Abstract
The microbiota-gut-brain axis is a complex interconnected system altered in schizophrenia. The antioxidant N-acetylcysteine (NAC) has been proposed as an adjunctive therapy to antipsychotics in clinical trials, but its role in the microbiota-gut-brain axis has not been sufficiently explored. We aimed to describe the effect of NAC administration during pregnancy on the gut-brain axis in the offspring from the maternal immune stimulation (MIS) animal model of schizophrenia. Pregnant Wistar rats were treated with PolyI:C/Saline. Six groups of animals were studied according to the study factors: phenotype (Saline, MIS) and treatment (no NAC, NAC 7 days, NAC 21 days). Offspring were subjected to the novel object recognition test and were scanned using MRI. Caecum contents were used for metagenomics 16S rRNA sequencing. NAC treatment prevented hippocampal volume reduction and long-term memory deficits in MIS-offspring. In addition, MIS-animals showed lower bacterial richness, which was prevented by NAC. Moreover, NAC7/NAC21 treatments resulted in a reduction of proinflammatory taxons in MIS-animals and an increase in taxa known to produce anti-inflammatory metabolites. Early approaches, like this one, with anti-inflammatory/anti-oxidative compounds, especially in neurodevelopmental disorders with an inflammatory/oxidative basis, may be useful in modulating bacterial microbiota, hippocampal size, as well as hippocampal-based memory impairments.
Keywords: Poly I:C, schizophrenia, microbiota-gut-brain axis, inflammation, oxidative stress
1. Introduction
The microbiota-gut-brain axis is a complex interconnected system that appears to be altered in the schizophrenia (SCZ) [1]. Certain psychiatric disorders have purportedly not only brain-related but also systemic causes, based on an inflammatory response of the organism in which bacterial components of the gut microbiota may participate [2]. The alteration of the bidirectional homeostasis between the intestine and the brain, which involves neurological, metabolic, hormonal, and immunological signaling pathways, can favor the appearance of metabolic, immunological, or CNS disorders, with behavioral changes, among other clinical manifestations. In this sense, first episode psychosis (FEP) patients show gut microbiota alterations with differences in the composition and number of microbes, which in turn are associated with their symptoms severity [3]. Furthermore, patients with acute SCZ have shown no changes in microbiota richness but decreased microbial biodiversity [4], indicating an altered gut microbiota.
Serological markers of gut bacterial translocation have also been reported to be substantially increased in subjects with psychosis and significantly correlated with systemic inflammatory markers [5]. In turn, cytokine levels correlate with the severity of clinical symptoms, and it has been suggested that the resulting neuroinflammation is directly involved in the pathogenesis of SCZ [6]. Similarly, gut microbiota changes have been observed in different animal models of psychiatric disorders, including autism and SCZ [7,8]. In this regard, the maternal immune stimulation (MIS) model is one of the most widely used animal models for SCZ. This is a well-validated animal model produced by maternal exposure to a viral infection with Polyinosinic:polycytidylic acid (Poly I:C), a viral-mimicking agent which stimulates toll-like receptor 3 (TLR3) during pregnancy. The MIS model is based on the known association between maternal viral infection during pregnancy and increased risk of the onset of SCZ in offspring [9,10,11]. Prenatal exposure to Poly I:C leads to the development of a broad schizophrenic spectrum that includes behavioral, neurological, immunological, structural and metabolic deficits, mainly characterized by behavioral and in vivo imaging techniques [10,11,12,13,14]. However, the impact of Poly I:C on offspring’s gut microbiota has been barely explored. Only a few studies have revealed the microbiota composition in the MIS model [7,15,16]. MIS model studies carried out in rats show an increase in specific bacterial taxa, such as E. coli, Lactobacillus spp., Bifidobacterium spp. and Bacteroides spp. [16], which is consistent with clinical studies [3,17]. In mice, the MIS model has failed to show differences in alpha diversity (calculated as Shannon index) [15] but detected large differences in the composition of gut bacteria between MIS offspring and control animals, mainly in the bacterial classes Clostridia and Bacteroidia, with an increase in bacterial families, such as Lachnospiraceae and Prevotellaceae [7]. Although these changes could suggest differences in the Firmicutes/Bacteroidetes ratio, significant differences were not found between MIS and control animals with respect to this ratio [15].
It is important to note that gut microbiota undergoes important maturation and development processes throughout life, being the perinatal, postnatal and adolescence stages critical periods for its progress [18]. In this sense, manipulation of the gut microbiota-brain axis through diet in these critical time windows appears to be an innovative therapeutic tool to prevent the risk of neurodevelopmental disorders that may affect lifelong health. In this regard, the likely involvement of inflammation/oxidative stress (IOS) in the pathophysiology of SCZ has raised the potential use of anti-oxidant and anti-inflammatory (anti-IOS) compounds as therapeutic strategies in this disorder [19]. Among them, the use of N-acetylcysteine (NAC) has received particular attention. NAC, a precursor of the amino acid L-cysteine (L-Cys), has remarkable antioxidant properties as it increases the availability of L-Cys, facilitating the synthesis of glutathione (GSH), which is the main non-enzymatic antioxidant defense that protects cells from reactive oxygen species [20,21]. This ability to regulate GSH biosynthesis is the key to its therapeutic efficacy. NAC is also able to reduce proinflammatory cytokines, such as IL-6, TNF-α and IL-1β [21]. In clinical trials, NAC is often administered as adjunctive treatment to conventional treatment with antipsychotics [22]. Overall, there is evidence that NAC has beneficial effects on SCZ symptoms beyond those induced by antipsychotics alone [22,23]. However, to our knowledge, no study has evaluated the effects of NAC on the interconnection between gut microbiota and the brain in SCZ. In this regard, few studies have demonstrated that NAC therapy may alleviate gut dysbiosis in several pathologies, such as diabetes and metabolic diseases [24,25,26], hypertension [27], and cardiovascular disease [28]. Moreover, three recent studies have evaluated maternal NAC treatment via regulating the gut microecology to alleviate maternal placental oxidative stress and inflammatory response in sows [29,30] and to prevent hypertension in spontaneously hypertensive rat offspring [27], which highlights its possible use in other pathologies with inflammatory and neurodevelopmental basis, such as SCZ.
Thus, this study aimed to investigate the composition, taxonomy, and functional diversity of the gut microbiota in an MIS animal model of SCZ and to evaluate the anti-IOS potential of NAC in the reversion of the gut microbiota dysbiosis produced in the MIS model. In addition, this study benefits from the study of the changes in hippocampal volume and hippocampal-based memory, measured by magnetic resonance imaging and novel object recognition (NOR) task, respectively, as well as the association between gut microbiota and hippocampal volume.
2. Materials and Methods
2.1. Animals
Seventy-two male Wistar rats were maintained at constant temperature (24 ± 0.5 °C) under a 12-h light/dark cycle, with free access to chow/water. All animal procedures were conducted in conformity with the European Communities Council Directive 2010/63/EU, following the ARRIVE guidelines [31] and approved by the Ethics Committee for Animal Experimentation of Universidad Rey Juan Carlos and Gregorio Marañón Hospital (ES280790000087). Two batches of animals were used: 44 animals underwent behavioral studies, and 69 animals underwent imaging and metagenomics. The number of animals per cohort was calculated for the 6 experimental groups, considering the 3Rs principle in animal experimentation and assuming independent groups, the detection of large effect (1 SD), and a power of 80%.
2.2. Treatments
MIS model: On gestational day 15 (GD15), Poly I:C (4 mg/kg, Sigma-Aldrich, Madrid, Spain) or saline solution was administered i.v. to pregnant Wistar rats. This protocol is associated with a high risk of the onset of behavioral and neurological deficits in the offspring at adulthood, similar to those found in patients with SCZ [11,14]. On post-natal day (PND) 21, male-offspring were weaned and housed 2–4 per cage. Females were not studied because they do not reproduce the well-characterized SCZ-like deficits in males of the MIS model at PND100 [32].
NAC treatment during gestation: NAC (500 mg/kg, Sigma-Aldrich, Madrid, Spain) diluted in drinking water (VH) was administered at two different time periods during gestation: throughout gestation (NAC21, 21 days of administration) or after the Poly I:C/saline administration (NAC7, 7 days of administration, from gestational days 15 to 21). Water intake was monitored to calculate the dose of NAC ingested by animals and freshly prepared to prevent NAC degradation. The dose of NAC ingested daily by each animal was similar in all groups (minimum dose: 518 mg/kg, maximum dose: 552 mg/kg).
Animals were divided into six groups (6–12 animals per group) according to the factors of the study: phenotype (Saline, MIS) and treatment (water without NAC, water with NAC for 7 days–NAC7, water with NAC for 21 days–NAC21).
2.3. Novel Object Recognition (NOR) Test
A behavioral test was performed at PND70 during their active phase (at night). On day 1, rats were habituated for 10 min to an open-field Plexiglas arena (45 cm × 45 cm × 45 cm) in the absence of objects. On day 2, they underwent a 10-min familiarization phase for NOR with identical objects located in opposite corners. On day 3, they underwent the test phase, in which long-term memory at 24 h was examined. During this phase, one familiar (F) object was replaced by a novel one (N), and rats were allowed to explore the arena for 10 min [33]. The objects were of different shapes, colors and textures, and they were thoroughly cleaned with 1% acetic acid between animals to ensure the absence of any olfactory cues. All sessions were videotaped and manually scored by a blind experimenter. The time of exploration of the N and F objects was recorded. The difference in the exploration time of N and F objects was calculated using the discrimination index (DI) as (time N − time F)/(time N + time F).
2.4. Magnetic Resonance Imaging (MRI)
Animals were scanned using a 7-Tesla Biospec 70/20 scanner (Bruker, Ettlingen, Germany). A coronal T2-weighted spin-echo sequence was acquired with TE = 33 ms, TR = 3732 ms, averages 2 and slice thickness 0.4 mm. Matrix size was 256 × 256 pixels at a FOV of 3.5 × 3.5 cm2 [12,13,14]. T2 images were registered to a common spatial reference using the rigid registration algorithms described in [34]. Then, the hippocampus was manually segmented on each MRI image, according to [35].
2.5. Genomic DNA Extraction and 16S Ribosomal RNA Sequencing for Metagenomics
Frozen tissue samples (−80 °C) from the intestinal cecum were used for the study of microbiota. Genomic DNA (gDNA) was extracted from 200 mg of frozen cecum feces using E.Z.N.A.® DNA Stool Kit (VWR, Madrid, Spain) and provided at least 200 μL of genomic DNA. These gDNA samples were then quantified using a BioPhotometer® (Eppendorf, Madrid, Spain) and their concentrations were finally diluted to 6 ng/μL. The diluted samples were used for performing polymerase chain reaction (PCR) amplification, following the protocol of the Ion 16 Metagenomics kit (Thermo Fischer Scientific, Madrid, Spain) [36].
PCR amplification products were used to create a library using the Ion Plus Fragment Library kit for AB Library Builder System (Thermo Fischer Scientific, Madrid, Spain), with sample indexing using the Ion Xpress Barcode Adapters 1–96 kit (Thermo Fischer Scientific, Madrid, Spain).
Template preparation was performed using the ION OneTouch 2 System and the ION PGM Hi-Q OT2 kit (Thermo Fischer Scientific, Madrid, Spain). Metagenomics sequencing was performed using the ION PGM Hi-Q Sequencing kit (Thermo Fischer Scientific, Madrid, Spain) on the ION PGM System. The chips used were the ION 314 v2, 316 v2 or 318 v2 Chips (Thermo Fischer Scientific, Madrid, Spain) with various barcoded samples per chip.
2.6. Phylogenetic Analysis
The consensus spreadsheet for each metagenomics sequencing was downloaded from ION Reporter software (version 5.6, Life Technologies Holdings Pte Ltd., Singapore). This spreadsheet included the percentages for each taxonomic level and was used for comparing frequencies between individuals and groups. Analyses were carried out using QIIME-2 software (version 2017.6.0).
2.7. Statistical Analysis of Data
Normality and homoscedasticity of each variable were tested using Shapiro-Wilk’s and Levene’s test, respectively. Data were analyzed by means of 2-way ANOVAs (considering MIS and NAC7 or MIS and NAC21 as factors) followed by Tukey posthoc test or Kruskal–Wallis analysis followed by Dunn’s multiple comparison test when normality and homoscedasticity were not met. A p value < 0.05 was considered statistically significant. When a trend was observed between control animals (Saline-VH) and MIS-VH animals, a Mann–Whitney or t-test was also performed. Analysis of the microbiome community was carried out by non-supervised multivariate analysis (PCA) using R software (v3.2.4). For LDA analysis, tab-delimited files were generated in GraphPad and computed at the family level. The Kendall rank correlation coefficient was used to measure the ordinal association between gut microbiota and hippocampal volume. Graphic representations of all the data were generated with GraphPad Prism software (version 8, GraphPad Software, San Diego, CA, USA). Data were expressed as mean ± standard error of the mean (SEM). Whenever results are statistically significant, it is indicated in the figures.
3. Results
3.1. Memory
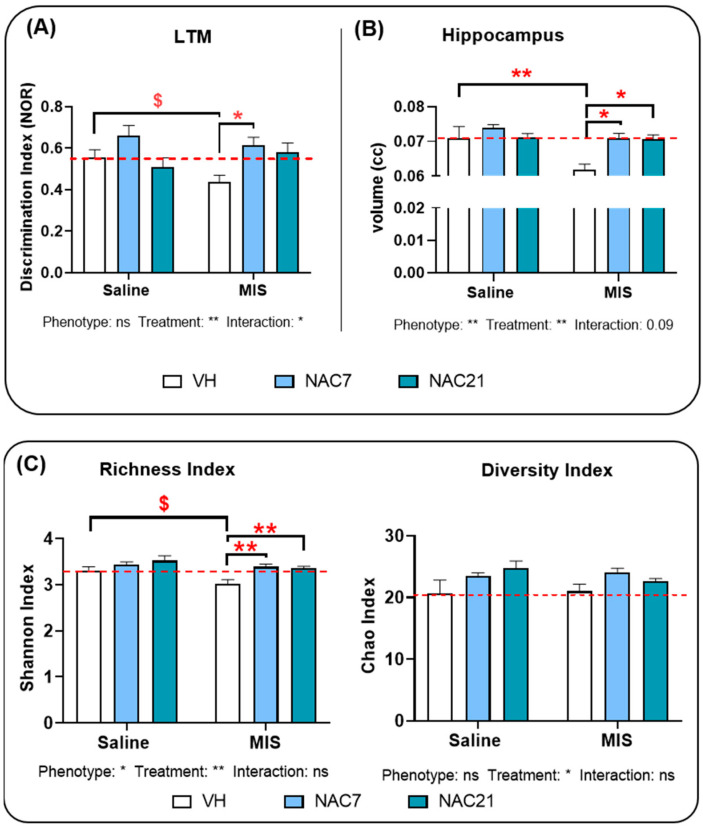
Two-way ANOVA analysis revealed that NAC led to significant differences in the discrimination index for long-term memory (p < 0.01) (Figure 1A). Thus, there was a significant reduction in the discrimination index in MIS-VH animals (pathological animals without NAC treatment) relative to Saline-VH (healthy control animals without NAC treatment) (p < 0.05), which was prevented by NAC treatment from the immune stimulation to delivery (p < 0.05). In addition, an interaction between Phenotype and NAC21 was found (p < 0.05).
Figure 1.
(A) Discrimination index. The effect of NAC treatment on the long-term memory (LTM) discrimination index between objects in the novel object recognition test (NOR) (Saline VH, N = 9; Saline NAC7, N = 6; Saline NAC21, N = 8; MIS VH, N = 7; MIS NAC7, N = 8; MIS NAC21, N = 6). (B). Hippocampal volume. The effect of NAC on hippocampal volume in the Saline (control) and MIS (pathological) animal groups, measured by MRI. (Saline VH, N = 7; Saline NAC7, N = 12; Saline NAC21, N = 12; MIS VH, N = 6; MIS NAC7, N = 12; MIS NAC21, N = 12). (C). Bacterial diversity and richness. Representation of the changes in bacterial diversity and richness, as measured by Shannon and Chao Indexes in Saline and MIS animals after a preventive treatment with NAC during pregnancy. (Saline VH, N = 8; Saline NAC7, N = 11; Saline NAC21, N = 11; MIS VH, N = 11; MIS NAC7, N = 10; MIS NAC21, N = 9). [Data is shown as mean ± SEM. 2-way ANOVA followed by a posthoc test, Tukey’s multiple comparison (* p < 0.05, ** p < 0.01) and unpaired t-test ($ p < 0.05) are shown. ns: non-significant]. The red dotted line indicates the mean value of the control group (Saline-VH).
3.2. Hippocampal Volumetric Changes
ANOVA analysis of hippocampal volume measurements showed a significant effect of MIS in this brain area, with reduced volume in MIS-VH versus the Saline-VH group (p < 0.01) (Figure 1B). In addition, a significant effect of NAC treatment was found, with increased hippocampal volume in MIS NAC-treated animals (7 and 21 d) (p < 0.01).
3.3. Microbiota Changes in MIS Offspring
ANOVA analysis of bacterial richness as measured by the Shannon index (Figure 1C) showed a significant effect of MIS (p < 0.05) and NAC (p < 0.01), with reduced bacterial richness in MIS-VH compared to Saline-VH animals (p < 0.05). NAC treatment during pregnancy increased bacterial richness in MIS animals, regardless of whether NAC was administered throughout gestation or from the immune stimulation to delivery.
ANOVA analysis of bacterial diversity as measured by the Chao index (Figure 1C) showed a significant effect of NAC (p < 0.05). No significant effect of MIS or any interaction was found (p > 0.05).
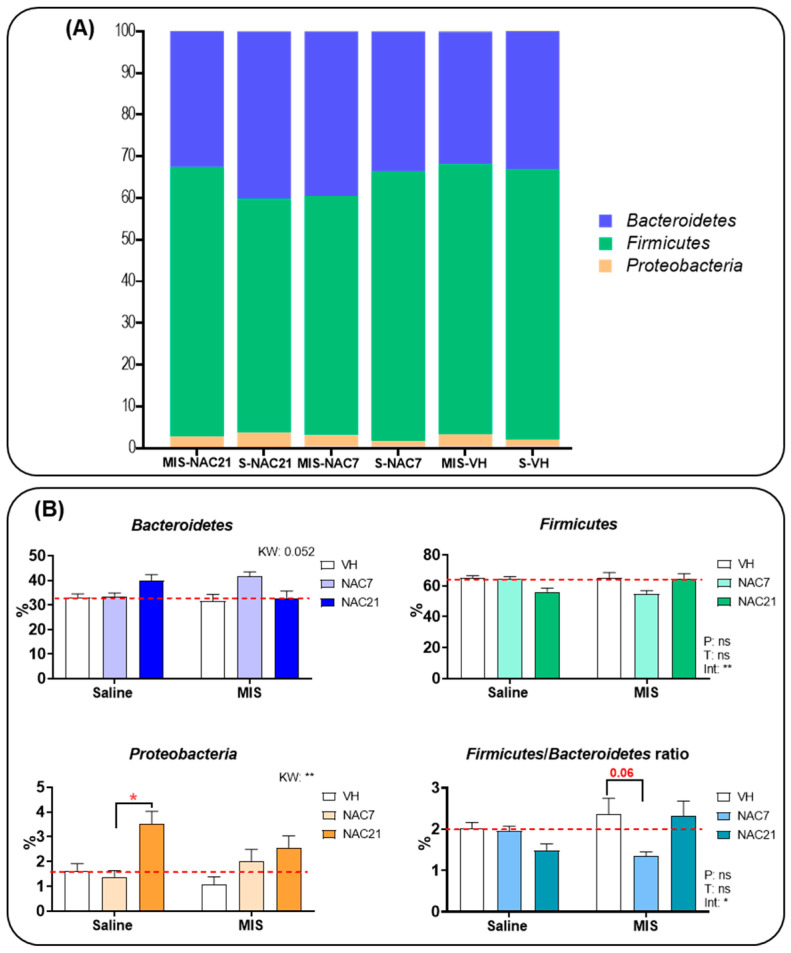
At the phylum level (Figure 2, Supplementary Table S1), Bacteroidetes and Firmicutes populations were the most abundant phyla in all groups. Proteobacteria was the third most common phylum, followed by Actinobacteria. Statistical analysis found differences between groups in the percentage of Firmicutes, Firmicutes/Bacteroidetes ratio, Proteobacteria and Deferribacteres (Supplementary Table S1). Posthoc analysis showed a Proteobacteria population increase in the Saline-NAC 21d group vs. Saline-NAC 7d (p < 0.05). NAC treatment during 21 d increased Deferribacteres in both Saline and MIS animals. In addition, the analysis of the Firmicutes/Bacteroidetes ratio, which has been suggested as an important index for healthy gut microbiota [34], showed a reduced ratio in MIS-NAC7 compared to MIS-VH (p < 0.09). No other statistical differences were observed in any other phyla (Supplementary Table S1).
Figure 2.
(A) The nested bar at the phylum level. The nested bar graph shows the mean percentage composition of the most representative phyla for each group. Bacteroidetes (purple), Firmicutes (green) and Proteobacteria (orange) were the most common phyla. (B) Gut microbiota composition at the phylum level. Graphs show the mean percentage composition of the 3 most representative phyla and the Firmicutes/Bacteroidetes ratio in the Saline and MIS animals treated with NAC during gestation. (Saline VH, N = 8; Saline NAC7, N = 11; Saline NAC21, N = 11; MIS VH, N = 11; MIS NAC7, N = 11; MIS NAC21, N = 11). Data are shown as mean ± SEM. 2-way ANOVAs followed by Tukey posthoc test or Kruskal–Wallis (KW) analysis followed by Dunn’s multiple comparison are shown (* p < 0.05, ** p < 0.01) [ns: non-significant, P: Phenotype, T: Treatment, int: Interaction]. The red dotted line indicates the mean value of the control group (Saline-VH).
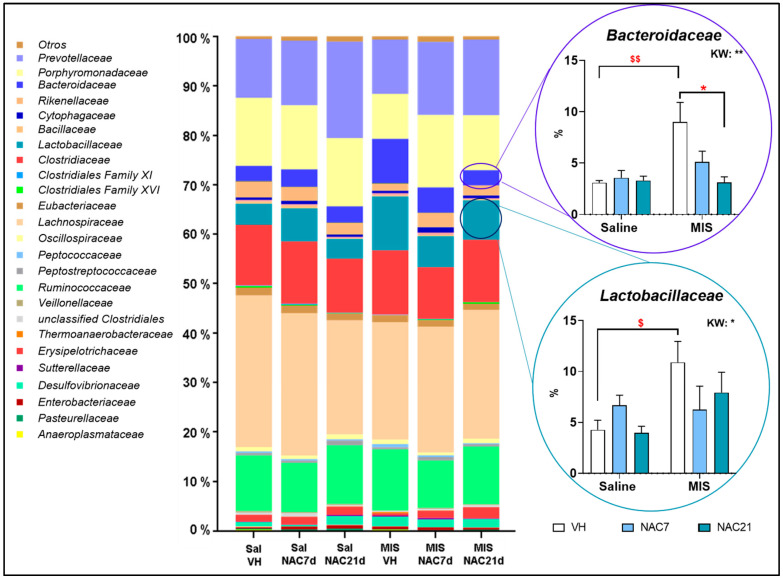
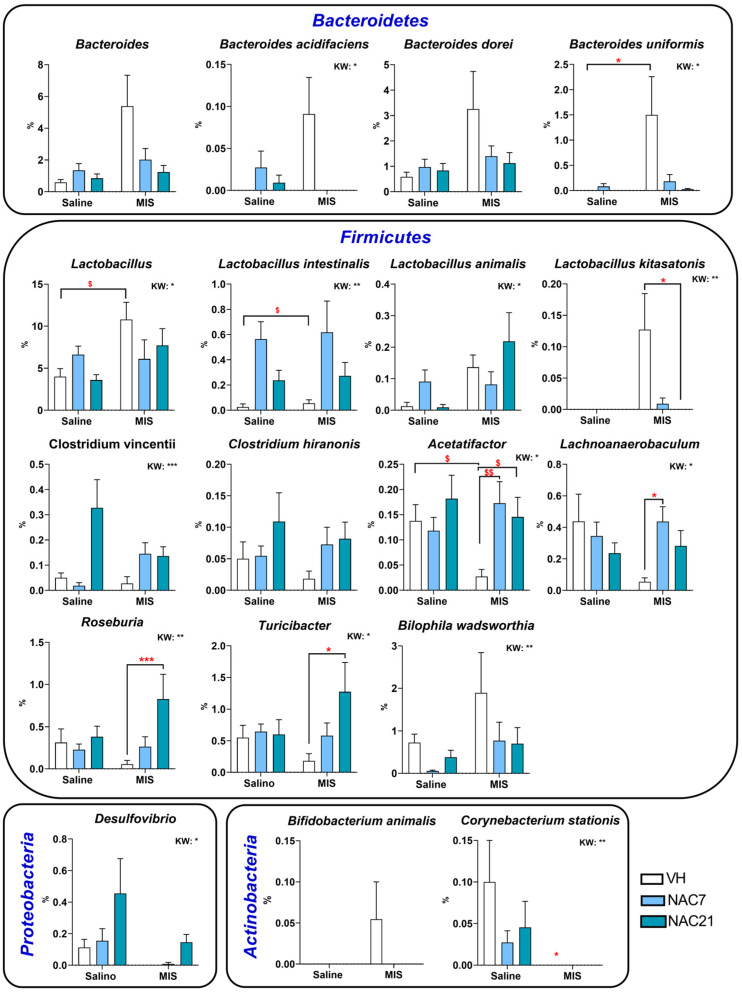
At the family level (Figure 3), in the control group (Saline-VH), the most abundant families were Lachnospiraceae (30.7%), Porphyromonadaceae (13.7%), Clostridiaceae (12.2%), Prevotellaceae (11.9%) and Ruminococcaceae (11.3%). In the MIS-VH group, the most abundant ones were Lachnospiraceae (23.7%), Clostridiaceae (12.9 %), Ruminococcaceae (12.4%), Prevotellaceae (11.0%), Lactobacillaceae (10.9%), Porphyromonadaceae (9.1%) and Bacteroidaceae (9.0%). These proportions were almost unchanged by NAC treatment (7 d and 21 d) except for Prevotellaceae, which increased with NAC treatment, Lactobacillaceae and Bacteroidaceae, which decreased with NAC in a dose-dependent manner.
Figure 3.
Nested bar plot showing gut microbiota composition at the family level. Differences in average intestinal microbiota composition at the family level. The nested bar graph shows the mean percentage composition of the most representative families for each group. A higher proportion of Bacteroidaceae and Lactobacillaceae families was found in MIS-VH animals compared to the control group (Sal-VH), which was prevented by NAC treatment. Kruskal–Wallis (KW) analysis followed by Dunn’s multiple comparison test (* p < 0.05, ** p < 0.01) and Mann–Whitney test ($ p < 0.05, $$ p < 0.01) are shown.
These compositions at a family level were relatively homogeneous between animals in each group, as can be seen in Supplementary Figure S1.
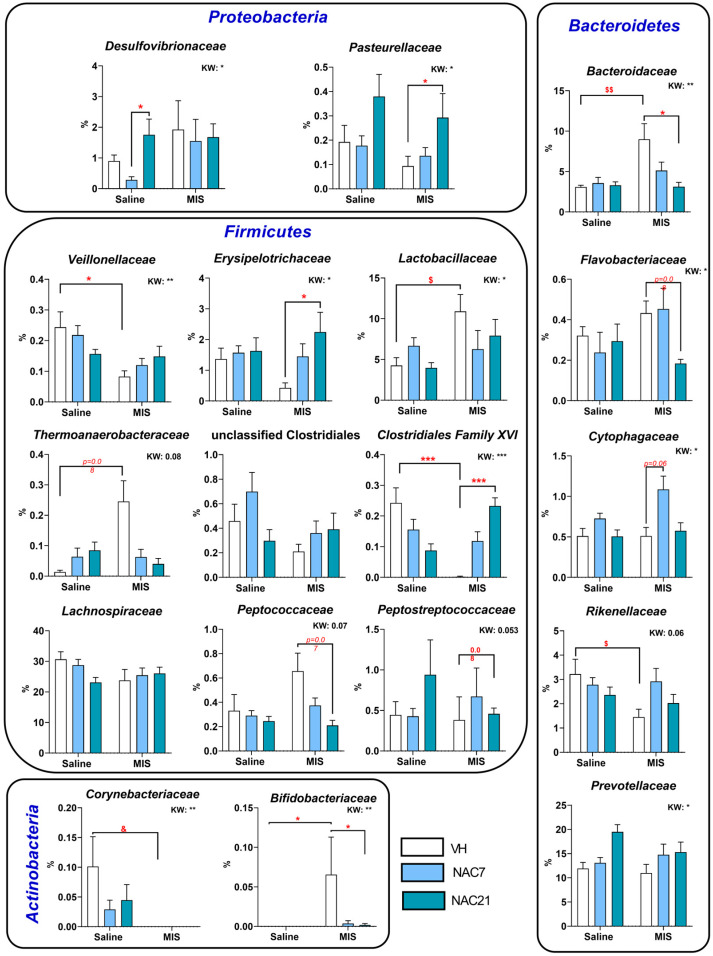
Likewise, at the family level (Supplementary Table S2), statistically significant differences between groups were found in several taxa, including the Firmicutes taxon Clostridiales XVI family, which is strongly reduced in the MIS-VH vs. Saline-VH group (p < 0.001), although NAC treatment during whole pregnancy restored this reduction (Figure 4). For Veillonellaceae, there was a statistically significant reduction in MIS-VH vs. Saline-VH animals (p < 0.05), with a certain tendency for its restoration under NAC treatment in MIS animals. Erysipelotrichaceae populations were strongly reduced in MIS-VH animals, but NAC for 21 d increased these levels significantly (p < 0.05). The Lactobacillaceae family showed a significant increase in MIS-VH vs. Saline-VH groups (*p < 0.05, ** p < 0.01).
Figure 4.
Gut microbiota composition at the family level. Selection of the most representative microbiota families altered in the MIS model and modified by NAC treatment. Each column shows the average of % changes in the composition of microbiota at the family level. (Saline-VH: 8, Saline-NAC7: 11, Saline-NAC21: 11, MIS-VH: 11, MIS-NAC7: 11, MIS-NAC21: 11). Data is shown as mean ± SEM. Kruskal–Wallis (KW) analysis followed by Dunn’s multiple comparison test (* p < 0.05, ** p < 0.01, *** p < 0.001) and Mann–Whitney test ($ p < 0.05, $$ p < 0.01) are shown.
As for the families of the phylum Bacteroidetes, the MIS challenge significantly affected two families. Bacteroidaceae populations were strongly increased in MIS-VH vs. Saline-VH animals (p < 0.01), an effect that was restored to normal conditions with NAC treatment in a dose-dependent manner. The Rikenellaceae family was reduced in MIS-VH vs. Saline-VH group, and NAC treatment restored these populations. With respect to Proteobacteria families, the Pasteurellaceae taxon increased in MIS-NAC21 vs. MIS-VH animals. Finally, regarding the Actinobacteria phylum, the Corynebacteriaceae taxon was not present in the MIS groups. Additionally, in the case of Bifidobacteriaceae, no populations were detected in the Saline groups but increased in MIS-VH animals, while these populations were strongly reduced in the MIS-NAC7 and MIS-NAC21 groups.
At the genus and species level (Figure 5 and Supplementary Table S3), statistically, significant differences were mainly found in Firmicutes phylum taxa. Lactobacillus was increased in MIS-VH vs. Saline-VH animals, and the same phenomenon occurred in L. intestinalis and L. kitasatonis species. Importantly, NAC7 and NAC21 treatments were able to reduce these increases in MIS animals, although the statistical significance of this reduction was only observed in the L. kitasatonis MIS-NAC21 group. Acetatifactor populations were strongly reduced in MIS-VH vs. Saline-VH animals, but both NAC treatments prevented this reduction. The genus Lachnoanaerobaculum was also reduced in MIS animals, and, as above, NAC7 treatment returned this decrease to normal levels. The behavior of Roseburia and Turicibacter was similar, with an increase in their populations in the MIS animals with NAC treatments, above the ones in Saline animals.
Figure 5.
Selection of the most representative genus and species altered in the MIS model and modified by NAC treatment. Each column shows the average of % changes in the composition of microbiota at the genera and species level. Data are shown as mean ± SEM. Kruskal–Wallis (KW) analysis followed by Dunn’s multiple comparison test (* p < 0.05, ** p < 0.01, *** p < 0.001) and Mann–Whitney test ($ p < 0.05, $$ p < 0.01) are shown.
For the phylum Bacteroidetes, in general, MIS-VH animals showed a strong increase in populations of the genus Bacteroides (such as in B. uniformis), which showed a tendency to decrease in a dose-dependent manner with NAC treatments.
In the phylum Proteobacteria, no Desulfovibrio populations were detected in MIS-VH animals, but NAC increased them in a dose-dependent manner in both Saline and MIS animals.
Finally, in the phylum Actinobacteria, the species Corynebacterium stations disappeared in MIS-VH animals and did not recover with NAC treatments.
3.4. Principal Component Analysis of the Bray-Curtis Distance of the Microbiota
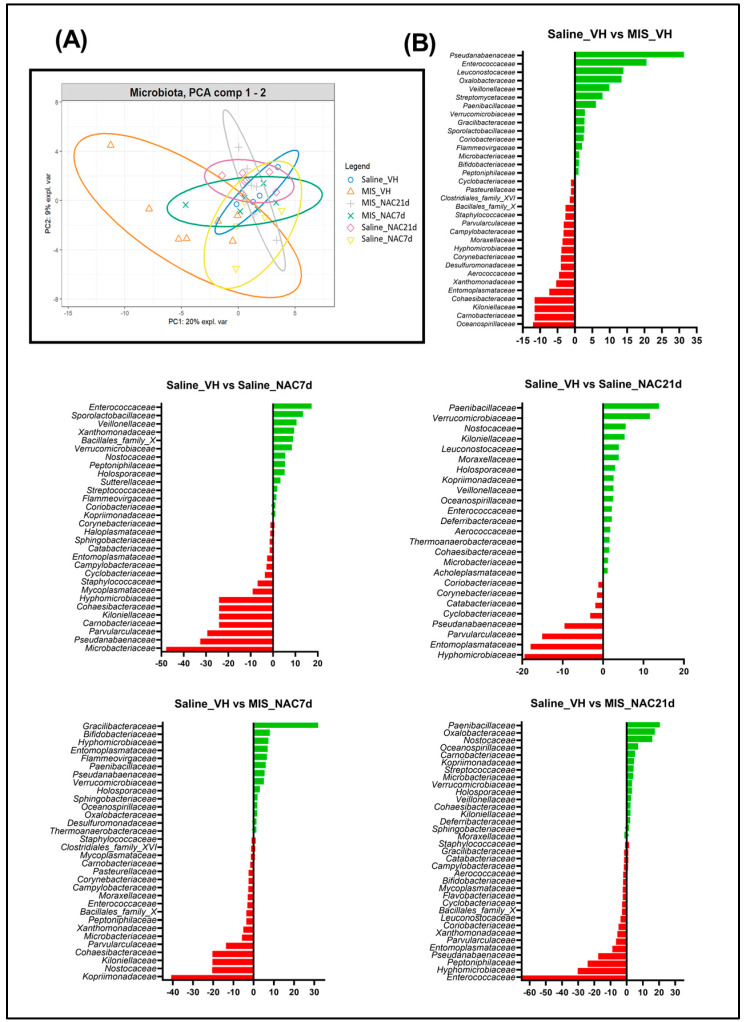
To determine whether overall gut microbiome composition differed between groups, we performed a principal component analysis (PCA) of the Bray-Curtis distance (Figure 6A). The PCA1 and PCA2 components explained 29% of the variance between the different animal groups. We found that MIS-VH animals presented the highest data dispersion compared to the other groups, indicating differences in the gut microbiota composition associated with the maternal immune challenge. Indeed, those animal groups receiving NAC treatment, regardless of its duration (7 or 21 days), showed a distribution of microbiota taxa more similar to that of the healthy animals’ group (Saline_VH) than to the pathological group (MIS_VH), thus suggesting that NAC treatment was able to return the variability of gut microbiota composition to similar parameters in MIS animals as in Saline animals.
Figure 6.
PCA and LDA analysis. (A). PCA cluster analysis of gut microbiota, which explains the 29% of the observed gut microbiota composition variance among groups. (B). LDA analysis among pairs of the different experimental groups, showing the families that better discriminate between the groups (Red: Saline-VH animals, Green: rest of groups).
3.5. Linear Discriminant Analysis of the Microbiota
Bacterial families with significant differences in their relative abundances between Saline and MIS animals are indicated in the linear discriminant analysis (LDA) (Figure 6B).
The main bacterial families that better discriminate between healthy and pathological controls (Saline_VH and MIS_VH) include Pseudanabaenaceae, Enterococcaceae and Leuconostocaceae (among others) for MIS_VH group, and Oceanospirillaceae, Carnobacteraceae and Kiloniellaceae (among others) for Saline_VH group. The main bacterial family that better discriminated between those groups that were treated with NAC during 21 days is Paenibacillaceae, which belongs to the Firmicutes phylum.
3.6. Kendall Correlation between Gut Microbiota Taxa and Hippocampal Volume
The taxa associated with MIS were negatively correlated with hippocampal volume (Thermoanaerobacteraceae) at a trend level (p = 0.06). 7-day and 21-day treatment with NAC during pregnancy-induced changes in the different taxa in control and MIS animals. Thus, the taxa associated with controls from dams treated with NAC for seven days were negatively correlated with hippocampal volume (Lachnospiraceae, p < 0.05), whereas in MIS animals, a positive correlation was found in Lachnospiraceae (p < 0.05), and a negative correlation was found in Sutterellaceae (p < 0.05). Moreover, NAC treatment during the whole pregnancy was negatively correlated with the hippocampal volume in control animals (Pasteuralleaceae, p < 0.05; Veillonellaceae, p < 0.05) and MIS animals (Sphingobacteriaceae, p < 0.01).
4. Discussion
In this study, we aimed to describe the effect of NAC administration during pregnancy on the gut-brain axis in the offspring of the MIS animal model of schizophrenia. First, we evaluated changes in hippocampal size and long-term memory after NAC treatment. Second, we evaluated changes in bacterial composition, taxonomy, and functional diversity of the gut microbiota. Finally, we analyzed the potential correlation between microbiome and hippocampal data.
The present study confirmed: (1) the existence of gut microbiota alterations in the offspring from Poly I:C-treated dams similar to those found in patients with schizophrenia, such as lower bacterial richness and an increase in Lactobacillaceae, Bifidobacteriaceae and Bacteroidaceae families; (2) the efficacy of NAC treatment during pregnancy to prevent hippocampal volume reduction, long-term memory deficits, and partially recover the alterations in the gut microbiota. Therefore, changes in brain volume, behavior, and microbiome in MIS offspring may be mediated by reduced oxidative stress levels, which led to an attenuated inflammatory response due to NAC treatment.
In recent years, much attention has been paid to the bidirectional homeostasis between the gut and the brain [37]. Communication between them involves neuroimmunoendocrine mediators through the central, autonomic, and enteric nervous systems and the hypothalamic-pituitary-adrenal (HPA) axis [38]. Gut dysbiosis, i.e., the imbalance in the proportions of the different elements that compose the bacterial microbiome, has been associated with the onset of different psychiatric and neurodevelopmental disorders, such as schizophrenia [38]. Furthermore, these patients may also display an increased intestinal permeability [38]. To date, nearly a dozen studies have reported differences in the microbiome between healthy individuals and patients at different stages of the disease [39,40]. Nevertheless, their results are not consistent, especially when analyzing bacterial components in a higher taxonomic order.
As expected, we found lower gut microbiota diversity in MIS animals compared to healthy animals; however, no changes in gut microbiota richness were found. In schizophrenia, results are not consistent so far. Recent studies reported disturbances in gut bacterial taxon composition, with lower gut microbiome diversity index, leading to lower numbers and species [41], but its clinical significance is not well described [42]. Nevertheless, a recent meta-analysis showed that microbial diversity was well preserved [43]. In terms of the gut microbiota richness index, this has been found to be generally low in patients with schizophrenia [42,43]. Microbial richness is involved in many cellular processes, such as the inflammatory response or those related to the maintenance of cell junction integrity. Thus, alterations in the richness index may suggest a weakness in epithelial barrier functions [44]. In our study, microbial richness was reduced in MIS-offspring, suggesting that this rat model may present altered epithelial barrier function, although further studies are needed to corroborate this fact. In addition, we found an effect of NAC treatment on both diversity and richness indices, increasing both indices in MIS-treated animals and suggesting a beneficial effect of NAC treatment on maintaining a healthy, resilient gut.
At the phylum level, Firmicutes and Proteobacteria taxa have shown consistent alterations in patients with schizophrenia [45]. Furthermore, the Firmicutes:Bacteroidetes (F:B) ratio, accepted as an important index in the normal intestinal homeostasis for the two dominant phyla in our gut microbiome, is also altered in schizophrenia, with enrichment of Firmicutes members and a decrease of Bacteroidetes [45,46]. In our study, we found no differences in the F:B ratio between healthy and MIS animals, as has been noted by other authors [15]. Nonetheless, although an increased F:B ratio has been linked to schizophrenia, some studies suggest that its alteration could be due to the metabolic disorder induced by antipsychotic medication since disorders, such as obesity or diabetes, in which inflammation and oxidative stress play an important role, also show an altered F:B ratio [47,48,49].
At the family taxa, the most consistent alteration in patients with schizophrenia is enrichment within the Lactobacillaceae family [37]. This imbalance has been associated with the evolution of the disorder and the severity of the symptoms, especially in the first episode of psychosis [50]. Interestingly, we successfully corroborated this finding in our MIS animals, and remarkably, NAC treatment during pregnancy prevented this enrichment. Specifically, we observed changes in the following species: Lactobacillus animalis, L. johnsonii, L. kitasatonis, L. murinus, L. reuteri and L. vaginalis (see Supplementary Table S3). This result is somehow surprising, especially considering that Lactobacillus has traditionally been suggested as a probiotic-based treatment. This apparent contradiction could be explained by the fact that Lactobacillus, as a probiotic treatment, does not colonize the digestive tract, as does the commensal microbiota.
The Bacteroidaceae family has also been extensively characterized in this disorder. Although some discrepancies are observed between different studies, most authors reported an increase in this bacterial family in patients with schizophrenia at different stages of the disease [37,50]. In our study, we corroborated this increase in Bacteroidaceae in MIS-offspring, and again, NAC treatment successfully prevented this effect, especially when administered for 21 days. Furthermore, we surprisingly observed differential effects of NAC on Clostridiales, Veillonellaceae and Lachnospiraceae families in control and MIS animals, with a reduction of bacteria from these families in healthy animals and an enrichment in MIS animals. These results could suggest a differential modulatory effect of NAC as a function of the different inflammatory and oxidative statuses in the animal. Interestingly, NAC7 and NAC21 treatments resulted in a reduction of proinflammatory bacterial taxons in MIS animals, such as in the case of reduced Bilophila wadsworthia, or in an increase in taxons known to produce anti-inflammatory metabolites (e.g., short-chain fatty acids), such as the genera Acetatifactor, Roseburia, Lachnoaerobaculum or Turicibacter; and families, such as Prevotellaceae. This finding is of particular relevance considering the key role of inflammation and oxidative stress in the pathogenesis of schizophrenia [51] and in our rat model [14]. Thus, changes in the microbiome are probably mediated by reduced oxidative stress levels due to NAC treatment, which led to an attenuated inflammatory response due to NAC treatment. Nevertheless, further studies evaluating the ability of NAC to modulate direct inflammatory markers are needed to fully demonstrate its anti-inflammatory role.
As stated above, regulation of the HPA axis is influenced by the state of the gut microbiome. Thus, alterations in different components of the HPA axis and the bacterial microbiota may reciprocally modulate each other [52]. Furthermore, alterations of this neural axis have been repeatedly linked to the schizophrenia pathophysiology, although its exact role in the course of this disorder remains to be clarified [53]. In this sense, one important brain region involved in the inhibitory regulation of this axis is the hippocampus, which is remarkably altered through changes in the microbiota, as demonstrated by Tang et al. [54]. In addition, the hippocampus is functionally and anatomically altered in patients with schizophrenia, showing a reduction in the volume of this structure in most patients [55,56]. Of relevance, a similar reduction in hippocampal volume has been found in animals of the MIS model induced by the viral mimetic Poly I:C [10,11,12,13,14]. Here, we replicated this hippocampal reduction and, more importantly, we demonstrated, for the first time, that NAC treatment during pregnancy was able to fully prevent this alteration. Given that inflammation in the hippocampus is key to vulnerability and recovery from psychiatric disorders. [54], these results are of great interest. On the other hand, the hippocampus is the center of learning and memory [57]. In this regard, schizophrenia is associated with cognitive deficits, including problems with short- and long-term working memory [58]. Prenatal Poly I:C challenge also affects behavior at the memory level, as previously demonstrated by our group and others [33,59]. In our setting, MIS animals showed memory impairments in the novel object recognition task, with a significant reduction in the object discrimination index for long-term memory, which was prevented by NAC treatment from the immune stimulation to delivery, although a similar trend was found for NAC treatment throughout pregnancy. Our results suggest that microbiota-based interventions with anti-IOS compounds could potentially be applied to prevent hippocampal-based memory impairments in neuropsychiatric disorders.
Lastly, gut microbiota composition has been associated with the severity of psychotic symptoms [60], but no relationship between brain structural changes by means of MRI and gut microbiota after NAC treatment has been described. Here, we found some correlations between hippocampal volume and gut microbiome after NAC treatment during pregnancy. Particularly, we found a correlation in the Lachnospiraceae taxa in the offspring from dams treated with NAC for seven days. Importantly, this taxon has been associated with the onset of negative symptoms in patients with schizophrenia [3,61] and is known to increase after antipsychotic treatment [4,62]. Thus, the positive correlation of Lachnospiraceae taxa could be related to the beneficial effect of NAC treatment in reducing the inflammatory basis of the MIS model. In addition, we also found a negative correlation between hippocampal volume and Sphingobacteriaceae taxa in MIS offspring from dams treated with NAC for 21 days. Alterations in this family have been linked to the pathogenesis of schizophrenia and bipolar disorder [63], as well as other inflammatory-based diseases, such as inflammatory bowel disease (IBD) [64,65], in which anxiety and depression are also present, and these are two distinctive features also described in the MIS model [66,67]. Of note, a large majority of cognitive impairments in the MIS model are possible through changes in the hippocampal function [68]. In this regard, we showed the existence of a reduced hippocampal volume in MIS animals, which may be responsible for the hippocampal-based memory impairment in the object discrimination index. Thus, an improvement in the hippocampal size would correlate with a reduction in the Sphingobacteriaceae taxa.
Finally, this study had some limitations. First, the small number of animals probably restricted statistical power, especially in the correlation tests. We believe that, with a larger number of animals, more statistically significant differences could have been found. Second, the study suffers from the inherent limitations of any psychiatry animal model. In this sense, the MIS model is considered a valid model for neurodevelopmental disorders, but we are aware that no animal model can fully mimic the specific characteristics of this type of pathology in humans. Third, we did not correct the statistical analyses for multiple comparisons to prevent type I errors, but the exploratory nature of this study warranted the suitability of this methodological choice. This is a common practice in studies of an exploratory nature, as in our case, and also noted by other authors [69]. Future dedicated studies are necessary to validate our findings. Finally, this study has only been conducted in males. Future studies, including both males and females, are warranted.
5. Conclusions
The current state of research on the microbiome in human disorders is at an extremely early stage. There are few certainties and large discrepancies in the existing data, probably due to the still low number of studies and the heterogeneity of the methodologies used. Beyond the few certainties we currently have, what does seem evident is that there is a relationship between alterations in the microbiome and the course of schizophrenia. Our study confirmed the existence of gut microbiota alterations in the offspring from Poly I:C-treated dams, similar to those found in patients with schizophrenia. Interestingly, these gut microbiota alterations were partially prevented by NAC treatment during pregnancy. In addition, we demonstrated the efficacy of NAC treatment in preventing hippocampal volume reduction and long-term memory deficits. These results suggest that early approaches with anti-IOS compounds, especially in neurodevelopmental disorders with an inflammatory/oxidative basis, may be useful in modulating bacterial microbiota, hippocampal size and hippocampal-based memory impairments.
Acknowledgments
Authors acknowledge the technical assistance of the Biostatistical and Epidemiology Unit of ISPA (Principado de Asturias, Spain).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antiox12040970/s1, Figure S1: Nested bar plot showing gut microbiota composition at the family level in all study groups; Table S1: Data from phyla populations; Table S2: Data from family populations; Table S3: Genus and species proportions.
Author Contributions
D.R.-M., M.C.-V. and J.F. contributed equally to this work. D.R.-M.: Methodology, formal analysis, writing—original draft preparation, writing—review and editing. M.C.-V.: Methodology, review and editing. J.F.: Methodology, review and editing. N.L.-R.; Methodology, review and editing. V.G.-R.: Methodology. C.G.-R.: Methodology, review and editing. C.S.-M.: Methodology, review and editing. C.J.V.: Methodology, review and editing. F.L.: Review and editing. R.A.; Review and editing. M.D.: Review and editing, funding acquisition. M.L.S.-M.: Conceptualization, methodology, writing—original draft preparation, writing—review and editing, project administration, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The animal study protocol was approved by the Ethics Committee for Animal Experimentation of Universidad Rey Juan Carlos and Gregorio Marañón Hospital and the Review Board at Comunidad de Madrid (PROEX number 231/17).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
M.L.S.-M. was supported by the Ministerio de Ciencia e Innovación, Instituto de Salud Carlos III (project number PI17/01766, and grant number BA21/00030), co-financed by the European Regional Development Fund (ERDF), “A way to make Europe”; project PID2021-128862OB-I00 funded by MCIN/AEI/10.13039/501100011033/FEDER, UE, CIBER de Salud Mental-Instituto de Salud Carlos III (project number CB07/09/0031); Delegación del Gobierno para el Plan Nacional sobre Drogas (project number 2017/085, 2022/008917); and Fundación Alicia Koplowitz. D.R.-M. was supported by Consejería de Educación e investigación, Comunidad de Madrid, co-funded by the European Social Fund “Investing in your future” (grant, PEJD-2018-PRE/BMD-7899). M.C.-V. was supported by a predoctoral grant from Fundación Tatiana Pérez de Guzmán el Bueno. N.L.-R. was supported by the Instituto de investigación Sanitaria Gregorio Marañón, “Programa Intramural de Impulso a la I+D+I 2019”. V.G.-R was supported by Consejería de Educación e investigación, Comunidad de Madrid, co-funded by the European Social Fund “Investing in your future” (grant, PEJD-2017-TL/BMD-7385). C.G.-R. was supported by Universidad Rey Juan Carlos (PREDOC20-054). R.A. was supported by Ministerio de Ciencia, Innovación y Universidades (grant number PID2019-111510RB-I00) and Grupo Español de Motilidad Digestiva (Beca Allergan, 2017). M.D. work was supported by Ministerio de Ciencia e Innovación (MCIN) and Instituto de Salud Carlos III (PT20/00044). The CNIC is supported by the Instituto de Salud Carlos III (ISCIII), the Ministerio de Ciencia e Innovación (MCIN) and the Pro CNIC Foundation and is a Severo Ochoa Center of Excellence (SEV-2015-0505). J.F. was supported by “Contrato Intramural Postdoctoral” from FINBA (Principado de Asturias, Spain). F.L. was supported by Ayudas para grupos de investigación de organismos del Principado de Asturias (SV-PA-21-AYUD/2021/51347).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Gupta L., Hoffman K.W. Exploring the intersection of the microbiome and the developing brain: Impacts on schizophrenia risk. Schizophr. Res. 2022;247:92–100. doi: 10.1016/j.schres.2021.08.010. [DOI] [PubMed] [Google Scholar]
- 2.Martín-Hernández D., Caso J.R., Bris G., Maus S.R., Madrigal J.L., García-Bueno B., MacDowell K.S., Alou L., Gómez-Lus M.L., Leza J.C. Bacterial translocation affects intracellular neuroinflammatory pathways in a depression-like model in rats. Neuropharmacology. 2016;103:122–133. doi: 10.1016/j.neuropharm.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Schwarz E., Maukonen J., Hyytiäinen T., Kieseppä T., Orešič M., Sabunciyan S., Mantere O., Saarela M., Yolken R., Suvisaari J. Analysis of microbiota in first episode psychosis identifies preliminary associations with symptom severity and treatment response. Schizophr. Res. 2017;192:398–403. doi: 10.1016/j.schres.2017.04.017. [DOI] [PubMed] [Google Scholar]
- 4.Zhang X., Pan L.Y., Zhang Z., Zhou Y.Y., Jiang H.Y., Ruan B. Analysis of gut mycobiota in first-episode, drug-naive Chinese patients with schizophrenia: A pilot study. Behav. Brain Res. 2020;379:112374. doi: 10.1016/j.bbr.2019.112374. [DOI] [PubMed] [Google Scholar]
- 5.Severance E.G., Gressitt K.L., Stallings C.R., Origoni A.E., Khushalani S., Leweke F.M., Dickerson F.B., Yolken R.H. Discordant patterns of bacterial translocation markers and implications for innate immune imbalances in schizophrenia. Schizophr. Res. 2013;148:130–137. doi: 10.1016/j.schres.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rogers G.B., Keating D., Young R., Wong M.-L., Licinio J., Wesselingh S. From gut dysbiosis to altered brain function and mental illness: Mechanisms and pathways. Mol. Psychiatry. 2016;21:738–748. doi: 10.1038/mp.2016.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsiao E.Y., McBride S.W., Hsien S., Sharon G., Hyde E.R., McCue T., Codelli J.A., Chow J., Reisman S.E., Petrosino J.F., et al. Microbiota Modulate Behavioral and Physiological Abnormalities Associated with Neurodevelopmental Disorders. Cell. 2013;155:1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vuong H.E., Yano J.M., Fung T.C., Hsiao E.Y. The Microbiome and Host Behavior. Annu. Rev. Neurosci. 2017;40:21–49. doi: 10.1146/annurev-neuro-072116-031347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bikovsky L., Hadar R., Soto-Montenegro M.L., Klein J., Weiner I., Desco M., Pascau J., Winter C., Hamani C. Deep brain stimulation improves behavior and modulates neural circuits in a rodent model of schizophrenia. Exp. Neurol. 2016;283:142–150. doi: 10.1016/j.expneurol.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hadar R., Bikovski L., Soto-Montenegro M.L., Schimke J., Maier P., Ewing S., Voget M., Wieske F., Götz T., Desco M., et al. Early neuromodulation prevents the development of brain and behavioral abnormalities in a rodent model of schizophrenia. Mol. Psychiatry. 2018;23:943–951. doi: 10.1038/mp.2017.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hadar R., Soto-Montenegro M.L., Götz T., Wieske F., Sohr R., Desco M., Hamani C., Weiner I., Pascau J., Winter C. Using a maternal immune stimulation model of schizophrenia to study behavioral and neurobiological alterations over the developmental course. Schizophr. Res. 2015;166:238–247. doi: 10.1016/j.schres.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casquero-Veiga M., García-García D., MacDowell K.S., Pérez-Caballero L., Torres-Sánchez S., Fraguas D., Berrocoso E., Leza J.C., Arango C., Desco M., et al. Risperidone administered during adolescence induced metabolic, anatomical and inflammatory/oxidative changes in adult brain: A PET and MRI study in the maternal immune stimulation animal model. Eur. Neuropsychopharmacol. 2019;29:880–896. doi: 10.1016/j.euroneuro.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Casquero-Veiga M., Romero-Miguel D., MacDowell K.S., Torres-Sanchez S., Garcia-Partida J.A., Lamanna-Rama N., Gomez-Rangel V., Romero-Miranda A., Berrocoso E., Leza J.C., et al. Omega-3 fatty acids during adolescence prevent schizophrenia-related behavioural deficits: Neurophysiological evidences from the prenatal viral infection with PolyI: C. Eur. Neuropsychopharmacol. 2021;46:14–27. doi: 10.1016/j.euroneuro.2021.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Romero-Miguel D., Casquero-Veiga M., MacDowell K.S., Torres-Sanchez S., Garcia-Partida J.A., Lamanna-Rama N., Romero-Miranda A., Berrocoso E., Leza J.C., Desco M., et al. A Characterization of the Effects of Minocycline Treatment during Adolescence on Structural, Metabolic, and Oxidative Stress Parameters in a Maternal Immune Stimulation Model of Neurodevelopmental Brain Disorders. Int. J. Neuropsychopharmacol. 2021;24:734–748. doi: 10.1093/ijnp/pyab036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Juckel G., Manitz M.P., Freund N., Gatermann S. Impact of Poly I: C induced maternal immune activation on offspring’s gut microbiome diversity—Implications for schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2021;110:110306. doi: 10.1016/j.pnpbp.2021.110306. [DOI] [PubMed] [Google Scholar]
- 16.Li W., Chen M., Feng X., Song M., Shao M., Yang Y., Zhang L., Liu Q., Lv L., Su X. Maternal immune activation alters adult behavior, intestinal integrity, gut microbiota and the gut inflammation. Brain Behav. 2021;11:e02133. doi: 10.1002/brb3.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castro-Nallar E., Bendall M., Perez-Losada M., Sabuncyan S., Severance E.G., Dickerson F.B., Schroeder J.R., Yolken R.H., Crandall K.A. Composition, taxonomy and functional diversity of the oropharynx microbiome in individuals with schizophrenia and controls. PeerJ. 2015;3:e1140. doi: 10.7717/peerj.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De la Fuente M., González-Pinto A., Pérez-Miralles F.C. Documento de Consenso Sobre la Microbiota y el uso de Probióticos/Prebióticos en Patologías Neurológicas y Psiquiátricas. Neuraxpharm; Madrid, Spain: 2021. Sociedad Española de Psiquiatría Biológica (SEPB), Probióticos y Prebióticos (SEMiPyP) y Sociedad Española de Neurología (SEN) Coordinadores. [Google Scholar]
- 19.Kulak A., Steullet P., Cabungcal J.-H., Werge T., Ingason A., Cuenod M., Do K.Q. Redox Dysregulation in the Pathophysiology of Schizophrenia and Bipolar Disorder: Insights from Animal Models. Antioxid. Redox Signal. 2013;18:1428–1443. doi: 10.1089/ars.2012.4858. [DOI] [PubMed] [Google Scholar]
- 20.Berk M., Malhi G.S., Gray L.J., Dean O.M. The promise of N-acetylcysteine in neuropsychiatry. Trends Pharmacol. Sci. 2013;34:167–177. doi: 10.1016/j.tips.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Bošković M., Vovk T., Saje M., Goričar K., Dolžan V., Plesničar B.K., Grabnar I. Association of SOD2, GPX1, CAT, and TNF Genetic Polymorphisms with Oxidative Stress, Neurochemistry, Psychopathology, and Extrapyramidal Symptoms in Schizophrenia. Neurochem. Res. 2013;38:433–442. doi: 10.1007/s11064-012-0937-4. [DOI] [PubMed] [Google Scholar]
- 22.Sepehrmanesh Z., Heidary M., Akasheh N., Akbari H., Heidary M. Therapeutic effect of adjunctive N-acetyl cysteine (NAC) on symptoms of chronic schizophrenia: A double-blind, randomized clinical trial. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2018;82:289–296. doi: 10.1016/j.pnpbp.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Rapado-Castro M., Berk M., Venugopal K., Bush A.I., Dodd S., Dean O.M. Towards stage specific treatments: Effects of duration of illness on therapeutic response to adjunctive treatment with N-acetyl cysteine in schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2015;57:69–75. doi: 10.1016/j.pnpbp.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Zheng J., Yuan X., Zhang C., Jia P., Jiao S., Zhao X., Yin H., Du Y., Liu H. N-Acetylcysteine alleviates gut dysbiosis and glucose metabolic disorder in high-fat diet-fed mice. J. Diabetes. 2019;11:32–45. doi: 10.1111/1753-0407.12795. [DOI] [PubMed] [Google Scholar]
- 25.Ding Q., Guo R., Pei L., Lai S., Li J., Yin Y., Xu T., Yang W., Song Q., Han Q., et al. N-Acetylcysteine alleviates high fat diet-induced hepatic steatosis and liver injury via regulating the intestinal microecology in mice. Food Funct. 2022;13:3368–3380. doi: 10.1039/D1FO03952K. [DOI] [PubMed] [Google Scholar]
- 26.German M.N., Musto J., Lucey M.R. Novel treatments for alcoholic hepatitis. Curr. Opin. Gastroenterol. 2021;37:179–186. doi: 10.1097/MOG.0000000000000725. [DOI] [PubMed] [Google Scholar]
- 27.Hsu C.-N., Hou C.-Y., Chang-Chien G.-P., Lin S., Tain Y.-L. Maternal N-Acetylcysteine Therapy Prevents Hypertension in Spontaneously Hypertensive Rat Offspring: Implications of Hydrogen Sulfide-Generating Pathway and Gut Microbiota. Antioxidants. 2020;9:856. doi: 10.3390/antiox9090856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsu C.N., Tain Y.L. Preventing Developmental Origins of Cardiovascular Disease: Hydrogen Sulfide as a Potential Target? Antioxidant. 2021;10:247. doi: 10.3390/antiox10020247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo Z., Xu X., Sho T., Luo W., Zhang J., Xu W., Yao J., Xu J. Effects of n-acetyl-cysteine supplementation in late gestational diet on maternal-placental redox status, placental NLRP3 inflammasome, and fecal microbiota in sows1. J. Anim. Sci. 2019;97:1757–1771. doi: 10.1093/jas/skz058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ding S., Fang J., Liu G., Veeramuthu D., Abdullah A.-D.N., Yin Y. The impact of different levels of cysteine on the plasma metabolomics and intestinal microflora of sows from late pregnancy to lactation. Food Funct. 2019;10:691–702. doi: 10.1039/C8FO01838C. [DOI] [PubMed] [Google Scholar]
- 31.Percie du Sert N., Hurst V., Ahluwalia A., Alam S., Avey M.T., Baker M., Browne W.J., Clark A., Cuthill I.C., Dirnagl U., et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020;18:e3000410. doi: 10.1371/journal.pbio.3000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Casquero Veiga M., Lamanna Rama N., Romero Miguel D., Rojas Márquez H., Alcaide J., Beltran M., Nacher J., Desco M., Soto Montenegro M.L. The Poly(I:C) maternal immune stimulation model shows unique patterns of brain metabolism, morphometry, and plasticity in female rats. Front. Behav. Neurosci. 2023;16:1022622. doi: 10.3389/fnbeh.2022.1022622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garcia-Partida J.A., Torres-Sanchez S., MacDowell K., Fernández-Ponce M.T., Casas L., Mantell C., Soto-Montenegro M.L., Romero-Miguel D., Lamanna-Rama N., Leza J.C., et al. The effects of mango leaf extract during adolescence and adulthood in a rat model of schizophrenia. Front. Pharmacol. 2022;13:886514. doi: 10.3389/fphar.2022.886514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gasull-Camós J., Soto-Montenegro M.L., Casquero-Veiga M., Desco M., Artigas F., Castañé A. Differential Patterns of Subcortical Activity Evoked by Glial GLT-1 Blockade in Prelimbic and Infralimbic Cortex: Relationship to Antidepressant-Like Effects in Rats. Int. J. Neuropsychopharmacol. 2017;20:988–993. doi: 10.1093/ijnp/pyx067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paxinos G., Watson C. The Rat Brain in Stereotaxic Coordinates. 4th ed. Academic Press; San Diego, CA, USA: 2008. [Google Scholar]
- 36.Fernández J., García L., Monte J., Villar C.J., Lombó F. Functional Anthocyanin-Rich Sausages Diminish Colorectal Cancer in an Animal Model and Reduce Pro-Inflammatory Bacteria in the Intestinal Microbiota. Genes. 2018;9:133. doi: 10.3390/genes9030133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu J., Gorbovskaya I., Hahn M., Müller D. The Gut Microbiome in Schizophrenia and the Potential Benefits of Prebiotic and Probiotic Treatment. Nutrients. 2021;13:1152. doi: 10.3390/nu13041152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ishida I., Ogura J., Aizawa E., Ota M., Hidese S., Yomogida Y., Matsuo J., Yoshida S., Kunugi H. Gut permeability and its clinical relevance in schizophrenia. Neuropsychopharmacol. Rep. 2022;42:70–76. doi: 10.1002/npr2.12227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bonnechère B., Amin N., van Duijn C. The Role of Gut Microbiota in Neuropsychiatric Diseases—Creation of An Atlas-Based on Quantified Evidence. Front. Cell. Infect. Microbiol. 2022;12:831666. doi: 10.3389/fcimb.2022.831666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murray N., Al Khalaf S., Kaulmann D., Lonergan E., Cryan J.F., Clarke G., Khashan A., O’Connor K. Compositional and functional alterations in the oral and gut microbiota in patients with psychosis or schizophrenia: A systematic review. HRB Open Res. 2021;4:108. doi: 10.12688/hrbopenres.13416.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bioque M., González-Rodríguez A., Garcia-Rizo C., Cobo J., Monreal J.A., Usall J., Soria V., Labad J. Targeting the microbiome-gut-brain axis for improving cognition in schizophrenia and major mood disorders: A narrative review. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2021;105:110130. doi: 10.1016/j.pnpbp.2020.110130. [DOI] [PubMed] [Google Scholar]
- 42.Thirion F., Speyer H., Hansen T.H., Nielsen T., Fan Y., Le Chatelier E., Fromentin S., Berland M., Oñate F.P., Pons N., et al. Alteration of Gut Microbiome in Patients With Schizophrenia Indicates Links between Bacterial Tyrosine Biosynthesis and Cognitive Dysfunction. Biol. Psychiatry Glob. Open Sci. 2022;3:283–291. doi: 10.1016/j.bpsgos.2022.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nikolova V.L., Hall M.R., Hall L.J., Cleare A.J., Stone J.M., Young A.H. Perturbations in Gut Microbiota Composition in Psychiatric Disorders: A Review and Meta-analysis. JAMA Psychiatry. 2021;78:1343–1354. doi: 10.1001/jamapsychiatry.2021.2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rinninella E., Raoul P., Cintoni M., Franceschi F., Miggiano G.A.D., Gasbarrini A., Mele M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms. 2019;7:14. doi: 10.3390/microorganisms7010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Szeligowski T., Yun A.L., Lennox B.R., Burnet P.W.J. The Gut Microbiome and Schizophrenia: The Current State of the Field and Clinical Applications. Front. Psychiatry. 2020;11:156. doi: 10.3389/fpsyt.2020.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singh R., Stogios N., Smith E., Lee J., Maksyutynsk K., Au E., Wright D.C., De Palma G., Graff-Guerrero A., Gerretsen P., et al. Gut microbiome in schizophrenia and antipsychotic-induced metabolic alterations: A scoping review. Ther. Adv. Psychopharmacol. 2022;12:20451253221096525. doi: 10.1177/20451253221096525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Belkaid Y., Hand T.W. Role of the Microbiota in Immunity and Inflammation. Cell. 2014;157:121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Magne F., Gotteland M., Gauthier L., Zazueta A., Pesoa S., Navarrete P., Balamurugan R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients. 2020;12:1474. doi: 10.3390/nu12051474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Larsen N., Vogensen F.K., Van Den Berg F.W.J., Nielsen D.S., Andreasen A.S., Pedersen B.K., Al-Soud W.A., Sørensen S.J., Hansen L.H., Jakobsen M. Gut Microbiota in Human Adults with Type 2 Diabetes Differs from Non-Diabetic Adults. PLoS ONE. 2010;5:e9085. doi: 10.1371/journal.pone.0009085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsamakis K., Galinaki S., Alevyzakis E., Hortis I., Tsiptsios D., Kollintza E., Kympouropoulos S., Triantafyllou K., Smyrnis N., Rizos E. Gut Microbiome: A Brief Review on Its Role in Schizophrenia and First Episode of Psychosis. Microorganisms. 2022;10:1121. doi: 10.3390/microorganisms10061121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Müller N., Weidinger E., Leitner B., Schwarz M.J. The role of inflammation in schizophrenia. Front. Neurosci. 2015;9:372. doi: 10.3389/fnins.2015.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Misiak B., Łoniewski I., Marlicz W., Frydecka D., Szulc A., Rudzki L., Samochowiec J. The HPA axis dysregulation in severe mental illness: Can we shift the blame to gut microbiota? Prog. Neuropsychopharmacol. Biol. Psychiatry. 2020;102:109951. doi: 10.1016/j.pnpbp.2020.109951. [DOI] [PubMed] [Google Scholar]
- 53.Mikulska J., Juszczyk G., Gawrońska-Grzywacz M., Herbet M. HPA Axis in the Pathomechanism of Depression and Schizophrenia: New Therapeutic Strategies Based on Its Participation. Brain Sci. 2021;11:1298. doi: 10.3390/brainsci11101298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tang W., Meng Z., Li N., Liu Y., Li L., Chen D., Yang Y. Roles of Gut Microbiota in the Regulation of Hippocampal Plasticity, Inflammation, and Hippocampus-Dependent Behaviors. Front. Cell. Infect. Microbiol. 2020;10:611014. doi: 10.3389/fcimb.2020.611014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heckers S., Konradi C. Hippocampal neurons in schizophrenia. J. Neural Transm. 2002;109:891–905. doi: 10.1007/s007020200073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pujol N., Penadés R., Junqué C., Dinov I., Fu C.H.Y., Catalán R., Ibarretxe-Bilbao N., Bargalló N., Bernardo M., Toga A., et al. Hippocampal abnormalities and age in chronic schizophrenia: Morphometric study across the adult lifespan. Br. J. Psychiatry. 2014;205:369–375. doi: 10.1192/bjp.bp.113.140384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lisman J., Buzsáki G., Eichenbaum H., Nadel L., Ranganath C., Redish A.D. Viewpoints: How the hippocampus contributes to memory, navigation and cognition. Nat. Neurosci. 2017;20:1434–1447. doi: 10.1038/nn.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guo J.Y., Ragland J.D., Carter C.S. Memory and cognition in schizophrenia. Mol. Psychiatry. 2019;24:633–642. doi: 10.1038/s41380-018-0231-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mattei D., Ivanov A., Ferrai C., Jordan P., Guneykaya D., Buonfiglioli A., Schaafsma W., Przanowski P., Deuther-Conrad W., Brust P., et al. Maternal immune activation results in complex microglial transcriptome signature in the adult offspring that is reversed by minocycline treatment. Transl. Psychiatry. 2017;7:e1120. doi: 10.1038/tp.2017.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kraeuter A.-K., Phillips R., Sarnyai Z. The Gut Microbiome in Psychosis From Mice to Men: A Systematic Review of Preclinical and Clinical Studies. Front. Psychiatry. 2020;11:799. doi: 10.3389/fpsyt.2020.00799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zheng P., Zeng B., Liu M., Chen J., Pan J., Han Y., Liu Y., Cheng K., Zhou C., Wang H., et al. The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice. Sci. Adv. 2019;5:eaau8317. doi: 10.1126/sciadv.aau8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Flowers S.A., Evans S.J., Ward K.M., McInnis M.G., Ellingrod V.L. Interaction between Atypical Antipsychotics and the Gut Microbiome in a Bipolar Disease Cohort. Pharmacotherapy. 2017;37:261–267. doi: 10.1002/phar.1890. [DOI] [PubMed] [Google Scholar]
- 63.Chen Y., Zhou C., Yu H., Wu W., Wang Y., Liu L., Hu G., Li B., Peng Z., Wang H. Gut microbial signatures and differences in bipolar disorder and schizophrenia of emerging adulthood. CNS Neurosci. Ther. 2022 doi: 10.1111/cns.14044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nelson T., Holmes S., Alekseyenko A., Shenoy M., DeSantis T.Z., Wu C.H., Andersen G., Winston J.H., Sonnenburg J.L., Pasricha P.J., et al. PhyloChip microarray analysis reveals altered gastrointestinal microbial communities in a rat model of colonic hypersensitivity. Neurogastroenterol. Motil. 2011;23:169–177. doi: 10.1111/j.1365-2982.2010.01637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kawoos Y., Wani Z., Kadla S., Shah I., Hussain A., Dar M.M., Margoob M., Sideeq K. Psychiatric Co-morbidity in Patients with Irritable Bowel Syndrome at a Tertiary Care Center in Northern India. J. Neurogastroenterol. Motil. 2017;23:555–560. doi: 10.5056/jnm16166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Talukdar P.M., Abdul F., Maes M., Binu V., Venkatasubramanian G., Kutty B.M., Debnath M. Maternal Immune Activation Causes Schizophrenia-like Behaviors in the Offspring through Activation of Immune-Inflammatory, Oxidative and Apoptotic Pathways, and Lowered Antioxidant Defenses and Neuroprotection. Mol. Neurobiol. 2020;57:4345–4361. doi: 10.1007/s12035-020-02028-8. [DOI] [PubMed] [Google Scholar]
- 67.Pendyala G., Chou S., Jung Y., Coiro P., Spartz E., Padmashri R., Li M., Dunaevsky A. Maternal Immune Activation Causes Behavioral Impairments and Altered Cerebellar Cytokine and Synaptic Protein Expression. Neuropsychopharmacology. 2017;42:1435–1446. doi: 10.1038/npp.2017.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wolff A.R., Cheyne K.R., Bilkey D.K. Behavioural deficits associated with maternal immune activation in the rat model of schizophrenia. Behav. Brain Res. 2011;225:382–387. doi: 10.1016/j.bbr.2011.07.033. [DOI] [PubMed] [Google Scholar]
- 69.Althouse A.D. Adjust for Multiple Comparisons? It’s Not That Simple. Ann. Thorac. Surg. 2016;101:1644–1645. doi: 10.1016/j.athoracsur.2015.11.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.