Abstract
Dogs are one of few species that naturally develop prostate cancer (PCa), which clinically resembles aggressive, advanced PCa in humans. Moreover, PCa-tumor samples from dogs are often androgen receptor (AR)-negative and may enrich our understanding of AR-indifferent PCa in humans, a highly lethal subset of PCa for which few treatment modalities are available This narrative review discusses the molecular similarities between dog PCa and specific human-PCa variants, underscoring the possibilities of using the dog as a novel pre-clinical animal model for human PCa, resulting in new therapies and diagnostics that may benefit both species.
Keywords: androgen receptor, androgen-indifferent, androgen-receptor indifferent, castration-resistant prostate cancer, dog, prostate cancer
1. Introduction
The initiation and progression of prostate cancer (PCa) in humans is initially reliant on androgen receptor (AR) signaling [1,2,3]. Directly targeting androgen ligands with androgen-inhibiting drugs (e.g., chemical castration) or decreasing their production via surgical castration has been utilized over the last 80 years in an attempt to suppress AR signaling and PCa-tumor progression [4,5,6]. Unfortunately, androgen-deprivation therapies (ADT) eventually fail for a subset of patients, and despite the presence of castrate levels of androgens, PCa progresses to an incurable form, termed castration-resistant prostate cancer (CRPC) [7,8,9]. This form of prostate cancer often continues AR signaling that is not reliant on androgen ligands (i.e., androgen independent) by way of multiple mechanisms, including mutations in the receptor or copy-number variations, and is typically treated with androgen-receptor inhibitors (ARIs) [10,11,12,13]. Eventually, new driver mutations develop in various genes in CRPC tumors, which leads to the abandonment of AR signaling altogether. Once PCa progresses, irrespective of AR signaling, it is termed androgen-receptor-indifferent PCa, a highly aggressive lethal form of the disease with poor outcomes [14,15]. Recently, the molecular characterization of CPRC and androgen-indifferent prostate cancer (AIPC) have improved our understanding of the drivers of these variants, which is critical for the identification of novel therapeutics. However, animal models for advanced forms of PCa are lacking and are wrought with limitations [16,17], which may make the approval of novel therapeutics challenging and the translation of results between species inconsistent.
The prostate of the dog, unlike that of rodents, is morphologically, histologically, and physiologically similar to that of humans and is under the control of androgens via androgen-receptor signaling [18,19,20]. Dogs also naturally develop other pathologic prostatic conditions with age, such as benign prostatic hyperplasia (BPH), in contrast to rodents, which typically develop prostatic atrophy with age [21,22]. More importantly, dogs are the among the only animals that spontaneously develop PCa, and often present with highly aggressive metastatic disease. The neutering or castration of male dogs is common in developed countries, with the majority of the male dogs in the United States undergoing this procedure [23,24]. It has been shown that castration influences PCa progression as castrated dogs develop PCa at higher rates and experience more metastases than intact dogs [25,26,27,28,29,30].
This review aims to discuss the molecular similarities between dog and specific human PCa variants to further examine the use of dogs as a suitable animal research model.
2. Androgen-Dependent PCa
The AR is a ligand-dependent nuclear receptor transcription factor that mediates the action of androgen ligands (e.g., testosterone) in androgen-dependent tissues in health and plays an important role in prostate organogenesis and maintenance in adulthood [31,32,33,34]. Initially, PCa is reliant on androgens and AR signaling for tumor growth and progression and is characterized by a rising prostate-specific antigen (PSA) level in humans, a marker not typically expressed in dog PCa [33,34]. This requirement for androgen-dependent growth is clinically exploited to combat advanced or recurrent PCa (following initial treatment with surgery and radiation) with the use of androgen-deprivation therapies (ADT) (i.e., androgen-ablation therapies, chemical castration) to inhibit the production or actions of androgens [35,36]. Invariably, PCa becomes resistant to ADT, and is then termed CRPC, where AR continues to signal irrespective of the presence of androgens [37,38]. Because of this continued signaling, AR targeting remains a valid treatment strategy using androgen-signaling inhibitors (ASIs) (e.g., androgen-synthesis inhibitors, androgen-receptor inhibitors). Dog PCa is most often low or null for AR expression, as well as for androgen-regulated proteins, such as NKX3.1 [39,40] (Table 1). However, opposing reports show some AR expression in intact dogs with PCa and cytoplasmic sequestration or the loss of AR in castrated dogs with PCa [41,42], making intact dogs with PCa potential models for androgen-dependent disease. As noted over 50 years ago in landmark studies performed by Huggins et al., dogs have similar pathophysiologies with respect to the androgen-dependent growth of the prostate and may still be of value to researchers [6,43].
Table 1.
Summary of molecular characteristics of dog and human PCa.
| PCa Variants | Marker of Pathway | Dog | Humans |
|---|---|---|---|
|
Androgen-
dependent |
|||
| AR + | No | Yes | |
| NKX3.1 + | No | Yes | |
| PSA + | No | Yes | |
| CRPC | |||
| PI3K-AKT-mTOR overexpression | Yes | Yes | |
| ERβ downregulation, ERα overexpression | No ‡ | Yes | |
| Markers of CSCs + | Yes | Yes | |
| Markers of EMT + | Yes | Yes | |
| Wnt/β-catenin overexpression | Yes | Yes | |
| AIPC | |||
| DNPC | AR (-) | Yes | Yes |
| Markers of NE (-) | Yes | Yes | |
| NEPC | Markers of NE + | No | Yes |
| AVPC | TP53 (-) or mutated | No | Yes |
| RB1 (-) or mutated | Unknown | Yes | |
| PTEN (-) or mutated | Yes | Yes |
‡ Estrogen-receptor expression in dog PCa is present but is not identical to human PCa. Abbreviations: +, positive for expression; (-), negative for expression; AR, androgen receptor, PSA, prostate-specific antigen; CSC, cancer stem cell; ER, estrogen receptor; EMT, epithelial mesenchymal transition; AIPC, androgen-indifferent prostate cancer; DNPC, double-negative prostate cancer; NE, neuroendocrine; NEPC, neuroendocrine prostate cancer; AVPC, aggressive-variant prostate cancer.
2.1. Androgen-Receptor Structure
The AR in humans is a protein of 919 amino acids consisting of several functional domains, including an N-terminal domain (NTD), DNA binding domain (DBD), and a ligand binding domain (LBD) at the C-terminus [44,45]. In dogs, the AR is approximately 907 amino acids, and has homologous DBD and LBD, which are highly conserved across evolution in various species and are activated upon binding to androgen ligands [45,46].
The NTD of the AR is essential for function and the least evolutionarily conserved region of AR; however, there are still similarities between humans and dogs. The polyglutamine (i.e., polyQ, CAG) repeat region of the NTD has an average of 21–23 Qs in humans. Longer polyQ repeats are related to decreased AR transcriptional activity, while shorter polyQ repeats are related to increased AR transcriptional activity and are often associated with increased PCa risk [47,48,49]. This finding has also been recapitulated in dogs in vitro, where the introduction of AR with fewer polyQ repeats resulted in higher AR activity [50]. However, the association between shorter polyQ repeats regions and PCa is unclear in dogs: while some studies reveal that a shorter polyQ length does not predispose dogs to PCa, others show that certain breeds with shorter polyQ lengths are predisposed to developing PCa [25,51,52,53].
2.2. Androgen-Receptor Co-Chaperones
In the absence of the agonist ligand, the AR is bound to heat-shock proteins (HSP40, HSP70 and HSP90) and other co-chaperone proteins in a complex known as the foldosome [54,55]. Many small co-chaperone proteins with tetratricopeptide repeats (TPR), such as CYP60, PP5, FKBP51, FKB52, PP5, CHIP, and SGTA, have been shown to interact with the AR-foldosome complex [56]. The small glutamine-rich tetratricopeptide repeat-containing protein α (SGTA), is a co-chaperone of interest in PCa, and is known to stabilize the apo-AR structure in the cytoplasm prior to ligand binding. In human PCa, the SGTA, a steroid-receptor molecular co-chaperone that influences hormone action, is known to regulate AR function. The AR:SGTA ratio is increased compared to patient-matched BPH, and it is also increased when metastatic PCa tumors are compared with their primary tumors [57,58]. It is hypothesized that AR thereby overwhelms the capacity for SGTA to limit AR response to ligands and ensure the appropriate cellular localization of AR in vivo. In addition, in vitro work from this study showed that SGTA overexpression blunted the AR’s response to androgen ligands [58].
This concept has also been explored in multiple studies in dogs, which have shown that the overexpression of SGTA in vitro abates AR signaling [59,60]. Therefore, androgen-independent disease was hypothesized to be attributed to SGTA overexpression in dog-PCa-patient tumor samples by some researchers, who also subsequently showed that interference with SGTA dimerization in vitro rescues AR signaling [61].
3. CRPC
The CRPC is a hormone-independent but AR-dependent progression of PCa, in which ADT has failed, yet AR signaling axis is still functional and increases growth and progression. Often, this stage is characterized by more aggressive tumor behavior, including the initiation of cancer stem cell (CSC) signaling, epithelial–mesenchymal transition (EMT), and metastasis. A subset of patients develop CRPC secondary to uncontrolled AR signaling due to a mutation in the AR. Splice variants of the AR lacking the ligand-binding domain, such as ARV7, are responsible for this uncontrolled signaling in humans [4]. To date, splice variants have not been identified in dog PCa, and the use of dogs as a model for splice-variant-driven CRPC is undetermined. Moreover, TMPRSS2–ERG fusion and its alternative splice variants have also not been investigated in dog PCa, and their value as a model for this event is yet to be determined.
Additionally, mutations in AR-independent pathways are more frequent in CRPC and are thought to drive CRPC to become AR-indifferent PCa after ASI therapy. Therefore, AR presence and signaling coupled with new driver mutations in other pathways, EMT, and metastasis is are hallmarks of this progression of PCa (metastatic CRPC (mCRPC)). Because dogs often present with low or null AR expression, are castrated at an early age, develop PCa without the influence of androgens, and often present with metastasis, it has been argued that CRPC in dogs closely resembles CRPC in humans.
3.1. PI3K-AKT-mTOR and PTEN
The PI3K–AKT–mTOR signaling axis has been shown to play a crucial role in the development and maintenance of CRPC [62,63,64]. This pathway integrates growth signals with downstream processes that increase cell survival and proliferation [64]. The activation of PI3K by growth-factor-transmembrane signaling leads to the activation of AKT and, in turn, the autophosphorylation and activation of mTOR [65]. Subsequently, mTOR joins raptor (to form mTORC1) or rictor (to form mTORC2). The former is implicated in cellular proliferation and protein synthesis and the latter is implicated in cytoskeletal organization and cellular metabolism [66,67]. Upon activation, mTORC1 increases mRNA translation by phosphorylating eIF4E-BP1 and p70S6, with the former leading to the release of eIF4E from its hold and allowing it to join the translation-initiation complex, and the latter leading to the enhanced translation of mRNA transcripts that encode for ribosomal proteins and insulin growth factor 2 [68]. This pathway is of great interest as the overactivation of the PI3K–AKT–mTOR pathway has been implicated in cell survival in many cancer types, including PCa [69,70]. Many factors contribute to the aberrant activation of the PI3K–AKT–mTOR pathway, such as PI3K amplification, PTEN loss of function or deletion, and AKT, p-4EBP1, and eIF4E overexpression [69].
This entire pathway has also been observed to be upregulated in samples of dog PCa tissue when compared to the non-malignant prostatic tissue of normal intact dogs [40]. Moreover, another study found that p-mTOR and eIF4E were overexpressed in dog PCa tissues compared to normal prostate tissue, and this overexpression was correlated with a higher Gleason score in these dog-PCa-histology sections, which was similar to what has been reported in human PCa [71,72]. The deletion or loss of function pf PTEN has been implicated in the aberrant activation of this pathway in human PCa, and has also been shown to be lost in dog PCa [73]. Because PI3K pathway inhibitors, as well as eIF4E inhibitors, are a class of emerging therapeutics in CRPC and are being used in current clinical trials [74,75,76], dogs may be useful models for testing drugs targeting this pathway.
3.2. Estrogen Receptors
Estrogens have been linked to CRPC progression, and increased levels of estrogens have been correlated with more aggressive PCa [77,78,79,80]. Estrogens serve as ligands for two nuclear-receptor isoforms, estrogen receptor alpha (ERα) and estrogen-receptor beta (ERβ), and ERα has been implicated in oncogenic functions and the increased proliferation of cancer cells, while ERβ has tumor-suppressor functions because its loss leads to hyperplasia of the prostate and the initiation of PCa [81,82,83,84]. Moreover, PCa has been shown to have increased expression of ERα and decreased expression of ERβ [85,86,87]. Although ERα expression is typically observed in tumor stromal cells, one study found that ERα is also expressed in small selections of patients’ tumor epithelial cells, although this had no effect on the clinical or biochemical recurrence of disease [88]. Estrogen-receptor expression in dog PCa was explored in one study, in which the ERα was strongly expressed in normal prostate epithelium and the epithelium in BPH samples, but had decreased expression in PCa-tumor epithelium and no expression in the tumor stroma [73]. This appears to be in contrast to what is found in the majority of cases of human PCa, although additional studies are needed to confirm these findings.
3.3. Stem-Cell Markers
Cancer stem cells (CSC), or tumor progenitor cells, have been linked to the driving of clonal evolution and tumor heterogeneity, growth, and progression via their ability to self-renew, resist apoptosis, and differentiate [89,90,91], and have been implicated in the development of CRPC [92]. During development, basal epithelial stem cells of the prostate differentiate into luminal epithelial cells under the influence of androgens and the AR [93]. These multipotent basal progenitor cells remain quiescent in the basal epithelial layer, acting only to replenish defunct or apoptotic luminal cells [92]. However, it is believed that CSCs probably arise from these basal epithelial cells in PCa, although there is evidence that suggests that luminal-cell populations have their own lineage-restricted stem cells [92]. Classic markers of pluripotency include OCT3/4, SOX2, KLF4, c-Myc, and NANOG, which have been observed in PCa [94]. In addition, PCa CSC populations of CD44+ and CD20- often express CD133 and ABCG2 in humans. These markers are often associated with more aggressive tumors, higher Gleason scores, metastasis, and chemoresistance in humans [95,96,97,98,99].
Stem-cell-marker expression has also been explored in dog PCa [100,101]. In patient-derived dog PCa cell lines, one study found the increased expression of CD44, CD133, ITGA6, and DDX5 [100]. Moreover, these findings were recapitulated and expanded on in another study using patient-derived tumor spheroids, which were found to express OCT3/4, Nestin, NANOG, and CD44 [101]. Two additional factors, SOX9 and survivin, have also been associated with CSCs in PCa [91,102]. The factor SOX9 is repressed by androgens and is often overexpressed in CRPC as a result, while survivin is an inhibitor of the apoptosis protein (IAP) family member and is overexpressed in many cancers [103,104,105,106]. Both of these factors have also been observed to have increased expression in dog PCa [107]. Altogether, dogs appear to develop PCa with the presence of CSCs, and may serve as models for the therapeutic targeting of these tumor-progenitor cells.
3.4. Epithelial–Mesenchymal Transition (EMT) Markers
Epithelial cells can undergo EMT, a phenomenon that is characterized by phenotypic changes in epithelial cells, which begin to appear more spindle-shaped, in the manner of mesenchymal cells. Additionally, this phenotypic change is accompanied by biochemical changes, in which epithelial markers associated with adhesion, such as E-cadherin, are downregulated and mesenchymal lineage markers, such as vimentin, become upregulated [108]. This event leads to the higher metastatic potential of cancer cells and plays a critical role in mCRPC [109,110]. The induction of EMT has been associated with multiple molecular drivers, including TGF-β and the WNT signaling pathways [110,111,112]. This signaling induces the expression of multiple transcription factors that are known as classic regulators of EMT, such as Snail, Twist1, and zinc-finger E homeobox-binding 1 and 2 (ZEB1/2) [110].
Epithelial–-mesenchymal transition has also been shown to occur in dog PCa. One study showed that dog PCa tumors with a more undifferentiated appearance and metastatic lesions had increased TGF-β nuclear positivity on immunohistochemistry (IHC) [113]. E-cadherin has been shown to be decreased in dog PCa and is associated with higher Gleason scores in dog-PCa-histology sections, and neoplastic luminal epithelial cells have been shown to have increased expression of vimentin when compared to non-neoplastic lesions [39,114,115]. Because dogs with PCa often have aggressive disease and metastatic lesions [114], EMT is an unsurprising occurrence in their PCa progression, and this may offer insights into this process in human PCa.
3.5. Canonical Wnt Signaling
The canonical Wnt-signaling pathway is integral to EMT and has been shown to be important in the development of CRPC and the proliferation of CSCs in the PCa microenvironment [116]. Briefly, Wnts are secreted glycoproteins that direct development, tissue homeostasis, and stem-cell proliferation. Classically, Wnts bind to the frizzled and lipoprotein receptor-related protein (Fz/LRP) co-receptor complex of the cell membrane, which leads to the release of β-catenin from a group of regulatory proteins (i.e., the destruction complex) that encourage its ubiquitination and destruction in the proteosome. Once released, β-catenin increases in the cytosol, which allows its migration to the nucleus, where it regulates target-gene expression by interacting with the T-cell-specific factor (TCF)/lymphoid enhancer-binding factor (LEF) family of transcription factors, ultimately leading to the transcription of target genes increasing cell differentiation and proliferation [117].
For decades, Wnt signaling has been implicated in human PCa progression, and it is critical for CRPC. Furthermore, Wnt signaling has been shown to increase the proliferation and differentiation of prostate cells and promote EMT, leading to more aggressive and invasive disease [118]. This pathway has also been shown to have cross-talk with AR and is activated after ADT, encouraging and maintaining CRPC development and progression [119]. One study found increased cytoplasmic β-catenin staining, but not nuclear staining, in pre-neoplastic and neoplastic lesions compared to BPH lesions in dog PCa [114]. In contrast, a more recent study showed the overexpression of nuclear β-catenin and the loss of membranous β-catenin in dog PCa, with these findings exacerbated in metastatic lesions [120]. This was not due to the hypermethylation of APC [120], as is found in human PCa, and a different mechanism is likely to be responsible for aberrant Wnt signaling in dog PCa. Coupled with E-cadherin, membranous downregulation and increased nuclear TGF-β expression were also observed in dog PCa [39,113,114,115], Additionally, Wnt signaling is likely to be a key event in the progression of PCa in this species; therefore, dog PCa may be a good model for phases of human PCa that are reliant on this aberrant pathway (i.e., CRPC and mCRPC).
4. Androgen-Indifferent PCa Variants (AIPC) of mCRPC
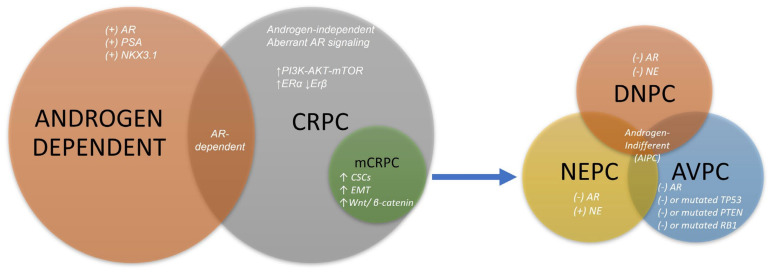
Androgen-indifferent prostate cancer (AIPC) is a form of PCa with treatment-resistant phenotypes that do not rely on AR signaling and typically arise from mCRPC following treatment with ASIs [121,122], although they may arise de novo in treatment-naïve PCa [123,124]. They are associated with an aggressive clinical course and poor treatment outcomes [14,124]. The AIPC variants of mCRPC include aggressive-variant prostate cancer (AVPC), neuroendocrine prostate cancer (NEPC), and double-negative prostate cancer (DNPC), each of which have their own distinct molecular signatures [14,125] (Figure 1). Multiple reports have shown that some dogs with PCa have molecular signatures similar to some AIPC variants. These are outlined below.
Figure 1.
Molecular characteristics of each PCa variant or subtype. Prostate cancer is initially reliant on androgens binding to the AR and promoting growth. Genes commonly regulated and expressed secondary to this signaling include NKX3.1 and PSA. Castration-resistant is also reliant on aberrant AR signaling, which does not require androgen ligands. It also accumulates additional driver mutations in other genes and pathways, including PI3K-AKT-mTOR. A subtype of CRPC, mCRPC, is also known to acquire the overexpression of Wnt/B-catenin signaling, as well as increased CSCs and evidence of EMT. Lastly, AIPC is a group of PCas that are androgen-indifferent and usually AR-indifferent as well, and often arise from mCRPC. Subtypes of AIPC have specific molecular signatures. The DNPC has loss of AR- and NE-marker expression; AVPC has a loss of AR expression and mutation or loss in TP53, PTEN, or RB1; and NEPC usually has loss of AR expression and gain in NE markers, such as chromogranin A or synaptophysin. Abbreviations: AR, androgen receptor; PSA, prostate-specific antigen; ER, estrogen receptor; CRPC, castration-resistant prostate cancer; CSC, cancer stem cell; EMT, epithelial–mesenchymal transition; NE, neuroendocrine; DNPC, double-negative prostate cancer; NEPC, neuroendocrine prostate cancer; AVPC, aggressive-variant prostate cancer.
4.1. DNPC
The DNPC is characterized by a loss of AR and neuroendocrine (NE) markers, and is sometimes referred to as CRPC subtype DNPC (CR-DNPC) [126]. The DNPC is frequently characterized by an increase in fibroblast growth factor (FGF) and mitogen-activated protein kinase (MAPK) signaling and has upregulated genes for EMT [122]. This has been shown to occur secondary to polycomb repressor complex 1 (PRC1) activity, a histone methylase upregulating CCL2 and promoting stemness and metastasis in DNPC [127].
Similarly, dog PCa is often AR- and NE-negative with increased FGF and MAPK signaling [40], and has demonstrated the upregulation of indirect markers of EMT [39,114,115]. Therefore, dog PCa may be considered molecularly similar to human DNPC. Whether DNPC in dog PCa is reliant on PRC1 and CCL2 signaling, as it is in human PCa, is yet to be explored.
4.2. NEPC
Neuroendocrine prostate cancer may occur de novo or as a consequence of selection pressures on CRPC tumors from ASIs, which is termed treatment-emergent NEPC (t-NEPC) or castration-resistant NEPC (CR-NEPC) [127,128]. These tumors often lack AR and AR-regulated genes, and have an increased expression of NE markers, including pro-neural transcription factors, such as BRN2, and immunohistochemical markers, such as chromogranin A (CHGA), synaptophysin (SYP), neuron-specific enolase 2 (ENO2), and neural cell adhesion molecule 1 (NCAM1, CD56) [128,129]. In contrast, dog PCa rarely expresses NE markers [130] and NEPC has not yet been reported. This may be partly because pharmaceutical androgen-signaling therapies are not clinically used to treat dogs with PCa, thus avoiding the selection pressures that may influence CSCs to differentiate into NE cells or harbor tumor microenvironments in which NE or NE-like clones proliferate. Moreover, there are conflicting reports on whether dogs contain NE cells in the prostate at all [131,132], likely rendering the dog an unrewarding model for de novo NEPC, as well as t-NEPC.
4.3. AVPC
The AVPC criteria were created to identify patients predicted to have an aggressive disease that is unlikely to respond to ASI therapy and may arise de novo or after pharmaceutical treatment. Any morphological variant of PCa (e.g., small-cell prostate cancer (SCPC), adenocarcinoma, adenocarcinoma with NE differentiation) may qualify as AVPC if it has a particular molecular signature and/or meets the criteria for an aggressive clinical course [132], including signs of visceral and lytic bone metastases, lymphadenopathy, and androgen-independent progression within 6 months of starting treatment [133]. The AVPC is characterized by the deleterious loss or mutation of at least two of the three molecular tumor suppressors, TP53, PTEN, and RB1, and is typically responsive to platinum drugs [125]. Moreover, a study on circulating tumor cells from patients with AVPC showed that these cells have losses not only in PTEN, RB1, and TP53, but also in BRCA2 [134].
Dog PCa is highly aggressive and clinically similar to AVPC, but whether it has the same molecular hallmarks as human AVPC is still unclear. One study showed the decreased expression of p53 in dog PCa tissues when compared to normal and inflammatory prostatic lesions, but not complete loss [42], while another study showed no significant increase or decrease in P53 [40]. The PTEN is either downregulated or completely lost according to a few studies on dog PCa, similar to AVPC in humans [42,73]. Interestingly, RB1 has not yet been studied in dog PCa. The loss of BRCA1 has been documented in dog PCa, but BRCA2 was not lost or downregulated according to another study utilizing RNA sequencing [40,135]. Because ASIs are not used in veterinary medicine, dog PCa may not completely match the molecular picture of human AVPC, but the molecular pictures is still incomplete, and further investigation is warranted.
5. Conclusions
Undeniably, there are molecular similarities between dog PCa and specific human PCa variants, although there are differences and areas that are understudied. Because the majority of dogs are neutered, present with advanced disease, and have AR null tumors, they are probably not feasible models for androgen-dependent PCa, with the exception of intact dogs in the early stages of the disease. Because of the lack of screening methods for PCa in dogs (i.e., PSA levels), establishing which intact dogs have early PCa would be challenging. Dog PCa is clinically and molecularly similar to CRPC and mCRPC, with upregulation in the PI3K-AKT pathway, Wnt signaling, EMT, CSCs, and indifference to AR, although ER expression appears to be different in this disease. Moreover, the importance of splice variants is yet to be determined in dog PCa. Lastly, regarding AIPC, it has been shown that dogs molecularly model DNPC, and may also model AVPC, but are unlikely to recapitulate NEPC. Altogether, dogs are likely to be suitable models for certain variants of advanced PCa, but not all, and additional studies are warranted to further characterize the molecular characteristics of dog PCa.
Author Contributions
Conceptualization, D.M.V. and C.A.L.; writing—original draft preparation, D.M.V.; writing—review and editing, C.A.L. and P.M.G.; supervision, P.M.G. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable. No new data were created in this study.
Conflicts of Interest
The authors declare no conflict of interest.
Disclaimer
The contents reported/presented within do not represent the views of the Department of Veterans Affairs or the United States Government.
Funding Statement
D.M.V. was supported by training fellowship NIH T32 OD011147. This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Sharma N.L., Massie C.E., Ramos-Montoya A., Zecchini V., Scott H.E., Lamb A.D., MacArthur S., Stark R., Warren A.Y., Mills I.G., et al. The Androgen Receptor Induces a Distinct Transcriptional Program in Castration-Resistant Prostate Cancer in Man. Cancer Cell. 2013;23:35–47. doi: 10.1016/j.ccr.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 2.Cai C., He H.H., Chen S., Coleman I., Wang H., Fang Z., Chen S., Nelson P.S., Liu X.S., Brown M., et al. Androgen Receptor Gene Expression in Prostate Cancer Is Directly Suppressed by the Androgen Receptor through Recruitment of Lysine-Specific Demethylase 1. Cancer Cell. 2011;20:457–471. doi: 10.1016/j.ccr.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dai C., Heemers H., Sharifi N. Androgen Signaling in Prostate Cancer. Cold Spring Harb. Perspect. Med. 2017;7:a030452. doi: 10.1101/cshperspect.a030452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Westaby D., Viscuse P.V., Ravilla R., de la Maza M.D.L.D.F., Hahn A., Sharp A., de Bono J., Aparicio A., Fleming M.T. Beyond the Androgen Receptor: The Sequence, the Mutants, and New Avengers in the Treatment of Castrate-Resistant Metastatic Prostate Cancer. Am. Soc. Clin. Oncol. Educ. Book. 2021;41:e190–e202. doi: 10.1200/EDBK_321209. [DOI] [PubMed] [Google Scholar]
- 5.Sharifi N., Gulley J.L., Dahut W.L. Androgen Deprivation Therapy for Prostate Cancer. JAMA. 2005;294:238–244. doi: 10.1001/jama.294.2.238. [DOI] [PubMed] [Google Scholar]
- 6.Huggins C., Clark P.J. Quantitative Studies Of Prostatic Secretion: II. The Effect of Castration and of Estrogen Injection on the Normal and on the Hyperplastic Prostate Glands of Dogs. J. Exp. Med. 1940;72:747–762. doi: 10.1084/jem.72.6.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramalingam S., Ramamurthy V.P., Njar V.C.O. Dissecting Major Signaling Pathways in Prostate Cancer Development and Progression: Mechanisms and Novel Therapeutic Targets. J. Steroid Biochem. Mol. Biol. 2017;166:16–27. doi: 10.1016/j.jsbmb.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heidenreich A., Bastian P.J., Bellmunt J., Bolla M., Joniau S., van der Kwast T., Mason M., Matveev V., Wiegel T., Zattoni F., et al. European Association of Urology. EAU Guidelines on Prostate Cancer. Part II: Treatment of Advanced, Relapsing, and Castration-Resistant Prostate Cancer. Eur. Urol. 2014;65:467–479. doi: 10.1016/j.eururo.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Katzenwadel A., Wolf P. Androgen Deprivation of Prostate Cancer: Leading to a Therapeutic Dead End. Cancer Lett. 2015;367:12–17. doi: 10.1016/j.canlet.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 10.Messner E.A., Steele T.M., Tsamouri M.M., Hejazi N., Gao A.C., Mudryj M., Ghosh P.M. The Androgen Receptor in Prostate Cancer: Effect of Structure, Ligands and Spliced Variants on Therapy. Biomedicines. 2020;8:422. doi: 10.3390/biomedicines8100422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharp A., Coleman I., Yuan W., Sprenger C., Dolling D., Rodrigues D.N., Russo J.W., Figueiredo I., Bertan C., Seed G., et al. Androgen Receptor Splice Variant-7 Expression Emerges with Castration Resistance in Prostate Cancer. J. Clin. Investig. 2019;129:192–208. doi: 10.1172/JCI122819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saad F., Bögemann M., Suzuki K., Shore N. Treatment of Nonmetastatic Castration-Resistant Prostate Cancer: Focus on Second-Generation Androgen Receptor Inhibitors. Prostate Cancer Prostatic Dis. 2021;24:323–334. doi: 10.1038/s41391-020-00310-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mateo J., Smith A., Ong M., de Bono J.S. Novel Drugs Targeting the Androgen Receptor Pathway in Prostate Cancer. Cancer Metastasis Rev. 2014;33:567–579. doi: 10.1007/s10555-013-9472-2. [DOI] [PubMed] [Google Scholar]
- 14.Berchuck J.E., Viscuse P.V., Beltran H., Aparicio A. Clinical Considerations for the Management of Androgen Indifferent Prostate Cancer. Prostate Cancer Prostatic Dis. 2021;24:623–637. doi: 10.1038/s41391-021-00332-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Handle F., Prekovic S., Helsen C., Van den Broeck T., Smeets E., Moris L., Eerlings R., Kharraz S.E., Urbanucci A., Mills I.G., et al. Drivers of AR Indifferent Anti-Androgen Resistance in Prostate Cancer Cells. Sci. Rep. 2019;9:13786. doi: 10.1038/s41598-019-50220-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma P., Schreiber-Agus N. Mouse Models of Prostate Cancer. Oncogene. 1999;18:5349–5355. doi: 10.1038/sj.onc.1203037. [DOI] [PubMed] [Google Scholar]
- 17.Nascimento-Goncalves E., Seixas F., da Costa R.M.G., Pires M.J., Neuparth M.J., Moreira-Goncalves D., Fardilha M., Faustino-Rocha A.I., Colaco B., Ferreira R., et al. Appraising Animal Models of Prostate Cancer for Translational Research: Future Directions. Anticancer Res. 2023;43:275–281. doi: 10.21873/anticanres.16160. [DOI] [PubMed] [Google Scholar]
- 18.Ryman-Tubb T., Lothion-Roy J.H., Metzler V.M., Harris A.E., Robinson B.D., Rizvanov A.A., Jeyapalan J.N., James V.H., England G., Rutland C.S., et al. Comparative Pathology of Dog and Human Prostate Cancer. Vet. Med. Sci. 2022;8:110–120. doi: 10.1002/vms3.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oliveira D.S.M., Dzinic S., Bonfil A.I., Saliganan A.D., Sheng S., Bonfil R.D. The Mouse Prostate: A Basic Anatomical and Histological Guideline. Bosn. J. Basic Med. Sci. 2016;16:8–13. doi: 10.17305/bjbms.2016.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jesik C.J., Holland J.M., Lee C. An Anatomic and Histologic Study of the Rat Prostate. Prostate. 1982;3:81–97. doi: 10.1002/pros.2990030111. [DOI] [PubMed] [Google Scholar]
- 21.Heber D. Prostate Enlargement: The Canary in the Coal Mine? Am. J. Clin. Nutr. 2002;75:605–606. doi: 10.1093/ajcn/75.4.605. [DOI] [PubMed] [Google Scholar]
- 22.Creasy D., Bube A., de Rijk E., Kandori H., Kuwahara M., Masson R., Nolte T., Reams R., Regan K., Rehm S., et al. Proliferative and Nonproliferative Lesions of the Rat and Mouse Male Reproductive System. Toxicol. Pathol. 2012;40((Suppl. S6)):40S–121S. doi: 10.1177/0192623312454337. [DOI] [PubMed] [Google Scholar]
- 23.Nolen R.S. When Should We Neuter Dogs? American Veterinary Medical Association. [(accessed on 15 November 2022)]. Available online: https://www.avma.org/javma-news/2021-03-01/when-should-we-neuter-dogs.
- 24.Hoffman J.M., Creevy K.E., Promislow D.E.L. Reproductive Capability Is Associated with Lifespan and Cause of Death in Companion Dogs. PLoS ONE. 2013;8:e61082. doi: 10.1371/journal.pone.0061082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bryan J.N., Keeler M.R., Henry C.J., Bryan M.E., Hahn A.W., Caldwell C.W. A Population Study of Neutering Status as a Risk Factor for Canine Prostate Cancer. Prostate. 2007;67:1174–1181. doi: 10.1002/pros.20590. [DOI] [PubMed] [Google Scholar]
- 26.Sorenmo K.U., Goldschmidt M., Shofer F., Goldkamp C., Ferracone J. Immunohistochemical Characterization of Canine Prostatic Carcinoma and Correlation with Castration Status and Castration Time. Vet. Comp. Oncol. 2003;1:48–56. doi: 10.1046/j.1476-5829.2003.00007.x. [DOI] [PubMed] [Google Scholar]
- 27.Bell F.W., Klausner J.S., Hayden D.W., Feeney D.A., Johnston S.D. Clinical and Pathologic Features of Prostatic Adenocarcinoma in Sexually Intact and Castrated Dogs: 31 Cases (1970–1987) J. Am. Vet. Med. Assoc. 1991;199:1623–1630. [PubMed] [Google Scholar]
- 28.Cornell K.K., Bostwick D.G., Cooley D.M., Hall G., Harvey H.J., Hendrick M.J., Pauli B.U., Render J.A., Stoica G., Sweet D.C., et al. Clinical and Pathologic Aspects of Spontaneous Canine Prostate Carcinoma: A Retrospective Analysis of 76 Cases. Prostate. 2000;45:173–183. doi: 10.1002/1097-0045(20001001)45:2<173::AID-PROS12>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 29.Lai C.-L., van den Ham R., van Leenders G., van der Lugt J., Mol J.A., Teske E. Histopathological and Immunohistochemical Characterization of Canine Prostate Cancer. Prostate. 2008;68:477–488. doi: 10.1002/pros.20720. [DOI] [PubMed] [Google Scholar]
- 30.Dehm S.M., Tindall D.J. Androgen Receptor Structural and Functional Elements: Role and Regulation in Prostate Cancer. Mol. Endocrinol. 2007;21:2855–2863. doi: 10.1210/me.2007-0223. [DOI] [PubMed] [Google Scholar]
- 31.Shah K., Gagliano T., Garland L., O’Hanlon T., Bortolotti D., Gentili V., Rizzo R., Giamas G., Dean M. Androgen Receptor Signaling Regulates the Transcriptome of Prostate Cancer Cells by Modulating Global Alternative Splicing. Oncogene. 2020;39:6172–6189. doi: 10.1038/s41388-020-01429-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharifi N., Auchus R.J. Steroid Biosynthesis and Prostate Cancer. Steroids. 2012;77:719–726. doi: 10.1016/j.steroids.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 33.Koivisto P., Kolmer M., Visakorpi T., Kallioniemi O.P. Androgen Receptor Gene and Hormonal Therapy Failure of Prostate Cancer. Am. J. Pathol. 1998;152:1–9. [PMC free article] [PubMed] [Google Scholar]
- 34.McEntee M., Isaacs W., Smith C. Adenocarcinoma of the Canine Prostate: Immunohistochemical Examination for Secretory Antigens. Prostate. 1987;11:163–170. doi: 10.1002/pros.2990110207. [DOI] [PubMed] [Google Scholar]
- 35.Crawford E.D., Heidenreich A., Lawrentschuk N., Tombal B., Pompeo A.C.L., Mendoza-Valdes A., Miller K., Debruyne F.M.J., Klotz L. Androgen-Targeted Therapy in Men with Prostate Cancer: Evolving Practice and Future Considerations. Prostate Cancer Prostatic Dis. 2019;22:24–38. doi: 10.1038/s41391-018-0079-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oefelein M.G., Feng A., Scolieri M.J., Ricchiutti D., Resnick M.I. Reassessment of the Definition of Castrate Levels of Testosterone: Implications for Clinical Decision Making. Urology. 2000;56:1021–1024. doi: 10.1016/S0090-4295(00)00793-7. [DOI] [PubMed] [Google Scholar]
- 37.Ehsani M., David F.O., Baniahmad A. Androgen Receptor-Dependent Mechanisms Mediating Drug Resistance in Prostate Cancer. Cancers. 2021;13:1534. doi: 10.3390/cancers13071534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schweizer M.T., Antonarakis E.S., Wang H., Ajiboye A.S., Spitz A., Cao H., Luo J., Haffner M.C., Yegnasubramanian S., Carducci M.A., et al. Effect of Bipolar Androgen Therapy for Asymptomatic Men with Castration-Resistant Prostate Cancer: Results from a Pilot Clinical Study. Sci. Transl. Med. 2015;7:269ra2. doi: 10.1126/scitranslmed.3010563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fonseca-Alves C.E., Rodrigues M.M.P., de Moura V.M.B.D., Rogatto S.R., Laufer-Amorim R. Alterations of C-MYC, NKX3.1, and E-Cadherin Expression in Canine Prostate Carcinogenesis. Microsc. Res. Tech. 2013;76:1250–1256. doi: 10.1002/jemt.22292. [DOI] [PubMed] [Google Scholar]
- 40.Thiemeyer H., Taher L., Schille J.T., Packeiser E.-M., Harder L.K., Hewicker-Trautwein M., Brenig B., Schütz E., Beck J., Nolte I., et al. An RNA-Seq-Based Framework for Characterizing Canine Prostate Cancer and Prioritizing Clinically Relevant Biomarker Candidate Genes. Int. J. Mol. Sci. 2021;22:11481. doi: 10.3390/ijms222111481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lai C.-L., van den Ham R., Mol J., Teske E. Immunostaining of the Androgen Receptor and Sequence Analysis of Its DNA-Binding Domain in Canine Prostate Cancer. Vet. J. 2009;181:256–260. doi: 10.1016/j.tvjl.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 42.Rivera-Calderón L.G., Fonseca-Alves C.E., Kobayashi P.E., Carvalho M., Drigo S.A., de Oliveira Vasconcelos R., Laufer-Amorim R. Alterations in PTEN, MDM2, TP53 and AR Protein and Gene Expression Are Associated with Canine Prostate Carcinogenesis. Res. Vet. Sci. 2016;106:56–61. doi: 10.1016/j.rvsc.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 43.Huggins C., Hodges C.V. Studies on Prostatic Cancer. I. The Effect of Castration, of Estrogen and Androgen Injection on Serum Phosphatases in Metastatic Carcinoma of the Prostate. CA Cancer J. Clin. 1972;22:232–240. doi: 10.3322/canjclin.22.4.232. [DOI] [PubMed] [Google Scholar]
- 44.Jamroze A., Chatta G., Tang D.G. Androgen Receptor (AR) Heterogeneity in Prostate Cancer and Therapy Resistance. Cancer Lett. 2021;518:1–9. doi: 10.1016/j.canlet.2021.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davey R.A., Grossmann M. Androgen Receptor Structure, Function and Biology: From Bench to Bedside. Clin. Biochem. Rev. 2016;37:3–15. [PMC free article] [PubMed] [Google Scholar]
- 46.Lu B., Smock S.L., Castleberry T.A., Owen T.A. Molecular Cloning and Functional Characterization of the Canine Androgen Receptor. Mol. Cell. Biochem. 2001;226:129–140. doi: 10.1023/A:1012752107129. [DOI] [PubMed] [Google Scholar]
- 47.Dos Santos M.L., Sarkis A.S., Nishimoto I.N., Nagai M.A. Androgen Receptor CAG Repeat Polymorphism in Prostate Cancer from a Brazilian Population. Cancer Detect. Prev. 2003;27:321–326. doi: 10.1016/S0361-090X(03)00106-5. [DOI] [PubMed] [Google Scholar]
- 48.Giovannucci E., Stampfer M.J., Krithivas K., Brown M., Dahl D., Brufsky A., Talcott J., Hennekens C.H., Kantoff P.W. The CAG Repeat within the Androgen Receptor Gene and Its Relationship to Prostate Cancer. Proc. Natl. Acad. Sci. USA. 1997;94:3320–3323. doi: 10.1073/pnas.94.7.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stanford J.L., Just J.J., Gibbs M., Wicklund K.G., Neal C.L., Blumenstein B.A., Ostrander E.A. Polymorphic Repeats in the Androgen Receptor Gene: Molecular Markers of Prostate Cancer Risk. Cancer Res. 1997;57:1194–1198. [PubMed] [Google Scholar]
- 50.Ochiai K., Sutijarit S., Uemura M., Morimatsu M., Michishita M., Onozawa E., Maeda M., Sasaki T., Watanabe M., Tanaka Y., et al. The Number of Glutamines in the N-Terminal of the Canine Androgen Receptor Affects Signalling Intensities. Vet. Comp. Oncol. 2021;19:399–403. doi: 10.1111/vco.12663. [DOI] [PubMed] [Google Scholar]
- 51.Teske E., Naan E.C., van Dijk E.M., Van Garderen E., Schalken J.A. Canine Prostate Carcinoma: Epidemiological Evidence of an Increased Risk in Castrated Dogs. Mol. Cell. Endocrinol. 2002;197:251–255. doi: 10.1016/S0303-7207(02)00261-7. [DOI] [PubMed] [Google Scholar]
- 52.L’Eplattenier H., Teske E., Van Sluijs F., Mol J.A. CAG-Repeats in the Androgen Receptor Gene Relate with Plasma Androgen Levels in the Bouvier Des Flandres. In Vivo. 2014;28:1051–1055. [PubMed] [Google Scholar]
- 53.Lai C.-L., L’Eplattenier H., van den Ham R., Verseijden F., Jagtenberg A., Mol J.A., Teske E. Androgen Receptor CAG Repeat Polymorphisms in Canine Prostate Cancer. J. Vet. Intern. Med. 2008;22:1380–1384. doi: 10.1111/j.1939-1676.2008.0181.x. [DOI] [PubMed] [Google Scholar]
- 54.Philp L.K., Butler M.S., Hickey T.E., Butler L.M., Tilley W.D., Day T.K. SGTA: A New Player in the Molecular Co-Chaperone Game. Horm. Cancer. 2013;4:343–357. doi: 10.1007/s12672-013-0151-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cano L.Q., Lavery D.N., Bevan C.L. Mini-Review: Foldosome Regulation of Androgen Receptor Action in Prostate Cancer. Mol. Cell. Endocrinol. 2013;369:52–62. doi: 10.1016/j.mce.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 56.Pratt W.B., Galigniana M.D., Harrell J.M., DeFranco D.B. Role of Hsp90 and the Hsp90-Binding Immunophilins in Signalling Protein Movement. Cell Signal. 2004;16:857–872. doi: 10.1016/j.cellsig.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 57.Trotta A.P., Need E.F., Selth L.A., Chopra S., Pinnock C.B., Leach D.A., Coetzee G.A., Butler L.M., Tilley W.D., Buchanan G. Knockdown of the Cochaperone SGTA Results in the Suppression of Androgen and PI3K/Akt Signaling and Inhibition of Prostate Cancer Cell Proliferation. Int. J. Cancer. 2013;133:2812–2823. doi: 10.1002/ijc.28310. [DOI] [PubMed] [Google Scholar]
- 58.Buchanan G., Ricciardelli C., Harris J.M., Prescott J., Yu Z.C.-L., Jia L., Butler L.M., Marshall V.R., Scher H.I., Gerald W.L., et al. Control of Androgen Receptor Signaling in Prostate Cancer by the Cochaperone Small Glutamine Rich Tetratricopeptide Repeat Containing Protein Alpha. Cancer Res. 2007;67:10087–10096. doi: 10.1158/0008-5472.CAN-07-1646. [DOI] [PubMed] [Google Scholar]
- 59.Azakami D., Nakahira R., Kato Y., Michishita M., Kobayashi M., Onozawa E., Bonkobara M., Kobayashi M., Takahashi K., Watanabe M., et al. The Canine Prostate Cancer Cell Line CHP-1 Shows over-Expression of the Co-Chaperone Small Glutamine-Rich Tetratricopeptide Repeat-Containing Protein α. Vet. Comp. Oncol. 2017;15:557–562. doi: 10.1111/vco.12199. [DOI] [PubMed] [Google Scholar]
- 60.Kato Y., Ochiai K., Michishita M., Azakami D., Nakahira R., Morimatsu M., Ishiguro-Oonuma T., Yoshikawa Y., Kobayashi M., Bonkobara M., et al. Molecular Cloning of Canine Co-Chaperone Small Glutamine-Rich Tetratricopeptide Repeat-Containing Protein α (SGTA) and Investigation of Its Ability to Suppress Androgen Receptor Signalling in Androgen-Independent Prostate Cancer. Vet. J. 2015;206:143–148. doi: 10.1016/j.tvjl.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 61.Kato Y., Ochiai K., Kawakami S., Nakao N., Azakami D., Bonkobara M., Michishita M., Morimatsu M., Watanabe M., Omi T. Canine REIC/Dkk-3 Interacts with SGTA and Restores Androgen Receptor Signalling in Androgen-Independent Prostate Cancer Cell Lines. BMC Vet. Res. 2017;13:170. doi: 10.1186/s12917-017-1094-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rybak A.P., Bristow R.G., Kapoor A. Prostate Cancer Stem Cells: Deciphering the Origins and Pathways Involved in Prostate Tumorigenesis and Aggression. Oncotarget. 2015;6:1900–1919. doi: 10.18632/oncotarget.2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Karantanos T., Corn P.G., Thompson T.C. Prostate Cancer Progression after Androgen Deprivation Therapy: Mechanisms of Castrate Resistance and Novel Therapeutic Approaches. Oncogene. 2013;32:5501–5511. doi: 10.1038/onc.2013.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Edlind M.P., Hsieh A.C. PI3K-AKT-MTOR Signaling in Prostate Cancer Progression and Androgen Deprivation Therapy Resistance. Asian J. Androl. 2014;16:378–386. doi: 10.4103/1008-682X.122876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.D’Abronzo L.S., Ghosh P.M. EIF4E Phosphorylation in Prostate Cancer. Neoplasia. 2018;20:563–573. doi: 10.1016/j.neo.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Laplante M., Sabatini D.M. MTOR Signaling in Growth Control and Disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oh W.J., Jacinto E. MTOR Complex 2 Signaling and Functions. Cell Cycle. 2011;10:2305–2316. doi: 10.4161/cc.10.14.16586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Faivre S., Kroemer G., Raymond E. Current Development of MTOR Inhibitors as Anticancer Agents. Nat. Rev. Drug Discov. 2006;5:671–688. doi: 10.1038/nrd2062. [DOI] [PubMed] [Google Scholar]
- 69.Pópulo H., Lopes J.M., Soares P. The MTOR Signalling Pathway in Human Cancer. Int. J. Mol. Sci. 2012;13:1886–1918. doi: 10.3390/ijms13021886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shorning B.Y., Dass M.S., Smalley M.J., Pearson H.B. The PI3K-AKT-MTOR Pathway and Prostate Cancer: At the Crossroads of AR, MAPK, and WNT Signaling. Int. J. Mol. Sci. 2020;21:4507. doi: 10.3390/ijms21124507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kremer C.L., Klein R.R., Mendelson J., Browne W., Samadzedeh L.K., Vanpatten K., Highstrom L., Pestano G.A., Nagle R.B. Expression of MTOR Signaling Pathway Markers in Prostate Cancer Progression. Prostate. 2006;66:1203–1212. doi: 10.1002/pros.20410. [DOI] [PubMed] [Google Scholar]
- 72.Rivera-Calderón L.G., Fonseca-Alves C.E., Kobayashi P.E., Carvalho M., Vasconcelos R.O., Laufer-Amorim R. P-MTOR, p-4EBP-1 and EIF4E Expression in Canine Prostatic Carcinoma. Res. Vet. Sci. 2019;122:86–92. doi: 10.1016/j.rvsc.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 73.Kobayashi P.E., Rodrigues M.M.P., Gartner F., Rema A., Fonseca-Alves C.E., Laufer-Amorim R. Association between Decreased Expression of Estrogen Receptor Alpha, Androgen Receptor and Phosphatase and Tensin Homolog Immunoexpression in the Canine Prostate. Pesqui. Vet. Bras. 2019;39:40–46. doi: 10.1590/1678-5150-pvb-5699. [DOI] [Google Scholar]
- 74.Saad F., Shore N., Zhang T., Sharma S., Cho H.K., Jacobs I.A. Emerging Therapeutic Targets for Patients with Advanced Prostate Cancer. Cancer Treat. Rev. 2019;76:1–9. doi: 10.1016/j.ctrv.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 75.Massard C., Chi K.N., Castellano D., de Bono J., Gravis G., Dirix L., Machiels J.-P., Mita A., Mellado B., Turri S., et al. Phase Ib Dose-Finding Study of Abiraterone Acetate plus Buparlisib (BKM120) or Dactolisib (BEZ235) in Patients with Castration-Resistant Prostate Cancer. Eur. J. Cancer. 2017;76:36–44. doi: 10.1016/j.ejca.2017.01.024. [DOI] [PubMed] [Google Scholar]
- 76.Armstrong A.J., Halabi S., Healy P., Alumkal J.J., Winters C., Kephart J., Bitting R.L., Hobbs C., Soleau C.F., Beer T.M., et al. Phase II Trial of the PI3 Kinase Inhibitor Buparlisib (BKM-120) with or without Enzalutamide in Men with Metastatic Castration Resistant Prostate Cancer. Eur. J. Cancer. 2017;81:228–236. doi: 10.1016/j.ejca.2017.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Leach D.A., Powell S.M., Bevan C.L. Women in Cancer Thematic Review: New Roles for Nuclear Receptors in Prostate Cancer. Endocr. Relat. Cancer. 2016;23:T85–T108. doi: 10.1530/ERC-16-0319. [DOI] [PubMed] [Google Scholar]
- 78.Bosland M.C., Mahmoud A.M. Hormones and Prostate Carcinogenesis: Androgens and Estrogens. J. Carcinog. 2011;10:33. doi: 10.4103/1477-3163.90678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Salonia A., Gallina A., Briganti A., Suardi N., Capitanio U., Abdollah F., Bertini R., Freschi M., Rigatti P., Montorsi F. Circulating Estradiol, but Not Testosterone, Is a Significant Predictor of High-Grade Prostate Cancer in Patients Undergoing Radical Prostatectomy. Cancer. 2011;117:5029–5038. doi: 10.1002/cncr.26136. [DOI] [PubMed] [Google Scholar]
- 80.Liang Z., Cao J., Tian L., Shen Y., Yang X., Lin Q., Zhang R., Liu H., Du X., Shi J., et al. Aromatase-Induced Endogenous Estrogen Promotes Tumour Metastasis through Estrogen Receptor-α/Matrix Metalloproteinase 12 Axis Activation in Castration-Resistant Prostate Cancer. Cancer Lett. 2019;467:72–84. doi: 10.1016/j.canlet.2019.09.001. [DOI] [PubMed] [Google Scholar]
- 81.Chakravarty D., Sboner A., Nair S.S., Giannopoulou E., Li R., Hennig S., Mosquera J.M., Pauwels J., Park K., Kossai M., et al. The Oestrogen Receptor Alpha-Regulated LncRNA NEAT1 Is a Critical Modulator of Prostate Cancer. Nat. Commun. 2014;5:5383. doi: 10.1038/ncomms6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Takizawa I., Lawrence M.G., Balanathan P., Rebello R., Pearson H.B., Garg E., Pedersen J., Pouliot N., Nadon R., Watt M.J., et al. Estrogen Receptor Alpha Drives Proliferation in PTEN-Deficient Prostate Carcinoma by Stimulating Survival Signaling, MYC Expression and Altering Glucose Sensitivity. Oncotarget. 2014;6:604–616. doi: 10.18632/oncotarget.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bonkhoff H. Estrogen Receptor Signaling in Prostate Cancer: Implications for Carcinogenesis and Tumor Progression. Prostate. 2018;78:2–10. doi: 10.1002/pros.23446. [DOI] [PubMed] [Google Scholar]
- 84.Warner M., Huang B., Gustafsson J.-A. Estrogen Receptor β as a Pharmaceutical Target. Trends Pharmacol. Sci. 2017;38:92–99. doi: 10.1016/j.tips.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 85.Fixemer T., Remberger K., Bonkhoff H. Differential Expression of the Estrogen Receptor Beta (ERbeta) in Human Prostate Tissue, Premalignant Changes, and in Primary, Metastatic, and Recurrent Prostatic Adenocarcinoma. Prostate. 2003;54:79–87. doi: 10.1002/pros.10171. [DOI] [PubMed] [Google Scholar]
- 86.Bonkhoff H., Fixemer T., Hunsicker I., Remberger K. Estrogen Receptor Expression in Prostate Cancer and Premalignant Prostatic Lesions. Am. J. Pathol. 1999;155:641–647. doi: 10.1016/S0002-9440(10)65160-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bonkhoff H., Berges R. The Evolving Role of Oestrogens and Their Receptors in the Development and Progression of Prostate Cancer. Eur. Urol. 2009;55:533–542. doi: 10.1016/j.eururo.2008.10.035. [DOI] [PubMed] [Google Scholar]
- 88.Grindstad T., Skjefstad K., Andersen S., Ness N., Nordby Y., Al-Saad S., Fismen S., Donnem T., Khanehkenari M.R., Busund L.-T., et al. Estrogen Receptors α and β and Aromatase as Independent Predictors for Prostate Cancer Outcome. Sci. Rep. 2016;6:33114. doi: 10.1038/srep33114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Qin J., Liu X., Laffin B., Chen X., Choy G., Jeter C.R., Calhoun-Davis T., Li H., Palapattu G.S., Pang S., et al. The PSA(-/Lo) Prostate Cancer Cell Population Harbors Self-Renewing Long-Term Tumor-Propagating Cells That Resist Castration. Cell Stem Cell. 2012;10:556–569. doi: 10.1016/j.stem.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Beck B., Blanpain C. Unravelling Cancer Stem Cell Potential. Nat. Rev. Cancer. 2013;13:727–738. doi: 10.1038/nrc3597. [DOI] [PubMed] [Google Scholar]
- 91.Aguilar-Medina M., Avendaño-Félix M., Lizárraga-Verdugo E., Bermúdez M., Romero-Quintana J.G., Ramos-Payan R., Ruíz-García E., López-Camarillo C. SOX9 Stem-Cell Factor: Clinical and Functional Relevance in Cancer. J. Oncol. 2019;2019:6754040. doi: 10.1155/2019/6754040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Moad M., Hannezo E., Buczacki S.J., Wilson L., El-Sherif A., Sims D., Pickard R., Wright N.A., Williamson S.C., Turnbull D.M., et al. Multipotent Basal Stem Cells, Maintained in Localized Proximal Niches, Support Directed Long-Ranging Epithelial Flows in Human Prostates. Cell Rep. 2017;20:1609–1622. doi: 10.1016/j.celrep.2017.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cunha G.R., Lung B. The Possible Influence of Temporal Factors in Androgenic Responsiveness of Urogenital Tissue Recombinants from Wild-Type and Androgen-Insensitive (Tfm) Mice. J. Exp. Zool. 1978;205:181–193. doi: 10.1002/jez.1402050203. [DOI] [PubMed] [Google Scholar]
- 94.Bae K.-M., Su Z., Frye C., McClellan S., Allan R.W., Andrejewski J.T., Kelley V., Jorgensen M., Steindler D.A., Vieweg J., et al. Expression of Pluripotent Stem Cell Reprogramming Factors by Prostate Tumor Initiating Cells. J. Urol. 2010;183:2045–2053. doi: 10.1016/j.juro.2009.12.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Klarmann G.J., Hurt E.M., Mathews L.A., Zhang X., Duhagon M.A., Mistree T., Thomas S.B., Farrar W.L. Invasive Prostate Cancer Cells Are Tumor Initiating Cells That Have a Stem Cell-like Genomic Signature. Clin. Exp. Metastasis. 2009;26:433–446. doi: 10.1007/s10585-009-9242-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Collins A.T., Berry P.A., Hyde C., Stower M.J., Maitland N.J. Prospective Identification of Tumorigenic Prostate Cancer Stem Cells. Cancer Res. 2005;65:10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 97.Guzel E., Karatas O.F., Duz M.B., Solak M., Ittmann M., Ozen M. Differential Expression of Stem Cell Markers and ABCG2 in Recurrent Prostate Cancer. Prostate. 2014;74:1498–1505. doi: 10.1002/pros.22867. [DOI] [PubMed] [Google Scholar]
- 98.Patrawala L., Calhoun T., Schneider-Broussard R., Li H., Bhatia B., Tang S., Reilly J.G., Chandra D., Zhou J., Claypool K., et al. Highly Purified CD44+ Prostate Cancer Cells from Xenograft Human Tumors Are Enriched in Tumorigenic and Metastatic Progenitor Cells. Oncogene. 2006;25:1696–1708. doi: 10.1038/sj.onc.1209327. [DOI] [PubMed] [Google Scholar]
- 99.Ni J., Cheung B.B., Beretov J., Duan W., Bucci J., Malouf D., Graham P., Li Y. CD44 Variant 6 Is Associated with Prostate Cancer Growth and Chemo-/Radiotherapy Response In Vivo. Exp. Cell Res. 2020;388:111850. doi: 10.1016/j.yexcr.2020.111850. [DOI] [PubMed] [Google Scholar]
- 100.Moulay M., Liu W., Willenbrock S., Sterenczak K.A., Carlson R., Ngezahayo A., Murua Escobar H., Nolte I. Evaluation of Stem Cell Marker Gene Expression in Canine Prostate Carcinoma- and Prostate Cyst-Derived Cell Lines. Anticancer Res. 2013;33:5421–5431. [PubMed] [Google Scholar]
- 101.Costa C.D., Justo A.A., Kobayashi P.E., Story M.M., Palmieri C., Laufer Amorim R., Fonseca-Alves C.E. Characterization of OCT3/4, Nestin, NANOG, CD44 and CD24 as Stem Cell Markers in Canine Prostate Cancer. Int. J. Biochem. Cell Biol. 2019;108:21–28. doi: 10.1016/j.biocel.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 102.Warrier N.M., Agarwal P., Kumar P. Emerging Importance of Survivin in Stem Cells and Cancer: The Development of New Cancer Therapeutics. Stem Cell Rev. Rep. 2020;16:828–852. doi: 10.1007/s12015-020-09995-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yie S.-M., Luo B., Ye N.-Y., Xie K., Ye S.-R. Detection of Survivin-Expressing Circulating Cancer Cells in the Peripheral Blood of Breast Cancer Patients by a RT-PCR ELISA. Clin. Exp. Metastasis. 2006;23:279–289. doi: 10.1007/s10585-006-9037-7. [DOI] [PubMed] [Google Scholar]
- 104.Cao M., Yie S.-M., Wu S.-M., Chen S., Lou B., He X., Ye S.-R., Xie K., Rao L., Gao E., et al. Detection of Survivin-Expressing Circulating Cancer Cells in the Peripheral Blood of Patients with Esophageal Squamous Cell Carcinoma and Its Clinical Significance. Clin. Exp. Metastasis. 2009;26:751–758. doi: 10.1007/s10585-009-9274-7. [DOI] [PubMed] [Google Scholar]
- 105.Liao C.-P., Adisetiyo H., Liang M., Roy-Burman P. Cancer Stem Cells and Microenvironment in Prostate Cancer Progression. Horm. Cancer. 2010;1:297–305. doi: 10.1007/s12672-010-0051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yuan X., Cai C., Chen S., Chen S., Yu Z., Balk S.P. Androgen Receptor Functions in Castration-Resistant Prostate Cancer and Mechanisms of Resistance to New Agents Targeting the Androgen Axis. Oncogene. 2014;33:2815–2825. doi: 10.1038/onc.2013.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bongiovanni L., Caposano F., Romanucci M., Grieco V., Malatesta D., Brachelente C., Massimini M., Benazzi C., Thomas R.E., Salda L.D. Survivin and Sox9: Potential Stem Cell Markers in Canine Normal, Hyperplastic, and Neoplastic Canine Prostate. Vet. Pathol. 2019;56:200–207. doi: 10.1177/0300985818794161. [DOI] [PubMed] [Google Scholar]
- 108.Micalizzi D.S., Farabaugh S.M., Ford H.L. Epithelial-Mesenchymal Transition in Cancer: Parallels between Normal Development and Tumor Progression. J. Mammary Gland. Biol. Neoplasia. 2010;15:117–134. doi: 10.1007/s10911-010-9178-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Grant C.M., Kyprianou N. Epithelial Mesenchymal Transition (EMT) in Prostate Growth and Tumor Progression. Transl. Androl. Urol. 2013;2:202–211. doi: 10.3978/j.issn.2223-4683.2013.09.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Haider M., Zhang X., Coleman I., Ericson N., True L.D., Lam H.-M., Brown L.G., Ketchanji M., Nghiem B., Lakely B., et al. Epithelial Mesenchymal-like Transition Occurs in a Subset of Cells in Castration Resistant Prostate Cancer Bone Metastases. Clin. Exp. Metastasis. 2016;33:239–248. doi: 10.1007/s10585-015-9773-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kim K., Lu Z., Hay E.D. Direct Evidence for a Role of Beta-Catenin/LEF-1 Signaling Pathway in Induction of EMT. Cell Biol. Int. 2002;26:463–476. doi: 10.1006/cbir.2002.0901. [DOI] [PubMed] [Google Scholar]
- 112.Zavadil J., Böttinger E.P. TGF-Beta and Epithelial-to-Mesenchymal Transitions. Oncogene. 2005;24:5764–5774. doi: 10.1038/sj.onc.1208927. [DOI] [PubMed] [Google Scholar]
- 113.Rodrigues M.M.P., Santis G.W.D., Moura V.M.B.D.D., Amorim R.L. COX-2 and TGF-β Expression in Proliferative Disorders of Canine Prostate. Braz. J. Vet. Pathol. 2010;3:31–36. [Google Scholar]
- 114.Rodrigues M.M.P., Rema A., Gartner M.F., Laufer-Amorim R. Role of Adhesion Molecules and Proliferation Hyperplasic, Pre Neoplastic and Neoplastic Lesions in Canine Prostate. Pak. J. Biol. Sci. 2013;16:1324–1329. doi: 10.3923/pjbs.2013.1324.1329. [DOI] [PubMed] [Google Scholar]
- 115.Fonseca-Alves C.E., Kobayashi P.E., Rivera-Calderón L.G., Laufer-Amorim R. Evidence of Epithelial-Mesenchymal Transition in Canine Prostate Cancer Metastasis. Res. Vet. Sci. 2015;100:176–181. doi: 10.1016/j.rvsc.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 116.Murillo-Garzón V., Kypta R. WNT Signalling in Prostate Cancer. Nat. Rev. Urol. 2017;14:683–696. doi: 10.1038/nrurol.2017.144. [DOI] [PubMed] [Google Scholar]
- 117.Patel S., Alam A., Pant R., Chattopadhyay S. Wnt Signaling and Its Significance Within the Tumor Microenvironment: Novel Therapeutic Insights. Front. Immunol. 2019;10:2872. doi: 10.3389/fimmu.2019.02872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kypta R.M., Waxman J. Wnt/β-Catenin Signalling in Prostate Cancer. Nat. Rev. Urol. 2012;9:418–428. doi: 10.1038/nrurol.2012.116. [DOI] [PubMed] [Google Scholar]
- 119.Luo J., Wang D., Wan X., Xu Y., Lu Y., Kong Z., Li D., Gu W., Wang C., Li Y., et al. Crosstalk between AR and Wnt Signaling Promotes Castration-Resistant Prostate Cancer Growth. OncoTargets Ther. 2020;13:9257–9267. doi: 10.2147/OTT.S245861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kobayashi P.E., Fonseca-Alves C.E., Rivera-Calderón L.G., Carvalho M., Kuasne H., Rogatto S.R., Laufer-Amorim R. Deregulation of E-Cadherin, β-Catenin, APC and Caveolin-1 Expression Occurs in Canine Prostate Cancer and Metastatic Processes. Res. Vet. Sci. 2018;118:254–261. doi: 10.1016/j.rvsc.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 121.Aggarwal R., Huang J., Alumkal J.J., Zhang L., Feng F.Y., Thomas G.V., Weinstein A.S., Friedl V., Zhang C., Witte O.N., et al. Clinical and Genomic Characterization of Treatment-Emergent Small-Cell Neuroendocrine Prostate Cancer: A Multi-Institutional Prospective Study. J. Clin. Oncol. 2018;36:2492–2503. doi: 10.1200/JCO.2017.77.6880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bluemn E.G., Coleman I.M., Lucas J.M., Coleman R.T., Hernandez-Lopez S., Tharakan R., Bianchi-Frias D., Dumpit R.F., Kaipainen A., Corella A.N., et al. Androgen Receptor Pathway-Independent Prostate Cancer Is Sustained through FGF Signaling. Cancer Cell. 2017;32:474–489.e6. doi: 10.1016/j.ccell.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Beltran H., Rickman D.S., Park K., Chae S.S., Sboner A., MacDonald T.Y., Wang Y., Sheikh K.L., Terry S., Tagawa S.T., et al. Molecular Characterization of Neuroendocrine Prostate Cancer and Identification of New Drug Targets. Cancer Discov. 2011;1:487–495. doi: 10.1158/2159-8290.CD-11-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hamid A.A., Gray K.P., Shaw G., MacConaill L.E., Evan C., Bernard B., Loda M., Corcoran N.M., Van Allen E.M., Choudhury A.D., et al. Compound Genomic Alterations of TP53, PTEN, and RB1 Tumor Suppressors in Localized and Metastatic Prostate Cancer. Eur. Urol. 2019;76:89–97. doi: 10.1016/j.eururo.2018.11.045. [DOI] [PubMed] [Google Scholar]
- 125.Aparicio A.M., Shen L., Tapia E.L.N., Lu J.-F., Chen H.-C., Zhang J., Wu G., Wang X., Troncoso P., Corn P., et al. Combined Tumor Suppressor Defects Characterize Clinically Defined Aggressive Variant Prostate Cancers. Clin. Cancer Res. 2016;22:1520–1530. doi: 10.1158/1078-0432.CCR-15-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Labrecque M.P., Alumkal J.J., Coleman I.M., Nelson P.S., Morrissey C. The Heterogeneity of Prostate Cancers Lacking AR Activity Will Require Diverse Treatment Approaches. Endocr. Relat. Cancer. 2021;28:T51–T66. doi: 10.1530/ERC-21-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shen M.M. A Positive Step toward Understanding Double-Negative Metastatic Prostate Cancer. Cancer Cell. 2019;36:117–119. doi: 10.1016/j.ccell.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 128.Bishop J.L., Thaper D., Vahid S., Davies A., Ketola K., Kuruma H., Jama R., Nip K.M., Angeles A., Johnson F., et al. The Master Neural Transcription Factor BRN2 Is an Androgen Receptor-Suppressed Driver of Neuroendocrine Differentiation in Prostate Cancer. Cancer Discov. 2017;7:54–71. doi: 10.1158/2159-8290.CD-15-1263. [DOI] [PubMed] [Google Scholar]
- 129.Merkens L., Sailer V., Lessel D., Janzen E., Greimeier S., Kirfel J., Perner S., Pantel K., Werner S., von Amsberg G. Aggressive Variants of Prostate Cancer: Underlying Mechanisms of Neuroendocrine Transdifferentiation. J. Exp. Clin. Cancer Res. 2022;41:46. doi: 10.1186/s13046-022-02255-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.De Brot S., Lothion-Roy J., Grau-Roma L., White E., Guscetti F., Rubin M.A., Mongan N.P. Histological and Immunohistochemical Investigation of Canine Prostate Carcinoma with Identification of Common Intraductal Carcinoma Component. Vet. Comp. Oncol. 2022;20:38–49. doi: 10.1111/vco.12704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ismail A H.R., Landry F., Aprikian A.G., Chevalier S. Androgen Ablation Promotes Neuroendocrine Cell Differentiation in Dog and Human Prostate. Prostate. 2002;51:117–125. doi: 10.1002/pros.10066. [DOI] [PubMed] [Google Scholar]
- 132.Angelsen A., Mecsei R., Sandvik A.K., Waldum H.L. Neuroendocrine Cells in the Prostate of the Rat, Guinea Pig, Cat, and Dog. Prostate. 1997;33:18–25. doi: 10.1002/(SICI)1097-0045(19970915)33:1<18::AID-PROS4>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 133.Aparicio A.M., Harzstark A.L., Corn P.G., Wen S., Araujo J.C., Tu S.-M., Pagliaro L.C., Kim J., Millikan R.E., Ryan C., et al. Platinum-Based Chemotherapy for Variant Castrate-Resistant Prostate Cancer. Clin. Cancer Res. 2013;19:3621–3630. doi: 10.1158/1078-0432.CCR-12-3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Malihi P.D., Graf R.P., Rodriguez A., Ramesh N., Lee J., Sutton R., Jiles R., Ruiz Velasco C., Sei E., Kolatkar A., et al. Single-Cell Circulating Tumor Cell Analysis Reveals Genomic Instability as a Distinctive Feature of Aggressive Prostate Cancer. Clin. Cancer Res. 2020;26:4143–4153. doi: 10.1158/1078-0432.CCR-19-4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Laufer-Amorim R., Fonseca-Alves C.E., Villacis R.A.R., Linde S.A.D., Carvalho M., Larsen S.J., Marchi F.A., Rogatto S.R. Comprehensive Genomic Profiling of Androgen-Receptor-Negative Canine Prostate Cancer. Int. J. Mol. Sci. 2019;20:1555. doi: 10.3390/ijms20071555. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable. No new data were created in this study.