Abstract
Background and Objective
Erythropoietin (EPO) is a candidate neuroprotective drug. We assessed its long-term safety and efficacy as an adjunct to methylprednisolone in patients with optic neuritis and focused on conversions to multiple sclerosis (MS).
Methods
The TONE trial randomized 108 patients with acute optic neuritis but without previously known MS to either 33,000 IU EPO or placebo in conjunction with 1,000 mg methylprednisolone daily for 3 days. After reaching the primary end point at 6 months, we conducted an open-label follow-up 2 years after randomization.
Results
The follow-up was attended by 83 of 103 initially analyzed patients (81%). There were no previously unreported adverse events. The adjusted treatment difference of peripapillary retinal nerve fiber layer atrophy in relation to the fellow eye at baseline was 1.27 µm (95% CI −6.45 to 8.98, p = 0.74). The adjusted treatment difference in low-contrast letter acuity was 2.87 on the 2.5% Sloan chart score (95% CI −7.92 to 13.65). Vision-related quality of life was similar in both treatment arms (National Eye Institute Visual Functioning Questionnaire median score [IQR]: 94.0 [88.0 to 96.9] in the EPO and 93.4 [89.5 to 97.4] in the placebo group). The rate of multiple sclerosis–free survival was 38% in the placebo and 53% in the EPO group (hazard ratio: 1.67, 95% CI 0.96 to 2.88, p = 0.068).
Discussion
In line with the results at 6 months, we found neither structural nor functional benefits in the visual system of patients with optic neuritis as a clinically isolated syndrome, 2 years after EPO administration. Although there were fewer early conversions to MS in the EPO group, the difference across the 2-year window was not statistically significant.
Classification of Evidence
This study provides Class II evidence that for patients with acute optic neuritis, EPO as an adjunct to methylprednisolone is well tolerated and does not improve long-term visual outcomes.
Trial Registration Information
The trial was preregistered before commencement at clinicaltrials.gov (NCT01962571).
Optic neuritis is the most common optic neuropathy in young adults and leads to retinal nerve fiber and ganglion cell layer thinning. It has a close relationship to multiple sclerosis (MS): It is the presenting symptom of MS in about 25% of patients, 70% of patients with MS will experience optic neuritis within the disease course,1 and 50% of patients with optic neuritis develop MS within 15 years.2 Visual recovery in optic neuritis is accelerated by high-dose methylprednisolone,3 but no therapy has been established to improve long-term outcomes. Thus, as in MS, there remains an unmet need of clinical long-term neuroprotection.
The role of the visual system in treatment assessment of neuroinflammatory diseases has been recently reviewed.4 One candidate neuroprotective agent gaining interest is the human cytokine erythropoietin (EPO). It crosses the blood-brain barrier5,6 and confers anti-inflammatory, anti-apoptotic, and neuroprotective effects in preclinical models of autoimmune neuroinflammation.7 Following ambiguous results from smaller clinical studies,8-10 we conducted the TONE trial (treatment of optic neuritis with erythropoietin) to assess retinal ganglion cell neuroprotection.11 The primary end point was set at 6 months as most atrophy occurs in the first 4 months, plateauing thereafter.12 Because conversions to MS occur within years after optic neuritis,2 we scheduled an additional long-term open-label assessment 2 years after treatment, the results of which are reported herein. The primary research question was to assess the safety and efficacy of erythropoietin in improving visual outcomes 2 years after acute optic neuritis.
Methods
The TONE study protocol,13 baseline characteristics, and the 6-month results11 have previously been described in detail. In brief summary, 108 patients within 10 days of onset of a first episode of acute optic neuritis and high-contrast visual acuity <3/6 in the affected eye were randomized to receive either placebo (saline solution) or 33,000 IU EPO as an adjunct to high-dose methylprednisolone daily for 3 consecutive days. Recruitment took place at 12 German academic tertiary referral centers between November 25, 2014, and October 9, 2017. Patients with previously known MS were not included, but conversion as a result of the subsequent neurologic workup did not lead to exclusion. Patients with positive serum tests for aquaporin-4 antibodies were excluded.
Outcomes and Procedures
The first primary outcome was the atrophy of the peripapillary retinal nerve fiber layer (pRNFL), defined as the change in pRNFL thickness in relation to the fellow eye at baseline. It was measured using spectral domain optical coherence tomography (OCT) along a peripapillary circle 3.5 mm in diameter. The second primary outcome was low-contrast letter acuity, measured as the 2.5% Sloan Chart score.
Structural changes were assessed by spectral domain OCT imaging (Nsite Analytics, version 6.0, and Spectralis-OCT, Heidelberg Engineering, Heidelberg, Germany), with reporting in adherence to the APOSTEL 2.0 recommendations.14 They included the peripapillary RNFL thickness in predefined nasal superior, superior, temporal superior, temporal inferior, inferior, and nasal inferior sectors and in the papillomacular bundle, each measured along circles of 3.5 mm, 4.1 mm, and 4.7 mm diameters. The central retina was assessed using the 1/3/6 mm early treatment of diabetic retinopathy (ETDRS) grid on volume scans derived from 61 B scans. Each of the 12 study centers served as an OCT operating site. All OCT scans were uploaded to the Bern Photographic Reading Center (Bern, Switzerland), which served as the single grader. Quality control according to the OSCAR IB15 criteria led to exclusion of 28 central retinal scans and 4 circumpapillary scans. Retinal layer thicknesses were measured by manual segmentation. The functional secondary endpoints consisted of high-contrast visual acuity (measured by ETDRS charts) contrast sensitivity (Mars charts), and static automated perimetry of the central 60° visual field with recording of the mean defect, the square root of loss variance, false-positive and false-negative answers. Electrophysiologic secondary end points were the P100 amplitudes and peak times of visually evoked potentials (VEPs). Neurologic secondary end points were the Expanded Disability Status Scale (EDSS) and the time to conversion to MS according to the 2010 McDonald criteria.16 Vision-related quality of life was assessed by the German version of the National Eye Institute Visual Functioning Questionnaire (NEI-VFQ). The current report is of the open-label follow-up of the study cohort, performed 24 months after randomization.
Statistical Analysis
The analysis was conducted in the full analysis set of all randomized patients with optic neuritis who received at least 1 dose of study medication and had at least 1 postinterventional OCT measurement. The primary and secondary outcomes were assessed by linear regression models with the outcome after 24 months as the dependent variable and treatment assignment, baseline measurement of the fellow eye, and study site as independent variables. The rates of multiple sclerosis–free survival and optic neuritis relapse-free survival were estimated by assigned treatment with the Kaplan-Meier method and an unadjusted Cox proportional hazards model. Missing data from the 24-month follow-up were addressed by characterizing the populations who did and did not attend the follow-up.
Post Hoc Analyses
Postacute changes were defined as the difference in the 24 months and 6 months observations of the study outcomes. Adjusted treatment differences for such changes were calculated with linear regression models as described above.
Sample Size Calculation and Statistical Reporting
The sample size calculation for the TONE trial was based on the first primary end point at week 26 and has been described in detail.11,13 The current report of long-term open-label outcomes is observational and adheres to the STROBE guidelines.17 In the spirit of the American Statistical Association's statement on p values18 and recent addendum,19 we report outcomes as adjusted treatment differences with 95% CIs. p Values are included for the main study outcome and rate of MS-free survival to facilitate comparisons with the results at week 26.
Standard Protocol Approvals, Registrations, and Patient Consents
The study was approved by the ethics committee of the University of Freiburg, Germany, and the institutional review boards of all participating sites. Written informed consent was obtained from all participating patients. The trial was preregistered before commencement at clinicaltrials.gov (NCT01962571).
Data Availability
Individual participant data of 2-year results, including data dictionaries, will be made available together with previous study data of the TONE trial to researchers with a methodologically sound proposal.
Results
Patient Characteristics and Compliance With Long-term Follow-up
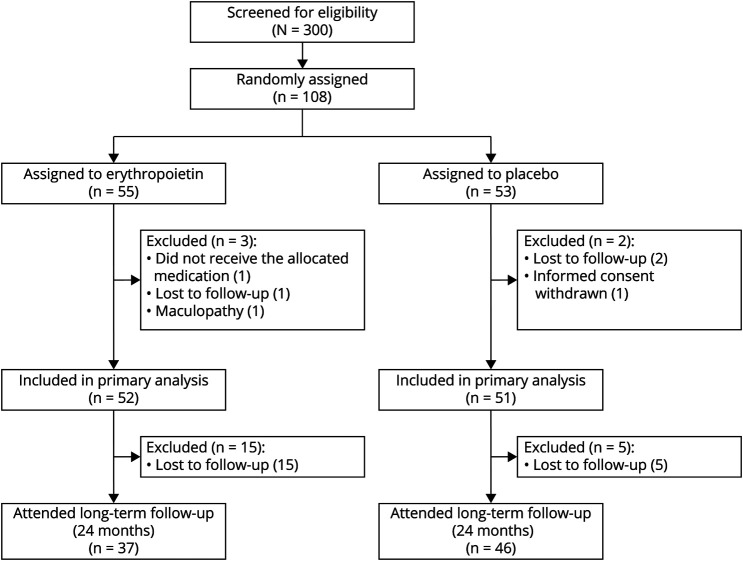
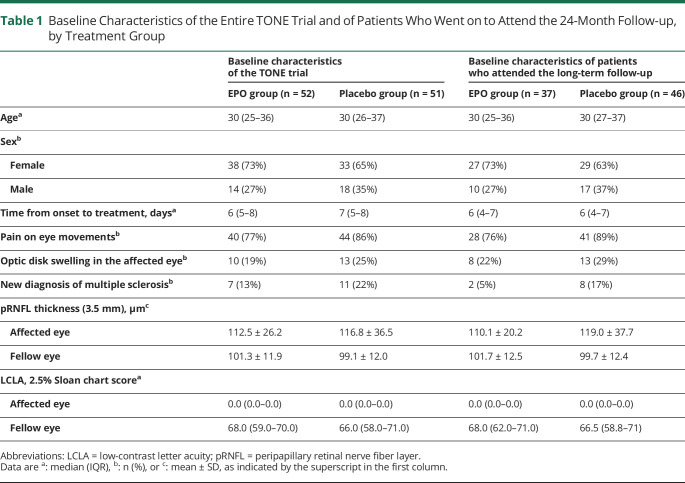
The 2-year follow-up was completed by 37/52 patients in the EPO (71%) and 46/51 patients (90%) in the placebo arm (Figure 1). The baseline characteristics of these patients were akin to those of the initial study population (Table 1). Patients with long-term follow-up had a similar age distribution between treatment groups. The female:male ratio was approximately 4:1 in the EPO and 3:1 in the placebo arm. The median time from onset to treatment was equal in both groups. Optic disk swelling and pain on eye movements were slightly more common in the placebo than in the EPO arm, as was a new diagnosis of MS because of baseline diagnostics. The pRNFL of the affected eye was on average 9 µm thicker in the placebo than in the EPO group. Scores for low-contrast letter acuity were similar.
Figure 1. Patient Flowchart.
Table 1.
Baseline Characteristics of the Entire TONE Trial and of Patients Who Went on to Attend the 24-Month Follow-up, by Treatment Group
Patients who did not attend the 2-year visit were slightly younger than the general study population (median age: 28 vs 30 years) and had a similar sex distribution (69% vs 67% female). At 6 months, they had less pRNFL atrophy (median: 5.5 µm vs 11 µm) but similar low-contrast visual acuity compared with the general study population (median 56 on the 2.5% Sloan chart score in both groups). Dropouts who had received EPO had worse low-contrast letter acuity at month 6 compared with placebo recipients who dropped out (median 54 vs 61) but similar pRNFL atrophy (median 6 µm vs 5 µm). The median age of dropouts in the EPO group was higher than in the placebo group (28 years vs 22 years); the sex distribution of dropouts was similar between treatment arms (data not shown).
Safety
In patients with available long-term data, no previously unreported severe adverse events occurred.
Long-term Outcome
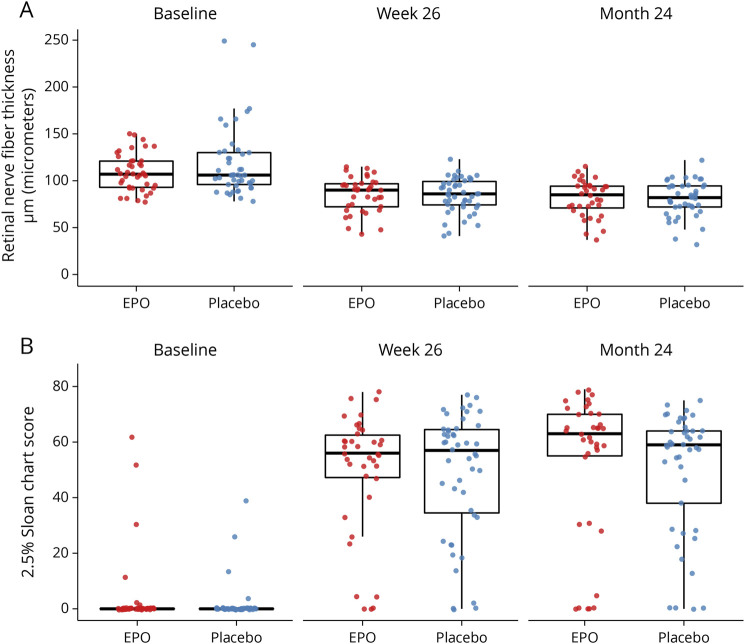
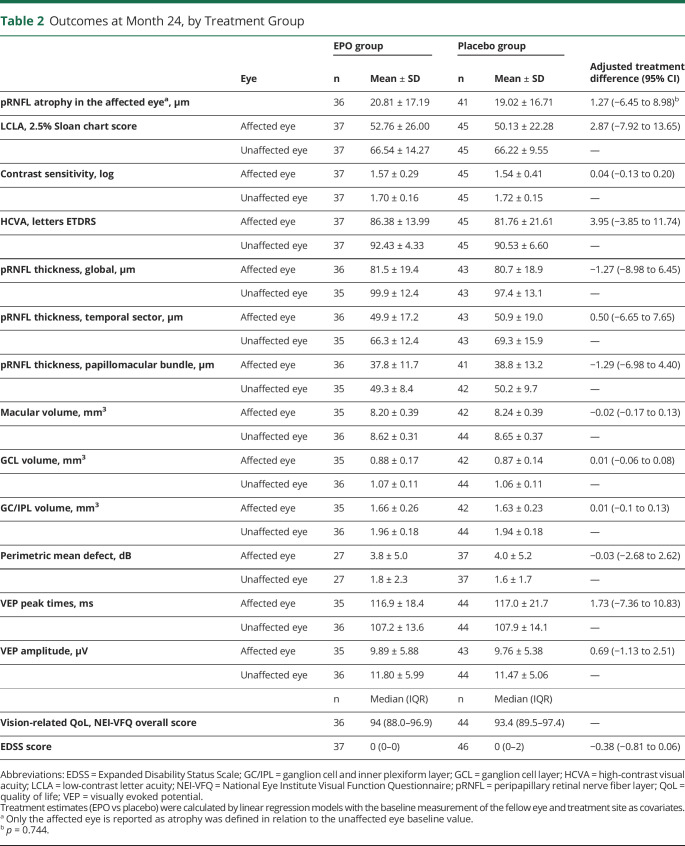
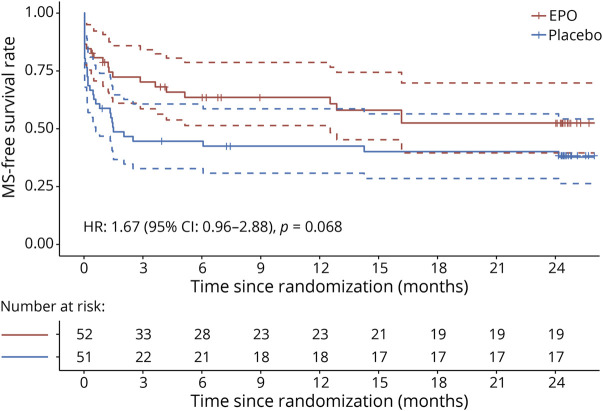
At 24 months, the amount of pRNFL atrophy was similar between treatment groups (adjusted treatment difference: 1.27 µm, 95% CI −6.45 to 8.98, p = 0.74), as was low-contrast letter acuity (adjusted treatment difference: 2.87 on the 2.5% Sloan chart score, 95% CI −7.92 to 13.65) (Figure 2). All secondary psychophysical and structural outcomes were similar between the treatment groups (Table 2, Figure 2). The rate of MS diagnosis-free survival is depicted in Figure 3. At 24 months, the rate was 0.38 in the placebo and 0.53 in the EPO group (hazard ratio: 1.67, 95% CI 0.96–2.88, p = 0.068). Of note, the 2 survival curves separated early within the first 3 months after optic neuritis and continued in parallel thereafter (Figure 3). Optic neuritis relapses occurred in 4 EPO and 3 placebo recipients. The optic neuritis relapse-free rate at 24 months was 0.9 in the EPO and 0.94 in the placebo group (hazard ratio: 0.69, 95% CI 0.15–3.08). Vision-related quality of life was similar in both trial arms (Table 2).
Figure 2. Visual Outcomes by Treatment Group.
(A) Retinal nerve fiber layer thickness in the affected eye. (B) Low-contrast letter acuity in the affected eye. Data are shown for the baseline visit, the primary end point at 26 weeks, and the long-term follow-up at 24 months. Dots are individual data points; horizontal bars are medians, and boxes are interquartile ranges. Whiskers extend to the largest/smallest value no further than 1.5 * IQR from the hinge.
Table 2.
Outcomes at Month 24, by Treatment Group
Figure 3. Kaplan-Meier Plot of Multiple Sclerosis–Free Survival by Treatment Group.
Diagnoses were made by the 2010 McDonald criteria. Vertical bars depict censored data. Dashed lines indicate the 95% CI of estimated MS-free survival. HR = hazard ratio.
Postacute Changes
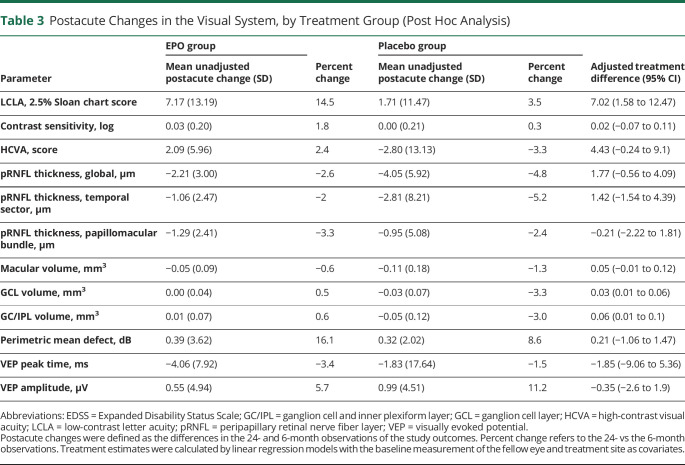
Compared with the 6-month observations, patients in both treatment arms had continued structural atrophy but functional improvement. Improved function was seen in both treatment arms in low-contrast letter acuity and VEP peak times, whereas VEP amplitudes, high-contrast visual acuity, and visual fields did not change. Continued but limited structural atrophy was observed for pRNFL thickness and macular volume in both treatment arms and combined ganglion cell and inner plexiform layer (GC/IPL) volume in placebo recipients. EPO recipients had a higher gain in low-contrast letter acuity (adjusted treatment difference: 7.02 on the 2.5% Sloan chart score, 95% CI 1.58–12.47) and less reduction of GC/IPL volume (adjusted treatment difference: 0.06, 95% CI 0.01–0.1) compared with patients in the placebo group (Table 3).
Table 3.
Postacute Changes in the Visual System, by Treatment Group (Post Hoc Analysis)
Discussion
High-dose erythropoietin did not result in long-term improvement in functional or structural outcomes in the visual pathway of patients with acute optic neuritis. In the postacute phase, patients in both treatment arms showed continuous but mild improvement in low-contrast letter acuity and VEP peak times, whereas structural measures continued to deteriorate. This postacute structural-functional discrepancy has previously been observed20 and is consistent with recent observations.21,22 It is likely to result from a resolution of conduction block by mechanisms of repair and remyelination resulting in increased function, while the neuroaxonal loss does not recover but slowly continues. Compared with patients in the placebo group, EPO recipients presented better trajectories in 2.5% low-contrast letter acuity and, less pronounced, GC/IPL atrophy between 6 and 24 months, possibly due to the lower percentage of patients with MS in the EPO group. However, there was no long-term visual benefit of EPO therapy as outcomes at 24 months were equal between treatment arms.
A notable result at the 6-month end point of the TONE trial was a difference in MS-free survival favoring the EPO group (p = 0.032 for the 6-month interval11). We previously stated that this finding might be explained by baseline imbalances of previous (undiagnosed) MS or predisposing factors, an effect of EPO on subclinical lesions and gadolinium enhancement outside the visual system, or a combination thereof. With 2 years' follow-up, the difference between treatment groups did not increase further and lost statistical significance (p = 0.068, Figure 3). This observation would be in line with both baseline imbalances or with an effect of EPO, as any treatment effect would likely be limited in time and, once worn off, lead to a similar rate of MS conversion. The fact that similar observations were made in the Vision Protect study,8 which included 40 patients with an intervention protocol similar to TONE, argues against baseline imbalances in TONE and for a true effect of EPO. In Vision Protect, standardized MRI was performed at baseline, week 4, week 8, and week 16. Here, subsequent conversions from clinically isolated syndrome to MS occurred more frequently in the placebo than in the EPO group, with a pronounced difference in early conversions.23 A smaller trial reported randomized 10 patients with optic neuritis as a clinically isolated syndrome and at least 3 hyperintense lesions on T2-weighted and fluid-attenuated inversion recovery (FLAIR) MRI to either 20′000 IE EPO/d or placebo as an adjunct to methylprednisolone pulse therapy for 5 days. One patient in the placebo group and no patients in the EPO group fulfilled the McDonald criteria for the diagnosis of MS within 1 year.24 The same treatment protocol was used in a randomized study comprising 20 participants with a severe motor relapse of relapsing-remitting MS (RR-MS), reported by Varzaneh and colleagues. Over a follow-up of 3 months, the authors observed lower ambulatory index and EDSS values and fewer hyperintense lesions on T2-weighted MRI in EPO recipients compared with patients in the placebo group.25 Most recently, a randomized controlled trial including 50 patients with progressive, primary, or secondary MS found no significant treatment effect of weekly then biweekly application of high-dose (48′000 IU) EPO on a composite outcome of hand dexterity, maximum gait distance, and cognition.26 Taken together and considering the preclinical evidence, a limited case might be made for beneficial systemic effects of EPO in acute but not chronic activity of clinical or preclinical demyelinating disease.
The long-term data of this study are limited by possible biases introduced by losses to follow-up. The higher percentage of males, patients with MS, and optic disk swelling in placebo recipients may point toward more severe disease in this group. However, if so, such a bias would likely have exacerbated, not masked, any treatment differences. Data on MS conversions in the TONE trial should be interpreted cautiously due to baseline differences and lack of standardized MR imaging. Other limitations of this study have been previously discussed and include the unknown influence of previous subclinical disease activity and trans-synaptic retrograde degeneration and the possible inclusion of a small number of patients with anti–MOG-related optic neuritis.
In conclusion, the open-label follow-up of the TONE trial provides Class II evidence that high-dose erythropoietin as an adjunct to methylprednisolone does not improve long-term visual outcomes in acute optic neuritis. Its efficacy in systemic demyelinating disease remains unclear and warrants further investigation.
Acknowledgment
The authors thank all patients who participated in the TONE trial and follow-up observation.
Glossary
- EDSS
Expanded Disability Status Scale
- EPO
erythropoietin
- ETDRS
early treatment of diabetic retinopathy
- NEI-VFQ
National Eye Institute Visual Functioning Questionnaire
- OCT
optical coherence tomography
- pRNFL
peripapillary retinal nerve fiber layer
- VEP
visually evoked potential
Appendix 1. Authors
Appendix 2. Coinvestigators
Footnotes
Class of Evidence: NPub.org/coe
Contributor Information
Collaborators: for the TONE Study Group, Fanni Eszter Moln´ar, Sebastian Rauer, Bettina Wabbels, Marion Noll, Marcus M¨uller, Tanja Guthoff, Rainer Guthoff, Orhan Aktas, Freidrich E Kruse, Konstantin Huhn, Florian T Nickel, Ralf A Linker, Dirk Fitzner, Katharina Hein, Christian van Oterendorp, Frauke Zipp, Timo Uphaus, Vinzenz Fleischer, Nelly Siller, Katrin Lorenz, Anna Beck, Susanna Pitz, Heike Elflein, Kathrin Hartmann, Tanja K¨umpfel, Elisabeth Mulazzani, Helmut Wilhelm, Ulf Zielmann, Christoph Heesen, Patrick Stellmann, and Sina Rosenkranz
Study Funding
This study was funded by German Federal Ministry for Education and Research (BMBF), Grant No. 01KG1306 Freunde der Universitäts-Augenklinik e.V.
Disclosure
S. Küchlin: doctoral fellowship with funding from the University of Freiburg and the Else Kröner-Fresenius Foundation. G. Ihorst: funding from Novartis and Janssen Pharmaceuticals. B. Grotejohann and F. Beisse: nothing to report. S.P. Heinrich: funding from the DFG, German Federal Institute for Sport Science, and German Ophthalmologic Society. P. Albrecht: grant and personal fees and nonfinancial support from Allergan, Biogen, Celgene, Ipsen, Janssen Cilag, Merck, Merz Pharmaceuticals, Novartis, Roche, and Teva, outside the submitted work. J. Ungewiss: employee at the Aalen University of Applied Sciences, employee at Carl Zeiss Vision International GmbH, doctoral fellowship with funding from the Ministry of Science Research and Arts Baden-Wuerttemberg as part of the HAW-Prom program, speakers' honorary from AMO Ireland (affiliated to Johnson & Johnson Vision), and coinventor of patents/patent applications with the numbers 10 2017 126 741, WO 2020/089284 A1, and 20 174 551.0. M. Wörner is a managing partner of Blickshift GmbH and coinventor of patent applications WO 2020/089284 A1 and 20 174 551.0. M.J. Hug: grants from Bristol-Myers Squibb and speaker's honoraria from Amgen, Baxter Deutschland, CSL Behring, Biotest Pharma, Fresenius Kabi, Leo Pharma, Merck Serono, Novartis Pharma, Pfizer, Roche Pharma, and Sun Pharmaceuticals Industries. S. Wolf has served as a consultant for Bayer, Novartis, Chengdu Kanghong Biotech, Zeiss, and Roche; has received grant support from Zeiss; and has received grant support from Heidelberg Engineering. W.A. Lagrèze has received grants from the German Federal Ministry for Education and Research (BMBF), grants from the German Research Foundation (DFG), and speakers' honoraria from and worked on advisory boards for Alcon, Allergan, Santhera, Boehringer Ingelheim, and Merz Pharma. Go to Neurology.org/NN for full disclosure.
References
- 1.Toosy AT, Mason DF, Miller DH. Optic neuritis. Lancet Neurol. 2014;13(1):83-99. doi: 10.1016/s1474-4422(13)70259-x [DOI] [PubMed] [Google Scholar]
- 2.The Optic Neuritis Study Group. Multiple sclerosis risk after optic neuritis: final optic neuritis treatment trial follow-up. Arch Neurol. 2008;65(6):727-732. doi: 10.1001/archneur.65.6.727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beck RW, Cleary PA, Backlund JyC; Optic Neuritis Study Group. The course of visual recovery after optic neuritis: experience of the optic neuritis treatment trial. Ophthalmology. 1994;101(11):1771-1778. doi: 10.1016/s0161-6420(94)31103-1 [DOI] [PubMed] [Google Scholar]
- 4.Graves JS, Oertel FC, Van der Walt A, et al. . Leveraging visual outcome measures to advance therapy development in neuroimmunologic disorders. Neurol Neuroimmunol Neuroinflamm. 2022;9(2):e1126. doi: 10.1212/nxi.0000000000001126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ehrenreich H, Aust C, Krampe H, et al. . Erythropoietin: novel approaches to neuroprotection in human brain disease. Metab Brain Dis. 2004;19(3/4):195-206. doi: 10.1023/b:mebr.0000043969.96895.3c [DOI] [PubMed] [Google Scholar]
- 6.Brines ML, Ghezzi P, Keenan S, et al. . Erythropoietin crosses the blood–brain barrier to protect against experimental brain injury. Proc Natl Acad Sci U S A. 2000;97(19):10526-10531. doi: 10.1073/pnas.97.19.10526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cervellini I, Ghezzi P, Mengozzi M. Therapeutic efficacy of erythropoietin in experimental autoimmune encephalomyelitis in mice, a model of multiple sclerosis. Methods Mol Biol. 2013:982:163-173. [DOI] [PubMed] [Google Scholar]
- 8.Sühs K-W, Hein K, Sättler MB, et al. . A randomized, double-blind, phase 2 study of erythropoietin in optic neuritis. Ann Neurol. 2012;72:199-210. doi: 10.1002/ana.23573 [DOI] [PubMed] [Google Scholar]
- 9.Soltan Sanjari M, Pakdel F, Moosavi F, et al. . Visual outcomes of adding erythropoietin to methylprednisolone for treatment of Retrobulbar optic neuritis. J Ophthalmic Vis Res. 2019;14(3):299-305. doi: 10.18502/jovr.v14i3.4786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shayegannejad V, Shahzamani S, Dehghani A, Dast Borhan Z, Rahimi M, Mirmohammadsadeghi A. A double-blind, placebo-controlled trial of adding erythropoietin to intravenous methylprednisolone for the treatment of unilateral acute optic neuritis of unknown or demyelinative origin. Graefes Arch Clin Exp Ophthalmol. 2015;253(5):797-801. doi: 10.1007/s00417-014-2925-7 [DOI] [PubMed] [Google Scholar]
- 11.Lagrèze WA, Küchlin S, Ihorst G, et al. . Safety and efficacy of erythropoietin for the treatment of patients with optic neuritis (TONE): a randomised, double-blind, multicentre, placebo-controlled study. Lancet Neurol. 2021;20(12):991-1000. doi: 10.1016/s1474-4422(21)00322-7 [DOI] [PubMed] [Google Scholar]
- 12.Gabilondo I, Martínez-Lapiscina EH, Fraga-Pumar E, et al. . Dynamics of retinal injury after acute optic neuritis: retinal Injury in ON. Ann Neurol. 2015;77(3):517-528. doi: 10.1002/ana.24351 [DOI] [PubMed] [Google Scholar]
- 13.Diem R, Molnar F, Beisse F, et al. . Treatment of optic neuritis with erythropoietin (TONE): a randomised, double-blind, placebo-controlled trial—study protocol. BMJ Open. 2016;6(3):e010956. doi: 10.1136/bmjopen-2015-010956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aytulun A, Cruz-Herranz A, Aktas O, et al. . APOSTEL 2.0 recommendations for reporting quantitative optical coherence tomography studies. Neurology. 2021;97(2):68-79. doi: 10.1212/wnl.0000000000012125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schippling S, Balk L, Costello F, et al. . Quality control for retinal OCT in multiple sclerosis: validation of the OSCAR-IB criteria. Mult Scler. 2015;21(2):163-170. doi: 10.1177/1352458514538110 [DOI] [PubMed] [Google Scholar]
- 16.Polman CH, Reingold SC, Banwell B, et al. . Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292-302. doi: 10.1002/ana.22366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453-1457. doi: 10.1016/S0140-6736(07)61602-X [DOI] [PubMed] [Google Scholar]
- 18.Wasserstein RL, Lazar NA. The ASA statement on p-values: context, process, and purpose. Am Statistician. 2016;70:129-133. [Google Scholar]
- 19.Wasserstein RL, Schirm AL, Lazar NA. Moving to a world beyond “p < 0.05”. Am Statistician. 2019;73:1-19. [Google Scholar]
- 20.Klistorner A, Arvind H, Garrick R, Graham SL, Paine M, Yiannikas C. Interrelationship of optical coherence tomography and multifocal visual-evoked potentials after optic neuritis. Invest Ophthalmol Vis Sci. 2010;51(5):2770. doi: 10.1167/iovs.09-4577 [DOI] [PubMed] [Google Scholar]
- 21.Andorrà M, Alba-Arbalat S, Camos-Carreras A, et al. . Using acute optic neuritis trials to assess neuroprotective and remyelinating therapies in multiple sclerosis. JAMA Neurol. 2020;77(2):234-244. doi: 10.1001/jamaneurol.2019.3283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Louzi OA, Bhargava P, Newsome SD, et al. . Outer retinal changes following acute optic neuritis. Mult Scler. 2016;22(3):362-372. doi: 10.1177/1352458515590646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sühs K-W, Papanagiotou P, Hein K, et al. . Disease activity and conversion into multiple sclerosis after optic neuritis is treated with erythropoietin. Int J Mol Sci. 2016;17(10):1666. doi: 10.3390/ijms17101666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borhani-Haghighi A, Ghodsi M, Razeghinejad MR, et al. . Erythropoietin for acute multiple sclerosis in patients with optic neuritis as a first demyelination event. Neurosciences (Riyadh). 2012;17(2):151-155. [PubMed] [Google Scholar]
- 25.Najmi Varzaneh F, Najmi Varzaneh F, Azimi AR, Rezaei N, Sahraian MA. Efficacy of combination therapy with erythropoietin and methylprednisolone in clinical recovery of severe relapse in multiple sclerosis. Acta Neurol Belg. 2014;114(4):273-278. doi: 10.1007/s13760-014-0286-y [DOI] [PubMed] [Google Scholar]
- 26.Schreiber K, Magyari M, Sellebjerg F, et al. . High-dose erythropoietin in patients with progressive multiple sclerosis: a randomized, placebo-controlled, phase 2 trial. Mult Scler. 2017;23(5):675-685. doi: 10.1177/1352458516661048 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Individual participant data of 2-year results, including data dictionaries, will be made available together with previous study data of the TONE trial to researchers with a methodologically sound proposal.