Abstract
Thyme oil (TO) is derived from the flowers of various plants belonging to the genus Thymus. It has been used as a therapeutic agent since ancient times. Thymus comprises numerous molecular species exhibiting diverse therapeutic properties that are dependent on their biologically active concentrations in the extracted oil. It is therefore not surprising that oils extracted from different thyme plants present different therapeutic properties. Furthermore, the phenophase of the same plant species has been shown to yield different anti-inflammatory properties. Given the proven efficacy of TO and the diversity of its constituents, a better understanding of the interactions of the various components is warranted. The aim of this review is to gather the latest research findings regarding TO and its components with respect to their immunomodulatory properties. An optimization of the various components has the potential to yield more effective thyme formulations with increased potency.
Keywords: thyme oil, carvacrol, thymol, γ-terpinene, p-cymene, linalool, inflammation, cancer
1. Introduction
Thymus is a perennial evergreen herb of the angiosperm plant family Lamiaceae (mint plant family) that has 350 species and 36 subspecies and is native to Europe, North Africa, and Asia [1]. Because of its distinct aroma, the plant is a popular culinary herb. Wild thyme (Thymus serphyllum) is the wild relative of all cultivated species [2]. Garden thyme is the most popular and commonly utilized plant for TO (Thymus vulgaris L.). Leaf morphology is one of the distinct features unique to the genus Thymus. The leaves are typically tiny (less than 1/8 inch long and 1/16 inch wide), narrow and elliptical, greenish-gray in color, and grouped in whorled phyllotaxy. Thyme flowers are white, yellow, or purple whorls that terminate branches. Some of the widely used cultivars of thymes for TO are Thymus rumidicus hispánicos, Thymus zygis, Thymus vulgaris, Thymus hyemalis, Thymus mastichina, Thymus citríodotus, Thymus corydothymus, Thymus loscossi, Thymus pipirella, and Thymus communis.
Thyme oil (TO) is a complex mixture of at least 70 different species [3]. Thymol tends to be among the most prevalent molecular species in TO in species such as Thymus vulgaris and Thymus magnus Nakai (Table 1). It is also the one that has been studied the most and has been shown to exert a range of therapeutic properties that includes antimicrobial, antitumoral, antifungal, antiparasitic, antioxidative and anti-inflammatory. P-cymene is another compound present in TO at relatively high concentrations with antiviral, antioxidant, and antitumoral properties particularly when conjugated to Ruthenium [4,5,6,7]. γ-Terpinene is another prevalent constituent in TO that belongs to a group of compounds called monoterpenes and has been shown to inhibit growth of helminthic and protozoan infections but only as a component in Australian tea tree oil [8]. Carvacrol, which is structurally related to thymol, has potent antimicrobial and antifungal properties [9,10]. Besides TO, carvacrol is present in essential oils derived from oregano, pepperwort, and wild bergamot plants. Its relative abundance can vary significantly depending on the source. (E)-β-Caryophellene is also a constituent of TO with anti-inflammatory and proapoptotic properties in tumor cells [11]. Consistent with thymol and carvacrol, (E)-β-Caryophellene exhibits antibacterial growth properties [12]. Linalool, a terpene alcohol also found in flowers and spices, is another TO component. Linalool exhibits antioxidant properties and reverses oxidative. In addition, linalool exhibits proapoptotic properties in cancer cells similar to other components of TO [13].
Table 1.
Prevalence of main thyme oil molecular species in different thyme plants.
|
Thymus spp. (T.) |
Thymol (%) | Carvacrol (%) | Linalool (%) | γ-Terpinene (%) | P-Cymene (%) |
Reference |
|---|---|---|---|---|---|---|
|
T. capitatus (L.) Hofmgg. Link |
8–61 | 14.2–81.2 | 0–0.4 | 2.6–33.4 | 5–22.8 | [14] |
| T. serpyllum | 0.6–0.8 | 6–20 | 0.4–63 | 2.5–4.0 | 2–9.1 | [15] |
| T. pulegoides | 0.2–2.2 | 9.5–18 | 0.6–13 | 1.5–8 | 1.6–6 | [15] |
| T. marschtallianus | 2–2.2 | 6.5–9 | 3.5–4.5 | 4.0 | 1.0 | [15] |
| T. vulgaris L. | 54.2–55.8 | 2.3–2.9 | 1.46–2.1 | 0.82–1.4 | 12.89–20.6 | [3] |
| T. atticus | 0.7 | 0.3 | 1.0 | Trace | 0.2 | [16] |
| T. leucotrichus | 2.7 | 0.6 | 1.8 | Trace | 0.2 | [16] |
| T. striatus | 1.9 | 4.3 | 0.9 | Trace | 0.2 | [16] |
| T. perinicus | 20.9 | 1.1 | 4.6 | 0.5 | 4.8 | [16] |
| T. zygioides | 51.2 | 2.9 | 0.1 | 1.1 | 6.5 | [16] |
| T. quinquecostatus Celak. | 39.8 | 2.6 | 0.1 | 10 | 9.2 | [16] |
| T. magnus Nakai | 54.7 | 3.2 | Trace | 6.4–15.0 | 3.5–6.7 | [17] |
2. Thymol
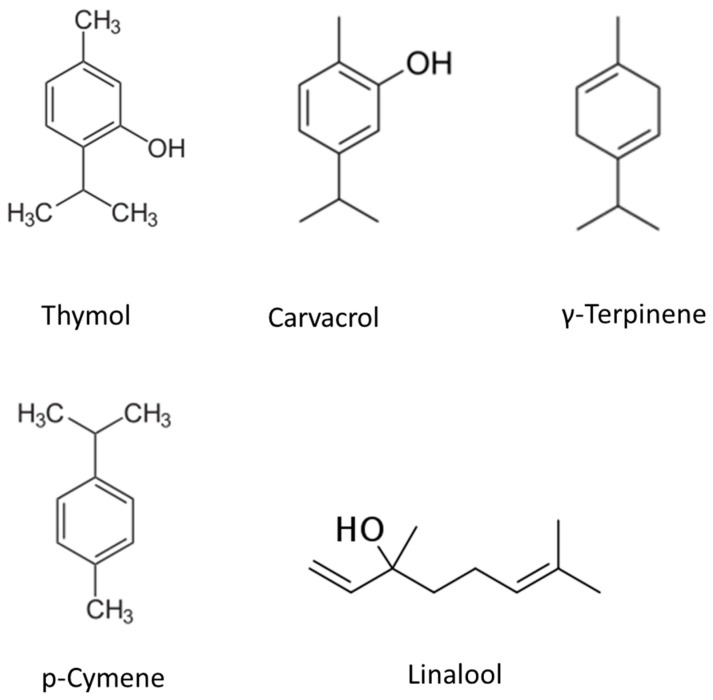
Thymol is one of the main constituents of TO (see Figure 1 for common TO constituents). Depending on the species (over 350 species are known per POWO), levels of thymol in the extracted oil can vary considerably. Thymol exhibits a variety of therapeutic properties and is among the most investigated components of TO.
Figure 1.
Chemical structures of thymol, carvacrol, p-cymene, γ-terpinene, and linalool.
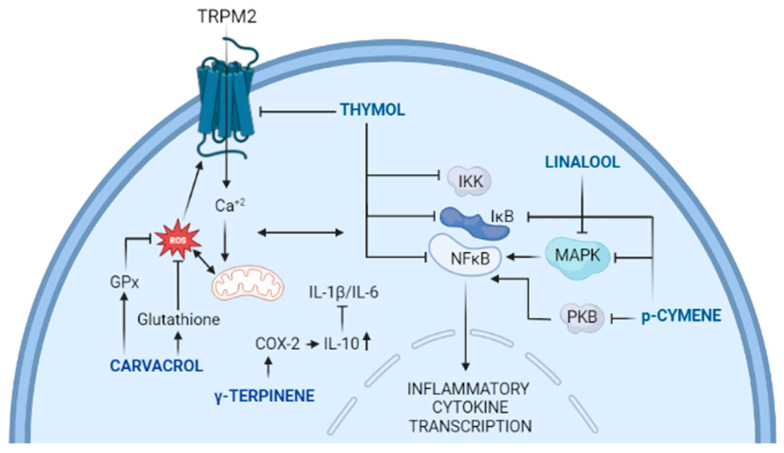
Multiple studies highlight the anti-inflammatory properties of thymol [18,19,20]. In a study utilizing Staphyloccocus aureus membrane vesicles and keratinocytes, thymol reduced expression levels of IL-1β, IL-6, TNF-α, IL-8, and MCP-1 [19]. The anti-inflammatory effect was observed in a mouse in vivo system as well, where thymol suppressed Th1-, Th2-, and Th17-mediated inflammatory responses. Using the human peritoneal mesothelial cell line HMrSV5, Wang Q et al. (2018) showed that thymol reduced phosphorylation of multiple mediators involved in the NFκB inflammatory pathway [18]. Thymol reduced phosphorylation of IKK, IκΒα, and ΝFκΒp65 and resulted in reduction of MCP-1, TNF-α, and IL-6. The anti-inflammatory outcome of thymol was investigated in a human clinical trial involving gingival inflammation [20]. Varnish containing thymol/chlorhexidine was used in 25 individuals with fixed orthodontic appliances and gingival inflammation. The thymol/chlorhexidine varnish reduced levels of Prostaglandin E2 (PGE2) within 8 days. In a different study, a combination of thymol and carvacrol reduced production of the inflammatory cytokines IL-25, IL-33, and Thymic Stromal Lymphopoietin (TSLP) in BEAS-2B-transformed human bronchial epithelial cells stimulated with chitin [21]. SHIP-1 and SOCS-1, known downregulators of TLR-2 and TLR-4 inflammatory signaling, were elevated with thymol/carvacrol.
A recent study utilizing U87 human malignant glioblastoma cells has shown that thymol induces apoptosis, increases Reactive Oxygen Species (ROS), and increases levels of the proapoptotic factors Bax and p53 [22]. Similar proapoptotic effects were observed in bladder cancer cells by activating the apoptotic intrinsic pathway via caspase-3, caspase-9, release of cytochrome c, and reduction of the antiapoptotic protein bcl-2 [23]. Thymol was effective in reducing cell proliferation and inducing apoptosis of colorectal cancer cells [24]. The antitumoral properties of thymol were linked to the activation of the Bax/bcl-2 pathway. Similar findings were seen using the acute promyelotic cancer cell line HL-60 [25]. As with previous studies, an increase in caspase-3, 8, and 9 was observed accompanied with an increase in Bax and a reduction in bcl-2.
Thymol has also been evaluated for its potential application as an antimicrobial agent. A thymol concentration of 100 μg/mL reduced viability of the dental caries causing bacteria Streptococcus mutans by 50% [26]. Thymol can inhibit the growth of S. mutans by inducing autolysis, stress growth inhibition, and reduced biofilm formation. Currently, thymol-rich extracts are generally used as an expectorant in coughs associated with cold and also in dentistry as a disinfectant [27]. Thymol is effective against both gram-positive and gram-negative bacteria [28]. The study of Thoshar et al. (2013) tested different essential oils rich in phenols and thyme oil for minimal inhibitory concentration (MIC) and determined that 2 µL/mL was effective against E. coli. However, for E. faecalis and S. aureus, growth inhibition required a higher concentration. Antibacterial properties of thyme oil rich in thymol was also tested against the methicillin resistant (MRSA) strain [29,30]. Kryvtsova et al. (2019) tested thyme oil extracted from T. vulgaris to study the antimicrobial properties using MRSA strain S. aureus isolated from the oral cavity of patients suffering from periodontitis and pharyngitis. Thyme oil concentration of 0.01% (v/v) and 0.05% effectively reduced the growth up to 53% and 76%, respectively. The main components of volatile oil used in this research were phenolic monoterpenes, including thymol. In a similar study by Tohidpour et al. (2010), thymol extracted from T. vulgaris was more effective than essential oil from Eucalyptus globulus Labill in controlling the growth of MRSA [31]. Surprisingly, the antibacterial properties of TO also depend on different growth ages of thyme plants [3]. For example, thyme oil before flowering and after flowering has significant differences in controlling the growth of Pseudomonas aeruginosa even though the thymol level difference was nonsignificant (55.81% and 54.21%, respectively). Recently, thymol-rich Thyme species are gaining attention for their antifungal properties. For example, studies on antifungal properties of thyme oil extract rich in thymol has been shown to be effective against growth of the fungal genus Cryptococcus, which is a pathogen responsible for cryptococcosis [32]. In this study, among six tested compounds, the combination of thymol and carvacrol was effective four to eight times higher than the standard therapeutic drugs. The potential mode of action of thymol against fungi is based on the target-specific effects of thymol against ergosterol, which is the unique sterol specific to fungal cells [27].
Recently, Liggri et al. (2023) have shown that both thymol and carvacrol bind to the novel mosquito protein AgamOBP5, an odorant-binding protein that is involved in the odorant perception process of mosquitoes [33]. This was the first study to show the binding of a natural plant-derived insect repellent to an odorant-binding protein. Furthermore, the study provides a mechanistic explanation for the insect repellent properties of TO and specifically thymol and carvacrol.
3. Carvacrol
Carvacrol content in TO also varies significantly depending on the thyme species. TO derived from Thymus capitatus plants was shown to be very high in carvacrol (79.9%) [34]. On the other hand, TO derived from Thymus vulgaris plants was shown to be much lower in carvacrol (1.56%) [3]. Similar to thymol, carvacrol exhibits anti-inflammatory properties and has been shown to protect retinal pigment epithelial cells against inflammation, oxidative stress, and apoptosis induced by high glucose levels [35]. Mechanistically, the transient potential melastatin 2 (TRPM2) cation channel was inhibited by carvacrol and thus provided protection against excessive calcium influx and ROS species. In addition, Glutathione and Glutathione Peroxidase were elevated with carvacrol, providing protection against the ROS species generated from high glucose levels. Similar anti-inflammatory findings were seen with human tonsil epithelial cells stimulated with Lipoteichoic Acid and Peptidoglycan [36]. IL-6, IL-8, ENA-78, and GCP-2 were all suppressed with carvacrol. Furthermore, PGE2 and COX-2 levels were reduced with carvacrol.
Carvacrol showed analogous antitumoral properties as thymol in a study involving oral squamous carcinoma cells [37]. Keap1/Nrf2 and NALP3 were upregulated in the carcinoma cells. Treatment with carvacrol inhibited both Keap1/Nrf2 and the NALP3 proteins and resulted in suppression of clone formation and migration capacity. Antiproliferative effects of carvacrol were observed in a study with osteosarcoma cells [38]. Carvacrol induced apoptosis in U2OS and 143B cells. Similar to thymol, a reduction in bcl-2 and an increase in Bax were detected after carvacrol treatment. A reduction in migration and invasion of the U2OS and 143B cells was correlated with a reduction in MMP-9 expression. Treatment with carvacrol showed similar anticancer effects in a study with breast cancer cells [39]. The effect was related to carvacrol’s ability to inhibit the transient receptor potential melastatin-like 7 channel (TRPM7), which regulates the cell cycle of cells. At 200 μΜ, carvacrol increased the number of cells in the G1/G0 phase and decreased the number of cells in the S and G2/M phases. Treatment with carvacrol reduced tumor size in mice transplanted with glioblastoma cancer cells [40]. Carvacrol’s inhibition of TRPM2 and TRPV4 is recognized in several studies [41]. Its ability to inhibit these channels makes it suitable as an anticancer agent and an antioxidative agent. Elevation of Glutathione and Glutathione Peroxidase in the presence of carvacrol in conjunction with the inhibition of the TRPM2 and TRPV4 channels act together to confer cellular protection against oxidative stress. The antioxidative nature of carvacrol and its therapeutic effects have been demonstrated in a mouse in vivo model of Alzheimer’s disease [42]. Intraperitoneal administration of carvacrol resulted in increased cellular viability in the experimental animal model of Alzheimer’s disease that uses Aβ1-42 bilateral intrahippocampal injection. Memory impairment assessed with a passive avoidance test was improved, providing support for carvacrol as a promising agent in neurodegenerative diseases. In a human clinical trial involving moderately asthmatic patients, carvacrol was effective in reducing the levels of inflammatory cytokines and oxidative stress markers and shown to improve pulmonary function tests [43]. The study was a randomized, placebo-controlled, double blind study involving 33 moderately asthmatic patients. The carvacrol-treated group received 1.2 mg/kg/day for a total of 2 months. Improvement of respiratory symptoms were observed by the first month, providing further support that carvacrol is a natural antioxidant and anti-inflammatory agent with therapeutic benefits in several diseases.
In addition to its anti-inflammatory properties, carvacrol has been extensively studied for its antibacterial properties. Several studies investigating the effects of carvacrol extracted from different Thyme species on various bacterial strains have been carried out. Carvacrol extracted from Thymus vulgaris L. has been shown to exhibit potent antibacterial activity against several strains of bacteria, including Escherichia coli, Staphylococcus aureus, and Pseudomonas aeruginosa [44]. In their study, Soković et al. (2010) reported that carvacrol extracted from T. vulgaris L. had a minimum inhibitory concentration (MIC) of 0.5–1.5 mg/mL against these bacterial strains. Similar results have been observed by Gedikoğlu et al. (2019) in evaluating the antimicrobial properties of T. vulgaris L. on a series of gram-positive and gram-negative bacteria (Bacillus cereus, Staphylococcus aureus, Staphylococcus epidermidis, Escherichia coli, Salmonella enteritidis, and Salmonella typhimurium) [45]. Surprisingly, extraction methods might affect the antibacterial properties of bioactive compounds. TO extracted by microwave-assisted extraction displayed significantly (p < 0.05) higher antibacterial activity against bacteria than did the hydrodistilled essential oil extraction. Antibiotic-resistant pathogenic bacteria are gaining more attention and various plant derived species are being explored as alternative antimicrobial agents. A comparative study on essential oil extract from different herbs including T. vulgaris reported the synergistic effectiveness of carvacrol against 32 erythromycin-resistant Group A Streptococci [46]. The Minimum Inhibitory Concentration (MIC) of carvacrol was found to be 64 μg/mL, which was effective against inhibiting growth Group A Streptococci. The same study evaluated the antibacterial properties of carvacrol alone and in combination with erythromycin. Surprisingly, carvacrol from T. vulgaris alone was effective in both treatment types’ ability to inhibit growth of Group A Streptococci, further enhancing the potential use of carvacrol as an effective phytotherapeutic agent against antibiotic-resistant bacteria [46]. Carvacrol from other Thyme species was also evaluated to determine the effectiveness of antibacterial properties.
Among different chemotypes, hydrocarbon monoterpenes in essential oils extracted from Thymus spp. show the lowest antibacterial activity compared to oxygenated compounds, especially phenol-type compounds such as thymol and carvacrol [44]. The antibacterial properties of carvacrol extract from other Thyme spp. were also evaluated. Carvacrol extracted from T. zygis L. was found to be effective against several bacterial strains, including S. aureus and E. coli. An MIC of 0.16–0.63 mg/mL carvacrol extracted from T. zygis L. was found to be effective against controlling the growth of these bacterial strains. The antimicrobial properties of essential oil from the genus Thymus could be explained by their high percentage of phenol components. Carvacrol might interfere with the activity of cell wall enzymes, thus inhibiting bacterial growth by damaging their cell wall. A recent report investigating the antibacterial properties of carvacrol provided the possible mechanism of carvacrol against Streptococcus pyogenes [47]. Carvacrol exhibits rapid antibactericidal activities by inducing morphological changes, cytoplasmic leakage, and, consequently, cell damage.
The potential application of carvacrol from various Thymus cultivars as antifungal agents has been reported recently [10,48,49,50]. Boukhatem et al. (2020) reported the antifungal activity of essential oil extracted from T. vulgaris against eight yeast species and eight filamentous fungi isolated from mucocutaneous fungal infection pathogens for skin diseases. For this study, TO was extracted from fresh leaves and floral heads. Out of 25 bioactive compounds in the thyme oil, carvacrol (56.8%) was detected in high amounts, followed by p-cymene (12.8%), γ-terpinene (11.17%), and thymol (3.99%). A series of fungal species were used for the sensitivity assay, which included different strains of yeast (Candida albicans, C. tropicalis, C. parapsilosis, Trichosporon, and Rhodotorula sp.), and filamentous fungus (Aspergillus terreus, A. flavus, A. niger, A. fumigatus, Mucor, and Penicillium). Out of three concentrations (20, 40 and 60 μL/disc), TO concentration of 60 μL was effective against all tested yeast strains as compared to the positive control (1% Hexamidine). Candida tropicalis and C. parapsilosis were the most vulnerable strains to the TO, with zones of inhibition of 50 mm and 60 mm. Among filamentous fungi, TO was more effective against Aspergillus terreus and A. fumigatus (zones of inhibition 55 and 45 mm, respectively). Similar antifungal properties of carvacrol against Candida tropicalis were reported by other studies as well [51,52]. In addition, a number of studies have confirmed the antifungal properties of essential oils and extracts from different varieties of the genus Thymus rich in monoterpenic alcohols and/or volatile phenols [53,54]. Another application of thyme oil rich in carvacrol acting as an antifungal agent has been reported in controlling Botrytis cinerea, a primary pathogen causing stem and fruit rot during pre- and postharvest of ornamental crops, fruits, and vegetables [10]. Out of several concentrations tested at 140 µL/L, carvacrol was effective in disrupting the mycelia. A possible mechanism of carvacrol-rich TO extract is a drop in extracellular pH, thus inducing membrane damage and a decrease in cellular lipid content. Other Thymus species such as T. zygis and T. vulgaris, with higher quantities of phenols, also demonstrated a wide spectrum of inhibitory growth against a range of pathogenic filamentous fungi and yeasts, with decreased sensibility to antifungal drug [53,54,55]. However, carvacrol was more active against dermatophyte strains, in a similar manner to the extracted oil.
4. P-Cymene
P-cymene is typically found at lower concentrations in TO compared to thymol and is a component of other essential oils including cinnamon and cumin [56]. P-cymene has an overall anti-inflammatory character and has been shown to reduce allergy-associated immunological markers in both human and murine allergy models [57]. Treatment of human monocyte-derived mature dendritic cells from grass or birch pollen allergic donors with cinnamon extract, p-cymene, or trans cinnamaldehyde inhibited their maturation. When cocultured with autologous CD4+ in the presence of specific antigen-pulsed dendritic cells, CD4+ proliferation and Th1/Th2 cytokine production was inhibited. P-cymene showed similar characteristics by inhibiting IL-8 release in LPS-stimulated THP-1 monocytes [58]. Synergism between p-cymene and trans cinnamaldehyde has been observed. Reduction in phosphorylation of Akt and IκB seem to play a key role in the observed anti-inflammatory effects. In the colitis rat model induced with trinitrobenzene sulphonic acid (TNBS), p-cymene with rosmarinic acid reduced levels of malondialdehyde (MDA) and myeloperoxidase while restoring glutathione levels [59]. Levels of IL-1β and TNF-α were also reduced. COX-2 levels in spleen, mesenteric lymph node, and colon samples declined as well. Overall, p-cymene and rosmarinic acid provided intestinal cytoprotection and maintained the mucous layer.
P-cymene’s antitumoral effects were reported in a high-fat diet colorectal cancer rat model [60]. A significant reduction in cancerous nodules in the p-cymene group was observed. Leptin and IL-1 levels were decreased, but interestingly, IL-6 levels were increased. In a similar colorectal cancer study involving the hyperlipidemia/dimethyl hydrazine rat model, p-cymene’s anticancer properties were linked to its antioxidant and anti-inflammatory properties [61]. Reduction in IL-6, COX-2, IL-1, and adiponectin was observed. Similar reduction in the oxidative markers Superoxide Dismutase and Malondialdehyde was detected. In addition to being utilized alone as an anticancer molecule, p-cymene has been used as a ligand for the metal Ruthenium, which gives rise to a number of organometallic compounds with promising anticancer properties [5]. Besides its antitumoral properties, p-cymene exhibits antinociceptive effects [62]. A reduction in mechanical hyperalgesia, spontaneous nociception, and nociception induced by nonnoxious palpation was achieved with p-cymene. Calcium channel current modulation was proposed as a mechanism for the antinociception.
5. γ-Terpinene
γ-Terpinene belongs to a group of isomeric compounds referred to as monoterpenes. Their difference lies in the position of the two carbon–carbon double bonds (see Figure 1). γ-Terpinene is the precursor molecule for both thymol and carvacrol. The structure and characteristics of the enzyme responsible for its synthesis from geranyl diphosphate, γ-terpinene synthase, has been elucidated and studied in detail [63]. In TO, γ-Terpinene is the most prevalent among α, β, and δ isomers. Species such as Thymus vulgaris have a much lower content in relation to the Thymus capitatus species [3,34]. It is unclear if the variation in γ-Terpinene translates to different therapeutic properties.
There is much less research on γ-Terpinene compared to thymol, carvacrol, and p-cymene. The antimicrobial properties of Thymbra capitata TO and, by extension, its components were mostly attributed to p-cymene and carvacrol where strong synergism was reported against Gardnerella spp [64]. Nonetheless, a number of studies have shown that γ-terpinene exhibits anti-inflammatory characteristics similar to carvacrol and thymol but through a different mechanism. Ramalho et al. (2016) showed that murine LPS-stimulated peritoneal macrophages produced less IL-1β and IL-6 when treated with γ-Terpinene but more of the anti-inflammatory cytokine IL-10 [65]. Interestingly, the increase in IL-10 was due to the elevation of the COX-2 enzyme and its downstream arachidonic acid metabolite PGE2. Inhibition of the COX-2 enzyme with nimeluside (selective COX-2 inhibitor) abolished the IL-10 increase. Furthermore, with diminished IL-10 production, the inhibition of IL-1β and IL-6 was lost. γ-Terpinee in IL-10-deficient mice did not produce any inhibition on IL-1β and IL-6, suggesting the PGE2/IL-10 pathway as a mechanism for its anti-inflammation. This pathway is not utilized by carvacrol, thymol, and p-cymene and makes γ-terpinene unique particularly in terms of the induction of IL-10.
6. Linalool
Linalool is another constituent of TO, but with lower prevalence than thymol, carvacrol, and p-cymene. A terpene alcohol, linalool is found in a variety of flowers and seeds and their essential oils [66]. Linalool has a pleasant smell and is widely used in the fragrance industry [67].
Multiple studies have investigated linalool’s anti-inflammatory qualities in a variety of systems. Cigarette smoke-induced inflammation was inhibited by linalool in mice [68]. Reduction in levels of the inflammatory cytokines/chemokines TNF-α, IL-6, IL-8, IL-1β, and MCP-1 were detected by ELISA. The inhibition was mediated via the NF-κB pathway. Similar findings were reported in a study involving LPS-stimulated RAW 264.7 cells and LPS-induced in vivo lung injury mouse models [69]. Analogous to the cigarette smoke-induced inflammation study, levels of TNF-α and IL-6 were reduced with linalool. The investigators showed that phosphorylation of IkBα was reduced along with p38 and c-Jun.
Protective effects of linalool have been reported in neurodegenerative animal models as well [70]. The animals used were a triple transgenic Alzheimer’s disease model (3xTg-AD) in which the linalool-treated group (25 mg/kg, every 48 h, 3 months) showed a reduction in the inflammatory markers p38 MAPK, NOS2, COX-2, and IL-1β in the hippocampi and amygdalae. Reduction in extracellular β-amyloidosis, tauopathy, astrogliosis, and microgliosis was accompanied by improved learning and spatial memory in the elevated plus maze test. Linalool’s anti-inflammatory properties were also observed in mice with allergic asthma [71]. A decrease in iNOS expression and PKB activation in lung tissues occurred with linalool. Similar to previous findings, downregulation of NFκB and MAPK and diminished signaling were responsible for the anti-inflammation. In another study involving streptozotocin-induced diabetic rats, linalool provided renoprotection [72]. Expression of NF-κB and TGF-β1 was reduced in the kidneys, and an overall reduction in diabetes-induced nephropathy was observed. Anti-inflammatory effects with linalool were reported in a UVB acute radiation-induced inflammation mouse skin model [73]. Topical application of linalool or intraperitoneal injection reversed the expression of COX-2 and ornithine decarboxylase. Furthermore, lipid peroxidation and antioxidant depletion were reduced. In the chronic UVB radiation model, there was a reduction in the classical inflammatory mediators NF-κΒ, TNF-α, IL-6, and COX-2 along with a reduction in tumor incidence. Tumor development was confirmed histopathologically in terms of dysplasia and squamous cell carcinoma presence. Related to its anti-inflammatory nature, linalool behaves as an antioxidant. In the neuronal HT-22 cell line, glutamate-induced mitochondrial oxidative stress was reduced in the presence of 100 μM linalool [74]. Mitochondrial membrane potential (ΔΨ) preservation accompanied by a reduction in mitochondrial ROS and calcium levels translated into increased neuronal survival during glutamate-induced toxicity.
7. Discussion
Thyme oil (TO) has a proven record of therapeutic properties and characterized in detail anti-inflammatory mechanisms (see Figure 2). However, it is composed of a very heterogenious mixture of a large number of molecular species. Adding to its heterogeneity is the large number of different plants from which TO can be extracted from. Furthermore, the phenophase of the thyme plant has a direct impact on the concentrations of the various constituents comprising the TO, which can yield different properties. Even the extraction process can yield TO oil with uneven characteristics. Nonetheless, thymol, carvacrol, p-cymene, γ-terpinene, and linalool are very likely among the key components of TO with the most potent therapeutic properties. Given the observed synergism and antagonism that the different molecular species can exhibit, it is rational to consider employing defined formulations of the key components of TO that are likely to be more potent than the natural product. This approach will require testing and validation of concentrations of various species that normally do not exist in the natural product. The potential of harnessing more potent formulations with more consistent therapeutic characteristics is worth the effort.
Figure 2.
A simplified schematic depiction of the anti-inflammatory pathways utilized by thymol, carvacrol, p-cymene, γ-terpinene, and linalool.
In addition, future research directions relating to TO and its derivatives may include synergistic formulations with other drugs. For example, the anti-inflammatory properties of thymol, carvacrol, p-cymene, γ-terpinene, and linalool can be used in conjunction with widely used corticosteroids. Formulations composed of synthetic corticosteroids and TO constituents may allow for lower concentrations of the synthetic component and achieve a reduction in side effects typically seen when using only the synthetic drugs. A similar approach may be employed with antibiotics where the use of TO components along with antibiotics may permit a reduction in concentration of the synthetic antibiotic and a reduction in side effects and antibiotic resistance. This strategy has been used for many years now with clavulanic acid and amoxicillin. Another future direction worth investigating is the use of carvacrol and thymol in combination with known synthetic insect repellants where the formulation will permit a lower concentration of the synthetic repellants. The natural scent of carvacrol and thymol will be an added benefit.
In conclusion, there is ample scientific evidence that TO and its constituents have diverse therapeutic properties. However, it is unlikely that they can compete or replace synthetic drugs with similar and often more potent properties. Future research should focus on optimizing formulations of TO constituents by themselves and with synthetic drugs to maximize their potency while reducing their toxicity.
Author Contributions
Conceptualization, E.V.; writing—original draft preparation, E.V., O.A., A.D. and S.M.; writing—review and editing, E.V., O.A., A.D. and S.M. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Jalas J. Notes on Thymus L. (Labiateae) in Europe. Bot. J. Linn. Soc. 1971;64:199–215. [Google Scholar]
- 2.Schauer T. A Field Guide to the Wild Flowers of Britain and Europe. Collins; London, UK: 1978. [Google Scholar]
- 3.Pandur E., Micalizzi G., Mondello L., Horváth A., Sipos K., Horváth G. Antioxidant and Anti-Inflammatory Effects of Thyme (Thymus vulgaris L.) Essential Oils Prepared at Different Plant Phenophases on Pseudomonas aeruginosa LPS-Activated THP-1 Macrophages. Antioxidants. 2022;11:1330. doi: 10.3390/antiox11071330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Panagiotopoulos A., Tseliou M., Karakasiliotis I., Kotzampasi D.M., Daskalakis V., Kesesidis N., Notas G., Lionis C., Kampa M., Pirintsos S., et al. p-cymene impairs SARS-CoV-2 and Influenza A (H1N1) viral replication: In silico predicted interaction with SARS-CoV-2 nucleocapsid protein and H1N1 nucleoprotein. Pharmacol. Res. Perspect. 2021;9:e00798. doi: 10.1002/prp2.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pujante-Galián M.A., Pérez S.A., Montalbán M.G., Carissimi G., Fuster M.G., Víllora G., García G. p-Cymene Complexes of Ruthenium (II) as Antitumor Agents. Molecules. 2020;25:5063. doi: 10.3390/molecules25215063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao X.L., Sparling M., Dabeka R. p-Cymene, a natural antioxidant, in Canadian total diet foods: Occurrence and dietary exposures. J. Sci. Food Agric. 2019;99:5606–5609. doi: 10.1002/jsfa.9854. [DOI] [PubMed] [Google Scholar]
- 7.Salimi A., Khodaparast F., Bohlooli S., Hashemidanesh N., Baghal E., Rezagholizadeh L. Linalool reverses benzene-induced cytotoxicity, oxidative stress and lysosomal/mitochondrial damages in human lymphocytes. Drug Chem. Toxicol. 2022;45:2454–2462. doi: 10.1080/01480545.2021.1957563. [DOI] [PubMed] [Google Scholar]
- 8.Lam N.S., Long X., Su X.Z., Lu F. Melaleuca alternifolia (tea tree) oil and its monoterpene constituents in treating protozoan and helminthic infections. Biomed. Pharmacother. 2020;130:110624. doi: 10.1016/j.biopha.2020.110624. [DOI] [PubMed] [Google Scholar]
- 9.Demirci F., Karaca N., Tekin M., Demirci B. Anti-inflammatory and antibacterial evaluation of Thymus sipyleus Boiss. subsp. sipyleus var. sipyleus essential oil against rhinosinusitis pathogens. Microb. Pathog. 2018;122:117–121. doi: 10.1016/j.micpath.2018.06.025. [DOI] [PubMed] [Google Scholar]
- 10.Zhang J., Ma S., Du S., Chen S., Sun H. Antifungal activity of thymol and carvacrol against postharvest pathogens Botrytis cinerea. J. Food Sci. Technol. 2019;56:2611–2620. doi: 10.1007/s13197-019-03747-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dahham S.S., Tabana Y., Asif M., Ahmed M., Babu D., Hassan L.E., Ahamed M.B.K., Sandai D., Barakat K., Siraki A., et al. β-Caryophyllene Induces Apoptosis and Inhibits Angiogenesis in Colorectal Cancer Models. Int. J. Mol. Sci. 2021;22:10550. doi: 10.3390/ijms221910550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woo H.J., Yang J.Y., Lee M.H., Kim H.W., Kwon H.J., Park M., Kim S.K., Park S.Y., Kim S.H., Kim J.B. Inhibitory Effects of β-Caryophyllene on Helicobacter pylori Infection In Vitro and In Vivo. Int. J. Mol. Sci. 2020;21:1008. doi: 10.3390/ijms21031008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elbe H., Ozturk F., Yigitturk G., Baygar T., Cavusoglu T. Anticancer activity of linalool: Comparative investigation of ultrastructural changes and apoptosis in breast cancer cells. Ultrastruct. Pathol. 2022;46:348–358. doi: 10.1080/01913123.2022.2091068. [DOI] [PubMed] [Google Scholar]
- 14.Casiglia S., Bruno M., Scandolera E., Senatore F., Senatore F. Influence of harvesting time on composition of the essential oil of Thymus capitatus (L.) Hoffmanns. & Link. growing wild in northern Sicily and its activity on microorganisms affecting historical art crafts. Arab. J. Chem. 2019;12:2704–2712. [Google Scholar]
- 15.Kryvtsova M., Hrytsyna M., Salamon I., Skybitska M., Novykevuch O. Chemotypes of Species of the Genus Thymus L. in Carpathians Region of Ukraine—Their Essential Oil Qualitative and Quantitative Characteristics and Antimicrobial Activity. Horticulturae. 2022;8:1218. doi: 10.3390/horticulturae8121218. [DOI] [Google Scholar]
- 16.Trendafilova A., Todorova M., Ivanova V., Zhelev P., Aneva I. Essential Oil Composition of Five Thymus Species from Bulgaria. Chem. Biodivers. 2021;18:e2100498. doi: 10.1002/cbdv.202100498. [DOI] [PubMed] [Google Scholar]
- 17.Kim Y.H., Lee J.C., Choi Y.H. Essential oils of Thymus quinquecostatus celakov. and Thymus magnus Nakai. Korean J. Med. Crop Sci. 1994;2:234–240. [Google Scholar]
- 18.Wang Q., Cheng F., Xu Y., Zhang J., Qi J., Liu X., Wang R. Thymol alleviates lipopolysaccharide-stimulated inflammatory response via downregulation of RhoA-mediated NF-κB signalling pathway in human peritoneal mesothelial cells. Eur. J. Pharmacol. 2018;833:210–220. doi: 10.1016/j.ejphar.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Kwon H.I., Jeong N.H., Kim S.Y., Kim M.H., Son J.H., Jun S.H., Kim S., Jeon H., Kang S.C., Kim S.H., et al. Inhibitory effects of thymol on the cytotoxicity and inflammatory responses induced by Staphylococcus aureus extracellular vesicles in cultured keratinocytes. Microb. Pathog. 2019;134:103603. doi: 10.1016/j.micpath.2019.103603. [DOI] [PubMed] [Google Scholar]
- 20.Sköld K., Twetman S., Hallgren A., Yucel-Lindberg T., Modéer T. Effect of a chlorhexidine/thymol-containing varnish on prostaglandin E2 levels in gingival crevicular fluid. Eur. J. Oral. Sci. 1998;106:571–575. doi: 10.1046/j.0909-8836.1998.eos106106.x. [DOI] [PubMed] [Google Scholar]
- 21.Khosravi A.R., Erle D.J. Chitin-Induced Airway Epithelial Cell Innate Immune Responses are Inhibited by Carvacrol/Thymol. PLoS ONE. 2016;11:e0159459. doi: 10.1371/journal.pone.0159459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qoorchi Moheb Seraj F., Heravi-Faz N., Soltani A., Ahmadi S.S., Shahbeiki F., Talebpour A., Afshari A.R., Ferns G.A., Bahrami A. Thymol has anticancer effects in U-87 human malignant glioblastoma cells. Mol. Biol. Rep. 2022;49:9623–9632. doi: 10.1007/s11033-022-07867-3. [DOI] [PubMed] [Google Scholar]
- 23.Li Y., Wen J.M., Du C.J., Hu S.M., Chen J.X., Zhang S.G., Zhang N., Gao F., Li S.J., Mao X.W., et al. Thymol inhibits bladder cancer cell proliferation via inducing cell cycle arrest and apoptosis. Biochem. Biophys. Res. Commun. 2017;491:530–536. doi: 10.1016/j.bbrc.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 24.Zeng Q., Che Y., Zhang Y., Chen M., Guo Q., Zhang W. Thymol Isolated from Thymus vulgaris L. Inhibits Colorectal Cancer Cell Growth and Metastasis by Suppressing the Wnt/β-Catenin Pathway. Drug Des. Devel. Ther. 2020;14:2535–2547. doi: 10.2147/DDDT.S254218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deb D.D., Parimala G., Saravana Devi S., Chakraborty T. Effect of thymol on peripheral blood mononuclear cell PBMC and acute promyelotic cancer cell line HL-60. Chem. Biol. Interact. 2011;193:97–106. doi: 10.1016/j.cbi.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 26.Khan S.T., Khan M., Ahmad J., Wahab R., Abd-Elkader O.H., Musarrat J., Alkhathlan H.Z., Al-Kedhairy A.A. Thymol and carvacrol induce autolysis, stress, growth inhibition and reduce the biofilm formation by Streptococcus mutans. AMB Express. 2017;7:49. doi: 10.1186/s13568-017-0344-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kowalczyk A., Przychodna M., Sopata S., Bodalska A., Fecka I. Thymol and Thyme Essential Oil-New Insights into Selected Therapeutic Applications. Molecules. 2020;25:4125. doi: 10.3390/molecules25184125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thosar N., Basak S., Bahadure R.N., Rajurkar M. Antimicrobial efficacy of five essential oils against oral pathogens: An in vitro study. Eur. J. Dent. 2013;7:S071–S077. doi: 10.4103/1305-7456.119078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perez A.P., Perez N., Lozano C.M.S., Altube M.J., de Farias M.A., Portugal R.V., Buzzola F., Morilla M.J., Romero E.L. The anti MRSA biofilm activity of Thymus vulgaris essential oil in nanovesicles. Phytomedicine. 2019;57:339–351. doi: 10.1016/j.phymed.2018.12.025. [DOI] [PubMed] [Google Scholar]
- 30.Kryvtsova M.V., Salamon I., Koscova J., Bucko D., Spivak M. Antimicrobial, antibiofilm and biochemichal properties of Thymus vulgaris essential oil against clinical isolates of opportunistic infections. Biosyst. Divers. 2019;27:270–275. doi: 10.15421/011936. [DOI] [Google Scholar]
- 31.Tohidpour A., Sattari M., Omidbaigi R., Yadegar A., Nazemi J. Antibacterial effect of essential oils from two medicinal plants against Methicillin-resistant Staphylococcus aureus (MRSA) Phytomedicine. 2010;17:142–145. doi: 10.1016/j.phymed.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 32.Kumari P., Mishra R., Arora N., Chatrath A., Gangwar R., Roy P., Prasad R. Antifungal and Anti-Biofilm Activity of Essential Oil Active Components against Cryptococcus neoformans and Cryptococcus laurentii. Front. Microbiol. 2017;8:2161. doi: 10.3389/fmicb.2017.02161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liggri P.G.V., Tsitsanou K.E., Stamati E.C.V., Saitta F., Drakou C.E., Leonidas D.D., Fessas D., Zographos S.E. The structure of AgamOBP5 in complex with the natural insect repellents Carvacrol and Thymol: Crystallographic, fluorescence and thermodynamic binding studies. Int. J. Biol. Macromol. 2023;237:124009. doi: 10.1016/j.ijbiomac.2023.124009. [DOI] [PubMed] [Google Scholar]
- 34.Gonçalves J.C., de Meneses D.A., de Vasconcelos A.P., Piauilino C.A., Almeida F.R., Napoli E.M., Ruberto G., de Araújo D.A. Essential oil composition and antinociceptive activity of Thymus capitatus. Pharm. Biol. 2017;55:782–786. doi: 10.1080/13880209.2017.1279672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daldal H., Nazıroğlu M. Carvacrol protects the ARPE19 retinal pigment epithelial cells against high glucose-induced oxidative stress, apoptosis, and inflammation by suppressing the TRPM2 channel signaling pathways. Graefe’s Arch. Clin. Exp. Ophthalmol. 2022;260:2567–2583. doi: 10.1007/s00417-022-05731-5. [DOI] [PubMed] [Google Scholar]
- 36.Wijesundara N.M., Lee S.F., Davidson R., Cheng Z., Rupasinghe H.P.V. Carvacrol Suppresses Inflammatory Biomarkers Production by Lipoteichoic Acid- and Peptidoglycan-Stimulated Human Tonsil Epithelial Cells. Nutrients. 2022;14:503. doi: 10.3390/nu14030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu H., Xu X., Wu R., Bi L., Zhang C., Chen H., Yang Y. Antioral Squamous Cell Carcinoma Effects of Carvacrol via Inhibiting Inflammation, Proliferation, and Migration Related to Nrf2/Keap1 Pathway. Biomed. Res. Int. 2021;2021:6616547. doi: 10.1155/2021/6616547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang S., He L., Shang J., Chen L., Xu Y., Chen X., Li X., Jiao Q., Jin S., Hu X., et al. Carvacrol Suppresses Human Osteosarcoma Cells via the Wnt/β-Catenin Signaling Pathway. Anti-Cancer Agents Med. Chem. 2022;22:1714–1722. doi: 10.2174/1871520621666210901111932. [DOI] [PubMed] [Google Scholar]
- 39.Li L., He L., Wu Y., Zhang Y. Carvacrol affects breast cancer cells through TRPM7 mediated cell cycle regulation. Life Sci. 2021;266:118894. doi: 10.1016/j.lfs.2020.118894. [DOI] [PubMed] [Google Scholar]
- 40.Alanazi R., Nakatogawa H., Wang H., Ji D., Luo Z., Golbourn B., Feng Z.P., Rutka J.T., Sun H.S. Inhibition of TRPM7 with carvacrol suppresses glioblastoma functions in vivo. Eur. J. Neurosci. 2022;55:1483–1491. doi: 10.1111/ejn.15647. [DOI] [PubMed] [Google Scholar]
- 41.Nazıroğlu M. A novel antagonist of TRPM2 and TRPV4 channels: Carvacrol. Metab. Brain Dis. 2022;37:711–728. doi: 10.1007/s11011-021-00887-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Celik Topkara K., Kilinc E., Cetinkaya A., Saylan A., Demir S. Therapeutic effects of carvacrol on beta-amyloid-induced impairments in in vitro and in vivo models of Alzheimer’s disease. Eur. J. Neurosci. 2022;56:5714–5726. doi: 10.1111/ejn.15565. [DOI] [PubMed] [Google Scholar]
- 43.Ghorani V., Alavinezhad A., Rajabi O., Boskabady M.H. Carvacrol improves pulmonary function tests, oxidant/antioxidant parameters and cytokine levels in asthmatic patients: A randomized, double-blind, clinical trial. Phytomedicine. 2021;85:153539. doi: 10.1016/j.phymed.2021.153539. [DOI] [PubMed] [Google Scholar]
- 44.Soković M., Glamočlija J., Marin P.D., Brkić D., van Griensven L.J. Antibacterial effects of the essential oils of commonly consumed medicinal herbs using an in vitro model. Molecules. 2010;15:7532–7546. doi: 10.3390/molecules15117532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gedikoğlu A., Sökmen M., Çivit A. Evaluation of Thymus vulgaris and Thymbra spicata essential oils and plant extracts for chemical composition, antioxidant, and antimicrobial properties. Food Sci. Nutr. 2019;7:1704–1714. doi: 10.1002/fsn3.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Magi G., Marini E., Facinelli B. Antimicrobial activity of essential oils and carvacrol, and synergy of carvacrol and erythromycin, against clinical, erythromycin-resistant Group A Streptococci. Front. Microbiol. 2015;6:165. doi: 10.3389/fmicb.2015.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wijesundara N.M., Lee S.F., Cheng Z., Davidson R., Rupasinghe H.P.V. Carvacrol exhibits rapid bactericidal activity against Streptococcus pyogenes through cell membrane damage. Sci. Rep. 2021;11:1487. doi: 10.1038/s41598-020-79713-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boukhatem M.N., Darwish N.H.E., Sudha T., Bahlouli S., Kellou D., Benelmouffok A.B., Chader H., Rajabi M., Benali Y., Mousa S.A. In Vitro Antifungal and Topical Anti-Inflammatory Properties of Essential Oil from Wild-Growing Thymus vulgaris (Lamiaceae) Used for Medicinal Purposes in Algeria: A New Source of Carvacrol. Sci. Pharm. 2020;88:33. doi: 10.3390/scipharm88030033. [DOI] [Google Scholar]
- 49.Satyal P., Murray B.L., McFeeters R.L., Setzer W.N. Essential Oil Characterization of Thymus vulgaris from Various Geographical Locations. Foods. 2016;5:70. doi: 10.3390/foods5040070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nazzaro F., Fratianni F., Coppola R., Feo V. Essential Oils and Antifungal Activity. Pharmaceuticals. 2017;10:86. doi: 10.3390/ph10040086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Giordani R., Regli P., Kaloustian J., Mikaïl C., Abou L., Portugal H. Antifungal effect of various essential oils against Candidaalbicans. Potentiation of antifungal action of amphotericin B by essential oil from Thymus vulgaris. Phytother. Res. 2004;18:990–995. doi: 10.1002/ptr.1594. [DOI] [PubMed] [Google Scholar]
- 52.Pina-Vaz C., Gonçalves Rodrigues A., Pinto E., Costa-de-Oliveira S., Tavares C., Salgueiro L., Cavaleiro C., Gonçalves M.J., Martinez-de-Oliveira J. Antifungal activity of Thymus oils and their major compounds. J. Eur. Acad. Dermatol. Venereol. 2004;18:73–78. doi: 10.1111/j.1468-3083.2004.00886.x. [DOI] [PubMed] [Google Scholar]
- 53.Pinto E., Pina-Vaz C., Salgueiro L., Gonçalves M.J., Costa-de-Oliveira S., Cavaleiro C., Palmeira A., Rodrigues A., Martinez-de-Oliveira J. Antifungal activity of the essential oil of Thymus pulegioides on Candida, Aspergillus and dermatophyte species. J. Med. Microbiol. 2006;55:1367–1373. doi: 10.1099/jmm.0.46443-0. [DOI] [PubMed] [Google Scholar]
- 54.Baser K.H. Biological and pharmacological activities of carvacrol and carvacrol bearing essential oils. Curr. Pharm. Des. 2008;14:3106–3119. doi: 10.2174/138161208786404227. [DOI] [PubMed] [Google Scholar]
- 55.Bouyahya A., Abrini J., Dakka N., Bakri Y. Essential oils of Origanum compactum increase membrane permeability, disturb cell membrane integrity, and suppress quorum-sensing phenotype in bacteria. J. Pharm. Anal. 2019;9:301–311. doi: 10.1016/j.jpha.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Altun M., Yapici B.M. Determination of chemical compositions and antibacterial effects of selected essential oils against human pathogenic strains. An. Acad. Bras. Ciências. 2022;94:e20210074. doi: 10.1590/0001-3765202220210074. [DOI] [PubMed] [Google Scholar]
- 57.Ose R., Tu J., Schink A., Maxeiner J., Schuster P., Lucas K., Saloga J., Bellinghausen I. Cinnamon extract inhibits allergen-specific immune responses in human and murine allergy models. Clin. Exp. Allergy. 2020;50:41–50. doi: 10.1111/cea.13507. [DOI] [PubMed] [Google Scholar]
- 58.Schink A., Naumoska K., Kitanovski Z., Kampf C.J., Fröhlich-Nowoisky J., Thines E., Pöschl U., Schuppan D., Lucas K. Anti-inflammatory effects of cinnamon extract and identification of active compounds influencing the TLR2 and TLR4 signaling pathways. Food Funct. 2018;9:5950–5964. doi: 10.1039/C8FO01286E. [DOI] [PubMed] [Google Scholar]
- 59.Formiga R.O., Alves Júnior E.B., Vasconcelos R.C., Guerra G.C.B., Antunes de Araújo A., Carvalho T.G., Garcia V.B., de Araújo Junior R.F., Gadelha F., Vieira G.C., et al. p-Cymene and Rosmarinic Acid Ameliorate TNBS-Induced Intestinal Inflammation Upkeeping ZO-1 and MUC-2: Role of Antioxidant System and Immunomodulation. Int. J. Mol. Sci. 2020;21:5870. doi: 10.3390/ijms21165870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jin H., Leng Q., Zhang C., Zhu Y., Wang J. P-cymene prevent high-fat diet-associated colorectal cancer by improving the structure of intestinal flora. J. Cancer. 2021;12:4355–4361. doi: 10.7150/jca.57049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang S., Wang X., Wang Y.U., Leng Q., Sun Y.U., Hoffman R.M., Jin H. The Anti-oxidant Monoterpene p-Cymene Reduced the Occurrence of Colorectal Cancer in a Hyperlipidemia Rat Model by Reducing Oxidative Stress and Expression of Inflammatory Cytokines. Anticancer Res. 2021;41:1213–1218. doi: 10.21873/anticanres.14878. [DOI] [PubMed] [Google Scholar]
- 62.Santos W.B.R., Melo M.A.O., Alves R.S., de Brito R.G., Rabelo T.K., Prado L.D.S., Silva V., Bezerra D.P., de Menezes-Filho J.E.R., Souza D.S., et al. p-Cymene attenuates cancer pain via inhibitory pathways and modulation of calcium currents. Phytomedicine. 2019;61:152836. doi: 10.1016/j.phymed.2019.152836. [DOI] [PubMed] [Google Scholar]
- 63.Rudolph K., Parthier C., Egerer-Sieber C., Geiger D., Muller Y.A., Kreis W., Müller-Uri F. Expression, crystallization and structure elucidation of γ-terpinene synthase from Thymus vulgaris. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2016;72:16–23. doi: 10.1107/S2053230X15023043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sousa L.G.V., Castro J., Cavaleiro C., Salgueiro L., Tomás M., Palmeira-Oliveira R., Martinez-Oliveira J., Cerca N. Synergistic effects of carvacrol, α-terpinene, γ-terpinene, ρ-cymene and linalool against Gardnerella species. Sci. Rep. 2022;12:4417. doi: 10.1038/s41598-022-08217-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ramalho T.R., Filgueiras L.R., Pacheco de Oliveira M.T., Lima A.L., Bezerra-Santos C.R., Jancar S., Piuvezam M.R. Gamma-Terpinene Modulation of LPS-Stimulated Macrophages is Dependent on the PGE2/IL-10 Axis. Planta Med. 2016;82:1341–1345. doi: 10.1055/s-0042-107799. [DOI] [PubMed] [Google Scholar]
- 66.National Center for Biotechnology Information PubChem Compound Summary for CID 6549, L.R.M. [(accessed on 19 March 2023)];2023 Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Linalool.
- 67.Letizia C.S., Cocchiara J., Lalko J., Api A.M. Fragrance material review on linalool. Food Chem. Toxicol. 2003;41:943–964. doi: 10.1016/S0278-6915(03)00015-2. [DOI] [PubMed] [Google Scholar]
- 68.Ma J., Xu H., Wu J., Qu C., Sun F., Xu S. Linalool inhibits cigarette smoke-induced lung inflammation by inhibiting NF-κB activation. Int. Immunopharmacol. 2015;29:708–713. doi: 10.1016/j.intimp.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 69.Huo M., Cui X., Xue J., Chi G., Gao R., Deng X., Guan S., Wei J., Soromou L.W., Feng H., et al. Anti-inflammatory effects of linalool in RAW 264.7 macrophages and lipopolysaccharide-induced lung injury model. J. Surg. Res. 2013;180:e47–e54. doi: 10.1016/j.jss.2012.10.050. [DOI] [PubMed] [Google Scholar]
- 70.Sabogal-Guáqueta A.M., Osorio E., Cardona-Gómez G.P. Linalool reverses neuropathological and behavioral impairments in old triple transgenic Alzheimer’s mice. Neuropharmacology. 2016;102:111–120. doi: 10.1016/j.neuropharm.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim M.G., Kim S.M., Min J.H., Kwon O.K., Park M.H., Park J.W., Ahn H.I., Hwang J.Y., Oh S.R., Lee J.W., et al. Anti-inflammatory effects of linalool on ovalbumin-induced pulmonary inflammation. Int. Immunopharmacol. 2019;74:105706. doi: 10.1016/j.intimp.2019.105706. [DOI] [PubMed] [Google Scholar]
- 72.Altinoz E., Oner Z., Elbe H., Uremis N., Uremis M. Linalool exhibits therapeutic and protective effects in a rat model of doxorubicin-induced kidney injury by modulating oxidative stress. Drug Chem. Toxicol. 2022;45:2024–2030. doi: 10.1080/01480545.2021.1894751. [DOI] [PubMed] [Google Scholar]
- 73.Gunaseelan S., Balupillai A., Govindasamy K., Muthusamy G., Ramasamy K., Shanmugam M., Prasad N.R. The preventive effect of linalool on acute and chronic UVB-mediated skin carcinogenesis in Swiss albino mice. Photochem. Photobiol. Sci. 2016;15:851–860. doi: 10.1039/c6pp00075d. [DOI] [PubMed] [Google Scholar]
- 74.Sabogal-Guáqueta A.M., Hobbie F., Keerthi A., Oun A., Kortholt A., Boddeke E., Dolga A. Linalool attenuates oxidative stress and mitochondrial dysfunction mediated by glutamate and NMDA toxicity. Biomed. Pharmacother. 2019;118:109295. doi: 10.1016/j.biopha.2019.109295. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.