Abstract
Photoreceptors are highly specialized sensory neurons with unique metabolic and physiological requirements. These requirements are partially met by Müller glia and cells of the retinal pigment epithelium (RPE), which provide essential metabolites, phagocytose waste, and control the composition of the surrounding microenvironment. A third vital supporting cell type, the retinal microglia, can provide photoreceptors with neurotrophic support or exacerbate neuroinflammation and hasten neuronal cell death. Understanding the physiological requirements for photoreceptor homeostasis and the factors that drive microglia to best promote photoreceptor survival has important implications for the treatment and prevention of blinding degenerative diseases like retinitis pigmentosa and age-related macular degeneration.
Keywords: neuroinflammation, rod, retina, degeneration, macrophage, monocyte
1. INTRODUCTION
Retinal cell death is the primary cause of permanent vision loss worldwide. Blinding diseases like age-related macular degeneration (AMD) and retinitis pigmentosa (RP) involve the loss of rod and cone photoreceptor cells, which transduce incident photons into electrical signals that are relayed to and interpreted by the rest of the visual system. Photoreceptors, by nature of their function and physiological construction, are uniquely susceptible to light-induced damage and metabolic stress, which exacerbate the senescence, neuroinflammation, and degenerative conditions that typically plague the central nervous system (CNS).
Photoreceptor stress and degeneration can be broadly categorized as arising from two main types of insults: (a) disruption of cell-autonomous homeostasis or (b) loss of function in critical support cells, like the cells of the retinal pigment epithelium (RPE) and Müller glia (Figure 1a). In both cases, photoreceptor stress and degeneration trigger a complex, dynamic response from the innate immune system, namely, the resident microglia and, in a subset of cases, infiltrating monocytes from peripheral circulation. Both microglia and monocytes migrate to neurons in distress and are capable of high-capacity, large-scale phagocytosis. Separating the effect of the neuroimmune response from degeneration itself is difficult and lies at the heart of the debate about the helpful versus harmful roles that microglia play in neurodegenerative disease. Fortunately, photoreceptors, by nature of their abundance, relative homogeneity, and well-defined function, as well as their place behind the naturally transparent biological window of the cornea and lens, offer a uniquely accessible experimental system to investigate how the immune system interacts with and impacts the health and survival of neurons. The goal of this review is to combine what is known about photoreceptor function and degeneration with what is known about retinal microglia and neuroinflammation to articulate future directions for understanding the interdependence of the two.
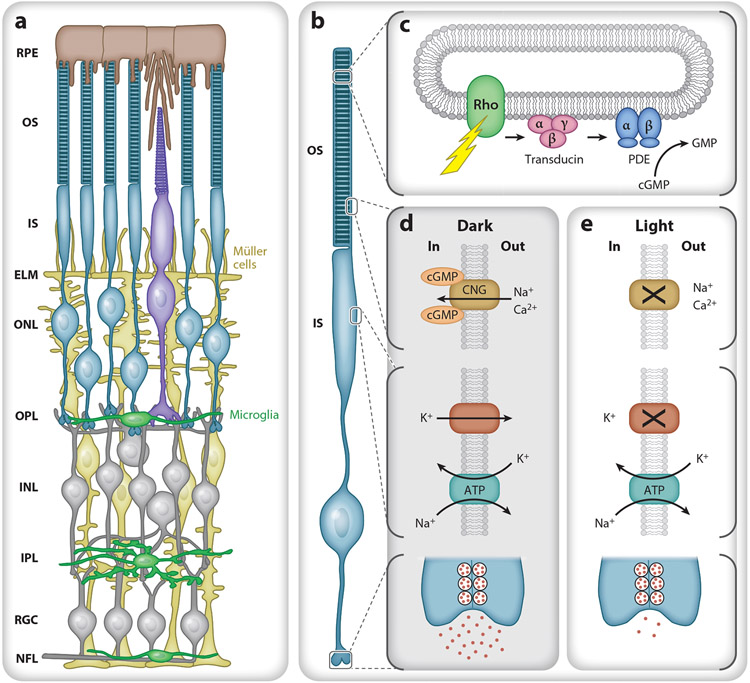
Figure 1.
Retinal morphology and basic photoreceptor physiology. (a) Schematic of the retina from the outer (top) to inner (bottom) in cross section. The retinal pigment epithelium (RPE) (brown) lies between the vascular choriocapillaris and photoreceptor outer segments (OS). Müller cells (yellow) span the retina from the nerve fiber layer (NFL) to the photoreceptor inner segments (IS) and form the external limiting membrane (ELM) that helps to establish the subretinal space as a distinct microenvironment. Normally, microglia (green) reside in the outer plexiform layer (OPL), inner plexiform layer (IPL), retinal ganglion cell layer (RGC), and NFL. (b) Rod photoreceptor compartment. From top to bottom: OS containing stacks of discs, IS with metabolic and biosynthetic machinery, cell body, and synaptic terminal. (c) A photon causes rhodopsin to change conformation, activating transducin (Gαtβ1γ1). Each GTP-bound Gαt then binds and activates cGMP phosphodiesterase 6 (PDE), allowing it to hydrolyze cGMP, decreasing intracellular cGMP levels. (d) In the dark, cGMP opens a cyclic nucleotide gated (CNG) channel in the OS plasma membrane, allowing cationic influx (Na+, Ca2+) that is balanced by an efflux of cations, mostly potassium (K+), from the IS. The IS Na+/K+ transporter uses ATP to maintain the electrochemical gradient and depolarized membrane potential, leading to the continual release of glutamate. (e) In light, cGMP levels fall, and the CNG channels close, reducing the influx of Na+ and Ca2+ and causing the cell to hyperpolarize and reduce glutamate release. Additional abbreviations: INL, inner nuclear layer; ONL, outer nuclear layer.
2. PHOTORECEPTOR FUNCTION IN THE HEALTHY RETINA
All vertebrate photoreceptors are comprised of four main functional subcellular compartments: an outer segment containing the light transduction machinery, an inner segment containing metabolic and biosynthetic machinery, a cell body, and the synaptic terminal (Figure 1b). In the mouse, both the cellular dimensions and the densities of rods and cones are similar to those in the human retina outside the fovea (Carter-Dawson & LaVail 1979). Photoreceptors across the vertebrate kingdom also utilize highly similar physiological properties. In the dark, a standing circulating current (known as the dark current) keeps the membrane potential of photoreceptors more depolarized than most neurons (Hagins et al. 1970, Penn & Hagins 1972), at approximately −35 mV in the mouse (Cangiano et al. 2012). The dark current arises from an influx of sodium (Na+) and calcium (Ca2+) ions through open cyclic nucleotide gated (CNG) channels in the outer segment and an efflux of cations, mostly potassium (K+), from the inner segment. The Na+/K+ transporter in the inner segment uses the energy of ATP to maintain the electrochemical gradients of ions and sustain the membrane potential. Together, the inward and outward movement of ions results in the standing circulating current and the depolarized steady state of photoreceptors in the dark, causing them to constantly release glutamate from their synapses (Figure 1d).
When a photon is absorbed and causes isomerization of rhodopsin’s bound chromophore, 11-cis to all-trans retinaldehyde, rhodopsin changes conformation to a form (metarhodopsin II) that initiates a sequence of biochemical events known as the phototransduction cascade (for review, see Arshavsky & Burns 2014). Each photoexcited rhodopsin activates many copies of the G-protein transducin (Gαtβ1γ1), and each GTP-bound Gαt then binds and activates cGMP phosphodiesterase 6, which hydrolyzes cGMP at a high rate (Figure 1c). The fall in cGMP allows the CNG channels in the outer segment to close, interrupting the influx of Na+ and Ca2+ and allowing the cell to hyperpolarize, reducing glutamate release (Figure 1e). The fall in intracellular calcium in the outer segment activates guanylate cyclase via guanylate cyclase activating proteins (GCAPs), leading to increased cGMP synthesis and more rapid restoration of the circulating current. Timely recovery also requires rhodopsin deactivation, which occurs when the carboxy-terminus is phosphorylated by rhodopsin kinase (Grk1), allowing high-affinity binding of Arrestin-1 (also known as visual arrestin or S-antigen/Sag). Mutations in proteins of the phototransduction cascade and its regulation often lead to the degeneration of photoreceptors (Wright et al. 2010).
All phototransduction occurs in the outer segments, which are packed with membranous intracellular (rods) or invaginating (cones) discs that contain phototransduction proteins at high density. Outer segments lack lysosomes, endosomes, endoplasmic reticulum (ER), and mitochondria, and they are thought to obtain the ATP needed to support phototransduction by aerobic glycolysis and diffusion of ATP from the inner segment (Hurley et al. 2015, Léveillard & Sahel 2017).
The inner segment is a less well-understood compartment. It is connected to the outer segment by the connecting cilium, through which all outer segment components ultimately must pass (for review, see Baehr et al. 2019). It can be structurally divided into two regions: the ellipsoid, closest to the connecting cilium, which is densely packed with mitochondria, and the myoid, which has fewer mitochondria and contains abundant ER, Golgi, and ribosomes. The ellipsoid region provides ATP to the outer segment and supports the Na+/K+ ATPase located in the inner segment plasma membrane (Narayan et al. 2017).
In addition to the metabolic need required to sustain the pumps that maintain the electrochemical equilibrium for the circulating current, photoreceptors must also synthesize, transport, and assemble one-tenth of the outer segment daily (Young 1967). The birth of new discs at the base of the outer segment is accompanied by the shedding of an equal number at the tips, which are then phagocytosed by the RPE. Thus, outer segment function (phototransduction and the circulating current) and structural renewal (disc biosynthesis and shedding) require a tremendous amount of energy for normal homeostasis. It is therefore not surprising that physiological stressors that further increase metabolic demand for a sustained period readily lead to degeneration. Still, precisely how physiological and cell biological stressors feed into cell death pathways is poorly understood.
2.1. Intrinsic and Cell-Autonomous Causes of Photoreceptor Death
Photoreceptor stress ultimately arises from a breakdown in cellular homeostasis. Common causes of stress in photoreceptors include protein misfolding or mistrafficking, prolonged signaling, and oxidative damage. Sustained periods of stress can converge on a handful of molecular pathways to trigger photoreceptor degeneration (for review, see Power et al. 2019, Wright et al. 2010). To better understand the degeneration process, we begin with examples of animal models of human diseases that lead to photoreceptor dysfunction and death.
Many congenital photoreceptor degenerations are caused by protein misfolding and mistrafficking. One common example is the P23H mutation in the rhodopsin gene, which is one of the most prevalent causes of human autosomal-dominant RP. In mice, the P23H rhodopsin mutation recapitulates the human disease, causing rhodopsin misfolding, structural disorganization of the outer segment, thinning of the outer nuclear layer (ONL), and progressive loss of rod function as measured by electroretinography (ERG) (Sakami et al. 2011). The abundant, misfolded P23H rhodopsin protein ultimately stimulates the unfolded protein response (UPR), leading to proapoptotic signaling that results in photoreceptor degeneration (Power et al. 2019, Wright et al. 2010). Similarly, loss of the transducin subunit Gγ1 leads to accumulation of large amounts of Gβ1 protein that overwhelm the ubiquitin-proteasomal system and cause slow photoreceptor degeneration (Lobanova et al. 2013). Increasing the proteasomal capacity in both P23H rhodopsin mutation and Gγ1 knockout mice alleviates ER-associated stress and slows the rate of degeneration (Lobanova et al. 2013, 2018; Qiu et al. 2019), and it is likely to be an effective treatment for many misfolding and mistrafficking diseases.
Photoreceptor degeneration can also be caused by signaling deficits. For example, loss-of-function mutations in proteins that deactivate rhodopsin signaling, such as rhodopsin kinase and Arrestin-1, cause Oguchi disease and RP (Zahid et al. 2018). In mice, the degeneration phenotype can be rescued by preventing the need for signal deactivation, either by dark-rearing the mice or concurrently knocking out the G-protein transducin, presumably because these perturbations prevent sustained rhodopsin signaling (Chen et al. 1999, Hao et al. 2002). Conversely, several lack-of-function mutations (e.g., rd1 and rd10 in mice) in the genes that encode the α- and β-subunits of the cGMP phosphodiesterase (PDE6) render the photoreceptors incapable of cGMP hydrolysis (for review, see Power et al. 2019, Tolone et al. 2019), leading to exceedingly high levels of cGMP (Farber & Lolley 1974, Farber et al. 1994). High levels of cGMP activate protein kinase G (PKG), which phosphorylates several downstream targets that perturb calcium homeostasis, depleting the cell of energy and inducing apoptotic factors, ultimately leading to photoreceptor death (Paquet-Durand et al. 2009, Power et al. 2019, Wang et al. 2017). In humans, mutations in PDE subunits are the most common cause of RP (Dryja et al. 1999, Huang et al. 1995, Tsang et al. 2008). In animal models with PDE mutations, photoreceptor loss can be prevented by AAV-mediated delivery of a functional PDE gene, and a similar viral gene replacement therapy for PDE6β is currently in clinical trials (Jiang et al. 2018).
Finally, oxidative damage arises when free-radical, singlet-reactive oxygen species steal electrons from other sources, including proteins and lipids, leading to a loss of cellular function. The high density of mitochondria in the ellipsoid region of the inner segment produces reactive oxygen species during oxidative phosphorylation, which can then attack the abundant polyunsaturated fatty acids (Brennan & Kantorow 2009). Oxidative stress can also trigger ER stress, leading to a reduction in functional proteins, resulting in cell death (Power et al. 2019). Several studies in animal models have focused on the administration of antioxidants to mitigate or delay photoreceptor degeneration, e.g., lutein and zeaxanthin (Sahin et al. 2019), edaravone (Imai et al. 2010), HM-10/10 (Su et al. 2019), and systemic taurine treatment (Tao et al. 2019). However, the data from several large-scale studies on antioxidant dietary compounds and supplements in humans have not been clear cut (Chew 2017). In individuals with intermediate AMD, lutein and zeaxanthin may reduce the risk of progression to late AMD by 10–25%, but there is currently no general agreement on antioxidant dietary recommendations to prevent the development of AMD (AREDS2 Res. Group et al. 2014).
Understanding the causes of cell-autonomous photoreceptor degeneration and the common downstream pathways reveals several overlapping therapeutic target points, some of which are highlighted above. Furthermore, given the eye’s accessibility, immune privilege, and compartmentalization, targeted recovery or replacement of mutated or deleted genes, especially with viral vectors, is now a reality. Since a number of comprehensive reviews on this topic have recently been published (e.g., Hori et al. 2019, Ludwig et al. 2019), we instead turn our attention to what happens when stress and degeneration affect nearby support cells, including the RPE, Müller glia cells, and, ultimately, retinal microglia.
2.2. Photoreceptor Death Due to Functional Loss of Critical Support Cells
Photoreceptors and their immediate microenvironment are maintained by three cell types: RPE, Müller glia cells, and microglia. Each cell type contributes to supplying photoreceptors with nutrients, processing photoreceptor waste, and ensuring that photoreceptors can effectively signal to downstream bipolar cells. Loss or disruption of any of these functions can result in photoreceptor malfunction or death.
2.2.1. Retinal pigment epithelium.
The RPE lies between one of the densest capillary beds in the body, the choriocapillaris, and the highly metabolically active photoreceptors, contributing to the outer blood–retinal barrier and playing several roles in maintaining the health of the outer retinal environment (Figure 1a). First, the RPE apical microvilli extend into the interphotoreceptor matrix and ensheath the photoreceptor outer segments, delivering glucose, retinoids, and other nutrients from the bloodstream, while blood cells and other substances are prevented from entering the retina by Bruch’s membrane and the intercellular tight junctions that lie between neighboring RPE cells. Second, the RPE phagocytoses the distal tips of the outer segments daily, consuming old discs that are continually replaced by newly formed ones at the outer segment base. Because there are no endocytotic or lysosomal pathways in the outer segment, the constant shedding and synthesis is the only way for the cell to rid itself of misfolded or damaged proteins and lipids, thereby preventing the build-up of toxic byproducts from light exposure. Finally, the RPE is essential for the retinoid cycle, supplying the 11-cis retinoid back to the outer segments, where it recombines with apo-opsin to regenerate the light-sensitive visual pigments.
Photoreceptor degeneration can occur secondarily following the loss of the RPE and any of the vital functions listed above. One common genetic form of secondary photoreceptor degeneration is caused by loss-of-function mutations in RPE65, the retinoid isomerase expressed by RPE cells (Wright et al. 2015). Loss of the 11-cis chromophore causes degeneration through loss of its stabilizing influence on opsin folding, through the constitutive signaling of the resulting apo-opsins, or both (Insinna et al. 2012, Kiser & Palczewski 2016, Sato et al. 2019). In both humans and canine models, virally mediated RPE65 gene restoration improves vision in the short term but does not prevent degeneration (Cideciyan et al. 2013; for review, see Pierce & Bennett 2015). Presumably, the exogenous gene successfully synthesizes enough retinoid that the surviving photoreceptors become light sensitive, but not enough to prevent their progressive demise, likely due to cumulative stress arising from defects in opsin folding and trafficking or continued low-level constitutive signaling.
Finally, photomechanical damage can result from very brief, high-energy light irradiation that generates heat when absorbed by the RPE melanosomes (Youssef et al. 2011). Absorption of high photothermal energy over very short time intervals can create thermoelastic expansion, directly damaging both the RPE and the photoreceptor outer segments by photomechanical forces. Increased temperature also causes protein denaturation and the fluidization of cell membranes. Irreversible damage to a cell occurs when the temperature of the retina is increased by at least 10°C; larger increases lead first to apoptosis and then to immediate necrosis (Glickman 2002, Yarmolenko et al. 2011, Youssef et al. 2011).
2.2.2. Müller glia cells.
On the other side of the subretinal space is the barrier created by the Müller glia cells, which are the radial glia of the retina (Figure 1a). The close apposition of Müller glia to photoreceptors, as well as their sensitivity to ion gradients and metabolites, makes them perfectly poised to detect physiological changes accompanying photoreceptor stress (Sarthy & Ripps 2001, Sparrow et al. 2010, Strauss 2005).
Müller cells form adherens junctions between their apical processes and photoreceptors, contributing to the barrier qualities of the matrix that forms the external limiting membrane (ELM) (Figure 1a). Müller cells play several critical roles that are essential for photoreceptor survival: They control the concentration of ions in the extracellular space to maintain osmolarity and electrochemical gradients, sense mechanical distortions of the tissue to provide structural support, contribute to the blood–retinal barrier at the retinal surface through their interactions with retinal vessels and the secretion of the inner limiting membrane, prevent the extrasynaptic diffusion of glutamate to preserve visual resolution, and provide a source of 11-cis retinal chromophore to cones in the noncanonical visual cycle (Reichenbach & Bringmann 2013, Wang & Kefalov 2011).
When photoreceptors become stressed, Müller glia are among the first cells to respond, entering a state of gliosis and undergoing a series of changes aimed at protecting the retina from further damage and restoring homeostasis (Bringmann & Wiedemann 2012). Müller glia release antioxidants, as well as an assortment of angiogenic and neurotrophic factors, such as vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) (Graca et al. 2018). This reactive gliosis can be detected by the upregulation of glial fibrillary acidic protein (GFAP), nestin, and vimentin (Bringmann & Wiedemann 2012). However, if the reactive state persists too long, or if the blood–retinal barrier is damaged, then Müller cells release cytokines and inflammatory factors like tumor necrosis factor-alpha (TNF-α), monocyte chemoattractant protein-1 (CCL2), and interleukins (ILs), triggering neuroinflammation (Bringmann & Wiedemann 2012, Graca et al. 2018, Karlen et al. 2018). Gliotic Müller cells work with astrocytes, microglia, and vascular cells to induce fibrovascular scarring, a response similar to the wound healing found in other parts of the body but devastating to vision when it occurs inside the eye (Friedlander 2007). Such scarring is associated with neovascularization, retinal thickening, and edema. Both the formation of fibrotic scars and neovascularization can ultimately lead to photoreceptor death (for review, see Saika et al. 2008).
During degeneration, Müller cells are phagocytic and help to clear dead or dying photoreceptors in both cold-blooded vertebrates (Bejarano-Escobar et al. 2017) and mammals (Sakami et al. 2019). Because phagocytosis of dying photoreceptors can stimulate Müller cell proliferation (Bejarano-Escobar et al. 2017), and under some conditions, proliferating Müller cells can be coaxed to differentiate into photoreceptors (Ueki et al. 2015), much current effort is focused on the regenerative potential of Müller glia for future therapeutic use.
3. FUNCTIONS OF RETINAL MICROGLIA
Microglia are the resident immune cells of the CNS. Evolutionarily, microglia are present in the CNS throughout the vertebrate lineage, with mammalian gene expression patterns that are particularly well conserved, suggesting that microglia in different species share similar functions (Geirsdottir et al. 2019). Unlike neurons and neuroglia, microglia are not ectodermally derived, but rather are myeloid cells derived from early mesodermal tissue (Ginhoux & Garel 2018) that take up permanent residence in the CNS around the time that the vasculature forms. Lineage tracing studies have shown that, once established, microglia make up a stable, long-lived population that undergoes apoptosis and proliferation at low rates (Askew et al. 2017, O’Koren et al. 2016, Sheng et al. 2015, Tay et al. 2017). Survival of microglia is dependent on colony stimulating factor 1, a receptor present on myeloid cells that, when genetically ablated or pharmacologically blocked, leads to loss of nearly all microglia (Elmore et al. 2014, Hickman et al. 2018). While the basal turnover of microglia is stochastic and slow, pathology can initiate clonal expansion (Askew et al. 2017, Kierdorf & Prinz 2017). Generally, it is thought that microglia numbers do not receive reinforcement from the peripheral monocyte population except under circumstances that elicit breakdown of the blood–retinal or blood–brain barrier (Ginhoux & Garel 2018).
Under normal conditions, microglia are confined to the synaptic and ganglion cell layers of the retina (Figure 1a). From this location, they survey the surrounding tissue with dynamic processes, playing an essential role in synaptic homeostasis and plasticity. Furthermore, they actively communicate with photoreceptors and other support cells to maintain retinal health.
3.1. Microglia Surveillance of the Healthy Retina
Microglia are typically ramified in healthy tissue, with small cell bodies and several thin, branching processes that actively participate in synapse formation and pruning during development and in synaptic maintenance throughout life (Kettenmann et al. 2013, Silverman & Wong 2018). The baseline dynamics of microglial processes appear to differ between CNS regions (Davalos et al. 2005, Miller et al. 2019, Nimmerjahn et al. 2005, Park et al. 2017). In the brain, in vivo two-photon imaging of the neocortex through thin-skull transcranial windows has shown microglia processes to be active and highly motile without any accompanying movement of the soma (Nimmerjahn et al. 2005, Park et al. 2017, Wake et al. 2009). In the retina, recent real-time in vivo scanning laser ophthalmoscopy (SLO) has found both highly motile subsets of ameboid cells (Miller et al. 2019) and rapid extensions and retractions of tertiary processes of highly branched cells (Wahl et al. 2019) in the healthy retina. The surveillance dynamics may depend on neuronal activity, as shown recently for cortical microglia (Liu et al. 2019).
Microglia are essential for photoreceptor synaptic function. Sustained depletion of microglia in the retina causes a significant decrease in the ERG b-wave amplitude (Wang et al. 2016), a measure of synaptic strength between photoreceptors and ON-bipolar cells. Furthermore, electron microscopy has shown that microglia depletion causes cone presynaptic terminals to develop a dystrophic morphology with indistinct vesicles and abnormal synaptic ribbons, reminiscent of models of synaptic degeneration (Wang et al. 2016).
The mechanisms that mediate microglia–synapse interactions are not yet clear. Microglia are capable of expressing many types of neurotransmitter receptors, including ionotropic and metabotropic glutamate receptors, GABAB receptors, and cholinergic receptors, as well as receptors for cannabinoids, dopamine, neuropeptides, and purines (Pocock & Kettenmann 2007). Retinal microglia can directly respond to ATP through metabotropic P2Y12 purinoceptors, and they detect local application of glutamatergic or GABA agonists indirectly, altering their morphology and dynamic behavior accordingly (Fontainhas et al. 2011, Wong et al. 2011).
Microglia also directly monitor the extracellular concentrations of ions, especially K+ (Izquierdo et al. 2019), which is very high in the subretinal space and critical for photoreceptor physiology (Figure 1d,e). During homeostatic surveillance, microglia have a negative membrane potential (−20 to −60 mV), with tonic activity of the two-pore domain K+ channel THIK-1, allowing for K+ efflux. Changes in channel activity or K+ concentration can drive decreases in ramification and surveillance activity (Bernier et al. 2019; Madry et al. 2018a,b). Since light stimulation can drive large changes in the concentration of extracellular potassium in the outer retina and subretinal space (Newman 1996), electrical activity of photoreceptors might directly impact the surveillance state of microglia, creating the possibility for differential interaction and regulation of synapses. Furthermore, disease conditions where potassium becomes dysregulated can lead to inflammasome activation and the release of IL-1β by microglia (Madry et al. 2018b).
Overall, microglia have a myriad of ways of monitoring the health and function of their environment to ensure that photoreceptors are working properly. However, microglia are not simply passive observers that react to insult; there is important bidirectional communication among microglia, photoreceptors, RPE, and Müller cells that maintains the health of the retina.
3.2. Microglia Communication with Photoreceptors, Retinal Pigment Epithelium, and Müller Glia
Under normal conditions, photoreceptors express several factors that help to keep microglia in their basal surveillance state. The surface of photoreceptors is ensheathed with a specialized glycocalyx that includes the surface glycoprotein CD200, for which microglia express the receptor CD200R. CD200–CD200R signaling inhibits Ras (Walker & Lue 2013) and appears to regulate the permeability of the blood–brain barrier (Denieffe et al. 2013). In addition, CD200 knockout mice have stronger inflammatory responses in a model of AMD (Horie et al. 2013, Rashid et al. 2019). The glycocalyx of photoreceptors also contains sialic acids that microglia can detect through several sialic acid binding immunoglobulin-like lectins (Siglec), including Siglec-11, Siglec-5, and Siglec-E. These Siglecs signal through immunoreceptor tyrosine-based inhibition motifs, resulting in suppression of microglial activation and phagocytosis (Linnartz-Gerlach et al. 2014, Rashid et al. 2019).
All neurons, including photoreceptors, express the chemokine fractalkine (Cx3CL1), which, when bound to the Cx3CR1 receptor expressed by microglia (and monocytes), can reduce the propensity for microglia to become activated (for review, see Rashid et al. 2019). In many models of neurodegeneration, disruption of Cx3CL1–Cx3CR1 signaling causes increased microglial activation. For example, in a mouse model of RP (rd10), loss of Cx3CR1 expression exacerbated the rate of photoreceptor degeneration and increased microglial phagocytosis, while injection of Cx3CL1 had the opposite, calming effect (Zabel et al. 2016). More recently, loss of Cx3CL1–Cx3CR1 signaling between microglia and photoreceptors led to defects in outer segment elongation and cone photoreceptor cell death during development (Jobling et al. 2018). In a local laser injury model, chronic loss of Cx3CR1 had no effect on the time course of microglia activation or photoreceptor clearance but did change the number and spatial distribution of microglia in the healthy retina (Miller et al. 2019).
In addition to photoreceptors, microglia also communicate with Müller cells and RPE. For example, microglia produce neurotrophic factors such as brain derived neurotrophic factor (BDNF) that act upon Müller cells, altering the release of other factors such as bFGF, glia-derived neurotrophic factor (GDNF), and leukemia inhibitory factor (LIF) that can impinge directly on photoreceptors, promoting prosurvival signaling cascades (Rashid et al. 2019). Conversely, activated microglia release TNF-α that decreases OTX2 expression in RPE cells in a strong, dose-dependent manner, leading to the downregulation of RPE genes like TTR and RDH5, which are critical for the visual cycle (Mathis et al. 2017). Not surprisingly, healthy RPE release several factors, such as Fas ligand (FasL), transforming growth factor-β (TGF-β), thrombospondin-1, and somatostatin, that prevent microglia and monocytes from taking up residence in the subretinal space (Mathis et al. 2017, Rashid et al. 2019). In AMD, this regulation appears to be broken, leading to chronic inflammation and accumulation of subretinal microglia and/or macrophages (e.g., Calippe et al. 2017, Combadière et al. 2007, McMenamin et al. 2009, Zhang et al. 2015).
4. MICROGLIA ACTIVATION AND THE NEUROINFLAMMATORY RESPONSE
After photoreceptor degeneration is triggered, the immune system is recruited to clean up dying cells and restrict the spread of damage to neighboring cells. During photoreceptor stress and degeneration, microglia lose their branched morphology and migrate to the afflicted region (Figure 2a). Once on the scene, they can be cytoprotective, decreasing the stress of surrounding cells, or cytotoxic, ramping up the destructive cytokine signaling.
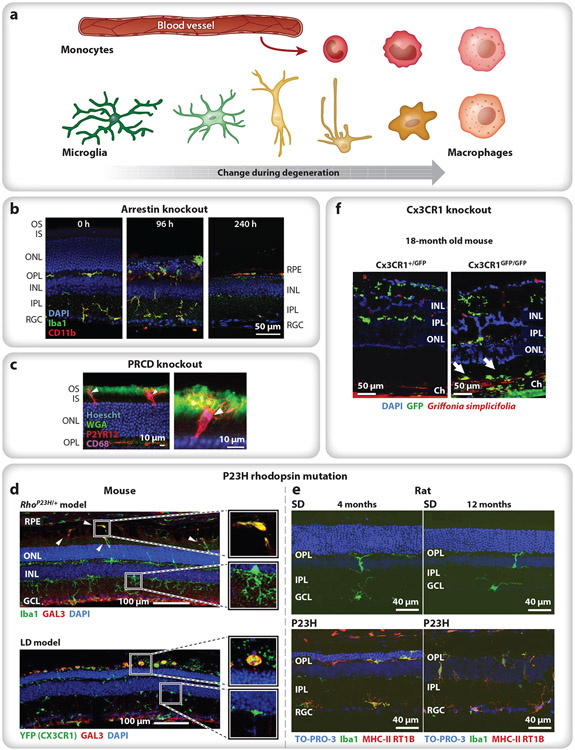
Figure 2.
Variations in the response of microglia and infiltrating monocytes during photoreceptor degeneration. (a) Microglia are typically ramified in healthy tissue, with small cell bodies and numerous thin processes (green). Activated microglia lose their branched morphology and migrate (yellow) to the afflicted region of damage, often transitioning into a macrophage phenotype (tangerine). Infiltrating bone marrow–derived monocytes (red) rapidly differentiate into macrophages (pink), becoming difficult to distinguish from resident immune cells. (b) In Arrestin-1 knockout mice, light onset causes microglia and monocytes (green, green + red) to migrate to the ONL and phagocytose photoreceptor cells bodies (96 h), eliminating the ONL within a week and leaving a handful of subretinal macrophages between the RPE and the ELM (240 h). Iba1 (green) labels microglia and macrophages; CD11b (red) labels microglia and leukocytes (e.g., monocytes); and nuclei are stained with DAPI (blue). Panel adapted from Karlen et al. (2018) (CC BY-SA 4.0). (c) In PRCD knockout mice, microglia (red) primarily target the inner (unlabeled)–outer (green) segment junction, with their processes extending toward the cilium (green). P2YR12 (red) labels microglia; CD68 (purple) labels lysosomes; WGA (green) labels the OS; and nuclei are stained with Hoescht (blue). Panel adapted with permission from Spencer et al. (2019). (d) In P23H rhodopsin mice (top) and LD mice (bottom), microglia with upregulated Gal3 (green + red) accumulate in the subretinal space, whereas infiltrating monocytes (green) remain in the retina. Iba1 (top, green) labels microglia and macrophages; YFP (Cx3CR1) (bottom, green) labels Cx3CR1+ cells (e.g., microglia, macrophages); GAL3 (red) labels subretinal macrophages; and nuclei are stained with DAPI (blue). Panel adapted with permission from O’Koren et al. (2019). (e) In P23H rhodopsin rats, microglia (green, green + red) remain in the subretinal space throughout adulthood, indicating chronic neuroinflammation after photoreceptor degeneration has been completed; compare 4 months (left) to 12 months (right) in controls (SD, top) and P23H rhodopsin mutants (bottom). Iba1 (green) labels microglia and macrophages; MHC-II RT1B (red) label macrophages; and nuclei are stained with TO-PRO-3 iodide (blue). Panel adapted from Noailles et al. (2016) (CC BY-SA 4.0). (f) In aged, 18-month Cx3CR1 knockout mice exposed to normal 12 h on/off light levels, microglia (green) appear in the inner retina (both conditions), and additional microglia accumulate in the subretinal space of the knockout (Cx3CR1GFP/GFP) (arrows). GFP (green) indicates Cx3CR1-positive cells; Griffonia simplicifolia-positive (red) labels vascular endothelial cells; and nuclei are stained with DAPI (blue). Panel adapted with permission from Combadière et al. (2007). Abbreviations: ELM, external limiting membrane; GFP, green fluorescent protein; INL, inner nuclear layer; IPL, inner plexiform layer; IS, inner segment; LD, light damage; ONL, outer nuclear layer; OPL, outer plexiform layer; OS, outer segment; PRCD, progressive rod–cone degeneration; RGC, reginal ganglion cell; RPE, retinal pigment epithelium; SD, Sprague-Dawley control rats; WGA, wheat germ agglutinin.
4.1. Two Sides of Microglia Activation: Helpful and Harmful
Microglia constantly monitor the extracellular microenvironment with a diverse armament of physiological sensors. In addition to the specialized neurotransmitter and potassium channels mentioned above for surveillance, microglia also express receptors specialized for damage. Microglia produce pattern recognition receptors that detect pathogen- and damage-associated molecular patterns (i.e., PAMPs and DAMPs) and a host of cytokine receptors, notably those for TNFα and ILs (Canton et al. 2013, Hickman et al. 2018). These receptors activate many intracellular pathways, commonly converging on nuclear factor-kappa B (NF-κB) and interferon regulatory factor 3 (IRF3), which can activate the expression of inflammatory signals. Likewise, scavenger receptors like CD68, which can bind oxidized lipids, cause the production of cytokines and play a role in phagocytosis (Canton et al. 2013).
Signaling through such receptors leads to functional and morphological changes, transforming microglia into an activated phenotype that can be pro- or anti-inflammatory (Town et al. 2005). One receptor from the immunoglobulin (Ig) superfamily, triggering receptor expressed on myeloid cells-2 (TREM2), binds to anionic ligands like DNA and phospholipids and signals through adaptor proteins DNAX-activation protein-10 and -12 (DAP10 and DAP12). This signaling utilizes the immunoreceptor tyrosine-based activation motif (ITAM), which drives cytoskeletal rearrangement, integrin activation, and calcium mobilization that promote an ameboid morphology with increased motility and phagocytic capacity (Ulland & Colonna 2018). Ec receptors, also of the Ig superfamily, are widely expressed by microglia and provide another avenue for ITAM signaling regulation (ElAli & Rivest 2016).
Changes in gene expression accompanying microglial activation can be highly diverse, leading to the release of factors that can provide neurotrophic support or that can hasten neuronal cell death (Colonna & Butovsky 2017). In photoreceptor degeneration, activated microglia migrate to the outer retina and can release inflammatory factors like CCL2, CCL3, TNFα, and IL1β (Appelbaum et al. 2017, Kohno et al. 2013, Zeng et al. 2005). This type of response is considered proinflammatory; it leads to a cytokine cascade that promotes neural damage and recruits additional inflammatory cells from the periphery, creating a positive feedback loop leading to runaway neuroinflammation and phagocytosis of stressed-but-viable neurons (Brown & Neher 2014, Silverman & Wong 2018).
Conversely, the microglia response can be neuroprotective, reducing cytokines and inhibiting the inflammatory response. Activated microglia can, in some instances, inhibit neuronal apoptosis and promote survival through the release of trophic factors like TGF-β, BDNF, and GDNF (Colonna & Butovsky 2017). Microglia can also indirectly limit the spread of damage through controlled phagocytosis, preventing necrosis of the surrounding tissue. Ablating microglia in the retina during excitotoxic damage exacerbates the upregulation of inflammatory cytokines and increases the number of dying cells, further supporting the conclusion that microglia can be neuroprotective (Todd et al. 2019). Clearly, describing microglia with a binary helpful/harmful classification schema is insufficient for describing the spectrum of functional states and their transcriptomic profiles (Colonna & Butovsky 2017, Ransohoff 2016). Understanding the mechanisms and signals that direct microglia to resolve neurodegeneration in an anti-inflammatory, proneuronal survival manner is an active and important area of study (for more details, see Jin et al. 2019, L. Zhang et al. 2018).
4.2. Monocyte Recruitment
When microglia activation is widespread and/or the blood–tissue barrier is compromised, bone marrow–derived monocytes can infiltrate the CNS from the bloodstream (Han et al. 2017, Jin et al. 2017, Waisman et al. 2015) (Figure 2a). These infiltrating cells can graft themselves into the CNS (Ajami et al. 2011,Mildner et al. 2007, Paschalis et al. 2018) and show differences in transcriptomic expression from the native, yolk sac–derived microglia (O’Koren et al. 2016, Prinz et al. 2017, Ronning et al. 2019). Because infiltrating monocytes rapidly differentiate into macrophages that become difficult to distinguish from resident immune cells (Figure 2a), determining the roles of these populations in the progression of degeneration has been difficult. Specifically, monocytes and microglia express many of the same markers, including CD11b and CD45, but at different levels that likely fluctuate during degeneration (Baufeld et al. 2017; O’Koren et al. 2016, 2019; Ronning et al. 2019; Saban 2018). Recently, some markers have been proposed to be microglia specific, including TREM2, Tmem119, and P2RY12, though expression can change depending on disease state, limiting the use of markers for identification (Hickman et al. 2018).
In the retina, as elsewhere, increased local expression of CCL2 is a particularly potent signal for recruiting CCR2+ inflammatory monocytes to the region (Karlen et al. 2018; Rutar et al. 2011, 2012; Sennlaub et al. 2013). Blocking monocyte infiltration can, in some instances, slow degeneration, suggesting that monocytes contribute to overzealous phagocytosis or escalation of neurotoxic cytokine expression (Guo et al. 2012). In other cases, monocytes play a reparative function (London et al. 2011, 2013) or have no effect at all on the time course of degeneration (Karlen et al. 2018). Thus, like microglia, the role of monocytes is likely to be context dependent, underscoring the importance of knowing the time course of infiltration; the time course of monocyte differentiation to macrophages; and the true, etiological identities of microglia, monocytes, and macrophages over time as degeneration progresses. Single-cell approaches like scRNAseq and high-resolution in vivo retinal imaging will undoubtedly make a vital contribution to that understanding, allowing us to monitor when and where monocytes enter the CNS, what they do, and when and where they leave.
4.3. Variations in Microglia and Monocyte Response During Photoreceptor Degeneration
In the retina, the breadth of photoreceptor degeneration etiologies is echoed in the apparent diversity of types of immune responses to these insults. For example, loss of three separate outer segment proteins leads to photoreceptor degeneration that elicits microglial responses with differing features. In the Arrestin-1 knockout mouse, light onset induces a rapid photoreceptor degeneration over the course of about a week, and both microglia and monocytes migrate to the ONL, where they aggressively phagocytose photoreceptor cells bodies, rather than first targeting the outer segments themselves (Karlen et al. 2018, Levine et al. 2014) (Figure 2b). Indeed, microglia and monocytes do not appear distal to the ELM until later times, when the ONL is substantially depleted. Conversely, when another small outer segment protein, progressive rod–cone degeneration (PRCD), is knocked out, the degeneration is slower, and microglia primarily target the inner segment–outer segment junction, with their processes extending towards the cilium (Spencer et al. 2019) (Figure 2c). In yet another example, the P23H rhodopsin mouse, microglia migrate to the ONL and later into the subretinal space, whereas infiltrating monocytes remain in the retina and appear to avoid the subretinal space altogether (O’Koren et al. 2019), a distribution that the authors noted was similar to their light damage model (Figure 2d). In a comparable P23H rhodopsin model in rats, microglia populated the subretinal space throughout adulthood, maintaining a level of chronic retinal neuroinflammation that persisted even after photoreceptor depletion was complete (Noailles et al. 2016) (Figure 2e).
Normal aging may also lead to an accumulation of subretinal macrophages (e.g., Chinnery et al. 2012; but see also Aredo et al. 2015). This is exacerbated in aged Cx3CR1 knockout mice, in which normal 12 h on/off light levels cause slow photoreceptor degeneration with a large accumulation of microglia in the subretinal space (Combadière et al. 2007) (Figure 2f). The Cx3CR1 knockout mice also develop drusen-like deposits of lipid-rich microglia and macrophages in the subretinal space that are similar to lipid accumulations in AMD patients (Sennlaub et al. 2013). Indeed, some patients with AMD have Cx3CR1 polymorphisms (e.g., Zhang et al. 2015), suggesting that Cx3CR1 signaling in microglia may play a causative role in AMD and that subretinal deposits may be directly related to the accumulation of subretinal macrophages. The differences in the localization and appearance of microglia and monocytes in these different models of photoreceptor degeneration are, as yet, unexplained.
5. THE NEW NORMAL: REESTABLISHING HOMEOSTASIS AFTER DEGENERATION
Following degeneration, it is critical for neuroinflammation to resolve and for the tissue to reestablish homeostasis, yet how this resolution occurs is still poorly understood. To investigate the return to homeostasis, two general approaches have emerged: (a) perturbing the function of specific proteins and (b) utilizing ablation and repopulation techniques. The first approach focuses on the role of individual proteins in neuroinflammatory resolution. For example, translocator protein (TSPO) is expressed by activated microglia (Wang et al. 2014) and binds a polypeptide called diazepam-binding inhibitor (DBI), expressed by gliotic Müller cells that reduce inflammation and other features associated with microglia activation (Choi et al. 2011). During degeneration, DBI and TSPO are concurrently upregulated in gliotic Müller cells and activated microglia, decreasing microglia activation, which suggests a coordinated Müller cell–microglia signaling mechanism that contributes to the resolution of retinal inflammation (Wang et al. 2014). Interestingly, other TSPO receptor ligands, such as XBD173, have been linked to reduced mRNA levels of the cytokines CCL2 and IL6, increased phagocytic capacity, reduced microglia migration, and decreased proliferation (Karlstetter et al. 2014), indicating that the interaction of specific ligands with the TSPO protein may culminate in different downstream effects (Klee et al. 2019).
Another molecule implicated in chronic inflammation is complement factor H (CFH), which is the most widely studied genetic risk factor for AMD. CFH is a soluble plasma factor secreted by microglia and macrophages that binds to CD11b, increasing myeloid cell adhesion (Kopp et al. 2012) and disrupting the CD11b–CD47 interaction that leads to macrophage elimination (Copland et al. 2018). Thus, when CFH binds to CD11b in inflamed tissue, microglia and macrophages are retained, preventing the retina from returning to homeostasis (Calippe et al. 2017). Conversely, in CFH knockout mice, there is no accumulation of extraneous immune cells in the subretinal space. Interestingly, CFH targets the rate of extraneous immune cell elimination, independent of the number of immune cells recruited (Calippe et al. 2017). The clinical relevance of this has been observed in patients with CFH(Y402H), a high-AMD-risk variant of CFH; CFH(Y402H) binds more efficiently to CD11b, thereby decreasing the elimination of macrophages from the subretinal space and preventing the reestablishment of homeostasis (for more details, see Copland et al. 2018).
A second approach to studying recovery is to use microglia ablation and repopulation. CSF1R inhibitors, such as PLX5622, can deplete >99% of microglia for sustained periods of time. Upon treatment removal, microglia will repopulate the CNS in a Cx3CL1–Cx3CR1-signaling-dependent manner (Spangenberg et al. 2019, Y. Zhang et al. 2018). In the brain, small local Nestin-positive microglia subpopulations are responsible for the proliferation required to repopulate the neuronal tissue (Huang et al. 2018b). However, in the retina, there are two regions that contain residual microglial populations from which new microglia are born: the optic nerve and the iris and ciliary body (Huang et al. 2018a). Repopulated retinal microglia recapitulate the morphology and distribution observed in the original population and have similar baseline motility dynamics and responses to neuronal injury (Y. Zhang et al. 2018). Prolonged microglia depletion can result in impairments in retinal signaling, as ERG amplitudes diminish over time in the absence of microglia and can only return to predepletion levels after short depletion periods (Y. Zhang et al. 2018). Despite these challenges, microglia depletion is still a valuable tool for investigating potential roles of microglia at distinct moments during degeneration.
Finally, there is some evidence that local removal and repopulation of microglia occur naturally in the CNS. A recent study investigating myelin restoration in the brain found that the naturally occurring death of inflammatory microglia by necroptosis was necessary for remyelination (Lloyd et al. 2019). When necrostatin-1, a small molecule that prevents necroptosome activity, was delivered preferentially to macrophages, it prevented the loss of CD68+ microglia and hindered remyelination. Furthermore, when necroptosis was not blocked, a population of Nestin+ Iba1+ microglia appeared between 3–7 days postlesion, indicating that microglia repopulation can occur naturally following focal damage. This suggests that, in the brain, targeted loss or removal of inflammatory microglia can induce the remaining population to fill in and promote a healing, proregenerative state.
In the retina, less is known about how the resident population transitions from an activated, inflammatory state back into a basal surveillance mode. Variations in the outcomes between degeneration models suggest that there are multiple paths to a new normal: de-escalating or reversing proinflammatory microglia in some scenarios and using cell death and proliferation to repopulate the population in others. The relative ease with which the new resident population can again become activated and whether such reactivation produces a different balance of pro- or anti-inflammatory responses remain important areas of future work. Understanding resolution is critical for avoiding chronic inflammation and ensuring a healthy microglia population capable of working in concert with other support cells to maintain a healthy microenvironment where photoreceptors and other neuronal cell types can thrive.
SUMMARY POINTS.
Photoreceptors and their unique niche in the posterior eye offer a highly accessible experimental system to investigate how the immune system interacts with and impacts the health and survival of neurons.
Photoreceptor stress and degeneration can be broadly categorized as arising from either (a) the disruption of cell-autonomous homeostasis (e.g., protein misfolding or mistrafficking, prolonged signaling, oxidative damage) or (b) loss of function in critical support cells, such as RPE and Müller glia.
Under normal conditions, microglia are confined to the synaptic and ganglion cell layers of the retina, playing an essential role in synaptic homeostasis and plasticity and actively communicating with photoreceptors and other support cells to maintain retinal health.
During periods of photoreceptor stress and degeneration, microglia transform and migrate to the outer retina, where they phagocytose debris and dying cells. In some instances, monocytes infiltrate from retinal blood vessels and contribute to the inflammatory response.
The functions of microglia and monocytes in the degenerating retina likely vary during disease progression and may be as diverse as the photoreceptor degeneration etiologies themselves. Separating the effect of the inflammatory response from degeneration is difficult and lies at the heart of the debate about the helpful versus harmful roles that microglia play in neurodegenerative disease.
Following degeneration, it is critical for the resident population to return to a basal surveillance mode and for the tissue to reestablish homeostasis. Failure to do so results in chronic inflammation that often leads to permanent vision loss.
FUTURE ISSUES.
Microglia communicate with photoreceptors, RPE, and Müller glia cells. Understanding the molecular signals that underlie this communication is the key to understanding normal homeostatic interactions and preventing chronic inflammation.
Characterizing microglia with a binary helpful/harmful classification schema is insufficient for describing the spectrum of their morphological and transcriptomic profiles; the integrated signals that tip microglia toward cytotoxic or cytoprotective functions need to be better understood.
Reliably differentiating resident and infiltrating cells in real time in vivo will be fundamental to determining the roles that these populations play in the progression of photoreceptor degeneration.
Variations in the localization and molecular hallmarks of microglia and monocytes in different models of photoreceptor degeneration underscore the importance of understanding the spatial and temporal signals driving cell position and morphology.
Little is known about how the resident population transitions from an activated, inflammatory state back into a basal surveillance mode; nevertheless, this transformation is critical to avoiding chronic inflammation and ensuring a healthy microglia population.
ACKNOWLEDGMENTS
The authors thank Kaitryn E. Ronning for helpful discussions and comments on the manuscript and the NIH National Eye Institute (grant R01EY24320) for financial support of ongoing research in this field.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- Ajami B, Bennett JL, Krieger C, McNagny KM, Rossi FM. 2011. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat. Neurosci, 14:1142–49 [DOI] [PubMed] [Google Scholar]
- Appelbaum T, Santana E, Aguirre GD. 2017. Strong upregulation of inflammatory genes accompanies photoreceptor demise in canine models of retinal degeneration. PLOS ONE 12:e0177224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aredo B, Zhang K, Chen X, Wang CX-Z, Li T, Ufret-Vincenty RL. 2015. Differences in the distribution, phenotype and gene expression of subretinal microglia/macrophages in C57BL/6N (Crb1 rd8/rd8) versus C57BL6/J (Crb1 wt/wt) mice. J. Neuroinflamm 12:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AREDS2 Res. Group, Chew EY, Clemons TE, Sangiovanni JP, Danis RP, et al. 2014. Secondary analyses of the effects of lutein/zeaxanthin on age-related macular degeneration progression: AREDS2 report No. 3. JAMA Ophthalmol.132:142–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arshavsky VY, Burns ME. 2014. Current understanding of signal amplification in phototransduction. Cell Logist. 4:e29390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askew K, Li K, Olmos-Alonso A, Garcia-Moreno F, Liang Y, et al. 2017. Coupled proliferation and apoptosis maintain the rapid turnover of microglia in the adult brain. Cell Rep. 18:391–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehr W, Hanke-Gogokhia C, Sharif A, Reed M, Dahl T, et al. 2019. Insights into photoreceptor ciliogenesis revealed by animal models. Prog. Retin. Eye Res 71:26–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baufeld C, O’Loughlin E, Calcagno N, Madore C, Butovsky O. 2017. Differential contribution of microglia and monocytes in neurodegenerative diseases. J. Neural Transm 125:809–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bejarano-Escobar R, Sánchez-Calderón H, Otero-Arenas J, Martín-Partido G, Francisco-Morcillo J. 2017. Müller glia and phagocytosis of cell debris in retinal tissue. J. Anat, 231:471–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier L-P, Bohlen CJ, York EM, Choi HB, Kamyabi A, et al. 2019. Nanoscale surveillance of the brain by microglia via cAMP-regulated filopodia. Cell Rep. 27:2895–908.e4 [DOI] [PubMed] [Google Scholar]
- Brennan LA, Kantorow M. 2009. Mitochondrial function and redox control in the aging eye: role of MsrA and other repair systems in cataract and macular degenerations. Exp. Eye Res 88:195–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bringmann A, Wiedemann P. 2012. Müller glial cells in retinal disease. Ophthalmologica 227:1–19 [DOI] [PubMed] [Google Scholar]
- Brown GC, Neher JJ. 2014. Microglial phagocytosis of live neurons. Nat. Rev. Neurosci 15:209–16 [DOI] [PubMed] [Google Scholar]
- Calippe B, Augustin S, Beguier F, Charles-Messance H, Poupel L, et al. 2017. Complement factor H inhibits CD47-mediated resolution of inflammation. Immunity 46:261–72 [DOI] [PubMed] [Google Scholar]
- Cangiano L, Asteriti S, Cervetto L, Gargini C. 2012. The photovoltage of rods and cones in the dark-adapted mouse retina. J. Physiol 590:3841–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canton J, Neculai D, Grinstein S. 2013. Scavenger receptors in homeostasis and immunity. Nat. Rev. Immunol 13:621–34 [DOI] [PubMed] [Google Scholar]
- Carter-Dawson LD, LaVail MM. 1979. Rods and cones in the mouse retina. I. Structural analysis using light and electron microscopy. J. Comp. Neurol 188:245–62 [DOI] [PubMed] [Google Scholar]
- Chen J, Simon MI, Matthes MT, Yasumura D, LaVail MM. 1999. Increased susceptibility to light damage in an arrestin knockout mouse model of Oguchi disease (stationary night blindness). Investig. Ophthalmol. Vis. Sci 40:2978–82 [PubMed] [Google Scholar]
- Chew EY. 2017. Nutrition, genes, and age-related macular degeneration: What have we learned from the trials? Ophthalmologica 238(1–2):1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnery HR, McLenachan S, Humphries T, Kezic JM, Chen X, et al. 2012. Accumulation of murine subretinal macrophages: effects of age, pigmentation and CX3CR1. Neurobiol. Aging 33:1769–76 [DOI] [PubMed] [Google Scholar]
- Choi J, Ifuku M, Noda M, Guilarte TR. 2011. Translocator protein (18 kDa)/peripheral benzodiazepine receptor specific ligands induce microglia functions consistent with an activated state. Glia 59:219–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cideciyan AV, Jacobson SG, Beltran WA, Sumaroka A, Swider M, et al. 2013. Human retinal gene therapy for Leber congenital amaurosis shows advancing retinal degeneration despite enduring visual improvement. PNAS 110:E517–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonna M, Butovsky O. 2017. Microglia function in the central nervous system during health and neurodegeneration. Annu. Rev. Immunol 35:441–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combadière C, Feumi C, Raoul W, Keller N, Rodéro M, et al. 2007. CX3CR1-dependent subretinal microglia cell accumulation is associated with cardinal features of age-related macular degeneration. J. Clin. Investig 117:2920–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copland DA, Theodoropoulou S, Liu J, Dick AD. 2018. A perspective of AMD through the eyes of immunology. Investig. Ophthalmol. Vis. Sci 59:83–92 [DOI] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, et al. 2005. ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci 8:752–58 [DOI] [PubMed] [Google Scholar]
- Denieffe S, Kelly RJ, McDonald C, Lyons A, Lynch MA. 2013. Classical activation of microglia in CD200-deficient mice is a consequence of blood brain barrier permeability and infiltration of peripheral cells. Brain Behav. Immun 34:86–97 [DOI] [PubMed] [Google Scholar]
- Dryja TP, Rucinski DE, Chen SH, Berson EL. 1999. Frequency of mutations in the gene encoding the alpha subunit of rod cGMP-phosphodiesterase in autosomal recessive retinitis pigmentosa. Investig. Ophthalmol. Vis. Sci 40:1859–65 [PubMed] [Google Scholar]
- ElAli A, Rivest S. 2016. Microglia ontology and signaling. Front. Cell Dev. Biol 4:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore MRP, Najafi AR, Koike MA, Dagher NN, Spangenberg EE, et al. 2014. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron 82:380–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber DB, Flannery JG, Bowes-Rickman C. 1994. The rd mouse story: seventy years of research on an animal model of inherited retinal degeneration. Prog. Retin. Eye Res, 13:31–64 [Google Scholar]
- Farber DB, Lolley RN. 1974. Cyclic guanosine monophosphate: elevation in degenerating photoreceptor cells of the C3H mouse retina. Science 186:449–51 [DOI] [PubMed] [Google Scholar]
- Fontainhas AM, Wang M, Liang KJ, Chen S, Mettu P, et al. 2011. Microglial morphology and dynamic behavior is regulated by ionotropic glutamatergic and GABAergic neurotransmission. PLOS ONE 6:e15973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlander M 2007. Fibrosis and diseases of the eye. J. Clin. Investig 117:576–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geirsdottir L, David E, Keren-Shaul H, Weiner A, Bohlen SC, et al. 2019. Cross-species single-cell analysis reveals divergence of the primate microglia program. Cell 179:1609–22.e16 [DOI] [PubMed] [Google Scholar]
- Ginhoux F, Garel S. 2018. The mysterious origins of microglia. Nat. Neurosci 21:897–99 [DOI] [PubMed] [Google Scholar]
- Glickman RD. 2002. Phototoxicity to the retina: mechanisms of damage. Int. J. Toxicol 21:473–90 [DOI] [PubMed] [Google Scholar]
- Graca AB, Hippert C, Pearson RA. 2018. Müller glia reactivity and development of gliosis in response to pathological conditions. In Retinal Degenerative Diseases, ed. Ash JD, Anderson RE, LaVail MM, Bowes Rickman C, Hollyfield JG, Grimm C, pp. 303–8. Berlin: Springer; [DOI] [PubMed] [Google Scholar]
- Guo C, Otani A, Oishi A, Kojima H, Makiyama Y, et al. 2012. Knockout of ccr2 alleviates photoreceptor cell death in a model of retinitis pigmentosa. Exp. Eye Res 104:39–47 [DOI] [PubMed] [Google Scholar]
- Hagins WA, Penn RD, Yoshikami S. 1970. Dark current and photocurrent in retinal rods. Biophys. J 10:380–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Harris RA, Zhang X-M. 2017. An updated assessment of microglia depletion: current concepts and future directions. Mol. Brain 10:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao W, Wenzel A, Obin MS, Chen C-K, Brill E, et al. 2002. Evidence for two apoptotic pathways in light-induced retinal degeneration. Nat. Genet 32:254–60 [DOI] [PubMed] [Google Scholar]
- Hickman S, Izzy S, Sen P, Morsett L, El Khoury J. 2018. Microglia in neurodegeneration. Nat. Neurosci 21:1359–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori T, Fukutome M, Koike C. 2019. Adeno associated virus (AAV) as a tool for clinical and experimental delivery of target genes into the mammalian retina. Biol. Pharm. Bull 42:343–47 [DOI] [PubMed] [Google Scholar]
- Horie S, Robbie SJ, Liu J, Wu W-K, Ali RR, et al. 2013. CD200R signaling inhibits pro-angiogenic gene expression by macrophages and suppresses choroidal neovascularization. Sci. Rep 3:3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SH, Pittler SJ, Huang X, Oliveira L, Berson EL, Dryja TP. 1995. Autosomal recessive retinitis pigmentosa caused by mutations in the alpha subunit of rod cGMP phosphodiesterase. Nat. Genet 11:468–71 [DOI] [PubMed] [Google Scholar]
- Huang Y, Xu Z, Xiong S, Qin G, Sun F, et al. 2018a. Dual extra-retinal origins of microglia in the model of retinal microglia repopulation. Cell Discov.4:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Xu Z, Xiong S, Sun F, Qin G, et al. 2018b. Repopulated microglia are solely derived from the proliferation of residual microglia after acute depletion. Nat. Neurosci 21:530–40 [DOI] [PubMed] [Google Scholar]
- Hurley JB, Lindsay KJ, Du J. 2015. Glucose, lactate, and shuttling of metabolites in vertebrate retinas. J. Neurosci. Res 93:1079–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai S, Inokuchi Y, Nakamura S, Tsuruma K, Shimazawa M, Hara H. 2010. Systemic administration of a free radical scavenger, edaravone, protects against light-induced photoreceptor degeneration in the mouse retina. Eur. J. Pharmacol 642:77–85 [DOI] [PubMed] [Google Scholar]
- Insinna C, Daniele LL, Davis JA, Larsen DD, Kuemmel C, et al. 2012. An S-opsin knock-in mouse (F81Y) reveals a role for the native ligand 11-cis-retinal in cone opsin biosynthesis. J. Neurosci 32:8094–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo P, Attwell D, Madry C. 2019. Ion channels and receptors as determinants of microglial function. Trends Neurosci. 42:278–92 [DOI] [PubMed] [Google Scholar]
- Jiang DJ, Xu CL, Tsang SH. 2018. Revolution in gene medicine therapy and genome surgery. Genes 9:575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin N, Gao L, Fan X, Xu H. 2017. Friend or foe? Resident microglia versus bone marrow-derived microglia and their roles in the retinal degeneration. Mol. Neurobiol 54:4094–112 [DOI] [PubMed] [Google Scholar]
- Jin X, Liu M-Y, Zhang D-F, Zhong X, Du K, et al. 2019. Natural products as a potential modulator of microglial polarization in neurodegenerative diseases. Pharmacol. Res 145:104253. [DOI] [PubMed] [Google Scholar]
- Jobling AI, Waugh M, Vessey KA, Phipps JA, Trogrlic L, et al. 2018. The role of the microglial Cx3cr1 pathway in the postnatal maturation of retinal photoreceptors. J. Neurosci 38:4708–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlen SJ, Miller EB, Wang X, Levine ES, Zawadzki RJ, Burns ME. 2018. Monocyte infiltration rather than microglia proliferation dominates the early immune response to rapid photoreceptor degeneration. J. Neuroinflamm 15:344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlstetter M, Nothdurfter C, Aslanidis A, Moeller K, Horn F, et al. 2014. Translocator protein (18 kDa) (TSPO) is expressed in reactive retinal microglia and modulates microglial inflammation and phagocytosis. J. Neuroinflamm 11:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenmann H, Kirchhoff F, Verkhratsky A. 2013. Microglia: new roles for the synaptic stripper. Neuron 77:10–18 [DOI] [PubMed] [Google Scholar]
- Kierdorf K, Prinz M. 2017. Microglia in steady state. J. Clin. Investig 127:3201–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiser PD, Palczewski K. 2016. Retinoids and retinal diseases. Annu. Rev. Vis. Sci 2:197–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee K, Storti F, Barben M, Samardzija M, Langmann T, et al. 2019. Systemic knockout of Tspo in mice does not affect retinal morphology, function and susceptibility to degeneration. Exp. Eye Res 188:107816. [DOI] [PubMed] [Google Scholar]
- Kohno H, Chen Y, Kevany BM, Pearlman E, Miyagi M, et al. 2013. Photoreceptor proteins initiate microglial activation via Toll-like receptor 4 in retinal degeneration mediated by all-trans-retinal. J. Biol. Chem 288:15326–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp A, Hebecker M, Svobodova E, Jozsi M. 2012. Factor h: a complement regulator in health and disease, and a mediator of cellular interactions. Biomolecules 2:46–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léveillard T, Sahel J-A. 2017. Metabolic and redox signaling in the retina. Cell Mol. Life Sci 74:3649–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine ES, Zam A, Zhang P, Pechko A, Wang X, et al. 2014. Rapid light-induced activation of retinal microglia in mice lacking Arrestin-1. Vis. Res 102:71–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnartz-Gerlach B, Kopatz J, Neumann H. 2014. Siglec functions of microglia. Glycobiology 24:794–99 [DOI] [PubMed] [Google Scholar]
- Liu YU, Ying Y, Li Y, Eyo UB, Chen T, et al. 2019. Neuronal network activity controls microglial process surveillance in awake mice via norepinephrine signaling. Nat. Neurosci 22:1771–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd AF, Davies CL, Holloway RK, Labrak Y, Ireland G, et al. 2019. Central nervous system regeneration is driven by microglia necroptosis and repopulation. Nat. Neurosci 22:1046–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobanova ES, Finkelstein S, Li J, Travis AM, Hao Y, et al. 2018. Increased proteasomal activity supports photoreceptor survival in inherited retinal degeneration. Nat. Commun 9:1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobanova ES, Finkelstein S, Skiba NP, Arshavsky VY. 2013. Proteasome overload is a common stress factor in multiple forms of inherited retinal degeneration. PNAS 110:9986–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- London A, Cohen M, Schwartz M. 2013. Microglia and monocyte-derived macrophages: functionally distinct populations that act in concert in CNS plasticity and repair. Front. Cell Neurosci 7:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London A, Itskovich E, Benhar I, Kalchenko V, Mack M, et al. 2011. Neuroprotection and progenitor cell renewal in the injured adult murine retina requires healing monocyte-derived macrophages. J. Exp. Med 208:23–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig PE, Freeman SC, Janot AC. 2019. Novel stem cell and gene therapy in diabetic retinopathy, age related macular degeneration, and retinitis pigmentosa. Int. J. Retina Vitr 5:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madry C, Arancibia-Carcamo IL, Kyrargyri V, Chan VTT, Hamilton NB, Attwell D. 2018a. Effects of the ecto-ATPase apyrase on microglial ramification and surveillance reflect cell depolarization, not ATP depletion. PNAS 115:E1608–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madry C, Kyrargyri V, Arancibia-Carcamo IL, Jolivet R, Kohsaka S, et al. 2018b. Microglial ramification, surveillance, and interleukin-1beta release are regulated by the two-pore domain K+ channel THIK-1. Neuron 97:299–312.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis T, Housset M, Eandi C, Beguier F, Touhami S, et al. 2017. Activated monocytes resist elimination by retinal pigment epithelium and downregulate their OTX2 expression via TNF-α. Aging Cell 16:173–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMenamin PG, Humphries TG, Kezic J, Cherepanoff S, Sarks SH. 2009. Accumulation of macrophages in the subretinal space: correlation with age, pigmentation and Cx3Cr1 genotype in the mouse eye and with age/AMD pathology in humans. Investig. Ophthalmol. Vis. Sci 50:3868 [Google Scholar]
- Mildner A, Schmidt H, Nitsche M, Merkler D, Hanisch UK, et al. 2007. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat. Neurosci 10:1544–53 [DOI] [PubMed] [Google Scholar]
- Miller EB, Zhang P, Ching K, Pugh EN, Burns ME. 2019. In vivo imaging reveals transient microglia recruitment and functional recovery of photoreceptor signaling after injury. PNAS 116:16603–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan DS, Chidlow G, Wood JP, Casson RJ. 2017. Glucose metabolismin mammalian photoreceptor inner and outer segments. Clin. Exp. Ophthalmol 45:730–41 [DOI] [PubMed] [Google Scholar]
- Newman EA. 1996. Regulation of extracellular K+ and pH by polarized ion fluxes in glial cells: the retinal Müller cell. Neuroscientist 2:109–17 [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. 2005. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308:1314–18 [DOI] [PubMed] [Google Scholar]
- Noailles A, Maneu V, Campello L, Gómez-Vicente V, Lax P, Cuenca N. 2016. Persistent inflammatory state after photoreceptor loss in an animal model of retinal degeneration. Sci. Rep, 6:33356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Koren EG, Mathew R, Saban DR. 2016. Fate mapping reveals that microglia and recruited monocyte-derived macrophages are definitively distinguishable by phenotype in the retina. Sci. Rep 6:20636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Koren EG, Yu C, Klingeborn M, Wong AYW, Prigge CL, et al. 2019. Microglial function is distinct in different anatomical locations during retinal homeostasis and degeneration. Immunity 50:723–37.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquet-Durand F, Hauck SM, van Veen T, Ueffing M, Ekstrom P. 2009. PKG activity causes photoreceptor cell death in two retinitis pigmentosa models. J. Neurochem 108:796–810 [DOI] [PubMed] [Google Scholar]
- Park J-H, Kong L, Zhou Y, Cui M. 2017. Large-field-of-view imaging by multi-pupil adaptive optics. Nat. Methods 14:581–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschalis EI, Lei F, Zhou C, Kapoulea V, Dana R, et al. 2018. Permanent neuroglial remodeling of the retina following infiltration of CSF1R inhibition-resistant peripheral monocytes. PNAS 115:E11359–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn RD, Hagins WA. 1972. Kinetics of the photocurrent of retinal rods. Biophys. J 12:1073–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce EA, Bennett J. 2015. The status of RPE65 gene therapy trials: safety and efficacy. Cold Spring Harb. Perspect. Med, 5:a017285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocock JM, Kettenmann H. 2007. Neurotransmitter receptors on microglia. Trends Neurosci. 30:527–35 [DOI] [PubMed] [Google Scholar]
- Power M, Das S, Schutze K, Marigo V, Ekstrom P, Paquet-Durand F. 2019. Cellular mechanisms of hereditary photoreceptor degeneration: focus on cGMP. Prog. Retin. Eye Res, 30:100772. [DOI] [PubMed] [Google Scholar]
- Prinz M, Erny D, Hagemeyer N. 2017. Ontogeny and homeostasis of CNS myeloid cells. Nat. Immunol, 18:385–92 [DOI] [PubMed] [Google Scholar]
- Qiu Y, Yao J, Jia L, Thompson DA, Zacks DN. 2019. Shifting the balance of autophagy and proteasome activation reduces proteotoxic cell death: a novel therapeutic approach for restoring photoreceptor homeostasis. Cell Death Dis. 10:547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM. 2016. A polarizing question: Do M1 and M2 microglia exist? Nat. Neurosci 19:987–91 [DOI] [PubMed] [Google Scholar]
- Rashid K, Akhtar-Schaefer I, Langmann T. 2019. Microglia in retinal degeneration. Front. Immunol 10:1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenbach A, Bringmann A. 2013. New functions of Müller cells. Glia 61:651–78 [DOI] [PubMed] [Google Scholar]
- Ronning KE, Karlen SJ, Miller EB, Burns ME. 2019. Molecular profiling of resident and infiltrating mononuclear phagocytes during rapid adult retinal degeneration using single-cell RNA sequencing. Sci. Rep 9:4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutar M, Natoli R, Provis JM. 2012. Small interfering RNA-mediated suppression of Ccl2 in Müller cells attenuates microglial recruitment and photoreceptor death following retinal degeneration. J. Neuroinflamm 9:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutar M, Natoli R, Valter K, Provis JM. 2011. Early focal expression of the chemokine Ccl2 by Müller cells during exposure to damage-inducing bright continuous light. Investig. Ophthalmol. Vis. Sci 52:2379–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saban DR. 2018. New concepts in macrophage ontogeny in the adult neural retina. Cell Immunol. 330:79–85 [DOI] [PubMed] [Google Scholar]
- Sahin K, Gencoglu H, Akdemir F, Orhan C, Tuzcu M, et al. 2019. Lutein and zeaxanthin isomers may attenuate photo-oxidative retinal damage via modulation of G protein-coupled receptors and growth factors in rats. Biochem. Biophys. Res. Commun 516:163–70 [DOI] [PubMed] [Google Scholar]
- Saika S, Yamanaka O, Sumioka T, Miyamoto T, Miyazaki K, et al. 2008. Fibrotic disorders in the eye: targets of gene therapy. Prog. Retin. Eye Res 27:177–96 [DOI] [PubMed] [Google Scholar]
- Sakami S, Imanishi Y, Palczewski K. 2019. Muller glia phagocytose dead photoreceptor cells in a mouse model of retinal degenerative disease. FASEB J. 33:3680–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakami S, Maeda T, Bereta G, Okano K, Golczak M, et al. 2011. Probing mechanisms of photoreceptor degeneration in a new mouse model of the common form of autosomal dominant retinitis pigmentosa due to P23H opsin mutations. J. Biol. Chem 286:10551–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarthy V, Ripps H. 2001. The Retinal Muller Cell: Structure and Function. New York: Kluwer Acad./Plenum Publ. [Google Scholar]
- Sato S, Jastrzebska B, Engel A, Palczewski K, Kefalov VJ. 2019. Apo-opsin exists in equilibrium between a predominant inactive and a rare highly active state. J. Neurosci 39:212–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sennlaub F, Auvynet C, Calippe B, Lavalette S, Poupel L, et al. 2013. CCR2+ monocytes infiltrate atrophic lesions in age-related macular disease and mediate photoreceptor degeneration in experimental subretinal inflammation in Cx3cr1 deficient mice. EMBO Mol. Med 5:1775–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng J, Ruedl C, Karjalainen K. 2015. Most tissue-resident macrophages except microglia are derived from fetal hematopoietic stem cells. Immunity 43:382–93 [DOI] [PubMed] [Google Scholar]
- Silverman SM, Wong WT. 2018. Microglia in the retina: roles in development, maturity, and disease. Annu. Rev. Vis. Sci 4:45–77 [DOI] [PubMed] [Google Scholar]
- Spangenberg E, Severson PL, Hohsfield LA, Crapser J, Zhang J, et al. 2019. Sustained microglial depletion with CSF1R inhibitor impairs parenchymal plaque development in an Alzheimer’s disease model. Nat. Commun 10:3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow JR, Hicks D, Hamel CP. 2010. The retinal pigment epithelium in health and disease. Curr. Mol. Med 10:802–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer WJ, Ding J-D, Lewis TR, Yu C, Phan S, et al. 2019. PRCD is essential for high-fidelity photoreceptor disc formation. PNAS 116:13087–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss O 2005. The retinal pigment epithelium in visual function. Physiol. Rev 85:845–81 [DOI] [PubMed] [Google Scholar]
- Su F, Spee C, Araujo E, Barron E, Wang M, et al. 2019. A novel HDL-mimetic peptide HM-10/10 protects RPE and photoreceptors in murine models of retinal degeneration. Int. J. Mol. Sci 20:4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y, He M, Yang Q, Ma Z, Qu Y, et al. 2019. Systemic taurine treatment provides neuroprotection against retinal photoreceptor degeneration and visual function impairments. Drug Des. Dev. Ther 13:2689–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay TL, Mai D, Dautzenberg J, Fernandez-Klett F, Lin G, et al. 2017. A new fate mapping system reveals context-dependent random or clonal expansion of microglia. Nat. Neurosci 20:793–803 [DOI] [PubMed] [Google Scholar]
- Todd L, Palazzo I, Suarez L, Liu X, Volkov L, et al. 2019. Reactive microglia and ILβ/IL-1R1-signaling mediate neuroprotection in excitotoxin-damaged mouse retina. J. Neuroinflamm 16:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolone A, Belhadj S, Rentsch A, Schwede F, Paquet-Durand F. 2019. The cGMP pathway and inherited photoreceptor degeneration: targets, compounds, and biomarkers. Genes 10:453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Town T, Nikolic V, Tan J. 2005. The microglial “activation” continuum: from innate to adaptive responses. J. Neuroinflamm 2:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang SH, Tsui I, Chou CL, Zernant J, Haamer E, et al. 2008. A novel mutation and phenotypes in phosphodiesterase 6 deficiency. Am. J. Ophthalmol 146:780–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki Y, Wilken MS, Cox KE, Chipman L, Jorstad N, et al. 2015. Transgenic expression of the proneural transcription factor Ascl1 in Müller glia stimulates retinal regeneration in young mice. PNAS 112:13717–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulland TK, Colonna M. 2018. TREM2: a key player in microglial biology and Alzheimer disease. Nat. Rev. Neurol 14:667–75 [DOI] [PubMed] [Google Scholar]
- Wahl DJ, Ng R, Ju MJ, Jian Y, Sarunic MV. 2019. Sensorless adaptive optics multimodal en-face small animal retinal imaging. Biomed. Opt. Express 10:252–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waisman A, Ginhoux F, Greter M, Bruttger J. 2015. Homeostasis of microglia in the adult brain: review of novel microglia depletion systems. Trends Immunol. 36:625–36 [DOI] [PubMed] [Google Scholar]
- Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. 2009. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J. Neurosci 29:3974–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DG, Lue L-F. 2013. Understanding the neurobiology of CD200 and the CD200 receptor: a therapeutic target for controlling inflammation in human brains? Future Neurol. 8:321–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J-S, Kefalov VJ. 2011. The cone-specific visual cycle. Prog. Retin. Eye Res 30:115–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Wang X, Zhao L, Ma W, Rodriguez IR, et al. 2014. Macroglia-microglia interactions via TSPO signaling regulates microglial activation in the mouse retina. J. Neurosci 34:3793–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Tsang SH, Chen J. 2017. Two pathways of rod photoreceptor cell death induced by elevated cGMP. Hum. Mol. Genet 26:2299–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhao L, Zhang J, Fariss RN, Ma W, et al. 2016. Requirement for microglia for the maintenance of synaptic function and integrity in the mature retina. J. Neurosci 36:2827–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong WT, Wang M, Li W. 2011. Regulation of microglia by ionotropic glutamatergic and GABAergic neurotransmission. Neuron Glia Biol. 7:41–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright AF, Chakarova CF, Abd El-Aziz MM, Bhattacharya SS. 2010. Photoreceptor degeneration: genetic and mechanistic dissection of a complex trait. Nat. Rev. Genet 11:273–84 [DOI] [PubMed] [Google Scholar]
- Wright CB, Redmond TM, Nickerson JM. 2015. A history of the classical visual cycle. Prog. Mol. Biol. Transl. Sci 134:433–48 [DOI] [PubMed] [Google Scholar]
- Yarmolenko PS, Moon EJ, Landon C, Manzoor A, Hochman DW, et al. 2011. Thresholds for thermal damage to normal tissues: an update. Int. J. Hyperthermia 27:320–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RW. 1967. The renewal of photoreceptor cell outer segments. J. Cell Biol, 33:61–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssef PN, Sheibani N, Albert DM. 2011. Retinal light toxicity. Eye 25:1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabel MK, Zhao L, Zhang Y, Gonzalez SR, Ma W, et al. 2016. Microglial phagocytosis and activation underlying photoreceptor degeneration is regulated by CX3CL1-CX3CR1 signaling in a mouse model of retinitis pigmentosa. Glia 64:1479–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahid S, Branham K, Schlegel D, Pennesi ME, Michaelides M, et al. 2018. SAG. In Retinal Dystrophy Gene Atlas, ed. Zahid S, Branham K, Schlegel D, Pennesi ME, Michaelides M, Heckenlively J, Jayasundera T, p. 251. Berlin: Springer [Google Scholar]
- Zeng H-Y, Zhu X-A, Zhang C, Yang L-P, Wu L-M, Tso MOM. 2005. Identification of sequential events and factors associated with microglial activation, migration, and cytotoxicity in retinal degeneration in rd mice. Investig. Ophthalmol. Vis. Sci 46:2992–99 [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhang J, You Z. 2018. Switching of the microglial activation phenotype is a possible treatment for depression disorder. Front. Cell Neurosci 12:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Wang L-Y, Wang Y-F, Wu C-R, Lei C-L, et al. 2015. Associations between the T280M and V249I SNPs in CX3CR1 and the risk of age-related macular degeneration. Investig. Ophthalmol. Vis. Sci 56:5590–98 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhao L, Wang X, Ma W, Lazere A, et al. 2018. Repopulating retinal microglia restore endogenous organization and function under CX3CL1-CX3CR1 regulation. Sci. Adv 4:eaap8492. [DOI] [PMC free article] [PubMed] [Google Scholar]