Abstract
The steroid 5α-reductase (5α-R) plays an important physiological role in the conversion of steroid hormones such as androgen and progesterone to their 5α-reduced derivatives. 5α-R type II (5α-R2), one of two 5α-R isoforms, is thought to be a key enzyme in the generation of neuroactive steroids in the brain, particularly allopregnanolone (AP), via the production of its precursor dihydroprogesterone from progesterone. In the present study, we investigated possible regulatory mechanisms of 5α-R2 gene expression by steroid hormones in the female mouse brain. We first cloned mouse 5α-R2 (m5α-R2) cDNA by degenerate PCR, and found that progesterone induced 5α-R2 gene expression to levels detectable by in situ hybridization in female mouse brains. Functional analysis of the m5α-R2 gene promoter by a transient expression assay with human progesterone receptor (PR) and androgen receptor (AR) expression vectors identified a progesterone and androgen regulatory element (m5α-R2 PRE/ARE). Results of an electrophoretic mobility shift assay revealed that both PR and AR homodimers bound directly to m5α-R2 PRE/ARE sequence. These findings suggest that the gene expression of m5α-R2 is transcriptionally regulated by progesterone in female brains.
INTRODUCTION
The steroid 5α-reductase (5α-R) is a key enzyme in the conversion of several Δ4-3keto steroids such as testosterone, progesterone, aldosterone and corticosterone, into their respective 5α-reduced derivatives (1–3). Two isoforms of 5α-R have been identified. While these isoforms catalyze the same reaction, they possess different biochemical and pharmacological properties, and display distinct expression patterns in tissues (4–7). Type I (5α-R1) is widely distributed throughout the body, and is most abundant in the liver where it catabolizes steroids. Type II (5α-R2) is primarily expressed in target tissues for androgens, such as the prostate and seminal vesicles, and is responsible for converting testosterone into dihydrotestosterone (DHT), a potent natural androgen. Impaired 5α-R2 activity caused by mutations in the human 5α-R2 gene results in insufficient DHT production, leading to male pseudohermaphroditism (5,8). Recently, it was found that 5α-R2 was expressed in male rat brains at detectable levels after testosterone was given. This raised the possibility that the testosterone-induced up-regulation of 5α-R2 gene expression and resultant enhanced production of DHT was important for mediating the male sexual behavior and aggression that occurred during a very critical period (9). In contrast, the physiological role of 5α-R2 in the female brain remains largely unknown.
5α-R2 is also known to metabolize progesterone into dihydroprogesterone (DHP), and DHP is further converted into allopregnanolene (AP) by 3α-hydroxysteroid dehydrogenase (3α-HSD) (10,11). AP is thought to exert anti-convulsant and anxiolytic-like actions (12,13) by binding to allosteric sites on neurotransmitter-gated ion channels, such as the γ-amino butyric acid type A (GABAA) receptor (14–16). When brain progesterone and AP levels are increased during pregnancy or transient stress, expression of genes for GABAA receptor inhibitory subunits α4 (17–20) were shown to be down-regulated, possibly resulting in the potentiation of AP action through enhanced GABAA receptor function. Nonetheless, there is little information concerning the effects of progesterone on 5α-R2 gene expression in the female brain.
Androgens and progesterone are believed to mediate their effects through the transcriptional control of specific sets of target genes via nuclear receptors (21–23). Androgen receptor (AR) and progesterone receptor (PR) are members of the nuclear receptor superfamily, and act as hormone-inducible transcription factors that bind specific DNA elements as homodimers (24–28). The DNA elements that bind AR and PR share the common sequence 5′-AGAACANNNTGTTCT-3′, known as the consensus progesterone/androgen response element (PRE/ARE) (29). As both hormone receptors recognize the same sequence, it is likely that the same genes are regulated when either AR or PR are present in a given tissue or cell (30–33). Therefore, the fact that the transcriptional control of 5α-R2 is regulated by the DHT–AR system in the male brain raises the possibility that progesterone may act as a positive regulator for 5α-R2 gene expression in the female brain.
To test this hypothesis, the present study was undertaken to examine 5α-R2 gene expression in female mice. First, the cDNA of mouse 5α-R2 (m5α-R2) was amplified by degenerate PCR and cloned. The 5α-R2 gene expression was induced by the treatment of either progesterone or androgen in female mouse brains. In situ analyses of female brains detected the 5α-R2 transcript in the hippocampus of the mice treated with progesterone. Furthermore, the putative PRE/ARE was identified in the m5α-R2 gene promoter. Thus, our results suggested that the progesterone-induced expression of 5α-R2 promoted AP production in the female brain.
MATERIALS AND METHODS
RNA isolation and molecular cloning of 5α-R2 cDNA in the mouse kidney
Total RNA was isolated from kidneys of 10-week-old female mice (ICR CD1; Charles River Laboratories, Inc.) using isogen solution (Nippongene) and reverse transcribed using an oligo(dT)18 primer and SuperScript II reverse transcriptase (Life Technologies, Inc.). Degenerate PCR was then performed using specific primers based on homologous regions of rat and human 5α-R2 sequences (1,4,8): 5′-ATGCAGATTGTCTGCCAKCAG-3′ (nucleotides 1–21) and 5′-TTAAAAGATGAATGGAAT-3′ (nucleotides 745–765). PCR products of the expected size (765 bp) were subcloned into the pcDNA3 expression vector under control of the cytomegalovirus promoter (Invitrogen), and subjected to sequence analysis using a Prism 377 DNA (Applied Biosystems).
Plasmid construction
cDNA encoding m5α-R2 and the mutant G34R (34) was subcloned into the pcDNA3 (Invitrogen) expression vector. A series of deletion mutants of the m5α-R2 gene promoter, consensus PRE/ARE (5′-AGAACATCCTGTTCT-3′) (×2) with the thymidine kinase (tk) short promoter, and m5α-R2 PRE/ARE ×2 with the tk short promoter were subcloned into the pGL3 reporter plasmid (Invitrogen). The 5′-flanking region of the m5α-R2 gene was isolated by the inverse PCR. Primers recognizing the predicted start of exon 1 (5′-CCAAAGCACAGGATCAGGGT-3′), and predicted end of exon 1 5′-TTGGACCTCCGGGGAATGTG-3′) were used to amplify product from circularized genomic DNA.
Converting activity of 5α-R2
5α-R2 activity was assayed using the nuclear receptor-mediated transactivation method (35), COS-1 cells were maintained in DMEM without phenol red and supplemented with 5% FBS stripped with dextran-coated charcoal. Cells cultured with precursors were transiently transfected with 50 and 5 ng expression vectors for nuclear receptors and m5α-R2, respectively, and 0.5 µg reporter plasmid (Firefly luciferase) using the Lipofectin reagent (Gibco BRL). Bluescribe M13+ (Stratagene) was used as a carrier to adjust the total amount of DNA to 1.5 µg for transfection and 1 nM steroid hormone used as a positive control. Transfection efficiency was normalized according to Renilla luciferase activity (derived from pRL-CMV) as an internal control. The m5α-R2 enzyme activity was assayed also by in vitro converting method (36) with the cell extract (10 mM Tris–HCl pH 5.0, 150 mM NaCl, 1 mM EDTA, 10% glycerol) of COS-1 cells transfected with the m5α-R2, rat 5α-R2 or mutant G34R expression plasmids. The cell extracts (30 µg protein) were incubated in 0.1 M Tris–HCl buffer pH 5.0 containing either 5 µM [14C]teststerone or progesterone (NEN™ Life Science Product) and 5 mM NADPH (Sigma) for 1 h at 37°C. The labeled steroids were extracted into methylene chroride, resuspended in chloroform–methanol (3:1, v/v) and developed by chloroform–ethylacetate (2:1, v/v) onto Silica Gel 60 F254 TLC plates (Merck) with the detection of converted steroids by autoradiography.
Animals and experimental protocols
Ten-week-old female mice, weighing ∼20 g, were fed a standard rodent chow with free access to food and tap water. Four hours after subcutaneous injection of progesterone (500 µg/mouse) or testosterone (500 µg/mouse), the mice were sacrificed according to principles and procedures outlined in the NIH Guide for the Care and Use of Laboratory Animals.
In situ hybridization
Brain tissues were fixed in 4% paraformaldehyde, and dehydrated with increasing concentrations of ethanol prior to paraffin embedding. Antisense and sense riboprobes were generated from nucleotides 1–765 of m5α-R2 cDNA in pcDNA3 using the DIG RNA labeling kit (Boehringer Mannhein). Paraffin-embedded tissue sections of adult mouse brain were placed on microscope slides, hybridized to riboprobes, and incubated with horseradish peroxidase-conjugated anti-DIG antibody (Boehringer Mannhein) using the ISHR starting kit (Nippongene). For in situ hybridization signal amplification, the slides were incubated with biotinyl tyramide and horseradish peroxidase-conjugated streptavidin using the TSA™-INDIRECT kit (NEN™ Life Science Products) and stained with diaminobenzidine.
Semi-quantitative RT–PCR
Aliquots of total RNA (3 µg) extracted from mouse brains were reverse-transcribed using the SuperScript pre-amplification system (Gibco BRL) according to the manufacturer’s instructions. PCR was performed in a 25 µl reaction volume containing 1 µl 1/20 reverse transcription mix solution, 0.4 µM primers, 0.25 mM dNTPs and 0.125 U AmpliTaq Gold DNA polymerase (Perkin-Elmer). After 5 min pre-incubation at 96°C, amplification of mouse 5α-R2, histidine decarboxylase (HDC) (37) or β-actin cDNA was performed for 35, 35 or 25 cycles, respectively. Cycles consisted of 1 min denaturation at 96°C, 1 min annealing at 56°C and 1 min extension at 72°C. Primers sets used were as follows: 5′-TTGGACCTCCGGGGAATGTG-3′ and 5′-GGCTGGAACAGACCAAGTGG-3′ for m5α-R2, 5′-CATCAAGCAGCCAGGAGCCAGTCTG-3′ and 5′-GCACGGTAGCTGGCGAGCACACTG-3′ for mouse HDC, 5′-TGGAATCCTGTGGCATCCATGAAACT-3′ and 5′-TAAAACGCAGCTCAGTAACTGTCCG-3′ for mouse β-actin.
Primer extension analysis
Poly(A)+ RNA was isolated from total RNA by oligo(dT) affinity chromatography and 1 µg mixed with 2 pmol 5′-32P-labeled primer corresponding to nucleotides 55 bp downstream from the translational start site (5′-TGGCCAATGTGGCGCTACCT-3′). Reactions were denatured by heating at 60°C for 60 min, then annealed at room temperature for 90 min. Primer extension proceeded at 37°C for 60 min with 15 U/µl of SuperScript II reverse transcriptase (Gibco BRL), 1× first strand buffer, 75 mM KCl, 3 mM MgCl2, 10 mM DTT and 0.4 mM dNTPs. After ethanol precipitation, products were analyzed on 8% acrylamide–7 M urea sequence gels. The subcloned 5′-flanking region was sequenced using the same primer using the Sequencing PRO DNA Sequencing Kit (Toyobo) and electrophoresed on the same gel (38).
Electrophoretic mobility shift assay (EMSA)
Nuclear extracts were prepared from COS-1 cells transfected with the expression plasmids. Reaction mixtures in the absence or presence of cold consensus PRE/ARE were incubated for 30 min on ice in binding buffer (5 mM Tris–HCl pH 8.0, 40 mM KCl, 6% glycerol, 1 mM DTT, 0.05% Nonidet P-40), and 2 µg of poly(deoxyinosinic-deoxycytidylic) acid in a final volume of 20 µl. Double-stranded consensus PRE/ARE (5′-ggggtaccAGAACATCCTGTTCTggtacccc-3′) and m5α-R2 PRE/ARE (5′-catgtgaGGGACAAACTTTTCTccaaggct-3′) DNA fragments were end-labeled using [γ-32P]ATP and T4 polynucleotide kinase and used as probes. PRE/ARE DNA fragments were added to the mixtures, and further incubated for 20 min at room temperature. Entire reaction mixtures (20 µl) were then loaded onto 4.5% polyacrylamide gels in 0.25× TAE buffer and electrophoresed at 4°C. The gels were dried on filter paper and exposed to X-ray film (39,40).
RESULTS
cDNA cloning of mouse 5α-reductase type II (5α-R2)
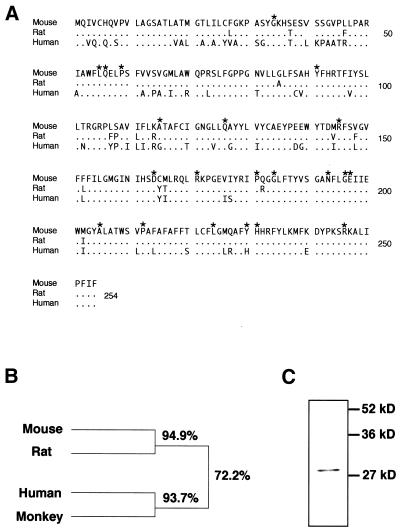
m5α-R2 cDNA was isolated by PCR amplification using degenerate primers based on homologous sequences between human and rat 5α-R2 cDNAs. The isolated cDNA (765 bp) contained an ORF that encoded a putative 254 amino acid protein that showed high similarity to rat (94.9%) and human (75.7%) 5α-R2 sequences (Fig. 1A and B) (1). Amino acids critical for enzymatic function assumed from human genetic diseases were mostly identical to those mouse and rat sequences (indicated by asterisks, Fig. 1A) (5,34). The cloned m5α-R2 cDNA was in vitro translated resulting in a 29 kDa protein (Fig. 1C), in agreement with the predicted molecular size.
Figure 1.
Molecular cloning of m5α-R2 cDNA. (A) Predicted amino acid sequence comparison of 5α-R2 protein between mouse and other species. Asterisks indicate the point mutation sites found in human 5α-R2 deficiency disease (34). (B) Homology between the mouse, rat, human and monkey 5α-R2 proteins (2). (C) In vitro-translated m5α-R2 protein. m5α-R2 protein in vitro translated in the presence of 35S-labeled methionine using the reticulocyte lysate system (Promega) was analyzed by 10% SDS–PAGE.
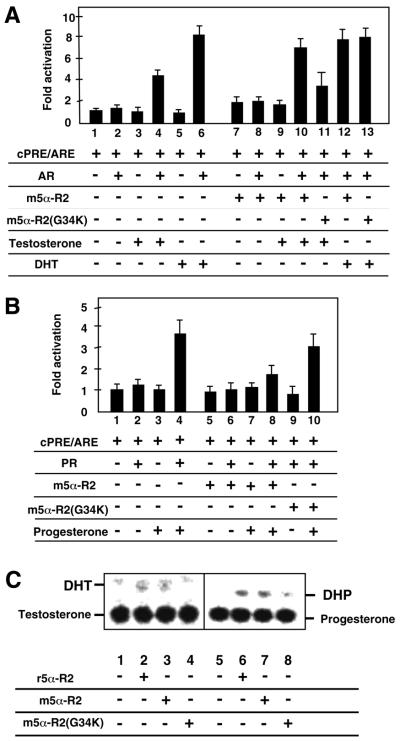
We then tested the enzymatic activity of the recombinant m5α-R2 using a nuclear receptor-mediated transactivation system (35). This system is based on the principle that the transactivation function of a nuclear receptor is initiated only by its endogenous ligand, and not by ligand precursors or metabolites (16). In the present study, the ability of m5α-R2 to metabolize androgen and progesterone was assessed with respect to the ligand-induced transactivation functions of AR and PR. While AR transactivation function was clearly induced by DHT, only a slight induction by testosterone was seen (Fig. 2A). The expression of m5α-R2 led to a 2-fold increase in testosterone-induced AR transactivation function (Fig. 2A, compare lanes 4 and 10). However, a putative inactivation mutant of m5α-R2 (G34R) failed to potentiate testosterone-induced AR transactivation function (34). Thus, our results suggested that the enzymatic conversion of testosterone into DHT was mediated by m5α-R2. Unlike the enhanced transactivation of testosterone-bound AR by m5α-R2, the progesterone-induced transactivation function of PR was reduced by the presence of m5α-R2 (Fig. 2B, compare lanes 4 and 8). This was due to the metabolic inactivation of progesterone into DHP, as mutant 5α-R2 (G34R) did not exhibit this effect. Moreover, we performed the in vitro converting assay (36) to confirm the 5α-R2 enzyme activity (Fig. 2C). The conversions of testosterone into DHT and progesterone into DHP by 5α-R2 were detected with equal efficiency by the rat 5α-R2 enzyme (Fig. 2C, compare lanes 2 and 3, or lanes 6 and 7). Thus, the cloned m5α-R2 appeared to possess the expected activities in steroid metabolism.
Figure 2.
Enzyme activity of m5α-R2 transiently transfected in COS-1 cells. Conversion of testosterone, serving as an AR ligand, into DHT, a potent ligand for AR (A); and progesterone, serving as a PR ligand, into the inactive ligand DHP (B) by m5α-R2. COS-1 cells were co-transfected with the luciferase reporter plasmid containing two copies of consensus PRE/ARE (cPRE/ARE), and the expression plasmids containing AR, PR, m5α-R2 or m5α-R2 mutant (G34R), with or without the indicated ligands. Human 5α-R2 mutant G34R results in reduced enzyme activity (34). Fold luciferase activities corresponding to means ± SEM for three independent experiments are shown. In vitro converting method (C) was described in the Materials and Methods (36). The conversions of testosterone into DHT and progesterone into DHP by 5α-R2 are shown in anes 1–4 or 5–8, respectively. r5α-R2, rat 5α-R2 (4).
Progesterone-induced 5α-R2 gene expression in the hippocampus of female brains
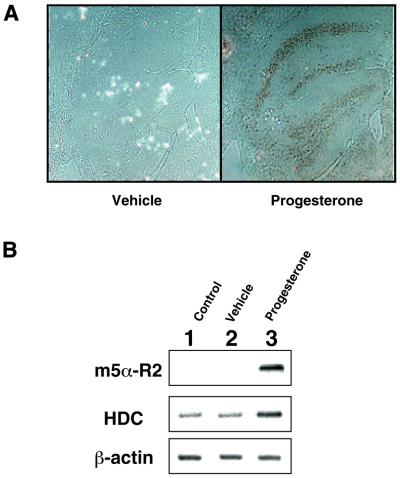
As m5α-R2 is thought to metabolize steroids into neurosteroids in the brain, the expression of 5α-R2 was examined in male and female mouse brains. No expression was detected in female brains by in situ hybridization. However, 4 h after the administration of progesterone (500 µg per mouse), clear m5α-R2 mRNA expression was detected in the hippocampus (Fig. 3A). RT–PCR (Fig. 3B) and northern blotting analyses confirmed the induction of the m5α-R2 gene in female brains by progesterone, while androgen induced the m5α-R2 gene in the male brain (data not shown) as expected from previous reports (9).
Figure 3.
Induction of m5α-R2 gene expression in adult female mice brains by the administration of progesterone. m5α-R2 gene expression was detected by in situ hybridization (A), and semi-quantitative RT–PCR (B) in the brains of female adult mice to which 500 µg of progesterone had been administrated 4 h previously. (A) m5α-R2 gene expression induced by progesterone was detected in the hippocampus. (B) Bands were obtained by semi-quantitative RT–PCR on total RNA extracted from individual mouse brains with or without the administration of progesterone. HDC was used as a positive control for progesterone administration (37). m5α-R2 gene expression was induced by progesterone in the female adult brain.
Isolation of the 5′-flanking region of the m5α-R2 gene and identification of the transcription initiation site
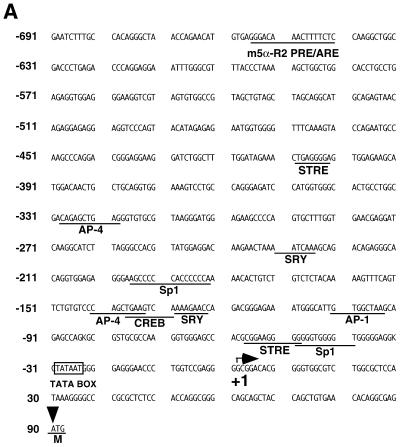
To address whether m5α-R2 gene induction in female brains by progesterone involved transcriptional or post-transcriptional mechanisms, we directly assessed the promoter function of the m5α-R2 gene. We first isolated the 5′-flanking region of the 5α-R2 by inverse-PCR using primers that corresponded to the exon 1 sequence, and a genomic fragment 780 bp upstream of the putative translation start site (ATG codon) was cloned (Fig. 4A) (41). Using this cloned genomic fragment, the transcription start site was determined by the primer extension method (38). Results indicated that the start site was located 89 bp upstream of the ORF, with a TATA box 30 bp upstream (Fig. 4A and B). A sequence closely related to the consensus PRE/ARE sequence (designated as m5α-R2 PRE/ARE hereafter) was present 657 bp upstream of the transcription start site, indicating possible regulation by progesterone and androgen via this element.
Figure 4.
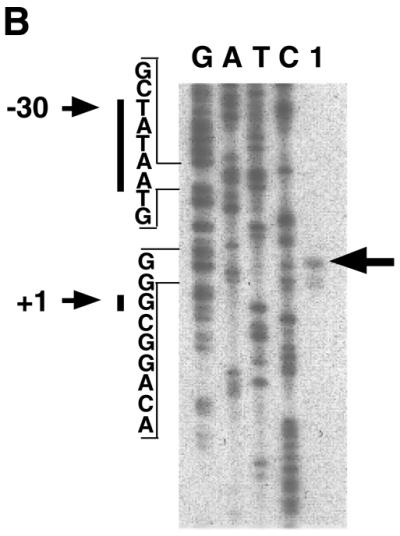
Promoter analysis of the m5α-R2 gene. (A) Nucleotide sequence of the 5′-flanking region of m5α-R2. Transcription start site is designated as +1, and putative cis regulatory elements are underlined. Translational start codon is indicated by closed triangle (+90). STRE, stress-response element; AP-4, activator protein 4; SRY, sex-determining region Y gene product; Sp1, stimulating protein 1; CREB, cAMP-responsive element binding protein; AP-1, activator protein 1; M, initiation codon. (B) Identification of the transcription start site of the m5α-R2 gene. Primer extension analysis of mouse kidney 5α-R2 mRNA was performed using 1 µg poly(A)+ RNA (lane 1) using a labeled primer. Primer extension products were co-electrophoresed with sequencing reaction products using the same primer to determine the precise start site. The major transcription start site (+1) is indicated by an arrow on the right, and the position of the TATA box (–30) is indicated by an arrow on the left.
Identification of a progesterone/audrogen response element (PRE/ARE) in the m5α-R2 gene promoter
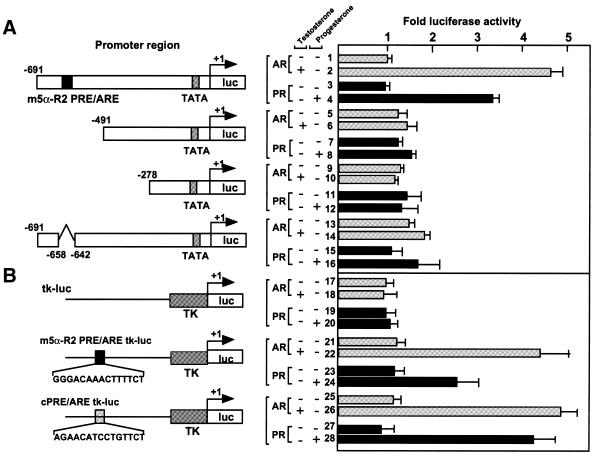
We then tested the function of the PRE/ARE-like sequence of m5α-R2 using a transient expression assay with expression vectors for PR and AR in COS-1 cells, and luciferase reporter plasmids bearing a series of the deletion mutants of the m5α-R2 gene promoter (Fig. 4A). Promoters with the intact m5α-R2 PRE/ARE conferred both androgen- and progesterone-specific responses in the presence of cognate receptor. However, deletion of m5α-R2 PRE/ARE caused complete loss of responsiveness to progesterone and androgen without reduction of basal m5α-R2 gene promoter activity (Fig. 5A). Like consensus PRE/ARE, m5α-R2 PRE/ARE conferred responsiveness to both hormones even when driven by the basal thymidine kinase (tk) promoter (Fig. 5B).
Figure 5.
Identification of the positive regulatory region for progesterone or testosterone in the m5α-R2 gene promoter. COS-1 cells were transiently co-transfected with the luciferase reporter plasmid containing the indicated promoter regions of the m5α-R2 gene (A), putative m5α-R2 PRE/ARE in the promoter region, or cPRE/ARE (B), and AR or PR expression plasmids with 10 nM testosterone or progesterone, respectively. Fold increases in luciferase activity are based on each of the activities without ligand. Fold luciferase activities corresponding to means ± SEM for three independent experiments are shown.
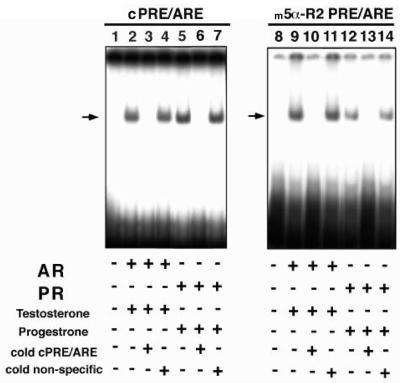
We then examined whether AR or PR homodimers directly bound to the identified m5α-R2 PRE/ARE. An EMSA was performed using the consensus PRE/ARE prepared for the transient expression assay (Fig. 5B) as a positive control. As shown in Figure 6, both AR and PR effectively bound both consensus and m5α-R2 PRE/ARE sequences (Fig. 6, lanes 2 and 5; 9 and 12). Moreover, in the presence of excess cold consensus PRE/ARE DNA, AR and PR binding was abolished (Fig. 6, lanes 3 and 6; 10 and 13). Thus, taken together, our results suggested that both hormone-bound steroid receptors controlled m5α-R2 gene transcription through m5α-R2 PRE/ARE.
Figure 6.
m5α-R2 PRE/ARE binding by AR and PR. EMSA was performed using [γ-32P]ATP-labeled consensus PRE/ARE (lanes 1–7) or m5α-R2 PRE/ARE (lanes 8–14). COS-1 cells were transfected with AR (lanes 2, 3, 4, 9, 10 and 11) or PR (lanes 5, 6, 7, 12, 13 and 14) expression plasmid, and nuclear extracts were assayed. After incubation at room temperature with the indicated labeled probes, the nuclear extracts were electrophoresed on 4.5% polyacrylamide gels under non-denaturing conditions. Arrows indicate bound AR or PR. Cold consensus PRE/ARE and cold non-specific indicate non-labeled probes used for competition assay (lanes 3, 6, 10 and 13) and negative control (lanes 4, 7, 11 and 14), respectively.
DISCUSSION
To clarify the physiological roles of 5α-R2 in the activity of hormonal and neuroactive steroids in the brain, we studied the possible regulation of m5α-R2 gene expression by one of its enzymatic substrates, progesterone. We first cloned the m5α-R2 cDNA by degenerate PCR, and found that recombinant m5α-R2 protein expressed in cultured cells metabolized progesterone and androgen as shown using a nuclear receptor-mediated assay (35). Using the cDNA clone as an in situ hybridization probe, we found that m5α-R2 gene expression was undetectable in the female brain, but was induced up to detectable levels in the hippocampus of female mice by progesterone. A progesterone regulatory element was identified as a highly related sequence to the consensus PRE/ARE sequence in the m5α-R2 promoter, and this element, m5α-R2 PRE/ARE, was found to confer responsiveness to both progesterone and androgen, similar to consensus PRE/ARE. Thus, transcriptional control of m5α-R2 gene expression by progesterone and androgen appeared to be mediated through m5α-R2 PRE/ARE.
Neuroactive steroids, like AP, are thought to exert behavioral effects such as convulsant and anxiolytic-like actions through the modulation of neurotransmitter-gated iron channel functions through direct binding of their allosteric sites (43). The effects of neuroactive steroids, especially progesterone derivatives, appear to be more prominent in females with premenstrual syndrome (PMS) (18) or under transient stress (44). Lowered serum progesterone levels may lead to PMS due to insufficient production of neuroactive steroids in the female brain. In contrast, enhanced production of progesterone during pregnancy is thought to promote the production of neuroactive steroids. As 5α-R2 converts progesterone into DHP, the precursor of AP, 5α-R2 enzymatic activity in the female brain is likely to be physiologically important for the action of neuroactive steroids. The significant induction of m5α-R2 by progesterone in female brains was detected only in the hippocampus. Therefore, the physiological role of 5α-R2 in the hippocampus needs to be verified by specific disruption of the 5α-R2 gene in the hippocampus in further animal experiments.
The promoter of the m5α-R2 gene harbored several potential binding sites for distinct classes of transcription regulatory factors in addition to PR/AR. Among these, the presence of two SRY binding sites raises the possibility that m5α-R2 gene expression is induced to convert testosterone into the more active form DHT during embryogenesis to develop male reproductive organs, when SRY plays a critical role in sex determination (45). Moreover, due to the identification of a PRE/ARE in the m5α-R2 gene promoter presented in this study, it is possible that positive feedback regulation of m5α-R2 gene expression by androgen occurs during male reproductive organ development and may occur in adult males to maintain reproductive organ function. Indeed, genetic mutations in 5α-R2 that impair its enzymatic activity are known to cause the hereditary disease male pseudohermaphroditism (5,8), clearly indicating the indispensability of this enzyme in males. However, the physiological role of 5α-R2 in females remains to be examined in more detail during embryogenesis and in adulthood.
Acknowledgments
ACKNOWLEDGEMENTS
We thank S. Kitanaka, T. Yoshizawa, T. Kawano, Y. Yamamoto, Y. Kodera, M. Suzawa and T. Matsumoto for helpful technical advice, and J. Yanagisawa, S. Sasagawa, T. Watanabe and T. Hashimoto for valuable discussions and technical assistance.
DDBJ/EMBL/GenBank accession nos AB049455 and AB049456
REFERENCES
- 1.Andersson S., Berman,D.M., Jenkins,E.P. and Russell,D.W. (1991) Deletion of steroid 5 alpha-reductase 2 gene in male pseudohermaphroditism. Nature, 354, 159–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levy M.A., Brandt,M., Sheedy,K.M., Holt,D.A., Heaslip,J.I., Trill,J.J., Ryan,P.J., Morris,R.A., Garrison,L.M. and Bergsma,D.J. (1995) Cloning, expression and functional characterization of type 1 and type 2 steroid 5 alpha-reductases from Cynomolgus monkey: comparisons with human and rat isoenzymes. J. Steroid Biochem. Mol. Biol., 52, 307–319. [DOI] [PubMed] [Google Scholar]
- 3.Poletti A., Coscarella,A., Negri,C.P., Colciago,A., Celotti,F. and Martini,L. (1998) 5 Alpha-reductase isozymes in the central nervous system. Steroids, 63, 246–251. [DOI] [PubMed] [Google Scholar]
- 4.Normington K. and Russell,D.W. (1992) Tissue distribution and kinetic characteristics of rat steroid 5 alpha-reductase isozymes. Evidence for distinct physiological functions. J. Biol. Chem., 267, 19548–19554. [PubMed] [Google Scholar]
- 5.Wilson J.D., Griffin,J.E. and Russell,D.W. (1993) Steroid 5 alpha-reductase 2 deficiency. Endocrine Rev., 14, 577–593. [DOI] [PubMed] [Google Scholar]
- 6.Russell D.W. and Wilson,J.D. (1994) Steroid 5 alpha-reductase: two genes/two enzymes. Annu. Rev. Biochem., 63, 25–61. [DOI] [PubMed] [Google Scholar]
- 7.Mahendroo M.S., Cala,K.M. and Russell,D.W. (1996) 5 Alpha-reduced androgens play a key role in murine parturition. Mol. Endocrinol., 10, 380–392. [DOI] [PubMed] [Google Scholar]
- 8.Andersson S. and Russell,D.W. (1990) Structural and biochemical properties of cloned and expressed human and rat steroid 5 alpha-reductases. Proc. Natl Acad. Sci. USA, 87, 3640–3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poletti A., Negri,C.P., Rabuffetti,M., Colciago,A., Celotti,F. and Martini,L. (1998) Transient expression of the 5alpha-reductase type 2 isozyme in the rat brain in late fetal and early postnatal life. Endocrinology, 139, 2171–2178. [DOI] [PubMed] [Google Scholar]
- 10.Celotti F., Negri,C.P. and Poletti,A. (1997) Steroid metabolism in the mammalian brain: 5alpha-reduction and aromatization. Brain Res. Bullet., 44, 365–375. [DOI] [PubMed] [Google Scholar]
- 11.Guidotti A. and Costa,E. (1998) Can the antidysphoric and anxiolytic profiles of selective serotonin reuptake inhibitors be related to their ability to increase brain 3 alpha, 5 alpha-tetrahydroprogesterone (allopregnanolone) availability? Biol. Psychiatry, 44, 865–873. [DOI] [PubMed] [Google Scholar]
- 12.Mendelson W.B., Martin,J.V., Perlis,M., Wagner,R., Majewska,M.D. and Paul,S.M. (1987) Sleep induction by an adrenal steroid in the rat. Psychopharmacology (Berl.), 93, 226–229. [DOI] [PubMed] [Google Scholar]
- 13.Paul S.M. and Purdy,R.H. (1992) Neuroactive steroids. FASEB J., 6, 2311–2322. [PubMed] [Google Scholar]
- 14.Majewska M.D., Harrison,N.L., Schwartz,R.D., Barker,J.L. and Paul,S.M. (1986) Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science, 232, 1004–1007. [DOI] [PubMed] [Google Scholar]
- 15.Harrison N.L., Majewska,M.D., Harrington,J.W. and Barker,J.L. (1987) Structure-activity relationships for steroid interaction with the gamma-aminobutyric acid A receptor complex. J. Pharmacol. Exp. Ther., 241, 346–353. [PubMed] [Google Scholar]
- 16.Rupprecht R., Reul,J.M., Trapp,T., van,S.B., Wetzel,C., Damm,K., Zieglgansberger,W. and Holsboer,F. (1993) Progesterone receptor-mediated effects of neuroactive steroids. Neuron, 11, 523–530. [DOI] [PubMed] [Google Scholar]
- 17.Concas A., Mostallino,M.C., Porcu,P., Follesa,P., Barbaccia,M.L., Trabucchi,M., Purdy,R.H., Grisenti,P. and Biggio,G. (1998) Role of brain allopregnanolone in the plasticity of gamma-aminobutyric acid type A receptor in rat brain during pregnancy and after delivery. Proc. Natl Acad. Sci. USA, 95, 13284–13289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith S.S., Gong,Q.H., Hsu,F.C., Markowitz,R.S., French,M.J. and Li,X. (1998) GABA(A) receptor alpha4 subunit suppression prevents withdrawal properties of an endogenous steroid. Nature, 392, 926–930. [DOI] [PubMed] [Google Scholar]
- 19.Concas A., Follesa,P., Barbaccia,M.L., Purdy,R.H. and Biggio,G. (1999) Physiological modulation of GABA(A) receptor plasticity by progesterone metabolites. Eur. J. Pharmacol., 375, 225–235. [DOI] [PubMed] [Google Scholar]
- 20.Fujii E. and Mellon,S.H. (2001) Regulation of uterine gamma-aminobutyric acid(A) receptor subunit expression throughout pregnancy. Endocrinology, 142, 1770–1777. [DOI] [PubMed] [Google Scholar]
- 21.Beato M., Herrlich,P. and Schutz,G. (1995) Steroid hormone receptors: many actors in search of a plot. Cell, 83, 851–857. [DOI] [PubMed] [Google Scholar]
- 22.Mangelsdorf D.J., Thummel,C., Beato,M., Herrlich,P., Schutz,G., Umesono,K., Blumberg,B., Kastner,P., Mark,M., Chambon,P. et al. (1995) The nuclear receptor superfamily: the second decade. Cell, 83, 835–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chambon P. (1996) A decade of molecular biology of retinoic acid receptors. FASEB J., 10, 940–954. [PubMed] [Google Scholar]
- 24.Yamamoto A., Hashimoto,Y., Kohri,K., Ogata,E., Kato,S., Ikeda,K. and Nakanishi,M. (2000) Cyclin E as a coactivator of the androgen receptor. J. Cell Biol., 150, 873–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hovland A.R., Powell,R.L., Takimoto,G.S., Tung,L. and Horwitz,K.B. (1998) An N-terminal inhibitory function, IF, suppresses transcription by the A-isoform but not the B-isoform of human progesterone receptors. J. Biol. Chem., 273, 5455–5460. [DOI] [PubMed] [Google Scholar]
- 26.Glass C.K. (1994) Differential recognition of target genes by nuclear receptor monomers, dimers and heterodimers. Endocrinol. Rev., 15, 391–407. [DOI] [PubMed] [Google Scholar]
- 27.Yanagisawa J., Yanagi,Y., Masuhiro,Y., Suzawa,M., Watanabe,M., Kashiwagi,K., Toriyabe,T., Kawabata,M., Miyazono,K. and Kato,S. (1999) Convergence of transforming growth factor-beta and vitamin D signaling pathways on SMAD transcriptional coactivators. Science, 283, 1317–1321. [DOI] [PubMed] [Google Scholar]
- 28.Watanabe M., Yanagisawa,J., Kitagawa,H., Takeyama,K., Ogawa,S., Arao,Y., Suzawa,M., Kobayashi,Y., Yano,T., Yoshikawa,H., Masuhiro,Y. and Kato,S. (2001) A subfamily of RNA-binding DEAD-box proteins acts as an estrogen receptor alpha coactivator through the N-terminal activation domain (AF-1) with an RNA coactivator, SRA. EMBO J., 20, 1341–1352. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Khorasanizadeh S. and Rastinejad,F. (2001) Nuclear-receptor interactions on DNA-response elements. Trends Biochem. Sci., 26, 384–390. [DOI] [PubMed] [Google Scholar]
- 30.Tan J.A., Marschke,K.B., Ho,K.C., Perry,S.T., Wilson,E.M. and French,F.S. (1992) Response elements of the androgen-regulated C3 gene. J. Biol. Chem., 267, 4456–4466. [PubMed] [Google Scholar]
- 31.Lydon J.P., DeMayo,F.J., Funk,C.R., Mani,S.K., Hughes,A.R., Montgomery,C.J., Shyamala,G., Conneely,O.M. and O’Malley,B.W. (1995) Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev., 9, 2266–2278. [DOI] [PubMed] [Google Scholar]
- 32.Schoenmakers E., Verrijdt,G., Peeters,B., Verhoeven,G., Rombauts,W. and Claessens,F. (2000) Differences in DNA binding characteristics of the androgen and glucocorticoid receptors can determine hormone-specific responses. J. Biol. Chem., 275, 12290–12297. [DOI] [PubMed] [Google Scholar]
- 33.Claessens F., Verrijdt,G., Schoenmakers,E., Haelens,A., Peeters,B., Verhoeven,G. and Rombauts,W. (2001) Selective DNA binding by the androgen receptor as a mechanism for hormone-specific gene regulation. J. Steroid Biochem. Mol. Biol., 76, 23–30. [DOI] [PubMed] [Google Scholar]
- 34.Wigley W.C., Prihoda,J.S., Mowszowicz,I., Mendonca,B.B., New,M.I., Wilson,J.D. and Russell,D.W. (1994) Natural mutagenesis study of the human steroid 5 alpha-reductase 2 isozyme. Biochemistry, 33, 1265–1270. [DOI] [PubMed] [Google Scholar]
- 35.Takeyama K., Kitanaka,S., Sato,T., Kobori,M., Yanagisawa,J. and Kato,S. (1997) 25-Hydroxyvitamin D3 1alpha-hydroxylase and vitamin D synthesis. Science, 277, 1827–1830. [DOI] [PubMed] [Google Scholar]
- 36.Mahendroo M.S., Cala,K.M., Hess,D.L. and Russell,D.W. (2001) Unexpected virilization in male mice lacking steroid 5 alpha-reductase enzymes. Endocrinology, 142, 4652–4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mulac J.B., Mullinax,R.A., DeMayo,F.J., Lydon,J.P. and Conneely,O.M. (2000) Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science, 289, 1751–1754. [DOI] [PubMed] [Google Scholar]
- 38.Murayama A., Takeyama,K., Kitanaka,S., Kodera,Y., Hosoya,T. and Kato,S. (1998) The promoter of the human 25-hydroxyvitamin D3 1 alpha-hydroxylase gene confers positive and negative responsiveness to PTH, calcitonin and 1 alpha,25(OH)2D3. Biochem. Biophys. Res. Commun., 249, 11–16. [DOI] [PubMed] [Google Scholar]
- 39.Kodera Y., Takeyama,K., Murayama,A., Suzawa,M., Masuhiro,Y. and Kato,S. (2001) Ligand type-specific interactions of peroxisome proliferator-activated receptor gamma with transcriptional coactivators. J. Biol. Chem., 275, 33201–33204. [DOI] [PubMed] [Google Scholar]
- 40.Shen R., Sumitomo,M., Dai,J., Hardy,D.O., Navarro,D., Usmani,B., Papandreou,C.N., Hersh,L.B., Shipp,M.A., Freedman,L.P. and Nanus,D.M. (2000) Identification and characterization of two androgen response regions in the human neutral endopeptidase gene. Mol. Cell. Endocrinol., 170, 131–142. [DOI] [PubMed] [Google Scholar]
- 41.Matsui D. (2001) GenBank accession no. AB049456.
- 42.Matsui D. (2001) GenBank accession no. AB049455.
- 43.Rupprecht R. and Holsboer,F. (1999) Neuroactive steroids: mechanisms of action and neuropsychopharmacological perspectives. Trends Neurosci., 22, 410–416. [DOI] [PubMed] [Google Scholar]
- 44.Purdy R.H., Morrow,A.L., Moore,P.J. and Paul,S.M. (1991) Stress-induced elevations of gamma-aminobutyric acid type A receptor-active steroids in the rat brain. Proc. Natl Acad. Sci. USA, 88, 4553–4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Swain A. and Lovell,B.R. (1999) Mammalian sex determination: a molecular drama. Genes Dev., 13, 755–767. [DOI] [PubMed] [Google Scholar]