Abstract
Heat-induced formation of 8-oxoguanine was demonstrated in DNA solutions in 10–3 M phosphate buffer, pH 6.8, by enzyme-linked immunosorbent assays using monoclonal antibodies against 8-oxoguanine. A radiation-chemical yield of 3.7 × 10–2 µmol J–1 for 8-oxoguanine production in DNA upon γ-irradiation was used as an adequate standard for quantitation of 8-oxoguanine in whole DNA. The initial yield of heat-induced 8-oxoguanine exhibits first order kinetics. The rate constants for 8-oxoguanine formation were determined at elevated temperatures; the activation energy was found to be 27 ± 2 kcal/mol. Extrapolation to 37°C gave a value of k37 = 4.7 × 10–10 s–1. Heat-induced 8-oxoguanine formation and depurination of guanine and adenine show similarities of the processes, which implies that heat-mediated generation of reactive oxygen species (ROS) should occur. Heat-induced production of H2O2 in phosphate buffer was shown. The sequence of reactions of thermally mediated ROS formation have been established: activation of dissolved oxygen to the singlet state, generation of superoxide radicals and their dismutation to H2O2. Gas saturation (O2, N2 and Ar), D2O, scavengers of 1O2, O2–• and OH• radicals and metal chelators influenced heat-induced 8-oxoguanine formation as they affected thermal ROS generation. These findings imply that heat acts via ROS attack leading to oxidative damage to DNA.
INTRODUCTION
Reactive oxygen species (ROS) generated by the action of ionizing radiation, chemical mutagens and carcinogens, as well as during normal cellular aerobic metabolism and during inflammation, are among the most important environmental genotoxic factors (1). ROS cause predominantly base damage in DNA, forming thymine glycols, 5-hydroxycytosine, 7,8-dihydro-8-oxoguanine (8-oxoguanine), 8-oxoadenine (8-hydroxyadenine), the formamidopyrimidine derivatives of purines, etc. Recent investigations have established that guanine is the main target for ROS in DNA, with 8-oxoguanine being the most frequent base lesion. Therefore, formation of 8-oxoguanine is an important biomarker of oxidative damage to DNA. 8-Oxoguanine exhibits ambiguous encoding properties in the biosynthesis of nucleic acids, behaving as guanine and thymine during replication (2–4) and as guanine and uracil during transcription (5,6), because it can pair with cytosine and mispair with adenine in the syn conformation. The miscoding properties of 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodG) have been implicated in such biological processes as mutagenesis (1,7–10), producing predominantly G→T transversions (2,3,7,9–11), carcinogenesis (12–16), aging (17,18) and some age-dependent diseases (11,16,19). This type of DNA damage is repaired by specific enzymes which excise 8-oxodG from DNA (20,21).
Damage to the primary structure of DNA by ROS is likely to be a major factor involved in mutagenesis, carcinogenesis and aging (22). To date, several heat-induced DNA lesions have been described: DNA strand breaks (23), hydrolysis of glycosyl bonds, predominantly with the release of purine bases (22,24,25), and deamination of cytosine (26,27). The disruption of glycosyl bonds leading to base release from the DNA and formation of apurinic and apyrimidinic sites is one of the best studied types of heat-induced genetic instability at the molecular level. In this work we demonstrate that heat causes formation of 8-oxoguanine in DNA and we compare this process with those of guanine and adenine depurination. Gas saturation (O2, N2 and Ar), D2O, scavengers of 1O2, O2–• and OH• radicals and metal chelators influence heat-induced 8-oxoguanine formation as they affect thermal ROS generation. These findings imply that heat acts via ROS attack leading to oxidative damage to DNA.
MATERIALS AND METHODS
Chemicals
8-OxodG was synthesized by Dr B. K. Chernov (Institute of Molecular Biology, Russian Academy of Sciences, Moscow) by his own technique and kindly provided for our study. The identity of 8-oxodG was proved by NMR and UV spectroscopy. The concentration of 8-oxodG was determined from the UV spectra using molar absorption coefficients of 12 300 M–1 cm–1 at 245 nm and 10 300 M–1 cm–1 at 293 nm (28). High molecular weight DNA from chicken erythrocytes (Reanal, Hungary) and salmon sperm (ICN, USA) was used. Solutions were prepared using Na2HPO4·12H2O, NaH2PO4·2H2O (analytical grade; Reakhim, Russia), and NaCl (extra purity grade; Soyuzkhlor, Russia). Bovine serum albumin (BSA), horseradish peroxidase, superoxide dismutase (SOD), catalase, DNase I, alkaline phosphatase, Tris–HCl, Tween-20, o-phenylenediamine hydrochloride and anti-mouse IgG–horseradish peroxidase conjugates were purchased from Sigma (USA), 4-iodophenol from Aldrich (USA) and 1,10-phenanthroline, Tiron (4,5-dihydroxy-1,3-benzene-disulfonic acid) and neocuproine (2,9-dimethyl-1,10-phenanthroline) from ICN (USA). We also used guanosine (Reanal, Hungary) and deuterium oxide (99.9%) (Izotop, Russia). Luminol (Chemical Reagent plant, Yerevan, Armenia) was additionally purified according to Hengen (29). The initial concentration of hydrogen peroxide was determined spectrophotometrically using a molar absorption coefficient of 43.6 M–1 cm–1 at 240 nm. Monoclonal antibodies specific for 8-oxoguanine were obtained using methods previously described in detail (30). The affinity constant determined by enzyme-linked immunosorbent assay (ELISA) was 1.3 × 106 M–1 and exceeded the binding constants of possible cross-structural analogs by more than three orders of magnitude. The initial concentration of monoclonal antibodies determined by the Lowry method was 2.85 × 10–4 M. Monoclonal antibodies in 0.05 M phosphate buffer, pH 7.4, were mixed with an equal volume of glycerol and stored at –20°C until used. With this mode of storage, the monoclonal antibodies were stable for at least 2 years.
N2 and Ar (99%) were used without further purification. O2 was obtained by thermal decomposition of KMnO4 and was purified with a purging water trap. Employment of medical oxygen (99.5%) in the following experiments produced identical effects.
Determination of DNA concentration
The concentration of DNA solutions was determined with a Specord UV-VIS spectrophotometer (Carl Zeiss Jena, Germany). A concentration of 50 µg/ml was taken as one unit of optical absorption of double-helical DNA at 260 nm with an optical path of 1 cm.
Determination of guanine depurination
High molecular weight DNA from chicken erythrocytes and salmon sperm in 10–3 M phosphate buffer, pH 6.8, with or without 0.14 M NaCl, at a concentration of 400 µg/ml (20 ml samples) was heated for various time periods ranging up to several days at temperatures from 60 to 90°C in a U10 ultrathermostat (Prufgerate-Werk Medingen, Germany). The heat-treated DNA was concentrated to 1.5 ml in a vacuum rotary evaporator and the products of glycosyl bond breakage were separated on a Toyopearl HW-40 (Toyosoda, Japan) column 90 cm in length and 1.2 cm in diameter. Elution was performed with bi-distilled water at a flow rate of 1 ml/min; the optical density was recorded at 254 nm on a UV-cord device. The eluents corresponding to the guanine and adenine peaks were concentrated in a vacuum rotary evaporator and the base concentration was measured spectrophotometrically using the available molar extinction values (31). Guanine was taken to be 20.5% and adenine 28.8% in chicken erythrocyte DNA and, on average, guanine was 21.4% and adenine 28.7% in salmon sperm DNA (32).
Determination of 8-oxoguanine in DNA
Competitive ELISA. DNA solutions (200 µg/ml in 10–3 M sodium phosphate buffer, pH 6.8) were heated in 0.5 ml plastic tubes with screw caps (QSP, USA) at various temperatures, denatured for 5 min in a boiling water bath and rapidly cooled to 0°C on ice. Denatured DNA samples (350 µl) were mixed with 36 µl of a solution containing 1 M Tris–HCl, pH 7.4, and 1 M phosphate-buffered saline (PBS). Then 68 µl of monoclonal antibodies in a BSA solution (20 µg/ml in 0.1 M PBS, pH 7.4) was added to the final concentration of 6 × 10–10 M. The mixture was incubated for 15 h at 20°C. Wells of stripped polystyrene immunoassay plates (Mosmedpolymer, Russia) were sensitized with an 8-oxoguanosine–casein conjugate solution, as described by Bruskov et al. (30). The sensitized wells were sequentially washed with 0.1 M PBS, pH 7.4, containing 0.05% Tween-20 and with the same buffer without Tween-20. The incubation mixture was applied to the wells (100 µl/well) in quadruplicate for each assay. The plates were incubated for 30 min at 20°C and washed with 0.1 M PBS, pH 7.4, containing 0.05% Tween-20. Then 100 µl of the anti-mouse IgG–peroxidase conjugate diluted 500 times in blocking solution (0.1 M PBS, pH 7.4, containing 5 mg/ml BSA and 0.05% Tween-20) was introduced into each well and incubated for 2 h at 37°C. After washing with 0.1 M PBS, pH 7.4, containing 0.05% Tween-20, the wells were filled with 100 µl of o-phenylenediamine hydrochloride solution (0.7 mg/ml in 0.1 M citrate–phosphate buffer, pH 5.3) with 6 mM hydrogen peroxide. Following 30 min incubation at room temperature, the reaction was stopped by adding 50 µl of 1 M H2SO4 to each well. The optical density at 490 nm was measured in the wells on an ELISA reader-photometer (Scientific Manufacturing Association Biopribor, Russia) with determination of mean values and standard deviations.
Non-competitive ELISA. The DNA samples were denatured in a water bath for 5 min and cooled on ice for 3–4 min. Aliquots of 40 µl (350 µg/ml) were transferred to the wells of immunoassay plates (Costar, USA). DNA was immobilized by a simple dry adsorption procedure (33) with incubation for 3 h at 80°C until the solution had evaporated.
The blocking of non-specific sites was performed using 300 µl of solution containing 1% skimmed milk powder in 0.15 M Tris–HCl buffer, pH 8.7, and 0.15 M NaCl. Then the plates were incubated for 3 h at 37°C. Antigen–antibody complexes (100 µl/well) of monoclonal antibodies against 8-oxoguanine (1:2000) were formed in the blocking solution at ambient temperature by incubation overnight (14–18 h). Two washes (300 µl/well) with solutions of 50 mM Tris–HCl buffer, pH 8.7, and 0.15 M NaCl with 0.1% Triton X-100 (v/v) (Koch-Light, UK) were performed after 20 min incubation. Next, a complex was formed with anti-mouse IgG–peroxidase conjugate (Sigma, USA) diluted 1:1000 in blocking solution (80 µl/well) by incubation for 1.5 h at 37°C. Then three washes were carried out as described above. The substrate [18.2 mM 2,2′-azino-bis(3-ethylbenz-thiazoline-6-sulfonic acid); Sigma, USA] and hydrogen peroxide (2.6 mM) in 75 mM sodium citrate buffer, pH 4.2, were added (100 µl/well). After green color development the reaction was stopped by addition of an equal volume of 1.5 mM NaN3 in 0.1 M sodium citrate buffer, pH 4.3. The optical density of the samples was measured with a microwell plate reader at 405 nm (Titertek Multiscan, Finland).
To avoid the influence of non-specific binding of monoclonal antibodies to DNA, 8-oxoguanine detection was performed at a constant DNA concentration.
Calibration procedures
The samples of DNA before irradiation were cooled in a refrigerator at 4°C to produce a standard set of conditions. DNA solutions were γ-irradiated at room temperature in a 137Cs source (27 TBq) with doses of 1, 2, 5, 10, 20 and 30 Gy, at a dose rate of 2.05 Gy/min, and used to derive a standard dose–response curve for determination of 8-oxoguanine in the heat-treated samples. Immediately after irradiation the DNA was heat denatured. Competitive or direct ELISA was used for determination of 8-oxoguanine. The heat-treated DNA was usually used without hydrolyzation but in some experiments with competitive ELISA it was hydrolyzed to nucleosides prior to determination of 8-oxoguanine. The irradiated and control DNA samples were hydrolyzed according to Dizdaroglu (34). The radiation-chemical yield value obtained from a standard curve was used to determine 8-oxodG. Initial calibration of the standard curve was derived by addition of definite quantities of authentic 8-oxodG to the hydrolyzate of non-irradiated DNA.
The concentrations of iron and copper ions in the sodium phosphate buffer were quantitated by atomic absorption spectroscopy in a M-303 spectrophotometer (Perkin-Elmer, USA).
Measurement of enhanced chemiluminescence
We used a sensitive method for quantitative measurement of hydrogen peroxide employing enhanced chemiluminescence in a peroxidase–luminol–p-iodophenol system. The reaction was quantified with a Beta-1 liquid scintillation counter (Manufacturing Association Medapparatura, Ukraine). The counter was used in single photon counting mode with one photomultiplier and the coincidence scheme disengaged. The efficiency of counting of the tritium standard in this regime was 34% at a background of 5000 c.p.m. Samples (10 ml) of 10–3 M phosphate buffer, pH 6.8, were heated for varying time intervals (up to 1 h) at temperatures ranging from 40 to 90°C in 20 ml glass liquid scintillation counter vials. The vials were screw capped and hermetically sealed with additional teflon gaskets. After heating, the vials were rapidly cooled to ambient temperature in an ice bath. To study the influence of SOD and catalase on heat-induced ROS formation, 4 × 10–5 U SOD (∼10–12 M) and 4 × 10–3 U catalase were added to each sample. For elucidation of the ‘oxygen effect’, phosphate buffer was bubbled with O2, N2 and Ar for 30 min prior to heating. Determination of hydrogen peroxide in buffer solutions with additional substances used standard curves with correction for quenching or enhancement of chemiluminescence by the substances.
Samples were placed in polyethylene vials (Beckman, USA) and 10 ml of ‘count solution’ containing 10–2 M Tris–HCl buffer, pH 8.5, 5 × 10–5 M 4-iodophenol, 5 × 10–5 M luminol and horseradish peroxidase (10–9 M for nanomolar hydrogen peroxide measurement) was added. The ‘count solution’ was prepared immediately prior to measurement. Usually, three independent samples were measured and the mean and standard deviation were determined.
RESULTS
Guanine depurination
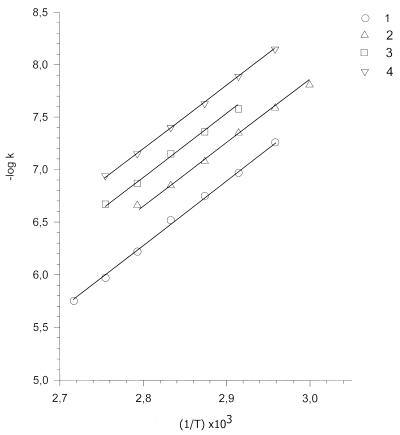
Figure 1 shows separation of the products of heat-induced DNA base release on a Toyopearl HW-40 column. The bases T, C, G and A were eluted following DNA elution. Depurination follows first order reaction kinetics. For short-term heating, the initial rate was linearly dependent on time t: B = B0 × kt, where B is the concentration of a base released due to breakage of glycosyl bonds, B0 is the concentration of this base in the DNA and k is the rate constant. The rate constants for guanine depurination at different temperatures determined at low ionic strength and in 0.14 M NaCl are presented in Table 1. The activation energy was calculated from Arrhenius plots as the slope of the linear dependence of –logk versus the inverse of the temperature: –logk = –logk0 + Ea/2.3RT, where Ea is the activation energy, R is the gas constant and T is the temperature in kelvins (Fig. 2). Heating induces mainly depurination of bases with an activation energy of 28 ± 2 kcal/mol. Guanine is released from DNA approximately 1.4 times faster than adenine, which agrees well with previously published data (24,25). This process depends strongly on the ionic strength of the solution. The rate constants decreased about 3.5 times in the presence of 0.14 M NaCl (Table 1 and Fig. 2).
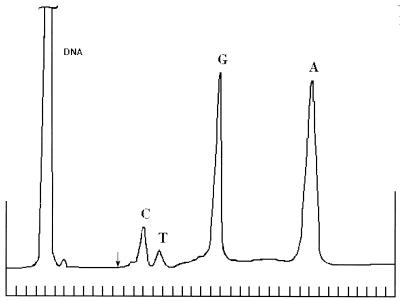
Figure 1.
Chromatographic separation of bases released from heat-treated DNA on a Toyopearl HW-40 column. DNA from chicken erythrocytes was dissolved at 355 µg/ml in 10–3 M phosphate buffer, pH 6.8, and heated for 22 h at 90°C. Abscissa, time (min); marks on the axis correspond to 12 min intervals. Ordinate, optical density determined at 254 nm. The arrow indicates a 20-fold increase in recording sensitivity.
Table 1. Rate constants for 8-oxoguanine formation and guanine depurination determined at different temperatures in DNA solutions in 10–3 M phosphate buffer, pH 6.8, with or without 0.14 M NaCl, and activation energies for these processes.
| Temperature (°C) | Rate constant k (s–1) | |||
|---|---|---|---|---|
| 8-Oxoguanine formation | Guanine depurination | |||
| Without NaCl | 0.14 M NaCl | Without NaCl | 0.14 M NaCl | |
| 60 | 9.8 × 10–9 | |||
| 65 | 1.67 × 10–8 | 4.6 × 10–9 | 5.5 × 10–8 | |
| 70 | 2.9 × 10–8 | 8.57 × 10–9 | 1.07 × 10–7 | 2.69 × 10–8 |
| 75 | 5.4 × 10–8 | 1.52 × 10–8 | 1.78 × 10–7 | 4.37 × 10–8 |
| 80 | 9.16 × 10–8 | 2.64 × 10–8 | 3.0 × 10–7 | 7.08 × 10–8 |
| 85 | 1.56 × 10–7 | 4.49 × 10–8 | 6.03 × 10–7 | 1.35 × 10–7 |
| 90 | 7.47 × 10–8 | 1.07 × 10–6 | 2.14 × 10–7 | |
| 95 | 1.78 × 10–6 | |||
| 37a | 4.7 × 10–10 | 1.3 × 10–10 | 1.3 × 10–9 | 3.7 × 10–10 |
| Ea (kcal/mol) | 27 ± 2 | 27 ± 2 | 28 ± 2 | 27 ± 2 |
Rate constants for 8-oxoguanine formation obtained by competitive ELISA.
aRate constants at 37°C were derived from Arrhenius plots (Fig. 2) by extrapolation of experimental data obtained at elevated temperatures.
Figure 2.
Arrhenius plots of –logk versus inverse temperature 1/T (k is the rate constant, s–1; T is the temperature, K). 1, Guanine depurination in 10–3 M phosphate buffer, pH 6.8; 2, 8-oxoguanine formation in 10–3 M phosphate buffer, pH 6.8; 3, guanine depurination in 10–3 M phosphate buffer, pH 6.8, with 0.14 M NaCl; 4, 8-oxoguanine formation in 10–3 M phosphate buffer, pH 6.8, with 0.14 M NaCl.
Formation of 8-oxoguanine
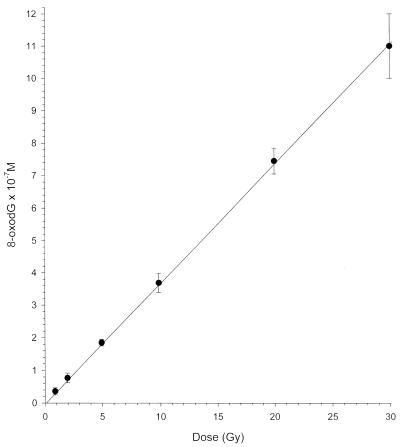
A dose–response curve for the formation of 8-oxodG in γ-irradiated DNA is shown in Figure 3. The amount of 8-oxoguanine formed in DNA upon γ-irradiation is linearly dependent on the dose. This allows the dose dependence of 8-oxodG formation in γ-irradiated DNA to be used as a convenient reference standard for determination of 8-oxoguanine. The radiation-chemical yield (G value) for 8-oxodG formation in DNA was 3.7 × 10–2 µmol J–1 (G = 0.37 molecules/100 eV absorbed radiation energy) (Fig. 3).
Figure 3.
Dose–yield relationship for the formation of 8-oxoguanine in γ-irradiated salmon sperm DNA obtained by competitive ELISA. Doses are 1, 2, 5, 10, 20 and 30 Gy. Background 8-oxoguanine level was subtracted from the observed data.
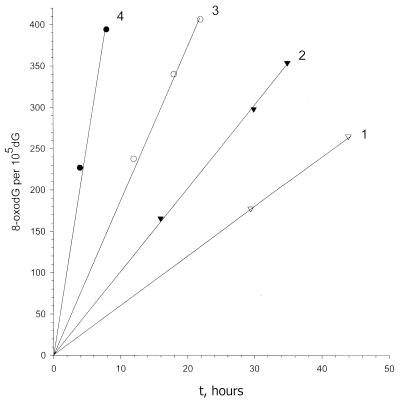
ELISA revealed that 8-oxoguanine is formed in DNA on heating. Its accumulation in DNA depends on temperature and duration of heating in a manner similar to that of depurination and the reaction obeys first order kinetics (Fig. 4). Table 1 and Figure 2 show the rate constants for 8-oxoguanine formation in 10–3 M phosphate buffer, pH 6.8, determined at different temperatures with or without 0.14 M NaCl. The activation energy for this process was 27 ± 2 kcal/mol. Extrapolation of the rate constants for 8-oxoguanine formation to 37°C gives a value for k37 of 4.7 × 10–10 s–1. Phosphate buffer with 0.14 M NaCl decreased the rate constants by about 3.5 times (k37 = 1.3 × 10–10 s–1). Raising the temperature by only 1°C increases the rate constants of 8-oxoguanine formation by ∼35%. The rate constants for guanine depurination are about 3 and 3.5 times higher than those for 8-oxoguanine formation in phosphate buffer with and without 0.14 M NaCl, respectively.
Figure 4.
Kinetics of 8-oxoguanine formation in salmon sperm DNA on heating at different temperatures obtained by competitive ELISA: 1, 65°C; 2, 70°C; 3, 75°C; 4, 85°C. Background 8-oxoguanine level was subtracted from the observed data.
The quantitative assessment of 8-oxodG by direct immunoblot assay of unhydrolyzed DNA has been reported earlier (35). In the present work we also used unhydrolyzed DNA to study heat-induced formation of 8-oxoguanine, having calibrated its content with γ-irradiated DNA (36). Heat-induced DNA depurination had an insignificant effect on the quantity of 8-oxoguanine determined by ELISA analysis. The same amounts of 8-oxodG were determined in the DNA both before and after enzymatic hydrolysis to nucleosides when the influence of DNA depurination was negated. Formation of 8-oxoguanine, a biomarker of oxidative damage to DNA, upon heating of DNA samples implies that heat-induced generation of ROS takes place.
Heat-induced formation of hydrogen peroxide
To detect the heat-induced ROS, a sensitive method of quantitative measurement of hydrogen peroxide has been developed employing enhanced chemiluminescence in a peroxidase–luminol–p-iodophenol system. A liquid scintillation counter for β-radiation measurement was used for the quantitative estimation of chemiluminescence. The sensitivity of the method permits the detection of <1 nM hydrogen peroxide (<10 pmol H2O2 in a sample). Figure 5 shows a typical standard curve for determination of hydrogen peroxide at nanomolar concentrations. Within this range, a linear relationship between chemiluminescence intensity and hydrogen peroxide concentration was observed. The linear dependence of chemiluminescence on hydrogen peroxide concentration can be achieved in the millimolar to nanomolar concentration range by changing the horseradish peroxidase concentration. Using this method, we observed heat-induced formation of hydrogen peroxide in phosphate buffer. Our results confirm the presence of an ‘oxygen effect’: an influence of dissolved oxygen concentration on H2O2 production upon heating. The level of H2O2 rises in oxygen-saturated phosphate buffer solution by a factor of 1.8 and decreases 2-fold in nitrogen-purged solution and more than 2-fold after argon bubbling (Table 2). Addition of 0.14 M NaCl decreases heat-induced H2O2 formation in phosphate buffer by about 3-fold (Table 2). The pronounced enhancing effect of deuterated phosphate buffer as well as inhibition of the process by the singlet oxygen traps sodium azide and guanosine point to 1O2 as the precursor species that is responsible for H2O2 production (Table 2).
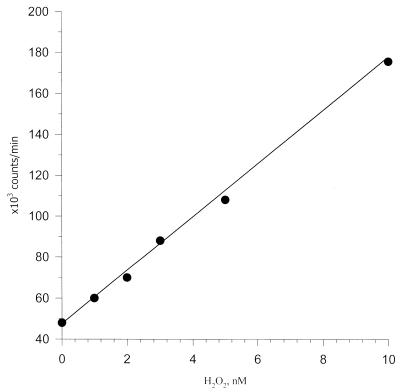
Figure 5.
Standard calibration curve for quantitation of hydrogen peroxide in the nanomolar concentration range in 10–3 M phosphate buffer, pH 6.8, obtained by competitive ELISA. Counts per minute (c.p.m.) versus nM H2O2.
Table 2. The influence of some substances on the level of heat-induced hydrogen peroxide production upon heating of 10–3 M phosphate buffer, pH 6.8, at 40°C for 4 h.
| Test sample | H2O2 concentration (nM) | |
|---|---|---|
| 1 | 10–3 M phosphate buffer, pH 6.8 | 3.2 ± 0.2 |
| 2 | 10–3 M phosphate buffer, pH 6.8, O2 saturateda | 5.7 ± 0.5 |
| 3 | 10–3 M phosphate buffer, pH 6.8, N2 saturateda | 1.7 ± 0.7 |
| 4 | 10–3 M phosphate buffer, pH 6.8, Ar saturateda | 1.45 ± 0.3 |
| 5 | 10–3 M phosphate buffer, pH 6.8, 0.14 M NaCl | 1.1 ± 0.5 |
| 6 | 10–3 M phosphate buffer, pH 6.8, 50% D2O | 22.4 ± 15.1 |
| 7 | 10–3 M phosphate buffer, pH 6.8, 100% D2O | 96.6 ± 21.4 |
| 8 | 10–3 M phosphate buffer, pH 6.8, 10 mM NaN3 | 0.7 ± 0.1 |
| 9 | 10–3 M phosphate buffer, pH 6.8, 1 mM guanosine | 0 |
| 10 | 10–3 M phosphate buffer, pH 6.8, 0.1 mM Tiron | 0.4 ± 0.2 |
| 11 | 10–3 M phosphate buffer, pH 6.8, 10–4 M 1,10-phenanthroline | 3.25 ± 0.2 |
| 12 | 10–3 M phosphate buffer, pH 6.8, 0.1 mM EDTA | 0.5 ± 0.1 |
| 13 | 10–3 M phosphate buffer, pH 6.8, 0.1 mM neocuproine | 0 |
aThe buffer was bubbled for 30 min prior to heating.
Generation of hydrogen peroxide is probably preceded by conversion of singlet oxygen to the superoxide anion radical, yielding hydrogen peroxide as a result of superoxide dismutation.
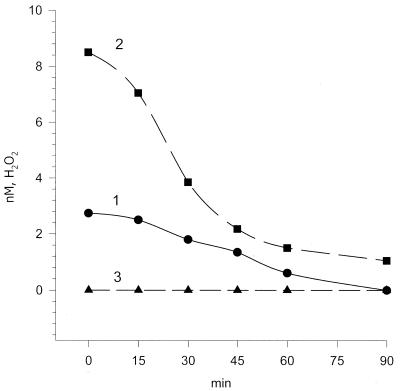
Figure 6 shows the time course for H2O2 formation on heating of phosphate buffer and the effects of SOD and catalase on the process. The effect of SOD on thermal H2O2 production testifies to the fact that formation of H2O2 results from the superoxide radical dismutation.
Figure 6.
Effects of SOD and catalase on heat-induced H2O2 formation in 10–3 M phosphate buffer, pH 6.8. Samples of the phosphate buffer were heated at 75°C for 4 h and rapidly cooled to ambient temperature, then enzyme was added. The moment of enzyme addition corresponds to the zero point on the abscissa. 1, Phosphate buffer (control); 2, phosphate buffer with SOD; 3, phosphate buffer with catalase. Abscissa, incubation time at ambient temperature (26°C) (min). Ordinate, concentration of H2O2 (nM).
In the presence of SOD one can observe a 3-fold increase in chemiluminescence intensity due to dismutation of superoxide, while the presence of catalase reduces chemiluminescence to the background level (Fig. 6). In the presence of 0.1 mM Tiron, a selective scavenger of the superoxide radical (37), the level of heat-induced hydrogen peroxide was decreased 8-fold (Table 2, row 10).
A powerful chelator of iron, 1,10-phenanthroline, does not affect the level of H2O2 production, while ethylendiaminetetraacetic acid (EDTA) and a selective chelator of copper ions, neocuproine, suppressed thermally induced H2O2 production, especially neocuproine (Table 2, row 13). We failed to estimate the influence of another iron chelator, deferoxamine, because of high quenching of the chemiluminescence. Decomposition of hydrogen peroxide in the time course is known to result from the Fenton reaction. Addition of hydrogen peroxide to an unheated blank sample allowed us to observe the process of H2O2 decay (data not shown). It was difficult to estimate the exact activation energy value for heat-induced H2O2 formation in phosphate buffer due to its complex nature and the distinct oscillatory behavior of the reaction at certain temperatures. This leads to rather wide variations in initial rate constants and, as a consequence, the activation energy for the process varies from 23 to 33 kcal/mol according to our estimates. The mean value of 27 kcal/mol closely agrees with the activation energies for depurination and for 8-oxodG formation.
Heat acts via ROS attack leading to 8-oxoguanine formation in DNA
Several large-scale supplemental trials on heat-induced 8-oxoguanine formation were performed to elucidate that the effect is produced principally through thermally induced ROS generation. The effects of gas saturation (O2, N2 and Ar), D2O, free radical scavengers (guanosine, NaN3, Tiron, mannitol and ethanol) and chelators (EDTA, 1,10-phenanthroline, deferoxamine and neocuproine) were scrutinized. The effects of these agents on 8-oxoguanine formation in DNA were in good agreement with their action on H2O2 production (Table 3). Experiments with different gas saturation levels clearly revealed an oxygen effect in so far as thermally induced formation of 8-oxoguanine depends on the concentration of dissolved oxygen (Table 3, rows 2–4). The enhancing effect of deuterated phosphate buffer and its decrease by singlet oxygen scavengers indicate that 1O2 participates in the process. Deuterium oxide, which leads to an increase in the lifetime of 1O2, caused a pronounced increase in the amount of 8-oxodG in DNA upon heating, whereas supplementation with singlet oxygen scavengers (sodim azide and guanosine) protected DNA from this thermally mediated damage.
Table 3. The influence of gas saturation, D2O, scavengers of 1O2, superoxide and OH• radicals and metal chelators on the level of heat-induced formation of 8-oxoguanine in salmon sperm DNA in 10–3 M phosphate buffer, pH 6.8, upon heating at 70°C for 24 h.
| Sample | 8-oxodG per 105 dG | |
|---|---|---|
| 1 | DNA in 10–3 M phosphate buffer pH 6.8 | 96 ± 13 |
| 2 | DNA in 10–3 M phosphate buffer pH 6.8, O2 saturateda | 195 ± 52 |
| 3 | DNA in 10–3 M phosphate buffer pH 6.8, N2 saturateda | 18.2 ± 9.8 |
| 4 | DNA in 10–3 M phosphate buffer pH 6.8, Ar saturateda | 9.1 ± 6.5 |
| 5 | DNA in 10–3 M phosphate buffer pH 6.8, 50% D2O | 180 ± 10 |
| 6 | DNA in 10–3 M phosphate buffer pH 6.8, 75% D2O | 360 ± 20 |
| 7 | DNA in 10–3 M phosphate buffer pH 6.8, 0.1 mM NaN3 | 23 ± 13 |
| 8 | DNA in 10–3 M phosphate buffer pH 6.8, 1 mM NaN3 | 10.4 ± 9.8 |
| 9 | DNA in 10–3 M phosphate buffer pH 6.8, 1 mM guanosine | 15 ± 9.8 |
| 10 | DNA in 10–3 M phosphate buffer pH 6.8, 0.1 mM Tiron | 4.6 ± 6.5 |
| 11 | DNA in 10–3 M phosphate buffer pH 6.8, 0.2 M mannitol | 6.5 ± 6.5 |
| 12 | DNA in 10–3 M phosphate buffer pH 6.8, 0.5 M ethanol | 15.6 ± 6.5 |
| 13 | DNA in 10–3 M phosphate buffer pH 6.8, 1 M ethanol | 8.5 ± 6.5 |
| 14 | DNA in 10–3 M phosphate buffer pH 6.8, 0.1 mM EDTA | 16.9 ± 6.5 |
| 15 | DNA in 10–3 M phosphate buffer pH 6.8, 10–4 M 1,10-phenanthroline | 23.4 ± 6.5 |
| 16 | DNA in 10–3 M phosphate buffer pH 6.8, 1 mM deferoxamine | 0 |
| 17 | DNA in 10–3 M phosphate buffer pH 6.8, 0.1 mM neocuproine | 0 |
8-Oxoguanine formation was determined by non-competitive ELISA.
aThe solution was bubbled for 30 min prior to heating.
In the presence of 0.1 mM Tiron, a scavenger of superoxide radicals (37), heat-induced production of 8-oxodG in DNA dropped to the background level (Table 3, row 10). Mannitol and ethanol, scavengers of hydroxyl radicals, suppressed formation of 8-oxodG in DNA upon heating (Table 3, rows 11–13). All chelators of metal ions used (EDTA, 1,10-phenanthroline and neocuproine) protect to a variable degree against heat-induced formation of 8-oxodG in DNA. The more marked effect of EDTA as compared to 1,10-phenanthroline and, especially, the action of neocuproine, a selective chelator of copper ions, which prevents 8-oxodG formation, indicated a more appreciable effect of copper compared to iron on the process of oxidative damage to DNA upon heating.
DISCUSSION
High pressure liquid chromatography with electrochemical detection and gas chromatography coupled to mass spectrometry are the two main methods used for the determination of 8-oxoguanine, but they require preliminary DNA hydrolysis. The quantitative assessment of 8-oxoguanine by immunoblot assay of whole DNA has been reported (35). We also used whole DNA and ELISA to study heat-induced formation of 8-oxoguanine, calibrating its content with γ-irradiated DNA (36). A G value (radiation-chemical yield) for 8-oxodG formation in calf thymus DNA of 0.3 molecules/100 eV absorbed radiation energy has been reported (34). Later, a value of 5.9 × 10–2 µmol J–1 for 8-oxoguanine formation was obtained (37). In the present study we estimated the G value for 8-oxodG as 0.37 molecules/100 eV absorbed radiation energy (3.7 × 10–2 µmol J–1). The linear dose–yield relationship for the formation of 8-oxodG in DNA upon γ-irradiation makes possible its use as an adequate standard for determination of amount of 8-oxodG in DNA.
The formation of 8-oxoguanine by thermal damage to guanyl nucleotides was originally shown by Bruskov and Petrov (38). The autooxidation of dGMP producing 8-oxoguanine upon heating to 70°C has also been reported (39). Our findings in the present study demonstrate heat-induced formation of 8-oxoguanine, a key biomarker of DNA damage by ROS, in DNA. It is likely that the DNA partially melted within the range of elevated temperatures we used. The yield of 8-oxoguanine under high intensity UV laser photolysis was about 3-fold lower in heat-denatured DNA than in double-stranded DNA (40). However, according to some studies the yield of 8-oxoguanine was not influenced greatly by DNA conformation (28,37). Exposure of heat-denatured DNA to γ-irradiation in our experiments reduced 8-oxoguanine formation by no more than 20% compared to double-stranded DNA. The influence of DNA conformation may only be a source of underestimation of the amount of 8-oxoguanine in double-stranded DNA upon heating. The rate constant of 8-oxoguanine formation in DNA at 37°C obtained by extrapolation of our experimental data at elevated temperatures will be refined in subsequent direct experimental determination of the value. Several modifications of bases weaken the glycosyl bond and accelerate the liberation of modified bases from DNA. This process may cause errors in the determination of modified bases in DNA. However, it has been shown that the glycosyl bond of 8-oxoguanine is ∼10 times more resistant to depurination as compared to that of guanine (41). In Table 4 the kinetic data available from the literature for various types of heat-induced damage, single-strand breaks (23), depurination (24,25) and deamination of cytosine (26,27), are compared with our data on guanine release and 8-oxoguanine formation. The data on adenine and guanine depurination at low ionic strength as well as the influence of ionic strength on depurination are in good agreement with those previously published (26,27).
Table 4. Kinetic data for various types of heat-induced DNA damage.
| Sample | Rate constant at 37°C (s–1) | Activation energy (kcal/mol) | Source |
|---|---|---|---|
| DNA strand breaks | |||
| Denatured DNA | 1.1 × 10–10 | 25 ± 2 | (23) |
| Depurination | |||
| Denatured calf thymus DNAa | 1 × 10–9 | 28 | (24) |
| Native Bacillus subtilis DNA | 3 × 10–11 | 31 ± 2 | (25) |
| Chicken erythrocyte DNAa | 1.3 × 10–9 | 28 ± 2 | This work |
| Salmon sperm DNA, 0.14 M NaCl | 3.7 × 10–10 | 27 ± 2 | This work |
| Cytosine deamination | |||
| dCMP, poly(dC), single-stranded DNA | 2 × 10–10 | 28–29 | (26) |
| Single-stranded DNA in vivo | 1 × 10–10 | 28 ± 1 | (27) |
| 8-Oxoguanine formation | |||
| dGMP | 5.8 × 10–10 | 24 | (38) |
| Salmon sperm DNA a | 4.7 × 10–10 | 27 ± 2 | This work |
| Salmon sperm DNA, 0.14 M NaCl | 1.3 × 10–10 | 27 ± 2 | This work |
aDenotes DNA in low ionic strength solution.
It was found that the measurement of oxidative DNA damage by gas chromatography–mass spectrometry, which requires a derivatization procedure at high temperature in the presence of air, causes ‘artifactual’ base oxidation leading to an overestimation of oxidation products, especially 8-oxoguanine (42). Our results point to the possibility that thermally related oxidative damage may be a cause of artifactual DNA oxidation during its isolation and analysis. The non-competitive ELISA employed in this study also led to additional heat-induced formation of 8-oxoguanine in DNA due to DNA heating during denaturation and immobilization. However, these effects were corrected by subtraction of the 8-oxoguanine value in the control samples subjected to the same treatment.
Comparative analysis of our data on heat-induced guanine depurination and 8-oxoguanine formation in DNA shows surprising similarities between the two processes: their activation energies are the same within the limits of experimental error and NaCl influences them in a similar way.
This study provides several lines of evidence indicating that heat-induced damage to DNA is caused by ROS. Our results on thermally mediated H2O2 production indicate that heat, a permanent environmental factor, activates dissolved atmospheric oxygen and leads to generation of ROS [singlet oxygen, superoxide radicals (anionic and protonated), hydrogen peroxide and hydroxyl radicals] in the sequence of oxygen reducing reactions: O2→1O2→O2–•→HO2•→H2O2→OH•. We have demonstrated an ‘oxygen effect’ in so far as the yield of H2O2 can be altered by saturation of the solution with different types of gases prior to heating.
Heat-mediated production of H2O2 is initiated by thermal activation of dissolved oxygen to the singlet state. It is known that the lifetime of singlet oxygen is more than an order of magnitude higher in D2O than in H2O (38). The enhancing effect of D2O on thermal H2O2 production as well as inhibition of the process by the singlet oxygen scavengers sodium azide and guanosine (43) point to 1O2 as the precursor species that is responsible for H2O2 production. Our results with SOD and Tiron suggest that the process is caused by superoxide anion radical generation. Dismutation of superoxide anion radicals leads to H2O2 production.
Our results reveal that 0.14 M NaCl approximately equally decreases heat-induced H2O2 production, depurination and 8-oxoguanine formation, which may be due to scavenging of ROS by chloride ions. The influence of different chelators (1,10-phenanthroline, EDTA and neocuproine) on H2O2 production upon heating may indicate that copper ions are the predominant electron donors leading to superoxide anion formation from singlet oxygen.
Gas saturation (O2, N2 and Ar), D2O, scavengers of 1O2, O2–• and OH• radicals and chelators of metal ions influenced heat-induced 8-oxoguanine formation as they affected thermal ROS generation. These findings imply that heat acts via ROS attack leading to oxidative damage to DNA. Our results also demonstrate an ‘oxygen effect’ for heat-induced 8-oxoguanine formation; an influence of concentration of dissolved oxygen on the process. It was shown earlier that the yield of radiation-induced 8-oxoguanine rises as the oxygen concentration increases (44). Heat-induced cell death in Saccharomyces cerevisiae is a consequence of oxidative stress, while anaerobic conditions cause a significant increase in thermotolerance of the yeast (45). It has been suggested that the concentration of O2 in the nucleus of a cell must be exceedingly low (46), although its concentration in this compartment has not yet been determined. Support for this assumption would imply an additional mode of nuclear DNA defense against thermal damage.
Ferrous ion complexes with phosphates are capable of reacting with molecular oxygen to generate ROS during autooxidation (47). Heat-induced DNA damage and 8-oxoguanine formation may be attributed to the action of singlet oxygen (38,43) and to OH• radical production by the iron-mediated Fenton reaction (30,35,48) and to a similar process due to copper ions. Superoxide radical and hydrogen peroxide seem incapable of reacting with DNA directly (15). It is known that DNA molecules always contain mixed iron and copper ions in complexes with phosphate groups. We found that in our 10–3 M phosphate buffer, pH 6.8, the concentration of iron evaluated by atomic absorption spectroscopy was equal to 0.4 µM, and to 0.06 µM for copper. Trace levels of iron and copper ions are ubiquitous in salt and buffer solutions (49). Iron and copper are present in the cell nucleus and play an important role in the production of OH• radicals responsible for several mutagenic modifications in DNA, including formation of 8-oxoguanine (50). Hydroxyl radical formation in vivo will be ‘site specific’ for points where selective binding of iron or copper ions to DNA takes place (51) and may be hot spots of mutagenesis.
Damage to single-stranded DNA by oxygen radicals during aerobic incubation with 10 µM Fe2+ ions produces a high frequency of mutations (52). The mutagenic spectrum of the damage revealed G→C and G→T transversions. G→T transversions are the specific type of mutation induced by 8-oxodG (2,3,7,9–11), which can pair with A. This mutation could also stem from depurination (52). The most frequent transversion, G→C, could be caused by misinsertion of dGMP opposite an oxidized form of 8-oxoguanine during replication (53). 8-OxodG in duplex DNA could be further oxidized, possibly to 2-aminoimidazolone as a major oxidation product (54). 2-Aminoimidazolone demonstrates specific dGMP incorporation and implies G:C→C:G transversion under oxidative conditions (54).
Over the past decade, several lines of evidence have indicated that ROS play an important role in aging. The free radical theory of aging proposes that damage by ROS is critical in defining lifespan (1). Heat-induced ROS formation may be an additional factor that provides molecular changes in DNA, proteins, lipids and other biological molecules which contribute to senescence. Environmental temperature influences longevity in poikilotherms. It is supposed that this is a straightforward consequence of altered metabolic rate (55), because a 10°C increase in temperature is equivalent to a 3-fold increase in metabolic rate (56). However, as shown in this report, a very similar dependence is evident for the formation of ROS and DNA damage.
Formation of 8-oxoguanine in DNA is one of the likely reasons for heat-induced generation of G:C→T:A transversions (57), since this guanine lesion produces corresponding base pair substitutions during replication (2,3,7,9). At present, it is difficult to evaluate the role of heating in formation of 8-oxodG in vivo, because the validity of 8-oxodG measurements in cellular DNA is still not clearly understood (58). However, our results provide support for the suggestion that heat is a potential source of continued generation of ROS and damage to DNA.
Because the haploid genome contains ∼1.4 × 109 G:C base pairs (57) and assuming that the rate constant for 8-oxoguanine formation is 1.3 × 10–10 s–1 (Table 1), there will be ∼1.6 × 104 adducts per cell per day provided that cell DNA is as sensitive to heat-induced ROS as DNA in solution. The estimated rate of excision of oxidized DNA bases (which are then excreted in the urine) by repair enzymes is 1.2 × 104 adducts per cell per day for humans (59). As can be seen, these values are closely related to each other. Thus it is possible that ROS-related thermal damage to DNA may be a significant cause of ‘spontaneous’ mutation. The increase in temperature during inflammation could increase ROS formation and be one of the means of cell defense against infection.
The exact role of heat and its relative contribution to DNA damage compared to production of ROS by other sources in the cell remains to be determined. Further investigations are apparently required to further our understanding of heat-induced damage to DNA and its biological significance.
Acknowledgments
ACKNOWLEDGEMENTS
We thank O. S. Morenkov for help in obtaining monoclonal antibodies and B. K. Chernov for the kind gift of 8-oxo-2′-deoxyguanosine. We are grateful to Youri Pavlov for helpful words of advice. This work was supported in part by the Russian Fund for Basic Research, grant no. 00-04-48124.
REFERENCES
- 1.Beckman K.B. and Ames,B.N. (1998) The free radical theory of aging matures. Physiol. Rev., 78, 547–581. [DOI] [PubMed] [Google Scholar]
- 2.Kuchino Y., Mori,F., Kasai,H., Inone,H., Iwai,S., Miure,K., Ohtsuka,E. and Nishimura,S. (1987) Misreading of DNA templates containing 8-hydroxydeoxyguanosine at the modified base and at adjacent residues. Nature, 327, 77–79. [DOI] [PubMed] [Google Scholar]
- 3.Shibutani S., Takeshita,M. and Grollman,A.P. (1991) Insertion of specific bases during DNA synthesis past the oxidation-damaged base 8-oxoG. Nature, 349, 431–434. [DOI] [PubMed] [Google Scholar]
- 4.Pavlov Y.I., Minnick,D.T., Izuta,S. and Kunkel,T.A. (1994) DNA replication fidelity with 8-oxodeoxyguanosine triphosphate. Biochemistry, 33, 4695–4701. [DOI] [PubMed] [Google Scholar]
- 5.Bruskov V.I. and Kuklina,O.V. (1988) 8-Oxo-GTP displays substrate properties of UTP in polynucleotide synthesis catalyzed by Escherichia coli RNA polymerase on a poly[d(A-T)]·poly[d(A-T)] template. Mol. Biol., 22, 580–584. [PubMed] [Google Scholar]
- 6.Bruskov V.I., Kurjavy V.V. and Usacheva,A.M. (1990) Ambiguity of the substrate-code properties of 8-hydroxy-GTP in the reaction of synthesis of oligonucleotides by Escherichia coli RNA polymerase on a DNA template from the phage mutant T7ΔD111. Dokl. Akad. Nauk SSSR, 307, 183–186. [PubMed] [Google Scholar]
- 7.Cheng K.C., Cahill,D.S., Kasai,H., Nishimura,S. and Loeb,L.A. (1992) 8-Hydroxyguanine, an abundant form of oxidative DNA damage, causes G-T and A-C substitutions. J. Biol. Chem., 267, 166–172. [PubMed] [Google Scholar]
- 8.Bruskov V.I., Yurov,S.S., Chernov,B.K. and Bezlepkin,V.G. (1992) Mutagenic action on Escherichia coli bacteria by 8-hydroxy-2′-deoxyguanosine, a product of DNA base damage induced by oxygen radicals and ionizing radiation. Genetika, 28, 859–865. [PubMed] [Google Scholar]
- 9.Wood M.L., Dizdaroglu,M., Gajewski,E. and Essigmann,J.M. (1990) Mechanistic studies of ionizing radiation and oxidative mutagenesis: genetic effect of a single 8-hydroxyguanine (7-hydro-8-oxoguanine) residue inserted at a unique site in a viral genome. Biochemistry, 29, 7024–7032. [DOI] [PubMed] [Google Scholar]
- 10.Moriya M., Ou,C., Bodepudi,V., Johnson,F., Takeshita,M. and Grollman,A.P. (1991) Site-specific mutagenesis using a gapped duplex vector: a study of translesion synthesis past 8-oxodeoxyguanosine in E. coli. Mutat. Res., 254, 281–288. [DOI] [PubMed] [Google Scholar]
- 11.Moriya M. (1993) Single-stranded shuttle phagemid for mutagenesis studies in mammalian cells: 8-oxoguanine in DNA induces targeted G·C→T·A transversions in simian kidney cells. Proc. Natl Acad. Sci. USA, 90, 1122–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kasai H. (1997) Analysis of a form of oxidative DNA damage, 8-hydroxy-2′-deoxyguanosine, as a marker of cellular oxidative stress during carcinogenesis. Mutat. Res., 387, 147–163. [DOI] [PubMed] [Google Scholar]
- 13.Chen Q., Fischer,A., Reagan,J.D., Yan,L.J.and Ames,B.N. (1995) Oxidative DNA damage and senescence of human diploid fibroblast cells. Proc. Natl Acad. Sci. USA, 92, 4337–4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shigenaga M.K., Hagen,T.M. and Ames,B.N. (1994) Oxidative damage and mitochondrial decay in aging. Proc. Natl Acad. Sci. USA, 91, 10771–10778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halliwell B. and Aruoma,O.I. (1991) DNA damage by oxygen-derived species. Its mechanism and measurement in mammalian systems. FEBS Lett., 281, 9–19. [DOI] [PubMed] [Google Scholar]
- 16.Wiseman H. and Halliwell,B. (1996) Damage to DNA by reactive oxygen and nitrogen species. Role in inflammatory disease and progression to cancer. Biochem. J., 313, 17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cutler R.G. (1991) Human longevity and aging: possible role of reactive oxygen species. Ann. N. Y. Acad. Sci., 621, 1–28. [DOI] [PubMed] [Google Scholar]
- 18.Ames B.N., Shigenaga,M.K. and Hagen,T.M. (1993) Oxidants, antioxidants and degenerative diseases of aging. Proc. Natl Acad. Sci. USA, 90, 7915–7922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loft S. and Poulsen,H.E. (1996) Cancer risk and oxidative DNA damage in man. J. Mol. Med., 74, 297–312. [DOI] [PubMed] [Google Scholar]
- 20.Grollman A.P. and Moria,M. (1993) Mutagenesis by 8-oxoguanine: an enemy within. Trends Genet., 9, 246–249. [DOI] [PubMed] [Google Scholar]
- 21.Park E.M., Shigenaga,M.K., Degan,P., Korn,T.S., Kitzler,J.W., Wehr,C.M., Kolachana,P. and Ames,B.N. (1992) Assay of excised oxidative DNA lesions: isolation of 8-oxoguanine and its nucleoside derivatives from biological fluids with a monoclonal antibody column. Proc. Natl Acad. Sci. USA, 89, 3375–3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lindahl T. (1993) Instability and decay of the primary structure of the DNA. Nature, 362, 709–715. [DOI] [PubMed] [Google Scholar]
- 23.Eigner J.E., Boldker,H. and Michaels,G. (1961) The thermal degradation of nucleic acids. Biochim. Biophys. Acta, 51, 165–168. [DOI] [PubMed] [Google Scholar]
- 24.Greer S. and Zamenhof,S.J. (1962) Studies on depurination of DNA by heat. J. Mol. Biol., 4, 123–141. [DOI] [PubMed] [Google Scholar]
- 25.Lindahl T. and Nyberg,B. (1972) Rate of depurination of native deoxyribonucleic acid. Biochemistry, 11, 3610–3618. [DOI] [PubMed] [Google Scholar]
- 26.Lindahl T. and Nyberg,B. (1974) Heat-induced deamination of cytosine residues in deoxyribonucleic acid. Biochemistry, 13, 3405–3410. [DOI] [PubMed] [Google Scholar]
- 27.Frederico L.A., Kunkel,T.A. and Shaw,B.R. (1990) A sensitive genetic assay for the detection of cytosine deamination: determination of rate constants and the activation energy. Biochemistry, 29, 2532–2537. [DOI] [PubMed] [Google Scholar]
- 28.Kasai H. and Nishimura,S. (1984) Hydroxylation of deoxyguanosine at the C-8 position by ascorbic acid and other reducing agents. Nucleic Acids Res., 12, 2137–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hengen P.N. (1997) Chemiluminescent detection methods. Trends Biochem. Sci., 22, 313–314. [DOI] [PubMed] [Google Scholar]
- 30.Bruskov V.I., Gaziev,A.I., Malakhova,L.V., Mantsygin, Yu.A. and Morenkov,O.S. (1996) Monoclonal antibodies to 8-oxo-2′-deoxyguanosine (8-hydroxyguanosine): characterization and use in determining damage to DNA by reactive oxygen species. Biokhimiya, 61, 535–540. [PubMed] [Google Scholar]
- 31.Dawson R.M.C., Elliott,D.C., Elliott,W.H. and Jones,K.M. (1986) Data for Biochemical Research, 3rd Edn. Clarendon Press, Oxford, UK, pp. 103–114.
- 32.Sober H.A. (ed.) (1970) Handbook of Biochemistry, 2nd Edn. The Chemical Rubber Co., Cleveland, OH, pp. H-96–H-97.
- 33.Hirayama H., Tamaoka,J. and Horikoshi,K. (1996) Improved immobilization of DNA to microwell plates for DNA–DNA hybridization. Nucleic Acids Res., 24, 4098–4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dizdaroglu M. (1985) Formation of an 8-hydroxyguanine moiety in deoxyribonucleic acid on γ-irradiation in aqueous solution. Biochemistry, 24, 4476–4481. [DOI] [PubMed] [Google Scholar]
- 35.Musarrat J. and Wani,A.A. (1994) Quantitative immunoanalysis of promutagenic 8-hydroxy-2′-deoxyguanosine in oxidized DNA. Carcinogenesis, 15, 2037–2043. [DOI] [PubMed] [Google Scholar]
- 36.Bruskov V.I., Masalimov,Zh.K. and Usacheva,A.M. (1999) Chemiluminescence enzyme immunoassay of 8-oxoguanine in DNA. Biokhimiya, 64, 803–808. [PubMed] [Google Scholar]
- 37.Fuciarelli A.F., Wegher,B.J., Blakely,W.F. and Dizdaroglu,M. (1990) Yields of radiation-induced base products in DNA: effects of DNA conformation and gassing conditions. Int. J. Radiat. Res., 58, 397–415. [DOI] [PubMed] [Google Scholar]
- 38.Bruskov V.I. and Petrov,A.I. (1992) Kinetics of heat-induced formation of 8-oxo-2′-deoxyguanosine 5′-monophosphate: estimation of rate constants and activation energy. Mol. Biol., 26, 898–902. [PubMed] [Google Scholar]
- 39.Helbook H.J., Beckman,K.B., Shigenaga,M.K., Walter,P.B., Woodall,A.A., Yeo,H.C. and Ames,B.N. (1998) DNA oxidation matters: the HPLC-electrochemical detection assay of 8-oxo-deoxyguanosine and 8-oxo-guanine. Proc. Natl Acad. Sci. USA, 95, 288–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Angelov D., Spassky,A., Berger,M. and Cadet,J. (1997) High-intensity UV laser photolysis of DNA and purine 2′-deoxyribonucleosides: formation of 8-oxopurine damage and oligonucleotide strand cleavage as revealed by HPLC and gel electrophoresis studies. J. Am. Chem. Soc., 119, 11373–11380. [Google Scholar]
- 41.Chernikov A.V., Usacheva,A.M. and Bruskov,V.I. (1996) Depurination of 8-oxo-7,8-dihydroguanine (8-hydroxyguanine) nucleosides. Biokhimiya, 61, 35–38. [Google Scholar]
- 42.Jenner A., England,T.G., Aruoma,O.I. and Halliwell,B. (1998) Measurement of oxidative DNA damage by gas chromatography-mass spectrometry: ethanethiol prevents artifactual generation of oxidized DNA bases. Biochem. J., 331, 365–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Devasagayam T.P.A., Steenken,S., Obendorf,M.S.W., Schulz,W.A. and Sies,H. (1991) Formation of 8-hydroxy(deoxy)guanosine and generation of strand breaks at guanine residues in DNA by singlet oxygen. Biochemistry, 30, 6283–6289. [DOI] [PubMed] [Google Scholar]
- 44.Peskin A.V., Labas,Yu.A. and Tikhonov,A.N. (1998) Superoxide radical production by sponges Sycon sp. FEBS Lett., 434, 201–204. [DOI] [PubMed] [Google Scholar]
- 45.Davidson J.F., Whyte,B., Bissinger,P.H. and Schiestl R.H. (1996) Oxidative stress is involved in heat-induced cell death in Saccharomyces cerevisiae. Proc. Natl Acad. Sci. USA, 93, 5116–5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Joenje H. (1989) Genetic toxicology of oxygen. Mutat. Res., 219, 193–208. [DOI] [PubMed] [Google Scholar]
- 47.Kachur A.V., Tuttle,S.W. and Biaglow,J.E. (1998) Autoxidation of ferrous ion complexes: a method for the generation of hydroxyl radicals. Radiat. Res., 150, 475–482. [PubMed] [Google Scholar]
- 48.Imlay J.A., Sherman,M.C. and Linn,S. (1988) Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science, 240, 640–642. [DOI] [PubMed] [Google Scholar]
- 49.Buettner G.R. (1988) Catalytic metals, ascorbate and free radicals. Combination to avoid. J. Biochem. Biophys. Methods, 16, 27–40. [DOI] [PubMed] [Google Scholar]
- 50.Meneghini R. (1997) Iron homeostasis, oxidative stress and DNA damage. Free Radic. Biol. Med., 23, 783–792. [DOI] [PubMed] [Google Scholar]
- 51.Halliwell B. and Gutteridge,J.M.C. (1986) Oxygen free radicals and iron in relation to biology and medicine: some problems and concepts. Arch. Biochem. Biophys., 246, 501–514. [DOI] [PubMed] [Google Scholar]
- 52.McBride T.J., Preston,B.D. and Loeb,L.A. (1991) Mutagenic spectrum resulting from DNA damage by oxygen radicals. Biochemistry, 30, 207–213. [DOI] [PubMed] [Google Scholar]
- 53.Duarte V., Muller,J.G. and Burrows,C.J. (1999) Insertion of dGMP and dAMP during in vitro DNA synthesis opposite an oxidized form of 7,8-dihydro-8-oxoguanine. Nucleic Acids Res., 27, 496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kino K. and Sugiyama,H. (2001) Possible cause of G:C→C:G transversion mutation by guanine oxidation product, imidazolone. Chem. Biol., 8, 369–378. [DOI] [PubMed] [Google Scholar]
- 55.Kirkwood T.B.L. and Austad,S.N. (2000) Why do we age? Nature, 408, 233–238. [DOI] [PubMed] [Google Scholar]
- 56.Sacher G.A. (1978) Longevity and aging in vertebrate evolution. Bioscience, 28, 497–501. [Google Scholar]
- 57.Kriker M.C. and Drake,J.W. (1990) Heat mutagenesis in bacteriophage T4: another walk down the transversion pathway. J. Bacteriol., 172, 3037–3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Halliwell B. (1999) Oxygen and nitrogen are pro-carcinogens. Damage to DNA by reactive oxygen, chlorine and nitrogen species: measurement, mechanism and the effects of nutrition. Mutat. Res., 443, 37–52. [DOI] [PubMed] [Google Scholar]
- 59.Beckman K.B. and Ames,B.N. (1997) Oxidative decay of DNA. J. Biol. Chem., 272, 19633–19636. [DOI] [PubMed] [Google Scholar]