Abstract
Simple Summary
The systemic treatment of advanced sarcoma remains challenging. Conventional chemotherapy and anti-angiogenic agents, even in the most responsive histologic subtypes, result in short responses and poor clinical outcomes. In a context where new therapeutic approaches are required, several strategies of immunotherapy have emerged as promising options, such as immune checkpoint inhibitors, vaccines, and adoptive cell therapy. In this review, we aim to summarize the current state and challenges of immunotherapy in patients with advanced bone and soft-tissue sarcomas.
Abstract
There is substantial heterogeneity between different subtypes of sarcoma regarding their biological behavior and microenvironment, which impacts their responsiveness to immunotherapy. Alveolar soft-part sarcoma, synovial sarcoma and undifferentiated pleomorphic sarcoma show higher immunogenicity and better responses to checkpoint inhibitors. Combination strategies adding immunotherapy to chemotherapy and/or tyrosine–kinase inhibitors globally seem superior to single-agent schemes. Therapeutic vaccines and different forms of adoptive cell therapy, mainly engineered TCRs, CAR-T cells and TIL therapy, are emerging as new forms of immunotherapy for advanced solid tumors. Tumor lymphocytic infiltration and other prognostic and predictive biomarkers are under research.
Keywords: bone sarcoma, soft-tissue sarcoma, immunotherapy, checkpoint inhibitors, TCR, TIL, vaccines, adoptive cell therapy
1. Introduction
Sarcomas are a heterogeneous group of malignant tumors of mesenchymal origin. Their incidence in adults is low, comprising less than 1% of cancer diagnoses versus up to 15% of malignancies in the pediatric population [1]. More than 70 histologic subtypes have been identified, and they can be broadly classified into bone sarcomas (BS) and soft-tissue sarcomas (STS). The most frequent types of BS are osteosarcoma, chondrosarcoma (CS) and Ewing sarcoma (ES), whereas liposarcoma, leiomyosarcoma and undifferentiated pleomorphic sarcoma (UPS) are the most common subtypes of STS [2].
Conventional chemotherapy (CT) is still the standard treatment for unresectable or metastatic STS. Anthracyclines-based regimens, usually adriamycin plus ifosfamide, remain the upfront treatment [3], whereas other cytotoxic drugs are usually used in further lines (gemcitabine plus docetaxel [4], trabectedin [5,6], eribulin [7] or dacarbazine [8]). Several oral tyrosine–kinase inhibitors (TKI) have also demonstrated activity for STS, including multi-TKI pazopanib for non-adipocytic STS [9], anaplastic lymphoma kinase (ALK) inhibitors for myofibroblastic tumors with ALK fusions [10], and cediranib for alveolar soft part sarcoma (ASPS) [11].
In BS, multimodal treatment with CT, radiotherapy (RT) and radical surgery is recommended. For high-grade osteosarcoma, preoperative CT with a MAP regimen (doxorubicin, cisplatin, and high-dose methotrexate) is usually the front-line treatment for young patients [12]. In progressive disease, conventional CT with ifosfamide or cyclophosphamide plus carboplatin or etoposide is commonly used, with less evidence for other drugs, such as docetaxel and gemcitabine [13]. In ES, perioperative CT is indicated, usually with an interval VDC/IE scheme (vincristine, doxorubicin, cyclophosphamide, ifosfamide and etoposide) [14]. Topotecan plus cyclophosphamide and high-dose ifosfamide are the preferable options for further lines, followed by irinotecan plus temozolomide and docetaxel plus gemcitabine [15]. Several multi-TKI have also shown efficacy in advanced BS, mainly regorafenib, cabozantinib and apatinib for osteosarcoma [16].
Despite the recent incorporation of TKI and other drugs beyond conventional CT, the long-term prognosis of advanced sarcoma remains poor, with a median survival of 12–18 months for advanced STS [3], a 5-year survival rate <20% for osteosarcoma [17] and <40% for advanced ES [18]. The need for new therapeutic approaches, especially relevant given the predominance of these tumors in very young populations, explains the recurrent attempts to incorporate immunotherapy into the arsenal against advanced sarcoma.
The history of immunotherapy in sarcoma began with Coley’s inoculations of erysipelas, inducing tumor regression in some patients [19], though its development was at a standstill for many decades. High-dose interleukin-2 (IL-2) therapy, approved for advanced melanoma and renal cell carcinoma in 1998 [20,21], demonstrated some activity in pretreated pediatric sarcoma [22], though its use was restricted due to the high incidence of severe toxicity (cytokine-induced capillary leak syndrome [23]).
Modern immunotherapy with immune checkpoint inhibitors (ICI) has revolutionized the treatment of solid tumors. Though the results of ICI in monotherapy are poorer in sarcoma than in other malignancies, their combination with other agents seems to have synergistic effects, and promising strategies, such as vaccines and adoptive cell therapy, are emerging. However, there is a wide clinical heterogeneity between different histologic subtypes, disease settings and treatment categories [24]. This review aims to summarize the biological basis, current state, and future challenges of immunotherapy in advanced sarcoma.
2. Immunogenicity of Sarcoma: Anti-Tumor Response and Biomarkers
2.1. Innate Immunity and Release of Neoantigens
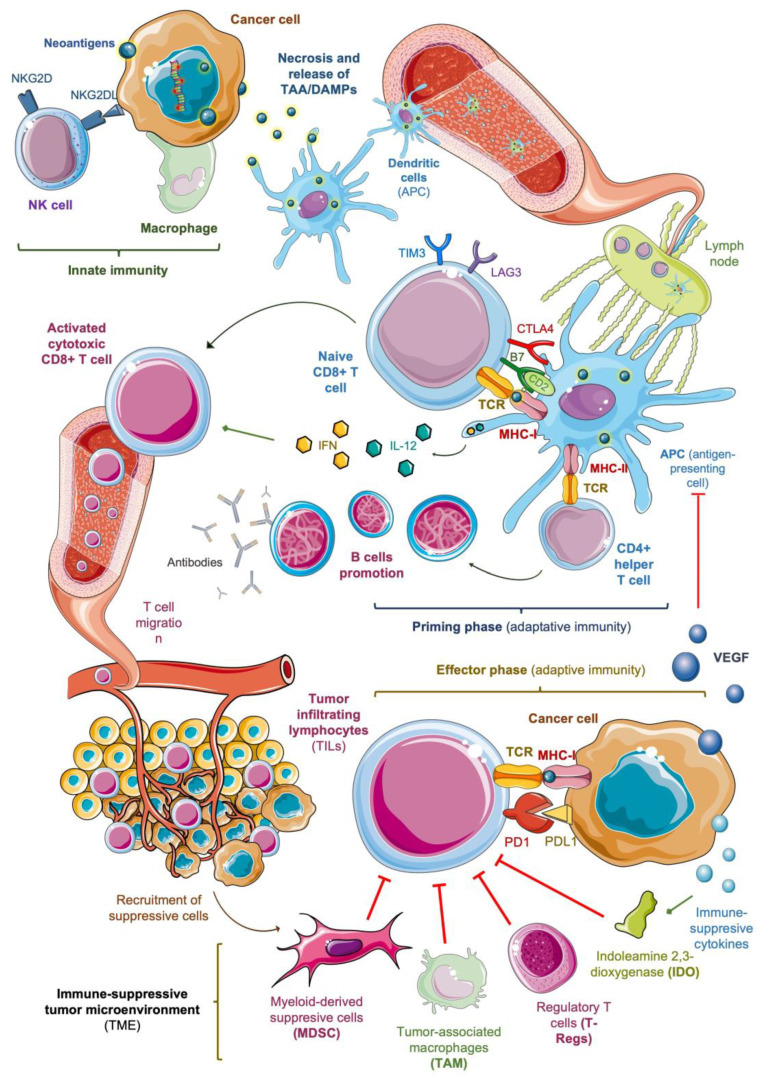
In sarcoma, as in other solid tumors, the activation and migration of cytotoxic T lymphocytes play a key role in the anti-tumor immune response [25] (Figure 1). Cancer cells are initially attacked by macrophages and natural killer (NK) cells, the main components of innate immunity, leading to cell death. Some ligands on the membrane of sarcoma cells activate NK cells, mainly through NK cell group 2D receptors (NKG2D) [26], and facilitate apoptosis. However, the essential mechanism of cell death is necrosis, with the subsequent release of tumor-associated antigens (TAA) with damage-associated molecular patterns (DAMPs), including aberrant ‘neoantigens’ produced as a result of accumulative somatic mutations [27].
Figure 1.
Mechanisms of anti-tumor response and potential immune biomarkers in solid tumors. NK: natural killer; TAA: tumor-associated antigen; DAMPs: damage-associated molecular patterns; TCR: T cell receptor; MHC: major histocompatibility complex; IL-12: interleukin-12; IFN: interferons; VEGF: vascular endothelial growth factor.
Tumors with a higher mutational burden (TMB) have an increased level of neoantigens and a higher immunogenicity [28]. Sarcoma is a heterogeneous disease with significant variability in TMB among different subtypes, as demonstrated by Chalmers et al. [29]. The median TMB exceeds 20 mutations per Mb of DNA in angiosarcoma, leiomyosarcoma and UPS but is lower than two mutations per Mb in myxofibrosarcoma, liposarcoma and synovial sarcoma. However, the neoantigens burden related to the TMB is not the only factor that determines tumor immunogenicity. The presence of certain chromosomal translocations in tumor cells give rise to fusion proteins that bind to major histocompatibility complex class I (MHC-I) molecules and activate cytotoxic CD8+ T cells, working as powerful neoantigens [30]. Worley et al. [31] showed that some subtypes of sarcoma, such as clear cell, synovial and desmoplastic round cell tumors, can be highly immunogenic due to these genetic alterations, despite their low median TMB.
Kakimoto et al. [32] revealed that some high-grade sarcoma aberrantly express testis antigens (CTA), usually present in germ cells, such as melanoma-associated antigen (MAGE)-A4 and New York esophageal squamous cell carcinoma (NY-ESO)-1. MAGE-A4 was detected in 59% of synovial sarcoma and 56% of myxoid liposarcoma. NY-ESO-1 was found in 53% of synovial sarcomas.
The expression of NY-ESO-1 was associated with a better prognosis in high-grade sarcoma (5-year overall survival of 81% in the NY-ESO1+ group vs. 53% in NY-ESO-1- group, p < 0.05), presumably due to the powerful immunogenicity conferred by these fusion proteins.
Tumors with a higher mutational burden (TMB) have an increased level of neoantigens and a higher immunogenicity [28]. Sarcoma is a heterogeneous disease with significant variability in TMB among different subtypes, as demonstrated by Chalmers et al. [29]. The median TMB exceeds 20 mutations per Mb of DNA in angiosarcoma, leiomyosarcoma and UPS but is lower than two mutations per Mb in myxofibrosarcoma, liposarcoma and synovial. However, the neoantigens burden related to the TMB is not the only factor that determines tumor immunogenicity. The presence of certain chromosomal translocations in tumor cells gives rise to fusion proteins that bind to major histocompatibility complex class I (MHC-I) molecules and activate cytotoxic CD8+ T cells, working as powerful neoantigens [30]. Worley et al. [31] showed that some subtypes of sarcoma, such as clear cell, synovial and desmoplastic round cell tumors, can be highly immunogenic due to these genetic alterations, despite their low median TMB.
2.2. Antigenic Presentation and Activation of T Cells
After necrosis, dendritic cells phagocytose the released DAMPs and migrate to lymph nodes, where they work as antigen-presenting cells (APC). APC are essential for unleashing adaptive immunity by activating naïve CD8+ T lymphocytes (priming phase). This process needs the interaction of T-cell receptors (TCR) with MHC-I molecules on the APC surface, through which the phagocytosed antigens are presented [33].
T-cell activation also requires co-stimulatory signals, such as the coupling between co-receptor B7 (CD80) and the ligand CD28 on the APC surface. Certain cytokines released by APCs, mainly type I interferons (IFN) and interleukin-12 (IL-12), promote the activity of cytotoxic T cells and contribute to the activation of CD4+ helper T cells -following the coupling of TCR and MHC class II-. This facilitates B cell promotion and antibody production. Zhou et al. [34] showed that IL-12 up-regulates the expression of Fas receptors in osteosarcoma and ES cells, increasing their sensitivity to Fas-induced apoptosis. Type I IFN (IFN-α/β) have antiangiogenic and antiproliferative properties, which have been studied in models of Kaposi sarcoma [35] and angiosarcoma [36].
On the other side, some competitive co-receptors on the T cell membrane, such as CTLA4, LAG3 and TIM3, work as co-inhibitory signals that control this process in negative feedback [37]. Dancsok et al. [38] studied 1072 sarcoma specimens, revealing LAG3 and TIM3 expression on the infiltrating T-cells of nearly 50% of them, reaching 80% in some subtypes, dedifferentiated liposarcoma, myxofibrosarcoma and UPS. These ‘immune checkpoints’ are pathologically stimulated by tumor cells as a mechanism of immune escape, which sets the rationale for the use of ICI.
2.3. Tumor Infiltration of Activated Lymphocytes
Activated cytotoxic T lymphocytes reach the tumor via blood vessels and kill malignant cells in the peripheral tissues (effector phase). The successful trafficking of T cells to the tumor site is a key component of an effective immune response [25]. Once cytotoxic T cells are primed, they undergo a shift in the expression of surface proteins, losing CD62L and CCR7, which mediate their access to lymph nodes, and gaining molecules that facilitate their migration to diseased tissues. These include selectins, which facilitate the rolling of T cells on the endothelium, and receptors for inflammatory chemokines (CXCL9, CXCL10) that mediate their extravasation [39].
Tumor-infiltrating lymphocytes (TILs) comprise different subtypes of lymphocytes with high immunogenicity against tumor cells CD4+, CD8+, CD20+ and FoxP3+ TILs. CD8+ TILs interact through their TCR with antigens presented by MHC class I molecules on the surface of cancer cells, unleashing the cytotoxic cascade that leads to necrosis. In this phase, as in the priming phase, the anti-tumor response is controlled by immune checkpoints and can be suppressed by the activation of inhibitory co-receptors of the lymphocyte, such as programmed cell death receptor PD1, due to the interaction with immune-suppressive proteins expressed by tumor cells and cells from the tumor microenvironment (TME), such as PD1 ligand PDL1- [40].
D’Angelo et al. [41] analyzed the variability in PDL1 expression in tumors, lymphocytes and macrophages among different subtypes of sarcoma. The expression of PDL1 was more frequent in lymphocytes and macrophages than in the tumor cells, where it was detected just in three histologic subtypes, gastrointestinal stromal tumor (GIST), radiation-associated pleomorphic sarcoma and spindle cell sarcoma. Globally, the PDL1 expression was positive in six samples among 50 (12%) and was significantly associated with a high density of CD8+ TILs.
A meta-analysis by Zheng et al. [42], containing 15 studies and 1451 patients with bone and soft-tissue sarcoma, concluded that high expression levels of PDL1 were associated with poorer overall survival (HR 1.27, p < 0.001) and events-free survival (HR 2.05, p < 0.001), confirming the negative prognostic role of PDL1 expression. This is consistent with other studies; Que et al. [43] showed that a positive PDL1 expression is associated with Foxp3+ T-regs infiltration and a poor clinical prognosis in STS.
Whereas the ‘tumor killing’ role mainly corresponds to cytotoxic CD8+ TILs, CD4+ cells contribute to their priming and proliferation [44]. A higher CD4+ and CD8+ TILs infiltration is related to better prognosis in several solid tumors [45]. On the contrary, a high density of TILs expressing transcription factor forkhead box protein 3 (FoxP3), a marker of immune-suppressive regulatory T cells (T-regs), has shown a positive correlation with poor clinical prognosis [46,47].
Globally, sarcoma has lower TILs infiltration than other solid tumors, with huge heterogeneity among histological subtypes. D’Angelo et al. [41] also analyzed the percentage of TILs subsets (CD3+, CD4+, CD8+ and FOXP3+) among different subtypes of sarcoma. A ‘high density’, defined as >5%- of CD3+ cells, was frequently found in GIST (41%), angiosarcoma (14%) and spindle cell sarcoma (14%). A high density of CD4+ cells was present in GIST (50%), angiosarcoma (25%) and pleomorphic rhabdomyosarcoma (25%), whereas a high density of CD8+ cells prevailed in GIST (27%) and spindle cell sarcoma (18%). The prognostic impact of TILs in sarcoma is not fully clear, though some studies suggest better survival rates in patients with higher infiltration levels of CD4+ TILs [48] and CD8+ TILs [49].
Though T cells have been the focus of anti-tumor immunity research, B cells are progressively gaining strong attention. The development of B cells in the TME depends on the maturity of tertiary lymphoid structures (TLS). TLS are ectopic lymphoid organs developed in tissues under chronic inflammation, including tumors. In immature TLS, B cells evolve as T-regs and release immune-suppressive cytokines, whereas, in mature TLS with a germinal center, B cells undergo affinity maturation and isotypic switching, resulting in plasmatic cells that secrete anti-tumor antibodies [50]. As a favorable lymphocytic infiltration, the presence of mature TLS has been associated with better clinical outcomes, and strategies to induce TLS neogenesis in immune-low tumors represent a promising pathway for cancer immunotherapy [51].
A favorable B population seems to have a key role in the response against sarcoma. Sorbye et al. [52] showed that a higher density of CD20+ TILs in STS is an independent positive prognostic factor. Petitprez et al. [53] proposed an immune-based classification of STS based on the TME composition, identifying an immune-high class E (SIC E) whose specimens were particularly rich in CD20+ TILs. They analyzed 47 patients with STS from the SARC028 trial [54] and found that a high infiltration of CD20+ cells determined the highest response rate and progression-free survival (PFS) to PD1 blockade, even in tumors with low CD8+ TILs infiltration.
2.4. Immune-Suppressive Tumor Microenvironment
The TME dynamics are affected by complex reciprocal interactions between immune-stimulatory and immune-suppressive cells. The recognition of tumor immunogenic epitopes by TILs promotes tumor regression by activating tumors into a T-cell-inflamed ‘hot’ state. On the contrary, immune-suppressive cells such as T-regs, myeloid-derived suppressor cells (MDSCs) and tumor-associated macrophages (TAMs) interfere with effector T cells and facilitate tumor immune escape [55]. The predominance of these immune-suppressive cells leads to non-T-cell inflamed or ‘cold’ tumors, promoting tumor progression and impoverishing clinical prognosis.
T-regs suppress the function of effector cells by direct contact through the interaction between granzymes and perforins with the CD8+ T cell membrane [56] but also by indirect mechanisms, such as the release of inhibitory cytokines growth factor β (TGF-β), interleukin-10 (IL-10), IL-35 and prostaglandin E2 (PGE2) [55] and the suppression of APCs through the downregulation of CD80 and other stimulatory coreceptors [57]. Smolle et al. [58] analyzed the infiltration of CD3+ FoxP3+ T-regs in 192 surgical samples of STS and found an increased risk of local recurrence in tumors with CD3+ FoxP3+ T-regs. An increasing prevalence of T cells with a regulatory phenotype (CD4+, FoxP3+) has been found in the advanced stages of Ewing [59] and Kaposi sarcoma [60].
MDSCs facilitate epithelial-to-mesenchymal transition [61], act as mediators of neo-angiogenesis through the release of vascular endothelial growth factor (VEGF), fibroblast growth factors (FGF) and matrix metalloproteinase 9 (MMP9) [62] and induce TME remodeling by establishing a ‘pre-metastatic niche’ [63]. The MDSCs trafficking to the tumor is mainly mediated by the CXCR2 receptor, which has been proposed as a potential target to alter the TME and attenuate tumor progression [64]. Sarcoma cells can produce CXCR2 ligands, such as CXCL8, that facilitate the arrival of MDSCs to the TME. Highfill et al. [65] showed that pediatric patients with advanced sarcoma display elevated serum levels of CXCL8, which are associated with poor survival rates. In murine models, the blockade of CXCR2 seems to suppress MDSCs trafficking and enhance the anti-tumor activity of PD1 blockade. These findings suggest that strategies to prevent the trafficking of MDSCs to the tumor bed may improve the efficacy of checkpoint inhibitors.
Monocyte-related MDSCs (M-MDSCs), together with circulating monocytes and tissue-resident macrophages, after being recruited to the tumor site in response to colony-stimulating factors (CSF) and several chemokines, can differentiate into TAMs [66]. TAMs can be polarized to M1-like (classically activated) or M2-like (alternatively activated) macrophages. TAMs with a M1-like phenotype display anti-tumor functions, whereas the M2-like phenotype is associated with pro-tumorigenic activity [67]. In fact, a high density of M2-like TAMs in the TME has been associated with poor clinical outcomes in many solid tumors [68].
The unfavorable prognostic role of M2-like TAMs has been established in STS. Higher levels of TAMs expressing M2-related markers (CD163+/CD204+) have been associated with poorer survival and higher disease stage in leiomyosarcoma [69], myxoid liposarcoma [70], synovial sarcoma [71] and UPS [72]. The prognostic significance of the M1/M2-phenotype in bone sarcoma is more controversial [73]. Some studies suggest a positive impact of polarized macrophages with an M1 phenotype [74], but others have found no clear correlation with survival [75]. Some studies have even reported longer survival rates in osteosarcoma [76] and ES [77] patients with a high density of M2-like TAMs.
Several cytokines released by malignant cells promote the production of indoleamine 2,3-dioxygenase (IDO1) and VEGF. IDO1 is an intracellular enzyme that reduces the activity of effector T cells through the suppression of the tryptophan pathway [25]. Some studies have suggested that the IDO1 pathway could contribute to the immune-suppressive phenotype of sarcoma cells and be a relevant mechanism of their primary resistance to PD1 blockade [78]. In fact, a high IDO1 expression may be used as a biomarker of poor response to anti-PD1 agents in sarcoma [79]. Around 39% of human sarcoma express IDO1, especially when the CD8+ TILs infiltration is high, setting a rationale for the dual blockade of IDO1 and immune checkpoints [80].
VEGF interferes with an antigenic presentation by inhibiting the maturation of dendritic cells [81], restricts the migration of lymphocytes into the tumor compartment [82], and favors the recruitment of T-regs, MDSCs and TAMs, contributing to a highly immune-suppressive TME [83]. The growth and dissemination of sarcoma strongly depend on angiogenesis, and VEGF circulating levels correlate with stage, grade, and risk of metastasis [84]. Overcoming these barriers of the TME remains a major challenge to move immunotherapy forward in advanced sarcoma.
3. Immunotherapy for Sarcoma: Clinical Results
3.1. Immune Checkpoint Inhibitors (ICIs)
The first studies with single-agent immunotherapy failed to demonstrate a significant anti-tumor activity (see Table 1). Anti-CTLA4 antibody ipilimumab in monotherapy showed negative results in recurrent synovial sarcoma [85] and pediatric sarcoma [86]. Anti-PD1 nivolumab was tried in the third line in 12 patients with advanced uterine leiomyosarcoma, with no objective responses [87].
Table 1.
Published results of immunotherapy in sarcoma. AEs: adverse effects; irAEs: immune-related adverse effects; ORR: objective response rate (RECIST criteria); mPFS: median progression-free survival; mOS: median overall survival; m: months; w: weeks; PR: partial response; SD: stable disease; DCR: disease control rate (PR+SD); mDR: median duration of response; NA: not available; AST: aspartate aminotransferase; ALT: alanine aminotransferase; BS: bone sarcoma; STS: soft-tissue sarcoma; OST: osteosarcoma; ES: Ewing sarcoma; CS: chondrosarcoma; LMS: leiomyosarcoma; LPS: liposarcoma; SS: synovial sarcoma; UPS: undifferentiated pleomorphic sarcoma; KS: Kaposi sarcoma; ALP: alkaline phosphatase; CK: creatine kinase; mCP: metronomic cyclophosphamide; AS: angiosarcoma; ASPS: alveolar soft-part sarcoma; DDLPS: dedifferentiated liposarcoma; PPS: palmar-plantar syndrome; LDH: lactate dehydrogenase; MDSCs: myeloid-derived suppressor cells; MRCL: myxoid/round cell liposarcoma; NY-ESO1: New York esophageal squamous cell carcinoma 1; MAGE-A4: melanoma-associated antigen A4; DC: dendritic cells; MLS: myxoid liposarcoma; CRS: cytokine release syndrome; RMS: rhabdomyosarcoma; adj: adjuvant; maint: maintenance; mono: monotherapy.
| Clinical Trial | Agent | Tumor | N | Age Range | Outcomes | Reported G3/G4 AEs |
|---|---|---|---|---|---|---|
| Immune checkpoint inhibitors (ICI) | ||||||
| Maki et al. (phase I/II) [85] |
Ipilimumab | Synovial sarcoma | 6 | 23–57 | ORR 0% mPFS 1.5 m mOS 8.8 m |
Nausea (50%), diarrhea (33.3%), lymphopenia (33.3%), hyperbilirubinemia (16.7%), thrombopenia (16.7%) |
| Merchant et al. (phase I) [86] | Ipilimumab | Pediatric sarcoma | 17 | 2–17 | ORR 0%; DCR 17.6% (3 SD) mPFS/mOS: NA |
Diarrhea (9%), AST/ALT increase (6%), endocrinopathies (3%), other irAEs (9%) |
| Ben-Ami et al. (phase II) [87] | Nivolumab | Uterine leiomyo-sarcoma | 12 | 29–73 | ORR 0% mPFS 1.8 m; mOS NA |
Lipase/amylase increase (8.3%), fatigue (8.3%), abdominal pain (8.3%) |
| Tawbi et al. (phase II) (SARC028) [54] | Pembrolizumab | BS cohort (22 OST, 13 ES, 5 CS) |
40 | 16–70 | mPFS 8 w; mOS 52 w OST: ORR 5%; DCR 32% (1 PR, 6 SD) ES: ORR 0%; DCR 15% (2 SD) CS: ORR 20%; DCR 40% (1 PR, 1 SD) |
Interstitial nephritis (2%), infectious pneumonia (2%), bone pain (2%), pleural effusion (2%), hypoxia (2%) |
| STS cohort (10 LMS, 10 LPS, 10 SS, 10 UPS) | 40 | 18–81 | mPFS 18 w; mOS 49 w LMS: ORR 0%; DCR 60% (6 SD) / LPS: ORR 20%; DCR 60% (2 PR, 4 SD)/SS: ORR 10%; DCR 30% (1 PR, 2 SD)/UPS: ORR 40%; DCR 70% (1 CR, 3 PR, 3 SD) |
Pulmonary embolism (2%), adrenal insufficiency (2%), pneumonitis (2%) |
||
| Blay et al. (phase II) (AcSé) [88] | Pembro-lizumab | Advanced rare sarcoma | 98 | >18 (NA) | ORR 15.3%; DCR 49% (1 CR, 14 PR, 33 SD); mDR 8.2 m; mPFS 2.8 m; mOS 19.7 m | NA |
| Delyon et al. (phase II) [89] | Pembrolizumab | Classic/ endemic KS |
17 | NA | ORR 70.1%; DCR 88% (1 CR, 10 PR, 4 SD) |
Reversible acute cardiac decompensation (6%) |
| D’Angelo et al. (phase II) (Alliance A091401) [90] |
Nivo/ipi vs. ipi | Advanced sarcoma (BS and STS) | 85 | 21–81 | ORR 16% vs. 5%; mDR 6.2 mmPFS 4.1 m vs. 1.7 m mOS 14.3 m vs. 10.7 m |
Pain (7% vs. 5%), thrombopenia (0% vs. 2%), pulmonary edema (2% vs. 0%), respiratory failure (5% vs. 5%), skin infection (2% vs. 0%), intestinal obstruction (2% vs. 2%), spinal fracture (0% vs. 2%), thrombo-embolic event (2% vs. 2%), urinary tract infection (7% vs. 2%), urinary obstruction (0% vs. 5%), fistula (2% vs. 0%), vomiting (0% vs. 2%) |
| Somaiah et al. (phase II) [91] | Durva-lumab + tremeli-mumab | Advanced sarcoma (BS and STS) | 57 | 35–59 | mPFS 2.8 m; mOS: 21.6 m; PFS at 12 m (all): 28%; PFS at 12 m (ASPS): 80% ORR (irRECIST) (all): 12%; ORR (irRECIST) (ASPS): 40% |
Lipase increase (7%), pneumonitis (6%), colitis (6%), myocarditis (4%), autoimmune disorders (4%), endocrine disorders (2%), diarrhea (2%), gastrointestinal disorders (2%), lung infection (2%), ALP increase (2%), amylase increase (2%), myositis (2%) |
| Immune checkpoint inhibitors (ICI) + conventional CT | ||||||
| Livingston et al. (phase II) [92] | Pembro + doxorubicin | Anthracy-cline naïve STS |
30 | NA | ORR 36.7%; DCR 80% (1 CR, 10 PR, 13 SD) mPFS 5.7 m; mOS 17 m PFS at 6 m: 44% PFS at 12 m: 62% |
Neutropenia (36.7%), anemia (26.7%), febrile neutropenia (16.7%), arthralgia (13.3%), lymphopenia (13.3%), nausea (13.3%), fatigue (10.0%), hyponatremia (10.0%), vomiting (10.0%), lung infection (10.0%), muscle weakness (10.0%) |
| Pollack et al. (phase I/II) [93] |
Pembro + doxorubicin | Anthracy-cline naïve STS |
37 | 25–80 | ORR 19%; DCR 78% (7 PR, 22 SD); mPFS 8.1 m; mOS 27.6 m PFS at 12 m: 27% |
Neutropenia (27.0%), oral mucositis (8.1%), anemia (5.4%), febrile neutropenia (5.4%), lymphopenia (5.4%), ejection fraction decrease (5.4%), anorexia (5.4%), diarrhea (2.7%), hypothyroidism (2.7%), nausea (2.7%), weight loss (2.7%) |
| Toulmonde et al. (phase II) [79] | Pembro + mCP | Advanced STS | 50 | 18–84 | ORR 2%; DCR 34% (1 PR, 16 SD); PFS at 6 m: 0% (LMS, UPS), 11.1% (GIST), 14.3% (others) | Anemia (7.0%), fatigue (3.5%), lymphopenia (3.5%), oral mucositis (3.5%) |
| Gordon et al. (phase I/II) (SAINT) [94] | Ipi/nivo + trabectedin (trab) | Advanced STS | 79 | NA | ORR 25.3%; DCR 87.3% (6 CR *, 14 PR, 49 SD) mPFS 6.7 m; mOS 24.6 m * One surgical CR |
ALT increase (25%), fatigue (8.7%), AST increase (8.7%), decreased neutrophil count (5.4%), anemia (4.6%) |
| Pink et al. (phase II) (NITRA-SARC) [95] | Nivo + trab | Advanced STS | 25 | NA | ORR 8%; DCR 48% (2 PR, 10 SD); mPFS 4 m | Leukopenia (47.2%), neutropenia (41.7%), thrombopenia (33.3%), increased ALT (30.6%), anemia (27.8%) |
| Smrke et al. (phase I) [96] | Pembro + gemcitabine | LMS, UPS | 13 | 40–67 | LMS (11): DCR 73% (8 SD) UPS (2): DCR 100% (2 PR) | NA |
| Nathenson et al. (phase II) [97] | Pembro + eribulin | LMS cohort | 19 | 48–80 | ORR 5.3%; DCR 26.3% (1 PR, 5 SD); mPFS 11 w | Most commonly, neutropenia, anemia, weight loss, diarrhea, lipase/ALP increase |
| Wagner et al. (phase I/II) [98] | Avelumab + trab | LMS, LPS | 23 | NA | ORR 13%; DCR 56% (3 PR, 10 SD); mPFS 8.3 m | NA |
| Toulmonde et al. (phase Ib) [99] | Durva + trab | Advanced STS cohort | 16 | NA | ORR 7%; PFS at 6 m: 28.6% | NA |
| Immune checkpoint inhibitors (ICI) + tyrosine–kinase inhibitors/antiangiogenic drugs | ||||||
| Martin-Broto et al. (phase I/II) (IMMU-NOSARC) | Nivo + suni-tinib | BS cohort (17 OST, 14 CS, 8 ES, 1 UPS) [100] | 40 | 21–74 | ORR 5%; DCR 60% (1 CR, 1 PR, 22 SD) mPFS 3.7 m; mOS 14.2 m |
Neutropenia (10%), anemia (10%), AST/ALT increase (7.5%), fatigue (5%), oral mucositis (5%), hemorrhage (2.5%), dysphagia (2.5%), thrombopenia (2.5%), malaise (2.5%), thromboembolism (2.5%), pneumonitis (2.5%) |
| STS cohort [101] | 43 | 19–77 | ORR 9.3%; DCR 69.3% (1 CR, 3 PR, 26 SD); mPFS 5.9 m; mOS not reached (follow up 6.1 m) |
AST increase (11.8%), ALT increase (9.8%), neutropenia (9.8%), fatigue (5.9%), thrombopenia (3.9%), diarrhea (3.9%), renal failure (3.9%) | ||
| Wilky et al. (phase II) [102] | Pembro + axitinib | 12 ASPS, 6 LMS, 5 UPS, 2 DDLPS, 8 others (2 BS) | 33 | 27–62 | ORR 25%; DCR 53.1% (8 PR, 9 SD); mPFS (all): 4.7 m; mOS (all): 18.7 m; mPFS (ASPS): 12.4 m; mPFS (others): 3.0 m. | Hypertension (15%), autoimmune toxic effects (15%), nausea (6%), ALT/AST increase (3%), oral mucositis (3%), diarrhea (3%), abdominal pain (3%), hemoptysis (3%), hyperlipidemia (3%) |
| Xie et al. (phase II) [103] | Camre-lizumab + apatinib | CT-refractory OST | 43 | 11–43 | ORR 20.1% (9 PR) mDR 6.2 m mPFS 6.2 m; mOS 11.3 m |
Wound dehiscence (14%), ALP increase (9.3%), AST/ALT increase (9.3%), blood bilirubin increase (9.3%), hypertriglyceridemia (7.0%), anorexia (7.0%), weight loss (7.0%), pneumothorax (7.0%), platelet count decrease (4.7%), diarrhea (4.7%), PPS (4.7%), limb pain (4.7%), leukopenia (4.7%), rash (4.7%), oral mucositis (4.7%), hypertension (4.7%), toothache (4.7%), nausea (4.7%), non-cardiac chest pain (4.7%), hypothyroidism (2.3%), LDH increase (2.3%), proteinuria (2.3%), cough (2.3%), hemorrhage (2.3%), fatigue (2.3%), peripheral neuroinflammation (2.3%) |
| Kim et al. (phase II) [104] | Durva + pazopanib | Advanced STS | 47 | NA | ORR 28.3% (1 CR, 12 PR) mPFS 8.6 m |
NA |
| Cousin et al. (phase II) [105] | Avelumab + regorafenib | Advanced STS | 43 | NA | ORR 9.3%; DCR 48.8% (4 PR, 17 SD); mDR 7.8 m; mPFS 1.8 m; mOS 15.1 m | PPS (12.2%), fatigue (10.2%), diarrhea (10.2%) |
| Kelly et al. (phase II) [106] | Pembro + epacadostat | Advanced STS | 29 | 24–78 | ORR 3%; DCR 48% (1 PR, 13 SD); mPFS 8 w; PFS at 24 w 27.9%; mOS NA | AST increase (10%), ALT increase (3%), anemia (3%), hypophosphatemia (3%), lipase increase (3%) |
| Schöffski et al. (phase Ib) [107] | Pembro + olaratumab | Advanced STS | 28 | NA | ORR 21.4%; DCR 53.5%; mDR 16.2 m; mPFS 2.7 m; mOS 14.8 m | NA |
| Immune checkpoint inhibitors (ICI) + other agents | ||||||
| Kelly et al. (phase II) [108] | Pembro + T-VEC | Advanced STS | 20 | 24–90 | ORR 35%; DCR 70% (7 PR, 7 SD); mPFS 17.1 w | Pneumonitis (5%), fever (5%), anemia (5%) |
| Chawla et al. (phase II) [109] | Trabectedin + nivo + T-VEC | Advanced sarcoma | 36 | NA | ORR 8.3%; DCR 86.1% (3 PR, 27 SD); mPFS 5.5 m; mOS 9.0 m; OS at 6 m 73% | Anemia (33.3%), ALT increase (22.2%), fatigue (11.1%), thrombopenia (11.1%), neutropenia (11.1%) |
| D’Angelo et al. (phase I) [110] | Nivo + bempegal-desleukin | Advanced STS | 84 | 13–80 | ORR 10.4% (PR: 3/8 in AS, 1/4 in ASPS, 2/10 in UPS, 1/10 in LMS, 1/10 in CS); mDR 9.3 m | Anemia (10%), lipase increase (10%), amylase increase (7%), hypertension (7%), pain (8%), thromboembolic events (5%) |
| Somaiah et al. (phase I) [111] | LV305 | STS (13 SS, 6 MRCL) | 24 | 25–72 | ORR 4.2%; DCR 62.5% (1 PR, 14 SD); mPFS 4.6 m; mOS 33 m |
No G3/G4 adverse events |
| Rosenbaum et al. (phase I) [112] | Avelumab + DCC-3014 | Advanced STS (7 LMS) | 13 | 32–71 | DCR 23% (3 SD); decreased circulating MDSCs in 5/7 patients (median 26.9%) | ALT/AST increase (31%), CK increase (23%), amylase/lipase increase (16%), anemia (8%), hypertension (8%) |
| Chawla et al. (phase I) [113] | Avelumab + SNK01 | Advanced sarcoma | 15 | 20–75 | ORR 13.3%; DCR 33.3% (2 PR, 3 SD); mPFS 11.1 w | No G3/G3 adverse events related to SNK01 |
| Therapeutic vaccines | ||||||
| Kawaguchi et al. (phase I) [114] | SYT-SSX vaccine | SS | 21 | 21–69 | DCR 50% (6 SD out of 12 assessable patients) | NA |
| Takahashi et al. (phase II) [115] | Peptide vaccine | Advanced sarcoma | 20 | 23–75 | DCR 30% (6 SD); mOS 9.6 m | NA |
| Pipia et al. (phase I/II) [116] | DC vaccine | Advanced STS | 74 | NA | Cohort 1 (adj/maint): mOS 24.4 m; cohort 2 (mono): mOS 14.2 m | NA |
| Chawla et al. (phase II) [117] | Atezo +/− CMB305 | SS, MLS | 89 | NA | mPFS 2.6 m vs. 1.6 m mOS 18 m in both groups |
4 G3/G4 events in each group (not specified) |
| Adoptive cell therapy | ||||||
| Robbins et al. (phase II) [118] | NY-ESO1 TCR | SS | 18 | 19–65 | ORR 61% (1 CR, 10 PR); PFS 3–47 m; estimated 3-y OS: 38% | No toxicities attributed to the transferred cells * |
| D’Angelo et al. (phase II) [119] | NY-ESO1 TCR | SS | 45 | NA | ORR 33% (1 CR, 14 PR); mPFS 8.6–22.4 w | No toxicities attributed to the transferred cells * |
| Van Tine et al. (phase I) [120] | MAGE-A4 TCR | SS | 8 | NA | ORR 50%; DCR 87.5% (4 PR, 3 SD) |
No toxicities attributed to the transferred cells * |
| D’Angelo et al. (phase II) [121] | MAGE-A4 TCR | STS (23 SS,2 MLS) | 25 | 24–73 | ORR 40%; DCR 84% (2 CR, 8 PR, 11 SD) |
CRS (5%) * |
| Ahmed et al. (phase I/II) [122] | Her2-CAR T cells | Advanced sarcoma (16 OST, 3 others) | 19 | 7–29 | DCR 23.5% (4 SD); mOS 10.3 m | Anemia (5.3%), muscle weakness (5.3%), back pain (5.3%) |
| Navai et al. (phase I) [123] | Her2-CAR T cells | Advanced sarcoma (5 OST, 5 others) | 10 | 4–54 | ORR 20%; DCR 50% (2 CR, 3 SD) (CR in 1 OST and 1 RMS) | No toxicities attributed to the transferred cells * |
* All patients experienced transient neutropenia and thrombopenia induced by the lymphodepleting CT with fludarabine plus cyclophosphamide and the transient toxicities associated with IL-2 infusion.
The first immunotherapy trial with positive results in sarcoma was phase II SARC028 with anti-PD1 pembrolizumab, including a cohort for BS and a cohort for STS [54]. In the BS cohort, there were only two objective responses (one osteosarcoma and one CS). In the STS cohort, with a total of 40 patients, there were seven responses (17.5%): four in UPS (including one complete response), two in dedifferentiated liposarcoma (DDLPS) and one in synovial sarcoma. Two expansion cohorts in UPS and DDLPS reported an objective response rate (ORR) of 23% and 10%, respectively [124]. Keung et al. [125] showed that patients from SARC028 who responded to pembrolizumab had higher densities of activated CD8+ TILs and an increased pre-treatment percentage of TAMs expressing PDL1 compared to non-responders.
Liu et al. [126] confirmed the activity of pembrolizumab in advanced STS in a real-world study, reporting an overall ORR of 19.4%.
Following the results of SARC028, a phase II randomized trial is currently studying neoadjuvant pembrolizumab combined with RT in high-risk UPS or DDLPS of the extremities (SUC2C-SARC032) [127]. Another phase II trial (STEREOSARC) is evaluating concomitant RT with atezolizumab in oligometastatic STS [128]. There is a rationale for combining ICIs and RT since RT induces the release of TAA following immunogenic cell death, which activates TILs and leads to the recruitment of more effector cells to the TME [129]. The primary endpoint is the progression-free survival rate at 6 months. If the results of these studies are favorable, ICIs may also gain ground in the context of early-stage disease.
ICIs have also been tested in less common histologic subtypes. The phase II trial AcSé evaluates pembrolizumab in different cohorts of patients with rare cancers. A total of 98 patients were enrolled in the sarcoma cohort [88], including 34 with chordoma, 14 with ASPS, 11 with SMARCA4-deficient malignant rhabdoid tumor (SMRT), 8 with desmoplastic small round cell tumor (DSRCT) and 31 with other histotypes. There were seven objective responses in ASPS (50%), three in SMRT (27%), one in DSCRT (12.5%), three in chordoma (8.8%) and one in other histotypes (3.2%). The greatest rates of PFS at 12 months were observed in ASPS (35.7%), chordoma (31.2%) and SMRT (18.2%).
SMARCA4-deficient thoracic sarcoma is a newly described entity of thoracic sarcomas that is associated with a poor prognosis. Partial responses to PD1/PDL1 blockade have been reported in PDL1-positive SMARCA4-deficient thoracic sarcomas with pembrolizumab [130,131,132], nivolumab [133] and atezolizumab plus bevacizumab combined with CT [134,135]. Marcrom et al. [136] reported a complete response of mediastinal clear cell sarcoma to pembrolizumab combined with RT. Yu et al. [137] reported a major response to pembrolizumab in an adult patient with an undifferentiated embryonal sarcoma of the liver. In metastatic myxofibrosarcoma, partial responses have been reported with pembrolizumab [138] and atezolizumab plus temozolomide [139]. These data, together with the data from AcSé trial, suggest that ICIs may be useful for certain subtypes of rare sarcoma, especially when selected by immune biomarkers such as the PDL1 expression.
A phase II trial with pembrolizumab in 17 patients with classic/endemic Kaposi sarcoma (KS) (71% of them pretreated with CT) showed 12 objective responses (ORR 70.1%), with 2 complete responses (CR) and 4 partial responses (PR) [89]. Interestingly, the lack of PDL1 expression on tumor and immune cells was associated with worse outcomes. Tabata et al. [140] reported a maintained response to ipilimumab plus nivolumab in a HIV-negative KS.
Nivolumab with and without ipilimumab was studied by D’Angelo et al. [90] in a phase II trial (Alliance A091401) in 85 patients with advanced sarcoma (BS and STS) after at least one previous line of treatment. The primary endpoint was the response rate. There were six objective responses in the combination group (16% vs. 5% in the monotherapy group), with a median duration of response of 6.2 months. There was a significant benefit for the combination group in terms of median PFS (4.1 m vs. 1.7 m) and median overall survival (OS) (14.3 m vs. 10.7 m). Responses occurred in UPS, LMS, myxofibrosarcoma, and angiosarcoma. Grade 3–4 toxicity was higher in the combined treatment (14 vs. 7%). Zhou et al. [141] reported two cases of PDL1-negative STS (DDLPS and myxofibrosarcoma) with long-term responses to ipilimumab plus nivolumab.
In the first-line setting, a retrospective study of nivolumab with or without ipilimumab in PDL1-positive STS found a significant benefit for the combination group in terms of ORR (13% vs. 7%), median PFS (4.1 m vs. 2.2 m) and median OS (12.2 m vs. 9.2 m) [142]. A similar ORR (15%) was observed in a retrospective study with ipilimumab plus nivolumab for advanced STS [143]. The combination ipilimumab plus nivolumab is also being evaluated by a phase II trial in patients with pre-treated classic KS (NCT03219671), with a promising ORR of 50% in an interim analysis [144].
A recent phase II trial has analyzed the combination of anti-CTLA4 tremelimumab plus anti-PDL1 durvalumab in 57 patients with advanced STS and BS after at least one line of systemic treatment [91]. The most represented subtypes were ASPS (18%), LPS (10%) and vascular tumors (18%). The median PFS and OS for all subtypes were 2.8 months and 21.6 months, respectively. The median PFS at 12 weeks (the primary endpoint) was 49% (95% CI 36–61). Global ORR was 12%, though there were significant differences among histological subtypes, with the greatest benefit observed in the ASPS subgroup (ORR 40%, including 2 CR). The authors concluded that tremelimumab and durvalumab is an active treatment for advanced sarcoma.
Lewin et al. have reported two ASPS with a durable response to durvalumab alone or combined with tremelimumab, confirming their activity in this subtype [145]. A randomized phase II trial (MEDI-SARC) is currently comparing durvalumab plus tremelimumab to CT (doxorubicin) in naïve-treatment STS [146]. Anti-PDL1 avelumab has been tried in recurrent osteosarcoma with negative results [147].
The combined blockade of PD1 and LAG3 is another encouraging strategy. A basket phase II study of anti-PD1 spartalizumab plus anti-LAG3 LAG525 in advanced solid tumors included a cohort of 10 patients with advanced sarcoma, reporting a disease control rate (DCR) of 40% at 24 weeks [148]. The combination nivolumab plus anti-LAG3 relatlimab, clearly superior to nivolumab alone in untreated melanoma according to the phase III trial RELATIVITY-047 [149], is being evaluated in metastatic STS by a phase II study (NCT04095208).
According to these results, there is not enough evidence to support the use of ICIs as monotherapy in the first-line setting of advanced sarcoma since the previous trials included mainly pre-treated patients. However, given the scarcity of therapeutic alternatives for CT-refractory patients, the authors consider that treatment with ICIs might be considered after progression to standard CT, especially in patients with immunogenic subtypes of STS (classic/endemic KS, UPS, synovial sarcoma and dedifferentiated liposarcoma). Dual blockade of CTLA4 and PD(L)1 with nivolumab plus ipilimumab or durvalumab plus tremelimumab seems to offer higher response rates and may be preferable to single-agent immunotherapy in fit patients. Although imperfect, predictive biomarkers such as high TMB, high PDL1 expression or dense lymphocytic infiltration may help clinicians decide to use ICIs in this subgroup of patients.
Several additional trials with monotherapy or a combination of ICIs are currently ongoing [25], such as anti-PDL1 atezolizumab alone or plus bevacizumab in ASPS (NCT03141684) and ipilimumab plus nivolumab in classical Kaposi sarcoma (NCT03219671).
3.2. Combination of ICIs and Conventional Chemotherapy
The combination of immunotherapy with CT is a promising approach to enhance antitumor activity in sarcoma. The DNA damage caused by cytotoxic drugs results in cell death, with the subsequent release of DAMPs and proteins that work as ‘danger signals’, upregulating PD1 and enhancing the activity of effector lymphocytes [150].
A phase II trial that studied pembrolizumab in combination with doxorubicin in 30 patients with unresectable STS, with no previous anthracycline therapy, showed interesting results, with a DCR of 80% and a global ORR of 36.7% [92]. The subtypes with the highest ORR were UPS (4/4 patients), epithelioid angiosarcoma (1/1 patient), leiomyosarcoma (4/10 patients) and liposarcoma (2/7 patients). The median PFS and OS were 5.7 months and 17 months, respectively. In this study, PDL1 expression was associated with improved ORR. The authors concluded that the combination of pembrolizumab and doxorubicin has manageable toxicity and promising activity in advanced STS.
Pollack et al. [93] performed another phase I/II trial with pembrolizumab plus doxorubicin in 37 anthracycline-naïve patients with advanced STS, reporting an ORR of 19%, a median PFS of 8.1 months and a median OS of 27.6 months. Similarly, durable partial responses were observed in two of three patients with UPS and two of four patients with DDLPS. A retrospective study of pembrolizumab plus doxorubicin in 21 patients with STS showed a similar DCR (71.4%) [151]. As in the clinical trials, patients with UPS, synovial sarcoma and angiosarcoma showed higher response rates.
The combination of pembrolizumab plus metronomic cyclophosphamide showed limited activity in the phase II trial PEMBROSARC [79], with just 1 partial response among 50 advanced STS. Interestingly, the response was observed in the only case, a solitary fibrous tumor, with a PDL1 expression >10% in immune cells.
Italiano et al. [152] demonstrated that the presence of tertiary lymphoid structures (TLS) was a powerful predictor of response among the patients from the PEMBROSARC trial, with an ORR of 40% in TLS+ and 26.7% in TLS- tumors. New strategies are needed to induce TLS neogenesis and sensitize TLS-negative tumors to immunotherapy. In fact, a cohort of 20 TLS- patients from the PEMBROSARC study were treated with pembrolizumab, low-dose cyclophosphamide and intra-tumoral injection of the toll-like receptor 4 (TLR4) agonist G100, which would potentially enhance the immune response against a TLS-negative TME [153]. G100 seemed to modulate the TME, increasing TILS infiltration, though the ratio CD8+/FoxP3+ decreased in 11 out of 14 assessable cases, suggesting a predominant recruitment of T-regs-, a finding that may explain the modest clinical results (PFS of 11.8% at 6 months).
A phase I/II clinical trial (SAINT) has analyzed the dual CTLA4/PD1 blockade with ipilimumab plus nivolumab added to trabectedin in advanced STS, with encouraging results. Among 79 patients enrolled in the phase II study, there was a DCR of 87.3% (6 CR, 14 PR, 49 SD), with a median PFS of 6.7 months and a median OS of 24.6 months [94]. A retrospective study of nivolumab alone combined with trabectedin in 28 pre-treated STS found a DCR of 72.7% (4 PR and 12 SD among 22 assessable patients), with a median PFS at 6 months of 68.2% [154].
The results of trabectedin combined with dual immune blockade (ipi/nivo) seem better than its combination with nivolumab alone, which obtained modest results in the phase II trial NITRASARC (2 PR among 25 evaluable patients, with an mPFS of 4 months) [95].
The phase I trial GEMMK [96] is studying the combination of pembrolizumab plus gemcitabine in STS; among 13 patients included, there were 11 leiomyosarcomas (LMS) (with 8 SD and 3 PD) and 2 UPS (with 2 PR). A phase II trial of pembrolizumab plus eribulin in STS (NCT 03899805) has reported preliminary data from the LMS cohort (19 patients), with limited efficacy (ORR 5.3% and DCR 26.3% after 12 weeks) [97].
A phase I/II study has evaluated anti-PDL1 avelumab combined with trabectedin in 33 patients with advanced liposarcoma and LMS, with an ORR of 13%, a DCR of 56% (3 PR, 10 SD) and a median PFS of 8.3 months [98]. Trabectedin has also been combined with durvalumab by a phase Ib study (TRAMUNE) in 16 patients with STS, with an ORR of 7% and a 6-month PFS of 28.6% [99].
The heterogeneity in the selection of patients may explain the significant differences in the results of these studies with combination strategies. The best results seem to be obtained with the early use of ICI+CT, particularly with dual immune blockade (CTLA4/PD1). As monotherapy with ICIs, the combination of ICI+CT seems to obtain better response rates in certain histologic subtypes of STS (such as liposarcoma and UPS). In our opinion, there are promising data supporting the combination of nivo/ipi plus trabectedin for advanced STS in anthracycline-refractory patients, as well as pembro plus doxorubicin in anthracycline-naïve patients. Phase III trials comparing these schemes to the standard treatment would be helpful to confirm these results and incorporate the ICI+CT combinations into the first-line of systemic treatment.
Several ongoing phase I/II clinical trials are studying combinations of different cytotoxic drugs with pembrolizumab (NCT 03899805, NCT 03123276, NCT 04332874), nivolumab (NCT 04535713, NCT 03590210) and durvalumab (NCT 03802071) [25]. Their results will hopefully help us identify proper ICI/CT synergistic combinations with manageable toxicity.
3.3. Combination of ICIs and Tyrosine–Kinase Inhibitors
Tyrosine–kinase inhibitors (TKIs) are progressively gaining ground in CT-refractory sarcoma. In addition to blocking the immune-suppressive effect of VEGF [83], multi-target TKIs, especially lenvatinib and cabozantinib, seem to decrease the arrival of MDSCs/TAMs to the TME, and increase the infiltration of dendritic cells, NK cells and CD8+ lymphocytes [155]. This favorable immune-modulating effect provides a rationale for their combination with ICIs.
The combination of nivolumab and sunitinib in advanced sarcoma has been studied by the phase I/II trial IMMUNOSARC, including 40 patients with BS and 50 patients with STS. In the BS cohort, there was 1 CR (2.5%), 1 PR (2.5%) and 22 SD (55%) among 40 assessable patients, with a median PFS of 3.7 months [100]. In the STS cohort, there was 1 CR (2.3%), 3 PR (7%) and 26 SD (60%) among 43 evaluable patients, with a median PFS of 5.9 months [101].
A phase II clinical trial [102] has evaluated pembrolizumab plus axitinib in 33 patients with advanced sarcoma, mainly STS (12 ASPS, 6 LMS, 5 UPS, 2 DDLPS and 8 other subtypes, including 2 BS). 51% of patients had received prior treatment with TKIs, and 15% of them with immunotherapy. Among 32 assessable patients, the ORR was 25% (8 PR) and the median PFS was 4.7 months in the intention-to-treat analysis. Six of the eight patients with a PR had ASPS, a subgroup in which this combination seems especially active (ORR 50%), as confirmed by a post-hoc analysis, median PFS of 12.4 months in the ASPS subgroup. Dorman et al. [156] reported a case of ASPS with a durable (15 months) response to pembrolizumab plus axitinib, in consonance with the previous data. The rarity of ASPS, which comprise just 1% of STS, and its unique biology, which seems to make it especially responsive to ICI/TKI combinations, have led some authors to warn about the potential skew of unselected STS studies that include a high proportion of this subtype.
The phase II trial APFAO [103] evaluated anti-PD1 camrelizumab in combination with apatinib in 43 CT-refractory osteosarcomas, with an ORR of 20.9% and a median PFS of 6.2 months. A PDL1 tumor proportion score >5% and lung metastasis correlated with longer PFS. A retrospective study in 33 STS (8 ASPS, 5 LMS, 3 UPS and 17 others) showed an interesting activity of this combination added to local therapy with radiofrequency ablation or transarterial chemoembolization [157].
Durvalumab plus pazopanib have also shown encouraging activity in advanced STS in a phase II trial with 47 patients, with an ORR of 28.3% (1 CR and 12 PR), a median duration of response of 11 months and a median PFS of 8.6 months [104]. Combinations of pazopanib with PD1 inhibitors have been explored by retrospective studies. Paoluzzi et al. [158] treated 28 patients (24 STS and 4 BS) with nivolumab (plus pazopanib in 18 cases), with a DCR of 50% and 3 PR (one CS, one epithelioid sarcoma and one maxillary osteosarcoma, last two patients on pazopanib). Arora et al. [159] reported a sustained response to pembrolizumab plus pazopanib in a patient with advanced UPS.
The combination of avelumab plus regorafenib has been tried by a phase II basket trial in advanced solid tumors (REGOMUNE), with an STS cohort including 49 patients (22 LMS, 9 synovial sarcomas, 4 liposarcomas, 4 UPS and 10 other subtypes) [105]. The results were similar to other combinations of ICI + TKIs, with 4 PR among 43 evaluable patients and a DCR of 48.8%.
Given the known immune-suppressive role of IDO1 in sarcoma, explained above in detail, there is a rationale for combining ICIs with TKI against IDO1. A phase II trial with pembrolizumab plus IDO1 inhibitor epacadostat showed modest efficacy in 29 pre-treated advanced sarcomas (ORR 3%, DCR 48%) [106]. A phase I trial has shown promising results of pembrolizumab plus olaratumab PDGFR inhibitor in 28 patients with advanced STS (ORR 21.4%, DCR 53.5%) [107].
In our opinion, the combination of ICI/TKI (nivolumab/sunitinib, pembrolizumab/axitinib, durvalumab/pazopanib or avelumab/regorafenib) should be considered in patients with advanced STS after progression to standard CT. The authors believe that further research is required to evaluate the benefits of these combinations compared to ICI or TKI monotherapy. It would also be interesting to evaluate this strategy in the first-line setting of patients unfit for anthracycline-based CT.
Several ongoing trials will assess the combination of ICIs with TKIs and other antiangiogenic drugs [25], including lenvatinib plus pembrolizumab (NCT 04784247), cabozantinib plus nivolumab and ipilimumab (NCT 04551430), anlotinib plus toripalimab (NCT 04172805), olaparib or cediranib plus durvalumab (NCT 03851614), epacadostat plus pembrolizumab (NCT 03414229), bevacizumab plus atezolizumab (NCT 03141684), tivozanib plus atezolizumab (NCT 05000294), dasatinib plus ipilimumab (NCT 01643278) and axitinib plus avelumab (NCT04258956).
3.4. Combination of ICIs and Other Agents
Various studies have explored the combination of ICIs with other immunomodulatory therapies. One of the most promising agents is talimogene laherparepvec (T-VEC), a modified immune-enhanced herpes simplex virus (HSV) type 1, engineered for intratumoral injection. It contains the coding sequence for granulocyte–macrophage colony-stimulating factor (GM-CSF) and proinflammatory cytokines, inducing an immune response against tumor cells. Preoperative RT plus T-VEC has been studied in 30 locally advanced STS, with modest results [160].
A phase II trial has evaluated T-VEC plus pembrolizumab in 20 cases of advanced sarcoma (after at least one standard therapy), with promising results (7 PR, with a median duration of response of 56 weeks, and 7 SD) and an acceptable safety profile. The most represented subtypes were LMS (25%), cutaneous angiosarcoma (15%) and UPS (10%) [108]. The mean TIL score was higher in responsive patients. A phase II trial has analyzed T-VEC plus nivolumab and trabectedin in 36 advanced sarcomas, including chordoma and desmoid tumor heavily pre-treated patients, with a median of 4 previous lines, with encouraging results: 3 PR, 27 SD (DCR 86.1%) and a median PFS of 5.5 months (being the median PFS of the immediately prior therapy 2.0 months) [109].
Bempegaldesleukin (NKTR-214), an interleukin-2 (IL-2) agonist, increases TIL infiltration and has shown encouraging activity in several refractory tumors [161], setting a rationale for its addition to PD1 inhibitors. A pilot study combining this agent with nivolumab in 84 patients showed positive results in angiosarcoma (3 PR out of 8), ASPS (1 PR out of 4) and UPS (2 PR out of 10) [110]. There was a correlation between the ORR, CD8+ TIL infiltration and PDL1 expression. Interestingly, the reduced expression of the Hedgehog signaling pathway was associated with better clinical outcomes, suggesting that the Hedgehog pathway enhances immune-suppressive mechanisms as it has been found in breast cancer [162] and skin basal cell carcinoma [163] and setting a rationale for combining immunotherapy with Hedgehog inhibitors.
Oleclumab is a monoclonal antibody that selectively binds and blocks the activity of CD73, a key enzyme for the extracellular generation of adenosine, which is a relevant biochemical component of the immunosuppressive TME [164]. An ongoing phase II study is evaluating the combination of durvalumab and oleclumab in specific sarcoma subtypes selected according to CD73 staining on the TME cells [165]. A basket phase II trial (CAIRE) is evaluating durvalumab plus tazemetostat, a molecule that inhibits EZH2, which leads to a functional alteration of Tregs and favors an effector-like profile in advanced solid tumors, including a cohort for STS [166].
Another approach is the modulation of immune cells in vivo. LV305 is a modified lentivirus-based vector designed to selectively transduce dendritic cells and promote the expression of NY-ESO1, unleashing an immune response against NY-ESO1-expressing cells. Somaiah et al. [111] tried LV305 in 24 patients (13 SS, 6 MRCL and 5 other subtypes), reporting 1 PR (SS) and 14 SD. NY-ESO1 expression was >75% in 67% of the subjects. A phase I study has evaluated avelumab plus SNK01, a therapy based on autologous modified NK cells, with enhanced cytotoxicity, in 15 patients with advanced sarcoma (6 LMS, 2 osteosarcoma and 7 other subtypes) [113], with 2 PR and 3 SD.
Other strategies to modulate the immune-suppressive TME of sarcoma have been explored in preclinical studies and early trials. Colony-stimulating factor 1 receptor (CSF1R) signaling regulates the infiltration of MDSCs and promotes their differentiation towards an M2 phenotype [167]. A phase Ib study has explored the combination of durvalumab with DCC-3014 (vimseltinib), a selective inhibitor of CSF1R, in 13 patients with advanced STS (7 leiomyosarcoma, 2 UPS, 2 DDLPS, 1 synovial sarcoma and 1 liposarcoma) [112]. There was a median decrease of 26.9% in circulating MDSCs, with disease stabilization in three patients.
3.5. Therapeutic Vaccines
Cancer vaccines are usually based on the exogenous administration of selected tumor antigens combined with adjuvants to induce the activity of APCs, mainly dendritic cells, aiming to stimulate the adaptive immune system against cancer cells [168].
Sato et al. [169] demonstrated that peptides derived from SYT-SSX fusion genes, resultant from chromosomal translocation t(X:18) specific to synovial sarcoma (SS), are recognized by circulating CD8+ T-cells in HLA-A24+ patients, and induce tumor-specific cytotoxic responses. They developed an SYT-SSX peptide vaccine and tried it on six patients with advanced SS, with no response [114]. However, they performed a second study adding interferon-alfa to the vaccine in 12 patients, observing 6 SD and a transient response in 1 patient [170].
Takahashi et al. [115] conducted a phase II clinical trial with a personalized vaccine in 20 patients with refractory BS and STS. A maximum of four HLA-matched peptides were selected, based on their high peptide-specific IgG responses in pre-vaccination plasma, and administered to each patient weekly for 6 weeks and each 2 weeks thereafter; six patients achieved SD, including one minor response in a malignant fibrous histiocytoma, and one durable SD (33 months) in a mediastinal SS. The authors concluded that this treatment could be feasible for the vast majority of refractory sarcoma patients, with high rates of immunological responses.
Neoantigens such as cancer–testis antigens (CTA), aberrantly expressed in a high percentage of high-grade sarcoma, mainly MAGE and NY-ESO, as explained above in detail, might also be used to design personalized vaccines [32]. A phase I/II trial has evaluated an autologous dendritic-cell vaccine based on CTA (CaTeVac) in 74 patients with advanced STS, with a cohort receiving the vaccine as adjuvant or maintenance treatment after the first or second line of systemic therapy and another cohort receiving it in monotherapy after at least one CT line [116]. Median OS was 24.4 months in the first cohort and 14.2 months in the second one, suggesting a positive impact on overall survival. A phase I study tried a dendritic cell vaccine-targeting MAGE-A1, MAGE-A3 and NY-ESO1 combined with decitabine in 10 children with neuroblastoma, Ewing’s sarcoma, osteosarcoma and rhabdomyosarcoma. Six of nine patients developed a response to MAGE-A1, MAGE-A3 or NY-ESO-1 peptides post-vaccine, concluding that the chemoimmunotherapy approach using DAC/DC-CT vaccine is feasible [171].
More recently, Chawla et al. [117] have published a phase II randomized study of CMB305 and atezolizumab compared with atezolizumab alone in STS expressing NY-ESO1. CMB305 is a vaccination regimen designed to prime the CD8+ T-cell population specific for NY-ESO1 and then use a TLR4 agonist to unleash the antitumor immune response. The study recruited 89 patients with SS and myxoid liposarcoma (MLS). Though the PFS increase was not significant, the patients treated with the combination acquired a higher rate of NY-ESO1-specific T-cells, which was associated with longer OS in a post hoc analysis.
In addition to vaccines based on specific peptides, a potential approach to induce tumor recognition is the production of vaccines derived from whole tumor cells combined with immune-enhancing adjuvants (such as IFN-γ and GM-CSF), with interesting preliminary data in a cohort of STS [172].
3.6. Adoptive Cell Therapy
Adoptive cell therapy (ACT) encompasses several strategies to improve the activity of autologous T cells, which are obtained through leukapheresis and genetically engineered to overcome tumor immune evasion. Modified T cells are reinfused into the patient, usually in combination with adjuvant IL-2, after lymphodepleting CT (commonly fludarabine plus cyclophosphamide). ACT includes chimeric antigen receptor (CAR) T cell therapy, engineered TCRs and TIL therapy [173].
3.6.1. Engineered TCR
In sarcoma, the aberrant expression of CTA not present in normal cells makes TCR therapy particularly attractive since T cells can be equipped with newly engineered TCRs transduced through retroviral vectors that enable them to target these specific neoantigens.
Robbins et al. [118] conducted a phase II trial with letetresgene autoleucel (Lete-cel), a therapy based on T cells transduced with a TCR against NY-ESO1, plus adjuvant IL-2- in HLA-A02 patients with metastatic melanoma and synovial sarcoma. Among 18 patients with NY-ESO1+ SS, 11 objective responses were documented (61%), including 1 CR and 10 PR (ranging from 3 to 47 months). The estimated overall 3-year OS rate was 38%. The authors concluded that the adoptive transfer of autologous T cells transduced with a retrovirus encoding a TCR against an HLA-A*0201 restricted NY-ESO-1 epitope can be an effective therapy for synovial cell sarcomas. Considering that all the patients were heavily pre-treated and refractory to standard CT, the authors consider it improbable that the lymphodepleting therapy could significantly contribute to the clinical responses.
More recently, Lete-cel has been evaluated in 45 patients with advanced SS after standard first-line CT, enrolled in 4 cohorts according to NY-ESO1 tumor expression [119]. Objective responses were documented in all cohorts, with a total of 1 CR and 14 PR (ORR 20–50%). The median PFS ranged from 8.6 to 22.4 weeks between cohorts. A post-hoc analysis revealed that responders had higher pre-infusion levels of IL-15, which may be used as a predictive biomarker [174]. NY-ESO1-targeted TCR therapy is also being evaluated in myxoid/round cell liposarcoma (MRCL) [175].
A first-in-human study with T cells equipped with afamitresgene autoleucel (Afami-cel), a genetically engineered autologous specific peptide enhanced affinity receptor (SPEAR) targeting MAGE-A4, reported 4 PR and 3 SD among eight assessed patients with advanced SS [120]. Following these results, Afami-cel was evaluated by a phase II trial (SPEARHEAD-1) in 32 eligible patients (HLA-A02) with MAGE-A4-expressing STS (87.5% SS, 12,5% MLS) [121]. Among 25 evaluable subjects (23 SS and 2 MLS), there were 2 CR, 8 PR and 11 SD (DCR 84%). The safety profile was favorable, with mainly low-grade cytokine release syndrome (CRS) and reversible hematologic toxicities due to lymphodepleting CT. A pooled efficacy analysis of phase I and II trials with Afami-cel revealed that the baseline tumor burden, prior systemic treatment history and MAGE-04 expression levels are potential predictors of response [176].
3.6.2. CAR T Cells
The use of CAR T cells has also been explored in sarcoma, with the advantage over TCR therapy of not being restricted to HLA-A02 carriers. In contrast to hematologic malignancies, the efficacy of CAR T cells in solid tumors is limited by their intense antigenic heterogeneity derived from their polyclonal expansion and accumulative mutations, which makes it hard to find homogeneously expressed targets, particularly without unacceptable off-tumor toxicity [177].
Human epidermal growth factor receptor 2 (Her2) is the most studied target for CAR T cells in sarcoma. A phase I/II trial evaluated Her2-CAR T cells in 19 Her2+ sarcoma (16 of them osteosarcoma), with modest activity (4 SD ranging from 12 weeks to 14 months). Her2-CAR T cells persisted for at least 6 weeks in seven of the nine evaluable patients. Three of these patients had their tumors removed, with one showing ≥90% necrosis. The median overall survival was 10.3 months (ranging 5.1 to 29.1 months) [122].
In a phase I trial with Her2-CAR T cells in 10 Her2+ sarcoma, there were 2 long-term CR, 1 in osteosarcoma and 1 in rhabdomyosarcoma, added to 3 SD [123]. A phase I study is currently using the combination of Her2-CAR T therapy with PD1 blockade pembrolizumab or nivolumab in advanced Her2+ sarcoma (NCT 049955003).
Other phase I trials are currently evaluating CAR T cells targeting epidermal growth factor receptor (EGFR) (NCT 03618381) and tumor antigen GD2 (NCT 04539366, NCT 03635632, NCT 02107963, NCT 03721068) [177]. Preclinical studies have also suggested a promising activity of CAR T cells targeting the type I insulin-like growth factor receptor (IGF1R) and the tyrosine–kinase-like orphan receptor 1 (ROR1), which are highly expressed in sarcoma cell lines [178]. Other potential targets for CAR T therapy in sarcoma are CD44v6 [179] and NK cell activating receptor group 2-member D ligand (NKG2DL) [180,181].
3.6.3. TIL Therapy
TIL therapy is based on the extraction of tumor-infiltrating lymphocytes for ex vivo expansion and reinfusion to the patient, after lymphodepleting conditioning, in combination with immune-enhancing adjuvants such as IL-2. It has an exciting potential, being the only ACT-using cells with multiple TCR clones able to cover the antigenic heterogeneity of solid tumors in contrast to engineered TCRs and CAR T cells, which target specific antigens [182]. Up to date, TIL therapy has been mainly developed in melanoma with a positive phase III trial versus ipilimumab in the first-line setting [183] and has promising results in cervical cancer [184,185,186], non-small cell lung cancer [187], ovarian cancer [188], colorectal cancer [189], breast cancer [190] and cholangiocarcinoma [191].
Mullinax et al. [192] demonstrated the feasibility of TIL therapy in STS. They successfully propagated TILs from 70 STS surgical specimens, cocultured them with tumor cells, and expanded them using a rapid expansion protocol, observing that nearly all specimens generated TILs, mainly CD3+, which were responsive to the autologous tumor. Ko et al. [193] published another preclinical study that suggests that certain sarcoma subtypes can potentially yield an appropriate number of cells for TIL therapy.
Zhou et al. [194] conducted a retrospective study of 60 patients with CT-resistant metastatic osteosarcoma who were treated with TIL therapy plus nivolumab. Of the patients, 83.3% had lung metastasis, and 83.3% had presented a poor response to neoadjuvant CT. The results were encouraging, with an ORR of 36.7% (2 CR and 20 PR), a DCR of 80% and a median PFS of 5.8 months. An infusion of ≥ 5 × 10 [9] TIL cells, a percentage of CD8+ TIL ≥ 60% and a percentage of CD4+/FoxP3+ TIL <20% were significantly associated with response to treatment. Overall, OS was 23.7 months in responders versus 8.7 months in non-responders (p < 0.0001).
Despite these interesting preclinical and retrospective data, the use of TIL therapy in sarcoma is not endorsed by any clinical trial to date. A phase I study of TILs in STS (NCT 04052334) and two phase II basket trials, one with a cohort for carcinosarcoma (NCT 03610490) and another with a cohort for STS (NCT 03935893), are ongoing [177]. Further research is required to understand how to overcome the challenges still faced by TIL therapy, such as the negative impact of the immunosuppressive TME or its application in ‘cold’ tumors, including many sarcoma subtypes.
4. Conclusions
Immunotherapy is progressively acquiring a role in the treatment of advanced sarcoma, though the biological heterogeneity among histologic subtypes impacts their clinical response. Globally, soft tissue sarcomas (STS) are more responsive than bone sarcomas (BS). Undifferentiated pleomorphic (UPS), alveolar soft part (ASPS), synovial and Kaposi sarcomas seem especially immunogenic, followed by some liposarcoma and leiomyosarcoma. The presence of tertiary lymphoid structures (TLS), a high density of infiltrating CD8+ T cells and a high PDL1 expression in cells of the TME have been identified as prognostic and predictive biomarkers.
Immune checkpoint inhibitors (ICI) have poor results in monotherapy, except for certain subtypes. The SARC028 trial is the most relevant study with an ICI alone (pembrolizumab), with an ORR of 40% in UPS and 20% in liposarcoma and chondrosarcoma but <10% in the other subtypes. Kaposi and other rare forms of sarcoma, such as chordoma and SMARCA4-deficient rhabdoid tumors, might also benefit from PD1 inhibitors. Dual blockade with ipilimumab plus nivolumab showed superiority compared to single immunotherapy (Alliance A091401), and durvalumab plus tremelimumab seems especially active in ASPS.
Multiple studies have evaluated ICI combined with conventional CT, trying to achieve a synergistic effect. Pembrolizumab plus doxorubicin has positive results in anthracycline-naïve STS, and some other combinations, ipi/nivo plus trabectedin, pembro plus gemcitabine and avelumab plus trabectedin, have promising results in CT-refractory STS. The IMMUNOSARC trial is the main study combining ICI with tyrosine–kinase inhibitors (nivolumab plus sunitinib), reaching a DCR >60% both in STS and BS, though other combinations have shown similar outcomes in phase I and II trials. ICI has also been combined with immunomodulatory agents, with promising results of oncolytic viral therapy (T-VEC) added to PD1 blockade.
Going beyond checkpoint inhibitors, therapeutic vaccines and adoptive cell therapy are incorporated into the arsenal of immunotherapy, with encouraging results. Engineered TCR targeting aberrant neoantigens NY-ESO1 (Lete-cel) and MAGE-A4 (Afami-cel) have shown ORR 40–60% in synovial sarcoma, with a favorable safety profile. Her2-targeted CAR-T cells and TIL therapy have promising preclinical and retrospective data in advanced sarcoma, with the potential benefit of their combination with PD1 inhibitors. Further research is still needed to overcome the theoretical and practical obstacles faced by adoptive cell therapy, especially in such aggressive tumors with a powerful immunosuppressive microenvironment.
Author Contributions
V.A.—conception of the work, writing and review of literature; J.P., J.C., D.I.R., M.S.R., P.G., P.P.d.A., J.C.C., C.G.d.Q. and C.G.—contribution to writing and review of literature; M.L.V., M.Á.V.—provision of study material and supervision of the work. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 2.Brennan M.F., Antonescu C.R., Moraco N., Singer S. Lessons learned from the study of 10,000 patients with soft tissue sarcoma. Ann. Surg. 2014;260:416–421. doi: 10.1097/SLA.0000000000000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Judson I., Verweij J., Gelderblom H., Hartmann J.T., Schöffski P., Blay J.-Y., Kerst J.M., Sufliarsky J., Whelan J., Hohenberger P., et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: A randomised controlled phase 3 trial. Lancet Oncol. 2014;15:415–423. doi: 10.1016/S1470-2045(14)70063-4. [DOI] [PubMed] [Google Scholar]
- 4.Seddon B., Strauss S.J., Whelan J., Leahy M., Woll P.J., Cowie F., Rothermundt C., Wood Z., Benson C., Ali N., et al. Gemcitabine and docetaxel versus doxorubicin as first-line treatment in previously untreated advanced unresectable or metastatic soft-tissue sarcomas (GeDDiS): A randomised controlled phase 3 trial. Lancet Oncol. 2017;18:1397–1410. doi: 10.1016/S1470-2045(17)30622-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demetri G.D., von Mehren M., Jones R.L., Hensley M.L., Schuetze S.M., Staddon A., Milhem M., Elias A., Ganjoo K., Tawbi H., et al. Efficacy and Safety of Trabectedin or Dacarbazine for Metastatic Liposarcoma or Leiomyosarcoma After Failure of Conventional Chemotherapy: Results of a Phase III Randomized Multicenter Clinical Trial. J. Clin. Oncol. 2016;34:786–793. doi: 10.1200/JCO.2015.62.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawai A., Araki N., Sugiura H., Ueda T., Yonemoto T., Takahashi M., Morioka H., Hiraga H., Hiruma T., Kunisada T., et al. Trabectedin monotherapy after standard chemotherapy versus best supportive care in patients with advanced, translocation-related sarcoma: A randomised, open-label, phase 2 study. Lancet Oncol. 2015;16:406–416. doi: 10.1016/S1470-2045(15)70098-7. [DOI] [PubMed] [Google Scholar]
- 7.Schöffski P., Chawla S., Maki R.G., Italiano A., Gelderblom H., Choy E., Grignani G., Camargo V., Bauer S., Rha S.Y., et al. Eribulin versus dacarbazine in previously treated patients with advanced liposarcoma or leiomyosarcoma: A randomised, open-label, multicentre, phase 3 trial. Lancet. 2016;387:1629–1637. doi: 10.1016/S0140-6736(15)01283-0. [DOI] [PubMed] [Google Scholar]
- 8.García-Del-Muro X., López-Pousa A.L., Maurel J., Martín J., Martínez-Trufero J., Casado A., Gómez-España A., Fra J., Cruz J., Poveda A., et al. Randomized Phase II Study Comparing Gemcitabine Plus Dacarbazine Versus Dacarbazine Alone in Patients With Previously Treated Soft Tissue Sarcoma: A Spanish Group for Research on Sarcomas Study. J. Clin. Oncol. 2011;29:2528–2533. doi: 10.1200/JCO.2010.33.6107. [DOI] [PubMed] [Google Scholar]
- 9.Van Der Graaf W.T., Blay J.Y., Chawla S.P., Kim D.W., Bui-Nguyen B., Casali P.G., Aglietta M., Staddon A.P., Beppu Y., Cesne A.L., et al. Faculty Opinions recommendation of Pazopanib for metastatic soft-tissue sarcoma (PALETTE): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet Lond. E. 2012;379:1879–1886. doi: 10.1016/S0140-6736(12)60651-5. [DOI] [PubMed] [Google Scholar]
- 10.Butrynski J.E., D’Adamo D.R., Hornick J.L., Cin P.D., Antonescu C.R., Jhanwar S.C., Ladanyi M., Capelletti M., Rodig S.J., Ramaiya N., et al. Crizotinib in ALK-Rearranged Inflammatory Myofibroblastic Tumor. N. Engl. J. Med. 2010;363:1727–1733. doi: 10.1056/NEJMoa1007056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Judson I., Morden J.P., Kilburn L., Leahy M., Benson C., Bhadri V., Campbell-Hewson Q., Cubedo R., Dangoor A., Fox L., et al. Cediranib in patients with alveolar soft-part sarcoma (CASPS): A double-blind, placebo-controlled, randomised, phase 2 trial. Lancet Oncol. 2019;20:1023–1034. doi: 10.1016/S1470-2045(19)30215-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marina N.M., Smeland S., Bielack S.S., Bernstein M., Jovic G., Krailo M.D., Hook J.M., Arndt C., van den Berg H., Brennan B., et al. Comparison of MAPIE versus MAP in patients with a poor response to preoperative chemotherapy for newly diagnosed high-grade osteosarcoma (EURAMOS-1): An open-label, international, randomised controlled trial. Lancet Oncol. 2016;17:1396–1408. doi: 10.1016/S1470-2045(16)30214-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palmerini E., Torricelli E., Cascinu S., Pierini M., De Paolis M., Donati D., Ferrari S. Is there a role for chemotherapy after local relapse in high-grade osteosarcoma? Pediatr. Blood Cancer. 2019;66:e27792. doi: 10.1002/pbc.27792. [DOI] [PubMed] [Google Scholar]
- 14.Womer R.B., West D.C., Krailo M.D., Dickman P.S., Pawel B.R., Grier H.E., Marcus K., Sailer S., Healey J.H., Dormans J.P., et al. Randomized Controlled Trial of Interval-Compressed Chemotherapy for the Treatment of Localized Ewing Sarcoma: A Report From the Children’s Oncology Group. J. Clin. Oncol. 2012;30:4148–4154. doi: 10.1200/JCO.2011.41.5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Felix A., Berlanga P., Toulmonde M., Landman-Parker J., Dumont S., Vassal G., Gaspar N. Systematic review of phase-I/II trials enrolling refractory and recurrent Ewing sarcoma: Actual knowledge and future directions to optimize the research. Cancer Med. 2021;10:1589–1604. doi: 10.1002/cam4.3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Albarrán V., Villamayor M.L., Chamorro J., Rosero D.I., Pozas J., Román M.S., Calvo J.C., de Aguado P.P., Moreno J., Guerrero P., et al. Receptor Tyrosine Kinase Inhibitors for the Treatment of Recurrent and Unresectable Bone Sarcomas. Int. J. Mol. Sci. 2022;23:13784. doi: 10.3390/ijms232213784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrari S., Briccoli A., Mercuri M., Bertoni F., Picci P., Tienghi A., Del Prever A.B., Fagioli F., Comandone A., Bacci G. Postrelapse Survival in Osteosarcoma of the Extremities: Prognostic Factors for Long-Term Survival. J. Clin. Oncol. 2003;21:710–715. doi: 10.1200/JCO.2003.03.141. [DOI] [PubMed] [Google Scholar]
- 18.Cotterill S., Ahrens S., Paulussen M., Jürgens H., Voûte P., Gadner H., Craft A. Prognostic Factors in Ewing’s Tumor of Bone: Analysis of 975 Patients From the European Intergroup Cooperative Ewing’s Sarcoma Study Group. J. Clin. Oncol. 2000;18:3108–3114. doi: 10.1200/JCO.2000.18.17.3108. [DOI] [PubMed] [Google Scholar]
- 19.Coley W.B. II Contribution to the Knowledge of Sarcoma. Ann. Surg. 1891;14:199–220. doi: 10.1097/00000658-189112000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenberg S.A. IL-2: The First Effective Immunotherapy for Human Cancer. J. Immunol. 2014;192:5451–5458. doi: 10.4049/jimmunol.1490019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fyfe G.A., Fisher R.I., Rosenberg S.A., Sznol M., Parkinson D.R., Louie A.C. Long-term response data for 255 patients with met-astatic renal cell carcinoma treated with high-dose recombinant interleukin-2 therapy. J. Clin. Oncol. Oncol. 1996;14:2410–2411. doi: 10.1200/JCO.1996.14.8.2410. [DOI] [PubMed] [Google Scholar]
- 22.Schwinger W., Klass V., Benesch M., Lackner H., Dornbusch H.J., Sovinz P., Moser A., Schwantzer G., Urban C. Feasibility of high-dose interleukin-2 in heavily pretreated pediatric cancer patients. Ann. Oncol. 2005;16:1199–1206. doi: 10.1093/annonc/mdi226. [DOI] [PubMed] [Google Scholar]
- 23.Baluna R., Vitetta E.S. Vascular leak syndrome: A side effect of immunotherapy. Immunopharmacology. 1997;37:117–132. doi: 10.1016/S0162-3109(97)00041-6. [DOI] [PubMed] [Google Scholar]
- 24.Saerens M., Brusselaers N., Rottey S., Decruyenaere A., Creytens D., Lapeire L. Immune checkpoint inhibitors in treatment of soft-tissue sarcoma: A systematic review and meta-analysis. Eur. J. Cancer. 2021;152:165–182. doi: 10.1016/j.ejca.2021.04.034. [DOI] [PubMed] [Google Scholar]
- 25.Dajsakdipon T., Siripoon T., Ngamphaiboon N., Ativitavas T., Dejthevaporn T. Immunotherapy and Biomarkers in Sarcoma. Curr. Treat. Options Oncol. 2022;23:415–438. doi: 10.1007/s11864-022-00944-6. [DOI] [PubMed] [Google Scholar]
- 26.Chen D.S., Mellman I. Oncology Meets Immunology: The Cancer-Immunity Cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 27.Rosin D.L., Okusa M.D. Dangers within: DAMP responses to damage and cell death in kidney disease. J. Am. Soc. Nephrol. JASN. 2011;22:416–425. doi: 10.1681/ASN.2010040430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fumet J.-D., Truntzer C., Yarchoan M., Ghiringhelli F. Tumour mutational burden as a biomarker for immunotherapy: Current data and emerging concepts. Eur. J. Cancer. 2020;131:40–50. doi: 10.1016/j.ejca.2020.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chalmers Z.R., Connelly C.F., Fabrizio D., Gay L., Ali S.M., Ennis R., Schrock A., Campbell B., Shlien A., Chmielecki J., et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017;9:34. doi: 10.1186/s13073-017-0424-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bocchia M., Wentworth P.A., Southwood S., Sidney J., McGraw K., Scheinberg D.A., Sette A. Specific binding of leukemia oncogene fusion protein peptides to HLA class I molecules. Blood. 1995;85:2680–2684. doi: 10.1182/blood.V85.10.2680.bloodjournal85102680. [DOI] [PubMed] [Google Scholar]
- 31.Worley B.S., Broeke L.T.V.D., Goletz T.J., Pendleton C.D., Daschbach E.M., Thomas E.K., Marincola F.M., Helman L.J., Berzofsky J.A. Antigenicity of fusion proteins from sarcoma-associated chromosomal translocations. Cancer Res. 2001;61:6868–6875. [PubMed] [Google Scholar]
- 32.Kakimoto T., Matsumine A., Kageyama S., Asanuma K., Matsubara T., Nakamura T., Sudo A. Immunohistochemical expression and clinicopathological assessment of the cancer testis antigens NY-ESO-1 and MAGE-A4 in high-grade soft-tissue sarcoma. Oncol. Lett. 2019;17:3937–3943. doi: 10.3892/ol.2019.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dustin M.L. The immunological synapse. Cancer Immunol. Res. 2014;2:1023–1033. doi: 10.1158/2326-6066.CIR-14-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou Z., Lafleur E.A., Koshkina N.V., Worth L.L., Lester M.S., Kleinerman E.S. Interleukin-12 Up-Regulates Fas Expression in Human Osteosarcoma and Ewing’s Sarcoma Cells by Enhancing Its Promoter Activity. Mol. Cancer Res. 2005;3:685–692. doi: 10.1158/1541-7786.MCR-05-0092. [DOI] [PubMed] [Google Scholar]
- 35.Marchisone C., Benelli R., Albini A., Santi L., Noonan D.M. Inhibition of Angiogenesis by Type I Interferons in Models of Kaposi’S Sarcoma. Int. J. Biol. Markers. 1999;14:257–262. doi: 10.1177/172460089901400411. [DOI] [PubMed] [Google Scholar]
- 36.Taylor K.L., Oates R.K., Grane R., Leaman D.W., Borden E.C., Lindner D.J. IFN-alpha1,8 inhibits tumor-induced angiogenesis in murine angiosarcomas. J. Interferon Cytokine Res. 2006;26:353–361. doi: 10.1089/jir.2006.26.353. [DOI] [PubMed] [Google Scholar]
- 37.Chen L., Flies D.B. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat. Rev. Immunol. 2013;13:227–242. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dancsok A.R., Setsu N., Gao D., Blay J.-Y., Thomas D., Maki R.G., Nielsen T.O., Demicco E.G. Expression of lymphocyte immunoregulatory biomarkers in bone and soft-tissue sarcomas. Mod. Pathol. 2019;32:1772–1785. doi: 10.1038/s41379-019-0312-y. [DOI] [PubMed] [Google Scholar]
- 39.Slaney C.Y., Kershaw M.H., Darcy P.K. Trafficking of T Cells into Tumors. Cancer Res. 2014;74:7168–7174. doi: 10.1158/0008-5472.CAN-14-2458. [DOI] [PubMed] [Google Scholar]
- 40.Kornepati A.V.R., Vadlamudi R.K., Curiel T.J. Programmed death ligand 1 signals in cancer cells. Nat. Rev. Cancer. 2022;22:174–189. doi: 10.1038/s41568-021-00431-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.D’Angelo S.P., Shoushtari A.N., Agaram N.P., Kuk D., Qin L.-X., Carvajal R.D., Dickson M.A., Gounder M., Keohan M.L., Schwartz G.K., et al. Prevalence of tumor-infiltrating lymphocytes and PD-L1 expression in the soft tissue sarcoma microenvironment. Hum. Pathol. 2014;46:357–365. doi: 10.1016/j.humpath.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zheng C., You W., Wan P., Jiang X., Chen J., Zheng Y., Li W., Tan J., Zhang S. Clinicopathological and prognostic significance of PD-L1 expression in sarcoma: A systematic review and meta-analysis. Medicine. 2018;97:e11004. doi: 10.1097/MD.0000000000011004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Que Y., Xiao W., Guan Y.-X., Liang Y., Yan S.-M., Chen H.-Y., Li Q.-Q., Xu B.-S., Zhou Z.-W., Zhang X. PD-L1 Expression Is Associated with FOXP3+ Regulatory T-Cell Infiltration of Soft Tissue Sarcoma and Poor Patient Prognosis. J. Cancer. 2017;8:2018–2025. doi: 10.7150/jca.18683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bremnes R.M., Busund L.-T., Kilvær T.L., Andersen S., Richardsen E., Paulsen E.E., Hald S., Khanehkenari M.R., Cooper W.A., Kao S.C., et al. The Role of Tumor-Infiltrating Lymphocytes in Development, Progression, and Prognosis of Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2016;11:789–800. doi: 10.1016/j.jtho.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 45.Hadrup S., Donia M., Straten P.T. Effector CD4 and CD8 T Cells and Their Role in the Tumor Microenvironment. Cancer Microenviron. 2012;6:123–133. doi: 10.1007/s12307-012-0127-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saleh R., Elkord E. FoxP3+ T regulatory cells in cancer: Prognostic biomarkers and therapeutic targets. Cancer Lett. 2020;490:174–185. doi: 10.1016/j.canlet.2020.07.022. [DOI] [PubMed] [Google Scholar]
- 47.Chen B., Li H., Liu C., Xiang X., Wang S., Wu A., Shen Y., Li G. Prognostic value of the common tumour-infiltrating lymphocyte subtypes for patients with non-small cell lung cancer: A meta-analysis. PLoS ONE. 2020;15:e0242173. doi: 10.1371/journal.pone.0242173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bi Q., Liu Y., Yuan T., Wang H., Li B., Jiang Y., Mo X., Lei Y., Xiao Y., Dong S., et al. Predicted CD4+ T cell infiltration levels could indicate better overall survival in sarcoma patients. J. Int. Med. Res. 2021;49:300060520981539. doi: 10.1177/0300060520981539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fujii H., Arakawa A., Utsumi D., Sumiyoshi S., Yamamoto Y., Kitoh A., Ono M., Matsumura Y., Kato M., Konishi K., et al. CD8+tumor-infiltrating lymphocytes at primary sites as a possible prognostic factor of cutaneous angiosarcoma. Int. J. Cancer. 2013;134:2393–2402. doi: 10.1002/ijc.28581. [DOI] [PubMed] [Google Scholar]
- 50.Fridman W.H., Sibéril S., Pupier G., Soussan S., Sautès-Fridman C. Activation of B cells in Tertiary Lymphoid Structures in cancer: Anti-tumor or anti-self? Semin. Immun. 2023;65:101703. doi: 10.1016/j.smim.2022.101703. [DOI] [PubMed] [Google Scholar]
- 51.Sautès-Fridman C., Petitprez F., Calderaro J., Fridman W.H. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat. Rev. Cancer. 2019;19:307–325. doi: 10.1038/s41568-019-0144-6. [DOI] [PubMed] [Google Scholar]
- 52.Sorbye S.W., Kilvaer T., Valkov A., Donnem T., Smeland E., Al-Shibli K., Bremnes R.M., Busund L.-T. Prognostic Impact of Lymphocytes in Soft Tissue Sarcomas. PLoS ONE. 2011;6:e14611. doi: 10.1371/journal.pone.0014611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Petitprez F., de Reyniès A., Keung E.Z., Chen T.W.-W., Sun C.-M., Calderaro J., Jeng Y.-M., Hsiao L.-P., Lacroix L., Bougoüin A., et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature. 2020;577:556–560. doi: 10.1038/s41586-019-1906-8. [DOI] [PubMed] [Google Scholar]
- 54.Tawbi H.A., Burgess M., Bolejack V., Van Tine B.A., Schuetze S.M., Hu J., D’Angelo S., Attia S., Riedel R.F., Priebat D.A., et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): A multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. 2017;18:1493–1501. doi: 10.1016/S1470-2045(17)30624-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shimizu K., Iyoda T., Okada M., Yamasaki S., Fujii S. Immune suppression and reversal of the suppressive tumor microenvi-ronment. Int. Immunol. 2018;30:445–455. doi: 10.1093/intimm/dxy042. [DOI] [PubMed] [Google Scholar]
- 56.Cao X., Cai S.F., Fehniger T.A., Song J., Collins L.I., Piwnica-Worms D.R., Ley T.J. Granzyme B and Perforin Are Important for Regulatory T Cell-Mediated Suppression of Tumor Clearance. Immunity. 2007;27:635–646. doi: 10.1016/j.immuni.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 57.Mahnke K., Ring S., Johnson T.S., Schallenberg S., Schönfeld K., Storn V., Bedke T., Enk A.H. Induction of immunosuppressive functions of dendritic cellsin vivo by CD4+CD25+ regulatory T cells: Role of B7-H3 expression and antigen presentation. Eur. J. Immunol. 2007;37:2117–2126. doi: 10.1002/eji.200636841. [DOI] [PubMed] [Google Scholar]
- 58.Smolle M.A., Herbsthofer L., Granegger B., Goda M., Brcic I., Bergovec M., Scheipl S., Prietl B., Pichler M., Gerger A., et al. T-regulatory cells predict clinical outcome in soft tissue sarcoma patients: A clinico-pathological study. Br. J. Cancer. 2021;125:717–724. doi: 10.1038/s41416-021-01456-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brinkrolf P., Landmeier S., Altvater B., Chen C., Pscherer S., Rosemann A., Ranft A., Dirksen U., Juergens H., Rossig C. A high proportion of bone marrow T cells with regulatory phenotype (CD4+CD25hiFoxP3+) in Ewing sarcoma patients is associated with metastatic disease. Int. J. Cancer. 2009;125:879–886. doi: 10.1002/ijc.24461. [DOI] [PubMed] [Google Scholar]
- 60.Camarillo D., Leslie K., Unemori P., Yashi A.K., McCune-Smith K., Maurer T. Regulatory T cells are present in Kaposi’s sarcoma and increasingly frequent in advanced disease. Infect. Agents Cancer. 2009;4:P12–1. doi: 10.1186/1750-9378-4-S2-P12. [DOI] [Google Scholar]
- 61.Colomiere M., Ward A.C., Riley C., Trenerry M.K., Cameron-Smith D., Findlay J., Ackland L., Ahmed N. Cross talk of signals between EGFR and IL-6R through JAK2/STAT3 mediate epi-thelial-mesenchymal transition in ovarian carcinomas. Br. J. Cancer. 2009;100:134–144. doi: 10.1038/sj.bjc.6604794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tartour E., Pere H., Maillere B., Terme M., Merillon N., Taieb J., Sandoval F., Quintin-Colonna F., Lacerda K., Karadimou A., et al. Angiogenesis and immunity: A bidirectional link potentially relevant for the monitoring of antiangiogenic therapy and the development of novel therapeutic combination with immunotherapy. Cancer Metastasis Rev. 2011;30:83–95. doi: 10.1007/s10555-011-9281-4. [DOI] [PubMed] [Google Scholar]
- 63.Wang Y., Ding Y., Guo N., Wang S. MDSCs: Key Criminals of Tumor Pre-metastatic Niche Formation. Front. Immunol. 2019;10:172. doi: 10.3389/fimmu.2019.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Han X., Shi H., Sun Y., Shang C., Luan T., Wang D., Ba X., Zeng X. CXCR2 expression on granulocyte and macrophage progenitors under tumor conditions con-tributes to mo-MDSC generation via SAP18/ERK/STAT3. Cell Death Dis. 2019;10:598. doi: 10.1038/s41419-019-1837-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Highfill S.L., Cui Y., Giles A.J., Smith J.P., Zhang H., Morse E., Kaplan R.N., Mackall C.L. Disruption of CXCR2-Mediated MDSC Tumor Trafficking Enhances Anti-PD1 Efficacy. Sci. Transl. Med. 2014;6:237ra67. doi: 10.1126/scitranslmed.3007974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mantovani A., Marchesi F., Malesci A., Laghi L., Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 2017;14:399–416. doi: 10.1038/nrclinonc.2016.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Malfitano A.M., Pisanti S., Napolitano F., Di Somma S., Martinelli R., Portella G. Tumor-Associated Macrophage Status in Cancer Treatment. Cancers. 2020;12:1987. doi: 10.3390/cancers12071987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jung K.Y., Cho S.W., Kim Y., Kim D., Oh B.-C., Park D.J., Park Y.J. Cancers with Higher Density of Tumor-Associated Macrophages Were Associated with Poor Survival Rates. J. Pathol. Transl. Med. 2015;49:318–324. doi: 10.4132/jptm.2015.06.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee C.-H., Espinosa I., Vrijaldenhoven S., Subramanian S., Montgomery K.D., Zhu S., Marinelli R.J., Peterse J.L., Poulin N., Nielsen T.O., et al. Prognostic Significance of Macrophage Infiltration in Leiomyosarcomas. Clin. Cancer Res. 2008;14:1423–1430. doi: 10.1158/1078-0432.CCR-07-1712. [DOI] [PubMed] [Google Scholar]
- 70.Nabeshima A., Matsumoto Y., Fukushi J., Iura K., Matsunobu T., Endo M., Fujiwara T., Iida K., Hatano M., Yokoyama N., et al. Tumour-associated macrophages correlate with poor prognosis in myxoid liposarcoma and promote cell motility and invasion via the HB-EGF-EGFR-PI3K/Akt pathways. Br. J. Cancer. 2015;112:547–555. doi: 10.1038/bjc.2014.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Oike N., Kawashima H., Ogose A., Hotta T., Hatano H., Ariizumi T., Sasaki T., Yamagishi T., Umezu H., Endo N. Prognostic impact of the tumor immune microenvironment in synovial sarcoma. Cancer Sci. 2018;109:3043–3054. doi: 10.1111/cas.13769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Komohara Y., Takeya H., Wakigami N., Kusada N., Bekki H., Ishihara S., Takeya M., Nakashima Y., Oda Y. Positive correlation between the density of macrophages and T-cells in undif-ferentiated sarcoma. Med. Mol. Morphol. 2019;52:44–51. doi: 10.1007/s00795-018-0201-3. [DOI] [PubMed] [Google Scholar]
- 73.Fujiwara T., Healey J., Ogura K., Yoshida A., Kondo H., Hata T., Kure M., Tazawa H., Nakata E., Kunisada T., et al. Role of Tumor-Associated Macrophages in Sarcomas. Cancers. 2021;13:1086. doi: 10.3390/cancers13051086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dumars C., Ngyuen J.-M., Gaultier A., Lanel R., Corradini N., Gouin F., Heymann D., Heymann M.-F. Dysregulation of macrophage polarization is associated with the metastatic process in osteosarcoma. Oncotarget. 2016;7:78343–78354. doi: 10.18632/oncotarget.13055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Buddingh E.P., Kuijjer M.L., Duim R.A., Bürger H., Agelopoulos K., Myklebost O., Serra M., Mertens F., Hogendoorn P.C., Lankester A.C., et al. Tumor-Infiltrating Macrophages Are Associated with Metastasis Suppression in High-Grade Osteosarcoma: A Rationale for Treatment with Macrophage Activating Agents. Clin. Cancer Res. 2011;17:2110–2119. doi: 10.1158/1078-0432.CCR-10-2047. [DOI] [PubMed] [Google Scholar]
- 76.Gomez-Brouchet A., Illac C., Gilhodes J., Bouvier C., Aubert S., Guinebretiere J.M., Marie B., Larousserie F., Entz-Werlé N., de Pinieux G., et al. CD163-positive tumor-associated macrophages and CD8-positive cytotoxic lymphocytes are powerful diagnostic markers for the therapeutic stratification of osteosarcoma patients: An immuno-histochemical analysis of the biopsies fromthe French OS2006 phase 3 trial. OncoImmunology. 2017;6:e1331193. doi: 10.1080/2162402X.2017.1331193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Handl M., Hermanova M., Hotarkova S., Jarkovsky J., Mudry P., Shatokhina T., Vesela M., Sterba J., Zambo I. Clinicopathological correlation of tumor-associated macrophages in Ewing sarcoma. Biomed. Pap. 2018;162:54–60. doi: 10.5507/bp.2017.049. [DOI] [PubMed] [Google Scholar]
- 78.Lussier D.M., O’Neill L., Nieves L.M., McAfee M.S., Holechek S.A., Collins A.W., Dickman P., Jacobsen J., Hingorani P., Blattman J.N. Enhanced T-Cell Immunity to Osteosarcoma Through Antibody Blockade of PD-1/PD-L1 Interactions. J. Immun. 2015;38:96–106. doi: 10.1097/CJI.0000000000000065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Toulmonde M., Penel N., Adam J., Chevreau C., Blay J.Y., Le Cesne A., Bompas E., Piperno-Neumann S., Cousin S., Grellety T., et al. Use of PD-1 Targeting, Macrophage Infiltration, and IDO Pathway Activation in Sarcomas: A Phase 2 Clinical Trial. JAMA Oncol. 2018;4:93–97. doi: 10.1001/jamaoncol.2017.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nafia I., Toulmonde M., Bortolotto D., Chaibi A., Bodet D., Rey C., Velasco V., Larmonier C.B., Cerf L., Adam J., et al. IDO Targeting in Sarcoma: Biological and Clinical Implications. Front. Immunol. 2020;11:274. doi: 10.3389/fimmu.2020.00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mimura K., Kono K., Takahashi A., Kawaguchi Y., Fujii H. Vascular endothelial growth factor inhibits the function of human mature dendritic cells mediated by VEGF receptor-2. Cancer Immunol. Immunother. 2006;56:761–770. doi: 10.1007/s00262-006-0234-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Oelkrug C., Ramage J.M. Enhancement of T cell recruitment and infiltration into tumours. Clin. Exp. Immunol. 2014;178:1–8. doi: 10.1111/cei.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang J., Yan J., Liu B. Targeting VEGF/VEGFR to Modulate Antitumor Immunity. Front. Immunol. 2018;9:978. doi: 10.3389/fimmu.2018.00978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dubois S., Demetri G. Markers of angiogenesis and clinical features in patients with sarcoma. Cancer. 2007;109:813–819. doi: 10.1002/cncr.22455. [DOI] [PubMed] [Google Scholar]
- 85.Maki R.G., Jungbluth A.A., Gnjatic S., Schwartz G.K., D’Adamo D.R., Keohan M.L., Wagner M.J., Scheu K., Chiu R., Ritter E., et al. A Pilot Study of Anti-CTLA4 Antibody Ipilimumab in Patients with Synovial Sarcoma. Sarcoma. 2013;2013:1–8. doi: 10.1155/2013/168145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Merchant M.S., Wright M., Baird K., Wexler L.H., Rodriguez-Galindo C., Bernstein D., Delbrook C., Lodish M., Bishop R., Wolchok J.D., et al. Phase I Clinical Trial of Ipilimumab in Pediatric Patients with Advanced Solid Tumors. Clin. Cancer Res. 2016;22:1364–1370. doi: 10.1158/1078-0432.CCR-15-0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ben-Ami E., Barysauskas C.M., Solomon S., Tahlil K., Malley R., Hohos M., Polson K., Loucks M., Severgnini M., Patel T., et al. Immunotherapy with single agent nivolumab for advanced leiomyosarcoma of the uterus: Results of a phase 2 study: Nivolumab for Uterine Leiomyosarcoma. Cancer. 2017;123:3285–3290. doi: 10.1002/cncr.30738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Blay J.-Y., Penel N., Ray-Coquard I.L., Cousin S., Bertucci F., Bompas E., Eymard J.C., Saada-Bouzid E., Soulie P., Boudou-Rouquette P., et al. High clinical activity of pembrolizumab in chordoma, alveolar soft part sarcoma (ASPS) and other rare sarcoma histotypes: The French AcSé pembrolizumab study from Unicancer. J. Clin. Oncol. 2021;39((Suppl. 15)):11520. doi: 10.1200/JCO.2021.39.15_suppl.11520. [DOI] [Google Scholar]
- 89.Delyon J., Resche-Rigon M., Renaud M., Le Goff J., Dalle S., Heidelberger V., Da Meda L., Allain V., Toullec L., Carcelain G., et al. 1077MO PD1 blockade with pembrolizumab in classic and endemic Kaposi sarcoma: A multicenter phase II study. Ann. Oncol. 2020;31:S732. doi: 10.1016/j.annonc.2020.08.1201. [DOI] [PubMed] [Google Scholar]
- 90.D’Angelo S.P., Mahoney M.R., Van Tine B.A., Atkins J., Milhem M.M., Jahagirdar B.N., Antonescu C.R., Horvath E., Tap W.D., Schwartz G.K., et al. Nivolumab with or without ipilimumab treatment for metastatic sarcoma (Alliance A091401): Two open-label, non-comparative, randomised, phase 2 trials. Lancet Oncol. 2018;19:416–426. doi: 10.1016/S1470-2045(18)30006-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Somaiah N., Conley A.P., Parra E.R., Lin H., Amini B., Soto L.S., Salazar R., Barreto C., Chen H., Gite S., et al. Durvalumab plus tremelimumab in advanced or metastatic soft tissue and bone sarcomas: A single-centre phase 2 trial. Lancet Oncol. 2022;23:1156–1166. doi: 10.1016/S1470-2045(22)00392-8. [DOI] [PubMed] [Google Scholar]
- 92.Livingston M.B., Jagosky M.H., Robinson M.M., Ahrens W.A., Benbow J.H., Farhangfar C.J., Foureau D.M., Maxwell D.M., Baldrige E.A., Begic X., et al. Phase II Study of Pembrolizumab in Combination with Doxorubicin in Metastatic and Unresectable Soft-Tissue Sarcoma. Clin. Cancer Res. 2021;27:6424–6431. doi: 10.1158/1078-0432.CCR-21-2001. [DOI] [PubMed] [Google Scholar]
- 93.Pollack S.M., Redman M.W., Baker K.K., Wagner M.J., Schroeder B.A., Loggers E.T., Trieselmann K., Copeland V.C., Zhang S., Black G., et al. Assessment of Doxorubicin and Pembrolizumab in Patients With Advanced Anthracycline-Naive Sarcoma: A Phase 1/2 Nonrandomized Clinical Trial. JAMA Oncol. 2020;6:1778. doi: 10.1001/jamaoncol.2020.3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gordon E.M., Chawla S.P., Tellez W.A., Younesi E., Thomas S., Chua-Alcala V.S., Chomoyan H., Valencia C., Brigham D.A., Moradkhani A., et al. SAINT: A Phase I/Expanded Phase II Study Using Safe Amounts of Ipilimumab, Nivolumab and Trabectedin as First-Line Treatment of Advanced Soft Tissue Sarcoma. Cancers. 2023;15:906. doi: 10.3390/cancers15030906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pink D., Andreou D., Flörcken A., Golf A., Richter S., Kessler T., Kortüm M., Schmidt C.A., Kasper B., Wardelmann E., et al. Efficacy and safety of nivolumab and trabectedin in pretreated patients with advanced soft tissue sarcomas (STS): Preliminary results of a phase II trial of the German Interdisciplinary Sarcoma Group (GISG-15, NitraSarc) for the non-L sarcoma cohort. J. Clin. Oncol. 2021;39:11545. doi: 10.1200/JCO.2021.39.15_suppl.11545. [DOI] [Google Scholar]
- 96.Smrke A., Ostler A., Napolitano A., Vergnano M., Asare B., Fotiadis N., Thway K., Zaidi S., Miah A., van der Graaf W., et al. 1526MO GEMMK: A phase I study of gemcitabine (gem) and pembrolizumab (pem) in patients (pts) with leiomyosarcoma (LMS) and undifferentiated pleomorphic sarcoma UPS) Ann. Oncol. 2021;32:S1114. doi: 10.1016/j.annonc.2021.08.856. [DOI] [Google Scholar]
- 97.Nathenson M., Choy E., Carr N.D., Hibbard H.D., Mazzola E., Catalano P.J., Thornton K.A., Morgan J.A., Cote G.M., Merriam P., et al. Phase II study of eribulin and pembrolizumab in patients (pts) with metastatic soft tissue sarcomas (STS): Report of LMS cohort. J. Clin. Oncol. 2020;38:11559. doi: 10.1200/JCO.2020.38.15_suppl.11559. [DOI] [Google Scholar]
- 98.Wagner M.J., Zhang Y., Cranmer L.D., Loggers E.T., Black G., McDonnell S., Maxwell S., Johnson R., Moore R., Hermida de Viveiros P., et al. A Phase 1/2 Trial Combining Avelumab and Trabectedin for Advanced Lipo-sarcoma and Leiomyosarcoma. Clin. Cancer Res. 2022;28:2306–2312. doi: 10.1158/1078-0432.CCR-22-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Toulmonde M., Brahmi M., Giraud A., Chakiba C., Bessede A., Kind M., Toulza E., Pulido M., Albert S., Guégan J.-P., et al. Trabectedin plus Durvalumab in Patients with Advanced Pretreated Soft Tissue Sarcoma and Ovarian Carcinoma (TRAMUNE): An Open-Label, Multicenter Phase Ib Study. Clin. Cancer Res. 2021;28:1765–1772. doi: 10.1158/1078-0432.CCR-21-2258. [DOI] [PubMed] [Google Scholar]
- 100.Palmerini E., Lopez-Pousa A., Grignani G., Redondo A., Hindi N., Stacchiotti S., Sebio A., Lopez-Martin J.A., Morales C.M.V., Martinez-Trufero J., et al. IMMUNOSARC: A collaborative Spanish (GEIS) and Italian (ISG) sarcoma groups phase I/II trial of sunitinib and nivolumab in advanced soft tissue and bone sarcoma: Results from the phase II part, bone sarcoma cohort. J. Clin. Oncol. 2020;38:11522. doi: 10.1200/JCO.2020.38.15_suppl.11522. [DOI] [Google Scholar]
- 101.Broto J.M., Hindi N., Grignani G., Trufero J.M., Redondo A., Valverde C., Pousa A.L., Stacchiotti S., Palmerini E., De Alava E., et al. IMMUNOSARC: A collaborative Spanish (GEIS) and Italian (ISG) sarcoma groups phase I/II trial of sunitinib plus nivolumab in advanced soft tissue and bone sarcomas: Results of the phase II- soft-tissue sarcoma cohort. Ann. Oncol. 2019;30:v684. doi: 10.1093/annonc/mdz283.002. [DOI] [Google Scholar]
- 102.Wilky B.A., Trucco M.M., Subhawong T.K., Florou V., Park W., Kwon D., Wieder E.D., Kolonias D., Rosenberg A.E., Kerr D.A., et al. Axitinib plus pembrolizumab in patients with advanced sarcomas including alveolar soft-part sarcoma: A single-centre, single-arm, phase 2 trial. Lancet Oncol. 2019;20:837–848. doi: 10.1016/S1470-2045(19)30153-6. [DOI] [PubMed] [Google Scholar]
- 103.Xie L., Xu J., Sun X., Guo W., Gu J., Liu K., Zheng B., Ren T., Huang Y., Tang X., et al. Apatinib plus camrelizumab (anti-PD1 therapy, SHR-1210) for advanced osteosarcoma (APFAO) progressing after chemotherapy: A single-arm, open-label, phase 2 trial. J. Immunother. Cancer. 2019;8:e000798. doi: 10.1136/jitc-2020-000798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kim H.S., Cho H.J., Yun K.-H., Lee Y.H., Kim S.H., Baek W., Jeon M.K. Durvalumab and pazopanib in patients with advanced soft tissue sarcoma: A single-center, single-arm, phase 2 trial. J. Clin. Oncol. 2021;39:11551. doi: 10.1200/JCO.2021.39.15_suppl.11551. [DOI] [Google Scholar]
- 105.Cousin S., Bellera C., Guegan J.-P., Valentin T., Bahleda R., Metges J.-P., Cassier P., Cantarel C., Ceruso M.S., Kind M., et al. 1494P Regomune-a phase II study of regorafenib + avelumab in solid tumors: Results of the soft tissue sarcoma (STS) cohort. Ann. Oncol. 2022;33:S1230. doi: 10.1016/j.annonc.2022.07.1597. [DOI] [Google Scholar]
- 106.Kelly C.M., Chi P., Dickson M.A., Gounder M.M., Keohan M.L., Qin L.-X., Adamson T., Condy M.M., Biniakewitz M., Phelan H., et al. A phase II study of epacadostat and pembrolizumab in patients with advanced sarcoma. J. Clin. Oncol. 2019;37:11049. doi: 10.1200/JCO.2019.37.15_suppl.11049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schöffski P., Bahleda R., Wagner A., Burgess M., Junker N., Chisamore M., Peterson P., Ceccarelli M., William T. 154P Results of an open-label, phase Ia/Ib study of olaratumab plus pembrolizumab in patients with unresectable, locally advanced or metastatic soft tissue sarcoma. Ann. Oncol. 2021;32:S1447. doi: 10.1016/j.annonc.2021.10.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kelly C.M., Antonescu C.R., Bowler T., Munhoz R., Chi P., Dickson M.A., Gounder M.M., Keohan M.L., Movva S., Dholakia R., et al. Objective Response Rate Among Patients With Locally Advanced or Metastatic Sarcoma Treated With Talimogene Laherparepvec in Combination With Pembrolizumab: A Phase 2 Clinical Trial. JAMA Oncol. 2020;6:402. doi: 10.1001/jamaoncol.2019.6152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chawla N.S., Kim T., Sherman T., Dang J., Chua V.S., Moradkhani A., Bhuiyan I., Krkyan N., Fernando M., Colletti E., et al. A phase 2 study of talimogene laherparepvec, nivolumab, and trabectedin (TNT) in advanced sarcoma. J. Clin. Oncol. 2021;39:11567. doi: 10.1200/JCO.2021.39.15_suppl.11567. [DOI] [Google Scholar]
- 110.D’Angelo S.P., Richards A.L., Conley A.P., Woo H.J., Dickson M.A., Gounder M., Kelly C., Keohan M.L., Movva S., Thornton K., et al. Pilot study of bempegaldesleukin in combination with nivolumab in patients with metastatic sarcoma. Nat. Commun. 2022;13:1–11. doi: 10.1038/s41467-022-30874-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Somaiah N., Block M.S., Kim J.W., Shapiro G.I., Do K.T., Hwu P., Eder J.P., Jones R.L., Lu H., ter Meulen J.H., et al. First-in-Class, First-in-Human Study Evaluating LV305, a Dendritic-Cell Tropic Lentiviral Vector, in Sarcoma and Other Solid Tumors Expressing NY-ESO-1. Clin. Cancer Res. 2019;25:5808–5817. doi: 10.1158/1078-0432.CCR-19-1025. [DOI] [PubMed] [Google Scholar]
- 112.Rosenbaum E., Movva S., Kelly C.M., Dickson M.A., Keohan M.L., Gounder M.M., Thornton K.A., Chi P., Chan J.E., Nacev B., et al. A phase 1b study of avelumab plus DCC-3014, a potent and selective inhibitor of colony stimulating factor 1 receptor (CSF1R), in patients with advanced high-grade sarcoma. J. Clin. Oncol. 2021;39:11549. doi: 10.1200/JCO.2021.39.15_suppl.11549. [DOI] [Google Scholar]
- 113.Chawla S.P., Chua-Alcala V.S., Gordon E.M., Kim T.T., Feske W., Gibson B.L., Chang P.Y., Robinson D., Song P.Y. Interim analysis of a phase I study of SNK01 (Autologous Nongenetically Modified Natural Killer Cells with Enhanced Cytotoxicity) and avelumab in advanced refractory sarcoma. J. Clin. Oncol. 2022;40:11517. doi: 10.1200/JCO.2022.40.16_suppl.11517. [DOI] [Google Scholar]
- 114.Kawaguchi S., Wada T., Ida K., Sato Y., Nagoya S., Tsukahara T., Kimura S., Sahara H., Ikeda H., Shimozawa K., et al. Phase I vaccination trial of SYT-SSX junction peptide in patients with disseminated synovial sarcoma. J. Transl. Med. 2005;3:1. doi: 10.1186/1479-5876-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Takahashi R., Ishibashi Y., Hiraoka K., Matsueda S., Kawano K., Kawahara A., Kage M., Ohshima K., Yamanaka R., Shichijo S., et al. Phase II study of personalized peptide vaccination for refractory bone and soft tissue sarcoma patients. Cancer Sci. 2013;104:1285–1294. doi: 10.1111/cas.12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pipia N., Baldueva I., Nekhaeva T., Novik A., Danilova A., Avdonkina N., Protsenko S., Komarov Y., Girdyuk D., Oganesyan A., et al. Autologous dendritic-cell vaccine based on cancer-testis antigens “CaTeVac” in the treatment of soft tissue sarcoma. Ann. Oncol. 2018;29:viii410–viii411. doi: 10.1093/annonc/mdy288.026. [DOI] [Google Scholar]
- 117.Chawla S.P., Van Tine B.A., Pollack S.M., Ganjoo K.N., Elias A.D., Riedel R.F., Attia S., Choy E., Okuno S.H., Agulnik M., et al. Phase II Randomized Study of CMB305 and Atezolizumab Compared With Atezolizumab Alone in Soft-Tissue Sarcomas Expressing NY-ESO-1. J. Clin. Oncol. 2022;40:1291–1300. doi: 10.1200/JCO.20.03452. [DOI] [PubMed] [Google Scholar]
- 118.Robbins P.F., Kassim S.H., Tran T.L.N., Crystal J.S., Morgan R.A., Feldman S.A., Yang J.C., Dudley M.E., Wunderlich J.R., Sherry R.M., et al. A Pilot Trial Using Lymphocytes Genetically Engineered with an NY-ESO-1–Reactive T-cell Receptor: Long-term Follow-up and Correlates with Response. Clin. Cancer Res. 2015;21:1019–1027. doi: 10.1158/1078-0432.CCR-14-2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.D’Angelo S., Demetri G., Tine B.V., Druta M., Glod J., Chow W., Araujo D. Regular and Young Investigator Award Abstracts. BMJ Publishing Group Ltd.; London, UK: 2020. [(accessed on 13 April 2023)]. 298 Final analysis of the phase 1 trial of NY-ESO-1–specific T-cell receptor (TCR) T-cell therapy (letetresgene autoleucel; GSK3377794) in patients with advanced synovial sarcoma (SS) [Internet] p. A182–3. Available online: https://jitc.bmj.com/lookup/doi/10.1136/jitc-2020-SITC2020.0298. [Google Scholar]
- 120.Van Tine B.A., Butler M.O., Araujo D., Johnson M.L., Clarke J., Liebner D., Odunsi K., Olszanski A.J., Basu S., Brophy F., et al. ADP-A2M4 (MAGE-A4) in patients with synovial sarcoma. Ann. Oncol. 2019;30:v684–5. doi: 10.1093/annonc/mdz283.003. [DOI] [Google Scholar]
- 121.D’Angelo S.P., Van Tine B.A., Attia S., Blay J.-Y., Strauss S.J., Morales C.M.V., Razak A.R.A., Van Winkle E., Trivedi T., Biswas S., et al. SPEARHEAD-1: A phase 2 trial of afamitresgene autoleucel (Formerly ADP-A2M4) in patients with advanced synovial sarcoma or myxoid/round cell liposarcoma. J. Clin. Oncol. 2021;39:11504. doi: 10.1200/JCO.2021.39.15_suppl.11504. [DOI] [Google Scholar]
- 122.Ahmed N., Brawley V.S., Hegde M., Robertson C., Ghazi A., Gerken C., Liu E., Dakhova O., Ashoori A., Corder A., et al. Human Epidermal Growth Factor Receptor 2 (HER2) –Specific Chimeric Antigen Receptor–Modified T Cells for the Immunotherapy of HER2-Positive Sarcoma. J. Clin. Oncol. 2015;33:1688–1696. doi: 10.1200/JCO.2014.58.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Navai S.A., Derenzo C., Joseph S., Sanber K., Byrd T., Zhang H., Mata M., Gerken C., Shree A., Mathew P.R., et al. Abstract LB-147: Administration of HER2-CAR T cells after lymphodepletion safely improves T cell expansion and induces clinical responses in patients with advanced sarcomas. Cancer Res. 2019;79((Suppl. 13)):LB-147. doi: 10.1158/1538-7445.AM2019-LB-147. [DOI] [Google Scholar]
- 124.Burgess M.A., Bolejack V., Schuetze S., Van Tine B.A., Attia S., Riedel R.F., Hu J.S., Davis L.E., Okuno S.H., Priebat D.A., et al. Clinical activity of pembrolizumab (P) in undifferentiated pleomorphic sarcoma (UPS) and dedifferentiated/pleomorphic liposarcoma (LPS): Final results of SARC028 expansion cohorts. J. Clin. Oncol. 2019;37:11015. doi: 10.1200/JCO.2019.37.15_suppl.11015. [DOI] [Google Scholar]
- 125.Keung E.Z., Burgess M., Salazar R., Parra E.R., Rodrigues-Canales J., Bolejack V., Tawbi H.A. Correlative Analyses of the SARC028 Trial Reveal an Association Between Sar-coma-Associated Immune Infiltrate and Response to Pembrolizumab. Clin. Cancer Res. 2020;26:1258–1266. doi: 10.1158/1078-0432.CCR-19-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liu J., Fan Z., Bai C., Li S., Xue R., Gao T., Zhang L., Tan Z., Fang Z. Real-world experience with pembrolizumab in patients with advanced soft tissue sarcoma. Ann. Transl. Med. 2021;9:339. doi: 10.21037/atm-21-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Saif A., Verbus E.A., Sarvestani A.L., Teke M.E., Lambdin J., Hernandez J.M., Kirsch D.G. A Randomized Trial of Pembrolizumab & Radiotherapy Versus Radiotherapy in High-Risk Soft Tissue Sarcoma of the Extremity (SU2C-SARC032) Ann. Surg. Oncol. 2022;30:683–685. doi: 10.1245/s10434-022-12762-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.le Guevelou J., Debaigt C., Saada-Bouzid E., Viotti J., Khalladi N., Thibouw D., Thariat J. Phase II study of concomitant radiotherapy with atezolizumab in oli-gometastatic soft tissue sarcomas: STEREOSARC trial protocol. BMJ Open. 2020;10:e038391. doi: 10.1136/bmjopen-2020-038391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Keung E.Z., Tsai J.-W., Ali A.M., Cormier J.N., Bishop A.J., Guadagnolo B.A., Roland C.L. Analysis of the immune infiltrate in undifferentiated pleomorphic sarcoma of the extremity and trunk in response to radiotherapy: Rationale for combination neoadjuvant immune checkpoint inhibition and radio-therapy. OncoImmunology. 2018;7:e1385689. doi: 10.1080/2162402X.2017.1385689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Takada K., Sugita S., Murase K., Kikuchi T., Oomori G., Ito R., Hayasaka N., Miyanishi K., Iyama S., Ikeda H., et al. Exceptionally rapid response to pembrolizumab in a SMARCA4-deficient thoracic sarcoma overexpressing PD-L1: A case report. Thorac. Cancer. 2019;10:2312–2315. doi: 10.1111/1759-7714.13215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Anžič N., Krasniqi F., Eberhardt A.-L., Tzankov A., Haslbauer J.D. Ipilimumab and Pembrolizumab Mixed Response in a 41-Year-Old Patient with SMARCA4-Deficient Thoracic Sarcoma: An Interdisciplinary Case Study. Case Rep. Oncol. 2021;14:706–715. doi: 10.1159/000515416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Henon C., Blay J.-Y., Massard C., Mir O., Bahleda R., Dumont S., Postel-Vinay S., Adam J., Soria J.-C., Le Cesne A. Long lasting major response to pembrolizumab in a thoracic malignant rhabdoid-like SMARCA4-deficient tumor. Ann. Oncol. 2019;30:1401–1403. doi: 10.1093/annonc/mdz160. [DOI] [PubMed] [Google Scholar]
- 133.Iijima Y., Sakakibara R., Ishizuka M., Honda T., Shirai T., Okamoto T., Tateishi T., Sakashita H., Tamaoka M., Takemoto A., et al. Notable response to nivolumab during the treatment of SMARCA4-deficient thoracic sarcoma: A case report. Immunotherapy. 2020;12:563–569. doi: 10.2217/imt-2019-0142. [DOI] [PubMed] [Google Scholar]
- 134.Kawachi H., Kunimasa K., Kukita Y., Nakamura H., Honma K., Kawamura T., Inoue T., Tamiya M., Kuhara H., Nishino K., et al. Atezolizumab with bevacizumab, paclitaxel and carboplatin was effective for patients with SMARCA4-deficient thoracic sarcoma. Immunotherapy. 2021;13:799–806. doi: 10.2217/imt-2020-0311. [DOI] [PubMed] [Google Scholar]
- 135.Kunimasa K., Okami J., Takenaka S., Honma K., Kukita Y., Nagata S., Kawamura T., Inoue T., Tamiya M., Kuhara H., et al. Conversion Surgery for Advanced Thoracic SMARCA4-Deficient Undifferentiated Tumor With Atezolizumab in Combination With Bevacizumab, Paclitaxel, and Carboplatin Treatment: A Case Report. JTO Clin. Res. Rep. 2021;2:100235. doi: 10.1016/j.jtocrr.2021.100235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Marcrom S., De Los Santos J.F., Conry R.M. Complete response of mediastinal clear cell sarcoma to pembrolizumab with ra-diotherapy. Clin. Sarcoma Res. 2017;7:14. doi: 10.1186/s13569-017-0079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yu X.-H., Huang J., Ge N.-J., Yang Y.-F., Zhao J.-Y. Recurrent undifferentiated embryonal sarcoma of the liver in adult patient treated by pembrolizumab: A case report. World J. Clin. Cases. 2021;9:2281–2288. doi: 10.12998/wjcc.v9.i10.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Song H.-N., Kang M.G., Park J.R., Hwang J.-Y., Kang J.H., Lee W.S., Lee G.-W. Pembrolizumab for Refractory Metastatic Myxofibrosarcoma: A Case Report. Cancer Res. Treat. 2018;50:1458–1461. doi: 10.4143/crt.2017.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lambden J.P., Kelsten M.F., Schulte B.C., Pa S.A., Hayes J.P., Villaflor V., Agulnik M. Metastatic Myxofibrosarcoma with Durable Response to Temozolomide Followed by Atezolizumab: A Case Report. Oncologist. 2021;26:549–553. doi: 10.1002/onco.13728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tabata M.M., Novoa R.A., Bui N.Q., Zaba L.C. Successful treatment of HIV-negative Kaposi sarcoma with ipilimumab and nivolumab and concurrent management of baseline psoriasis and bullous pemphigoid. JAAD Case Rep. 2020;6:447–449. doi: 10.1016/j.jdcr.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhou M., Bui N., Lohman M., van de Rjin M., Hwang G., Ganjoo K. Long-Term Remission with Ipilimumab/Nivolumab in Two Patients with Different Soft Tissue Sarcoma Subtypes and No PD-L1 Expression. Case Rep. Oncol. 2021;14:459–465. doi: 10.1159/000512828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chen Y., Liu X., Liu J., Liang D., Zhao M., Yu W., Chen P. Nivolumab plus ipilimumab versus nivolumab in individuals with treatment-naive programmed death-ligand 1 positive metastatic soft tissue sarcomas: A multicentre retrospective study. BMC Cancer. 2021;21:1–8. doi: 10.1186/s12885-021-07843-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhou M., Bui N., Bolleddu S., Lohman M., Becker H.-C., Ganjoo K. Nivolumab plus ipilimumab for soft tissue sarcoma: A single institution retrospective review. Immunotherapy. 2020;12:1303–1312. doi: 10.2217/imt-2020-0155. [DOI] [PubMed] [Google Scholar]
- 144.Zer A., Icht O., Joseph L., Avram D., Jacobi O., Fenig E., Shamai S., Frommer R.S., Bernstine H., Weitzen R., et al. A phase II single-arm study of nivolumab and ipilimumab (Nivo/Ipi) in previously treated Classic Kaposi sarcoma (CKS) J. Clin. Oncol. 2019;37:11064. doi: 10.1200/JCO.2019.37.15_suppl.11064. [DOI] [Google Scholar]
- 145.Lewin J., Davidson S., Anderson N.D., Lau B.Y., Kelly J., Tabori U., Salah S., Butler M.O., Aung K.L., Shlien A., et al. Response to Immune Checkpoint Inhibition in Two Patients with Alveolar Soft-Part Sarcoma. Cancer Immunol. Res. 2018;6:1001–1007. doi: 10.1158/2326-6066.CIR-18-0037. [DOI] [PubMed] [Google Scholar]
- 146.Grünwald V., Bauer S., Hermes B., Ivanyi P., Lindner L.H., Pink D., Reichardt P., Richter S., Tuchscherer A. A randomized phase II study of durvalumab and tremelimumab compared to dox-orubicin in patients with advanced or metastatic soft tissue sarcoma (MEDISARC, AIO-STS 0415) J. Clin. Oncol. 2019;37((Suppl. 15)):TPS11075. doi: 10.1200/JCO.2019.37.15_suppl.TPS11075. [DOI] [Google Scholar]
- 147.Bishop M.W., Kaste S.C., Sykes A., Pan H., Cruz F.S.D., Whittle S., Mascarenhas L., Thomas P.G., Youngblood B., Harman J.L., et al. OSTPDL1: A phase II study of avelumab, a monoclonal antibody targeting programmed death-ligand 1 (PD-L1) in adolescent and young adult patients with recurrent or progressive osteosarcoma. J. Clin. Oncol. 2020;38:10521. doi: 10.1200/JCO.2020.38.15_suppl.10521. [DOI] [Google Scholar]
- 148.Uboha N.V., Milhem M.M., Kovacs C., Amin A., Magley A., Das Purkayastha D., Piha-Paul S.A. Phase II study of spartalizumab (PDR001) and LAG525 in advanced solid tumors and hematologic malignancies. J. Clin. Oncol. 2019;37:2553. doi: 10.1200/JCO.2019.37.15_suppl.2553. [DOI] [Google Scholar]
- 149.Tawbi H.A., Schadendorf D., Lipson E.J., Ascierto P.A., Matamala L., Castillo Gutiérrez E., Long G.V. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Mela-noma. N. Engl. J. Med. 2022;386:24–34. doi: 10.1056/NEJMoa2109970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rivera Vargas T., Apetoh L. Danger signals: Chemotherapy enhancers? Immunol. Rev. 2017;280:175–193. doi: 10.1111/imr.12581. [DOI] [PubMed] [Google Scholar]
- 151.Tian Z., Yang Y., Yang J., Zhang P., Zhang F., Du X., Li C., Wang J. Safety and Efficacy of PD-1 Inhibitors Plus Chemotherapy in Advanced Soft Tissue Sarcomas: A Retrospective Study. Cancer Manag. Res. 2020;12:1339–1346. doi: 10.2147/CMAR.S237300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Italiano A., Bessede A., Bompas E., Piperno-Neumann S., Chevreau C., Penel N., Bertucci F., Toulmonde M., Bellera C.A., Guegan J.-P., et al. PD1 inhibition in soft-tissue sarcomas with tertiary lymphoid structures: A multicenter phase II trial. J. Clin. Oncol. 2021;39:11507. doi: 10.1200/JCO.2021.39.15_suppl.11507. [DOI] [Google Scholar]
- 153.Spalato-Ceruso M., Bouteiller F., Guegan J.-P., Toulmonde M., Bessede A., Kind M., Cousin S., Buy X., Palussiere J., Le Loarer F., et al. Pembrolizumab combined with low-dose cyclophosphamide and in-tra-tumoral injection of the toll-like receptor 4 agonist G100 in patients with advanced pretreated soft tissue sarcoma: Results from the PEMBROSARC basket study. J. Hematol. Oncol. J. Hematol. Oncol. 2022;15:157. doi: 10.1186/s13045-022-01377-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chawla S.P., Sankhala K.K., Ravicz J., Kang G., Liu S., Stumpf N., Leong B., Kim S., Arasheben S., Tseng W.W., et al. Clinical experience with combination chemo-/immunotherapy using trabectedin and nivolumab for advanced soft tissue sarcoma. J. Clin. Oncol. 2018;36((Suppl. 15)):e23568. doi: 10.1200/JCO.2018.36.15_suppl.e23568. [DOI] [Google Scholar]
- 155.Fleuren E.D.G., Terry R.L., Meyran D., Omer N., Trapani J.A., Haber M., Neeson P.J., Ekert P.G. Enhancing the Potential of Immunotherapy in Paediatric Sarcomas: Breaking the Immunosuppressive Barrier with Receptor Tyrosine Kinase Inhibitors. Biomedicines. 2021;9:1798. doi: 10.3390/biomedicines9121798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Dorman K., Burkhard-Meier A., Di Gioia D., Kunz W.G., Knösel T., Holch J.W., Lindner L.H. Treatment of metastatic alveolar soft part sarcoma with axitinib and pembrolizumab in an 80-year-old patient with a history of autoimmune disorders. Anti-Cancer Drugs. 2022;34:311–316. doi: 10.1097/CAD.0000000000001398. [DOI] [PubMed] [Google Scholar]
- 157.Li H.-L., Li Y., Hu H.-T., Shao S.-S., Chen C.-S., Guo C.-Y., Zhao Y., Yao Q.-J. Clinical observation of local intervention combined with camrelizumab and apatinib in the treatment of metastatic soft-tissue sarcoma. J. Cancer Res. Ther. 2021;17:1718. doi: 10.4103/jcrt.jcrt_1310_21. [DOI] [PubMed] [Google Scholar]
- 158.Paoluzzi L., Cacavio A., Ghesani M., Karambelkar A., Rapkiewicz A., Weber J., Rosen G. Response to anti-PD1 therapy with nivolumab in metastatic sarcomas. Clin. Sarcoma Res. 2016;6:1–7. doi: 10.1186/s13569-016-0064-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Arora S., Rastogi S., Shamim S.A., Barwad A., Sethi M. Good and sustained response to pembrolizumab and pazopanib in advanced undifferentiated pleomorphic sarcoma: A case report. Clin. Sarcoma Res. 2020;10:1–6. doi: 10.1186/s13569-020-00133-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Monga V., Miller B.J., Tanas M., Boukhar S., Allen B., Anderson C., Stephens L., Hartwig S., Varga S., Houtman J., et al. Intratumoral talimogene laherparepvec injection with concurrent preoperative radiation in patients with locally advanced soft-tissue sarcoma of the trunk and extremities: Phase IB/II trial. J. Immunother. Cancer. 2021;9:e003119. doi: 10.1136/jitc-2021-003119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Diab A., Tannir N.M., Bentebibel S.-E., Hwu P., Papadimitrakopoulou V., Haymaker C., Kluger H.M., Gettinger S.N., Sznol M., Tykodi S.S., et al. Bempegaldesleukin (NKTR-214) plus Nivolumab in Patients with Advanced Solid Tumors: Phase I Dose-Escalation Study of Safety, Efficacy, and Immune Activation (PIVOT-02) Cancer Discov. 2020;10:1158–1173. doi: 10.1158/2159-8290.CD-19-1510. [DOI] [PubMed] [Google Scholar]
- 162.Hanna A., Metge B.J., Bailey S.K., Chen D., Chandrashekar D.S., Varambally S., Samant R.S., Shevde L.A. Inhibition of Hedgehog signaling reprograms the dysfunctional immune microenvironment in breast cancer. Oncoimmunology. 2019;8:1548241. doi: 10.1080/2162402X.2018.1548241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Grund-Gröschke S., Ortner D., Szenes-Nagy A.B., Zaborsky N., Weiss R., Neureiter D., Wipplinger M., Risch A., Hammerl P., Greil R. Epidermal activation of Hedgehog signaling establishes an immuno-suppressive microenvironment in basal cell carcinoma by modulating skin immunity. Mol. Oncol. 2020;14:1930–1946. doi: 10.1002/1878-0261.12758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yang H., Yao F., Davis P.F., Tan S.T., Hall S.R.R. CD73, Tumor Plasticity and Immune Evasion in Solid Cancers. Cancers. 2021;13:177. doi: 10.3390/cancers13020177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Somaiah N., Livingston J.A.A., Ravi V., Lin H.Y., Amini B., Solis L.M., Conley A.P., Zarzour M.A., Ludwig J.A., Ratan R., et al. A phase II multi-arm study to test the efficacy of oleclumab and durvalumab in specific sarcoma subtypes. J. Clin. Oncol. 2022;40((Suppl. 16)):TPS11594. doi: 10.1200/JCO.2022.40.16_suppl.TPS11594. [DOI] [Google Scholar]
- 166.Italiano A., Isambert N., Metges J.-P., Toulmonde M., Cousin S., Pernot S., Spalato M., Grellety T., Auzanneau C., Lortal B., et al. CAIRE: A basket multicenter open-label phase 2 study evaluating the EZH2 inhibitor tazemetostat in combination with durvalumab in patients with advanced solid tumors. J. Clin. Oncol. 2022;40((Suppl. 16)):TPS2703. doi: 10.1200/JCO.2022.40.16_suppl.TPS2703. [DOI] [Google Scholar]
- 167.Lin C.-C. Clinical Development of Colony-Stimulating Factor 1 Receptor (CSF1R) Inhibitors. J. Immunother. Precis. Oncol. 2021;4:105–114. doi: 10.36401/JIPO-20-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Saxena M., van der Burg S.H., Melief C.J.M., Bhardwaj N. Therapeutic cancer vaccines. Nat. Rev. Cancer. 2021;21:360–378. doi: 10.1038/s41568-021-00346-0. [DOI] [PubMed] [Google Scholar]
- 169.Sato Y., Nabeta Y., Tsukahara T., Hirohashi Y., Syunsui R., Maeda A., Sahara H., Ikeda H., Torigoe T., Ichimiya S., et al. Detection and Induction of CTLs Specific for SYT-SSX-Derived Peptides in HLA-A24+ Patients with Synovial Sarcoma. J. Immunol. 2002;169:1611–1618. doi: 10.4049/jimmunol.169.3.1611. [DOI] [PubMed] [Google Scholar]
- 170.Kawaguchi S., Tsukahara T., Ida K., Kimura S., Murase M., Kano M., Emori M., Nagoya S., Kaya M., Torigoe T., et al. SYT-SSX breakpoint peptide vaccines in patients with synovial sarcoma: A study from the Japanese Musculoskeletal Oncology Group. Cancer Sci. 2012;103:1625–1630. doi: 10.1111/j.1349-7006.2012.02370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Krishnadas D.K., Shusterman S., Bai F., Diller L., Sullivan J.E., Cheerva A.C., George R.E., Lucas K.G. A phase I trial combining decitabine/dendritic cell vaccine targeting MAGE-A1, MAGE-A3 and NY-ESO-1 for children with relapsed or therapy-refractory neuroblastoma and sarcoma. Cancer Immunol. Immunother. 2015;64:1251–1260. doi: 10.1007/s00262-015-1731-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Dillman R.O., Wiemann M., Nayak S.K., DeLeon C., Hood K., DePriest C. Interferon-gamma or granulocyte-macrophage col-ony-stimulating factor administered as adjuvants with a vaccine of irradiated autologous tumor cells from short-term cell line cultures: A randomized phase 2 trial of the cancer biotherapy research group. J. Immun. Hagerstown Md. 2003;26:367–373. doi: 10.1097/00002371-200307000-00009. [DOI] [PubMed] [Google Scholar]
- 173.Kershaw M., Westwood J.A., Darcy P. Gene-engineered T cells for cancer therapy. Nat. Rev. Cancer. 2013;13:525–541. doi: 10.1038/nrc3565. [DOI] [PubMed] [Google Scholar]
- 174.Gyurdieva A., Zajic S., Chang Y.-F., Houseman E.A., Zhong S., Kim J., Nathenson M., Faitg T., Woessner M., Turner D.C., et al. Biomarker correlates with response to NY-ESO-1 TCR T cells in patients with synovial sarcoma. Nat. Commun. 2022;13:1–18. doi: 10.1038/s41467-022-32491-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.D’Angelo S.P., Druta M., Liebner D.A., Schuetze S., Somaiah N., Van Tine B.A., Tap W.D., Pulham T., Chagin K., Norry E., et al. Pilot study of NY-ESO-1c259 T cells in advanced myxoid/round cell liposarcoma. J. Clin. Oncol. 2018;36:3005. doi: 10.1200/JCO.2018.36.15_suppl.3005. [DOI] [Google Scholar]
- 176.D’Angelo S.P., Attia S., Blay J.-Y., Strauss S.J., Morales C.M.V., Razak A.R.A., Van Tine B.A. Identification of response stratification factors from pooled efficacy analyses of afami-tresgene autoleucel (“Afami-cel” [Formerly ADP-A2M4]) in metastatic synovial sarcoma and myxoid/round cell liposarcoma phase 1 and phase 2 trials. J. Clin. Oncol. 2022;40((Suppl. 16)):11562. [Google Scholar]
- 177.Moreno Tellez C., Leyfman Y., D’Angelo S.P., Wilky B.A., Dufresne A. Immunotherapy in Sarcoma: Where Do Things Stand? Surg. Oncol. Clin. N. Am. 2022;31:381–397. doi: 10.1016/j.soc.2022.03.004. [DOI] [PubMed] [Google Scholar]
- 178.Huang X., Park H., Greene J., Pao J., Mulvey E., Zhou S.X., Albert C.M., Moy F., Sachdev D., Yee D., et al. IGF1R-and ROR1-Specific CAR T Cells as a Potential Therapy for High Risk Sarcomas. PLoS ONE. 2015;10:e0133152. doi: 10.1371/journal.pone.0133152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Leuci V., Casucci G.M., Grignani G., Rotolo R., Rossotti U., Vigna E., Gammaitoni L., Mesiano G., Fiorino E., Donini C., et al. CD44v6 as innovative sarcoma target for CAR-redirected CIK cells. Oncoimmunology. 2018;7:e1423167. doi: 10.1080/2162402X.2017.1423167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lehner M., Götz G., Proff J., Schaft N., Dörrie J., Full F., Ensser A., Muller Y., Cerwenka A., Abken H., et al. Redirecting T Cells to Ewing’s Sarcoma Family of Tumors by a Chimeric NKG2D Receptor Expressed by Lentiviral Transduction or mRNA Transfection. PLoS ONE. 2012;7:e31210. doi: 10.1371/journal.pone.0031210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Fernández L., Metais J.-Y., Escudero A., Vela M., Valentín J., Vallcorba I., Leivas A., Torres J., Valeri A., Patiño-García A., et al. Memory T Cells Expressing an NKG2D-CAR Efficiently Target Osteosarcoma Cells. Clin. Cancer Res. 2017;23:5824–5835. doi: 10.1158/1078-0432.CCR-17-0075. [DOI] [PubMed] [Google Scholar]
- 182.Zhao Y., Deng J., Rao S., Guo S., Shen J., Du F., Wu X., Chen Y., Li M., Chen M., et al. Tumor Infiltrating Lymphocyte (TIL) Therapy for Solid Tumor Treatment: Progressions and Challenges. Cancers. 2022;14:4160. doi: 10.3390/cancers14174160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Rohaan M.W., Borch T.H., van den Berg J.H., Met Ö., Kessels R., Foppen M.H.G., Granhøj J.S., Nuijen B., Nijenhuis C., Jedema I., et al. Tumor-Infiltrating Lymphocyte Therapy or Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2022;387:2113–2125. doi: 10.1056/NEJMoa2210233. [DOI] [PubMed] [Google Scholar]
- 184.Huang H., Nie C.-P., Liu X.-F., Song B., Yue J.-H., Xu J.-X., He J., Li K., Feng Y.-L., Wan T., et al. Phase I study of adjuvant immunotherapy with autologous tumor-infiltrating lymphocytes in locally advanced cervical cancer. J. Clin. Investig. 2022;132:e157726. doi: 10.1172/JCI157726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Stevanović S., Helman S.R., Wunderlich J.R., Langhan M.M., Doran S.L., Kwong M.L.M., Somerville R.P., Klebanoff C.A., Kammula U.S., Sherry R.M., et al. A Phase II Study of Tumor-infiltrating Lymphocyte Therapy for Human Papillomavirus–associated Epithelial Cancers. Clin. Cancer Res. 2019;25:1486–1493. doi: 10.1158/1078-0432.CCR-18-2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Jazaeri A.A., Zsiros E., Amaria R.N., Artz A.S., Edwards R.P., Wenham R.M., Slomovitz B.M., Walther A., Thomas S.S., Chesney J.A., et al. Safety and efficacy of adoptive cell transfer using autologous tumor infiltrating lymphocytes (LN-145) for treatment of recurrent, metastatic, or persistent cervical carcinoma. J. Clin. Oncol. 2019;37:2538. doi: 10.1200/JCO.2019.37.15_suppl.2538. [DOI] [Google Scholar]
- 187.Creelan B., Wang C., Teer J., Toloza E., Yao J., Kim S., Landin A., Mullinax J., Saller J., Saltos A., et al. Tumor-infiltrating lymphocyte treatment for anti-PD-1-resistant metastatic lung cancer: A phase 1 trial. Immune ACCESS. 2021;27:1410–1418. doi: 10.1038/s41591-021-01462-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Pedersen M., Westergaard M., Milne K., Nielsen M., Borch T.H., Poulsen L.G., Hendel H.W., Kennedy M., Briggs G., Ledoux S., et al. Adoptive cell therapy with tumor-infiltrating lymphocytes in patients with metastatic ovarian cancer: A pilot study. Oncoimmunology. 2018;7:e1502905. doi: 10.1080/2162402X.2018.1502905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Tran E., Robbins P.F., Lu Y.-C., Prickett T.D., Gartner J.J., Jia L., Pasetto A., Zheng Z., Ray S., Groh E.M., et al. T-Cell Transfer Therapy Targeting Mutant KRAS in Cancer. N. Engl. J. Med. 2016;375:2255–2262. doi: 10.1056/NEJMoa1609279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zacharakis N., Chinnasamy H., Black M., Xu H., Lu Y.C., Zheng Z., Feldman S.A. Immune recognition of somatic mutations leading to complete durable re-gression in metastatic breast cancer. Nat. Med. 2018;24:724–730. doi: 10.1038/s41591-018-0040-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Tran E., Turcotte S., Gros A., Robbins P.F., Lu Y.C., Dudley M.E., Wunderlich J.R., Somerville R.P., Hogan K., Hinrichs C.S., et al. Cancer Immunotherapy Based on Mutation-Specific CD4+ T Cells in a Patient with Epi-thelial Cancer. Science. 2014;344:641–645. doi: 10.1126/science.1251102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Mullinax J.E., Hall M., Beatty M., Weber A.M., Sannasardo Z., Svrdlin T., Hensel J., Bui M., Richards A., Gonzalez R.J., et al. Expanded Tumor-infiltrating Lymphocytes From Soft Tissue Sarcoma Have Tu-mor-specific Function. J. Immunother Hagerstown Md. 2021;44:63–70. doi: 10.1097/CJI.0000000000000355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ko A., Coward V.S., Gokgoz N., Dickson B.C., Tsoi K., Wunder J.S., Andrulis I.L. Investigating the Potential of Isolating and Expanding Tumour-Infiltrating Lymphocytes from Adult Sarcoma. Cancers. 2022;14:548. doi: 10.3390/cancers14030548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zhou X., Wu J., Duan C., Liu Y. Retrospective Analysis of Adoptive TIL Therapy plus Anti-PD1 Therapy in Patients with Chemotherapy-Resistant Metastatic Osteosarcoma. J. Immunol. Res. 2020;2020:1–12. doi: 10.1155/2020/7890985. [DOI] [PMC free article] [PubMed] [Google Scholar]