Abstract
Background
To prevent importations of wild polioviruses into a polio free region a high level of population immunity must be kept. Standard methodology for determination of polio antibodies is a feature aimed at obtaining consistent results. An International Standard Serum for polio antibodies exists, but no protective level in International Units is defined.
Methods
A representative study was carried out in order to determine the serological status against poliomyelitis in Germany (n = 2564, age 18–79 years). Furthermore, sera from persons aged less than 18 years were included (n = 881). Microneutralization test has been used for determination of antibody levels. Results have been expressed in International Units.
Results
The results of this study indicate that the cut-off level for polio antibodies is 0.075 IU/ml for Polio 1, 0.180 IU/ml for Polio 2 and 0.080 IU/ml for Polio 3. Neutralizing antibodies against poliovirus type 1, 2 and 3 were detected in 96.2%, 96.8% and 89.6% of samples, respectively.
Conclusions
Overall, this seroprevalence indicates a very high level of immunity of the general population. It must be kept after the switch of immunization strategy from attenuated to inactivated vaccine in Germany.
Background
One of the strategic objectives of the WHO is the eradication of poliomyelitis in all regions of the world within the next years. According to the WHO plans the transmission of wild poliovirus has to be interrupted worldwide by the end of 2002. The global certification will be held in 2005.
Although the European region is actually polio – free, it remains subject to the risk of importation from endemic regions. The most recent cases of poliomyelitis due to an importation of wild polio type 1 occurred in unvaccinated Bulgarian children [1]. To prevent such importations it is necessary to keep a high level of population immunity.
The last indigenous case of poliomyelitis was diagnosed in Germany in 1990. The last imported wild viruses were detected in patients with travel history to India and Egypt in 1992. Since that period of time there were found only 0 – 3 cases of vaccine-associated paralytic poliomyelitis (VAPP) per year. In order to prevent these VAPP – cases the vaccination strategy has been changed in Germany. Since 1998 it is recommended to use only the inactivated vaccine (IPV).
The neutralizing antibody test is the method of choice in conducting serological surveys to identify epidemiologically important immunity gaps in the population. It is considered essential to provide the precise procedure for carrying out polio neutralizing antibody tests. Use of standard reagents, the International Standard Serum (ISS), standard Sabin strains, and expression of the results in International Units (IU) is a feature aimed at obtaining consistent results. This will assure that comparison between studies can be made with confidence. An International Standard Serum does exist, but WHO recommendations do not indicate a protective level in IU [2,3].
Materials and methods
Serum samples
The first German Health Survey 1997/98 was a representative study of the health status of the population in unified Germany. In this project which has been carried out by the Robert Koch Institute on behalf of the Ministry of Health about 7200 study participants aged between 18 and 79 years were going through a medical check-up and interviewed as to health – relevant issues. Germans as well as foreigners, who lived in Germany, were included in the survey. As a result of various selection criteria (age, sex, and size of town) a representative collection of study participants was obtained [4].
A representative panel of 2564 serum samples from the total amount of sera from the Health Survey was investigated for the presence of antibodies to all three poliovirus serotypes.
Additionally, 881 sera from children less than 18 years obtained from six large laboratories in Germany were included in this study. These laboratories performed wide-range diagnostics without any visible bias within the group of patients. The sera were collected in 1996/97 and stored at -20°C until testing.
Neutralization test
The microneutralization test using 100 ID50 of challenge virus (Sabin strains) was performed according to the WHO-guidelines [5][6]. An In-House Reference serum (IHR) of known neutralizing activity was included in each test to control reproducibility of results. Each test serum was investigated in duplicate. If the final serum dilutions of these duplicates differed more than one stage the test was rejected.
The International Standard Serum (ISS, a preparation of pooled human sera) containing 25 IU, 50 IU, and 5 IU for Poliovirus 1, 2 and 3, respectively has been used to calibrate the potency of the IHR. ISS and IHR were titrated in parallel on ten separate occasions using eight replicates per serum dilution (starting from serum dilution of 1/4).
Calculation of the antibody concentration in International Units (IU/ml)
The calculation of the concentration with
![]()
resulted in CIHR1 = 3.125 IU/ml, CIHR2 = 6.25 IU/ml, CIHR3 = 0,625 IU/ml for Polio 1, 2, 3, respectively for the used IHR (example for Polio 1: a serum dilution ValISS = 1/256, and ValIHR = 1/32 would result in CIHR = 3.125 IU/ml).
The calculation of the antibody concentration in a given test serum sample was done analogously.
![]()
In contrast to WHO – recommendations not the geometric mean (GMT) of all measured IHR's was used but the concentration of the IHR in the given test only. Thus each test was calibrated in respect to the appropriate IHR.
A given test was only accepted if the final serum dilution did not exceed a one-stage range of the GMT of all tested and accepted IHR's. For an accepted test the following condition holds:
![]()
The threshold values Co were calculated as follows:
![]()
with
![]()
SDev(C) = StandardDeviation{C, with serumdil. < 1/4}.
Results
In the past the cut-off value for "sufficiently protected" against poliomyelitis was set if the final serum dilution giving protection against 100 ID50 of challenge virus was greater or equal to 1/4 (titer 8). This condition should be substituted by expressing results in IU/ml.
The cut-off values (Co) for the protective level were calculated as follows:
for Polio 1: C0 = 0.080 IU/ml
for Polio 2: C0 = 0.180 IU/ml
for Polio 3: C0 = 0.075 IU/ml
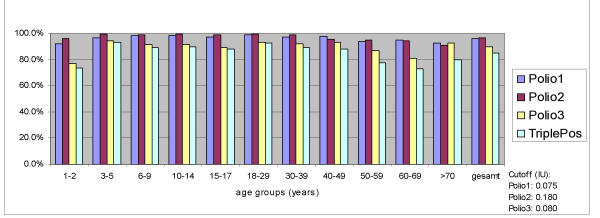
Neutralizing antibodies against poliovirus type 1, 2 and 3 were detected in 96.2% (95% confidence interval CI: 95.5%-96.9%), 96.8% (CI: 96.1%-97.5%) and 89.6% (CI: 88.4%-90.8%) of samples, respectively (Fig. 1). 85% of all tested persons had antibodies against all three virus types. Only 0.3% of tested persons had no detectable antibodies against all polioviruses.
Figure 1.
Prevalence of antibodies to polio viruses in Germany, n = 3445
Statistically significant regional differences in the immunity level between the adult populations in the East and West of Germany have been detected for Polio 3 (86,6% / 90,9%; p = 0,001).
Within the given data set the decision "sufficiently protected" slightly changes according to Tab. 1 (expressing results in IU vs. serumdilution). Thus for Polio 3 about 3% (82/2564) of the samples were additionally classified as "not protected sufficiently " against disease.
Table 1.
Differences in numbers of samples classified as "sufficiently protected" (serumdilution vs. concentration C in IU/ml), n = 2564
| Polio 1 | C<C0 | C>=C0 |
| Serum dil.< 1/4 | 90 | 11 |
| Serum dil.>=1/4 | 12 | 2451 |
| Polio 2 | ||
| Serum dil.< 1/4 | 97 | 17 |
| Serum dil.>=1/4 | 1 | 2449 |
| Polio 3 | ||
| Serum dil.< 1/4 | 182 | 0 |
| Serum dil.>=1/4 | 82 | 2300 |
Discussion / Conclusions
The goal of eradication of poliomyelitis has been complicated by some events in 2000–2001. First, the discovery of circulation of a vaccine virus strain that mutated to regain its disease-causing ability in Hispaniola. This outbreak in the Dominican Republic and Haiti was the first in the Americas since 1991 and occurred in areas of very low vaccination coverage [7]. Second, the polio cases in Bulgaria. There had been no reported cases of polio in Europa since November 1998. In spring 2001 two gypsy unvaccinated children were paralyzed by a wild poliovirus in Bulgaria, which was imported from India [1]. These outbreaks send a warning to all countries to maintain their guard against polio, despite the rapid decline in its incidence. Sufficient surveillance systems and high levels of population immunity can prevent polio outbreaks.
The level of immunity to poliomyelitis has been checked in Germany at regular intervals.
For the first time in Germany the results were expressed in International Units. A representative serum panel from the German Health Survey was investigated. Additionally, sera from children less than 18 years old were included in this study. These sera were not representative for all selection criteria because they originated from diagnostic laboratories. A representative study of the health status of persons under an age of 18 years is prepared by the Robert Koch Institute. The pilot phase has started in 2001. Within the next years valid data will be obtained for the younger population of Germany.
The given threshold values of 0.075 lU/ml, 0.180 IU/ml, and 0.080 IU/ml were calculated for Polio 1,2,3 respectively. The calculation was based on the assumption that the new criteria (threshold in IU/ml) should give comparable results with respect to the old one (threshold of serum dilution).
Overall, neutralizing antibodies against poliovirus type 1, 2 and 3 were detected in 96.2%, 96.8% and 89.6% of samples, respectively. This seroprevalence indicates a very high level of immunity of the German population. Although the prevalence of antibodies against poliovirus was generally somewhat lower for type 3 than for types 1 and 2, it was still close to 90%.
Data presented in this study are concordant to those obtained in other countries [8,9].
A previous seroepidemiological study in Germany as well as this one revealed substantial regional variations of immunity levels to Polio 3 [10]. Compared to the results of this study other German studies have shown remarkable lower antibody prevalence to Polio 3 among blood donors in Berlin and in hospitalized patients [11,12].
Limitations in some of these serological studies due to non-representative samples or methodological reasons resulted in different conclusions regarding immunity status. Standardization of the neutralization test as well as expressing results of serological studies in International Units will permit better comparability of polio immune status of populations in different studies, and in different countries.
Immunity to poliomyelitis is largely dependent on humoral neutralizing antibodies, both after natural infection and after vaccination. It is unclear at present whether all those persons with a low level or no detectable antibodies are susceptible to infection; some, particularly the elderly, may be protected by memory immunity, an accelerated antibody response because the immune system has been primed previously. It has also been shown that enterovirus infections induce T-cell immunity [13]. The production of local secretory IgA antibodies in the gut mucosa may play a major role in protection [14,15].
Although IPV offers excellent protection against disease, it is less effective in preventing poliovirus infection because of limited mucosal immunity. IPV recipients may contribute to the circulation of outbreak virus. Extensive circulation of Polio 3 was observed during 1984 outbreak in Finland. A study from the Netherlands has also shown that poliovirus circulation occurred during the early phase of the 1992–93 poliomyelitis outbreak. Most infected children had not been vaccinated [16]. A high level of immunity of the German population, including immigrants, must be monitored after the switch from attenuated to inactivated polio vaccine (OPV to IPV).
Competing interests
None declared
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgments
Acknowledgements
The authors are grateful to Helga Schickhoff for her excellent technical assistance.
Contributor Information
Sabine Diedrich, Email: DiedrichS@rki.de.
Hermann Claus, Email: ClausH@rki.de.
Eckart Schreier, Email: SchreierE@rki.de.
References
- Schrope M. Plans to eradicate polio hit by virus outbreak in Bulgaria. Nature. 2001;411:405. doi: 10.1038/35078219. [DOI] [PubMed] [Google Scholar]
- Wood DJ, Heath AB. Comparability of poliovirus neutralizing antibody tests. Biologicals. 1992;20:293–300. doi: 10.1016/s1045-1056(05)80050-8. [DOI] [PubMed] [Google Scholar]
- Wood DJ, Heath AB. The Second International Standard for Anti-Poliovirus Sera Types 1, 2 and 3. Biologicals. 1992;20:203–211. doi: 10.1016/s1045-1056(05)80039-9. [DOI] [PubMed] [Google Scholar]
- Bellach BM, Knopf H, Thefeld W. Der Bundes-Gesundheitssurvey 1997/98. Das Gesundheitswesen. 1998;60:S59–S68. [PubMed] [Google Scholar]
- WHO Poliovirus neutralizing antibody assay. Bull WHO. 1992;70:669. [Google Scholar]
- WHO Manual for the virological investigation of polio. WHO/EPI/GEN/9701 44-51. 1997.
- Update: Outbreak of poliomyelitis – Dominican Republic and Haiti, 2000–2001. MMWR. 1997;50:855–856. [PubMed] [Google Scholar]
- Mastroeni I, Patti AM, Fabrizi A, Santi AL, Manduca AM, Vescia N, et al. Immunity status against poliomyelitis in persons 13–14 years old living in Rome. Vaccine. 1997;15:747–750. doi: 10.1016/S0264-410X(96)00208-3. [DOI] [PubMed] [Google Scholar]
- Conyn-van Spaendonck MA, de Melker HE, Abbink F, Elzinga-Gholizadea N, Kimman TG, van Loon T. Immunity to poliomyelitis in the Netherlands. Am J Epidemiol. 2001;153:207–214. doi: 10.1093/aje/153.3.207. [DOI] [PubMed] [Google Scholar]
- Diedrich S, Schreier E. Immunitätslage gegen Poliomyelitis (Polio-Serosurvey 1993)]. Dtsch Med Wochenschr. 1995;120:239–244. doi: 10.1055/s-2008-1055339. [DOI] [PubMed] [Google Scholar]
- Stark K, Schonfeld C, Barg J, Molz B, Vornwald A, Bienzle U. Seroprevalence and determinants of diphtheria, tetanus and poliomyelitis antibodies among adults in Berlin, Germany. Vaccine. 1999;17:844–850. doi: 10.1016/S0264-410X(98)00269-2. [DOI] [PubMed] [Google Scholar]
- Franck S, Allwinn R, Rabenau HF, Doerr HW. Epidemiological analysis of immunity to poliovirus after termination of an era of vaccination with OPV in Germany. An analysis of the German Association Against Viral Diseases (DVV). Zentralbl Bakteriol. 1999;289:475–481. doi: 10.1016/s0934-8840(99)80086-3. [DOI] [PubMed] [Google Scholar]
- Juhela S, Hyoety H, Lönnrot M, Roivainen M, Simell O, Ilonen J. Enterovirus infections and enterovirus specific T-cell responses in infancy. J Med Virol. 1998;54:226–232. doi: 10.1002/(SICI)1096-9071(199803)54:3<226::AID-JMV14>3.3.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Fiore L, Ridolfi B, Genovese D, Buttinelli G, Lucioli S, Lahm A, et al. Poliovirus Sabin type 1 neutralization epitopes recognized immunoglobulin A monoclonal antibodies. Virol. 1997;71:6905–6912. doi: 10.1128/jvi.71.9.6905-6912.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valtanen S, Roivainen M, Piirainen L, Stenvik M, Hovi T. Poliovirus-Specific Intestinal Antibody Responses Coincide with Decline of Poliovirus Excretion. J Infect Dis. 2000;182:1–5. doi: 10.1086/315684. [DOI] [PubMed] [Google Scholar]
- Oostvogel PM, Rumke HC, Conyn-van Spaendonck MA, van der Avoort HG, Leeuwenburg J, van Loon AM. Poliovirus circulation among schoolchildren during the early phase of the 1992–1993 poliomyelitis outbreak in The Netherlands. J Infect Dis. 2001;184:1451–1455. doi: 10.1086/324327. [DOI] [PubMed] [Google Scholar]