Abstract
Precision and organization govern the cell cycle, ensuring normal proliferation. However, some cells may undergo abnormal cell divisions (neosis) or variations of mitotic cycles (endopolyploidy). Consequently, the formation of polyploid giant cancer cells (PGCCs), critical for tumor survival, resistance, and immortalization, can occur. Newly formed cells end up accessing numerous multicellular and unicellular programs that enable metastasis, drug resistance, tumor recurrence, and self-renewal or diverse clone formation. An integrative literature review was carried out, searching articles in several sites, including: PUBMED, NCBI-PMC, and Google Academic, published in English, indexed in referenced databases and without a publication time filter, but prioritizing articles from the last 3 years, to answer the following questions: (i) “What is the current knowledge about polyploidy in tumors?”; (ii) “What are the applications of computational studies for the understanding of cancer polyploidy?”; and (iii) “How do PGCCs contribute to tumorigenesis?”
Keywords: polyploid giant cancer cells (PGCCs), bioinformatics, systems biology, tumor evolution
1. Introduction
The cell cycle is a series of events that occur to accurately replicate genetic material and cellular contents into daughter cells. This regulation is precisely monitored and controlled [1]. Nevertheless, some cells can undergo alternative cellular divisions or mitotic cycle variations, generating aneuploid (polyploid) genomes in cells with varying cell sizes [1].
Polyploidy, a cell that has three or more chromosomal sets [2], in cancer is complex, primarily because the initiation and progression of cancer involves multiple integrated processes [3]. The relationship between PGCCs (cell subpopulation with a chromosome content greater than 2n) and cancer has recently reached interesting results regarding the fundamental role of these cells for tumor survival, immortalization, aggressiveness, and progression [4].
Fei et al. [3], Glassmann et al. [5], and Fei et al. [6] showed the presence of PGCCs in breast, colorectal, and ovarian cancer, correlating with poor survival and prognosis. These cells are considered an intermediate product of genomic instability [7].
Recent data demonstrated that PGCCs may form from oncogenic and therapeutic stress, generating reprogrammed cancer cells [8] and being a source of cancer stem cells (CSC) [9], influencing tumor resistance [10], and auto renewal [11]. PGCCs exhibit elevated aneuploidy and are connected to the tumor microenvironment evolution [12]; they have a cell cycle of their own, called “giant cell cycle” [1]. They are also able to: (i) create or repopulate macroscopic spheroids in vitro [13], (ii) generate tumors when inoculated in mice [14], and (iii) convert into different phenotypes, showing high plasticity [15], which has been included as a new hallmark of cancer.
Bharadwaj et al. [16], Mirzayans, Andrais, and Murray [17], Mirzayans and Murray [18,19], and Bharadwaj and Mandal [20] showed the connection between senescence and PGCCs, as cancer cells can escape from premature senescence, possibly leading to the formation of a multinucleated gigantic cell. Zhang et al. [21,22], Lin et al. [23], Sirois et al. [24], White-Gilbertson et al. [25], and Voelkel-Johnson [26] highlighted the metabolic and biophysical aspects of PGCCs, including cytoskeleton and biochemical modifications, that sustain cell cycle dysregulation, stress responses, and dedifferentiation.
Numerous studies have begun to use high throughput approaches through multiple techniques, including next generation sequencing (NGS), multi-omics, and phylostratigraphy, among others. Additionally, this review seeks to integrate theories, show different hypotheses, emphasize the need for multidisciplinary and collaborative approaches, demonstrate possible therapeutic options, and highlight the impact of ploidy on different stages of carcinogenesis. Our goal is to discuss the following questions: (i) “What is the current knowledge about polyploidy in tumors?”; (ii) “What are the applications of computational studies for the understanding of cancer polyploidy?”; and (iii) “How do PGCCs contribute to tumorigenesis?”
2. Polyploidy in Cancer
Cell cycle deregulation and/or failures in mitosis can lead to polyploidy, the upregulation of genes that positively impact the cell cycle progression, and cytostatic genes downregulation [27,28] in physiological and pathological manners [29]. Polyploidy, or whole genome duplication (WGDs), results from the cell cycle prematurely ending or from cellular fusion [30], increasing genetic variation, stress tolerance, the ability to colonize different environments, and mutation burden relief. The evolutionary selective advantages of WGD offer a theoretical base for cyclic ploidy, mitotic slippage, and cancer cell fusion [31].
Polyploid cells are vulnerable during meiosis due to chromosome pairing difficulties and genomic instability [30]; this can be solved by a transient reversion of polyploidy (depolyploidization) [32], multipolar division [33], chromosomal rearrangement, and DNA divergence. A high degree of polyploidy or aneuploidy are detrimental to the cell, but can increase cellular adaptability and plasticity, fueling intratumoral heterogeneity [34,35].
3. A Brief Introduction to PGCCs
PGCCs have been observed for at least 180 years in diverse cancer types [36,37,38,39]. Some were observed after chemotherapy, in cells with irregular nuclei, increased migratory potential, genetic instability, altered response to hypoxia, drug resistance, or higher mortality rates [40].
PGCCs are a special subpopulation of tumor cells containing a large cytoplasm and multiple nuclei [41], undergoing an altered giant cell cycle, creating genetic heterogeneity, and allowing cancer cells to overcome multiple micro-environment challenges [42].
3.1. PGCC’s Giant Cell Cycle
Stress factors such as chemotherapy, antimitotic drugs, radiotherapy, hypoxia, or a deficient microenvironment may induce PGCCs formation [28] by means of a giant cell cycle (cycle linked to polyploidization and depolyploidization processes together with neosis) used for dedifferentiation of somatic cells, which can generate stem cells for tumor initiation. Such a cell cycle is divided in four phases: (i) Initiation: stressed tumor cells undergo catastrophic mitosis or cell death. Surviving cells assume a tetraploid or polyploid transient state [1,11]; (ii) Auto-renovation: tetraploid cells start endocycling to produce mononuclear or multinuclear PGCCs. Some multinuclear cells go through cyto-fission to create smaller polyploid cells [1,11]; (iii) Termination: PGCCs undergo depolyploidization (genome reduction division) and generate diploid nuclei by budding. Others form a structure that resembles a reproductive cyst [1,11]; and (iv) Stability: diploid descendent cells with altered genotypes continue to differentiate into a variety of aneuploid cell types with proliferative capacity [1,11].
Giant Cell Cycle Possible Outcomes and Fates
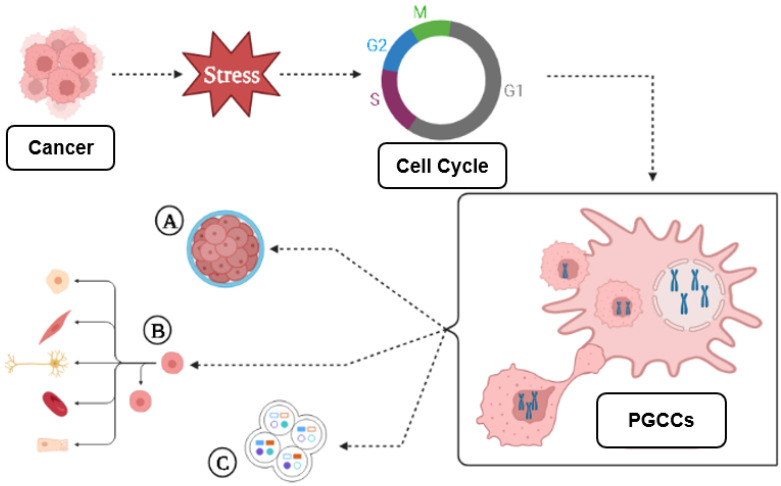
There is a correlation between endoreplication cell cycles and the amount of DNA replication mistakes, followed by complete dedifferentiation. In parallel, cell division stagnation reflects the degree of tumor malignancy [11]. As a result of polyploidization, cancer cells can: (i) initiate metastasis; (ii) survive “lethal” drugs; or (iii) divide asymmetrically to generate cell multilineages with higher therapy resistance [39,43]. The giant cell cycle can lead to tumor evolution, numbness, resistance, recurrence, and regeneration, as shown in Figure 1.
Figure 1.
Giant cell cycle outcomes. Under stress, the cell responds in various ways to maintain balance. When chemotherapy treated tumor cells are isolated, large numbers of cells die; surviving cells may start an altered cell cycle, capable of generating PGCCs, which follow four phases or other unusual divisions; PGCCs exhibit abnormal shapes similar to single-celled organisms such as amoebas, or embryonic cells at the blastomere stage; that (A) can undergo diapause, dormancy, relapse, and generate multilineages; (B) tumor cells use these means to survive and generate resistant daughter cells by budding, fragmentation (bursting), sporulation or encystment; (C) new genetic and epigenetic characteristics create unlimited potential for more aggressive, resistant and immortal phenotypes. Terms are shown in Box A1 (Appendix A). Note: created with BioRender.com (accessed on 15 March 2023).
Genotoxic agents used in cancer therapy are associated with the induction of cell stress, ploidy, and cell size variation; these can lead to the generation of 3D structures similar to a blastocyst [44,45]. These blastocyst-like multicellular structures with a reprogrammed genome can generate resistant daughter-cells of different lineages that can acquire the capacity to go through embryonic latency, a reversible state of numbness, to survive environmental stress factors [44,46].
PGCCs can rejuvenate by way of amitotic division, generating cancer stem cells like blastomeres, that may transdifferentiate into different lineages, while the mother cell retains undifferentiated cancer properties [45].
3.2. PGCCs Functionalities: Plasticity, Metabolism and Resistance to Therapy
The diversity of PGCCs functions suggests polyploidy as an evolutionary source of tissue regeneration and plasticity, being explored by cancer cells to promote survival [47] and stress response [29,48], recap embryonic stages [49], exhibit adaptive exploration of multiple gene regulatory networks [48,50], and regulate biochemical and biophysical pathways [51,52].
The Warburg effect is the utilization of aerobic glycolysis for ATP synthesis, despite having abundant oxygen available. PGCCs probably use a Warburg effect similar to the embryo in state of pre-implantation by means of glucose repression of oxidative phosphorylation, as seen in the yeast Crabtree effect. The occurrence of polyploidy or aneuploidy in PGCCs ends up passing through gene losses that could alter the relative amount of enzymes favoring glycolysis, and, consequently, investing in rapid cell division [47,52].
The functionalities developed by the PGCCs also influence tumor resistance and recurrence; these are still big challenges for modern therapies, especially because they can produce stem cells able to repopulate the tumor microenvironment [53].
Thus, polyploid tumor cells can be used as a molecular model for better understanding fundamental evolutive paradigms [54,55]; evolution studies may help us answer questions about cancer resistance and success [56].
3.3. PGCC’s Role in Tumor Evolution
Tumor evolution is a process by which tumor cells change across time, acquiring advantageous characteristics for their survival and perpetuation, surviving immune attack, drug treatments, and diverse anti-proliferative signals [57,58]. Key mechanisms and processes altered from tumor initiation to progression include proliferation, motility, metabolism, autocrine signaling, activated intracellular pathways, inflammation, plasticity, senescence, transdifferentiation, and cell cannibalism [58].
PGCCs also share features of the Mendel and McClintock models [46], showing independent segregation for different characteristics. Stressed cells respond by redefining their genomic structure to promote tumor success [46], primarily because PGCCs exhibit diverse gene expression, which leads to neosis [48]. The stress previously suffered directs tumor cells to certain paths, including apoptosis, necrosis, senescence, mitotic catastrophe, or a mixture of these processes [48]. However, when the tumor overcomes the death threshold through alterations in regulatory networks [59,60,61], polyploidization becomes possible in a generative and reversible process [48].
In parallel to this mechanism of ploidy regulation, numerous other processes are shared by PGCCs, including endoreplication (endocycle and endomitosis), cell fusion, cytokinesis failure, cell cannibalism (entosis), emperipolesis, reductive mitosis, budding, fragmentation, nucleophagy, and sporulation (asexual reproduction), leading to a quite unlimited potential for PGCCs to resist extinction and achieve immortalization [48,62], as shown in Figure 2.
Figure 2.
PGCC mediated tumor evolution. Cell division errors, such as mitotic arrest, can cause whole-genome duplications (WGD), which is represented by a reversible polyploidy, originated from biological processes such as endocycling, endomitosis, cell fusion, cellular cannibalism, emperipolesis, cytokinesis failure, reductive mitosis, budding, fragmentation (bursting), nucleophagy, and sporulation. Reversible polyploidy may create resistance to tumor extinction. Terms are shown in Box A1 (Appendix A).
Vladimir Niculescu has also highlighted the potential of “pre-existing PGCCs” (before response to therapies) that can turn into multinucleated genome repair structures (MGRNs) and, subsequently, originate PGCCs [38,63,64].
3.4. Genome Chaos
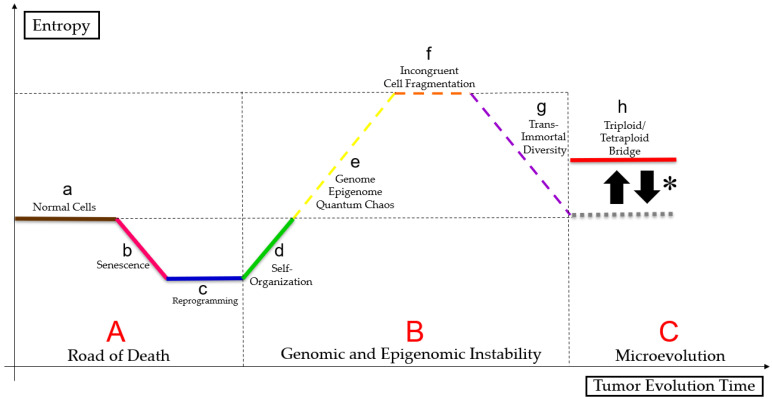
Liu [29] suggests that cell size regulation may control micro- and macroevolution, as somatic cells can follow the Waddington epigenetic landscape. PGCCs can overcome stress signals by rapidly adapting, living close to the genome, epigenome, and quantum chaos, altering cell fates, reprogramming, phylogenetically regressing, and senescing [48,65,66]. Therefore, that clonal selection enables better-adapted tumor cell survival, as shown in the Figure 3 (figure based on a bibliographic compilation by Erenpreisa and Cragg [51], Erenpreisa et al. [52], Heng and Heng [67], Heng and Heng [68], Heng and Heng [69], Liu et al. [70], Baramiya and Baranov [71] and Baramiya et al. [72]).
Figure 3.
Paradoxical cancer life cycle. Normal cells (a) follow a linear ontogenetic process towards cell death interspersed with senescence (b), in a pathway called “road of death” (A). Some tumor cells may undergo reprogramming (c) and self-organization (d) either by mitotic catastrophe or by neosis or by mitotic slippage, because of genome, epigenome and quantum chaos (e), resulting in changes in cell fate. Tumor undergoes a macroevolutionary process (genomic and epigenomic instability (B)). Polyploid tumor cells undergo epigenetic and genetic regression, causing incongruent cell fragmentation (f), to achieve trans-immortal diversity (access to immortalization capacity, resistance to extinction and heterogeneous profile in the formation of multilineages of more apt tumor aneuploid cells) (g) new tumor cells follow a Darwinian evolution (microevolution (C)) and maintain a “triploid/tetraploid bridge” (*), between states 2n and pn–“triploid/tetraploid bridge” (h). Terms shown in Box A1 (Appendix A).
3.5. PGCCs Characterization in Diverse Cancer Types
Experimental studies with tumor cell lines suggest that targeted PGCCs therapies in cancer may be more efficient than conventional therapies. Even under similar stresses, PGCCs may exhibit tumor specific features and responses, which are shown below according to tumor type:
3.5.1. Breast and Ovarian Cancer
Lin et al. [23] showed that chemotherapy-originated PGCCs upregulated the expression of Zinc finger E-box binding homeobox 1 (ZEB1) gene, correlating with metastases. Liu et al. [73] and Wang et al. [74], researching PGCCs in breast and ovarian cancer cell lines, identified a relationship between metastasis from resistant PGCCs offspring and higher lymph node grades, as well as an association between asymmetric divisions and tumor resistance.
3.5.2. Colorectal Cancer
Lopez-Sánchez et al. [75], Zhang et al. [21], Fei et al. [15], Fei et al. [76], Fei et al. [77], Fei et al. [78], and Fei et al. [6] highlighted that, in colorectal cancer cell lines, PGCCs were associated with overexpression of HIF-1α, CK7, cathepsin B, E-cadherin, fibronectin, Snail, Slug, Twist-1, Syncytin 1, CD9, CD47, JNK1, cyclin B1, S100A4, and downregulation of CDC25A, CDC25B, and CDC25C, affecting cell cycle regulation, vasculogenic mimicry, migration, and invasion.
3.5.3. Glioblastoma
Qu et al. [7] and Liu et al. [79], studying glioblastoma cell lines, observed a relationship between PGCCs’ number and higher tumor grade, hypoxia, red cytoplasmic inclusions, budding vasculogenic mimicry, tumor immunosuppressive microenvironment, and more aggressive phenotypes.
3.5.4. Lung Cancer
Tagal and Roth [14] and Glassmann et al. [5] showed an association between staurosporine generated PGCCs and polyploid and multinucleated growth traits in lung cancer cell lines.
3.5.5. Prostate Cancer and Melanoma
White-Gilbertson et al. [25,80], working with prostate cancer and melanoma cell lines, found that ASAH1 inhibition and cholesterol regulation are associated with PGCCs generation, being a bridge to tumor survival.
3.5.6. Only Ovarian Cancer
Zhang et al. [81], Lv et al. [82], Zhang et al. [83], Zhang et al. [84], Niu et al. [1], Niu, Mercado-Uribe, and Liu [49], and Liu et al. [85] revealed associations between PGCCs and cell cycle, motility, metabolism, vasculogenic mimetism, auto-renovation, nuclear fragmentation, and tumor aggressiveness using ovarian cancer cell lines.
3.5.7. Only Breast Cancer
Zhang, Mercado-Uribe, and Liu [13], Fei et al. [3], and Sirois et al. [24], studying breast cancer cell lines, suggested that PGCCs offspring can form organotypic structures in vitro, stromal transdifferentiation, chemoresistant cells, higher migratory capacity, and metabolic reprogramming.
Ultimately, the primary purposes of tumor polyploidization are to activate survival and aggressiveness (metastasis).
3.6. Autophagy, Senescence and PGCCs
Autophagy seems to be involved in the generation of PGCCs, since autophagy inhibitors before chemotherapy decreases the formation of PGCCs [86]. This process modulates PGCCs colony formation by stoppage, representing paradoxical roles both beneficial and harmful to PGCCs.
Senescence and PGCCs have been related through comparative transcriptome studies, revealing the altered expression of meiotic cell cycle genes, spermatogenesis, and EMT [87,88]. Senescence induced by chemotherapy can halter tumor proliferation, but this response also causes polyploidization, leading to PGCCs formation [89,90].
Cellular damage triggered by genotoxic stress induces senescence which, under the influence of senescence-associated secretory phenotype (SASP) and immune response evasion, facilitates reversible polyploidy through mitotic slippage, circumventing mitotic catastrophe and terminal senescence, followed by endoreplication and damage repair; this, in turn, directs new polyploid tumor cells to generate aneuploid offspring [45,50]. There mechanisms are still little understood, but they result in more aggressive tumor profiles [91,92].
4. Reaching New Paths
Histological, physiological, and morphological PGCCs studies used (i) CoCl2 and traditional cancer treatments for PGCCs induction [75,91]; (ii) classic tumor cell lineages [92,93]; (iii) PGCCs generated by budding [94,95]; and (iv) protein expression studies [96], showing that PGCCs’ acquired features are similar to classic tumor marks, described by Hanahan and Weinberg [97], Hanahan and Weinberg [98], and Hanahan [99], contributing to needed cancer traits for utter success [93].
The complexity of PGCCs motivated recent computational approaches, providing new insights into essential questions about such cells. Single cell [100], multi-omics (genomics, transcriptomics, proteomics, metabolomics, electroma) [101,102], bioinformatics, NGS [103], Big Data, and Artificial Intelligence [104], together with laboratory studies, have provided a better interpretation of polyploidy, elucidating its multiple cellular states [100], epigenetic and genetic profiles [101], spatial distributions [102], microenvironmental interactions [103], and translational advances [103,104].
5. Computational Perspectives
5.1. Bioinformatics/Computational Studies in Oncology
Modern oncology studies are using highly complex computational tools to improve data interpretation [105,106]. Furthermore, with the increase in evidence related to the role of the interaction between genes and proteins in tumor mechanisms, there was a need to integrate a new concept of medicine based on computational language to better explore all the processes involved in the emergence of cancer [107,108].
High-performance software is now used in genomics, proteomics, cell biology, physiology, pathology, therapeutics, clinical trials, and epidemiology [108]; multi-omic data processing still needs to translate in silico findings into in vivo scenarios, and is a future tool for precision medicine [107,108].
Bioinformatics also reduced the time from lab experiments to clinical oncology studies [106]. Computational biology provided a reduction in the time required to extract information due to the rapid growth of oncological data made available on online servers [106]. In this way, the increasing number of computational tools specialized in cancer has been associated with more detailed approaches to working with data [108,109]. In view of this, specialized bioinformatics in cancer are expected to play a central role in advances in translational oncology studies [106].
Novel Precision Medicine therapies, such as BRAF V600E inhibitors in patients with melanoma and PD1/PD-L1 immunotherapy for melanoma, lung, kidney, and other types of cancer [109,110], are enabling more efficient treatment with minimized side effects. In the following years, with the evolution of translational bioinformatics, advances in data sharing and integration are expected [111], improving collaborative and multidisciplinary teams [112], advances in data mining [113], expanding the ability to generate and analyze data [114], and planning therapies through better diagnosis [115] with the identification of new biomarkers and drug targets [116,117].
Artificial intelligence in oncology can refine, integrate, classify, and guide modeling prediction, classification, and screening of molecules with potential use in cancer treatment [118]. In this way, it is worth highlighting numerous other studies that express the application of computing in oncology, as shown in Table 1.
Table 1.
Computational studies in oncology.
| Cancer Type | Study Description | Author |
|---|---|---|
| Breast Cancer | Computational studies about differentially expressed genes, omics, and systems biology data provided the identification of new gene signatures-NUSAP1, MELK, CENPF, TOP2A, and PPARG-genes related to chemoresistance, potential biomarkers, and new therapeutic targets associated with tumor polyploidy. | Alam et al. [119], Kaur et al. [120], Mukherjee et al. [121], Yadav et al. [122], and Yang et al. [123] |
| Prostate Cancer | Systems biology and bioinformatics identification of key genes as biomarkers of diagnostics, prognosis, and treatment (EGFR, MYC, VEGFA, PTEN). | Khan et al. [124] |
| Melanoma | Mathematical modeling after therapeutic regression was able to identify a triphasic signaling pathway in tumor regression. | Kumari et al. [125] |
| Pan-cancer | Identified the relationship between SLC7A11 expression and tumor microenvironment, using bioinformatics in 20 tumors. | Lin et al. [126] |
| Colorectal and Uterine Cancer | Found potential genes and their signaling pathways using bioinformatics and systems biology. | Nguyen et al. [127] andReza et al. [128] |
| Lung Cancer | An integrated systems biology and bioinformatics approach provided the detection of genetic correlations between COVID-19 and small cell lung cancer and the interaction of biological pathways associated with tumor polyploidization. | Roudi et al. [129] and Zhuang et al. [130] |
5.2. Computational Studies about Polyploidization and PGCCs
Bioinformatics in oncology has highlighted new ways to elucidate the role of chromosomal aberrations, cell cycle errors, intratumoral heterogeneity, and tumor evolution in the face of new, more aggressive cancer phenotypes [131]. In parallel, multimodal, multi-omics, pan-cancer, and single cell analyses integrate data from diverse biological systems, clarifying tumor peculiarities, as for polyploidy [132].
Computational tools have been tested for different purposes to study cancer and polyploidy [133,134]. Among these approaches, we would like to highlight studies on nucleotide polymorphisms (SNPs), structural variants (SVs), unique somatic tumor-specific variants [135], genome annotation, mutation detection, evolutionary analysis, gene function, comparative genomics [136,137], identification of haplotypes, subgenomes, and assembly of whole genomes [137].
Among the computational approaches to tumor polyploidy, Anatskaya et al. [138] built a protein-protein interaction network (PPIs) of bivalent genes using the STRING server to investigate the regulation of polyploidy-associated gene expression, suggesting a role for polyploidy in the upregulation of oncogenes and downregulation of tumor suppressor genes. Furthermore, down-regulation of the DNA damage response (DDR) has been shown to increase damage tolerance and inhibit apoptosis.
Furthermore, Czarnecka-Herok et al. [88] performed gene enrichment analysis (GSEA) in doxorubicin-induced senescent/polyploid HCT116 and MCF-7 cell lines. In that study, an enrichment analysis revealed changes in genes involved in controlling meiosis and mitosis. Interestingly, enrichment of spermatogenesis genes was also observed, supporting the premise that genetic alterations in tumors may be associated with germline expression of normally silenced genes, a characteristic that may be related to tumor malignancy.
Additionally, Yang et al. [139] used GEO2R software, genetic ontology (GO), and the Kyoto Encyclopedia of Genes and Genomes (KEGG) to analyze databases for integrated annotation, visualization, and discovery (DAVID) of differentially expressed genes in cervical cancer cells using Affymetrix Human Genome arrays. In addition, they used the STRING software to build protein-protein interaction (PPI) and to analyze differentially expressed genes involved in cancer prognosis, with the help of UNLCAN and Oncomine browsers. In total, 57 differentially expressed genes were recognized, especially those enriched in the mitotic cell cycle G1/S transition, participating in cytokine-cytokine receptor interaction, including WD Repeat and HMG-Box DNA Binding Protein 1 (WDHD1), a gene involved in cancer promotion and polyploidy induction.
Moreover, using the Gene Expression Omnibus (GEO) database, Yan et al. [140] analyzed different gene expression profiles (GSE54238 and GSE84004) in the development of hepatocellular carcinoma from data taken from GPL16955 and GPL22109. STRING and Cytoscape software were used to visualize the integrated regulatory networks and raw data were analyzed using the multi-array averaging algorithm in the R Affy package. The PPI network they constructed highlighted five main core genes with the highest degree of connectivity, including AURKA. This gene showed a role in regulating the mitosis G2/M transition and its overexpression is associated with polyploidy and genomic instability.
In addition, Wang et al. [141] investigated the variation of GSE38241, GSE69223, GSE46602, and GSE104749 in the development of prostate cancer by using the GPL570 Affymetrix Human Genome U133 Plus 2.0 Array and GPL4133 Agilent-014850 Whole Human Genome microarray 4 × 44 K G4112F platforms. For this purpose, GEO software was used. Furthermore, a PPI network was also built using STRING and MCODE and the enrichment of the GO function was conducted with the DAVID software. The study revealed 20 genes related to mitosis and cell division and the process of carcinogenesis, including cyclin B1 (CCNB1). Elevated levels of cyclin B1 have been associated with polyploid cell uptake and shown to be a potential marker for diagnosing prostate cancer.
Another comparative study using bioinformatics for transcriptome analysis of polyploid versus diploid cells from normal mammalian tissues highlighted a potential downregulation of circadian clock genes by c-Myc (a cell cycle regulator), whereas polyploidization correlated with deletion of bivalent genes responsible for circadian rhythm regulation [142].
Structural variants (SVs) are crucial to understanding cancer polyploidy. Kosugi et al. [143] evaluated the performance of 69 SV detection algorithms using whole exome sequencing (WES) datasets, using the GRIDSS, Lumpy, SVseq2, SoftSV, Manta, and Wham algorithms to improve variant detection accuracy, focusing on SV size. This means that careful selection of algorithms for each type and size range of SVs is crucial for accurate SV detection. Therefore, it results in the most accurate information possible regarding the correlation between tumor structural alterations and polyploidization.
In addition to such basic research applications, computing linked to tumor polyploidy also highlights new paths for clinical studies and strengthened by the advancement of high-throughput technology integration. Among the main computational approaches, machine learning algorithms stand out [144], showing promise for the evolution of translational research in oncology and initiating a revolution in tumor mechanistic knowledge [145].
In a search on polyploidy clinical trials around the world at the website https://clinicaltrials.gov (accessed on 15 February 2023), only two studies were found for the terms “cancer” and “polyploidy”. The distribution of clinical trials around the globe demonstrated a study recruiting patients (USA) and another already completed (Germany), confirming that research on PGCCs needs to be further developed worldwide.
Although there are still obstacles to the implementation of computational PGCC analyses in clinical practice, some tools demonstrate new trends towards faster and more personalized diagnoses and treatments [146]. In addition, an expansion of PGCCs computational models will provide valuable data for the understanding of this complex phenotype and its translational relevance in cancer [147].
Multiple analyses, including multi-omics, comparative phylostratigraphy, mathematical modeling, and others, will provide a better understanding of cell subtype and state. Table 2 demonstrates the impact of computation in the area of tumor polyploidy.
Table 2.
Computational biology applied to polyploidy studies.
| Cancer Type | Study Description and Key Findings | Author |
|---|---|---|
| Ovary Cancer | Computational research based on NGS, total RNA, and microarray sequencing, using primary tumors, cell lines, and tumor chemoresistance highlighted the association with tumor polyploidy. | Adibi, Moein and Gheisari [148], Quinton et al. [149], Rohnalter et al. [150] |
| Lung Cancer | Investigated the role of natural and synthetic mutations in tumor migration and invasion. | Alwash et al. [151] |
| Several types of cancer | Molecular mechanisms associated with polyploidy, cell plasticity, unicellularity, energy metabolism, tumor DNA damage in tumors, phylogenetic approaches, and molecular modeling were used study the effects of PGCCs on gene expression, tumor microenvironment, and p53. | Anatskaya and Vinogradov [138], Anatskaya et al. [152], Anatskaya et al. [142], Kimmel et al. [153], and Potapova et al. [154] |
| Breast Cancer | Studied formation of PGCCs by mechanical stress.In silico studies to detect gene signatures related to PGCC formation. | Buehler et al. [155] and Rantala et al. [156] |
| Colorectal Cancer | S100A10 expression changes caused by differential SUMOylation during the migration of PGCCs. | Fu et al. [157] and Zhao et al. [158] |
| Gastric Cancer | MiRNA sequencing to study the role of epigenetic in regulation of Aurora kinase A (AURKA) expression. | Gomaa et al. [159] |
| Cervical cancer, Breast Cancer and Burkitt Lymphoma | In silico studies using Mitelman’s database to uncover PGCC’s role in DNA repair, genetic variation, and tumor survival. | Salmina et al. [160] |
| Prostate Cancer and Melanoma | Transcriptome analysis of PGCCs after ASAH1 treatment leading to cholesterol metabolism alterations. | White-Gilbertson et al. [80] |
| Nasopharyngeal Cancer | Induction of PGCC by autophagy. | You et al. [86] |
The computational era has provided simulation tools to better understand cell-to-cell interactions in normal and altered cell cycles, relying on specific and detailed cell cycle models, with the aim of understanding the dynamics of tumor formation and the potential outcomes associated with polyploidy [161]. Such innovations and improvements in oncology are only possible through the integration and multidisciplinarity of research groups.
As highlighted in this review, advances in computing permeate artificial intelligence approaches, machine learning algorithms [162], personalized targeting [163], and several other approaches. Broad access to low cost computational tools will expand specialized computational models in the study of cancer and PGCCs [162,163].
5.3. Future Directions
Applications of systems biology, computational biology, and bioinformatics, as shown by the studies in Table 1 and Table 2, allowed the analysis of high-throughput samples through artificial intelligence, machine learning, and other techniques [162,163], resulting in data about the identification of genetic signatures [164], new biomarkers [163,165], multiscale modeling [164,165], and visualization and interpretation of cellular data [164].
Research with artificial intelligence will embody the personalization of prevention, diagnosis, and therapy [162,166]. New biotechnological approaches will provide better cancer treatment, especially in cases of resistance, numbness, metastasis, and recurrence correlated to the polyploidization process. Molecular computers inside tumor cells acting as sensors, digital pathology, personalized biopsies, analysis of microscopic images and exams, synthesis of biological structures, and pharmacological bioassays will increase the computational area as required to solve demands in these complex studies [161,162,167].
In addition, machine learning will provide new answers for studies in oncology and tumor polyploidy (especially for the understanding of PGCCs), and will further extend its sophisticated approaches with studies in microrobotics for cell manipulation [168,169], ploidy inference based on co-sequencing of DNA and RNA in individual nuclei [169], multi-omics [170,171], NGS [170], single-cell [171], and other approaches capable of bringing new paths to the understanding of polyploidization in cancer (as indicates Figure 4).
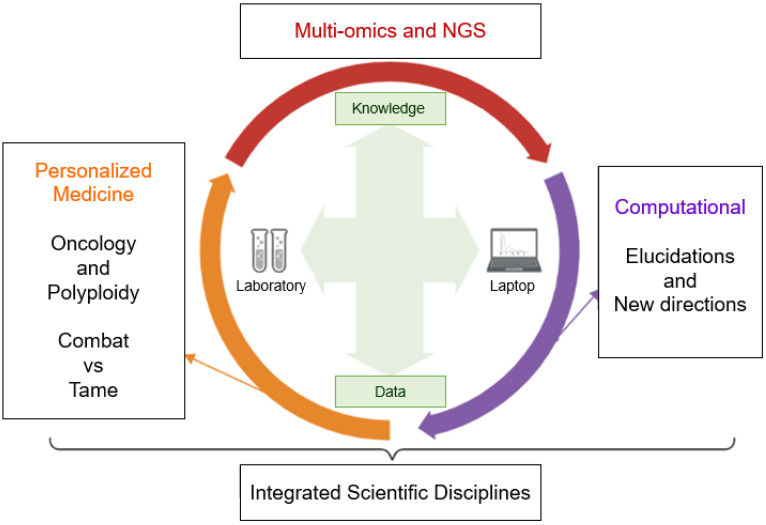
Figure 4.
Integrated scheme of computational resources in oncology and polyploidy. The biological computational era has provided numerous advances on the amount of biological data and their significance. Crosstalk between computing and research laboratory is needed to create personalized medicine through multi-omics. Note: created with BioRender.com (accessed on 15 March 2023).
Computational tools provide ideal support for understanding self-organization in face of cellular chaos, linked to the complex genomic functioning of the polyploid cells [172,173]. Single-cell RNA sequencing approaches will increasingly add new light to polyploidy, as shown in studies on hypertranscription [164,172,174]. In the long term, an increasingly prominent role for bioinformatics in elucidating polyploidy in tumors is expected. To achieve this goal, scientists from different fields are expected to come together to understand, tame, and fight cancer [172,173].
6. Conclusions
Polyploidization in tumors runs through multiple mechanisms phylogenetically shared between unicellular and multicellular organisms, resulting in evolutionary advantages for the tumor, thus reverberating an intrinsic complexity that is highly difficult to access through isolated studies. Thus, the need for integrated and multidisciplinary approaches capable of elucidating polyploidy in tumors becomes clear. Computational biology, bioinformatics, NGS, single-cell analysis, multi-omics studies, and systems biology reveal a growing and promising potential in the understanding of polyploidization as well as its innovative potentials to create personalized, comprehensive, and multifactorial therapies in cancer treatment.
Acknowledgments
We would like to thank Patricia A. Possik (Instituto Nacional de Câncer—INCA) and Roger Chammas (Instituto do Câncer de São Paulo—ICESP) for helping review the manuscript. In addition, thanks to Agnia A. Pinevich (Russian Research Center for Radiology and Surgical Technologies), Jekaterina Erenpreisa (Latvian Biomedical Research and Study Centre), Razmik Mirzayans (University of Alberta) and Vladimir F. Niculescu (University of Bucharest) for answering questions during the writing of this review.
Abbreviations
| ASAH1 | N-acylsphingosine amidohydrolase (acid ceramidase) 1 |
| ATP | Adenosine triphosphate |
| AURKA | Aurora kinase A |
| BRAF V600E | Mutation of the BRAF gene |
| CD47 | Integrin-associated protein |
| CD9 | Motility-Related Protein |
| CDC25A | M-phase inducer phosphatase 1 |
| CDC25B | M-phase inducer phosphatase 2 |
| CDC25C | M-phase inducer phosphatase 3 |
| CENPF | Centromere Protein F |
| CK7 | Cytokeratin 7 |
| CoCl2 | Cobalt chloride |
| CSC | Cancer stem cells |
| EGFR | Epidermal growth factor receptor |
| EMT | Epithelial-Mesenchymal Transition |
| ERK | Extracellular signal-regulated kinase |
| EZH2 | Enhancer of zeste homolog 2 |
| HIF-1α | Hypoxia-inducible factor-1 |
| JNK | c-Jun N-terminal kinase |
| JNK1 | c-Jun N-terminal kinase 1 |
| LOH | Loss of heterozygosity |
| MELK | Maternal embryonic leucine zipper kinase |
| MYC | MYC Proto-Oncogene |
| NUSAP1 | Nucleolar and spindle associated protein 1 |
| p38MAPK | p38 mitogen-activated protein kinase |
| PACCs | Poly-aneuploid cancer cells |
| PD1/PD-L1 | Programmed cell death protein-1 |
| PGCCs | Polyploid giant cancer cells |
| PPARG | Peroxisome proliferator activated receptor γ |
| PTEN | Phosphatase and tensin homologue |
| S100A4 | S100 calcium binding protein A4 |
| SKP2 | S-phase kinase associated protein 2 |
| SLC7A11 | solute carrier family 7 member 11 |
| Slug | Snail family transcriptional repressor 2 |
| Snail | Snail family transcriptional repressor 1 |
| SSP | Staurosporine |
| STC1 | Stanniocalcin-1 |
| TNBC | Triple negative breast cancer |
| TOP2A | DNA Topoisomerase II α |
| TPL | Triptolide |
| Twist-1 | Twist family bHLH transcription factor 1 |
| VEGFA | Vascular endothelial growth factor A |
| VM | Vasculogenic mimicry |
| WGDs | Whole-genome duplications |
| ZEB1 | Zinc finger E-box binding homeobox 1 |
Appendix A
Box A1. Important terms and definitions.
Aneuploidy: autosomal abnormality that occurs mainly during meiosis and affects the number of chromosomes.
Bivalent chromatin: segments of DNA linked to histone proteins that have epigenetic regulators repressors and activators in the same region. These regulators work to increase or silence gene expression.
Endocycle/Endocycling: nuclear genome replication in the absence of mitosis, which leads to high nuclear genetic content and polyploidy.
Endomitosis: chromosome duplication within the intact nuclear membrane, without telophase or cytokinesis, resulting in a single cell with twice the chromosomal content.
Endoreplication/Endoreduplication/Endopolyploidy (Mitotic Cycle variant forms): increased number of chromosomes in cells.
Euploidy: numerical chromosomal variation affecting the entire chromosomal set (n).
Incongruous partition: interconversion phase between ploidy and mitosis cycles capable of inducing depolyploidization.
Ploidy: complete numerical set of chromosomes in a cell (n).
Progressive Partial Endoreduplication/DNA Overreplication: process of almost perfect linearity between DNA content and the number of endoreduplication cycles.
Transcendent Regression: ability to recover primordial phylogenetic features due to deep homology between multicellular and unicellular organisms ((formation of PGCCs, polyaneuploid cancer cells (PACCs), multinucleated tumor cells/multinucleated genome repair structures (MNGCs), micronucleated tumor cells, cells tumor hybrids (cell fusion) and supergiant “nurse” cells, following the amoeba-cancer model and the cancer life cycle)).
Trans-Immortal Diversity: access to immortalization capacity (sustained by a constant renewal of telomerase function/Hayflick limit renewal), resistance to extinction and heterogeneous profile in the formation of multilineages of more apt tumor aneuploid cells after a process of depolyploidization (after ploidy cycle).
Types of endopolyploidy: endocycles, endomitosis and progressive partial endoreduplication.
Author Contributions
Conceptualization, M.C.C. and D.D.M.; methodology, M.C.C. and D.D.M.; data curation, M.C.C., D.D.M., L.N.R.A., A.S.S.Z., B.C.d.A., D.R.C.d.S., F.M.G., R.F.R.B. and R.S.d.R.T.; writing—original draft preparation, M.C.C., D.D.M. and R.F.R.B.; review and editing, M.C.C., D.D.M., E.C.F.C., E.F.d.C., E.d.V.W.d.S., F.d.P., G.L., S.S.B. and I.D.L.; translation, G.M.S., T.E.S.L., L.S.L. and I.D.L.; supervision, M.C.C., E.F.d.C., D.D.M. and I.D.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Niu N., Zhang J., Zhang N., Mercado-Uribe I., Tao F., Han Z., Pathak S., Multani A.S., Kuang J., Yao J., et al. Linking genomic reorganization to tumor initiation via the giant cell cycle. Oncogenesis. 2016;5:e281. doi: 10.1038/oncsis.2016.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Griffiths A.J., Wessler S.R., Lewontin R.C., Gelbart W.M., Suzuki D.T., Miller J.H. Introdução à Genética. Grupo GEN; Guanabara Koogan, SP, Brazil: 2022. pp. 1–768. [Google Scholar]
- 3.Fei F., Zhang D., Yang Z., Wang S., Wang X., Wu Z., Wu Q., Zhang S. The number of polyploid giant cancer cells and epithelial-mesenchymal transition-related proteins are associated with invasion and metastasis in human breast cancer. J. Exp. Clin. Cancer Res. 2015;34:158. doi: 10.1186/s13046-015-0277-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niculescu V.F. The reproductive life cycle of cancer: Hypotheses of cell of origin, TP53 drivers and stem cell conversions in the light of the atavistic cancer cell theory. Med. Hypotheses. 2019;123:19–23. doi: 10.1016/j.mehy.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Glassmann A., Garcia C.C., Janzen V., Kraus D., Veit N., Winter J., Probstmeier R. Staurosporine induces the generation of polyploid giant cancer cells in non-small-cell lung carcinoma A549 cells. Anal. Cell. Pathol. 2018;2018:7. doi: 10.1155/2018/1754085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fei F., Liu K., Li C., Du J., Wei Z., Li B., Li Y., Zhang Y., Zhang S. Molecular mechanisms by which S100A4 regulates the migration and invasion of PGCCs with their daughter cells in human colorectal cancer. Front. Oncol. 2020;10:182. doi: 10.3389/fonc.2020.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qu Y., Zhang L., Rong Z., He T., Zhang S. Number of glioma polyploid giant cancer cells (PGCCs) associated with vasculogenic mimicry formation and tumor grade in human glioma. J. Exp. Clin. Cancer Res. 2013;32:75. doi: 10.1186/1756-9966-32-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen J., Niu N., Zhang J., Qi L., Shen W., Donkena K.V., Feng Z., Liu J. Polyploid giant cancer cells (PGCCs): The evil roots of cancer. Curr. Cancer Drug Targets. 2019;19:360–367. doi: 10.2174/1568009618666180703154233. [DOI] [PubMed] [Google Scholar]
- 9.Liu G., Wang Y., Fei F., Wang X., Li C., Liu K., Du J., Cao Y., Zhang S. Clinical characteristics and preliminary morphological observation of 47 cases of primary anorectal malignant melanomas. Melanoma Res. 2018;28:592–599. doi: 10.1097/CMR.0000000000000491. [DOI] [PubMed] [Google Scholar]
- 10.Arun R.P., Sivanesan D., Patra B., Varadaraj S., Verma R.S. Simulated microgravity increases polyploid giant cancer cells and nuclear localization of YAP. Sci. Rep. 2019;9:10684. doi: 10.1038/s41598-019-47116-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu J. Seminars in Cancer Biology. Volume 60. Academic Press; Cambridge, MA, USA: 2020. The “life code”: A theory that unifies the human life cycle and the origin of human tumors; pp. 380–397. [DOI] [PubMed] [Google Scholar]
- 12.Pienta K.J., Hammarlund E.U., Austin R.H., Axelrod R., Brown J.S., Amend S.R. Seminars in Cancer Biology. Volume 81. Academic Press; Cambridge, MA, USA: 2020. Cancer cells employ an evolutionarily conserved polyploidization program to resist therapy; pp. 145–159. [DOI] [PubMed] [Google Scholar]
- 13.Zhang S., Mercado-Uribe I., Liu J. Tumor stroma and differentiated cancer cells can be originated directly from polyploid giant cancer cells induced by paclitaxel. Int. J. Cancer. 2014;134:508–518. doi: 10.1002/ijc.28319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tagal V., Roth M.G. Loss of Aurora kinase signaling allows lung cancer cells to adopt endoreplication and form polyploid giant cancer cells that resist antimitotic drugs. Cancer Res. 2021;81:400–413. doi: 10.1158/0008-5472.CAN-20-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fei F., Li C., Cao Y., Liu K., Du J., Gu Y., Wang X., Li Y., Zhang S. CK7 expression associates with the location, differentiation, lymph node metastasis, and the Dukes’ stage of primary colorectal cancers. J. Cancer. 2019;10:2510. doi: 10.7150/jca.29397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bharadwaj D., Parekh A., Das S., Jena B.C., Mandal M. Polyploid giant cancer cells induce growth arrest and cytoskeletal rearrangement in breast cancer cells. New Biotechnol. 2018;44:S141. doi: 10.1016/j.nbt.2018.05.1111. [DOI] [Google Scholar]
- 17.Mirzayans R., Andrais B., Murray D. Roles of polyploid/multinucleated giant cancer cells in metastasis and disease relapse following anticancer treatment. Cancers. 2018;10:118. doi: 10.3390/cancers10040118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mirzayans R., Murray D. Do TUNEL and other apoptosis assays detect cell death in preclinical studies? Int. J. Mol. Sci. 2020;21:9090. doi: 10.3390/ijms21239090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mirzayans R., Murray D. Intratumor heterogeneity and therapy resistance: Contributions of dormancy, apoptosis reversal (anastasis) and cell fusion to disease recurrence. Int. J. Mol. Sci. 2020;21:1308. doi: 10.3390/ijms21041308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bharadwaj D., Mandal M. Senescence in polyploid giant cancer cells: A road that leads to chemoresistance. Cytokine Growth Factor Rev. 2020;52:68–75. doi: 10.1016/j.cytogfr.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Zhang D., Yang X., Yang Z., Fei F., Li S., Qu J., Zhang M., Li Y., Zhang X., Zhang S. Daughter cells and erythroid cells budding from PGCCs and their clinicopathological significances in colorectal cancer. J. Cancer. 2017;8:469. doi: 10.7150/jca.17012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang H., Ma H., Yang X., Fan L., Tian S., Niu R., Yan M., Zheng M., Zhang S. Cell Fusion-Related Proteins and Signaling Pathways, and Their Roles in the Development and Progression of Cancer. Front. Cell Dev. Biol. 2021;9:809668. doi: 10.3389/fcell.2021.809668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin K.C., Torga G., Sun Y., Axelrod R., Pienta K.J., Sturm J.C., Austin R.H. The role of heterogeneous environment and docetaxel gradient in the emergence of polyploid, mesenchymal and resistant prostate cancer cells. Clin. Exp. Metastasis. 2019;36:97–108. doi: 10.1007/s10585-019-09958-1. [DOI] [PubMed] [Google Scholar]
- 24.Sirois I., Aguilar-Mahecha A., Lafleur J., Fowler E., Vu V., Scriver M., Buchanan M., Chabot C., Ramanathan A., Balachandran B., et al. A unique morphological phenotype in chemoresistant triple-negative breast cancer reveals metabolic reprogramming and PLIN4 expression as a molecular vulnerability. Mol. Cancer Res. 2019;17:2492–2507. doi: 10.1158/1541-7786.MCR-19-0264. [DOI] [PubMed] [Google Scholar]
- 25.White-Gilbertson S., Lu P., Jones C.M., Chiodini S., Hurley D., Das A., Delaney J.R., Norris J.S., Voelkel-Johnson C. Tamoxifen is a candidate first-in-class inhibitor of acid ceramidase that reduces amitotic division in polyploid giant cancer cells—Unrecognized players in tumorigenesis. Cancer Med. 2020;9:3142–3152. doi: 10.1002/cam4.2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Voelkel-Johnson C. Seminars in Cancer Biology. Volume 81. Academic Press; Cambridge, MA, USA: 2021. Sphingolipids in embryonic development, cell cycle regulation, and stemness–Implications for polyploidy in tumors; pp. 206–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shu Z., Row S., Deng W.-M. Endoreplication: The good, the bad, and the ugly. Trends Cell Biol. 2018;28:465–474. doi: 10.1016/j.tcb.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang D., Wang Y., Zhang S. Asymmetric cell division in polyploid giant cancer cells and low eukaryotic cells. BioMed Res. Int. 2014;2014:432652. doi: 10.1155/2014/432652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu J. Seminars in Cancer Biology. Volume 53. Academic Press; Cambridge, MA, USA: 2018. The dualistic origin of human tumors; pp. 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anatskaya O.V., Vinogradov A.E. Polyploidy as a Fundamental Phenomenon in Evolution, Development, Adaptation and Diseases. Int. J. Mol. Sci. 2022;23:3542. doi: 10.3390/ijms23073542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Archetti M. Polyploidy as an Adaptation against Loss of Heterozygosity in Cancer. Int. J. Mol. Sci. 2022;23:8528. doi: 10.3390/ijms23158528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu C.S., Lu W.H., Hung M.C., Huang Y.S., Chao H.W. From polyploidy to polyploidy reversal: Its role in normal and disease states. Trends Genet. 2022;38:991–995. doi: 10.1016/j.tig.2022.05.007. [DOI] [PubMed] [Google Scholar]
- 33.Bukkuri A., Pienta K.J., Austin R.H., Hammarlund E.U., Amend S.R., Brown J.S. A life history model of the ecological and evolutionary dynamics of polyaneuploid cancer cells. Sci. Rep. 2022;12:13713. doi: 10.1038/s41598-022-18137-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anatskaya O.V., Vinogradov A.E. Whole-Genome Duplications in Evolution, Ontogeny, and Pathology: Complexity and Emergency Reserves. Mol. Biol. 2021;55:813–827. doi: 10.1134/S0026893321050022. [DOI] [PubMed] [Google Scholar]
- 35.Lukow D.A., Sheltzer J.M. Chromosomal instability and aneuploidy as causes of cancer drug resistance. Trends Cancer. 2022;8:43–53. doi: 10.1016/j.trecan.2021.09.002. [DOI] [PubMed] [Google Scholar]
- 36.Mukherjee S., Ali A.M., Murty V.V., Raza A. Mutation in SF3B1 gene promotes formation of polyploid giant cells in Leukemia cells. Med. Oncol. 2022;39:65. doi: 10.1007/s12032-022-01652-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kasperski A. Life Entrapped in a Network of Atavistic Attractors: How to Find a Rescue. Int. J. Mol. Sci. 2022;23:4017. doi: 10.3390/ijms23074017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Niculescu V.F. Cancer genes and cancer stem cells in tumorigenesis: Evolutionary deep homology and controversies. Genes Dis. 2022;9:1234–1247. doi: 10.1016/j.gendis.2022.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amend S.R., Torga G., Lin K.C., Kostecka L.G., de Marzo A., Austin R.H., Pienta K.J. Polyploid giant cancer cells: Unrecognized actuators of tumorigenesis, metastasis, and resistance. Prostate. 2019;79:1489–1497. doi: 10.1002/pros.23877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.White-Gilbertson S., Voelkel-Johnson C. Giants and monsters: Unexpected characters in the story of cancer recurrence. Adv. Cancer Res. 2020;148:201–232. doi: 10.1016/bs.acr.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alameddine R.S., Hamieh L., Shamseddine A. From sprouting angiogenesis to erythrocytes generation by cancer stem cells: Evolving concepts in tumor microcirculation. BioMed Res. Int. 2014;2014:986768. doi: 10.1155/2014/986768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang Z., Yao H., Fei F., Li Y., Qu J., Li C., Zhang S. Generation of erythroid cells from polyploid giant cancer cells: Re-thinking about tumor blood supply. J. Cancer Res. Clin. Oncol. 2018;144:617–627. doi: 10.1007/s00432-018-2598-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Amend S.R., Pienta K.J. Ecology meets cancer biology: The cancer swamp promotes the lethal cancer phenotype. Oncotarget. 2015;6:9669. doi: 10.18632/oncotarget.3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu J., Niu N., Li X., Zhang X., Sood A.K. Seminars in Cancer Biology. Volume 81. Academic Press; Cambridge, MA, USA: 2021. The life cycle of polyploid giant cancer cell and dormancy in cancer: Opportunities for novel therapeutic interventions; pp. 132–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu J., Erenpreisa J., Sikora E. Seminars in Cancer Biology. Volume 81. Academic Press; Cambridge, MA, USA: 2021. Polyploid giant cancer cells: An emerging new field of cancer biology; pp. 1–4. [DOI] [PubMed] [Google Scholar]
- 46.Liu J. Seminars in Cancer Biology. Volume 81. Academic Press; Cambridge, MA, USA: 2021. Giant cells: Linking McClintock’s heredity to early embryogenesis and tumor origin throughout millennia of evolution on Earth; pp. 176–192. [DOI] [PubMed] [Google Scholar]
- 47.Song Y., Zhao Y., Deng Z., Zhao R., Huang Q. Stress-Induced Polyploid Giant Cancer Cells: Unique Way of Formation and Non-Negligible Characteristics. Front. Oncol. 2021;11:3390. doi: 10.3389/fonc.2021.724781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Erenpreisa J., Salmina K., Anatskaya O., Cragg M.S. Seminars in Cancer Biology. Volume 81. Academic Press; Cambridge, MA, USA: 2020. Paradoxes of cancer: Survival at the brink; pp. 119–131. [DOI] [PubMed] [Google Scholar]
- 49.Niu N., Mercado-Uribe I., Liu J. Dedifferentiation into blastomere-like cancer stem cells via formation of polyploid giant cancer cells. Oncogene. 2017;36:4887–4900. doi: 10.1038/onc.2017.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Erenpreisa J., Cragg M.S. Three steps to the immortality of cancer cells: Senescence, polyploidy and self-renewal. Cancer Cell Int. 2013;13:92. doi: 10.1186/1475-2867-13-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Erenpreisa J., Salmina K., Anatskaya O., Vinogradov A., Cragg M.S. The Enigma of cancer resistance to treatment. Organisms. J. Biol. Sci. 2022;5:71–75. [Google Scholar]
- 52.Mayfield-Jones D., Washburn J.D., Arias T., Edger P.P., Pires J.C., Conant G.C. Seminars in Cell & Developmental Biology. Volume 24. Academic Press; Cambridge, MA, USA: 2013. Watching the grin fade: Tracing the effects of polyploidy on different evolutionary time scales; pp. 320–331. [DOI] [PubMed] [Google Scholar]
- 53.Moein S., Adibi R., Meirelles L.d.S., Nardi N.B., Gheisari Y. Cancer regeneration: Polyploid cells are the key drivers of tumor progression. Biochim. Biophys. Acta (BBA)-Rev. Cancer. 2020;1874:188408. doi: 10.1016/j.bbcan.2020.188408. [DOI] [PubMed] [Google Scholar]
- 54.Dasari K., Somarelli J.A., Kumar S., Townsend J.P. The somatic molecular evolution of cancer: Mutation, selection, and epistasis. Prog. Biophys. Mol. Biol. 2021;165:56–65. doi: 10.1016/j.pbiomolbio.2021.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Somarelli J.A., Gardner H., Cannataro V.L., Gunady E.F., Boddy A.M., Johnson N.A., Fisk J.N., Gaffney S.G., Chuang J.H., Li S., et al. Molecular biology and evolution of cancer: From discovery to action. Mol. Biol. Evol. 2020;37:320–326. doi: 10.1093/molbev/msz242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dujon A.M., Aktipis A., Alix-Panabières C., Amend S.R., Boddy A.M., Brown J.S., Capp J.P., DeGregori J., Ewald P., Gatenby R., et al. Identifying key questions in the ecology and evolution of cancer. Evol. Appl. 2021;14:877–892. doi: 10.1111/eva.13190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Casás-Selves M., DeGregori J. How cancer shapes evolution and how evolution shapes cancer. Evol. Educ. Outreach. 2011;4:624–634. doi: 10.1007/s12052-011-0373-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fortunato A., Boddy A., Mallo D., Aktipis A., Maley C.C., Pepper J.W. Natural selection in cancer biology: From molecular snowflakes to trait hallmarks. Cold Spring Harb. Perspect. Med. 2017;7:a029652. doi: 10.1101/cshperspect.a029652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arendt D. The evolution of cell types in animals: Emerging principles from molecular studies. Nat. Rev. Genet. 2008;9:868–882. doi: 10.1038/nrg2416. [DOI] [PubMed] [Google Scholar]
- 60.Arendt D., Musser J.M., Baker C.V., Bergman A., Cepko C., Erwin D.H., Wagner G.P. The origin and evolution of cell types. Nat. Rev. Genet. 2016;17:744–757. doi: 10.1038/nrg.2016.127. [DOI] [PubMed] [Google Scholar]
- 61.Tarashansky A.J., Musser J.M., Khariton M., Li P., Arendt D., Quake S.R., Wang B. Mapping single-cell atlases throughout Metazoa unravels cell type evolution. eLife. 2021;10:e66747. doi: 10.7554/eLife.66747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Was H., Borkowska A., Olszewska A., Klemba A., Marciniak M., Synowiec A., Kieda C. Seminars in Cancer Biology. Volume 81. Academic Press; Cambridge, MA, USA: 2022. Polyploidy formation in cancer cells: How a Trojan horse is born; pp. 24–36. [DOI] [PubMed] [Google Scholar]
- 63.Niculescu V.F. aCLS cancers: Genomic and epigenetic changes transform the cell of origin of cancer into a tumorigenic pathogen of unicellular organization and lifestyle. Gene. 2020;726:144174. doi: 10.1016/j.gene.2019.144174. [DOI] [PubMed] [Google Scholar]
- 64.Niculescu V.F. Is an ancient genome repair mechanism the Trojan Horse of cancer. Nov Appro Can Study. 2021;5:555–557. doi: 10.31031/NACS.2021.05.000625. [DOI] [Google Scholar]
- 65.Baker S.G., Kramer B.S. Paradoxes in carcinogenesis: New opportunities for research directions. BMC Cancer. 2007;7:151. doi: 10.1186/1471-2407-7-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Baker S.G. Paradoxes in carcinogenesis should spur new avenues of research: An historical perspective. Disruptive Sci. Technol. 2012;1:100–107. doi: 10.1089/dst.2012.0011. [DOI] [Google Scholar]
- 67.Heng J., Heng H.H. Two-phased evolution: Genome chaos-mediated information creation and maintenance. Prog. Biophys. Mol. Biol. 2021;165:29–42. doi: 10.1016/j.pbiomolbio.2021.04.003. [DOI] [PubMed] [Google Scholar]
- 68.Heng J., Heng H.H. Seminars in Cancer Biology. Volume 81. Academic Press; Cambridge, MA, USA: 2020. Genome chaos: Creating new genomic information essential for cancer macroevolution; pp. 160–175. [DOI] [PubMed] [Google Scholar]
- 69.Heng J., Heng H.H. Genome Chaos, Information Creation, and Cancer Emergence: Searching for New Frameworks on the 50th Anniversary of the “War on Cancer”. Genes. 2021;13:101. doi: 10.3390/genes13010101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu J., Mercado-Uribe I., Niu N., Sun B., Kuang J., Zhang S. Re-thinking the concept of cancer stem cells: Polyploid giant cancer cells as mother cancer stem cells. Cancer Res. 2014;74:1917. doi: 10.1158/1538-7445.AM2014-1917. [DOI] [Google Scholar]
- 71.Baramiya M.G., Baranov E. From cancer to rejuvenation: Incomplete regeneration as the missing link (Part I: The same origin, different outcomes) Future Sci. AO. 2020;6:FSO450. doi: 10.2144/fsoa-2019-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baramiya M.G., Baranov E., Saburina I., Salnikov L. From cancer to rejuvenation: Incomplete regeneration as the missing link (part II: Rejuvenation circle) Future Sci. AO. 2020;6:FSO610. doi: 10.2144/fsoa-2020-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu K., Zheng M., Zhao Q., Zhang K., Li Z., Fu F., Zhang H., Du J., Li Y., Zhang S. Different p53 genotypes regulating different phosphorylation sites and subcellular location of CDC25C associated with the formation of polyploid giant cancer cells. J. Exp. Clin. Cancer Res. 2020;39:83. doi: 10.1186/s13046-020-01588-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang X., Zheng M., Fei F., Li C., Du J., Liu K., Li Y., Zhang S. EMT-related protein expression in polyploid giant cancer cells and their daughter cells with different passages after triptolide treatment. Med. Oncol. 2019;36:82. doi: 10.1007/s12032-019-1303-z. [DOI] [PubMed] [Google Scholar]
- 75.Lopez-Sanchez L.M., Jimenez C., Valverde A., Hernandez V., Penarando J., Martinez A., Lopez-Pedrera C., Muñoz-Castañeda J.R., Haba-Rodríguez J.R.D., Aranda E., et al. CoCl2, a mimic of hypoxia, induces formation of polyploid giant cells with stem characteristics in colon cancer. PLoS ONE. 2014;9:e99143. doi: 10.1371/journal.pone.0099143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fei F., Zhang M., Li B., Zhao L., Wang H., Liu L., Li Y., Ding P., Gu Y., Zhang X., et al. Formation of polyploid giant cancer cells involves in the prognostic value of neoadjuvant chemoradiation in locally advanced rectal cancer. J. Oncol. 2019;2019:2316436. doi: 10.1155/2019/2316436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fei F., Li C., Wang X., Du J., Liu K., Li B., Yao P., Li Y., Zhang S. Syncytin 1, CD9, and CD47 regulating cell fusion to form PGCCs associated with cAMP/PKA and JNK signaling pathway. Cancer Med. 2019;8:3047–3058. doi: 10.1002/cam4.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fei F., Qu J., Liu K., Li C., Wang X., Li Y., Zhang S. The subcellular location of cyclin B1 and CDC25 associated with the formation of polyploid giant cancer cells and their clinicopathological significance. Lab. Investig. 2019;99:483–498. doi: 10.1038/s41374-018-0157-x. [DOI] [PubMed] [Google Scholar]
- 79.Liu Y., Shi Y., Wu M., Liu J., Wu H., Xu C., Chen L. Hypoxia-induced polypoid giant cancer cells in glioma promote the transformation of tumor-associated macrophages to a tumor-supportive phenotype. CNS Neurosci. Ther. 2022;28:1326–1338. doi: 10.1111/cns.13892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.White-Gilbertson S., Lu P., Esobi I., Echesabal-Chen J., Mulholland P.J., Gooz M., Ogretmen B., Stamatikos A., Voelkel-Johnson C. Polyploid giant cancer cells are dependent on cholesterol for progeny formation through amitotic division. Sci. Rep. 2022;12:8971. doi: 10.1038/s41598-022-12705-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang S., Mercado-Uribe I., Hanash S., Liu J. iTRAQ-based proteomic analysis of polyploid giant cancer cells and budding progeny cells reveals several distinct pathways for ovarian cancer development. PLoS ONE. 2013;8:e80120. doi: 10.1371/journal.pone.0080120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lv H., Shi Y., Zhang L., Zhang D., Liu G., Yang Z., Li Y., Fei F., Zhang S. Polyploid giant cancer cells with budding and the expression of cyclin E, S-phase kinase-associated protein 2, stathmin associated with the grading and metastasis in serous ovarian tumor. BMC Cancer. 2014;14:576. doi: 10.1186/1471-2407-14-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang S., Mercado-Uribe I., Xing Z., Sun B., Kuang J., Liu J. of cancer stem-like cells through the formation of polyploid giant cancer cells. Oncogene. 2014;33:116–128. doi: 10.1038/onc.2013.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang L., Ding P., Lv H., Zhang D., Liu G., Yang Z., Li Y., Liu J., Zhang S. Number of polyploid giant cancer cells and expression of EZH2 are associated with VM formation and tumor grade in human ovarian tumor. BioMed Res. Int. 2014;2014:903542. doi: 10.1155/2014/903542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu K., Lu R., Zhao Q., Du J., Li Y., Zheng M., Zhang S. Association and clinicopathologic significance of p38MAPK-ERK-JNK-CDC25C with polyploid giant cancer cell formation. Med. Oncol. 2020;37:6. doi: 10.1007/s12032-019-1330-9. [DOI] [PubMed] [Google Scholar]
- 86.You B., Xia T., Gu M., Zhang Z., Zhang Q., Shen J., Fan Y., Yao H., Pan S., Lu Y., et al. AMPK–mTOR–Mediated Activation of Autophagy Promotes Formation of Dormant Polyploid Giant Cancer Cells. Cancer Res. 2022;82:846–858. doi: 10.1158/0008-5472.CAN-21-2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bowers R.R., Andrade M.F., Jones C.M., White-Gilbertson S., Voelkel-Johnson C., Delaney J.R. Autophagy modulating therapeutics inhibit ovarian cancer colony generation by polyploid giant cancer cells (PGCCs) BMC Cancer. 2022;22:410. doi: 10.1186/s12885-022-09503-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Czarnecka-Herok J., Sliwinska M.A., Herok M., Targonska A., Strzeszewska-Potyrala A., Bojko A., Wolny A., Mosieniak G., Sikora E. Therapy-Induced Senescent/Polyploid Cancer Cells Undergo Atypical Divisions Associated with Altered Expression of Meiosis, Spermatogenesis and EMT Genes. Int. J. Mol. Sci. 2022;23:8288. doi: 10.3390/ijms23158288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ye J.C., Horne S., Zhang J.Z., Jackson L., Heng H.H. Therapy induced genome chaos: A novel mechanism of rapid cancer drug resistance. Front. Cell Dev. Biol. 2021;9:676344. doi: 10.3389/fcell.2021.676344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Paul D. The systemic hallmarks of cancer. J. Cancer Metastasis Treat. 2020;6:29. doi: 10.20517/2394-4722.2020.63. [DOI] [Google Scholar]
- 91.Zhang J., Qiao Q., Xu H., Zhou R., Liu X. Seminars in Cancer Biology. Volume 81. Academic Press; Cambridge, MA, USA: 2021. Human cell polyploidization: The good and the evil; pp. 54–63. [DOI] [PubMed] [Google Scholar]
- 92.Zhang S., Xu X., Zhu S., Liu J. PGCCS generating erythrocytes to form VM structure contributes to tumor blood supply. BioMed Res. Int. 2015;2015:402619. doi: 10.1155/2015/402619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang S., Zhang D., Yang Z., Zhang X. Tumor budding, micropapillary pattern, and polyploidy giant cancer cells in colorectal cancer: Current status and future prospects. Stem Cells Int. 2016;2016:4810734. doi: 10.1155/2016/4810734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang Z., Feng X., Deng Z., Cheng J., Wang Y., Zhao M., Zhao Y., He S., Huang Q. Irradiation-induced polyploid giant cancer cells are involved in tumor cell repopulation via neosis. Mol. Oncol. 2021;15:2219–2234. doi: 10.1002/1878-0261.12913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu H.T., Xia T., You Y.W., Zhang Q.C., Ni H.S., Liu Y.F., Liu Y.R., Xu Y.Q., You B., Zhang Z.X. Characteristics and clinical significance of polyploid giant cancer cells in laryngeal carcinoma. Laryngoscope Investig. Otolaryngol. 2021;6:1228–1234. doi: 10.1002/lio2.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu L.L., Long Z.J., Wang L.X., Zheng F.M., Fang Z.G., Yan M., Xu D.F., Chen J.J., Wang S.W., Lin D.J., et al. Inhibition of mTOR Pathway Sensitizes Acute Myeloid Leukemia Cells to Aurora Inhibitors by Suppression of Glycolytic Metabolism. Mol. Cancer Res. 2013;11:1326–1336. doi: 10.1158/1541-7786.MCR-13-0172. [DOI] [PubMed] [Google Scholar]
- 97.Hanahan D., Weinberg R.A. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/S0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 98.Hanahan D., Weinberg R.A. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 99.Hanahan D. Hallmarks of cancer: New dimensions. Cancer Discov. 2022;12:31–46. doi: 10.1158/2159-8290.CD-21-1059. [DOI] [PubMed] [Google Scholar]
- 100.Nam A.S., Chaligne R., Landau D.A. Integrating genetic and non-genetic determinants of cancer evolution by single-cell multi-omics. Nat. Rev. Genet. 2021;22:3–18. doi: 10.1038/s41576-020-0265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hasanzad M., Sarhangi N., Ehsani Chimeh S., Ayati N., Afzali M., Khatami F., Nikfar S., Aghaei Meybodi H.R. Precision medicine journey through omics approach. J. Diabetes Metab. Disord. 2021;21:881–888. doi: 10.1007/s40200-021-00913-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lewis S.M., Asselin-Labat M.L., Nguyen Q., Berthelet J., Tan X., Wimmer V.C., Merino D., Rogers K.L., Naik S.H. Spatial omics and multiplexed imaging to explore cancer biology. Nat. Methods. 2021;18:997–1012. doi: 10.1038/s41592-021-01203-6. [DOI] [PubMed] [Google Scholar]
- 103.Dotolo S., Esposito A.R., Roma C., Guido D., Preziosi A., Tropea B., Palluzzi F., Giacò L., Normanno N. Bioinformatics: From NGS Data to Biological Complexity in Variant Detection and Oncological Clinical Practice. Biomedicines. 2022;10:2074. doi: 10.3390/biomedicines10092074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jiang P., Sinha S., Aldape K., Hannenhalli S., Sahinalp C., Ruppin E. Big data in basic and translational cancer research. Nat. Rev. Cancer. 2022;22:625–639. doi: 10.1038/s41568-022-00502-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bourke P.M., Voorrips R.E., Visser R.G., Maliepaard C. Tools for genetic studies in experimental populations of polyploids. Front. Plant Sci. 2018;9:513. doi: 10.3389/fpls.2018.00513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Desany B., Zhang Z. Bioinformatics and cancer target discovery. Drug Discov. Today. 2004;9:795–802. doi: 10.1016/S1359-6446(04)03224-6. [DOI] [PubMed] [Google Scholar]
- 107.Wu D., Rice C.M., Wang X. Cancer bioinformatics: A new approach to systems clinical medicine. BMC Bioinform. 2012;1:71. doi: 10.1186/1471-2105-13-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dopazo J. Bioinformatics and cancer: An essential alliance. Clin. Transl. Oncol. 2006;8:409–415. doi: 10.1007/s12094-006-0194-6. [DOI] [PubMed] [Google Scholar]
- 109.Zheng H., Zhang G., Zhang L., Wang Q., Li H., Han Y., Xie L., Yan Z., Li Y., An Y., et al. Comprehensive review of web servers and bioinformatics tools for cancer prognosis analysis. Front. Oncol. 2020;10:68. doi: 10.3389/fonc.2020.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gómez-López G., Valencia A. Bioinformatics and cancer research: Building bridges for translational research. Clin. Transl. Oncol. 2008;10:85–95. doi: 10.1007/s12094-008-0161-5. [DOI] [PubMed] [Google Scholar]
- 111.Menyhárt O., Győrffy B. Multi-omics approaches in cancer research with applications in tumor subtyping, prognosis, and diagnosis. Comput. Struct. Biotechnol. J. 2021;19:949–960. doi: 10.1016/j.csbj.2021.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Brenner C. Applications of bioinformatics in cancer. Cancers. 2019;11:1630. doi: 10.3390/cancers11111630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Voshall A., Moriyama E.N. Next-generation transcriptome assembly and analysis: Impact of ploidy. Methods. 2020;1:14–24. doi: 10.1016/j.ymeth.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 114.Jiménez-Santos M.J., García-Martín S., Fustero-Torre C., Di Domenico T., Gómez-López G., Al-Shahrour F. Bioinformatics roadmap for therapy selection in cancer genomics. Mol. Oncol. 2022;16:3881–3908. doi: 10.1002/1878-0261.13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hanauer D.A., Rhodes D.R., Sinha-Kumar C., Chinnaiyan A.M. Bioinformatics approaches in the study of cancer. Curr. Mol. Med. 2007;7:133–141. doi: 10.2174/156652407779940431. [DOI] [PubMed] [Google Scholar]
- 116.Hernández-Lemus E., Martínez-García M. Pathway-based drug-repurposing schemes in cancer: The role of translational bioinformatics. Front. Oncol. 2021;10:605680. doi: 10.3389/fonc.2020.605680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nasser F.K., Behadili S.F. A Review of Data Mining and Knowledge Discovery Approaches for Bioinformatics. Iraqi J. Sci. 2022;63:3169–3188. doi: 10.24996/ijs.2022.63.7.37. [DOI] [Google Scholar]
- 118.Yang H.H., Lee M.P. Application of bioinformatics in cancer epigenetics. Ann. N. Y. Acad. Sci. 2004;1020:67–76. doi: 10.1196/annals.1310.008. [DOI] [PubMed] [Google Scholar]
- 119.Alam M.S., Sultana A., Reza M.S., Amanullah M., Kabir S.R., Mollah M.N.H. Integrated bioinformatics and statistical approaches to explore molecular biomarkers for breast cancer diagnosis, prognosis and therapies. PLoS ONE. 2022;17:e0268967. doi: 10.1371/journal.pone.0268967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kaur B., Mukhlis Y., Natesh J., Penta D., Musthapa Meeran S. Identification of hub genes associated with EMT-induced chemoresistance in breast cancer using integrated bioinformatics analysis. Gene. 2022;809:146016. doi: 10.1016/j.gene.2021.146016. [DOI] [PubMed] [Google Scholar]
- 121.Mukherjee N., Browne A., Ivers L., Santra T., Cremona M., Hennessy B.T., O’Donovan N., Crown J., Kolch W., Fey D., et al. A Systems Biology Approach to Investigate Kinase Signal Transduction Networks That Are Involved in Triple Negative Breast Cancer Resistance to Cisplatin. J. Pers. Med. 2022;12:1277. doi: 10.3390/jpm12081277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yadav D.K., Sharma A., Dube P., Shaikh S., Vaghasia H., Rawal R.M. Identification of crucial hub genes and potential molecular mechanisms in breast cancer by integrated bioinformatics analysis and experimental validation. Comput. Biol. Med. 2022;149:106036. doi: 10.1016/j.compbiomed.2022.106036. [DOI] [PubMed] [Google Scholar]
- 123.Yang G., Lu T., Weisenberger D.J., Liang G. The Multi-Omic Landscape of Primary Breast Tumors and Their Metastases: Expanding the Efficacy of Actionable Therapeutic Targets. Genes. 2022;13:1555. doi: 10.3390/genes13091555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Khan M.M., Mohsen M.T., Malik M.Z., Bagabir S.A., Alkhanani M.F., Haque S., Serajuddin M., Bharadwaj M. Identification of Potential Key Genes in Prostate Cancer with Gene Expression, Pivotal Pathways and Regulatory Networks Analysis Using Integrated Bioinformatics Methods. Genes. 2022;13:655. doi: 10.3390/genes13040655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kumari B., Sakode C., Raghavendran L., Kumar Roy P. Systems biology basis of permanent tumor regression with normal tissue protection: Experimentally validated signaling pathway framework. ASCO Annu. Meet. I. 2022;40:e15066. doi: 10.1200/JCO.2022.40.16_suppl.e15066. [DOI] [Google Scholar]
- 126.Lin Y., Dong Y., Liu W., Fan X., Sun Y. Pan-Cancer Analyses Confirmed the Ferroptosis-Related Gene SLC7A11 as a Prognostic Biomarker for Cancer. Int. J. Gen. Med. 2022;15:2501. doi: 10.2147/IJGM.S341502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nguyen T.B., Do D.N., Nguyen-Thi M.L., Hoang-The H., Tran T.T., Nguyen-Thanh T. Identification of potential crucial genes and key pathways shared in Inflammatory Bowel Disease and cervical cancer by machine learning and integrated bioinformatics. Comput. Biol. Med. 2022;149:105996. doi: 10.1016/j.compbiomed.2022.105996. [DOI] [PubMed] [Google Scholar]
- 128.Reza M.S., Harun-Or-Roshid M., Islam M.A., Hossen M.A., Hossain M.T., Feng S., Xi W., Mollah M.N.H., Wei Y. Bioinformatics Screening of Potential Biomarkers from mRNA Expression Profiles to Discover Drug Targets and Agents for Cervical Cancer. Int. J. Mol. Sci. 2022;23:3968. doi: 10.3390/ijms23073968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Roudi R., Beikzadeh B., Roviello G., D’angelo A., Hadizadeh M. Identification of hub genes, modules and biological pathways associated with lung adenocarcinoma: A system biology approach. Gene Rep. 2022;27:101638. doi: 10.1016/j.genrep.2022.101638. [DOI] [Google Scholar]
- 130.Zhuang Z., Zhong X., Chen Q., Chen H., Liu Z. Bioinformatics and System Biology Approach to Reveal the Interaction Network and the Therapeutic Implications for Non-Small Cell Lung Cancer Patients With COVID-19. Front. Pharmacol. 2022;13:857730. doi: 10.3389/fphar.2022.857730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shimizu K.K. Robustness and the generalist niche of polyploid species: Genome shock or gradual evolution? Curr. Opin. Plant Biol. 2022;69:102292. doi: 10.1016/j.pbi.2022.102292. [DOI] [PubMed] [Google Scholar]
- 132.Baudoin N.C., Bloomfield M. Karyotype aberrations in action: The evolution of cancer genomes and the tumor microenvironment. Genes. 2021;12:558. doi: 10.3390/genes12040558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kaigorodova E.V., Kozik A.V., Zavaruev I.S., Grishchenko M.Y. Hybrid/Atypical Forms of Circulating Tumor Cells: Current State of the Art. Biochemistry. 2022;87:380–390. doi: 10.1134/S0006297922040071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Soraggi S., Rhodes J., Altinkaya I., Tarrant O., Balloux F., Fisher M.C., Fumagalli M. HMMploidy: Inference of ploidy levels from short-read sequencing data. Peer Community J. 2022;2:e60. doi: 10.24072/pcjournal.178. [DOI] [Google Scholar]
- 135.Van Belzen I.A.E.M., Schönhuth A., Kemmeren P., Hehir-Kwa J.Y. Structural variant detection in cancer genomes: Computational challenges and perspectives for precision oncology. NPJ Precis. Oncol. 2021;5:15. doi: 10.1038/s41698-021-00155-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Spence T., Dubuc A.M. Copy Number Analysis in Cancer Diagnostic Testing. Clin. Lab. Med. 2022;42:451–468. doi: 10.1016/j.cll.2022.05.003. [DOI] [PubMed] [Google Scholar]
- 137.Zhang T., Zhou J., Gao W., Jia Y., Wei Y., Wang G. Complex genome assembly based on long-read sequencing. Brief. Bioinform. 2022;23:bbac305. doi: 10.1093/bib/bbac305. [DOI] [PubMed] [Google Scholar]
- 138.Anatskaya O.V., Vinogradov A.E. Polyploidy and Myc Proto-Oncogenes Promote Stress Adaptation via Epigenetic Plasticity and Gene Regulatory Network Rewiring. Int. J. Mol. Sci. 2022;23:9691. doi: 10.3390/ijms23179691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yang H.J., Xue J.M., Li J., Wan L.H., Zhu Y.X. Identification of key genes and pathways of diagnosis and prognosis in cervical cancer by bioinformatics analysis. Mol. Genet. Genom. Med. 2020;8:e1200. doi: 10.1002/mgg3.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yan G., Liu Z. Identification of differentially expressed genes in hepatocellular carcinoma by integrated bioinformatic analysis. bioRxiv. 2019:570846. doi: 10.1101/570846. [DOI] [Google Scholar]
- 141.Wang Y., Wang J., Yan K., Lin J., Zheng Z., Bi J. Identification of core genes associated with prostate cancer progression and outcome via bioinformatics analysis in multiple databases. PeerJ. 2020;8:e8786. doi: 10.7717/peerj.8786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Anatskaya O.V., Vinogradov A.E., Vainshelbaum N.M., Giuliani A., Erenpreisa J. Phylostratic shift of whole-genome duplications in normal mammalian tissues towards unicellularity is driven by developmental bivalent genes and reveals a link to cancer. Int. J. Mol. Sci. 2020;21:8759. doi: 10.3390/ijms21228759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kosugi S., Momozawa Y., Liu X., Terao C., Kubo M., Kamatani Y. Comprehensive evaluation of structural variation detection algorithms for whole genome sequencing. Genome Biol. 2019;20:117. doi: 10.1186/s13059-019-1720-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rahman M.M., Islam M.R., Rahman F., Rahaman M.S., Khan M.S., Abrar S., Ray T.K., Uddin M.B., Kali M.S.K., Dua K., et al. Emerging promise of computational techniques in anti-cancer research: At a glance. Bioengineering. 2022;9:335. doi: 10.3390/bioengineering9080335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lobato-Delgado B., Priego-Torres B., Sanchez-Morillo D. Combining Molecular, Imaging, and Clinical Data Analysis for Predicting Cancer Prognosis. Cancers. 2022;14:3215. doi: 10.3390/cancers14133215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Collin C.B., Gebhardt T., Golebiewski M., Karaderi T., Hillemanns M., Khan F.M., Salehzadeh-Yazdi A., Kirschner M., Krobitsch S., EU-STANDS4PM consortium et al. Computational models for clinical applications in personalized medicine—Guidelines and recommendations for data integration and model validation. J. Pers. Med. 2022;12:166. doi: 10.3390/jpm12020166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Shao D., Dai Y., Li N., Cao X., Zhao W., Cheng L., Rong Z., Huang L., Wang Y., Zhao J. Artificial intelligence in clinical research of cancers. Brief. Bioinform. 2022;23:bbab523. doi: 10.1093/bib/bbab523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Adibi R., Moein S., Gheisari Y. Cisplatin resistant ovarian cancer cells reveal a polyploid phenotype with remarkable activation of nuclear processes. Res. Sq. 2021;1:rs-440506. doi: 10.4103/abr.abr_348_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Quinton R.J., DiDomizio A., Vittoria M.A., Kotýnková K., Ticas C.J., Patel S., Koga Y., Vakhshoorzadeh J., Hermance N., Kuroda T., et al. Whole-genome doubling confers unique genetic vulnerabilities on tumour cells. Nature. 2021;590:492–497. doi: 10.1038/s41586-020-03133-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rohnalter V., Roth K., Finkernagel F., Adhikary T., Obert J., Dorzweiler K., Bensberg M., Müller-Brüsselbach S., Müller R. A multi-stage process including transient polyploidization and EMT precedes the emergence of chemoresistent ovarian carcinoma cells with a dedifferentiated and pro-inflammatory secretory phenotype. Oncotarget. 2015;6:40005. doi: 10.18632/oncotarget.5552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Alwash B.H., Al-Rubaye J.R.A., Alaaraj M.M., Ebrahim A.Y. Cancer Bioinformatics. Intechopen; London, UK: 2022. Control of Cytoskeletal Dynamics in Cancer through a Combination of Cytoskeletal Components; pp. 167–211. Chapter 3. [Google Scholar]
- 152.Anatskaya O.V., Erenpreisa J.A., Nikolsky N.N., Vinogradov A.E. Pairwise comparison of mammalian transcriptomes associated with the effect of polyploidy on the expression activity of developmental gene modules. Cell Tissue Biol. 2016;10:122–132. doi: 10.1134/S1990519X16020024. [DOI] [Google Scholar]
- 153.Kimmel G.J., Dane M., Heiser L.M., Altrock P.M., Andor N. Integrating mathematical modeling with high-throughput imaging explains how polyploid populations behave in nutrient-sparse environments. Cancer Res. 2020;80:5109–5120. doi: 10.1158/0008-5472.CAN-20-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Potapova T.A., Seidel C.W., Box A.C., Rancati G., Li R. Transcriptome analysis of tetraploid cells identifies cyclin D2 as a facilitator of adaptation to genome doubling in the presence of p53. Mol. Biol. Cell. 2016;27:3065–3084. doi: 10.1091/mbc.e16-05-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Buhler A., Kruger R., Monavari M., Fuentes-Chandia M., Palmisano R., Schodel J., Boccaccini A.R., Boberhoff A.K., Kappelmann-Fenzl M., Letort G., et al. When Mechanical Stress Matters: Generation of Polyploid Giant Cancer Cells in Tumor-like Microcapsules. BioRxiv. 2022 doi: 10.1101/2022.09.22.508846. [DOI] [Google Scholar]
- 156.Rantala J.K., Edgren H., Lehtinen L., Wolf M., Kleivi K., Vollan H.K., Aaltola A.R., Laasola P., Kilpinen S., Saviranta P., et al. Integrative functional genomics analysis of sustained polyploidy phenotypes in breast cancer cells identifies an oncogenic profile for GINS2. Neoplasia. 2010;12:877-IN14. doi: 10.1593/neo.10548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Fu F., Chen L., Yang X., Fan L., Zhang M., Chen S., Zheng M., Gao M., Zhang S. SPLK4 is a key molecule in the formation of PGCCs and promotes invasion and migration of progeny cells derived from PGCCs. J. Cancer. 2022;13:2954. doi: 10.7150/jca.74211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhao Q., Zhang K., Li Z., Zhang H., Fu F., Fu J., Zheng M., Zhang S. High migration and invasion ability of PGCCs and their daughter cells associated with the nuclear localization of S100A10 modified by SUMOylation. Front. Cell Dev. Biol. 2021;9:696871. doi: 10.3389/fcell.2021.696871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gomaa A., Peng D., Chen Z., Soutto M., Abouelezz K., Corvalan A., El-Rifai W. Epigenetic regulation of AURKA by miR-4715-3p in upper gastrointestinal cancers. Sci. Rep. 2019;9:1–11. doi: 10.1038/s41598-019-53174-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Salmina K., Gerashchenko B.I., Hausmann M., Vainshelbaum N.M., Zayakin P., Erenpreiss J., Freivalds T., Cragg M.S., Erenpreisa J. When three isn’ta crowd: A digyny concept for treatment-resistant, near-triploid human cancers. Genes. 2019;10:2019. doi: 10.3390/genes10070551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hambali M.A., Oladele T.O., Adewole K.S. Microarray cancer feature selection: Review, challenges and research directions. Int. J. Cogn. Comput. Eng. 2020;1:78–97. doi: 10.1016/j.ijcce.2020.11.001. [DOI] [Google Scholar]
- 162.Sebastian A.M., Peter D. Artificial Intelligence in Cancer Research: Trends, Challenges and Future Directions. Life. 2022;12:1991. doi: 10.3390/life12121991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Csikasz-Nagy A. Computational systems biology of the cell cycle. Brief. Bioinform. 2009;10:424–434. doi: 10.1093/bib/bbp005. [DOI] [PubMed] [Google Scholar]
- 164.Malhotra A., Wang Y., Waters J., Chen K., Meric-Bernstam F., Hall I.M., Navin N.E. Ploidy-Seq: Inferring mutational chronology by sequencing polyploid tumor subpopulations. Genome Med. 2015;7:6. doi: 10.1186/s13073-015-0127-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Peng H., Tan H., Zhao W., Jin G., Sharma S., Xing F., Watabe K., Zhou X. Computational systems biology in cancer brain metastasis. Front. Biosci. 2016;8:169. doi: 10.2741/s456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Arjmand B., Hamidpour S.K., Tayanloo-Beik A., Goodarzi P., Aghayan H.R., Adibi H., Larijani B. Machine learning: A new prospect in multi-omics data analysis of cancer. Front. Genet. 2022;13:76. doi: 10.3389/fgene.2022.824451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Brown J.B., Okuno Y. Systems biology and systems chemistry: New directions for drug discovery. Chem. Biol. 2012;19:23–28. doi: 10.1016/j.chembiol.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 168.Agarwal D., Thakur A.D., Thakur A. A feedback-based manoeuvre planner for nonprehensile magnetic micromanipulation of large microscopic biological objects. Robot. Auton. Syst. 2022;148:103941. doi: 10.1016/j.robot.2021.103941. [DOI] [Google Scholar]
- 169.Olsen T.R., Talla P., Furnari J., Bruce J.N., Canoll P., Zha S., Sims P.A. Scalable co-sequencing of RNA and DNA from individual nuclei. bioRxiv. 2023;2023:527940. [Google Scholar]
- 170.Zhang C.Z., Pellman D. Cancer genomic rearrangements and copy number alterations from errors in cell division. Annu. Rev. Cancer Biol. 2022;6:245–268. doi: 10.1146/annurev-cancerbio-070620-094029. [DOI] [Google Scholar]
- 171.Funnell T., O’Flanagan C.H., Williams M.J., McPherson A., McKinney S., Kabeer F., Lee H., Salehi S., Vázquez-García I., Shi H., et al. Single-cell genomic variation induced by mutational processes in cancer. Nature. 2022;612:106–115. doi: 10.1038/s41586-022-05249-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Erenpreisa J., Giuliani A., Yoshikawa K., Falk M., Hildenbrand G., Salmina K., Freivalds T., Vainshelbaum N., Weidner J., Sievers A., et al. Spatial-Temporal Genome Regulation in Stress-Response and Cell-Fate Change. Int. J. Mol. Sci. 2023;24:2658. doi: 10.3390/ijms24032658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Mirzayans R., Murray D. What Are the Reasons for Continuing Failures in Cancer Therapy? Are Misleading/Inappropriate Preclinical Assays to Be Blamed? Might Some Modern Therapies Cause More Harm than Benefit? Int. J. Mol. Sci. 2022;23:13217. doi: 10.3390/ijms232113217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kim Y.K., Cho B., Cook D.P., Trcka D., Wrana J.L., Ramalho-Santos M. Absolute scaling of single-cell transcriptomes identifies pervasive hypertranscription in adult stem and progenitor cells. Cell Rep. 2023;42:111978. doi: 10.1016/j.celrep.2022.111978. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.