Abstract
Background
Crocus sativus L. (saffron) is used in folk medicine, for example as an antiedematogenic agent. We aimed to evaluate the antinociceptive and anti-inflammatory activity of saffron extracts in mice.
Results
We used aqueous and ethanolic maceration extracts of Crocus sativus L. stigma and petals. Antinociceptive activity was examined using the hot plate and writhing tests. The effect of extracts against acute inflammation was studied using xylene induced ear edema in mice. The activity of the extracts against chronic inflammation was assessed by formalin-induced edema in the rat paw. In the hot plate tests, intraperitoneal injection of both extracts showed no significant antinociceptive activity in mice. The extracts exhibited antinociceptive activity against acetic acid induced writhing. Naloxone partially blocked only the antinociceptive activity of the stigma aqueous extract. Only the stigma extracts showed weak to moderate effect against acute inflammation. In chronic inflammation, both aqueous and ethanolic stigma extracts, as well as ethanolic petal extract, exerted anti-inflammatory effects.
Conclusions
We conclude that aqueous and ethanolic extracts of saffron stigma and petal have an antinociceptive effect, as well as acute and/or chronic anti-inflammatory activity.
Background
Crocus sativus L. (Iridaceae), commonly known as saffron is used in folk medicine for various purposes such as an aphrodisiac, antispasmodic and expectorant [1]. Modern pharmacological studies have demonstrated that saffron extracts have antitumour [2-4], radical scavenger, hypolipaemic [5], anticonvulsant effects [6] and improve activity on learning and memory [5,7].
Chemical studies on C. sativus have shown the presence of constituents such as crocin, crocetin, safranal and picrocrocin [8-10]. Among the constituents of saffron extract, crocetin is mainly responsible for these pharmacological activities [5].
In traditional medicine, the stigma of this plant is used as an antiedematogenic remedy [1]. The aim of this study was to validate the anti-inflammatory and antinociceptive effect of the saffron stigma and petal extracts.
Results
The maximum non-fatal doses of stigma aqueous and ethanolic extracts were 0.8 g/kg and 2 g/kg (i.p.), respectively.
The maximum non-fatal doses of petal aqueous and ethanolic extracts were 3.6 g/kg and 8 g/kg (i.p.), respectively. LD50 values of the petal aqueous and ethanolic extracts were 6.67 g/kg, i.p. (4.95, 8.99) and 9.99 g/kg, i.p. (8.13,12.28), respectively.
Phytochemical screening of the extracts indicated the presence of flavonoids, tannins and anthocyanins in the petal aqueous and ethanolic extracts. Alkaloids and saponins were found in the stigma aqueous and ethanolic extracts (Table 1)
Table 1.
Phytochemical screening of Crocus sativus petal and stigma aqueous and ethanolic extracts.
| Extract | Alkaloids | Flavonoids | Tannins | Saponins | Anthocyanins |
| Petal | |||||
| Aqueous | - | + | + | - | + |
| Ethanolic | - | + | + | - | + |
| Stigma | |||||
| Aqueous | + | - | - | + | - |
| Ethanolic | + | - | - | + | - |
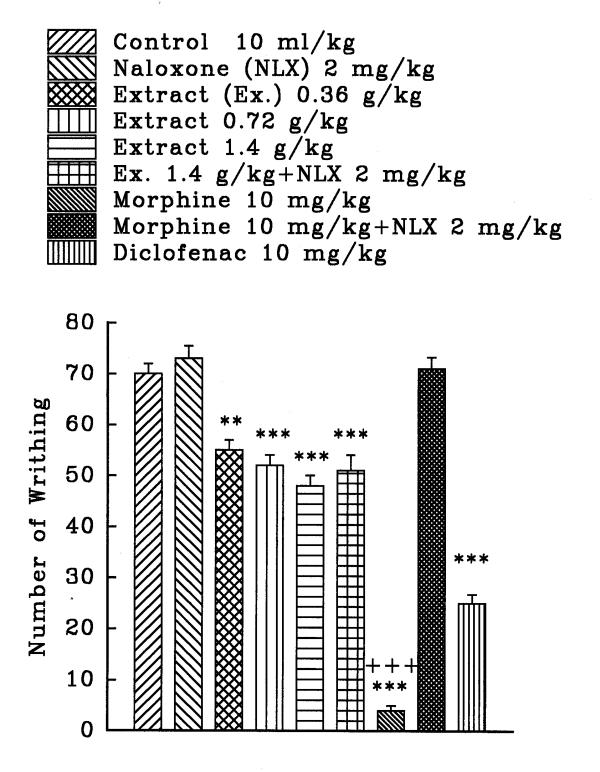
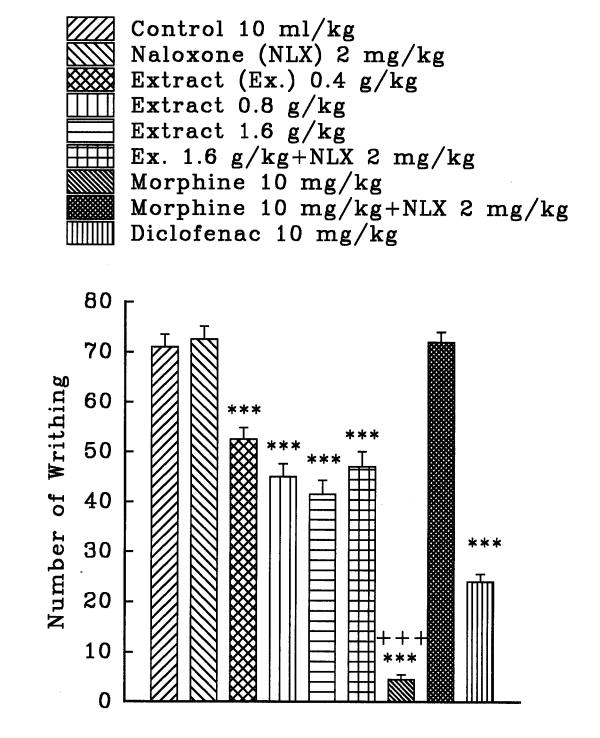
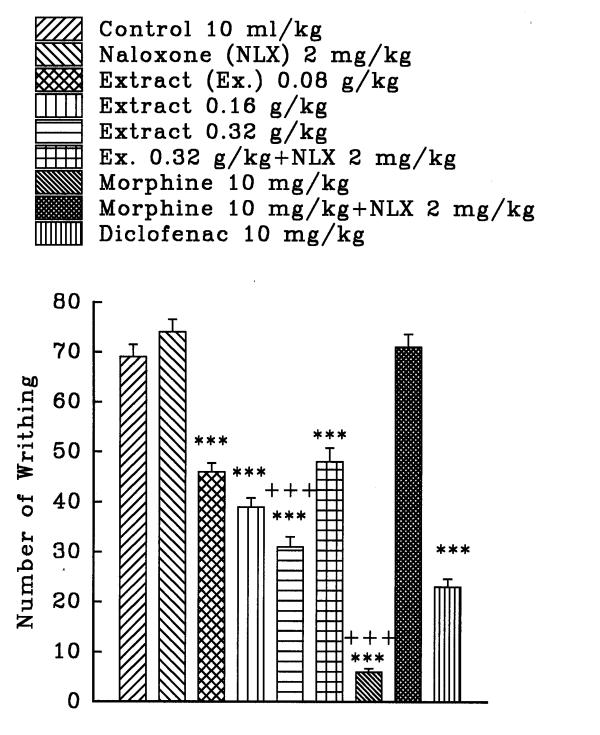
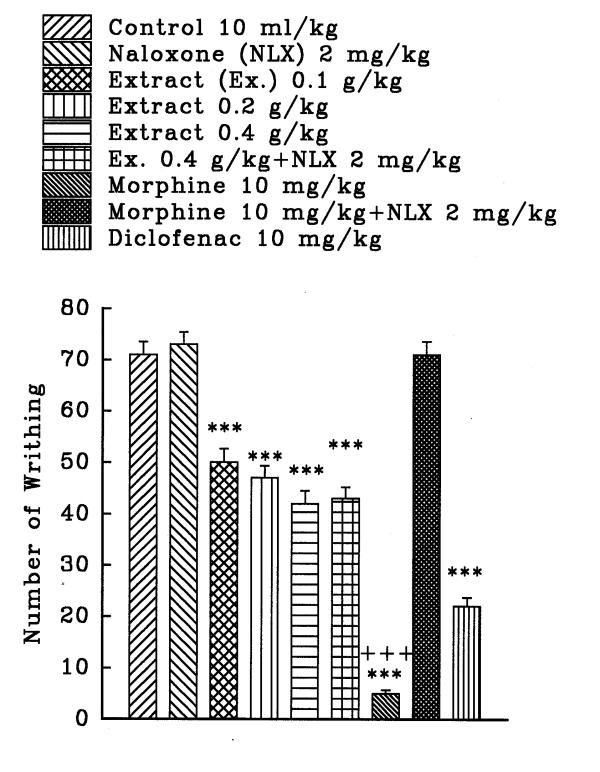
The aqueous and ethanolic extracts of C. sativus petal and stigma significantly reduce the number of mouse abdominal constrictions induced by a 0.7% acetic acid solution in a dose dependent manner (P<0.001, except for the dose of 0.36 g/kg of aqueous stigma which was P<0.01). Morphine and diclofenac induced a protection against abdominal constriction (P<0.001). Naloxone, (2 mg/kg, s.c.) pretreatment after i.p. injection of the extracts practically did not inhibit the antinociceptive activity of both extracts (figures 1, 2, 3, and 4). Only, the antinociceptive effect of aqueous stigma extract, 0.32 g/kg, was partially blocked by naloxone (figure 3). Naloxone completely antagonized the antinociceptive activity of morphine.
Figure 1.
Effect of a subcutaneous injection of naloxone on the antinociceptive effect of intraperitoneally administered Crocus sativus petal aqueous extract, morphine and diclofenac on acetic acid-induced writhing test in mice. Values are the mean ± S.E.M. of writhes number for 8 mice, **P<0.01, ***P<0.001, compared to control (normal saline); +++P<0.001, compared to morphine plus naloxone, Tukey-Kramer test.
Figure 2.
Effect of a subcutaneous injection of naloxone on the antinociceptive effect of intraperitoneally administered Crocus sativus petal ethanolic extract, morphine and diclofenac on acetic acid-induced writhing test in mice. Values are the mean ± S.E.M. of writhes number for 8 mice, ***P<0.001, compared to control (normal saline); +++P<0.001, compared to morphine plus naloxone, Tukey-Kramer test.
Figure 3.
Effect of a subcutaneous injection of naloxone on the antinociceptive effect of intraperitoneally administered Crocus sativus stigma aqueous extract, morphine and diclofenac on acetic acid-induced writhing test in mice. Values are the mean ± S.E.M. of writhes number for 8 mice, ***P<0.001, compared to control (normal saline); +++P<0.001, compared to morphine (or extract) plus naloxone, Tukey-Kramer test.
Figure 4.
Effect of a subcutaneous injection of naloxone on the antinociceptive effect of intraperitoneally administered Crocus sativus stigma ethanolic extract, morphine and diclofenac on acetic acid-induced writhing test in mice. Values are the mean ± S.E.M. of writhes number for 8 mice, ***P<0.001, compared to control (normal saline); +++P<0.001, compared to morphine plus naloxone, Tukey-Kramer test.
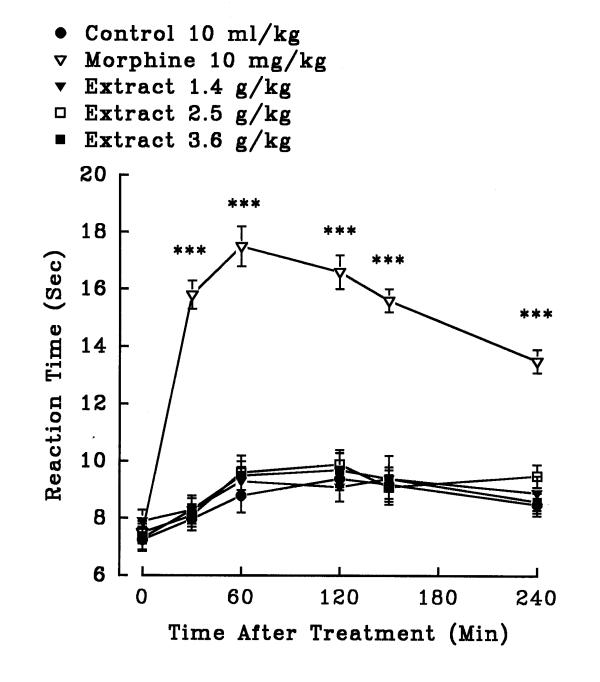
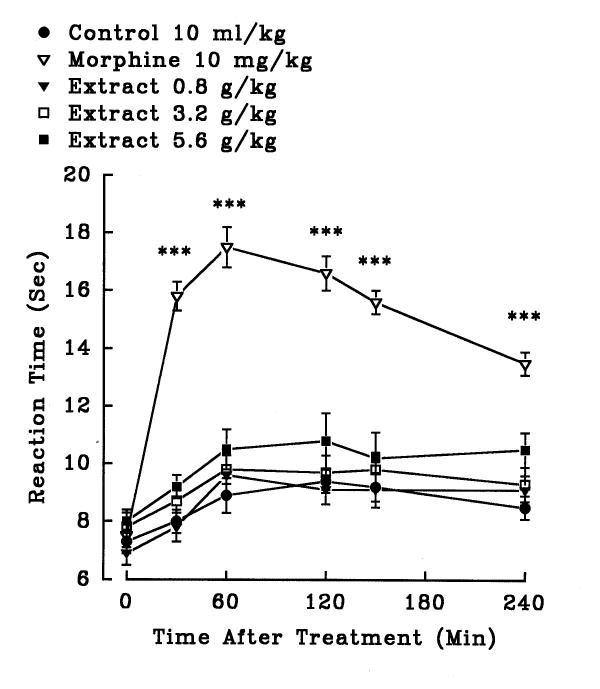
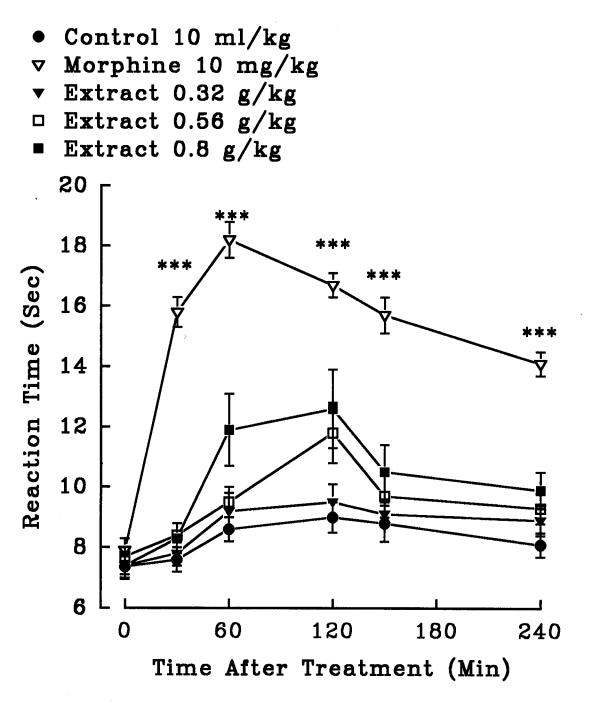
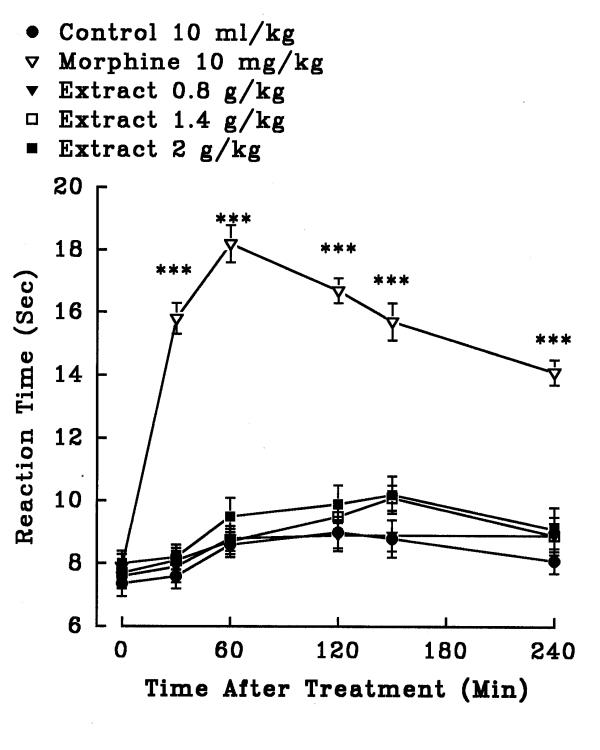
In hot plate test, aqueous (1.4, 2.5 and 3.6 g/kg, i.p.) and ethanolic (0.8, 3.2 and 5.6 g/kg, i.p.) petal extracts showed no significant antinociceptive activity (P>0.05)(figures 5 and 6). The aqueous (0.32, 0.56 and 0.8 g/kg, i.p.) and ethanolic (0.8, 1.4 and 2 g/kg, i.p.) stigma extracts also exerted no significant analgesic activity (P>0.05)(figures 7 and 8). Morphine, a positive reference, (10 mg/kg, i.p.) showed significant analgesic effect in the hot-plate test beginning 30 min after treatment (P<0.001). In the xylene induced ear edema, the aqueous and ethanolic extracts of petal showed no significant anti-inflammatory activity but diclofenac and dexamethasone reduced the edema about 50% (Tables 2 and 3). In higher doses, the aqueous (0.56 g/kg, P<0.05; 0.8 g/kg, P<0.01) and ethanolic (1.4 g/kg, P<0.05; 2 g/kg, P<0.05) extracts of stigma showed significant activity against the acute inflammation (Tables 4 and 5).
Figure 5.
Effect of the aqueous extract of Crocus sativus petal and morphine (i.p.) on pain threshold of mice in the hot-plate test. Each point represents the mean ± S.E.M. of reaction time for n = 8 experiments on mice. ***P<0.001, compared to control (normal saline), Tukey-Kramer test.
Figure 6.
Effect of the ethanolic extract of Crocus sativus petal and morphine (i.p.) on pain threshold of mice in the hot-plate test. Each point represents the mean ± S.E.M. of reaction time for n = 8 experiments on mice. ***P<0.001, compared to control (normal saline), Tukey-Kramer test.
Figure 7.
Effect of the aqueous extract of Crocus sativus stigma and morphine (i.p.) on pain threshold of mice in the hot-plate test. Each point represents the mean ± S.E.M. of reaction time for n = 8 experiments on mice. ***P<0.001, compared to control (normal saline), Tukey-Kramer test.
Figure 8.
Effect of the ethanolic extract of Crocus sativus stigma and morphine (i.p.) on pain threshold of mice in the hot-plate test. Each point represents the mean ± S.E.M. of reaction time for n = 8 experiments on mice. ***P<0.001, compared to control (normal saline), Tukey-Kramer test.
Table 2.
Effect of the intraperitoneal doses of Crocus sativus petal aqueous extract, diclofenac and dexamethasone on xylene-induced ear swelling in mice.
| Treatment | Dose | Ear swelling (mg) | Inhibition (%) |
| Control | 10 ml/kg | 7.6 ± 0.6 | – |
| Diclofenac | 15 mg/kg | 3.9 ± 0.4*** | 49.1 |
| Dexamethasone | 15 mg/kg | 3.2 ± 0.3*** | 58.2 |
| Extract | 1.4 g/kg | 6.8 ± 0.3 | 11.7 |
| Extract | 2.5 g/kg | 6.4 ± 0.4 | 15.9 |
| Extract | 3.6 g/kg | 6.3 ± 0.3 | 17.2 |
The increase in weight caused by the irritant (xylene) was measured by subtracting the weight of the untreated left ear section from that of the treated right ear sections. Values are the mean ± S.E.M. for 7 mice, ***P<0.001, compared to control (normal saline), Tukey-Kramer.
Table 3.
Effect of the intraperitoneal doses of Crocus sativus petal ethanolic extract, diclofenac and dexamethasone on xylene-induced ear swelling in mice.
| Treatment | Dose | Ear swelling (mg) | Inhibition (%) |
| Control | 10 ml/kg | 6.8+0.5 | – |
| Diclofenac | 15 mg/kg | 3.7 ± 0.6** | 45.3 |
| Dexamethasone | 15 mg/kg | 3.3 ± 0.5** | 51.4 |
| Extract | 0.8 g/kg | 5.5 ± 0.6 | 19.2 |
| Extract | 1.6 g/kg | 5.5 ± 0.5 | 19.4 |
| Extract | 2.5 g/kg | 5.1 ± 0.6 | 25.8 |
| Extract | 3.2 g/kg | 4.8 ± 0.6 | 29.0 |
The increase in weight caused by the irritant (xylene) was measured by subtracting the weight of the untreated left ear section from that of the treated right ear sections. Values are the mean ± S.E.M. for 7 mice, **P<0.01, compared to control (normal saline), Tukey-Kramer.
Table 4.
Effect of the intraperitoneal doses of Crocus sativus stigma aqueous extract, diclofenac and dexamethasone on xylene-induced ear swelling in mice.
| Treatment | Dose | Ear swelling (mg) | Inhibition (%) |
| Control | 10 ml/kg | 7.6 ± 0.6 | – |
| Diclofenac | 15 mg/kg | 3.9 ± 0.4*** | 53.6 |
| Dexamethasone | 15 mg/kg | 32+0 3*** | 63.1 |
| Extract | 0.32 g/kg | 5.0 ± 0.4 | 20.9 |
| Extract | 0.56 g/kg | 4.3 ± 0.4* | 32.6 |
| Extract | 0.8 g/kg | 3.5 ± 0.5** | 44.1 |
The increase in weight caused by the irritant (xylene) was measured by subtracting the weight of the untreated left ear section from that of the treated right ear sections. Values are the mean ± S.E.M. for 7 mice, *P<0.05, **P<0.01, ***P<0.001, compared to control (normal saline), Tukey-Kramer.
Table 5.
Effect of the intraperitoneal doses of Crocus sativus stigma ethanolic extract on xylene-induced ear swelling in mice.
| Treatment | Dose | Ear swelling (mg) | Inhibition (%) |
| Control | 10 ml/kg | 6.2 ± 0.3 | – |
| Diclofenac | 15 mg/kg | 2.6 ± 0.4*** | 58.3 |
| Dexamethasone | 15 mg/kg | 3.1 ± 0.4*** | 49.5 |
| Extract | 0.2 g/kg | 5.1 ± 0.3 | 18.2 |
| Extract | 0.8 g/kg | 4.8 ± 0.3 | 22.4 |
| Extract | 1.4 g/kg | 4.4 ± 0.4* | 29.3 |
| Extract | 2.0 g/kg | 4.5 ± 0.6* | 27.8 |
The increase in weight caused by the irritant (xylene) was measured by subtracting the weight of the untreated left ear section from that of the treated right ear sections. Values are the mean ± S.E.M. for 7 mice, *P<0.05, ***P<0.001, compared to control (normal saline), Tukey-Kramer.
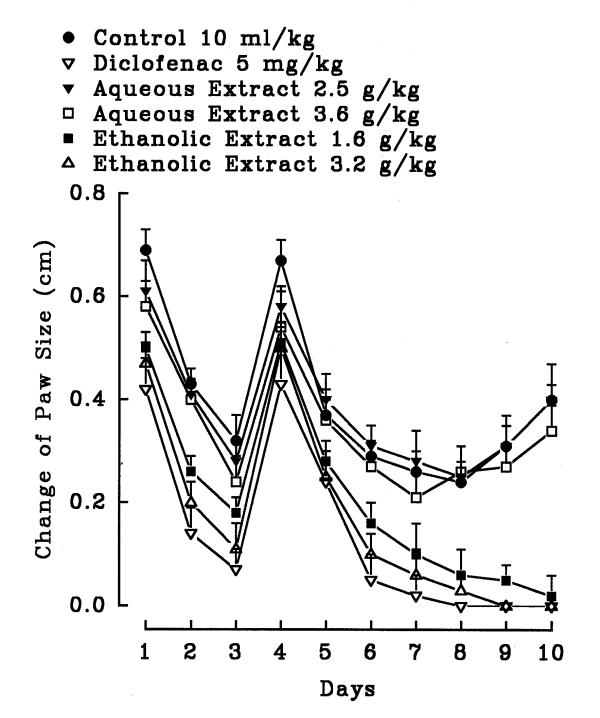
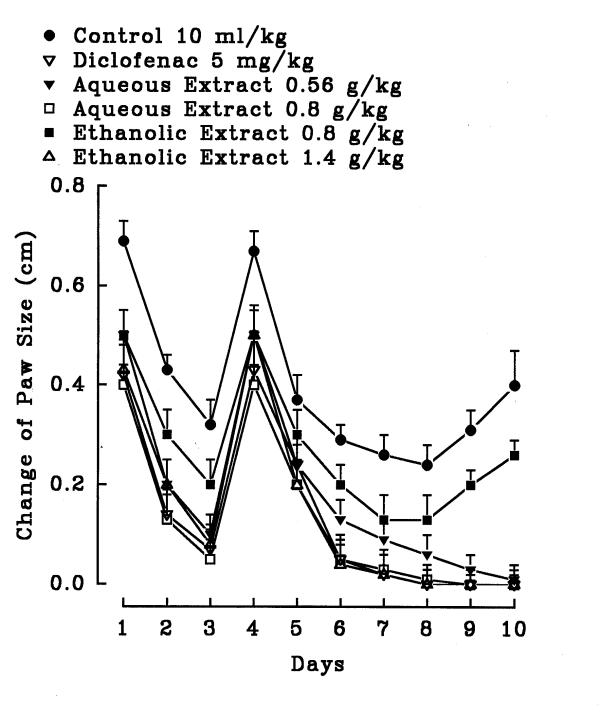
In the chronic inflammation (formalin test), the aqueous petal extracts did not exhibit significant anti-inflammatory activity (P>0.05) but the ethanolic petal extract showed significant activity (figure 9). Both extracts of the stigma exerted significant anti-inflammatory activity (figure 10). In diclofenac and the ethanolic stigma extract (1.4 g/kg) groups; the hind paw edema of rat disappeared after 6 days (P<0.001) (figure 10). Diclofenac as well as the aqueous (0.8 g/kg) and ethanolic stigma extract (1.4 g/kg) showed anti-inflammatory effect on day one (P<0.05) (figure 10). On days 4 and 5, both diclofenac and the extracts did not demonstrate anti-inflammatory activity (P>0.05).
Figure 9.
Effect of petal Crocus sativus aqueous and ethanolic extracts and diclofenac on formaldehyde induced arthritis in hind paw of rats. The inflammation was produced by subaponeurotic injection of 0.1 ml of 2% formaldehyde in the right hind paw of the rats on the first and third day. The animals were treated daily with the extracts or diclofenac intraperitoneally for 10 days. All agents were administered intraperitoneally. Each point represents the mean ± S.E.M. of change of hind paw size for 6 rats. Only the ethanolic extracts and diclofenac were effective compared to control (normal saline), Tukey-Kramer.
Figure 10.
Effect of stigma Crocus sativus aqueous and ethanolic extracts and diclofenac on formaldehyde induced arthritis in hind paw of rats. The inflammation was produced by subaponeurotic injection of 0.1 ml of 2% formaldehyde in the right hind paw of the rats on the first and third day. The animals were treated daily with the extracts or diclofenac intraperitoneally for 10 days. All agents were administered intraperitoneally. Each point represents the mean ± S.E.M. of change of paw size for 6 rats. Both extracts and diclofenac were effective compared to control (normal saline), Tukey-Kramer.
Discussion
The present results indicate that aqueous and ethanolic extracts of C. sativus petal and stigma have antinociceptive activity in chemical pain tests. The stigma extracts showed activity against acute and chronic inflammation. The petal ethanolic extract showed anti-inflammatory activity in the chronic inflammatory test.
In respect to LD50 values and maximum non-fatal doses, the stigma extracts were more toxic than the petal extracts. LD50 values of aqueous and ethanolic stigma extracts have been reported as 1.6 g/kg, i.p. (1.16, 2.22) and 3.38 g/kg, i.p. (2.55, 4.52) in mice [6]. According to a toxicity classification [11], stigma and petal extracts are "relatively toxic" and "low toxic", respectively. Treatment with the C. sativus extract also significantly prolonged the life span of cisplatin-treated mice almost three-fold [12]. Toxicity studies showed that the hematological and biochemical parameters were within normal range with saffron extract [2].
As preliminary phytochemical results indicated, it could be suggested that the antinociceptive and anti-inflammatory effects of the petal extracts may be due to their content of flavonoids, tannins and anthocyanins. Other studies have demonstrated that various flavonoids such as rutin, quercetin, luteolin, hesperidin and biflavonoids produced significant antinociceptive and/or anti-inflammatory activities [13-16]. There are few reports on the role of tannins in antinociceptive and anti-inflammatory activities [17]. Recently, it has been shown that crocins, Crocus glycosides, exhibited an anti-inflammatory effect in some models of inflammation [18].
In the present study, morphine, a centrally acting analgesic drug, produced an inhibitory effect on the nociceptive response in the hot plate test, a central antinociceptive test [19], while the aqueous and ethanolic extracts showed no antinociceptive activity in this test. Thus, the extracts may not act via central mechanisms, although agents that alter the motor performance of animals may increase the latency time on the hot plate test without acting on the central nervous system [19]. Antinociceptive activity of opioid agonists, opioid partial agonists, and non-steroidal anti-inflammatory agents can be determined by the writhing test [20]. It has also been shown that some plants such as Hunteria zeylanica[21] and Ocotea suaveolens[22] decrease stretching induced by acid acetic acid but have no effect on heat-induced pain. The association of both antinociceptive activity and moderate anti-inflammatory effect observed with the extracts has also been shown in non-steroidal anti-inflammatory drugs (NSAIDs). It is a well-established fact that NSAIDs exert their analgesic and anti-inflammatory activity by the inhibition of cyclo-oxygenase activity [23]. As the antinociceptive activity of most extracts in the writhing test was not inhibited by naloxone, the extracts may not act via opioid receptors and may exert their activity via a peripheral mechanism. The stigma extracts and the ethanolic petal extracts significantly diminished in a dose dependent way the induced paw edema in rats. The extracts were able to reduce the inflammation in acute and chronic phases.
It is concluded that saffron stigma and petal aqueous and ethanolic maceration extracts have antinociceptive effects in chemical pain tests and have acute and/or chronic anti-inflammatory activity. The antinociceptive and anti-inflammatory effects of the extracts may be due to their content of flavonoids, tannins, anthocyanins, alkaloids and saponins. However, the chemical constituents and mechanism(s) responsible for the pharmacologoical activities remain to be investigated.
Materials and Methods
Animals
Male and female albino mice 25–30 g and Wistar rats weighing 150–210 g were obtained from a random bred colony in the animal house of Mashhad University of Medical Sciences. Animals were housed in colony room 12/12 hr light/dark cycle at 21 ± 2°C and had free access to water and food.
Plant material
Plants were collected from Torabat Hydarieh (in south of Khorassan province, I.R. of Iran) in October 1998 and dried in shadow and ground. The C. sativus L. was identified by Ferdowsi University (Ms. Molaei) and voucher samples were preserved for reference in the herbarium of School of Pharmacy, Mashhad, IR. Iran (143-0319-1).
Preparation of extracts
The powder of stigma or petal was extracted using maceration with ethanol or water. The powdered plant was macerated in water or ethanol (80 %, v/v) for 3 days and, subsequently, the mixture was filtered and concentrated under reduced pressure at 40°C. The yield (w/w) of aqueous and ethanolic extracts of stigma was 50.8% and 56.6%, respectively. The yield (w/w) of aqueous and ethanolic extracts of petal was 15.5% and 19%, respectively. The extracts were dissolved in normal saline.
Phytochemical screening
Phytochemical screening of the extract was performed using the following reagents and chemicals: Alkaloids with Dragendorffs reagent, flavonoids with the use of Mg and HCl; tannins with 1% gelatin and 10% NaCl solutions and saponins with ability to produce suds [24].
The maximum non-fatal dose (MNFD) and acute toxicity
Different doses of the extracts were injected to separate groups of five animals. After 48 h, the highest dose that did not induce any mortality was considered as the maximum non-fatal dose. The number of deaths was counted at 48 h after treatment. LD50 values and the corresponding confidence limits (CL, 95%) were determined by the Litchfield and Wilcoxon method (PHARM/PCS Version 4). Doses of 10, 40 (50), 70 and 100 % MNFD were chosen for most tests. Lower doses were found to be effective in the writhing test.
Antinociceptive study
Hot-plate test
The hot-plate test was assessed on groups of 8 mice. The temperature of a metal surface was maintained at 55 ± 0.2°C. Latency to a discomfort reaction (licking paws or jumping) was determined before and after drug administration. The cut-off time was 20 s. The latency was recorded before and 30, 60, 120, 150 and 240 min following intraperitoneal administration of the agents. The prolongation of the latency times compared with the values of the control was used for statistical comparison. Control received normal saline (10 ml/kg, i.p.) and morphine (10 mg/kg, i.p.) was used as reference drug [25].
Writhing test
Groups of 8 mice were used for controls and test mice. One hour after the administration of the extract, the mice were given an intraperitoneal injection of 0.7% v/v acetic acid solution (volume of injection 0.1 ml/10 g). The mice were placed individually into glass beakers and five min were allowed to elapse. The number of writhes produced in these animals was counted for 30 min. For scoring purposes, a writhe is indicated by stretching of the abdomen with simultaneous stretching of at least one hind limb. Control received normal saline (10 ml/kg, i.p.), diclofenac (10 mg/kg, i.p.) and morphine (10 mg/kg, i.p.) were used as reference drugs. Naloxone (2 mg/kg, s.c.) was administered 15 min prior to the extracts or morphine injections [25].
Anti-inflammatory study
Xylene-induced ear edema
Mice were divided into groups of seven. Thirty minutes after i.p. injection of the extract, diclofenac and dexamethasone, 0.03 ml of xylene was applied to the anterior and posterior surfaces of the right ear. The left ear was considered as control. Two hours after xylene application, mice were killed and both ears were removed. Circular sections were taken, using a cork borer with a diameter of 7 mm, and weighed. The increase in weight caused by the irritant was measured by subtracting the weight of the untreated left ear section from that of the treated right ear sections. The formula for computing percent inhibition was: average writhes in the control group (normal saline) minus writhes in the drug group divided by writhes in the control group times 100%. Control received normal saline (10 ml/kg, i.p.), diclofenac (10 mg/kg, i.p.) and dexamethasone (15 mg/kg, i.p.) were used as reference drugs [25].
Formalin induced inflammation
Rats were divided into groups of six. The inflammation was produced by subaponeurotic injection of 0.1 ml of 2% formaldehyde in the right hind paw of the rats on the first and third day. The animals were treated daily with the extracts or diclofenac intraperitoneally for 10 days. The daily changes in paw size were measured by wrapping a piece of cotton thread round the paw and measuring the circumference with a meter rule [26].
Materials
The following reagents were used: morphine and dexamethasone (Dam Pakhsh, I.R. Iran), naloxone hydrochloride (Tolid Daru, I.R. Iran), diclofenac (Zahrawi, I.R. Iran), acetic acid, xylene and formaldehyde (Merck).
Statistical analysis
The data were expressed as mean values ± S.E.M. and tested with analysis of variance followed by the multiple comparison test of Tukey-Kramer.
Acknowledgments
Acknowledgments
The authors are thankful to T.W. Stone .Professor of Pharmacology, Division of Neuroscience and Biomedical Systems, University of Glasgow, Scotland, for his linguistic help.
Contributor Information
Hossein Hosseinzadeh, Email: hosseinzadehh@yahoo.com.
Hani M Younesi, Email: school-pharmacy@mums.ac.ir.
References
- Zargari A. Medicinal Plants. Tehran, University Press. 1990;4:574–578. [Google Scholar]
- Nair SC, Pannikar B, Panikkar KR. Antitumour activity of saffron (Crocus sativus). Cancer Lett. 1991;57:109–114. doi: 10.1016/0304-3835(91)90203-t. [DOI] [PubMed] [Google Scholar]
- Salomi MJ, Nair SC, Panikkar KR. Inhibitory effects of Nigella sativa and saffron (Crocus sativus) on chemical carcinogenesis in mice. Nutr Cancer. 1991;16:67–72. doi: 10.1080/01635589109514142. [DOI] [PubMed] [Google Scholar]
- Nair SC, Kurumboor SK, Hasegawa JH. Saffron chemoprevention in biology and medicine: a review. Cancer Biother. 1995;10:257–264. doi: 10.1089/cbr.1995.10.257. [DOI] [PubMed] [Google Scholar]
- Abe K, Saito H. Effects of saffron extract and its constituent crocin on learning behaviour and long-term potentiation. Phytother Res. 2000;14:149–152. doi: 10.1002/(SICI)1099-1573(200005)14:3<149::AID-PTR665>3.3.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Hosseinzadeh H, Khosravan V. Anticonvulsant effect of Crocus sativus L. stigmas aqueous and ethanolic extracts in mice. Arch Irn Med. 2001.
- Zhang Y, Shoyama Y, Sugiura M, Saito H. Effects of Crocus sativus L. on the ethanol-induced impairment of passive avoidance performances in mice. Biol Pharm Bull. 1994;17:217–221. doi: 10.1248/bpb.17.217. [DOI] [PubMed] [Google Scholar]
- Tarantilis PA, Tsoupras G, Polissiou M. Determination of saffron (Crocus sativus L.) components in crude plant extract using high-performance liquid chromatography-UV-visible photodiode-array detection-mass spectrometry. J Chromatography. 1995;699:107–118. doi: 10.1016/0021-9673(95)00044-N. [DOI] [PubMed] [Google Scholar]
- Escribano J, Alonso GL, Coca-Prados M, Fernandez JA. Crocin, safranal and picrocrocin from saffron (Crocus sativus L.) inhibit the growth of human cancer cells in vitro. Cancer Lett. 1996;100:23–30. doi: 10.1016/0304-3835(95)04067-6. [DOI] [PubMed] [Google Scholar]
- Lozano P, Delgado D, Gomez D, Rubio M, Iborra JL. A non-destructive method to determine the safranal content of saffron (Crocus sativus L.) by supercritical carbon dioxide extraction combined with high-performance liquid chromatography and gas chromatography. J Biochem Biophys methods. 2000;43:367–378. doi: 10.1016/S0165-022X(00)00090-7. [DOI] [PubMed] [Google Scholar]
- Loomis TA. Essential of Toxicology. Philladelphia, Lea and Febiger. 1968. pp. 67–78.
- Nair SC, Salomi MJ, Panikkar B, Pannikkar KR. Modulatory effects of Crocus-sativus and Nigella-sativa extracts on cisplatin-induced toxicity in mice. J Ethnopharmacol. 1991;31:75–84. doi: 10.1016/0378-8741(91)90146-5. [DOI] [PubMed] [Google Scholar]
- Bittar M, de Souza MM, Yunes RA, Lento R, Delle Monache F, Cechinel Filho V. Antinociceptive activity of I3,II8-binaringenin, a biflavonoid present in plants of the guttiferae. Planta Med. 2000;66:84–86. doi: 10.1055/s-0029-1243118. [DOI] [PubMed] [Google Scholar]
- Calixto JB, Beirith A, Ferreira J, Santos AR, Cechinel Filho V, Yunes RA. Naturally occurring antinociceptive substances from plants. Phytother Res. 2000;14:401–418. doi: 10.1002/1099-1573(200009)14:6<401::AID-PTR762>3.3.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Galati EM, Monforte MT, Kirjavainen S, Forestieri AM, Trovato A, Tripodo MM. Biological effects of hesperidin, a citrus flavonoid. (Note I): anti-inflammatory and analgesic activity. Farmaco. 1994;40:709–712. [PubMed] [Google Scholar]
- Ramesh M, Rao YN, Rao AV, Prabhakar MC, Rao CS, Muralidhar N, Reddy BM. Antinociceptive and anti-inflammatory activity of a flavonoid isolated from Caralluma attenuata. J Ethnopharmacol. 1998;62:63–66. doi: 10.1016/S0378-8741(98)00048-8. [DOI] [PubMed] [Google Scholar]
- Starec M, Waitzov'a D, Elis J. Evaluation of the analgesic effect of RG-tannin using the "hot plate" and "tail flick" method in mice. Cesk Farm. 1988;37:319–321. [PubMed] [Google Scholar]
- Ma S, Zhou S, Shu B, Zhou J. Pharmacological studies on Crocus glycosides I. Effects on antiinflammatory and immune function. Zhongcaoyao. 1998;29:536–539. [Google Scholar]
- Parkhouse J, Pleuvry BJ. Analgesic Drug. Oxford, Black Well. 1979. pp. 1–5.
- Vogel HG, Vogel WH. Drug Discovery and Evaluation, Pharmacological Assays. Berlin, Springer, 1997. pp. 402–403.
- Reanmongkol W, Subhadhirasakul S, Kongsang J, Tanchong M, Kitti J. Analgesic and antipyretic activity of n-butanol alkaloids extracted from the stem bark Hunteria zeylanica and its major constituent, strictosidinic acid, in mice. Pharm Biol. 2000;38:68–73. doi: 10.1076/1388-0209(200001)38:1;1-B;FT068. [DOI] [PubMed] [Google Scholar]
- Beirith A, Santos ARS, Calixto JB, Hess SC, Messana I, Ferrari F, Yunes RA. Study of the antinociceptive action of the ethanolic ectract and the triterpene 24-hydroxytormentic acid isolated from the stem bark of Ocotea suaveolens. Planta Med. 1999;65:50–55. doi: 10.1055/s-1999-13962. [DOI] [PubMed] [Google Scholar]
- Vane JR. Inhibition prostaglandine synthesis as a mechanism of action for aspirin-like drugs. Nature New Biol. 1971;231:232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- Trease GE, Evans WC. Pharmacognosy. London, Bailliere Tindall Press. 1983. pp. 309–706.
- Hosseinzadeh H, Ramezani M, Salmani G-A. Antinociceptive, anti-inflammatory and acute toxicity effects of Zataria multiflora Boiss extracts in mice and rats. J Ethnopharmacol. 2000;73:379–385. doi: 10.1016/S0378-8741(00)00238-5. [DOI] [PubMed] [Google Scholar]
- Saxena RS, Gupta B, Saxena KK, Singh RC, Prasad DN. Study of anti-inflammatory activity in the leaves of Nyctanthes arbor tristis linn.: an indian medicinal plant. J Ethnopharmacol. 1984;11:319–330. doi: 10.1016/0378-8741(84)90077-1. [DOI] [PubMed] [Google Scholar]