Abstract
Background: Autoimmune glial fibrillary acidic protein (GFAP) astrocytopathy usually presents as meningoencephalomyelitis. Many patients developed flu-like symptoms preceding the neurologic symptoms. Reversible lesion in the splenium of the corpus callosum (SCC) is a clinical and radiological syndrome secondary to many kinds of etiologies, including infections, which is termed RESLES. Case presentation: we reported a case developing irregularly high fever, both temporal pain, low limbs fatigue with frequent urination admitted to our neurology department. CSF test showed GFAP-IgG positive, elevated WBC counts and protein, with low glucose and chlorine, while MRI showed a reversible lesion on SCC, leading us to diagnose autoimmune GFAP autocytopathy accompanied with RESLES. The boy had significantly improved after anti-virus and steroids therapy. Discussion: Autoimmune GFAP autocytopathy accompanied with RESLES is rarely seen, and pathogenesis for the co-existence has not been clarified. Autoimmune GFAP autocytopathy and RESLES are both related to viral infection. Our case covered infectious symptoms and improved after antiviral treatment, suggesting virus infection may perform a key role in pathogenesis.
Keywords: GFAP, astrocytopathy, reversible splenial lesion syndrome, autoantibody
1. Introduction
Autoimmune glial fibrillary acidic protein (GFAP) astrocytopathy is a spectrum of autoimmune inflammatory central nervous system (CNS) disorders that were first defined by Fang et al. (Mayo Clinic) in 2016 [1]. GFAP is a specific intermediate fibrin of astrocytes. The disruption of astrocytes causing by GFAP-specific CDT cells mediated cellular immunity leads to inflammatory cells accumulate, and allow autoimmune attacks spreading to healthy nerve tissues, manifesting as encephalitis and meningitis, followed by myelitis and optic neuritis. CSF usually shows a specific GFAP antibody, and MRI reveals abnormal hyperintensity on T2-weighted images and gadolinium enhancement on T1. Previous studies reveal 30–40% of patients developed flu-like symptoms before neurologic symptoms [2].
Reversible splenial lesion syndrome (RESLES) is a clinical and radiological syndrome, patients usually develop headache, altered consciousness and seizure, followed by mental abnormality, focal neurological deficit, personality change, and visual disturbance [3]. Current studies indicate infections, epileptic/antiepileptic factors, or metabolic diseases cause SCC myelin sheath edema, leading to abnormal signal on MRI, and lesions can totally disappeared or significantly shrink during follow-up.
Autoimmune GFAP autocytopathy accompanied with RESLES is rarely seen, only three cases have reported the same situation, but they are different in etiology, clinical, and MRI features [4,5,6]. The relationship between autoimmune GFAP astrocytopathy and RESLES remains unclear. Here, we report a case of autoimmune GFAP astrocytopathy coexist with RESLES, and review the previous literature, to probe into the possible link between autoimmune GAFP autocytopathy and RESLES.
2. Case Report
A 16-year-old boy became irregularly fever, both temporal pain, low limbs fatigue with frequent urination since 28 October 2021. The maximum body temperature reached 40.5 °C. Urinary output was normal. The patient’s first visit to the local hospital, initial blood testing revealed hypokalemia, hyponatremia, and hypochloremia (K+ 3.19 mmol/L, Na+ 132.9 mmol/L, Cl− 94.7 mmol/L). A chest computed tomography (CT) scan was performed but showed no significant findings. The local hospital gave the treatment of Amoxicillin and antipyretic for few days, but he still felt ill.
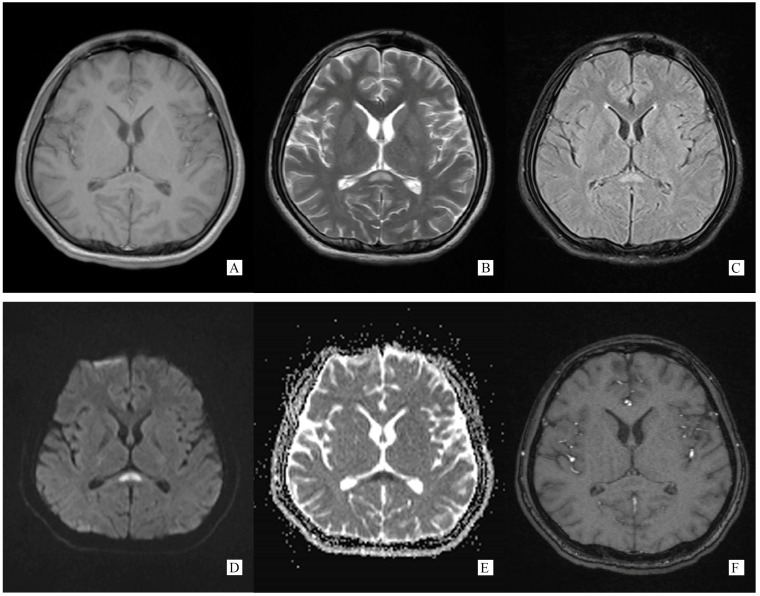
A total of 6 days after onset, on 3 November 2021, he turned to a higher-level hospital for further evaluation and treatment. Lumbar puncture showed CSF ICP 350 mmH2O, glucose 2.25 mmol/L, chlorine 118.2 mmol/L, and protein 1.27 g/L. WBC counts were normal. Cerebral MRI demonstrated an abnormal signal on SCC (Figure 1). Based on these results, he was considered as meningoencephalomyelitis, and was given ceftriaxone to anti-infective. However, there was no improvement in the condition.
Figure 1.
(Performed on 5 November 2021). (A) Axial T1 weight brain MRI image showing an oval hypointense lesion on SCC. (B) Axial T2 weight brain MRI image showing an oval hyperintense lesion on SCC. (C) Axial T2 flair weight brain MRI image showing an oval hyperintense lesion on SCC. (D) Axial DWI weight (b = 1000) brain MRI image showing an oval hyperintense lesion on SCC. (E) Axial ADC weight brain MRI image showing an oval hyperintense lesion on SCC. (F) Axial post-gadolinium T1-weighted brain MRI showing no enhancement on SCC lesion.
A total of 9 days after onset, on 6 November 2021, he was admitted to the infectious department of our hospital. Physical examination at hospital admission revealed fever, headache, mild weakness of muscle force (MRC grade 5), mild neck rigidity, mild misnicturition, and frequent urination, with weakened low limbs deep reflect and suspicious positive of Babinski’s sign without other meningeal syndrome and cranial nerves dysfunction.
Laboratory examinations indicated normal serum blood cell counts and the inflammatory work-up (CRP, ESR). No infectious agents were found including hepatitis virus, HIV virus, EB virus, cytomegalovirus, plasmodium, bacterial, and fungus by routine serum microbiological studies. Antinuclear antibody and rheumatoid factor were both negative. CSF analysis clued an increase in WBC counts (20 × 106/L), high intracranial pressure, hypoglycorrhachia, and hyperproteinorachia (ICP 80 drops per minutes, Glu 1.93 mmol/L, Pro 1.27 g/L), CSF adenosine deaminase (ADA) was 8.50 U/L. Infection agents in CSF were not found by general smear and culture. No remarkable abnormality among chest CT and abdominal ultrasonography.
According to the results described above, he was primarily diagnosed with intracranial infections, and was given treatment by ceftriaxone and trimethoprim-sulfamethoxazole to anti-bacterial infection lasted for 5 days, but he was still symptomatic. For further diagnosis and treatment, he then transferred to our neurology department on 10 November 2021. Thinking there is no sufficient evidence of bacterial infection, we stopped anti-bacterial infection and starting with acyclovir to antivirus on 11 November 2021.
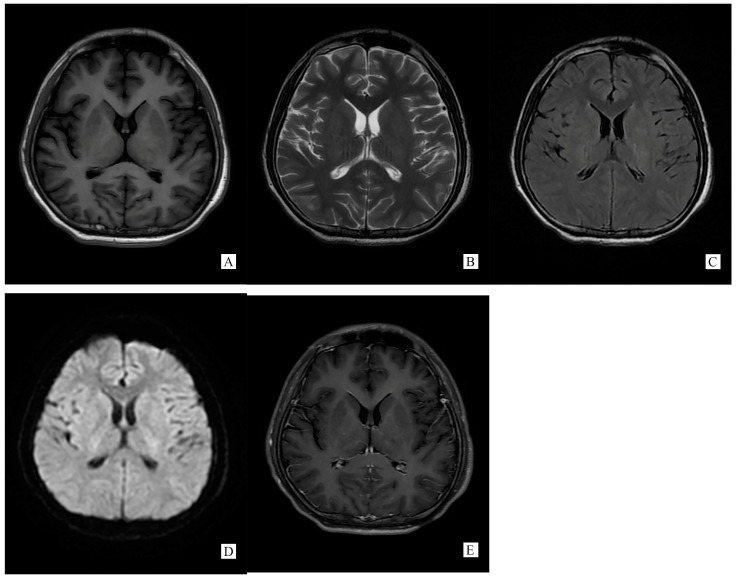
A repeated spinal tap was performed on 11 November 2021, which showed increased intracranial pressure, CSF glucose was still below normal contents, while CSF protein and chloride was lower than before and still abnormal (ICP 330 mmH2O, Glu 2.14 mmol/L, Cl− 119.50 mmol/L, Pro 1.05 g/L). WBC counts increased to 500 × 106/L. CSF screen using next-generation sequencing (NGS) indicated no infection. The CSF test of neural auto-antibodies revealed positive anti-GFAP antibodies (antibody titer 1:32), while the serum test was negative. CSF Ig electrophoresis revealed positive IgG oligoclonal bands (≥2 bands). A second cerebral MRI was performed on 13 November 2021 showing totally disappeared on SCC lesion (Figure 2).
Figure 2.
(Performed on 13 November 2021) SCC lesion totally disappeared on brain MRI ((A). T1WI, (B). T2WI, (C). T2 flair, (D). DWI, (E). Post-gadolinium T1-weighted).
Based on the combination of clinical features, CSF test, and cerebral MRI changes, we finally diagnosed the patient with autoimmune GFAP astrocytopathy accompanied with RESLES. On 12 November 2021, the second day of antiviral treatment, his body temperature returned to normal without headache. However, difficulty and frequency of urination still persisted. We then gave a high-dose intravenous steroid starting on 15 November 2021 at 1 g/day for 4 days followed by 0.5 g/day for 1 day and 0.25 g/day for 3 days. Rechecked spinal tap on 17 November 2021 revealed CSF WBC counts 240 × 106/L, normal CSF protein, glucose, and chloride (Pro 0.45 g/L, Glu 3.61 mmol/L, Cl− 128.00 mmol/L). On 22 November 2021, the patient was discharged from hospital with oral corticosteroids and go home to outpatient follow-up. During follow-up, his neurological symptoms were completely recovered within 3 months. Steroid therapy was progressively tapered over 6 months until complete withdrawal. A lumbar puncture was performed on 11 May 2022 before steroid withdrawal showed CSF WBC counts for 16 × 106/L, normal CSF protein, glucose, and chloride levels. All the CSF results during hospitalization and outpatient visits are listed in Table 1.
Table 1.
Characteristics of the patient’s CSF.
| CSF CharacteristicsTime |
4 November 2021 | 8 November 2021 | 11 November 2021 | 17 November 2021 | 8 December 2021 | 11 May 2022 |
|---|---|---|---|---|---|---|
| Day7 | Day11 | Day14 | Day17 | Day41 | Day195 | |
| ICP | 350 | 80 drops/min | 330 mmH2O | 215 mmH2O | 300 mmH2O | 230 mmH2O |
| Appearance | clear and colorless | clear and colorless | clear and colorless | clear and colorless | clear and colorless | clear and colorless |
| WBC counts | 0 | 206 | 500 | 240 | 12 | 16 |
| Glu(mmol/L) | 2.25 | 1.93 | 2.14 | 3.61 | 3.37 | 3.3 |
| Cl−(mmol/L) | 118.2 | 121.2 | 119.5 | 128 | 126 | 127.5 |
| Pro(mg/L) | 1270 | 1277.5 | 1051.9 | 499.2 | 307.6 | 337.7 |
| ADA(U/L) | Unknown | 8.5 | 8.8 | 6 | 2.7 | 2.2 |
| CK(U/L) | Unknown | Unknown | 12 | 8 | Unknown | Unknown |
| LDH(U/L) | Unknown | Unknown | 89 | 20 | Unknown | Unknown |
| AST(U/L) | Unknown | Unknown | 24 | 20 | Unknown | Unknown |
| Ca2+(mmol/L) | Unknown | Unknown | 1.1 | 1.1 | Unknown | Unknown |
| Mg2+(mmol/L) | Unknown | Unknown | 0.86 | 0.98 | Unknown | Unknown |
| UA(μmol/L) | Unknown | Unknown | 52 | 24 | Unknown | Unknown |
| Alb(mg/L) | Unknown | Unknown | 604.5 | 299 | Unknown | Unknown |
| IgG(mg/L) | Unknown | Unknown | 111.1 | 63.4 | Unknown | Unknown |
| GFAP IgG | Unknown | Unknown | Positive (antibody titer 1:32) | Unknown | Unknown | Unknown |
| Oligoclonal bands | Unknown | Unknown | Positive (≥2 bands) | Unknown | Unknown | Unknown |
3. Discussion
Autoimmune GFAP astrocytopathy is a spectrum of immunotherapy-responsive autoimmune inflammatory CNS disorders, with GFAP-IgG detected in CSF. RESLES is a class of diseases caused by infectious or non-infectious factors presenting as mild encephalitis/encephalopathy with a reversible splenial lesion on brain MRI. Autoimmune GFAP astrocytopathy accompanied with RESLES is rare and only three cases have been reported so far. To our knowledge, our case is the first reported in China.
Generally, typical features of autoimmune GFAP astrocytopathy present as a linear, radial perivascular enhancement pattern on brain MRI, and central longitudinally extensive enhancement pattern on spinal cord MRI [7]. RESLES is typically classified into two patterns on MRI: type I is an isolated lesion on SCC, type II is a lesion in SCC expending to callosal fibers, cerebral white matters or anterior portion of corpus callosum. No matter type I or type II, lesions can be significantly shrunk or totally disappeared within a month, accompanied with the relief of symptoms [8]. In the MRI of our case, the lesion was located in the SCC, manifested a hypointense signal on T1WI and hyperintense on T2WI and DWI, reversibly, which is similar to type I RESLES, but unlike the typical autoimmune GFAP astrocytopathy.
We made a brief review of the three GFAP-RESLES cases (Table 2). One is a 10-year-old boy, with a comparatively similar MRI characteristic as our case, an isolated and reversible lesion on SCC. The boy had fever, heachache and vomiting for two days before admission, then the neurological symptoms. No possible pathogens were found in his CSF. The boy did not receive antiviral therapy but improved significantly after Ig IV infusion [5]. Another case is an adult patient whose abnormal signals on MRI spread to white matter and meninges, not only located in SCC. The patient also suffered transient fever before neurological symptoms onset. No possible pathogens were found in CSF either. In hospital, she was first treated with acyclovir, amoxicillin, and cephalosporin, but became worse, which is different from our case. Then, they give the treatment of high-dose corticosteroids and her condition improved [4]. The one left of the cases was confirmed to be coexisted with primary central nervous system lymphoma, MRI demonstrated multiple abnormal enhancement lesions in bilateral basal ganglia and around the third ventricle, and reversible lesion on SCC, also different with our case. He never had any flu-like symptoms such as fever [6].
Table 2.
Review of published cases of autoimmune GFAP autocytopathy coexist with RESLES.
| Author, Year | Diagnosis | Flu-Like Symptoms | Pathogens | Neurological Symptoms | CSF Features | MRI Features | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|
| Oger et al., 2019 [5] | Autoimmune GFAP autocytopathy with RESLES | Exist | Not found | Progressively, alteration of consciousness, dysmetria, nystagmus, gait difficulties | WBC cell counts: 380 cells/mm3, proteins: 1.33 g/L, oligoclonal bands negative, GFAP antibody positive | Reversible SCC lesion with hyperintensity on T2 and DWI with increased ADC, disappeared within a week | IGIV infusion (1 g/kg/J) for 2 days | Completely recovered within 12 months |
| He’raud et al., 2021 [4] | Autoimmune GFAP autocytopathy with RESLES | Exist | Not found | progressive and worsening fatigue, headaches, diplopia, walking difficulties, multidirectional nystagmus, right side apraxia and anosognosia, multiple cranial nerve palsy (left sixth cranial nerve, right facial and bilateral trigeminal hypoesthesia), proprioceptive ataxia of the lower limbs | WBC cell counts: 121 cells/μL, proteins: 1.51 g/L, glucose: 1.93 mmol/L, GFAP antibody positive | Reversible SCC lesion with hyperintensity on T2 and diffusion sequences with hypointensity on T1, linear gadolinium enhancement of the Virchow-Robin perivascular spaces, SCC lesion disappeared within 29 days; hyperintensities on C2, C3, and C6 levels, diffuse leptomeningeal gadolinium enhancement | Started with amoxicillin and cepha losporin (worsening presented by altered consciousness, left facial paralysis, acute urinary retention, dysarthria, and complete visual loss in the left eye), then transferred to steroids therapy after diagnosed autoimmune GFAP autocytopathy | Completely recovered within 4 months |
| Fang et al., 2022 [6] | Primary central nervous system lymphoma coexistent with autoimmune GFAP astrocytopathy | No | Not found | Somnolence, memory declination | WBC cell counts: 12 cells/mm3, proteins: 0.455 g/L, GFAP antibody positive | Multiple abnormal enhancement lesions in bilateral basal ganglia and around the third ventricle, ands transient T2-weighted hyperintensity lesions at the SCC, SCC lesion attenuated in 2 months | Steroids therapy for about 2 months (worsening presented by poor response and even worsened clinical manifestations when the dose of prednisone reduced to 45 mg), then transferred to rituximab and methotrexate after diagnosed large B-cell lymphoma | Significantly improved after three courses of chemotherapy |
The previous 3 cases did not delve into the mechanisms of the two diseases co-occurring, so the underlying mechanism of autoimmune GFAP austrocytopathy with RESLES is still unclear. Based on previous studies, we speculate there may be two possible explanations for the disease occurring. One explanation is that virus infection may perform a key role in pathogenesis, since autoimmune GFAP astrocytopathy and RESLES can both be secondary to virus infection. When pathogens invade, the body’s inflammatory response to pathogens may activate cellular immunity mediated by GFAP-specific CDT cells [9,10,11,12,13,14]. CDT cells attack astrocytes then release chemokine which leads to accumulation of inflammatory cells, allowing autoimmune attacks to spread to healthy nerve tissues and cause cellular edema [15,16,17]. Due to the high water content and insufficient self-protection, cellular edema is more likely to occur on SCC. Distinctively, cellular edema of SCC belongs to intra-myelin sheath edema, which does not damage fibers on SCC, so the lesions are reversible [18]. Another possible explanation is that the reversible lesion on SCC may be secondary to autoimmune GFAP astrocytopathy, since several articles have reported cases of RESLES with some auto-immune diseases [19,20]. However, regretfully, no research proves that GFAP antibodies can result in reversible SCC lesion so far. Since we have not further studied the internal relationship between these two in pathology, we can only explain the relationship clinically; thus, in-depth research and more cases are essential to correctly illustrate the inner relations. The case we reported, the boy covered flu-like symptom with abnormal high fever before neurological symptoms onset, his body temperature dropped to normal after antiviral therapy for two days, with lesion in SCC disappeared, demonstrate viral infection is the common pathogenesis basis of the two disease. Of course, viral infection is the basic, but not the only pathogenic mechanism, and there should be secondary autoimmune response to virus infection.
Treatments of autoimmune GFAP autocytopathy in acute stage mainly depends on high-dose corticosteroids, other treatments, including intravenous immunoglobulin (Ig IV) and plasma exchange. Treatment in remission is the maintenance of oral steroids and immunosuppressant. In both our case and the previous cases, patients had significant improvement after given high-dose corticosteroids or Ig IV infusion, confirmed that hormone therapy and Ig IV infusion are still effective way for autoimmune GFAP astrocytopathy accompanied with RESLES. In addition, since viral infection is the common basis of both autoimmune GFAP astrocytopathy and RESLES, antiviral therapy may be helpful for patients with flu-like symptoms, but further studies are still needed to verify.
4. Conclusions
Autoimmune GFAP astrocytopathy accompanied with RESLES is rare, its clinical features, and CSF and MRI characteristics are consistent with autoimmune GFAP astrocytopathy and RESLES. The pathogenesis for the co-existence has not been clarified. Our case covered infectious symptoms and improved after antiviral treatment, suggesting virus infection may perform a key role in pathogenesis.
Acknowledgments
The authors thank all the participants in the diagnosis and treatment of the case.
Author Contributions
S.W. wrote the manuscript. J.Y. analyzed the clinical data. J.L. provided professional comments on the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of The First Affiliated Hospital of Guangxi Medical University (protocol code 2023-E123-01 and date of approval 4 April 2023).
Informed Consent Statement
Informed consent has been obtained from the patient before publication. This study was supported by the National Natural Science Foundation of China (No. 82160236).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare that they have no competing interests.
Funding Statement
This research was funded by [National Natural Science Foundation of China] grant number [82160236] and [Guangxi Natural Science Foundation] grant number [2021GXNSFAA196035]. The APC was funded by [National Natural Science Foundation of China] and [Guangxi Natural Science Foundation].
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Fang B., McKeon A., Hinson S.R., Kryzer T.J., Pittock S.J., Aksamit A.J., Lennon V.A. Autoimmune Glial Fibrillary Acidic Protein Astrocytopathy: A Novel Meningoencephalomyelitis. JAMA Neurol. 2016;73:1297–1307. doi: 10.1001/jamaneurol.2016.2549. [DOI] [PubMed] [Google Scholar]
- 2.Kunchok A., Zekeridou A., McKeon A. Autoimmune glial fibrillary acidic protein astrocytopathy. Curr. Opin. Neurol. 2019;32:452–458. doi: 10.1097/WCO.0000000000000676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garcia-Monco J.C., Cortina I.E., Ferreira E., Martínez A., Ruiz L., Cabrera A., Beldarrain M.G. Reversible splenial lesion syndrome (RESLES): What’s in a name? J. Neuroimaging. 2011;21:e1–e14. doi: 10.1111/j.1552-6569.2008.00279.x. [DOI] [PubMed] [Google Scholar]
- 4.Heraud C., Capet N., Levraut M., Hattenberger R., Bourg V., Thomas P., Mondot L., Lebrun-Frenay C. Glial Fibrillary Acidic Protein (GFAP) Astrocytopathy Presenting as Mild Encephalopathy with Reversible Splenium Lesion. Neurol. Ther. 2022;11:499–505. doi: 10.1007/s40120-021-00302-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oger V., Bost C., Salah L., Yazbeck E., Maurey H., Bellesme C., Sevin C., Adamsbaum C., Chrétien P., Benaiteau M., et al. Mild Encephalitis/Encephalopathy with reversible splenial lesion syndrome: An unusual presentation of anti-GFAP astrocytopathy. Eur. J. Paediatr. Neurol. 2020;26:89–91. doi: 10.1016/j.ejpn.2020.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Fang J., Tong Z., Lu W. Case Report: Need for Caution in the Diagnosis of GFAP Astrocytopathy-A Case of GFAP Astrocytopathy Coexistent With Primary Central Nervous System Lymphoma. Front. Neurol. 2022;13:806224. doi: 10.3389/fneur.2022.806224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shan F., Long Y., Qiu W. Autoimmune Glial Fibrillary Acidic Protein Astrocytopathy: A Review of the Literature. Front. Immunol. 2018;9:2802. doi: 10.3389/fimmu.2018.02802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tetsuka S. Reversible lesion in the splenium of the corpus callosum. Brain Behav. 2019;9:e01440. doi: 10.1002/brb3.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flanagan E.P., Hinson S.R., Lennon V.A., Fang B., Aksamit A.J., Morris P.P., Basal E., Honorat J.A., Alfugham N.B., Linnoila J.J., et al. Glial fibrillary acidic protein immunoglobulin G as biomarker of autoimmune astrocytopathy: Analysis of 102 patients. Ann. Neurol. 2017;81:298–309. doi: 10.1002/ana.24881. [DOI] [PubMed] [Google Scholar]
- 10.Fu M.L., Han N., Wang W. Cytomegalovirus-Associated Mild Encephalopathy/Encephalitis With Reversible Splenial Lesion. Neurologist. 2021;26:172–174. doi: 10.1097/NRL.0000000000000334. [DOI] [PubMed] [Google Scholar]
- 11.Guo Y., Wang S., Jiang B., Li J., Liu L., Wang J., Zhao W., Jia J. Encephalitis with reversible splenial and deep cerebral white matter lesions associated with Epstein-Barr virus infection in adults. Neuropsychiatr. Dis. Treat. 2017;13:2085–2092. doi: 10.2147/NDT.S135510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laizane G., Smane L., Nokalna I., Gardovska D., Feemster K.A. Rotavirus-associated seizures and reversible corpus callosum lesion. Acta Med. Litu. 2019;26:113–117. doi: 10.6001/actamedica.v26i2.4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sathananthasarma P., Weeratunga P.N., Chang T. Reversible splenial lesion syndrome associated with dengue fever: A case report. BMC Res. Notes. 2018;11:412. doi: 10.1186/s13104-018-3491-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ventresca S., Guiducci C., Tagliani S., Bo S.D., Ricciardelli P., Cenni P., Marchetti F. Clinically Mild Encephalopathy with a Reversible Splenial Lesion Caused by Influenza B Virus in an Unvaccinated Child. Pediatr. Rep. 2021;13:72–75. doi: 10.3390/pediatric13010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuan Z., Li H., Huang L., Fu C., Chen Y., Zhi C., Qiu W., Long Y. CD8(+) T-cell predominance in autoimmune glial fibrillary acidic protein astrocytopathy. Eur. J. Neurol. 2021;28:2121–2125. doi: 10.1111/ene.14778. [DOI] [PubMed] [Google Scholar]
- 16.Sasaki K., Bean A., Shah S., Schutten E., Huseby P.G., Peters B., Shen Z.T., Vanguri V., Liggitt D., Huseby E.S. Relapsing-remitting central nervous system autoimmunity mediated by GFAP-specific CD8 T cells. J. Immunol. 2014;192:3029–3042. doi: 10.4049/jimmunol.1302911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zekeridou A., McKeon A., Flanagan E.P. A path to understanding autoimmune GFAP astrocytopathy. Eur. J. Neurol. 2018;25:421–422. doi: 10.1111/ene.13527. [DOI] [PubMed] [Google Scholar]
- 18.Anneken K., Evers S., Mohammadi S., Schwindt W., Deppe M. Transient lesion in the splenium related to antiepileptic drug: Case report and new pathophysiological insights. Seizure. 2008;17:654–657. doi: 10.1016/j.seizure.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 19.De Greef J., Jaumotte C., Quivron B., Derue G. Reversible splenial lesion in auto-immune thyroid disease: A case report. Acta Clin. Belg. 2014;69:208–209. doi: 10.1179/2295333713Y.0000000002. [DOI] [PubMed] [Google Scholar]
- 20.Gilder T.R., Hawley J.S., Theeler B.J. Association of reversible splenial lesion syndrome (RESLES) with Anti-VGKC autoantibody syndrome: A case report. Neurol. Sci. 2016;37:817–819. doi: 10.1007/s10072-015-2464-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.