Abstract
Simple Summary
The identification of clinical and tumor factors to identify patients who most benefit from oncological treatments is a crucial clinical unmet need. We studied differences in the tumor microenvironment assessed using an easy method called immunohistochemistry between cancers in patients who did or did not respond to an immunotherapy called nivolumab. The tumors in those patients who most benefitted from nivolumab were characterized by the following: a poor presence of specific immune cells (CD4+), which are likely to have an immunosuppressive role; a high expression of the CD56 marker on tumor cells, which plays a role in cell cytotoxicity; and a high expression of the phosphorylated form of the mTOR protein in tumor cells, which regulates the function of intra-tumor inflammatory cells and cancer cells. Further immunohistochemical and genomic analyses are planned to deeply examine the prognostic role of the tumor microenvironment.
Abstract
Background: Prognostic and predictive factors for patients with metastatic renal cell carcinoma (mRCC) treated with immunotherapy are highly warranted, and the immune tumor microenvironment (I-TME) is under investigation. Methods: The Meet-URO 18 was a multicentric retrospective study assessing the I-TME in mRCC patients treated with ≥2nd-line nivolumab, dichotomized into responders and non-responders according to progression-free survival (≥12 months and ≤3 months, respectively). The primary objective was to identify differential immunohistochemical (IHC) patterns between the two groups. Lymphocyte infiltration and the expressions of different proteins on tumor cells (CD56, CD15, CD68, and ph-mTOR) were analyzed. The expression of PD-L1 was also assessed. Results: A total of 116 tumor tissue samples from 84 patients (59% were primary tumors and 41% were metastases) were evaluated. Samples from responders (N = 55) were significantly associated with lower expression of CD4+ T lymphocytes and higher levels of ph-mTOR and CD56+ compared with samples from non-responders (N = 61). Responders also showed a higher CD3+ expression (p = 0.059) and CD8+/CD4+ ratio (p = 0.084). Non-responders were significantly associated with a higher percentage of clear cell histology and grading. Conclusions: Differential IHC patterns between the tumors in patients who were responders and non-responders to nivolumab were identified. Further investigation with genomic analyses is planned.
Keywords: renal cell carcinoma, immunotherapy, immune checkpoint inhibitor, nivolumab, tumor microenvironment, immunohistochemistry, immune infiltration, CD56, lymphocyte
1. Introduction
In recent years, the treatment landscape of metastatic renal cell carcinoma (mRCC) has been revolutionized with the approval of immune checkpoint inhibitors (ICIs) as monotherapy for pretreated patients and in combination therapies for untreated patients [1]. In 2015, nivolumab was the first FDA-approved ICI after its survival advantage over everolimus, as reported in the Checkmate 025 study [2]. Despite the survival advantage observed with ICI-based therapies, only a small percentage of patients respond to immunotherapy or maintain a durable clinical benefit over time [3]. The identification of prognostic and/or predictive biomarkers is crucial for therapeutic selection and sequencing, maximizing efficacy, sparing patients from unnecessary toxicities, and minimizing costs [3].
Prognostic and/or predictive factors, which are under investigation in mRCC patients treated with immunotherapies, include peripheral blood inflammatory indices, clinical factors, and tumor microenvironment (TME) biomarkers [3,4]. More recently, new frontiers in the investigation of predictive biomarkers for immunotherapy, such as mitochondrial metabolism, have been explored and are of high interest [5].
On the other hand, peripheral blood inflammatory indices, clinical factors, and their combinations in clinical models are of great interest because of their low cost and are readily available and thus easily integrated into therapeutic decision making [6].
The TME is heterogeneous and consists of many types of immune cells (lymphocytes, macrophages, and neutrophils), stromal cells, and the activation of different molecular pathways [4]. Numerous studies have shown the association of the immune compartment of the TME (I-TME), which includes tumor-infiltrating lymphocytes (TILs), tumor-associated macrophages (TAMs), and neutrophils (TANs), with prognoses and treatment responses, including ICIs. Nevertheless, the I-TME is far from being routinely used [4,7,8]. Recently, efforts have been made to implement the understanding of the I-TME and responses to immunotherapy, also considering genomic and transcriptomic signatures [9,10].
The multicenter retrospective Meet-URO 15 study was a large study assessing the prognostic role of peripheral blood inflammatory indices and clinical factors, leading to the development of a new prognostic model (the Meet-URO score) [11]. These results led to the idea of focusing on the differences in the I-TME between patients who most (i.e., responders) and least (i.e., non-responders) benefited from immunotherapy with nivolumab. We, therefore, derived a follow-up study (the Meet-URO 18 study) assessing the I-TMEs of responders and non-responders patients performing immunohistochemical (IHC) and molecular analyses. Herein, we report the IHC analyses of this study, while the molecular analyses are still ongoing and will be presented separately.
2. Materials and Methods
The Meet-URO 18 study was a multicentric retrospective translational study designed to assess the prognostic role of the I-TME of primary tumors and metastases in patients with mRCC treated with ≥second-line nivolumab and divided into two cohorts according to clinical benefit from immunotherapy.
This study was approved by the Regional Ethical Committee (Regional Ethical Committee of Liguria; registration number: 209/2020-DB ID 10531). The first analyses in this study included 7 Italian centers. They were performed according to the Declaration of Helsinki, Good Clinical Practice, and local ethical guidelines. Written informed consent was signed by all living patients enrolled in this study.
2.1. Study Population
The main inclusion criteria included the following: histological diagnosis of renal cell carcinoma; advanced disease; at least one completed infusion of nivolumab given as standard clinical practice and as a second or further treatment line; progression-free survival ≤3 months (non-responders) or ≥12 months (responders); availability of sufficient histological material of primary tumor and/or metastasis to perform immunohistochemical and molecular analyses; and availability of pre-treatment complete blood count values and the “International Metastatic RCC Database Consortium” (IMDC) score.
2.2. Study Procedures
The IHC analyses included the grading, histological revision, and digital multitarget analysis of lymphocyte infiltration and tumor cells (TCs).
The histological revision was performed according to the last WHO 2016 classification of RCC morphology reassessing and immunohistochemical and molecular characteristics. The qualitative and quantitative analysis of lymphocyte infiltration included the morphological and immunophenotypic evaluation of tumor-infiltrating lymphocytes (TILs) within the tumor and at the tumor margin, including CD8+, CD4+, and FOXP3+ T cells, the CD8+/CD4+ ratio, and peri/intra-tumoral T cells.
Assessment of the expressions of CD56, CD15, CD68, and phosphorylated mTOR (ph-mTOR) in TCs was performed. The expression of PD-L1 (SP263) staining in both TCs and tumor-infiltrating immune cells (ICs) was also assessed.
Percentages of immunoreactive cells were counted and aligned to a ×200 (0.933 mm2) microscopic field.
2.3. Study Objectives
The primary objective of this study was the identification of differential IHC and molecular patterns in the I-TMEs between responder and non-responder patients treated with nivolumab. Secondary objectives included investigating the correlation of the IHC and molecular patterns in the I-TMEs between primary tumors and metastases to identify potential inter-tumor heterogeneity. The present analysis refers only to the primary objective.
2.4. Statistical Analysis
The I-TME parameters were evaluated according to the median plus interquartile range (IQR), and the cut-offs were identified via the receiver operating curves (ROCs) based on the PFS of the whole sample.
The chi-square test or the Fisher test was used for percentage comparisons, while Student’s t-test or the Mann–Whitney test, when appropriate, was used for the comparisons of the means and the medians, respectively. Statistical significance was defined as p < 0.05. All statistical analyses were performed using the software Stata v.16 (StataCorp., College Station, TX, USA, 2019).
3. Results
3.1. Patients’ Characteristics
A total of 84 patients with mRCC were included in the analysis. The patients’ characteristics are summarized in Table 1.
Table 1.
Patients’ and tumor samples’ characteristics.
| Characteristic | N (%) | p-Value | ||
|---|---|---|---|---|
| Patients N = 84 | All Patients |
Responders (N = 37, 42.5%) |
Non-Responders (N = 47, 57.5%) |
|
| Gender | ||||
| Male | 62 (73.8) | 25 (67.6) | 37 (78.7) | 0.25 |
| Female | 22 (26.2) | 12 (32.4) | 10 (21.3) | |
| Age (median, range) | 69, 27–84 | 73, 50–84 | 66, 27–82 | 0.004 |
| Histology | ||||
| ccRCC | 65 (77.4) | 25 (67.6) | 40 (85.1) | 0.056 |
| Other | 19 (22.6) | 12 (32.4) | 7 (14.9) | |
| Nephrectomy | ||||
| Yes | 72 (86.8) | 34 (94.4) | 38 (80.9) | 0.070 |
| No | 11 (13.2) | 2 (5.6) | 9 (19.2) | |
| IMDC score | ||||
| Favorable | 17 (20.2) | 12 (32.4) | 5 (10.6) | 0.030 |
| Intermediate | 52 (61.9) | 21 (56.8) | 31 (66.0) | |
| Poor | 15 (17.9) | 4 (10.8) | 11 (23.4) | |
| Meet-URO score | ||||
| 1 | 18 (22.5) | 8 (44.4) | 10 (55.6) | 0.21 |
| 2 | 28 (35.0) | 14 (50) | 14 (50) | |
| 3 | 17 (21.3) | 7 (41.2) | 10 (58.8) | |
| 4 | 10 (12.5) | 4 (40.0) | 6 (60.0) | |
| 5 | 7 (8.7) | 0 (0) | 7 (100.0) | |
| Treatment line | ||||
| 2nd line | 60 (71.4) | 26 (70.3) | 34 (72.3) | 0.84 |
| ≥3rd line | 24 (28.6) | 11 (29.7) | 13 (27.7) | |
| Samples N = 116 | All samples |
Samples of Responders
(N = 55, 47%) |
Samples of Non-responders
(N = 61, 53%) |
|
| Type of tumor sample | ||||
| Primitive tumor | 68 (59) | 32 (58.2) | 36 (59.0) | 0.93 |
| Metastasis | 48 (41) | 23 (41.8) | 25 (41.0) | |
N—number, ccRCC—clear cell renal cell carcinoma, and IMDC—International Metastatic RCC Database Consortium.
Most patients were male (73.8%), and the median age was 69 years. A total of 77.4% of patients had clear cell histology, and most of the patients had previously undergone nephrectomy (86.1%). From December 2015 to July 2022, 71.4% of patients started nivolumab as a 2nd-line treatment and 28.6% as a ≥3rd-line one. According to the IMDC risk scores at the time of nivolumab treatment, 21.3% of patients had favorable risk, while 61.3% and 17.5% had intermediate or poor risk. Moreover, lymph node metastases were present in 51.3% (N = 41) of patients, visceral metastasis in 88.8% (N = 71), and bone metastasis in 50% (N = 40).
Notably, non-responder patients were significantly younger (p = 0.004) and had a worse prognosis according to the IMDC risk score (p = 0.030) compared with responder patients.
3.2. IHC Analysis of Primary Tumors and Metastases (Responders vs. Non-Responders)
Overall, 116 tumor tissue samples (59% of which were primary tumors and 41% metastases) were assessed. The overall results are shown in Table 2.
Table 2.
IHC results of primary tumor and metastases samples between responder and non-responder mRCC patients.
| Parameter (Cut-Off) |
Responders N (%) |
Non-Responders N (%) |
OR (95% CI) |
p-Value |
|---|---|---|---|---|
| Histology | ||||
| ccRCC | 34 (61.8) | 52 (85.3) | 3.57 (1.46–8.71) | 0.005 |
| Other | 21 (38.2) | 9 (14.7) | 1.00 (ref.) | |
| Grading | ||||
| 1–2 | 17 (48.6) | 10 (19.6) | 1.00 (ref.) | |
| 3 | 12 (34.3) | 26 (51.0) | 3.68 (1.30–10.40) | 0.014 |
| 4 | 6 (17.1) | 15 (29.4) | 4.25 (1.25–14.50) | 0.021 |
| CD3+ IC | ||||
| Median (IQR) | 90 (34–200) | 45 (25–210) | - | 0.77 |
| <40 | 15 (27.3) | 27 (44.3) | 1.00 (ref.) | 0.059 |
| ≥40 | 40 (72.7) | 34 (55.7) | 0.47 (0.22–1.03) | |
| CD8+ IC | ||||
| Median (IQR) | 100 (25–150) | 105 (25–139) | - | 0.76 |
| CD4+ IC | ||||
| Median (IQR) | 45 (12–70) | 60 (15–88) | - | 0.22 |
| <70 | 41 (74.6) | 32 (52.5) | 1.00 (ref.) | 0.015 |
| ≥70 | 14 (25.5) | 29 (47.5) | 2.65 (1.21–5.83) | |
|
CD8+/CD4+ ratio Median, IQR |
1.74 (0.54–3.71) | 1.20 (0.32–2.39) | - | 0.084 |
| Peri/intra-tumoral T cells | ||||
| Absent | 24 (43.6) | 26 (42.6) | 1.00 (ref.) | 0.91 |
| Present | 31 (56.4) | 35 (57.4) | 1.04 (0.50–2.18) | |
| CD56 TC | ||||
| Median (IQR) | 0 (0–40) | 0 (0–10) | - | 0.23 |
| <40 | 40 (72.7) | 54 (88.5) | 1.00 (ref.) | 0.035 |
| ≥40 | 15 (27.3) | 7 (11.5) | 0.35 (0.13–0.93) | |
| CD15 TC | ||||
| Median (IQR) | 1 (0–10) | 1 (0–5) | - | 0.70 |
| <30 | 48 (87.3) | 48 (78.7) | 1.00 (ref.) | 0.23 |
| ≥30 | 7 (12.7) | 13 (21.3) | 1.86 (0.68–5.06) | |
| CD68 TC | ||||
| Median (IQR) | 0 (0–40) | 0 (0–10) | - | 0.77 |
| ph-mTOR TC | ||||
| Median (IQR) | 20 (10–70) | 15 (0–70) | - | 0.25 |
| <15 | 16 (29.1) | 30 (49.2) | 1.00 (ref.) | 0.029 |
| ≥15 | 39 (70.9) | 31 (50.8) | 0.42 (0.20–0.91) | |
| PD-L1 TC/IC | ||||
| Median (IQR) | 3 (0–10) | 0 (0–5) | - | 0.46 |
| <10 | 40 (72.7) | 51 (83.6) | 1.00 (ref.) | 0.16 |
| ≥10 | 15 (27.3) | 10 (16.4) | 0.52 (0.21–1.29) |
N—number of patients, OR—odds ratio, CI—confidence interval, ccRCC—clear cell renal cell carcinoma, ref.—reference, IQR—interquartile range, TC—tumor cell, and IC—immune cell.
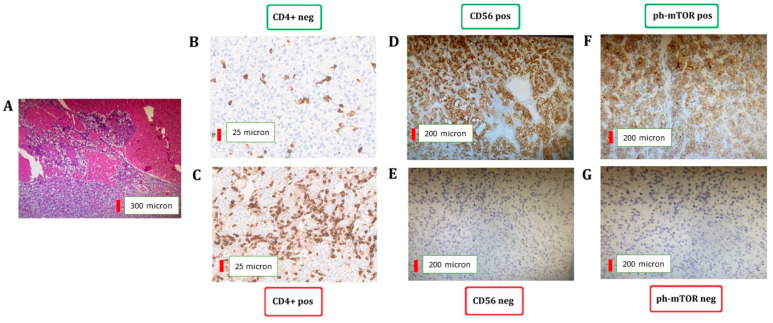
Responder patients were significantly associated with lower expression of CD4+ T lymphocytes (<70: 74.6% vs. 52.5%; p = 0.015), higher levels of ph-mTOR (≥15: 70.9% vs. 50.8%; p = 0.029), and CD56+ in TCs (≥40: 27.3% vs. 11.5%; p = 0.035) compared with non-responder patients (Figure 1).
Figure 1.
IHC assessment of CD4+ T lymphocytes and CD56 and ph-mTOR in TCs. Representative hematoxylin and eosin staining of clear cell renal cell carcinoma (A). CD4 (B,C), CD56 (D,E), and ph-mTOR (F,G) expression between responders (B,D,F) and non-responders (C,E,G).
Responders also showed a numerical difference in CD3 expression (≥40: 72.7% vs. 55.7%; p = 0.059) and CD8+/CD4+ ratios (median 1.74 vs. 1.20; p = 0.084) in ICs compared with non-responder patients.
Non-responder patients presented a higher percentage of clear cell histology (ccRCC) (85.3% vs. 61.8%; p = 0.005) and higher tumors gradings (G3–4: 80.4% vs. 51.4%; p < 0.05).
No differences between responders and non-responders were observed according to CD8+ and peritumoral T lymphocytes, the expression of CD15 and CD68 in TCs, and PD-L1 expression.
The IHC assessment of FOXP3+ T cells and CD56+ NK cells showed high heterogeneity in single tumor expression between pathologists; thus, no interpretation and clinical pathological correlation was performed due to a lack of robustness in categorization reporting.
Moreover, according to the ROCs, we were not able to identify an optimal cutoff for the expression of CD8+ T lymphocytes and CD68.
4. Discussion
The personalization of cancer therapy is a crucial clinical issue that drives the preclinical and clinical assessment of prognostic and predictive factors in mRCC patients treated with immunotherapy [12]. Clinical factors have the advantage of being easily assessable and applicable, but great interest and effort have been spent on translational research [4]. In this context, the analysis of the composition of the I-TME is essential to understand tumor immunology, which is ultimately involved in the response to these treatment breakthroughs.
Tumor-infiltrating immune cells (TI-ICs) have a central role in pro- and anti-tumorigenic processes and correlate with clinical outcomes, in many cancer types, and responses to different types of treatments, including immunotherapies [13].
This is true for RCC especially, which is among the most immune and vascularly infiltrated cancer types [4]. Therefore, there is great interest in understanding the I-TME in advanced cancer patients treated with immunotherapy, and we reported a comprehensive evaluation of pre-treatment tumor-intrinsic immune cell infiltration in mRCC patients assessed according to the clinical benefit of immunotherapy.
To identify consistent I-TME differences that may be correlated with responses to immunotherapy, the extremes of the real-world context were explored: the patients with mRCC who highly benefited from nivolumab immunotherapy with a PFS of >12 months (responders) and those who did not have a PFS of <3 months (non-responders). In our analysis, different aspects of the I-TME and tumors were assessed: TI-ICs, the mTOR pathway, tumor histotype, and grading.
The most studied TI-ICs are TILs, which have been associated with the prognosis and response to different types of therapies [14]. More recently, an increased lymphocyte infiltrate was shown to be a positive prognostic factor and a potential predictor of responses to ICIs, including in mRCC patients [15].
Generally, CD8+ cytotoxic T cells and T helper 1 (Th1) CD4+ T cells promote anti-tumor immunity, while regulatory CD4+ T cells (Treg) and T helper 2 (Th2) CD4+ T cells are associated with immune evasion [4]. RCC is generally characterized by rich intra-tumoral T cell infiltration compared with other cancer types; however, contrasting with other tumors, increased CD8+ infiltration is often found to confer a worse prognosis due to the possibly high infiltration of exhausted CD8+ and immunosuppressive TILs [4,16].
In our analysis, responder patients were significantly associated with lower CD4+ T lymphocyte expression and showed higher CD3+ T lymphocyte expression and CD8+/CD4+ ratios. These results could possibly suggest the characterization of these CD4+ T cells as being immune-suppressive (Th2 or Treg) in origin [17].
Interestingly, we found a significant correlation between CD56 expression in TCs and the response to nivolumab immunotherapy. CD56, also known as neural cell adhesion molecule (NCAM), is a member of the immunoglobulin superfamily, and its expression is typically associated with NK cells [18,19]. However, it has been detected in other ICs, and it is aberrantly expressed in TCs of hematological malignancies (e.g., multiple myeloma and leukemia) as well as solid tumors (e.g., lung, ovarian, and renal cell cancers) [19]. In this context, a new role of CD56 in cancer and immune cell functioning has been observed. CD56 homodimerization between immune cells is implicated in communication and organization within the TME [20]. In addition, CD56 expression plays a role in cell cytotoxicity, providing the interaction between effector ICs and cancer cells via CD56-CD56 homophilic interactions (the so-called “kiss of death”) [20].
Molecular pathways in the TME have also been studied as prognostic factors, and the mTOR pathway is one of the most investigated ones in RCC patients [21,22]. The mTOR pathway regulates metabolism and thus the functions of numerous intra-tumor inflammatory cells and cancer cells [23]. We found that tumors in responder patients had higher expression of ph-mTOR. In fact, in addition to the regulation of the survival, differentiation, and migration of cancer cells, the activation of the mTOR pathway is associated with the immunoregulation of ICs and increased PD-L1 expression [23,24], which has been correlated with a higher benefit from immunotherapy in RCC patients [25]. Moreover, PD-L1 expressed in TCs may activate antiapoptotic signals, enhancing the PI3K–Akt-mTOR pathway and tumor-intrinsic glycolysis [26]. It can be assumed that the higher expression of ph-mTOR in the tumors of responder patients may be an indirect sign of the crucial role of PD-1/PD-L1 interaction; as a consequence, the action of nivolumab in these tumors may inhibit an essential manner of growth and proliferation. This hypothesis is in accordance with the numerically higher expression of PD-L1 in the tumors of responder patients.
Finally, we observed that the tumors of non-responder patients presented a higher grading (G3-G4). This result lies in the fact that the tumor grade is a well-known factor correlated with poor prognosis in patients with RCC [27]. Unexpectedly, we observed a higher probability of clear cell histology in the tumors of non-responder patients; we trust that the already planned molecular analyses will elucidate this finding.
In summary, responder patients seem to have a more immunological phenotype compared with non-responder patients, which seems to be correlated with the poor prognostic features generally associated with an angiogenetic phenotype. In this context, the different molecular profiles of mRCC are under investigation as potential drivers for the therapeutic choice. The BIONIKK trial is the first biomarker-driven trial that randomized mRCC patients to different treatments (immunotherapy monotherapy, immuno-combination, and TKI) according to tumor molecular characteristics according to a 35-gene expression mRNA signature [28]. What emerged from this study is that “immune-high” tumors benefit from immunotherapy monotherapy, “immune-low” tumors from immuno-combination, and “angio-high” tumors from TKI [28].
We acknowledge that among the limitations of the present analysis are its retrospective nature, the small size of the population, the lack of some tumor details (i.e., sarcomatoid differentiation), and the assessment of two different tumor specimens (primary tumors and metastases) with an underestimation of possible heterogeneity. However, the distribution of the primary tumor and metastasis specimens is similar between responders vs. non-responders (p-value = 0.93).
Moreover, another limitation regards the use of a less-used PD-L1 assay (Ventana PD-L1-SP263) compared with the PD-L1 IHC 22C3 pharmDx assay and the Dako PD-L1 staining, even though no standard PD-L1 assessment has been established in international guidelines and clinical practice.
The strength of our study was the attempt to identify molecular factors related to both the I-TME and tumors, the potential prediction of nivolumab treatment benefit, and the employment of easy-to-use and easily reproducible methods such as IHC.
Further analyses with a larger population are planned to confirm our IHC results. Moreover, the assessment of the gene expression profiles of angiogenic and immunosuppressive features in the two groups (responders and non-responders) is currently ongoing to assess the prognostic role of transcriptomic biomarkers and their correlation with the I-TME.
5. Conclusions
Many efforts have been made to investigate the prognostic and predictive role of the I-TME in cancer patients treated with immunotherapy, but no biomarkers have been established in clinical practice. This study aimed to identify potential differential I-TME and tumor features between responder and non-responder patients, which could help predict the response to immunotherapy. Further IHC and genomic analyses are planned to deeply examine the prognostic role of the I-TME.
Acknowledgments
S.E.R. and G.F. would like to thank LILT-Lega Italiana per la Lotta ai Tumori for supporting this research (Bando 5 × 1000 2019) and the Italian Ministry of Health (Ricerca Corrente 2018–2021 grants), which financially supports their clinical research focused on the identification of prognostic and predictive markers for patients with genitourinary tumors.
Author Contributions
S.E.R. and M.B. contributed equally to this work; S.B. and G.F. contributed equally as senior authors. Study concept and design: S.E.R., G.F., S.B. and M.B.; methodology: S.E.R., G.F., S.B., M.B. and A.S.; acquisition and curation of data: all authors; statistical analysis: A.S.; interpretation of data: S.E.R., M.B., G.F., S.B., F.G., V.G.V., A.S., G.G., P.R. and M.M. (Marco Maruzzo); writing—original draft preparation: S.E.R., A.S., F.C. and A.D.; writing—review and editing: S.E.R., M.B., S.B., P.R. and G.L.B.; supervision, S.E.R. and G.F. All authors had full access to all the data in this study and take responsibility for the integrity and the accuracy of the data. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Regional Ethical Committee of Liguria, registration number 209/2020-DB ID 10531.
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
All the data are accessible and available upon request from the corresponding author.
Conflicts of Interest
S.E.R. received honoraria as a speaker at scientific events and travel accommodation from BMS, Amgen, GSK, Janssen, Astellas, and MSD. G.F. serves on the advisory boards of Astellas, Janssen, Pfizer, Bayer, MSD, and Merck and received travel accommodation from Astellas, Janssen, and Bayer. S.B. received honoraria as a speaker at scientific events and an advisory role from BMS, Pfizer, MSD, Ipsen, Roche, Eli Lilly, AstraZeneca, Pierre-Fabre, and Novartis. P.R. serves on the advisory boards of MSD, AstraZeneca, Gilead, BMS, and Janssen and received honoraria from MSD, AstraZeneca, and Janssen. G.L.B. reports personal fees from AstraZeneca, Janssen-Cilag, Boehringer Ingelheim, and Roche, and non-financial support from BMS, AstraZeneca, MedImmune, Pierre Fabre, and IPSEN, and has received grant consultancies from Astrazeneca and Astellas Pharma. The other authors declare no conflicts of interest. The funders had no role in the design of this study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
Funding Statement
This research received external funding from the nonprofit organization LILT-Lega Italiana per la Lotta ai Tumori–Bando 5 × 1000 2019, Investigator Grant (Funding number: M357A). For this project, S.E.R. won the “Giovanni Gardin Award—AIOM Liguria” in September 2021.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Zhuang T.Z., Case K., Olsen T.A., Brown J.T., Carthon B.C., Kucuk O., Nazha B. Metastatic Clear-Cell Renal Cell Carcinoma in the Era of Immune Checkpoint Inhibitors: Therapies and Ongoing Trials. Cancers. 2022;14:2867. doi: 10.3390/cancers14122867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Motzer R.J., Escudier B., McDermott D.F., George S., Hammers H.J., Srinivas S., Sharma P. Nivolumab versus everolimus in advanced renal-cell carcinoma. N. Engl. J. Med. 2015;373:1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rebuzzi S.E., Perrone F., Bersanelli M., Bregni G., Milella M., Buti S. Prognostic and predictive molecular biomarkers in metastatic renal cell carcinoma patients treated with immune checkpoint inhibitors: A systematic review. Expert Rev. Mol. Diagn. 2019;20:169–185. doi: 10.1080/14737159.2019.1680286. [DOI] [PubMed] [Google Scholar]
- 4.Simonaggio A., Epaillard N., Pobel C., Moreira M., Oudard S., Vano Y.A. Tumor Microenvironment Features as Predictive Biomarkers of Response to Immune Checkpoint Inhibitors (ICI) in Metastatic Clear Cell Renal Cell Carcinoma (mccRCC) Cancers. 2021;13:231. doi: 10.3390/cancers13020231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Houshyari M., Taghizadeh-Hesary F. Is Mitochondrial Metabolism a New Predictive Biomarker for Antiprogrammed Cell Death Protein-1 Immunotherapy? JCO Oncol. Pract. 2023;19:123–124. doi: 10.1200/OP.22.00733. [DOI] [PubMed] [Google Scholar]
- 6.Yanagisawa T., Mori K., Katayama S., Mostafaei H., Quhal F., Laukhtina E., Shariat S.F. Hematological prognosticators in metastatic renal cell cancer treated with immune checkpoint inhibitors: A meta-analysis. Immunotherapy. 2022;14:709–725. doi: 10.2217/imt-2021-0207. [DOI] [PubMed] [Google Scholar]
- 7.Li J., Byrne K.T., Yan F., Yamazoe T., Chen Z., Baslan T., Stanger B.Z. Tumor Cell-Intrinsic Factors Underlie Heterogeneity of Immune Cell Infiltration and Response to Immunotherapy. Immunity. 2018;49:178–193.e7. doi: 10.1016/j.immuni.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu G., Pei L., Yin H., Lin F., Li X., Zhu X., Gou X. Profiles of tumor-infiltrating immune cells in renal cell carcinoma and their clinical implications. Oncol Lett. 2019;18:5235–5242. doi: 10.3892/ol.2019.10896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anker J., Miller J., Taylor N., Kyprianou N., Tsao C.K. From Bench to Bedside: How the Tumor Microenvironment Is Impacting the Future of Immunotherapy for Renal Cell Carcinoma. Cells. 2021;10:3231. doi: 10.3390/cells10113231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin E., Liu X., Liu Y., Zhang Z., Xie L., Tian K., Liu J., Yu Y. Roles of the Dynamic Tumor Immune Microenvironment in the Individualized Treatment of Advanced Clear Cell Renal Cell Carcinoma. Front. Immunol. 2021;12:653358. doi: 10.3389/fimmu.2021.653358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rebuzzi S.E., Signori A., Banna G.L., Maruzzo M., De Giorgi U., Pedrazzoli P., Fornarini G. Inflammatory indices and clinical factors in metastatic renal cell carcinoma patients treated with nivolumab: The development of a novel prognostic score (Meet-URO 15 study) Ther. Adv. Med. Oncol. 2021;13:17588359211019642. doi: 10.1177/17588359211019642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ged Y., Voss M.H. Novel emerging biomarkers to immunotherapy in kidney cancer. Ther. Adv. Med. Oncol. 2021;13:17588359211059367. doi: 10.1177/17588359211059367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naser R., Fakhoury I., El-Fouani A., Abi-Habib R., El-Sibai M. Role of the tumor microenvironment in cancer hallmarks and targeted therapy (Review) Int. J. Oncol. 2023;62:23. doi: 10.3892/ijo.2022.5471. [DOI] [PubMed] [Google Scholar]
- 14.Badalamenti G., Fanale D., Incorvaia L., Barraco N., Listi A., Maragliano R., Russo A. Role of tumor-infiltrating lymphocytes in patients with solid tumors: Can a drop dig a stone? Cell Immunol. 2019;343:103753. doi: 10.1016/j.cellimm.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 15.Presti D., Dall’Olio F.G., Besse B., Ribeiro J.M., Di Meglio A., Soldato D. Tumor infiltrating lymphocytes (TILs) as a predictive biomarker of response to checkpoint blockers in solid tumors: A systematic review. Crit. Rev. Oncol. Hematol. 2022;177:103773. doi: 10.1016/j.critrevonc.2022.103773. [DOI] [PubMed] [Google Scholar]
- 16.Braun D.A., Street K., Burke K.P., Cookmeyer D.L., Denize T., Pedersen C.B., Wu C.J. Progressive immune dysfunction with advancing disease stage in renal cell carcinoma. Cancer Cell. 2021;39:632–648.e8. doi: 10.1016/j.ccell.2021.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Basu A., Ramamoorthi G., Albert G., Gallen C., Beyer A., Snyder C., Kodumudi K. Differentiation and Regulation of TH Cells: A Balancing Act for Cancer Immunotherapy. Front. Immunol. 2021;12:669474. doi: 10.3389/fimmu.2021.669474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ćirović S., Vještica J., Mueller C.A., Tatić S., Vasiljević J., Milenković S., Marković-Lipkovski J. NCAM and FGFR1 coexpression and colocalization in renal tumors. Int. J. Clin. Exp. Pathol. 2014;7:1402–1414. [PMC free article] [PubMed] [Google Scholar]
- 19.Van Acker H.H., Capsomidis A., Smits E.L., Van Tendeloo V.F. CD56 in the Immune System: More Than a Marker for Cytotoxicity? Front. Immunol. 2017;8:892. doi: 10.3389/fimmu.2017.00892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Acker H.H., Van Acker Z.P., Versteven M., Ponsaerts P., Pende D., Berneman Z.N., Smits E.L. CD56 Homodimerization and Participation in Anti-Tumor Immune Effector Cell Functioning: A Role for Interleukin-15. Cancers. 2019;11:1029. doi: 10.3390/cancers11071029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conciatori F., Bazzichetto C., Falcone I., Pilotto S., Bria E., Cognetti F., Ciuffreda L. Role of mTOR Signaling in Tumor Microenvironment: An Overview. Int. J. Mol. Sci. 2018;19:2453. doi: 10.3390/ijms19082453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Voss M.H., Molina A.M., Motzer R.J. mTOR inhibitors in advanced renal cell carcinoma. Hematol. Oncol. Clin. N. Am. 2011;25:835–852. doi: 10.1016/j.hoc.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caforio M., de Billy E., De Angelis B., Iacovelli S., Quintarelli C., Paganelli V., Folgiero V. PI3K/Akt Pathway: The Indestructible Role of a Vintage Target as a Support to the Most Recent Immunotherapeutic Approaches. Cancers. 2021;13:4040. doi: 10.3390/cancers13164040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Braun C., Weichhart T. mTOR-dependent immunometabolism as Achilles’ heel of anticancer therapy. Eur. J. Immunol. 2021;51:3161–3175. doi: 10.1002/eji.202149270. [DOI] [PubMed] [Google Scholar]
- 25.Carretero-González A., Lora D., Martin Sobrino I., Saez Sanz I., Bourlon M.T., Anido Herranz U., de Velasco G. The Value of PD-L1 Expression as Predictive Biomarker in Metastatic Renal Cell Carcinoma Patients: A Meta-Analysis of Randomized Clinical Trials. Cancers. 2020;12:1945. doi: 10.3390/cancers12071945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boussiotis V.A. Molecular and Biochemical Aspects of the PD-1 Checkpoint Pathway. N. Engl. J. Med. 2016;375:1767–1778. doi: 10.1056/NEJMra1514296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petitprez F., Ayadi M., de Reyniès A., Fridman W.H., Sautès-Fridman C., Job S. Review of Prognostic Expression Markers for Clear Cell Renal Cell Carcinoma. Front. Oncol. 2021;11:643065. doi: 10.3389/fonc.2021.643065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vano Y.A., Elaidi R., Bennamoun M., Chevreau C., Borchiellini D., Pannier D., Oudard S. Nivolumab, nivolumab-ipilimumab, and VEGFR-tyrosine kinase inhibitors as first-line treatment for metastatic clear-cell renal cell carcinoma (BIONIKK): A biomarker-driven, open-label, non-comparative, randomised, phase 2 trial. Lancet Oncol. 2022;23:612–624. doi: 10.1016/S1470-2045(22)00128-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data are accessible and available upon request from the corresponding author.