Abstract
Adenocarcinoma of the esophagus (EAC) and gastroesophageal junction (GEJ-AC) is associated with poor prognosis, treatment resistance and limited systemic therapeutic options. To deeply understand the genomic landscape of this cancer type, and potentially identify a therapeutic target in a neoadjuvant chemotherapy non-responder 48-year-old man, we adopted a multi-omic approach. We simultaneously evaluated gene rearrangements, mutations, copy number status, microsatellite instability and tumor mutation burden. The patient displayed pathogenic mutations of the TP53 and ATM genes and variants of uncertain significance of three kinases genes (ERBB3, CSNK1A1 and RPS6KB2), along with FGFR2 and KRAS high copy number amplification. Interestingly, transcriptomic analysis revealed the Musashi-2 (MSI2)-C17orf64 fusion that has never been reported before. Rearrangements of the RNA-binding protein MSI2 with a number of partner genes have been described across solid and hematological tumors. MSI2 regulates several biological processes involved in cancer initiation, development and resistance to treatment, and deserves further investigation as a potential therapeutic target. In conclusion, our extensive genomic characterization of a gastroesophageal tumor refractory to all therapeutic approaches led to the discovery of the MSI2-C17orf64 fusion. The results underlie the importance of deep molecular analyses enabling the identification of novel patient-specific markers to be monitored during therapy or even targeted at disease evolution.
Keywords: gastroesophageal cancer, chemotherapy resistance, poorly-cohesive, fusion, MSI2
1. Introduction
Adenocarcinoma of the esophagus (EAC) and gastroesophageal junction (GEJ-AC) is an aggressive disease usually diagnosed at advanced stages, with limited curative options, a median life expectancy of 12 months and a 5-year survival rate of 12–20% in Western populations [1,2,3]. Its incidence has increased several-fold in Western countries in recent decades, being now the eighth most common cancer and the sixth leading cause of death in the world.
Several strong epidemiologic risk factors have been identified including reflux symptoms, obesity and smoking, and an etiologic role for inherited genetics more recently emerged, including candidate risk genes and pathways [4].
Patients with locally advanced or oligometastatic tumors are mostly candidates for multimodal therapy. Neoadjuvant chemotherapy or chemoradiotherapy, followed by radical surgery, is the standard of care for these patients, aiding tumor shrinkage, a higher rate of complete resection and eradication of circulating malignant cells [2]. The individual response is unpredictable, however 80% of patients achieve only an incomplete or absent response and their survival remains very poor [5,6]. In particular, poorly differentiated gastroesophageal adenocarcinomas, eventually characterized by the presence of signet ring cells (SRC), are generally resistant to current oncological therapies and are associated with poor prognosis [6]. Moreover, large-scale genomic studies have shown that EAC and GEJ-AC mainly resemble gastric cancer with chromosomal instability, though among cases not clearly of esophageal origin, positivity for microsatellite instability (MSI) and Epstein–Barr Virus (EBV) have been identified [7]. Their high mutational burden and mutation rates, the high frequency of copy number alterations and somatic structural rearrangements give rise to a significant heterogeneity among patients and within the same tumor, the latter potentially correlated to a poor response to standard chemotherapy treatments and to a worse outcome [6].
Therefore, there is an urgent need to find prognostic and predictive markers guiding the selection of the most appropriate treatments for patients affected by these tumors, also taking into consideration that, in the localized setting, novel systemic therapeutic regimens, such as immunotherapy, are underway.
While the analysis of HER2 in localized settings do not have a clear recommendation, the evaluation of MSI, EBV and PD-L1, are becoming more important. Accordingly, CLDN18.2 and FGFR might be interesting targets in the near future [8].
In this context, a wide genomic characterization of each case by next-generation sequencing (NGS) may be very helpful in the identification of biomarkers for the response to therapy, actionable alterations and new clinically-relevant targeted drugs.
Among molecular aberrations, the discovery and characterization of new fusion genes can both improve patient diagnosis and precision medicine, as it has been recently demonstrated for a number of novel rearrangements [9].
In the context of gastroesophageal cancer, some recurrent in-frame fusions have been associated with diffuse gastric cancer (DGC) and an aggressive disease phenotype [10]. In particular, the most common fusion, CLDN18–ARHGAP26, is more prevalent in early-onset DGC and correlates with poor survival and chemoresistance [11].
The aim of this report was to characterize fusion genes in gastroesophageal cancer, aiding in a clinically useful molecular refinement of this tumor subtype.
2. Case Presentation
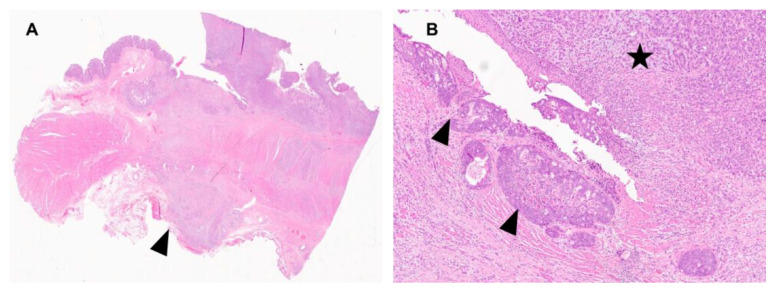
A 48-year-old Caucasian patient affected by GEJ-AC (clinical stage cT3N0M0) received fluorouracil plus leucovorin, oxaliplatin, and docetaxel chemotherapy (FLOT4 regimen) followed by total gastrectomy with an extended lymphadenectomy (ypT4aN3M0 G3). As reported in Figure 1, the tumor presented two different components, including a glandular and a poorly-cohesive one. The poorly-cohesive component was prevalent (90%), with focal SRC areas (<25%). No tumor regression was observed following neoadjuvant chemotherapy (regression score 5 according to Mandard classification). Cancer recurred during adjuvant therapy and the patient died 7 months after surgery.
Figure 1.
Hematoxylin and Eosin staining of the surgical specimen. (A) Low power view (2× magnification) showing the serosa invasion (arrowhead). (B) The glandular (intestinal-type) and the poorly-cohesive components are marked by arrowheads and an asterisk, respectively (10× magnification).
The nucleic acids were extracted from the formalin-fixed paraffin-embedded (FFPE) surgical specimen, with macrodissection of all different components of the tumor before combining them together. Genetic analyses were performed by Next Generation Sequencing using the Trusight Oncology (TSO) 500 panel (Illumina, San Diego, CA, USA) on a NextSeq 550 sequencer (Illumina). TSO 500 allows detection of the mutational status of more than 500 genes, copy number variation (CNV) for 59 genes, as well as MSI and the tumor mutational burden (TMB) with an analytical sensitivity > 96% (for all variant types at 5% variant allele frequency (VAF)) and specificity > 99.9% (Illumina data sheet M-GL-00173 v4.0). Data were processed through the TSO500 Local App pipeline which provides quality metrics for each sample, TMB, MSI, small variant calling and CNV detection. To select clinically-relevant variants, we filtered those retained according to the software small variant default settings as follows: (a) we excluded variants with a population frequency higher than 0.01 into at least one of the public human polymorphism databases (esp5400, https://evs.gs.washington.edu/EVS/ accessed on 22 November 2011; ExAC, http://exac.broadinstitute.org/;GnomAD accessed on 23 April 2016, https://gnomad.broadinstitute.org/ v2.1.1, accessed on 18 March 2019); (b) we discarded synonymous, 3′/5′ untranslated region, intronic and intergenic variants; (c) we excluded variants with a coverage lower than 100× or a VAF lower than 5%; (d) we held back variants annotated by ClinVar as pathogenic or likely pathogenic and rejected benign/likely benign variants (https://www.ncbi.nlm.nih.gov/clinvar/ accessed on 16 March 2020); (e) we integrated Varsome premium information (v. 11.6.1; https://varsome.com/ accessed on 2 February 2023) in the characterization of variants not annotated or marked as “uncertain significance” by ClinVar. RNA sequencing analysis of 1385 cancer genes was performed by the TruSight RNA Pancancer Panel (Illumina) on MiSeq (Illumina). Illumina panel sensitivity tests were reported on their website. Transcriptome data were analyzed by an in-house pipeline to identify true positive transcripts. In detail, we used FusionCatcher, STAR-Fusion and two Basespace applications (RNA-Seq Alignment and TopHat Alignment; Illumina). Each tool used its own aligner except for FusionCatcher which combines BLAT, STAR, Bowtie and Bowtie2. The four tool outputs were unified and filtered by a multistep strategy to identify true positive transcripts. Briefly, retained fusions were detected by at least three tools and we introduced two further criteria to retain or reject fusions detected by two or one tool specified in patent request (PCT/EP2021/065692).
The DNA analysis revealed very high FGFR2 and KRAS gene amplifications (estimated copy number of 20.6 and 19.9, respectively; Table S1), a status of microsatellite stability (MSI < 20%) and of a low TMB (<10 mutations/megabase). Moreover, as reported in Table 1, two pathogenic mutations have been detected: a hotspot variant in the TP53 gene (TP53:p.R248W; VAF = 46.3%) and a mutation of the ATM gene (ATM: p.Gly2023Arg; VAF = 46.8%). Both variants were also detected at the RNA level. We identified three additional mutations, with uncertain significance, targeting three different kinases: Erb-B2 receptor tyrosine kinase 3 (ERBB3), casein kinase 1 α 1 (CSNK1A1) and ribosomal protein S6 kinase B2 (RPS6KB2; Table 1 and Table S2).
Table 1.
Mutations identified by DNA sequencing.
| Gene | Chr | HGVSC | HGVSP | Varsome Classification | AF |
|---|---|---|---|---|---|
| TP53 | 17 | c.742C>T | p.(Arg248Trp) | pathogenic | 46.85 |
| ATM | 11 | c.6067G>A | p.(Gly2023Arg) | pathogenic | 46.31 |
| ERBB3 | 12 | c.172G>A | p.(Val58Met) | VUS | 7.12 |
| CSNK1A1 | 5 | c.702_703insAACATGGAATCA | p.(Ser234_Leu235insAsnMetGluSer) | VUS | 15.99 |
| RPS6KB2 | 11 | c.358C>T | p.(Arg120Trp) | VUS | 54.59 |
Chr = chromosome. HGVSC = human genome variation society-coding. HGVSP = human genome variation society-protein. VUS = variant of uncertain significance; educational use only. AF = allele frequency.
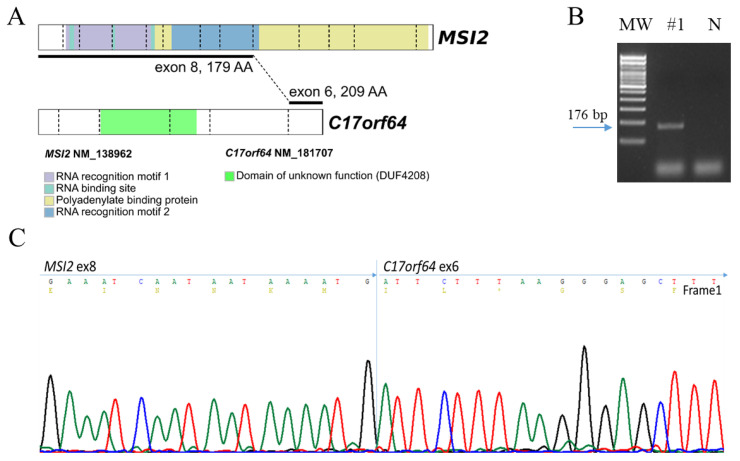
By transcriptome sequencing we obtained 4.025.762 reads and 99.32% of them were aligned reads. Fusion analysis excluded the presence of the diffuse-type associated fusions CLDN18–ARHGAP26 and CTNND1–ARHGAP26 that are frequently found in SRC. Conversely, all fusion calling tools detected a novel transcript, namely Musashi-2 (MSI2)–C17orf64 (MSI2, chr17:55674311, ex8; C17orf64, chr17:58508542, ex6; Figure 2A). The resulting in-frame chimera caused the loss of all MSI2 polyadenylate binding protein domains and of the C17orf64 DUF4208 domain (Figure 2A). The fusion transcript was confirmed by RT-PCR and Sanger sequencing (Figure 2B,C).
Figure 2.
MSI2–C17orf64 fusion. (A) MSI2 and C17orf64 protein diagrams, domain annotations and fusion scheme between MSI2 exon 8 and C17orf64 exon 6 (MSI2, NM_138962, chr17:55674311, +; C17orf64, NM_181707, chr17:58508542, +; Human hg19). (B) Agarose gel electrophoresis of tumor sample (#1). Lane MW: DNA marker (100 bp ladder). Samples 1: MSI2-C17orf64 PCR amplified product; N = negative. (C) Sanger sequencing chromatogram of MSI2 exon 8–C17orf64 exon 6 fusion.
3. Discussion
The patient described in the present case report was affected by a mixed adenocarcinoma with two distinct histological components [12,13]: a poorly-cohesive one (90% of the tumor) including SRC, and a glandular one (10% of the tumor). The proportion of SRCs in poorly cohesive subtypes is a marker of differentiation able to predict tumor prognosis [14], thus suggesting a potential connection between low (<25%) SRC proportion in the analyzed tumor and the poor overall survival of the patient. Moreover, it has been reported that chemotherapy and chemoradiotherapy are more effective in intestinal type carcinomas compared with poorly-cohesive/mixed type carcinomas [5].
The resistance to conventional therapies is a recurring issue in EAC and GEJ-AC, since only a small percentage of patients achieve a complete pathological response, leading to a better survival [6]. The definition of predictive biomarkers is needed to tailor treatments for patients and to identify additional targets for novel therapeutic approaches.
EAC and of GEJ-AC resemble chromosomally unstable gastric adenocarcinoma in their genetic makeup [7]. TP53 is the most frequently mutated gene in these tumors, being mostly prevalent in GEJ-AC [15]. In particular, the TP53R248W variant found in our case, is the most common site-specific mutation in all cancers. It induces loss of function by preventing p53 binding to its target promoters, leading to a consequent p53 dysfunction known to be directly related to poor response to chemotherapy [16]. The disruption of the DNA damage response pathway in the present tumor is also reinforced by the pathogenic variant found in the ATM gene, which could affect its expression, impacting on the patient survival [17]. Indeed, a potential benefit from combined chemotherapy and PARP-inhibitor treatment has been suggested [18].
Moreover, the high level of FGFR2 amplification is of particular interest due to its association with poor patient prognosis [19] and therapeutic implications as a potential target of tyrosine kinase inhibitors [20,21]. FGFR2 amplification in gastroesophageal cancers has been found with a frequency ranging from 2.5% to 7.4%, it is often associated with FGFR2 overexpression [22] and other receptor tyrosine kinases’ alterations, such as KRAS amplification. Both the level of FGFR copy number and the putative co-occurring alterations which can modify FGFR2-directed therapy should be considered for a rational combination of treatment options and patients’ selection.
Several fusion transcripts have been repeatedly reported as drivers of gastroesophageal cancer, being mainly described in young patients and in diffuse-type cancers and SRC carcinomas [10,11]. However, none of them have been found in the present tumor.
However, we detected a new rearrangement, involving the MSI2 and C17orf64 genes that, to our knowledge, has never been previously described.
Little is known about C17orf64, a poorly characterized protein showing a testis-specific expression pattern and heavy methylation of the promoter in high-grade serous ovarian carcinoma [23,24]. Conversely, MSI2 is a crucial regulator of cancer stem cell programs by acting on the stability and translation of target mRNAs that encode key proteins of oncogenic signaling pathways (e.g., TGFβR1/SMAD3, NUMB/Notch, PTEN/mTOR, MET, and MYC) [25,26,27,28,29].
MSI2 has been deeply studied across cancers. Its expression strongly correlated with poor clinical prognosis in hematological malignancies [30,31]. Moreover, it is overexpressed in many solid tumors, including glioblastoma, breast cancer, cervical cancer, pancreatic cancer, gastric cancer (GC), colorectal cancer (CRC) and hepatocellular c arcinoma (HCC) [28,32]. High MSI2 levels were associated with poor tumor differentiation, and poor prognosis. Indeed, functional studies demonstrated a role of MSI2 in maintaining stemness properties, metastatic capacity, in vitro and in vivo tumorigenic ability and promoting drug resistance either in HCC or pancreatic tumors [33,34,35,36]. Interestingly, in pancreatic cancer, a high proportion of circulating tumor cells (CTCs) expressed Msi2 (Msi2+), and were more tumorigenic than Msi2− CTCs, posing a greater risk for tumor dissemination [33]. MSI2 is also a central component of oncogenic pathways promoting intestinal transformation [37], its expression is further elevated during CRC progression, and is associated with poor prognosis [38]. In GC the expression level of MSI2 was positively associated with invasion depth, stage, degree of differentiation and tumor size [39].
The association between MSI2 overexpression and poor prognosis has been recently established across different malignancies, thus indicating that MSI2 could be a novel prognostic biomarker and therapeutic target [40].
Finally, recent findings pointed to MSI2 as a promising therapeutic target for solid and hematological malignancies. Small molecules inhibiting its oncogenic activity are currently under preclinical investigation [41,42,43] and their potential role in treating cases carrying MSI2 rearrangements deserves a case-by-case evaluation.
A rare MSI2–HOXA9 translocation has been previously identified in patients progressing from chronic myeloid leukemia to blast crisis [44]. More recently, a MSI2–PC rearrangement has been described in myelodysplastic syndrome [45]. These fusions suggest a potential role of MSI2 as a driver of enforced expression of the partner gene.
The novel MSI2–C17orf64 that we describe resulted in the loss of the MSI2 C-terminal region and poly-A binding protein (PABP)-interaction domain, which regulates the translation of a subset of MSI2 target genes [28]. In this case, it is not clear whether the rearrangement activates the aberrant expression of an unexplored C17orf64 domain or whether it generates a functional, dysfunctional or even inactive chimeric protein. Although one limitation of our work is the analysis of a single case carrying the fusion, the consequences of the rearrangement on MSI2 activity deserve further investigation.
4. Conclusions
This is the first case reporting an MSI2–C17orf64 fusion in tumors in general, and in gastroesophageal adenocarcinoma, in particular. The MSI2 gene is involved in intestinal and hematological stem cell pathways and promotes tumor progression, dissemination and drug resistance in several solid and hematological malignancies. Future studies will highlight the biological and clinical significance of this novel fusion. This finding opens an interesting new perspective on the importance of a deep genomic characterization of gastroesophageal tumors that are actually refractory to every therapeutic approach.
5. Patents
PCT application No. PCT/EP2021/065692 (10 June 2021): Method to identify linked genetic fusions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/genes14040918/s1, Table S1: Copy number (CN) alterations; Table S2: TruSight Oncology 500 (Illumina) detailed output on the selected mutations.
Author Contributions
A.F. designed the research study. A.F., E.F., D.A. and C.M. analyzed genetic data. A.F. and C.D. performed the research. S.M. and G.M. contributed to patient enrolment. A.F., G.D.R., E.B., R.F., S.M., G.M. and C.M. contributed to data interpretation and A.F., R.F. and C.M. contributed to manuscript writing. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the or Ethics Committee Ethics Committee “Comitato Etico IRST IRCCS AVR (CEIIAV)”- Italy (Reg. Sper. 109/2016 Protocol 7353/51/2016, approved on 23 November 2016).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. GM has competing interests with Novartis, BMS, Roche, Pfizer, ARIAD, MSD.
Funding Statement
This work was partly supported thanks to the contribution of Ricerca Corrente by the Italian Ministry of Health within the research line “Precision, gender and ethnicity-based medicine and geroscience: genetic-molecular mechanisms in the development, characterization and treatment of tumors”.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Olsen C.M., Pandeya N., Green A.C., Webb P.M., Whiteman D.C. Population attributable fractions of adenocarcinoma of the esophagus and gastroesophageal junction. Am. J. Epidemiol. 2011;174:582–590. doi: 10.1093/aje/kwr117. [DOI] [PubMed] [Google Scholar]
- 2.Chevallay M., Bollschweiler E., Chandramohan S.M., Schmidt T., Koch O., Demanzoni G., Mönig S., Allum W. Cancer of the gastroesophageal junction: A diagnosis, classification, and management review. Ann. N. Y. Acad. Sci. 2018;1434:132–138. doi: 10.1111/nyas.13954. [DOI] [PubMed] [Google Scholar]
- 3.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 4.Chen J., Ali M.W., Yan L., Dighe S.G., Dai J.Y., Vaughan T.L., Casey G., Buas M.F. Prioritization and functional analysis of GWAS risk loci for Barrett’s esophagus and esophageal adenocarcinoma. Hum. Mol. Genet. 2022;31:410–422. doi: 10.1093/hmg/ddab259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Batran S.-E., Hofheinz R.D., Pauligk C., Kopp H.-G., Haag G.M., Luley K.B., Meiler J., Homann N., Lorenzen S., Schmalenberg H., et al. Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): Results from the phase 2 part of a multicentre, open-label, randomised phase 2/3 trial. Lancet Oncol. 2016;17:1697–1708. doi: 10.1016/S1470-2045(16)30531-9. [DOI] [PubMed] [Google Scholar]
- 6.Smyth E.C., Lagergren J., Fitzgerald R.C., Lordick F., Shah M.A., Lagergren P., Cunningham D. Oesophageal cancer. Nat. Rev. Dis. Prim. 2017;3:17048. doi: 10.1038/nrdp.2017.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cancer Genome Atlas Research Network Integrated genomic characterization of oesophageal carcinoma Molecular separation of ESCC and EAC. Nature. 2017;541:169–175. doi: 10.1038/nature20805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Puhr A.I.-M.H.C., Reiter T.J., Preusser M., Prager G.W. Recent Advances in the Systemic Treatment of Localized Gastroesophageal Cancer. Cancers. 2023;15:1900. doi: 10.3390/cancers15061900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao Q., Liang W.-W., Foltz S.M., Mutharasu G., Jayasinghe R.G., Cao S., Liao W.-W., Reynolds S.M., Wyczalkowski M.A., Yao L., et al. Driver Fusions and Their Implications in the Development and Treatment of Human Cancers. Cell Rep. 2018;23:227–238.e3. doi: 10.1016/j.celrep.2018.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang H., Hong D., Cho S.Y., Park Y.S., Ko W.R., Kim J.H., Hur H., Lee J., Kim S.-J., Kwon S.Y., et al. RhoGAP domain-containing fusions and PPAPDC1A fusions are recurrent and prognostic in diffuse gastric cancer. Nat. Commun. 2018;9:4439. doi: 10.1038/s41467-018-06747-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shu Y., Zhang W., Hou Q., Zhao L., Zhang S., Zhou J., Song X., Zhang Y., Jiang D., Chen X., et al. Prognostic significance of frequent CLDN18-ARHGAP26/6 fusion in gastric signet-ring cell cancer. Nat. Commun. 2018;9:2447. doi: 10.1038/s41467-018-04907-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO . Digestive System Tumours. 5th ed. Volume 1 WHO; Geneva, Switzerland: 2019. Classification of Tumours Editorial Board. (WHO Classification of Tumours Series). [Google Scholar]
- 13.Fiocca R., Mastracci L., Lugaresi M., Grillo F., D’errico A., Malvi D., Spaggiari P., Tomezzoli A., Albarello L., Ristimäki A., et al. The prognostic impact of histology in esophageal and esophago-gastric junction adenocarcinoma. Cancers. 2021;13:5211. doi: 10.3390/cancers13205211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roviello F., Marano L., Ambrosio M.R., Resca L., D'Ignazio A., Petrelli F., Petrioli R., Costantini M., Polom K., Macchiarelli R., et al. Signet ring cell percentage in poorly cohesive gastric cancer patients: A potential novel predictor of survival. Eur. J. Surg. Oncol. 2022;48:561–569. doi: 10.1016/j.ejso.2021.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Wood A.C., Zhang Y., Mo Q., Cen L., Fontaine J., Hoffe S.E., Frakes J., Dineen S.P., Pimiento J.M., Walko C.M., et al. Evaluation of Tumor DNA Sequencing Results in Patients with Gastric and Gastroesophageal Junction Adenocarcinoma Stratified by TP53 Mutation Status. Oncologist. 2022;27:307–313. doi: 10.1093/oncolo/oyac018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sihag D.M.S., Nussenzweig S.C., Walch H.S., Hsu M., Tan K.S., De La Torre S., Janjigian Y.Y., Maron S.B., Ku G.Y., Tang L.H., et al. The Role of the TP53 Pathway in Predicting Response to Neoadjuvant Therapy in Esophageal Adenocarcinoma. Clin. Cancer Res. 2022;28:2669–2678. doi: 10.1158/1078-0432.CCR-21-4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim H.S., Kim M.A., Hodgson D., Harbron C., Wellings R., O’connor M.J., Womack C., Yin X., Bang Y.-J., Im S.-A., et al. Concordance of ATM (ataxia telangiectasia mutated) immunohistochemistry between biopsy or metastatic tumor samples and primary tumors in gastric cancer patients. Pathobiology. 2013;80:127–137. doi: 10.1159/000346034. [DOI] [PubMed] [Google Scholar]
- 18.Bang Y.-J., Im S.-A., Lee K.-W., Cho J.Y., Song E.-K., Lee K.H., Kim Y.H., Park J.O., Chun H.G., Zang D.Y., et al. Randomized, double-blind phase II trial with prospective classification by ATM protein level to evaluate the efficacy and tolerability of olaparib plus paclitaxel in patients with recurrent or metastatic gastric cancer. J. Clin. Oncol. 2015;33:3858–3865. doi: 10.1200/JCO.2014.60.0320. [DOI] [PubMed] [Google Scholar]
- 19.Su X., Zhan P., Gavine P.R., Morgan S., Womack C.J., Ni X., Shen D., Bang Y.-J., Im S.-A., Kim W.H., et al. FGFR2 amplification has prognostic significance in gastric cancer: Results from a large international multicentre study. Br. J. Cancer. 2014;110:967–975. doi: 10.1038/bjc.2013.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lengyel C.G., Hussain S., Seeber A., Nidhamalddin S.J., Trapani D., Habeeb B.S., Elfaham E., Mazher S.A., Seid F., Khan S.Z., et al. FGFR Pathway Inhibition in Gastric Cancer: The Golden Era of an Old Target? Life. 2022;12:81. doi: 10.3390/life12010081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hyung S., Han B., Jung J., Kim S.T., Hong J.Y., Park S.H., Zang D.Y., Park J.O., Park Y.S., Kim K.M., et al. Incidence of FGFR2 Amplification and FGFR2 Fusion in Patients with Metastatic Cancer Using Clinical Sequencing. J. Clin. Oncol. 2022;2022:9714570. doi: 10.1155/2022/9714570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klempner S.J., Madison R., Pujara V., Ross J.S., Miller V.A., Ali S.M., Schrock A.B., Kim S.T., Maron S.B., Dayyani F., et al. FGFR2-Altered Gastroesophageal Adenocarcinomas Are an Uncommon Clinicopathologic Entity with a Distinct Genomic Landscape. Oncologist. 2019;24:1462–1468. doi: 10.1634/theoncologist.2019-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pisanic T.R., Cope L.M., Lin S.-F., Yen T.-T., Athamanolap P., Asaka R., Nakayama K., Fader A.N., Wang T.-H., Shih I.-M., et al. Methylomic Analysis of Ovarian Cancers Identifies Tumor-Specific Alterations Readily Detectable in Early Precursor Lesions. Clin. Cancer Res. 2018;24:6536–6547. doi: 10.1158/1078-0432.CCR-18-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dai Y., Nie J., Luo Z., Nie D. Expression profile analysis of a new testis-specifically expressed gene C17ORF64 and its association with cell apoptosis in MCF-7 cells. Mol. Biol. Rep. 2021;48:1521–1529. doi: 10.1007/s11033-021-06191-6. [DOI] [PubMed] [Google Scholar]
- 25.Kudinov A.E., Deneka A., Nikonova A.S., Beck T.N., Ahn Y.-H., Liu X., Martinez C.F., Schultz F.A., Reynolds S., Yang D.-H., et al. Musashi-2 (MSI2) supports TGF-β signaling and inhibits claudins to promote non-small cell lung cancer (NSCLC) metastasis. Proc. Natl. Acad. Sci. USA. 2016;113:6955–6960. doi: 10.1073/pnas.1513616113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang C., Zhang W., Wang L., Kazobinka G., Han X., Li B., Hou T. Musashi-2 promotes migration and invasion in bladder cancer via activation of the JAK2/STAT3 pathway. Lab. Investig. 2016;96:950–958. doi: 10.1038/labinvest.2016.71. [DOI] [PubMed] [Google Scholar]
- 27.Kharas M.G., Lengner C.J. Stem Cells, Cancer, and MUSASHI in Blood and Guts. Trends Cancer. 2017;3:347–356. doi: 10.1016/j.trecan.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kudinov A.E., Karanicolas J., Golemis E.A., Boumber Y. Musashi RNA-Binding Proteins as Cancer Drivers and Novel Therapeutic Targets. Clin. Cancer Res. 2017;23:2143–2153. doi: 10.1158/1078-0432.CCR-16-2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dong W., Liu X., Yang C., Wang D., Xue Y., Ruan X., Zhang M., Song J., Cai H., Zheng J., et al. Glioma glycolipid metabolism: MSI2–SNORD12B–FIP1L1–ZBTB4 feedback loop as a potential treatment target. Clin. Transl. Med. 2021;11:e411. doi: 10.1002/ctm2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Byers R.J., Currie T., Tholouli E., Rodig S.J., Kutok J.L. MSI2 protein expression predicts unfavorable outcome in acute myeloid leukemia. Blood. 2011;118:2857–2867. doi: 10.1182/blood-2011-04-346767. [DOI] [PubMed] [Google Scholar]
- 31.Mu Q., Wang Y., Chen B., Qian W., Meng H., Tong H., Chen F., Ma Q., Ni W., Chen S., et al. High expression of Musashi-2 indicates poor prognosis in adult B-cell acute lymphoblastic leukemia. Leuk. Res. 2013;37:922–927. doi: 10.1016/j.leukres.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 32.Zong Z., Zhou T., Rao L., Jiang Z., Li Y., Hou Z., Yang B., Han F., Chen S. Musashi2 as a novel predictive biomarker for liver metastasis and poor prognosis in colorectal cancer. Cancer Med. 2016;5:623–630. doi: 10.1002/cam4.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo K., Cui J., Quan M., Xie D., Jia Z., Wei D., Wang L., Gao Y., Ma Q., Xie K. The novel KLF4/MSI2 signaling pathway regulates growth and metastasis of pancreatic cancer. Clin. Cancer Res. 2017;23:687–696. doi: 10.1158/1078-0432.CCR-16-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fang T., Lv H., Wu F., Wang C., Li T., Lv G., Tang L., Guo L., Tang S., Cao D., et al. Musashi 2 contributes to the stemness and chemoresistance of liver cancer stem cells via LIN28A activation. Cancer Lett. 2017;384:50–59. doi: 10.1016/j.canlet.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 35.Wang X., Wang R., Bai S., Xiong S., Li Y., Liu M., Zhao Z., Wang Y., Zhao Y., Chen W., et al. Musashi2 contributes to the maintenance of CD44v6+ liver cancer stem cells via notch1 signaling pathway. J. Exp. Clin. Cancer Res. 2019;38:505. doi: 10.1186/s13046-019-1508-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao H., Bi M., Lou M., Yang X., Sun L. Downregulation of SOX2-OT Prevents Hepatocellular Carcinoma Progression Through miR-143-3p/MSI2. Front. Oncol. 2021;11:685912. doi: 10.3389/fonc.2021.685912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang S., Li N., Yousefi M., Nakauka-Ddamba A., Li F., Parada K., Rao S., Minuesa G., Katz Y., Gregory B.D., et al. Transformation of the intestinal epithelium by the MSI2 RNA-binding protein. Nat. Commun. 2015;6:6517. doi: 10.1038/ncomms7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kharin L., Bychkov I., Karnaukhov N., Voloshin M., Fazliyeva R., Deneka A., Frantsiyants E., Kit O., Golemis E., Boumber Y. Prognostic role and biologic features of Musashi-2 expression in colon polyps and during colorectal cancer progression. PLoS ONE. 2021;16:e0252132. doi: 10.1371/journal.pone.0252132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang Z., Li J., Shi Y., Li L., Guo X. Increased musashi 2 expression indicates a poor prognosis and promotes malignant phenotypes in gastric cancer. Oncol. Lett. 2019;17:2599–2606. doi: 10.3892/ol.2019.9889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu W., Li J., Dong D., Dou F., Lin Y., Yang X., Zhou Y. Prognostic value of MSI2 expression in human malignancies: A PRISMA-compliant meta-analysis. Medicine. 2022;101:e32064. doi: 10.1097/MD.0000000000032064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Minuesa G., Albanese S.K., Xie W., Kazansky Y., Worroll D., Chow A., Schurer A., Park S.-M., Rotsides C.Z., Taggart J., et al. Small-molecule targeting of MUSASHI RNA-binding activity in acute myeloid leukemia. Nat. Commun. 2019;10:2691. doi: 10.1038/s41467-019-10523-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mohibi S., Chen X., Zhang J. Cancer the‘RBP’eutics–RNA-binding proteins as therapeutic targets for cancer. Pharmacol. Ther. 2019;203:107390. doi: 10.1016/j.pharmthera.2019.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang X., Su K., Liu Y., Zhu D., Pan Y., Ke X., Qu Y. Small Molecule Palmatine Targeting Musashi-2 in Colorectal Cancer. Front. Pharmacol. 2022;12:793449. doi: 10.3389/fphar.2021.793449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barbouti A., Höglund M., Johansson B., Lassen C., Nilsson P.-G., Hagemeijer A., Mitelman F., Fioretos T. A novel gene, MSI2, encoding a putative RNA-binding protein is recurrently rearranged at disease progression of chronic myeloid leukemia and forms a fusion gene with HOXA9 as a result of the cryptic t(7;17)(p15;q23) Cancer Res. 2003;63:1202–1206. [PubMed] [Google Scholar]
- 45.Zhang Y., Liu Y., Wang T., Wang H., Chen X., Cao P., Ma X., Liu M., Xu P., Bi H., et al. Competitive evolved sub-clonal BCR::ABL1 and novel MSI2::PC fusion genes in myelodysplastic syndrome with isolated del(5q) Hematol. Oncol. 2023;41:178–181. doi: 10.1002/hon.3095. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used and/or analyzed during the current study are available from the corresponding author on reasonable request.