Abstract
Polycystic ovary syndrome (PCOS) is an endocrine and metabolic disorder affecting women of reproductive age. Research has shown that epigenetic alterations such as DNA methylation may play a role in the development and progression of abnormal ovarian function and metabolic disorders in PCOS. Studies have identified specific genes (related with insulin signaling and steroid hormone metabolism) that are methylated in women with PCOS. DNA methylation appears to respond to various interventions aimed at altering health and lifestyle factors. We tested the efficacy of a mindfulness-based stress reduction program (MBSR) in PCOS patients. We examined its effects on anthropometric measurements, mental health and wellbeing, and alterations in DNA methylation in peripheral blood. MBSR was associated with a reduction in body mass index, waist circumference and blood glucose level, an improvement in subjectively perceived general health, emotional role limitation, and levels of pain, as well as mindfulness-like traits. MBSR reduced the expression of anxious symptomatology and subjectively perceived stress. Methylation changes were observed in four genes: COMT, FST, FKBP51, and MAOA. We conclude that MBSR may be a useful supplementary therapy to mitigate the deleterious effects of PCOS on mental health.
Keywords: mindfulness-based stress reduction program, polycystic ovary syndrome, epigenetics, DNA methylation, candidate genes, depression, anxiety
1. Introduction
Polycystic ovary syndrome (PCOS) is a common endocrine and metabolic disorder that occurs in 5–18% of women of reproductive age [1,2]. It is characterized by the presence of heterogeneous reproductive, metabolic, and androgenizing symptoms that may manifest in complex phenotypes. The most common clinical symptoms are obesity, androgenization, menstrual cycle disorders, and infertility [3]. Various signs of metabolic syndrome may be present [4]. The expression of symptoms is strongly influenced by obesity, which is present in 40–80% of PCOS patients [5]. Obesity increases insulin resistance (IR) [6], which, together with impaired insulin sensitivity and beta cell function, leads to accelerated development of prediabetes and type 2 diabetes in 30–45% of patients [7]. In the presence of metabolic syndrome, patients are also at risk of developing cardiovascular disease, non-alcoholic fatty liver disease, endometrial hyperplasia and cancer, as well as some other cancers [7,8]. Comorbid mental disorders are also common, especially depression, which occurs in 40% of patients [9]. The development of depression and anxiety disorders is significantly influenced by patients’ low self-esteem due to physical symptoms, which cause problems in social life and sexuality [10]. The exact pathogenesis of depression in PCOS may be more complex and dependent on other pathological mechanisms of the disorder [11].
The etiologic mechanisms of PCOS are not clear. In addition to hypothalamic and ovarian dysfunction [2,11], androgenization [12], dysfunction of adipose tissue [13], insulin resistance in skeletal muscle [14], elevated serum levels of inflammatory markers [15], and changes in the endometrium are present [16]. The cause of PCOS is complex and includes genetic and epigenetic susceptibility. Several studies have shown the influence of genetic and environmental factors, starting early in life [1,17]. Epigenetic alterations such as DNA methylation, histone modifications, and changes in noncoding RNA activity have been detected in various tissues of patients with PCOS [18]. DNA methylation appears to play an important role in the pathogenesis of PCOS. Studies have reported alterations in DNA methylation in ovarian [18] and adipose tissue [19], skeletal muscle [20], as well as in peripheral blood [21] and umbilical cord blood [22]. These DNA methylation changes likely participate in the deregulation of genes involved in inflammation, hormone synthesis and signaling, and glucose and lipid metabolism [18].
Epigenetic changes can be altered by various environmental factors such as physical activity, stress, or medical interventions such as medication, psychotherapy, or various meditation-based interventions [23,24]. Although epigenetic marks are considered relatively stable, their changes can be very dynamic and respond rapidly to stress, diet, exercise, or lifestyle-modifying therapeutic interventions [25]. In PCOS, recent studies in animal models and patient populations have shown that therapeutic interventions can influence DNA methylation and phenotypic changes in tissues affected by the disease [26,27], raising the possibility of therapeutic interventions Recently, there has been a resurgence of interest in traditional mind–body therapeutic practices as psychological interventions for various chronic medical conditions [28]. Mind–body interventions (MBI) are a group of diverse techniques that focus on the interactions between mind, brain, body, and behavior and aim to reduce stress and increase acceptance through long-term personality changes [29]. Some of them include an active physical component [30], while others focus mainly on meditation and relaxation techniques [31,32]. The majority of MBIs for medical disorders appear to be based on mindfulness-related approaches. Mindfulness can refer to both a dispositional trait and a meditation practice that cultivates awareness of the present moment [33,34]. Health interventions based on mindfulness can include formal programs such as Mindfulness-Based Stress Reduction (MBSR) and Mindfulness-Based Cognitive Therapy [35], or be part of other therapeutic techniques such as Acceptance and Commitment Therapy [36]. Like other MBIs, they can reduce stress, regulate emotional processes in depression and anxiety, increase motivation, and influence cognitive processes to promote adaptations and compliance in chronic disease management [37,38]. Mindfulness, when practiced over time, promotes trait-related changes in attention, awareness, perception, appraisal, and self-regulation [39]. At the cellular level, significant epigenetic changes in DNA methylation patterns may occur in genes related to the immune system, inflammation, and aging [40,41].
Management of weight and lifestyle factors such as diet, physical activity, and psychological well-being are first-line therapies in evidence-based guidelines for PCOS [42]. Mindfulness-based stress management programs have been proposed and successfully used in women with PCOS to reduce their levels of stress, anxiety, and depression [43,44] and to improve their self-efficacy related to diet and physical activity [45]. To our knowledge, no study to date has attempted to examine the effects of mindfulness on epigenetic changes in PCOS. We conducted a randomized clinical trial to determine the efficacy of a mindfulness-based stress reduction program in PCOS patients and to investigate its effects on changes in DNA methylation in peripheral blood. Candidate genes were selected considering those shown to be affected in PCOS and compared with genes affected in stress-related disorders (see Section 2).
2. Materials and Methods
2.1. Participants
Forty-two female patients with a mean age of 40.1 years (SD = 7.3) and an average of 14.1 years of education (SD = 1.7) were recruited from the Department of Endocrinology, Diabetes and Metabolic Diseases at University Medical Centre Ljubljana and randomly assigned to the experimental group participating in MBSR program (N = 21) or to the control group on the waiting list (N = 21) (see Table 1 for descriptive statistics on demographic, clinical and laboratory characteristics of participants at baseline). All patients were diagnosed with PCOS according to the ASRM-ESHRE Rotterdam Consensus Criteria (Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group, 2004). Diagnoses were confirmed by experienced clinicians (MJ and AJ) involved in the study. The following exclusion criteria applied to participants: current moderate or severe depressive episode, neurologic disorder or traumatic brain injury, substance abuse, current metformin therapy, pregnancy, chronic kidney or liver disease. Infertility was present in 10 patients. Metabolic syndrome was present in 28 patients according to the modified NCEP-ATP III criteria [46]. In accordance with the exclusion criteria, none of the participants were formally diagnosed with a psychiatric disorder such as depression or anxiety at the beginning of the study or in the past.
Table 1.
Demographic, clinical, and laboratory characteristics of participants at baseline. Baseline characteristics were compared either using independent samples t-test or Mann–Whitney test as appropriate.
| Participant Characteristics | Experimental Group | Control Group | p-Value |
|---|---|---|---|
| Age (mean (SD)) | 42.5 y (6.4) | 37.7 y (7.4) | 0.028 |
| Education (mean (SD)) | 14.1 y (1.8) | 14.1 y (1.6) | 0.687 |
| Currently unemployed (n) | 3 | 1 | |
| Metformin treatment (n) | 1 | 1 | |
| Infertility (n) | 6 | 4 | |
| Metabolic syndrome (n) | 16 | 12 | |
| Body weight (mean (SD)) | 103.8 kg (22.9) | 93.0 kg (15.4) | 0.084 |
| Body height (mean (SD)) | 167.8 cm (4.9) | 165.0 cm (6.2) | 0.105 |
| BMI (mean (SD)) | 36.2 (7.8) | 34.3 (6.3) | 0.381 |
| Waist circumference (mean (SD)) | 115.9 cm (13.4) | 108.7 cm (12.2) | 0.073 |
| Systolic blood pressure (mean (SD)) | 126.6 mm Hg (15.4) | 123.4 mm Hg (17.4) | 0.158 |
| Diastolic blood pressure (mean (SD)) | 87.1 mm Hg (12.8) | 83.3 mm Hg (14.3) | 0.364 |
| Heart rate (mean (SD)) | 77.5 bpm (9.4) | 77.8 bpm (10.2) | 0.925 |
| Glucose—0 min (mean (SD)) | 6.2 mmol/L (2.6) | 5.6 mmol/L (1.7) | 0.279 |
| Glucose—120 min (mean (SD)) | 7.8 mmol/L (2.5) | 7.4 mmol/L (3.5) | 0.389 |
| Cholesterol (mean (SD)) | 5.1 mmol/L (0.9) | 5.1 mmol/L (1.1) | 0.904 |
| HDL (mean (SD)) | 1.3 mmol/L (0.3) | 1.2 mmol/L (0.2) | 0.989 |
| LDL (mean (SD)) | 3.1 mmol/L (0.9) | 3.2 mmol/L (0.9) | 0.638 |
| Triglycerides (mean (SD)) | 1.8 mmol/L (0.8) | 1.3 mmol/L (0.6) | 0.018 |
| BAI score (mean (SD)) | 15.3 (12.5) | 12.0 (8.8) | 0.579 |
| BDI-II score (mean (SD)) | 10.4 (9.2) | 8.9 (9.2) | 0.3650 |
| Perceived Stress score (mean (SD)) | 7.7 (3.1) | 7.4 (2.8) | 0.756 |
| RAND-36 (mean (SD)) | |||
| Physical functioning | 74.8 (23.7) | 83.1 (17.5) | 0.238 |
| Physical role limitations | 70.2 (35.0) | 76.2 (33.0) | 0.598 |
| Emotional role limitations | 57.1 (38.2) | 77.8 (32.2) | 0.067 |
| Energy/Fatigue | 50.2 (15.8) | 53.3 (14.9) | 0.519 |
| Emotional well-being | 62.9 (12.0) | 61.7 (12.3) | 0.762 |
| Social functioning | 76.2 (23.7) | 79.8 (19.1) | 0.786 |
| Pain | 65.0 (27.6) | 73.6 (22.6) | 0.416 |
| General health | 53.1 (23.2) | 60.7 (17.8) | 0.239 |
| FFMQ (mean (SD)) | |||
| Observing | 3.1 (0.8) | 2.8 (0.7) | 0.244 |
| Describing (R) | 3.0 (1.0) | 3.2 (0.9) | 0.510 |
| Acting (R) | 2.7 (0.9) | 2.7 (0.8) | 0.886 |
| Non-judging (R) | 2.4 (0.8) | 2.4 (0.7) | 0.961 |
| Non-reactivity | 2.8 (0.7) | 2.7 (0.6) | 0.658 |
BMI: body mass index; HDL: high density lipoprotein; LDL: low density lipoprotein; BAI: Beck Anxiety Inventory; BDI-II: Beck Depression Inventory-II; RAND-36: RAND-36 Measure of Health-Related Quality of Life questionnaire; FFMQ: Five-Facet Mindfulness Questionnaire; R: reverse score; SD: standard deviation; n: number of participants in the group.
The study was approved by the Medical Ethics Committee and all participants signed an informed consent form (see Institutional Review Board Statement).
2.2. Study Design
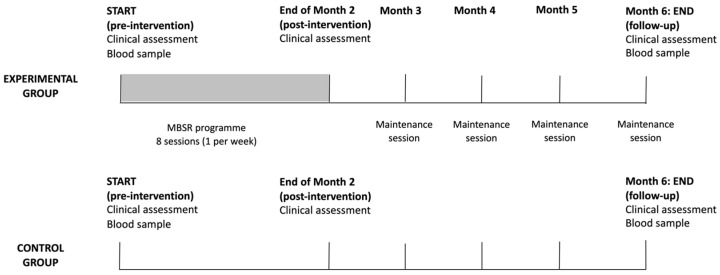
We conducted a clinical assessment of all patients at three time points in the study—at baseline (pre-intervention), at the end of the MBSR program at 2 months (post-intervention), and at the end of the study at 6 months (follow-up) (see Figure 1). Clinical assessment included measurements of height, weight, waist circumference, systolic and diastolic blood pressure (from which we calculated mean arterial blood pressure, MAP), and heart rate. Mental health status was assessed with clinical scales for depression (Beck Depression Inventory-II [47]) and anxiety (Beck Anxiety Inventory [48]), perceived stress (Stress Questionnaire [49]), quality of life (RAND-36 [50,51]), and trait-like tendencies of mindfulness in daily life (Five-Facet Mindfulness Questionnaire [52]). Perceived stress was measured as a cumulative score on a 20-item Stress Questionnaire, with possible scores ranging from 0 to 20 [49].
Figure 1.
Study design.
Blood samples were collected at the beginning and end of the study. We determined serum levels of cholesterol, HDL, LDL, triglycerides, fasting glucose, and postprandial glucose after 120 min.
Participant properties of the experimental and control groups are summarized in Table 1. Control group was, on average, statistically significantly younger, and had a lower level of triglycerides.
2.3. Mindfulness-Based Stress Reduction Program
Patients in the experimental group participated in a mindfulness-based stress reduction (MBSR) program (see Figure 1). Therapeutic sessions were held once a week for 8 weeks at the Department of Mental Health at the University Psychiatric Clinic Ljubljana. They were led by experienced therapists who were not otherwise involved in the study. The content of the standard MBSR program at the department consists of 8 group meetings (once a week, two hours) and one individual meeting to clarify dilemmas and open questions and to anchor the practice of mindfulness in everyday life. The structure of the group meeting is as follows: Beginning with formal practice, review of practice and experience at home, discussion and group practice related to the theoretical theme of the weekly meeting and concluding with mindfulness practice. Participants performed specific exercises and techniques for developing mindfulness, such as practicing mindfulness in relation to loving kindness and breathing, in everyday activities and walking, awareness of body sensations (body scan) and simple body positions. To eliminate confounding factors, participants were instructed to maintain their usual diet, medications, and exercise levels during the mindfulness-based treatment.
2.4. DNA Methylation Analysis
2.4.1. Selection of Candidate Genes and Target Sequences
DNA methylation analysis was performed for several candidate genes selected for their association with reproduction, obesity, metabolic syndrome, and diabetes or their relevance to stress-related disorders and mood disorders. The genes and their functional significance are listed in Table 2.
Table 2.
Amplicon positions of candidate genes and their functional significance.
| Amplicon | Position (Human Genome Build 19) and Length of Target Sequence | Number of CpG Islands |
Functional Significance |
|---|---|---|---|
| BDNF-81_1 | chr11:27744260-27744605 (−), 346 | 22 | Brain-derived neurotrophic factor gene; regulates growth, differentiation, maintenance, death/survival and plasticity of neurons [54] |
| BDNF-81_2 | chr11:27743702-27743960 (−), 259 | 10 | |
| BDNF-81_3 | chr11:27743454-27743762 (−), 309 | 20 | |
| BDNF-14_1 | chr11:27741988-27742250 (−), 263 | 13 | |
| BDNF-58_1 | chr11:27740916-27741131 (−), 216 | 16 | |
| BDNF-58_2 | chr11:27740607-27740901 (−), 295 | 30 | |
| BDNF-95_1 | chr11:27721638-27721854 (−), 217 | 19 | |
| BDNF-95_2 | chr11:27722466-27722696 (−), 231 | 13 | |
| BDNF-95_5 | chr11:27722209-27722487 (−), 279 | 23 | |
| CEBPB_1 | chr20:48807584-48807968 (+), 385 | 52 | Transcription factor gene; participates in the ovarian follicle development and insulin signaling [55] |
| CEBPB_2 | chr20:48807389-48807650 (+), 262 | 23 | |
| COMT_1 | chr22:19951071-19951343, 273 | 14 | Catechol-O-methyl transferase (COMT) gene, determines prefrontal dopaminergic availability [56] |
| COMT_2 | chr22:19929042-19929349, 308 | 36 | |
| COMT_4 | chr22:19950002-19950320, 319 | 13 | |
| EPHX1_2 | chr1:225998005-225998262 (+), 258 | 25 | Epoxide hydrolase-1 gene; regulates steroid synthesis pathways associated with PCOS [57] |
| EPM2A_1 | chr6:146056330-146056621 (−), 292 | 36 | Dual-specificity phosphatase gene; involved in the regulation of glycogen metabolism [55] |
| FKBP51_1 | chr6:35656629-35656978 (−), 350 | 38 | FK506 binding protein 5 gene; regulates glucocorticoid activity and acute stress response [58] |
| FST_1 | chr5:52775484-52775780 (+), 297 | 23 | Follistatin, an activin-binding protein gene; associated with PCOS [59] |
| FST_2 | chr5:52776111-52776302 (+), 192 | 13 | |
| FST_3 | chr5:52776279-52776668 (+), 390 | 54 | |
| HTR1A_2 | chr5:63257662-63257938 (−),277 | 15 | Serotonin receptor 1A gene; regulates serotonin system function [60] |
| HTR1A_3 | chr5:63256777-63257065 (−), 289 | 20 | |
| IGFBP1_1 | chr7:45928046-45928301 (+), 256 | 21 | Insulin-like growth factor binding protein gene; regulates cell migration and metabolism [55] |
| IGFBP1_2 | chr7:45928280-45928533 (+), 254 | 35 | |
| INSR_1 | chr19:7293426-7293764 (−), 339 | 36 | Insulin receptor gene; associated with insulin resistance in PCOS [61] |
| LHCGR_1 | chr2:48982757-48983019 (−), 263 | 16 | Luteinizing hormone/choriogonadotropin receptor gene; involved in human gonadal maturation and function [62] |
| MAOA_2 | chrX:43513981-43514236, 256 | 18 | Monoamine oxidase A gene; involved in serotonin degradation [60] |
| MAOA_3 | chrX:43515510-43515787, 278 | 13 | |
| NR3C1_1 | chr5:142783586-142783906 (−), 321 | 39 | Glucocorticoid receptor gene; associated with regulation of HPA axis and stress response [63] |
| PPARG1A_1 | chr4:23890471-23890804 (−), 334 | 9 | Peroxisome proliferator-activated receptor gamma 1 gene; regulates ovarian function [64] |
| SLC6A4_3 | chr17:28562753-28563050 (−), 298 | 29 | Serotonin transporter gene; associated with depression treatment outcomes [58] |
| SLC6A4_5 | chr17:28563277-28563552 (−), 276 | 7 | |
| TBKBP1_1 | chr17:45772538-45772753 (+), 216 | 9 | TBKBP1 gene; involved in the TNF-α/NF-κB pathway activated under conditions of acute and chronic psychological stress [40] |
| TPH2_1 | chr12:72332514-72332805 (+), 292 | 9 | Tryptophan hydroxylase 2 gene; serotonin synthesis rate limiting enzyme [60] |
Mapping of target sequences was performed using the UCSC Genome Browser, Human Genome Build 19 (GRCh37/hg19) [53]. Target sequences in candidate genes were located in the CpG islands (CGI), with additional 500-base-pair flanking regions upstream and downstream of the CGI. For genes lacking specific CpG islands, the target sequence was located near the transcription start site, where sequences with potential regulatory function are located.
2.4.2. DNA Isolation and Bisulfite Conversion
Venous blood tubes were labeled with a code to ensure confidentiality of the data. They were stored at −70 °C until DNA isolation. DNA was isolated from 100 μL whole blood using the QIAcube robotic workstation and the commercially available QIAmp DNA Mini Kit (Qiagen, Venlo, The Netherlands) according to the supplier’s instructions and eluted in 100 μL of ultra clean water. A total of 1000 ng of DNA was bisulfite converted using the EpiTect Fast Bisulfite Kit (Qiagen, Venlo, The Netherlands) and eluted in 50 μL of elution buffer, yielding DNA at a final concentration of 20 ng/μL.
2.4.3. Primer Design
Primers were designed using Methyl Primer Express v1.0 [65], which allows the design of primers for bisulfite-converted DNA. Each primer pair was designed to amplify regions approximately 200–350 basepairs long. If the CGI were greater than 300 bp, two or more primer pairs were designed to cover the CGI sequence as much as possible. Table 2 shows the amplicon positions, amplicon lengths and the number of CpGs.
Primer properties and specificity were determined using BiSearch [66] and the IDT Oligo Analyzer [67]. In the final step of primer design, the Illumina adapter overhang sequences were added to the 5′ end of the DNA sequence-specific primer and the final primer sequences were rechecked for their properties and specificity.
2.4.4. Amplicon Generation and Sequencing
Amplicon library preparation followed Illumina’s 16S protocol with some modifications [68]. The target sequences were amplified in two rounds of PCR. The first round of PCR was used to generate target sequences (complete primer pair sequences and annealing temperatures in Supplementary Table S1). The total volume of PCR reactions was 25 μL and was composed of 12.5 μL KAPA HiFi HotStart Uracil+ ReadyMix (Roche, KAPA Biosystems Ltd., Cape Town, South Africa), 1 μM primer, and 20 ng DNA. The PCR protocol was performed as follows: Activation for 5 min at 95 °C, followed by 35 amplification cycles (denaturation for 30 s at 98 °C, annealing for 15 s at a temperature dependent on the primer pair and extension for 15 s at 72 °C), and concluded by a final extension for 1 min at 72 °C and holding at 4 °C. Annealing temperatures for each specific primer pair are listed in Supplementary Table S1. Amplicons from the first round of PCR were visualized on 2% agarose gel electrophoresis to confirm amplification of fragments of the correct length. Shorter, nonspecific amplification fragments were removed using AMPure XP beads (Beckman Coulter, Brea, CA, USA). Purified amplicons from each subject were pooled in a tube to form an equimolar pool (concentrations were measured using Quant-iT PicoGreen dsDNA (Thermo Scientific, Life Technologies, Waltham, MA, USA)).
The second round of PCR was used to label each subject with a specific identifying sequence that would allow multiplexing during the sequencing run. Nextera XT v2 Index Set A and Set D primers (Illumina, San Diego, CA, USA) were used to label the pooled samples. The total volume of PCR reactions was 50 μL and was composed of 25 μL KAPA HiFi HotStart Uracil+ ReadyMix (Roche, Basel, Switzerland), Nextera XT v2 primers, and 4 ng of equimolar amplicon pool. The PCR protocol was performed: Activation for 45 s at 98 °C, 10 amplification cycles (denaturation for 15 s at 98 °C, annealing for 30 s at 55 °C, and extension for 30 s at 72 °C), followed by a final extension for 1 min at 72 °C. Generation of amplicons of appropriate length was again confirmed by 2% agarose gel electrophoresis.
2.4.5. Library Preparation and Sequencing
Amplicons from the second round of PCR amplification were subjected to size selection (AMPure XP paramagnetic beads, Beckman Coulter, Brea, CA, USA), followed by concentration determination. The concentration of each subject library was measured using an ultrasensitive fluorescent nucleic acid dye that allowed quantification of double-stranded DNA (PicoGreen dsDNA quantification reagent, Thermo Fisher, Waltham, MA, USA). The final library was prepared by equimolar pooling of the individual subject libraries to a final library with a molar concentration of 10 nM. The final library was diluted and denatured according to the recommendations in the Illumina MiniSeq System Denature and Dilute Libraries Guide. The final library was sequenced on the Illumina MiniSeq sequencer using the MiniSeq Mid Output Kit (300 cycles) with 150 bp paired-end reads.
2.5. Bioinformatic and Statistical Analysis
Raw sequencing reads (in FASTQ format) were quality checked using the FastQC tool (v0.14.1) [69]. Trim galore (v0.6.7) [70] was used to trim bases of insufficient quality (Q score below 30) and adapter sequences. Bismark (v0.21.0) [71] was used to align these trimmed sequences to the reference genome UCSC Genome Browser (Homo sapiens version hg19).
Aligned reads were further analyzed using the R environment (v4.0.4) [72], the methylKit package [73], and the methylSig package [74], with data corrected for age and multiple testing. Differentially methylated CpGs (DMC) were identified by comparing the percent DNA methylation for each CpG cytosine between our two assay groups. The average DNA methylation values of the amplicon were calculated using the values of all CpGs in that amplicon and compared between the two groups of subjects studied. The normality of the distribution of CpG values was tested using the Shapiro–Wilk normality test. Because the distribution was nonparametric for most of the amplicon data, differences in the percentages of mean methylation of the amplicons between groups were calculated using the Mann–Whitney U test and the Benjamini–Hochberg correction. Corrected p values ofless than 0.05 were considered statistically significant.
Statistical analysis was performed using custom scripts written in R (version x64 4.1.2) [72] and RStudio (version 4.1.2) [75]. A between-group and within-subjects design was used. Accordingly, data were analyzed with mixed ANOVA. Data were first tested for assumption violation. The identify outlier function from the R package rstatix was used to automatically detect outliers. Shapiro–Wilk test was used to test for normality, followed by a visual inspection of the QQ plot. Levene’s test was used to test for homogeneity of variance. Box’s M test was used to test homogeneity of covariance. Mauchly’s test was used to test sphericity. When parametric assumptions were met, a two-way mixed method ANOVA was used to test for differences between groups and within subjects. When parametric assumptions were not met, the robust mixed ANOVA implemented in the R package WRS2 [76] was used.
When a significant interaction was detected, post hoc tests were performed. Bonferroni correction for multiple comparisons was applied to each test. The effect of group at each time point and the effect of time at each level of the grouping variable were estimated using one-way ANOVA. Pairwise comparisons between grouping levels were performed using a pairwise T test. Finally, a pairwise t test was used for pairwise comparisons between time points at each level of the grouping variable.
3. Results
3.1. Effects of the MBSR Program on Physical Health and Quality of Life
Eleven clinical and laboratory measures were obtained. A two-way mixed ANOVA was performed to evaluate the effects of time and group on each measurement. The results are summarized in Table 3 and Figure 2.
Table 3.
p-values for interactions between group and time for clinical and laboratory measurements. Statistically significant p-values are marked in bold.
| Participant Characteristics | p-Value (ANOVA) |
|---|---|
| BMI | 0.011 |
| Waist circumference | 0.024 |
| Mean arterial blood pressure | 0.581 |
| Heart rate | 0.631 |
| Glucose—0 min | 0.0460 |
| Glucose—120 min | 0.039 |
| Cholesterol | 0.811 |
| S-HDL | 0.461 |
| S-LDL | 0.693 |
| Triglycerides | 0.5387 |
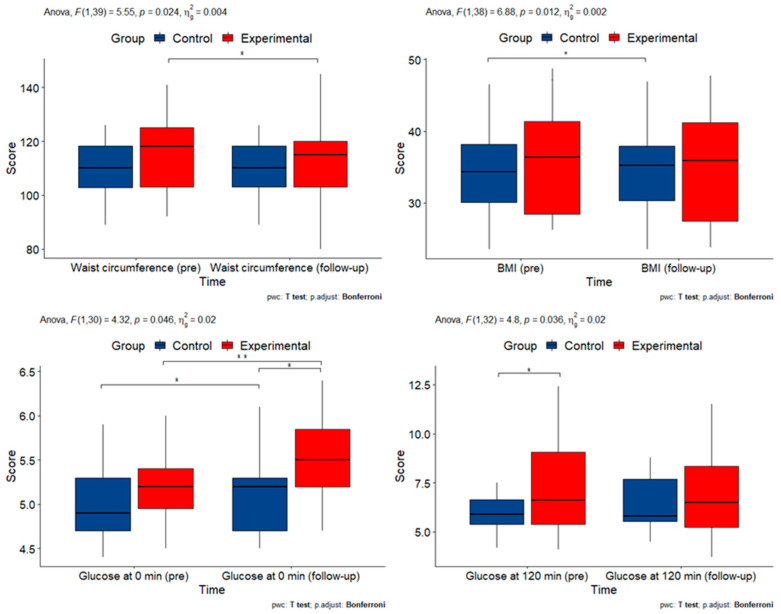
Figure 2.
Box plots of clinical and laboratory measurements with statistically significant interactions (BMI: body mass index; min: minutes; pre: pre-intervention). Asterisks denote statistically significant pairwise comparisons.
A two-way mixed ANOVA was performed to evaluate the effects of time and group on all clinical and laboratory measurements except triglycerides, for which a robust mixed ANOVA was used because the data were nonparametric. For weight (F(1, 40) = 5.032, p = 0.030), BMI (F(1, 39) = 7.196, p = 0.011), waist circumference (F(1, 39) = 5.546, p = 0.024), P-glucose at 0 min (F(1, 30) = 4.316, p = 0.046) and P-glucose at 120 min (F(1, 33) = 4.643, p = 0.039) were statistically significant. This indicates that the intervention affected these measures, but not MAP, heart rate, cholesterol, S-HDL, and S-LDL. For triglycerides, we can assume an effect of the intervention because the groups were significantly different at baseline and did not differ after the intervention.
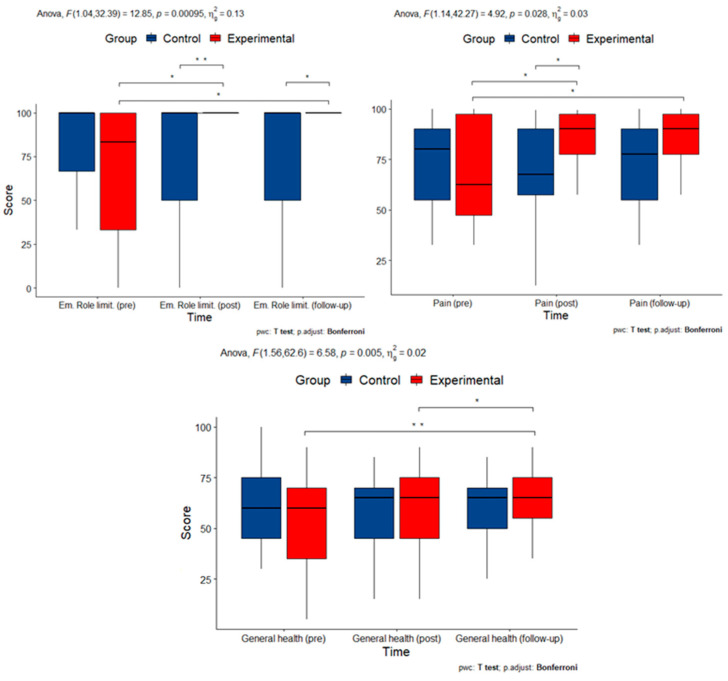
Quality of life was assessed using the SF-36 questionnaire (RAND-36 version), which consists of eight subscales: physical functioning, physical role limitations, emotional role limitations, energy/fatigue, emotional well-being, social functioning, pain, and general health. There was a statistically significant interaction between time and group on measures of emotional role limitations (F(1.04, 32.39) = 12.85, p < 0.001), pain (F(1.14, 42.27) = 4.923, p = 0.034), and general health (F(1.56, 62.6) = 6.581, p = 0.005), but not physical well-being (F(1, 66) = 48.09, p = 0.509) or robust estimation of physical limitations (p = 0.593), energy/fatigue (F(1.31, 52.22) = 3.106, p = 0.073), and emotional well-being (F(1.39, 55.43) = 2.525, p = 0.107). To determine whether the intervention had a longitudinal effect, pairwise comparisons were performed between time points at each group level after a Bonferroni correction for multiple comparisons. Scores for emotional limitations differed significantly between the beginning of the study (pre) and after the intervention (post) (p = 0.014) and between the beginning of the study and at follow-up (p = 0.014) between the experimental and control groups. Pain scores differed significantly between the experimental and control groups between the beginning of the study and after the intervention (p = 0.033) and between the beginning of the study and at follow-up (p = 0.03). General health scores differed significantly between the experimental and control groups between the beginning of the study and after the intervention (p = 0.011) and between the end of the intervention and at follow-up (p = 0.002). This suggests that there was a longitudinal effect of the intervention on pain, general health status, and emotional limitations.
Measurements with statistically significant interactions are summarized in Figure 3.
Figure 3.
Box plots of measurements of quality-of-life indices with statistically significant interactions (Em. Role: Emotional Role subscale; pre: pre-intervention; post: post-intervention). Asterisks denote statistically significant pairwise comparisons.
3.2. Effects and Interactions of the MBSR Program on Psychological Well-Being
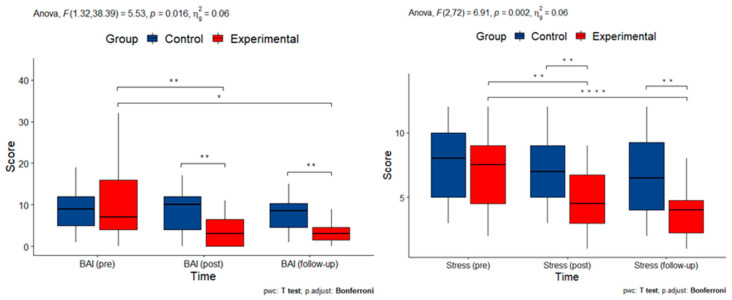
The severity of anxious symptomatology was assessed with BAI. There was a statistically significant two-way interaction between time and group (F(1, 39) = 5.525, p = 0.016). Considering the Bonferroni-adjusted p-value and using pairwise paired t-test comparisons, it can be seen that for the experimental group, the mean BAI score was statistically significantly different between the beginning of the study and after the intervention (p = 0.009) and between the beginning of the study and at follow-up (p = 0.022). This indicates an immediate reduction in anxiety symptomatology that remained stable over time.
Depressive symptomatology was measured by BDI-II. Scores from BDI-II were nonparametric. To assess the effects of time and group on the scores of BDI-II, the robust estimation mixed ANOVA was used. There were no statistically significant interactions between time and group (p = 0.929) or main effects of group (p = 0.163) or time (p = 0.492).
Finally, there was a statistically significant two-way interaction between time and group (F(2, 72) = 6.911, p = 0.002) on scores of perceived stress. Considering the Bonferroni adjusted p-value and using pairwise paired t-test comparisons, we note that for the experimental group, the mean stress score was statistically significantly different between the beginning of the study and after the intervention (p = 0.003) and between the beginning of the study and at follow-up (p = 0.000).
Measurements with statistically significant interactions are summarized in Figure 4.
Figure 4.
Box plots of measurements of mental health indices with statistically significant interactions (BAI: Beck Anxiety Inventory; Stress: Perceived Stress score; pre: pre-intervention; post: post-intervention). Asterisks denote statistically significant pairwise comparisons.
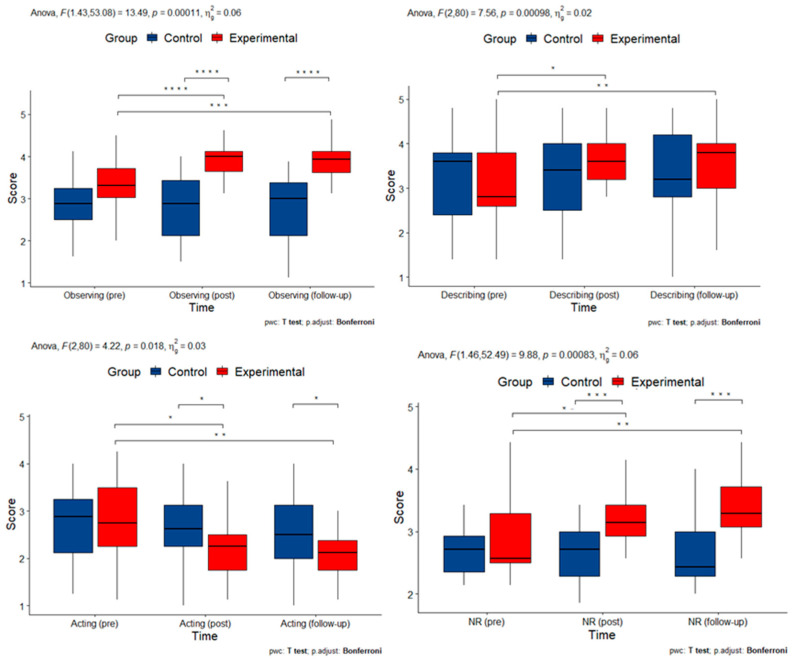
3.3. Effects and Interactions of the MBSR Program on Mindfulness-Like Traits
Mindfulness-like traits were assessed with the FFMQ questionnaire, which includes five subscales: Observing, Describing, Acting, Non-judging, and Non-reactivity. There was a statistically significant interaction between time and group on measures of Observing (F(1.43, 53.98) = 13.495, p < 0.001), Describing (F(2, 80) = 7.563, p = 0.018), Acting (F(2, 80) = 4.217, p = 0.026), and Non-reactivity (F(1.63, 47.31) = 14.976, p < 0.001), but not on Non-judging (F(1.65, 62.85) = 1.088, p = 0.333). To determine whether the intervention had a longitudinal effect, pairwise comparisons were performed between time points at each group level after a Bonferroni correction for multiple comparisons. Observing scores were statistically significantly different between the beginning of the study and after the intervention (p < 0.001) and between the beginning of the study and at follow-up (p < 0.001) in the experimental group. The scores for Describing were statistically significantly different between the beginning of the study and after the intervention (p < 0.001) and between the beginning of the study and at follow-up (p = 0.008) in the experimental group. The scores for Acting were statistically significantly different between the beginning of the study and after the intervention (p = 0.016) and between the beginning of the study and at follow-up (p = 0.001) in the experimental group. Non-reactivity scores differed statistically significantly between the beginning of the study and after the intervention for the experimental group (p < 0.001) and between the beginning of the study and at follow-up (p < 0.001). These results suggest that mindfulness-like traits of observing, describing, acting, and non-reactivity were improved by the intervention and remained stable over time.
Measurements with statistically significant interactions are summarized in Figure 5.
Figure 5.
Box plots of measurements of mindfulness-like traits with statistically significant interactions (pre: pre-intervention; post: post-intervention; NR: Non-reactivity subscale). Asterisks denote statistically significant pairwise comparisons.
3.4. DNA Methylation Alterations in Candidate Genes
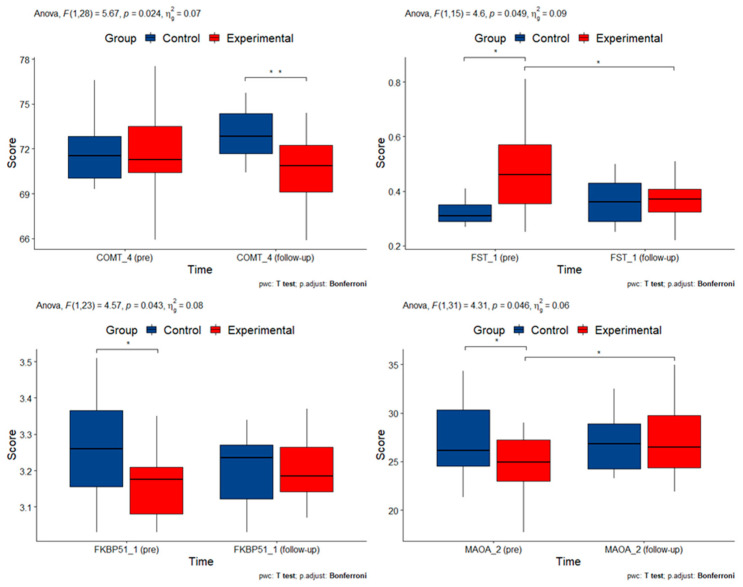
Mixed ANOVA or robust mixed ANOVA estimation (in the case of nonparametric data) were used to test for differences in the level of DNA methylation of candidate gene amplicons six months after the start of the therapeutic intervention. Table 4 summarizes the p-values for the significance of interactions of group and time for candidate gene methylation. Several of the original amplicons could not be included in the analysis due to missing methylation data. A mixed two-way ANOVA was performed to evaluate the effects of time and group on methylation levels. There was a statistically significant two-way interaction between time and group on the methylation of several gene amplicons: COMT_4 (F(1, 28) = 5.667, p = 0.075), FST_1 (F(1, 15) = 4.597, p = 0.049), FKBP51_1 (F(1, 23) = 4.569, p = 0.043) and MAOA_2 (F(1, 31) = 4.313, p = 0.046).
Table 4.
p-values for significant interaction of group and time for methylation of candidate gene amplicons. Table contains only amplicons where the average DNA methylation values could be calculated from available data. Statistically significant p-values are marked in bold.
| Candidate Gene Amplicon | p-Value (ANOVA) |
|---|---|
| BDNF_95_5 | 0.797 |
| BDNF_95_2 | 0.382 |
| BDNF_58_2 | 0.731 |
| BDNF_58_1 | 0.845 |
| BDNF_14_1 | 0.439 |
| BDNF_81_3 | 0.564 |
| BDNF_81_1 | 0.795 |
| CEBPB_2 | 0.696 |
| CEBPB_1 | 0.557 |
| COMT_2 | 0.532 |
| COMT_4 | 0.024 |
| COMT_1 | 0.717 |
| EPHX1_2 | 0.929 |
| EPM2A_1 | 0.63 |
| FKBP51_1 | 0.043 |
| FST_1 | 0.049 |
| FST_3 | 0.491 |
| HTR1A_3 | 0.115 |
| HTR1A_2 | 0.684 |
| IGFBP1_1 | 0.611 |
| IGFBP1_2 | 0.169 |
| INSR_1 | 0.91 |
| LHCGR_1 | 0.952 |
| MAOA_2 | 0.046 |
| MAOA_3 | 0.862 |
| PPRG1A_1 | 0.85 |
| SLC6A4_3 | 0.58 |
| SLC6A4_5 | 0.403 |
| TPH2_1 | 0.896 |
p-values for all candidate genes are summarized in Table 4. Measurements with statistically significant interactions are summarized in Figure 6.
Figure 6.
Box plots of measurements of DNA methylation changes with statistically significant interactions. Asterisks denote statistically significant pairwise comparisons.
4. Discussion
In this study, we investigated the effects of mindfulness-based therapy on clinical presentation and DNA methylation changes in patients with PCOS and high metabolic risk. We expected that patients in the experimental group would have fewer clinical symptoms of PCOS, anxiety, and depression and achieve a higher quality of life after the mindfulness intervention. We also expected that the mindfulness intervention would affect methylation of certain genes associated with PCOS.
PCOS is often associated with a metabolic syndrome defined by central obesity, high triglyceride and low S-HDL levels, hypertension, and inability to control blood glucose. We collected a range of clinical and laboratory data to evaluate the effects of mindfulness intervention on the metabolic syndrome in PCOS. Interestingly, we observed a statistically significant decrease in body weight/BMI and waist circumference. Although there was a statistically significant increase in fasting blood serum glucose level at 0 min in both groups, the change was not clinically meaningful, since all values remained within prediabetes range (5.60 to 6.21 mmol/L). There were no statistically significant differences in MAP, heart rate, cholesterol, S-HDL, or S-LDL. Triglyceride levels, which were significantly higher in the experimental group at baseline, did not differ anymore after the intervention.
Patients who participated in the mindfulness intervention experienced statistically significant improvements in emotional role limitations and measures of general health, as well as significant decreases in measures of pain, as measured by RAND-36. All of these measures of well-being remained stable over time at a six-month follow-up after the intervention. In contrast to the other measures, general health continued to improve, as indicated by the statistically significant difference in this subscale between the end of the intervention and the follow-up.
RAND-36 is a common tool used to assess the effectiveness of mindfulness-based interventions in clinical settings on quality of life [77]. However, results have been mixed. In the study by Morledge and colleagues, mindfulness-based interventions improved physical functioning, general health, social functioning, and emotional role limitations in the normative population as measured by RAND-36 [78]. Van Berkel and colleagues used RAND-36 as a general measure of mental health, and there were no statistically significant effects of a mindfulness-based intervention in the normative population [79]. Mindfulness-based interventions used as adjunctive treatment improved measures of pain, emotional limitations, physical limitations, well-being, energy, and social functioning, but not overall health in addiction patients [80]. Allexandre and colleagues reported statistically significant differences in the normative population in emotional well-being, emotional role functioning, and energy [81]. A mindfulness-based intervention for cancer survivors at metabolic risk reported by Lucas and colleagues showed statistically significant change in RAND-36 subscales for physical well-being but not for psychological well-being [82]. It is likely that mindfulness-based interventions influence psychological and physical well-being in a nonlinear manner.
We expected a statistically significant improvement in the severity of anxious and depressive symptomatology between patients who participated in the MBSR program and the control group that would last six months after the start of therapy. We also hypothesized that the MBSR program would help participants experience and tolerate stress more efficiently. The mindfulness intervention was indeed associated with improvement in anxiety symptomatology as well as subjectively perceived stress, but not in depressive symptomatology. It is possible that the mindfulness intervention provided participants in the experimental group with a new coping mechanism with which to regulate stress, a possibility that is well described in the literature [83,84,85,86]. Another possibility, also supported in the literature, is that mindfulness-based interventions indirectly affect emotion regulation by increasing emotional differentiation [87]. Importantly, this pattern remained stable over time and was still present six months after the intervention. The differential effect of mindfulness intervention on depression and anxiety is surprising, as reviews of MBI efficacy suggest that they alleviate depressive rather than anxiety symptomatology [88].
Interestingly, although there was improvement between the first and second measurement (immediately after therapy) and the first and third measurement (six months after the start of therapy) for anxiety and subjectively perceived stress, as well as for measures of emotional role limitations, pain, and general health, there was no difference between the second measurement after therapy and the six-month follow-up. This suggests that the mindfulness intervention is associated with one-time, long-lasting improvements in psychological well-being, but not with ongoing improvements in mental health.
Our hypothesis was also that six months after the start of therapy, there will be a statistically significant difference in the expression of mindfulness-like traits between patients who underwent the MBSR program and the control group. Indeed, the mindfulness intervention was associated with a statistically significant increase in all mindfulness-like traits, except for non-judging. These improvements remained stable over time but did not increase further.
The results of studies on various cognitive-behavioral and mind–body interventions in women with PCOS suggest that these treatments reduce stress [44,89], anxiety [89,90], depression [89,90,91], quality of life [42,89], eating problems [90], and decrease in body mass index [91], and treatment by a multidisciplinary team is effective in improving healthy lifestyle and achieving long-term weight loss in these women [92]. In addition, when combined with lifestyle modification, these treatments had a greater effect on weight loss and improvement in depression symptoms and quality of life in obese or overweight women with PCOS compared with lifestyle modification alone [93].
Although the effects of mindfulness on adult women with PCOS have not been extensively researched, the results are consistently positive. In a study published in the journal Stress, women with PCOS were randomly assigned to participate in an 8-week mindfulness program for stress management that included 30 min of instruction. Twenty-three and fifteen women with PCOS were randomly assigned to the intervention and control groups, respectively. At the end of the study, the women were determined to experience less stress, depression, and anxiety. The women’s salivary cortisol levels also decreased, and life satisfaction and quality of life scores increased only in the intervention group. There was no significant “placebo” effect on outcome measures [89]. Another study examined mindfulness yoga as a popular form of mindfulness intervention. Twenty-two women with PCOS participated in the three-month intervention, 13 in the mindful yoga group and 9 in the control group. The women who participated in the mindful yoga intervention had significantly lower free testosterone levels. In addition, improvements in anxiety and depression were noted, with no weight loss [94].
Regarding the DNA methylation levels of the candidate genes, we expected changes between patients in the experimental and control groups six months after the start of therapy. Candidate genes were selected from two different points of view: Genes already associated with PCOS and its clinical features (CEBPB, EPHX1, EPM2A, FST, IGFBP1, INSR, LHCGR, PPARG1A) and genes involved in the stress response system (SLC6A4, COMT, MAOA, BDNF, NR3C1, FKBP51, TBKBP1) and neurotransmitter signaling (HTR1A, TPH2). We observed a statistically significant effect of mindfulness intervention on three candidate genes selected as stress-associated genes: COMT, MAOA, and FKBP51, and one gene already associated with PCOS, FST. The COMT and MAOA genes encode enzymes that degrade neurotransmitters such as dopamine, serotonin, and norepinephrine, thereby regulating normal brain function. Alterations in COMT and MAOA DNA methylation have previously been linked to stress [95] and various stress-related disorders, such as depression and anxiety [56,96]. Our results on COMT amplicon are similar to those in [95], as stress correlates with increasing DNA methylation. In our study, mindfulness intervention decreased stress and COMT methylation. In the review of stress-related disorders [97] and MAOA methylation, decreased methylation levels were detected in patients compared to controls or in patients before treatment compared to the same patients after treatment. These results are similar to those observed in our study, where MAOA amplicon methylation increased after the mindfulness intervention.
One gene involved in HPA axis function that has been frequently studied in DNA methylation studies is FKBP51. It regulates glucocorticoid activity and response to acute stress through a negative feedback loop in a dynamic process of methylation and demethylation. The protein of FKBP51, FK506-binding protein 5, binds to the glucocorticoid receptor complex, impeding its translocation to the nucleus. In the study of mindfulness-based stress reduction in posttraumatic stress disorder patients, a significant decrease in methylation was observed in patients who responded to the intervention and an increase was observed in patients who did not respond to the intervention. These results suggest that the mediation intervention may affect stress-related molecular pathways at the molecular level [58]. Similarly, a decrease in methylation has been observed in responders and an increase in non-responders of PTSD patients after psychotherapy [63]. In our case, we analyzed similar CpGs as [63], but we observed exactly the opposite pattern of methylation—increase after intervention, while we observed decrease in controls. The only gene selected from the group of PCOS-associated genes that showed a statistically significant change in methylation patterns before and after intervention was FST, follistatin. FST regulates follicular development by binding and neutralizing activins [59], and increased serum levels of FST were detected in PCOS women compared to controls [98]. We attempted to cover the CpG island of FST as much as possible and were able to design three primer pairs. For the primer pair covering the beginning of the island, FST_1, we observed a decrease after the intervention, whereas in the control group, the methylation level remained the same after six months compared with the initial methylation status. On the contrary, in another study with PCOS women, the methylation level of FST showed an increase in methylation at the beginning of the CpG island, but any changes in gene expression could not be detected [59].
5. Limitations
Because our sample size was small, the study was exploratory in nature. A series of measurements of DNA methylation of candidate genes was performed, and then the effect of the mindfulness intervention on these genes was estimated. Follow-up studies targeting only the specific measurements that showed significant effects should be conducted to consolidate these results.
6. Conclusions
Mindfulness techniques seem promising in ameliorating stress, anxiety, depression, and the quality of life in women with PCOS and could be used as an adjunct method to the conventional management of these women. Drug treatment is crucial for the management of PCOS, but it only targets the symptoms associated with the disease, while the causative treatment for this syndrome is still unknown. Some of these symptoms could also be affected by mindfulness therapy only, which would reduce the number of medications the patient needs to take. It would also be important to determine which aspects of mindfulness therapy are most beneficial in PCOS. With consumer interest in holistic healthcare rising, healthcare providers are required to broaden their knowledge pertaining to the safe and appropriate utilization of these therapies as adjuncts to conventional medical management [42].
Acknowledgments
The authors want to thank the participants, without whom this study would not have been possible. We also thank the MBSR therapists from University Psychiatric Clinic Ljubljana that carried out the MBSR program with participants. K.K. wishes to acknowledge her current place of employment, the Institute of Microbiology and Immunology, Faculty of Medicine, University of Ljubljana, Ljubljana, Slovenia.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cimb45040178/s1, Table S1: Amplicon primer sequences and annealing temperatures for candidate genes. Reference [99] is cited in the Supplementary Materials.
Author Contributions
Conceptualization, H.D., A.V.P. and J.B.; methodology, H.D., A.V.P., K.K., A.O., M.J., B.Š. and J.B.; formal analysis, H.D., A.V.P., M.K., K.K., A.O. and J.B.; investigation, H.D., A.V.P., M.K., B.Š.; resources, A.V.P., M.J., A.J., B.Š. and J.B.; data curation, A.O.; writing—original draft preparation, H.D., A.V.P., A.O. and J.B.; writing—review and editing, H.D., A.V.P., K.K., A.O., M.J., A.J., B.Š. and J.B.; visualization, A.O.; supervision, A.V.P. and J.B.; project administration, H.D.; funding acquisition, A.V.P. and J.B. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Medical Ethics Committee of the Republic of Slovenia (protocol code 0120-239/2018/6, date of approval 31 May 2018).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on reasonable request from the corresponding author. The data are not publicly available due to privacy and ethical restriction.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Funding Statement
This research was funded by Javna Agencija za Raziskovalno Dejavnost RS (grants P5-0110, P1-0390, J3-1763).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Escobar-Morreale H.F. Polycystic ovary syndrome: Definition, aetiology, diagnosis and treatment. Nat. Rev. Endocrinol. 2018;14:270–284. doi: 10.1038/nrendo.2018.24. [DOI] [PubMed] [Google Scholar]
- 2.Joham A.E., Norman R.J., Stener-Victorin E., Legro R.S., Franks S., Moran L.J., Boyle J., Teede H.J. Polycystic ovary syndrome. Lancet Diabetes Endocrinol. 2022;10:668–680. doi: 10.1016/S2213-8587(22)00163-2. [DOI] [PubMed] [Google Scholar]
- 3.Witchel S.F., Oberfield S.E., Peña A.S. Polycystic Ovary Syndrome: Pathophysiology, Presentation, and Treatment with Emphasis on Adolescent Girls. J. Endocr. Soc. 2019;3:1545–1573. doi: 10.1210/js.2019-00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pasquali R. Metabolic Syndrome in Polycystic Ovary Syndrome. In: Popovic V., Korbonits M., editors. Frontiers of Hormone Research. Volume 49. S. Karger AG; Basel, Switzerland: 2018. pp. 114–130. [DOI] [PubMed] [Google Scholar]
- 5.Wang Z., Groen H., Cantineau A.E.P., Van Elten T.M., Karsten M.D.A., Van Oers A.M., Mol B.W.J., Roseboom T.J., Hoek A. Dietary Intake, Eating Behavior, Physical Activity, and Quality of Life in Infertile Women with PCOS and Obesity Compared with Non-PCOS Obese Controls. Nutrients. 2021;13:3526. doi: 10.3390/nu13103526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herman R., Sikonja J., Jensterle M., Janez A., Dolzan V. Insulin Metabolism in Polycystic Ovary Syndrome: Secretion, Signaling, and Clearance. Int. J. Mol. Sci. 2023;24:3140. doi: 10.3390/ijms24043140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu T., Cui J., Goodarzi M.O. Polycystic Ovary Syndrome and Risk of Type 2 Diabetes, Coronary Heart Disease, and Stroke. Diabetes. 2021;70:627–637. doi: 10.2337/db20-0800. [DOI] [PubMed] [Google Scholar]
- 8.Meczekalski B., Pérez-Roncero G.R., López-Baena M.T., Chedraui P., Pérez-López F.R. The polycystic ovary syndrome and gynecological cancer risk. Gynecol. Endocrinol. 2020;36:289–293. doi: 10.1080/09513590.2020.1730794. [DOI] [PubMed] [Google Scholar]
- 9.Dokras A., Stener-Victorin E., Yildiz B.O., Li R., Ottey S., Shah D., Epperson N., Teede H. Androgen Excess-Polycystic Ovary Syndrome Society: Position statement on depression, anxiety, quality of life, and eating disorders in polycystic ovary syndrome. Fertil. Steril. 2018;109:888–899. doi: 10.1016/j.fertnstert.2018.01.038. [DOI] [PubMed] [Google Scholar]
- 10.Castelo-Branco C., Naumova I. Quality of life and sexual function in women with polycystic ovary syndrome: A comprehensive review. Gynecol. Endocrinol. 2020;36:96–103. doi: 10.1080/09513590.2019.1670788. [DOI] [PubMed] [Google Scholar]
- 11.Xing L., Xu J., Wei Y., Chen Y., Zhuang H., Tang W., Yu S., Zhang J., Yin G., Wang R., et al. Depression in polycystic ovary syndrome: Focusing on pathogenesis and treatment. Front. Psychiatry. 2022;13:1001484. doi: 10.3389/fpsyt.2022.1001484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walters K.A., Gilchrist R.B., Ledger W.L., Teede H.J., Handelsman D.J., Campbell R.E. New Perspectives on the Pathogenesis of PCOS: Neuroendocrine Origins. Trends Endocrinol. Metab. 2018;29:841–852. doi: 10.1016/j.tem.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Visser J.A. The importance of metabolic dysfunction in polycystic ovary syndrome. Nat. Rev. Endocrinol. 2021;17:77–78. doi: 10.1038/s41574-020-00456-z. [DOI] [PubMed] [Google Scholar]
- 14.Stepto N.K., Moreno-Asso A., McIlvenna L.C., Walters K.A., Rodgers R.J. Molecular Mechanisms of Insulin Resistance in Polycystic Ovary Syndrome: Unraveling the Conundrum in Skeletal Muscle? J. Clin. Endocrinol. Metab. 2019;104:5372–5381. doi: 10.1210/jc.2019-00167. [DOI] [PubMed] [Google Scholar]
- 15.Rudnicka E., Suchta K., Grymowicz M., Calik-Ksepka A., Smolarczyk K., Duszewska A.M., Smolarczyk R., Meczekalski B. Chronic Low Grade Inflammation in Pathogenesis of PCOS. Int. J. Mol. Sci. 2021;22:3789. doi: 10.3390/ijms22073789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang N.-X., Li X.-L. The Disorders of Endometrial Receptivity in PCOS and Its Mechanisms. Reprod. Sci. 2022;29:2465–2476. doi: 10.1007/s43032-021-00629-9. [DOI] [PubMed] [Google Scholar]
- 17.Parker J., O’Brien C., Hawrelak J., Gersh F.L. Polycystic Ovary Syndrome: An Evolutionary Adaptation to Lifestyle and the Environment. Int. J. Environ. Res. Public Health. 2022;19:1336. doi: 10.3390/ijerph19031336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vázquez-Martínez E.R., Gómez-Viais Y.I., García-Gómez E., Reyes-Mayoral C., Reyes-Muñoz E., Camacho-Arroyo I., Cerbón M. DNA methylation in the pathogenesis of polycystic ovary syndrome. Reproduction. 2019;158:R27–R40. doi: 10.1530/REP-18-0449. [DOI] [PubMed] [Google Scholar]
- 19.Rawat K., Sandhu A., Gautam V., Saha P.K., Saha L. Role of genomic DNA methylation in PCOS pathogenesis: A systematic review and meta-analysis involving case-controlled clinical studies. Mol. Hum. Reprod. 2022;28:gaac024. doi: 10.1093/molehr/gaac024. [DOI] [PubMed] [Google Scholar]
- 20.Nilsson E., Benrick A., Kokosar M., Krook A., Lindgren E., Källman T., Martis M.M., Højlund K., Ling C., Stener-Victorin E. Transcriptional and Epigenetic Changes Influencing Skeletal Muscle Metabolism in Women With Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2018;103:4465–4477. doi: 10.1210/jc.2018-00935. [DOI] [PubMed] [Google Scholar]
- 21.Echiburú B., Milagro F., Crisosto N., Pérez-Bravo F., Flores C., Arpón A., Salas-Pérez F., Recabarren S.E., Sir-Petermann T., Maliqueo M. DNA methylation in promoter regions of genes involved in the reproductive and metabolic function of children born to women with PCOS. Epigenetics. 2020;15:1178–1194. doi: 10.1080/15592294.2020.1754674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abbott D.H., Kraynak M., Dumesic D.A., Levine J.E. In utero Androgen Excess: A Developmental Commonality Preceding Polycystic Ovary Syndrome? In: Pasquali R., Pignatelli D., editors. Frontiers of Hormone Research. Volume 53. S. Karger AG; Basel, Switzerland: 2019. pp. 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaliman P. Epigenetics and meditation. Curr. Opin. Psychol. 2019;28:76–80. doi: 10.1016/j.copsyc.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 24.Venditti S., Verdone L., Reale A., Vetriani V., Caserta M., Zampieri M. Molecules of Silence: Effects of Meditation on Gene Expression and Epigenetics. Front. Psychol. 2020;11:1767. doi: 10.3389/fpsyg.2020.01767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andersen G.B., Tost J. A Summary of the Biological Processes, Disease-Associated Changes, and Clinical Applications of DNA Methylation. In: Tost J., editor. DNA Methylation Protocols. Volume 1708. Springer; New York, NY, USA: 2018. pp. 3–30. Methods in Molecular Biology. [DOI] [PubMed] [Google Scholar]
- 26.Cui P., Ma T., Tamadon A., Han S., Li B., Chen Z., An X., Shao L.R., Wang Y., Feng Y. Hypothalamic DNA methylation in rats with dihydrotestosterone-induced polycystic ovary syndrome: Effects of low-frequency electro-acupuncture. Exp. Physiol. 2018;103:1618–1632. doi: 10.1113/EP087163. [DOI] [PubMed] [Google Scholar]
- 27.Kokosar M. Polycystic Ovary Syndrome. Androgen Excess and Insulin Resistance in Women: Identification of Molecular Targets to Improve Glucose Homeostasis. Göteborgs Universitet; Göteborg, Sweden: 2018. [Google Scholar]
- 28.Tran B.X., Harijanto C., Vu G.T., Ho R.C.M. Global mapping of interventions to improve quality of life using mind-body therapies during 1990–2018. Complement. Ther. Med. 2020;49:102350. doi: 10.1016/j.ctim.2020.102350. [DOI] [PubMed] [Google Scholar]
- 29.Bandealy S.S., Sheth N.C., Matuella S.K., Chaikind J.R., Oliva I.A., Philip S.R., Jones P.M., Hoge E.A. Mind-Body Interventions for Anxiety Disorders: A Review of the Evidence Base for Mental Health Practitioners. Focus. 2021;19:173–183. doi: 10.1176/appi.focus.20200042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ebner S.A., Meikis L., Morat M., Held S., Morat T., Donath L. Effects of Movement-Based Mind-Body Interventions on Physical Fitness in Healthy Older Adults: A Meta-Analytical Review. Gerontology. 2021;67:125–143. doi: 10.1159/000512675. [DOI] [PubMed] [Google Scholar]
- 31.Buric I., Farias M., Jong J., Mee C., Brazil I.A. What Is the Molecular Signature of Mind–Body Interventions? A Systematic Review of Gene Expression Changes Induced by Meditation and Related Practices. Front. Immunol. 2017;8:670. doi: 10.3389/fimmu.2017.00670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kohls N., Esch T., Gerber L., Adrian L., Wittmann M. Mindfulness Meditation and Fantasy Relaxation in a Group Setting Leads to a Diminished Sense of Self and an Increased Present Orientation. Behav. Sci. 2019;9:87. doi: 10.3390/bs9080087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kropp A., Sedlmeier P. What Makes Mindfulness-Based Interventions Effective? An Examination of Common Components. Mindfulness. 2019;10:2060–2072. doi: 10.1007/s12671-019-01167-x. [DOI] [Google Scholar]
- 34.Lutz A., Jha A.P., Dunne J.D., Saron C.D. Investigating the phenomenological matrix of mindfulness-related practices from a neurocognitive perspective. Am. Psychol. 2015;70:632–658. doi: 10.1037/a0039585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Querstret D., Morison L., Dickinson S., Cropley M., John M. Mindfulness-based stress reduction and mindfulness-based cognitive therapy for psychological health and well-being in nonclinical samples: A systematic review and meta-analysis. Int. J. Stress Manag. 2020;27:394–411. doi: 10.1037/str0000165. [DOI] [Google Scholar]
- 36.Wersebe H., Lieb R., Meyer A.H., Hofer P., Gloster A.T. The link between stress, well-being, and psychological flexibility during an Acceptance and Commitment Therapy self-help intervention. Int. J. Clin. Health Psychol. 2018;18:60–68. doi: 10.1016/j.ijchp.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kraines M.A., Peterson S.K., Tremont G.N., Beard C., Brewer J.A., Uebelacker L.A. Mindfulness-Based Stress Reduction and Mindfulness-Based Cognitive Therapy for Depression: A Systematic Review of Cognitive Outcomes. Mindfulness. 2022;13:1126–1135. doi: 10.1007/s12671-022-01841-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rogers J.M., Ferrari M., Mosely K., Lang C.P., Brennan L. Mindfulness-based interventions for adults who are overweight or obese: A meta-analysis of physical and psychological health outcomes: Mindfulness for adults who are overweight or obese. Obes. Rev. 2017;18:51–67. doi: 10.1111/obr.12461. [DOI] [PubMed] [Google Scholar]
- 39.Black D.S., Christodoulou G., Cole S. Mindfulness meditation and gene expression: A hypothesis-generating framework. Curr. Opin. Psychol. 2019;28:302–306. doi: 10.1016/j.copsyc.2019.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chaix R., Fagny M., Cosin-Tomás M., Alvarez-López M., Lemee L., Regnault B., Davidson R.J., Lutz A., Kaliman P. Differential DNA methylation in experienced meditators after an intensive day of mindfulness-based practice: Implications for immune-related pathways. Brain Behav. Immun. 2020;84:36–44. doi: 10.1016/j.bbi.2019.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaliman P., Álvarez-López M.J., Cosín-Tomás M., Rosenkranz M.A., Lutz A., Davidson R.J. Rapid changes in histone deacetylases and inflammatory gene expression in expert meditators. Psychoneuroendocrinology. 2014;40:96–107. doi: 10.1016/j.psyneuen.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cowan S., Lim S., Alycia C., Pirotta S., Thomson R., Gibson-Helm M., Blackmore R., Naderpoor N., Bennett C., Ee C., et al. Lifestyle management in polycystic ovary syndrome—Beyond diet and physical activity. BMC Endocr. Disord. 2023;23:14. doi: 10.1186/s12902-022-01208-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moradi F., Ghadiri-Anari A., Dehghani A., Reza Vaziri S., Enjezab B. The effectiveness of counseling based on acceptance and commitment therapy on body image and self-esteem in polycystic ovary syndrome: An RCT. Int. J. Reprod. Biomed. 2020;18:243–252. doi: 10.18502/ijrm.v13i4.6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Phimphasone-Brady P., Palmer B., Vela A., Johnson R.L., Harnke B., Hoffecker L., Coons H.L., Epperson C.N. Psychosocial interventions for women with polycystic ovary syndrome: A systematic review of randomized controlled trials. FS Rev. 2022;3:42–56. doi: 10.1016/j.xfnr.2021.11.004. [DOI] [Google Scholar]
- 45.Young C.C., Monge M., Minami H., Rew L., Conroy H., Peretz C., Tan L. Outcomes of a Mindfulness-Based Healthy Lifestyle Intervention for Adolescents and Young Adults with Polycystic Ovary Syndrome. J. Pediatr. Adolesc. Gynecol. 2022;35:305–313. doi: 10.1016/j.jpag.2021.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grundy S.M., Cleeman J.I., Daniels S.R., Donato K.A., Eckel R.H., Franklin B.A., Gordon D.J., Krauss R.M., Savage P.J., Smith S.C., et al. Diagnosis and Management of the Metabolic Syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 47.Beck A.T., Steer R.A., Brown G. Beck Depression Inventory–II. Psychol. Assess. :2011. doi: 10.1037/t00742-000. [DOI] [Google Scholar]
- 48.Beck A.T., Epstein N., Brown G., Steer R.A. An inventory for measuring clinical anxiety: Psychometric properties. J. Consult. Clin. Psychol. 1988;56:893–897. doi: 10.1037/0022-006X.56.6.893. [DOI] [PubMed] [Google Scholar]
- 49.Dernovšek M.Z., Gorenc M., Jeriček Klanšček H. Ko te Strese Stres: Kako Prepoznati in Zdraviti Stresne, Anksiozne in Depresivne Motnje. Ponatis.; Inštitut za Varovanje Zdravja Republike Slovenije; Ljubljana, Slovenia: 2012. [Google Scholar]
- 50.Hays R.D., Morales L.S. The RAND-36 measure of health-related quality of life. Ann. Med. 2001;33:350–357. doi: 10.3109/07853890109002089. [DOI] [PubMed] [Google Scholar]
- 51.Logar Zakrajšek B., Bren A., Sočan G., Pajek J. Pilotna raziskava psihometričnih lastnosti vprašalnikov SF-36v2 in ESRD-SCL-TM za merjenje z zdravjem povezane kakovosti življenja bolnikov po presaditvi ledvice. Psihol. Obz. Horiz. Psychol. 2018;12:1–11. doi: 10.20419/2018.27.479. [DOI] [Google Scholar]
- 52.Baer R.A., Smith G.T., Hopkins J., Krietemeyer J., Toney L. Using self-report assessment methods to explore facets of mindfulness. Assessment. 2006;13:27–45. doi: 10.1177/1073191105283504. [DOI] [PubMed] [Google Scholar]
- 53.Kent W.J., Sugnet C.W., Furey T.S., Roskin K.M., Pringle T.H., Zahler A.M., Haussler A.D. The Human Genome Browser at UCSC. Genome Res. 2002;12:996–1006. doi: 10.1101/gr.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen D., Meng L., Pei F., Zheng Y., Leng J. A review of DNA methylation in depression. J. Clin. Neurosci. 2017;43:39–46. doi: 10.1016/j.jocn.2017.05.022. [DOI] [PubMed] [Google Scholar]
- 55.Shen H., Qiu L., Zhang Z., Qin Y., Cao C., Di W. Genome-Wide Methylated DNA Immunoprecipitation Analysis of Patients with Polycystic Ovary Syndrome. PLoS ONE. 2013;8:e64801. doi: 10.1371/journal.pone.0064801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Na K.-S., Won E., Kang J., Kim A., Choi S., Tae W.-S., Kim Y.-K., Lee M.-S., Joe S.-H., Ham B.-J. Differential effect of COMT gene methylation on the prefrontal connectivity in subjects with depression versus healthy subjects. Neuropharmacology. 2018;137:59–70. doi: 10.1016/j.neuropharm.2018.04.030. [DOI] [PubMed] [Google Scholar]
- 57.Sang Q., Li X., Wang H., Wang H., Zhang S., Feng R., Xu Y., Li Q., Zhao X., Xing Q., et al. Quantitative Methylation Level of the EPHX1 Promoter in Peripheral Blood DNA Is Associated with Polycystic Ovary Syndrome. PLoS ONE. 2014;9:e88013. doi: 10.1371/journal.pone.0088013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bishop J.R., Lee A.M., Mills L.J., Thuras P.D., Eum S., Clancy D., Erbes C.R., Polusny M.A., Lamberty G.J., Lim K.O. Methylation of FKBP5 and SLC6A4 in Relation to Treatment Response to Mindfulness Based Stress Reduction for Posttraumatic Stress Disorder. Front. Psychiatry. 2018;9:418. doi: 10.3389/fpsyt.2018.00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sang Q., Zhang S., Zou S., Wang H., Feng R., Li Q., Jin L., He L., Xing Q., Wang L. Quantitative analysis of follistatin (FST) promoter methylation in peripheral blood of patients with polycystic ovary syndrome. Reprod. BioMedicine Online. 2013;26:157–163. doi: 10.1016/j.rbmo.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 60.Kouter K., Zupanc T., Videtič Paska A. Targeted sequencing approach: Comprehensive analysis of DNA methylation and gene expression across blood and brain regions in suicide victims. World J. Biol. Psychiatry. 2022;24:12–23. doi: 10.1080/15622975.2022.2046291. [DOI] [PubMed] [Google Scholar]
- 61.Zhong X., Jin F., Huang C., Du M., Gao M., Wei X. DNA methylation of AMHRII and INSR gene is associated with the pathogenesis of Polycystic Ovary Syndrome (PCOS) Technol. Health Care. 2021;29:11–25. doi: 10.3233/THC-218002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Selig J., Troppmann B., Gromoll J. Impact of DNA methylation on the regulation of the luteinizing hormone/choriogonadotropin receptor expression. Exp. Clin. Endocrinol. Diabetes. 2013;121:OP5_28. doi: 10.1055/s-0033-1336636. [DOI] [Google Scholar]
- 63.Yehuda R., Daskalakis N.P., Desarnaud F., Makotkine I., Lehrner A.L., Koch E., Flory J.D., Buxbaum J.D., Meaney M.J., Bierer L.M. Epigenetic Biomarkers as Predictors and Correlates of Symptom Improvement Following Psychotherapy in Combat Veterans with PTSD. Front. Psychiatry. 2013;4:118. doi: 10.3389/fpsyt.2013.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Qu F., Wang F.-F., Yin R., Ding G.-L., El-prince M., Gao Q., Shi B.-W., Pan H.-H., Huang Y.-T., Jin M., et al. A molecular mechanism underlying ovarian dysfunction of polycystic ovary syndrome: Hyperandrogenism induces epigenetic alterations in the granulosa cells. J. Mol. Med. 2012;90:911–923. doi: 10.1007/s00109-012-0881-4. [DOI] [PubMed] [Google Scholar]
- 65.ThermoFisher Methyl Primer Express v1.0. [(accessed on 24 November 2021)]. Available online: https://resource.thermofisher.com/page/WE28396_2/
- 66.Institute of Enzymology BiSearch v2.63. [(accessed on 24 November 2021)]. Available online: http://bisearch.enzim.hu/
- 67.Integrated DNA Technologies IDT Oligo Analyzer. [(accessed on 24 November 2021)]. Available online: https://eu.idtdna.com/pages/tools/oligoanalyzer.
- 68.Illumina PCR, Amplicon PCR and Index PCR: 16S Metagenomic Sequencing Library Preparation. [(accessed on 21 February 2023)]. Available online: https://www.illumina.com/content/dam/illumina-support/documents/documentation/chemistry_documentation/16s/16s-metagenomic-library-prep-guide-15044223-b.pdf.
- 69.Andrews S. Fastqc: A Quality Control Tool for High Throughput Sequence Data. [(accessed on 20 March 2022)]. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/
- 70.Krueger F. Trimgalore. [(accessed on 20 March 2022)]. Available online: https://github.com/FelixKrueger/TrimGalore.
- 71.Krueger F., Andrews S.R. Bismark: A flexible aligner and methylation caller for Bisulfite-Seq applications. Bioinformatics. 2011;27:1571–1572. doi: 10.1093/bioinformatics/btr167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.R Core Team R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. [(accessed on 21 February 2023)]. Available online: https://www.R-project.org/
- 73.Altuna Akalin MethylKit. [(accessed on 22 February 2023)]. Available online: https://bioconductor.org/packages/methylKit.
- 74.Park Y., Cavalcante R. MethylSig. [(accessed on 22 February 2023)]. Available online: https://bioconductor.org/packages/methylSig.
- 75.Allaire J. RStudio: Integrated Development Environment for R. Volume 770. RStudio; Boston, MA, USA: 2012. pp. 165–171. [Google Scholar]
- 76.Mair P., Wilcox R. Robust statistical methods in R using the WRS2 package. Behav. Res. Methods. 2020;52:464–488. doi: 10.3758/s13428-019-01246-w. [DOI] [PubMed] [Google Scholar]
- 77.Grahn Kronhed A.-C., Enthoven P., Spångeus A., Willerton C. Mindfulness and Modified Medical Yoga as Intervention in Older Women with Osteoporotic Vertebral Fracture. J. Altern. Complement. Med. 2020;26:610–619. doi: 10.1089/acm.2019.0450. [DOI] [PubMed] [Google Scholar]
- 78.Morledge T.J., Allexandre D., Fox E., Fu A.Z., Higashi M.K., Kruzikas D.T., Pham S.V., Reese P.R. Feasibility of an Online Mindfulness Program for Stress Management—A Randomized, Controlled Trial. Ann. Behav. Med. 2013;46:137–148. doi: 10.1007/s12160-013-9490-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Van Berkel J., Boot C.R.L., Proper K.I., Bongers P.M., Van Der Beek A.J. Effectiveness of a Worksite Mindfulness-Related Multi-Component Health Promotion Intervention on Work Engagement and Mental Health: Results of a Randomized Controlled Trial. PLoS ONE. 2014;9:e84118. doi: 10.1371/journal.pone.0084118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cooperman N.A., Hanley A.W., Kline A., Garland E.L. A pilot randomized clinical trial of mindfulness-oriented recovery enhancement as an adjunct to methadone treatment for people with opioid use disorder and chronic pain: Impact on illicit drug use, health, and well-being. J. Subst. Abus. Treat. 2021;127:108468. doi: 10.1016/j.jsat.2021.108468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Allexandre D., Bernstein A.M., Walker E., Hunter J., Roizen M.F., Morledge T.J. A Web-Based Mindfulness Stress Management Program in a Corporate Call Center: A Randomized Clinical Trial to Evaluate the Added Benefit of Onsite Group Support. J. Occup. Environ. Med. 2016;58:254–264. doi: 10.1097/JOM.0000000000000680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lucas A.R., Klepin H.D., Porges S.W., Rejeski W.J. Mindfulness-Based Movement: A Polyvagal Perspective. Integr. Cancer Ther. 2018;17:5–15. doi: 10.1177/1534735416682087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chambers R., Gullone E., Allen N.B. Mindful emotion regulation: An integrative review. Clin. Psychol. Rev. 2009;29:560–572. doi: 10.1016/j.cpr.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 84.Chiesa A., Serretti A., Jakobsen J.C. Mindfulness: Top–down or bottom–up emotion regulation strategy? Clin. Psychol. Rev. 2013;33:82–96. doi: 10.1016/j.cpr.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 85.Guendelman S., Medeiros S., Rampes H. Mindfulness and Emotion Regulation: Insights from Neurobiological, Psychological, and Clinical Studies. Front. Psychol. 2017;8:220. doi: 10.3389/fpsyg.2017.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Roemer L., Williston S.K., Rollins L.G. Mindfulness and emotion regulation. Curr. Opin. Psychol. 2015;3:52–57. doi: 10.1016/j.copsyc.2015.02.006. [DOI] [Google Scholar]
- 87.Hill C.L.M., Updegraff J.A. Mindfulness and its relationship to emotional regulation. Emotion. 2012;12:81–90. doi: 10.1037/a0026355. [DOI] [PubMed] [Google Scholar]
- 88.Hempel S., Taylor S.L., Marshall N.J., Miake-Lye I.M., Beroes J.M., Shanman R., Solloway M.R., Shekelle P.G. Evidence Map of Mindfulness. Department of Veterans Affairs (US); Washington, DC, USA: 2014. VA Evidence-based Synthesis Program Reports. [PubMed] [Google Scholar]
- 89.Stefanaki C., Bacopoulou F., Livadas S., Kandaraki A., Karachalios A., Chrousos G.P., Diamanti-Kandarakis E. Impact of a mindfulness stress management program on stress, anxiety, depression and quality of life in women with polycystic ovary syndrome: A randomized controlled trial. Stress. 2015;18:57–66. doi: 10.3109/10253890.2014.974030. [DOI] [PubMed] [Google Scholar]
- 90.Correa J.B., Sperry S.L., Darkes J. A case report demonstrating the efficacy of a comprehensive cognitive-behavioral therapy approach for treating anxiety, depression, and problematic eating in polycystic ovarian syndrome. Arch. Women’s Ment. Health. 2015;18:649–654. doi: 10.1007/s00737-015-0506-3. [DOI] [PubMed] [Google Scholar]
- 91.Abdollahi L., Mirghafourvand M., Babapour Kheyradin J., Mohammadi M. The Effect of Cognitive Behavioral Therapy on Depression and Obesity in Women with Polycystic Ovarian Syndrome: A Randomized Controlled Clinical Trial. Iran Red Crescent Med. J. 2018;20:e62735. doi: 10.5812/ircmj.62735. [DOI] [Google Scholar]
- 92.Jiskoot G., Benneheij S.H., Beerthuizen A., de Niet J.E., de Klerk C., Timman R., Busschbach J.J., Laven J.S.E. A three-component cognitive behavioural lifestyle program for preconceptional weight-loss in women with polycystic ovary syndrome (PCOS): A protocol for a randomized controlled trial. Reprod Health. 2017;14:34. doi: 10.1186/s12978-017-0295-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cooney L.G., Milman L.W., Hantsoo L., Kornfield S., Sammel M.D., Allison K.C., Epperson C.N., Dokras A. Cognitive-behavioral therapy improves weight loss and quality of life in women with polycystic ovary syndrome: A pilot randomized clinical trial. Fertil. Steril. 2018;110:161–171.e1. doi: 10.1016/j.fertnstert.2018.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Patel S. Polycystic ovary syndrome (PCOS), an inflammatory, systemic, lifestyle endocrinopathy. J. Steroid Biochem. Mol. Biol. 2018;182:27–36. doi: 10.1016/j.jsbmb.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 95.Wiegand A., Blickle A., Brückmann C., Weller S., Nieratschker V., Plewnia C. Dynamic DNA Methylation Changes in the COMT Gene Promoter Region in Response to Mental Stress and Its Modulation by Transcranial Direct Current Stimulation. Biomolecules. 2021;11:1726. doi: 10.3390/biom11111726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Peng H., Zhu Y., Strachan E., Fowler E., Bacus T., Roy-Byrne P., Goldberg J., Vaccarino V., Zhao J. Childhood Trauma, DNA Methylation of Stress-Related Genes, and Depression: Findings From Two Monozygotic Twin Studies. Psychosom. Med. 2018;80:599–608. doi: 10.1097/PSY.0000000000000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ziegler C., Domschke K. Epigenetic signature of MAOA and MAOB genes in mental disorders. J. Neural Transm. 2018;125:1581–1588. doi: 10.1007/s00702-018-1929-6. [DOI] [PubMed] [Google Scholar]
- 98.Norman R.J., Milner C.R., Groome N.P., Robertson D.M. Circulating follistatin concentrations are higher and activin concentrations are lower in polycystic ovarian syndrome. Hum. Reprod. 2001;16:668–672. doi: 10.1093/humrep/16.4.668. [DOI] [PubMed] [Google Scholar]
- 99.Kouter K., Nedic G., Erjavec T., Milos L., Tudor S., Uzun N.M., Pivac N., Videtič Paska A. Difference in methylation and expression of brain-derived neurotrophic factor in Alzheimer’s disease and mild cognitive impairment. Biomedicines. 2023;11:235. doi: 10.3390/biomedicines11020235. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on reasonable request from the corresponding author. The data are not publicly available due to privacy and ethical restriction.