 (2.0) |
| HER2 status | ||||
| Positive | 307 (7.1) | 87 (5.5) | 220 (8.0) | 50 (4.3) |
| Negative | 2500 (57.6) | 825 (51.8) | 1675 (61.0) | 698 (60.0) |
| Missing | 1531 (35.3) | 680 (42.7) | 851 (31.0) | 416 (35.7) |
| Tumor size, cm | ||||
| <1 | 877 (20.2) | 333 (20.9) | 544 (19.8) | 489 (42.0) |
| 1-<2 | 2004 (46.2) | 767 (48.2) | 1237 (45.1) | 461 (39.6) |
| 2-<5 | 1275 (29.4) | 449 (28.2) | 826 (30.1) | 192 (16.5) |
| ≥5 | 149 (3.4) | 27 (1.7) | 122 (4.4) | 15 (1.3) |
| Missing | 33 (0.8) | 16 (1.0) | 17 (0.6) | 7 (0.6) |
| Grade | ||||
| Well differentiated | 1518 (35.0) | 639 (40.1) | 879 (32.0) | 583 (50.1) |
| Moderately differentiated | 2042 (47.1) | 740 (46.5) | 1302 (47.4) | 433 (37.2) |
| Poorly differentiated/undifferentiated | 778 (17.9) | 213 (13.4) | 565 (20.6) | 148 (12.7) |
| Surgical procedure | ||||
| BCS | 280 (6.5) | 111 (7.0) | 169 (6.2) | 198 (17.0) |
| BCS + radiotherapy | 2591 (59.7) | 964 (60.6) | 1627 (59.2) | 637 (54.7) |
| Mastectomy | 1467 (33.8) | 517 (32.5) | 950 (34.6) | 329 (28.3) |
| Chemotherapy | ||||
| No | 3158 (72.8) | 1248 (78.4) | 1910 (69.6) | 1071 (92.0) |
| Yes | 1180 (27.2) | 344 (21.6) | 836 (30.4) | 93 (8.0) |
Abbreviations: HR - Hormone receptor; ER - Estrogen receptor; PR – Progesterone receptor; HER2 - Human epidermal growth factor receptor 2; BCS - Breast conserving surgery (lumpectomy/partial mastectomy)
Includes any use of tamoxifen, letrozole, anastrozole, exemestane within 12 months of breast cancer diagnosis
Defined as no use of endocrine therapy at any time.
Endocrine therapy initiation over time
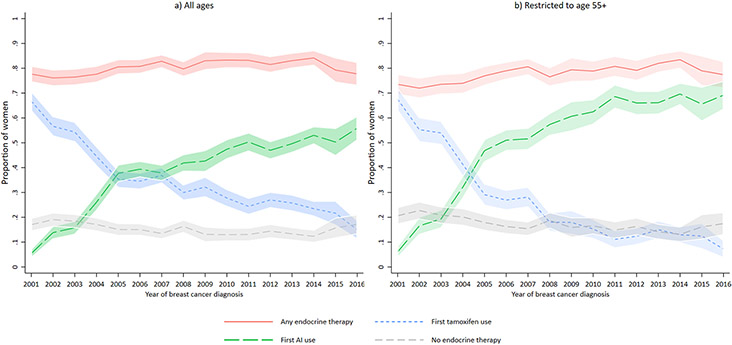
Adjusted proportions of endocrine therapy use increased slightly over time from a low of 76% in 2002 (Figure 2a and Supplementary Table 1) to a high of 84% in 2014 with a slight decline to 78% in 2016. Adjusted proportions of no endocrine therapy initiation decreased slightly from 19% in 2002 to 12% in 2014, and then increased to 17% in 2016, although years 2015-2016 included only one study site. First tamoxifen use declined from a high of 67% in 2001 (Figure 2a and Supplementary Table 1) to 15% in 2016 in all women, and 68% in 2001 (Figure 2b and Supplementary Table 2) to 7% in 2016 in women aged ≧55 years. First AI use increased from 6% in 2001 to 69% in 2016 in women aged ≥55 years.
Fig. 2.
Adjusted proportion (with 95% confidence intervals) of endocrine therapy use within 12 months of diagnosis over time, Kaiser Permanente Breast Cancer Survivors Cohort, 2001-2016. This figure shows endocrine therapy initiation within 12 months of breast cancer diagnosis by year of diagnosis. Figure 2a includes all ages and Figure 2b includes women aged 55 and older. The lines represent the proportion of women who initiated any endocrine therapy (solid line), tamoxifen first (small dash line), aromatase inhibitors first (big dash line), and no endocrine therapy (medium dash line). Each line is adjusted for age at diagnosis (<45, 45-<55, 55-<65, 65-<75, 75+), stage (I,II,III), grade (well differentiated, moderately differentiated, poorly differentiated/undifferentiated), HR status (ER+/PR+, ER+/PR−, ER−/PR+), and study site (KP CO, NW and WA). Study sites contributed different calendar periods to the analysis: Kaiser Permanente (KP) Colorado (years of diagnosis 2001-2014), KP Northwest (years of diagnosis 2001-2008), and KP Washington (years of diagnosis 2001-2016)
Initiation of any endocrine therapy by women’s characteristics
Compared to a reference group aged 55-64 (Table 3, model 2), women ≥65 were less likely to initiate any endocrine therapy (65-<74 RR=0.96, 95%CI=0.93-0.98 and ≥75 RR=0.81, 95%CI=0.77-0.85) after model adjustment. Black women were less likely to initiate endocrine therapy (RR=0.93, 95%CI=0.87-1.00) compared with white women. Women diagnosed with discordant hormone receptor disease were less likely to initiate endocrine therapy (ER+/PR− RR=0.96, 95%CI=0.93-0.99; ER−/PR+ RR=0.48, 95%CI=0.37-0.63) compared with women with ER+/PR+ disease. Women with more favorable tumor characteristics (stage I disease and/or tumor size <1cm) and less aggressive treatment (BCS without radiation and no chemotherapy) were less likely to initiate endocrine therapy even after adjustment for confounders.
Table 3.
Adjusted associations between any endocrine therapy, tamoxifen or AI initiation within 12 months of breast cancer diagnosis
| Characteristic | Any endocrine therapya vs. none | Tamoxifen first vs. none | AIs first vs. none (ages ≥55) | |||
|---|---|---|---|---|---|---|
| Model 1b RR (95% CI) |
Model 2c RR (95% CI) |
Model 1b RR (95% CI) |
Model 2c RR (95% CI) |
Model 1b RR (95% CI) |
Model 2c RR (95% CI) |
|
| Total number of patients | 6329 vs. 1448 | 6329 vs. 1448 | 3105 vs. 1448 | 3105 vs. 1448 | 2746 vs 1164 | 2746 vs 1164 |
| Age at diagnosis | ||||||
| <45 | 0.96 (0.93-0.99) | 0.95 (0.91-0.98) | 1.21 (1.14-1.28) | 1.17 (1.10-1.24) | -- | -- |
| 45-<55 | 1.01 (0.99-1.03) | 1.00 (0.98-1.03) | 1.23 (1.17-1.29) | 1.20 (1.14-1.26) | -- | -- |
| 55-<65 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| 65-<75 | 0.93 (0.91-0.96) | 0.96 (0.93-0.98) | 0.87 (0.82-0.93) | 0.90 (0.85-0.96) | 0.90 (0.87-0.94) | 0.92 (0.89-0.96) |
| ≥75 | 0.75 (0.72-0.78) | 0.81 (0.77-0.85) | 0.68 (0.62-0.73) | 0.74 (0.68-0.81) | 0.65 (0.61-0.69) | 0.70 (0.65-0.75) |
| Year of diagnosis | ||||||
| 2001-2004 | 0.94 (0.91-0.97) | 0.92 (0.89-0.95) | 1.29 (1.20-1.38) | 1.25 (1.17-1.34) | 0.56 (0.51-0.61) | 0.55 (0.50-0.60) |
| 2005-2008 | 0.97 (0.94-1.00) | 0.96 (0.93-0.99) | 1.14 (1.06-1.22) | 1.11 (1.04-1.19) | 0.87 (0.83-0.92) | 0.87 (0.82-0.91) |
| 2009-2012 | 0.99 (0.96-1.02) | 0.98 (0.95-1.01) | 1.04 (0.97-1.12) | 1.03 (0.95-1.11) | 0.95 (0.91-1.00) | 0.95 (0.91-0.99) |
| 2013-2016 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Race | ||||||
| White | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Black | 0.93 (0.87-1.00) | 0.93 (0.87-1.00) | 0.91 (0.80-1.02) | 0.90 (0.80-1.02) | 0.86 (0.75-0.99) | 0.86 (0.75-0.99) |
| American Indian/Alaska Native | 1.00 (0.90-1.12) | 1.00 (0.90-1.12) | 1.05 (0.88-1.27) | 1.05 (0.89-1.25) | 1.00 (0.79-1.27) | 1.00 (0.79-1.27) |
| Asian/Pacific Islander | 1.01 (0.96-1.06) | 1.01 (0.96-1.05) | 1.06 (0.98-1.15) | 1.05 (0.97-1.14) | 1.01 (0.92-1.10) | 1.01 (0.93-1.10) |
| Other | 0.95 (0.82-1.10) | 0.96 (0.84-1.11) | 0.86 (0.65-1.13) | 0.89 (0.70-1.14) | 1.05 (0.86-1.28) | 1.03 (0.85-1.26) |
| Unknown | 0.83 (0.72-0.96) | 0.84 (0.72-0.97) | 0.78 (0.62-0.97) | 0.78 (0.62-0.98) | 0.71 (0.52-0.97) | 0.72 (0.53-0.98) |
| Stage | ||||||
| I | 0.84 (0.82-0.86) | 0.88 (0.85-0.91) | 0.81 (0.76-0.85) | 0.87 (0.81-0.92) | 0.74 (0.70-0.78) | 0.77 (0.73-0.82) |
| II | 0.98 (0.96-1.01) | 1.00 (0.97-1.02) | 1.01 (0.96-1.07) | 1.04 (0.98-1.09) | 0.94 (0.90-0.99) | 0.95 (0.91-1.01) |
| III | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Histology | ||||||
| Ductal | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Lobular | 1.02 (0.99-1.05) | 1.02 (0.99-1.05) | 1.04 (0.98-1.10) | 1.04 (0.98-1.10) | 1.02 (0.97-1.08) | 1.03 (0.97-1.08) |
| Mixed | 1.02 (0.98 to 1.05) | 1.02 (0.99 to 1.05) | 1.01 (0.95 to 1.07) | 1.01 (0.96 to 1.07) | 1.07 (1.01 to 1.13) | 1.07 (1.01 to 1.14) |
| Other | 0.86 (0.81 to 0.92) | 0.87 (0.82 to 0.93) | 0.83 (0.75 to 0.92) | 0.84 (0.75 to 0.93) | 0.78 (0.69 to 0.89) | 0.79 (0.70 to 0.90) |
| HR Status | ||||||
| ER+/PR+ | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| ER+/PR− | 0.96 (0.93-0.99) | 0.96 (0.93-0.99) | 0.92 (0.86-0.98) | 0.91 (0.86-0.97) | 0.96 (0.91-1.02) | 0.96 (0.91-1.01) |
| ER−/PR+ | 0.49 (0.37-0.64) | 0.48 (0.37-0.63) | 0.44 (0.31-0.63) | 0.43 (0.30-0.62) | 0.36 (0.21-0.64) | 0.36 (0.21-0.64) |
| HER2 status | ||||||
| Positive | 1.03 (1.00-1.07) | 1.02 (0.98-1.05) | 1.04 (0.97-1.12) | 1.03 (0.96-1.10) | 1.06 (1.00-1.13) | 1.04 (0.98-1.11) |
| Negative | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Missing | 0.99 (0.96-1.01) | 0.99 (0.96-1.02) | 0.92 (0.87-0.97) | 0.93 (0.88-0.98) | 1.02 (0.97-1.07) | 1.02 (0.97-1.08) |
| Tumor size, cm | ||||||
| <1 cm | 0.87 (0.83-0.90) | 0.87 (0.84-0.91) | 0.78 (0.73-0.84) | 0.79 (0.73-0.85) | 0.84 (0.78-0.91) | 0.84 (0.78-0.90) |
| 1-<2 | 1.03 (1.01-1.06) | 1.03 (1.00-1.05) | 1.03 (0.98-1.08) | 1.02 (0.97-1.07) | 1.08 (1.03-1.14) | 1.07 (1.02-1.13) |
| 2-<5 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| ≥5 | 1.01 (0.97-1.05) | 1.02 (0.98-1.06) | 0.98 (0.90-1.07) | 0.99 (0.91-1.07) | 1.03 (0.96-1.11) | 1.04 (0.97-1.12) |
| Missing | 0.92 (0.81-1.04) | 0.92 (0.82-1.04) | 0.88 (0.72-1.08) | 0.88 (0.72-1.07) | 0.93 (0.73-1.20) | 0.94 (0.73-1.21) |
| Grade | ||||||
| Well differentiated | 0.95 (0.92-0.98) | 0.97 (0.94-1.00) | 0.98 (0.92-1.03) | 1.01 (0.95-1.06) | 0.87 (0.83-0.92) | 0.90 (0.85-0.95) |
| Moderately differentiated | 1.03 (1.00-1.05) | 1.04 (1.02-1.07) | 1.09 (1.04-1.14) | 1.11 (1.06-1.16) | 1.01 (0.97-1.06) | 1.03 (0.98-1.07) |
| Poorly differentiated/undifferentiated | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Surgical procedure | ||||||
| BCS | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| BCS + radiotherapy | 1.29 (1.20-1.39) | 1.29 (1.20-1.39) | 1.55 (1.35-1.78) | 1.55 (1.35-1.78) | 1.31 (1.17-1.47) | 1.31 (1.17-1.47) |
| Mastectomy | 1.26 (1.16-1.36) | 1.25 (1.16-1.35) | 1.55 (1.35-1.78) | 1.54 (1.34-1.77) | 1.24 (1.11-1.40) | 1.24 (1.10-1.40) |
| Chemotherapy | ||||||
| No | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Yes | 1.09 (1.06-1.12) | 1.09 (1.06-1.11) | 1.12 (1.06-1.17) | 1.11 (1.06-1.16) | 1.09 (1.05-1.14) | 1.09 (1.05-1.14) |
Abbreviations: HR - Hormone receptor; ER - Estrogen receptor; PR – Progesterone receptor; HER2 - Human epidermal growth factor receptor 2; BCS - Breast conserving surgery (lumpectomy/partial mastectomy)
Includes any use of tamoxifen, letrozole, anastrozole, or exemestane within 12 months of breast cancer diagnosis
Adjusted for age at diagnosis (<45, 45-<55, 55-<65, 65-<75, 75+), year of diagnosis (2001-2004, 2005-2008, 2009-2012, 2013-2016), stage (I,II,III), grade (well differentiated, moderately differentiated, poorly differentiated/undifferentiated), HR status (ER+/PR+, ER+/PR−, ER−/PR+), and study site (KP CO, NW and WA)
Adjusted for variables in model 1 + chemotherapy (yes, no), and surgery type (BCS, BCS + radiotherapy, mastectomy)
Initiation by type of endocrine therapy
Women 65 and older were less likely to initiate tamoxifen (65-74 years RR=0.90, 95%CI=0.85-0.96 and ≥75 years RR=0.74, 95%CI=0.68-0.81, Table 3, model 2) compared with women ages 55-<65. Similarly, women 65 and older were less likely to use AIs first (65-74 years RR=0.92, 95%CI=0.89-0.96 and ≥75 years RR=0.70, 95%CI=0.65-0.75). Black women were less likely to use tamoxifen (RR=0.90, 95%CI=0.80-1.02) or AIs (RR=0.86, 95%CI=0.75-0.99) compared with white women. Women with stage I disease were less likely to use tamoxifen (RR=0.87, 95%CI=0.81-0.92) or AIs (RR=0.77, 95%CI=0.73-0.82) compared with women with stage III disease, and women with tumor sizes <1cm were less likely to use tamoxifen (RR=0.79, 95%CI=0.73-0.85) or AIs (RR=0.84, 95%CI=0.78-0.90) compared with women with tumor sizes 2-<5cm. Women with hormone receptor status ER−/PR+ were unlikely to initiate tamoxifen (RR=0.43, 95%CI=0.30-0.62) or AIs (RR=0.36, 95%CI=0.21-0.64) compared with women with ER+/PR+ disease. Women who had more aggressive treatment (mastectomy, BCS with radiotherapy, and/or chemotherapy) were more likely to initiate tamoxifen or AIs compared to women who did not receive these treatments. In a sensitivity analysis restricting all sites to the same study years (2001-2008), results were slightly attenuated but similar to overall results.
When comparing initiation of AIs first versus tamoxifen first among patients age 55+ years at diagnosis (Table 4, model 2), women were less likely to initiate AIs if they were ≥75 years (RR=0.88, 95%CI=0.82-0.94) after adjusting for demographics, tumor characteristics, and other treatment. Women were less likely to initiate AIs first if they were diagnosed with stage I (RR=0.87, 95%CI=0.81-0.94) or stage II disease (RR=0.90, 95%CI=0.84-0.97), or well differentiated (RR=0.93, 95%CI=0.88-0.99) or moderately differentiated grade tumors (RR=0.94, 95%CI=0.89-0.98). Women were more likely to initiate AIs first if they also received chemotherapy (RR=1.08, 95%CI=1.03-1.14).
Table 4.
Adjusted associations between AI vs tamoxifen initiation among hormone receptor positive breast cancer patients age 55+ years at diagnosis (2001-2016) within 12 months of breast cancer diagnosisa
| Characteristic | AIs first vs. Tamoxifen first | |
|---|---|---|
| Model 1b RR (95% CI) |
Model 2c RR (95% CI) |
|
| Total number of patients | 2746 vs 1592 | 2746 vs 1592 |
| Age at diagnosis | ||
| <45 | -- | -- |
| 45-<55 | -- | -- |
| 55-<65 | 1.00 (Ref) | 1.00 (Ref) |
| 65-<75 | 0.95 (0.91-0.99) | 0.97 (0.93-1.01) |
| ≥75 | 0.85 (0.80-0.90) | 0.88 (0.82-0.94) |
| Year of diagnosis | ||
| 2001-2004 | 0.28 (0.25-0.32) | 0.28 (0.25-0.31) |
| 2005-2008 | 0.74 (0.69-0.78) | 0.73 (0.69-0.78) |
| 2009-2012 | 0.97 (0.93-1.01) | 0.97 (0.93-1.01) |
| 2013-2016 | 1.00 (Ref) | 1.00 (Ref) |
| Race | ||
| White | 1.00 (Ref) | 1.00 (Ref) |
| Black | 1.06 (0.94-1.19) | 1.06 (0.94-1.19) |
| American Indian/Alaska Native | 0.90 (0.67-1.20) | 0.89 (0.66-1.20) |
| Asian/Pacific Islander | 0.91 (0.82-1.01) | 0.91 (0.82-1.01) |
| Other | 1.10 (0.88-1.38) | 1.11 (0.89-1.39) |
| Missing | 1.01 (0.75-1.36) | 1.01 (0.75-1.36) |
| Stage | ||
| I | 0.85 (0.79-0.90) | 0.87 (0.81-0.94) |
| II | 0.90 (0.84-0.95) | 0.90 (0.84-0.97) |
| III | 1.00 (Ref) | 1.00 (Ref) |
| Histology | ||
| Ductal | 1.00 (Ref) | 1.00 (Ref) |
| Lobular | 1.02 (0.96-1.09) | 1.03 (0.96-1.09) |
| Mixed | 1.08 (1.01-1.16) | 1.09 (1.01-1.17) |
| Other | 0.97 (0.86-1.08) | 0.97 (0.87-1.09) |
| HR Status | ||
| ER+/PR+ | 1.00 (Ref) | 1.00 (Ref) |
| ER+/PR− | 1.04 (0.98-1.10) | 1.03 (0.98-1.09) |
| ER−/PR+ | 0.84 (0.59-1.19) | 0.83 (0.59-1.18) |
| HER2 status | ||
| Positive | 1.03 (0.97-1.11) | 1.02 (0.95-1.09) |
| Negative | 1.00 (Ref) | 1.00 (Ref) |
| Missing | 1.05 (0.99-1.11) | 1.05 (0.99-1.11) |
| Tumor size, cm | ||
| <1 | 1.02 (0.94-1.11) | 1.01 (0.94-1.10) |
| 1-<2 | 1.04 (0.98-1.11) | 1.03 (0.96-1.10) |
| 2-<5 | 1.00 (Ref) | 1.00 (Ref) |
| ≥5 | 1.15 (1.05-1.25) | 1.16 (1.06-1.26) |
| Missing | 0.99 (0.73-1.33) | 0.99 (0.73-1.34) |
| Grade | ||
| Well differentiated | 0.91 (0.87-0.97) | 0.93 (0.88-0.99) |
| Moderately differentiated | 0.92 (0.88-0.97) | 0.94 (0.89-0.98) |
| Poorly differentiated/undifferentiated | 1.00 (Ref) | 1.00 (Ref) |
| Surgical procedure | ||
| BCS | 1.00 (Ref) | 1.00 (Ref) |
| BCS + radiotherapy | 1.04 (0.94-1.14) | 1.04 (0.95-1.15) |
| Mastectomy | 1.00 (0.90-1.10) | 1.00 (0.90-1.10) |
| Chemotherapy | ||
| No | 1.00 (Ref) | 1.00 (Ref) |
| Yes | 1.08 (1.02-1.14) | 1.08 (1.03-1.14) |
Abbreviations: HR - Hormone receptor; ER - Estrogen receptor; PR – Progesterone receptor; HER2 - Human epidermal growth factor receptor 2; BCS - Breast conserving surgery (lumpectomy/partial mastectomy)
Analyses are limited to women ages 55+ who initiated tamoxifen or AIs within 12 months of diagnosis (N=4,338)
Adjusted for age at diagnosis (55-<65, 65-<75, 75+), year of diagnosis (2001-2004, 2005-2008, 2009-2012, 2013-2016), stage (I,II,III), grade (I,II,III,IV), HR status (ER+/PR+, ER+/PR−, ER−/PR+), and study site (KP CO, NW and WA)
Adjusted for variables in model 1 + chemotherapy (yes, no), and surgery type (BCS, BCS + radiotherapy, mastectomy)
Discussion
In this population-based cohort, approximately 80% of women diagnosed with invasive, hormone receptor-positive breast cancer between 2001-2016 initiated endocrine therapy within 12 months of diagnosis with a slight increase over time. Starting in 2005, AIs were used more often as the first mode of endocrine therapy, while tamoxifen use declined over time. This change in therapy over time reflects clinical trial results presented between 2002-2005[5,7,9,48,49] and guideline changes in 2004 and 2006[50,51] to recommend AIs as the preferred mode of endocrine therapy for postmenopausal women due to increased effectiveness and better side effect profile.[19,20,52] The 18% of women who did not initiate endocrine therapy decreased slightly over time and were more likely to be older, Black, and have less aggressive tumor characteristics at diagnosis. While it may be reassuring to see the proportion who did not initiate endocrine therapy decrease over time, these findings raise concerns about a potential racial disparity and inconsistency of treatment recommendations and decisions with national clinical guidelines.[20] They also highlight opportunities for improving endocrine therapy uptake in breast cancer survivors.
Endocrine therapy dispensings were lower among Black women in our study, consistent with several previous studies.[34,35,38,41,42] Although our results were statistically significant even after adjustment for stage and other treatment, the number of Black women (N=213) was small, and these results should be interpreted with caution. There may be many reasons that women of any race did not initiate endocrine therapy including low perceived risk of recurrence and/or second primary breast cancer, not being offered treatment, lack of shared decision making about treatment risks and benefit, concerns about side effects, potential cost of treatment, and personal preference.[38,53,54] We could not evaluate reasons individuals did not receive any endocrine therapy dispensings in our study. All women were enrolled in an integrated healthcare system, suggesting that cost of treatment or access to care should have been less of a barrier than in other community settings. However, even with insurance, healthcare access within Kaiser Permanente still has significant variability in insurance generosity. Disparities in pharmacy co-pays or high deductible plans, which have increased over time, may have dissuaded some women from starting a long-term treatment.[55] Black women having lower endocrine therapy initiation is consistent with other studies showing disparities in treatment and/or survival among Black women with breast cancer.[56-60] Understanding and dismantling existing structural barriers for Black women to obtain treatment beyond having healthcare and insurance access deserves more study. In addition, there may be a need for greater attention to patient-provider decision making to increase patient understanding of endocrine therapy effectiveness and adequately address concerns of Black women.
Current NCCN guidelines for invasive breast cancer state that women who are “ER or PR-positive should be considered for adjuvant endocrine therapy regardless of patient age, lymph node status, or whether adjuvant chemotherapy is to be administered.”[20] A recent review confirmed the benefit of endocrine therapy treatment in breast cancer survivors 65 years and older, estimating that women who received breast conserving surgery plus endocrine therapy (without radiotherapy) had 7-9% estimated cumulative risk of ipsilateral breast cancer and 1-2% cumulative risk of contralateral breast cancer within 10 years.[61] However, the NCCN guidelines for Older Adult Oncology acknowledge that treatment of older adults should consider different issues including life expectancy, quality of life, performance status, supportive care, and tolerance to therapy.[62] Lower endocrine therapy initiation in our study among women 65-74 and particularly among women ≥75 may reflect all these considerations plus comorbid conditions and patient choice – neither of which we could account for in our study. These results are consistent with previous studies that have shown 60-75% of women 75 and older initiate endocrine therapy.[34,35,40]
Women diagnosed with less aggressive tumors (stage I, tumor size <1cm, and/or low grade) or who had less treatment (breast conserving surgery without radiation or chemotherapy) were also less likely to have any endocrine therapy dispensings compared to women with more aggressive tumors or treatment. This result is concerning because it suggests some women with early-stage breast cancer who would have particularly good prognosis if treated with endocrine therapy[4,6,63,64] are not receiving treatment according to clinical guidelines. Lower initiation in this group may be the result of patient-provider decision making to weigh the risks and benefits of endocrine therapy incorporating factors that we did not evaluate, such as OncotypeDX breast cancer recurrence risk score and other pre-existing comorbidities. This result is consistent with some studies that have shown less endocrine therapy use among women with early stage, low-grade, and node-negative disease.[34,39,40,65] However, findings are inconsistent with two studies that have shown higher initiation rates among women with stage I and low-grade disease.[37,66] These two studies were limited to women <65 years, which may explain some of the inconsistency with our results because younger women in our study tended to initiate endocrine therapy more often than older women regardless of tumor characteristics. Our study is consistent with one other that reported more initiation among women who received chemotherapy,[38] but inconsistent with others, including two large studies that used SEER-Medicare data with diagnoses through 2011.[34,35,37] Evaluating the influence of other breast cancer treatments on endocrine therapy use requires additional contemporary studies with detailed treatment data.
The continued increase in AI use over time, well beyond 2008 when our previous study ended,[11] is consistent with evolving clinical guidelines and has not been evaluated in prior studies. Some studies have reported an increase in endocrine therapy initiation over time but have not evaluated tamoxifen and AIs separately.[41,43] AIs are only recommended for postmenopausal women or women with suppressed ovarian function (we used age ≥55 as a proxy).[19,20] When we compared AI initiation directly to tamoxifen initiation among women ≥55 years, AIs were more likely to be used first among women aged 55-64 years (compared with older women) and women with worse disease prognosis (stage III, tumor size ≥5cm, and poorly differentiated tumor grade). The lower use of AIs among older women ≥75 may reflect concerns about low bone density – a contraindication to AI use.[67]
A major strength of our study is that we had a well-characterized, large cohort of breast cancer survivors with electronic prescription record data on endocrine therapy use within a general community healthcare setting over a 16-year period. Our study limitations included the lack of information on patient or provider discussions, comorbidities, OncotypeDx, and menopausal status – all of which may have impacted treatment decisions. While we leveraged automated pharmacy dispensings to capture endocrine therapy use, there is no guarantee that women with a dispensing used the medication. We did not include 369 women who initiated treatment >12 months after their initial diagnosis. Expanding this time frame beyond 12 months could have included women with endocrine therapy use for breast cancer recurrence but it also could have included some women who waited to complete trastuzumab or other treatment before starting endocrine therapy. We extracted chemotherapy data from the tumor registry but did not have chemotherapy type on every woman to evaluate whether trastuzumab affected delays in our data. Our results also showed that women who had more aggressive treatment were more likely to initiate endocrine therapy within a year. Finally, our results may not be generalizable to communities outside of integrated health plans. While all women in our analysis had access to care and treatment through their health system, we were unable to evaluate whether factors associated with insurance generosity, such as high deductible plans, pharmacy co-pays, or low-cost generic prescriptions obtained from outside the healthcare system, influenced initiation. Additionally, only one site contributed to the last two years of study data, which may explain the apparent decrease in any endocrine therapy initiation in 2015-2016. We adjusted for study site in our analysis; however, there may be residual confounding that resulted in endocrine therapy administration being higher in 2014 than 2016. The overall proportion of women who initiated any endocrine therapy (80%) was still within the 60-90% range reported in previous studies.[34-39]
In conclusion, our study showed that approximately 80% of women initiated endocrine therapy within 12 months of breast cancer diagnosis, with a slight increase between 2001-2014. As of 2005, AIs were more likely to be initiated over tamoxifen. Despite the increase in AI use over time, approximately one in six eligible women remained at risk for not starting any endocrine therapy. It is critical to ensure that all women eligible for endocrine therapy are given opportunities to discuss the risks and benefits.[2-4,6-9] Understanding those barriers is critical to developing targeted interventions that may help improve uptake of endocrine therapy in breast cancer survivors.
Supplementary Material
Funding
This work was supported by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics of the US National Cancer Institute. Data collected at Kaiser Permanente Colorado was supported by contracts from the National Cancer Institute (contract numbers HHSN 261201800469P, HHSN 261201700708P, HHSN 261201600711P) and a subcontract with RTI International (contract number HHSN 26120090017C). Data collected at Kaiser Permanente Washington was supported by grants from the National Institutes of Health (grant numbers 1R01CA120562, P01CA154292, and R50CA211115 [to EJAB]) and contracts from the National Cancer Institute (contract numbers HHSN 261201700564P, HHSN75N91019P00076, HHSN 5N91020P00327). Data collected at Kaiser Permanente Northwest was supported by several National Cancer Institute subcontracts with RTI International (contract numbers 20-312-0212208, 17-312-0212208). Cancer incidence data used in this study was supported by the Cancer Surveillance System of the Fred Hutchinson Cancer Research Center, which is funded by Contract No. N01-CN-67009 and N01-PC-35142 from the Surveillance, Epidemiology and End Results (SEER) Program of the National Cancer Institute with additional support from the Fred Hutchinson Cancer Research Center and the State of Washington.
Abbreviations:
- AI
aromatase inhibitor
- BCS
breast conserving surgery
- CI
confidence interval
- ER
estrogen receptor
- HR
hormone receptor
- HER2
human epidermal growth factor receptor 2
- KP
Kaiser Permanente
- NCCN
National Comprehensive Cancer Network
- PR
progesterone receptor
- RR
relative risk
- SEER
Surveillance Epidemiology and End Results
Footnotes
Conflicts of interest
The authors have no conflicts of interest to disclose.
Ethics approval
This study received approval from the NCI Special Studies Institutional Review Board, and the Institutional Review Boards of Kaiser Permanente Washington, Kaiser Permanente Colorado, and Kaiser Permanente Northwest.
Consent to participate
This study received a waiver of written informed consent to access and analyze participant data based on the minimal risk of this electronic linkage-based research.
Data availability
The data analyzed in the current study are not publicly available because they contain potentially identifiable information (e.g. dates of diagnoses and treatment) that cannot be shared openly without human subjects approval and data use agreements but are available from the corresponding author on reasonable request.
References
- 1.Early Breast Cancer Trialists' Collaborative Group (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet, 365(9472):1687–1717. [DOI] [PubMed] [Google Scholar]
- 2.Goss PE, Ingle JN, Martino S, Robert NJ, Muss HB, Piccart MJ, Castiglione M, Tu D, Shepherd LE, Pritchard KI, Livingston RB, Davidson NE, Norton L, Perez EA, Abrams JS, Cameron DA, Palmer MJ, Pater JL (2005) Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. J Natl Cancer Inst, 97(17):1262–1271. [DOI] [PubMed] [Google Scholar]
- 3.Arimidex Tamoxifen Alone or in Combination Trialists' Group, Forbes JF, Cuzick J, Buzdar A, Howell A, Tobias JS, Baum M (2008) Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol, 9(1):45–53. [DOI] [PubMed] [Google Scholar]
- 4.Davies C, Pan H, Godwin J, Gray R, Arriagada R, Raina V, Abraham M, Medeiros Alencar VH, Badran A, Bonfill X, Bradbury J, Clarke M, Collins R, Davis SR, Delmestri A, Forbes JF, Haddad P, Hou MF, Inbar M, Khaled H, Kielanowska J, Kwan WH, Mathew BS, Mittra I, Müller B, Nicolucci A, Peralta O, Pernas F, Petruzelka L, Pienkowski T, Radhika R, Rajan B, Rubach MT, Tort S, Urrútia G, Valentini M, Wang Y, Peto R, Adjuvant Tamoxifen: Longer Against Shorter Collaborative G (2013) Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet, 381(9869):805–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coombes RC, Hall E, Gibson LJ, Paridaens R, Jassem J, Delozier T, Jones SE, Alvarez I, Bertelli G, Ortmann O, Coates AS, Bajetta E, Dodwell D, Coleman RE, Fallowfield LJ, Mickiewicz E, Andersen J, Lonning PE, Cocconi G, Stewart A, Stuart N, Snowdon CF, Carpentieri M, Massimini G, Bliss JM (2004) A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med, 350(11):1081–1092. [DOI] [PubMed] [Google Scholar]
- 6.Kaufmann M, Jonat W, Hilfrich J, Eidtmann H, Gademann G, Zuna I, von Minckwitz G (2007) Improved overall survival in postmenopausal women with early breast cancer after anastrozole initiated after treatment with tamoxifen compared with continued tamoxifen: the ARNO 95 Study. J Clin Oncol, 25(19):2664–2670. [DOI] [PubMed] [Google Scholar]
- 7.Goss PE, Ingle JN, Martino S, Robert NJ, Muss HB, Piccart MJ, Castiglione M, Tu D, Shepherd LE, Pritchard KI, Livingston RB, Davidson NE, Norton L, Perez EA, Abrams JS, Therasse P, Palmer MJ, Pater JL (2003) A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med, 349(19):1793–1802. [DOI] [PubMed] [Google Scholar]
- 8.Howell A, Cuzick J, Baum M, Buzdar A, Dowsett M, Forbes JF, Hoctin-Boes G, Houghton J, Locker GY, Tobias JS (2005) Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years' adjuvant treatment for breast cancer. Lancet, 365(9453):60–62. [DOI] [PubMed] [Google Scholar]
- 9.Thurlimann B, Keshaviah A, Coates AS, Mouridsen H, Mauriac L, Forbes JF, Paridaens R, Castiglione-Gertsch M, Gelber RD, Rabaglio M, Smith I, Wardley A, Price KN, Goldhirsch A (2005) A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med, 353(26):2747–2757. [DOI] [PubMed] [Google Scholar]
- 10.Fallowfield L, Cella D, Cuzick J, Francis S, Locker G, Howell A (2004) Quality of life of postmenopausal women in the Arimidex, Tamoxifen, Alone or in Combination (ATAC) Adjuvant Breast Cancer Trial. J Clin Oncol, 22(21):4261–4271. [DOI] [PubMed] [Google Scholar]
- 11.Bowles EJ, Buist DS, Chubak J, Yu O, Johnson J, Chestnut J, Boudreau DM (2012) Endocrine therapy initiation from 2001 to 2008 varies by age at breast cancer diagnosis and tumor size. J Oncol Pract, 8(2):113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whelan TJ, Goss PE, Ingle JN, Pater JL, Tu D, Pritchard K, Liu S, Shepherd LE, Palmer M, Robert NJ, Martino S, Muss HB (2005) Assessment of quality of life in MA.17: a randomized, placebo-controlled trial of letrozole after 5 years of tamoxifen in postmenopausal women. J Clin Oncol, 23(28):6931–6940. [DOI] [PubMed] [Google Scholar]
- 13.Eastell R, Adams JE, Coleman RE, Howell A, Hannon RA, Cuzick J, Mackey JR, Beckmann MW, Clack G (2008) Effect of anastrozole on bone mineral density: 5-year results from the anastrozole, tamoxifen, alone or in combination trial 18233230. J Clin Oncol, 26(7):1051–1057. [DOI] [PubMed] [Google Scholar]
- 14.Goss PE, Ingle JN, Pater JL, Martino S, Robert NJ, Muss HB, Piccart MJ, Castiglione M, Shepherd LE, Pritchard KI, Livingston RB, Davidson NE, Norton L, Perez EA, Abrams JS, Cameron DA, Palmer MJ, Tu D (2008) Late extended adjuvant treatment with letrozole improves outcome in women with early-stage breast cancer who complete 5 years of tamoxifen. J Clin Oncol, 26(12):1948–1955. [DOI] [PubMed] [Google Scholar]
- 15.Mouridsen H, Keshaviah A, Coates AS, Rabaglio M, Castiglione-Gertsch M, Sun Z, Thürlimann B, Mauriac L, Forbes JF, Paridaens R, Gelber RD, Colleoni M, Smith I, Price KN, Goldhirsch A (2007) Cardiovascular adverse events during adjuvant endocrine therapy for early breast cancer using letrozole or tamoxifen: safety analysis of BIG 1-98 trial. J Clin Oncol, 25(36):5715–5722. [DOI] [PubMed] [Google Scholar]
- 16.Rabaglio M, Sun Z, Price KN, Castiglione-Gertsch M, Hawle H, Thürlimann B, Mouridsen H, Campone M, Forbes JF, Paridaens RJ, Colleoni M, Pienkowski T, Nogaret JM, Láng I, Smith I, Gelber RD, Goldhirsch A, Coates AS, Collaborative BIG, International Breast Cancer Study G (2009) Bone fractures among postmenopausal patients with endocrine-responsive early breast cancer treated with 5 years of letrozole or tamoxifen in the BIG 1-98 trial. Ann Oncol, 20(9):1489–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, Vogel V, Robidoux A, Dimitrov N, Atkins J, Daly M, Wieand S, Tan-Chiu E, Ford L, Wolmark N (1998) Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst, 90(18):1371–1388. [DOI] [PubMed] [Google Scholar]
- 18.Gail MH, Costantino JP, Bryant J, Croyle R, Freedman L, Helzlsouer K, Vogel V (1999) Weighing the risks and benefits of tamoxifen treatment for preventing breast cancer. J Natl Cancer Inst, 91(21):1829–1846. [DOI] [PubMed] [Google Scholar]
- 19.Visvanathan K, Fabian CJ, Bantug E, Brewster AM, Davidson NE, DeCensi A, Floyd JD, Garber JE, Hofstatter EW, Khan SA, Katapodi MC, Pruthi S, Raab R, Runowicz CD, Somerfield MR (2019) Use of Endocrine Therapy for Breast Cancer Risk Reduction: ASCO Clinical Practice Guideline Update. J Clin Oncol, 37(33):3152–3165. [DOI] [PubMed] [Google Scholar]
- 20.NCCN Clinical Practice Guidelines in Oncology - v.8.2021, Breast Cancer. http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf Accessed 8/23/2021. [Google Scholar]
- 21.Howlader N, Altekruse SF, Li CI, Chen VW, Clarke CA, Ries LA, Cronin KA (2014) US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst, 106(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.American Cancer Society. Breast cancer facts & figures, 2019-2020. In. Atlanta. [Google Scholar]
- 23.Blanchette PS, Lam M, Richard L, Allen B, Shariff SZ, Vandenberg T, Pritchard KI, Chan KKW, Louie AV, Desautels D, Raphael J, Earle CC (2020) Factors associated with endocrine therapy adherence among post-menopausal women treated for early-stage breast cancer in Ontario, Canada. Breast Cancer Res Treat, 179(1):217–227. [DOI] [PubMed] [Google Scholar]
- 24.Farias AJ, Du XL (2017) Association Between Out-Of-Pocket Costs, Race/Ethnicity, and Adjuvant Endocrine Therapy Adherence Among Medicare Patients With Breast Cancer. J Clin Oncol, 35(1):86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farias AJ, Hansen RN, Zeliadt SB, Ornelas IJ, Li CI, Thompson B (2016) Factors Associated with Adherence to Adjuvant Endocrine Therapy Among Privately Insured and Newly Diagnosed Breast Cancer Patients: A Quantile Regression Analysis. J Manag Care Spec Pharm, 22(8):969–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kroenke CH, Hershman DL, Gomez SL, Adams SR, Eldridge EH, Kwan ML, Ergas IJ, Kubo A, Kushi LH (2018) Personal and clinical social support and adherence to adjuvant endocrine therapy among hormone receptor-positive breast cancer patients in an integrated health care system. Breast Cancer Res Treat, 170(3):623–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lambert LK, Balneaves LG, Howard AF, Gotay CC (2018) Patient-reported factors associated with adherence to adjuvant endocrine therapy after breast cancer: an integrative review. Breast Cancer Res Treat, 167(3):615–633. [DOI] [PubMed] [Google Scholar]
- 28.Lundgren C, Lindman H, Rolander B, Ekholm M (2018) Good adherence to adjuvant endocrine therapy in early breast cancer - a population-based study based on the Swedish Prescribed Drug Register. Acta Oncol, 57(7):935–940. [DOI] [PubMed] [Google Scholar]
- 29.Sheppard VB, He J, Sutton A, Cromwell L, Adunlin G, Salgado TM, Tolsma D, Trout M, Robinson BE, Edmonds MC, Bosworth HB, Tadesse MG (2019) Adherence to Adjuvant Endocrine Therapy in Insured Black and White Breast Cancer Survivors: Exploring Adherence Measures in Patient Data. J Manag Care Spec Pharm, 25(5):578–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weaver KE, Camacho F, Hwang W, Anderson R, Kimmick G (2013) Adherence to adjuvant hormonal therapy and its relationship to breast cancer recurrence and survival among low-income women. Am J Clin Oncol, 36(2):181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cavazza M, Banks H, Ercolanoni M, Cukaj G, Bianchi G, Capri G, Longo F (2020) Factors influencing adherence to adjuvant endocrine therapy in breast cancer-treated women: using real-world data to inform a switch from acute to chronic disease management. Breast Cancer Res Treat, 183(1):189–199. [DOI] [PubMed] [Google Scholar]
- 32.Lambert-Côté L, Bouhnik AD, Bendiane MK, Bérenger C, Mondor M, Huiart L, Lauzier S (2020) Adherence trajectories of adjuvant endocrine therapy in the five years after its initiation among women with non-metastatic breast cancer: a cohort study using administrative databases. Breast Cancer Res Treat, 180(3):777–790. [DOI] [PubMed] [Google Scholar]
- 33.Lee C, Check DK, Manace Brenman L, Kushi LH, Epstein MM, Neslund-Dudas C, Pawloski PA, Achacoso N, Laurent C, Fehrenbacher L, Habel LA (2020) Adjuvant endocrine therapy for breast cancer patients: impact of a health system outreach program to improve adherence. Breast Cancer Res Treat, 180(1):219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Camacho FT, Tan X, Alcalá HE, Shah S, Anderson RT, Balkrishnan R (2017) Impact of patient race and geographical factors on initiation and adherence to adjuvant endocrine therapy in medicare breast cancer survivors. Medicine (Baltimore), 96(24):e7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farias AJ, Du XL (2016) Ethnic differences in initiation and timing of adjuvant endocrine therapy among older women with hormone receptor-positive breast cancer enrolled in Medicare Part D. Med Oncol, 33(2):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nekhlyudov L, Li L, Ross-Degnan D, Wagner AK (2011) Five-year patterns of adjuvant hormonal therapy use, persistence, and adherence among insured women with early-stage breast cancer. Breast Cancer Res Treat, 130(2):681–689. [DOI] [PubMed] [Google Scholar]
- 37.Reeder-Hayes KE, Meyer AM, Dusetzina SB, Liu H, Wheeler SB (2014) Racial disparities in initiation of adjuvant endocrine therapy of early breast cancer. Breast Cancer Res Treat, 145(3):743–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sheppard VB, de Mendoza AH, He J, Jennings Y, Edmonds MC, Oppong BA, Tadesse MG (2018) Initiation of Adjuvant Endocrine Therapy in Black and White Women With Breast Cancer. Clin Breast Cancer, 18(5):337–346 e331. [DOI] [PubMed] [Google Scholar]
- 39.Svahn TH, Niland JC, Carlson RW, Hughes ME, Ottesen RA, Theriault RL, Edge SB, Schott AF, Bookman MA, Weeks JC (2009) Predictors and temporal trends of adjuvant aromatase inhibitor use in breast cancer. J Natl Compr Canc Netw, 7(2):115–121. [DOI] [PubMed] [Google Scholar]
- 40.Ma S, Shepard DS, Ritter GA, Martell RE, Thomas CP (2020) The impact of the introduction of generic aromatase inhibitors on adherence to hormonal therapy over the full course of 5-year treatment for breast cancer. Cancer, 126(15):3417–3425. [DOI] [PubMed] [Google Scholar]
- 41.Bedi JS, Mayo RM, Truong K, Chen L, Dickes L, Sherrill WW, Jones K (2018) Endocrine therapy use in the twenty-first century: usage rates and temporal trends illustrate opportunities for improvement for South Carolina Medicaid women. Breast Cancer Res Treat, 171(3):759–765. [DOI] [PubMed] [Google Scholar]
- 42.Ko NY, Qureshi MM, Oladeru OT, Cassidy MR, Oshry L, Truong MT, Hirsch AE (2020) Racial differences in genomic testing and receipt of endocrine therapy in early-stage breast cancer. Breast Cancer Res Treat, 184(3):849–859. [DOI] [PubMed] [Google Scholar]
- 43.Emanuel G, Henson KE, Broggio J, Charman J, Horgan K, Dodwell D, Darby SC (2019) Endocrine therapy in the years following a diagnosis of breast cancer: A proof of concept study using the primary care prescription database linked to cancer registration data. Cancer Epidemiol, 61:185–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feigelson HS, Bodelon C, Powers JD, Curtis RE, Buist DSM, Veiga LHS, Bowles EJA, Berrington de Gonzalez A, Gierach GL (2021) Body Mass Index and Risk of Second Cancer Among Women With Breast Cancer. J Natl Cancer Inst, 113(9):1156–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ross TR, Ng D, Brown JS, Pardee R, Hornbrook MC, Hart G, Steiner JF (2014) The HMO Research Network Virtual Data Warehouse: A Public Data Model to Support Collaboration. EGEMS (Washington, DC), 2(1):1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gold EB, Crawford SL, Avis NE, Crandall CJ, Matthews KA, Waetjen LE, Lee JS, Thurston R, Vuga M, Harlow SD (2013) Factors related to age at natural menopause: longitudinal analyses from SWAN. Am J Epidemiol, 178(1):70–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Phipps AI, Ichikawa L, Bowles EJ, Carney PA, Kerlikowske K, Miglioretti DL, Buist DS (2010) Defining menopausal status in epidemiologic studies: A comparison of multiple approaches and their effects on breast cancer rates. Maturitas, 67(1):60–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boccardo F, Rubagotti A, Puntoni M, Guglielmini P, Amoroso D, Fini A, Paladini G, Mesiti M, Romeo D, Rinaldini M, Scali S, Porpiglia M, Benedetto C, Restuccia N, Buzzi F, Franchi R, Massidda B, Distante V, Amadori D, Sismondi P (2005) Switching to anastrozole versus continued tamoxifen treatment of early breast cancer: preliminary results of the Italian Tamoxifen Anastrozole Trial. J Clin Oncol, 23(22):5138–5147. [DOI] [PubMed] [Google Scholar]
- 49.Jakesz R, Jonat W, Gnant M, Mittlboeck M, Greil R, Tausch C, Hilfrich J, Kwasny W, Menzel C, Samonigg H, Seifert M, Gademann G, Kaufmann M, Wolfgang J (2005) Switching of postmenopausal women with endocrine-responsive early breast cancer to anastrozole after 2 years' adjuvant tamoxifen: combined results of ABCSG trial 8 and ARNO 95 trial. Lancet, 366(9484):455–462. [DOI] [PubMed] [Google Scholar]
- 50.Winer EP, Hudis C, Burstein HJ, Wolff AC, Pritchard KI, Ingle JN, Chlebowski RT, Gelber R, Edge SB, Gralow J, Cobleigh MA, Mamounas EP, Goldstein LJ, Whelan TJ, Powles TJ, Bryant J, Perkins C, Perotti J, Braun S, Langer AS, Browman GP, Somerfield MR (2005) American Society of Clinical Oncology technology assessment on the use of aromatase inhibitors as adjuvant therapy for postmenopausal women with hormone receptor-positive breast cancer: status report 2004. J Clin Oncol, 23(3):619–629. [DOI] [PubMed] [Google Scholar]
- 51.Aiello EJ, Buist DS, Wagner EH, Tuzzio L, Greene SM, Lamerato LE, Field TS, Herrinton LJ, Haque R, Hart G, Bischoff KJ, Geiger AM (2008) Diffusion of aromatase inhibitors for breast cancer therapy between 1996 and 2003 in the Cancer Research Network. Breast Cancer Res Treat, 107(3):397–403. [DOI] [PubMed] [Google Scholar]
- 52.Early Breast Cancer Trialists' Collaborative Group (2015) Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet, 386(10001):1341–1352. [DOI] [PubMed] [Google Scholar]
- 53.Bluethmann SM, Murphy CC, Tiro JA, Mollica MA, Vernon SW, Bartholomew LK (2017) Deconstructing Decisions to Initiate, Maintain, or Discontinue Adjuvant Endocrine Therapy in Breast Cancer Survivors: A Mixed-Methods Study. Oncol Nurs Forum, 44(3):E101–E110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wheeler SB, Roberts MC, Bloom D, Reeder-Hayes KE, Espada M, Peppercorn J, Golin CE, Earp JA (2016) Oncology providers' perspectives on endocrine therapy prescribing and management. Patient Prefer Adherence, 10:2007–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cole MB, Ellison JE, Trivedi AN (2020) Association Between High-Deductible Health Plans and Disparities in Access to Care Among Cancer Survivors. JAMA Netw Open, 3(6):e208965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Collin LJ, Yan M, Jiang R, Gogineni K, Subhedar P, Ward KC, Switchenko JM, Lipscomb J, Miller-Kleinhenz J, Torres MA, Lin J, McCullough LE (2021) Receipt of Guideline-Concordant Care Does Not Explain Breast Cancer Mortality Disparities by Race in Metropolitan Atlanta. J Natl Compr Canc Netw. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.DeSantis CE, Ma J, Gaudet MM, Newman LA, Miller KD, Goding Sauer A, Jemal A, Siegel RL (2019) Breast cancer statistics, 2019. CA Cancer J Clin, 69(6):438–451. [DOI] [PubMed] [Google Scholar]
- 58.Emerson MA, Golightly YM, Aiello AE, Reeder-Hayes KE, Tan X, Maduekwe U, Johnson-Thompson M, Olshan AF, Troester MA (2020) Breast cancer treatment delays by socioeconomic and health care access latent classes in Black and White women. Cancer, 126(22):4957–4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Enewold L, Penn DC, Stevens JL, Harlan LC (2018) Black/white differences in treatment and survival among women with stage IIIB-IV breast cancer at diagnosis: a US population-based study. Cancer Causes Control, 29(7):657–665. [DOI] [PubMed] [Google Scholar]
- 60.Heiney SP, Truman S, Babatunde OA, Felder TM, Eberth JM, Crouch E, Wickersham KE, Adams SA (2020) Racial and Geographic Disparities in Endocrine Therapy Adherence Among Younger Breast Cancer Survivors. Am J Clin Oncol, 43(7):504–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Freedman RA, Minami CA, Winer EP, Morrow M, Smith AK, Walter LC, Sedrak MS, Gagnon H, Perilla-Glen A, Wildiers H, Wildes TM, Lichtman SM, Loh KP, Brain EGC, Ganschow PS, Hunt KK, Mayer DK, Ruddy KJ, Jagsi R, Lin NU, Canin B, LeStage BK, Revette AC, Schonberg MA, Keating NL (2021) Individualizing Surveillance Mammography for Older Patients After Treatment for Early-Stage Breast Cancer: Multidisciplinary Expert Panel and International Society of Geriatric Oncology Consensus Statement. JAMA Oncol, 7(4):609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.NCCN Clinical Practice Guildeines in Oncology - v.1.2021, Older Adult Oncology. https://www.nccn.org/professionals/physician_gls/pdf/senior.pdf Accessed 10/1/21 2021.
- 63.Coombes RC, Kilburn LS, Snowdon CF, Paridaens R, Coleman RE, Jones SE, Jassem J, Van de Velde CJ, Delozier T, Alvarez I, Del Mastro L, Ortmann O, Diedrich K, Coates AS, Bajetta E, Holmberg SB, Dodwell D, Mickiewicz E, Andersen J, Lønning PE, Cocconi G, Forbes J, Castiglione M, Stuart N, Stewart A, Fallowfield LJ, Bertelli G, Hall E, Bogle RG, Carpentieri M, Colajori E, Subar M, Ireland E, Bliss JM, Intergroup Exemestane S (2007) Survival and safety of exemestane versus tamoxifen after 2-3 years' tamoxifen treatment (Intergroup Exemestane Study): a randomised controlled trial. Lancet, 369(9561):559–570. [DOI] [PubMed] [Google Scholar]
- 64.Showalter SL, Meneveau MO, Keim-Malpass J, Camacho TF, Squeo G, Anderson RT (2021) Effects of Adjuvant Endocrine Therapy Adherence and Radiation on Recurrence and Survival Among Older Women with Early-Stage Breast Cancer. Ann Surg Oncol. [DOI] [PubMed] [Google Scholar]
- 65.Friese CR, Pini TM, Li Y, Abrahamse PH, Graff JJ, Hamilton AS, Jagsi R, Janz NK, Hawley ST, Katz SJ, Griggs JJ (2013) Adjuvant endocrine therapy initiation and persistence in a diverse sample of patients with breast cancer. Breast Cancer Res Treat, 138(3):931–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.O'Neill SC, Isaacs C, Lynce F, Graham DM, Chao C, Sheppard VB, Zhou Y, Liu C, Selvam N, Schwartz MD, Potosky AL (2017) Endocrine therapy initiation, discontinuation and adherence and breast imaging among 21-gene recurrence score assay-eligible women under age 65. Breast Cancer Res, 19(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goss PE, Ingle JN, Pritchard KI, Robert NJ, Muss H, Gralow J, Gelmon K, Whelan T, Strasser-Weippl K, Rubin S, Sturtz K, Wolff AC, Winer E, Hudis C, Stopeck A, Beck JT, Kaur JS, Whelan K, Tu D, Parulekar WR (2016) Extending Aromatase-Inhibitor Adjuvant Therapy to 10 Years. N Engl J Med, 375(3):209–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data analyzed in the current study are not publicly available because they contain potentially identifiable information (e.g. dates of diagnoses and treatment) that cannot be shared openly without human subjects approval and data use agreements but are available from the corresponding author on reasonable request.