Abstract
Background
Bi-directional flow of nutrients between marine and terrestrial ecosystems can provide essential resources that structure communities in transitional habitats. On the Pacific coast of North America, anadromous salmon (Oncorhynchus spp.) constitute a dominant nutrient subsidy to aquatic habitats and riparian vegetation, although the contribution to terrestrial habitats is not well established. We use a dual isotope approach of δ15N and δ13C to test for the contribution of salmon nutrients to multiple trophic levels of litter-based terrestrial invertebrates below and above waterfalls that act as a barrier to salmon migration on two watersheds in coastal British Columbia.
Results
Invertebrates varied predictably in δ15N with enrichment of 3–8‰ below the falls compared with above the falls in all trophic groups on both watersheds. We observed increasing δ15N levels in our invertebrate groups with increasing consumption of dietary protein. Invertebrates varied in δ13C but did not always vary predictably with trophic level or habitat. From 19.4 to 71.5% of invertebrate total nitrogen was originally derived from salmon depending on taxa, watershed, and degree of fractionation from the source.
Conclusions
Enrichment of δ15N in the invertebrate community below the falls in conjunction with the absence of δ13C enrichment suggests that enrichment in δ15N occurs primarily through salmon-derived nitrogen subsidies to litter, soil and vegetation N pools rather than from direct consumption of salmon tissue or salmon tissue consumers. Salmon nutrient subsidies to terrestrial habitats may result in shifts in invertebrate community structure, with subsequent implications for higher vertebrate consumers, particularly the passerines.
Background
Nutrient cycling between geographically distinctive ecosystems can produce zones of major productivity and biodiversity. It is generally recognized that downstream transport of terrestrial nutrients into marine estuaries produces one of the world's most productive habitats, but recent investigations suggest that the reverse flow, from marine to terrestrial habitats, may also be exceptionally important in structuring highly diverse coastal ecosystems [1].
Every year in the Pacific Northwest anadromous salmon (Oncorhynchus spp.) transport marine-derived nutrients from the North Pacific Ocean into coastal ecosystems. This salmon nutrient subsidy extends from aquatic habitats into riparian forests, and is thought to be ecologically equivalent to the migration of the wildebeest on the Serengeti [2]. Stable isotope studies in aquatic and terrestrial ecosystems reveal that salmon contribute highly to yearly protein intake for many vertebrates [1,3-5] and invertebrates [6,7], and provide substantial nutrient inputs to limnetic food webs [6,8-10], and riparian vegetation [7,11-13], emphasizing the ecological magnitude of this keystone resource for coastal communities.
Transfer of salmon nutrients into terrestrial habitats occurs primarily through bear (Ursus spp.) mediated salmon carcass transfer [14-16] and urine deposition [12], but can also occur as a result of flooding events [11], hyporheic zone transfer [5], or the activities of other scavengers and predators [3,5]. Since nitrogen is often limiting in coastal temperate rainforests of the Pacific Northwest [17], this salmon nutrient pulse to riparian forests can provide a significant proportion of plant total nitrogen [11-13], and is thought to increase riparian primary productivity, vegetation and litter quality, and soil nutrient capital [13].
Studies in forest ecosystems adjacent to salmon streams have so far been limited to vegetational use of salmon nutrients and have ignored other potential food web beneficiaries, particularly terrestrial invertebrates. Macro-invertebrates of coastal coniferous forests of the Pacific Northwest, including insects, arachnids, myriapods, annelid worms, isopods and gastropods, comprise the base of the myriad of nutrient and energy pathways from primary producers through to higher vertebrate consumers, and are highly important in many ecosystem processes including herbivory, litter decomposition, and nutrient cycling [18-20].
We use a dual isotope approach of δ15N and δ13C to assess: a) the extent of utilization of salmon-derived nitrogen and carbon by various trophic groups in a terrestrial invertebrate forest litter community and b) the mechanism of salmon nutrient utilization by invertebrates; either directly through salmon tissue consumption, or indirectly through utilization of salmon nitrogen sequestered into riparian vegetation or soil N pools. We compare the cycling of nutrients above and below waterfalls as a means of examining ecological discontinuities that may occur in litter-based macro-invertebrates between salmon and salmon-free forest sites, and speculate on possible implications to invertebrate community structure and higher vertebrate consumers. We also discuss components of invertebrate isotopic variability as it relates to microspatial variability in δ15N, invertebrate trophic structure, and invertebrate niche.
Results
Invertebrate trophic groups varied predictably with respect to δ15N. The nested ANOVA analysis demonstrated that the majority of variance in δ15N was due to falls within watersheds (F = 9.191; p = 0.031; R2 = 0.819) and taxonomic group within all other factors (F = 13.71; p < 0.001; R2 = 0.689). Variation in δ15N that occurred between watersheds or distance of collection from the stream contributed little to total variance and was insignificant in the model (See methods for violations). Invertebrates were enriched by 3–8‰ along salmon spawning reaches compared to similar groups collected above the falls, and showed a gradient of increasing values with increased trophic level at both salmon and non-salmon sites (Figure 1). There were highly significant differences in δ15N (t-tests: p < 0.01) above and below waterfalls for all trophic groups at both watersheds. Multiple comparison tests (Tukey's post hoc) revealed distinct trophic separation in δ15N between at least two invertebrate groups depending on site of collection (Table 1). Millipede detritivores had higher δ15N values than root feeding weevils on all sites but only on the Clatse above the falls was this trend significant. Carabid beetles demonstrated higher δ15N values than millipedes at all sites with significant differences on the Clatse River below and above the falls and on the Neekas River above the falls. Spider predators were significantly more enriched than carabid beetles on the Neekas River on both salmon and non-salmon sites, but demonstrated only marginally higher δ15N values than these beetles on the Clatse River. Carabid beetle omnivores and spider predators demonstrated significantly higher variance in δ15N below the falls than above on both watersheds (Carabidae Clatse: F14,6 = 14.61, p < 0.005; Carabidae Neekas: F21,6= 21.94, p < 0.001; Araneae Clatse: F18,16 = 5.41, p < 0.002; Araneae Neekas: F17,11 = 4.94, p < 0.02) (F-ratio tests).
Figure 1.
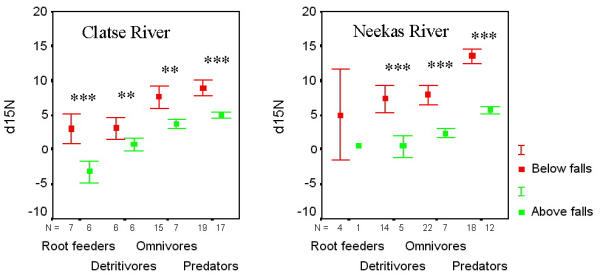
δ15N values in four trophic groupings of litter-based invertebrates collected above and below waterfall barriers to salmon migration on the Clatse and Neekas Rivers, British Columbia. Invertebrates are ranked (left to right) based on increasing consumption of animal protein (see methods). t-test results: ** denotes p < 0.01; *** denotes p < 0.001.
Table 1.
Tukey's multiple comparison post hoc tests for δ15N values in four invertebrate trophic groups collected above and below waterfalls on the Clatse and Neekas Rivers, British Columbia. N/A indicates post hoc tests not possible due to low sample sizes.
| Clatse River | |||||
|---|---|---|---|---|---|
| Below falls | Above falls | ||||
| Trophic grouping (1) | Trophic grouping (J) | Mean Difference (I-J) | Significance | Mean Difference (I-J) | Significance |
| Predators | Root Feeders | 5.93 | <0.001 | 8.31 | <0.001 |
| Predators | Detritivores | 5.81 | <0.001 | 4.39 | <0.001 |
| Predators | Omnivores | 1.28 | 0.615 | 1.30 | 0.123 |
| Omnivores | Root Feeders | 4.65 | 0.002 | 7.01 | <0.001 |
| Omnivores | Detritivores | 4.52 | 0.005 | 3.09 | <0.001 |
| Detritivores | Root Feeders | 0.13 | 1.000 | 3.92 | <0.001 |
| Neekas River | |||||
| Below falls | Above falls | ||||
| Trophic grouping (1) | Trophic grouping (J) | Mean Difference (I-J) | Significance | Mean Difference (I-J) | Significance |
| Predators | Root Feeders | 8.55 | <0.001 | 5.33 | N/A |
| Predators | Detritivores | 6.14 | <0.001 | 5.32 | <0.001 |
| Predators | Omnivores | 5.84 | <0.001 | 3.34 | <0.001 |
| Omnivores | Root Feeders | 2.7 | 0.548 | 1.99 | N/A |
| Omnivores | Detritivores | 0.3 | 0.999 | 1.98 | 0.006 |
| Detritivores | Root Feeders | 2.4 | 0.693 | 0.01 | N/A |
Invertebrate groups varied in δ13C but did not always vary predictably with trophic level or habitat (Figure 2). Nested ANOVA analysis using δ13C indicated significant variability only in taxonomic groupings (F = 11.801; p < 0.001; R2 = 0.657), with all other levels insignificant. Relatively high δ13C values were observed in millipedes from both watersheds in salmon and non-salmon sites, most likely a reflection of inorganic carbon content. Multiple comparisons revealed trophic separation for spiders over carabid beetles in all sites (Table 2). Spiders were enriched over root feeders on the Clatse River above the falls and on the Neekas below the falls. Carabids and root feeders did not differ in their δ13C values. Carabid beetles collected on the Neekas River were the only group to demonstrate isotopic enrichment below the falls (p = 0.042). Spiders on the Clatse River were found to be higher in δ13C above the falls than below (p= 0.016).
Figure 2.
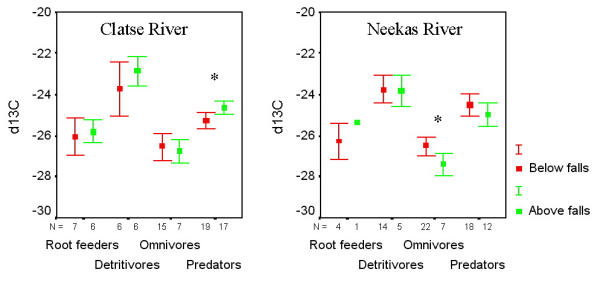
δ13C values in four trophic groupings of litter-based invertebrates collected above and below waterfall barriers to salmon migration on the Clatse and Neekas Rivers, British Columbia. Invertebrates are ranked (left to right) based on increasing consumption of animal protein (see methods). t-test results: * denotes 0.01 < p < 0.05
Table 2.
Tukey's multiple comparison post hoc tests for δ13C values in four invertebrate trophic groups collected above and below waterfall barriers to salmon migration on the Clatse and Neekas Rivers, British Columbia. N/A indicates post hoc tests not possible due to low sample sizes.
| Clatse River | |||||
|---|---|---|---|---|---|
| Below falls | Above falls | ||||
| Trophic grouping (1) | Trophic grouping (J) | Mean Difference (I-J) | Significance | Mean Difference (I-J) | Significance |
| Predators | Root Feeders | 0.77 | 0.555 | 1.17 | 0.004 |
| Predators | Omnivores | 1.27 | 0.036 | 2.14 | <0.001 |
| Omnivores | Root Feeders | -0.49 | 0.847 | -0.92 | 0.058 |
| Neekas River | |||||
| Below falls | Above falls | ||||
| Trophic grouping (1) | Trophic grouping (J) | Mean Difference (I-J) | Significance | Mean Difference (I-J) | Significance |
| Predators | Root Feeders | 1.80 | 0.033 | 0.90 | N/A |
| Predators | Omnivores | 2.02 | <0.001 | 2.46 | <0.001 |
| Omnivores | Root Feeders | -0.22 | 0.984 | -2.08 | N/A |
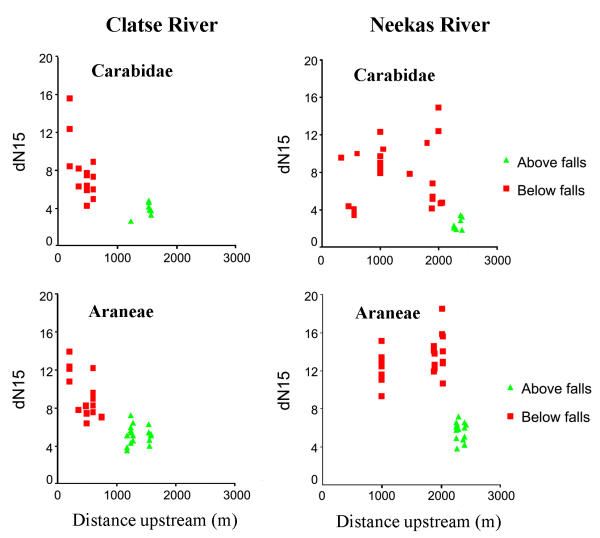
We examined isotopic levels in relation to distance upstream from the ocean. At Clatse River, δ15N declined with increased distance upstream with the lowest levels occurring above the waterfalls. However, at Neekas River, δ15N levels were high but variable throughout the stream channel below the waterfall, above which there was a striking reduction in δ15N over short distance delineated by the geological barrier to salmon (Figure 3).
Figure 3.
δ15N values in ground beetles (Carabidae) and spiders (Araneae) with distance of collection upstream from the estuary (m) on the Clatse and Neekas Rivers, British Columbia.
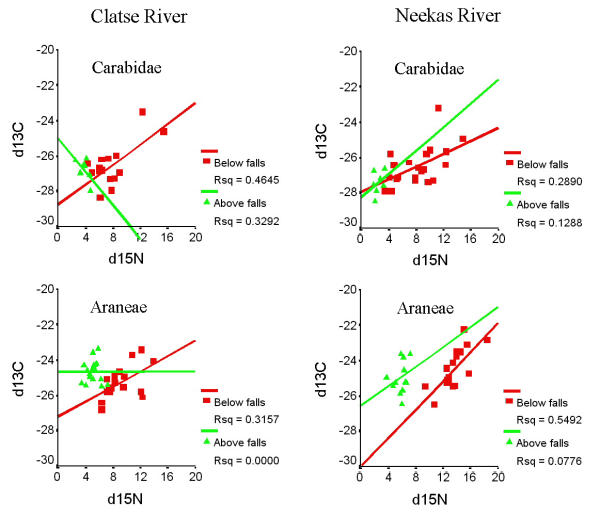
In order to assess niche differences within and among groups, we examined the relationships between δ15N and δ13C. Below the falls, there were significant positive correlations between δ15N and δ13C in spiders on the Clatse (R = 0.562; p = 0.012) and on the Neekas (R = 0.741; p = 0.001), and in carabid beetles on the Clatse (R = 0.682; p = 0.005) and on the Neekas (R = 0.538; p = 0.010) (Figure 4). None of the remaining correlations were significant in groups collected below the falls, and there were no significant correlations between δ15N and δ13C for any group collected above the falls.
Figure 4.
δ15N and δ13C values in ground beetles (Carabidae) and spiders (Araneae) below and above waterfalls on the Clatse and Neekas Rivers, British Columbia.
We estimated contribution of marine-derived nitrogen to the total nitrogen content among invertebrate groups on both watersheds (Table 3). At Clatse River, assuming no fractionation, values ranged from 19% in millipedes to 49% in weevils (with fractionation: 28% in millipedes to 71% in weevils). At Neekas River, assuming no fractionation, values ranged from 35% in ground beetles to 51% in spiders (with fractionation 47% in ground beetles to 70% in spiders).
Table 3.
% Marine-derived nitrogen (MDN) estimates in invertebrates collected above and below waterfall barriers to salmon migration on the Clatse and Neekas Rivers, British Columbia. Estimates of % MDN were made under conditions of no fractionation from the source and maximum fractionation of 4‰ from the source nitrogen by primary producers.
| Clatse River | ||
|---|---|---|
| Invertebrate family(ies) | % MDN (No Fractionation) | %MDN (Max Fractionation) |
| Curculionidae | 49.1% | 71.5% |
| Parajulidae | 19.4% | 28.2% |
| Carabidae | 30.6% | 44.6% |
| Agelenidae/Antrodiaetidae | 30.4% | 44.3% |
| Neekas River | ||
| Invertebrate family(ies) | %MDN (No Fractionation) | %MDN (Max Fractionation) |
| Parajulidae | 45.8% | 62.3% |
| Carabidae | 34.8% | 47.3% |
| Agelenidae/Antrodiaetidae | 51.2% | 69.6% |
Discussion
We demonstrate isotopic evidence for substantive incorporation of salmon-derived nitrogen into multiple trophic levels of terrestrial litter-based invertebrates from two salmon bearing watersheds. Enrichment in δ15N in terrestrial invertebrates occurs through two possible pathways: 1) direct consumption of salmon tissue and/or predation off of direct salmon consumers such as larval blowflies; or 2) indirect enrichment through δ15N enriched soil and vegetation N pools. Here, the use of the dual isotope method provides insight into the mechanism of salmon nitrogen utilization by terrestrial invertebrates. Direct consumption of salmon, with approximate δ15N and δ13C values of +11.2‰ [21] and -21‰ [9] respectively, would lead to enriched signatures of δ15N and δ13C in animal tissues. For example, consumption of salmon carcasses by larval blowflies (Calliphoridae) has been documented through the dual isotope method [7]. However, terrestrially derived carbon through C3 photosynthesis dominates δ13C pools in coniferous forest soils and salmon-derived carbon is assumed to contribute little to total carbon in litter and soil. The process of indirect utilization of salmon-derived nitrogen by animals has been observed previously in small mammals [11], whereby individuals were enriched in δ15N but not δ13C. Because we found little differences in δ13C in all trophic groups collected above versus below the waterfalls, this suggests that the primary mechanism of δ15N enrichment is by indirect processes through salmon-derived nitrogen subsidies to soil and vegetation N pools.
δ15N / δ14N ratios of forest nitrogen pools are influenced by the isotopic values of nitrogen inputs and outputs and fractionation that occurs during nitrogen transformations within ecosystems [22]. Nitrogen inputs to typical Pacific coast forest ecosystems include atmospheric deposition and biological nitrogen fixation. In the case of forests adjacent to salmon streams there is substantial evidence that marine-derived nitrogen from salmon is transferred to forest ecosystems through predator activity [11,12,14-16], flooding events [11] and hyporheic zone transfer [5], and is incorporated into soil N pools through uptake by vegetation [6,7,11-13].
Vegetation δ15N values tend to parallel those in the soil and litter across multiple sites and are typically slightly depleted in δ15N relative to the soil source [22,23]. Recent estimates for the contribution of marine-derived nitrogen from salmon in riparian ecosystems to total plant nitrogen have ranged from 15.5–24% [6,12,13]. These values may be conservative as they are based on the assumption of no plant fractionation from the original source nitrogen. In the case of high nitrogen inputs from salmon, vegetation may preferentially assimilate isotopically light nitrogen (even though it is also originally from salmon). However, in nutrient rich habitats fractionation from the source is potentially not as marked compared with nutrient poor soils [23,24], making %MDN estimates challenging. %MDN estimates from hemlock (Mathewson & Reimchen unpublished data), possibly constituting a large percentage of litter biomass, vary from 23–34% on the Clatse River and 49–66% on the Neekas River depending on degree of fractionation from the source. These estimates are higher than previously reported, yet remain the baseline for comparison with %MDN estimates in our litter-based invertebrate community.
Ponsard and Arditi [25] observed substantial site variation in litter and soil δ15N due to variations in soil processes and nitrogen sources across small scales (< 1 km). Soil and litter δ15N and δ13C values are not yet available for our sites. However, δ15N values in litter-based terrestrial invertebrates are known to parallel the δ15N values in the litter and soil [25,26]. We suspect that because vegetation and all invertebrates collected below the waterfall barrier to salmon migration are enriched in δ15N, that soil and litter δ15N are also enriched at these sites. Our data demonstrates that terrestrial invertebrates exhibit a substantial shift in δ15N over a sharp ecological discontinuity (ca. 250 m) in the source of nitrogen to the forest community, as a consequence of a distinct salmon-derived nitrogen subsidy to litter, soil and vegetation N pools. We estimate that %MDN to multiple trophic levels of litter-based invertebrates ranges from 19–71% on the Clatse River and 34–70% on the Neekas River depending on trophic grouping, and on the extent of fractionation from the original source nitrogen. These values are similar to %MDN estimates of hemlock and indicate that salmon-derived nitrogen is cycled from primary producers through multiple trophic levels of litter-based terrestrial invertebrates.
Grouping all invertebrate samples over the entire 100 m riparian zone may have reduced the extent of statistical differences for δ15N in our comparisons above and below falls. This occurs because of a potential isotopic gradient of decreasing δ15N from salmon in terrestrial vegetation with increasing distance from the stream over a relatively small scale (< 100 meters) [11-13]. Nevertheless, our %MDN estimates are higher than any other study investigating salmon nutrient transfer into terrestrial ecosystems and emphasizes the magnitude of the discontinuity that occurs across the waterfall barrier to salmon migration in these watersheds.
These %MDN estimates assume salmon tissue δ15N as the marine end-member in the model. However, there are other factors that can influence these estimates. Vertebrate urine, particularly from bears (Ursus spp.) [12], faeces and guano deposition may contribute highly to nitrogen inputs during the salmon spawning season. Despite the fact that these inputs are ultimately from salmon tissue consumption, high fractionation during multiple transformation steps prior to nitrogen availability, such as ammonia volatilization [22], may lead to unknown shifts in the δ15N levels of the source nitrogen. This may increase the microspatial variability in δ15N in litter, soil, and vegetation, and subsequently invertebrates, along the salmon spawning channel.
Variation in δ15N in carabid beetles and spiders collected below the waterfall barrier was substantially greater than above the falls. It was only marginally higher (non-significant) in root feeding weevils and millipede detritivores, possibly due to low sample sizes. This may indicate higher microspatial variability in δ15N in soil, litter and vegetation N pools, increased range of prey resources below the falls, and/ or invertebrate dispersal from other habitats into the zone of substantial salmon transfer.
We detected variation in δ15N at different stream reaches, most likely as a function of abundance and species of spawning salmon. On the Clatse River, δ15N values decreased with increasing distance upstream. Potentially, this might result from a gradient in marine subsidies other than salmon as a function of distance from the estuary [27]. However, this trend was not observed on the Neekas River where δ15N values remain high, even at 2 km upstream. The difference between these two watersheds in the distribution of marine-derived nitrogen appears to be due to topography and the species and distribution spawning salmon. Clatse River is pink salmon dominated, with the majority of spawning, and subsequent predator activity, occurring in the lower 500 meters of the spawning channel [28] (personal observations). Above 600 meters the stream narrows and the riparian profile becomes increasingly steep on both sides. The Neekas River has high density chum spawning to the base of the falls with high salmon nutrient transfer and predator activity occurring in this region [28] (personal observations). Chum salmon contain twice the biomass of nitrogen than pink salmon, and this may partly explain the higher %MDN estimates obtained on the Neekas River compared to the Clatse. The distribution of δ15N in these terrestrial invertebrate groups thus appears to be directly correlated to salmon spawning density and biomass, and subsequent predator activity, a pattern that has been observed for δ15N in ground beetles (Carabidae) occurring between watersheds on Vancouver Island [7].
Differences in the variance of isotopic signatures within a population provide insight as to the range of diet available to the individual. For example, this has been found in stable isotope studies of marine mammals and chimpanzees [29,30]. In the case of carabid beetles and spiders, high variability in δ15N along the salmon-spawning channel compared to above the falls, may indicate higher prey variability in this region. Variance in isotopic signatures can also indicate mobility between habitats [31,32]. Carabid beetles, particularly on the Neekas River, exhibited high variance in signatures. The carabid beetle species collected, although brachypterous, can move freely between habitats [33], and captured individuals may not have obtained their nutrition along the salmon spawning channel for their entire life history.
Correlations between δ15N and δ13C values provide further resolution into individual niche variability. We observed a significant positive correlation between δ15N and δ13C values in carabid beetles and spiders below waterfalls, with access to salmon nutrients, but not above falls. Both groups feed on a diverse array of prey including primary and secondary consumers, and in the case of the ground beetles, vegetative matter as well. Individuals within each group that fed at a higher average trophic level would be expected to exhibit more enrichment for δ15N and δ13C [34,35]. Alternatively, individuals that fed on salmon directly or on prey that fed on salmon would also demonstrate isotopic enrichment in both isotopes [3-7]. Positive relationships in δ15N and δ13C below the falls and the absence of that relationship above the falls hints that direct consumption of salmon or salmon consumers below the falls may be a factor for some individuals of these species. However, increased range of food resources below the falls would also be consistent with this finding. Furthermore, smaller sample sizes above the falls may have reduced our ability to detect relationships. For the majority of the spiders and ground beetles, direct uptake of the marine isotopes most likely contributes only a minor component to yearly protein intake, as uptake of marine-derived nitrogen occurs by indirect means. The use of dual isotope model becomes most relevant when investigating terrestrial organisms that use salmon protein as a major contributor to diet. This is the case for several terrestrial necrophages including flies (Diptera: Calliphoridae, Scathophagidae, Anthomyiidae), and beetles (Coleoptera: Silphidae, Leiodidae, Staphylinidae) [7] (Hocking unpublished data).
Animals are isotopically enriched in δ15N and δ13C relative to their dietary intake as a consequence of preferential excretion of the lighter isotope in metabolism [36], and this allows insight into relative trophic position within a community. Isotopic enrichment varies widely by body tissue, but there is an approximate stepwise enrichment of 3.4 ± 1.1‰ for δ15N [35,37] and 0.4 ± 1.4‰ for δ13C [34,38] for each sequential trophic level. Ponsard & Arditi [25] suggest that there are on average two trophic levels within litter-based invertebrate communities. We also find general evidence for two general trophic levels within the litter-based community at Clatse and Neekas Rivers usually consisting of: 1) root feeders and detritivores (weevils and millipedes) as primary consumers of plant material, and 2) predators (carabid beetles and spiders) that feed on these and other presumed plant feeders within the litter community. Our data, however, provides substantial evidence for a gradient in trophic level among our litter-based invertebrates rather than two distinct trophic groupings, a finding that coincides with that of Scheu & Faica [26]. Millipedes, for instance, were often found to be enriched in δ15N compared to root feeders, a finding that suggests that either weevils (Curculionidae) feed on roots that are somewhat depleted in δ15N compared to litter, or that millipede detritivores utilize some δ15N enriched protein food sources such as bacteria in their guts, or both [25]. Spiders were enriched in δ15N in all cases over those in carabid beetles, and below the falls on the Neekas this constituted a mean difference greater than a single trophic level. Evidence for omnivory is emerging in the carabid beetles [33,39-42] and the observed discrepancy between spiders and carabid beetles is most likely a result of the purely predaceous versus omnivorous life histories of these groups. Spiders also demonstrated trophic enrichment in δ13C over carabid beetles at all sites. However, spiders were not consistently enriched over root feeders at each site and carabid beetles exhibited the lowest δ13C values. We conclude that, in general, carbon is a poor trophic level indicator [25]. Overall, this suggests that increased trophic and individual niche resolution in stable isotope studies will more likely extend from a detailed taxonomic separation rather than with guild analyses [26].
Implications
With the use of stable isotopes (δ15N and δ13C), spawning salmon have been shown to provide substantial nutrient inputs to limnetic food webs [6,8-10], with implications for stream primary productivity and subsequent juvenile salmonid survivorship. Young salmon may in fact derive a large proportion of their required nitrogen and carbon from the death and decomposition of their parents, through food web utilization of salmon nutrients by algae and aquatic invertebrates.
Other than inputs to terrestrial vegetation, salmon nutrient effects in forest food webs are poorly known. Input of salmon-derived nitrogen contributes to total available N in the soil and thereby increases forest primary productivity and vegetation and litter quality [11-13]. Nutrient subsidies (other than salmon) to terrestrial invertebrate communities can result in shifts in invertebrate community structure and abundance as a consequence of bottom-up ecosystem effects [27,43,44]. Soils in coniferous forests of low nutrient status are typically dominated by fungi as the primary decomposers of organic material, and thick humus layers quickly accumulate due to slow rates of nutrient turnover [45]. In nutrient-rich conditions, fungi are replaced by bacteria and invertebrates as the dominant decomposers, resulting in higher net rates of nitrogen mineralization and total available nitrogen [44,45]. Shifts in invertebrate community structure and abundance due to a nutrient subsidy may have further implications for higher invertebrate and vertebrate consumers such as predaceous beetles, spiders, hymenopteran parasitoids, small mammals, amphibians and passerines. For example, in another form of marine subsidy, spider densities have been reported to be 4–5 times higher on islands with marine bird colonies than those without [46]. Furthermore, avian populations in boreal forests have been observed to respond to experimental nitrogen fertilization [47], a pattern that also may well be true in the case of nutrient inputs to forest communities along salmon streams [48]. Shifts in litter-based invertebrate community structure and abundance could have particular benefits for ground foraging birds such as the resident and migratory sparrows, thrushes and wrens. The widespread enrichment in salmon derived nitrogen among multiple trophic levels also hints at an ecosystem level effect that has further implications for shrub and canopy level invertebrate communities and their various vertebrate consumers [1,5,48].
Conclusions
The increasing evidence for the coast-wide decline in salmon abundance on the Pacific coast of North America [49] may have substantially more ecological implications to terrestrial forest food webs than previously recognized [5]. We present evidence for major uptake of salmon-derived nitrogen into a terrestrial invertebrate food web, with a sharp reduction in uptake across a waterfall barrier to salmon migration. These results supplement the conclusions of a diversity of recent contributions that have focused on the ecological consequences of the decline of salmon on the west coast of North America [1,2,5-13,48].
Methods
Site Description
Two salmon bearing streams were investigated – the Clatse (52° 20.6'N; 127° 50.3'W) and Neekas Rivers (52° 28.4'N; 128° 8.0'W), on the mid-coast of British Columbia, near Bella Bella, Canada. Both watersheds occur in the Coastal Western Hemlock Biogeoclimatic Zone along the boundary between the central very wet hyper-maritime (CWHvh2) and sub-montane very-wet maritime (CWHvm1) subzones [50]. Climate is considered cool and wet with mean annual temperature of approximately 8°C, and mean annual precipitation above 4000 mm (Environment Canada 2001). Dominant tree species include Western Hemlock (Tsuga heterophylla), Sitka spruce (Picea sitchensis), Amabilis fir (Abies amabilis), Western redcedar (Thuja plicata), and Red alder (Alnus rubra). Common understory species include Alaskan blueberry (Yaccinium alaskaense), red huckleberry (V. parvifolium), false azalea (Menziesia feruginea), deer fern (Blechnum spicant), bunchberry (Cornus canadensis), lanky moss (Rhytidiadelphus loreus), step moss (Hylocomium splendens), and common green sphagnum (Sphagnum girgensohnii) on zonal sites, and salmonberry (Rubus spectabilis), red elderberry (Sambucus racemosa), stink current (Ribies bracteosum), and spiny-wood fern (Dryopteris expansa) on nutrient rich sites. Deep acidic soils predominate with high organic matter content due to low rates of decomposition. Soil deposits are typically alluvial or glacial in origin, are heavily leached, and often contain iron deposits in the B layer. Mor humus types are most common with a thick layer of moss, but moder/mull humus forms occur in nutrient rich sites along the salmon spawning channel.
Both the Clatse and Neekas watersheds are dominated by high-density returns of pink (Oncorhynchus gorbuscha) and chum (O. keta) salmon, with minor runs of coho (O. kisutch) and the occasional sockeye (O. nerka). In the last ten years, pink and chum salmon returns on the Clatse River average 17000 and 5000 individuals respectively. Chum salmon constitute the majority of spawning biomass on the Neekas (mean = 30000). Mean pink salmon returns on the Neekas River vary from an average of 33000 on even years to an average of 2700 on odd years (Department of Fisheries and Oceans Escapement data: 1990–1999). Suitable spawning habitat extends for 2.1 km on the Neekas River, roughly twice that of the Clatse (1 km), whereby both are interrupted by waterfalls that act as a barrier to salmon migration [28].
Invertebrate samples
In August of 2000 terrestrial macro-invertebrates were collected in each watershed through passive pitfall trapping and hand collection from the soil and course woody debris. Invertebrate sampling occurred above and below the waterfall barrier and up to 100 meters from the stream. On the Clatse River, main invertebrate sampling occurred from 200 to 800 meters upstream from the mouth, and again above the falls at 1200 and 1600 meters. The majority of invertebrate trapping on the Neekas occurred at 1 km, and again at 2 km, just below the falls. Control samples from the Neekas were collected just above the falls from 2250 to 2400 meters upstream from the mouth.
Pitfall arrays were arranged in a three-way branching fashion. This included a central 10 cm diameter pitfall connected via three 24-inch by 6-inch aluminium drift fences (separated by 120°) to a perimeter pitfall at the end of each fence [7]. Pitfall arrays were cleared from four to five days after initial set-up, and to prevent rotting of invertebrate tissue 70% ethanol was used as a field preservative within each pitfall cup. Hand collection of invertebrates occurred more randomly as individuals were discovered in the riparian area. All specimens were stored in 70% ethanol prior to identification and isotopic analysis.
Stable Isotope Analysis
Whole invertebrate specimens were dried at 60°C for at least 48 hours and ground into a fine powder with a Wig-L-Bug grinder (Crescent Dental Co., Chicago, 111). Approximately 1 mg dry weight per ground specimen was then sub-sampled for continuous-flow isotope ratio mass spectrometry (CF-IRMS) analysis of nitrogen and carbon. Mass spectrometry analysis of δ15N and δ13C was conducted at the stable isotope facility, University of Saskatchewan, Saskatoon, Canada using a Europa Scientific ANCA NT gas/solid/liquid preparation module coupled to a Europa Scientific Tracer/ 20 mass spectrometer.
Isotopic contents are expressed in 'δ' (delta) notation representing the difference between the isotopic content of the sample and known isotopic standards (atmospheric N2 for nitrogen and PeeDee Belemnite (PDB) limestone for carbon). This is expressed in parts per thousand (‰) according to the formula (1):
1) δ15N Or δ13C (‰) = (Rsample / Rstandard - 1) * 1000
where R is the ratio of the heavy isotope (15N or 13C) / light isotope (14N or 12C).
Data Analysis
Individual terrestrial macro-invertebrates processed for δ15N or δ13C were separated into four main groups based on taxonomic similarity and ranked according to degree of animal protein consumption, thus providing a proxy for relative trophic level within the litter based food chain: 1) Root feeders (Coleoptera: Curculionidae) [18,51]; 2) Detritivores (Julida: Parajulidae) [25,52]; 3) Omnivores (Coleoptera: Carabidae) [33,39-42]; 4) Predators (Araneae: Agelenidae, Antrodiaetidae) [53,54] (Table 4).
Table 4.
Family and species level designations by trophic grouping for invertebrates collected on the Clatse and Neekas Rivers, British Columbia, in August 2000.
| Trophic grouping | Family | Species |
|---|---|---|
| 1) Root Feeders | Curculionidae | Steremnius carinatus Boh. Steremnius tuberosus Gyll. |
| 2) Detritivores | Parajulidae | Unknown |
| 3) Omnivores | Carabidae | Pterostichus crenicollis LeC. Scaphinotus angusticollis Mann. Zacotus matthewsii LeC. |
| 4) Predators | Agelenidae Antrodiaetidae | Cybaeus reticulatus Simon Antrodiaetus pacificus Simon |
Curculionid beetles of the genus Steremnius feed as larvae and adults on the roots and slash of conifers and are assigned the lowest trophic rank, as there is no current evidence that these beetles utilize animal protein [18,51]. Millipedes are detritivores, feeding primarily on dead plant material and fragments of organic matter. This potentially includes small amounts of animal protein from faeces, dead animals or microorganisms that occur on the litter material [25,52]. The Parajulidae are indigenous to the forest ecosystems of the Pacific Northwest but are poorly known at the species level [55]. A priori, we assume here that the parajulid millipedes include minor contributions of organic matter derived from animal protein in diet. Carabid beetles of the genera Pterostichus, Scaphinotus and Zacotus are generalist forest floor predators on a variety of soil invertebrates including snails and slugs (Gastropoda), millipedes (Diplopoda), isopods (Isopoda), worms (Oligochaeta) and springtails (Collembola) [33,40,56]. However, documented observations of carabids feeding on plant material including seeds and fruit suggest that these beetles may be omnivorous rather than purely predaceous [39,41,42]. Arachnids of the genera Cybaeus and Antrodiaetus are known to be funnel-web [54] and trap-door spiders [53] respectively, feeding exclusively on animals including various insects, myriapods, isopods, other spiders and even small vertebrates [57].
Independent sample t-tests (two-tailed) were used to test for differences between invertebrate groups collected above and below the falls for δ15N and δ13C on each watershed (equal variances not assumed in all tests). All invertebrates collected within 100 meters of the stream were pooled for the analysis, and those collected less than 200 meters from the estuary were removed since these were assumed to possess ambiguous isotopic signatures where marine incursions other than salmon input may particularly obscure soil N pools [27]. F-ratio tests (two-tailed) were conducted for δ15N between invertebrate groups collected above versus below the falls under the null hypothesis of equal variances. We also performed separate Nested ANOVA's on δ15N and δ13C to examine the effects of trophic group, distance from the stream, above and below falls and watershed [model: watershed, watershed(falls), watershed(falls(distance)), watershed(falls(distance (invertebrate group)))]. However, assumptions of normality and homoscedasticity were not met and as such, we place more emphasis on the t-test comparisons. Tukey HSD multiple comparison post hoc tests were performed for δ15N and δ13C within sites under the null hypothesis that all invertebrate groups were isotopically indistinct. Since inorganic carbon in the form of CaCO3, present in the exoskeleton of our millipedes [52], is enriched in δ13C relative to organic forms [25], we removed millipedes from the post hoc analysis of δ13C among feeding groups. Pearson's Correlation Coefficients were used to examine the relationships between δ15N and δ13C within trophic groups at different sites to investigate the individual niche variability.
Estimating % MDN
δ15N values in animals are influenced by the δ15N value of the principal N sources, and fractionation during nitrogen transformations within ecosystems. Principal N sources to riparian ecosystems include atmospheric N2 with a δ15N value of 0‰ [36], and salmon N with a δ15N value of approximately 11.2 ± 1.0‰ [21]. Variations in δ15N with trophic level appear to be relatively predictable such that biota are enriched by 3.4 ± 1.1‰ more than their food [37], a pattern that seems to hold true for soil macro-invertebrates [25,26]. Estimates for % marine-derived nitrogen (MDN) in our litter-based macro-invertebrate food chain were obtained based on a combination of a limnetic trophic model proposed by Kline et al.[8] and a terrestrial vegetation model utilized by Helfield and Naiman [13] and is expressed mathematically by (2):
2) %MDN = [(Obs - TEM) / (MEMTL - TEM) ] * 100%
where Obs is the observed δ15N value of a particular taxa below the waterfall barrier to salmon, TEM is the terrestrial end-member (the isotopic value obtained for the same taxa above the falls in absence of salmon input), MEM is the marine end-member (δ15N value of salmon of 11.2‰ [21] which should equal maximum vegetation δ15N values), and TL refers to the trophic level correction factor that applies to the marine end-member in the model. Since variability in utilization of MDN by the various invertebrate groups below the falls might obscure relative trophic level, we used invertebrate δ15N values above the falls on each watershed to provide an indication of relative trophic position. The trophic level correction factor was thus calculated by subtracting mean δ15N values in hemlock above the falls, (Mathewson & Reimchen unpublished data: Clatse mean δ15N = -1.55‰; Neekas mean δ15N = -3.93‰) from mean δ15N values in each invertebrate group above the falls on each watershed. This simplifies the above equation to (3):
3) % MDN = [(Obs - TEM) / (MEM - VEGabv) ] * 100%
where MEM equals salmon tissue [21] and VEGabv equals mean vegetation δ15N values above the falls. We also calculated %MDN for vegetation below the falls (Mathewson & Reimchen unpublished data: Clatse mean δ15N = +1.43‰; Neekas mean δ15N = +3.44‰) as a benchmark comparison to our invertebrate estimates. We were not able to assess the extent of fractionation occurring in the situation of 100% MDN at the level of primary producers (See assumptions in [13]). As such, we calculated two %MDN estimates based on no fractionation (MEM = 11.2‰) and maximum fractionation of 4‰ (MEM = 7.2‰), which is a typical maximum level of fractionation in vegetation from atmospheric N2 that is observed in the Clatse-Neekas non-salmon habitats (Mathewson & Reimchen unpublished data). This model assumes that invertebrate trophic level does not differ above and below the falls and that the marine end-member for vegetation δ15N values is represented by salmon tissue.
Author's contributions
MDH conducted the field research, sorted and processed the invertebrate samples, performed the statistical analyses, and drafted the manuscript. TER conceived of the study, participated in its design and coordination, and contributed to the manuscript preparation. All authors read and approved the final draft.
Contributor Information
Morgan D Hocking, Email: morganhocking@hotmail.com.
Thomas E Reimchen, Email: reimchen@uvic.ca.
Acknowledgements
We would like to thank Carsten Brinkmeier, Mike Windsor, Dan Windsor and Chester Starr for field assistance, Dr. Richard Ring for laboratory support and discussion, Myles Stocki for stable isotope analysis Dr. Bristol Foster for the zodiac, Larry Jorgenson for logistical assistance, and Rob Bennett for identification of the Arachnids. Thanks to the Heiltsuk First Nations and the Raincoast Conservation Society for local project support. This work was supported by funds from the David Suzuki Foundation and the Natural Sciences and Engineering Research operating grant (NRC2354) to TER, and from an NSERC – Industrial Post-graduate Scholarship to MDH.
References
- Willson MF, Halupka KC. Anadromous fish as keystone species in vertebrate communities. Conserv Biol. 1995;2:489–497. doi: 10.1046/j.1523-1739.1995.09030489.x. [DOI] [Google Scholar]
- Reimchen TE. Estuaries, energy flow and biomass extraction in Gwaii Haanas. Sea Wind. 1995;2:26. [Google Scholar]
- Ben-David M, Flynn RW, Schell DM. Annual and seasonal changes in the diet of martens: evidence from stable isotope analysis. Oecologia. 1997;2:280–291. doi: 10.1007/s004420050236. [DOI] [PubMed] [Google Scholar]
- Hilderbrand GV, Schwartz CC, Robbins CT, Jacoby ME, Hanley TA, Arthur SM, Servheen C. The importance of meat, particularly salmon, to body size, population productivity, and conservation of North American brown bears. Can J Zool. 1999;2:132–138. doi: 10.1139/cjz-77-1-132. [DOI] [Google Scholar]
- Cederholm CJ, Johnson DH, Bilby RE, Dominguez LG, Garrett AM, Graeber WH, Greda EL, Kunze MD, Marcot BG, Palmisano JF, Pacific Salmon and Wildlife – Ecological Contexts, Relationships and Implications for Management. Olympia, Washington, Washington Department of Fish and Wildlife, Special Edition Technical Report, Prepared for D. H. Johnson and T. A. O'Neil, Wildlife – Habitat Relationships in Oregon and Washington. 2000.
- Bilby RE, Fransen BR, Bisson PA. Incorporation of nitrogen and carbon from spawning coho salmon into the trophic system of small streams: evidence from stable isotopes. Can J Fish Aquat Sci. 1996;2:1909–1918. [Google Scholar]
- Reimchen TE, Mathewson D, Hocking MD, Moran J, Harris D. Isotopic evidence for enrichment of salmon-derived nutrients in vegetation, soil, and insects in riparian zones in coastal British Columbia. Fisheries. 2002. in press .
- Kline TC, Goering JJ, Mathisen OA, Poe PH. Recycling of elements transported upstream by runs of Pacific Salmon: I. δ15N and δ13C evidence in the Sashin creek, Southeastern Alaska. Can J Fish Aquat Sci. 1990;2:136–144. [Google Scholar]
- Kline TC, Goering JJ, Mathisen OA, Poe PH, Parker PL, Scalan RS. Recycling of elements transported upstream by runs of Pacific Salmon: II. δ15N and δ13C evidence in the Kvichak river watershed, Bristol Bay, Southwestern Alaska. Can J Fish Aquat Sci. 1993;2:2350–2365. [Google Scholar]
- Wipfli MS, Hudson J, Caouette J. Influence of salmon carcasses on stream productivity: response of biofilm and benthic macroinvertebrates in southeastern Alaska, Can J Fish Aquat Sci. 1998;2:1503–1511. doi: 10.1139/cjfas-55-6-1503. [DOI] [Google Scholar]
- Ben-David M, Hanley TA, Schell DM. Fertilization of terrestrial vegetation by spawning Pacific salmon: the role of flooding and predator activity. Oikos. 1998;2:47–55. [Google Scholar]
- Hilderbrand GV, Hanley TA, Robbins CT, Schwartz CC. Role of brown bears (Ursus arctos) in the flow of marine nitrogen into a terrestrial ecosystem. Oecologia. 1999;2:546–550. doi: 10.1007/s004420050961. [DOI] [PubMed] [Google Scholar]
- Helfield JM, Naiman RJ. Effects of salmon-derived nitrogen on riparian forest growth and implications for stream productivity. Ecology. 2001;2:2403–2409. [Google Scholar]
- Reimchen TE. Mammal and bird utilization of adult salmon in stream and estuarine habitats at Bag Harbour, Moresby Island. Queen Charlotte City, British Columbia, Canadian Parks Service, Technical Report. 1992.
- Reimchen TE. Further studies of black bear and chum salmon in stream and estuarine habitats at Bag Harbour, Gwaii Haanas. Queen Charlotte City, British Columbia, Canadian Parks Service, Technical Report. 1994.
- Reimchen TE. Some ecological and evolutionary aspects of bear-salmon interactions in coastal British Columbia. Can J Zool. 2000;2:448–457. doi: 10.1139/cjz-78-3-448. [DOI] [Google Scholar]
- Waring RH, Franklin JF. Evergreen coniferous forests of the Pacific Northwest. Science. 1979;2:1380–1386. doi: 10.1126/science.204.4400.1380. [DOI] [PubMed] [Google Scholar]
- Fumiss RL, Carolin VM. Western forest insects: Miscellaneous publication no. 1339. U.S. Department of Agriculture, Forest Service. 1977.
- Spiers GA, Gagnon D, Nason GE, Pakee EC, Lousier JD. Effects and importance of indigenous earthworms on decomposition and nutrient recycling in coastal forest ecosystems. Can J For Res. 1986;2:983–989. [Google Scholar]
- Cárcamo HA, Abe TA, Prescott CE, Holl FB, Chanway CP. Influence of millipedes on litter decomposition, N mineralization, and microbial communities in a coastal forest in British Columbia, Canada. Can J For Res. 2000;2:817–826. doi: 10.1139/cjfr-30-5-817. [DOI] [Google Scholar]
- Mathisen OA, Parker PL, Goering JJ, Kline TC, Poe PH, Scalan RS. Recycling of marine elements transported into freshwater by anadromous salmon. Verh Int Ver Limnol. 1988;2:1089–1095. [Google Scholar]
- Nadelhoffer KJ, Fry B. In: Stable isotopes in ecology and environmental science. Lathja K, Michener RH, editor. Oxford, UK, Blackwell Scientific; 1994. Nitrogen isotope studies in forest ecosystems. pp. 22–44. [Google Scholar]
- Evans DR. Physiological mechanisms influencing plant nitrogen isotope composition. Trends Plant Sci. 2001;2:121–126. doi: 10.1016/S1360-1385(01)01889-1. [DOI] [PubMed] [Google Scholar]
- Hobbie EA, Macko SA, Williams M. Correlations between foliar δ15N and nitrogen concentrations may indicate plant-mycorrhizal interactions. Oecologia. 2000;2:273–283. doi: 10.1007/PL00008856. [DOI] [PubMed] [Google Scholar]
- Ponsard S, Arditi R. What can stable isotopes (δ15N and δ13C) tell about the food web of soil macro-invertebrates? Ecology. 2000;2:852–864. [Google Scholar]
- Scheu S, Falca M. The soil food web of two beech forests (Fagus sylvatica) of contrasting humus type: stable isotope analysis of a macro- and mesofauna-dominated community. Oecologia. 2000;2:285–296. doi: 10.1007/s004420051015. [DOI] [PubMed] [Google Scholar]
- Polis GA, Hurd SD. Linking marine and terrestrial food webs: allochthonous input from the ocean supports high secondary productivity on small islands and coastal land communities. Am Nat. 1996;2:396–423. doi: 10.1086/285858. [DOI] [Google Scholar]
- Manzon CI, Marshall DE. Catalogue of salmon streams and spawning escapements of statistical area 7 (Bella Bella): Fisheries and marine service data report no. 159. Vancouver, British Columbia, Enhancement Services Branch, Department of Fisheries and Oceans. 1981.
- Schoenmger MJ, Moore J, Sept JM. Subsistence strategies of two "savanna" chimpanzee populations: the stable isotope evidence. Am J Primatol. 1999;2:297–314. doi: 10.1002/(SICI)1098-2345(199912)49:4<297::AID-AJP2>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Das K, Lepoint G, Loizeau V, Debacker V, Dauby P, Bouquegneau JM. Tuna and dolphin associations in the North-east Atlantic: evidence of different ecological niches from stable isotope and heavy metal measurements. Mar Pollut Bull. 2000;2:102–109. doi: 10.1016/S0025-326X(99)00178-2. [DOI] [Google Scholar]
- Hobson KA. Tracing origins and migration of wildlife using stable isotopes: a review. Oecologia. 1999;2:314–326. doi: 10.1007/s004420050865. [DOI] [PubMed] [Google Scholar]
- Hobson KA, McFarland KP, Wassenaar LI, Rimmer CC, Goetz JE. Linking breeding and wintering grounds of Bicknell's thrushes using stable isotope analyses of feathers. Auk. 2001;2:16–23. [Google Scholar]
- LaBonte JR. Terrestrial riparian arthropod investigations in the Big Beaver creek research natural area, North Cascades National Park Service Complex, 1995–1996: Part II, Coleoptera. United States Department of the Interior, National Park Service, Pacific West Region, Technical Report NPS/NRNOCA/NRTR/98-02. 1998.
- DeNiro MJ, Epstein S. Influence of diet on the distribution of carbon isotopes in animals. Geochim Cosmochim Acta. 1978;2:495–506. doi: 10.1016/0016-7037(78)90199-0. [DOI] [Google Scholar]
- DeNiro MJ, Epstein S. Influence of diet on the distribution of nitrogen isotopes in animals. Geochim Cosmochim Acta. 1981;2:341–351. doi: 10.1016/0016-7037(81)90244-1. [DOI] [Google Scholar]
- Peterson BJ, Fry B. Stable isotopes in ecosystem studies. Ann Rev Ecol Syst. 1987;2:293–320. doi: 10.1146/annurev.es.18.110187.001453. [DOI] [Google Scholar]
- Minigawa M, Wada E. Stepwise enrichment of 15N along food chains: further evidence and relation between δ15N and animal age. Geochim Cosmochim Acta. 1984;2:1135–1140. doi: 10.1016/0016-7037(84)90204-7. [DOI] [Google Scholar]
- Gearing JN, Gearing PJ, Rudnick DT, Requejo AG, Hutchins MJ. Isotopic variability of organic carbon in a phytoplankton-based estuary. Geochim Cosmochim Acta. 1984;2:1089–1098. doi: 10.1016/0016-7037(84)90199-6. [DOI] [Google Scholar]
- Johnson NE, Lawrence WE, Ellis ID. Seasonal occurrence of ground beetles (Coleoptera: Carabidae) in three habitats in southwestern Washington. Annals of the Entomological Society of America. 1966;2:1055–1059. [Google Scholar]
- Larochelle A. Notes on the food of the Cychrini (Coleoptera: Carabidae). The Great Lakes Entomologist. 1972;2:81–83. [Google Scholar]
- Thiele H-U. Carabid beetles in their environments: a study on habitat selection by adaptations in physiology and behavior. Springer-Verlag, Berlin, Heidelberg, New York. 1977.
- Lövei GL, Sunderland KD. Ecology and behavior of ground beetles (Coleoptera: Carabidae). Annu Rev Entomol. 1996;2:231–256. doi: 10.1146/annurev.en.41.010196.001311. [DOI] [PubMed] [Google Scholar]
- Kytö M, Niemelä P, Larsson S. Insects on trees: population and individual response to fertilization. Oikos. 1996;2:148–159. [Google Scholar]
- Forge TA, Simard SW. Short-term effects of nitrogen and phosphorus fertilizers on nitrogen mineralization and trophic structure of the soil ecosystem in forest clearcuts in the southern interior of British Columbia. Can J Soil Sci. 2001;2:11–20. [Google Scholar]
- Prescott CE, Chappell HN, Vesterdall L. Nitrogen turnover in forest floors of coastal Douglas-fir at sites differing in soil nitrogen capital. Ecology. 2000;2:1878–1886. [Google Scholar]
- Polis GA, Hurd SD. Extraordinarily high spider densities on islands: flow of energy from the marine to terrestrial food webs and the absence of predation. Proc Natl Acad Sci. 1995;2:4382–4386. doi: 10.1073/pnas.92.10.4382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkard NFG, Smith JNM. Evidence for bottom-up effects in the boreal forest: do passerine birds respond to large-scale experimental fertilization? Can J Zool. 1995;2:2231–2237. [Google Scholar]
- Willson MF, Gende SM, Marston BH. Fishes and the forest: expanding perspectives on fish-wildlife interactions. BioScience. 1998;2:455–462. [Google Scholar]
- Finney BP, Gregory-Eaves I, Sweetman J, Douglas MSV, Smol JP. Impacts of climate change and fishing on Pacific salmon abundance over the past 300 years. Science. 2000;2:795–799. doi: 10.1126/science.290.5492.795. [DOI] [PubMed] [Google Scholar]
- Green RN, Klinka K. A field guide to site identification and interpretation for the Vancouver forest region. Victoria, British Columbia, Research Branch of the Ministry of Forests. 1994.
- Hatch MH. In: University of Washington Publications in Biology. 5. Seattle WA, editor. Vol. 2. University of Washington Press; 1971. The Beetles of the Pacific Northwest. Part V: Rhipiceroidea, Sternoxi, Phytophaga, Rhynchophora, and Lamellicornia. [Google Scholar]
- Hopkin SP, Reid HJ. The biology of millipedes. Oxford, New York, Tokyo, Oxford University Press, 1992.
- Gertsh WJ. American spiders. Toronto, New York, London, D. 1949.
- Glesne RS. Terrestrial riparian arthropod investigations in the Big Beaver creek research natural area, North Cascades National Park Service Complex, 1995–1996: Part III, Arachnida: Araneae. United States Department of the Interior, National Park Service, Pacific West Region, Technical Report NPS/NRNOCA/NRTR/98-03, 1998.
- Shelley RM. A new millipede of the genus Metaxycheir from the Pacific coast of Canada (Polydesmida: Xystodesmidae), with remarks on the tribe Chonaphini and the western Canadian and Alaskan diplopod fauna. Can J Zool. 1990;2:2310–2322. [Google Scholar]
- Hatch MH. Seattle, Washington, University of Washington Publications in Biology. 1. Vol. 2. University of Washington Press; 1953. The Beetles of the Pacific Northwest. Part I: Introduction and Adephaga. [Google Scholar]
- Cloudsley-Thompson JL. Spiders, scorpions, centipedes and mites: the ecology and natural history of woodlice, 'myriapods' and arachnids. Pergamon Press, New York. 1958.