Abstract
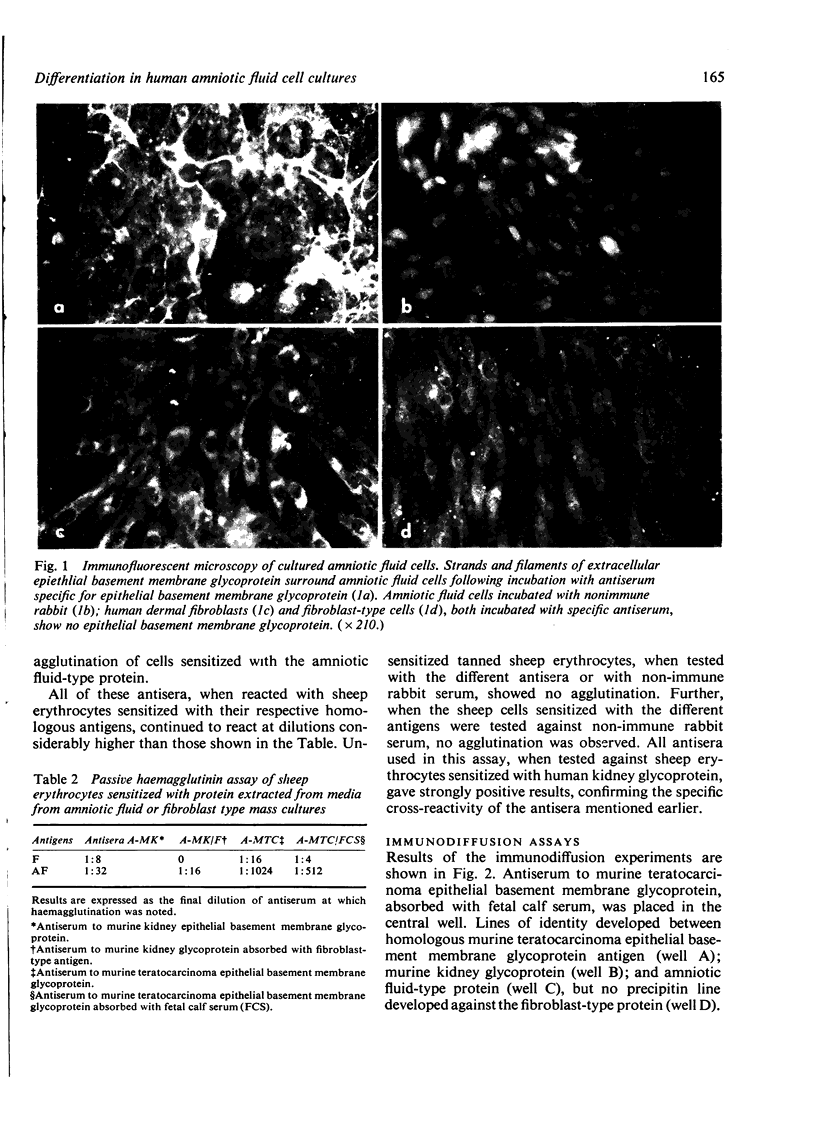
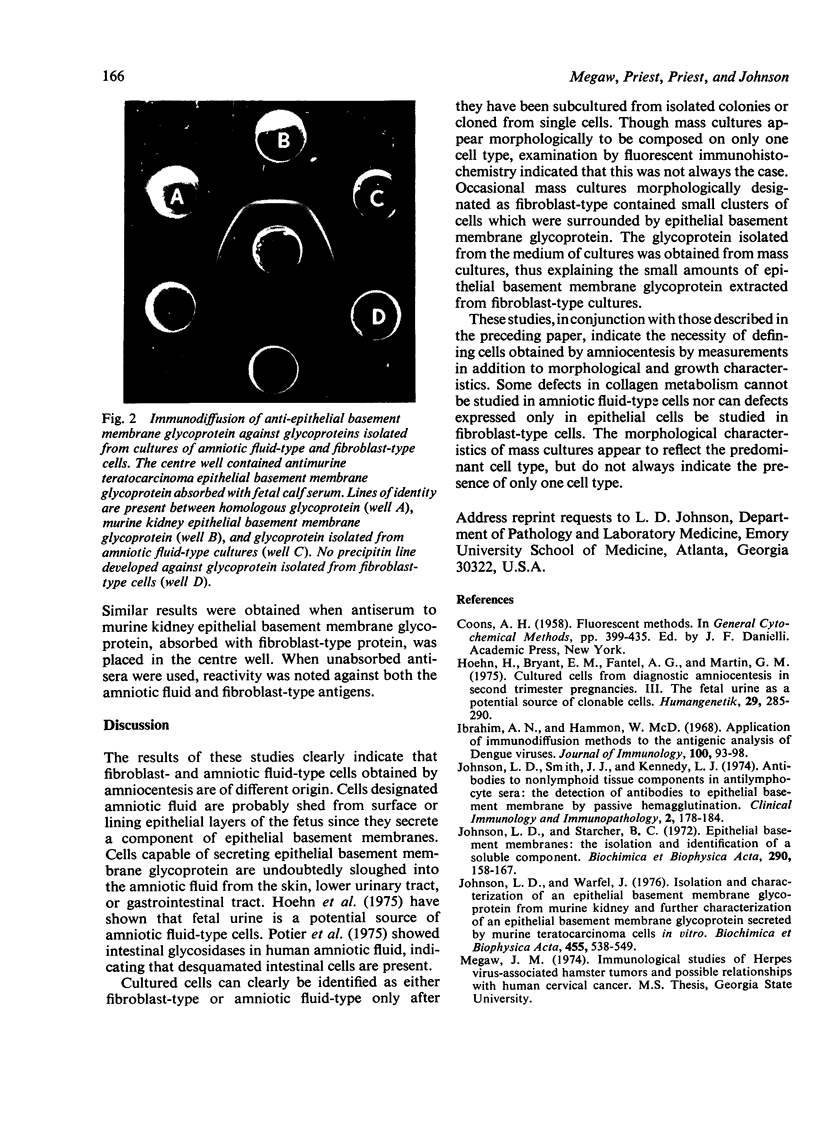
Cells obtained by amniocentesis for prenatal diagnosis were grown in vitro and examined for the presence of a glycoprotein component epithelial basement membrane. Isolated colonies or clones of amniotic fluid-type cells secrete the glycoprotein, which was identified in association with the cells using indirect immunofluorescent antibody techniques. In addition, the glycoprotein was isolated from tissue culture medium and identified as a component of epithelial basement membranes by passive haemagglutination (PHA) and immunodiffusion assays. Fibroblast-type cells do not secrete the glycoprotein. These results correlate well with the synthesis of type IV collagen by amniotic fluid cells reported in the accompanying paper (Priest et al., 1977) and indicate that amniotic fluid cells are epithelial in origin.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hoehn H., Bryant E. M., Fantel A. G., Martin G. M. Cultivated cells from diagnostic amniocentesis in second trimester pregnancies. III. The fetal urine as a potential source of clonable cells. Humangenetik. 1975 Oct 7;29(4):285–290. doi: 10.1007/BF00394190. [DOI] [PubMed] [Google Scholar]
- Ibrahim A. N., Hammon W. M. Application of immunodiffusion methods to the antigenic analysis of dengue viruses. II. Immunoelectrophoresis. J Immunol. 1968 Jan;100(1):93–98. [PubMed] [Google Scholar]
- Johnson L. D., Smith J. J., 3rd, Kennedy L. J., Jr Antibodies to nonlymphoid tissue components in antilymphocyte sera. The detection of antibodies to epithelial basement membrane by passive hemagglutination. Clin Immunol Immunopathol. 1974 Jan;2(2):178–184. doi: 10.1016/0090-1229(74)90037-3. [DOI] [PubMed] [Google Scholar]
- Johnson L. D., Starcher B. C. Epithelial basement membranes: the isolation and identification of a soluble component. Biochim Biophys Acta. 1972 Dec 1;290(1):158–167. doi: 10.1016/0005-2736(72)90060-0. [DOI] [PubMed] [Google Scholar]
- Johnson L. D., Warfel J. Isolation and characterization of an epithelial basement membrane glycoprotein from murine kidney and further characterization of an epithelial basement membrane glycoprotein secreted by murine teratocarcinoma cells in vitro. Biochim Biophys Acta. 1976 Dec 2;455(2):538–549. doi: 10.1016/0005-2736(76)90323-0. [DOI] [PubMed] [Google Scholar]
- Potier M., Dallaire L., Melançon S. B. Occurrence and properties of fetal intestinal glycosidases (disaccharidases) in human amniotic fluid. Biol Neonate. 1975;27(3-4):141–152. doi: 10.1159/000240771. [DOI] [PubMed] [Google Scholar]
- Priest R. E., Priest J. H., Moinuddin J. F., Keyser A. J. Differentiation in human amniotic fluid cell cultures: I: Collagen production. J Med Genet. 1977 Jun;14(3):157–162. doi: 10.1136/jmg.14.3.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STAVITSKY A. B. Micromethods for the study of proteins and antibodies. I. Procedure and general applications of hemagglutination and hemagglutination-inhibition reactions with tannic acid and protein-treated red blood cells. J Immunol. 1954 May;72(5):360–367. [PubMed] [Google Scholar]