Abstract
Phototherapeutic agent-based phototherapies activated by light have proven to be safe modalities for the treatment of various malignant tumor indications. The two main modalities of phototherapies include photothermal therapy, which causes localized thermal damage to target lesions, and photodynamic therapy, which causes localized chemical damage by generated reactive oxygen species (ROS). Conventional phototherapies suffer a major shortcoming in their clinical application due to their phototoxicity, which primarily arises from the uncontrolled distribution of phototherapeutic agents in vivo. For successful antitumor phototherapy, it is essential to ensure the generation of heat or ROS specifically occurs at the tumor site. To minimize the reverse side effects of phototherapy while improving its therapeutic performance, extensive research has focused on developing hydrogel-based phototherapy for tumor treatment. The utilization of hydrogels as drug carriers allows for the sustained delivery of phototherapeutic agents to tumor sites, thereby limiting their adverse effects. Herein, we summarize the recent advancements in the design of hydrogels for antitumor phototherapy, offer a comprehensive overview of the latest advances in hydrogel-based phototherapy and its combination with other therapeutic modalities for tumor treatment, and discuss the current clinical status of hydrogel-based antitumor phototherapy.
Keywords: hydrogel, photodynamic therapy, photothermal therapy, antitumor
1. Introduction
Malignant tumors have emerged as the foremost contributor to mortality rates worldwide, serving as a significant impediment to the progressive trend of increasing life expectancy [1]. They are projected to continue as the primary etiology of premature death, defined as death before the age of 70, on a global scale. Malignant tumor tissue exhibits distinctive features such as vascular malformation, low pH, hypoxia, and others. Concurrently, malignant tumor cells possess unique properties including unrestrained proliferation, facile metastasis, and drug resistance [2]. At present, the foremost approaches for malignant tumor therapy comprise surgery, chemotherapy, and radiotherapy. Although surgical procedures can be useful in the management of tumors, complete removal of all malignant tumor cells may not be feasible [3,4,5]. Chemotherapy and radiotherapy, while effective in eliminating tumor cells, can also result in collateral damage to normal tissue [3,4,5].
The use of phototherapy in tumor treatment has garnered significant attention due to its minimal invasiveness, high effectiveness, and low probability of drug resistance [6]. Phototherapy has been employed for tumor management for a considerable duration of time, dating back to the early 1960s, shortly after its initial investigation in the treatment of retinal detachment [7]. The achievement of effective tumor ablation in phototherapy necessitates the utilization of high-powered lasers, ranging in magnitude up to hundreds of watts. This poses challenges related to its safety and logistical feasibility. Thus, to circumvent these limitations, phototherapies that utilize externally administered phototherapeutic agents to augment the efficacy of light-based treatments have been devised. Based on the distinct properties of different techniques and phototherapeutic agents involved, phototherapy can be broadly categorized into two main types, namely photothermal therapy (PTT) and photodynamic therapy (PDT) [8].
PTT is a treatment modality that utilizes photothermal agents, encompassing both endogenous chromophores within human tissues and exogenous agents [7]. The exogenous photothermal agents can be classified into various types, including inorganic materials (e.g., gold nanoparticles and graphene oxide) and organic materials (e.g., indocyanine green (ICG) and polydopamine) [9,10]. These agents can facilitate photothermal conversion when exposed to light radiation of specific wavelengths, leading to the release of heat that elevates the temperature of the affected region, consequently inducing cell death [11].
In contrast to PTT, PDT relies on the production of reactive oxygen species (ROS) to mediate its cytotoxic effects [12]. The three primary mechanisms of ROS-mediated tumor tissue destruction are direct tumor cell killing, induction of vascular damage, and activation of an immune response against the tumor [13,14,15]. PDT necessitates three key elements, namely a photosensitizer, molecular oxygen, and light [16,17,18]. The majority of photosensitizers employed in clinical settings are derived from porphyrins, chlorines, or dyes, and are structurally based on the tetrapyrrole framework [19]. The photothermal agents and photosensitizers can absorb light in the visible range (wavelengths of 400–700 nm) or near-infrared (NIR) range (700–1350 nm), and commercially available lasers, such as alexandrite lasers (720–800 nm), dye lasers (390–1000 nm), and diode lasers (630–1100 nm) can excite these agents to generate heat or ROS [20]. The placement of light can be further controlled using interventional techniques such as optical fibers and endoscopy, which can minimize off-target toxicity to surrounding tissues and avoid invasive procedures such as laparotomy and thoracotomy [20].
Although phototherapy has achieved significant success, its extended clinical application is limited by the evident phototoxicity of traditional phototherapeutic methods [7,21,22,23,24]. The main cause of phototoxicity is the uncontrolled distribution of phototherapeutic agents, which can cause off-target effects in normal tissues, including the skin, blood vessels, and liver, when exposed to natural light, ultimately resulting in the damage of normal cells. Additionally, the uncontrolled distribution of phototherapeutic agents can lead to lower accumulation in tumor cells, limiting the efficacy of phototherapy. Secondly, from a technical standpoint, selectively irradiating tumor cells is a challenge due to the proximity of normal tissues. As a result, healthy cells near the tumor may also be damaged. To overcome these limitations, there is a need to develop a method for the controlled and sustained delivery of phototherapeutic agents directly to tumor sites to facilitate effective antitumor phototherapy. Therefore, advancements in drug delivery systems, such as liposomes, micelles, nanoparticles, microspheres, and hydrogels, have provided significant opportunities for improving antitumor phototherapy. Among these systems, hydrogels have gained prominence due to their high biocompatibility, and have emerged as the preferred options [25].
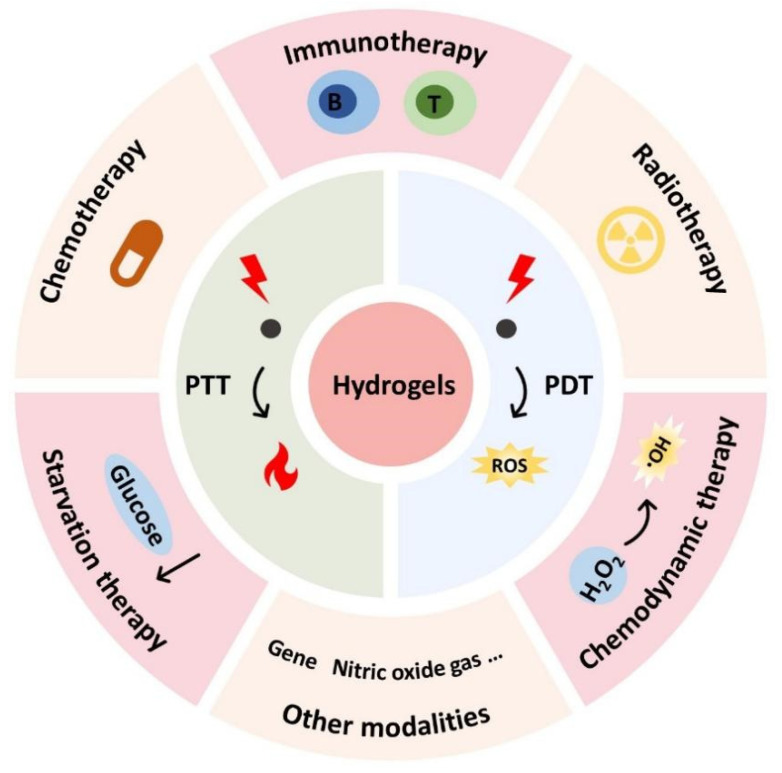
Hydrogels are insoluble biomaterial scaffolds with three-dimensional (3D) networks that can be formed through physical or chemical cross-linking [26,27]. Their unique features such as high biocompatibility, biodegradability, high water content, and flexible mechanical properties have made them extensively utilized in various biomedical applications, including tissue engineering, cell culture, drug delivery, biosensors, and antitumor therapy [28,29,30,31,32]. In recent years, hydrogels have garnered significant interest as drug carriers in antitumor phototherapy owing to their ability to achieve localized delivery of phototherapeutic agents while minimizing their distribution in non-target sites [33,34,35,36,37]. As a result, this approach can enhance therapeutic efficacy while reducing phototoxicity from phototherapy [38,39,40]. In addition, hydrogels also offer a versatile platform that shows significant potential to complement other tumor treatments such as immunotherapy, chemotherapy, and radiotherapy, and exhibit excellent synergistic antitumor efficacy (Figure 1) [41,42,43,44].
Figure 1.
Schematic illustration of the hydrogels-based combinatorial phototherapy.
In this review, we will provide a summary of recent advancements in the design of hydrogels for antitumor phototherapy and offer a comprehensive overview of the latest advances in hydrogel-based phototherapy and its combination with other therapeutic modalities for tumor treatment. To illustrate these advances, representative examples will be presented. Lastly, the potential applications of hydrogels in clinical antitumor phototherapy will be discussed.
2. Hydrogels
Hydrogels are 3D networks of polymers that contain a significant amount of water or other fluids. Due to their high water content and cross-linked structure, hydrogels have potential for the encapsulation and delivery of photosensitizers/photothermal agents [41,42,43,44]. Furthermore, the characteristics of hydrogels, such as surface topography, porosity, and mechanical strength, depend largely on the properties of the polymers used and the mechanism of polymeric cross-linking [45,46]. By controlling various features of hydrogels, it is expected that a wide range of selectivities and diversities can be achieved for antitumor phototherapy. Hydrogels can be classified based on different parameters, including the source of materials (natural or synthetic), polymeric composition (homopolymeric, copolymeric, or multipolymeric), crosslinking mechanism (physical or chemical), sample size (nanogels, microgels, macrogels, or bulk hydrogels), and degradability (degradable or nondegradable) [47,48,49,50]. Considering the numerous excellent review articles on the introduction, classification, preparation, and application of hydrogels that have already been published [51,52,53,54,55,56,57,58], we will refrain from providing an exhaustive classification of hydrogels in this work.
For localized antitumor phototherapy, an injectable hydrogel is the preferred option for local administration. Compared to traditional hydrogels with pre-fabricated shapes, injectable hydrogels are better applied for filling irregular defects caused by tumor resection. They can also be injected into deep tissue through minimally invasive administration procedures [59,60,61]. Therefore, it is not surprising that most studies on hydrogel-based antitumor phototherapy are reported on the usage of injectable hydrogels for local hydrogel administration. Moreover, the use of conventional hydrogels in antitumor phototherapy is also limited by slow response rates, uncontrollable drug release, and difficulty in manipulating swelling and shrinking kinetics. To overcome these limitations, emerging hydrogels such as smart hydrogels and nanogels have garnered significant attention in antitumor phototherapy.
2.1. Smart Hydrogels
The distinctive characteristics of the tumor microenvironment, such as changes in overexpression of certain proteins and enzymes, acidic pH, hyperthermia, and redox potential have captured the attention of researchers who seek to utilize stimulus-responsive hydrogels, commonly known as smart hydrogels, for tumor treatment [62,63]. In contrast to conventional hydrogels, smart hydrogels have the unique ability to alter their physical or mechanical properties, such as swelling, shrinking, and undergoing a phase transition, in response to various stimuli, and thus make them suitable for various biomedical applications [64]. These stimuli can be external (such as temperature, magnetic fields, and light) or endogenous (such as enzymes, ionic strength, pH, and redox potential) [64,65,66,67,68,69,70,71]. This feature allows for on-demand drug release, leading to improved efficacy and reduced side effects from drug leakage [72,73,74,75,76]. Therefore, the utilization of smart hydrogels has become increasingly popular in the field of phototherapeutic agent-based phototherapies for tumor treatment [58]. In recent years, the widely used smart hydrogels for antitumor phototherapy are thermosensitive hydrogels, pH-sensitive hydrogels, photosensitive hydrogels, and redox-sensitive hydrogels.
Thermosensitive hydrogels. The application of thermosensitive hydrogels for antitumor phototherapy has undergone extensive research, with this type of smart hydrogel being the primary focus in the field. The unique sol–gel phase transition of thermosensitive hydrogels in response to temperature changes has led to their classification into two distinct groups: lower critical solution temperature (LCST) hydrogels and upper critical solution temperature (UCST) hydrogels [77,78]. The most commonly used thermosensitive hydrogels in recent years are LCST hydrogels, which are in a gel state above their LCST and a solution state below it. In the presence of temperatures lower than the LCST, the hydrophilic moieties present in the polymer chains of the hydrogel form hydrogen bonds with hydrophilic molecules present in their surroundings, causing the hydrogel to display high solubility. Upon temperature elevation, hydrogen bonds are weakened while the hydrophobic components of polymer chains experience increased interaction. This can lead to a substantial drop in polymer solubility, triggering matrix shrinkage or phase transition [79,80]. Furthermore, hydrogels with an LCST within the temperature range of room temperature to body temperature are highly suitable for in situ gelling upon injection. Additionally, the recent development of LCST hydrogels involves the incorporation of natural, such as chitosan [81], or synthetic macromolecules, such as poly-(N-isopropyl acrylamide) (PNIPAM) [82,83,84] and Pluronic F127 [85,86,87,88].
pH-sensitive hydrogels. Solid tumors are associated with an acidic tumor microenvironment, which is primarily due to the increased production of lactate by tumor cells resulting from their high rate of aerobic glycolysis [89]. The acidic nature of the tumor microenvironment has motivated the exploration of pH-sensitive drug delivery hydrogels to neutralize the microenvironment and impede tumor progression. Thus, pH-responsive hydrogels have been widely investigated as a localized drug delivery approach to achieve targeted antitumor phototherapy with reduced systematic toxicity. pH-responsive hydrogels can be divided into two main categories, namely anionic–cationic pH-responsive hydrogels and covalent pH-responsive hydrogels. The matrices of the anionic–cationic pH-responsive hydrogels typically comprise numerous weakly acidic or basic groups (e.g., carboxyl and amine groups) that can donate or accept protons. Alterations in the external pH can cause changes in the characteristics of these polymers, such as solubility and volume, which can lead to phase transitions and the release of drugs. In the case of in situ injection, the basic groups (such as amine groups) of the polymer undergo positive charge due to proton acceptance from the acidic external pH of the tumor microenvironment. Consequently, the polymer chains experience expansion and swelling from the electrostatic repulsion among charges. The enlarged mesh of the swollen hydrogels then facilitates the diffusion of drugs throughout the network. On the contrary, under acidic conditions, the presence of acidic groups (such as carboxyl group) in polymers causes the polymer chains to shrink. This shrinkage leads to the expulsion of water and drug molecules [90]. As for covalent pH-responsive hydrogels, the pH-sensitivity is attributed to degradation of acid-cleavable covalent bonds, such as ketals and hydra-zone bonds [91,92]. In addition, several natural polymers (such as chitosan, alginate, and cellulose) and synthetic polymers (such as polyamines and pyridine derivatives) have been extensively investigated for the production of pH-sensitive hydrogels [93]. Among these, chitosan and its derivatives are the most commonly employed polymers to develop pH-responsive hydrogels for antitumor phototherapy.
Photosensitive hydrogels. Photosensitive hydrogels can undergo property changes or a phase transition triggered by changes in their chemical structure or conformation under radiation, ultraviolet, or visible light [94,95]. Incorporating photothermal agents into hydrogel systems is a widely used method that involves photothermal conversion when exposed to light radiation, leading to a temperature increase in the system and a subsequent phase transition in thermosensitive hydrogels. This approach is also considered part of photothermal therapy. Another common method used to create light-responsive hydrogels involves polymers with a photoreactive moiety. This moiety can respond to light by undergoing photochemical reactions, such as photoisomerization, photocleavage, and crosslinking point photopolymerization. These reactions alter the properties of the hydrogel, including its crosslinking density, hydrophilicity, and charging state, which lead to phase transition and the release of drugs [93]. One example of a material used to prepare light-responsive hydrogels is gelatin methacryloyl (GelMA). This compound is created by incorporating methacryloyl groups into gelatin, which allows for photopolymerization by crosslinking with a photo-initiator [96]. Moreover, radiation can be used to trigger the swelling of hydrogels via ionizable functional groups. As a result of radiation-induced ionization, the osmotic pressure inside the hydrogel matrix increases, causing it to swell. This mechanism is similar to pH-sensitive hydrogels [95]. In recent years, the use of photosensitive hydrogels represents a promising approach for drug delivery in tumor treatment, as it enables noncontact drug delivery with high temporal and spatial precision. As a result, photosensitive hydrogels also have emerged as an attractive drug delivery system for antitumor phototherapy.
Redox-sensitive hydrogels. The current emphasis on redox-sensitive hydrogels is primarily centered on addressing tumor drug resistance and facilitating drug delivery. Redox-sensitive hydrogels can be designed to preferentially release drugs in the cytoplasm of tumor cells as opposed to the extracellular matrix and normal cells, primarily due to the higher levels of glutathione (GSH) in the former. Specifically, the cytoplasm contains a much higher concentration of GSH (2–10 mM) compared to the extracellular matrix (2–20 μM) [97]. In multidrug-resistant tumors, this difference is particularly pronounced, with the cytoplasmic GSH concentration being four times higher than in healthy tissues [98]. The incorporation of GSH-reactive groups (such as ditelluride, disulfide, and diselenide bonds) is a fundamental principle underlying such hydrogels, enabling their cleavage through the acceptance of electrons from the thiol groups in GSH [99,100].
2.2. Nanogels
Nanogels, which are colloidal particles of hydrogels at the nanoscale, have emerged as promising approaches for antitumor phototherapy [101]. Nanogels can be synthesized based on natural or synthetic polymers using a range of cross-linking techniques, including functional group cross-linking, radiation cross-linking, cross-linking polymerization, crystallization, and ionic cross-linking [102]. Furthermore, various nanogel configurations can be synthesized, such as yolk–shell architectures, double-walled structures, hollow nanogels, and core-shell nanoparticles [103,104,105,106,107]. Over the years, the biomedical applications of nanogels have been the subject of numerous review articles, many of which have examined the advancements made in drug delivery [108,109,110,111], regenerative medicine [112,113], imaging [114,115,116,117], diagnostics [118,119,120], theranostics [121,122], and tumor therapy [111,123,124,125].
It is worth noting that the ability of nanogels to cross biological barriers and deliver agents intracellularly through endocytosis makes them an attractive candidate for advanced drug delivery systems [126,127]. Nanogels also offer a solution to the problem of fast clearance of phototherapeutic agents in vivo by utilizing enhanced permeability and retention (EPR) effect to enhance their retention in tumors, thereby improving the therapeutic outcome of phototherapy for tumor treatment [7,21]. Specifically, the EPR effect is primarily attributed to the aggressive proliferation of tumor cells, which leads to the consumption of local nutrients and irregular blood vessel formation. In turn, the abnormal vessels contain porous openings that increase the ability of circulating nanogels to penetrate the tumor microenvironment. In contrast, non-malignant tissues are less penetrable due to the intact vasculature barrier. Furthermore, nanoparticles tend to accumulate selectively within tumor tissues due to the impaired lymphatic drainage system in those areas. In general, nanogels must have a size between 10 and 200 nm to achieve the EPR effect [128]. In addition to size, other intrinsic characteristics of nanogels, such as shape, electrical charge, hydrophilicity, and circulation time in the bloodstream, can impact the efficacy of the EPR effect [129]. While the effectiveness of EPR-based targeting has been demonstrated in preclinical tumor models in vivo, this approach has several drawbacks. These include limited efficacy for certain early-stage tumors due to their smaller size and more regular vasculature, heterogeneous throughout resulting from malformation of vessel fenestrations, and a lack of investigation of the EPR effect in human tumors [130,131].
Moreover, the large surface area of nanogels enables increased opportunities for multivalent bioconjugation and enhanced drug-loading capacity [132]. As a result of this unique nanostructure, nanogels demonstrate a rapid response to changes in the environment. Accordingly, smart nanogels, also called stimuli-responsive nanogels, can be designed to achieve triggered drug release in response to various stimuli, such as light, ion-exchange, pH, and temperature [133,134,135,136]. The use of smart nanogels as a drug delivery system for anti-tumor phototherapy holds great promise, as evidenced by recent reports on thermosensitive nanogels [137], pH-sensitive nanogels [138,139], photosensitive nanogels [140], and redox-sensitive hydrogels [139].
Ongoing research also indicates that smart nanogels could potentially revolutionize the field of tumor treatment by functioning not only as drug carriers but also as diagnostic, imaging, and theranostic agents [141]. Additionally, nanogels can be further customized to incorporate additional beneficial features, including prolonged circulation, targeted cell recognition, and multifunctionality through the integration of multiple features [126,142]. As a result, nanogels have emerged as one of the most promising platforms for phototherapeutic agent-based phototherapy.
3. Hydrogel-Based Phototherapy
3.1. Hydrogel-Based PTT
PTT involves the use of photothermal agents to generate localized heat [7]. Upon irradiation with light of a specific wavelength, the photothermal agents undergo photothermal conversion, which leads to an increase in kinetic energy and heating of the surrounding microenvironment. The extent of tissue damage induced by PTT depends on the temperature achieved. A heat-shock response is initiated at 41 °C [143], while irreversible tissue damage occurs at 42 °C [143,144]. Heating tissues to 42–46 °C for 10 min leads to cell necrosis, while cell death occurs almost instantaneously at tissue temperatures above 60 °C [145]. Although capable of eliminating established tumors, PTT may result in unintentional harm to healthy tissue adjacent to the tumor [146]. The incorporation of hydrogels in PTT can be beneficial in preserving the local temperature and reducing the risk of burns to healthy tissues. Furthermore, utilizing hydrogels to retain photothermal agents also enables repeated PTT sessions with a single administration of the hydrogel.
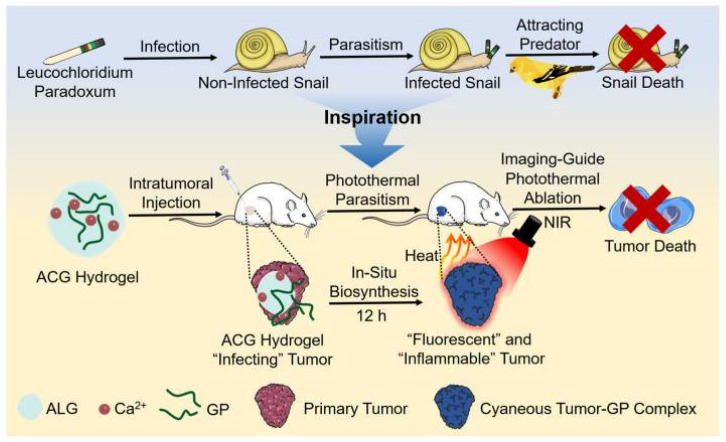
Hydrogels are widely used as carriers for investigating new photothermal agents for antitumor PTT. Yao et al. [85] achieved excellent antitumor PTT efficiency by incorporating titanium carbide nanoparticles as photothermal agents into thermosensitive Pluronic F127 hydrogels. Wang et al. [147] utilized an alginate-calcium hydrogel as a carrier to demonstrate the potential application of commercial copper sulfide as a photothermal agent for PTT. In another study, Wang et al. [148] prepared an alginate–calcium–genipin hydrogel that can achieve brilliant fluorescent and photothermal effects based on the fluorescent/photothermal features of the crosslinking product of genipin and protein (Figure 2). This hydrogel system provided a feasible solution for the homogenous dispersion of genipin and presented a novel methodological strategy for fluorescence imaging-guided antitumor phototherapy. However, it should be noted that the long-term biosafety of these photothermal agents should be carefully evaluated.
Figure 2.
Schematic illustration of in situ biosynthesis of the genipin–protein crosslinking product for fluorescence imaging-guided PTT. Reprinted with permission from [148].
Self-assembled injectable hydrogels have received great attention in antitumor PTT. Hsiao et al. [149] modified polyaniline side chains into chitosan derivatives, which can self-assemble to form micelles and rapidly form hydrogels under the stimulation of a local acidic environment (pH = 6.9–7.0) in tumors. These hydrogels can then be loaded with hollow gold nanospheres as photothermal agents, which function as a heating source under NIR light irradiation. The local temperature of the tumor can quickly reach and be maintained at 50–55 °C within 5 min, surpassing the photothermal effect of commonly used inorganic photothermal agents, such as gold nanospheres. Additionally, the hydrogel system can prevent the leakage of the micelles and enable multiple effective treatments, thereby achieving the elimination of tumors and preventing tumor relapse. Moreover, Fan et al. [150] prepared an iodine-loaded starch-g-PNIPAM polymer that can self-assemble into nanogels and then transform into an injectable thermosensitive hydrogel at body temperature. The hydrogel showed an excellent photothermal effect due to the “iodine-starch” complex’s photothermal effect. Furthermore, the hydrogel can also exhibit bactericidal effects due to the release of iodine. However, the stability of these self-assembled hydrogels in the presence of body fluids should be further evaluated. Moreover, the intricate synthesis procedure involved in creating supramolecular hydrogels can impede their reproducibility and practical utilization.
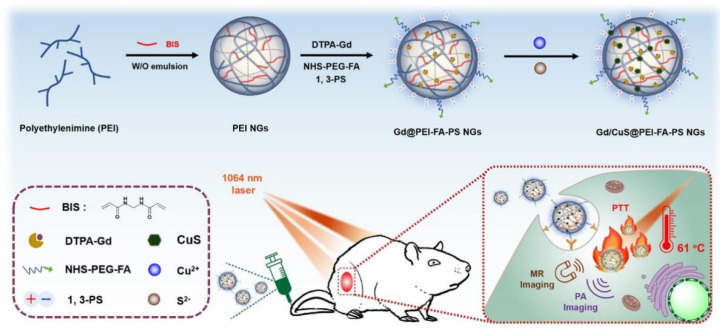
Nanogel-based PTT also has emerged as an attractive strategy for tumor treatment. Zhou et al. [151] reported the facile synthesis of a theranostic nanogel system for photoacoustic (PA) imaging-guided PTT of tumors. They used a double emulsion approach to synthesize γ-polyglutamic acid (γ-PGA) nanogels loaded with polyaniline (PANI). Then, they applied the carbodiimide coupling method to crosslink them with cystamine dihydrochloride (Cys). After loading with aniline monomers, the obtained γ-PGA/Cys nanogels underwent in situ polymerization to produce the final nanogel system. Additionally, this nanoplatform can be extended for diverse theranostic applications by functionalizing the surface carboxyl groups of the nanogels or by loading other theranostic elements. Furthermore, Zhang et al. [152] developed a polyethylenimine (PEI) nanogel system integrated with Gd and copper sulfide (CuS) for tumor-targeted PTT. The final cross-linked PEI nanogels were prepared using an inverse emulsion method. Under NIR light irradiation, the nanogel platform can realize magnetic resonance (MR) and PA imaging-guided antitumor PTT (Figure 3).
Figure 3.
Schematic illustration of the synthesis of the PEI nanogel platform for tumor-targeted PTT. Reprinted with permission from [152]. Copyright 2023 American Chemical Society.
Hydrogels for tumor therapy are expected to degrade after achieving the therapeutic purpose. Therefore, hydrogels with an adjustable degradation rate have emerged as highly appealing options for antitumor therapy. Wang et al. [153] achieved efficient PTT on tumors by using alginate–calcium hydrogel as the carrier of the prepared photothermal agent, dendrimer-encapsulated platinum nanoparticles. Under NIR light irradiation, the hydrogel system can quickly heat up to 47 °C and maintain this temperature through multiple photothermal treatments, demonstrating a strong immobilizing effect of the hydrogel. After achieving the desired curative effect, the alginate–calcium hydrogel can be degraded by injecting chelates (DTPA), and the nanoparticles can be quickly cleared from the body through renal secretion. The adjustable degradation of the hydrogel system can greatly reduce the risk of toxicity from the long-term retention of the hydrogel in the body.
Following the completion of PTT, some localized hydrogel can also be employed to facilitate the tissue repair process. Liao et al. [154] incorporated gold nanorods and nanohydroxyapatite into a hydrogel composed of methacrylated gelatin/methacrylated chondroitin sulfate, resulting in a hydrogel system with remarkable PTT effects and bone-regenerative properties. Chen et al. [86] utilized a thermosensitive Pluronic F127 hydrogel as a carrier for carrageenan-capped gold–silver nanoparticles as a photothermal agent, aiming for the prevention of tumor recurrence and promotion of wound healing. However, the long-term safety of gold nanoparticle-encapsulated hydrogels in living animals is questionable due to the unknown cytotoxicity associated with gold nanoparticles.
3.2. Hydrogel-Based PDT
PDT primarily induces tumor damage through the following mechanisms: (1) the direct killing effect of ROS on tumor cells, leading to apoptosis, necrosis, or autophagy; (2) photosensitizers target the tumor’s vascular system to form thrombi, resulting in hypoxic infarction of tumor tissues; and (3) the inflammatory response triggered by the release of inflammatory factors from apoptotic or necrotic tumor cells can promote an antitumor immune response [155,156,157,158]. The application of PDT necessitates the presence of three fundamental components: a photosensitizer, molecular oxygen, and light. Upon exposure to light, the photosensitizer absorbs photons, resulting in an excited electronic state. After excitation, the photosensitizer can transition to an excited singlet state and then undergo intersystem crossing to produce a long-lasting excited triplet state. The relaxation of the molecule can result in the emission of energy via fluorescence, heat, or other photophysical processes. Upon reaching the excited triplet state, the photosensitizer can induce the formation of ROS through two separate pathways. In the first pathway, the photosensitizer engages in electron transfer reactions to produce radicals and radical ions. In the second pathway, the photosensitizer transfers energy to triplet ground-state molecular oxygen (3O2), resulting in the production of highly reactive singlet oxygen (1O2) [159,160,161]. However, traditional PDT is limited by lack of targeting, aggregation in aqueous solutions, easy degradation, and instability of the photosensitizer, indicating the need for improvement to effectively kill tumor cells [162]. The incorporation of photosensitizers into hydrogels greatly enhances their localized concentration and biocompatibility, prolongs their residence time within the body, and ultimately augments the efficacy of PDT [163]. The incorporation of photosensitizers into hydrogels is a viable approach for enhancing the efficiency of photosensitizers [44,48].
The application of hydrogels as carriers has been extensively investigated for the evaluation of novel photosensitizers in the realm of antitumor PDT. Zhang et al. [164] incorporated TiO2 nanorods as a photosensitizer into PEGDA hydrogel. Under NIR light irradiation, TiO2 nanorods induced PEGDA molecules surrounding the tumor cells to wrap around the cell surface, while also generating a large amount of ROS to cause necrosis of the tumor cells. The presence of the hydrogel system also prevented TiO2 nanorods from flowing to normal tissues, reducing the possibility of side effects. In another study, Zhang et al. [81] synthesized a novel photosensitizer, NaYF4:Yb3+, Tm3+/Zn2GeO4:Mn2+/g-C3N4@hyaluronic acid (UZC@HA), and incorporated it into a thermosensitive chitosan hybrid hydrogel. UZC@HA exhibited tumor-targeting capability and could generate ROS upon irradiation with a 980 nm laser, leading to the destruction of tumor cells in the hydrogel. In a subcutaneous model of mouse breast cancer, intratumoral injection of the thermosensitive hydrogel demonstrated high antitumor efficacy with no side effects on normal tissues. However, the long-term toxicity of these novel photosensitizers should be thoroughly evaluated.
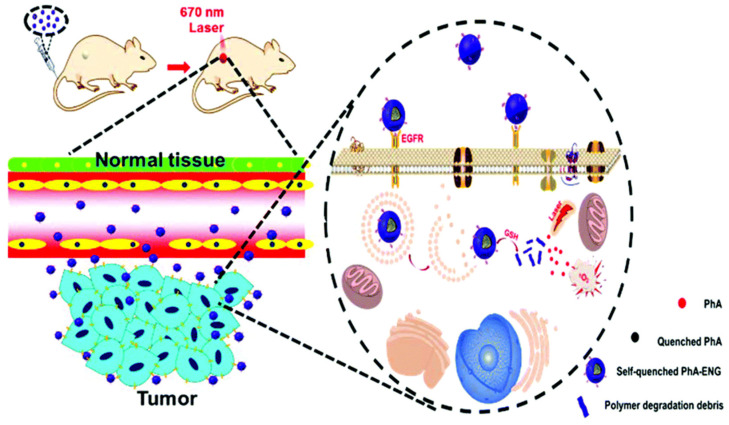
Nanogels are also applied in PTT for efficient antitumor PDT. In the study conducted by Palantoken and colleagues [165], a nanoplatform for antitumor PDT was developed utilizing Ce6-bearing pullulan nanogels. The resultant nanogel system demonstrated enhanced water solubility and stability of Ce6. Notably, the nanogel exhibited significantly greater anti-tumor effects compared to the commonly used photosensitizer photofrin, with a 780-fold increase in efficacy observed. He et al. [166] developed a smart self-quenched nanogel for targeted PDT (Figure 4), which is crosslinked using poly[(2-(pyridin-2-yldisulfanyl) ethyl acrylate)-co-[poly(ethylene glycol)]] (PDA-PEG) polymers conjugated with photosensitizers pheophorbide A (PhA) via a disulfide bond. The nanogel inhibited the generation of 1O2 through self-aggregation-induced fluorescence quenching of PhA. It is also decorated with an anti-fluorescence resonance energy transfer affibody to improve its tumor-homing ability. The nanogel was quenched in the bloodstream, where the GSH concentration was low, reducing phototoxicity to normal tissues. Upon accumulation in tumor tissues, the nanogel can be activated by an elevated GSH concentration and realize efficient antitumor PDT. However, the long-term degradability and toxicity of nanogels should be further evaluated.
Figure 4.
Schematic illustration of the smart self-quenched nanogel for targeted PDT. Reproduced from Ref. [166] with permission from the Royal Society of Chemistry.
Although PDT shows potential in antitumor treatment, it faces limitations in treating multidrug-resistant tumor, such as protective autophagy following sub-lethal PDT [167,168] and the short lifetime and limited diffusion distance of 1O2 [44,169,170], which is the primary form of ROS generated by PDT. Zhang et al. [138] designed a supramolecular nanogel system for the delivery of the photosensitizer tetraphenylporphinesulfonate (TPPS) to overcome multidrug resistance in tumor cells. The nanogel was prepared through the self-assembly of TPPS, organosilica nanodots, and methoxy-poly(ethylene glycol)113-block-poly(l-glutamic acid sodium salt)200. By exploiting the endo/lysosomal entrapment mechanism, the photosensitizer-loaded nanogels can evade drug efflux pumps and be internalized by drug-resistant tumor cells. The pH-sensitive nature of the nanogels allowed them to aggregate in the acidic endosomes/lysosomes, leading to their retention in cells. Furthermore, the nanogel-mediated damage to lysosomes after PDT inhibited protective autophagy, resulting in improved therapeutic performance. However, it should be noted that the long-term biosafety of the nanogel needs to be further investigated.
Moreover, current PDT approaches are suboptimal for treating large and deep-seated solid tumors due to poor light penetration depth [171]. To overcome this challenge, several chemical solutions for designing new-generation photosensitizers [172,173,174] and physical solutions for choosing alternative light sources [175,176,177,178,179,180] have been proposed and investigated to achieve more efficient PDT. Recently, Yang et al. [171] proposed a novel bacterial solution to address the poor light penetration depth of PDT. They used modified bioluminescent bacteria as an internal light source to activate the photosensitizer chlorin e6 (Ce6), enabling even illumination of whole tumors, and embedded it inside an alginate–calcium hydrogel. This hydrogel-based approach demonstrated superior therapeutic efficacy over conventional PDT.
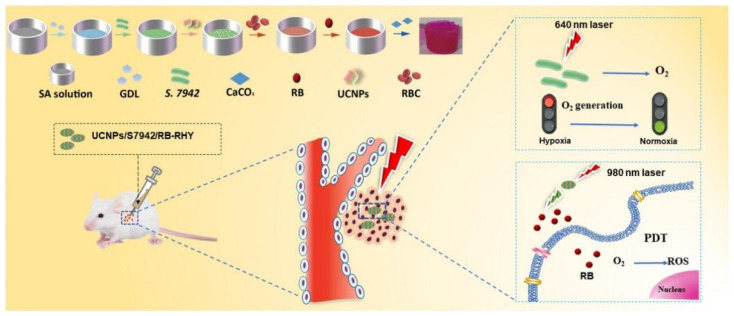
Furthermore, another inherent problem of PDT is that its therapeutic effects depend on oxygen availability. Hypoxia is a typical feature of most tumor microenvironments caused by the rapid proliferation of tumor cells and abnormal growth of tumor blood vessels [181,182,183,184,185,186]. Currently, most photosensitizers consume a large amount of oxygen during PDT, which undoubtedly exacerbates tumor hypoxia and, in turn, significantly weakens the therapy’s effectiveness. The anti-hypoxia hydrogel system developed by Zhang et al. [187] presented innovative approaches to addressing the challenge of hypoxia in antitumor PDT. They used an injectable hydrogel made of red blood cell (RBC) membrane and sodium alginate to carry the photosensitizer Rose Bengal (RB), cyanobacteria Synechococcus elongatus PCC 7942 (S. 7942), and upconversion nanoparticles (UCNPs). In this anti-hypoxia hydrogel system, S. 7942 acted as a source of oxygen for PDT, while UCNPs facilitated the conversion of 980 nm light to visible light, triggering RB activation to release ROS for antitumor treatment (Figure 5). However, it is imperative to thoroughly assess the long-term biosafety of bacterial hydrogels. Moreover, the intricate process involved in synthesizing bacterial hydrogels could potentially hinder their reproducibility and widespread practical application.
Figure 5.
Schematic illustration of the preparation process of the novel anti-hypoxia hydrogel system for antitumor PDT. Reprinted with permission from [187].
3.3. Hydrogel-Based PDT Combined with PTT
The combination of PDT and PTT has been investigated as an efficient approach for enhancing the efficacy of antitumor phototherapy. By inducing heat, PTT can facilitate the intracellular transportation of photosensitizers and elevate the oxygen concentration in tumor tissue, which can result in enhanced efficacy of PDT [188,189,190,191]. Moreover, PDT-generated ROS can hamper the protective effects of heat shock protein (HSP) in tumor cells during PTT [192].
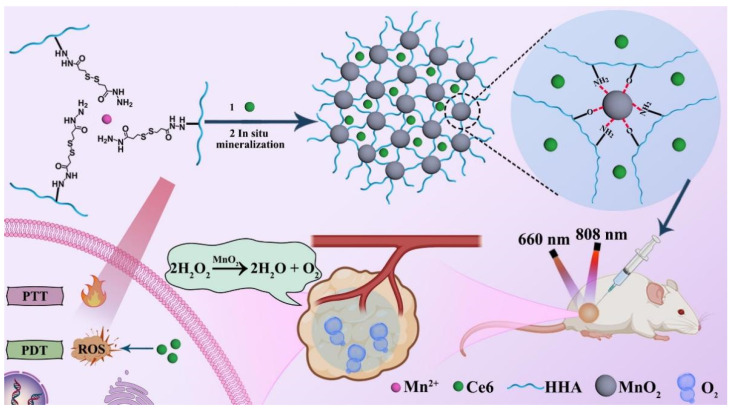
Hydrogels are widely used as a platform for synergistic PTT/PDT of tumors. Yue et al. [193] prepared an injectable and self-healing hydrogel by combining Eu-doped red fluorescent carbon dots (CDs) with oxidized hyaluronic acid. The CDs were used not only as a cross-linking agent to form a hydrogel network through the Schiff base reaction but also as a photosensitizer/photothermal agent to realize efficient PTT/PDT synergistic treatment. This injectable hydrogel also showed good biocompatibility and provided a promising method for effective injectable hydrogel-based phototherapy for tumor treatment. Similarly, Qi et al. [194] synthesized an injectable and self-healing hydrogel through the formation of Schiff base bonds between hydroxypropyl chitosan and oxidized sodium alginate. After loading bovine serum albumin-modified molybdenum disulfide nanoflakes into the hydrogel system, it could realize excellent synergistic PTT/PDT of the tumor. In another study, Wang et al. [195] incorporated Mn2+ and hydrazided hyaluronan (HHA) to develop an injectable and self-healing hydrogel system. The hydrogel can be formed in a physiological pH condition through a mineralization-triggered Mn-hydrazide crosslinking. Incorporating photosensitizer chlorin e6 and manganese dioxide (MnO2) nanoparticles into the hydrogel enabled the accomplishment of oxygen-enhanced antitumor PTT/PDT synergistic treatment (Figure 6). However, the long-term toxic concerns of MnO2 nanoparticles should be thoroughly evaluated.
Figure 6.
Schematic illustration of HHA-based hydrogel system for synergistic antitumor PTT/PDT. Reprinted with permission from [195].
Additionally, Hou et al. [196] investigated the development of a novel thermosensitive agarose-based hydrogel, which was introduced with sodium humate, MnO2, and Ce6 to achieve effective PDT and PTT of tumors. The inherent characteristics of the thermosensitive hydrogels enabled their intra-tumoral injection, deep tissue penetration, and exceptional retention at the tumor site. Under NIR light irradiation, the photothermal agent sodium humate generated heat to kill tumor cells. The efficacy of PDT was enhanced by the produced heat, which facilitated the release of photosensitizer Ce6 and strengthened the catalytic effects of manganese dioxide on H2O2 to alleviate hypoxia. Furthermore, in vivo experiments have provided additional evidence regarding the ultralow systemic toxicity induced by the hybrid hydrogel.
4. Hydrogel-Based Phototherapy Combined with Other Therapeutic Modalities
The combination of phototherapy with conventional tumor therapies such as immunotherapy [197], chemotherapy [198], and radiotherapy [199] has shown significant potential for improving treatment outcomes, demonstrating strong synergistic antitumor effects. The natural imaging abilities of most organic photosensitizers and photothermal agents can provide phototherapy with diverse imaging functions, including PA imaging [200,201], MR imaging [202], and NIR fluorescent imaging [198]. The combination of diagnostic and therapeutic approaches is a promising way to improve the precision and efficacy of tumor therapy. Hydrogels possess a distinctive network structure and high water content, which enable them to carry both hydrophilic and hydrophobic molecules to achieve synergistic antitumor effects. Thus, hydrogel-based phototherapies can be used in conjunction with other antitumor therapies to improve their efficacy in treating tumors.
4.1. Hydrogel-Based Phototherapy Combined with Immunotherapy
The effectiveness of combining phototherapy and immunotherapy in tumor treatment has been extensively studied in recent years and also has been proven to be a highly synergistic approach [41]. Phototherapy can directly eradicate the tumor mass through photodynamic or photothermal effects and augment the effectiveness of immunotherapy. Evidence suggests that PDT can efficiently induce the release of danger-associated molecular patterns and tumor-associated antigens (TAAs) from tumor cells, which play a critical role in eliciting strong immune responses [203,204,205,206,207,208]. Additionally, PTT has been shown to promote immune responses and enhance the effectiveness of immunotherapy by triggering immunogenic cell death [209].
Hydrogels have recently emerged as a promising drug delivery system for the local administration of various immunotherapeutic agents in tumor immunotherapy [210]. They enable the targeted delivery of drugs to tumors, resulting in enhanced antitumor immunity at lower doses. Therefore, the codelivery of immunotherapeutic agents and phototherapeutic agents via hydrogels is anticipated to improve therapeutic efficacy by producing a combinational phototherapy-immunotherapy effect. For example, Dong et al. [211] developed a self-assembled injectable hydrogel system for combined antitumor photothermal/immunotherapy. The hydrogel was formed through the self-assembly of a copolymer of PEG and α-cyclodextrin, which was conjugated with New Indocyanine Green (IR820). Cytosine–phosphate–guanine (CpG) nanoparticles were fabricated separately and then added to the hydrogel system. Under NIR light irradiation, IR820 generated heat, which destroyed the tumor and induced antigens releasement from tumor cells. Additionally, CpG nanoparticles can regulate the immune function of various cells, including CD8+ T cells, dendritic cells, regulatory T cells, and myeloid-derived suppressor cells, resulting in a significant improvement in the effectiveness of the combined photothermal/immunotherapy treatment compared to single-modality therapy. Additionally, Fei et al. [212] developed an injectable hydrogel composed of RBCs for combined antitumor photothermal/immunotherapy. The hydrogel can be activated by physiological signals such as platelets and thrombin, and it has a deep-red color that enables it to act as a photosensitizer when heated by NIR light. Intratumoral injection of the hydrogel can achieve photoablation of tumors, producing debris of tumor cells and TAAs that have the potential to stimulate adaptive immune responses against tumor. The administration of the immune adjuvant imiquimod within the RBCs can elicit long-lasting and potent immune responses, which can prevent the spread and recurrence of tumor. Furthermore, recent studies also investigated the use of hydrogels, such as alginate–calcium hydrogel [213], alginate–collagen hydrogel [214], and gellan gum hydrogel [215], as carriers for phototherapeutic agents and immune stimulators in the context of combined antitumor photothermal/immunotherapy.
4.2. Hydrogel-Based Phototherapy Combined with Chemotherapy
Despite its widespread usage, chemotherapy, the most common tumor treatment, is faced with multiple challenges, including severe systemic side effects, inability to target tumor heterogeneity, low bioavailability, off-target effects, and drug resistance. PTT can generate heat that enhances the permeability of extracellular matrix, cell membranes, and blood vessels [216,217,218,219]. As a result, this physiological alteration increases the efficacy of chemotherapy by increasing the concentration of antitumor drugs within the tumor tissue [220]. Chemotherapy can also aid in improving the sensitivity of tumor cells to PTT in return [221,222]. In addition, the utilization of hydrogels as a drug delivery platform can enable efficient phototherapy as well as address the limitations of chemotherapy by enhancing the efficacy and reducing the toxicity of chemotherapy drug molecules [77,79,141].
The configuration of the hydrogel scaffold used for phototherapy can be designed using the 3D printing technique. Wei et al. [223] presented a simple and effective technique for producing core-shell hydrogel fibers/scaffolds that had potential application in PTT-chemotherapy for residual breast cancer treatment. The core-shell hydrogel fibers/scaffolds were created through a coaxial 3D printing technique, where concentrated alginate inks mixed with polydopamine as a photothermal agent were used for the shell layer, while the core consisted of temperature-sensitive gelatin hydrogels loaded with therapeutic drug doxorubicin (DOX). The NIR irradiation could cause a temperature increase, leading to a gel-sol transition of the core hydrogels and resulting in on-demand drug release. Moreover, using DOX, polydopamine nanoparticles, gellan gum, and sodium alginate, Xu et al. [224] created a 3D-printable hydrogel scaffold designed to achieve antitumor photothermal chemotherapy and promote wound healing following surgical intervention.
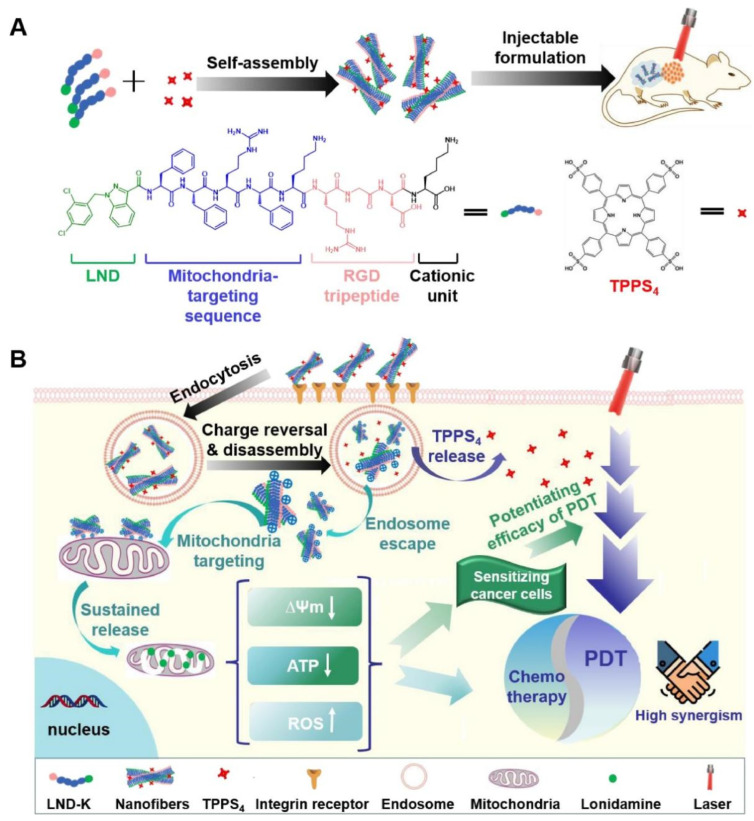
An injectable hydrogel is an ideal form of hydrogel for local hydrogel administration for phototherapy. The study conducted by Yang et al. [225] revealed that incorporating selenium as an anticancer agent and ICG as a photothermal agent into an injectable catecholamine-modified hyaluronic acid hydrogel can result in efficient PTT-chemotherapy for breast cancers. Additionally, Wu et al. [226] designed an injectable hydrogel composed of a lonidamine-conjugated peptide (LND-K) and photosensitizer TPPS4. The peptide self-assembled to form the hydrogel and enabled the targeted delivery of lonidamine to mitochondria for efficient photodynamic/chemotherapy combined therapy (Figure 7). In addition, Hou et al. [227] developed a nanogel system by combining an engineered polypeptide PC10A–arginine–glycine–aspartic acid (PC10ARGD) with Ag2S quantum dot as a photosensitizer. The nanogel system was subsequently loaded with DOX, a chemotherapy drug, and Bestatin, an immune-adjuvant drug, to transform into an injectable hydrogel. This strategy provides an effective combination of PTT and immunotherapy for the treatment of tumors. However, it should be noted that the long-term biosafety of Ag2S quantum dots needs to be further investigated.
Figure 7.
Schematic illustrations of self-assembled injectable hydrogels for combinational antitumor PDT-chemotherapy. (A) Injectable formulation of LND-K and TPPS4 for PDT. (B) Synergistic cancer therapy achieved by utilizing the self-assembled injectable hydrogel. Reprinted with permission from [226].
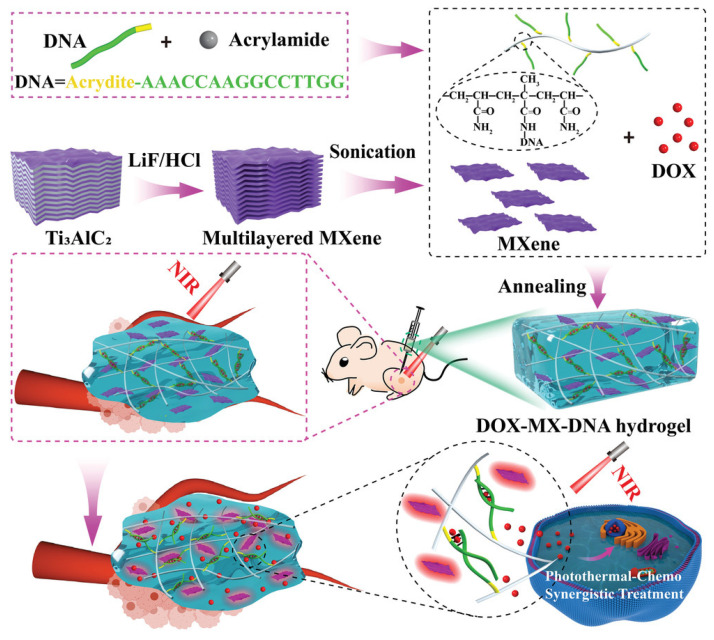
Thermosensitive hydrogels are the most popular smart hydrogels for synergistic PTT-chemotherapy. For hydrogels with good fluidity or thermosensitivity, PTT can regulate and control the release of chemotherapy drugs. For example, Huang et al. [87] developed a thermosensitive Pluronic F127 hydrogel platform incorporating self-assembled nanoparticles containing thermosensitive liposomes, gold–manganese oxide (Au–MnO) nanoparticles, and DOX. Under NIR light irradiation, the Au–MnO triggered the nanoparticles to release DOX into the hydrogel, while the sol–gel transition of the hydrogel at 37 °C avoided drug leakage and maintained sustained DOX release at the tumor site. Furthermore, Qi et al. [82] developed a thermosensitive hydrogel through the self-assembly of thermosensitive polymers PNIPAM, PEGDA, and 2,2-azobis [2-(2-imidazolin-2-yl) propane, which can undergo a sol–gel transition triggered by heat. After being loaded with ICG-methotrexate (MTX) nanomedicines, the hydrogel system exhibited additional desirable PTT efficiency and controlled release of MTX. Furthermore, Chang et al. [228] developed a thermosensitive hydrogel by combining thermosensitive polymers P(NIPAM-co-AH) and oxidized carboxymethyl cellulose. With the addition of a photothermal agent gold nanorods and DOX, the hydrogel system can realize the NIR-triggered photothermal effect and localized drug release of DOX. In another study, Zhang et al. [88] developed an injectable hydrogel system that incorporated chitosan polymeric micelles loaded with PTX and PEGylated gold nanorods in a thermosensitive Pluronic F127 hydrogel. This innovative approach enabled precise and selective thermal ablation of tumors while reducing the systemic toxicity of PTX. In addition, He et al. [229] synthesized a thermosensitive DNA hydrogel using acrydite-modified DNA and acrylamide monomers through conventional radical polymerization, which was integrated with DOX and a photothermal agent Ti3C2TX-based MXene nanosheets to establish the efficient antitumor PTT-chemotherapy (Figure 8). The gel-to-solution transition of the DOX-loaded MXene-DNA hydrogel can be triggered by a temperature rise resulting from the photothermal effect of MXene nanosheets upon NIR light irradiation. This process also involved the unwinding of DNA duplex crosslinking structures, enabling the on-demand release of DOX. Note that the long-term toxicity of Ti3C2TX-based MXene nanosheets should be thoroughly evaluated.
Figure 8.
Schematic illustrations of self-assembled injectable hydrogels for combinational antitumor PDT-chemotherapy. Reprinted with permission from [229].
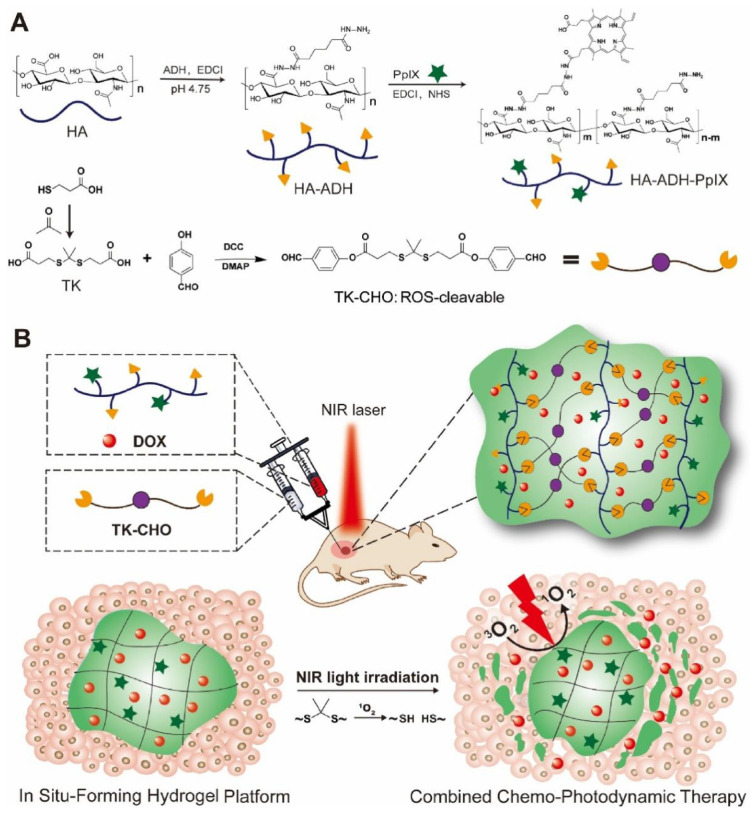
In addition to the aforementioned thermosensitive hydrogels, other types of stimulus-responsive hydrogels have also been investigated for combinational antitumor phototherapy-chemotherapy. Ghavaminejad et al. [83] incorporated DOX and photothermal agent dopamine nanoparticles, which were loaded with chemotherapy drugs bortezomib, into a thermosensitive pNIPAAm-co-pAAm hydrogel. Under the acidic tumor microenvironment, the boronic acid functionality of bortezomib would dissociate from the catechol groups of dopamine nanoparticles, thus leading to the release of this chemotherapy drug. As a result, the hydrogel can deliver drugs under an acidic environment as well as under NIR light irradiation. Zhang et al. [230] developed a silk sericin–chitosan hydrogel incorporating the chemotherapy drug tegafur (TF) and protoporphyrin IX (PpIX) heterodimers (TTP). The TTP formulation includes ROS-sensitive thioether bonds linking TF and the photosensitizer PpIX, allowing for gradual drug release upon the destruction of thioether bonds by high concentrations of ROS. The proposed approach achieved a synergistic effect between chemotherapy and PDT, while the on-demand drug release mechanism maximized the therapeutic effects of TF while minimizing its potential toxicity. Xu et al. [231] developed an injectable hyaluronic acid hydrogel that can be degraded in response to ROS and loaded it with PpIX as a photosensitizer and DOX as a chemotherapy drug. To achieve stable and efficient PDT, PpIX was covalently bonded to the hydrogel. Under NIR light irradiation, ROS generated by the photosensitizer can decompose the hydrogel, leading to an on-demand release of DOX (Figure 9).
Figure 9.
(A) Schematic illustrations for the synthesis of formulation of the ROS-sensitive hyaluronic acid hydrogel and (B) its application for combinational antitumor PDT-chemotherapy. Reprinted with permission from [231].
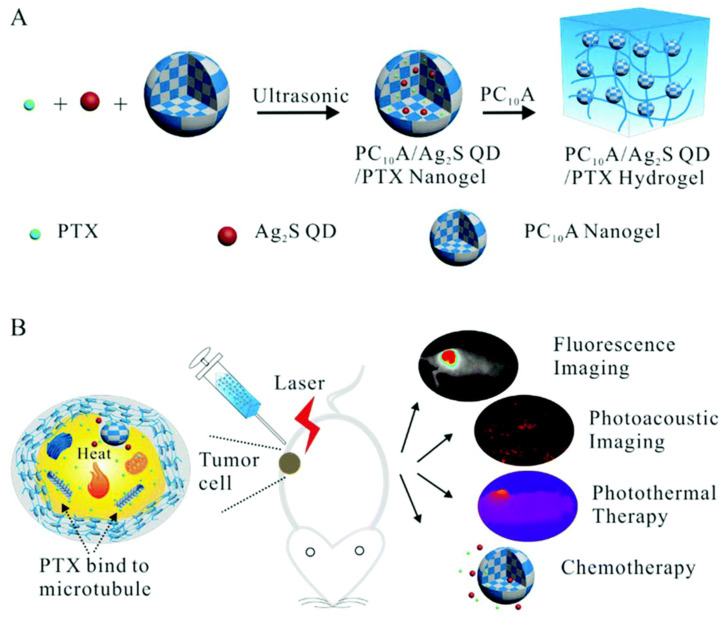
Nanogels as drug carriers also have been applied for combinational antitumor PDT-chemotherapy. The study by González-Ayón et al. [232] investigated the use of galacto-functionalized poly(N-vinylcaprolactam) (PNVCL)-based nanogels for encapsulating cisplatin or DOX and gold nanorods. The results demonstrated that the nanogels achieved an encapsulation efficiency of approximately 64% and 52% for cisplatin and DOX, respectively. This study highlighted the potential of PNVCL nanogels loaded with gold nanorods and cisplatin/DOX for enhancing PTT-chemotherapy. Jin et al. [233] developed an injectable multifunctional hydrogel as a theranostic platform for sustained antitumor PTT-chemotherapy. To achieve this, they prepared a nanogel system using an engineered polypeptide PC10A, which was loaded with oil-soluble PTX as a chemotherapy drug and Ag2S quantum dots (QDs) as photothermal agents. The PC10A hydrogel was then used to dissolve the nanogel system. The final hydrogel was found to be effective in inhibiting tumor cell growth, while NIR fluorescence and PA imaging enabled real-time monitoring of hydrogel degradation in vivo (Figure 10). However, the long-term biosafety of Ag2S QDs should be carefully evaluated.
Figure 10.
(A) Schematic illustration of the preparation of the injectable multifunctional PC10A hydrogel and (B) its application for combinational antitumor PTT-chemotherapy. Reproduced from Ref. [233] with permission from the Royal Society of Chemistry.
Smart nanogels as promising carriers for combinational antitumor PTT-chemotherapy also have been widely investigated. Arjamaet et al. [139] synthesized hydrogel nanocubes composed of carboxymethyl-chitosan and PEG that were loaded with DOX-ICG, exhibiting pH and redox-responsive drug release behavior. The hydrogel nanocubes also integrated endosomal/lysosomal escape and rapid degradation in response to intracellular GSH into their design. Theune et al. [84] designed a nanogel system using thermosensitive polymers PNIPAM/PNIPMAM that incorporated the photothermal agent polymer polypyrrole (PPY) for combinational antitumor PTT-chemotherapy. The system was tested with MTX as a model antitumor drug and was found to be an efficient drug delivery system for chemotherapy. Zhou et al. [137] synthesized a DNA nanogel system by combining DOX as a chemotherapy drug, polyethyleneimine-black phosphorus quantum dots as photosensitizers/photothermal agents, and X-shaped DNA molecules through hydrogen and electrostatic bonding. This innovative approach allowed for the efficient on-demand delivery of DOX and combined PDT/PTT upon NIR light irradiation. Howaili et al. [140] synthesized a plasmonic nanogel with dual thermo-pH-responsiveness as a drug carrier system by grafting PNIPAM to chitosan using a chemical crosslinker. The incorporation of gold nanoparticles loaded with curcumin into the nanogel yielded a stimuli-responsive nanocarrier system with the potential for effective PTT-chemotherapy in tumor treatment.
4.3. Hydrogel-Based Phototherapy Combined with Radiotherapy
Radiotherapy is a widely utilized and effective tumor treatment technique that involves the use of ionizing radiation (e.g., γ-rays and X-rays) to destroy tumors by generating cytotoxic ROS [234]. The limited tissue penetration of light in phototherapy hampers its effectiveness in treating deep-seated tumors. In contrast, radiotherapy using high-energy X-rays can effectively penetrate the tissue and eliminate tumor cells located deep within the tumor tissue. However, the regulation of ionizing radiation dosage for eliminating tumors is challenging due to tumor cell radiation-resistance, which is related to the tumor hypoxic microenvironment, and the need to shield healthy tissues from the harmful effects of radiation exposure [235,236,237]. Fortunately, phototherapy can enhance the therapeutic outcomes of radiotherapy, overcoming some of its limitations [199,238,239]. The therapeutic efficacy of PDT and radiotherapy can be heightened when they are used in combination, and thus PDT can facilitate a reduction in radiation dose or exposure duration [240]. Additionally, PTT raises the temperature of the tumor tissue, which can enhance tumor oxygenation and heighten the sensitivity of the tumor to X-ray radiation [241].
Hydrogels are widely used as a platform for synergistic antitumor phototherapy/radiotherapy. For example, Mirrahimi et al. [242] incorporated cisplatin as a radiosensitizer and gold nanoparticles as photosensitizers and photothermal agents into an alginate hydrogel, resulting in successful and efficient antitumor thermo-chemo-radiotherapy. In another study, Wang et al. [243] investigated the use of agarose hydrogel containing Prussian blue nanoparticles for combined radiotherapy and PTT. The Prussian blue nanoparticles exhibited a high-efficiency photothermal effect under NIR light irradiation and could react with endogenous H2O2 to generate oxygen, thereby counteracting the hypoxic tumor environment and enhancing the sensitivity of tumor cells to radiotherapy. Furthermore, Zhou et al. [244] synthesized Cys-crosslinked γ-PGA nanogels and then incorporated photothermal agent PPY via oxidation polymerization under the influence of Fe(III) ions. They found that performing X-ray radiation after laser irradiation significantly increased the sensitization of the tumor to PTT.
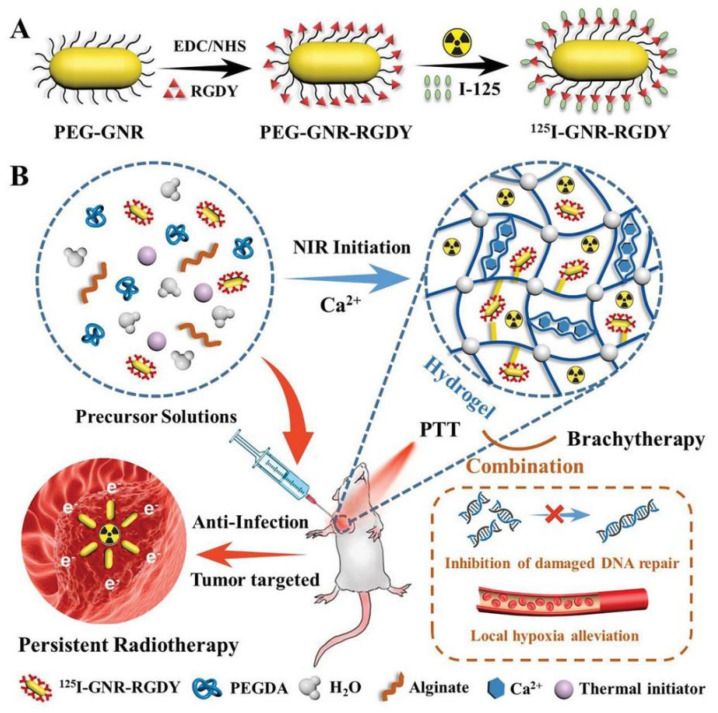
Brachytherapy is an important and useful type of radiotherapy that involves the implantation of small radioactive sources directly into or near the target tissue to destroy tumor cells [245]. Mukerji et al. [246] studied the use of photosensitizer Ce6 and radionuclide 125I in a hydrogel made from elastin-like polypeptides containing cysteine residues that can undergo ROS-mediated disulfide crosslinking. The hydrogel enabled stable fixation of 125I and improved safety in brachytherapy, while also showing a synergistic effect for PDT and brachytherapy. Moreover, Wu et al. [247] developed a PEGDA-alginate double-network nanocomposite hydrogel to provide a combinational PTT-brachytherapy therapy for preventing local recurrence and wound infection in postoperative breast cancer. They used 125I-labeled Arg–Gly–Asp–Tyr (RGDY)-modified gold nanorods as phototherapeutic agents to realize combinational PTT-brachytherapy effects. The double-network hydrogel was produced through a process that involved the initiation of PEGDA polymerization by heat and the formation of a second crosslink between alginate and Ca2+-alginate established in the tumor microenvironment (Figure 11). However, the development of nanocomposite hydrogels is a complex and multi-step process, and the long-term degradability and toxicity of the component should be thoroughly evaluated.
Figure 11.
(A) Schematic illustration of the preparation of the modified gold nanorods and (B) the PEGDA-alginate double-network nanocomposite hydrogel and their application for combinational PTT-brachytherapy. Reprinted with permission from [247].
4.4. Hydrogel-Based Phototherapy Combined with Starvation Therapy
Starvation therapy is a therapeutic approach aimed at blocking the energy supply to tumor cells. It is a treatment approach that commonly utilizes glucose oxidase (GOx) or its mimicking enzymes to catalyze glucose into gluconic acid and H2O2, ultimately leading to glucose depletion and reduced ATP levels [248,249,250,251]. By decreasing ATP levels in tumor tissues, starvation therapy can downregulate HSP expression in tumor cells, which can enhance the efficacy of PTT [252,253,254]. Moreover, the H2O2 produced, which serves as a source of oxygen, can further improve the efficacy of PDT [255]. Despite its potential, GOx-mediated starvation therapy faces significant challenges such as safety concerns, immunogenicity, low stability, and fragile enzymatic activity, which limit its clinical applicability [256,257]. Thus, hydrogels have recently gained significant attention as a platform for combining phototherapy and starvation therapy.
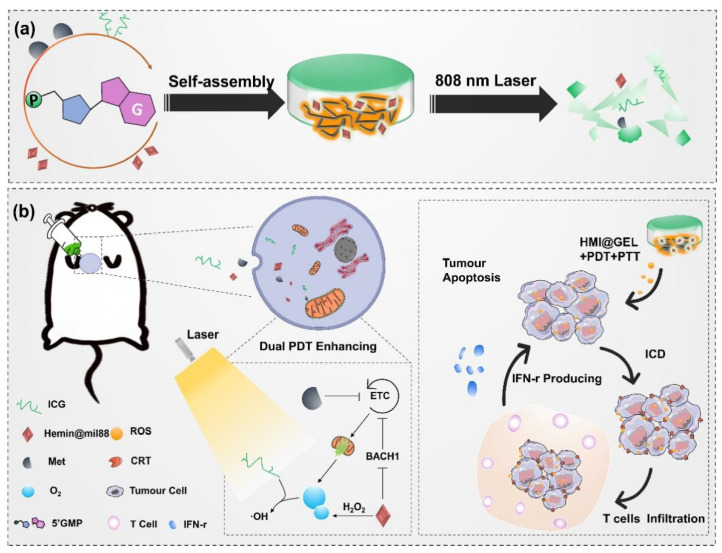
Recently, smart hydrogels have received much more preference. He et al. [258] developed a multifunctional hydrogel platform by incorporating GOx, a hydrogen peroxide catalytic active agent, and a photothermal agent prodrug into a thermosensitive PDLLA1500-PEG1500-PDLLA1500 hydrogel. This platform employed starvation therapy to block the energy supply of tumor tissue, thereby inhibiting the expression of HSP in tumor cells. By utilizing a relatively lower therapeutic temperature, the synergistic application of PTT and starvation therapy can achieve high tumor therapeutic efficacy while reducing the risk of burns to surrounding tissues and the local inflammatory response. Moreover, Sun et al. [259] fabricated a NIR light-responsive hydrogel by combining ICG as a phototherapeutic agent for PDT/PTT and 5′-guanosine monophosphate (5′GMP). Subsequently, they delivered a NIR light-responsive hydrogel system (HMI@GEL) after loading metformin (Met) to block the energy supply of the tumor and catalase-mimicking Hemin@mil88 to effectively alleviate tumor hypoxia. The hydrogel system achieved light-controlled local delivery of Met to the tumor site, which greatly reduced systemic side effects from Met leakage. In vivo antitumor experiments indicated that this hydrogel system could achieve excellent anti-tumor PDT/PTT efficacy (Figure 12).
Figure 12.
(a) Schematic illustration of the preparation of HMI@GEL hydrogel and (b) its application for combinational phototherapy-starvation therapy. Reprinted with permission from [259].
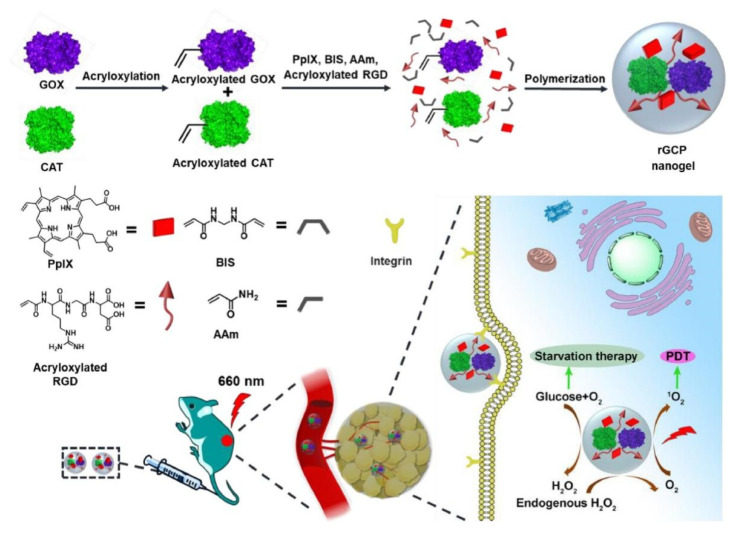
Nanogel-based platforms can also provide efficient combinational phototherapy-starvation therapy for tumor treatment. Luo et al. [260] and Fan et al. [261] created enzyme-immobilized nanogels with tumor tissue-targeting ability and self-supplying oxygen capability to counteract the rapid proliferation of cancer cells. The nanogel incorporating GOX and catalase (CAT) was fabricated via polymerization, utilizing PpIX and cancer-cell-specific Arg-Gly-Asp (RGD) as comonomers (Figure 13). Inside the nanogel, the cascade reaction efficiently utilized intracellular glucose catalyzed by GOX, while CAT safely decomposed the generated H2O2 to generate oxygen. The production of oxygen could further enhance glucose consumption by GOX and facilitate the generation of 1O2. Combining starvation therapy and PDT, the nanogel system exhibited a potent synergistic effect against cancer cells. However, the stability and degradability of the nanogel should be further evaluated.
Figure 13.
Schematic illustration of enzyme-immobilized nanogels for synergistic PDT and starvation therapy. Reprinted with permission from [261].
4.5. Hydrogel-Based Phototherapy Combined with Chemodynamic Therapy
Chemodynamic therapy is a promising tumor treatment modality that generates cytotoxic hydroxyl radicals via the catalysis of metal ions on H2O2 through a Fenton or Fenton-like reaction, and unlike oxygen-dependent PDT, it does not rely on the availability of oxygen [262,263,264,265]. For example, Chen et al. [266] incorporated Hu Kaiwen ink as a photothermal agent and dihydroartemisinin-Fe2+ as an inducer of ROS into an agarose hydrogel to achieve synergistic antitumor PTT-chemodynamic therapy. Moreover, Qin et al. [267] prepared an enzyme complex-loaded hybrid nanogel that could function in diverse biomedical applications related to ROS regulation and could serve as a safe and effective PDT-chemodynamic therapy for tumors.
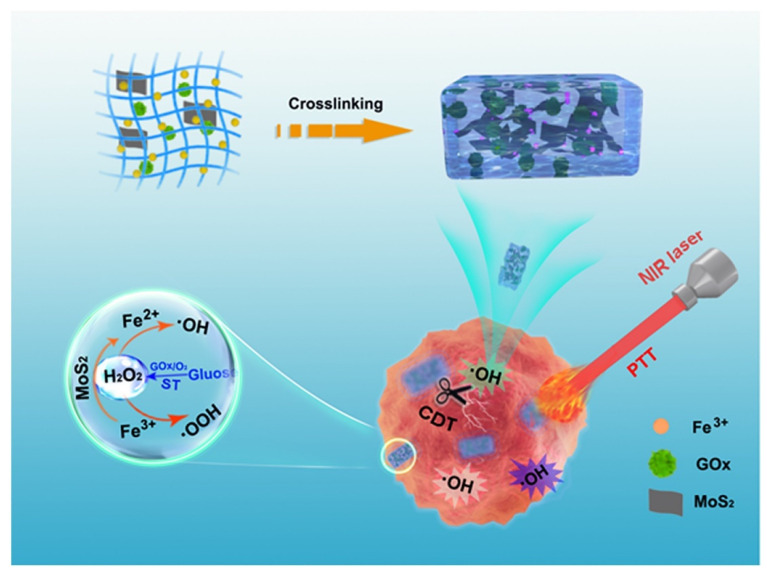
However, chemodynamic therapy alone is often insufficient to achieve a satisfactory antitumor effect due to the limited H2O2 in the tumor microenvironment [268,269,270,271,272]. Therefore, it is often combined with GOx-mediated starvation therapy, which can produce H2O2, to achieve the desired therapeutic outcome. For example, Zhou et al. [273] wisely designed a novel sodium-alginate hydrogel system for combined cancer photothermal, starvation, and chemodynamic therapy. This hydrogel encapsulates molybdenum dioxide (MoS2) nanosheets as a photothermal agent, GOx that mediates starvation therapy, and Fe3+ as a cross-linking agent. In this hydrogel system, MoS2 nanosheets had a strong photothermal ability and can convert Fe3+ to Fe2+. GOx can catalyze glucose to produce gluconic acid and H2O2, and the Fe2+ can undergo a Fenton reaction with excess H2O2 to produce hydroxyl radicals, achieving an enhanced therapeutic effect (Figure 14). In another study, Xu et al. [274] utilized a gelatin-hydroxyphenyl hydrogel as a carrier for the enzyme CoMnFe-layered double oxides (CoMnFe-LDO) and the GOx. Due to the cascaded catalytic reaction performed by CoMnFe-LDO and GOx, the composite hydrogel can realize satisfactory synergetic multifunctional tumor therapies, including chemodynamic therapy, starvation therapy, and PTT. Furthermore, Lee et al. [275] developed a hyaluronic acid-based hydrogel for the treatment of breast cancer through multiple approaches including ferroptosis, PTT, chemodynamic therapy, and starvation therapy.
Figure 14.
Schematic illustration of the sodium-alginate hydrogel system for combinational photothermal/starvation/chemodynamic therapy. Reprinted with permission from [273].
4.6. Hydrogel-Based Phototherapy Combined with Other Antitumor Modalities
In addition to the above-mentioned combinational strategies for tumor treatment, researchers have also explored antitumor modalities that combine phototherapy with other antitumor treatments, such as gene therapy, nitric oxide (NO) gas therapy, thermodynamic therapy, anti-angiogenic therapy, and epigenetic therapy.
Conde et al. [276] simultaneously loaded small interfering RNA, gold nanorods, and Avastin into a dendrimer/dextran hydrogel for a synergistic gene/drug/phototherapy. Their study demonstrated that modified gold nanorods as phototherapeutic agents, which were used as small interfering RNA carriers, could significantly knock down cancer-promoting genes. Additionally, modified gold nanorods were also used to load antitumor drugs Avastin and enhance targeting drug delivery. This three-mode synergistic treatment strategy of phototherapy-chemotherapy-gene therapy has the potential to eliminate tumors and prevent a recurrence, making it a promising candidate for clinical applications.
Sun et al. [277] developed an injectable and thermosensitive hydrogel system that enabled combined PDT-NO gas therapy. The hydrogel system was based on a poly(ε-caprolactone)-poly(ethylene glycol)-poly(ε-caprolactone) (PCL–PEG–PCL) hydrogel, which served as a carrier for poly(lactic-glycolic acid) (PLGA) nanoparticles encapsulating both the photosensitizer ICG and NO donor l-arginine. The system induced ROS production and promoted NO production, leading to cancer cell apoptosis and inhibition of cancer cell proliferation. However, the long-term biosafety of PCL–PEG–PCL copolymer should be thoroughly evaluated, and the large-scale production of this hydrogel system seemed to be challenging.
To combine PTT-thermodynamic therapy for hypoxic tumor treatment, Sun et al. [278] developed an innovative injectable hydrogel formed by copolymerizing hydrophilic glycidyl methacrylate-modified hyaluronic acid with hydrophobic N-isopropyl acrylamide. The addition of 2,2′-Azobis [2-(2-imidazalin-2-yl)propane] dihydrochloride (AIPH) as an alkyl-free radical source and gold nanorods as photothermal agents enabled the hydrogel to generate free radicals from AIPH and heat through the photothermal effects, providing an efficient strategy for treating hypoxic tumors.
To realize combined PTT and targeted anti-angiogenic therapy for tumor treatment, Wu et al. [279] designed an injectable gellan gum hydrogel that integrated ultra-small bismuth sulfide (Bi2S3) nanodots as photothermal agents and sorafenib as a targeted therapy drug. However, the long-term safety of Bi2S3 nanodots-encapsulated hydrogels is questionable due to the unknown cytotoxicity associated with Bi2S3 nanodots.
To achieve synergistic PDT/epigenetic therapy for prostate cancer, Liu et al. [280] devised a smart therapeutic nanoplatform that used a nanogel as a photosensitizer and drug carrier. By suppressing HIF-1α and VEGF pathways that contribute to PDT resistance, the inclusion of histone deacetylase inhibitors into PDT enhanced the synergistic PDT/epigenetic therapy for prostate cancer. The study highlighted the well-designed architecture of the nanoplatform, which functioned as a photodynamic agent without releasing Ce6 photosensitizer molecules in a responsive environment. It also allowed for the incorporation of diverse functional components for smart drug release and imaging-guided combination therapy.
5. Conclusions and Prospect
In recent years, significant progress has been made in the development of hydrogels for antitumor phototherapy, which shows great potential for clinical applications in tumor treatment. The use of hydrogels in antitumor phototherapy offers unique advantages, including (1) the ability to encapsulate phototherapeutic agents to enhance their therapeutic efficacy and reduce their side effects; (2) easy access to the target site via needle injection with injectable hydrogels; (3) the ability of smart hydrogels to release phototherapeutic agents on-demand; (4) the selective accumulation of nanogels in tumors through EPR effect; (5) the capability of nanogels to deliver phototherapeutic agents intracellularly, with potential tumor-targeting and multifunctionality features; and (6) the capacity of hydrogels as a carrier to synergistically combine with other therapeutic modalities to achieve a more effective therapeutic effect.
However, hydrogel-based phototherapies face some limitations in clinical tumor treatment. First, the in vivo stability and degradability of phototherapeutic hydrogel systems remain uncertain and concerning. The impact of prolonged exposure of hydrogels on tumor sites and the mechanism of its phototherapeutic effect are not yet to be fully understood. Additionally, there is a lack of clarity regarding the long-term toxic and side effects of phototherapeutic agents and hydrogels on organisms in current studies. Second, the therapeutic efficacy of most hydrogels-based phototherapies lacks rigorous assessment or verification in tumor models. Moreover, the commonly used mouse tumor models in preclinical experiments are not adequate to reflect the physiological conditions of clinical patients. To advance the clinical translation of hydrogels-based phototherapies, it is necessary to assess their therapeutic efficacy in larger animals, such as dogs, pigs, and monkeys.
Furthermore, the design, preparation, and application of phototherapeutic hydrogel systems are still in the experimental stage due to the current lack of clinical investigations. To date, no hydrogel-based antitumor phototherapy has been registered on the Clinical Trials Gov database (http://www.clinicaltrials.gov (accessed on 26 March 2023)). Furthermore, compared with bulk hydrogels, the clinical application of nanogels is a relatively new area of research, with only one nanogel-related therapeutic modality entered clinical trials (ClinicalTrials.gov Identifier: NCT05268718). Many therapeutic factors (such as questionable biosafety and efficacy) and non-therapeutic factors (such as lack of clear regulatory guidelines and standardized assays) hinder the clinical transition of nanogels [126]. Therefore, compared with nanogels, bulk hydrogels-based phototherapies are more promising to enter the clinical stage first.
In conclusion, hydrogels are promising carriers for antitumor phototherapies, but further in-depth research is required to establish the safety and effectiveness of hydrogel-based phototherapy for tumor clinical treatment. Although the current understanding is limited, there is a growing expectation that hydrogel-based phototherapy will soon experience a significant surge in clinical applications. Hence, it is crucial to continuously explore the possibilities offered by hydrogel-based phototherapy to optimize its future clinical applications in tumor treatment.
Acknowledgments
Not applicable.
Author Contributions
Conceptualization, J.L., S.G. and Y.W.; investigation, X.Z., Z.Z., M.Z. and L.L.; writing—original draft preparation, S.G. and Y.W.; writing—review and editing, J.L.; supervision, J.L. and W.C.; funding acquisition, W.C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was financially supported by the National Natural Science Foundation of China (81771049 and 82270957), the Department of Science and Technology of Sichuan Province (2020YJ0228), Med-X Innovation Programme of Med-X Center for Materials, Sichuan University (MCM202105), and Research and Develop Program of West China Hospital of Stomatology Sichuan University (LCYJ2019-13).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.He M., Sui J., Chen Y., Bian S., Cui Y., Zhou C., Sun Y., Liang J., Fan Y., Zhang X. Localized multidrug co-delivery by injectable self-crosslinking hydrogel for synergistic combinational chemotherapy. J. Mater. Chem. B. 2017;5:4852–4862. doi: 10.1039/C7TB01026E. [DOI] [PubMed] [Google Scholar]
- 3.Qi Y., Min H., Mujeeb A., Zhang Y., Han X., Zhao X., Anderson G.J., Zhao Y., Nie G. Injectable Hexapeptide Hydrogel for Localized Chemotherapy Prevents Breast Cancer Recurrence. ACS Appl. Mater. Interfaces. 2018;10:6972–6981. doi: 10.1021/acsami.7b19258. [DOI] [PubMed] [Google Scholar]
- 4.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 5.Pérez-Herrero E., Fernández-Medarde A. Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy. Eur. J. Pharm. Biopharm. Off. J. Arb. Fur Pharm. Verfahr. Ev. 2015;93:52–79. doi: 10.1016/j.ejpb.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 6.Liang C., Xu L., Song G., Liu Z. Emerging nanomedicine approaches fighting tumor metastasis: Animal models, metastasis-targeted drug delivery, phototherapy, and immunotherapy. Chem. Soc. Rev. 2016;45:6250–6269. doi: 10.1039/C6CS00458J. [DOI] [PubMed] [Google Scholar]
- 7.Li X., Lovell J.F., Yoon J., Chen X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 2020;17:657–674. doi: 10.1038/s41571-020-0410-2. [DOI] [PubMed] [Google Scholar]
- 8.Zhu H., Cheng P., Chen P., Pu K. Recent progress in the development of near-infrared organic photothermal and photodynamic nanotherapeutics. Biomater. Sci. 2018;6:746–765. doi: 10.1039/C7BM01210A. [DOI] [PubMed] [Google Scholar]
- 9.Jung H.S., Verwilst P., Sharma A., Shin J., Sessler J.L., Kim J.S. Organic molecule-based photothermal agents: An expanding photothermal therapy universe. Chem. Soc. Rev. 2018;47:2280–2297. doi: 10.1039/C7CS00522A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chitgupi U., Qin Y., Lovell J.F. Targeted Nanomaterials for Phototherapy. Nanotheranostics. 2017;1:38–58. doi: 10.7150/ntno.17694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen J., Ning C., Zhou Z., Yu P., Zhu Y., Tan G., Mao C. Nanomaterials as photothermal therapeutic agents. Prog. Mater. Sci. 2019;99:1–26. doi: 10.1016/j.pmatsci.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poderys V., Jarockyte G., Bagdonas S., Karabanovas V., Rotomskis R. Protein-stabilized gold nanoclusters for PDT: ROS and singlet oxygen generation. J. Photochem. Photobiol. B Biol. 2020;204:111802. doi: 10.1016/j.jphotobiol.2020.111802. [DOI] [PubMed] [Google Scholar]
- 13.Castano A.P., Mroz P., Hamblin M.R. Photodynamic therapy and anti-tumour immunity. Nat. Rev. Cancer. 2006;6:535–545. doi: 10.1038/nrc1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castano A.P., Demidova T.N., Hamblin M.R. Mechanisms in photodynamic therapy: Part three-Photosensitizer pharmacokinetics, biodistribution, tumor localization and modes of tumor destruction. Photodiagnosis Photodyn. Ther. 2005;2:91–106. doi: 10.1016/S1572-1000(05)00060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fingar V.H. Vascular effects of photodynamic therapy. J. Clin. Laser Med. Surg. 1996;14:323–328. doi: 10.1089/clm.1996.14.323. [DOI] [PubMed] [Google Scholar]
- 16.Lan M., Zhao S., Liu W., Lee C.S., Zhang W., Wang P. Photosensitizers for Photodynamic Therapy. Adv. Healthc. Mater. 2019;8:e1900132. doi: 10.1002/adhm.201900132. [DOI] [PubMed] [Google Scholar]
- 17.Liu M., Li C. Recent Advances in Activatable Organic Photosensitizers for Specific Photodynamic Therapy. ChemPlusChem. 2020;85:948–957. doi: 10.1002/cplu.202000203. [DOI] [PubMed] [Google Scholar]
- 18.Rejinold N.S., Choi G., Choy J.H. Recent Developments on Semiconducting Polymer Nanoparticles as Smart Photo-Therapeutic Agents for Cancer Treatments-A Review. Polymers. 2021;13:981. doi: 10.3390/polym13060981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huis In ‘t Veld R.V., Heuts J., Ma S., Cruz L.J., Ossendorp F.A., Jager M.J. Current Challenges and Opportunities of Photodynamic Therapy against Cancer. Pharmaceutics. 2023;15:330. doi: 10.3390/pharmaceutics15020330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seung Lee J., Kim J., Ye Y.S., Kim T.I. Materials and device design for advanced phototherapy systems. Adv. Drug Deliv. Rev. 2022;186:114339. doi: 10.1016/j.addr.2022.114339. [DOI] [PubMed] [Google Scholar]
- 21.Xu H., Nie W., Dai L., Luo R., Lin D., Zhang M., Zhang J., Gao F. Recent advances in natural polysaccharides-based controlled release nanosystems for anti-cancer phototherapy. Carbohydr. Polym. 2023;301:120311. doi: 10.1016/j.carbpol.2022.120311. [DOI] [PubMed] [Google Scholar]
- 22.Cui D., Huang J., Zhen X., Li J., Jiang Y., Pu K. A Semiconducting Polymer Nano-prodrug for Hypoxia-Activated Photodynamic Cancer Therapy. Angew. Chem. 2019;58:5920–5924. doi: 10.1002/anie.201814730. [DOI] [PubMed] [Google Scholar]
- 23.Habault J., Poyet J.L. Recent Advances in Cell Penetrating Peptide-Based Anticancer Therapies. Molecules. 2019;24:927. doi: 10.3390/molecules24050927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van der Meel R., Sulheim E., Shi Y., Kiessling F., Mulder W.J.M., Lammers T. Smart cancer nanomedicine. Nat. Nanotechnol. 2019;14:1007–1017. doi: 10.1038/s41565-019-0567-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raina N., Pahwa R., Bhattacharya J., Paul A.K., Nissapatorn V., de Lourdes Pereira M., Oliveira S.M.R., Dolma K.G., Rahmatullah M., Wilairatana P., et al. Drug Delivery Strategies and Biomedical Significance of Hydrogels: Translational Considerations. Pharmaceutics. 2022;14:574. doi: 10.3390/pharmaceutics14030574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y., Li X., Zhang Y., Wang L., Yang Z. A supramolecular hydrogel to boost the production of antibodies for phosphorylated proteins. Chem. Commun. 2019;55:12388–12391. doi: 10.1039/C9CC05633E. [DOI] [PubMed] [Google Scholar]
- 27.Deng H., Yu Z., Chen S., Fei L., Sha Q., Zhou N., Chen Z., Xu C. Facile and eco-friendly fabrication of polysaccharides-based nanocomposite hydrogel for photothermal treatment of wound infection. Carbohydr. Polym. 2020;230:115565. doi: 10.1016/j.carbpol.2019.115565. [DOI] [PubMed] [Google Scholar]
- 28.Zhuang Y., Kan Z., Jiang Y., He M., Zhang Y., Sun X., Du M., Wang J., Li Y., Li Y., et al. Constructing an on-demand drug release system composed of thermosensitive PPP hydrogel and drug-laden alginate/graphene microspheres to treat tumorous defect. J. Mater. Sci. 2022;57:4754–4770. doi: 10.1007/s10853-022-06907-4. [DOI] [Google Scholar]
- 29.Wang M., Chen M., Niu W., Winston D.D., Cheng W., Lei B. Injectable biodegradation-visual self-healing citrate hydrogel with high tissue penetration for microenvironment-responsive degradation and local tumor therapy. Biomaterials. 2020;261:120301. doi: 10.1016/j.biomaterials.2020.120301. [DOI] [PubMed] [Google Scholar]
- 30.Kumar S., Bajaj A. Advances in self-assembled injectable hydrogels for cancer therapy. Biomater. Sci. 2020;8:2055–2073. doi: 10.1039/D0BM00146E. [DOI] [PubMed] [Google Scholar]
- 31.Du H., Liu W., Zhang M., Si C., Zhang X., Li B. Cellulose nanocrystals and cellulose nanofibrils based hydrogels for biomedical applications. Carbohydr. Polym. 2019;209:130–144. doi: 10.1016/j.carbpol.2019.01.020. [DOI] [PubMed] [Google Scholar]
- 32.Zhou X., Li Z. Advances and Biomedical Applications of Polypeptide Hydrogels Derived from α-Amino Acid N-Carboxyanhydride (NCA) Polymerizations. Adv. Healthc. Mater. 2018;7:e1800020. doi: 10.1002/adhm.201800020. [DOI] [PubMed] [Google Scholar]
- 33.Darge H.F., Hanurry E.Y., Birhan Y.S., Mekonnen T.W., Andrgie A.T., Chou H.-Y., Lai J.-Y., Tsai H.-C. Multifunctional drug-loaded micelles encapsulated in thermo-sensitive hydrogel for in vivo local cancer treatment: Synergistic effects of anti-vascular and immuno-chemotherapy. Chem. Eng. J. 2021;406:126879. doi: 10.1016/j.cej.2020.126879. [DOI] [Google Scholar]
- 34.Chao Y., Chen Q., Liu Z. Smart Injectable Hydrogels for Cancer Immunotherapy. Adv. Funct. Mater. 2020;30:1902785. doi: 10.1002/adfm.201902785. [DOI] [Google Scholar]
- 35.Cirillo G., Spizzirri U.G., Curcio M., Nicoletta F.P., Iemma F. Injectable Hydrogels for Cancer Therapy over the Last Decade. Pharmaceutics. 2019;11:486. doi: 10.3390/pharmaceutics11090486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Q., Wang C., Zhang X., Chen G., Hu Q., Li H., Wang J., Wen D., Zhang Y., Lu Y., et al. In situ sprayed bioresponsive immunotherapeutic gel for post-surgical cancer treatment. Nat. Nanotechnol. 2019;14:89–97. doi: 10.1038/s41565-018-0319-4. [DOI] [PubMed] [Google Scholar]
- 37.Meng Z., Zhou X., Xu J., Han X., Dong Z., Wang H., Zhang Y., She J., Xu L., Wang C., et al. Light-Triggered In Situ Gelation to Enable Robust Photodynamic-Immunotherapy by Repeated Stimulations. Adv. Mater. 2019;31:e1900927. doi: 10.1002/adma.201900927. [DOI] [PubMed] [Google Scholar]
- 38.Jian W.H., Wang H.C., Kuan C.H., Chen M.H., Wu H.C., Sun J.S., Wang T.W. Glycosaminoglycan-based hybrid hydrogel encapsulated with polyelectrolyte complex nanoparticles for endogenous stem cell regulation in central nervous system regeneration. Biomaterials. 2018;174:17–30. doi: 10.1016/j.biomaterials.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 39.Xue B., Wang W., Qin J.J., Nijampatnam B., Murugesan S., Kozlovskaya V., Zhang R., Velu S.E., Kharlampieva E. Highly efficient delivery of potent anticancer iminoquinone derivative by multilayer hydrogel cubes. Acta Biomater. 2017;58:386–398. doi: 10.1016/j.actbio.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li J., Mooney D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016;1:16071. doi: 10.1038/natrevmats.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kong C., Chen X. Combined Photodynamic and Photothermal Therapy and Immunotherapy for Cancer Treatment: A Review. Int. J. Nanomed. 2022;17:6427–6446. doi: 10.2147/IJN.S388996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Z., Wang J., Xia W., Cao D., Wang X., Kuang Y., Luo Y., Yuan C., Lu J., Liu X. Application of Hydrogels as Carrier in Tumor Therapy: A Review. Chem. Asian J. 2022;17:e202200740. doi: 10.1002/asia.202200740. [DOI] [PubMed] [Google Scholar]
- 43.Xing R., Liu Y., Zou Q., Yan X. Self-assembled injectable biomolecular hydrogels towards phototherapy. Nanoscale. 2019;11:22182–22195. doi: 10.1039/C9NR06266A. [DOI] [PubMed] [Google Scholar]
- 44.Zhang X., Xia L.-Y., Chen X., Chen Z., Wu F.-G. Hydrogel-based phototherapy for fighting cancer and bacterial infection. Sci. China-Mater. 2017;60:487–503. doi: 10.1007/s40843-017-9025-3. [DOI] [Google Scholar]
- 45.Saludas L., Pascual-Gil S., Prósper F., Garbayo E., Blanco-Prieto M. Hydrogel based approaches for cardiac tissue engineering. Int. J. Pharm. 2017;523:454–475. doi: 10.1016/j.ijpharm.2016.10.061. [DOI] [PubMed] [Google Scholar]
- 46.Lakshmanan R., Krishnan U.M., Sethuraman S. Polymeric scaffold aided stem cell therapeutics for cardiac muscle repair and regeneration. Macromol. Biosci. 2013;13:1119–1134. doi: 10.1002/mabi.201300223. [DOI] [PubMed] [Google Scholar]
- 47.Lima C.S.A., Balogh T.S., Varca J., Varca G.H.C., Lugão A.B., Camacho-Cruz L.A., Bucio E., Kadlubowski S.S. An Updated Review of Macro, Micro, and Nanostructured Hydrogels for Biomedical and Pharmaceutical Applications. Pharmaceutics. 2020;12:970. doi: 10.3390/pharmaceutics12100970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu Y., Chen H., Fang Y., Wu J. Hydrogel Combined with Phototherapy in Wound Healing. Adv. Healthc. Mater. 2022;11:e2200494. doi: 10.1002/adhm.202200494. [DOI] [PubMed] [Google Scholar]
- 49.Patel P., Thareja P. Hydrogels differentiated by length scales: A review of biopolymer-based hydrogel preparation methods, characterization techniques, and targeted applications. Eur. Polym. J. 2022;163:110935. doi: 10.1016/j.eurpolymj.2021.110935. [DOI] [Google Scholar]
- 50.Ahmed E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015;6:105–121. doi: 10.1016/j.jare.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guo J., Yao H., Li X., Chang L., Wang Z., Zhu W., Su Y., Qin L., Xu J. Advanced Hydrogel systems for mandibular reconstruction. Bioact. Mater. 2023;21:175–193. doi: 10.1016/j.bioactmat.2022.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang X., Wei P., Yang Z., Liu Y., Yang K., Cheng Y., Yao H., Zhang Z. Current Progress and Outlook of Nano-Based Hydrogel Dressings for Wound Healing. Pharmaceutics. 2022;15:68. doi: 10.3390/pharmaceutics15010068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kass L.E., Nguyen J. Nanocarrier-hydrogel composite delivery systems for precision drug release. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022;14:e1756. doi: 10.1002/wnan.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang H., Wu S., Chen W., Hu Y., Geng Z., Su J. Bone/cartilage targeted hydrogel: Strategies and applications. Bioact. Mater. 2023;23:156–169. doi: 10.1016/j.bioactmat.2022.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trubelja A., Kasper F.K., Farach-Carson M.C., Harrington D.A. Bringing hydrogel-based craniofacial therapies to the clinic. Acta Biomater. 2022;138:1–20. doi: 10.1016/j.actbio.2021.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lei L., Bai Y., Qin X., Liu J., Huang W., Lv Q. Current Understanding of Hydrogel for Drug Release and Tissue Engineering. Gels. 2022;8:301. doi: 10.3390/gels8050301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mahmood A., Patel D., Hickson B., DesRochers J., Hu X. Recent Progress in Biopolymer-Based Hydrogel Materials for Biomedical Applications. Int. J. Mol. Sci. 2022;23:1415. doi: 10.3390/ijms23031415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mo C., Luo R., Chen Y. Advances in the Stimuli-Responsive Injectable Hydrogel for Controlled Release of Drugs. Macromol. Rapid Commun. 2022;43:e2200007. doi: 10.1002/marc.202200007. [DOI] [PubMed] [Google Scholar]
- 59.Du W., Zong Q., Guo R., Ling G., Zhang P. Injectable Nanocomposite Hydrogels for Cancer Therapy. Macromol. Biosci. 2021;21:e2100186. doi: 10.1002/mabi.202100186. [DOI] [PubMed] [Google Scholar]
- 60.Zhuang B., Chen T., Xiao Z., Jin Y. Drug-loaded implantable surgical cavity-adaptive hydrogels for prevention of local tumor recurrence. Int. J. Pharm. 2020;577:119048. doi: 10.1016/j.ijpharm.2020.119048. [DOI] [PubMed] [Google Scholar]
- 61.Yu S., He C., Chen X. Injectable Hydrogels as Unique Platforms for Local Chemotherapeutics-Based Combination Antitumor Therapy. Macromol. Biosci. 2018;18:e1800240. doi: 10.1002/mabi.201800240. [DOI] [PubMed] [Google Scholar]
- 62.Zhao J., Wang L., Zhang H., Liao B., Li Y. Progress of Research in In Situ Smart Hydrogels for Local Antitumor Therapy: A Review. Pharmaceutics. 2022;14:2028. doi: 10.3390/pharmaceutics14102028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhao Y., Ran B., Xie X., Gu W., Ye X., Liao J. Developments on the Smart Hydrogel-Based Drug Delivery System for Oral Tumor Therapy. Gels. 2022;8:741. doi: 10.3390/gels8110741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.El-Husseiny H.M., Mady E.A., Hamabe L., Abugomaa A., Shimada K., Yoshida T., Tanaka T., Yokoi A., Elbadawy M., Tanaka R. Smart/stimuli-responsive hydrogels: Cutting-edge platforms for tissue engineering and other biomedical applications. Mater. Today Bio. 2022;13:100186. doi: 10.1016/j.mtbio.2021.100186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sun H., He Y., Wang Z., Liang Q. An Insight into Skeletal Networks Analysis for Smart Hydrogels. Adv. Funct. Mater. 2022;32:2108489. doi: 10.1002/adfm.202108489. [DOI] [Google Scholar]
- 66.Sharifzadeh G., Hosseinkhani H. Biomolecule-Responsive Hydrogels in Medicine. Adv. Healthc. Mater. 2017;6:1700801. doi: 10.1002/adhm.201700801. [DOI] [PubMed] [Google Scholar]
- 67.Fan C., Shi J., Zhuang Y., Zhang L., Huang L., Yang W., Chen B., Chen Y., Xiao Z., Shen H., et al. Myocardial-Infarction-Responsive Smart Hydrogels Targeting Matrix Metalloproteinase for On-Demand Growth Factor Delivery. Adv. Mater. 2019;31:e1902900. doi: 10.1002/adma.201902900. [DOI] [PubMed] [Google Scholar]
- 68.Huang K.T., Ishihara K., Huang C.J. Polyelectrolyte and Antipolyelectrolyte Effects for Dual Salt-Responsive Interpenetrating Network Hydrogels. Biomacromolecules. 2019;20:3524–3534. doi: 10.1021/acs.biomac.9b00796. [DOI] [PubMed] [Google Scholar]
- 69.English M.A., Soenksen L.R., Gayet R.V., de Puig H., Angenent-Mari N.M., Mao A.S., Nguyen P.Q., Collins J.J. Programmable CRISPR-responsive smart materials. Science. 2019;365:780–785. doi: 10.1126/science.aaw5122. [DOI] [PubMed] [Google Scholar]
- 70.Papathanasiou K.E., Turhanen P., Brückner S.I., Brunner E., Demadis K.D. Smart, programmable and responsive injectable hydrogels for controlled release of cargo osteoporosis drugs. Sci. Rep. 2017;7:4743. doi: 10.1038/s41598-017-04956-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim Y.J., Matsunaga Y.T. Thermo-responsive polymers and their application as smart biomaterials. J. Mater. Chem. B. 2017;5:4307–4321. doi: 10.1039/C7TB00157F. [DOI] [PubMed] [Google Scholar]
- 72.Mohammadi M., Karimi M., Malaekeh-Nikouei B., Torkashvand M., Alibolandi M. Hybrid in situ- forming injectable hydrogels for local cancer therapy. Int. J. Pharm. 2022;616:121534. doi: 10.1016/j.ijpharm.2022.121534. [DOI] [PubMed] [Google Scholar]
- 73.Huang H., Wang X., Wang W., Qu X., Song X., Zhang Y., Zhong L., Yang D.P., Dong X., Zhao Y. Injectable hydrogel for postoperative synergistic photothermal-chemodynamic tumor and anti-infection therapy. Biomaterials. 2022;280:121289. doi: 10.1016/j.biomaterials.2021.121289. [DOI] [PubMed] [Google Scholar]
- 74.Chen J., Gu H., Fu S., Lu J., Tan H., Wei Q., Ai H. Multifunctional injectable hydrogels for three-in-one cancer therapy: Preoperative remission via mild photothermal-enhanced supramolecular chemotherapy and prevention of postoperative recurrence and adhesion. Chem. Eng. J. 2021;425:130377. doi: 10.1016/j.cej.2021.130377. [DOI] [Google Scholar]
- 75.Tan B., Tang Q., Zhong Y., Wei Y., He L., Wu Y., Wu J., Liao J. Biomaterial-based strategies for maxillofacial tumour therapy and bone defect regeneration. Int. J. Oral Sci. 2021;13:9. doi: 10.1038/s41368-021-00113-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Milcovich G., Lettieri S., Antunes F.E., Medronho B., Fonseca A.C., Coelho J.F.J., Marizza P., Perrone F., Farra R., Dapas B., et al. Recent advances in smart biotechnology: Hydrogels and nanocarriers for tailored bioactive molecules depot. Adv. Colloid Interface Sci. 2017;249:163–180. doi: 10.1016/j.cis.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 77.Xiao Y., Gu Y., Qin L., Chen L., Chen X., Cui W., Li F., Xiang N., He X. Injectable thermosensitive hydrogel-based drug delivery system for local cancer therapy. Colloids Surf. B Biointerfaces. 2021;200:111581. doi: 10.1016/j.colsurfb.2021.111581. [DOI] [PubMed] [Google Scholar]
- 78.Dethe M.R., Prabakaran A., Ahmed H., Agrawal M., Roy U., Alexander A. PCL-PEG copolymer based injectable thermosensitive hydrogels. J. Control. Release Off. J. Control. Release Soc. 2022;343:217–236. doi: 10.1016/j.jconrel.2022.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ma N., Yan Z. Research Progress of Thermosensitive Hydrogel in Tumor Therapeutic. Nanoscale Res. Lett. 2021;16:42. doi: 10.1186/s11671-021-03502-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xue B., Qu Y., Shi K., Zhou K., He X., Chu B., Qian Z. Advances in the Application of Injectable Thermosensitive Hydrogel Systems for Cancer Therapy. J. Biomed. Nanotechnol. 2020;16:1427–1453. doi: 10.1166/jbn.2020.2988. [DOI] [PubMed] [Google Scholar]
- 81.Zhang L., Yang M., Ji Y., Xiao K., Shi J., Wang L. UCPs/Zn(2)GeO(4):Mn(2+)/g-C(3)N(4) heterojunction engineered injectable thermosensitive hydrogel for oxygen independent breast cancer neoadjuvant photodynamic therapy. Biomater. Sci. 2021;9:2124–2136. doi: 10.1039/D0BM01876G. [DOI] [PubMed] [Google Scholar]
- 82.Qi D., Zhu H., Kong Y., Shen Q. Injectable Nanomedicine-Hydrogel for NIR Light Photothermal-Chemo Combination Therapy of Tumor. Polymers. 2022;14:5547. doi: 10.3390/polym14245547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.GhavamiNejad A., SamariKhalaj M., Aguilar L.E., Park C.H., Kim C.S. pH/NIR Light-Controlled Multidrug Release via a Mussel-Inspired Nanocomposite Hydrogel for Chemo-Photothermal Cancer Therapy. Sci. Rep. 2016;6:33594. doi: 10.1038/srep33594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Theune L.E., Buchmann J., Wedepohl S., Molina M., Laufer J., Calderón M. NIR- and thermo-responsive semi-interpenetrated polypyrrole nanogels for imaging guided combinational photothermal and chemotherapy. J. Control. Release Off. J. Control. Release Soc. 2019;311–312:147–161. doi: 10.1016/j.jconrel.2019.08.035. [DOI] [PubMed] [Google Scholar]
- 85.Yao J., Zhu C., Peng T., Ma Q., Gao S. Injectable and Temperature-Sensitive Titanium Carbide-Loaded Hydrogel System for Photothermal Therapy of Breast Cancer. Front. Bioeng. Biotechnol. 2021;9:791891. doi: 10.3389/fbioe.2021.791891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen X., Tao J., Zhang M., Lu Z., Yu Y., Song P., Wang T., Jiang T., Zhao X. Iota carrageenan gold-silver NPs photothermal hydrogel for tumor postsurgical anti-recurrence and wound healing. Carbohydr. Polym. 2022;298:120123. doi: 10.1016/j.carbpol.2022.120123. [DOI] [PubMed] [Google Scholar]
- 87.Huang S., Ma Z., Sun C., Zhou Q., Li Z., Wang S., Yan Q., Liu C., Hou B., Zhang C. An injectable thermosensitive hydrogel loaded with a theranostic nanoprobe for synergistic chemo-photothermal therapy for multidrug-resistant hepatocellular carcinoma. J. Mater. Chem. B. 2022;10:2828–2843. doi: 10.1039/D2TB00044J. [DOI] [PubMed] [Google Scholar]
- 88.Zhang N., Xu X., Zhang X., Qu D., Xue L., Mo R., Zhang C. Nanocomposite hydrogel incorporating gold nanorods and paclitaxel-loaded chitosan micelles for combination photothermal-chemotherapy. Int. J. Pharm. 2016;497:210–221. doi: 10.1016/j.ijpharm.2015.11.032. [DOI] [PubMed] [Google Scholar]
- 89.Peppicelli S., Andreucci E., Ruzzolini J., Laurenzana A., Margheri F., Fibbi G., Del Rosso M., Bianchini F., Calorini L. The acidic microenvironment as a possible niche of dormant tumor cells. Cell. Mol. Life Sci. CMLS. 2017;74:2761–2771. doi: 10.1007/s00018-017-2496-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bazban-Shotorbani S., Hasani-Sadrabadi M.M., Karkhaneh A., Serpooshan V., Jacob K.I., Moshaverinia A., Mahmoudi M. Revisiting structure-property relationship of pH-responsive polymers for drug delivery applications. J. Control. Release. 2017;253:46–63. doi: 10.1016/j.jconrel.2017.02.021. [DOI] [PubMed] [Google Scholar]
- 91.Marques A.C., Costa P.J., Velho S., Amaral M.H. Stimuli-responsive hydrogels for intratumoral drug delivery. Drug Discov. Today. 2021;26:2397–2405. doi: 10.1016/j.drudis.2021.04.012. [DOI] [PubMed] [Google Scholar]
- 92.Tabassum N., Ahmed S., Ali M.A. Chitooligosaccharides and their structural-functional effect on hydrogels: A review. Carbohydr. Polym. 2021;261:117882. doi: 10.1016/j.carbpol.2021.117882. [DOI] [PubMed] [Google Scholar]
- 93.Andrade F., Roca-Melendres M.M., Durán-Lara E.F., Rafael D., Schwartz S., Jr. Stimuli-Responsive Hydrogels for Cancer Treatment: The Role of pH.; Light, Ionic Strength and Magnetic Field. Cancers. 2021;13:1164. doi: 10.3390/cancers13051164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lim H.L., Hwang Y., Kar M., Varghese S. Smart hydrogels as functional biomimetic systems. Biomater. Sci. 2014;2:603–618. doi: 10.1039/C3BM60288E. [DOI] [PubMed] [Google Scholar]
- 95.Mahinroosta M., Farsangi Z.J., Allahverdi A., Shakoori Z. Hydrogels as intelligent materials: A brief review of synthesis, properties and applications. Mater. Today Chem. 2018;8:42–55. doi: 10.1016/j.mtchem.2018.02.004. [DOI] [Google Scholar]
- 96.Kurian A.G., Singh R.K., Patel K.D., Lee J.H., Kim H.W. Multifunctional GelMA platforms with nanomaterials for advanced tissue therapeutics. Bioact. Mater. 2022;8:267–295. doi: 10.1016/j.bioactmat.2021.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schafer F.Q., Buettner G.R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free. Radic. Biol. Med. 2001;30:1191–1212. doi: 10.1016/S0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 98.Kuppusamy P., Li H., Ilangovan G., Cardounel A.J., Zweier J.L., Yamada K., Krishna M.C., Mitchell J.B. Noninvasive imaging of tumor redox status and its modification by tissue glutathione levels. Cancer Res. 2002;62:307–312. [PubMed] [Google Scholar]
- 99.Zhang J., Jiang X., Wen X., Xu Q., Zeng H., Zhao Y., Liu M., Wang Z., Hu X., Wang Y. Bio-responsive smart polymers and biomedical applications. J. Phys. Mater. 2019;2:032004. doi: 10.1088/2515-7639/ab1af5. [DOI] [Google Scholar]
- 100.Krisch E., Messager L., Gyarmati B., Ravaine V., Szilágyi A. Redox- and pH-Responsive Nanogels Based on Thiolated Poly(aspartic acid) Macromol. Mater. Eng. 2016;301:260–266. doi: 10.1002/mame.201500119. [DOI] [Google Scholar]
- 101.Ahmed S., Alhareth K., Mignet N. Advancement in nanogel formulations provides controlled drug release. Int. J. Pharm. 2020;584:119435. doi: 10.1016/j.ijpharm.2020.119435. [DOI] [PubMed] [Google Scholar]
- 102.Yallapu M.M., Jaggi M., Chauhan S.C. Design and engineering of nanogels for cancer treatment. Drug Discov. Today. 2011;16:457–463. doi: 10.1016/j.drudis.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nguyen N.T., Bui Q.A., Nguyen H.H.N., Nguyen T.T., Ly K.L., Tran H.L.B., Doan V.N., Nhi T.T.Y., Nguyen N.H., Nguyen N.H., et al. Curcuminoid Co-Loading Platinum Heparin-Poloxamer P403 Nanogel Increasing Effectiveness in Antitumor Activity. Gels. 2022;8:59. doi: 10.3390/gels8010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu L., Du P., Zhao X., Zeng J., Liu P. Independent temperature and pH dual-stimuli responsive yolk/shell polymer microspheres for controlled release: Structural effect. Eur. Polym. J. 2015;69:540–551. doi: 10.1016/j.eurpolymj.2015.03.026. [DOI] [Google Scholar]
- 105.Sahu P., Kashaw S.K., Kushwah V., Sau S., Jain S., Iyer A.K. pH responsive biodegradable nanogels for sustained release of bleomycin. Bioorganic Med. Chem. 2017;25:4595–4613. doi: 10.1016/j.bmc.2017.06.038. [DOI] [PubMed] [Google Scholar]
- 106.Shoueir K.R., Atta A.M., Sarhan A.A., Akl M.A. Synthesis of monodisperse core shell PVA@P(AMPS-co-NIPAm) nanogels structured for pre-concentration of Fe(III) ions. Environ. Technol. 2017;38:967–978. doi: 10.1080/09593330.2016.1215351. [DOI] [PubMed] [Google Scholar]
- 107.Xing Z., Wang C., Yan J., Zhang L., Li L., Zha L. Dual stimuli responsive hollow nanogels with IPN structure for temperature controlling drug loading and pH triggering drug release. Soft Matter. 2011;7:7992–7997. doi: 10.1039/c1sm05925d. [DOI] [Google Scholar]
- 108.Topuz F., Uyar T. Advances in the development of cyclodextrin-based nanogels/microgels for biomedical applications: Drug delivery and beyond. Carbohydr. Polym. 2022;297:120033. doi: 10.1016/j.carbpol.2022.120033. [DOI] [PubMed] [Google Scholar]
- 109.Singh G., Majeed A., Singh R., George N., Singh G., Gupta S., Singh H., Kaur G., Singh J. CuAAC ensembled 1,2,3-triazole linked nanogels for targeted drug delivery: A review. RSC Adv. 2023;13:2912–2936. doi: 10.1039/D2RA05592A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dave R., Randhawa G., Kim D., Simpson M., Hoare T. Microgels and Nanogels for the Delivery of Poorly Water-Soluble Drugs. Mol. Pharm. 2022;19:1704–1721. doi: 10.1021/acs.molpharmaceut.1c00967. [DOI] [PubMed] [Google Scholar]
- 111.Attama A.A., Nnamani P.O., Onokala O.B., Ugwu A.A., Onugwu A.L. Nanogels as target drug delivery systems in cancer therapy: A review of the last decade. Front. Pharmacol. 2022;13:874510. doi: 10.3389/fphar.2022.874510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Aurora Grimaudo M., Concheiro A., Alvarez-Lorenzo C. Nanogels for regenerative medicine. J. Control. Release. 2019;313:148–160. doi: 10.1016/j.jconrel.2019.09.015. [DOI] [PubMed] [Google Scholar]
- 113.Jiang Y., Chen J., Deng C., Suuronen E.J., Zhong Z. Click hydrogels, microgels and nanogels: Emerging platforms for drug delivery and tissue engineering. Biomaterials. 2014;35:4969–4985. doi: 10.1016/j.biomaterials.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 114.Zhu J., Sun W., Shi X. Nanogels as Contrast Agents for Molecular Imaging. Chin. J. Chem. 2016;34:547–557. doi: 10.1002/cjoc.201500743. [DOI] [Google Scholar]
- 115.Sivaram A.J., Rajitha P., Maya S., Jayakumar R., Sabitha M. Nanogels for delivery, imaging and therapy. Wiley Interdiscip. Rev. -Nanomed. Nanobiotechnol. 2015;7:509–533. doi: 10.1002/wnan.1328. [DOI] [PubMed] [Google Scholar]
- 116.Ekkelenkamp A.E., Elzes M.R., Engbersen J.F.J., Paulusse J.M.J. Responsive crosslinked polymer nanogels for imaging and therapeutics delivery. J. Mater. Chem. B. 2018;6:210–235. doi: 10.1039/C7TB02239E. [DOI] [PubMed] [Google Scholar]
- 117.Chan M., Almutairi A. Nanogels as imaging agents for modalities spanning the electromagnetic spectrum. Mater. Horiz. 2016;3:21–40. doi: 10.1039/C5MH00161G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wu H.-Q., Wang C.-C. Biodegradable Smart Nanogels: A New Platform for Targeting Drug Delivery and Biomedical Diagnostics. Langmuir. 2016;32:6211–6225. doi: 10.1021/acs.langmuir.6b00842. [DOI] [PubMed] [Google Scholar]
- 119.Preman N.K., Jain S., Johnson R.P. “Smart” Polymer Nanogels as Pharmaceutical Carriers: A Versatile Platform for Programmed Delivery and Diagnostics. ACS Omega. 2021;6:5075–5090. doi: 10.1021/acsomega.0c05276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Preman N.K., Barki R.R., Vijayan A., Sanjeeva S.G., Johnson R.P. Recent developments in stimuli-responsive polymer nanogels for drug delivery and diagnostics: A review. Eur. J. Pharm. Biopharm. 2020;157:121–153. doi: 10.1016/j.ejpb.2020.10.009. [DOI] [PubMed] [Google Scholar]
- 121.Wang H., Picchio M.L., Calderon M. One stone, many birds: Recent advances in functional nanogels for cancer nanotheranostics. Wiley Interdiscip. Rev. -Nanomed. Nanobiotechnol. 2022;14:e1791. doi: 10.1002/wnan.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Li F., Liang Z., Ling D. Smart Organic-Inorganic Nanogels for Activatable Theranostics. Curr. Med. Chem. 2019;26:1366–1376. doi: 10.2174/0929867324666170920164614. [DOI] [PubMed] [Google Scholar]
- 123.Neerooa B.N.H.M., Ooi L.-T., Shameli K., Dahlan N.A., Islam J.M.M., Pushpamalar J., Teow S.-Y. Development of Polymer-Assisted Nanoparticles and Nanogels for Cancer Therapy: An Update. Gels. 2021;7:60. doi: 10.3390/gels7020060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mekuria S.L., Ouyang Z., Song C., Rodrigues J., Shen M., Shi X. Dendrimer-Based Nanogels for Cancer Nanomedicine Applications. Bioconjugate Chem. 2022;33:87–96. doi: 10.1021/acs.bioconjchem.1c00587. [DOI] [PubMed] [Google Scholar]
- 125.Li Z., Huang J., Wu J. pH-Sensitive nanogels for drug delivery in cancer therapy. Biomater. Sci. 2021;9:574–589. doi: 10.1039/D0BM01729A. [DOI] [PubMed] [Google Scholar]
- 126.Ali A.A., Al-Othman A., Al-Sayah M.H. Multifunctional stimuli-responsive hybrid nanogels for cancer therapy: Current status and challenges. J. Control. Release Off. J. Control. Release Soc. 2022;351:476–503. doi: 10.1016/j.jconrel.2022.09.033. [DOI] [PubMed] [Google Scholar]
- 127.Soni K.S., Desale S.S., Bronich T.K. Nanogels: An overview of properties, biomedical applications and obstacles to clinical translation. J. Control. Release Off. J. Control. Release Soc. 2016;240:109–126. doi: 10.1016/j.jconrel.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Allison R.R., Mota H.C., Bagnato V.S., Sibata C.H. Bio-nanotechnology and photodynamic therapy--state of the art review. Photodiagnosis Photodyn. Ther. 2008;5:19–28. doi: 10.1016/j.pdpdt.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 129.Bertrand N., Wu J., Xu X., Kamaly N., Farokhzad O.C. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 2014;66:2–25. doi: 10.1016/j.addr.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Danhier F. To exploit the tumor microenvironment: Since the EPR effect fails in the clinic, what is the future of nanomedicine? J. Control. Release. 2016;244:108–121. doi: 10.1016/j.jconrel.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 131.Fang J., Nakamura H., Maeda H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv. Drug Deliv. Rev. 2011;63:136–151. doi: 10.1016/j.addr.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 132.Oh J.K., Drumright R., Siegwart D.J., Matyjaszewski K. The development of microgels/nanogels for drug delivery applications. Prog. Polym. Sci. 2008;33:448–477. doi: 10.1016/j.progpolymsci.2008.01.002. [DOI] [Google Scholar]
- 133.Liu Y., Luo Y.N., Zhang P., Yang W.F., Zhang C.Y., Yin Y.L. The Preparation of Novel P(OEGMA-co-MEO(2)MA) Microgels-Based Thermosensitive Hydrogel and Its Application in Three-Dimensional Cell Scaffold. Gels. 2022;8:313. doi: 10.3390/gels8050313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Montané X., Bajek A., Roszkowski K., Montornés J.M., Giamberini M., Roszkowski S., Kowalczyk O., Garcia-Valls R., Tylkowski B. Encapsulation for Cancer Therapy. Molecules. 2020;25:1605. doi: 10.3390/molecules25071605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yang W.J., Zhou P., Liang L., Cao Y., Qiao J., Li X., Teng Z., Wang L. Nanogel-Incorporated Injectable Hydrogel for Synergistic Therapy Based on Sequential Local Delivery of Combretastatin-A4 Phosphate (CA4P) and Doxorubicin (DOX) ACS Appl. Mater. Interfaces. 2018;10:18560–18573. doi: 10.1021/acsami.8b04394. [DOI] [PubMed] [Google Scholar]
- 136.Xiong M.H., Bao Y., Yang X.Z., Wang Y.C., Sun B., Wang J. Lipase-sensitive polymeric triple-layered nanogel for “on-demand” drug delivery. J. Am. Chem. Soc. 2012;134:4355–4362. doi: 10.1021/ja211279u. [DOI] [PubMed] [Google Scholar]
- 137.Zhou L., Pi W., Hao M., Li Y., An H., Li Q., Zhang P., Wen Y. An injectable and biodegradable nano-photothermal DNA hydrogel enhances penetration and efficacy of tumor therapy. Biomater. Sci. 2021;9:4904–4921. doi: 10.1039/D1BM00568E. [DOI] [PubMed] [Google Scholar]
- 138.Zhang X., Chen X., Guo Y., Jia H.R., Jiang Y.W., Wu F.G. Endosome/lysosome-detained supramolecular nanogels as an efflux retarder and autophagy inhibitor for repeated photodynamic therapy of multidrug-resistant cancer. Nanoscale Horiz. 2020;5:481–487. doi: 10.1039/C9NH00643E. [DOI] [PubMed] [Google Scholar]
- 139.Arjama M., Mehnath S., Jeyaraj M. Self-assembled hydrogel nanocube for stimuli responsive drug delivery and tumor ablation by phototherapy against breast cancer. Int. J. Biol. Macromol. 2022;213:435–446. doi: 10.1016/j.ijbiomac.2022.05.190. [DOI] [PubMed] [Google Scholar]
- 140.Howaili F., Özliseli E., Küçüktürkmen B., Razavi S.M., Sadeghizadeh M., Rosenholm J.M. Stimuli-Responsive, Plasmonic Nanogel for Dual Delivery of Curcumin and Photothermal Therapy for Cancer Treatment. Front. Chem. 2020;8:602941. doi: 10.3389/fchem.2020.602941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sun Z., Song C., Wang C., Hu Y., Wu J. Hydrogel-Based Controlled Drug Delivery for Cancer Treatment: A Review. Mol. Pharm. 2020;17:373–391. doi: 10.1021/acs.molpharmaceut.9b01020. [DOI] [PubMed] [Google Scholar]
- 142.Yin Y., Hu B., Yuan X., Cai L., Gao H., Yang Q. Nanogel: A Versatile Nano-Delivery System for Biomedical Applications. Pharmaceutics. 2020;12:290. doi: 10.3390/pharmaceutics12030290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Richter K., Haslbeck M., Buchner J. The heat shock response: Life on the verge of death. Mol. Cell. 2010;40:253–266. doi: 10.1016/j.molcel.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 144.Zhang Y., Zhan X., Xiong J., Peng S., Huang W., Joshi R., Cai Y., Liu Y., Li R., Yuan K., et al. Temperature-dependent cell death patterns induced by functionalized gold nanoparticle photothermal therapy in melanoma cells. Sci. Rep. 2018;8:8720. doi: 10.1038/s41598-018-26978-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Knavel E.M., Brace C.L. Tumor ablation: Common modalities and general practices. Tech. Vasc. Interv. Radiol. 2013;16:192–200. doi: 10.1053/j.tvir.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Day E.S., Thompson P.A., Zhang L., Lewinski N.A., Ahmed N., Drezek R.A., Blaney S.M., West J.L. Nanoshell-mediated photothermal therapy improves survival in a murine glioma model. J. Neuro-Oncol. 2011;104:55–63. doi: 10.1007/s11060-010-0470-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wang X., Yang Z., Meng Z., Sun S.K. Transforming Commercial Copper Sulfide into Injectable Hydrogels for Local Photothermal Therapy. Gels. 2022;8:319. doi: 10.3390/gels8050319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wang Y., Pan H., Meng Z., Zhang C. In Situ Biosynthesis of Photothermal Parasite for Fluorescence Imaging-Guided Photothermal Therapy of Tumors. Gels. 2022;8:754. doi: 10.3390/gels8110754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hsiao C.W., Chuang E.Y., Chen H.L., Wan D., Korupalli C., Liao Z.X., Chiu Y.L., Chia W.T., Lin K.J., Sung H.W. Photothermal tumor ablation in mice with repeated therapy sessions using NIR-absorbing micellar hydrogels formed in situ. Biomaterials. 2015;56:26–35. doi: 10.1016/j.biomaterials.2015.03.060. [DOI] [PubMed] [Google Scholar]
- 150.Fan M., Li M., Wang X., Liao Y., Wang H., Rao J., Yang Y., Wang Q. Injectable Thermosensitive Iodine-Loaded Starch-g-poly(N-isopropylacrylamide) Hydrogel for Cancer Photothermal Therapy and Anti-Infection. Macromol. Rapid Commun. 2022;43:e2200203. doi: 10.1002/marc.202200203. [DOI] [PubMed] [Google Scholar]
- 151.Zhou Y., Hu Y., Sun W., Zhou B., Zhu J., Peng C., Shen M., Shi X. Polyaniline-loaded γ-polyglutamic acid nanogels as a platform for photoacoustic imaging-guided tumor photothermal therapy. Nanoscale. 2017;9:12746–12754. doi: 10.1039/C7NR04241H. [DOI] [PubMed] [Google Scholar]
- 152.Zhang C., Sun W., Wang Y., Xu F., Qu J., Xia J., Shen M., Shi X. Gd-/CuS-Loaded Functional Nanogels for MR/PA Imaging-Guided Tumor-Targeted Photothermal Therapy. ACS Appl. Mater. Interfaces. 2020;12:9107–9117. doi: 10.1021/acsami.9b23413. [DOI] [PubMed] [Google Scholar]
- 153.Wang C., Wang X., Dong K., Luo J., Zhang Q., Cheng Y. Injectable and responsively degradable hydrogel for personalized photothermal therapy. Biomaterials. 2016;104:129–137. doi: 10.1016/j.biomaterials.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 154.Liao J., Shi K., Jia Y., Wu Y., Qian Z. Gold nanorods and nanohydroxyapatite hybrid hydrogel for preventing bone tumor recurrence via postoperative photothermal therapy and bone regeneration promotion. Bioact. Mater. 2021;6:2221–2230. doi: 10.1016/j.bioactmat.2021.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Donohoe C., Senge M.O., Arnaut L.G., Gomes-da-Silva L.C. Cell death in photodynamic therapy: From oxidative stress to anti-tumor immunity. Biochim. Et Biophys. Acta Rev. Cancer. 2019;1872:188308. doi: 10.1016/j.bbcan.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 156.Meng Z., Hou W., Zhou H., Zhou L., Chen H., Wu C. Therapeutic Considerations and Conjugated Polymer-Based Photosensitizers for Photodynamic Therapy. Macromol. Rapid Commun. 2018;39:1700614. doi: 10.1002/marc.201700614. [DOI] [PubMed] [Google Scholar]
- 157.Van Straten D., Mashayekhi V., de Bruijn H.S., Oliveira S., Robinson D.J. Oncologic Photodynamic Therapy: Basic Principles, Current Clinical Status and Future Directions. Cancers. 2017;9:19. doi: 10.3390/cancers9020019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhang G.D., Harada A., Nishiyama N., Jiang D.L., Koyama H., Aida T., Kataoka K. Polyion complex micelles entrapping cationic dendrimer porphyrin: Effective photosensitizer for photodynamic therapy of cancer. J. Control. Release Off. J. Control. Release Soc. 2003;93:141–150. doi: 10.1016/j.jconrel.2003.05.002. [DOI] [PubMed] [Google Scholar]
- 159.Celli J.P., Spring B.Q., Rizvi I., Evans C.L., Samkoe K.S., Verma S., Pogue B.W., Hasan T. Imaging and photodynamic therapy: Mechanisms, monitoring, and optimization. Chem. Rev. 2010;110:2795–2838. doi: 10.1021/cr900300p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Juarranz A., Jaén P., Sanz-Rodríguez F., Cuevas J., González S. Photodynamic therapy of cancer. Basic principles and applications. Clinical & translational oncology: Official publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico. Clin. Transl. Oncol. 2008;10:148–154. doi: 10.1007/s12094-008-0172-2. [DOI] [PubMed] [Google Scholar]
- 161.Dolmans D.E., Fukumura D., Jain R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer. 2003;3:380–387. doi: 10.1038/nrc1071. [DOI] [PubMed] [Google Scholar]
- 162.Temizel E., Sagir T., Ayan E., Isik S., Ozturk R. Delivery of lipophilic porphyrin by liposome vehicles: Preparation and photodynamic therapy activity against cancer cell lines. Photodiagnosis Photodyn. Ther. 2014;11:537–545. doi: 10.1016/j.pdpdt.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 163.Belali S., Savoie H., O’Brien J.M., Cafolla A.A., O’Connell B., Karimi A.R., Boyle R.W., Senge M.O. Synthesis and Characterization of Temperature-Sensitive and Chemically Cross-Linked Poly(N-isopropylacrylamide)/Photosensitizer Hydrogels for Applications in Photodynamic Therapy. Biomacromolecules. 2018;19:1592–1601. doi: 10.1021/acs.biomac.8b00293. [DOI] [PubMed] [Google Scholar]
- 164.Zhang H., Shi R., Xie A., Li J., Chen L., Chen P., Li S., Huang F., Shen Y. Novel TiO2/PEGDA hybrid hydrogel prepared in situ on tumor cells for effective photodynamic therapy. ACS Appl. Mater. Interfaces. 2013;5:12317–12322. doi: 10.1021/am4025559. [DOI] [PubMed] [Google Scholar]
- 165.Kawasaki R., Ohdake R., Yamana K., Eto T., Sugikawa K., Ikeda A. Photodynamic therapy using self-assembled nanogels comprising chlorin e6-bearing pullulan. J. Mater. Chem. B. 2021;9:6357–6363. doi: 10.1039/D1TB00377A. [DOI] [PubMed] [Google Scholar]
- 166.He H., Nieminen A.L., Xu P. A bioactivatable self-quenched nanogel for targeted photodynamic therapy. Biomater. Sci. 2019;7:5143–5149. doi: 10.1039/C9BM01237K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Duan X., Chen B., Cui Y., Zhou L., Wu C., Yang Z., Wen Y., Miao X., Li Q., Xiong L., et al. Ready player one? Autophagy shapes resistance to photodynamic therapy in cancers. Apoptosis. 2018;23:587–606. doi: 10.1007/s10495-018-1489-0. [DOI] [PubMed] [Google Scholar]
- 168.Spring B.Q., Rizvi I., Xu N., Hasan T. The role of photodynamic therapy in overcoming cancer drug resistance. Photochem. Photobiol. Sci. 2015;14:1476–1491. doi: 10.1039/c4pp00495g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Cheng L., Wang C., Feng L., Yang K., Liu Z. Functional Nanomaterials for Phototherapies of Cancer. Chem. Rev. 2014;114:10869–10939. doi: 10.1021/cr400532z. [DOI] [PubMed] [Google Scholar]
- 170.Lucky S.S., Soo K.C., Zhang Y. Nanoparticles in Photodynamic Therapy. Chem. Rev. 2015;115:1990–2042. doi: 10.1021/cr5004198. [DOI] [PubMed] [Google Scholar]
- 171.Yang Z., Zhu Y., Dong Z., Hao Y., Wang C., Li Q., Wu Y., Feng L., Liu Z. Engineering bioluminescent bacteria to boost photodynamic therapy and systemic anti-tumor immunity for synergistic cancer treatment. Biomaterials. 2022;281:121332. doi: 10.1016/j.biomaterials.2021.121332. [DOI] [PubMed] [Google Scholar]
- 172.Nguyen V.N., Park S.J., Qi S., Ha J., Heo S., Yim Y., Baek G., Lim C.S., Lee D.J., Kim H.M., et al. Design and synthesis of efficient heavy-atom-free photosensitizers for photodynamic therapy of cancer. Chem. Commun. 2020;56:11489–11492. doi: 10.1039/D0CC04644B. [DOI] [PubMed] [Google Scholar]
- 173.Li X., Park E.Y., Kang Y., Kwon N., Yang M., Lee S., Kim W.J., Kim C., Yoon J. Supramolecular Phthalocyanine Assemblies for Improved Photoacoustic Imaging and Photothermal Therapy. Angew. Chem. 2020;59:8630–8634. doi: 10.1002/anie.201916147. [DOI] [PubMed] [Google Scholar]
- 174.Nguyen V.N., Qi S., Kim S., Kwon N., Kim G., Yim Y., Park S., Yoon J. An Emerging Molecular Design Approach to Heavy-Atom-Free Photosensitizers for Enhanced Photodynamic Therapy under Hypoxia. J. Am. Chem. Soc. 2019;141:16243–16248. doi: 10.1021/jacs.9b09220. [DOI] [PubMed] [Google Scholar]
- 175.Sun W., Luo L., Feng Y., Cai Y., Zhuang Y., Xie R.J., Chen X., Chen H. Aggregation-Induced Emission Gold Clustoluminogens for Enhanced Low-Dose X-ray-Induced Photodynamic Therapy. Angew. Chem. 2020;59:9914–9921. doi: 10.1002/anie.201908712. [DOI] [PubMed] [Google Scholar]
- 176.Yu X., Liu X., Wu W., Yang K., Mao R., Ahmad F., Chen X., Li W. CT/MRI-Guided Synergistic Radiotherapy and X-ray Inducible Photodynamic Therapy Using Tb-Doped Gd-W-Nanoscintillators. Angew. Chem. 2019;58:2017–2022. doi: 10.1002/anie.201812272. [DOI] [PubMed] [Google Scholar]
- 177.Overchuk M., Cheng M.H.Y., Zheng G. X-ray-Activatable Photodynamic Nanoconstructs. ACS Cent. Sci. 2020;6:613–615. doi: 10.1021/acscentsci.0c00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lioret V., Bellaye P.S., Arnould C., Collin B., Decréau R.A. Dual Cherenkov Radiation-Induced Near-Infrared Luminescence Imaging and Photodynamic Therapy toward Tumor Resection. J. Med. Chem. 2020;63:9446–9456. doi: 10.1021/acs.jmedchem.0c00625. [DOI] [PubMed] [Google Scholar]
- 179.Xu X., An H., Zhang D., Tao H., Dou Y., Li X., Huang J., Zhang J. A self-illuminating nanoparticle for inflammation imaging and cancer therapy. Sci. Adv. 2019;5:eaat2953. doi: 10.1126/sciadv.aat2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sun W., Shi T., Luo L., Chen X., Lv P., Lv Y., Zhuang Y., Zhu J., Liu G., Chen X., et al. Monodisperse and Uniform Mesoporous Silicate Nanosensitizers Achieve Low-Dose X-Ray-Induced Deep-Penetrating Photodynamic Therapy. Adv. Mater. 2019;31:e1808024. doi: 10.1002/adma.201808024. [DOI] [PubMed] [Google Scholar]
- 181.Wan Y., Fu L.-H., Li C., Lin J., Huang P. Conquering the Hypoxia Limitation for Photodynamic Therapy. Adv. Mater. 2021;33:2103978. doi: 10.1002/adma.202103978. [DOI] [PubMed] [Google Scholar]
- 182.Bhandari V., Hoey C., Liu L., Lalonde E., Ray J., Livingstone J., Lesurf R., Shiah Y.-J., Vujcic T., Huang X., et al. Molecular landmarks of tumor hypoxia across cancer types. Clin. Cancer Res. 2021;27:308–318. doi: 10.1158/1557-3265.RADSCI21-IA-018. [DOI] [PubMed] [Google Scholar]
- 183.Sun Y., Zhao D., Wang G., Wang Y., Cao L., Sun J., Jiang Q., He Z. Recent progress of hypoxia-modulated multifunctional nanomedicines to enhance photodynamic therapy: Opportunities, challenges, and future development. Acta Pharm. Sin. B. 2020;10:1382–1396. doi: 10.1016/j.apsb.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Jahanban-Esfahlan R., de la Guardia M., Ahmadi D., Yousefi B. Modulating tumor hypoxia by nanomedicine for effective cancer therapy. J. Cell. Physiol. 2018;233:2019–2031. doi: 10.1002/jcp.25859. [DOI] [PubMed] [Google Scholar]
- 185.Li X., Kwon N., Guo T., Liu Z., Yoon J. Innovative Strategies for Hypoxic-Tumor Photodynamic Therapy. Angew. Chem. Int. Ed. 2018;57:11522–11531. doi: 10.1002/anie.201805138. [DOI] [PubMed] [Google Scholar]
- 186.Fan W., Huang P., Chen X. Overcoming the Achilles’ heel of photodynamic therapy. Chem. Soc. Rev. 2016;45:6488–6519. doi: 10.1039/C6CS00616G. [DOI] [PubMed] [Google Scholar]
- 187.Zhang X., Zhang Y., Zhang C., Yang C., Tian R., Sun T., Zhang W., Chang J., Wang H. An injectable hydrogel co-loading with cyanobacteria and upconversion nanoparticles for enhanced photodynamic tumor therapy. Colloids Surf. B Biointerfaces. 2021;201:111640. doi: 10.1016/j.colsurfb.2021.111640. [DOI] [PubMed] [Google Scholar]
- 188.Li X., Fan H., Guo T., Bai H., Kwon N., Kim K.H., Yu S., Cho Y., Kim H., Nam K.T., et al. Sequential Protein-Responsive Nanophotosensitizer Complex for Enhancing Tumor-Specific Therapy. ACS Nano. 2019;13:6702–6710. doi: 10.1021/acsnano.9b01100. [DOI] [PubMed] [Google Scholar]
- 189.Han H.S., Choi K.Y., Lee H., Lee M., An J.Y., Shin S., Kwon S., Lee D.S., Park J.H. Gold-Nanoclustered Hyaluronan Nano-Assemblies for Photothermally Maneuvered Photodynamic Tumor Ablation. ACS Nano. 2016;10:10858–10868. doi: 10.1021/acsnano.6b05113. [DOI] [PubMed] [Google Scholar]
- 190.Xiao Q., Zheng X., Bu W., Ge W., Zhang S., Chen F., Xing H., Ren Q., Fan W., Zhao K., et al. A core/satellite multifunctional nanotheranostic for in vivo imaging and tumor eradication by radiation/photothermal synergistic therapy. J. Am. Chem. Soc. 2013;135:13041–13048. doi: 10.1021/ja404985w. [DOI] [PubMed] [Google Scholar]
- 191.Tian B., Wang C., Zhang S., Feng L., Liu Z. Photothermally enhanced photodynamic therapy delivered by nano-graphene oxide. ACS Nano. 2011;5:7000–7009. doi: 10.1021/nn201560b. [DOI] [PubMed] [Google Scholar]
- 192.Tang Z., Zhao P., Ni D., Liu Y., Zhang M., Wang H., Zhang H., Gao H., Yao Z., Bu W. Pyroelectric nanoplatform for NIR-II-triggered photothermal therapy with simultaneous pyroelectric dynamic therapy. Mater. Horiz. 2018;5:946–952. doi: 10.1039/C8MH00627J. [DOI] [Google Scholar]
- 193.Yue J., Miao P., Li L., Yan R., Dong W.F., Mei Q. Injectable Carbon Dots-Based Hydrogel for Combined Photothermal Therapy and Photodynamic Therapy of Cancer. ACS Appl. Mater. Interfaces. 2022;14:49582–49591. doi: 10.1021/acsami.2c15428. [DOI] [PubMed] [Google Scholar]
- 194.Qi Y., Yuan Y., Qian Z., Ma X., Yuan W., Song Y. Injectable and Self-Healing Polysaccharide Hydrogel Loading Molybdenum Disulfide Nanoflakes for Synergistic Photothermal-Photodynamic Therapy of Breast Cancer. Macromol. Biosci. 2022;22:e2200161. doi: 10.1002/mabi.202200161. [DOI] [PubMed] [Google Scholar]
- 195.Wang J., Xu W., Qian J., Wang Y., Hou G., Suo A., Ma Y. Injectable hyaluronan/MnO(2) nanocomposite hydrogel constructed by metal-hydrazide coordinated crosslink mineralization for relieving tumor hypoxia and combined phototherapy. J. Colloid Interface Sci. 2022;628:79–94. doi: 10.1016/j.jcis.2022.08.024. [DOI] [PubMed] [Google Scholar]
- 196.Hou M., Liu W., Zhang L., Zhang L., Xu Z., Cao Y., Kang Y., Xue P. Responsive agarose hydrogel incorporated with natural humic acid and MnO(2) nanoparticles for effective relief of tumor hypoxia and enhanced photo-induced tumor therapy. Biomater. Sci. 2020;8:353–369. doi: 10.1039/C9BM01472A. [DOI] [PubMed] [Google Scholar]
- 197.Jin F., Liu D., Xu X., Ji J., Du Y. Nanomaterials-Based Photodynamic Therapy with Combined Treatment Improves Antitumor Efficacy Through Boosting Immunogenic Cell Death. Int. J. Nanomed. 2021;16:4693–4712. doi: 10.2147/IJN.S314506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Yuan Y., Bo R., Jing D., Ma Z., Wang Z., Lin T.-y., Dong L., Xue X., Li Y. Excipient-free porphyrin/SN-38 based nanotheranostics for drug delivery and cell imaging. Nano Res. 2020;13:503–510. doi: 10.1007/s12274-020-2641-z. [DOI] [Google Scholar]
- 199.Wang G.D., Nguyen H.T., Chen H., Cox P.B., Wang L., Nagata K., Hao Z., Wang A., Li Z., Xie J. X-Ray Induced Photodynamic Therapy: A Combination of Radiotherapy and Photodynamic Therapy. Theranostics. 2016;6:2295–2305. doi: 10.7150/thno.16141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zhang J., Chen J., Ren J., Guo W., Li X., Chen R., Chelora J., Cui X., Wan Y., Liang X.J., et al. Biocompatible semiconducting polymer nanoparticles as robust photoacoustic and photothermal agents revealing the effects of chemical structure on high photothermal conversion efficiency. Biomaterials. 2018;181:92–102. doi: 10.1016/j.biomaterials.2018.07.042. [DOI] [PubMed] [Google Scholar]
- 201.Zhang S., Guo W., Wei J., Li C., Liang X.J., Yin M. Terrylenediimide-Based Intrinsic Theranostic Nanomedicines with High Photothermal Conversion Efficiency for Photoacoustic Imaging-Guided Cancer Therapy. ACS Nano. 2017;11:3797–3805. doi: 10.1021/acsnano.6b08720. [DOI] [PubMed] [Google Scholar]
- 202.Wang Z., Xue X., Lu H., He Y., Lu Z., Chen Z., Yuan Y., Tang N., Dreyer C.A., Quigley L., et al. Two-way magnetic resonance tuning and enhanced subtraction imaging for non-invasive and quantitative biological imaging. Nat. Nanotechnol. 2020;15:482–490. doi: 10.1038/s41565-020-0678-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Wang H., Liu H., Guo Y., Zai W., Li X., Xiong W., Zhao X., Yao Y., Hu Y., Zou Z., et al. Photosynthetic microorganisms coupled photodynamic therapy for enhanced antitumor immune effect. Bioact. Mater. 2022;12:97–106. doi: 10.1016/j.bioactmat.2021.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ji B., Wei M., Yang B. Recent advances in nanomedicines for photodynamic therapy (PDT)-driven cancer immunotherapy. Theranostics. 2022;12:434–458. doi: 10.7150/thno.67300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Sun Y., Zhang Y., Gao Y., Wang P., He G., Blum N.T., Lin J., Liu Q., Wang X., Huang P. Six Birds with One Stone: Versatile Nanoporphyrin for Single-Laser-Triggered Synergistic Phototheranostics and Robust Immune Activation. Adv. Mater. 2020;32:e2004481. doi: 10.1002/adma.202004481. [DOI] [PubMed] [Google Scholar]
- 206.Li Q., Zhang D., Zhang J., Jiang Y., Song A., Li Z., Luan Y. A Three-in-One Immunotherapy Nanoweapon via Cascade-Amplifying Cancer-Immunity Cycle against Tumor Metastasis, Relapse, and Postsurgical Regrowth. Nano Lett. 2019;19:6647–6657. doi: 10.1021/acs.nanolett.9b02923. [DOI] [PubMed] [Google Scholar]
- 207.Duan X., Chan C., Lin W. Nanoparticle-Mediated Immunogenic Cell Death Enables and Potentiates Cancer Immunotherapy. Angew. Chem. 2019;58:670–680. doi: 10.1002/anie.201804882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Huang A., Ma J., Huang L., Yang F., Cheng P. Mechanisms for enhanced antitumor immune responses induced by irradiated hepatocellular carcinoma cells engineered to express hepatitis B virus X protein. Oncol. Lett. 2018;15:8505–8515. doi: 10.3892/ol.2018.8430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ng C.W., Li J., Pu K. Recent Progresses in Phototherapy-Synergized Cancer Immunotherapy. Adv. Funct. Mater. 2018;28:1804688. doi: 10.1002/adfm.201804688. [DOI] [Google Scholar]
- 210.Xie Z., Shen J., Sun H., Li J., Wang X. Polymer-based hydrogels with local drug release for cancer immunotherapy. Biomed. Pharmacother. Biomed. Pharmacother. 2021;137:111333. doi: 10.1016/j.biopha.2021.111333. [DOI] [PubMed] [Google Scholar]
- 211.Dong X., Liang J., Yang A., Qian Z., Kong D., Lv F. Fluorescence imaging guided CpG nanoparticles-loaded IR820-hydrogel for synergistic photothermal immunotherapy. Biomaterials. 2019;209:111–125. doi: 10.1016/j.biomaterials.2019.04.024. [DOI] [PubMed] [Google Scholar]
- 212.Fei Z., Fan Q., Dai H., Zhou X., Xu J., Ma Q., Maruyama A., Wang C. Physiologically triggered injectable red blood cell-based gel for tumor photoablation and enhanced cancer immunotherapy. Biomaterials. 2021;271:120724. doi: 10.1016/j.biomaterials.2021.120724. [DOI] [PubMed] [Google Scholar]
- 213.Ding M., Fan Y., Lv Y., Liu J., Yu N., Kong D., Sun H., Li J. A prodrug hydrogel with tumor microenvironment and near-infrared light dual-responsive action for synergistic cancer immunotherapy. Acta Biomater. 2022;149:334–346. doi: 10.1016/j.actbio.2022.06.041. [DOI] [PubMed] [Google Scholar]
- 214.Hwang J., An E.K., Zhang W., Kim H.J., Eom Y., Jin J.O. Dual-functional alginate and collagen-based injectable hydrogel for the treatment of cancer and its metastasis. J. Nanobiotechnol. 2022;20:245. doi: 10.1186/s12951-022-01458-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Liu Y., Han Y.Y., Lu S., Wu Y., Li J., Sun X., Yan J. Injectable hydrogel platform with biodegradable Dawson-type polyoxometalate and R848 for combinational photothermal-immunotherapy of cancer. Biomater. Sci. 2022;10:1257–1266. doi: 10.1039/D1BM01835C. [DOI] [PubMed] [Google Scholar]
- 216.Raeesi V., Chan W.C. Improving nanoparticle diffusion through tumor collagen matrix by photo-thermal gold nanorods. Nanoscale. 2016;8:12524–12530. doi: 10.1039/C5NR08463F. [DOI] [PubMed] [Google Scholar]
- 217.Fay B.L., Melamed J.R., Day E.S. Nanoshell-mediated photothermal therapy can enhance chemotherapy in inflammatory breast cancer cells. Int. J. Nanomed. 2015;10:6931–6941. doi: 10.2147/IJN.S93031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Gormley A.J., Larson N., Banisadr A., Robinson R., Frazier N., Ray A., Ghandehari H. Plasmonic photothermal therapy increases the tumor mass penetration of HPMA copolymers. J. Control. Release Off. J. Control. Release Soc. 2013;166:130–138. doi: 10.1016/j.jconrel.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Gormley A.J., Greish K., Ray A., Robinson R., Gustafson J.A., Ghandehari H. Gold nanorod mediated plasmonic photothermal therapy: A tool to enhance macromolecular delivery. Int. J. Pharm. 2011;415:315–318. doi: 10.1016/j.ijpharm.2011.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Ye H., Wang K., Wang M., Liu R., Song H., Li N., Lu Q., Zhang W., Du Y., Yang W., et al. Bioinspired nanoplatelets for chemo-photothermal therapy of breast cancer metastasis inhibition. Biomaterials. 2019;206:1–12. doi: 10.1016/j.biomaterials.2019.03.024. [DOI] [PubMed] [Google Scholar]
- 221.Wu X., Liu J., Yang L., Wang F. Photothermally controlled drug release system with high dose loading for synergistic chemo-photothermal therapy of multidrug resistance cancer. Colloids Surf. B Biointerfaces. 2019;175:239–247. doi: 10.1016/j.colsurfb.2018.11.088. [DOI] [PubMed] [Google Scholar]
- 222.Zhang Y., Sha R., Zhang L., Zhang W., Jin P., Xu W., Ding J., Lin J., Qian J., Yao G., et al. Harnessing copper-palladium alloy tetrapod nanoparticle-induced pro-survival autophagy for optimized photothermal therapy of drug-resistant cancer. Nat. Commun. 2018;9:4236. doi: 10.1038/s41467-018-06529-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Wei X., Liu C., Wang Z., Luo Y. 3D printed core-shell hydrogel fiber scaffolds with NIR-triggered drug release for localized therapy of breast cancer. Int. J. Pharm. 2020;580:119219. doi: 10.1016/j.ijpharm.2020.119219. [DOI] [PubMed] [Google Scholar]
- 224.Xu L., Chen Y., Zhang P., Tang J., Xue Y., Luo H., Dai R., Jin J., Liu J. 3D printed heterogeneous hybrid hydrogel scaffolds for sequential tumor photothermal-chemotherapy and wound healing. Biomater. Sci. 2022;10:5648–5661. doi: 10.1039/D2BM00903J. [DOI] [PubMed] [Google Scholar]
- 225.Yang M., Lee S.Y., Kim S., Koo J.S., Seo J.H., Jeong D.I., Hwang C., Lee J., Cho H.J. Selenium and dopamine-crosslinked hyaluronic acid hydrogel for chemophotothermal cancer therapy. J. Control. Release Off. J. Control. Release Soc. 2020;324:750–764. doi: 10.1016/j.jconrel.2020.04.024. [DOI] [PubMed] [Google Scholar]
- 226.Wu C., Jiao Q., Wang C., Zheng Y., Pan X., Zhong W., Xu K. Nanofibrillar peptide hydrogels for self-delivery of lonidamine and synergistic photodynamic therapy. Acta Biomater. 2023;155:139–153. doi: 10.1016/j.actbio.2022.11.008. [DOI] [PubMed] [Google Scholar]
- 227.Hou X.L., Dai X., Yang J., Zhang B., Zhao D.H., Li C.Q., Yin Z.Y., Zhao Y.D., Liu B. Injectable polypeptide-engineered hydrogel depot for amplifying the anti-tumor immune effect induced by chemo-photothermal therapy. J. Mater. Chem. B. 2020;8:8623–8633. doi: 10.1039/D0TB01370F. [DOI] [PubMed] [Google Scholar]
- 228.Chang L., Liu X., Zhu J., Rao Y., Chen D., Wang Y., Zhao Y., Qin J. Cellulose-based thermo-responsive hydrogel with NIR photothermal enhanced DOX released property for anti-tumor chemotherapy. Colloids Surf. B Biointerfaces. 2022;218:112747. doi: 10.1016/j.colsurfb.2022.112747. [DOI] [PubMed] [Google Scholar]
- 229.He P.P., Du X., Cheng Y., Gao Q., Liu C., Wang X., Wei Y., Yu Q., Guo W. Thermal-Responsive MXene-DNA Hydrogel for Near-Infrared Light Triggered Localized Photothermal-Chemo Synergistic Cancer Therapy. Small. 2022;18:e2200263. doi: 10.1002/smll.202200263. [DOI] [PubMed] [Google Scholar]
- 230.Zhang Z., Li A., Min X., Zhang Q., Yang J., Chen G., Zou M., Sun W., Cheng G. An ROS-sensitive tegafur-PpIX-heterodimer-loaded in situ injectable thermosensitive hydrogel for photodynamic therapy combined with chemotherapy to enhance the tegafur-based treatment of breast cancer. Biomater. Sci. 2021;9:221–237. doi: 10.1039/D0BM01519A. [DOI] [PubMed] [Google Scholar]
- 231.Xu X., Zeng Z., Huang Z., Sun Y., Huang Y., Chen J., Ye J., Yang H., Yang C., Zhao C. Near-infrared light-triggered degradable hyaluronic acid hydrogel for on-demand drug release and combined chemo-photodynamic therapy. Carbohydr. Polym. 2020;229:115394. doi: 10.1016/j.carbpol.2019.115394. [DOI] [PubMed] [Google Scholar]
- 232.González-Ayón M.A., Licea-Rodriguez J., Méndez E.R., Licea-Claverie A. NVCL-Based Galacto-Functionalized and Thermosensitive Nanogels with GNRDs for Chemo/Photothermal-Therapy. Pharmaceutics. 2022;14:560. doi: 10.3390/pharmaceutics14030560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Jin R., Yang X., Zhao D., Hou X., Li C., Song X., Chen W., Wang Q., Zhao Y., Liu B. An injectable hybrid hydrogel based on a genetically engineered polypeptide for second near-infrared fluorescence/photoacoustic imaging-monitored sustained chemo-photothermal therapy. Nanoscale. 2019;11:16080–16091. doi: 10.1039/C9NR04630E. [DOI] [PubMed] [Google Scholar]
- 234.Marill J., Mohamed Anesary N., Paris S. DNA damage enhancement by radiotherapy-activated hafnium oxide nanoparticles improves cGAS-STING pathway activation in human colorectal cancer cells. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2019;141:262–266. doi: 10.1016/j.radonc.2019.07.029. [DOI] [PubMed] [Google Scholar]
- 235.Goel S., Ni D., Cai W. Harnessing the Power of Nanotechnology for Enhanced Radiation Therapy. ACS Nano. 2017;11:5233–5237. doi: 10.1021/acsnano.7b03675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Her S., Jaffray D.A., Allen C. Gold nanoparticles for applications in cancer radiotherapy: Mechanisms and recent advancements. Adv. Drug Deliv. Rev. 2017;109:84–101. doi: 10.1016/j.addr.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 237.Hainfeld J.F., Dilmanian F.A., Slatkin D.N., Smilowitz H.M. Radiotherapy enhancement with gold nanoparticles. J. Pharm. Pharmacol. 2008;60:977–985. doi: 10.1211/jpp.60.8.0005. [DOI] [PubMed] [Google Scholar]
- 238.Chen J., Li M., Yi X., Zhao Q., Chen L., Yang C., Wu J., Yang K. Synergistic Effect of Thermo-Radiotherapy Using Au@FeS Core–Shell Nanoparticles as Multifunctional Therapeutic Nanoagents. Part. Part. Syst. Charact. 2017;34:1600330. doi: 10.1002/ppsc.201600330. [DOI] [Google Scholar]
- 239.Zhou M., Chen Y., Adachi M., Wen X., Erwin B., Mawlawi O., Lai S.Y., Li C. Single agent nanoparticle for radiotherapy and radio-photothermal therapy in anaplastic thyroid cancer. Biomaterials. 2015;57:41–49. doi: 10.1016/j.biomaterials.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Park J., Park J., Ju E.J., Park S.S., Choi J., Lee J.H., Lee K.J., Shin S.H., Ko E.J., Park I., et al. Multifunctional hollow gold nanoparticles designed for triple combination therapy and CT imaging. J. Control. Release Off. J. Control. Release Soc. 2015;207:77–85. doi: 10.1016/j.jconrel.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 241.Yong Y., Cheng X., Bao T., Zu M., Yan L., Yin W., Ge C., Wang D., Gu Z., Zhao Y. Tungsten Sulfide Quantum Dots as Multifunctional Nanotheranostics for In Vivo Dual-Modal Image-Guided Photothermal/Radiotherapy Synergistic Therapy. ACS Nano. 2015;9:12451–12463. doi: 10.1021/acsnano.5b05825. [DOI] [PubMed] [Google Scholar]
- 242.Mirrahimi M., Beik J., Mirrahimi M., Alamzadeh Z., Teymouri S., Mahabadi V.P., Eslahi N., Ebrahimi Tazehmahalleh F., Ghaznavi H., Shakeri-Zadeh A., et al. Triple combination of heat, drug and radiation using alginate hydrogel co-loaded with gold nanoparticles and cisplatin for locally synergistic cancer therapy. Int. J. Biol. Macromol. 2020;158:617–626. doi: 10.1016/j.ijbiomac.2020.04.272. [DOI] [PubMed] [Google Scholar]
- 243.Wang Z., Zeng W., Chen Z., Suo W., Quan H., Tan Z.J. An intratumoral injectable nanozyme hydrogel for hypoxia-resistant thermoradiotherapy. Colloids Surf. B Biointerfaces. 2021;207:112026. doi: 10.1016/j.colsurfb.2021.112026. [DOI] [PubMed] [Google Scholar]
- 244.Zhou Y., Hu Y., Sun W., Lu S., Cai C., Peng C., Yu J., Popovtzer R., Shen M., Shi X. Radiotherapy-Sensitized Tumor Photothermal Ablation Using γ-Polyglutamic Acid Nanogels Loaded with Polypyrrole. Biomacromolecules. 2018;19:2034–2042. doi: 10.1021/acs.biomac.8b00184. [DOI] [PubMed] [Google Scholar]
- 245.Magnuson W.J., Mahal A., Yu J.B. Emerging Technologies and Techniques in Radiation Therapy. Semin. Radiat. Oncol. 2017;27:34–42. doi: 10.1016/j.semradonc.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 246.Mukerji R., Schaal J., Li X., Bhattacharyya J., Asai D., Zalutsky M.R., Chilkoti A., Liu W. Spatiotemporally photoradiation-controlled intratumoral depot for combination of brachytherapy and photodynamic therapy for solid tumor. Biomaterials. 2016;79:79–87. doi: 10.1016/j.biomaterials.2015.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Wu Y., Yao Y., Zhang J., Gui H., Liu J., Liu J. Tumor-Targeted Injectable Double-Network Hydrogel for Prevention of Breast Cancer Recurrence and Wound Infection via Synergistic Photothermal and Brachytherapy. Adv. Sci. 2022;9:e2200681. doi: 10.1002/advs.202200681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Gao S., Lin H., Zhang H., Yao H., Chen Y., Shi J. Nanocatalytic Tumor Therapy by Biomimetic Dual Inorganic Nanozyme-Catalyzed Cascade Reaction. Adv. Sci. 2019;6:1801733. doi: 10.1002/advs.201801733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Han L., Zhang H., Chen D., Li F. Protein-Directed Metal Oxide Nanoflakes with Tandem Enzyme-Like Characteristics: Colorimetric Glucose Sensing Based on One-Pot Enzyme-Free Cascade Catalysis. Adv. Funct. Mater. 2018;28:1800018. doi: 10.1002/adfm.201800018. [DOI] [Google Scholar]
- 250.Zhang R., Feng L., Dong Z., Wang L., Liang C., Chen J., Ma Q., Zhang R., Chen Q., Wang Y., et al. Glucose & oxygen exhausting liposomes for combined cancer starvation and hypoxia-activated therapy. Biomaterials. 2018;162:123–131. doi: 10.1016/j.biomaterials.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 251.Zhang Y.-H., Qiu W.-X., Zhang M., Zhang L., Zhang X.-Z. MnO2 Motor: A Prospective Cancer-Starving Therapy Promoter. ACS Appl. Mater. Interfaces. 2018;10:15030–15039. doi: 10.1021/acsami.8b01818. [DOI] [PubMed] [Google Scholar]
- 252.Tang W., Fan W., Zhang W., Yang Z., Li L., Wang Z., Chiang Y.-L., Liu Y., Deng L., He L., et al. Wet/Sono-Chemical Synthesis of Enzymatic Two-Dimensional MnO2 Nanosheets for Synergistic Catalysis-Enhanced Phototheranostics. Adv. Mater. 2019;31:1900401. doi: 10.1002/adma.201900401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Zhou J., Li M., Hou Y., Luo Z., Chen Q., Cao H., Huo R., Xue C., Sutrisno L., Hao L., et al. Engineering of a Nanosized Biocatalyst for Combined Tumor Starvation and Low-Temperature Photothermal Therapy. ACS Nano. 2018;12:2858–2872. doi: 10.1021/acsnano.8b00309. [DOI] [PubMed] [Google Scholar]
- 254.Van den Tempel N., Horsman M.R., Kanaar R. Improving efficacy of hyperthermia in oncology by exploiting biological mechanisms. Int. J. Hyperth. 2016;32:446–454. doi: 10.3109/02656736.2016.1157216. [DOI] [PubMed] [Google Scholar]
- 255.Yang G., Ji J., Liu Z. Multifunctional MnO2 nanoparticles for tumor microenvironment modulation and cancer therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021;13:e1720. doi: 10.1002/wnan.1720. [DOI] [PubMed] [Google Scholar]
- 256.Fan W., Lu N., Huang P., Liu Y., Yang Z., Wang S., Yu G., Liu Y., Hu J., He Q., et al. Glucose-Responsive Sequential Generation of Hydrogen Peroxide and Nitric Oxide for Synergistic Cancer Starving-Like/Gas Therapy. Angew. Chem. Int. Ed. 2017;56:1229–1233. doi: 10.1002/anie.201610682. [DOI] [PubMed] [Google Scholar]
- 257.Li S.-Y., Cheng H., Xie B.-R., Qiu W.-X., Zeng J.-Y., Li C.-X., Wan S.-S., Zhang L., Liu W.-L., Zhang X.-Z. Cancer Cell Membrane Camouflaged Cascade Bioreactor for Cancer Targeted Starvation and Photodynamic Therapy. ACS Nano. 2017;11:7006–7018. doi: 10.1021/acsnano.7b02533. [DOI] [PubMed] [Google Scholar]
- 258.He X., Hao Y., Chu B., Yang Y., Sun A., Shi K., Yang C., Zhou K., Qu Y., Li H., et al. Redox-activatable photothermal therapy and enzyme-mediated tumor starvation for synergistic cancer therapy. Nano Today. 2021;39:101174. doi: 10.1016/j.nantod.2021.101174. [DOI] [Google Scholar]
- 259.Sun Y., Fang K., Hu X., Yang J., Jiang Z., Feng L., Li R., Rao Y., Shi S., Dong C. NIR-light-controlled G-quadruplex hydrogel for synergistically enhancing photodynamic therapy via sustained delivery of metformin and catalase-like activity in breast cancer. Mater. Today Bio. 2022;16:100375. doi: 10.1016/j.mtbio.2022.100375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Luo Z., Fan X., Chen Y., Lai X., Li Z., Wu Y.L., He C. Mitochondria targeted composite enzyme nanogels for synergistic starvation and photodynamic therapy. Nanoscale. 2021;13:17737–17745. doi: 10.1039/D1NR06214J. [DOI] [PubMed] [Google Scholar]
- 261.Fan X., Luo Z., Chen Y., Yeo J.C.C., Li Z., Wu Y.L., He C. Oxygen self-supplied enzyme nanogels for tumor targeting with amplified synergistic starvation and photodynamic therapy. Acta Biomater. 2022;142:274–283. doi: 10.1016/j.actbio.2022.01.056. [DOI] [PubMed] [Google Scholar]
- 262.Yu P., Li X., Cheng G., Zhang X., Wu D., Chang J., Wang S. Hydrogen peroxide-generating nanomedicine for enhanced chemodynamic therapy. Chin. Chem. Lett. 2021;32:2127–2138. doi: 10.1016/j.cclet.2021.02.015. [DOI] [Google Scholar]
- 263.Lin H., Chen Y., Shi J. Nanoparticle-triggered in situ catalytic chemical reactions for tumour-specific therapy. Chem. Soc. Rev. 2018;47:1938–1958. doi: 10.1039/C7CS00471K. [DOI] [PubMed] [Google Scholar]
- 264.Huo M., Wang L., Chen Y., Shi J. Tumor-selective catalytic nanomedicine by nanocatalyst delivery. Nat. Commun. 2017;8:357. doi: 10.1038/s41467-017-00424-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Jia C., Guo Y., Wu F.G. Chemodynamic Therapy via Fenton and Fenton-Like Nanomaterials: Strategies and Recent Advances. Small. 2022;18:e2103868. doi: 10.1002/smll.202103868. [DOI] [PubMed] [Google Scholar]
- 266.Chen D., Chen C., Huang C., Chen T., Liu Z. Injectable Hydrogel for NIR-II Photo-Thermal Tumor Therapy and Dihydroartemisinin-Mediated Chemodynamic Therapy. Front. Chem. 2020;8:251. doi: 10.3389/fchem.2020.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Qin X., Wu C., Niu D., Qin L., Wang X., Wang Q., Li Y. Peroxisome inspired hybrid enzyme nanogels for chemodynamic and photodynamic therapy. Nat. Commun. 2021;12:5243. doi: 10.1038/s41467-021-25561-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Chang M., Feng W., Ding L., Zhang H., Dong C., Chen Y., Shi J. Persistent luminescence phosphor as in-vivo light source for tumoral cyanobacterial photosynthetic oxygenation and photodynamic therapy. Bioact. Mater. 2022;10:131–144. doi: 10.1016/j.bioactmat.2021.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Jiang F., Zhao Y., Yang C., Cheng Z., Liu M., Xing B., Ding B., Ma P., Lin J. A tumor microenvironment-responsive Co/ZIF-8/ICG/Pt nanoplatform for chemodynamic and enhanced photodynamic antitumor therapy. Dalton Trans. 2022;51:2798–2804. doi: 10.1039/D1DT04120G. [DOI] [PubMed] [Google Scholar]
- 270.Deng Y., Song P., Chen X., Huang Y., Hong L., Jin Q., Ji J. 3-Bromopyruvate-Conjugated Nanoplatform-Induced Pro-Death Autophagy for Enhanced Photodynamic Therapy against Hypoxic Tumor. ACS Nano. 2020;14:9711–9727. doi: 10.1021/acsnano.0c01350. [DOI] [PubMed] [Google Scholar]
- 271.Liu C., Wang D., Zhang S., Cheng Y., Yang F., Xing Y., Xu T., Dong H., Zhang X. Biodegradable Biomimic Copper/Manganese Silicate Nanospheres for Chemodynamic/Photodynamic Synergistic Therapy with Simultaneous Glutathione Depletion and Hypoxia Relief. ACS Nano. 2019;13:4267–4277. doi: 10.1021/acsnano.8b09387. [DOI] [PubMed] [Google Scholar]
- 272.Liu C., Cao Y., Cheng Y., Wang D., Xu T., Su L., Zhang X., Dong H. An open source and reduce expenditure ROS generation strategy for chemodynamic/photodynamic synergistic therapy. Nat. Commun. 2020;11:1735. doi: 10.1038/s41467-020-15591-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Zhou L., Zhao J., Chen Y., Zheng Y., Li J., Zhao J., Zhang J., Liu Y., Liu X., Wang S. MoS(2)-ALG-Fe/GOx hydrogel with Fenton catalytic activity for combined cancer photothermal, starvation, and chemodynamic therapy. Colloids and surfaces B Biointerfaces. 2020;195:111243. doi: 10.1016/j.colsurfb.2020.111243. [DOI] [PubMed] [Google Scholar]
- 274.Xu R., Zhang D., Tan J., Ge N., Liu D., Liu J., Ouyang L., Zhu H., Qiao Y., Qiu J., et al. A multifunctional cascade bioreactor based on a layered double oxides composite hydrogel for synergetic tumor chemodynamic/starvation/photothermal therapy. Acta Biomater. 2022;153:494–504. doi: 10.1016/j.actbio.2022.09.024. [DOI] [PubMed] [Google Scholar]
- 275.Lee S.Y., Park J., Jeong D.I., Hwang C., Lee J., Lee K., Kim H.J., Cho H.J. Ferrocene and glucose oxidase-installed multifunctional hydrogel reactors for local cancer therapy. J. Control. Release Off. J. Control. Release Soc. 2022;349:617–633. doi: 10.1016/j.jconrel.2022.07.017. [DOI] [PubMed] [Google Scholar]
- 276.Conde J., Oliva N., Zhang Y., Artzi N. Local triple-combination therapy results in tumour regression and prevents recurrence in a colon cancer model. Nat. Mater. 2016;15:1128–1138. doi: 10.1038/nmat4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Sun Z., Wang X., Liu J., Wang Z., Wang W., Kong D., Leng X. ICG/l-Arginine Encapsulated PLGA Nanoparticle-Thermosensitive Hydrogel Hybrid Delivery System for Cascade Cancer Photodynamic-NO Therapy with Promoted Collagen Depletion in Tumor Tissues. Mol. Pharm. 2021;18:928–939. doi: 10.1021/acs.molpharmaceut.0c00937. [DOI] [PubMed] [Google Scholar]
- 278.Sun X., Liu D., Xu X., Shen Y., Huang Y., Zeng Z., Xia M., Zhao C. NIR-triggered thermo-responsive biodegradable hydrogel with combination of photothermal and thermodynamic therapy for hypoxic tumor. Asian J. Pharm. Sci. 2020;15:713–727. doi: 10.1016/j.ajps.2019.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Wu Y., Liang Y., Liu Y., Hao Y., Tao N., Li J., Sun X., Zhou M., Liu Y.N. A Bi(2)S(3)-embedded gellan gum hydrogel for localized tumor photothermal/antiangiogenic therapy. J. Mater. Chem. B. 2021;9:3224–3234. doi: 10.1039/D1TB00257K. [DOI] [PubMed] [Google Scholar]
- 280.Liu N., Liu H., Chen H., Wang G., Teng H., Chang Y. Polyphotosensitizer nanogels for GSH-responsive histone deacetylase inhibitors delivery and enhanced cancer photodynamic therapy. Colloids Surf. B Biointerfaces. 2020;188:110753. doi: 10.1016/j.colsurfb.2019.110753. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.