Abstract
In the current review, we focused on identifying aliment compounds and micronutrients, as well as addressed promising bioactive nutrients that may interfere with NAFLD advance and ultimately affect this disease progress. In this regard, we targeted: 1. Potential bioactive nutrients that may interfere with NAFLD, specifically dark chocolate, cocoa butter, and peanut butter which may be involved in decreasing cholesterol concentrations. 2. The role of sweeteners used in coffee and other frequent beverages; in this sense, stevia has proven to be adequate for improving carbohydrate metabolism, liver steatosis, and liver fibrosis. 3. Additional compounds were shown to exert a beneficial action on NAFLD, namely glutathione, soy lecithin, silymarin, Aquamin, and cannabinoids which were shown to lower the serum concentration of triglycerides. 4. The effects of micronutrients, especially vitamins, on NAFLD. Even if most studies demonstrate the beneficial role of vitamins in this pathology, there are exceptions. 5. We provide information regarding the modulation of the activity of some enzymes related to NAFLD and their effect on this disease. We conclude that NAFLD can be prevented or improved by different factors through their involvement in the signaling, genetic, and biochemical pathways that underlie NAFLD. Therefore, exposing this vast knowledge to the public is particularly important.
Keywords: liver, NAFLD, antioxidants, bile acids, AMPK
1. Non-Alcoholic Fatty Liver Disease
The liver is a central organ that makes life possible for humans and which is at the center of vital metabolic functions. The health of the liver often reflects a person’s overall health. The liver tissue can be the target of different diseases, and all of them are able to change the functions of the liver. Nowadays, one of the most common causes of primary and chronic liver disorders is non-alcoholic fatty liver disease (NAFLD) [1], which is an important public health problem in different age groups [2]. NAFLD is defined as an excessive accumulation of fat, mainly in the form of triglycerides in the liver epithelial cells or hepatocytes [3]. The disease encompasses a wide range of liver disorders, from simple steatosis to non-alcoholic steatohepatitis (NASH), terminal liver failure, which can eventually lead to liver carcinoma [4], which can cause death [5]. If simple hepatic steatosis is not effectively treated, it may progress to cirrhosis, which may lead to liver failure and the development of liver carcinoma.
NAFLD is formed from fat deposits in the liver cells and is associated with metabolic syndrome, obesity, and oxidative stress. NAFLD constitutes a spectrum of liver diseases associated with collateral metabolic and cardiovascular disorders [6]. NAFLD is also characterized by atherogenic dyslipidemia, postprandial lipemia, and HDL lipoprotein dysfunction [7].
In the last decade, NAFLD has emerged as the most common cause of chronic liver disease in developed countries. NAFLD is a global epidemic that threatens human health. Its incidence rate reaches around thirty percent. Its prevalence increases significantly between 70 and 90% among people with obesity or type 2 diabetes [T2D] [7]. NAFLD is directly related to the metabolic syndrome: central obesity, hyperglycemia, type 2 diabetes, arterial hypertension, and hypertriglyceridemia, which are usual components of the metabolic syndrome and are also known risk factors for NAFLD [8].
The occurrence of NAFLD directly increases with the rising prevalence of obesity, metabolic syndrome (MetS), and type 2 diabetes (T2D). In recent times, the number of people with obesity has increased globally. The worldwide prevalence of obesity registered for NAFLD and NASH patients was 51 and 81%, respectively [9]. NAFLD prevalence fluctuates from 60 to 95% in obese people [10].
Diabetes is one of the fastest growing global health emergencies of the 21st century [11]. Around 463 million people worldwide have been diagnosed with diabetes in 2019, and a 51% increase is expected by 2045, raising the prevalence of diabetes to 700 million.
The association between T2D and NAFLD is well established and T2D is highly prevalent in NAFLD patients [12]. Dai et al. [13] analyzed the data of 24 studies which included 35,599 T2D patients and found a prevalence of 59.67% of NAFLD, which rose to 77.87% in those with T2D and obesity. The majority of the NAFLD patients with T2D fulfilled criteria for MetS, highlighting the relationship between these conditions in the metabolic risk continuum.
NAFLD is characterized by a wide variety of liver changes, ranging from simple steatosis to non-alcoholic steatohepatitis (NASH), cirrhosis, and liver carcinoma. NASH is described as steatosis combined with inflammation and thus has become the second leading liver disease that leads to liver transplantation in the US [14]. Approximately one-third of US adults who have NAFLD also have NASH, and 30% of these individuals have the potential to progress to advanced cirrhosis, hepatocellular carcinoma, and liver-related mortality [15]. The pathogenesis of NAFLD/NASH is complicated and involves lipid accumulation, insulin resistance, inflammation, and fibrogenesis. During the progression of NAFLD, oxygen-containing free radicals (ROS) are activated and cause oxidative stress.
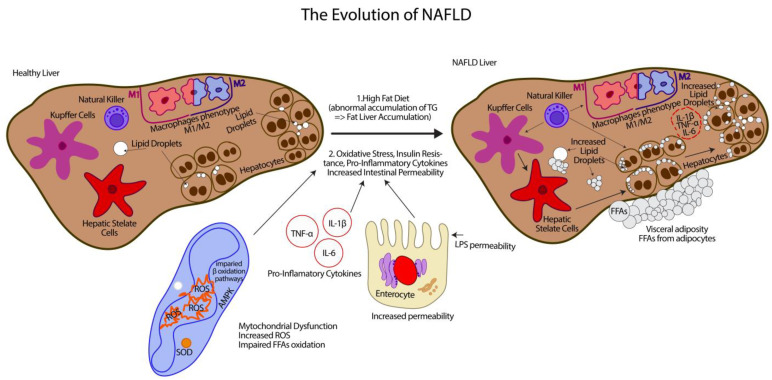
The biological mechanism of the onset of basic steatosis and progression to liver disease is not fully understood, which is likely due to a number of factors that manifest in addition to genetic predisposition. The central concept of NAFLD is the “parallel hits” hypothesis [16], which was developed from the two-hit theory proposed by Day et al. in 1998 [17]. The two-hit theory asserts that a high-fat diet or diabetes-induced steatosis (the first hit) will make the liver more susceptible to other risk factors associated with oxidative stress and cause severe lipid oxidation (the second hit) (Figure 1).
Figure 1.
Liver damage in NAFLD.
In addition, insulin resistance has been shown to facilitate the progression of NAFLD to NASH [18]. Hepatic lipid overload and/or hyperinsulinemia-driven de novo lipogenesis increases lipid peroxidation, which results in the production of reactive oxygen species (ROS).
From this, the “two hits” hypothesis can be put forward [17]: the first hit is determined by an abnormal accumulation of triglycerides (TGs) and causes fat accumulation in the liver (steatosis), and the second hit is mainly caused by oxidative stress and triggers the progression of steatosis to NASH (Figure 1). As mentioned, it includes oxidative stress, insulin resistance, the secretion of pro-inflammatory cytokines, increased intestinal permeability, and obesity, which are identified as the main factors involved in the pathogenesis of NAFLD [16]. These factors, accompanied by oxidative stress, can promote intrahepatic fat accumulation and lipotoxicity, and develop inflammation, liver cell apoptosis, and fibrogenesis responsible for disease progression (Figure 1) [19].
A current view has recently been developed and is based on a more complex “multiple parallel hits hypothesis”, which includes a wide spectrum of parallel hits, including insulin resistance, oxidative stress, genetic and epigenetic mechanisms, environmental elements, cytokines, and changes in the microbiota. The theory of multiple parallel hits states that the effect in the development of NAFLD is more comprehensive and includes more diverse factors than a simple effect of one or two factors [20].
With regard to treatment, lifestyle intervention is the only approach to NAFLD. Thus, there is still no accepted effective drug treatment. The basis for the effective treatment of NAFLD is the identification of various components that intervene in each of the processes to prevent the progression of the disease.
Human survival is associated with the consumption of various nutrients required for life, which consist of macronutrients such as carbohydrates, lipids, amino acids, dietary fiber, and micronutrients such as minerals and vitamins. In addition to these common nutrients, there are additional components in the diet such as coffee (rich in caffeine) and tea (comprising tea polyphenols), forming an important part of our daily diet.
Dietary components reach the digestive system for the sake of digestion and absorption and then they are transformed into small molecule metabolites. Intestinal microorganisms further decompose nutrients into smaller units, such as bile acids (BAs) [21], short-chain fatty acids (SCFAs) [22], free fatty acids (FFAs) [21], and generate a wide variety of biological responses. All these processes may affect the intestinal microbiota and therefore may impinge on intestinal absorption, dietary energy, BAs metabolism, and finally may affect the intestinal permeability. Gut microbiota strains widely fluctuate from one individual to the other. The identity of the microbiome population and their quantity are highly dependent on the host genotype, on the host nutritional practices, and others [23].
Immune cells such as Th1/Th2 lymphocytes and T regulatory cells (T regs), macrophages, and natural killer cells play key roles in the pathogenesis of NAFLD. Specifically, hepatic macrophages, which consist of resident Kupffer cells and recruited bone marrow-derived macrophages, are the major cells that produce inflammatory mediators, such as tumor necrosis factor (TNF)-α and interleukin (IL)-1β, causing systemic insulin resistance followed by NAFLD and, ultimately, NASH [24]. In tissues, macrophages undergo maturation via specific pathways following their stimulation by different triggers, leading them to obtain specialized functional phenotypes. Toll-like receptors (TLRs), such as lipopolysaccharide (LPS) and interferon gamma (IFN-γ), are ligands that stimulate the conventional activation of M1, while IL-4/IL-13 stimulate the activation of M2 [25]. The dysregulation and polarization of M1/M2 macrophages can lead to chronic inflammation, infection, cancer, obesity, and their associated disorders such as NAFLD [26]. A novel mechanism that may regulate the M1/M2 balance relies on apoptotic effects of M2 Kupffer cells toward their M1 counterparts was previously published [27]. Wan et al. suggested that, by promoting M2-induced apoptosis of M1 Kupffer cells, one may prove a relevant strategy to limit high fat-induced inflammation and hepatocyte injury in NAFLD (Figure 1). Many food-derived molecules are associated with M1 and/or M2 activation. Depending on the progressive stages of NAFLD, NK cells that belong to innate immunity are distinct in phenotypes and frequency. They are also involved in inflammatory processes, hepatic steatosis, and fibrosis [28]. In the current review, we will focus on identifying aliment compounds, micronutrients, or promising bioactive nutrients that may interfere with NAFLD advance and will finally affect the disease progress. We used relevant information from many research articles and reviews by accessing some international databases over a fairly long period of time, namely 1963–2022. However, most studies focused on the next coverage period of 2005–2022. In this way, we will reach our final goal. In this search, we used specific phrases in order to obtain information that was as objective as possible. Additionally, some words such as liver, NAFLD, antioxidants, bile acids, AMPK, sweeteners, cannabinoids, and finally caffeine helped us significantly in gathering information and stabilizing the final organization of the review. This review was divided into the general presentation of NAFLD pathology; clinical information regarding nutrients with bioactive potential; and the presentation of different enzymes, which have influenced the progression and development of this pathology. All these correspond to 20 issues.
The liver has its own immunity. The components that belong to innate immunity and influence the pathology of NAFLD are represented by Kupffer cells, hepatic stellate cells, and natural killers. Additionally, besides these, there are macrophages which, depending on the situation, can change their M1/M2 phenotype. According to the “two hits” hypothesis that sustains the NAFLD, the abnormal accumulation of triglycerides (TGs) causes fat storage in the liver (steatosis), and oxidative stress can trigger the progression of steatosis into NASH. In order to argue in favor of this hypothesis, there is a variety of cellular, molecular, and signaling changes. They include oxidative stress, insulin resistance, the secretion of pro-inflammatory cytokines, and increased intestinal permeability.
2. Potential Bioactive Nutrients That May Interfere with NAFLD
2.1. Dark Chocolate
One of the richest foods in bioflavonoids (flavonols, polyphenols, and theobromine) is dark chocolate (DC) [29]. DC has the highest level of antioxidants compared to other food sources [30]. Besides nutrients such as saturated fat (60%), monounsaturated fat (35%), and linoleic acid (3%), chocolate contains important minerals such as potassium and magnesium [31] as well as cocoa, which is the main ingredient in chocolate [32]. Cocoa and some of its derivatives are a very complex food [33] and a rich source of antioxidants flavonoids, catechin, and epicatechin [34]. The potential health benefits of chocolate consumption have mainly been discovered recently [35]. Several recent studies have suggested that DC may have positive and changing effects on the lipid profile, reducing total and LDL cholesterol levels and increasing HDL levels [36].
Several studies have indicated that cocoa from chocolate may contribute to protective effects through the effect of the many beneficial components they contain such as minerals, antioxidants [37], and especially polyphenolic compounds (antioxidant flavonoids), which exhibit anti-inflammatory and antithrombotic activities. The beneficial metabolites may all contribute to their protective effect [38]. Furthermore, these can improve insulin resistance through reducing oxidative stress, improving endothelial function, and/or altering glucose metabolism [39]. A previous study on a rat model induced with alcoholic steatohepatitis showed that cocoa supplementation had a beneficial effect on the disease by reducing the accumulation of fat in the liver, reducing inflammation, and reducing cell necrosis [40]. Recent studies have mainly argued the protective context of cocoa or chocolate consumption and different health indicators [41]. The results of these studies affirmed that cocoa and DC intake can reduce oxidative stress, stimulate brain function, fight cancer cells, improve blood circulation, improve the mood, memory, immune system, and even protect the heart [42] and the liver tissue [43].
Therefore, our assumption to administrate dark chocolate may also have an adequate potential in the prevention and control of NAFLD. Support for this assumption is provided in a recent study among NAFLD patients in which they wanted to evaluate the effect of constant consumption of 30 g per day of dark chocolate (DC) containing 83% cocoa for 12 weeks on blood lipid profile, on fasting blood sugar levels, on enzyme levels in the liver, as well as the inflammatory status and antioxidants among patients suffering from NAFLD [44]. The findings of the above study demonstrate that the consumption of DC in the amounts indicated above causes a decrease in weight, BMI, and aspartate aminotransferase (AST) levels in the serum of NAFLD patients. The therapeutic effects of DC as a protective substance for the liver are still not well understood. According to the results obtained in the study, a daily dose of 30 DC (83%) for 12 weeks in patients with NAFLD resulted in an important reduction in BMI and body weight. The mechanism of NAFLD development is related to the existence of overweight and obesity, as well as the place of fat storage in the body (abdominal). Many studies that have investigated the effect of DC on body weight revealed conflicting results. Similar to the results of the aforementioned study [44], Massolt and his coworkers [45] found that the effects of DC consumption (85% cocoa) comprised appetite suppression and possibly lost-weight gain after eating 30 g of chocolate for the 12 subjects. It appears that DC can suppress lipid synthesis and grow lipolysis in adipose tissue, likely by enhancing the bioavailability of nitric oxide (NO) and increasing glucose uptake, intensification of fatty acid catabolism, and glucose oxidation. Moreover, in another study [46], it was found that cocoa is effective in reducing abdominal fat tissue in rats, possibly by changing genes for enzyme expression and transport molecules involved in fatty acid synthesis and thermogenesis in the liver and white adipose tissue (Table 1).
The results of the study of Alavinejad et al. [44] confirmed that the administration of DC for 12 weeks at a dose of 30 g per day can increase the levels of high-density lipoprotein cholesterol (HDL-C) in the serum of NAFLD patients. However, the serum levels of cholesterol such as toral cholesterol (TC), low-density lipoprotein (LDL), and very low-density lipoprotein (VLDL) were not affected after DC supplementation (Table 1). It is clear that postprandial hepatic lipid metabolism may be altered in patients with NAFLD. Furthermore, the suppression of LDL oxidation may be key to its anti-atherogenic attribute. The findings of Alavinejad et al. [44] are consistent with those reported by Hamed et al. [47], who showed that seven days of DC consumption increased serum HDL levels by 9% in 28 healthy volunteers. Another study [48] observed that the daily consumption of 45 g of chocolate rich in flavonoids for 4 months registered a notable rise in serum HDL levels in type 2 diabetes (T2DM) pathology. Additionally, in another study [49], they observed the effects of cocoa supplementation (36.9 g dark chocolate bar and 30.95 g cocoa powder drink) on the lipid profile in healthy subjects for 6 weeks. It can be noted that cocoa components, such as flavonols, include epicatechin, catechin, and procyanidins. These flavonols exhibit anti-inflammatory properties, which can regulate TNF-κB gene expression and reduce inflammatory biomarkers [50] and ROS production.
Elevated levels of liver enzymes (trans-aminases) such as AST and ALT in serum are known markers of NAFLD and liver diseases [51]. In the study by Alavinejad et al. [44], DC consumption for 12 weeks significantly reduced serum AST levels in NAFLD patients. Support for the above study was published by the group of McKim et al. [52], who found that the administration of cocoa extract (400 mg/kg per day) continuously for one month stimulates liver functions together with a notable decrease in fat accumulation, inflammation, and necrosis in early liver injuries caused by administering alcohol in a laboratory model in mice [52].
2.2. Cocoa Butter
Chocolates and cocoa-based products are praised for their “health benefits”, due to their polyphenol content. However, the fat content in chocolate has a supposed connection to the health benefits of chocolate [53]. Palmitic, stearic, lauric, and myristic acids are considered saturated fatty acids, oleic acid is a monounsaturated fatty acid, while linoleic acid is a polyunsaturated fatty acid. In cocoa butter (CB), there are fats that are naturally found in the cocoa beans. CB, also called theobroma oil, is a light-yellow vegetable fat that comes from the cocoa bean and is responsible for the melting properties of chocolate. As mentioned, cocoa butter (CB) consists mainly of palmitic fatty acids (C16:0), stearic acid (C18:0), oleic acid (C18:1), and linoleic acid (C18:2), with low levels of lauric acid (C12: 0) and myristic acid (C14:0) for cocoa butter and a fatty acid profile of approximately 60% saturated fat, 35% monounsaturated fat, and 1% polyunsaturated fat [54]. Although saturated fat is generally thought to adversely raise total cholesterol and LDL cholesterol levels, early studies also suggested that stearic acid may be hypo-cholesterolemic [55]. CB stearic acid can turn in the liver into oleic acid, a monounsaturated fatty acid, wherein oleic acid lowers cholesterol levels (LDL) and increases cholesterol levels (HDL) (Table 1). In addition, CB is a suitable source of Vitamin E [56].
A recent study [57] on rats fed an ethanol-enriched diet that causes alcoholic liver damage in rats evaluated the role of saturated fats from cocoa butter (plant source) compared to lard (animal source). After 8 weeks of feeding, plasma aspartate aminotransferase (AST) and alanine aminotransferase (ALT) activity, liver triglyceride (TG) levels, intercellular adhesion molecule (ICAM)-1 levels, liver cytochrome P450 2E1 (CYP2E1), and liver protein expression were recorded. Interleukin (IL)-1β was significantly increased in ethanol-fed rats. In addition, liver histopathological scores of fatty changes, inflammatory cell infiltration, and atrophy and necrosis were significantly altered. However, fatty changes were significantly inhibited only in rats fed ethanol and cocoa butter as along with inflammatory cell infiltration, degeneration, and necrosis in the liver. In addition, plasma ICAM-1 and hepatic tumor necrosis factor (TNF)-α, IL-1β, IL-6, and IL-10 levels were significantly lower in rats fed ethanol and cocoa butter. Moreover, correlation analysis showed that liver histopathological scores of atrophy and necrosis were significantly positively correlated with erythrocytic oleic acid (C18:1) and negatively correlated with linoleic acid (C18:2). In conclusion, cocoa butter protected the liver from lipid accumulation and inflammation in rats fed chronic ethanol (Table 1).
In summary, the daily recommendation of cocoa butter consumption is one tablespoon of cocoa butter. This tablespoon contains 8 g of saturated fatty acids, including 4 g of stearic acid, 4 g of monounsaturated fatty acids, and 0.5 g of polyunsaturated fatty acids (mostly omega-6) [58].
2.3. Peanut Butter
Peanuts originated in Central America and were afterward spread out to other regions of the world [59]. Today, peanuts are among the most significant legume crops in the world and they are also considered as oilseeds due to their high lipids matter. In addition to oil, a great variety of peanut products such as flour, peanut butter, milk, and more have been developed [60].
Peanuts contain about 50% monounsaturated fatty acids (MUFAs), 33% polyunsaturated fatty acids (PUFAs), and 14% saturated fatty acids. The greatest quantity of fatty acids found in peanuts is represented bytriglycerides, which are from 93.3% to 95.5% of the total fatty acid weight [60]. The standard peanut varieties have an oil profile containing about 52% oleic acid and about 27% linoleic acid. Roasted peanuts contain about 21.5% carbohydrates with starch being the main carbohydrate [61]. Peanuts are considered to have a low glycemic index [62], and the consumption of peanuts or peanut oil is linked with a low risk of cardiovascular disease and may ameliorate the lipid profile [63]. A high intake of peanuts or peanut butter has been linked with protection against the development of diabetes [64]. Peanut butter and even peanut oil, in combination with a slimming diet, allow for maintaining a stable body weight in the long term [65]. Despite these adequate effects of peanut consumption, their effect on fatty liver disease has hardly been studied.
It is clear that some of the health properties of peanuts are related to their nutritional composition, particularly their fat profile. The fat content in conventional varieties is about 50% MUFAs and about 25% PUFAs. Dietary recommendations place great importance on consuming up to 20% of the total daily caloric intake from MUFA oils such as those found in olive oil. One of the main fats in peanut butter is oleic acid. Oleic acid contributes to balancing cholesterol levels, blood sugar, and blood pressure. Managing these levels in the body can lower the risk of heart disease [66].
Oleic acid has also been shown to reduce the body’s resistance to insulin, a condition that raises blood sugar and leads to diabetes. Research shows that the omega-6 content of peanut butter may have the same effect as well. Peanut butter also contains omega-6. This fatty acid decreases low cholesterol (LDL) and increases protective cholesterol (HDL) (Table 1). Additionally, peanuts are a natural source of arginine, an amino acid that may prevent cardiovascular disease by promoting adequate blood vessel function [67].
Peanuts are an excellent source of antioxidants such as manganese, Vitamin E, and B vitamins. These compounds work to prevent and repair cell damage, and this effect can reduce the risk of chronic diseases such as NAFLD [59,61]. Due to the high content of antioxidants and vitamins, peanut butter can improve antioxidant activity in the liver and promote its health. Along with peanuts, peanut butter is also a balanced source of protein that is especially useful for NAFLD patients, as they have many dietary restrictions. One of the most powerful antioxidants in peanut butter is coumaric acid [68]—and a study found that its activity is increased by 22% if the peanuts are roasted before turning them into butter. Peanuts also contain resveratrol, an antioxidant that has been shown to have anti-cancer effects and may lower the risk of obesity, heart disease and cognitive decline. In addition, foods rich in healthy fats, proteins, and fiber—such as peanut butter—take longer to digest, which can contribute to a longer feeling of satiety and reduce the risk of overeating [59].
The recommended daily dose of peanut butter is 2 tablespoons of natural peanut butter (about 28 g). This dose is equivalent to 160–200 calories, it is therefore true that this is a spread high in fat and calories, but at the same time, eating peanut butter will provide a long-lasting feeling of satiety, thus preventing the phenomenon of excessive snacking [69]. When you add snacks, blood sugar levels rise and remain high for a long time, which is not a desirable situation in the prevention of diabetes and NAFLD. In addition, peanut butter has the amino acid tryptophan, which helps improve the quality of sleep, prevents depression or anxiety, helps lose weight, and even improves exercise performance; therefore, peanut butter has become especially common among exercisers and health seekers.
Moreover, peanut butter contains reasonable amounts of available calcium that contributes to strengthening bones, hair, and nails and prevents osteoporosis, it has folic acid and is recommended for pregnant and lactating women, and it has magnesium and potassium that help muscles recover from training [61].
2.4. Caffeine
As we have previously shown, nutritional therapy is important for maintaining the state of satiety [70]. It is carried out in order to degrade glucose, fatty acids, and cholesterol, which influence the metabolism of toxic amyloid beta oligomers [71] with great importance in chronic diseases. Caffeine is used as an appetite suppressant [72], but with age, its delayed metabolism can be involved in triggering NAFLD and type 3 diabetes [73]. Daily drinking of at least one cup of coffee is a common habit for more and more people, which is why consumption has increased exponentially in the recent times [74]. In contrast to previous studies, Hayat et al., 2021, showed that regular and moderate coffee consumption in the healthy population is associated with a low risk of NAFLD. In addition, in patients with NAFLD, it reduces the risk of developing fibrosis [75]. This was evident by stimulating the apoptosis of hepatic stellate cells and the expression of cAMP, the suppression of actin synthesis, along with the inhibition of alpha-smooth muscle actin. Moreover, the expression of procollagen was also suppressed [76]. However, it was difficult to identify the source of caffeine responsible for these effects. Interestingly, of all the caffeinated beverages on the market such as energy drinks, expresso, tea, and soda, only moderate and frequent consumption of normal coffee has been shown to be associated with a significant decrease in fibrosis. In addition, it caused an improvement in liver function by decreasing the activity of marker enzymes such as ALT and GGT [77].
NAFLD in patients with type 2 diabetes is more frequent and severe, and the risk of cirrhosis and liver cancer is much higher. Coffee is a drink composed of several compounds, the main ones being caffeine and chlorogenic acid. Mansour et al. attempted to demonstrate the effects of chronic administration (6 months) of chlorogenic acid and/or caffeine in patients with type 2 diabetes who also presented NAFLD. After 6 months, no improvement in the activity of the liver markers ALT and GGT was observed [78]. Additionally, the supplementation of the two compounds in the modulation of insulin resistance—homeostasis model assessment–estimated insulin resistance (HOMA–IR)—had no effects. The exception was the decrease in cholesterol after caffeine administration and the increase in insulin in the group that received chlorogenic acid plus caffeine.
In the end, they concluded that the administration of 200 mg/day of chlorogenic acid and caffeine in patients with NAFLD did not significantly change the inflammatory, biochemical, and metabolic parameters [78].
MacKenzie et al. reported that caffeine in young adults can decrease insulin sensitivity (400 mg) [79]. Several mechanisms underlying this metabolic change have been suggested, not all of which are fully understood. On the one hand, caffeine inhibits the sensitivity of skeletal muscle for glucose uptake by competitively blocking adenosine receptors. Moreover, glycogen synthase activity is also inhibited [80]. On the other hand, these effects are attributed to the increased concentration of epinephrine and fatty acids that can increase insulin resistance after coffee consumption [80]. However, it remains to be understood whether the positive effects of coffee are the result of other ingredients that annihilate the effects of caffeine on insulin resistance [81]. In rats, it has been observed that the chlorogenic acid from coffee is responsible for lowering glucose concentration. In addition, insulin sensitivity is increased by quinides, which are metabolites of chlorogenic acid [82]. It competitively inhibits glucose absorption in enterocytes. At the same time, by suppressing glucose-6-phosphatase activity, it reduces the synthesis of glucose in the liver [82]. In summary, caffeine from regular coffee may be useful in fibrosis.
3. Sweeteners
3.1. Stevia
Stevia is a natural sweetener extracted from the Stevia rebaudiana plant [83]. This plant has been grown for its sweetness and medicinal purposes for centuries in South America. The plant compounds that provide sweetness are known as steviol glycosides. Rebaudioside A is a glycoside 200 times sweeter than sugar [84]. Of all the steviol glycosides in the plant, Rebaudioside A has the least bitterness.
The consumption of sugar-sweetened beverages is a greater risk factor for the evolution of non-alcoholic steatohepatitis (NASH). Natural sweeteners such as stevia are food additives that provide sweetness without calories and are considered safe and/or not metabolized by the liver [85]. The potential role of sweeteners such as sugar-sweetened beverages are now known to have serious implications for human health. As a result, non-caloric sweeteners such as aspartame, sucralose, saccharin, and Rebaudioside A have gained in popularity and use [86]. Rebaudioside A is an extract of the stevia leaf and provides sweetness without calories. It is worth noting that the literature has shown that Rebaudioside A may in fact play an important role in glucose metabolism and has even been described to improve the postprandial glucose-insulin index [87], and its consumption may result in weight loss (Table 1).
The effect of stevia consumption in the development of NASH began to be clarified recently in a groundbreaking study [88]. The study aims to determine the effect of sweeteners such as Rebaudioside A from stevia and sucralose on NASH using a murine model of NASH and obesity by a high-fat diet and by replacing fructose and sucrose with the aforementioned sweeteners in the drinking water [88]. The authors found that sweeteners had no effect on weight increase and energy balance in high-fat diet-induced obesity. However, compared to fructose and sucrose, Rebaudioside A significantly improved liver enzymes, liver steatosis, and liver fibrosis. In addition, Rebaudioside A induced enhanced gene expressions related to oxidative stress, improved fasting glucose levels, improved insulin sensitivity and caused an increased pancreatic beta cell mass, as well as caused changes in the composition of the microbiome. The findings allowed for the researchers to conclude that Rebaudioside A significantly improved the pathological expression of NASH in mice.
The known relationship between nutrition, human health, and gut microbiota is related to the fact that the microbiome is in tight connection with metabolism and immunity. Additionally, it participates in the development of NASH pathology [89]. The composition and function of the microbiome is rapidly modulated by nutrition, such as the fermentation of undigested carbohydrates [90]. It was recently published that Akkermansia muciniphila bacteria partially counteract obesity and related metabolic diseases [91]. The transplantation of fecal contents from saccharin-fed mice into germ-free mice has been reported to transfer the glucose tolerance phenotype to the recipient mice. In this way, the potential role of microbiome metabolic changes secondary to dietary sweetener consumption was shown [92]. Since Rebaudioside A is not absorbed in the gut [93], the role of the microbiome may be more significant to define the enhanced metabolic outcomes observed in the previous study [88].
The reported data [88] denote that substituting fructose and sucrose with Rebaudioside A as a sweetener may ensure liver protection. They report that the utilization of Rebaudioside A is related to improved glucose tolerance, lower liver fibrosis, and inflammation mediation through lowered oxidative stress (Table 1). For example, Rebaudioside A may have the potential to inhibit hepatic oxidative stress and NFκβ-mediated inflammatory response by upregulating the nuclear factor Nrf2. It was also reported [94] that Rebaudioside A protected human hepatocytes in HepG2 cell culture against carbon tetrachloride-induced oxidative stress and inhibited the development of fibrosis. Specifically, Bacteroides have been reported to efficiently hydrolyze Rebaudioside A to steviol [95]. An inverse relationship with Akkermansia abundance and body weight of mice and humans has also been reported [96]. The study by Xi et al. [88] initially found that the ratio between Akkermansia and Bacteroides was improved by Rebaudioside A compared to sucralose administration.
It can be concluded that stevia has many beneficial effects on the non-alcoholic fatty liver disease (NAFLD) of diabetic rats. Its effectiveness is mainly due to a decrease in oxidative stress and a hypoglycemic effect on the microbiome.
3.2. Sucralose and Saccharin
Compared to the above publications, there are several studies that report that the consumption of several sweeteners may disrupt the diversity of the microbiome in both rats and humans [97,98]. The aforementioned studies suggest that this may lead to glucose intolerance. At the same time, additional studies reported that the consumption of sweeteners has no effect on the abundance of the microbiome and gene function, but the consumption of several sweeteners changes the diversity of the microbiome [99]. Additional studies also showed that a change in microbial diversity may lead to metabolic changes [100]. These artificial sweeteners, as mentioned, have been reported to be associated with dysbiosis (Table 1). Dysbiosis, by definition, is “an imbalance in the bacterial community in the intestines associated with diseases” [101]. According to this definition, it can be safely said that, despite previous misconceptions, some sweeteners “unequivocally and irrefutably” disrupt the gut microbiota [95]. Nevertheless, different sweetener formulations may have different effects. Moreover, there are several questions about the extent and nature of what happens after consuming certain sweeteners.
Sucralose is one of the most widely consumed sweeteners worldwide. It is 600 times sweeter than sucrose. Studies indicate that sucralose may cause dysbiosis by reducing the total number of aerobic and anaerobic species, Bifidobacteria, Lactobacilli, Bacteriodes, and Clostridiales [102]. Another study showed that it can increase Clostridium XIVa strains in mice [103]. Saccharin, also one of the most common sweeteners in the world, has been studied for its possible role in dysbiosis. Recent data indicate that saccharin may inhibit the growth of six bacterial strains: three species of lactobacilli and three strains of E. coli [95] (Table 1). Another study found that saccharin increases the genus Bacteriodes and, similar to the previous study, decreases the number of lactobacilli [98]. An important issue that can be emphasized is that most of the available information is based on animals. There is a significant need to examine this possible relationship in human subjects with different dietary approaches due to the fact that, in humans, there are many factors that affect the gut microbiota, and the most important is the diet pattern. In a recent study, it was suggested that, by following a large group of human subjects, the researchers were able to find associations between the consumption of sweeteners and a disturbed microbiota [98]. In summary, the use of sucralose and saccharin sweeteners is not recommended.
3.3. Maltitol
Maltitol is a polyol produced by the hydrogenation of maltose [104]. It has a low caloric value and a low glycemic index [104]. Moreover, maltitol has sweetening and satiety effects equivalent to sugar and is currently used as a sugar substitute by diabetic patients.
The cholesterol levels may be suppressed by many indigestible components such as dietary fiber and beta-glucan, through the absorption in the intestine of bile acids [105]. Propionic acid produced by the intestinal bacterial flora, in addition to indigestion, may inhibit cholesterol synthesis in the liver, determining a decrease in blood cholesterol concentration [106,107] (Table 1). Additional studies demonstrate that indigestible fibers as well as soluble fibers, such as maltitol, absorb bile acids in the intestine and reduce circulating bile acid levels, which leads to the activation of bile receptors in the liver and an increase in circulating bile acid levels [108] (Table 1).
A study by Urushima et al. [109] demonstrated that supplementation with maltitol suppressed obesity, hyperglycemia, hypercholesterolemia, and fatty liver degeneration in mice fed a high-fat diet. Therefore, maltitol may be useful for treating patients in the initial stages of fatty liver disease to improve steatohepatitis. They demonstrated that maltitol ameliorates non-alcoholic fatty liver disease by activating the Nrf2 antioxidant capacity.
3.4. Erythritol
Erythritol (Ery) is a natural polyol sweetener derived from corn, wheat, and other starches. It has an extremely low energy value and a variety of biological functions. Erythritol contains 0.2 calories per gram and is about 60–80% sweeter compared to sugar. Studies have found that the long-term administration of Ery has no effect on the body weight and glucose tolerance of young/adolescent mice [110]. Another study found that Ery can alleviate metabolic disorders in mice induced by a high-fat diet (HFD), including dyslipidemia, impaired glucose tolerance, and obesity [111]. Ery also has an effect of reducing oxidative stress in diabetic rats [112]. It has been shown that erythritol can effectively inhibit hepatic lipid accumulation and alleviate hepatic oxidative damage in HepG2 cells induced by fatty acid treatment and in high-fat diet-induced NAFLD models [113] (Table 1). The potential mechanism of its protective effect is that erythritol exerts an antioxidant function by activating the Nrf2 signaling pathway, thus inhibiting endoplasmic reticulum stress and lipid accumulation and then playing a role in alleviating NAFLD (Table 1).
Erythritol is absorbed relatively rapidly in the small intestine. Due to the fact that it does not stay in the intestines for long, it cannot attract water—the main cause of watery diarrhea from sweeteners such as maltitol. This is why erythritol causes diarrhea less frequently than other sweeteners such as xylitol [114].
In 64 healthy young adults, erythritol caused fewer digestive problems (bloating, gas) even at a high dose (50 g), compared to xylitol [115]. Therefore, the maximum intake of erythritol is 0.7 to 1 g per kilogram of body weight. In summary, some sweeteners can be used as an adequate alternative replacement for sugar.
4. Glutathione and NAFLD
Glutathione, γ-L-glutamyl-L-cysteinyl-glycine, is a tripeptide present in every cell in the human body [116]. Although its roles are complex and remain the subject of ongoing research, glutathione is thought to play crucial roles in cellular detoxification and antioxidant systems, due to the fact that a reduction in glutathione levels in cells has been found to increase the risks of disease and poisoning. Accordingly, direct intravenous injection of glutathione has been used to treat patients with chronic liver diseases and poisonings [117].
Glutathione is formed in cells from glutamic acid, cysteine, and glycine. Cysteine and glycine are formed from methionine and serine, respectively, and glutamic acid is synthesized from α-ketoglutarate, a metabolite of glucose. These amino acids are usually supplied from food. It was reported that oral administration of glutathione did not change blood glutathione and glutathione disulfide levels [118]. It has been suggested that, when glutathione is administered orally, it breaks down into amino acid components and does not exert specific activity beyond the amino acid source. However, it has been reported that glutathione can pass through the Caco-2 cell layer (in vitro system) without degradation [119]. In addition, Park et al. demonstrated an increase in the protein-attached form of glutathione in human blood after oral consumption [120]. These studies indicate that glutathione administered orally is absorbed into the blood and can influence the redox state in the human body.
Glutathione has a long history of treating chronic liver disease by injecting it into a vein. A recent study [121] demonstrated the therapeutic effect of oral glutathione in patients with NAFLD. The main result of this study was a change in ALT levels. The patients with oral treatment of glutathione (300 mg per day) for 16 weeks exhibited a drop in ALT levels as well as low in triglycerides, NEFA, and ferritin levels (Table 2).
The findings of this study reveal the beneficial effects of glutathione administered through the mouth for NAFLD patients. As we mentioned before, the explanation for this is that glutathione is broken down into amino acids during digestion and absorption processes. The claim today is that, by intaking glutathione orally, it can be used as a source of amino acids in the synthesis of endogenous glutathione. The addition of large doses of glycine and serine (components of glutathione), can also attenuate the severity of NAFLD in humans [121]. In the above study, since the glutathione dose was 300 mg per day, the amount of cysteine potentially released from 300 mg glutathione is less than 120 mg, an amount which can be obtained from between 10 and 20 g of meat or 100 mL of milk. Therefore, it is highly unlikely that the above dose of oral glutathione attenuates the pathogenesis of NAFLD through an amino acid source for glutathione synthesis.
It has been indicated that the level of glutathione in its protein-bound form increases 1–2 h after glutathione ingestion, demonstrating that orally administered glutathione is absorbed into the blood [120]. This protein-bound glutathione may be transported to the liver, thus weakening the hepatitis.
Protein-bound glutathione levels have been reported to return to baseline levels after an overnight fast [122]. In a study by Park et al. [122], they found that the baseline level of the protein-bound form of glutathione decreased significantly after an overnight fast after 16 weeks of glutathione administration. Protein-bound glutathione levels in patients were significantly higher than those of healthy volunteers in previous studies [122] assessed using the same method. Glutathione treatment also lowered protein-bound glutathione to normal basal levels. These findings indicate that oral administration of glutathione may increase the incorporation of protein-bound glutathione in the liver or reduce the pathological secretion of glutathione from the liver.
NAFLD is a complex disease. Its pathogenesis involves various factors, including lipotoxicity, insulin resistance, gut/nutrient-derived signals, oxidative stress, adipocytokines, and genetic factors. In NAFLD, about 20–80% of patients reported dyslipidemia [123]. A previous study indicated that the administration of glutathione accelerates fatty acid utilization by increasing levels of the γ-activated receptor PPR-1α and mitochondrial DNA with reduced levels of nonesterified fatty acids in plasma [124].
An increase in body ferritin and iron stores has been frequently found in NAFLD patients [125]. Ferritin and iron can promote the development of NAFLD through oxidative stress [126]. Results from an experiment conducted on various populations showed that oral administration of the antioxidant Vitamin E improved liver dysfunction and the pathological conditions of NASH [127]. However, long-term treatment with Vitamin E has been associated with increased all-cause mortality and prostate cancer risk [128], suggesting the need to evaluate the efficacy and safety of this agent. In the aforementioned study [127], treatment with glutathione significantly decreased ferritin levels, but the mechanism behind the decrease remains unclear. Glutathione suppresses hyperferritinemia and oxidative stress and has therapeutic effects in patients with NAFLD. Liver fat assessed noninvasively showed that it tended to decrease in all patients and decreased significantly in responders in the decrease of the ALT enzyme after 4 months of glutathione treatment. However, the relationship between histological improvement of liver steatosis and reduction in liver fat values has not yet been determined, including whether glutathione administration may reduce liver steatosis.
Additionally, in the aforementioned work [127], they investigated other factors that could be related to the adequate effects of glutathione. They found that HDL cholesterol and LDL cholesterol levels were higher and that HbA1c levels were lower in patients who responded with a decrease in the ALT enzyme than in those who did not react (Table 2). In summary: glutathione has a therapeutic effect of oral glutathione in NAFLD patients.
5. Whole Milk or Low-Fat Milk for Fatty Liver
Milk is an important part of the diet of most people. According to the recommendations, there is no difference in choosing whole milk or low-fat milk if you drink up to one glass of milk per day; it should not pose a significant problem. The benefit of milk in fatty liver is reflected in the consumption of the protein found in milk. Milk protein consumption has been shown to be inversely related to the development of NAFLD [129].
Several possible mechanisms may explain the association of increased protein intake from milk with reduced risk of incident NAFLD. First, mitochondria are considered essential for the development of NAFLD [130]. Reduced β-oxidation, together with increased lipogenesis, production of reactive oxygen free radicals, and damage to hepatocytes lead to lipid accumulation as well as inflammation and fibrosis in hepatocytes [130]. Increased production of oxidative radicals and depletion of antioxidants such as glutathione, Vitamin E, β-carotene, or Vitamin C in the liver may occur in NASH [131]. In a study conducted on rats [132], a significant increase in glutathione was observed only when rats were fed whey protein hydrolysates and β-lactoglobulin. A higher intake of milk protein may (1) help suppress NAFLD synergistically with exercise, and (2) prevent sarcopenia, a known risk factor for NAFLD [133]. Insulin resistance is a key factor in the development of NAFLD. A prospective study in a population of young adults found an inverse relationship between the frequency of consumption of dairy products and the development of insulin resistance syndrome [134].
6. Soluble Dietary Fiber (FDS)
Many recently published studies have revealed that bacterial flora and dietary microbial metabolites, such as short-chain fatty acids (SCFAs), contribute to homeostasis as well as to the prevention of the development and progression of various diseases in humans including NAFLD and NASH [135]. Recently, an imbalance in the microbiota, in the bacterial flora of the digestive system, known as dysbiosis, has been indicated, and is involved in a variety of metabolic diseases, including fatty liver [136].
In NASH, dysbiosis can result from an unbalanced diet or obesity, and a reduction in SCFA production can lead to a dysfunctional intestinal mucosal barrier and immunological disturbances [137]. Studies have shown that a large number of pathogen-related molecules can reach the liver through the disrupted intestinal mucosal epithelial barrier, causing the hypersensitivity of Kupffer cells and potentially leading to NAFLD and NASH [138].
A recently published study attempted to explain the mechanism underlying the possible amelioration caused by the administration of fructo-oligosaccharide (FOS) in improving NASH disease in mice. They reported that increased SCFA production by the bacteria provided nutrients to intestinal epithelial cells, thus improving intestinal barrier function. The effect was also to increase immunoglobulin A production and suppress Kupffer cell activation in NASH-induced mice [139].
There are three other potential explanations for the observed beneficial effects of SCFA in the study in NASH. First, SCFAs stimulate the secretion of peptide-1 (GLP-1) from the L cells in the gastrointestinal tract [140], given that several recent clinical studies have demonstrated that treatment with GLP-1 can regulate the accumulation of lipids in the liver [141]. Additional studies have shown the effectiveness of GLP-1 in the treatment of NAFLD [142]. Second, adipocytes express G-type receptor (GPR43) SCFAs [143]. It was recently reported that GPR43 can act to suppress insulin signaling in adipocytes and inhibit fat accumulation in adipose tissue as well as promote lipid and glucose metabolism in the liver [144]. Third, SCFAs can act as potential ligands for the peroxisome proliferator-activated receptor γ- (PPARγ), and as a result can result in improved insulin sensitivity (Table 1). It was also reported that a significant reduction in the improvement of steatosis was caused by the administration of SCFAs in the liver in mice lacking PPARγ in the liver [145]. It was also reported that the addition of butyrate to the diet caused a decrease in pro-inflammatory markers such as interleukin-6 and nuclear factor-kappa-beta (NF-κB), thereby raising the threshold for inflammatory reactions in the liver of rats fed a high-fat diet [146] (Table 1).
Table 1.
The influence of bioactive aliment compounds on NAFLD.
| Nutrients/Category | Effects on Liver | Effects on Intestinal Microbiota | References |
|---|---|---|---|
| Dark chocolate | 1. Positive effects on the lipid profile, reducing total and LDL cholesterol levels and increasing HDL levels | [36] | |
| 2. Improve insulin resistance through reducing oxidative stress, improving endothelial function, and/or altering glucose metabolism | [39] | ||
| 3. Decrease aspartate aminotransferase (AST) levels in the serum of NAFLD patients | [44] | ||
| 4. Increase glucose uptake, increase fatty acid and glucose oxidation, inhibit lipid synthesis | [45] | ||
| 5. Anti-inflammatory properties, which can regulate the TNF-κB gene expression and reduce inflammatory biomarkers and ROS production | [50] | ||
| Cocoa butter | 1. Lowers cholesterol LDL levels and increases cholesterol HDL levels | [56] | |
| Peanut butter | 1. Lowers cholesterol LDL levels and increases protective cholesterol HDL levels. | [67] | |
| 2. Prevents cell damage and induces cell repair, effects associated with reduced risk of chronic diseases such as NAFLD | [59,61] | ||
| Caffeine | 1. Lowers the risk of NAFLD in healthy people | [75,77] | |
| 2. Reduces the risk of developing fibrosis | |||
| Sweeteners | |||
| Stevia | 1. Plays a role in glucose metabolism and has even been reported to improve the postprandial glucose–insulin index | [87] | |
| 2. Significant improvement of liver enzymes blood levels, improvement of liver steatosis and liver fibrosis | 1. Causes changes in the composition of the microbiome. | [88] | |
| 3. Decreased inflammation associated with oxidative stress | 2. An inverse relationship with Akkermansia abundance associated with body weight of mice and humans | [96] | |
| 4. Lower gene expression related to oxidative stress. Improve fasting glucose levels and improve insulin sensitivity | |||
| Sucralose and saccharin | 1. Sucralose may cause dysbiosis by reducing the total number of aerobic and anaerobic species, bifidobacteria, lactobacilli, Bacteriodes, and Clostridiales | [102] | |
| 2. Saccharin may inhibit the growth of six bacterial strains: three species of lactobacilli and three strains of E. coli in animal models | [95] | ||
| 3. Associations between the consumption of sweeteners and a disturbed microbiota | [98] | ||
| Maltitol | 1.Suppress cholesterol synthesis in the liver leading to a decrease in circulating cholesterol levels | [106,107] | |
| 2. Maltitol mimics indigestible fibers, absorbs bile acids in the intestine, and reduces circulating bile acid levels, which leads to the activation of bile receptors in the liver and an increase in circulating bile acid levels | [108] | ||
| 3. Prevents obesity, hyperglycemia, hypercholesterolemia, and fatty liver degeneration in mice fed a high-fat diet | [109] | ||
| Erythritol | 1. Long-term administration of Ery has no effect on body weight and glucose tolerance of young/adolescent mice | [110] | |
| 2. Alleviate metabolic disorders in mice induced by a high-fat diet (HFD), including dyslipidemia, impaired glucose tolerance, and obesity | [111] | ||
| 3. Inhibits hepatic lipid accumulation and alleviate hepatic oxidative damage in HepG2 cells induced by fatty acid treatment and in high-fat diet-induced NAFLD models | [113] | ||
| 4. Exerts an antioxidant function by activating the Nrf2 signaling pathway, thus inhibiting endoplasmic reticulum stress and lipid accumulation and then playing a role in alleviating NAFLD. | |||
| Soluble dietary fiber (FDS) | 1.Improvement caused by the administration of fructo-oligosaccharide (FOS) in improving NASH disease in mice | 1. Improvement of intestinal barrier function | [139] |
| 2. Regulate the accumulation of lipids in the liver | [141] | ||
| 3. Inhibit fat accumulation in adipose tissue as well as promote lipid and glucose metabolism in the liver | [144] | ||
| 4. Provide potential ligands for the peroxisome proliferator-activated receptor γ- (PPARγ), and as a result can result in improved insulin sensitivity | [145] | ||
| 5. Decrease expression of pro-inflammatory markers such as interleukin-6 and nuclear factor-kappa-beta (NF-κB), thereby raising the threshold for inflammatory reactions in the liver of rats fed a high-fat diet | [146] |
LDL, Low-density lipoprotein; HDL, High-density lipoprotein; VLDL, Very low-density lipoprotein; TNF-α, Tumor necrosis alpha; NF-κB, Nuclear Factor-Kappa beta; ROS, Reactive oxygen species; AST, Aspartate aminotransferase; ALT, Alanine transaminase; NASH, Non-alcoholic steatohepatitis; NAFLD, Non-alcoholic fatty liver disease; TGs, Triglycerides; MDA, Malondialdehyde; IL-1β, Interleukin-1 beta; IL-6, Interleukin-6.
A current study found that FDS significantly increased the concentration of propionic acid in the serum of NASH-induced mice and decreased the mRNA expression levels of the rate-determining enzyme for glycerolipid-glycerol-3-phosphate acyltransferase [147]. It has been reported that propionic acid decreases the hepatic mRNA and protein expression of lipid biosynthetic enzymes [139,148], increases the expression of glucose transporter type 4 (GLUT4), improves insulin sensitivity [149], and inhibits lipopolysaccharide (LPS)-stimulated TNF α release by neutrophils [150]. In summary, FDS inhibits fat accumulation in adipose tissue as well as promotes lipid and glucose metabolism in the liver.
7. Soy Lecithin as a Source for Choline and Inositol
Lecithin in the context of NAFLD is considered one of the most important sources of choline and inositol. There is a general lack of awareness about the importance of the essential nutrient choline. This is reflected in the consumption of less than recommended levels of choline by most people. In fact, this relatively low intake of choline by the general population may be directly linked to the great incidence of NAFLD in the Western world, as choline deficiency is known to cause “fatty liver” or steatosis in animals and therefore similarly in humans. Despite the current reported deficiencies, the importance of the nutrient choline has long been demonstrated [151]. Despite the possibility that choline can be obtained from its endogenous biosynthesis, the American Institute of Medicine recognized its nutritional importance and established the minimum values for choline already in 1998.
It is well known that optimal nutrition is extremely important in reducing the risk of metabolic disorders. Maintaining liver health has also been linked to adequate intake of certain nutrients; one of the most important is choline. Choline and phosphatidylcholine (PC), and therefore lecithin, are known to prevent the development of fatty liver [152]. There is now consensus that the potential for increased consumption of choline and lecithin may prevent metabolic pathologies of the liver and other parts of the body [153].
Dietary choline deficiency has multiple consequences for human health, including birth defects, neurological dysfunction, and the development of fatty liver [154]. With regard to steatosis, or fatty liver, choline deficiency has been shown to play an important role. Phosphatidylcholine (PC), found in lecithin, as mentioned above, is an essential structural component of VLDLs and is required for its secretion and the export of triglycerides (TGs) from the liver [155]. Therefore, choline and subsequent PC deficiency may cause fatty accumulation in the liver. In addition, the roles of betaine in homocysteine methylation, antioxidant activity, and AMP-activated protein kinase (AMPK) stimulation have been investigated in relation to NAFLD. In fact, choline and betaine have been shown in animal and human studies to prevent and even ameliorate NAFLD [155] (Table 2).
Currently, only low quantities of lecithin are used in processed foods, usually due to its role as an emulsifier. Increasing the lecithin content in foods for the purpose of improving human health may definitely be an advantage since many people rely on processed foods and do not consume natural sources of choline. A functional food enriched with lecithin will definitely benefit foods that are natural sources of choline, such as soy products, milk, and peanut butter [156]. This may also be an effective strategy in light of the research showing that the consumption of lecithin by humans effectively increases choline levels [157]. Increased levels of lecithin may play a direct role in the prevention of NAFLD based on the essential role of PC as an essential phospholipid in ensuring adequate TG export from the liver (Table 2). Thus, with increased lecithin intake, the incidence of NAFLD can be reduced in the general population. However, the success of functional food depends, at least in part, on consumer awareness of the aforementioned nutrient.
In summary, choline, phosphatidylcholine, and lecithin are associated with preventing the development of fatty liver.
8. Turmeric and Curcumin Extracts
Turmeric (Curcuma Longa) has active ingredients called curcuminoids with the most prominent curcuminoid being called curcumin. In vitro and animal studies, turmeric has demonstrated potent antioxidant, anti-inflammatory, and anti-fibrotic properties, as well as insulin-sensitizing effects [158]. As such, it may be promising in the treatment of patients with NAFLD.
Several controlled studies investigated the effects of curcumin consumption on anthropometric measures including BMI and body weight and in patients suffering from NAFLD [159,160]. The essential findings of these studies showed that curcumin supplementation (a dose ranging from 80 to 1500 mg per day) significantly dropped BMI and waist circumference in NAFLD patients [159,160].
Even though curcumin has a very low water solubility, it represents an option for use as a dietary supplement and drug [161]. Therefore, its effectiveness on health variables in randomized controlled trials is limited [162,163]. In recent years, researchers have attempted to develop a more water-soluble and more available form of curcumin such as an amorphous form, phospholipid complexes, the addition of piperine (black pepper), liposomal curcumin, and nanoparticles [164]. The nonlinear dose–response test showed the significant effects of nanocurcumin (80 and 400 mg/day) on abdominal obesity, while the natural forms of curcumin (1000 and 1500 mg/day) had less effect on this parameter [159].
Several mechanisms have been proposed for the effects of anti-inflammatory diets and their components, such as curcumin on obesity, and this includes the inhibition of lipogenesis and inflammation (reduction in pro-inflammatory cytokines), the suppression of angiogenesis in adipose tissue, the reduction in preadipocyte differentiation, the increase in lipolysis, and the activation of brown fat. Additionally, the increased energy metabolism of adipocytes and/or the induction of apoptosis increased the expression of neseptin levels in serum and probiotic-like effects [165,166,167]. The effects of nesceptin include loss of appetite, reduction in body fat, anti-inflammatory activities, anti-hyperglycemic activity, as well as metabolic and neuroendocrine regulation [168,169]. Thus, the improvements in NAFLD, anthropometric indices, inflammation, glucose, and lipid metabolism, and subsequently the increased levels of neseptin by curcumin supplementation can be noted. Additionally, curcumin may reduce total body fat by increasing the basal metabolic rate [170].
The enzyme 1β-hydroxysteroid dehydrogenase 1 is expressed in adipose tissue and the liver. This enzyme can increase the cortisol level in visceral (abdominal) fat by changing the inactive form of the cortisol hormone to its active form [171]. A high amount of cortisol hormone in adipocytes can cause adipogenesis and consequently central obesity [172]. Therefore, the inhibition of the enzyme 11β-hydroxysteroid dehydrogenase 1 can be effective for reducing visceral fat and treating the metabolic syndrome. According to a previous study, curcumin can act as an inhibitor of this enzyme [173].
Curcumin reduces body fat mass by inhibiting adipocyte differentiation through the suppression of peroxisome proliferator-activated receptor-γ and by increasing adenosine monophosphate-activated protein kinase, resulting in lipolysis [174]. From all of the above, it can be assumed that curcumin supplementation (especially nano-curcumin) may have a moderate effect on BMI in patients with NAFLD.
The current therapeutic strategies for the treatment of NAFLD and NASH are mainly aimed at correcting and changing risk factors such as obesity, diabetes, and hyperlipidemia. However, there are several studies that indicate that curcumin can also intervene in the oxidative stress that occurs in NAFLD [160]. Additionally, the turmeric plant (Curcuma longa) or its component is indeed likely able to protect the liver with an anti-oxidant mechanism; therefore, it has been used not only as a spice but also as a traditional medicine for many centuries, and its properties have been reported in the literature [175]. A clinical trial was recently conducted to evaluate the potential role of orally administered turmeric on liver enzymes, lipid profile, oxidative stress status, malonaldehyde (MDA), and degree of hepatic steatosis [176]. The study included 62 patients who were randomly divided into intervention groups or placebo (wheat flour). The participants in the intervention group received a turmeric supplement (2 g per day) as oral capsules and the other group received a placebo. The intervention period was 8 weeks and the subjects were advised to consume their capsules after the main meals to improve absorption in the small intestine due to the presence of fat in the diet. The study revealed that taking a supplement containing 2 g per day of turmeric for a period of 8 weeks resulted in a significant decrease in the degree of steatosis compared to the beginning as seen in the liver ultrasound tests. The results of the clinical study showed that supplementation with turmeric extracts reduces elevated serum ALT and AST levels in patients with NAFLD (Table 2). A decrease in these two enzymes can indicate an improvement in liver function. Therefore, it can be considered as an adequate therapeutic supplement with hypolipidemic and antioxidant properties for this disease. However, the reports in other studies do not always support the above results.
Previous studies have also highlighted the mechanism of the hepatoprotective effect of curcumin [177] in both in vitro and in vivo studies. The antioxidant capacity of curcumin in scavenging free oxygen radicals, reactive nitrogen molecules, and lipid radicals is one of the most important mechanisms [178]. The role of oxidative stress and inflammation in inducing hepatocyte injury and progression of NAFLD has long been established in previous studies [179]. Curcumin treatment also enhances the activities of detoxifying enzymes such as glutathione-S-transferase, glutathione peroxidase, glutathione reductase, catalase, and he-oxygenase-1, and thus suppresses oxidative stress in the liver [179,180,181]. Curcumin inhibits the activation of key mediators of cellular inflammation such as 5-lipoxygenase (5-LOX), NF-κB, and cyclooxygenase-2 (COX-2). These are involved in the stimulation of different genes including several pro-inflammatory and cytotoxic cytokines such as TNF-α, IL-1, IFN-γ, and NF-KB [182,183]. Curcumin also suppresses the activation and proliferation of stellate cells in the liver, which have a known role in the progression of liver fibrosis [184] (Table 2). A decrease in hydroxyproline content in the liver and a downregulation of collagen mRNA synthesis after curcumin administration supports this claim [185].
9. Silymarin
Silymarin is derived from an extract from milk thistle (Silybum marianum L.), a plant that originates from southern Europe through Asia with the active compound silymarin, which is a natural flavonoid. Silymarin consists of four flavonolignan isomers, namely silybin, silychristin, silydianin, and isolibin [186], and it is widely used as an over-the-counter preparation for liver diseases [187]. Its beneficial effect on the liver is attributed to possible anti-inflammatory, antioxidant, and anti-fibrotic activity. Silymarin also reduces insulin resistance [188]. It has been tested in the treatment of various liver diseases, exhibiting adequate results with remarkable safety [189].
Today, it is the most studied plant for the treatment of liver diseases and the most common over-the-counter treatment for liver diseases [190]. Several studies have indicated its promising antifibrotic activity in liver injury in experimental systems [191,192] (Table 2). Silymarin may reduce liver inflammation by inhibiting lipooxygenase activity and reducing the function of leukotrienes and their effect on Kupffer cells in the liver and by reducing oxidative stress by increasing glutathione levels [193].
Treatment with milk thistle extract significantly improved serum superoxide dismutase activity and malondaldehyde (MDA) levels in rats in which NAFLD was induced [192]. Milk thistle extract treatment reduced serum aspartate aminotransferase enzyme levels and levels of triglycerides (TGs), as well as cholesterol including VLDL in NAFLD-induced rats (Table 2). Treatment with milk thistle extract preparations also effectively protected the liver against histological changes. From these data, it can be concluded that treatment with milk thistle extract preparations can be a promising medicinal option for the treatment of NAFLD.
Recently, several clinical trials have been conducted to treat NAFLD with milk thistle extract preparations. A recent randomized controlled trial (RCT) [194] concluded that silymarin was effective in reducing alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels compared to the placebo treatment. In a double-blind, placebo-controlled RCT by Kheong et al. [195] with adults with NASH, it was demonstrated by biopsy that 48-week silymarin treatment did not lead to an improvement in the NAFLD activity score compared to the placebo group, but it did lead to a significant improvement in fibrosis after repeat liver biopsies. Another clinical trial in NAFLD patients, conducted by Solhi et al. [196], tested the effect of silymarin after a treatment period of 8 weeks. They demonstrated that there was a marked improvement in transaminase levels compared to placebo. Besides the mentioned RCTs that tested silymarin monotherapy in NAFLD/NASH versus placebo treatment, other studies with methodological shortcomings or not placebo-controlled were also performed [197,198].
In conclusion, turmeric, curcumin extracts, and silymarin may be therapeutic for NAFLD patients.
10. Selenium (Se)
There are animal studies that indicate a link between selenium (Se) supplementation and NAFLD. Although most indicate a beneficial effect, some report a negative effect of Se supplementation on NAFLD.
10.1. Evidence Suggesting a Beneficial Effect of Se on NAFLD
Rats fed a Se-deficient diet exhibited a decrease in reduced glutathione (GSH) in the liver and an increase in the ratio of n-6/n-3 fatty acids, which are considered to be detrimental to NAFLD [199]. Additionally, mice fed a Se-deficient diet exhibited an increase in lipid peroxidation and a decrease in glutathione peroxidase (GPx) and thioredoxin reductase activity in the liver, suggesting increased oxidative stress and impaired antioxidant capacity [200], both of which are strongly associated with the pathogenesis of NAFLD [201]. Furthermore, rats fed a Se-deficient diet had lower levels of Se in the liver, which resulted in changes in hepatocyte cells, such as abnormal chromatin and mitochondrial swelling, as well as more severe fibrosis around the portal vein, upregulation of metalloproteinases, and downregulation of tissue inhibitors of metalloproteinases type 1 and 3, all of these changes being associated with increased liver inflammation [202].
Liver fibrosis induced by N-Nitrosodimethylamine treatment in rats was associated with decreased circulating Se, decreased hepatic GSH and GPx, and increased circulating IL-6 and TGFβ1 cytokines. Another study demonstrated that low Se levels and consequently lower -GPx activity may impair cellular antioxidant defense, leading to oxidative stress and liver fibrosis [203]. In addition to the link between Se deficiency and NAFLD, there are also studies that indicate a positive effect of Se supplementation, alone or in combination with other drugs, on liver function tests and liver steatosis (Table 3).
Se supplementation improved abnormal liver functions induced by carbon tetrachloride (CCl4) treatment in rats [204]. The administration of Se plus magnesium prevented high-fat diet (HFD)-induced lipid increase in rats, possibly by enhancing the activity of several antioxidant enzymes [205]. Another study showed that the administration of a combination of Se and zinc improved the lipid profile, liver functions, and liver steatosis in rats [206]. Additionally, the administration of Se and probiotics reversed the negative effect of feeding in mice on HFD and improved liver functions and steatosis [207]. Another study demonstrated that administering selenomethionine (the organic form of selenium which is the same form found naturally in foods such as grains, soybeans, and yeast) to rats induced steatosis. The improvement was accompanied by increasing GPx activity, weakening the steatosis in the liver and allowing for the appearance of hepatocytes in a balloon-like configuration (a hallmark of hepatitis) [208]. The effect of Se supplementation on liver fibrosis, the principal histological prognostic factor for advanced disease observed in the study [208], appears to be even more important. Other studies demonstrated that Se supplementation decreased the number of hepatic stellate cells (HSCs) and liver fibrosis induced by CCl4 treatment in mice [209]. It should be emphasized that HSCs are considered to be key players in the pathogenesis of liver fibrosis [210] (Table 3).
10.2. Evidence Suggesting a Negative Effect of Se Administration on NAFLD
Contrary to the evidence presented above, some articles reported a negative effect of Se administration on NAFLD, which, at the same time, may be related to exposure to high doses of Se (higher doses and/or longer duration). Early reports suggested that oral administration of Se for 2 months in rats induced the formation of nodular regenerative hyperplasia with sinusoidal damage in certain areas of the liver [211]. These areas are found around nodules (perinodular areas) and in which atrophic hepatocytes appear around capillary sinusoids but without fibrosis [212]. Based on these findings, another study showed that a Se-enriched diet induced hepatic nodular hyperplasia in rats [211].
11. The Enzyme Stearoyl-CoA Desaturase 1 (SCD1) in NAFLD and the Use of Supplements That Lower Its Activity
In many tissues, stearoyl-CoA desaturase 1 (SCD1) catalyzes the biosynthesis of monounsaturated fatty acids (MUFAs), (i.e., palmitoleate and oleate) derived from their saturated fatty acid (SFA) counterparts (i.e., palmitate and stearate), leading to broad effects in terms of physiology in humans. In addition to its main role in fatties metabolism and body weight control, SCD1 has recently appeared as a potential new target for the treatment of various diseases, such as cancer, Alzheimer’s disease, skin disorders and, in our case, NAFLD [213].
Under normal conditions, lipogenesis and lipolysis are in dynamic balance. Signals arrive from both the central nervous system, as well as peripheral tissues, inducing the balance of synthesis and breakdown of triglycerides. There are two different sources of lipogenesis in the endogenous formation of fats (de novo lipogenesis). In tissues with a high metabolic rate, such as the liver or adipose tissue, de novo lipogenesis is more active, although every single cell is capable of performing lipogenesis. In particular, human adipose tissue appears to be the primary tissue in which de novo lipogenesis takes place [214]. This type of lipogenesis is characterized by the conversion of carbohydrates into fatty acids, which are then stored as triglycerides if the body does not need energy. This process begins with the glycolysis of carbohydrates to obtain acetyl-CoA. The enzyme acetyl-CoA carboxylase 1 (ACC1) converts acetyl-CoA into malonyl-CoA, which is then converted to palmitate by fatty acid synthase (FASN) [214]. Finally, the last step of de novo lipogenesis is carried out by stearoyl-CoA desaturase (SCD), the first rate-limiting enzyme involved in desaturation [215]. SCD catalyzes the reaction in converting palmitoyl-CoA to palmitoleoyl-CoA, in a reaction that also involves redox by nicotinamide adenine dinucleotide (NADH), flavoprotein cytochrome β5 reductase, after receiving the electrons from cytochrome β5 [216]. In addition to the palmitic acid reaction, stearic acid is also one of the major substrates of SCD, which is ultimately converted into oleic acid [217]. SCD also catalyzes the conversion of myristic acid into myristolic acid [218] but this reaction is carried out to a lesser extent.
De novo lipogenesis is triggered when blood glucose and insulin levels are increased [214]. These nutrients cause the activation of the transcription factor ChREBP by using another transcription factor, SREBP-1c, and the liver type receptor (X -LXR). These transcription factors are specific and their activation promotes lipogenesis again [215]. Thus, the hepatic expression of SCD-1 is induced after high-carbohydrate consumption through an SREBP-1c-dependent mechanism involving the binding of LXR to the LXR response to the promoter activation of SCD-1 through the transcriptional activation of SREBP-1c [219]. Pharmacologically administered SCD1 inhibitors have been tested with adequate results in NAFLD, diabetes, dyslipidemic failure, and hepatitis C virus infections [220]. For example, a drug called MK-8245 was developed and is currently in advanced clinical trials in humans, including against NAFLD [221].
Researchers have reported that an SCD inhibitor called Daiichi Sankyo [222] may be effective for the treatment of NASH. The Daiichi Sankyo compound was given orally once daily at a dose of 30 or 100 mg per kg to rats fed a methionine-deficient choline diet for 2 months before treatment of the SCD inhibitor. After 1 month of administration with the above compound (100 mg/kg), there was a reduction in the accumulation of triglycerides in the liver of rats suffering from NASH by 80% (Table 4). The Daiichi Sankyo compound also reduced the increase in aspartate aminotransferase (AST) enzyme levels and alanine transaminase (ALT) by 86% and 78%, respectively. Hepatic steatosis, hepatocellular degeneration, and inflammatory cell infiltration were also treated after treatment with this compound (Table 4).
Adverse Effects of SCD1 Inhibitor Treatments
It is important to note that SCD suppression can also produce unwanted effects in mammals. Tissues, where lipogenic mechanisms have to be active to function normally, can be affected by long-term systemic treatments with SCD1 inhibitors. For example, SCD1 knockout mice develop multiple skin eczemas accompanied by weight disturbances [223]. These animals exhibit dysfunction of the epidermal lipid barrier with subsequent thermoregulation failure, transepidermal water loss, and metabolic problems [224]. The SCD1-deficient mice also suffer from atherosclerosis accompanied by an increased inflammatory response of the macrophages [225]. In addition, treatments with SCD1 inhibitors usually cause the atrophy of sebocyte cells (epithelial cells in the skin), due to which hair loss and dry eyes are caused [226]. On the basis of these findings, the development of new SCD inhibitors with fewer adverse effects, as well as a better understanding of the mechanisms involved, are of crucial interest. Using nutritional supplements that perform the activity in a moderate way is possible. An example is sterculic acid (SA) (see below).
12. Sterculic Acid (SA)
Sterculic acid (SA) is a cyclopropane fatty acid with many biological activities. SA is a fatty acid mainly obtained from the seeds of Stracolia petida and it forms more than 50% of its oil composition. SA is known for the inhibitory effect it exerts on the SCD1 enzyme, both in vivo and in vitro [227,228]. Studies in adipocyte cells have shown that this inhibition occurs by regulating enzyme activity without affecting SCD mRNA levels or protein expression [229]. Thus, this inhibition could be due to the irreversible binding of the sulfhydryl groups of the enzyme with cyclopropane groups found in sterculic acid [230] or through the conversion of SA to stearoyl-CoA, which appears to be the active form [231].
SA is one of the main components of sterculic oil (SO) [232]. SO contains not only SA, which is its main component, but also malvalic acid, another cyclopropane acid with properties similar to that of SA, including an inhibitory ability of SCD [233]. The beneficial effects of SO include improvement in terms of glucose tolerance and blood pressure, reduction in body mass, and benefit in serum levels of triglycerides and adiponectin [228] (Table 2).
However, a number of side effects have also been described. These include hypercholesterolemia, reproductive problems in animals, and inhibition of the beneficial effect of CLA (conjugated linoleic acid) in rats due to disruption of SCD activity [234].
13. Aquamin
Aquamin is a multimineral complex containing calcium, magnesium, and 72 other marine minerals that are absorbed from the surrounding seawater. It can be used in foods, beverages, and nutritional supplements.
The effect of Aquamin treatment was tested in a murine model of NAFLD. Groups of mice were fed a high-fat diet with fructose added to the drinking water with and without the addition of Aquamin for a period of 16 weeks [235]. They proved that Aquamin, which is rich in calcium, magnesium, and other elements (derived from red algae), prevents and can even help stop the progression of NAFLD (Table 2). Previous studies by this group [236,237], were conducted with black mice that were fed a high-fat Western-style diet (HFWD) for up to 18 months. The long feeding period of HFWD allowed for the development of extensive liver damage in most animals, especially males. In addition to the widespread steatosis found in mice fed HFWD, the mice exhibited widespread inflammation of the liver, liver injury, and the development of collagen deposits (Table 2). The diet caused the appearance of large fibrotic nodules. It was even possible to identify liver tumors including liver adenomas and carcinomas in several animals. Their studies demonstrated that supplying an adequate level of calcium (estimated as 20–25 mg per day consumed) together with several other trace elements in the mineral supplement Aquamin dramatically reduced the formation of tumors [236,237]. Inflammation, damage to hepatocytes, and the appearance of collagen deposits were also reduced, but the steatosis indices themselves were largely unaffected.
Calcium, which is the distinct “driver” of epithelial cell differentiation [238], is the most abundant mineral in Aquamin. Thus, epithelial cell differentiation induced by calcium in the multimineral product may underlie the suppression of the precancerous and cancerous processes in the liver induced by the HFWD diet and prevented by Aquamin treatment. Furthermore, it is well documented that some of the trace elements in the Aquamin multimineral product can act as calcimimetic (calcium-like) agonists, promoting the calcium response. Support for this is that an extracellular calcium-sensing receptor is also expressed in rat hepatocytes [239]. Inadequate mineral intake is not limited to people consuming a Western-style diet. A recent study showed that most people living in many developing regions of the world also lack an adequate amount of calcium in their diet [240]. The question that is asked is whether the mineral supplement Aquamin may ensure the consumption of minerals at an adequate level. A recent 90-day pilot trial was conducted in which 30 healthy subjects were randomly divided into several groups: one group designed to receive Aquamin that should provide 800 mg of calcium per day, another group that received calcium carbonate at the same level, and a third group that received a placebo [241]. In conclusion, no safety or tolerability issues were seen with Aquamin. At the same time, the colon biopsies obtained before and after the treatment demonstrated the regulation of several proteins related to cell differentiation in the colon mucosa. In the calcium-only group, differentiation proteins were also formed, but the levels of increase were much lower compared to what was seen with Aquamin. Finally, a decrease in the levels of certain primary and secondary bile acids was also observed in subjects who received Aquamin in combination with a change in the intestinal bacterial profile. These metabolic and microbial changes were not observed with calcium alone. While the focus of these clinical studies was colon health, the same approach may also provide benefits in terms of liver health.
In conclusion, previous studies have clearly demonstrated the importance of adequate mineral intake to prevent the consequences of fat accumulation in fatty liver in a murine model. Current studies attempt to provide mechanistic insight into how mineral supplementation may contribute to a reduction in liver tumor formation in a murine model, one of the most devastating consequences of fatty liver disease versus steatosis. There is currently no evidence of the effectiveness of using Aquamin to prevent NAFLD in humans.
14. Oleic Acid
The main fatty acid provided by olive oil is oleic acid or oleate (C18:1 n-9), and it is known for being the main contributor to the beneficial effects of olive oil consumption (Hu, 2003).
Ducheix et al. [242] recently investigated the effects of dietary-supplied oleic acid on the regulation of gene expression in the liver. Indeed, while oleic acid can be synthesized de novo through the activity of Stearoyl-CoA desaturase 1 (SCD1), it is clear from the results obtained in mice lacking the SCD1 gene (general Scd1 KO) [243] as well as in liver-specific Scd1 KO mice [244] and in mice overexpressing Scd3 [245] that oleate can positively contribute to various physiological functions, mainly in the liver. Dietary-supplied olive oil [246] and oleic acid [247] have been shown to have beneficial effects in various experimental models of liver pathologies in NAFLD, ranging from steatosis to steatohepatitis (NASH). In addition to the effect that prevents the accumulation of lipids in the liver, oleic acid synthesized de novo also contributes to the protection of hepatocytes against insulin resistance [248].
Recently, it has been shown that oleic acid can modulate the activity of liver X receptors (LXRs) in human neutrophils [249] (Table 2). Moreover, LXR activity is sensitive to fatty acids as tested in vitro [250]. The LXRs are type II nuclear hepatocyte receptors [251]. They are sensitive to derivatives of oxidized cholesterol, the oxysterols, which bind to and activate both isotypes of LXR (β α, NR1H3 NR1H2). An increase in the oxysterol concentration stimulates the transcription of the LXR target genes. For example, LXR α and β regulate genes involved in lipogenesis [252]. LXRα binds to transcription factors responsible for the control of lipogenic genes such as Fasn (responsible for fatty acid synthesis) [253] or Scd1 [254] and directly promotes de novo fatty acid synthesis. Therefore, the pharmacological activation of LXR leads to the accumulation of neutral lipids in the liver, which is the hallmark of NAFLD [255]. LXRs are also involved in the reverse transport of cholesterol and the breakdown of cholesterol into bile acids. LXR is not only involved in cholesterol secretion [256] but also in suppressing inflammation [257], and thus may be involved in protecting the liver against inflammatory processes that may occur in NAFLD [258]. Indeed, the contribution of LXR to the modulation of lipogenesis, cholesterol metabolism, and liver inflammation by dietary oleic acid was recently investigated [242]. It was demonstrated that LXR is required for the lipogenic genetic response as well as for the decrease in cholesterol in response to a diet that provides a high content of oleic acid. Moreover, it was identified that, in this process, LXR protects against inflammation and liver damage caused by lipogenesis. The authors’ work reveals that LXR contributes to the effects induced by dietary oleic acid and protects the liver from inflammation while inducing lipogenesis. Support for the above findings was published in the in vivo work carried out by Moravcová et al. [259], who found that in in vitro models of steatosis, oleic acid protects against the cytotoxic activity of steatosis caused by treatment with palmitic acid in primary rat hepatocytes in culture (Table 2).
Table 2.
General and nutritional-associated compounds with beneficiary action on NAFLD.
| Compounds | Effects on Liver | Effects on Intestinal Microbiota | References | |
|---|---|---|---|---|
| Glutathione | 1. Therapeutic effect of oral glutathione in patients with NAFLD | [121] | ||
| 2. Improvement of ALT blood levels | [122] | |||
| 3. Decrease in triglycerides, NEFAs, and ferritin levels | [127] | |||
| 4. Lowers protein-bound glutathione to normal basal levels | ||||
| 5. Improvement of hyperferritinemia and oxidative stress, and exertion of therapeutic effects in patients with NAFLD | ||||
| Soy lecithin as a source of choline and inositol | 1. Lecithin in the context of NAFLD is considered one of the most important sources of choline and inositol | [151] | ||
| 2. Choline, phosphatidylcholine, and lecithin are associated with the prevention of the development of fatty liver | [152] | |||
| 3. Choline and betaine have been shown in animal and human studies to prevent and even ameliorate NAFLD | [155] | |||
| 4. Lecithin ensures adequate TG export from the liver | [157] | |||
| Turmeric and curcumin extracts | 1. Antioxidant, anti-inflammatory, and anti-fibrotic properties, as well as insulin-sensitizing effects | 1. Probiotic-like effects | [158] | |
| 2. Induces increased energy metabolism of adipocytes and/or induction of apoptosis, increased expression of neseptin levels in serum | [165,167] | |||
| 3. Loss of appetite, reduction in body fat, anti-inflammatory activities, anti-hyperglycemic activity, metabolic and neuroendocrine regulation | [168,169] | |||
| 4. Reduces body fat mass by inhibiting adipocyte differentiation through suppression of peroxisome proliferator-activated receptor-γ and by increasing adenosine monophosphate-activated protein kinase resulting in lipolysis | [174] | |||
| 5. Enhances the activities of detoxifying enzymes such as glutathione-S-transferase, glutathione peroxidase, glutathione reductase, catalase, and he-oxygenase-1 in the liver and thus suppresses oxidative stress in the liver | [179,180,181] | |||
| 6. Blocks the activation of key mediators of cellular inflammation such as NF-κB, 5-lipoxygenase (5-LOX), and cyclooxygenase-2 (COX-2) | [182,183] | |||
| 7. Inhibits the activation and proliferation of stellate cells in the liver, which have a known role in the progression of liver fibrosis | [184] | |||
| Silymarin | 1. Anti-inflammatory, antioxidant, and anti-fibrotic activity | [188] | ||
| 2. Reduces insulin resistance | [193] | |||
| 3. Reduces liver inflammation by inhibiting lipooxygenase activity and reducing the function of leukotrienes and their effect on Kupffer cells in the liver and by reducing oxidative stress by increasing glutathione levels | ||||
| 4. Improvement of serum superoxide dismutase activity and malondaldehyde (MDA) levels in rats in which NAFLD was induced | [192] | |||
| 5. Reduce serum aspartate aminotransferase enzyme levels and levels of triglycerides (TGs) and cholesterol including VLDL in NAFLD-induced rats | ||||
| Sterculic acid (SA) | 1. Improvement in glucose tolerance and blood pressure, reduction in body mass, and benefit in serum levels of triglycerides and adiponectin | [228] | ||
| Aquamin [241] | 1. Prevents and can even help stop the progression of NAFLD | 1. Regulates expression of several proteins related to cell differentiation in the colon mucosa | [235,241] | |
| 2. Reduce the formation of tumors | [236,237] | |||
| 3. Reduces inflammation, damage to hepatocytes, and the appearance of collagen deposits | 2. Change the intestinal bacterial profile | [241] | ||
| Oleic acid | 1. Prevents the accumulation of lipids in the liver | [248] | ||
| 2. De novo synthesized oleic acid contributes to the protection of hepatocytes against insulin resistance | ||||
| 3. Modulates the activity of liver X receptors (LXRs) | [249] | |||
| 4. Protects against the cytotoxic activity caused by treatment with palmitic acid-induced steatosis in primary rat hepatocytes in culture. | [259] | |||
| Bilirel (BIL) | 1. Rapid improvement in liver fat accumulation, improvement in glucose levels and metabolism | [260] | ||
| Cannabinoids | 1. Decreases fibrosis | [261] | ||
| 2. Stimulation of adipocyte metabolism | [262] | |||
| 3. Improvement on the insulin–glucose circuit and inhibition of weight gain | [263] | |||
| 4. Suppresses the development of NAFLD | [264,265,266] | |||
| 5. Decreases hepatic TG synthesis, as does VLDL synthesis, and increases insulin sensitivity |
LDL, Low-density lipoprotein; HDL, High-density lipoprotein; VLDL, Very low-density lipoprotein; TNF-α, Tumor necrosis alpha; NF-κB, Nuclear factor-Kappa beta; ROS, Reactive oxygen species; AST, Aspartate aminotransferase; ALT, Alanine transaminase; NASH, Non-alcoholic steatohepatitis; NAFLD, Non-alcoholic fatty liver disease; TGs, Triglycerides; MDA, Malondialdehyde; IL-1β, Interleukin-1 beta; IL-6, Interleukin-6; 5-LOX, 5-lipoxygenase; COX-2, cyclooxygenase-2.
15. Antioxidants and NAFLD
15.1. Oxidative Stress and NAFLD
Oxidative stress has a key role in the initiation of NAFLD as well as its development and progression to NASH. As mentioned above, the perturbation of lipid metabolism determines fat accumulation in hepatocytes. In this way, the intracellular organelles such as mitochondria, endoplasmic reticulum (ER), and NADPH oxidase are stimulated to generate acid radicals or reactive oxygen species (ROS). The increased oxidation of the fatty acids and the increased mitochondrial activity stimulate the generation of ROS within the components of the electron transport chain (I, II, and III) in the reaction chain of cytochrome C oxidase. In particular, increased β-oxidation of fatty acids in mitochondria and microsomes appears to generate more ROS in NAFLD [267]. The mitochondria produce ATP through phosphorylation in an oxidative process, and thus superoxide radicals are formed as a byproduct of the oxidative phosphorylation. Similarly, oxidative stress in NAFLD may be caused by the changes that occur in NADPH oxidase and ER stress [268]. Intracellular oxidative stress usually occurs when there is an imbalance between the levels of intracellular ROS and endogenous and enzymatic antioxidants. Clinically, a decrease in endogenous antioxidants has been reported in NAFLD patients [269]. In a clinical study, it was reported that the levels of catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPx), glutathione (GSH), and glutathione reductase (GR) in the serum/plasma of NAFLD patients were disturbed in patients with early and advanced disease characteristics [270]. Increased intracellular ROS provokes changes in insulin sensitivity and the alteration of various essential enzymes involved in lipid metabolism. In liver steatosis, oxidative stress is responsible for triggering immune responses [271]. The experimental and clinical studies demonstrated the infiltration of adaptive immune cells (T cells) into the liver during NASH and the presence of circulating antibodies targeting antigens derived from oxidative stress (Van Herck et al., 2019). In NAFLD, oxidative stress causes the activation of many redox-sensitive transcription factors such as NF-κB and pro-inflammatory mediators (TNF-α), interleukins (IL), etc., leading to liver inflammation, fibrosis, and cell death [272].
NAFLD is a multifactorial disease involving insulin resistance, oxidative stress, and excessive fat intake, and a carbohydrate-based diet that causes the accumulation of excess fat in the liver leading to steatosis [273]. Simple steatosis of the liver can cause intracellular ROS upregulation through induction of the CYP2E1 enzyme. The increased intracellular ROS causes oxidative stress [274]. Accumulations of fat, ROS, and decrease in intracellular antioxidants together cause lipotoxicity, mitochondrial dysfunction, and ER stress in the liver. Fat infiltration in hepatocytes leads to impaired β-oxidation and oxidative phosphorylation in mitochondria, while disturbances in β-oxidation in peroxisomes, and lysosome dysfunction lead to the accumulation of intracellular ROS and hydrogen peroxide radicals [275]. Thus, damaged lipid metabolism is involved in the alteration of oxidative and antioxidant homeostasis, causing redox imbalance and oxidative stress. Impaired lipid metabolism in hepatocytes increases fatty acid uptake via the CD36 transporter, and mitochondrial dysfunction may result in the accumulation of intracellular triglycerides. Redox imbalance in fatty liver increases endoplasmic reticulum (ER) stress by regulating protein response. The chronic stress of the ER and the activation of a prolonged protein response increase the expressions of ER stress proteins such as PKR-like ER kinase, the activation of HNF4α, which is transcription factor 4, a homologous protein that binds CCAAT-enhancer-enhancer, which leads to the activation of pro-inflammatory marker expression and to the activation of cell death pathways in hepatocytes [276]. Furthermore, sustained ER stress leads to activation of the SREBP1c protein to bind sterol elements. Its nuclear translocation can induce the transcription of genes related to lipogenesis. Thus, oxidative stress plays a central role in the initiation and exacerbation of NAFLD.
15.2. Antioxidants Effects on NAFLD
Raised lipid peroxidation and dropped antioxidant status have been correlated with NAFLD progression. Thus, oxidative stress is involved in NAFLD progression. For this reason, different antioxidants have been studied experimentally and clinically against NAFLD patients [277]. In the last decade, several clinical and experimental studies have involved oxidative stress in NAFLD conditions and aimed NAFLD with antioxidants. We will further address the effect of some of the entirely studied plant-derived and synthetic antioxidants and antioxidant vitamins to date against experimental and clinical NAFLD conditions. For example, antioxidants such as silybin or silibinin, silymarin vanillin (apocynin), resveratrol, pentoxifylline, and vitamins A, C, and E have come to clinical trials against NAFLD.
Apocynin, also known as acetonylon, is the natural organic compound structurally related to vanillin (natural vanilla). In a study, CCl4 was orally administrated to rats (1 mL/kg) twice a week for two weeks and they were treated with apocynin (100 mg/kg, orally) daily for two weeks. Apocynin notably lowers serum AST, ALT, and ALP activity and suppresses OS markers (MDA and NO levels) in CCl4-treated rats. Apocynin treatment also recovers catalase and SOD activity in CCl4-treated rats. Thus, apocynin has protective effects in CCl4-induced liver damage by inhibiting lipid oxidation and stimulating the cellular antioxidant system. Synthetic vanillin is a cheap and unhealthy alternative to real vanilla extract. Today, the vast majority of synthetically produced vanillin is made from eugenol or guaiacol, petrochemicals that are often derived from crude oil. Hence, this product is not recommended for use in general and especially not for fatty liver [278].
15.2.1. Bilirel (BIL)
Abenavoli et al. [260] reported the effect of a new antioxidant complex, called Bilirel (BIL) (Pharmaluce, Republic of San Marino), recently introduced on the Italian market. The composition of one pill of BIL was as follows: silymarin 75 mg, chlorogenic acid 3.75 mg, protopine 0.02 mg, L-methionine 75 mg, and L-glutathione 75 mg. They report a case series of seven overweight patients with NAFLD, in which the combination of an Italian Mediterranean diet, increased physical activity, and daily administration of two BIL pills for 6 weeks, resulted in rapid improvement in liver fat accumulation, improvement in glucose levels and metabolism, and weight reduction (Table 2).
15.2.2. Additional Antioxidants
As NAFLD is strongly associated with the presence of oxidative stress, mitochondrial dysfunction, and inflammation, antioxidants can therefore exert a significant ameliorative effect. The products of the plant Silybum marianum, especially silybin, have a specific role in regulating oxidative stress and lipid metabolism. The antioxidant effect of silybin was achieved by activating Nrf-2-related genes, and the lipid-lowering effect was achieved by promoting PPARα, while the anti-inflammatory effect was achieved by inhibiting NF-κB signaling. Resveratrol, a known SIRT 1 and AMPK activator, inhibits SREBP1c, which is responsible for de novo lipogenesis. Resveratrol also inhibits Nrf-2 promoter methylation and protects the NAFLD liver from epigenetic changes.
16. Vitamins with Antioxidant Activity in NAFLD
Vitamins control various fundamental enzymatic processes in the liver, and modifications in the metabolism of vitamins have a crucial role in the progression of NAFLD. Vitamins A, C, and E have been particularly studied in relation to NAFLD due to their antioxidant activity. Likewise, serum concentrations of vitamins D and B12 have been reported to have a strong correlation with NAFLD severity [279] (Figure 2). Hepatocyte cells (HSC) store most of the body’s retinol [280].
Figure 2.
The impact of vitamins on hepatocytes affected by NAFLD.
16.1. Vitamin A and NAFLD
At the same time, the impaired metabolism of Vitamin A caused its accumulation in hepatocytes and not in HSC in mice in which NAFLD was induced. Thus, NAFLD causes the amassment of Vitamin A in hepatocytes, which may provoke disease progression [281]. Retinoic acid (RA) treatments have been demonstrated to be effective and antioxidant by lowering mitochondrial ROS and enhancing SOD2 in mice (Table 3). Retinoic acid treatment also increased the expression of hepatic Sirt1 and inhibited SREBP1c expression in HFD-fed mice [282]. Moreover, through signaling regulation of lipid metabolism involving Retinoid X receptors alpha (RXRsα), the liver could be protected by RA. In this sense, the increased concentrations of circulating RA protect against hepatic steatosis itself, as well as against liver damage in NAFLD populations [283]. A considerable presence of lipid droplets in stellate cells of the liver can affect the release of retinol. It is known that once released, it converts into retinoic acid with a beneficial role regarding inflammation, fibrogenesis, and carcinogenesis. This process is closely related to the triggering of a pro-inflammatory and pro-fibrogenic phenotype in hepatic stellate cells [284] (Table 3). A reduction in the release of transforming growth factor-beta 1 (TGF-β1) induces the suppression of hepatic stellate cells, and ultimately of fibrogenesis caused by NAFLD due to the ability of RA to inhibit proto-oncogene tyrosine-protein kinase MER (MERTK) in Kupffer cells. Thus, the modulation of RA release may represent a common genetic pathway associated with NAFLD [285] (Figure 2).
16.2. Vitamin C and NAFLD
In vivo Vitamin C supplementation reduces the hepatic fatty acid load by promoting the gene expression of PPARα-dependent β-fatty acid genes in in mice fed HFDs and in which NAFLD is induced [286] (Table 3). It can be emphasized that the prophylactic treatment of Vitamin C (15 and 30 mg/kg/day) significantly dropped body weight and steatosis, and thus induced a decrease in the risk of NAFLD in mice. In a therapeutic study, the administration of 30 mg/kg/day of Vitamin C reduced steatosis and NAFLD in mice. However, the administration of Vitamin C overdoses (90 mg/kg/day) did not reduce the risk of NAFLD development. In fact, high-dose Vitamin C intake significantly increased body weight, inflammation, and adipose tissue mass. [287]. This study clearly shows that an accurate dose of Vitamin C must be determined in cases of NAFLD. In NAFLD rats with choline deficiency, the administration of Vitamin C (30 mg/kg/day) significantly suppressed steatosis and oxidative stress. In the MCD diet-induced NASH model, a megadose administration (2.5 g/kg/day) of Vitamin C reduced macro-vesicular steatosis. However, AST and ALT were also increased after overdose Vitamin C administration [122].
In contrast, a recent study reported that Vitamin C deficiency leads to the inhibition of NAFLD. Vitamin C-deficient knockout mice reduced NAFLD progression compared to control mice. Vitamin C-deficient mice exhibit increased levels of the transcription factor -SREBP-1c and decreased expression of FAS [288], suggesting that long-term Vitamin C deficiency may be useful for inhibiting de novo lipogenesis via the SREBP-1c protein. However, NAFLD inhibition mediated by Vitamin C deficiency should be carefully investigated. There are reports according to which long-term Vitamin C is beneficial by improving adiponectin and reducing liver TG level, and therefore the chances of NASH in NAFLD patients (Figure 2).
Wei et al., 2016, demonstrated that, in the middle-aged male population without obesity, there is a significant inverse association between ingested Vitamin C and NAFLD. [289]. They explained that estrogens are responsible for these sex differences because their beneficial roles against NAFLD in middle-aged women may inhibit Vitamin C effects [290]. A strong causal relation has been demonstrated between obesity and NAFLD. Regarding the relationship between Vitamin C, obesity, and NAFLD (Table 3), Ipsen et al. have speculated that the therapeutic effects of this vitamin can be counteracted by obesity [291].
16.3. Vitamin E and NAFLD
Regarding Vitamin E, it was demonstrated that three weeks of Vitamin E administration (0.5 g/kg) to HFD-induced phosphatidylethanolamine N methyltransferase-deficient NAFLD mice induced the normalization of cholesterol metabolism as well as reduced the inflammation and fibrosis associated with oxidative stress; however, it failed to reduce liver TG content [292] (Table 3). Additionally, Vitamin E administration reduced fructose diet-induced NAFLD by stimulating the Nrf2/carboxylesterase 1 pathway implicated in lipogenesis [293]. Therefore, as a strong antioxidant, Vitamin E has been extensively studied as an adjuvant along with other medicines against NAFLD pathology, but there are no unequivocal conclusions for human use. In mice who received a diet poor in methionine-choline (MCD), Vitamin E, due to decreasing liver markers and decreasing histological steatosis, can be considered an inhibitor of steatohepatitis [294]. As a result, SOD activity increased and malonaldehyde (MDA) concentration decreased. In addition, the genes responsible for fibrosis, inflammation, and apoptosis were inhibited. Additionally, the activation of hepatic stellate cells and the replenishment of hepatic glutathione are also therapeutic effects of Vitamin E in mice with NAFLD [295]. Abdel-Maboud et al. [296] found that, depending on the dose of Vitamin E, the levels of some liver markers such as ALT and AST improve. Among these, the most significant variation was observed in AST activity, with adequate repercussions on the NAFLD activity score (NAS). Moreover, this process strongly influenced anthropometric parameters such as weight and IMC [296]. As previously mentioned, the increase in oxidative stress is one of the most common changes in NAFLD. This result is represented by the accumulation of reactive oxygen species, concomitantly with the incapacity of the body to defend itself through the antioxidant systems, which ultimately leads to damage to DNA and tissues [297]. In this sense, it is known that Vitamin E is one of the most powerful antioxidants [298]. These effects are observed at the molecular level through cellular, biochemical, genetic, and signaling pathways, with results in the modulation of the inflammatory response and cell proliferation. Moreover, it is responsible for regulating the cellular signaling of different enzymes essential in molecular signal translation, such as 5-lipoxygenase, cyclooxygenase-2 (COX-2), protein kinase C (PKC), and protein phosphate 2A (PP2A) (Table 3). Additionally, there are certain factors such as mitogen-activated protein kinase (MAPK) that can be modulated by Vitamin E [299]. Wang et al. demonstrated that Vitamin E reduces liver fibrosis by suppressing TGF-β expression, [294]. In addition, due to its capacity to stimulate the activation and transcription of PPARγ in adipocytes in mice, Vitamin E stimulates the expression of adiponectin (Figure 2). It is well documented that the protein–hormone adiponectin is involved in the regulation of glucose concentration and the degradation of fatty acids [300]. The effects of Vitamin E on gut microbiota in NAFLD are discussed in several studies and seem to change the gut microbiota composition into a healthier one [301]. Similarly, in mice models in which colitis was induced, the changes provoked by Vitamin E administration were observed through favorable modifications in intestinally disturbed microbiota by the increases of portal LPS [302] (Table 3).
16.4. Vitamin D and NAFLD
Like all organs, the liver has its own typical immunity configuration. The components that belong to innate immunity and influence the pathology of NAFLD are represented by Kupffer cells, hepatic stellate cells, and natural killers. Besides these cells, there are macrophages and monocytes involved [303]. Recruited macrophages and Kupffer cells have been associated with insulin resistance and NASH, since they synthesize the well-known pro-inflammatory cytokines interleukin-1 beta (IL-1β), IL-6, and TNF-α, [304]. Regarding macrophages, they are divided into M1 and M2, the difference consisting of the fact that the M1 macrophages are classically activated, whereas the M2 macrophages are alternatively activated [305]. The polarization and dysregulation of M1-like/M2-like macrophages, in which M1-like initiate and sustain inflammation, whereas M2-like attenuate chronic inflammation, are linked to NAFLD pathology. Finally, all these processes determine insulin resistance and some metabolic diseases such as diabetes and obesity [304]. Due to the fact that liver cells express receptors for Vitamin D (VDR), the liver can be protected against the inflammation induced by chronic hepatitis following the administration of Vitamin D [306] (Figure 2). Moreover, by stimulating VDR expression, insulin sensitivity increases. This process involves the Glut-4 translocators of muscle cells which therefore transport glucose inside the cell and then decrease blood glucose concentration. In addition, the modulation of free fatty acids (FFAs) is associated with the improved expression of these receptors [307]. Additionally, Vitamin D treatment decreases the cytokeratin 18-associatedapoptotic fragment M30 and thus reduces liver damage; therefore, Vitamin D exerts anti-fibrotic, anti-inflammatory, and anti-cirrhotic effects [308,309]. Sharifi et al. demonstrated that tumor necrosis factor alpha (TNFα) and C-reactive protein were significantly affected by Vitamin D administration [310]. In the pathogenesis of NAFLD, insulin resistance is very critical and is considered a significant risk factor associated with NAFLD. As previously described, it is linked to the increase in oxidative stress and lipotoxicity [311]. Nuclear factor κ-β (NF-κB) represents a mediator through which, if activated, a pro-inflammatory modulation can be made, resulting in the release of pro-inflammatory cytokines such as IL-1β, IL-6, or TNF-α. In this way, Kupffer cells are finally activated [312]. Additionally, histological features of NASH can be induced by the activities of these cytokines. In humans, the increased gene expression of these cytokines is much more frequent in the liver of patients with NASH compared to the normal liver of obese patients. The higher the secretion of these cytokines, the greater the severity of NAFLD [313]. In this regard, Neyestani et al. 2012 observed that, in type 2 diabetic patients, supplementing a daily intake of 1000 IU of Vitamin D single or combined with calcium, for 12 weeks, led to a decrease in IL-1β and IL -6 pro-inflammatory cytokines secretion [314] (Table 3). In other studies, after 10 weeks of administration of 50,000 IU Vitamin D weekly, a notable decrease in TG was shown in patients with NAFLD [315] (Figure 2). Interestingly, regarding transaminases, Amiri et al., [316] discovered that the combination of 25 μg/day Vitamin D with 500 mg calcium carbonate during 12 weeks, decreases ALT and AST activities compared to patients who were administered only Vitamin D or the placebo group. Different studies have shown that the dietary intake of calcium influences the absorption of Vitamin D and through this changes the blood lipid profile. Thus, after 12 weeks of simultaneous administration of Vitamin D together with calcium, the ratios of LDL-C/HDL-C, and TC/HDL-C decreased. Concomitantly, non-HDL-C increased [317].
At the liver cells level, depending on the time and the administered dose, vitamins can have a positive or negative impact. Vitamin A can inhibit the release of the cytokine TGF-β1. Chronic administration of low doses of Vitamin C exerts antioxidant effects by suppressing ROS release. It stimulates PPAR γ, which in this way decreases the concentration of FFAs. The same effect is observed in the case of Vitamin D. However, chronic administration of high doses of Vitamin C activates lipogenesis, which ultimately leads to NAFLD. Another beneficial vitamin in this disease is D, which increases insulin sensitivity. Additionally, Vitamin B12 has the same properties. Vitamin E, known for its role in the fight against ROS, has stimulating effects on the activity of the SOD enzyme.
16.5. Vitamin B12 and NAFLD
DNA synthesis and repair are dependent on appropriate levels of Vitamin B12 [303]. Likewise, the disruption of mitochondrial metabolism, involved in the pathology of NAFLD, is influenced by Vitamin B12 [318]. Only a few studies evaluated the effect of Vitamin B12 and NAFLD. Even so, the results were contradictory. There are studies that discovered an association between NAFLD and hyperhomocysteinemia [319]. This can be demonstrated by the fact that intracellular lipid metabolism is altered at high serum concentrations of homocysteine, which in the hepatocyte causes in the endoplasmic reticulum an increase in oxidative stress. This affects the signaling pathway in response to sterols, and as a consequence, the progression of NAFLD occurs [319]. In this way, the daily administration of 1000 µg of cyanocobalamin for 3 months in NAFLD patients decreased the serum concentration of homocysteine [320]. In NAFLD patients, by administering Vitamin B12, the serum concentration of homocysteine decreased significantly. Therefore, Vitamin B12 supplementation exerts therapeutic effects regarding the pathology of NAFLD [320]. Moreover, in NAFLD pathology, it has been observed that MDA, which is a marker of lipid peroxidation, is always increased [321]. After the administration of Vitamin B12, the serum concentration of MDA decreased significantly. It is believed that the basis of this change could be the fact that Vitamin B12 lowers the levels of homocysteine, and in this way, changes the metabolism of MDA, in the sense of the decrease. There are studies that confirm this speculation since it was demonstrated that supplementation with folate or B12 decreased induced decrease in MDA activity [322] (Table 3). Unfortunately, it is not known whether Vitamin B12 action is direct or indirect. Regarding insulin resistance, it was observed that treatment with Vitamin B12 reduces fasting blood glucose (FBG) (Table 3). An inversely proportional relationship was observed in the study of Al-Daghri et al. [323] between Vitamin B12 and FBG [323]. Moreover, other studies have reported [324] that the combined daily administration of B12 (500 µg) with folic acid (5 mg) significantly improves the Homeostatic Model Assessment for Insulin Resistance (HOMA-IR), as well as FBG. The results are not very relevant because the vitamins therapy was not performed separately. Additionally, in this case, the mechanisms underlying the improvement of the carbohydrate profile target homocysteine [325].
Table 3.
Impact of micronutrients on NAFLD.
| Type of Micronutrient | Effects on Liver | Effects on Intestinal Microbiota | References |
|---|---|---|---|
| Se | 1. Administration of a combination of Se and zinc improved the lipid profile, liver functions, and liver steatosis in rats | [206] | |
| 2. Se and probiotics reversed the negative effect of feeding in mice on HFD and improved liver functions and steatosis | [207] | ||
| 3. Decreases the number of hepatic stellate cells (HSCs) and liver fibrosis induced by CCl4 treatment in mice | [209] | ||
| Vitamin A | 1. Retinoic acid (RA) administration has been shown to be an effective antioxidant by reducing mitochondrial ROS and by increasing SOD2 in mice | [282] | |
| 2. Protects the liver against hepatic steatosis itself, as well as against liver damage in NAFLD populations | [283] | ||
| 3. Reduces the release of transforming growth factor beta 1 (TGF-β1) | [285] | ||
| 4. Suppresses the activation of hepatic stellate cells and fibrogenesis | |||
| Vitamin C | 1. Reduces hepatic fatty acid load by promoting the gene expression of PPARα-dependent β-fatty acid genes in HFD-induced NAFLD mice | [286] | |
| 2. Attenuates steatosis and NAFLD in mice | |||
| 3. Improves adiponectin levels and reduces liver TG levels and thus prevents NASH progression in NAFLD patients | [288] | ||
| 4. Significant inverse association between ingested Vitamin C and NAFLD | [289] | ||
| Vitamin E | 1. Normalizes cholesterol metabolism and reduces inflammation and fibrosis associated with oxidative stress | [292] | |
| 2. Attenuates fructose diet-induced NAFLD by activating the Nrf2/carboxylesterase 1 pathway involved in lipogenesis | [293] | ||
| 3. Is considered an effective inhibitor of steatohepatitis | [295] | ||
| 4. Inhibits the expression of genes responsible for fibrosis, inflammation, and apoptosis | [296] | ||
| 5. Improves the blood levels of ALT and AST | [299] | ||
| 6. Responsible for regulating the cellular signaling of different enzymes essential in molecular signal translation, such as 5-lipoxygenase, cyclooxygenase-2 (COX-2), protein kinase C (PKC), and protein phosphate 2A (PP2A) | [300] | ||
| 7. Stimulates the expression of adiponectin | [302] | ||
| 8. Induces favorable modifications in intestinal disturbed microbiota by the increases of portal LPS | |||
| Vitamin D | 1. Protects the liver against the inflammation induced by different chronic hepatitises | [306] | |
| 2. Increases insulin sensitivity | [307] | ||
| 3. Induces anti-fibrotic, anti-inflammatory, and anti-cirrhotic properties | [308] | ||
| 4. Decreases secretion of the pro-inflammatory cytokines IL-1β and IL-6 | [314] | ||
| 5. Decreases TG levels in NAFLD patients | [315] | ||
| 6. Improves blood lipid profile | [317] | ||
| Vitamin B12 | 1. Affects the disruption of mitochondrial metabolism, involved in the pathology of NAFLD | [318] | |
| 2. Therapeutic effects regarding the pathology of NAFLD | [320] | ||
| 3. Significantly decreases the serum concentration of MDA | [322] | ||
| 4. Reduces fasting blood glucose (FBG) | [323] |
LDL, Low-density lipoprotein; HDL, High-density lipoprotein; VLDL, Very low-density lipoprotein; TNF-α, Tumor necrosis alpha; NF-κB, Nuclear Factor-Kappa beta; ROS, Reactive oxygen species; AST, Aspartate aminotransferase; ALT, Alanine transaminase; NASH, Non-alcoholic steatohepatitis; NAFLD, Non-alcoholic fatty liver disease; TGs, Triglycerides; MDA, Malondialdehyde; IL-1β, Interleukin-1 beta; IL-6, Interleukin-6; 5-LOX, 5-lipoxygenase; COX-2, cyclooxygenase-2; PKC, Protein kinase C; PP2A, Protein Phosphate 2A; LPS, lipopolysaccharide; PPARα, Peroxisome Proliferator Activated Receptor alpha.
In conclusion, when we take vitamins, we must take into account several aspects, namely the dose, the physiological and pathological state of humans, and the duration of the treatment.
17. Bile Acids and NAFLD
Regarding fat absorption, bile acids are essential [326]. They are obtained through the oxidation of cholesterol in hepatocytes. The process involves several stages and results in primary bile acids such as chenodeoxycholic and cholic acids. The synthesis pathways of primary cholic acids are defined as classical (75%) or alternative (25%) [327]. The primary bile acids obtained are then conjugated with taurine or glycine. These acids are water-soluble when entering the duodenum at a pH of 3–5. Then, they are capable of emulsifying and solubilizing fats [328]. The bile ducts store bile acids via the bile salt export pump. Then, through a biliary tree system, the bile with newly formed bile acids is stored in the gallbladder during the inter-digestive periods. During the digestive period, through the contraction of the gallbladder, the bile is transported into the duodenum [329]. Primary bile acids are converted into secondary acids (lithocholic acid, deoxycholic acid, and ursodeoxycholic acid) by the intestinal microbiota [330]. Enterocytes from the distal ileum carry out the reabsorption of bile acids in a proportion of over 95%. Then, they are transported back to the liver. The intestinal microbiota is responsible for the deconjugation of the remaining 5%, which is then excreted in the feces. However, a very small amount reaches the peripheral tissues, where it causes peripheral effects [329].
Additionally, bile acids are considered signaling molecules in charge of regulating glucose and lipid metabolism. This is achieved by activating several factors such as Takeda G protein-coupled receptor 5 (TGR5), farnesoid X receptor (FXR), and multipurpose nuclear receptor. In NAFLD patients, a disturbance of bile acid signaling as well as dysbiosis is observed [331]. The stimulation of bile acid signaling pathways regulates glucose and lipid homeostasis by changing the intestinal microbiota, resulting in the improvement of the metabolic phenotype [332]. The intestinal microbiota are characterized by four types of bacteria: Actinobacteria, Bacteroidetes, Firmicutes, and Proteobacteria [333]. The imbalance determined by the intestinal microbiota is called dysbiosis. Obesity is associated with an increase in Firmicutes and a decrease in Bacteroidetes, which results in the stimulation of dietary energy gain [334]. It was demonstrated that colonizing mice with a specific microbiota from obese individuals had as a result an increase in body fat. In addition, mice fed a Western diet had a lower Bacteroidetes/Firmicutes ratio in the distal intestine [335]. The influence of bile acids on the intestinal microbiota can be direct or indirect, when it is mediated by FXR. The acid with the greatest antibacterial influence on the intestinal microflora is deoxycholic acid [330]. Moreover, the antibacterial action of non-conjugated acids is much stronger compared to that of conjugated acids [336]. In addition, the activation of FXR results in the stimulation of peroxisome proliferator-activated receptor alpha (PPAR-alpha). This nuclear receptor regulates the metabolism of glucose, lipids, and anti-inflammatory activity [337]. Thus, bile acids via FXR activation can reduce TG concentration through a signaling mechanism involving sterol regulatory element-binding protein 1 (SREBP-1) and its small heterodimer partner (SHP). This modulates the transcription of lipogenic genes [338]. Thus, from what can be seen, the most important mechanism for the development of NAFLD is the damage to the acid bile-signaling pathways [325].
18. Imbalance of the Intestinal Microbiota, Choline Metabolism, and NAFLD
In the causal link that exists between NAFLD and the imbalance of the intestinal microbiota, the increase in the permeability of enterocytes, the energy from food, the decrease in the metabolism of choline, the excessive growth of intestinal bacteria, and finally, the suppression of the metabolism of bile acids can be highlighted. The most well-known mechanism involved in the deregulation of the intestinal microbiota with repercussions on NAFLD includes the amplification under the action of this destabilization of the energy from the diet. It has been shown that the synthesis of short-chain fatty acids is much higher in obese patients. These acids are the main fermentation product of the bacteria that metabolize dietary fibers [334]. Thus, the total energy produced by the diet is increased, due to the bacterial metabolism of fibers, which in physiological conditions would be eliminated through defecation [339]. Additionally, increased intestinal permeability is closely associated with NAFLD. Enterocytes are essential for adequate intestinal immunity. Between some pathogens and the lamina propria, intestinal cells represent a physical barrier. These cells communicate with each other through junctions. The dysregulation of the intestinal microbiota can be closely linked to certain signaling pathways, which can regulate the expression and distribution of proteins and thereby intestinal permeability [340]. Regarding the increased permeability, this is responsible for triggering an inflammatory response in the liver through the receptor for bacterial DNA (TLR9) and receptor for bacterial flagellin (TLR5), due to the fact that translocation of bacterial components takes place in intestinal cells [341]. The increase in enterocyte permeability, as well as the multiplication of bacteria, in NAFLD patients, was correlated with the severity of steatosis [342]. It is known that LPS is closely related to the TLR4/NF-κB signaling pathway [343].
The role of choline in the pathogenesis of NAFLD is major. It has been observed that the deficiency of choline and methionine is correlated with the increase in the release of interleukins IL-1β, hepatic inflammation, and finally with the occurrence of NAFLD [344]. This is possible due to the fact that phosphatidylcholine results from choline metabolism, which is involved in the degradation of lipids in the liver [345]. Moreover, there is an inversely proportional relationship between supplementing food intake with choline and NAFLD [346].
19. The Activity of the Enzyme AMPK (5′ Adenosine Monophosphate-Activated Protein Kinase) in NAFLD
Considering that most therapies for NAFLD have focused on caloric restriction and sports [347], treatment strategies have been proposed to improve this disease. The majority of the strategies are based on AMP-activated protein kinase (AMPK), which is linked to different metabolic mechanisms. Furthermore, many of the effects of therapeutic compounds are mediated by the modulation of AMPK activity [348,349].
AMPK is a heterotrimer consisting of two regulatory units (β and γ) and one with catalytic activity [350]. Each of the three subunits have several isomers. AMPK is an enzyme considered crucial in the maintenance of energy balance. It is activated by different stimuli that mainly lead to the consumption of ATP. Between both, there is a relation of inverse proportionality. This explains the activation of AMPK under physical and cellular stress conditions, in which ATP production is considerably low or ATP consumption is increased (physical activity) [351]. AMPK, in the liver, is controlled by liver kinase B1 (LKB1). In NAFLD patients, even if the ATP content is reduced, the activity of AMPK is altered [352].
In NAFLD, increasing the activity of AMPK can inhibit the synthesis of fatty acids and cholesterol by downregulating the expression of the adipogenesis gene. Simultaneously, by increasing the expression of fatty acid oxidation and lipid decomposition genes involved in fatty acid oxidation and lipid decomposition, the body’s natural lipid balance can be maintained.
Secondary factors also play an essential role in the control of AMPK activity. The most plausible explanation for this can be related to the fact that LPS and TNFα (inflammatory factors) can decrease the activity of AMPK [353].
At present, some AMPK activators are thought to be beneficial during adequate treatment. Therefore, the activation of the AMPK signaling pathway is a potential therapeutic target for disorders of the liver. In the pathology of NAFLD, the increased concentration of FFAs is the most important factor contributing to the increase in hepatic lipids [354]. Therefore, the control of the factors involved in the regulation of their metabolism represents an aspect that must be taken into account. Insulin is one of them, because, in healthy people (sensitive to insulin), it suppresses lipolysis in white adipose tissue. In this respect, in NAFLD patients who also have insulin resistance, the insulin loses its suppression capacity. The result is the release of FFAs into circulation. Considering that FFAs reach the portal circulation directly, the effect is more dangerous because they make deposits in the form of visceral fat [355]. In addition, a part of FFAs reach the liver, and under esterification they form TGs, leading to an increase at the liver level. However, the hepatic esterification process is independent of insulin and dependent on the concentration of FFAs [356]. Insulin resistance is correlated with reduced AMPK activity in obese persons. In this sense, AMPK in adipose tissue is a very important factor for NAFLD [357]. Mottillo et al., 358 in a knockout mouse model showed that the ablation of AMPK activity in adipose tissue is correlated with increased insulin resistance and lipid accumulation in the liver [358].
Moreover, AMPK from adipose tissue macrophages can inhibit different signaling pathways such as TNF-α and IL-1β (Table 4). This is why AMPK activation has been proposed to suppress inflammation [359] (Figure 2). Due to the fact that AMPK is responsible for suppressing primary inflammatory lesions and HSCs, it is used to mitigate fibrosis [360]. Thus, by phosphorylating transcription factors such as ChREBP and SREBP-1c, AMPK inhibits the transcription of lipogenic genes [361] (Table 4).
Contrary to what is believed, an excess in amino acids (AAs, high-protein diet) has been shown to inhibit AMPK activity [362]. Increased protein intake reduces AMPK phosphorylation. In the liver and hypothalamus, mTOR phosphorylation is increased. Thus, the increased concentration of AAs decreases the AMP/ATP ratio. In this way, AMPK activity is suppressed [363]. Moreover, AMPK improves NAFLD by stimulating metabolism at the mitochondrial level and intensifying the oxidation of fatty acids in the liver [364] (Figure 2).
Table 4.
The effects of different enzymes on NAFLD.
| Type of Enzyme | Positive Effects of Enzymes and/or Supplements on Liver | Adverse Effects of Enzymes Inhibitor Treatments | References |
|---|---|---|---|
| Stearoyl-CoA desaturase 1 (SCD1) inhibitors | 1. SCD1 inhibitors are associated with amelioration of NAFLD, diabetes, dyslipidemic failure, and hepatitis C virus infections | 1. Knockout mice develop multiple skin eczemas accompanied by weight disturbances [223] | [220,223] |
| 2. SCD1 inhibitors reduces the accumulation of triglycerides in the liver of rats suffering from NASH by 80% | 2. Failure of thermoregulation, transepidermal water loss, and metabolic problems [224] | [222] | |
| 3. Attenuate the increase in aspartate aminotransferase (AST) enzyme levels and alanine transaminase (ALT) by 86% and 78%, respectively | 3. Cause atrophy of sebocyte cells (epithelial cells in the skin), effects reflected in hair loss and dry eyes causation | [226] | |
| AMPK (5′ adenosine monophosphate-activated protein kinase) | 1. Inhibits different signaling pathways such as TNF-α and IL-1β | [359] | |
| 2. Suppresses inflammation | [361] | ||
| 3. Inhibits the transcription of lipogenic genes | [364] | ||
| 4. Improves NAFLD by stimulating metabolism at the mitochondrial level and intensifying the oxidation of fatty acids in the liver |
NASH, Non-alcoholic steatohepatitis; NAFLD, Non-alcoholic fatty liver disease; TNF, Tumor necrosis factor alpha; IL-1β, Interleukin-1 beta.
20. Cannabinoids and NAFLD
Since many nutritional and pharmacological therapies have been used to treat NAFLD, there are additional compounds that must be considered. One of them is the endocannabinoid system (ECS), which represents a physiological complex. The endocannabinoid system (ECS) represents an endogenous signaling system demonstrated to play a key role in the regulation of appetite, metabolic processes, and energy balance. The ECS is made up of bioactive lipids, the endocannabinoids, enzymes that regulate their production and degradation, and receptors through which they transmit their signal [365]. The most studied and well-known receptors of the ECS are the G-protein-coupled receptors: cannabinoid receptor 1 (CBr1) and 2 (CBr2). CBr1 is mainly expressed, but not limited to, the central nervous system (CNS) and centrally and peripherally regulates metabolic homeostasis. CBr2, on the other hand, is mainly expressed by immune cells and plays a role in inflammation processes [366]. It is well established that obesity is associated with dysregulation of the ECS, resulting in high endocannabinoid “tone”, which in turn leads to increased appetite, lipogenesis, adipogenesis, and decrease in energy expenditure, which further exacerbates adiposity, thus creating a vicious cycle. In addition to CB1 and CB2, endocannabinoids can also be related by other receptors such as G protein-coupled receptor 55 (GRP55) and peroxisome proliferator-activated nuclear receptors (PPARs). Therefore, the independent effects promoted by cannabinoids may be related to their high lipophilicity.
Moreover, in the endocannabinoid system there are other important compounds such as exocannabinoids or phytocannabinoids. They are used as a treatment for different metabolic diseases [367]. The most studied compounds from Cannabis sp. are delta-9-tetrahydrocannabinol (THC), cannabidiol (CBD), followed by tetrahydrocannabivarin (THCV). Even if they were performed on in vivo models in terms of the effects of their studies in certain metabolic pathologies [368], only a few studies focused on their effects on NAFLD patients [369].
Regarding the endocannabinoid system, there are studies in which CB1 and CB2 in animals, as well as in cell cultures, had fibrosis-stimulating effects. At the same time, CB2 had a pro-inflammatory effect and determined insulin resistance [370]. Insulin resistance is linked to the modification of CB2Rs-dependent signaling pathways, through a different mechanism than CB1Rs [264].
Animal studies have shown that decreasing food intake and increasing energy consumption are correlated with the antagonism of CB1. This effect results in the improvement of different metabolic diseases [365]. In addition, the stimulation of adipocyte metabolism is correlated with the reduction in CB1 activity in visceral fat [371].
In human cell cultures, the CB1 antagonist decreases fibrosis [261]. It is important to note that, regarding phytocannabinoids, their effects on different organs can be different because they can interact with each other. An important example relates to the fact that CBD can antagonistically affect THC through different CBR1 and non-CBR1 receptor-related mechanisms of action. Other effects can be complementary or synergistic [372]. Regarding the medicinal effects of this plant, the most important is the ratio THC: CBD: THCV [373]. In addition to the previously mentioned compounds, there are also precursor compounds such as cannabidiol acid (CBDA), ∆9 tetrahydrocannabinol acid (THCA), and tetrahydrocannabivarin acid (THCVA). They are recognized for their therapeutic properties in obese mice, such as their improvement effects on the insulin–glucose circuit and inhibition of weight gain [262] (Table 2). The dysregulation of the endocannabinoid system is one of the main factors in the development of NAFLD. It is expected that the consumption of cannabis will increase in the next years due to its therapeutic properties in different pathologies. However, it is known that the majority of metabolic disorders that lead to the development of NAFLD are associated with chronic consumption of cannabis. Additionally, it is well established that chronic consumption of cannabis causes an increase in appetite as well as in ingested calories [374]. In addition, with the increasing appetite, a large number of unhealthy foods rich in fats and refined carbohydrates are consumed. We cannot neglect the fact that there are many studies which have shown the therapeutic effects of cannabis suppressing the development of NAFLD (Table 2). In this sense, chronic consumption was associated with a decrease in the prevalence of metabolic syndrome and type 2 diabetes [263]. Moreover, chronic marijuana use has been associated with lower obesity among users [375]. The antagonistic action of CBD and THCV on CB1R may be one of the mechanisms by which cannabis consumption decreases the prevalence of NAFLD as well as other metabolic pathologies [376]. Through CB1R antagonism, hepatic TG synthesis decreases [264], as does VLDL synthesis [266] while insulin sensitivity increases [265] (Table 2). By improving IR, hepatic glucose homeostasis is restored, fat deposits decrease, and therefore cannabis inhibits the development of NAFLD [377]. Additionally, phytocannabinoids have anti-inflammatory roles. They may decrease adipokines and different cytokines, such as IL-6 and TNF-a. This can lead to NF-kB upregulation [378]. Another beneficial mechanism for NAFLD regarding cannabis consumption is represented by the ability of THC to downregulate CBR1 and develop tolerance to these receptors. In this way, an inversely proportional relationship between marijuana consumption and NAFLD develops [379]. In conclusion, cannabinoids may suppress the development of NAFLD.
21. Conclusions
We can conclude that, even though the mechanisms associated with NAFLD initiation and progression remain not fully deciphered in this review, we address a number of factors demonstrated to be involved in NAFLD including genetic and epigenetic modifications, signaling, molecular, and biochemical factors, effects of dietary factors, effects of microbiota, and additionally we address the effects of behavioral factors. All these effects can be related from the “two hits” hypothesis to the current “multiple parallel hits hypothesis”. In this context, we present specific compounds and the mechanisms associated with their effects which can ultimately prevent or diminish the progression of NAFLD. In this regard, we concentrate on the following:
1. Potential bioactive nutrients that may interfere with NAFLD. In this sense, dark chocolate, cocoa butter, and peanut butter may be involved in decreasing cholesterol concentration. Moreover, dark chocolate can affect glucose metabolism by reducing oxidative stress. Additionally, cocoa butter has anti-inflammatory effects. Usually, this pathology is accompanied by insulin resistance, which over time perpetuates with reaching type 2 diabetes. That is why it is important to know how it can be prevented and/or efficiently fought against.
2. In addition to physical exercise, the sweeteners used in coffee and other frequent beverages also play an important role. On the one hand, most of them are consumed without knowing their effects on the body. For this reason, is necessary to know that some sweeteners have negative effects on the intestinal microbiota, others have no effects, and some have beneficial effects. Thus, stevia has proven to be adequate for improving carbohydrate metabolism, liver steatosis, and liver fibrosis. Moreover, it can decrease inflammation through the suppression of oxidative stress. Maltitol is used to prevent obesity, hyperglycemia, hypercholesterolemia, and fatty liver degeneration. Another sweetener widely used is Erythritol, which may have a role in alleviating NAFLD.
3. On the other hand, there are adequate compounds demonstrated to exert beneficial actions on NAFLD. Most of them are used for therapeutic purposes in NAFLD. In order to lower the serum concentration of triglycerides, glutathione, soy lecithin, silymarin, Aquamin, and cannabinoids are used. In addition, turmeric and curcumin extracts have antioxidant, anti-inflammatory, and anti-fibrotic properties, as well as insulin-sensitizing effects. Bilirel decreases liver fat accumulation and improves glucose metabolism. Additionally, serum superoxide dismutase activity and malonaldehyde (MDA) levels are improved by silymarin.
4. We additionally address the effects of micronutrients, especially vitamins. The effects of micronutrients directly depend on the dose administered. Even if most studies demonstrate the beneficial role of vitamins in the pathology of NAFLD, there are exceptions. Thus, the widely used Vitamin C, especially in overdose during the COVID-19 and post-COVID-19 period, is no longer beneficial. In fact, high-dose Vitamin C administration significantly increased body weight, adipose tissue mass, and inflammation. Regarding the administration of selenium, even if in most studies there is a positive correlation between it and NAFLD, there are reports that indicate negative effects. A Se-enriched diet provoked hepatic nodular hyperplasia in rats. Vitamin A has a protective role against mitochondrial ROS and may suppress fibrogenesis. Vitamin E seems to alter the gut microbiota composition into a healthier one. Vitamin D exerts anti-fibrotic, anti-inflammatory, and anti-cirrhotic effects. Vitamin B12 significantly decreases the serum concentration of MDA.
5. Additionally, the activity of some enzymes is related to the pathology of NAFLD. For example, SCD1 inhibitors are recognized for their beneficial effects on NAFLD, diabetes, dyslipidemic failure, and hepatitis C virus infections. Specifically, they can significantly decrease the accumulation of triglycerides in the liver of rats suffering from NASH. As one of the key enzymes in human metabolism, AMPK can suppress inflammation by inhibiting different signaling pathways such as TNF-α and IL-1β. Moreover, it inhibits the transcription of lipogenic genes.
Having mentioned these, we conclude that NAFLD can be prevented or improved by different factors through their involvement in the signaling, genetic, and biochemical pathways that underlie NAFLD. Therefore, exposing this vast knowledge to the public is particularly important.
As future perspectives, through the information that this work provides, we hope that it can be used by clinicians as a therapeutic nutritional alternative, starting from the premise that an adequate diet can prevent, in healthy individuals, the occurrence of NAFLD. In the case of patients with this disease, proper nutrition can delay, and even suppress, its progression and development, as well as collateral diseases such as type 2 diabetes or cardiovascular diseases.
In addition, we believe that this paper contains information that could represent a sustainable alternative to public health policy. Considering the vast information that has been presented, and based on it, nutritional guides can be created for patients who are often lacking in awareness and scientifically correct information regarding the vital role of nutrition in this disease. Along with patients, the clinicians are the direct beneficiaries, helping them to have a better view of the mechanisms and interactions of the nutrients with each other, but also with the medication associated with NAFLD.
Author Contributions
Conceptualization, C.M. and B.S.; Methodology, C.M. and B.S. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Pouwels S., Sakran N., Graham Y., Leal A., Pintar T., Yang W., Kassir R., Singhal R., Mahwar K., Ramnarain D. Non-alcoholic fatty liver disease (NAFLD): A review of pathophysiology, clinical management and effects of weight loss. BMC Endocr. Disord. 2022;22:63. doi: 10.1186/s12902-022-00980-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Townsend S.A., Newsome P.N. Non-alcoholic fatty liver disease in 2016. Br. Med. Bull. 2016;119:143. doi: 10.1093/bmb/ldw031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu Q., Tian X., Wu H., Huang J., Li M., Mei Z., Zhou L., Xie H., Zheng S. Metabolic changes of hepatocytes in NAFLD. Front. Physiol. 2021;12:710420. doi: 10.3389/fphys.2021.710420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Powell E.E., Wong V.W.S., Rinella M. Non-alcoholic fatty liver disease. Lancet. 2021;397:2212–2224. doi: 10.1016/S0140-6736(20)32511-3. [DOI] [PubMed] [Google Scholar]
- 5.Bedossa P. Diagnosis of non-alcoholic fatty liver disease/non-alcoholic steatohepatitis: Why liver biopsy is essential. Liver Int. 2018;38:64–66. doi: 10.1111/liv.13653. [DOI] [PubMed] [Google Scholar]
- 6.Reccia I., Kumar J., Akladios C., Virdis F., Pai M., Habib N., Spalding D. Non-alcoholic fatty liver disease: A sign of systemic disease. Metabolism. 2017;72:94–108. doi: 10.1016/j.metabol.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 7.Katsiki N., Mikhailidis D.P., Mantzoros C.S. Non-alcoholic fatty liver disease and dyslipidemia: An update. Metabolism. 2016;65:1109–1123. doi: 10.1016/j.metabol.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Rinaldi L., Pafundi P.C., Galiero R., Caturano A., Morone M.V., Silvestri C., Giordano M., Salvatore T., Sasso F.C. Mechanisms of non-alcoholic fatty liver disease in the metabolic syndrome. A narrative review. Antioxidants. 2021;10:270. doi: 10.3390/antiox10020270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martins I.J. Non-Alcoholic Fatty Liver Disease-Molecular Bases, Prevention and Treatment. IntechOpen; London, UK: 2017. Caffeine with links to NAFLD and accelerated brain aging. [Google Scholar]
- 10.Perumpail B.J., Khan M.A., Yoo E.R., Cholankeril G., Kim D., Ahmed A. Clinical epidemiology and disease burden of nonalcoholic fatty liver disease. World J. Gastroenterol. 2017;23:8263. doi: 10.3748/wjg.v23.i47.8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tiwari P. Recent trends in therapeutic approaches for diabetes management: A comprehensive update. J. Diabetes Res. 2015 doi: 10.1155/2015/340838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Younossi Z.M., Golabi P., de Avila L., Paik J.M., Srishord M., Fukui N., Qui Y., Burns L., Afendy A., Nader F. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J. Hepatol. 2019;71:793–801. doi: 10.1016/j.jhep.2019.06.021. [DOI] [PubMed] [Google Scholar]
- 13.Dai W., Ye L., Liu A., Wen S.W., Deng J., Wu X., Lai Z. Prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus. Medicine. 2017;96:e8179. doi: 10.1097/MD.0000000000008179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parthasarathy G., Revelo X., Malhi H. Pathogenesis of nonalcoholic steatohepatitis: An overview. Hepatol. Commun. 2020;4:478–492. doi: 10.1002/hep4.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Younossi Z.M., Henry L. Epidemiology of non-alcoholic fatty liver disease and hepatocellular carcinoma. JHEP Rep. 2021;3:100305. doi: 10.1016/j.jhepr.2021.100305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buzzetti E., Pinzani M., Tsochatzis E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD) Metabolism. 2016;65:1038–1048. doi: 10.1016/j.metabol.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 17.Day C.P., James O.F. Steatohepatitis: A tale of two “hits”? Gastroenterology. 1998;114:842–845. doi: 10.1016/S0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 18.Kitade H., Chen G., Ni Y., Ota T. Nonalcoholic fatty liver disease and insulin resistance: New insights and potential new treatments. Nutrients. 2017;9:387. doi: 10.3390/nu9040387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma Y., Lee G., Heo S.Y., Roh Y.S. Oxidative stress is a key modulator in the development of nonalcoholic fatty liver disease. Antioxidants. 2021;11:91. doi: 10.3390/antiox11010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arrese M., Arab J.P., Barrera F., Kaufmann B., Valenti L., Feldstein A.E. Seminars in Liver Disease. Volume 41. Thieme Medical Publishers Inc.; Leipzig, Germany: 2021. Insights into nonalcoholic fatty-liver disease heterogeneity; pp. 421–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rowland I., Gibson G., Heinken A., Scott K., Swann J., Thiele I., Tuohy K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018;57:1–24. doi: 10.1007/s00394-017-1445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Portincasa P., Bonfrate L., Vacca M., De Angelis M., Farella I., Lanza E., Khalil M.Q.-H., Wang D., Sperandio M., Di Ciaula A. Gut microbiota and short chain fatty acids: Implications in glucose homeostasis. Int. J. Mol. Sci. 2022;23:1105. doi: 10.3390/ijms23031105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wells J.M., Brummer R.J., Derrien M., MacDonald T.T., Troost F., Cani P.D., Theodorou V., Dekker J., Meheust A., de Vos W.M., et al. Homeostasis of the gut barrier and potential biomarkers. Am. J. Physiol. Gastrointest. Liver Physiol. 2017;312:G171–G193. doi: 10.1152/ajpgi.00048.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dixon L.J., Barnes M., Tang H., Pritchard M.T., Nagy L.E. Kupffer cells in the liver. Compr. Physiol. 2013;3:785. doi: 10.1002/cphy.c120026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martinez F.O., Gordon S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000prime Rep. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lefere S., Tacke F. Macrophages in obesity and non-alcoholic fatty liver disease: Crosstalk with metabolism. JHEP Rep. 2019;1:30–43. doi: 10.1016/j.jhepr.2019.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wan J., Benkdane M., Teixeira-Clerc F., Bonnafous S., Louvet A., Lafdil F., Pecher F., Tran A., Gual P., Mallat A., et al. M2 Kupffer cells promote M1 Kupffer cell apoptosis: A protective mechanism against alcoholic and nonalcoholic fatty liver disease. Hepatology. 2014;59:130–142. doi: 10.1002/hep.26607. [DOI] [PubMed] [Google Scholar]
- 28.Gebru Y.A., Gupta H., Kim H.S., Eom J.A., Kwon G.H., Park E., Jeong J.J., Won S.M., Sharma S.P., Ganesan R., et al. T Cell Subsets and Natural Killer Cells in the Pathogenesis of Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2022;22:12190. doi: 10.3390/ijms222212190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jalil A.M.M., Ismail A. Polyphenols in cocoa and cocoa products: Is there a link between antioxidant properties and health? Molecules. 2008;13:2190–2219. doi: 10.3390/molecules13092190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petyaev I.M., Bashmakov Y.K. Dark Chocolate: Opportunity for an Alliance between Medical Science and the Food Industry? Front. Nutr. 2017;4:43. doi: 10.3389/fnut.2017.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steinberg F.M., Bearden M.M., Keen C.L. Cocoa and chocolate flavonoids: Implications for cardiovascular health. J. Am. Diet. Assoc. 2003;103:215–223. doi: 10.1053/jada.2003.50028. [DOI] [PubMed] [Google Scholar]
- 32.Barišić V., Kopjar M., Jozinović A., Flanjak I., Ačkar Đ., Miličević B., Šubarić D., Jokić S., Babić J. The chemistry behind chocolate production. Molecules. 2019;24:3163. doi: 10.3390/molecules24173163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katz D.L., Doughty K., Ali A. Cocoa and chocolate in human health and disease. Antioxid. Redox Signal. 2011;15:2779–2811. doi: 10.1089/ars.2010.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee K.W., Kim Y.J., Lee H.J., Lee C.Y. Cocoa has more phenolic phytochemicals and a higher antioxidant capacity than teas and red wine. J. Agric. Food Chem. 2003;51:7292–7295. doi: 10.1021/jf0344385. [DOI] [PubMed] [Google Scholar]
- 35.Tan T.Y.C., Lim X.Y., Yeo J.H.H., Lee S.W.H., Lai N.M. The health effects of chocolate and cocoa: A systematic review. Nutrients. 2021;13:2909. doi: 10.3390/nu13092909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Paula Silva T., Silva A.A., Toffolo M.C.F., de Aguiar A.S. The action of phytochemicals present in cocoa in the prevention of vascular dysfunction and atherosclerosis. J. Clin. Transl. Res. 2022;8:509. doi: 10.3390/ijms232214365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Magrone T., Russo M.A., Jirillo E. Cocoa and dark chocolate polyphenols: From biology to clinical applications. Front. Immunol. 2017;8:677. doi: 10.3389/fimmu.2017.00677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Samanta S., Sarkar T., Chakraborty R., Rebezov M., Shariati M.A., Thiruvengadam M., Rengasamy K.R. Dark chocolate: An overview of its biological activity, processing, and fortificationapproaches. Curr. Res. Food Sci. 2022;8:677. doi: 10.1016/j.crfs.2022.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gasmi A., Mujawdiya P.K., Noor S., Lysiuk R., Darmohray R., Piscopo S., Lenchyk L., Antonyak H., Dehtiarova K., Shanaida M., et al. Polyphenols in metabolic diseases. Molecules. 2022;27:6280. doi: 10.3390/molecules27196280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Janevski M., Antonas K.N., Sullivan-Gunn M.J., McGlynn M.A., Lewandowski P.A. The effect of cocoa supplementation on hepatic steatosis, reactive oxygen species and LFABP in a rat model of NASH. Comp. Hepatol. 2011;10:10–13. doi: 10.1186/1476-5926-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kwok C.S., Boekholdt S.M., Lentjes M.A., Loke Y.K., Luben R.N., Yeong J.K., Wareham J.N., Myint K.P., Khaw K.T. Habitual chocolate consumption and risk of cardiovascular disease among healthy men and women. Heart. 2015;101:1279–1287. doi: 10.1136/heartjnl-2014-307050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martin M.Á., Ramos S. Impact of cocoa flavanols on human health. Food Chem. Toxicol. 2021;151:112121. doi: 10.1016/j.fct.2021.112121. [DOI] [PubMed] [Google Scholar]
- 43.Wiese M., Bashmakov Y., Chalyk N., Nielsen D.S., Krych Ł., Kot W., Klochkov V., Pristensky D., Bandaletova T., Chernyshova M., et al. Prebiotic effect of lycopene and dark chocolate on gut microbiome with systemic changes in liver metabolism, skeletal muscles and skin in moderately obese persons. BioMed. Res. Int. 2019;2019:1–15. doi: 10.1155/2019/4625279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alavinejad P., Farsi F., Rezazadeh A., Mahmoodi M., Hajiani E., Masjedizadeh A.R., Mard Ali S., Neisi N., Hoseini H., Haghighizadeh M.H., et al. The effects of dark chocolate consumption on lipid profile, fasting blood sugar, liver enzymes, inflammation, and antioxidant status in patients with non-alcoholic fatty liver disease: A randomized, placebo-controlled, pilot study. J. Gastroenterol. Hepatol. Res. 2015;4:1858–1864. doi: 10.17554/j.issn.2224-3992.2015.04.589. [DOI] [Google Scholar]
- 45.Massolt E.T., Van Haard P.M., Rehfeld J.F., Posthuma E.F., van der Veer E., Schweitzer D.H. Appetite suppression through smelling of dark chocolate correlates with changes in ghrelin in young women. Regul. Pept. 2010;161:81–86. doi: 10.1016/j.regpep.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 46.Matsui N., Ito R., Nishimura E., Yoshikawa M., Kato M., Kamei M., Shibata H., Matsumoto I., Abe K., Hashizume S. Ingested cocoa can prevent high-fat diet-induced obesity by regulating the expression of genes for fatty acid metabolism. Nutrition. 2005;21:594–601. doi: 10.1016/j.nut.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 47.Hamed M.S., Gambert S., Bliden K.P., Bailon O., Singla A., Antonino M.J., Hamed F., Tantry S.U., Gurbel P.A. Dark chocolate effect on platelet activity, C-reactive protein and lipid profile: A pilot study. South. Med. J. 2008;101:1203–1208. doi: 10.1097/SMJ.0b013e31818859eb. [DOI] [PubMed] [Google Scholar]
- 48.Mellor D.D., Sathyapalan T., Kilpatrick E.S., Beckett S., Atkin S. High-cocoa polyphenol-rich chocolate improves HDL cholesterol in Type 2 diabetes patients. Diabet. Med. 2010;27:1318–1321. doi: 10.1111/j.1464-5491.2010.03108.x. [DOI] [PubMed] [Google Scholar]
- 49.Mathur S., Devaraj S., Grundy S.M., Jialal I. Cocoa products decrease low density lipoprotein oxidative susceptibility but do not affect biomarkers of inflammation in humans. J. Nutr. 2002;132:3663–3667. doi: 10.1093/jn/132.12.3663. [DOI] [PubMed] [Google Scholar]
- 50.Goya L., Martín M.Á., Sarriá B., Ramos S., Mateos R., Bravo L. Effect of cocoa and its flavonoids on biomarkers of inflammation: Studies of cell culture, animals and humans. Nutrients. 2016;8:212. doi: 10.3390/nu8040212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rector R.S., Thyfault J.P., Wei Y., Ibdah J.A. Non-alcoholic fatty liver disease and the metabolic syndrome: An update. World J. Gastroenterol. WJG. 2008;14:185. doi: 10.3748/wjg.14.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McKim S.E., Konno A., Gäbele E., Uesugi T., Froh M., Sies H., Thurman G.R., Arteel G.E. Cocoa extract protects against early alcohol-induced liver injury in the rat. Arch. Biochem. Biophys. 2002;406:40–46. doi: 10.1016/S0003-9861(02)00425-3. [DOI] [PubMed] [Google Scholar]
- 53.Cooper K.A., Donovan J.L., Waterhouse A.L., Williamson G. Cocoa and health: A decade of research. Br. J. Nutr. 2008;99:1–11. doi: 10.1017/S0007114507795296. [DOI] [PubMed] [Google Scholar]
- 54.Quek R.Y.C., Peh E.W.Y., Henry C.J. Effects of cocoa butter and cocoa butter equivalent in a chocolate confectionery on human blood triglycerides, glucose and insulin. Foods. 2020;9:455. doi: 10.3390/foods9040455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ding E.L., Hutfless S.M., Ding X., Girotra S. Chocolate and prevention of cardiovascular disease: A systematic review. Nutr. Metab. 2006;3:1–12. doi: 10.1186/1743-7075-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Loganathan R., Nagapan G., Teng K.T., Voon P.T., Yap S.Y., Ng Y.T., Choo M.Y., Ong H.S.A., Ong H.S., Selvaduray K.R. Diets enriched with palm olein, cocoa butter, and extra virgin olive oil exhibited similar lipid response: A randomized controlled study in young healthy adults. Nutr. Res. 2022;105:113–125. doi: 10.1016/j.nutres.2022.06.011. [DOI] [PubMed] [Google Scholar]
- 57.Chang H.Y., Chen J.R., Chen Y.H., Xiao Q., Chen Y.L., Yang S.C. The Preliminary Results for Evaluating Cocoa Butter’s Hepatoprotective Effects against Lipid Accumulation and Inflammation in Adult Male Rats Chronically Fed Ethanol. Bioengineering. 2022;9:526. doi: 10.3390/bioengineering9100526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Torres-Moreno M., Torrescasana E., Salas-Salvadó J., Blanch C. Nutritional composition and fatty acids profile in cocoa beans and chocolates with different geographical origin and processing conditions. Food Chem. 2015;166:125–132. doi: 10.1016/j.foodchem.2014.05.141. [DOI] [PubMed] [Google Scholar]
- 59.Arya S.S., Salve A.R., Chauhan S. Peanuts as functional food: A review. J. Food Sci. Technol. 2016;53:31–41. doi: 10.1007/s13197-015-2007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Akhtar S., Khalid N., Ahmed I., Shahzad A., Suleria H.A.R. Physicochemical characteristics, functional properties, and nutritional benefits of peanut oil: A review. Crit. Rev. Food Sci. Nutr. 2014;54:1562–1575. doi: 10.1080/10408398.2011.644353. [DOI] [PubMed] [Google Scholar]
- 61.Toomer O.T. Nutritional chemistry of the peanut (Arachis hypogaea) Crit. Rev. Food Sci. Nutr. 2018;58:3042–3053. doi: 10.1080/10408398.2017.1339015. [DOI] [PubMed] [Google Scholar]
- 62.Hou Y.Y., Ojo O., Wang L.L., Wang Q., Jiang Q., Shao X.Y., Wang X.H. A randomized controlled trial to compare the effect of peanuts and almonds on the cardio-metabolic and inflammatory parameters in patients with type 2 diabetes mellitus. Nutrients. 2018;10:1565. doi: 10.3390/nu10111565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Parilli-Moser I., Hurtado-Barroso S., Guasch-Ferre M., Lamuela-Raventos R.M. Effect of Peanut Consumption on Cardiovascular Risk Factors: A Randomized Clinical Trial and Meta-Analysis. Front. Nutr. 2022;9:853378. doi: 10.3389/fnut.2022.853378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Coates A.M., Hill A.M., Tan S.Y. Nuts and cardiovascular disease prevention. Curr. Atheroscler. Rep. 2018;20:1–9. doi: 10.1007/s11883-018-0749-3. [DOI] [PubMed] [Google Scholar]
- 65.Petersen K.S., Murphy J., Whitbread J., Clifton P.M., Keogh J.B. The Effect of a Peanut-Enriched Weight Loss Diet Compared to a Low-Fat Weight Loss Diet on Body Weight, Blood Pressure, and Glycemic Control: A Randomized Controlled Trial. Nutrients. 2022;14:2986. doi: 10.3390/nu14142986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shramko V.S., Polonskaya Y.V., Kashtanova E.V., Stakhneva E.M., Ragino Y.I. The short overview on the relevance of fatty acids for human cardiovascular disorders. Biomolecules. 2020;10:1127. doi: 10.3390/biom10081127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vassiliou E.K., Gonzalez A., Garcia C., Tadros J.H., Chakraborty G., Toney J.H. Oleic acid and peanut oil high in oleic acid reverse the inhibitory effect of insulin production of the inflammatory cytokine TNF-α both in vitro and in vivo systems. Lipids Health Dis. 2009;8:1–10. doi: 10.1186/1476-511X-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Parilli-Moser I., Domínguez-López I., Arancibia-Riveros C., Marhuenda-Muñoz M., Vallverdú-Queralt A., Hurtado-Barroso S., Lamuela-Raventós R.M. Effect of crushing peanuts on fatty acid and phenolic bioaccessibility: A long-term study. Antioxidants. 2022;11:423. doi: 10.3390/antiox11020423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Reis C.E., Ribeiro D.N., Costa N.M., Bressan J., Alfenas R.C., Mattes R.D. Acute and second-meal effects of peanuts on glycaemic response and appetite in obese women with high type 2 diabetes risk: A randomised cross-over clinical trial. Br. J. Nutr. 2013;109:2015–2023. doi: 10.1017/S0007114512004217. [DOI] [PubMed] [Google Scholar]
- 70.Martins I.J. Appetite Control with Relevance to Mitochondrial Biogenesis and Activation of Post-Prandial Lipid Metabolism in Obesity Linked Diabetes. Ann. Obes. Disord. 2016;1:1012. [Google Scholar]
- 71.Martins I.J. Diabetes and cholesterol dyshomeostasis involve abnormal α-synuclein and amyloid beta transport in neurodegenerative diseases. Austin Alzheimer’s J. Park. Dis. 2015;2:1020. [Google Scholar]
- 72.Jessen A., Buemann B., Toubro S., Skovgaard I.M., Astrup A. The appetite-suppressant effect of nicotine is enhanced by caffeine. Diabetes Obes. Metab. 2005;7:327–333. doi: 10.1111/j.1463-1326.2004.00389.x. [DOI] [PubMed] [Google Scholar]
- 73.Martins I.J. Nutritional and genotoxic stress contributes to diabetes and neurodegenerative diseases such as Parkinson’s and Alzheimer’s diseases. In: Rahman A., editor. Frontiers in Clinical Drug Research—CNS and Neurological Disorders. Volume 3. Bentham Science Publishers; Sharjah, United Arab Emirates: 2015. pp. 158–192. [Google Scholar]
- 74.Shen H., Rodriguez A.C., Shiani A., Lipka S., Shahzad G., Kumar A., Mustacchia P. Association between caffeine consumption and nonalcoholic fatty liver disease: A systemic review and meta-analysis. Ther. Adv. Gastroenterol. 2016;9:113–120. doi: 10.1177/1756283X15593700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hayat U., Siddiqui A.A., Okut H., Afroz S., Tasleem S., Haris A. The effect of coffee consumption on the non-alcoholic fatty liver disease and liver fibrosis: A meta-analysis of 11 epidemiological studies. Ann. Hepatol. 2021;20:100254. doi: 10.1016/j.aohep.2020.08.071. [DOI] [PubMed] [Google Scholar]
- 76.Shim S.G., Jun D.W., Kim E.K., Saeed W.K., Lee K.N., Lee H.L., Lee O.Y., Choi H.S., Yoon B.C. Caffeine attenuates liver fibrosis via defective adhesion of hepatic stellate cells in cirrhotic model. J. Gastroenterol. Hepatol. 2013;28:1877–1884. doi: 10.1111/jgh.12317. [DOI] [PubMed] [Google Scholar]
- 77.Molloy J.W., Calcagno C.J., Williams C.D., Jones F.J., Torres D.M., Harrison S.A. Association of coffee and caffeine consumption with fatty liver disease, nonalcoholic steatohepatitis, and degree of hepatic fibrosis. Hepatology. 2012;55:429–436. doi: 10.1002/hep.24731. [DOI] [PubMed] [Google Scholar]
- 78.Mansour A., Mohajeri-Tehrani M.R., Samadi M., Qorbani M., Merat S., Adibi H., Poustchi H., Hekmatdoost A. Effects of supplementation with main coffee components including caffeine and/or chlorogenic acid on hepatic, metabolic, and inflammatory indices in patients with non-alcoholic fatty liver disease and type 2 diabetes: A randomized, double-blind, placebo-controlled, clinical trial. Nutr. J. 2021;20:35. doi: 10.1186/s12937-021-00694-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.MacKenzie T., Comi R., Sluss P., Keisari R., Manwar S., Kim J., Larson R., Baron J.A. Metabolic and hormonal effects of caffeine: Randomized, double-blind, placebo-controlled crossover trial. Metab. Clin. Exp. 2007;56:1694–1698. doi: 10.1016/j.metabol.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 80.Thong F.S., Derave W., Kiens B., Graham T.E., Ursø B., Wojtaszewski J.F., Hansen B.F., Richter E.A. Caffeine-induced impairment of insulin action but not insulin signaling in human skeletal muscle is reduced by exercise. Diabetes. 2002;51:583–590. doi: 10.2337/diabetes.51.3.583. [DOI] [PubMed] [Google Scholar]
- 81.Arnlöv J., Vessby B., Risérus U. Coffee consumption and insulin sensitivity. JAMA. 2004;291:1199–1201. doi: 10.1001/jama.291.10.1199-b. [DOI] [PubMed] [Google Scholar]
- 82.van Dam R.M., Hu F.B. Coffee consumption and risk of type 2 diabetes: A systematic review. JAMA. 2005;294:97–104. doi: 10.1001/jama.294.1.97. [DOI] [PubMed] [Google Scholar]
- 83.Peteliuk V., Rybchuk L., Bayliak M., Storey K.B., Lushchak O. Natural sweetener Stevia rebaudiana: Functionalities, health benefits and potential risks. EXCLI J. 2021;20:1412. doi: 10.17179/excli2021-4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Magnuson B.A., Carakostas M.C., Moore N.H., Poulos S.P., Renwick A.G. Biological fate of low-calorie sweeteners. Nutr. Rev. 2016;74:670–689. doi: 10.1093/nutrit/nuw032. [DOI] [PubMed] [Google Scholar]
- 85.Ashwell M. Stevia, nature’s zero-calorie sustainable sweetener: A new player in the fight against obesity. Nutr. Today. 2015;50:129. doi: 10.1097/NT.0000000000000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tandel K.R. Sugar substitutes: Health controversy over perceived benefits. J. Pharmacol. Pharmacother. 2011;2:236–243. doi: 10.4103/0976-500X.85936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Anker C.C.B., Rafiq S., Jeppesen P.B. Effect of steviol glycosides on human health with emphasis on type 2 diabetic biomarkers: A systematic review and meta-analysis of randomized controlled trials. Nutrients. 2019;11:1965. doi: 10.3390/nu11091965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xi D., Bhattacharjee J., Salazar-Gonzalez R.M., Park S., Jang A., Warren M., Merritt R., Michail S., Bouret S., Kohli R. Rebaudioside affords hepatoprotection ameliorating sugar sweetened beverage-induced nonalcoholic steatohepatitis. Sci. Rep. 2020;10:6689. doi: 10.1038/s41598-020-63688-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kolodziejczyk A.A., Zheng D., Shibolet O., Elinav E. The role of the microbiome in NAFLD and NASH. EMBO Mol. Med. 2019;11:e9302. doi: 10.15252/emmm.201809302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang P. Influence of Foods and Nutrition on the Gut Microbiome and Implications for Intestinal Health. Int. J. Mol. Sci. 2022;23:9588. doi: 10.3390/ijms23179588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yan J., Sheng L., Li H. Akkermansia muciniphila: Is it the Holy Grail for ameliorating metabolic diseases? Gut Microbes. 2021;13:1984104. doi: 10.1080/19490976.2021.1984104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Richardson I.L., Frese S.A. Non-nutritive sweeteners and their impacts on the gut microbiome and host physiology. Front. Nutr. 2022;9:988144. doi: 10.3389/fnut.2022.988144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Renwick A.G., Tarka S.M. Microbial hydrolysis of steviol glycosides. Food Chem. Toxicol. 2008;46:S70–S74. doi: 10.1016/j.fct.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 94.Casas-Grajales S., Reyes-Gordillo K., Cerda-García-Rojas C.M., Tsutsumi V., Lakshman M.R., Muriel P. Rebaudioside A administration prevents experimental liver fibrosis: An in vivo and in vitro study of the mechanisms of action involved. J. Appl. Toxicol. 2019;39:1118–1131. doi: 10.1002/jat.3797. [DOI] [PubMed] [Google Scholar]
- 95.Ruiz-Ojeda F.J., Plaza-Díaz J., Sáez-Lara M.J., Gil A. Effects of sweeteners on the gut microbiota: A review of experimental studies and clinical trials. Adv. Nutr. 2019;10((Suppl. 1)):S31–S48. doi: 10.1093/advances/nmy037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xu Y., Wang N., Tan H.Y., Li S., Zhang C., Feng Y. Function of Akkermansia muciniphila in obesity: Interactions with lipid metabolism, immune response and gut systems. Front. Microbiol. 2020;11:219. doi: 10.3389/fmicb.2020.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nettleton J.E., Reimer R.A., Shearer J. Reshaping the gut microbiota: Impact of low calorie sweeteners and the link to insulin resistance? Physiol. Behav. 2016;164:488–493. doi: 10.1016/j.physbeh.2016.04.029. [DOI] [PubMed] [Google Scholar]
- 98.Suez J., Cohen Y., Valdés-Mas R., Mor U., Dori-Bachash M., Federici S., Zmora N., Leshem A., Heinemann M., Linevsky R., et al. Personalized microbiome-driven effects of non-nutritive sweeteners on human glucose tolerance. Cell. 2022;185:3307–3328. doi: 10.1016/j.cell.2022.07.016. [DOI] [PubMed] [Google Scholar]
- 99.Pang M.D., Goossens G.H., Blaak E.E. The impact of artificial sweeteners on body weight control and glucose homeostasis. Front. Nutr. 2021;7:598340. doi: 10.3389/fnut.2020.598340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Olofsson L.E., Bäckhed F. The metabolic role and therapeutic potential of the microbiome. Endocr. Rev. 2022;43:907–926. doi: 10.1210/endrev/bnac004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hrncir T. Gut Microbiota Dysbiosis: Triggers, Consequences, Diagnostic and Therapeutic Options. Microorganisms. 2022;10:578. doi: 10.3390/microorganisms10030578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Del Pozo S., Gómez-Martínez S., Díaz L.E., Nova E., Urrialde R., Marcos A. Potential effects of Sucralose and saccharin on gut microbiota: A review. Nutrients. 2022;14:1682. doi: 10.3390/nu14081682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Uebanso T., Ohnishi A., Kitayama R., Yoshimoto A., Nakahashi M., Shimohata T., Mawatari K., Takahashi A. Effects of low-dose non-caloric sweetener consumption on gut microbiota in mice. Nutrients. 2017;9:560. doi: 10.3390/nu9060560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Saraiva A., Carrascosa C., Raheem D., Ramos F., Raposo A. Maltitol: Analytical determination methods, applications in the food industry, metabolism and health impacts. Int. J. Environ. Res. Public Health. 2020;17:5227. doi: 10.3390/ijerph17145227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sima P., Vannucci L., Vetvicka V. β-glucans and cholesterol. Int. J. Mol. Med. 2018;41:1799–1808. doi: 10.3892/ijmm.2018.3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wright R.S., Anderson J.W., Bridges S.R. Propionate inhibits hepatocyte lipid synthesis. Proc. Soc. Exp. Biol. Med. 1990;195:26–29. doi: 10.3181/00379727-195-43113. [DOI] [PubMed] [Google Scholar]
- 107.Xue H., Chen X., Yu C., Deng Y., Zhang Y., Chen S., Chen X., Chen K., Yang Y., Ling W. Gut microbially produced indole-3-propionic acid inhibits atherosclerosis by promoting reverse cholesterol transport and its deficiency is causally related to atherosclerotic cardiovascular disease. Circ. Res. 2022;131:404–420. doi: 10.1161/CIRCRESAHA.122.321253. [DOI] [PubMed] [Google Scholar]
- 108.Bhuiyan M.J., Do H.V., Mun S., Jun H.J., Lee J.H., Kim Y.R., Lee S.J. Hypocholesterolemic and hypoglycemic effects of enzymatically modified carbohydrates from rice in high-fat-fed C57BL/6J mice. Mol. Nutr. Food Res. 2011;55:S214–S226. doi: 10.1002/mnfr.201100121. [DOI] [PubMed] [Google Scholar]
- 109.Urushima H., Sanada Y., Sakaue M., Matsuzawa Y., Ito T., Maeda K. Maltitol prevents the progression of fatty liver degeneration in mice fed high-fat diets. J. Med. Food. 2015;18:1081–1087. doi: 10.1089/jmf.2014.3380. [DOI] [PubMed] [Google Scholar]
- 110.Ortiz S.R., Field M.S. Chronic dietary erythritol exposure elevates plasma erythritol concentration in mice but does not cause weight gain or modify glucose homeostasis. J. Nutr. 2021;151:2114–2124. doi: 10.1093/jn/nxab130. [DOI] [PubMed] [Google Scholar]
- 111.Kawano R., Okamura T., Hashimoto Y., Majima S., Senmaru T., Ushigome E., Asano M., Yamazaki M., Takakuwa M., Sasano R., et al. Erythritol ameliorates small intestinal inflammation induced by high-fat diets and improves glucose tolerance. Int. J. Mol. Sci. 2021;22:5558. doi: 10.3390/ijms22115558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yokozawa T., Kim H.Y., Cho E.J. Erythritol attenuates the diabetic oxidative stress through modulating glucose metabolism and lipid peroxidation in streptozotocin-induced diabetic rats. J. Agric. Food Chem. 2002;50:5485–5489. doi: 10.1021/jf020168z. [DOI] [PubMed] [Google Scholar]
- 113.Jin M., Wei Y., Yu H., Ma X., Yan S., Zhao L., Ding L., Cheng J., Feng H. Erythritol improves nonalcoholic fatty liver disease by activating Nrf2 antioxidant capacity. J. Agric. Food Chem. 2021;69:13080–13092. doi: 10.1021/acs.jafc.1c05213. [DOI] [PubMed] [Google Scholar]
- 114.Oku T., Nakamura S. Threshold for transitory diarrhea induced by ingestion of xylitol and lactitol in young male and female adults. J. Nutr. Sci. Vitaminol. 2007;53:13–20. doi: 10.3177/jnsv.53.13. [DOI] [PubMed] [Google Scholar]
- 115.Storey D., Lee A., Bornet F., Brouns F.J.P.H. Gastrointestinal tolerance of erythritol and xylitol ingested in a liquid. Eur. J. Clin. Nutr. 2007;61:349–354. doi: 10.1038/sj.ejcn.1602532. [DOI] [PubMed] [Google Scholar]
- 116.Anderson M.E. Glutathione: An overview of biosynthesis and modulation. Chem. Biol. Interact. 1998;111:1–14. doi: 10.1016/S0009-2797(97)00146-4. [DOI] [PubMed] [Google Scholar]
- 117.Honda Y., Kessoku T., Sumida Y., Kobayashi T., Kato T., Ogawa Y., Tomeno W., Imajo K., Fujita K., Yoneda M. Efficacy of glutathione for the treatment of nonalcoholic fatty liver disease: An open-label, single-arm, multicenter, pilot study. BMC Gastroenterol. 2017;17:1–8. doi: 10.1186/s12876-017-0652-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Allen J., Bradley R.D. Effects of oral glutathione supplementation on systemic oxidative stress biomarkers in human volunteers. J. Altern. Complement. Med. 2011;17:827–833. doi: 10.1089/acm.2010.0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kovacs-Nolan J., Rupa P., Matsui T., Tanaka M., Konishi T., Sauchi Y., Sato K., Ono S., Mine Y. In vitro and ex vivo uptake of glutathione (GSH) across the intestinal epithelium and fate of oral GSH after in vivo supplementation. J. Agric. Food Chem. 2014;62:9499–9506. doi: 10.1021/jf503257w. [DOI] [PubMed] [Google Scholar]
- 120.Park E.Y., Shimura N., Konishi T., Sauchi Y., Wada S., Aoi W., Nakamura Y., Sato K. Increase in the protein-bound form of glutathione in human blood after the oral administration of glutathione. J. Agric. Food Chem. 2014;62:6183–6189. doi: 10.1021/jf501338z. [DOI] [PubMed] [Google Scholar]
- 121.Mardinoglu A., Bjornson E., Zhang C., Klevstig M., Söderlund S., Ståhlman M., Adiels M., Hakkarainen A., Lundbom N., Kiricarslan M., et al. Personal model-assisted identification of NAD+ and glutathione metabolism as intervention target in NAFLD. Mol. Syst. Biol. 2017;13:916. doi: 10.15252/msb.20167422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Park S.H., Han A.L., Kim N.H., Shin S.R. Liver histological improvement after administration of high-dose vitamin C in guinea pig with nonalcoholic steatohepatitis. Int. J. Vitam. Nutr. Res. 2019;88:263–269. doi: 10.1024/0300-9831/a000515. [DOI] [PubMed] [Google Scholar]
- 123.Souza M.R.D.A., Diniz M.D.F.F.D.M., Medeiros-Filho J.E.M.D., Araújo M.S.T.D. Metabolic syndrome and risk factors for non-alcoholic fatty liver disease. Arq. De Gastroenterol. 2012;49:89–96. doi: 10.1590/S0004-28032012000100015. [DOI] [PubMed] [Google Scholar]
- 124.Aoi W., Ogaya Y., Takami M., Konishi T., Sauchi Y., Park E.Y., Wada S., Sato K., Higashi A. Glutathione supplementation suppresses muscle fatigue induced by prolonged exercise via improved aerobic metabolism. J. Int. Soc. Sport. Nutr. 2015;12:7. doi: 10.1186/s12970-015-0067-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lapenna D., Pierdomenico S.D., Ciofani G., Ucchino S., Neri M., Giamberardino M.A., Cuccurullo F. Association of body iron stores with low molecular weight iron and oxidant damage of human atherosclerotic plaques. Free. Radic. Biol. Med. 2007;42:492–498. doi: 10.1016/j.freeradbiomed.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 126.Dongiovanni P., Valenti L., Fracanzani A.L., Gatti S., Cairo G., Fargion S. Iron depletion by deferoxamine up-regulates glucose uptake and insulin signaling in hepatoma cells and in rat liver. Am. J. Pathol. 2008;172:738–747. doi: 10.2353/ajpath.2008.070097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sanyal A.J., Chalasani N., Kowdley K.V., McCullough A., Diehl A.M., Bass N.M., Neuschwander-Tetri B.A., Lavine J.E., Tonascia J., Unalp A., et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N. Engl. D J. Med. 2010;362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lippman S.M., Klein E.A., Goodman P.J., Lucia M.S., Thompson I.M., Ford L.G., Parnes H.L., Minasian L.M., Gaziani J.M., Hartline J.A., et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: The Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lee J.H., Lee H.S., Ahn S.B., Kwon Y.J. Dairy protein intake is inversely related to development of non-alcoholic fatty liver disease. Clin. Nutr. 2021;40:5252–5260. doi: 10.1016/j.clnu.2021.08.012. [DOI] [PubMed] [Google Scholar]
- 130.Léveillé M., Estall J.L. Mitochondrial Dysfunction in the Transition from NASH to HCC. Metabolites. 2019;9:233. doi: 10.3390/metabo9100233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Irie M., Sohda T., Anan A., Fukunaga A., Takata K., Tanaka T., Yokoyama K., Morihara D., Takeyama Y., Shakado S., et al. Reduced glutathione suppresses oxidative stress in nonalcoholic fatty liver disease. Euroasian J. Hepato-Gastroenterol. 2016;6:13. doi: 10.5005/jp-journals-10018-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hamad E.M., Taha S.H., Abou Dawood A.G.I., Sitohy M.Z., Abdel-Hamid M. Protective effect of whey proteins against nonalcoholic fatty liver in rats. Lipids Health Dis. 2011;10:57. doi: 10.1186/1476-511X-10-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Morton R.W., Murphy K.T., McKellar S.R., Schoenfeld B.J., Henselmans M., Helms E., Helms E., Aragon A.A., Devries M.C., Banfield L., et al. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br. J. Sport. Med. 2018;52:376–384. doi: 10.1136/bjsports-2017-097608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pereira M.A., Jacobs D.R., Jr., Van Horn L., Slattery M.L., Kartashov A.I., Ludwig D.S. Dairy consumption, obesity, and the insulin resistance syndrome in young adults: The CARDIA Study. JAMA. 2002;287:2081–2089. doi: 10.1001/jama.287.16.2081. [DOI] [PubMed] [Google Scholar]
- 135.Duarte S.M., Stefano J.T., Oliveira C.P. Microbiota and nonalcoholic fatty liver disease/nonalcoholic steatohepatitis (NAFLD/NASH) Ann. Hepatol. 2019;18:416–421. doi: 10.1016/j.aohep.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 136.Song Q., Zhang X. The Role of Gut–Liver Axis in Gut Microbiome Dysbiosis Associated NAFLD and NAFLD-HCC. Biomedicines. 2022;10:524. doi: 10.3390/biomedicines10030524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Khan A., Ding Z., Ishaq M., Bacha A.S., Khan I., Hanif A., Li W., Guo X. Understanding the effects of gut microbiota dysbiosis on nonalcoholic fatty liver disease and the possible probiotics role: Recent updates. Int. J. Biol. Sci. 2021;17:818. doi: 10.7150/ijbs.56214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang H., Mehal W., Nagy L.E., Rotman Y. Immunological mechanisms and therapeutic targets of fatty liver diseases. Cell. Mol. Immunol. 2021;18:73–91. doi: 10.1038/s41423-020-00579-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Takai A., Kikuchi K., Ichimura M., Tsuneyama K., Moritoki Y., Matsumoto K., Tsunashima H., Onda T., Kuniyoshi N., Nariyama T., et al. Fructo-oligosaccharides ameliorate steatohepatitis, visceral adiposity, and associated chronic inflammation via increased production of short-chain fatty acids in a mouse model of non-alcoholic steatohepatitis. BMC Gastroenterol. 2020;20:1–10. doi: 10.1186/s12876-020-01194-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tolhurst G., Heffron H., Lam Y.S., Parker H.E., Habib A.M., Diakogiannaki E., Cameron J., Grosse J., Reimann F., Gribble F.M. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein–coupled receptor FFAR2. Diabetes. 2012;61:364–371. doi: 10.2337/db11-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yabut J.M., Drucker D.J. Glucagon-like peptide-1 receptor-based therapeutics for metabolic liver disease. Endocr. Rev. 2023;44:14–32. doi: 10.1210/endrev/bnac018. [DOI] [PubMed] [Google Scholar]
- 142.Seghieri M., Christensen A.S., Andersen A., Solini A., Knop F.K., Vilsbøll T. Future perspectives on GLP-1 receptor agonists and GLP-1/glucagon receptor co-agonists in the treatment of NAFLD. Front. Endocrinol. 2018;9:649. doi: 10.3389/fendo.2018.00649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kimura I., Inoue D., Hirano K., Tsujimoto G. The SCFA receptor GPR43 and energy metabolism. Front. Endocrinol. 2014;5:85. doi: 10.3389/fendo.2014.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kimura I., Ozawa K., Inoue D., Imamura T., Kimura K., Maeda T., Terasawa K., Kashihara D., Hirano K., Tani T., et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat. Commun. 2013;4:1829. doi: 10.1038/ncomms2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Den Besten G., Bleeker A., Gerding A., van Eunen K., Havinga R., van Dijk T.H., Oosterveer M.H., Jonker J.W., Groen A.K., Reijngound D.J., et al. Short-chain fatty acids protect against high-fat diet–induced obesity via a PPARγ-dependent switch from lipogenesis to fat oxidation. Diabetes. 2015;64:2398–2408. doi: 10.2337/db14-1213. [DOI] [PubMed] [Google Scholar]
- 146.Mattace Raso G., Simeoli R., Russo R., Iacono A., Santoro A., Paciello O., Ferrante M.C., Canani R.B., Calignano A., Meli R. Effects of sodium butyrate and its synthetic amide derivative on liver inflammation and glucose tolerance in an animal model of steatosis induced by high fat diet. PLoS ONE. 2013;8:e68626. doi: 10.1371/journal.pone.0068626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yu J., Loh K., Song Z.Y., Yang H.Q., Zhang Y., Lin S. Update on glycerol-3-phosphate acyltransferases: The roles in the development of insulin resistance. Nutr. Diabetes. 2018;8:34. doi: 10.1038/s41387-018-0045-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Weitkunat K., Schumann S., Nickel D., Kappo K.A., Petzke K.J., Kipp A.P., Blaut M., Klaus S. Importance of propionate for the repression of hepatic lipogenesis and improvement of insulin sensitivity in high-fat diet-induced obesity. Mol. Nutr. Food Res. 2016;60:2611–2621. doi: 10.1002/mnfr.201600305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Al-Lahham S., Roelofsen H., Rezaee F., Weening D., Hoek A., Vonk R., Venema K. Propionic acid affects immune status and metabolism in adipose tissue from overweight. Eur. J. Clin. Investig. 2012;42:357–364. doi: 10.1111/j.1365-2362.2011.02590.x. [DOI] [PubMed] [Google Scholar]
- 150.Tedelind S., Westberg F., Kjerrulf M., Vidal A. Anti-inflammatory properties of the short-chain fatty acids acetate and propionate: A study with relevance to inflammatory bowel disease. World J. Gastroenterol. 2007;13:2826. doi: 10.3748/wjg.v13.i20.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bedogni G., Miglioli L., Masutti F., Castiglione A., Croce L.S., Tiribelli C., Bellentani S. Incidence and natural course of fatty liver in the general population: The Dionysos study. Hepatology. 2007;46:1387–1391. doi: 10.1002/hep.21827. [DOI] [PubMed] [Google Scholar]
- 152.Singh S., Osna N.A., Kharbanda K.K. Treatment options for alcoholic and non-alcoholic fatty liver disease: A review. World J. Gastroenterol. 2017;23:6549. doi: 10.3748/wjg.v23.i36.6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sherriff J.L., O’Sullivan T.A., Properzi C., Oddo J.L., Adams L.A. Choline, its potential role in nonalcoholic fatty liver disease, and the case for human and bacterial genes. Adv. Nutr. 2016;7:5–13. doi: 10.3945/an.114.007955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Craig S.A. Betaine in human nutrition. Am. J. Clin. Nutr. 2004;80:539–549. doi: 10.1093/ajcn/80.3.539. [DOI] [PubMed] [Google Scholar]
- 155.Yao Z.M., Vance D.E. The active synthesis of phosphatidylcholine is required for very low density lipoprotein secretion from rat hepatocytes. J. Biol. Chem. 1988;263:2998–3004. doi: 10.1016/S0021-9258(18)69166-5. [DOI] [PubMed] [Google Scholar]
- 156.Zeisel S.H., Da Costa K.A. Choline: An essential nutrient for public health. Nutr. Rev. 2009;67:615–623. doi: 10.1111/j.1753-4887.2009.00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wiedeman A.M., Barr S.I., Green T.J., Xu Z., Innis S.M., Kitts D.D. Dietary choline intake: Current state of knowledge across the life cycle. Nutrients. 2018;10:1513. doi: 10.3390/nu10101513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lobo R., Prabhu K.S., Shirwaikar A., Shirwaikar A. Curcuma zedoaria Rosc.(white turmeric): A review of its chemical, pharmacological and ethnomedicinal properties. J. Pharm. Pharmacol. 2009;61:13–21. doi: 10.1211/jpp/61.01.0003. [DOI] [PubMed] [Google Scholar]
- 159.Baziar N., Parohan M. The effects of curcumin supplementation on body mass index, body weight, and waist circumference in patients with nonalcoholic fatty liver disease: A systematic review and dose–response meta-analysis of randomized controlled trials. Phytother. Res. 2020;34:464–474. doi: 10.1002/ptr.6542. [DOI] [PubMed] [Google Scholar]
- 160.White C.M., Lee J.Y. The impact of turmeric or its curcumin extract on nonalcoholic fatty liver disease: A systematic review of clinical trials. Pharm. Pract. 2019;17:477–483. doi: 10.18549/PharmPract.2019.1.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Anand P., Kunnumakkara A.B., Newman R.A., Aggarwal B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 162.Cicero A.F., Sahebkar A., Fogacci F., Bove M., Giovannini M., Borghi C. Effects of phytosomal curcumin on anthropometric parameters, insulin resistance, cortisolemia and non-alcoholic fatty liver disease indices: A double-blind, placebo-controlled clinical trial. Eur. J. Nutr. 2020;59:477–483. doi: 10.1007/s00394-019-01916-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Saadati S., Hatami B., Yari Z., Shahrbaf M.A., Eghtesad S., Mansour A., Poustchi H., Hedayati M., Aghajanpoor-Pasha M., Sadeghi A., et al. The effects of curcumin supplementation on liver enzymes, lipid profile, glucose homeostasis, and hepatic steatosis and fibrosis in patients with non-alcoholic fatty liver disease. Eur. J. Clin. Nutr. 2019;73:441–449. doi: 10.1038/s41430-018-0382-9. [DOI] [PubMed] [Google Scholar]
- 164.Liu W., Zhai Y., Heng X., Che F.Y., Chen W., Sun D., Zhai G. Oral bioavailability of curcumin: Problems and advancements. J. Drug Target. 2016;24:694–702. doi: 10.3109/1061186X.2016.1157883. [DOI] [PubMed] [Google Scholar]
- 165.Jin T., Song Z., Weng J., Fantus I.G. Curcumin and other dietary polyphenols: Potential mechanisms of metabolic actions and therapy for diabetes and obesity. Am. J. Physiol. -Endocrinol. Metab. 2018;314:E201–E205. doi: 10.1152/ajpendo.00285.2017. [DOI] [PubMed] [Google Scholar]
- 166.Marton L.T., Pescinini-e-Salzedas L.M., Camargo M.E.C., Barbalho S.M., Haber J.F.D.S., Sinatora R.V., Detregiachi C.R.P., Girio R.J.S., Buchaim D.V., Cincotto dos Santos Bueno P. The effects of curcumin on diabetes mellitus: A systematic review. Front. Endocrinol. 2021;12:669448. doi: 10.3389/fendo.2021.669448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Navekar R., Rafraf M., Ghaffari A., Asghari-Jafarabadi M., Khoshbaten M. Turmeric supplementation improves serum glucose indices and leptin levels in patients with nonalcoholic fatty liver diseases. J. Am. Coll. Nutr. 2017;36:261–267. doi: 10.1080/07315724.2016.1267597. [DOI] [PubMed] [Google Scholar]
- 168.Dong J., Xu H., Xu H., Wang P.F., Cai G.J., Song H.F., Wang C., Dong Z., Ju Y., Jiang Z.Y. Nesfatin-1 stimulates fatty-acid oxidation by activating AMP-activated protein kinase in STZ-induced type 2 diabetic mice. PLoS ONE. 2013;8:e83397. doi: 10.1371/journal.pone.0083397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Shen P., Han Y., Cai B., Wang Y. Decreased levels of serum nesfatin-1 in patients with obstructive sleep apnea syndrome. Sleep Breath. 2015;19:515–522. doi: 10.1007/s11325-014-1039-0. [DOI] [PubMed] [Google Scholar]
- 170.Wang S., Wang X., Ye Z., Xu C., Zhang M., Ruan B., Wei M., Jiang J., Zhang Y., Wang L., et al. Curcumin promotes browning of white adipose tissue in a norepinephrine-dependent way. Biochem. Biophys. Res. Commun. 2015;466:247–253. doi: 10.1016/j.bbrc.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 171.Masuzaki H., Paterson J., Shinyama H., Morton N.M., Mullins J.J., Seckl J.R., Flier J.S. A transgenic model of visceral obesity and the metabolic syndrome. Science. 2001;294:2166–2170. doi: 10.1126/science.1066285. [DOI] [PubMed] [Google Scholar]
- 172.Kumari M., Chandola T., Brunner E., Kivimaki M. A nonlinear relationship of generalized and central obesity with diurnal cortisol secretion in the Whitehall II study. J. Clin. Endocrinol. Metab. 2010;95:4415–4423. doi: 10.1210/jc.2009-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hu G.X., Lin H., Lian Q.Q., Zhou S.H., Guo J., Zhou H.Y., Chu Y., Ge R.S. Curcumin as a potent and selective inhibitor of 11β-hydroxysteroid dehydrogenase 1: Improving lipid profiles in high-fat-diet-treated rats. PLoS ONE. 2013;8:e49976. doi: 10.1371/journal.pone.0049976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bradford P.G. Curcumin and obesity. Biofactors. 2013;39:78–87. doi: 10.1002/biof.1074. [DOI] [PubMed] [Google Scholar]
- 175.Qin S., Huang L., Gong J., Shen S., Huang J., Tang Y., Ren H., Hu H. Meta-analysis of randomized controlled trials of 4 weeks or longer suggest that curcumin may afford some protection against oxidative stress. Nutr. Res. 2018;60:1–12. doi: 10.1016/j.nutres.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 176.Alavinejad P., Farsi F., Husain D., Rezazadeh A. The effect of turmeric on lipid profile, malondialdehyde, liver echogenicity and enzymes among patients with nonalcoholic fatty liver disease: A randomized double blind clinical trial. Diabetol. Metab. Syndr. 2021;13:1–9. doi: 10.1186/s13098-021-00731-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Rao M.N.A. Curcuminoids as potent inhibitors of lipid peroxidation. J. Pharm. Pharmacol. 1994;46:1013–1016. doi: 10.1111/j.2042-7158.1994.tb03258.x. [DOI] [PubMed] [Google Scholar]
- 178.Reddy A.C.P., Lokesh B.R. Studies on the inhibitory effects of curcumin and eugenol on the formation of reactive oxygen species and the oxidation of ferrous iron. Mol. Cell. Biochem. 1994;137:1–8. doi: 10.1007/BF00926033. [DOI] [PubMed] [Google Scholar]
- 179.Mantena S.K., King A.L., Andringa K.K., Eccleston H.B., Bailey S.M. Mitochondrial dysfunction and oxidative stress in the pathogenesis of alcohol-and obesity-induced fatty liver diseases. Free. Radic. Biol. Med. 2008;44:1259–1272. doi: 10.1016/j.freeradbiomed.2007.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Motterlini R., Foresti R., Bassi R., Green C.J. Curcumin, an antioxidant and anti-inflammatory agent, induces heme oxygenase-1 and protects endothelial cells against oxidative stress. Free. Radic. Biol. Med. 2000;28:1303–1312. doi: 10.1016/S0891-5849(00)00294-X. [DOI] [PubMed] [Google Scholar]
- 181.Piper J.T., Singhal S.S., Salameh M.S., Torman R.T., Awasthi Y.C., Awasthi S. Mechanisms of anticarcinogenic properties of curcumin: The effect of curcumin on glutathione linked detoxification enzymes in rat liver. Int. J. Biochem. Cell Biol. 1998;30:445–456. doi: 10.1016/S1357-2725(98)00015-6. [DOI] [PubMed] [Google Scholar]
- 182.Barnes P.J., Karin M. Nuclear factor-κB—A pivotal transcription factor in chronic inflammatory diseases. N. Engl. J. Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 183.Nanji A.A., Jokelainen K., Rahemtulla A., Miao L., Fogt F., Matsumoto H., Tahan S.R., Su G.L. Activation of nuclear factor kappa B and cytokine imbalance in experimental alcoholic liver disease in the rat. Hepatology. 1999;30:934–943. doi: 10.1002/hep.510300402. [DOI] [PubMed] [Google Scholar]
- 184.Kang H.C., Nan J.X., Park P.H., Kim J.Y., Lee S.H., Woo S.W., Zhao Y.Z., Park E.J., Sohn D.H. Curcumin inhibits collagen synthesis and hepatic stellate cell activation in-vivo and in-vitro. J. Pharm. Pharmacol. 2002;54:119–126. doi: 10.1211/0022357021771823. [DOI] [PubMed] [Google Scholar]
- 185.Zarei M., Acharya P., Talahalli R.R. Ginger and turmeric lipid-solubles attenuate heated oil-induced hepatic inflammation via the downregulation of NF-kB in rats. Life Sci. 2021;265:118856. doi: 10.1016/j.lfs.2020.118856. [DOI] [PubMed] [Google Scholar]
- 186.Graf T.N., Wani M.C., Agarwal R., Kroll D.J., Oberlies N.H. Gram-scale purification of flavonolignan diastereoisomers from Silybum marianum (Milk Thistle) extract in support of preclinical in vivo studies for prostate cancer chemoprevention. Planta Med. 2007;73:1495–1501. doi: 10.1055/s-2007-990239. [DOI] [PubMed] [Google Scholar]
- 187.Fehér J., Lengyel G. Silymarin in the prevention and treatment of liver diseases and primary liver cancer. Curr. Pharm. Biotechnol. 2012;13:210–217. doi: 10.2174/138920112798868818. [DOI] [PubMed] [Google Scholar]
- 188.Fried M.W., Navarro V.J., Afdhal N., Belle S.H., Wahed A.S., Hawke R.L., Doo E., Meyers C.M., Reddy K.R. Effect of silymarin (milk thistle) on liver disease in patients with chronic hepatitis C unsuccessfully treated with interferon therapy: A randomized controlled trial. JAMA. 2012;308:274–282. doi: 10.1001/jama.2012.8265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Polyak S.J., Ferenci P., Pawlotsky J.M. Hepatoprotective and antiviral functions of silymarin components in HCV infection. Hepatology. 2013;57:1262. doi: 10.1002/hep.26179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Abenavoli L., Izzo A.A., Milić N., Cicala C., Santini A., Capasso R. Milk thistle (Silybum marianum): A concise overview on its chemistry, pharmacological, and nutraceutical uses in liver diseases. Phytother. Res. 2018;32:2202–2213. doi: 10.1002/ptr.6171. [DOI] [PubMed] [Google Scholar]
- 191.Loguercio C., Andreone P., Brisc C., Brisc M.C., Bugianesi E., Chiaramonte M., Cursaro C., Danila M., de Sio I., Floreani A., et al. Silybin combined with phosphatidylcholine and vitamin E in patients with nonalcoholic fatty liver disease: A randomized controlled trial. Free. Radic. Biol. Med. 2012;52:1658–1665. doi: 10.1016/j.freeradbiomed.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 192.Mengesha T., Gnanasekaran N., Mehare T. Hepatoprotective effect of silymarin on fructose induced nonalcoholic fatty liver disease in male albino wistar rats. BMC Complement. Med. Ther. 2021;21:1–13. doi: 10.1186/s12906-021-03275-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ross S.M. Milk thistle (Silybum marianum): An ancient botanical medicine for modern times. Holist. Nurs. Pract. 2008;22:299–300. doi: 10.1097/01.HNP.0000334924.77174.6d. [DOI] [PubMed] [Google Scholar]
- 194.Navarro V.J., Belle S.H., D’Amato M., Adfhal N., Brunt E.M., Fried M.W., Reddy K.R., Wahed A.S., Harrison S. Silymarin in non-cirrhotics with non-alcoholic steatohepatitis: A randomized, double-blind, placebo controlled trial. PLoS ONE. 2019;14:e0221683. doi: 10.1371/journal.pone.0221683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kheong C.W., Mustapha N.R.N., Mahadeva S. A randomized trial of silymarin for the treatment of nonalcoholic steatohepatitis. Clin. Gastroenterol. Hepatol. 2017;15:1940–1949. doi: 10.1016/j.cgh.2017.04.016. [DOI] [PubMed] [Google Scholar]
- 196.Solhi H., Ghahremani R., Kazemifar A.M., Yazdi Z.H. Silymarin in treatment of non-alcoholic steatohepatitis: A randomized clinical trial. Casp. J. Intern. Med. 2014;5:9. [PMC free article] [PubMed] [Google Scholar]
- 197.Abenavoli L., Greco M., Nazionale I., Peta V., Milic N., Accattato F., Foti D., Gulletta E., Luzza F. Effects of Mediterranean diet supplemented with silybin–vitamin E–phospholipid complex in overweight patients with non-alcoholic fatty liver disease. Expert Rev. Gastroenterol. Hepatol. 2015;9:519–527. doi: 10.1586/17474124.2015.1004312. [DOI] [PubMed] [Google Scholar]
- 198.Loguercio C., Federico A., Trappoliere M., Tuccillo C., Sio I.D., Leva A.D., Niosi M., D’auria V.M., Capasso R., Blanci C.D.V., et al. The effect of a silybin-vitamin e-phospholipid complex on nonalcoholic fatty liver disease: A pilot study. Dig. Dis. Sci. 2007;52:2387–2395. doi: 10.1007/s10620-006-9703-2. [DOI] [PubMed] [Google Scholar]
- 199.Geillinger K.E., Rathmann D., Köhrle J., Fiamoncini J., Daniel H., Kipp A.P. Hepatic metabolite profiles in mice with a suboptimal selenium status. J. Nutr. Biochem. 2014;25:914–922. doi: 10.1016/j.jnutbio.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 200.Schäfer K., Kyriakopoulos A., Gessner H., Grune T., Behne D. Effects of selenium deficiency on fatty acid metabolism in rats fed fish oil-enriched diets. J. Trace Elem. Med. Biol. 2004;18:89–97. doi: 10.1016/j.jtemb.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 201.Polyzos S.A., Kountouras J., Zavos C. Nonalcoholic fatty liver disease: The pathogenetic roles of insulin resistance and adipocytokines. Curr. Mol. Med. 2009;9:299–314. doi: 10.2174/156652409787847191. [DOI] [PubMed] [Google Scholar]
- 202.Han J., Liang H., Yi J., Tan W., He S., Wang S., Li F., Wu X., Ma J., Shi X., et al. Long-term selenium-deficient diet induces liver damage by altering hepatocyte ultrastructure and MMP1/3 and TIMP1/3 expression in growing rats. Biol. Trace Elem. Res. 2017;175:396–404. doi: 10.1007/s12011-016-0781-5. [DOI] [PubMed] [Google Scholar]
- 203.George J. Determination of selenium during pathogenesis of hepatic fibrosis employing hydride generation and inductively coupled plasma mass spectrometry. Biol. Chem. 2018;399:499–509. doi: 10.1515/hsz-2017-0260. [DOI] [PubMed] [Google Scholar]
- 204.Bitiren M., Karakılçık A.Z., Zerin M., Aksoy N., Musa D. Effects of selenium on histopathological and enzymatic changes in experimental liver injury of rats. Exp. Toxicol. Pathol. 2004;56:59–64. doi: 10.1016/j.etp.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 205.Zhang Q., Qian Z.Y., Zhou P.H., Zhou X.L., Zhang D.L., He N., Zhang J., Liu Y.H., Gu Q. Effects of oral selenium and magnesium co-supplementation on lipid metabolism, antioxidative status, histopathological lesions, and related gene expression in rats fed a high-fat diet. Lipids Health Dis. 2018;17:165. doi: 10.1186/s12944-018-0815-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Shidfar F., Faghihi A., Amiri H.L., Mousavi S.N. Regression of nonalcoholic fatty liver disease with zinc and selenium co-supplementation after disease progression in rats. Iran. J. Med. Sci. 2018;43:26. [PMC free article] [PubMed] [Google Scholar]
- 207.Nido S.A., Shituleni S.A., Mengistu B.M., Liu Y., Khan A.Z., Gan F., Kumbhar S., Huang K. Effects of selenium-enriched probiotics on lipid metabolism, antioxidative status, histopathological lesions, and related gene expression in mice fed a high-fat diet. Biol. Trace Elem. Res. 2016;171:399–409. doi: 10.1007/s12011-015-0552-8. [DOI] [PubMed] [Google Scholar]
- 208.González-Reimers E., Monedero-Prieto M.J., González-Pérez J.M., Duran-Castellon M.C., Galindo-Martín L., Abreu-González P., Sanchez-Perez M.J., Santolaria-Fernández F. Relative and combined effects of selenium, protein deficiency and ethanol on hepatocyte ballooning and liver steatosis. Biol. Trace Elem. Res. 2013;154:281–287. doi: 10.1007/s12011-013-9734-4. [DOI] [PubMed] [Google Scholar]
- 209.Ding M., Potter J.J., Liu X., Torbenson M.S., Mezey E. Selenium supplementation decreases hepatic fibrosis in mice after chronic carbon tetrachloride administration. Biol. Trace Elem. Res. 2010;133:83–97. doi: 10.1007/s12011-009-8414-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Polyzos S.A., Kountouras J., Mantzoros C.S. Adipokines in nonalcoholic fatty liver disease. Metabolism. 2016;65:1062–1079. doi: 10.1016/j.metabol.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 211.Bioulac-Sage P., Dubuisson L., Bedin C., Gonzalez P., de Tinguy-Moreaud E., Garcin H., Balabaud C. Nodular regenerative hyperplasia in the rat induced by a selenium-enriched diet: Study of a model. Hepatology. 1992;16:418–425. doi: 10.1002/hep.1840160221. [DOI] [PubMed] [Google Scholar]
- 212.Dubuisson L., Boussarie L., Bedin C.A., Balabaud C., Bioulac-Sage P. Transformation of sinusoids into capillaries in a rat model of selenium-induced nodular regenerative hyperplasia: An immunolight and immunoelectron microscopic study. Hepatology. 1995;21:805–814. [PubMed] [Google Scholar]
- 213.Koeberle A., Löser K., Thürmer M. Stearoyl-CoA desaturase-1 and adaptive stress signaling. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Biol. Lipids. 2016;1861:1719–1726. doi: 10.1016/j.bbalip.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 214.Song Z., Xiaoli A.M., Yang F. Regulation and metabolic significance of de novo lipogenesis in adipose tissues. Nutrients. 2018;10:1383. doi: 10.3390/nu10101383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Poudyal H., Brown L. Stearoyl-CoA desaturase: A vital checkpoint in the development and progression of obesity. Endocr. Metab. Immune Disord. -Drug Targets (Former. Curr. Drug Targets-Immune Endocr. Metab. Disord.) 2011;11:217–231. doi: 10.2174/187153011796429826. [DOI] [PubMed] [Google Scholar]
- 216.Paton C.M., Ntambi J.M. Biochemical and physiological function of stearoyl-CoA desaturase. Am. J. Physiol. -Endocrinol. Metab. 2009;297:E28–E37. doi: 10.1152/ajpendo.90897.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Enoch H.G., Catalá A., Strittmatter P. Mechanism of rat liver microsomal stearyl-CoA desaturase. Studies of the substrate specificity, enzyme-substrate interactions, and the function of lipid. J. Biol. Chem. 1976;251:5095–5103. doi: 10.1016/S0021-9258(17)33223-4. [DOI] [PubMed] [Google Scholar]
- 218.Mosley E.E., McGuire M.A. Methodology for the In Vivo Measurement of the Δ9-Desaturation of Myristic, Palmitic, and Stearic Acids in Lactating Dairy Cattle. Lipids. 2007;42:939–945. doi: 10.1007/s11745-007-3085-x. [DOI] [PubMed] [Google Scholar]
- 219.Miyazaki M.A., Dobrzyn W.C., Man K., Chu H., Sampath H.J., Kim J.M. Ntambi. Stearoyl-CoA desaturase 1 gene expression is necessary for fructose-mediated induction of lipogenic gene expression by sterol regulatory element-binding protein-1c-dependent and -independent mechanisms. J. Biol. Chem. 2004;279:25164–25171. doi: 10.1074/jbc.M402781200. [DOI] [PubMed] [Google Scholar]
- 220.Ntambi J.M., Miyazaki M., Stoehr J.P., Lan H., Kendziorski C.M., Yandell B.S., Song Y., Cohen P., Friedman J.M., Attie A.D. Loss of stearoyl-CoA desaturase-1 function protects mice against adiposity. Proc. Natl. Acad. Sci. USA. 2002;99:11482–11486. doi: 10.1073/pnas.132384699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Oballa R.M., Belair L., Black W.C., Bleasby K., Chan C.C., Desroches C., Du X., Gordon R., Guay J., Guiral S., et al. Development of a liver-targeted stearoyl-CoA desaturase (SCD) inhibitor (MK-8245) to establish a therapeutic window for the treatment of diabetes and dyslipidemia. J. Med. Chem. 2011;54:5082–5096. doi: 10.1021/jm200319u. [DOI] [PubMed] [Google Scholar]
- 222.Uto Y., Ogata T., Kiyotsuka Y., Ueno Y., Miyazawa Y., Kurata H., Deguchi T., Watanabe N., Konishi M., Okuyama R., et al. Novel benzoylpiperidine-based stearoyl-CoA desaturase-1 inhibitors: Identification of 6-[4-(2-methylbenzoyl) piperidin-1-yl]pyridazine-3-carboxylic acid (2-hydroxy-2-pyridin-3-ylethyl)amide and its plasma triglyceride-lowering effects in Zucker fatty rats. Bioorg. Med. Chem. Lett. 2010;20:341–345. doi: 10.1016/j.bmcl.2009.10.101. [DOI] [PubMed] [Google Scholar]
- 223.Flowers M.T., Paton C.M., O’Byrne S.M., Schiesser K., Dawson J.A., Blaner W.S., Kendziorski C., Ntambi J.M. Metabolic changes in skin caused by Scd1 deficiency: A focus on retinol metabolism. PLoS ONE. 2011;9:e19734. doi: 10.1371/journal.pone.0019734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Brown J.M., Rudel L.L. Stearoyl-coenzyme A desaturase 1 inhibition and the metabolic syndrome: Considerations for future drug discovery. Curr. Opin. Lipidol. 2010;21:192–197. doi: 10.1097/MOL.0b013e32833854ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Brown J.M., Chung S., Sawyer J.K., Degirolamo C., Alger H.M., Nguyen T., Zhu X., Duong M.N., Wibley A.L., Shah R., et al. Inhibition of stearoyl-coenzyme A desaturase 1 dissociates insulin resistance and obesity from atherosclerosis. Circulation. 2008;118:1467–1475. doi: 10.1161/CIRCULATIONAHA.108.793182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Zhang Z., Dales N.A., Winther M.D. Opportunities and challenges in developing stearoyl-coenzyme A desaturase-1 inhibitors as novel therapeutics for human disease. J. Med. Chem. 2014;57:5039–5056. doi: 10.1021/jm401516c. [DOI] [PubMed] [Google Scholar]
- 227.Major C.A., Ryan K., Bennett A.J., Lock A.L., Bauman D.E., Salter A.M. Inhibition of stearoyl CoA desaturase activity induces hypercholesterolemia in the cholesterol-fed hamster. J. Lipid Res. 2008;49:1456–1465. doi: 10.1194/jlr.M700596-JLR200. [DOI] [PubMed] [Google Scholar]
- 228.Ortinau L.C., Nickelson K.J., Stromsdorfer K.L., Naik C.Y., Pickering R.T., Haynes R.A., Fritsche K.L., Perfield J.W., II Sterculic Oil, a natural inhibitor of SCD1, improves the metabolic state of obese OLETF rats. Obesity. 2013;21:344–352. doi: 10.1002/oby.20040. [DOI] [PubMed] [Google Scholar]
- 229.Gomez F.E., Bauman D.E., Ntambi J.M., Fox B.G. Effects of sterculic acid on stearoyl-CoA desaturase in differentiating 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 2003;300:316–326. doi: 10.1016/S0006-291X(02)02842-5. [DOI] [PubMed] [Google Scholar]
- 230.Raju P.K., Reiser R. Inhibition of fatty acyl desaturase by cyclopropene fatty acids. J. Biol. Chem. 1967;242:379–384. doi: 10.1016/S0021-9258(18)96281-2. [DOI] [PubMed] [Google Scholar]
- 231.Jeffcoat R., Pollard M.R. Studies on the inhibition of the desaturases by cyclopropenoid fatty acids. Lipids. 1977;12:480–485. doi: 10.1007/BF02535446. [DOI] [PubMed] [Google Scholar]
- 232.Ortinau L.C., Pickering R.T., Nickelson K.J., Stromsdorfer K.L., Naik C.Y., Haynes R.A., Bauman D.E., Rector R.S., Fritsche K.L. Sterculic Oil, a Natural SCD1 Inhibitor, Improves Glucose Tolerance in Obese ob/ob Mice. Perfield JW 2nd. ISRN Endocrinol. 2012;2012:947323. doi: 10.5402/2012/947323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Cao J.J., Blond P., Bézard J. Inhibition of fatty acid Δ6-and Δ5-desaturation by cyclopropene fatty acids in rat liver microsomes. Biochim. Biophys. Acta (BBA)-Lipids Lipid Metab. 1993;1210:27–34. doi: 10.1016/0005-2760(93)90045-B. [DOI] [PubMed] [Google Scholar]
- 234.Lock A.L., Corl B.A., Barbano D.M., Bauman D.E., Ip C. The anticarcinogenic effect of trans-11 18:1 is dependent on its conversion to cis-9, trans-11 CLA by delta9-desaturase in rats. J. Nutr. 2004;134:2698–2704. doi: 10.1093/jn/134.10.2698. [DOI] [PubMed] [Google Scholar]
- 235.Varani J., McClintock S.D., Knibbs R.N., Harber I., Zeidan D., Jawad-Makki M.A.H., Aslam M.N. Liver Protein Expression in NASH Mice on a High-Fat Diet: Response to Multi-Mineral Intervention. Front. Nutr. 2022;11:859292. doi: 10.3389/fnut.2022.859292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Aslam M.N., Bergin I., Naik M., Hampton A., Allen R., Kunkel S.L., Rush H., Varani J. A multi-mineral natural product inhibits liver tumor formation in C57BL/6 mice. Biol. Trace Elem. Res. 2012;147:267–274. doi: 10.1007/s12011-011-9316-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Aslam M.N., Bassis C.M., Zhang L., Zaidi S., Varani J., Bergin I.L. Correction: Calcium Reduces Liver Injury in Mice on a High-Fat Diet: Alterations in Microbial and Bile Acid Profiles. PLoS ONE. 2017;12:e0170136. doi: 10.1371/journal.pone.0170136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Stewart T.A., Yapa K.T., Monteith G.R. Altered calcium signaling in cancer cells. Biochim. Biophys. Acta. 2015;1848:2502–2511. doi: 10.1016/j.bbamem.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 239.Canaff L., Petit J.L., Kisiel M., Watson P.H., Gascon-Barré M., Hendy G.N. Extracellular calcium-sensing receptor is expressed in rat hepatocytes. coupling to intracellular calcium mobilization and stimulation of bile flow. J. Biol. Chem. 2001;276:4070–4079. doi: 10.1074/jbc.M009317200. [DOI] [PubMed] [Google Scholar]
- 240.Bai Y., Herforth A., Masters W.A. Global variation in the cost of a nutrient-adequate diet by population group: An observational study. Lancet Planet Health. 2022;6:e19–e28. doi: 10.1016/S2542-5196(21)00285-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Aslam M.N., Bassis C.M., Bergin I.L., Knuver K., Zick S.M., Sen A., Turgeon D.K., Varani J. A Calcium-Rich Multimineral Intervention to Modulate Colonic Microbial Communities and Metabolomic Profiles in Humans: Results from a 90-Day Trial. Cancer Prev. Res. 2020;13:101–116. doi: 10.1158/1940-6207.CAPR-19-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Ducheix S., Montagner A., Polizzi A., Lasserre F., Régnier M., Marmugi A., Benhamed F., Bertrand-Michel J., Mselli-Lakhal L., Loiseau N., et al. Dietary oleic acid regulates hepatic lipogenesis through a liver X receptor-dependent signaling. PLoS ONE. 2017;12:e0181393. doi: 10.1371/journal.pone.0181393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Chen C., Shah Y.M., Morimura K., Krausz K.W., Miyazaki M., Richardson T.A., Morgan E.T., Ntambi J.M., Idle J.R., Gonzalez F.J. Metabolomics reveals that hepatic stearoyl-CoA desaturase 1 downregulation exacerbates inflammation and acute colitis. Cell Metab. 2008;7:135–147. doi: 10.1016/j.cmet.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Flowers M.T., Ade L., Strable M.S., Ntambi J.M. Combined deletion of SCD1 from adipose tissue and liver does not protect mice from obesity. J. Lipid Res. 2012;53:1646–1653. doi: 10.1194/jlr.M027508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Burhans M.S., Flowers M.T., Harrington K.R., Bond L.M., Guo C.A., Anderson R.M., Ntambi J.M. Hepatic oleate regulates adipose tissue lipogenesis and fatty acid oxidation. J. Lipid Res. 2015;56:304–318. doi: 10.1194/jlr.M054429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Tanaka N., Kono H., Ishii K., Hosomura N., Fujii H. Dietary olive oil prevents carbon tetrachloride-induced hepatic fibrosis in mice. J. Gastroenterol. 2009;44:983–990. doi: 10.1007/s00535-009-0088-9. [DOI] [PubMed] [Google Scholar]
- 247.Lee J.Y., Moon J.H., Park J.S., Lee B.W., Kang E.S., Ahn C.W., Lee H.C., Cha B.S. Dietary oleate has beneficial effects on every step of non-alcoholic Fatty liver disease progression in a methionine- and choline-deficient diet-fed animal model. Diabetes Metab. J. 2011;35:489–496. doi: 10.4093/dmj.2011.35.5.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Benhamed F., Denechaud P.D., Lemoine M., Robichon C., Moldes M., Bertrand-Michel J., Ratziu V., Serfaty L., Housset C., Capeau J., et al. The lipogenic transcription factor ChREBP dissociates hepatic steatosis from insulin resistance in mice and humans. J. Clin. Investig. 2012;122:2176–2194. doi: 10.1172/JCI41636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Reyes-Quiroz M.E., Alba G., Saenz J., Santa-María C., Geniz I., Jiménez J., Ramírez R., Martín-Nieto J., Pintado E., Sobrino F. Oleic acid modulates mRNA expression of liver X receptor (LXR) and its target genes ABCA1 and SREBP1c in human neutrophils. Eur. J. Nutr. 2014;53:1707–1717. doi: 10.1007/s00394-014-0677-0. [DOI] [PubMed] [Google Scholar]
- 250.Ou J., Tu H., Shan B., Luk A., DeBose-Boyd R.A., Bashmakov Y., Goldstein J.L., Brown M.S. Unsaturated fatty acids inhibit transcription of the sterol regulatory element-binding protein-1c (SREBP-1c) gene by antagonizing ligand-dependent activation of the LXR. Proc. Natl. Acad. Sci. USA. 2001;98:6027–6032. doi: 10.1073/pnas.111138698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Janowski B.A., Willy P.J., Devi T.R., Falck J.R., Mangelsdorf D.J. An oxysterol signalling pathway mediated by the nuclear receptor LXR alpha. Nature. 1996;383:728–731. doi: 10.1038/383728a0. [DOI] [PubMed] [Google Scholar]
- 252.Calkin A.C., Tontonoz P. Transcriptional integration of metabolism by the nuclear sterol-activated receptors LXR and FXR. Nat. Rev. Mol. Cell Biol. 2012;13:213–224. doi: 10.1038/nrm3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Joseph S.B., Laffitte B.A., Patel P.H., Watson M.A., Matsukuma K.E., Walczak R., Collins J.L., Osborne T.F., Tontonoz P. Direct and indirect mechanisms for regulation of fatty acid synthase gene expression by liver X receptors. J. Biol. Chem. 2002;277:11019–11025. doi: 10.1074/jbc.M111041200. [DOI] [PubMed] [Google Scholar]
- 254.Chu K., Miyazaki M., Man W.C., Ntambi J.M. Stearoyl-coenzyme A desaturase 1 deficiency protects against hypertriglyceridemia and increases plasma high-density lipoprotein cholesterol induced by liver X receptor activation. Mol. Cell. Biol. 2006;26:6786–6798. doi: 10.1128/MCB.00077-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Grefhorst A., Elzinga B.M., Voshol P.J., Plösch T., Kok T., Bloks V.W., van der Sluijs F.H., Havekes L.M., Romijn J.A., Verkade H.J., et al. Stimulation of lipogenesis by pharmacological activation of the liver X receptor leads to production of large, triglyceride-rich very low density lipoprotein particles. J. Biol. Chem. 2002;277:34182–34190. doi: 10.1074/jbc.M204887200. [DOI] [PubMed] [Google Scholar]
- 256.Peet D.J., Turley S.D., Ma W., Janowski B.A., Lobaccaro J.M., Hammer R.E., Mangelsdorf D.J. Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXR alpha. Cell. 1998;93:693–704. doi: 10.1016/S0092-8674(00)81432-4. [DOI] [PubMed] [Google Scholar]
- 257.Zelcer N., Tontonoz P. Liver X receptors as integrators of metabolic and inflammatory signaling. J. Clin. Investig. 2006;116:607–614. doi: 10.1172/JCI27883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Beaven S.W., Wroblewski K., Wang J., Hong C., Bensinger S., Tsukamoto H., Tontonoz P. Liver X receptor signaling is a determinant of stellate cell activation and susceptibility to fibrotic liver disease. Gastroenterology. 2011;140:1052–1062. doi: 10.1053/j.gastro.2010.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Moravcová A., Červinková Z., Kučera O., Mezera V., Rychtrmoc D., Lotková H. The effect of oleic and palmitic acid on induction of steatosis and cytotoxicity on rat hepatocytes in primary culture. Physiol. Res. 2015;64((Suppl. 5)):S627–S636. doi: 10.33549/physiolres.933224. [DOI] [PubMed] [Google Scholar]
- 260.Abenavoli L., Peta V., Milic N. Lifestyle changes associated with a new antioxidant formulation in non-alcoholic fatty liver disease: A case series. Ann. Hepatol. 2015;14:121–126. doi: 10.1016/S1665-2681(19)30809-9. [DOI] [PubMed] [Google Scholar]
- 261.Wasmuth H.E., Trautwein C. CB1 cannabinoid receptor antagonism: A new strategy for the treatment of liver fibrosis. Hepatology. 2007;45:543–544. doi: 10.1002/hep.21527. [DOI] [PubMed] [Google Scholar]
- 262.Palomares B., Ruiz-Pino F., Garrido-Rodriguez M., Eugenia Prados M., Sánchez-Garrido M.A., Velasco I., Vazquez M.J., Nadal X., Ferreiro-Vera C., Morrugares R., et al. Tetrahydrocannabinolic acid A (THCA-A) reduces adiposity and prevents metabolic disease caused by diet-induced obesity. Biochem. Pharmacol. 2020;171:113693. doi: 10.1016/j.bcp.2019.113693. [DOI] [PubMed] [Google Scholar]
- 263.Rajavashisth T.B., Shaheen M., Norris K.C., Pan D., Sinha S.K., Ortega J., Friedman T.C. Decreased prevalence of diabetes in marijuana users: Cross-sectional data from the National Health and Nutrition Examination Survey (NHANES) III. BMJ Open. 2012;2:e000494. doi: 10.1136/bmjopen-2011-000494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Osei-Hyiaman D., DePetrillo M., Pacher P., Liu J., Radaeva S., Bátkai S., Har-vey-White J., Mackie K., Offertáler L., Wang L., et al. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J. Clin. Investig. 2005;115:1298–1305. doi: 10.1172/JCI200523057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Osei-Hyiaman D., Liu J., Zhou L., Godlewski G., Harvey-White J., Jeong W.I., Bátkai S., Marsicano G., Lutz B., Buettner C., et al. Hepatic CB1 receptor is required for development of diet-induced steatosis, dyslipidemia, and insulin and leptin resistance in mice. J. Clin. Investig. 2008;118:3160–3169. doi: 10.1172/JCI34827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Tam J., Liu J., Mukhopadhyay B., Cinar R., Godlewski G., Kunos G. Endocannabinoids in liver disease. Hepatology. 2011;53:346–355. doi: 10.1002/hep.24077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Chen Z., Tian R., She Z., Cai J., Li H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free. Radic. Biol. Med. 2020;152:116–141. doi: 10.1016/j.freeradbiomed.2020.02.025. [DOI] [PubMed] [Google Scholar]
- 268.Gabbia D., Cannella L., De Martin S. The Role of Oxidative Stress in NAFLD-NASH-HCC Transition-Focus on NADPH Oxidases. Biomedicines. 2021;9:687. doi: 10.3390/biomedicines9060687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Salomone F., Li Volti G., Rosso C., Grosso G., Bugianesi E. Unconjugated bilirubin, a potent endogenous antioxidant, is decreased in patients with non-alcoholic steatohepatitis and advanced fibrosis. J. Gastroenterol. Hepatol. 2013;28:1202–1208. doi: 10.1111/jgh.12155. [DOI] [PubMed] [Google Scholar]
- 270.Świderska M., Maciejczyk M., Zalewska A., Pogorzelska J., Flisiak R., Chabowski A. Oxidative stress biomarkers in the serum and plasma of patients with non-alcoholic fatty liver disease (NAFLD). Can plasma AGE be a marker of NAFLD? Oxidative stress biomarkers in NAFLD patients. Free. Radic. Res. 2019;53:841–850. doi: 10.1080/10715762.2019.1635691. [DOI] [PubMed] [Google Scholar]
- 271.Sutti S., Albano E. Adaptive immunity: An emerging player in the progression of NAFLD. Nature reviews. Gastroenterol. Hepatol. 2020;17:81–92. doi: 10.1038/s41575-019-0210-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Li Z., Yu P., Wu J., Tao F., Zhou J. Transcriptional Regulation of Early Growth Response Gene-1 (EGR1) is Associated with Progression of Nonalcoholic Fatty Liver Disease (NAFLD) in Patients with Insulin Resistance. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019;25:2293–3004. doi: 10.12659/MSM.914044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Abenavoli L., Larussa T., Corea A., Procopio A.C., Boccuto L., Dallio M., Federico A., Luzza F. Dietary Polyphenols and Non-Alcoholic Fatty Liver Disease. Nutrients. 2021;13:494. doi: 10.3390/nu13020494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Aljomah G., Baker S.S., Liu W., Kozielski R., Oluwole J., Lupu B., Baker R.D., Zhu L. Induction of CYP2E1 in non-alcoholic fatty liver diseases. Exp. Mol. Pathol. 2015;99:677–681. doi: 10.1016/j.yexmp.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Fu A., Shi X., Zhang H., Fu B. Mitotherapy for Fatty Liver by Intravenous Administration of Exogenous Mitochondria in Male Mice. Front. Pharmacol. 2017;8:241. doi: 10.3389/fphar.2017.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Koo J.H., Han C.Y. Signaling Nodes Associated with Endoplasmic Reticulum Stress during NAFLD Progression. Biomolecules. 2021;11:242. doi: 10.3390/biom11020242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Madan K., Bhardwaj P., Thareja S., Gupta S.D., Saraya A. Oxidant stress and antioxidant status among patients with nonalcoholic fatty liver disease (NAFLD) J. Clin. Gastroenterol. 2006;40:930–935. doi: 10.1097/01.mcg.0000212608.59090.08. [DOI] [PubMed] [Google Scholar]
- 278.Berenstein N. Making a global sensation: Vanilla flavor, synthetic chemistry, and the meanings of purity. Hist. Sci. 2016;54:399–424. doi: 10.1177/0073275316681802. [DOI] [PubMed] [Google Scholar]
- 279.Raza S., Tewari A., Rajak S., Sinha R.A. Vitamins and non-alcoholic fatty liver disease: A Molecular Insight⋆. Liver Res. 2021;5:62–71. doi: 10.1016/j.livres.2021.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Ezhilarasan D. Hepatic stellate cells in the injured liver: Perspectives beyond hepatic fibrosis. J. Cell. Physiol. 2022;237:436–449. doi: 10.1002/jcp.30582. [DOI] [PubMed] [Google Scholar]
- 281.Saeed A., Bartuzi P., Heegsma J., Dekker D., Kloosterhuis N., de Bruin A., Jonker J.W., van de Sluis B., Faber K.N. Impaired Hepatic Vitamin A Metabolism in NAFLD Mice Leading to Vitamin A Accumulation in Hepatocytes. Cell. Mol. Gastroenterol. Hepatol. 2021;11:309–325.e3. doi: 10.1016/j.jcmgh.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Geng C., Xu H., Zhang Y., Gao Y., Li M., Liu X., Gao M., Wang X., Liu X., Fang F., et al. Retinoic acid ameliorates high-fat diet-induced liver steatosis through sirt1. Sci. China Life Sci. 2017;60:1234–1241. doi: 10.1007/s11427-016-9027-6. [DOI] [PubMed] [Google Scholar]
- 283.Liu Y., Chen H., Wang J., Zhou W., Sun R., Xia M. Association of serum retinoic acid with hepatic steatosis and liver injury in nonalcoholic fatty liver disease. Am. J. Clin. Nutr. 2015;102:130–137. doi: 10.3945/ajcn.114.105155. [DOI] [PubMed] [Google Scholar]
- 284.Pingitore P., Dongiovanni P., Motta B.M., Meroni M., Lepore S.M., Mancina R.M., Pelusi S., Russo C., Caddeo A., Rossi G., et al. PNPLA3 overexpression results in reduction of proteins predisposing to fibrosis. Hum. Mol. Genet. 2016;25:5212–5222. doi: 10.1093/hmg/ddw341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Cai B., Dongiovanni P., Corey K.E., Wang X., Shmarakov I.O., Zheng Z., Kasikara C., Davra V., Meroni M., Chung R.T., et al. Macrophage MerTK Promotes Liver Fibrosis in Nonalcoholic Steatohepatitis. Cell Metab. 2020;31:406–421.e7. doi: 10.1016/j.cmet.2019.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Lee H., Ahn J., Shin S.S., Yoon M. Ascorbic acid inhibits visceral obesity and nonalcoholic fatty liver disease by activating peroxisome proliferator-activated receptor α in high-fat-diet-fed C57BL/6J mice. Int. J. Obes. 2019;43:1620–1630. doi: 10.1038/s41366-018-0212-0. [DOI] [PubMed] [Google Scholar]
- 287.Zeng Q., Zhao L., Meng C., Zhao X., Liu Y., Shi R., Han X., Wang T., Li J. Prophylactic and therapeutic effects of different doses of vitamin C on high-fat-diet-induced non-alcoholic fatty liver disease in mice. Biomed. Pharmacother. Biomed. Pharmacother. 2020;131:110792. doi: 10.1016/j.biopha.2020.110792. [DOI] [PubMed] [Google Scholar]
- 288.Lee S.W., Baek S.M., Kang K.K., Lee A.R., Kim T.U., Choi S.K., Roh Y.S., Hong I.H., Park S.J., Kim T.H., et al. Vitamin C Deficiency Inhibits Nonalcoholic Fatty Liver Disease Progression through Impaired de Novo Lipogenesis. Am. J. Pathol. 2021;191:1550–1563. doi: 10.1016/j.ajpath.2021.05.020. [DOI] [PubMed] [Google Scholar]
- 289.Wei J., Lei G.H., Fu L., Zeng C., Yang T., Peng S.F. Association between Dietary Vitamin C Intake and Non-Alcoholic Fatty Liver Disease: A Cross-Sectional Study among Middle-Aged and Older Adults. PLoS ONE. 2016;11:e0147985. doi: 10.1371/journal.pone.0147985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Zhang Z.C., Liu Y., Xiao L.L., Li S.F., Jiang J.H., Zhao Y., Qian S.W., Tang Q.Q., Li X. Upregulation of miR-125b by estrogen protects against non-alcoholic fatty liver in female mice. J. Hepatol. 2015;63:1466–1475. doi: 10.1016/j.jhep.2015.07.037. [DOI] [PubMed] [Google Scholar]
- 291.Ipsen D.H., Tveden-Nyborg P., Lykkesfeldt J. Does vitamin C deficiency promote fatty liver disease development? Nutrients. 2014;6:5473–5499. doi: 10.3390/nu6125473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Riederer P., Konradi C., Schay V., Kienzl E., Birkmayer G., Danielczyk W., Sofic E., Youdim M.B. Localization of MAO-A and MAO-B in human brain: A step in understanding the therapeutic action of L-deprenyl. Adv. Neurol. 1987;45:111–118. [PubMed] [Google Scholar]
- 293.He W., Xu Y., Ren X., Xiang D., Lei K., Zhang C., Liu D. Vitamin E Ameliorates Lipid Metabolism in Mice with Nonalcoholic Fatty Liver Disease via Nrf2/CES1 Signaling Pathway. Dig. Dis. Sci. 2019;64:3182–3191. doi: 10.1007/s10620-019-05657-9. [DOI] [PubMed] [Google Scholar]
- 294.Nan Y.M., Wu W.J., Fu N., Liang B.L., Wang R.Q., Li L.X., Zhao S.X., Zhao J.M., Yu J. Antioxidants vitamin E and 1-aminobenzotriazole prevent experimental non-alcoholic steatohepatitis in mice. Scand. J. Gastroenterol. 2009;44:1121–1131. doi: 10.1080/00365520903114912. [DOI] [PubMed] [Google Scholar]
- 295.Phung N., Pera N., Farrell G., Leclercq I., Hou J.Y., George J. Pro-oxidant-mediated hepatic fibrosis and effects of antioxidant intervention in murine dietary steatohepatitis. Int. J. Mol. Med. 2009;24:171–180. doi: 10.3892/ijmm_00000220. [DOI] [PubMed] [Google Scholar]
- 296.Abdel-Maboud M., Menshawy A., Menshawy E., Emara A., Alshandidy M., Eid M. The efficacy of vitamin E in reducing non-alcoholic fatty liver disease: A systematic review, meta-analysis, and meta-regression. Ther. Adv. Gastroenterol. 2020;13:1756284820974917. doi: 10.1177/1756284820974917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Birben E., Sahiner U.M., Sackesen C., Erzurum S., Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012;5:9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Peh H.Y., Tan W.S., Liao W., Wong W.S. Vitamin E therapy beyond cancer: Tocopherol versus tocotrienol. Pharmacol. Ther. 2016;162:152–169. doi: 10.1016/j.pharmthera.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 299.Rimbach G., Minihane A.M., Majewicz J., Fischer A., Pallauf J., Virgli F., Weinberg P.D. Regulation of cell signalling by vitamin E. Proc. Nutr. Soc. 2002;61:415–425. doi: 10.1079/PNS2002183. [DOI] [PubMed] [Google Scholar]
- 300.Landrier J.F., Gouranton E., El Yazidi C., Malezet C., Balaguer P., Borel P., Amiot M.J. Adiponectin expression is induced by vitamin E via a peroxisome proliferator-activated receptor gamma-dependent mechanism. Endocrinology. 2009;150:5318–5325. doi: 10.1210/en.2009-0506. [DOI] [PubMed] [Google Scholar]
- 301.Choi Y., Lee S., Kim S., Lee J., Ha J., Oh H., Lee Y., Kim Y., Yoon Y. Vitamin E (α-tocopherol) consumption influences gut microbiota composition. Int. J. Food Sci. Nutr. 2020;71:221–225. doi: 10.1080/09637486.2019.1639637. [DOI] [PubMed] [Google Scholar]
- 302.Ritze Y., Bárdos G., Claus A., Ehrmann V., Bergheim I., Schwiertz A., Bischoff S.C. Lactobacillus rhamnosus GG protects against non-alcoholic fatty liver disease in mice. PLoS ONE. 2014;9:e80169. doi: 10.1371/journal.pone.0080169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Li J., Cordero P., Nguyen V., Oben J.A. The Role of Vitamins in the Pathogenesis of Non-alcoholic Fatty Liver Disease. Integr. Med. Insights. 2016;11:19–25. doi: 10.4137/IMI.S31451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Nagashimada M., Ota T. Role of vitamin E in nonalcoholic fatty liver disease. IUBMB life. 2019;71:516–522. doi: 10.1002/iub.1991. [DOI] [PubMed] [Google Scholar]
- 305.Chen G., Ni Y., Nagata N., Xu L., Ota T. Micronutrient Antioxidants and Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2016;17:1379. doi: 10.3390/ijms17091379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Barchetta I., Carotti S., Labbadia G., Gentilucci U.V., Muda A.O., Angelico F., Silecchia G., Leonetti F., Fraioli A., Picardi A., et al. Liver vitamin D receptor, CYP2R1, and CYP27A1 expression: Relationship with liver histology and vitamin D3 levels in patients with nonalcoholic steatohepatitis or hepatitis C virus. Hepatology. 2012;56:2180–2187. doi: 10.1002/hep.25930. [DOI] [PubMed] [Google Scholar]
- 307.Zhou Q.G., Hou F.F., Guo Z.J., Liang M., Wang G.B., Zhang X. 1,25-Dihydroxyvitamin D improved the free fatty-acid-induced insulin resistance in cultured C2C12 cells. Diabetes/Metab. Res. Rev. 2008;24:459–464. doi: 10.1002/dmrr.873. [DOI] [PubMed] [Google Scholar]
- 308.Abramovitch S., Dahan-Bachar L., Sharvit E., Weisman Y., Ben Tov A., Brazowski E., Reif S. Vitamin D inhibits proliferation and profibrotic marker expression in hepatic stellate cells and decreases thioacetamide-induced liver fibrosis in rats. Gut. 2011;60:1728–1737. doi: 10.1136/gut.2010.234666. [DOI] [PubMed] [Google Scholar]
- 309.Feldstein A.E., Wieckowska A., Lopez A.R., Liu Y.C., Zein N.N., McCullough A.J. Cytokeratin-18 fragment levels as noninvasive biomarkers for nonalcoholic steatohepatitis: A multicenter validation study. Hepatology. 2009;50:1072–1078. doi: 10.1002/hep.23050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Sharifi N., Amani R., Hajiani E., Cheraghian B. Does vitamin D improve liver enzymes, oxidative stress, and inflammatory biomarkers in adults with non-alcoholic fatty liver disease? A randomized clinical trial. Endocrine. 2014;47:70–80. doi: 10.1007/s12020-014-0336-5. [DOI] [PubMed] [Google Scholar]
- 311.Bechmann L.P., Hannivoort R.A., Gerken G., Hotamisligil G.S., Trauner M., Canbay A. The interaction of hepatic lipid and glucose metabolism in liver diseases. J. Hepatol. 2012;56:952–964. doi: 10.1016/j.jhep.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 312.Cai D., Yuan M., Frantz D.F., Melendez P.A., Hansen L., Lee J., Shoelson S.E. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat. Med. 2005;11:183–190. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Crespo J., Cayón A., Fernández-Gil P., Hernández-Guerra M., Mayorga M., Domínguez-Díez A., Fernández-Escalante J.C., Pons-Romero F. Gene expression of tumor necrosis factor alpha and TNF-receptors, p55 and p75, in nonalcoholic steatohepatitis patients. Hepatology. 2001;34:1158–1163. doi: 10.1053/jhep.2001.29628. [DOI] [PubMed] [Google Scholar]
- 314.Neyestani T.R., Nikooyeh B., Alavi-Majd H., Shariatzadeh N., Kalayi A., Tayebinejad N., Heravifard S., Salekzamani S., Zahedirad M. Improvement of vitamin D status via daily intake of fortified yogurt drink either with or without extra calcium ameliorates systemic inflammatory biomarkers, including adipokines, in the subjects with type 2 diabetes. J. Clin. Endocrinol. Metab. 2012;97:2005–2011. doi: 10.1210/jc.2011-3465. [DOI] [PubMed] [Google Scholar]
- 315.Foroughi M., Maghsoudi Z., Ghiasvand R., Iraj B., Askari G. Effect of Vitamin D Supplementation on C-reactive Protein in Patients with Nonalcoholic Fatty Liver. Int. J. Prev. Med. 2014;5:969–975. [PMC free article] [PubMed] [Google Scholar]
- 316.Lorvand Amiri H., Agah S., Tolouei Azar J., Hosseini S., Shidfar F., Mousavi S.N. Effect of daily calcitriol supplementation with and without calcium on disease regression in non-alcoholic fatty liver patients following an energy-restricted diet: Randomized, controlled, double-blind trial. Clin. Nutr. 2017;36:1490–1497. doi: 10.1016/j.clnu.2016.09.020. [DOI] [PubMed] [Google Scholar]
- 317.Shidfar F., Mousavi S.N., Lorvand Amiri H., Agah S., Hoseini S., Hajimiresmail S.J. Reduction of Some Atherogenic Indices in Patients with Non-Alcoholic Fatty Liver by Vitamin D and Calcium Co-Supplementation: A Double Blind Randomized Controlled Clinical Trial. Iranian journal of pharmaceutical research. IJPR. 2019;18:496–505. [PMC free article] [PubMed] [Google Scholar]
- 318.Pickett-Blakely O., Young K., Carr R.M. Micronutrients in Nonalcoholic Fatty Liver Disease Pathogenesis. Cell. Mol. Gastroenterol. Hepatol. 2018;6:451–462. doi: 10.1016/j.jcmgh.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Ai Y., Sun Z., Peng C., Liu L., Xiao X., Li J. Homocysteine Induces Hepatic Steatosis Involving ER Stress Response in High Methionine Diet-Fed Mice. Nutrients. 2017;9:346. doi: 10.3390/nu9040346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Talari H.R., Molaqanbari M.R., Mokfi M., Taghizadeh M., Bahmani F., Tabatabaei S.M.H., Sharifi N. The effects of vitamin B12 supplementation on metabolic profile of patients with non-alcoholic fatty liver disease: A randomized controlled trial. Sci. Rep. 2022;12:14047. doi: 10.1038/s41598-022-18195-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Zelber-Sagi S., Ivancovsky-Wajcman D., Fliss-Isakov N., Hahn M., Webb M., Shibolet O., Kariv R., Tirosh O. Serum Malondialdehyde is Associated with Non-Alcoholic Fatty Liver and Related Liver Damage Differentially in Men and Women. Antioxidants. 2020;9:578. doi: 10.3390/antiox9070578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Racek J., Rusnáková H., Trefil L., Siala K.K. The influence of folate and antioxidants on homocysteine levels and oxidative stress in patients with hyperlipidemia and hyperhomocysteinemia. Physiol. Res. 2005;54:87–95. doi: 10.33549/physiolres.930520. [DOI] [PubMed] [Google Scholar]
- 323.Al-Daghri N.M., Rahman S., Sabico S., Yakout S., Wani K., Al-Attas O.S., Saravanan P., Tripathi G., McTernan P.G., Alokail M.S. Association of Vitamin B12 with Pro-Inflammatory Cytokines and Biochemical Markers Related to Cardiometabolic Risk in Saudi Subjects. Nutrients. 2016;8:460. doi: 10.3390/nu8090460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Kurt R., Yilmaz Y., Ermis F., Kalayoglu Besisik S., Polat N., Elitok A., Oflaz H., Karan M.A. Folic Acid and vitamin B12 supplementation improves coronary flow reserve in elderly subjects with vitamin B12 deficiency. Arch. Med. Res. 2010;41:369–372. doi: 10.1016/j.arcmed.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 325.Setola E., Monti L.D., Galluccio E., Palloshi A., Fragasso G., Paroni R., Magni F., Sandoli E.P., Lucotti P., Costa S., et al. Insulin resistance and endothelial function are improved after folate and vitamin B12 therapy in patients with metabolic syndrome: Relationship between homocysteine levels and hyperinsulinemia. Eur. J. Endocrinol. 2004;151:483–489. doi: 10.1530/eje.0.1510483. [DOI] [PubMed] [Google Scholar]
- 326.Hofmann A. The function of bile salts in fat absorption. The solvent properties of dilute micellar solutions of conjugated bile salts. Biochem. J. 1963;1963. 89:57. doi: 10.1042/bj0890057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Chow M.D., Lee Y.H., Guo G.L. The role of bile acids in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Mol. Asp. Med. 2017;56:34–44. doi: 10.1016/j.mam.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Chiang J.Y. Bile acids: Regulation of synthesis. J. Lipid Res. 2009;50:50,1955–1966. doi: 10.1194/jlr.R900010-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Chávez-Talavera O., Tailleux A., Lefebvre P., Staels B. Bile Acid Control of Metabolism and Inflammation in Obesity, Type 2 Diabetes, Dyslipidemia, and Nonalcoholic Fatty Liver Disease. Gastroenterology. 2017;152:1679–1694.e3. doi: 10.1053/j.gastro.2017.01.055. [DOI] [PubMed] [Google Scholar]
- 330.Ridlon J.M., Kang D.J., Hylemon P.B. Bile salt biotransformations by human intestinal bacteria. J. Lipid Res. 2006;47:241–259. doi: 10.1194/jlr.R500013-JLR200. [DOI] [PubMed] [Google Scholar]
- 331.Jiao N., Baker S.S., Chapa-Rodriguez A., Liu W., Nugent C.A., Tsompana M., Mastrandrea L., Buck M.J., Baker R.D., Genco R.J., et al. Suppressed hepatic bile acid signalling despite elevated production of primary and secondary bile acids in NAFLD. Gut. 2018;67:1881–1891. doi: 10.1136/gutjnl-2017-314307. [DOI] [PubMed] [Google Scholar]
- 332.Pierre J.F., Martinez K.B., Ye H., Nadimpalli A., Morton T.C., Yang J., Wang Q., Patno N., Chang E.B., Yin D.P. Activation of bile acid signaling improves metabolic phenotypes in high-fat diet-induced obese mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2016;311:G286–G304. doi: 10.1152/ajpgi.00202.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Eckburg P.B., Bik E.M., Bernstein C.N., Purdom E., Dethlefsen L., Sargent M., Gill S.R., Nelson K.E., Relman D.A. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Turnbaugh P.J., Ley R.E., Mahowald M.A., Magrini V., Mardis E.R., Gordon J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 335.Turnbaugh P.J., Bäckhed F., Fulton L., Gordon J.I. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 2008;3:213–223. doi: 10.1016/j.chom.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Sannasiddappa T.H., Lund P.A., Clarke S.R. In Vitro Antibacterial Activity of Unconjugated and Conjugated Bile Salts on Staphylococcus aureus. Front. Microbiol. 2017;8:1581. doi: 10.3389/fmicb.2017.01581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Pineda Torra I., Claudel T., Duval C., Kosykh V., Fruchart J.C., Staels B. Bile acids induce the expression of the human peroxisome proliferator-activated receptor alpha gene via activation of the farnesoid X receptor. Mol. Endocrinol. 2003;17:259–272. doi: 10.1210/me.2002-0120. [DOI] [PubMed] [Google Scholar]
- 338.Watanabe M., Houten S.M., Wang L., Moschetta A., Mangelsdorf D.J., Heyman R.A., Moore D.D., Auwerx J. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J. Clin. Investig. 2004;113:1408–1418. doi: 10.1172/JCI21025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Nishitsuji K., Xiao J., Nagatomo R., Umemoto H., Morimoto Y., Akatsu H., Tsuneyama K. Analysis of the gut microbiome and plasma short-chain fatty acid profiles in a spontaneous mouse model of metabolic syndrome. Sci. Rep. 2017;7:15876. doi: 10.1038/s41598-017-16189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Ulluwishewa D., Anderson R.C., McNabb W.C., Moughan P.J., Wells J.M., Roy N.C. Regulation of tight junction permeability by intestinal bacteria and dietary components. J. Nutr. 2011;141:769–776. doi: 10.3945/jn.110.135657. [DOI] [PubMed] [Google Scholar]
- 341.Pierantonelli I., Rychlicki C., Agostinelli L., Giordano D.M., Gaggini M., Fraumene C., Saponaro C., Manghina V., Sartini L., Mingarelli E., et al. Lack of NLRP3-inflammasome leads to gut-liver axis derangement, gut dysbiosis and a worsened phenotype in a mouse model of NAFLD. Sci. Rep. 2017;7:12200. doi: 10.1038/s41598-017-11744-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Miele L., Valenza V., La Torre G., Montalto M., Cammarota G., Ricci R., Mascianà R., Forgione A., Gabrieli M.L., Perotti G., et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49:1877–1887. doi: 10.1002/hep.22848. [DOI] [PubMed] [Google Scholar]
- 343.Kuzmich N.N., Sivak K.V., Chubarev V.N., Porozov Y.B., Savateeva-Lyubimova T.N., Peri F. TLR4 Signaling Pathway Modulators as Potential Therapeutics in Inflammation and Sepsis. Vaccines. 2017;5:34. doi: 10.3390/vaccines5040034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Csak T., Ganz M., Pespisa J., Kodys K., Dolganiuc A., Szabo G. Fatty acid and endotoxin activate inflammasomes in mouse hepatocytes that release danger signals to stimulate immune cells. Hepatology. 2011;54:133–144. doi: 10.1002/hep.24341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Noga A.A., Vance D.E. A gender-specific role for phosphatidylethanolamine N-methyltransferase-derived phosphatidylcholine in the regulation of plasma high density and very low density lipoproteins in mice. J. Biol. Chem. 2003;278:21851–21859. doi: 10.1074/jbc.M301982200. [DOI] [PubMed] [Google Scholar]
- 346.Xu X., Gammon M.D., Zeisel S.H., Bradshaw P.T., Wetmur J.G., Teitelbaum S.L., Neugut A.I., Santella R.M., Chen J. High intakes of choline and betaine reduce breast cancer mortality in a population-based study. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2009;23:4022–4028. doi: 10.1096/fj.09-136507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Nascimbeni F., Pais R., Bellentani S., Day C.P., Ratziu V., Loria P., Lonardo A. From NAFLD in clinical practice to answers from guidelines. J. Hepatol. 2013;59:859–871. doi: 10.1016/j.jhep.2013.05.044. [DOI] [PubMed] [Google Scholar]
- 348.Fullerton M.D., Galic S., Marcinko K., Sikkema S., Pulinilkunnil T., Chen Z.P., O’Neill H.M., Ford R.J., Palanivel R., O’Brien M., et al. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat. Med. 2013;19:1649–1654. doi: 10.1038/nm.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Hawley S.A., Fullerton M.D., Ross F.A., Schertzer J.D., Chevtzoff C., Walker K.J., Peggie M.W., Zibrova D., Green K.A., Mustard K.J., et al. The ancient drug salicylate directly activates AMP-activated protein kinase. Science. 2012;336:918–922. doi: 10.1126/science.1215327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Zhang Y.L., Guo H., Zhang C.S., Lin S.Y., Yin Z., Peng Y., Luo H., Shi Y., Lian G., Zhang C., et al. AMP as a low-energy charge signal autonomously initiates assembly of AXIN-AMPK-LKB1 complex for AMPK activation. Cell Metab. 2013;18:546–555. doi: 10.1016/j.cmet.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 351.Mihaylova M.M., Shaw R.J. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat. Cell Biol. 2011;13:1016–1023. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Scott J.W., Galic S., Graham K.L., Foitzik R., Ling N.X., Dite T.A., Issa S.M., Langendorf C.G., Weng Q.P., Thomas H.E., et al. Inhibition of AMP-Activated Protein Kinase at the Allosteric Drug-Binding Site Promotes Islet Insulin Release. Chem. Biol. 2015;22:705–711. doi: 10.1016/j.chembiol.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 353.Steinberg G.R., Michell B.J., van Denderen B.J., Watt M.J., Carey A.L., Fam B.C., Andrikopoulos S., Proietto J., Görgün C.Z., Carling D., et al. Tumor necrosis factor alpha-induced skeletal muscle insulin resistance involves suppression of AMP-kinase signaling. Cell Metab. 2006;4:465–474. doi: 10.1016/j.cmet.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 354.Donnelly K.L., Smith C.I., Schwarzenberg S.J., Jessurun J., Boldt M.D., Parks E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Investig. 2005;115:1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Nielsen S., Guo Z., Johnson C.M., Hensrud D.D., Jensen M.D. Splanchnic lipolysis in human obesity. J. Clin. Investig. 2004;113:1582–1588. doi: 10.1172/JCI21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Vatner D.F., Majumdar S.K., Kumashiro N., Petersen M.C., Rahimi Y., Gattu A.K., Bears M., Camporez J.P., Cline G.W., Jurczak M.J., et al. Insulin-independent regulation of hepatic triglyceride synthesis by fatty acids. Proc. Natl. Acad. Sci. USA. 2015;112:1143–1148. doi: 10.1073/pnas.1423952112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Xu X.J., Gauthier M.S., Hess D.T., Apovian C.M., Cacicedo J.M., Gokce N., Farb M., Valentine R.J., Ruderman N.B. Insulin sensitive and resistant obesity in humans: AMPK activity, oxidative stress, and depot-specific changes in gene expression in adipose tissue. J. Lipid Res. 2012;53:792–801. doi: 10.1194/jlr.P022905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Mottillo E.P., Desjardins E.M., Crane J.D., Smith B.K., Green A.E., Ducommun S., Henriksen T.I., Rebalka I.A., Razi A., Sakamoto K., et al. Lack of Adipocyte AMPK Exacerbates Insulin Resistance and Hepatic Steatosis through Brown and Beige Adipose Tissue Function. Cell Metab. 2016;24:118–129. doi: 10.1016/j.cmet.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Mancini S.J., White A.D., Bijland S., Rutherford C., Graham D., Richter E.A., Viollet B., Touyz R.M., Palmer T.M., Salt I.P. Activation of AMP-activated protein kinase rapidly suppresses multiple pro-inflammatory pathways in adipocytes including IL-1 receptor-associated kinase-4 phosphorylation. Mol. Cell. Endocrinol. 2017;440:44–56. doi: 10.1016/j.mce.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Caligiuri A., Bertolani C., Guerra C.T., Aleffi S., Galastri S., Trappoliere M., Vizzutti F., Gelmini S., Laffi G., Pinzani M., et al. Adenosine monophosphate-activated protein kinase modulates the activated phenotype of hepatic stellate cells. Hepatology. 2008;47:668–676. doi: 10.1002/hep.21995. [DOI] [PubMed] [Google Scholar]
- 361.Li Y., Xu S., Mihaylova M.M., Zheng B., Hou X., Jiang B., Park O., Luo Z., Lefai E., Shyy J.Y., et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011;13:376–388. doi: 10.1016/j.cmet.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Gleason C.E., Lu D., Witters L.A., Newgard C.B., Birnbaum M.J. The role of AMPK and mTOR in nutrient sensing in pancreatic beta-cells. J. Biol. Chem. 2007;282:10341–10351. doi: 10.1074/jbc.M610631200. [DOI] [PubMed] [Google Scholar]
- 363.Chotechuang N., Azzout-Marniche D., Bos C., Chaumontet C., Gausserès N., Steiler T., Gaudichon C., Tomé D. mTOR, AMPK, and GCN2 coordinate the adaptation of hepatic energy metabolic pathways in response to protein intake in the rat. Am. J. Physiol. Endocrinol. Metab. 2009;297:E1313–E1323. doi: 10.1152/ajpendo.91000.2008. [DOI] [PubMed] [Google Scholar]
- 364.Smith B.K., Marcinko K., Desjardins E.M., Lally J.S., Ford R.J., Steinberg G.R. Treatment of nonalcoholic fatty liver disease: Role of AMPK. American journal of physiology. Endocrinol. Metab. 2016;311:730–740. doi: 10.1152/ajpendo.00225.2016. [DOI] [PubMed] [Google Scholar]
- 365.Silvestri C., Di Marzo V. The endocannabinoid system in energy homeostasis and the etiopathology of metabolic disorders. Cell Metab. 2013;17:475–490. doi: 10.1016/j.cmet.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 366.Mazier W., Saucisse N., Gatta-Cherifi B., Cota D. The Endocannabinoid System: Pivotal Orchestrator of Obesity and Metabolic Disease. Trends Endocrinol. Metab. TEM. 2015;26:524–537. doi: 10.1016/j.tem.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 367.Assa-Glazer T., Gorelick J., Sela N., Nyska A., Bernstein N., Madar Z. Cannabis Extracts Affected Metabolic Syndrome Parameters in Mice Fed High-Fat/Cholesterol Diet. Cannabis Cannabinoid Res. 2020;5:202–214. doi: 10.1089/can.2020.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Di Marzo V., Piscitelli F., Mechoulam R. Cannabinoids and endocannabinoids in metabolic disorders with focus on diabetes. Handb. Exp. Pharmacol. 2011;203:75–104. doi: 10.1007/978-3-642-17214-4_4. [DOI] [PubMed] [Google Scholar]
- 369.Dibba P., Li A., Cholankeril G., Iqbal U., Gadiparthi C., Khan M.A., Kim D., Ahmed A. Mechanistic Potential and Therapeutic Implications of Cannabinoids in Nonalcoholic Fatty Liver Disease. Medicines. 2018;5:47. doi: 10.3390/medicines5020047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Mallat A., Lotersztajn S. Endocannabinoids and their role in fatty liver disease. Dig. Dis. 2010;28:261–266. doi: 10.1159/000282100. [DOI] [PubMed] [Google Scholar]
- 371.Jadoon K.A., Ratcliffe S.H., Barrett D.A., Thomas E.L., Stott C., Bell J.D., O’Sullivan S.E., Tan G.D. Efficacy and Safety of Cannabidiol and Tetrahydrocannabivarin on Glycemic and Lipid Parameters in Patients with Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled, Parallel Group Pilot Study. Diabetes Care. 2016;39:1777–1786. doi: 10.2337/dc16-0650. [DOI] [PubMed] [Google Scholar]
- 372.McPartland J.M., Duncan M., Di Marzo V., Pertwee R.G. Are cannabidiol and Δ(9) -tetrahydrocannabivarin negative modulators of the endocannabinoid system? A systematic review. Br. J. Pharmacol. 2015;172:737–753. doi: 10.1111/bph.12944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Wu J. Cannabis, cannabinoid receptors, and endocannabinoid system: Yesterday, today, and tomorrow. Acta Pharmacol. Sin. 2019;40:297–299. doi: 10.1038/s41401-019-0210-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Kirkham T.C. Cannabinoids and appetite: Food craving and food pleasure. Int. Rev. Psychiatry. 2009;21:163–171. doi: 10.1080/09540260902782810. [DOI] [PubMed] [Google Scholar]
- 375.Muniyappa R., Sable S., Ouwerkerk R., Mari A., Gharib A.M., Walter M., Courville A., Hall G., Chen K.Y., Volkow N.D., et al. Metabolic effects of chronic cannabis smoking. Diabetes Care. 2013;36:2415–2422. doi: 10.2337/dc12-2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Thomas A., Baillie G.L., Phillips A.M., Razdan R.K., Ross R.A., Pertwee R.G. Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br. J. Pharmacol. 2007;150:613–623. doi: 10.1038/sj.bjp.0707133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Mu W., Cheng X.F., Liu Y., Lv Q.Z., Liu G.L., Zhang J.G., Li X.Y. Potential Nexus of Non-alcoholic Fatty Liver Disease and Type 2 Diabetes Mellitus: Insulin Resistance Between Hepatic and Peripheral Tissues. Front. Pharmacol. 2019;9:1566. doi: 10.3389/fphar.2018.01566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Jorgačević B., Vučević D., Vesković M., Mladenović D., Vukićević D., Vukićević R.J., Todorović V., Radosavljević T. The effect of cannabinoid receptor 1 blockade on adipokine and proinflammatory cytokine concentration in adipose and hepatic tissue in mice with nonalcoholic fatty liver disease. Can. J. Physiol. Pharmacol. 2019;97:120–129. doi: 10.1139/cjpp-2018-0607. [DOI] [PubMed] [Google Scholar]
- 379.Hirvonen J., Goodwin R.S., Li C.T., Terry G.E., Zoghbi S.S., Morse C., Pike V.W., Volkow N.D., Huestis M.A., Innis R.B. Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Mol. Psychiatry. 2012;17:642–649. doi: 10.1038/mp.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]