Abstract
Over recent years, preclinical and clinical evidence has implicated myocardial inflammation (M-Infl) in the pathophysiology and phenotypes of traditionally genetic cardiomyopathies. M-Infl resembling myocarditis on imaging and histology occurs frequently as a clinical manifestation of classically genetic cardiac diseases, including dilated and arrhythmogenic cardiomyopathy. The emerging role of M-Infl in disease pathophysiology is leading to the identification of druggable targets for molecular treatment of the inflammatory process and a new paradigm in the field of cardiomyopathies. Cardiomyopathies constitute a leading cause of heart failure and arrhythmic sudden death in the young population. The aim of this review is to present, from bedside to bench, the current state of the art about the genetic basis of M-Infl in nonischemic cardiomyopathies of the dilated and arrhythmogenic spectrum in order to prompt future research towards the identification of novel mechanisms and treatment targets, with the ultimate goal of lowering disease morbidity and mortality.
Keywords: myocardial inflammation, cardiomyopathies, genetics, preclinical models, bench, dilated, arrhythmogenic, ventricular arrhythmias, desmosomes, sudden cardiac death, endomyocardial biopsy, cardiac magnetic resonance
1. Myocarditis and Primary Cardiomyopathies
1.1. Introduction
The World Health Organization defines myocarditis as an inflammatory disease of the myocardium diagnosed by established histological, immunological, and immunohistochemical criteria [1]. The main recognized etiologies of myocarditis include viral infections, toxic agents, and autoimmune mechanisms [2]. Although a genetic background has been hypothesized either as a predisposing [3] or accelerating factor [4], myocarditis is currently classified as a nongenetic disease [2]. Over the last decade, however, a growing body of evidence has attempted to identify a strict connection between genetics and myocarditis. In particular, pathogenic variants in cardiomyopathic genes have been identified in probands with familial myocarditis. On the other hand, bursts of active myocardial inflammation (M-Infl) [5] overlapping with classic myocarditis have been reported in patients with known genetic cardiomyopathies. Since the development of a manifest phenotype of each genetic disease depends on the interaction between genetics and environment, myocardial inflammation may represent the “cellular environment” that plays a primary role in the evolution of cardiomyopathies. The aim of this narrative review is to describe the state of the art regarding the genetic basis of myocarditis. In particular, the connection points between myocarditis and cardiomyopathies of the dilated and arrhythmogenic spectrum will be presented in both the clinical and preclinical settings. A brief overview of the current definitions of myocarditis and cardiomyopathies is presented in Table 1.
Table 1.
Inflammatory cardiomyopathies: key concepts and definitions.
| Term | Definition | References |
|---|---|---|
| Myocarditis | Inflammatory disease of the myocardium diagnosed by established Histological, immunological, and immunohistochemical criteria (WHO, ESC). In detail: -Histology: infiltrating inflammatory mononucleated cells with myocyte myocyte degeneration and necrosis of nonischemic origin (Dallas criteria). It is defined borderline myocarditis in the absence of necrosis. It is defined chronic myocarditis in the presence of replacement-type fibrosis. -Immunohistochemistry: ≥14 leucocytes/mm2 including up to 4 monocytes/mm2 with the presence of CD 3 positive T-lymphocytes ≥7 cells/mm2. Clinical classification based on symptom onset: -Acute (<1 month) -Subacute (1–3 months) -Chronic (>3 months) |
[6,7,8] |
| Cardiomyopathy | Myocardial disorders in which the heart muscle is structurally and functionally abnormal, in the absence of coronary artery disease, hypertension, valvular disease and congenital heart disease sufficient to cause the observed myocardial abnormality (ESC). It may also include electrical diseases prone to life-threatening arrhythmias (AHA). |
[9,10,11] |
| Dilated cardiomyopathy (DCM) | Dilation and impaired contraction of the left or both ventricles that is not explained by abnormal loading conditions or coronary artery disease. Phenotype classification:-Hypokinetic nondilated cardiomyopathy (LV systolic dysfunction with no dilation). -Overt DCM (LV dilation and systolic dysfunction). |
[6,12] |
| Arrhythmogenic cardiomyopathy (ACM) | Arrhythmogenic heart muscle disorder not explained by ischemic, hypertensive, or valvular heart disease. Phenotype classification: -Classic right ventricular ACM (modified Task Force Criteria). -Biventricular ACM. -Left-dominant ACM (Padua criteria). |
[13,14] |
| Inflammatory cardiomyopathy | Myocarditis in association with cardiac dysfunction (i.e., LV ejection fraction <50%) | [6,7] |
| Myocardial inflammation (M-Infl) | Evidence of myocardial inflammation fulfilling the histological (±myocyte necrosis, i.e., borderline myocarditis) and immunohistochemical criteria for myocarditis, in a patient with clinical diagnosis of ACM or DCM. | [3,5] |
Common definitions in the field of myocarditis and cardiomyopathies are shown. ACM = arrhythmogenic cardiomyopathy; AHA = American Heart Association; CD = cluster of differentiation; DCM = dilated cardiomyopathy; ESC = European Society of Cardiology; LV = left ventricular; M-Infl = myocardial inflammation; WHO = World Health Organization.
1.2. Diagnosis of Myocarditis
Based on the aforementioned definition and updated evidence [6,7], histology is the gold standard of diagnostics for myocarditis. In particular, the European Society of Cardiology (ESC) proposed specific histological and immunohistochemical criteria [6,8], reported in Table 1. In this setting, borderline myocarditis, defined as the absence of myocyte necrosis [6], is frequently indistinguishable from cardiomyopathy-associated M-Infl [5] (Table 2). Histology information can be obtained by means of endomyocardial biopsy (EMB), provided that tissue sampling is adequate and informative [6,15]. As a diagnostic tool complementary to EMB, cardiac magnetic resonance (CMR) allows noninvasive, multiplanar, and panoramic investigation of myocarditis [2]. In particular, the key pathophysiological components of myocarditis, namely hyperemia, edema, and necrosis, are mirrored by distinct CMR sequences. As defined by the updated Lake Louise criteria [16], while T2-weighted sequences point to M-Infl, late gadolinium enhancement (LGE) is observed both during the acute and the post-inflammatory stages of myocarditis [4]. In the chronic setting, LGE on CMR, as well as low-voltage areas on electro-anatomical mapping, are considered as rough equivalents of myocardial scar [5,9]. Among the other imaging techniques, 18F-fluorodeoxy-positron emission tomography (FDG-PET) constitutes an informative diagnostic test when CMR is not feasible, such as in cardiac device carriers [17]. The main techniques and diagnostic criteria for myocarditis are summarized in Figure 1.
Table 2.
Evidence of myocardial inflammation in human cardiomyopathies.
| Gene | Model | Main Findings | References |
|---|---|---|---|
| DSP, PKP2 | Clinical setting | Patients with DSP variant cardiomyopathy including 16/105 (15%) who had “acute myocardial injury episodes” akin to clinical myocarditis. | [18] |
| DSP | Clinical setting | Acute myocarditis reflects an active phase of ACM that leads to changes in phenotype and abrupt progression of ACM. | [19] |
| DSP | Clinical setting | Cohort of patients initially presenting with a classic myocarditis syndrome (chest pain, troponin elevation) who were subsequently diagnosed with ACM. Most patients had a DSP genetic variant. | [20,21,22] |
| DSP, LAMA4, LDB3, MYBPC3 DSC2, RYR2, SOS1, SCN5A, SGCD, LPL, PKP2, MYH1, GATA6, and DSG2 | Human heart specimens from autopsy cases | Minimal inflammatory foci may be an early sign of inherited cardiomyopathy. | [23] |
| DSP, FLNC, PKP2, TMPO, TTN | Human EMB | Retrospective multicenter study on patients with undefined LV ACM and extensive overlap between EMB-proven myocardial inflammation and rare genetic variants of the DCM/ACM spectrum. | [5] |
| Human EMB | Most asymptomatic relatives of dilated cardiomyopathy patients with mild left ventricular enlargement already showed infiltration of inflammatory cells, at levels that were similar to those of patients with established disease. | [24] | |
| SCN5A | Case report | Young SCN5A variant carrier with recurrent ventricular fibrillation and massive myocardial inflammation | [25] |
The main reports linking myocardial inflammation to primary cardiomyopathies in human subjects are shown.
Figure 1.
Diagnostic workup for myocarditis. The diagnostic workup for myocarditis is summarized, including gold standard techniques (EMB and CMR, yellow panel), additional imaging techniques applying to special patient subsets (FDG-PET and EAM, orange panel), and genetic testing to investigate the overlap with primary cardiomyopathies (blue panel). For each diagnostic exam, diagnostic criteria and main indications are reported. In each figure, the arrows point to remarkable findings. Representative examples are shown in the lower panel. ACM = arrhythmogenic cardiomyopathy; CMR = cardiac magnetic resonance; DCM = dilated cardiomyopathy; EAM = electroanatomic mapping; EMB = endomyocardial biopsy; FDG-PET = 18F-fluorodeoxypositron emission tomography; IHC = immunohistochemistry; LGE = late gadolinium enhancement; PCR = polymerase chain reaction; VA = ventricular arrhythmias.
1.3. Classification of Cardiomyopathies
Even in the absence of universal agreement about their definition (Table 1), cardiomyopathies are defined as myocardial disorders in which the heart muscle is structurally and/or functionally abnormal in the absence of alternative explanatory causes, including coronary artery disease, hypertension, valvular, and congenital heart disease [10,11]. Among a wide spectrum of nonischemic muscle diseases, dilated cardiomyopathy (DCM) is characterized by dilation and impaired contraction of the left or both ventricles [12], and is caused by rare genetic variants in nearly half of cases [26]. On the contrary, arrhythmogenic cardiomyopathy (ACM), in turn caused by gene variants in up to 50% of familial cases [13], has arrhythmic manifestations as the main clinical phenotype. In clinical practice, DCM and ACM constitute the main source of overlap with myocarditis and inflammatory cardiomyopathy [6,12,27,28]. In detail, while heart failure manifestations call for differential diagnosis with DCM [6,12], ventricular arrhythmias (VA) may suggest either classic or left dominant ACM [13,14]. In both cases, the nonischemic pattern of LGE is frequently found on the CMR [9]. Figure 2 shows the whole spectrum of overlapping phenotypes of DCM, ACM, and myocarditis. Although, in the vast majority of cases, specific diagnostic criteria allow a conclusive differential diagnosis among DCM, ACM, and myocarditis, in some cases a definitive diagnosis represents an inconclusive challenge due to a substantial overlap between clinical, instrumental, laboratory, and anatomical-histological data (Figure 2).
Figure 2.
Overlap between myocarditis and primary cardiomyopathies. The Venn diagram shows the overlaps between myocarditis and genetic cardiomyopathies of the arrhythmogenic and dilated spectrums. ACM = arrhythmogenic cardiomyopathy; CMR = cardiac magnetic resonance; DCM = dilated cardiomyopathy; EMB = endomyocardial biopsy.
2. Myocarditis as a Manifestation of Primary Cardiomyopathy: Clinical Scenarios
2.1. Familial Myocarditis
A family history of sudden death or cardiomyopathy is the main clue to suspect genetic cardiomyopathies [29]. However, a hereditary component has also been described for myocarditis [2]. In detail, it has been reported that genetic variants involved in familial cardiomyopathies are frequently found in subjects with childhood-onset disease [30] and EMB-proven lymphocytic myocarditis [31].
Among DCM-causing genes, lymphocytic myocarditis has been suggested as a driver for the disease progression [32] in patients with truncating variants of the titin gene (TTN), which constitute the most prevalent cause of familial DCM. Other cytoskeletal gene variants have been shown to increase susceptibility to viral myocarditis [33], accounting for inconstant viral clearance and heterogeneous evolution towards inflammatory DCM [31]. For instance, variants in genes encoding structural proteins such as DMD have been associated with the persistence of viral genomes and unfavorable outcomes [34,35], whereas variants affecting genes coding for non-structural proteins such as SCN5A and BAG3 lead to slowly progressing disease [33]. In a recent series of genotyped probands, a higher prevalence of pathogenic or likely pathogenic variants, in particular in the FLNC, RBM20, and BAG3 genes, was reported in association with severe forms of myocarditis, including cardiogenic shock and sustained VA [36].
As for ACM, pathogenic variants in desmosomal genes, namely plakoglobin (JUP), desmoplakin (DSP), plakophilin (PKP2), desmoglein (DSG2), and desmocollin (DSC), are depicted as the main cause [13], in turn displaying extensive overlap with myocarditis. A recent study reported variants in the DSP and DSG2 genes in six probands with familial myocarditis [20], allowing subsequent identification of up to 28 gene variant carriers, 39% of whom had a phenotype consistent with LV rather than classic right ventricular (RV) ACM. Myocarditis has been recently included within the typical phenotype of desmoplakin cardiomyopathy [18], where VA and recurrence of acute myocarditis are frequently associated [21,22].
A further challenging topic for familial myocarditis is the overlap between genetic background and autoimmunity. Recently, either pathogenic or likely pathogenic variants in genes associated with recessive immune disorders have been identified in pediatric patients with myocarditis [37]. Consistently with the complex gene-environment interactions underlying the pathophysiology of inflammatory cardiomyopathy [2,6], serum anti-heart autoantibodies and anti-intercalated disk autoantibodies have been found in the majority of patients with familial ACM [38], as well as in arrhythmic myocarditis [39]. Additional evidence includes the finding of circulating anti-myosin and anti-troponin I autoantibodies in DSP cardiomyopathy [40] and of anti-DSG2 autoantibodies in classic RV-dominant ACM [41].
2.2. Autoptic Findings from Sudden Death Victims
Both myocarditis and cardiomyopathies constitute relevant causes of sudden cardiac death in the young population [9,42]. Recent data suggest a relative contribution of 12% for myocarditis, 10% for ACM, and 4% for DCM [43]. Irrespective of the final diagnosis, the autoptic series revealed significant overlapping findings, including inflammatory infiltrates that constantly met definite criteria for myocarditis [6].
In DCM, common findings beyond LV volume dilation and wall thinning include myocyte atrophy, vacuolar degeneration, nuclear pleomorphism, and fibrosis [44]. In this setting, it has been suggested that minimal inflammatory foci may be an early sign of inherited cardiomyopathy [23,24]. In many DCM, however, inflammatory infiltrates are composed of lymphocytes, with no evidence of an activated phenotype or myocytolysis [44]. Conversely, positive staining for CD3 labeling the activated phenotype of T-lymphocytes has been mainly reported in classic myocarditis and inflammatory DCM [6]. Similar findings have been described in myocarditis patients known for systemic autoimmune diseases, such as systemic sclerosis [45].
As for ACM, the main histopathological feature is the progressive loss of ventricular myocardium and fibro-fatty replacement [44]. In the LV-dominant forms of ACM, fibrofatty or fibrous scars are typically located in the epicardial layers of the posterolateral free wall [46], mimicking the distribution pattern of classic myocarditis [4,5]. In ACM, myocardial atrophy results from injury and repair processes, which are often accompanied by patchy myocarditis containing CD45+ and CD43+ T-lymphocytes in up to 75% of hearts at autopsy [47]. Lymphocytic inflammatory infiltrates and focal fibrosis have been reported as concealed substrates even in patients carrying nondesmosomal variants of ACM overlapping with channelopathies such as Brugada syndrome [25,48]. Since myocyte necrosis fulfilling the Dallas criteria [8] is seldom evident on autopsy, the differential diagnosis between true myocarditis and cardiomyopathy-associated M-Infl remains unsolved for many patients [3].
Given the considerable overlap among ACM, DCM, and chronic myocarditis, differential diagnosis should rely on experienced cardiovascular pathologists and integrated clinical-pathological assessment [49].
2.3. Overlapping Phenotypes in Sporadic Disease
The clinical presentation of myocarditis is extremely variable and ranges from acute coronary syndrome-like chest pain to heart failure and arrhythmias [6]. In turn, the latter manifestations are frequently found in primary cardiomyopathies: while heart failure is more common in DCM, VA have been described in both ACM [13] and early-stage DCM [12,50,51]. To be noted, however, hot-phase bursts of M-Infl resembling an infarct-like clinical presentation of acute myocarditis have been reported even in ACM patients irrespectively of family history [19,21,22]. Given the complex and widely overlapping scenarios, differential diagnosis is challenging in sporadic diseases.
In this setting, CMR has been suggested as a valuable diagnostic tool. As a common trait, classic myocarditis, ACM, and DCM present with a nonischemic distribution of substrate abnormalities (Figure 3). However, the presence of LV LGE exceeding the degree of systolic dysfunction, especially when arrhythmic manifestations dominate heart failure symptoms, suggests primary ACM [51]. Furthermore, a ring-like pattern of LGE could better identify primary cardiomyopathies, in particular those secondary to DSP and FLNC pathogenic variants [52]. On the other hand, septal involvement and associated conduction system defects may point to LMNA cardiomyopathy and an adverse prognosis [50,53,54].
Figure 3.
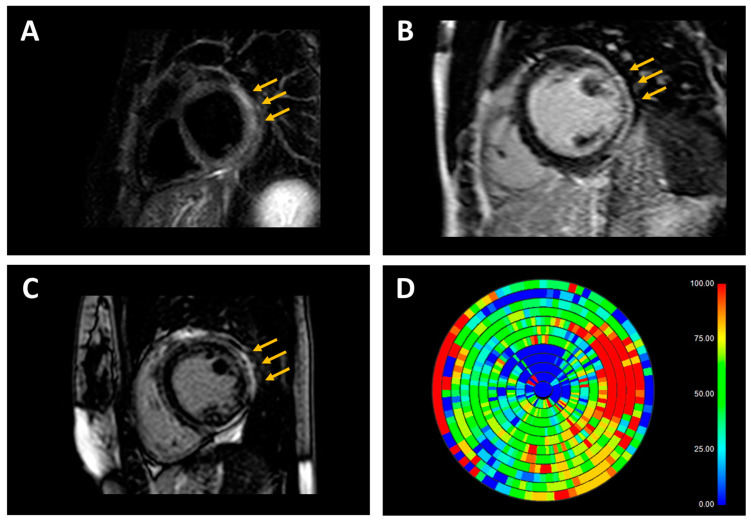
Representative examples of cardiac magnetic resonance (CMR) findings in patients with inflammatory and genetic cardiomyopathies. (A) T2-weighted short-tau inversion recovery (STIR) sequence in a patient with acute myocarditis and nonischemic distribution pattern of substrate abnormalities (subepicardial hyperintensity in inferolateral left ventricular wall, arrows). (B) Late gadolinium enhancement (LGE) sequence in a patient with genetic dilated cardiomyopathy (DCM; pathogenic variant in the FLNC gene) and nonischemic distribution pattern of substrate abnormalities (subepicardial hyperintensity in inferolateral left ventricular wall, arrows). (C) LGE sequence in a patient with genetic arrhythmogenic cardiomyopathy (ACM; pathogenic variant in the DSP gene) and nonischemic distribution pattern of substrate abnormalities (subepicardial hyperintensity mainly involving the inferolateral left ventricular wall, arrows). (D) In the patient with genetic ACM, the LGE map shows an extensive left ventricular scar burden (red = maximal scar burden; blue = healthy myocardium).
Once the suspicion of cardiomyopathy is confirmed by CMR, additional effort is needed to identify M-Infl. Very recent data suggest that EMB-proven myocardial inflammation is found in more than 50% of patients with clinically suspected ACM [5]. Consistently, the presence of associated abnormalities at T2-weighted sequences on CMR suggests M-Infl [16]. Additional diagnostic tools allowing M-Infl identification include FDG-PET [55] and ECG recording during arrhythmias. In detail, polymorphic and irregular VA appearances on the 12-lead ECG may point to M-Infl, in contrast with the scar-related regular and monomorphic arrhythmias observed both in healed myocarditis and primary cardiomyopathies [56,57,58].
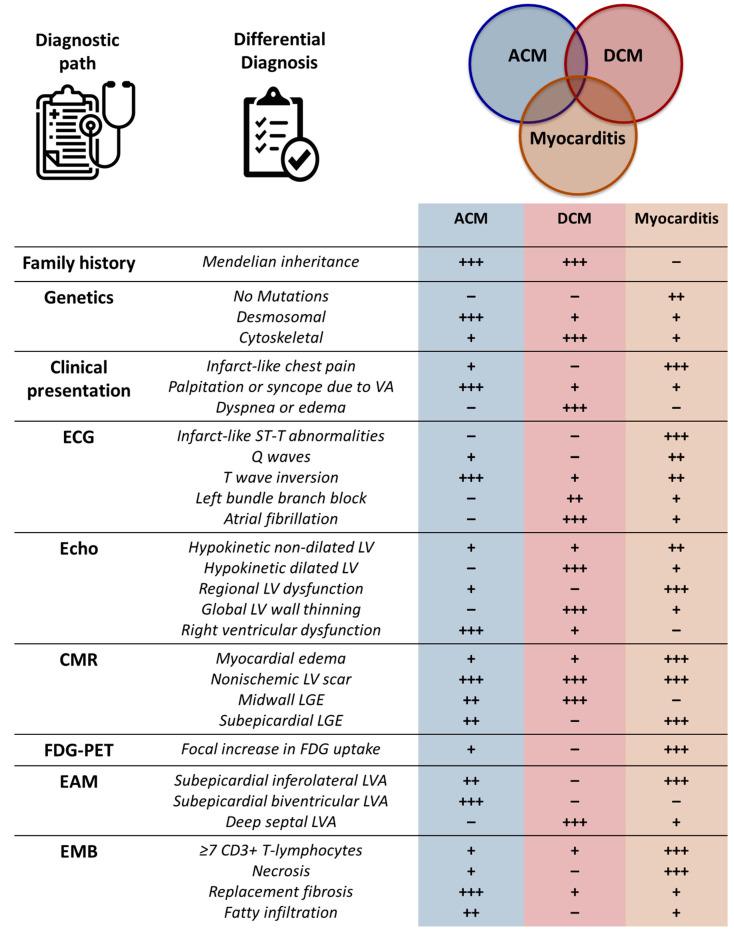
A complete overview allowing differentiation among myocarditis, DCM, and ACM is presented in Figure 4. As for etiology identification in sporadic disease, genetic testing might be considered in the presence of recurrent myocarditis bursts, persistent LV dysfunction or arrhythmias, or a special LGE pattern on CMR [21,22,52].
Figure 4.
Diagnostic path and differential diagnosis between myocarditis, arrhythmogenic, and dilated cardiomyopathies. Key elements in differential diagnosis between myocarditis, arrhythmogenic, and dilated are shown from a clinical to an instrumental viewpoint. A semiquantitative scale is used to highlight the main differences. ACM = arrhythmogenic cardiomyopathy; CD = cluster of differentiation; CMR = cardiac magnetic resonance; DCM = dilated cardiomyopathy; EAM = electroanatomic mapping; EMB = endomyocardial biopsy; FDG-PET = 18F-fluorodeoxy positron emission tomography; LV = left ventricular; LVA = low-volage areas; LGE = late gadolinium enhancement; VA = ventricular arrhythmias.
3. Modeling Myocardial Inflammation in Cardiomyopathies: Evidence from the Preclinical Setting
3.1. Molecular Mechanisms of Primary Cardiomyopathies
The main molecular players involved in cardiomyopathy pathophysiology are shown in Figure 5. Given their primary role in determining the architecture and contractile function of cardiac myocytes, genetic variants in a wide variety of proteins of the cytoskeleton, sarcomeres, and calcium handling system have been associated with DCM [59,60]. In detail, DCM phenotypes from structural changes and impaired mechanotransduction are observed secondary to pathogenic variants in a number of cytoskeletal genes, including TTN (titin), FLNC (filamin C), DES (desmin), DMD (dystrophin), LDB3 (ZASP protein), BAG3 (BCL2-associated athanogene 3), and the same desmosomal genes involved in ACM pathophysiology [61,62,63,64,65,66]. In addition, variants in genes encoding sarcomeric proteins, such as myosin binding proteins and troponin, or calcium-handling proteins such as phospholamban, encoded by the PLN gene, have been associated with DCM phenotypes [67,68].
Figure 5.
Genetic architecture of genetic cardiomyopathies. The main molecular players involved in the pathophysiology of either arrhythmogenic, or dilated cardiomyopathies are shown, together with the localization of single molecules in cellular compartments.
On the other hand, the electrical uncoupling between cardiac myocytes secondary to pathogenic gene variants involving the intercalated disk components has been proposed as the main arrhythmogenic mechanism in primary ACM [69,70]. With an estimated prevalence of 19–46%, PKP2 accounts for the majority of variants in adult-onset ACM [71]. As opposed, pediatric cohorts show prevalence of DSP [72], where C-terminal variants account for up to 16% of ACM, frequently with a LV-dominant variant [73]. Irrespective of the specific variant, the common pathophysiologic factors observed in ACM [69,74] include plakoglobin redistribution from the membrane to the intercellular pool [75], gap junction remodeling with loss of connexin 43 (Cx43) [75], and apoptosis [76]. Among nondemsosomal proteins, a relevant role is played by ion channels, which reside in the intercalated disks [69]. In particular, pathogenic variants in SCN5A, which encodes the pore-forming subunit of the sodium channel Nav1.5, have been associated with a number of arrhythmogenic disorders ranging from isolated channelopathies to structural forms of ACM or DCM [48,77].
Finally, the linker of nucleoskeleton and cytoskeleton (LINC) complex tethers the nuclear envelope to the cytoskeleton and controls the transcription of a broad range of genes, including those implicated in DCM and ACM phenotypes [78]. For instance, the nuclear proteins lamin A and C, encoded by alternative splicing by the LMNA gene, account for a life-threatening ACM/DCM with potential multisystemic involvement and age-related penetrance [53,79]. Among the other LINC proteins, while pathogenic variants in TMPO have been associated with DCM with a low prevalence of approximately 1% [80], variants in transmembrane protein 43 (TMEM43) account for a non-neglectable proportion of ACM cases [81].
3.2. Role of Systemic and Local Inflammation
Multiple clues suggest a critical role for both systemic inflammation and M-Infl in cardiomyopathy pathophysiology. For both DCM and ACM, preclinical evidence suggesting a role for inflammation is summarized in Table 3.
Table 3.
Preclinical models of inflammatory cardiomyopathies.
| Models of Genetic Etiologies Linked to DCM | |||
|---|---|---|---|
| Gene | Model | Phenotype | References |
| TTN | C57BL6/J mice | Cardiac inflammation | [82] |
| RBM20 | shRbm20 iPSCs | Dysregulation of genes involved in inflammation | [83] |
| DMD | Dmdmdx rat | Infiltration of leukocytes | [84] |
| DMD | Dmdmdx rat | Infiltration of macrophages | [85] |
| MYH | MyH-mutant mouse | Upregulation of inflammasome pathways | [86] |
| LMNA | HEK293 cells expressing LMNA-p. Leu140_Ala146dup | Upregulation of Hsp70 | [87] |
| LMNA | csPLA transgenic mouse | Inflammatory cardiomyopathy; activation of NF-κB | [88] |
| LMNA |
LmnaCMKO mouse |
Upregulation of pro-inflammatory gene expression programs | [89] |
| BAG3 | BAG3-deficient mice | Minimal cardiac muscle inflammation | [90] |
| MYBPC3 | cMyBP-C(t/t) mouse | Macrophage infiltration; upregulation of inflammatory pathways |
[91] |
| - | IKKMyHC
mouse |
Excessive inflammatory response and myocyte atrophy | [92] |
| Models of Genetic Etiologies Linked to ACM | |||
| Gene | Model | Phenotype | References |
| PKP2 | PKP2-Hz mouse | More sensitivity to experimental autoimmune myocarditis | [93] |
| PKP2 | C57BL/6 RiboTagflox mice | Abundance of transcripts involved in the inflammatory/immune response | [94] |
| PKP2 | hiPSC line from an ACM patient with a c.2013delC (p.Lys672Argfs*12) variant in plakophilin-2 (PKP2) | Secretion of inflammatory cytokines | [95] |
| DSG2 | N271Sdsg2 transgenic mouse | Presence of massive inflammatory infiltrates | [96] |
| DSG2 | Dsg2+/+ mouse, Dsg2+/− mouse, Dsg2−/− mouse | Activation of inflammatory pathways; upregulation of genes linked to specific macrophage populations | [97] |
| DSG2 | Dsg2MT mouse, Dsg2cKO mouse | Presence of inflammatory response, recruitment of immune cell | [98] |
| DSG2 | Dsg2mut/mut mouse | Activation of NFκB; increased levels of inflammatory cytokines and chemotactic molecules | [95] |
| DSC2 | DSC2 transgenic mouse | Upregulation of inflammatory and fibrotic remodeling pathways | [99] |
| JUP | Neonatal rat ventricular myocytes expressing 2057del2 plakoglobin | Release of inflammatory mediators, reduced by SB216763 treatment | [100] |
| JUP | Dsg2mut/mut mouse, JUP2157del2 mouse | Extensive inflammation, improved by SB216763 treatment | [101] |
| JUP | Neonatal rat ventricular myocytes expressing JUP2157del2 | Activation of NFκB | [95] |
| JUP | JUP mutant mouse | Expression of pro-inflammatory cytokines | [102] |
| JUP | PGTR mouse | Overexpression of IGFBP5 | [103] |
| DSP | Cardiac-specific Dsp knockout mouse | Fibro-fatty substitution of the myocardium; cardiomyocyte death | [104] |
| TMEM43 | Tmem43-S358L mutant mice | Activation of NFκB | [105] |
| TMEM43 | Tmem43-S358L KI | Infiltration of inflammatory cells | [106] |
| - | Boxer right ventricular ACM | Myocarditis | [107] |
| - | Cat right ventricular ACM | Signs of inflammatory infiltrates | [108] |
Cell and mouse models linking inflammatory mechanisms to ACM and DCM are presented.
In DCM, the cytokine network has been implicated in the pathophysiology of LV dilation and systolic dysfunction. It has been shown that systemic inflammation affects myocardial stiffness by multiple mechanisms, such as post-translational modifications of titin [109]. Remarkably, truncating variants in the TTN gene, encoding titin, account for 25% of familial DCM and 18% of idiopathic DCM [110]. Phosphorylation of the N2B segment by protein kinases decreases the distensibility of titin, while phosphorylation of the PEVK-domain has the opposite effect [111]. An arrhythmic phenotype of TTN-associated DCM has been described due to protease vulnerability [112]. Consistently, EMB-proven M-Infl has been documented in a patient presenting with severe heart failure and VA [113]. In this setting, titin might play a major role in immunity regulation. In a recent article, DCM patients with and without a truncating TTN variant showed an autoimmune/inflammatory trigger in 9% and 12%, respectively [114].
However, gene-environment interactions may be more complex. For instance, it has been shown that toll-like receptor 3 variants increase susceptibility to enteroviral myocarditis and DCM [115]. In patients with immune checkpoint inhibitor (ICI)-related myocarditis and myositis, anti-titin and anti-potassium channel Kv1.4 antibodies are often detected [116]. Remarkably, pathogenic titin variants in solid tumors are associated with higher immunogenicity and inflammatory infiltrates [117]. Recently, it has also been suggested that mimic peptides from commensal bacteria can promote inflammatory cardiomyopathy in genetically susceptible individuals [118]. Consistently, the use of immunosuppressive therapy [119,120], as well as IL-1 inhibitors [121], to target EMB-proven M-Infl, has been associated with LV reverse remodeling and improved systolic function in clinical practice.
As for ACM, high circulating levels of proinflammatory cytokines, as well as reduced levels of anti-inflammatory ones [122], have been reported irrespectively of the specific underlying genetic background. For instance, plakoglobin translocation from intercalated disks to the intracellular pool has been observed in response to low concentrations of proinflammatory cytokines, including IL-6 and TNF-α in granulomatous myocarditis and ACM but not in classic lymphocytic myocarditis [123]. Regardless of the underlying pathogenic variant, one of the main drivers of myocardial inflammation in the ACM is signaling by glycogen synthase kinase-3β (GSK3β) an ubiquitin protein involved in the proteasomal degradation system. In ACM hearts, GSK3β translocates from the cytoplasm to the intercalated disk, resulting in activation of nuclear factor-κB (NFκB), the master cellular regulator of inflammation and innate immune response [95]. Downstream NFκB, transforming growth factor-β3 (TGFβ3) induces a fibrotic response by promoting the expression of extracellular matrix genes and by suppressing the activity of matrix metalloproteinases [124]. As for humoral autoimmunity, anti-DSG2 antibodies have been shown to cause gap junction dysfunction regardless of the ACM genotype [41]. The proarrhythmogenic role of DSG2 autoantibodies is further enhanced by the positive correlation found between circulating levels and the burden of premature ventricular contractions [41].
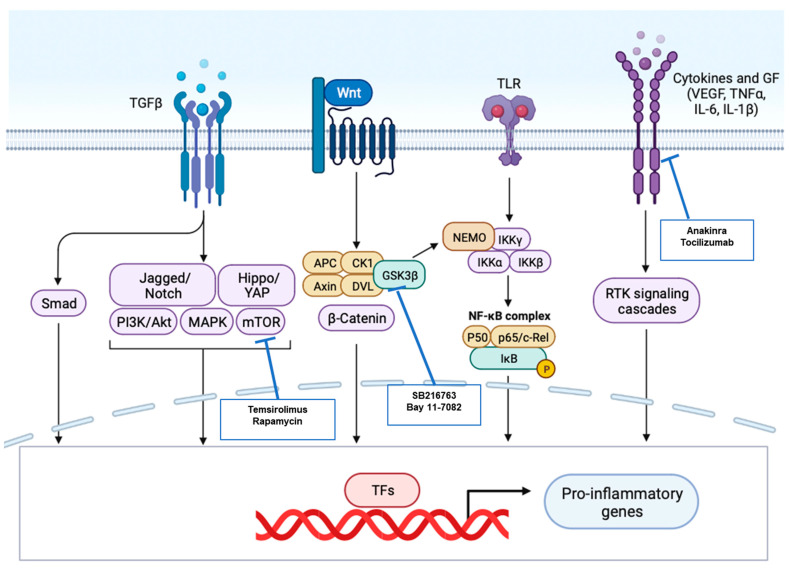
Since the whole process of M-Infl is too complex to be properly reproduced in vitro, cell and animal models have been developed to understand the molecular mechanisms of inflammatory cardiomyopathies [125]. In this setting, key targets for biological and immunomodulating therapy are summarized in Figure 6.
Figure 6.
Proinflammatory pathways upregulated in cardiomyopathies and targets for molecular therapy. Suitable molecular targets to mitigate myocardial inflammation in primary cardiomyopathies are shown, along with the proinflammatory pathways most commonly involved. Biological agents and immunomodulatory drugs currently available are in evidence.
3.3. Inflammation Models in Genetic Etiologies Linked to DCM
A common pathway for most of the described genetic forms of myocarditis susceptibility is NF-kB. It has been shown that NFκB activation in cardiomyocytes is per se sufficient to cause DCM. Inducible transgenic mice with cardiomyocyte-specific constitutively active IκB kinase (IKK; activator of NF-kB) induce an excessive inflammatory response and myocyte atrophy, leading to a reversible form of heart failure [92].
While TTN transgenic animal models have not been investigated for cardiac inflammatory patterns, so far, TTN protein has been indirectly linked to viral myocarditis pathogenesis. Indeed, in a model of Coxsackie virus B3 (CVB3)-induced myocarditis, impaired titin phosphorylation was observed, along with LV dysfunction, increased cardiac inflammation, including IL-6 levels, and fibrosis. In this setting, IL-6 receptor blockade led to a reduction in viral load paralleled by extracellular matrix regulation, and titin function improved, resulting in preserved LV function [82]. This concept can be further generalized. Viruses or other infecting agents release proteases that interfere with cytoskeleton/sarcomere proteins, as, for instance, desmin or cardiac troponin, worsening an already compromised situation when these proteins are defective due to a genetic variant [32].
Defects in phospholamban and other proteins involved in Ca2+-dependent signaling, have both a direct role in arrhythmia induction as well as in the maladaptive remodeling of the heart [126]. The impaired cellular Ca2+ content leads to the abnormal activation of key mediators, such as calmodulin-dependent kinase II (CaMKII) and calcineurin. Active CaMKII, among many functions, is known to increase inflammatory signaling [127]. However, myocardial inflammation has not been investigated in PLN animal models.
The RBM20 protein is an essential component of the RNA processing machinery and is required to regulate cardiac gene expression and processing. Using a human-induced pluripotent stem cell (hiPSC)-derived platform, the genes dysregulated by RBM20 pathogenic variants have been revealed. Among them, 16 are involved in heart diseases, such as cardiomyopathies and inflammation of the myocardium [83].
Dystrophin-deficient rats (Dmdmdx) are used to recapitulate the pathological phenotype of DMD patients. The first immunophenotype profile assessed in skeletal and cardiac muscles excised from Dmdmdx rats showed leukocyte infiltration at the age of 12 weeks, which consisted mostly of macrophages and T cells, including CD68+ and CD45RChigh T cells [84]. Accordingly, another study pointing at the characterization of the cardiovascular phenotype of the Dmdmdx rat demonstrated CD68+ macrophage infiltration in both left and right ventricles [85]. Collectively, these data suggest that the recruitment of inflammatory cells could be at the basis of the cardiac dysfunction of DMD patients, who indeed develop a progressive DCM characterized by inflammatory cell infiltration, necrosis, and cardiac fibrosis.
In MyH-mutant mouse hearts, which show heart failure and arrhythmias, the inflammasome pathway was upregulated [86].
It has been shown that expression of mutant LMNA (LMNA-p. Leu140_Ala146dup) in HEK293 cells led to increased expression of the pro-inflammatory protein Hsp70, mimicking its increase in serum exosomes of patients harboring this same variant [87]. Interestingly, the degree of inflammation, in terms of the amount of pro-inflammatory cytokines upregulated, correlated with the severity of the clinical manifestations associated with each patient. Besides heterologous systems, several mouse models carrying different pathogenic variants of LMNA have been obtained, which recapitulate the severe human cardiac structural and arrhythmic defects [128]. One in particular, a transgenic with cardiac-specific prelamin-A accumulation (csPLA-Tg) exhibited a phenotype consistent with inflammatory cardiomyopathy [88]. In the LMNA context, it was demonstrated that the trigger for inflammation is likely linked to premature myocardial senescence [129], due to the nuclear lamina disruption, and mediated by the senescence-associated secretory phenotype (SASP) [130]. Inflammaging was sustained by the high myocardial activation of NFκB in csPLA-Tg. Finally, in a LmnaCMKO mouse model, cardiomyocyte nuclear rupture occurred 2 weeks prior to the development of fibrosis and reduction in ejection fraction and was accompanied by upregulation of pro-inflammatory gene expression and cytoplasmic release of HMGB1, a potent pro-inflammatory protein normally localized in the nucleus [89]. This nuclear-driven pro-inflammatory machinery comes into play in the early stages of the disease, possibly accounting for the development of DCM. Consistently, in animal models, the inhibition of the mTOR pathway by temsirolimus or rapamycin was able to rescue the DCM phenotype [131].
Although cardiac muscle inflammation is minimal in BAG3-deficient mice [90], a lack of BAG3 is associated with a pathogen-dependent reaction. Indeed, NF-kB regulates BAG3 expression in the presence of lipopolysaccharide, the main component of the bacterial membrane, hinting at BAG3s’ implication in the bacteria-induced response, which is associated with enhanced expression of pro-inflammatory cytokines [132]. In addition, a mouse model overexpressing the BAG3 inhibitor Tat, is more sensitive to viral infection, developing virus-associated cardiomyopathy [133].
The association between myocardial inflammation and cardiac dysfunction in DCM caused by the rare MYBPC3 variant has been investigated in the cMyBP-C(t/t) mouse model of DCM at 3 months of age [91]. This study described the infiltration of activated (CD45+CD11b+Ly6C−MHCII+F480+) and pro-inflammatory M1 (CD45+CD11b+Ly6C−MHCII+F480+CD206−) macrophages, along with upregulation of inflammatory pathways, in the hearts of DCM animals.
3.4. Inflammation Models in Genetic Etiologies Linked to ACM
A transcriptome analysis in mice revealed that functional pathways related to viral infection, platelet activation, inflammation, and immune response networks were inversely correlated with PKP2 expression [94]. PKP2 haploinsufficiency in the murine heart does not lead to overt phenotypes but has been demonstrated to make the heart more sensitive to experimental autoimmune myocarditis (EAM). The induction of EAM resulted in more subepicardial fibrosis, loss of Cx43 expression, and larger non-myocyte areas [93]. The mouse model with cardiomyocyte-specific conditional homozygous knock-out of PKP2 (PKP2cKO), instead, phenotypically shows biventricular dilation and few spontaneous premature ventricular contractions, worsened by isoproterenol induction [134]. Transcriptome analyses revealed, among others, pathways enriched for inflammatory and pro-fibrotic genes. In particular, untranslated region variants in the TGFB3 gene in ACM can lead to increased myocardial fibrosis [135] via activation of the SMAD2/3 and mitogen-activated protein kinase signaling pathways [122,136]. In a mouse derived by crossing PKP2cKO with a RiboTag line, PKP2 loss in cardiomyocytes was transcriptionally linked to genes coding for host-response mechanisms even in the absence of an exogenous trigger [94]. In this model, infiltration of CD45+ cells in the sub-epicardial layer of PKP2cKO/RiboTag mouse hearts was seen in the early phases of the disease [94], hinting at a deficient cardiomyocyte-dependent recruitment of inflammatory cells from the bloodstream. Accordingly, hiPSC-derived cardiac myocytes from a carrier of a PKP2 variant are characterized by an over-activation of NFκB and increased cytokine expression [95], describing a cardiac activation of innate immunity. In this setting, small molecules such as the GSK3β inhibitor SB216763 and Bay 11-7082 have shown the capability of reversing the ACM phenotype [94,95,99,100]. In particular, SB216763 normalized the expression and localization of GSK3β as well as plakoglobin and Cx43 with positive effects on arrhythmogenesis [122].
In mice with cardiac-restricted inactivation of wild-type desmosomal cadherins, as well as in those with overexpression of mutant ones, inflammatory macrophagic infiltration has been described in nearby necrotic tissue [70,96,137]. In the first-generated mouse model of Dsg2-related right ventricular ACM (a transgenic mouse overexpressing dsg2-N271S), the progression of the disease phenotype has been finely reported [96]. It involves: (i) necrotic cell death that prompts an inflammatory response, mediated by massive inflammatory infiltrates, and calcification in the myocardium, (ii) injury repair with fibrous tissue replacement. Early activation of inflammatory-associated pathways, along with upregulation of genes linked to specific macrophage populations, was confirmed also in other Dsg2 mouse models [97], further strengthening the idea that activation of the pro-inflammatory machinery plays a pivotal role in generating the phenotype in Dsg2 mouse models of ACM. In addition to this, the contribution of inflammatory cells to all stages of murine ACM has been reported and comprises neutrophil granulocyte recruitment at the disease onset, recruitment and differentiation of macrophages during acute disease progression, and persistence of T cells during chronic disease progression [98]. The increased levels of inflammatory cytokines and chemotactic molecules were associated with activation of NFκB signaling, in a mouse model in which exons four and five of Dsg2 were excised to generate a frameshift variant [95]. Consistently, treatment of DSG2 mutant mice with Bay 11-7082 reduced the number of infiltrating inflammatory cells in the myocardium [95].
While DSC2 KO mice do not show a cardiac phenotype unless stressed [138], cardiac-specific overexpression of wild-type DSC2 causes biventricular cardiomyopathy. This includes severe fibrosis, cardiac necrosis, calcification, and cardiac inflammation [104]. Accordingly, gene expression data indicate up-regulation of genes responsible for acute sterile inflammation (cytokines, chemokines, and toll-like receptor signaling) and fibrotic remodeling [104].
Neonatal rat ventricular myocytes transfected to express the variant 2057del2 of plakoglobin showed typical cardiomyocyte-specific ACM phenotypes, including the release of different inflammatory mediators into the culture medium, such as IL-6, TNF-α, and MIP-1α [100]. These features were normalized when cells were incubated with the GSK3β inhibitor SB216763 [100,101]. In addition, the transfected cardiomyocytes with the JUP2157del2 construct showed nuclear signal for phospho-RelA/p65 (Ser536), indicating activation of NFκB, which was prevented by the anti-inflammatory Bay 11-7082. Accordingly, inflammatory cells, mostly neutrophils and macrophages, were observed in homozygous Jup mutant mice obtained by ablating Jup in cardiomyocytes. This agrees with the increased expression of pro-inflammatory cytokines IL-1β and IL-6 in Jup mutant hearts [102]. Among WNT pathway mediators, stromal progenitor cells from the hearts of plakoglobin transgenic mice (truncation variant of Jup) overexpressed IGFBP5, which has many biological functions, including in the inflammatory response [103,139].
A cardiac-specific Dsp knockout in the heterozygous form was obtained in mice. Haploinsufficiency gave rise to fibro-fatty substitution of the myocardium and cardiomyocyte death, leading to cardiac dysfunction and arrhythmogenesis [140]. Interestingly, among the differentially expressed genes for WNT pathway inhibitors and endothelial to mesenchymal transition mediators, those encoding for inflammatory proteins were highly expressed in the cardiomyocytes of these mice [140].
The hearts of Tmem43-S358L mutant mice are characterized by NFκB activation. However, this activation does not promote a typical cardiac inflammatory response, instead leading to TGFβ-mediated fibrosis [105]. On the other side, inflammatory cell infiltration was also observed in homozygous Tmem43-S358L KI rats [106]. Consistently, GSK3β inhibition in TMEM43 mutant mice resulted in a relative improvement in the contractile function [105,141].
Other animal models with different but still unknown genetic causes phenotypically display a cardiomyopathy with an inflammatory aspect. ACM in boxer dogs is inherited, but the genetic etiology is still debated [142,143]. Dogs show ventricular arrhythmia, ventricular dilation, fibro-fatty replacement, and myocarditis, characterized by patchy mononuclear cells infiltrating the RV [107]. Auto-antibodies against DSG2, proposed as a specific biomarker for all genetic forms of ACM and as a proof of autoimmunity resultant from ACM pathogenic gene variants, have been found in the sera of boxer dogs spontaneously manifesting the disease [41], but were never tested in ACM mouse models. Spontaneous feline ACM, characterized by right-ventricular disease, ventricular tachycardia, and fibro-fatty replacement of the myocardium, also has signs of inflammatory infiltrates [108].
4. Clinical Implications and Conclusive Remarks
To date, the clinical significance of M-Infl in genetic cardiomyopathies is still under investigation. Preliminary evidence suggests that the detection of M-Infl by multimodal workup is feasible and allows the subsequent use of immunomodulatory treatment [144]. However, the impact of immunomodulatory strategies on outcomes is still controversial. For instance, while M-Infl has been recognized as a major pathophysiological contributor to heart failure, the results of several trials attempting anti-inflammatory strategies in uncharacterized DCM did not result in a significant clinical improvement [145]. As opposed, in patients with EMB-proven inflammatory DCM, the use of immunosuppressive therapy has been proven safe and associated with LV reverse remodeling and recovery of the systolic function [6,119]. In light of its reversibility potential, M-Infl has been proposed as an indicator of good prognosis even in pediatric series [146]. In the setting of DCM, the use of IST is meant not only to prevent end-stage heart failure but also to avoid the improper application of implantable cardioverter defibrillators in primary prevention [4,147]. As for the arrhythmic manifestations, it has been suggested that M-Infl is capable of predicting ventricular arrhythmia recurrences in patients with classic lymphocytic myocarditis [58], as well as in cases with M-Infl in the context of rare genetic variants of the DCM/ACM spectrum [5]. In both conditions, preliminary data support a favorable role of immunosuppressive therapy on arrhythmic outcomes [5,39,144]. In light of the above, a careful assessment of the myocardial inflammatory status may result in patient-tailored strategies to prevent sudden arrhythmic death [4,148]. Additional evidence from larger multicenter trials is needed before such preliminary evidence can be translated into practical clinical guidelines.
In conclusion, based on current knowledge, M-Infl is expected to play a major role in the pathophysiology of cardiomyopathies. In detail, both clinical and preclinical evidence support multiple interconnections between cardiac inflammation and diseases of the DCM and ACM spectrum. As shown in Figure 4, genetic testing should be more extensively applied to distinguish specific etiologies among a spectrum of cardiomyopathies with overlapping phenotypes [149]. On the other hand, further studies using in vitro and in vivo models are needed to explore the causal relationship between M-Infl and inherited cardiomyopathy genes [150]. In this setting, inflammation could also be a general, aberrant, and unspecific response to the impairment of cardiomyocyte function, irrespective of the underlying etiology, microbial, chemical, or primarily genetic. The contribution of human leukocyte antigen (HLA) variants and immune genes deserves appropriate investigation as well. Finally, additional evidence is expected from the pharmacological targeting of inflammation in genetic diseases. Preliminary data from animal models [95,122] and clinical experience [5,39,119] showed that the use of anti-inflammatory and immunomodulating therapy was accompanied by an improvement in both the arrhythmic and mechanical manifestations of the diseases. These findings pave the way for novel therapeutic perspectives and trials in human disease.
Abbreviations
ACM = arrhythmogenic cardiomyopathy; CMR = cardiac magnetic resonance; DCM = dilated cardiomyopathy; EMB = endomyocardial biopsy; FDG-PET = 18F-fluorodeoxypositron emission tomography; LGE = late gadolinium enhancement; M-Infl = myocardial inflammation; VA = ventricular arrhythmias.
Author Contributions
Conceptualization, G.P. (Giovanni Peretto) and E.S.; methodology, G.P. (Giovanni Peretto) and L.T.C.; software, G.P. (Giovanni Peretto); validation, S.S., G.P. (Giovanni Peretto) and L.T.C.; formal analysis, G.P. (Giovanni Peretto), E.S., C.D.R., M.R. and A.V.; investigation, G.P. (Giovanni Peretto), E.S., C.D.R., M.R., A.V., D.L., S.S., G.P. (Giulio Pompilio) and L.T.C.; resources, C.D.R., G.P. (Giovanni Peretto) and L.T.C.; data curation, A.V. and D.L.; writing—original draft preparation, G.P. (Giovanni Peretto), E.S. and M.R.; writing—review and editing, C.D.R., D.L., S.S., G.P. (Giulio Pompilio) and L.T.C.; visualization, G.P. (Giovanni Peretto), E.S., C.D.R., M.R., A.V., D.L., S.S., G.P. (Giulio Pompilio) and L.T.C.; supervision, S.S., G.P. (Giovanni Peretto) and L.T.C.; project administration, C.D.R. and L.T.C.; funding acquisition, not applicable. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Richardson P., McKenna R.W., Bristow M., Maisch B., Mautner B., O’Connell J., Olsen E., Thiene G., Goodwin J., Gyarfas I., et al. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of Cardiomyopathies. Circulation. 1996;93:841–842. doi: 10.1161/01.CIR.93.5.841. [DOI] [PubMed] [Google Scholar]
- 2.Tschöpe C., Ammirati E., Bozkurt B., Caforio A.L.P., Cooper L.T., Felix S.B., Hare J.M., Heidecker B., Heymans S., Hübner N., et al. Myocarditis and Inflammatory Cardiomyopathy: Current Evidence and Future Directions. Nat. Rev. Cardiol. 2021;18:169–193. doi: 10.1038/s41569-020-00435-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peretto G., Sala S., della Bella P., Basso C., Cooper L.T. Reply: Genetic Basis for Acute Myocarditis Presenting with Ventricular Arrhythmias? J. Am. Coll. Cardiol. 2020;76:126–128. doi: 10.1016/j.jacc.2020.05.014. [DOI] [PubMed] [Google Scholar]
- 4.Peretto G., Sala S., Rizzo S., de Luca G., Campochiaro C., Sartorelli S., Benedetti G., Palmisano A., Esposito A., Tresoldi M., et al. Arrhythmias in Myocarditis: State of the Art. Heart Rhythm. 2019;16:793–801. doi: 10.1016/j.hrthm.2018.11.024. [DOI] [PubMed] [Google Scholar]
- 5.Peretto G., Casella M., Merlo M., Benedetti S., Rizzo S., Cappelletto C., Di Resta C., Compagnucci P., De Gaspari M., Dello Russo A., et al. Inflammation on Endomyocardial Biopsy Predicts Risk of Subsequent Adverse Cardiovascular Events in Undefined Left Ventricular Arrhythmogenic Cardiomyopathy. JACC Clin. Electrophysiol. 2022:S2405-500X(22)00949-5. doi: 10.1016/j.jacep.2022.10.032. [DOI] [PubMed] [Google Scholar]
- 6.Caforio A.L.P., Pankuweit S., Arbustini E., Basso C., Gimeno-Blanes J., Felix S.B., Fu M., Heliö T., Heymans S., Jahns R., et al. Current State of Knowledge on Aetiology, Diagnosis, Management, and Therapy of Myocarditis: A Position Statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013;34:2636–2648. doi: 10.1093/eurheartj/eht210. [DOI] [PubMed] [Google Scholar]
- 7.Ammirati E., Frigerio M., Adler E.D., Basso C., Birnie D.H., Brambatti M., Friedrich M.G., Klingel K., Lehtonen J., Moslehi J.J., et al. Management of Acute Myocarditis and Chronic Inflammatory Cardiomyopathy: An Expert Consensus Document. Circ. Heart Fail. 2020;13:e007405. doi: 10.1161/CIRCHEARTFAILURE.120.007405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aretz H.T., Billingham M.E., Edwards W.D., Factor S.M., Fallon J.T., Fenoglio J.J., Jr., Olsen E.G., Schoen F.J. Myocarditis. A Histopathologic Definition and Classification. Am. J. Cardiovasc. Pathol. 1987;1:3–14. [PubMed] [Google Scholar]
- 9.Zeppenfeld K., Tfelt-Hansen J., de Riva M., Winkel B.G., Behr E.R., Blom N.A., Charron P., Corrado D., Dagres N., de Chillou C., et al. 2022 ESC Guidelines for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death. Eur. Heart J. 2022;43:3997–4126. doi: 10.1093/eurheartj/ehac262. [DOI] [PubMed] [Google Scholar]
- 10.Elliott P., Andersson B., Arbustini E., Bilinska Z., Cecchi F., Charron P., Dubourg O., Kühl U., Maisch B., McKenna W.J., et al. Classification of the Cardiomyopathies: A Position Statement from the European Society Of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2008;29:270–276. doi: 10.1093/eurheartj/ehm342. [DOI] [PubMed] [Google Scholar]
- 11.Maron B.J., Towbin J.A., Thiene G., Antzelevitch C., Corrado D., Arnett D., Moss A.J., Seidman C.E., Young J.B. Contemporary Definitions and Classification of the Cardiomyopathies: An American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006;113:1807–1816. doi: 10.1161/CIRCULATIONAHA.106.174287. [DOI] [PubMed] [Google Scholar]
- 12.Pinto Y.M., Elliott P.M., Arbustini E., Adler Y., Anastasakis A., Böhm M., Duboc D., Gimeno J., de Groote P., Imazio M., et al. Proposal for a Revised Definition of Dilated Cardiomyopathy, Hypokinetic Non-Dilated Cardiomyopathy, and Its Implications for Clinical Practice: A Position Statement of the ESC Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2016;37:1850–1858. doi: 10.1093/eurheartj/ehv727. [DOI] [PubMed] [Google Scholar]
- 13.Towbin J.A., McKenna W.J., Abrams D.J., Ackerman M.J., Calkins H., Darrieux F.C.C., Daubert J.P., de Chillou C., DePasquale E.C., Desai M.Y., et al. 2019 HRS Expert Consensus Statement on Evaluation, Risk Stratification, and Management of Arrhythmogenic Cardiomyopathy. Heart Rhythm. 2019;16:e301–e372. doi: 10.1016/j.hrthm.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 14.Corrado D., Perazzolo Marra M., Zorzi A., Beffagna G., Cipriani A., de Lazzari M., Migliore F., Pilichou K., Rampazzo A., Rigato I., et al. Diagnosis of Arrhythmogenic Cardiomyopathy: The Padua Criteria. Int. J. Cardiol. 2020;319:106–114. doi: 10.1016/j.ijcard.2020.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Peretto G., Cappelletti A.M., Spoladore R., Slavich M., Rizzo S., Palmisano A., Esposito A., de Cobelli F., Margonato A., Basso C., et al. Right Ventricular Endomyocardial Biopsy in Patients with Cardiac Magnetic Resonance Showing Left Ventricular Myocarditis. J. Cardiovasc. Med. 2021;22:560–566. doi: 10.2459/JCM.0000000000001162. [DOI] [PubMed] [Google Scholar]
- 16.Ferreira V.M., Schulz-Menger J., Holmvang G., Kramer C.M., Carbone I., Sechtem U., Kindermann I., Gutberlet M., Cooper L.T., Liu P., et al. Cardiovascular Magnetic Resonance in Nonischemic Myocardial Inflammation: Expert Recommendations. J. Am. Coll. Cardiol. 2018;72:3158–3176. doi: 10.1016/j.jacc.2018.09.072. [DOI] [PubMed] [Google Scholar]
- 17.Peretto G., Busnardo E., Ferro P., Palmisano A., Vignale D., Esposito A., de Luca G., Campochiaro C., Sartorelli S., de Gaspari M., et al. Clinical Applications of FDG-PET Scan in Arrhythmic Myocarditis. JACC Cardiovasc. Imaging. 2022;15:1771–1780. doi: 10.1016/j.jcmg.2022.02.029. [DOI] [PubMed] [Google Scholar]
- 18.Smith E.D., Lakdawala N.K., Papoutsidakis N., Aubert G., Mazzanti A., McCanta A.C., Agarwal P.P., Arscott P., Dellefave-Castillo L.M., Vorovich E.E., et al. Desmoplakin Cardiomyopathy, a Fibrotic and Inflammatory Form of Cardiomyopathy Distinct From Typical Dilated or Arrhythmogenic Right Ventricular Cardiomyopathy. Circulation. 2020;141:1872–1884. doi: 10.1161/CIRCULATIONAHA.119.044934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopez-Ayala J.M., Pastor-Quirante F., Gonzalez-Carrillo J., Lopez-Cuenca D., Sanchez-Munoz J.J., Oliva-Sandoval M.J., Gimeno J.R. Genetics of Myocarditis in Arrhythmogenic Right Ventricular Dysplasia. Heart Rhythm. 2015;12:766–773. doi: 10.1016/j.hrthm.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Piriou N., Marteau L., Kyndt F., Serfaty J.M., Toquet C., le Gloan L., Warin-Fresse K., Guijarro D., le Tourneau T., Conan E., et al. Familial Screening in Case of Acute Myocarditis Reveals Inherited Arrhythmogenic Left Ventricular Cardiomyopathies. ESC Heart Fail. 2020;7:1520–1533. doi: 10.1002/ehf2.12686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bariani R., Cipriani A., Rizzo S., Celeghin R., Bueno Marinas M., Giorgi B., de Gaspari M., Rigato I., Leoni L., Zorzi A., et al. “Hot Phase” Clinical Presentation in Arrhythmogenic Cardiomyopathy. Europace. 2021;23:907–917. doi: 10.1093/europace/euaa343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ammirati E., Raimondi F., Piriou N., Sardo Infirri L., Mohiddin S.A., Mazzanti A., Shenoy C., Cavallari U.A., Imazio M., Aquaro G.D., et al. Acute Myocarditis Associated With Desmosomal Gene Variants. JACC Heart Fail. 2022;10:714–727. doi: 10.1016/j.jchf.2022.06.013. [DOI] [PubMed] [Google Scholar]
- 23.Hata Y., Hirono K., Yamaguchi Y., Ichida F., Oku Y., Nishida N. Minimal Inflammatory Foci of Unknown Etiology May Be a Tentative Sign of Early Stage Inherited Cardiomyopathy. Mod. Pathol. 2019;32:1281–1290. doi: 10.1038/s41379-019-0274-0. [DOI] [PubMed] [Google Scholar]
- 24.Mahon N.G., Madden B.P., Caforio A.L.P., Elliott P.M., Haven A.J., Keogh B.E., Davies M.J., McKenna W.J. Immunohistologic Evidence of Myocardial Disease in Apparently Healthy Relatives of Patients with Dilated Cardiomyopathy. J. Am. Coll. Cardiol. 2002;39:455–462. doi: 10.1016/S0735-1097(01)01762-4. [DOI] [PubMed] [Google Scholar]
- 25.Poller W., Escher F., Haas J., Heidecker B., Schultheiss H.P., Attanasio P., Skurk C., Haghikia A., Meder B., Klaassen S. Missense Variant E1295K of Sodium Channel SCN5A Associated With Recurrent Ventricular Fibrillation and Myocardial Inflammation. JACC Case Rep. 2022;4:280–286. doi: 10.1016/j.jaccas.2022.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hershberger R.E., Hedges D.J., Morales A. Dilated Cardiomyopathy: The Complexity of a Diverse Genetic Architecture. Nat. Rev. Cardiol. 2013;10:531–547. doi: 10.1038/nrcardio.2013.105. [DOI] [PubMed] [Google Scholar]
- 27.Casella M., Gasperetti A., Gaetano F., Busana M., Sommariva E., Catto V., Sicuso R., Rizzo S., Conte E., Mushtaq S., et al. Long-Term Follow-up Analysis of a Highly Characterized Arrhythmogenic Cardiomyopathy Cohort with Classical and Non-Classical Phenotypes-a Real-World Assessment of a Novel Prediction Model: Does the Subtype Really Matter. Europace. 2020;22:797–805. doi: 10.1093/europace/euz352. [DOI] [PubMed] [Google Scholar]
- 28.Peretto G., Mazzone P. Arrhythmogenic Cardiomyopathy: One, None and a Hundred Thousand Diseases. J. Pers. Med. 2022;12:1256. doi: 10.3390/jpm12081256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Charron P., Arad M., Arbustini E., Basso C., Bilinska Z., Elliott P., Helio T., Keren A., McKenna W.J., Monserrat L., et al. Genetic Counselling and Testing in Cardiomyopathies: A Position Statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2010;31:2715–2728. doi: 10.1093/eurheartj/ehq271. [DOI] [PubMed] [Google Scholar]
- 30.Kontorovich A.R., Patel N., Moscati A., Richter F., Peter I., Purevjav E., Selejan S.R., Kindermann I., Towbin J.A., Bohm M., et al. Myopathic Cardiac Genotypes Increase Risk for Myocarditis. JACC Basic Transl. Sci. 2021;6:584–592. doi: 10.1016/j.jacbts.2021.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Artico J., Merlo M., Delcaro G., Cannatà A., Gentile P., de Angelis G., Paldino A., Bussani R., Ferro M.D., Sinagra G. Lymphocytic Myocarditis: A Genetically Predisposed Disease? J. Am. Coll. Cardiol. 2020;75:3098–3100. doi: 10.1016/j.jacc.2020.04.048. [DOI] [PubMed] [Google Scholar]
- 32.Cannata A., Artico J., Gentile P., Merlo M., Sinagra G. Myocarditis Evolving in Cardiomyopathy: When Genetics and Offending Causes Work Together. Eur. Heart J. Suppl. 2019;21:B90–B95. doi: 10.1093/eurheartj/suz033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knowlton K.U. Myocarditis: An Intersection Between Genetic and Acquired Causes of Human Cardiomyopathy. J. Am. Coll. Cardiol. 2017;69:1666–1668. doi: 10.1016/j.jacc.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 34.Campuzano O., Fernández-Falgueras A., Sarquella-Brugada G., Sanchez O., Cesar S., Mademont I., Allegue C., Mates J., Pérez-Serra A., Coll M., et al. A Genetically Vulnerable Myocardium May Predispose to Myocarditis. J. Am. Coll. Cardiol. 2015;66:2913–2914. doi: 10.1016/j.jacc.2015.10.049. [DOI] [PubMed] [Google Scholar]
- 35.Xiong D., Lee G.H., Badorff C., Dorner A., Lee S., Wolf P., Knowlton K.U. Dystrophin Deficiency Markedly Increases Enterovirus-Induced Cardiomyopathy: A Genetic Predisposition to Viral Heart Disease. Nat. Med. 2002;8:872–877. doi: 10.1038/nm737. [DOI] [PubMed] [Google Scholar]
- 36.Tiron C., Campuzano O., Fernández-Falgueras A., Alcalde M., Loma-Osorio P., Zamora E., Caballero A., Sarquella-Brugada G., Cesar S., Garcia-Cuenllas L., et al. Prevalence of Pathogenic Variants in Cardiomyopathy-Associated Genes in Myocarditis. Circ. Genom. Precis. Med. 2022;15:E003408. doi: 10.1161/CIRCGEN.121.003408. [DOI] [PubMed] [Google Scholar]
- 37.Seidel F., Laser K.T., Klingel K., Dartsch J., Theisen S., Pickardt T., Holtgrewe M., Gärtner A., Berger F., Beule D., et al. Pathogenic Variants in Cardiomyopathy Disorder Genes Underlie Pediatric Myocarditis-Further Impact of Heterozygous Immune Disorder Gene Variants? J. Cardiovasc. Dev. Dis. 2022;9:216. doi: 10.3390/jcdd9070216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caforio A.L.P., Re F., Avella A., Marcolongo R., Baratta P., Seguso M., Gallo N., Plebani M., Izquierdo-Bajo A., Cheng C.Y., et al. Evidence From Family Studies for Autoimmunity in Arrhythmogenic Right Ventricular Cardiomyopathy: Associations of Circulating Anti-Heart and Anti-Intercalated Disk Autoantibodies with Disease Severity and Family History. Circulation. 2020;141:1238–1248. doi: 10.1161/CIRCULATIONAHA.119.043931. [DOI] [PubMed] [Google Scholar]
- 39.Peretto G., Sala S., de Luca G., Marcolongo R., Campochiaro C., Sartorelli S., Tresoldi M., Foppoli L., Palmisano A., Esposito A., et al. Immunosuppressive Therapy and Risk Stratification of Patients With Myocarditis Presenting With Ventricular Arrhythmias. JACC Clin. Electrophysiol. 2020;6:1221–1234. doi: 10.1016/j.jacep.2020.05.013. [DOI] [PubMed] [Google Scholar]
- 40.Poller W., Haas J., Klingel K., Kühnisch J., Gast M., Kaya Z., Escher F., Kayvanpour E., Degener F., Opgen-Rhein B., et al. Familial Recurrent Myocarditis Triggered by Exercise in Patients With a Truncating Variant of the Desmoplakin Gene. J. Am. Heart Assoc. 2020;9:e015289. doi: 10.1161/JAHA.119.015289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chatterjee D., Fatah M., Akdis D., Spears D.A., Koopmann T.T., Mittal K., Rafiq M.A., Cattanach B.M., Zhao Q., Healey J.S., et al. An Autoantibody Identifies Arrhythmogenic Right Ventricular Cardiomyopathy and Participates in Its Pathogenesis. Eur. Heart J. 2018;39:3932–3944. doi: 10.1093/eurheartj/ehy567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Al-Khatib S.M., Stevenson W.G., Ackerman M.J., Bryant W.J., Callans D.J., Curtis A.B., Deal B.J., Dickfeld T., Field M.E., Fonarow G.C., et al. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2018;72:e91–e220. doi: 10.1016/J.JACC.2017.10.054. [DOI] [PubMed] [Google Scholar]
- 43.Rizzo S., Carturan E., de Gaspari M., Pilichou K., Thiene G., Basso C. Update on Cardiomyopathies and Sudden Cardiac Death. Forensic Sci. Res. 2019;4:202–210. doi: 10.1080/20961790.2019.1631957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leone O., Veinot J.P., Angelini A., Baandrup U.T., Basso C., Berry G., Bruneval P., Burke M., Butany J., Calabrese F., et al. 2011 Consensus Statement on Endomyocardial Biopsy from the Association for European Cardiovascular Pathology and the Society for Cardiovascular Pathology. Cardiovasc. Pathol. 2012;21:245–274. doi: 10.1016/j.carpath.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 45.de Luca G., Campochiaro C., de Santis M., Sartorelli S., Peretto G., Sala S., Canestrari G., de Lorenzis E., Basso C., Rizzo S., et al. Systemic Sclerosis Myocarditis Has Unique Clinical, Histological and Prognostic Features: A Comparative Histological Analysis. Rheumatology. 2020;59:2523–2533. doi: 10.1093/rheumatology/kez658. [DOI] [PubMed] [Google Scholar]
- 46.Lobo V.F., Silver M.D., Butany J., Heggtveit H.A. Left Ventricular Involvement in Right Ventricular Dysplasia/Cardiomyopathy. Can. J. Cardiol. 1999;15:1239–1247. [PubMed] [Google Scholar]
- 47.Basso C., Thiene G., Corrado D., Angelini A., Nava A., Valente M. Arrhythmogenic Right Ventricular Cardiomyopathy. Dysplasia, Dystrophy, or Myocarditis? Circulation. 1996;94:983–991. doi: 10.1161/01.CIR.94.5.983. [DOI] [PubMed] [Google Scholar]
- 48.di Resta C., Berg J., Villatore A., Maia M., Pili G., Fioravanti F., Tomaiuolo R., Sala S., Benedetti S., Peretto G. Concealed Substrates in Brugada Syndrome: Isolated Channelopathy or Associated Cardiomyopathy? Genes. 2022;13:1755. doi: 10.3390/genes13101755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zampieri F., Zanatta A., Basso C., Thiene G. Cardiovascular Medicine in Morgagni’s De Sedibus: Dawn of Cardiovascular Pathology. Cardiovasc. Pathol. 2016;25:443–452. doi: 10.1016/j.carpath.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 50.Gigli M., Merlo M., Graw S.L., Barbati G., Rowland T.J., Slavov D.B., Stolfo D., Haywood M.E., Dal Ferro M., Altinier A., et al. Genetic Risk of Arrhythmic Phenotypes in Patients With Dilated Cardiomyopathy. J. Am. Coll. Cardiol. 2019;74:1480–1490. doi: 10.1016/j.jacc.2019.06.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cipriani A., Bauce B., de Lazzari M., Rigato I., Bariani R., Meneghin S., Pilichou K., Motta R., Aliberti C., Thiene G., et al. Arrhythmogenic Right Ventricular Cardiomyopathy: Characterization of Left Ventricular Phenotype and Differential Diagnosis With Dilated Cardiomyopathy. J. Am. Heart Assoc. 2020;9:e014628. doi: 10.1161/JAHA.119.014628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Augusto J.B., Eiros R., Nakou E., Moura-Ferreira S., Treibel T.A., Captur G., Akhtar M.M., Protonotarios A., Gossios T.D., Savvatis K., et al. Dilated Cardiomyopathy and Arrhythmogenic Left Ventricular Cardiomyopathy: A Comprehensive Genotype-Imaging Phenotype Study. Eur. Heart J. Cardiovasc. Imaging. 2020;21:326–336. doi: 10.1093/ehjci/jez188. [DOI] [PubMed] [Google Scholar]
- 53.Peretto G., di Resta C., Perversi J., Forleo C., Maggi L., Politano L., Barison A., Previtali S.C., Carboni N., Brun F., et al. Cardiac and Neuromuscular Features of Patients With LMNA-Related Cardiomyopathy. Ann. Intern. Med. 2019;171:458–463. doi: 10.7326/M18-2768. [DOI] [PubMed] [Google Scholar]
- 54.Peretto G., Sala S., Lazzeroni D., Palmisano A., Gigli L., Esposito A., de Cobelli F., Camici P.G., Mazzone P., Basso C., et al. Septal Late Gadolinium Enhancement and Arrhythmic Risk in Genetic and Acquired Non-Ischaemic Cardiomyopathies. Heart Lung Circ. 2020;29:1356–1365. doi: 10.1016/j.hlc.2019.08.018. [DOI] [PubMed] [Google Scholar]
- 55.Protonotarios A., Wicks E., Ashworth M., Stephenson E., Guttmann O., Savvatis K., Sekhri N., Mohiddin S.A., Syrris P., Menezes L., et al. Prevalence of 18F-Fluorodeoxyglucose Positron Emission Tomography Abnormalities in Patients with Arrhythmogenic Right Ventricular Cardiomyopathy. Int. J. Cardiol. 2019;284:99–104. doi: 10.1016/j.ijcard.2018.10.083. [DOI] [PubMed] [Google Scholar]
- 56.Peretto G., Sala S., Rizzo S., Palmisano A., Esposito A., de Cobelli F., Campochiaro C., de Luca G., Foppoli L., Dagna L., et al. Ventricular Arrhythmias in Myocarditis: Characterization and Relationships With Myocardial Inflammation. J. Am. Coll. Cardiol. 2020;75:1046–1057. doi: 10.1016/j.jacc.2020.01.036. [DOI] [PubMed] [Google Scholar]
- 57.Cronin E.M., Bogun F.M., Maury P., Peichl P., Chen M., Namboodiri N., Aguinaga L., Leite L.R., Al-Khatib S.M., Anter E., et al. 2019 HRS/EHRA/APHRS/LAHRS Expert Consensus Statement on Catheter Ablation of Ventricular Arrhythmias. Europace. 2019;21:1143–1144. doi: 10.1093/europace/euz132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peretto G., Sala S., Basso C., Rizzo S., Radinovic A., Frontera A., Limite L.R., Paglino G., Bisceglia C., de Luca G., et al. Inflammation as a Predictor of Recurrent Ventricular Tachycardia After Ablation in Patients With Myocarditis. J. Am. Coll. Cardiol. 2020;76:1644–1656. doi: 10.1016/j.jacc.2020.08.012. [DOI] [PubMed] [Google Scholar]
- 59.Bowles N.E., Bowles K.R., Towbin J.A. The “Final Common Pathway” Hypothesis and Inherited Cardiovascular Disease. The Role of Cytoskeletal Proteins in Dilated Cardiomyopathy. Herz. 2000;25:168–175. doi: 10.1007/s000590050003. [DOI] [PubMed] [Google Scholar]
- 60.Towbin J.A., Lorts A. Arrhythmias and Dilated Cardiomyopathy Common Pathogenetic Pathways? J. Am. Coll. Cardiol. 2011;57:2169–2171. doi: 10.1016/j.jacc.2010.11.061. [DOI] [PubMed] [Google Scholar]
- 61.Sequeira V., Nijenkamp L.L.A.M., Regan J.A., van der Velden J. The Physiological Role of Cardiac Cytoskeleton and Its Alterations in Heart Failure. Biochim. Biophys. Acta. 2014;1838:700–722. doi: 10.1016/j.bbamem.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 62.Bermúdez-Jiménez F.J., Carriel V., Brodehl A., Alaminos M., Campos A., Schirmer I., Milting H., Abril B.Á., Álvarez M., López-Fernández S., et al. Novel Desmin Mutation p.Glu401Asp Impairs Filament Formation, Disrupts Cell Membrane Integrity, and Causes Severe Arrhythmogenic Left Ventricular Cardiomyopathy/Dysplasia. Circulation. 2018;137:1595–1610. doi: 10.1161/CIRCULATIONAHA.117.028719. [DOI] [PubMed] [Google Scholar]
- 63.Towbin J.A., Hejtmancik J.F., Brink P., Gelb B., Zhu X.M., Chamberlain J.S., McCabe E.R.B., Swift M. X-Linked Dilated Cardiomyopathy. Molecular Genetic Evidence of Linkage to the Duchenne Muscular Dystrophy (Dystrophin) Gene at the Xp21 Locus. Circulation. 1993;87:1854–1865. doi: 10.1161/01.CIR.87.6.1854. [DOI] [PubMed] [Google Scholar]
- 64.Vatta M., Mohapatra B., Jimenez S., Sanchez X., Faulkner G., Perles Z., Sinagra G., Lin J.H., Vu T.M., Zhou Q., et al. Mutations in Cypher/ZASP in Patients with Dilated Cardiomyopathy and Left Ventricular Non-Compaction. J. Am. Coll. Cardiol. 2003;42:2014–2027. doi: 10.1016/j.jacc.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 65.Arimura T., Ishikawa T., Nunoda S., Kawai S., Kimura A. Dilated Cardiomyopathy-Associated BAG3 Mutations Impair Z-Disc Assembly and Enhance Sensitivity to Apoptosis in Cardiomyocytes. Hum. Mutat. 2011;32:1481–1491. doi: 10.1002/humu.21603. [DOI] [PubMed] [Google Scholar]
- 66.Vite A., Radice G.L. N-Cadherin/Catenin Complex as a Master Regulator of Intercalated Disc Function. Cell Commun. Adhes. 2014;21:169–179. doi: 10.3109/15419061.2014.908853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kamisago M., Sharma S.D., DePalma S.R., Solomon S., Sharma P., McDonough B., Smoot L., Mullen M.P., Woolf P.K., Wigle E.D., et al. Mutations in Sarcomere Protein Genes as a Cause of Dilated Cardiomyopathy. N. Engl. J. Med. 2000;343:1688–1696. doi: 10.1056/NEJM200012073432304. [DOI] [PubMed] [Google Scholar]
- 68.Schmitt J.P., Kamisago M., Asahi M., Hua Li G., Ahmad F., Mende U., Kranias E.G., MacLennan D.H., Seidman J.G., Seidman C.E. Dilated Cardiomyopathy and Heart Failure Caused by a Mutation in Phospholamban. Science. 2003;299:1410–1413. doi: 10.1126/science.1081578. [DOI] [PubMed] [Google Scholar]
- 69.Vermij S.H., Abriel H., van Veen T.A.B. Refining the Molecular Organization of the Cardiac Intercalated Disc. Cardiovasc. Res. 2017;113:259–275. doi: 10.1093/cvr/cvw259. [DOI] [PubMed] [Google Scholar]
- 70.Rizzo S., Lodder E.M., Verkerk A.O., Wolswinkel R., Beekman L., Pilichou K., Basso C., Remme C.A., Thiene G., Bezzina C.R. Intercalated Disc Abnormalities, Reduced Na(+) Current Density, and Conduction Slowing in Desmoglein-2 Mutant Mice Prior to Cardiomyopathic Changes. Cardiovasc. Res. 2012;95:409–418. doi: 10.1093/cvr/cvs219. [DOI] [PubMed] [Google Scholar]
- 71.Gerull B., Heuser A., Wichter T., Paul M., Basson C.T., McDermott D.A., Lerman B.B., Markowitz S.M., Ellinor P.T., MacRae C.A., et al. Mutations in the Desmosomal Protein Plakophilin-2 Are Common in Arrhythmogenic Right Ventricular Cardiomyopathy. Nat. Genet. 2004;36:1162–1164. doi: 10.1038/ng1461. [DOI] [PubMed] [Google Scholar]
- 72.te Riele A.S.J.M., James C.A., Sawant A.C., Bhonsale A., Groeneweg J.A., Mast T.P., Murray B., Tichnell C., Dooijes D., van Tintelen J.P., et al. Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy in the Pediatric Population: Clinical Characterization and Comparison With Adult-Onset Disease. JACC Clin. Electrophysiol. 2015;1:551–560. doi: 10.1016/j.jacep.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 73.Xu Z., Zhu W., Wang C., Huang L., Zhou Q., Hu J., Cheng X., Hong K. Genotype-Phenotype Relationship in Patients with Arrhythmogenic Right Ventricular Cardiomyopathy Caused by Desmosomal Gene Mutations: A Systematic Review and Meta-Analysis. Sci. Rep. 2017;7:srep41387. doi: 10.1038/srep41387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Marian A.J., Asatryan B., Wehrens X.H.T. Genetic Basis and Molecular Biology of Cardiac Arrhythmias in Cardiomyopathies. Cardiovasc. Res. 2020;116:1600–1619. doi: 10.1093/cvr/cvaa116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Asimaki A., Tandri H., Huang H., Halushka M.K., Gautam S., Basso C., Thiene G., Tsatsopoulou A., Protonotarios N., McKenna W.J., et al. A New Diagnostic Test for Arrhythmogenic Right Ventricular Cardiomyopathy. N. Engl. J. Med. 2009;360:1075–1084. doi: 10.1056/NEJMoa0808138. [DOI] [PubMed] [Google Scholar]
- 76.Mallat Z., Tedgui A., Fontaliran F., Frank R., Durigon M., Fontaine G. Evidence of Apoptosis in Arrhythmogenic Right Ventricular Dysplasia. N. Engl. J. Med. 1996;335:1190–1197. doi: 10.1056/NEJM199610173351604. [DOI] [PubMed] [Google Scholar]
- 77.Rook M.B., Evers M.M., Vos M.A., Bierhuizen M.F.A. Biology of Cardiac Sodium Channel Nav1.5 Expression. Cardiovasc. Res. 2012;93:12–23. doi: 10.1093/cvr/cvr252. [DOI] [PubMed] [Google Scholar]
- 78.Burke B., Stewart C.L. The Nuclear Lamins: Flexibility in Function. Nat. Rev. Mol. Cell Biol. 2013;14:13–24. doi: 10.1038/nrm3488. [DOI] [PubMed] [Google Scholar]
- 79.Peretto G., Sala S., Benedetti S., di Resta C., Gigli L., Ferrari M., Bella P.D. Updated Clinical Overview on Cardiac Laminopathies: An Electrical and Mechanical Disease. Nucleus. 2018;9:380–391. doi: 10.1080/19491034.2018.1489195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Taylor M.R.G., Slavov D., Gajewski A., Vlcek S., Ku L., Fain P.R., Carniel E., di Lenarda A., Sinagra G., Boucek M.M., et al. Thymopoietin (Lamina-Associated Polypeptide 2) Gene Mutation Associated with Dilated Cardiomyopathy. Hum. Mutat. 2005;26:566–574. doi: 10.1002/humu.20250. [DOI] [PubMed] [Google Scholar]
- 81.Sen-Chowdhry S., Syrris P., McKenna W.J. Genetics of Right Ventricular Cardiomyopathy. J. Cardiovasc. Electrophysiol. 2005;16:927–935. doi: 10.1111/j.1540-8167.2005.40842.x. [DOI] [PubMed] [Google Scholar]
- 82.Savvatis K., Müller I., Fröhlich M., Pappritz K., Zietsch C., Hamdani N., Grote K., Schieffer B., Klingel K., van Linthout S., et al. Interleukin-6 Receptor Inhibition Modulates the Immune Reaction and Restores Titin Phosphorylation in Experimental Myocarditis. Basic Res. Cardiol. 2014;109:449. doi: 10.1007/s00395-014-0449-2. [DOI] [PubMed] [Google Scholar]
- 83.Beraldi R., Li X., Fernandez A.M., Reyes S., Secreto F., Terzic A., Olson T.M., Nelson T.J. Rbm20-Deficient Cardiogenesis Reveals Early Disruption of RNA Processing and Sarcomere Remodeling Establishing a Developmental Etiology for Dilated Cardiomyopathy. Hum. Mol. Genet. 2014;23:3779–3791. doi: 10.1093/hmg/ddu091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ouisse L.H., Remy S., Lafoux A., Larcher T., Tesson L., Chenouard V., Guillonneau C., Brusselle L., Vimond N., Rouger K., et al. Immunophenotype of a Rat Model of Duchenne’s Disease and Demonstration of Improved Muscle Strength After Anti-CD45RC Antibody Treatment. Front. Immunol. 2019;10:2131. doi: 10.3389/fimmu.2019.02131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Szabó P.L., Ebner J., Koenig X., Hamza O., Watzinger S., Trojanek S., Abraham D., Todt H., Kubista H., Schicker K., et al. Cardiovascular Phenotype of the Dmdmdx Rat—A Suitable Animal Model for Duchenne Muscular Dystrophy. Dis. Model. Mech. 2021;14:dmm047704. doi: 10.1242/dmm.047704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vakrou S., Liu Y., Zhu L., Greenland G.V., Simsek B., Hebl V.B., Guan Y., Woldemichael K., Talbot C.C., Aon M.A., et al. Differences in Molecular Phenotype in Mouse and Human Hypertrophic Cardiomyopathy. Sci. Rep. 2021;11:13163. doi: 10.1038/s41598-021-89451-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gerbino A., Forleo C., Milano S., Piccapane F., Procino G., Pepe M., Piccolo M., Guida P., Resta N., Favale S., et al. Pro-Inflammatory Cytokines as Emerging Molecular Determinants in Cardiolaminopathies. J. Cell. Mol. Med. 2021;25:10902–10915. doi: 10.1111/jcmm.16975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brayson D., Frustaci A., Verardo R., Chimenti C., Russo M.A., Hayward R., Ahmad S., Vizcay-Barrena G., Protti A., Zammit P.S., et al. Prelamin A Mediates Myocardial Inflammation in Dilated and HIV-Associated Cardiomyopathies. JCI Insight. 2019;4:126315. doi: 10.1172/jci.insight.126315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ikegami K., Stutzman A., Ikegami S., Almakki O., Liu S., Burnicka-turek O., Moskowitz I. Abstract MP230: Nuclear Rupture In A Mouse Model Of Lmna -Related Cardiomyopathy Causes Cytoplasmic Exposure Of The Proinflammatory Signaling Protein Hmgb1. Circ. Res. 2021;129:AMP230. doi: 10.1161/res.129.suppl_1.MP230. [DOI] [Google Scholar]
- 90.Homma S., Iwasaki M., Shelton G.D., Engvall E., Reed J.C., Takayama S. BAG3 Deficiency Results in Fulminant Myopathy and Early Lethality. Am. J. Pathol. 2006;169:761–773. doi: 10.2353/ajpath.2006.060250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lynch T.L., Ismahil M.A., Jegga A.G., Zilliox M.J., Troidl C., Prabhu S.D., Sadayappan S. Cardiac Inflammation in Genetic Dilated Cardiomyopathy Caused by MYBPC3 Mutation. J. Mol. Cell. Cardiol. 2017;102:83–93. doi: 10.1016/j.yjmcc.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Maier H.J., Schips T.G., Wietelmann A., Krüger M., Brunner C., Sauter M., Klingel K., Böttger T., Braun T., Wirth T. Cardiomyocyte-Specific IκB Kinase (IKK)/NF-ΚB Activation Induces Reversible Inflammatory Cardiomyopathy and Heart Failure. Proc. Natl. Acad. Sci. USA. 2012;109:11794–11799. doi: 10.1073/pnas.1116584109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.van Opbergen C.J.M., Noorman M., Pfenniger A., Copier J.S., Vermij S.H., Li Z., van der Nagel R., Zhang M., de Bakker J.M.T., Glass A.M., et al. Plakophilin-2 Haploinsufficiency Causes Calcium Handling Deficits and Modulates the Cardiac Response Towards Stress. Int. J. Mol. Sci. 2019;20:4076. doi: 10.3390/ijms20174076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pérez-Hernández M., Marrón-Liñares G.M., Schlamp F., Heguy A., van Opbergen C.J.M., Mezzano V., Zhang M., Liang F.X., Cerrone M., Delmar M. Transcriptomic Coupling of PKP2 With Inflammatory and Immune Pathways Endogenous to Adult Cardiac Myocytes. Front. Physiol. 2021;11:623190. doi: 10.3389/fphys.2020.623190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chelko S.P., Asimaki A., Lowenthal J., Bueno-Beti C., Bedja D., Scalco A., Amat-Alarcon N., Andersen P., Judge D.P., Tung L., et al. Therapeutic Modulation of the Immune Response in Arrhythmogenic Cardiomyopathy. Circulation. 2019;140:1491–1505. doi: 10.1161/CIRCULATIONAHA.119.040676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pilichou K., Remme C.A., Basso C., Campian M.E., Rizzo S., Barnett P., Scicluna B.P., Bauce B., van den Hoff M.J.B., de Bakker J.M.T., et al. Myocyte Necrosis Underlies Progressive Myocardial Dystrophy in Mouse Dsg2-Related Arrhythmogenic Right Ventricular Cardiomyopathy. J. Exp. Med. 2009;206:1787–1802. doi: 10.1084/jem.20090641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ng K.E., Delaney P.J., Thenet D., Murtough S., Webb C.M., Zaman N., Tsisanova E., Mastroianni G., Walker S.L.M., Westaby J.D., et al. Early Inflammation Precedes Cardiac Fibrosis and Heart Failure in Desmoglein 2 Murine Model of Arrhythmogenic Cardiomyopathy. Cell Tissue Res. 2021;386:79–98. doi: 10.1007/s00441-021-03488-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lubos N., van der Gaag S., Gerçek M., Kant S., Leube R.E., Krusche C.A. Inflammation Shapes Pathogenesis of Murine Arrhythmogenic Cardiomyopathy. Basic. Res. Cardiol. 2020;115:42. doi: 10.1007/s00395-020-0803-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Asimaki A., Protonotarios A., James C.A., Chelko S.P., Tichnell C., Murray B., Tsatsopoulou A., Anastasakis A., te Riele A., Kléber A.G., et al. Characterizing the Molecular Pathology of Arrhythmogenic Cardiomyopathy in Patient Buccal Mucosa Cells. Circ. Arrhythm. Electrophysiol. 2016;9:e003688. doi: 10.1161/CIRCEP.115.003688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Asimaki A., Kapoor S., Plovie E., Arndt A.K., Adams E., Liu Z.Z., James C.A., Judge D.P., Calkins H., Churko J., et al. Identification of a New Modulator of the Intercalated Disc in a Zebrafish Model of Arrhythmogenic Cardiomyopathy. Sci. Transl. Med. 2014;6:240ra74. doi: 10.1126/scitranslmed.3008008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chelko S.P., Asimaki A., Andersen P., Bedja D., Amat-Alarcon N., DeMazumder D., Jasti R., MacRae C.A., Leber R., Kleber A.G., et al. Central Role for GSK3β in the Pathogenesis of Arrhythmogenic Cardiomyopathy. JCI Insight. 2016;1:e85923. doi: 10.1172/jci.insight.85923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li D., Liu Y., Maruyama M., Zhu W., Chen H., Zhang W., Reuter S., Lin S.F., Haneline L.S., Field L.J., et al. Restrictive Loss of Plakoglobin in Cardiomyocytes Leads to Arrhythmogenic Cardiomyopathy. Hum. Mol. Genet. 2011;20:4582–4596. doi: 10.1093/hmg/ddr392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lombardi R., da Graca Cabreira-Hansen M., Bell A., Fromm R.R., Willerson J.T., Marian A.J. Nuclear Plakoglobin Is Essential for Differentiation of Cardiac Progenitor Cells to Adipocytes in Arrhythmogenic Right Ventricular Cardiomyopathy. Circ. Res. 2011;109:1342–1353. doi: 10.1161/CIRCRESAHA.111.255075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Brodehl A., Belke D.D., Garnett L., Martens K., Abdelfatah N., Rodriguez M., Diao C., Chen Y.X., Gordon P.M.K., Nygren A., et al. Transgenic Mice Overexpressing Desmocollin-2 (DSC2) Develop Cardiomyopathy Associated with Myocardial Inflammation and Fibrotic Remodeling. PLoS ONE. 2017;12:e0174019. doi: 10.1371/journal.pone.0174019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zheng G., Jiang C., Li Y., Yang D., Ma Y., Zhang B., Li X., Zhang P., Hu X., Zhao X., et al. TMEM43-S358L Mutation Enhances NF-ΚB-TGFβ Signal Cascade in Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy. Protein Cell. 2019;10:104–119. doi: 10.1007/s13238-018-0563-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shinomiya H., Kato H., Kuramoto Y., Watanabe N., Tsuruda T., Arimura T., Miyashita Y., Miyasaka Y., Mashimo T., Takuwa A., et al. Aberrant Accumulation of TMEM43 Accompanied by Perturbed Transmural Gene Expression in Arrhythmogenic Cardiomyopathy. FASEB J. 2021;35:e21994. doi: 10.1096/fj.202100800R. [DOI] [PubMed] [Google Scholar]
- 107.Basso C., Fox P.R., Meurs K.M., Towbin J.A., Spier A.W., Calabrese F., Maron B.J., Thiene G. Arrhythmogenic Right Ventricular Cardiomyopathy Causing Sudden Cardiac Death in Boxer Dogs. Circulation. 2004;109:1180–1185. doi: 10.1161/01.CIR.0000118494.07530.65. [DOI] [PubMed] [Google Scholar]
- 108.Pugliese M., la Maestra R., la Gamba G., Lanteri G., Passantino A. Arrhythmogenic Right Ventricular Cardiomyopathy in a Cat. Atti Accad. Peloritana Pericolanti Cl. Sci. Med. Biol. 2021;109:1–6. doi: 10.6092/1828-6550/APMB.109.1.2021.CCS1. [DOI] [Google Scholar]
- 109.Paulus W.J., Zile M.R. From Systemic Inflammation to Myocardial Fibrosis. Circ. Res. 2021;128:1451–1467. doi: 10.1161/CIRCRESAHA.121.318159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Herman D.S., Lam L., Taylor M.R.G., Wang L., Teekakirikul P., Christodoulou D., Conner L., DePalma S.R., McDonough B., Sparks E., et al. Truncations of Titin Causing Dilated Cardiomyopathy. N. Engl. J. Med. 2012;366:619–628. doi: 10.1056/NEJMoa1110186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kötter S., Gout L., von Frieling-Salewsky M., Müller A.E., Helling S., Marcus K., dos Remedios C., Linke W.A., Krüger M. Differential Changes in Titin Domain Phosphorylation Increase Myofilament Stiffness in Failing Human Hearts. Cardiovasc. Res. 2013;99:648–656. doi: 10.1093/cvr/cvt144. [DOI] [PubMed] [Google Scholar]
- 112.Taylor M., Graw S., Sinagra G., Barnes C., Slavov D., Brun F., Pinamonti B., Salcedo E.E., Sauer W., Pyxaras S., et al. Genetic Variation in Titin in Arrhythmogenic Right Ventricular Cardiomyopathy-Overlap Syndromes. Circulation. 2011;124:876–885. doi: 10.1161/CIRCULATIONAHA.110.005405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mueller M., Zwinger L., Klaassen S., Poller W., Monserrat Iglesias L., Pablo Ochoa J., Klingel K., Landmesser U., Heidecker B. Severe Heart Failure in the Setting of Inflammatory Cardiomyopathy with Likely Pathogenic Titin Variant. Int. J. Cardiol. Heart Vasc. 2022;39:100969. doi: 10.1016/j.ijcha.2022.100969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Henkens M.T.H.M., Stroeks S.L.V.M., Raafs A.G., Sikking M.A., Tromp J., Ouwerkerk W., Hazebroek M.R., Krapels I.P.C., Knackstedt C., van den Wijngaard A., et al. Dynamic Ejection Fraction Trajectory in Patients With Dilated Cardiomyopathy With a Truncating Titin Variant. Circ. Heart Fail. 2022;15:E009352. doi: 10.1161/CIRCHEARTFAILURE.121.009352. [DOI] [PubMed] [Google Scholar]
- 115.Gorbea C., Makar K.A., Pauschinger M., Pratt G., Bersola J.L.F., Varela J., David R.M., Banks L., Huang C.H., Li H., et al. A Role for Toll-like Receptor 3 Variants in Host Susceptibility to Enteroviral Myocarditis and Dilated Cardiomyopathy. J. Biol. Chem. 2010;285:23208–23223. doi: 10.1074/jbc.M109.047464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ono R., Iwai Y., Yamazaki T., Takahashi H., Hori Y., Fukushima K., Saotome T. Nivolumab-Induced Myositis and Myocarditis with Positive Anti-Titin Antibody and Anti-Voltage-Gated Potassium Channel Kv1.4 Antibody. Intern. Med. 2022;61:2973–2979. doi: 10.2169/internalmedicine.8772-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang Z., Wang C., Lin S., Yu X. Effect of TTN Mutations on Immune Microenvironment and Efficacy of Immunotherapy in Lung Adenocarcinoma Patients. Front. Oncol. 2021;11:725292. doi: 10.3389/fonc.2021.725292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gil-Cruz C., Perez-Shibayama C., de Martin A., Ronchi F., van der Borght K., Niederer R., Onder L., Lütge M., Novkovic M., Nindl V., et al. Microbiota-Derived Peptide Mimics Drive Lethal Inflammatory Cardiomyopathy. Science. 2019;366:881–886. doi: 10.1126/science.aav3487. [DOI] [PubMed] [Google Scholar]
- 119.Frustaci A., Russo M.A., Chimenti C. Randomized Study on the Efficacy of Immunosuppressive Therapy in Patients with Virus-Negative Inflammatory Cardiomyopathy: The TIMIC Study. Eur. Heart J. 2009;30:1995–2002. doi: 10.1093/eurheartj/ehp249. [DOI] [PubMed] [Google Scholar]
- 120.de Luca G., Campochiaro C., Sartorelli S., Peretto G., Dagna L. Therapeutic Strategies for Virus-Negative Myocarditis: A Comprehensive Review. Eur. J. Intern. Med. 2020;77:9–17. doi: 10.1016/j.ejim.2020.04.050. [DOI] [PubMed] [Google Scholar]
- 121.de Luca G., Campochiaro C., Dinarello C.A., Dagna L., Cavalli G. Treatment of Dilated Cardiomyopathy With Interleukin-1 Inhibition. Ann. Intern. Med. 2018;169:819–820. doi: 10.7326/L18-0315. [DOI] [PubMed] [Google Scholar]
- 122.Asatryan B., Asimaki A., Landstrom A.P., Khanji M.Y., Odening K.E., Cooper L.T., Marchlinski F.E., Gelzer A.R., Semsarian C., Reichlin T., et al. Inflammation and Immune Response in Arrhythmogenic Cardiomyopathy: State-of-the-Art Review. Circulation. 2021;144:1646–1655. doi: 10.1161/CIRCULATIONAHA.121.055890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Asimaki A., Tandri H., Duffy E.R., Winterfield J.R., MacKey-Bojack S., Picken M.M., Cooper L.T., Wilber D.J., Marcus F.I., Basso C., et al. Altered Desmosomal Proteins in Granulomatous Myocarditis and Potential Pathogenic Links to Arrhythmogenic Right Ventricular Cardiomyopathy. Circ. Arrhythm. Electrophysiol. 2011;4:743–752. doi: 10.1161/CIRCEP.111.964890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Beffagna G., Occhi G., Nava A., Vitiello L., Ditadi A., Basso C., Bauce B., Carraro G., Thiene G., Towbin J.A., et al. Regulatory Mutations in Transforming Growth Factor-Beta3 Gene Cause Arrhythmogenic Right Ventricular Cardiomyopathy Type 1. Cardiovasc. Res. 2005;65:366–373. doi: 10.1016/j.cardiores.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 125.Błyszczuk P. Myocarditis in Humans and in Experimental Animal Models. Front. Cardiovasc. Med. 2019;6:64. doi: 10.3389/fcvm.2019.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Haghighi K., Kolokathis F., Gramolini A.O., Waggoner J.R., Pater L., Lynch R.A., Fan G.C., Tsiapras D., Parekh R.R., Dorn G.W., et al. A Mutation in the Human Phospholamban Gene, Deleting Arginine 14, Results in Lethal, Hereditary Cardiomyopathy. Proc. Natl. Acad. Sci. USA. 2006;103:1388–1393. doi: 10.1073/pnas.0510519103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rusciano M.R., Sommariva E., Douin-Echinard V., Ciccarelli M., Poggio P., Maione A.S. CaMKII Activity in the Inflammatory Response of Cardiac Diseases. Int. J. Mol. Sci. 2019;20:4374. doi: 10.3390/ijms20184374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chen S.N., Sbaizero O., Taylor M.R.G., Mestroni L. Lamin A/C Cardiomyopathy: Implications for Treatment. Curr. Cardiol. Rep. 2019;21:160. doi: 10.1007/s11886-019-1224-7. [DOI] [PubMed] [Google Scholar]
- 129.Bonello-Palot N., Simoncini S., Robert S., Bourgeois P., Sabatier F., Levy N., Dignat-George F., Badens C. Prelamin A Accumulation in Endothelial Cells Induces Premature Senescence and Functional Impairment. Atherosclerosis. 2014;237:45–52. doi: 10.1016/j.atherosclerosis.2014.08.036. [DOI] [PubMed] [Google Scholar]
- 130.Tchkonia T., Zhu Y., van Deursen J., Campisi J., Kirkland J.L. Cellular Senescence and the Senescent Secretory Phenotype: Therapeutic Opportunities. J. Clin. Investig. 2013;123:966–972. doi: 10.1172/JCI64098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bowles N.E., Ni J., Marcus F., Towbin J.A. The Detection of Cardiotropic Viruses in the Myocardium of Patients with Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy. J. Am. Coll. Cardiol. 2002;39:892–895. doi: 10.1016/S0735-1097(02)01688-1. [DOI] [PubMed] [Google Scholar]
- 132.Wang H.Q., Meng X., Liu B.Q., Li C., Gao Y.Y., Niu X.F., Li N., Guan Y., Du Z.X. Involvement of JNK and NF-ΚB Pathways in Lipopolysaccharide (LPS)-Induced BAG3 Expression in Human Monocytic Cells. Exp. Cell Res. 2012;318:16–24. doi: 10.1016/j.yexcr.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 133.Bruno A.P., de Simone F.I., Iorio V., de Marco M., Khalili K., Sariyer I.K., Capunzo M., Nori S.L., Rosati A. HIV-1 Tat Protein Induces Glial Cell Autophagy through Enhancement of BAG3 Protein Levels. Cell Cycle. 2014;13:3640–3644. doi: 10.4161/15384101.2014.952959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cerrone M., Montnach J., Lin X., Zhao Y.T., Zhang M., Agullo-Pascual E., Leo-Macias A., Alvarado F.J., Dolgalev I., Karathanos T.V., et al. Plakophilin-2 Is Required for Transcription of Genes That Control Calcium Cycling and Cardiac Rhythm. Nat. Commun. 2017;8:106. doi: 10.1038/s41467-017-00127-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rampazzo A., Beffagna G., Nava A., Occhi G., Bauce B., Noiato M., Basso C., Frigo G., Thiene G., Towbin J., et al. Arrhythmogenic Right Ventricular Cardiomyopathy Type 1 (ARVD1): Confirmation of Locus Assignment and Mutation Screening of Four Candidate Genes. Eur. J. Hum. Genet. 2003;11:69–76. doi: 10.1038/sj.ejhg.5200914. [DOI] [PubMed] [Google Scholar]
- 136.Dubash A.D., Kam C.Y., Aguado B.A., Patel D.M., Delmar M., Shea L.D., Green K.J. Plakophilin-2 Loss Promotes TGF-Β1/P38 MAPK-Dependent Fibrotic Gene Expression in Cardiomyocytes. J. Cell Biol. 2016;212:425–438. doi: 10.1083/jcb.201507018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kant S., Holthöfer B., Magin T.M., Krusche C.A., Leube R.E. Desmoglein 2-Dependent Arrhythmogenic Cardiomyopathy Is Caused by a Loss of Adhesive Function. Circ. Cardiovasc. Genet. 2015;8:553–563. doi: 10.1161/CIRCGENETICS.114.000974. [DOI] [PubMed] [Google Scholar]
- 138.Rimpler U. Ph.D. Thesis. Humboldt-Universität; Berlin, Germany: 2014. Funktionelle Charakterisierung von Desmocollin 2 Während Der Embryonalentwicklung Und Im Adulten Herzen in Der Maus (Functional Characterization of Desmocollin-2 during the Embryonic Development and in the Adult Murine Heart) [DOI] [Google Scholar]
- 139.Beattie J., Allan G.J., Lochrie J.D., Flint D.J. Insulin-like Growth Factor-Binding Protein-5 (IGFBP-5): A Critical Member of the IGF Axis. Biochem. J. 2006;395:1–19. doi: 10.1042/BJ20060086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Garcia-Gras E., Lombardi R., Giocondo M.J., Willerson J.T., Schneider M.D., Khoury D.S., Marian A.J. Suppression of Canonical Wnt/β-Catenin Signaling by Nuclear Plakoglobin Recapitulates Phenotype of Arrhythmogenic Right Ventricular Cardiomyopathy. J. Clin. Investig. 2006;116:2012–2021. doi: 10.1172/JCI27751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Padrón-Barthe L., Villalba-Orero M., Gómez-Salinero J.M., Domínguez F., Román M., Larrasa-Alonso J., Ortiz-Sánchez P., Martínez F., López-Olañeta M., Bonzón-Kulichenko E., et al. Severe Cardiac Dysfunction and Death Caused by Arrhythmogenic Right Ventricular Cardiomyopathy Type 5 Are Improved by Inhibition of Glycogen Synthase Kinase-3β. Circulation. 2019;140:1188–1204. doi: 10.1161/CIRCULATIONAHA.119.040366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Meurs K.M., Mauceli E., Lahmers S., Acland G.M., White S.N., Lindblad-Toh K. Genome-Wide Association Identifies a Deletion in the 3 Untranslated Region of Striatin in a Canine Model of Arrhythmogenic Right Ventricular Cardiomyopathy. Hum. Genet. 2010;128:315–324. doi: 10.1007/s00439-010-0855-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cattanach B.M., Dukes-McEwan J., Wotton P.R., Stephenson H.M., Hamilton R.M. A Pedigree-Based Genetic Appraisal of Boxer ARVC and the Role of the Striatin Mutation. Vet. Rec. 2015;176:492. doi: 10.1136/vr.102821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Peretto G., De Luca G., Villatore A., Di Resta C., Sala S., Palmisano A., Vignale D., Campochiaro C., Lazzeroni D., De Gaspari M., et al. Multimodal detection and targeting of biopsy-proven myocardial inflammation in genetic cardiomyopathies: A pilot report. JACC Basic Transl. Sci. 2023. ahead of print . [DOI] [PMC free article] [PubMed]
- 145.Reina-Couto M., Pereira-Terra P., Quelhas-Santos J., Silva-Pereira C., Albino-Teixeira A., Sousa T. Inflammation in Human Heart Failure: Major Mediators and Therapeutic Targets. Front. Physiol. 2021;12:746494. doi: 10.3389/fphys.2021.746494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Raimondi F., Iserin F., Raisky O., Laux D., Bajolle F., Boudjemline Y., Boddaert N., Bonnet D. Myocardial inflammation on cardiovascular magnetic resonance predicts left ventricular function recovery in children with recent dilated cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging. 2015;16:756–762. doi: 10.1093/ehjci/jev002. [DOI] [PubMed] [Google Scholar]
- 147.Peretto G., Mazzone P., Paglino G., Marzi A., Tsitsinakis G., Rizzo S., Basso C., Della Bella P., Sala S. Continuous Electrical Monitoring in Patients with Arrhythmic Myocarditis: Insights from a Referral Center. J. Clin. Med. 2021;10:5142. doi: 10.3390/jcm10215142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Peretto G., Barzaghi F., Cicalese M.P., Di Resta C., Slavich M., Benedetti S., Giangiobbe S., Rizzo S., Palmisano A., Esposito A., et al. Immunosuppressive therapy in childhood-onset arrhythmogenic inflammatory cardiomyopathy. Pacing Clin. Electrophysiol. 2021;44:552–556. doi: 10.1111/pace.14153. [DOI] [PubMed] [Google Scholar]
- 149.Kontorovich A.R. Approaches to Genetic Screening in Cardiomyopathies: Practical Guidance for Clinicians. JACC Heart Fail. 2023;11:133–142. doi: 10.1016/j.jchf.2022.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lota A.S., Hazebroek M.R., Theotokis P., Wassall R., Salmi S., Halliday B.P., Tayal U., Verdonschot J., Meena D., Owen R., et al. Genetic Architecture of Acute Myocarditis and the Overlap with Inherited Cardiomyopathy. Circulation. 2022;146:1123–1134. doi: 10.1161/CIRCULATIONAHA.121.058457. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.