Summary
Blood cell adhesion to P‐selectin and vascular cell adhesion molecule‐1 (VCAM‐1) contributes to the pathophysiology of vaso‐occlusion crisis (VOC) events in individuals with sickle cell disease (SCD). We evaluated the use of standardized flow adhesion biomarkers in a six‐month, 35‐subjects longitudinal study (ELIPSIS). Flow adhesion of whole blood on P‐selectin (FA‐WB‐Psel) and VCAM1 (FA‐WB‐VCAM), and of isolated white blood cells on P‐selectin (FA‐WBC‐Psel) and VCAM‐1 (FA‐WBC‐VCAM) were elevated on VOC days compared with non‐VOC days, but only FA‐WB‐Psel reached statistical significance (P = 0·015). Optimal cut‐off values were established with Cox regression models for FA‐WB‐Psel [46 cells/mm²; hazard ratio (HR): 2·3; 95% confidence interval (CI):1·4–4·0; P = 0·01] and FA‐WB‐VCAM (408 cells/mm², HR:1·8; 95% CI: 0·9–3·45; P = 0·01). A combined (FA‐WB‐Psel and FA‐WB‐VCAM) multimarker risk score was also significantly (P = 0·0006) correlated with VOC risk that was two‐fold higher for intermediate and 5·64‐fold higher for high score. The concordance (C)‐index for the multimarker score was 0·63 in the six‐month period (95% CI: 0·56–0·70), indicating a better ability to distinguish patient risk of VOC, compared to individual biomarkers FA‐WB‐VCAM (C‐index: 0·57; 95% CI: 0·49–0·65) or FA‐WB‐Psel (C‐index: 0·58; 95% CI: 0·53–0·62). The presented multimarker score can be used to risk‐stratify individuals with SCD during their steady state into low, intermediate, and high‐risk strata for self‐reported VOCs. Such risk stratification could help focus healthcare resources more efficiently to maintiain health, personalize treatment selection to each patient’s individual needs, and potentially reduce healthcare costs.
Keywords: flow adhesion, P‐selectin, vascular cell adhesion molecule‐1, biomarker, sickle cell disease
Introduction
Sickle cell disease (SCD) is one of the most common hereditary blood disorders affecting over 100,000 people in the United States and many more world‐wide. 1 The most common genotype is homozygous haemoglobin SS (HbSS). SCD is associated with a wide range of symptoms and adverse events including chronic anaemia, pulmonary hypertension and acute chest syndrome, stroke, splenic and renal dysfunction, and susceptibility to bacterial infections. Many of these symptoms are related to vaso‐occlusion crises (VOC), a common painful complication of SCD responsible for about 85% of all SCD‐related emergency room (ER) visits and hospitalizations. 2 Diagnosis may be complicated by the lack of objective metrics of pain severity, resulting in providers relying on subjective reports from SCD patients. Traditionally, treatment options have been limited, focusing primarily on alleviating symptoms with analgesics, with simple or exchange blood transfusions used in severe cases. Preventively, severe SCD is often managed with hydroxycarbamide (HC) which promotes fetal haemoglobin (HbF) formation and was shown to reduce the frequency and shorten the duration of hospitalization due to VOC. 3 , 4 While the precise pathways leading to VOC remain uncertain, and possibly vary between subjects, the formation of cellular aggregates, which interact with the endothelium to cause microvascular occlusion and local ischaemia, are believed to be major contributors to the microvascular blood flow disturbances that characterize VOCs. 5
Recently, a number of SCD‐modifying therapies have been approved to improve rheology and normalize RBC function. Those include L‐glutamine (Endari®, Emmaus Medical) which reduces oxidative damage on the RBC membrane, 6 crizanlizumab‐tmca (Adakveo®, Novartis) a P‐selectin‐blocking monoclonal antibody that inhibits multicellular adhesive interactions, and haemoglobin‐modifying voxelotor that reduces haemoglobin polymerization by increasing haemoglobin‐oxygen binding affinity. 7 Both L‐glutamine and crizanlizumab were shown to reduce the annualized frequency of VOCs, 8 , 9 and voxelotor increased haemoglobin concentration by targeting the primary polymerization mechanism, 10 which may improve overall RBC health and rheology.
VOC events are unpredictable in people with SCD, and there are no biomarkers that reliably predict VOC onset. While certain triggers (e.g., dehydration, inflammation, physical or emotional stress, cold temperature) had been shown to increase VOC risks, most episodes are not associated with an easily discernable cause. 11 As a result, it is difficult for a provider to effectively utilize and target measures to prevent VOCs. Thus, there is substantial interest in the development of prognostic biomarkers capable of stratifying individual patients at steady state based on their risk for developing VOCs.
The pathophysiology of VOC is complex and the mechanisms underlying its development remain to be fully elucidated. On the molecular level hypoxia‐induced polymerization of HbS induces membrane damage leading to decreased RBC survival in circulation though intravascular haemolysis and accelerated phagocytosis in the spleen. 12 At the same time, membrane damage results in calcium influx and potassium and water efflux from sickle RBC leading to cell dehydration and sickling. 13 Haemoglobin released upon cell lysis depletes NO, leading to vasoconstriction, 14 playing a role in elevation of normal and sickle RBC adhesion to endothelium 15 and also contributing to platelet activation. 16
Additionally, HbS undergoes autooxidation and precipitates on the inner membrane surface contributing to sickle RBC oxidative stress, 17 with generated reactive oxygen species (ROS) inducing lipid peroxidation and attacking membrane and cytoskeletal proteins. 18 , 19 Proximate adhesion of RBC to vascular endothelium, observed in SCD, exacerbated by membrane damage and cell lysis, was suggested as a triggering event for acute VOC development. 20 Exposure of endothelial cell to oxygen radicals, as well as to some other plasma factors like tumour necrosis factor α (TNF‐α), Interleukin‐1, or thrombin, brings about rapid mobilization of P‐selectin from Weibel–Palade bodies to the surface of endothelial cells through the oxidant‐dependent activation of the transcription factor NF‐κB. 21 Elevated levels of E‐selectin, vascular cell adhesion molecule‐1 (VCAM‐1), and intercellular adhesion molecule 1 (ICAM‐1) were reported during VOC, as compared to subjects with other SCD complications likely indicating their potential involvement in the development of VOCs. 22
The initiation of a VOC involves a multitude of molecules. 23 P‐selectin plays a key role in the adhesion of sickle erythrocytes and leucocytes to the endothelial wall. 24 The role of P‐selectin in the process of VOC was first demonstrated by a transgenic murine model that SCD mice deficient in P‐selectin, which displayed defective leukocyte recruitment to the vessel wall, were protected from vaso‐occlusion. 25 P‐selectin inhibition by anti‐P‐selectin aptamer in SCD mice decreased cell adhesion and increased microvascular velocities. 26 Surface‐expressed P‐selectin also mediated abnormal rolling and static adhesion of SS red blood cells to the vessel surface in vitro. 27 , 28 Translocation of endothelial P‐selectin to the cell surface also results in the prompt adhesion of sickle erythrocytes to vessels and the development of vascular occlusion in transgenic mice with SCD. 24 In humans with SCD, dose‐dependent inhibition of adhesion to P‐selectin was observed following pre‐exposure to crizanlizumab, an anti‐P‐selectin monoclonal antibody, 29 with VOC rates reduced upon crizanlizumab treatment in a recent double‐blind, placebo‐controlled study. 30 , 31
Very late antigen‐4 (VLA‐4) is highly expressed in sickle reticulocytes and supports cell adhesion to endothelium through the interaction with VCAM‐1 with VLA‐4/VCAM‐1 interactions proposed to contribute to SCD severity and VOC. A decrease in VLA‐4 expression on reticulocytes in SCD subjects treated with HC, a therapy known to reduce the clinical severity of SCD, was previously reported. 32 Recent positron emission tomography data indicate VLA‐4‐mediated hyper‐adhesion, primarily of SCD reticulocytes, during VOCs in the SCD Townes mouse model. 33 We have demonstrated that pulsatile flow increased VCAM‐1 – VLA‐4 adhesive interactions as compared to non‐pulsatile flow, 34 likely due to an increase in interaction time allowing for the development of additional adhesive points of attachment. 5 We developed a standardized, flow‐based adhesion bioassay to measure whole blood adhesion to VCAM‐1 and P‐selectin during a simulated physiologic flow. In this study, we evaluated the ability of four flow‐based adhesion biomarkers, including flow adhesion of whole blood to VCAM‐1 (FA‐WB‐VCAM), flow adhesion of whole blood to P‐selectin (FA‐WB‐Psel), flow adhesion of white blood cells to VCAM‐1 (FA‐WBC‐VCAM) and flow adhesion of WBC to P‐selectin (FA‐WBC‐Psel), to stratify individuals with SCD accordingly to their risk of self‐reported VOCs. The six‐month longitudinal study of subjects with SCD documented the natural history of self‐reported and hospital VOCs and assessed biomarkers in longitudinally collected blood samples before, during, and after VOC events.
Methods
Study subjects and sample collection
This protocol was approved by the Wayne State University Human Investigation Committee Institutional Review Board. Participants with a confirmed diagnosis of stable SCD (defined as no significant complications for at least one week prior to study entry such as VOC requiring in‐patient hospitalization, acute chest syndrome or any complication requiring in‐patient hospitalization, or no acute transfusions for at least one month prior to the baseline visit) with HbSS or S‐β thalassaemia0 (HbS‐β0) were identified at clinic visits and consented to participate in the study. Participants must have had two unplanned medical interventions with health care providers for VOCs, requiring opioid treatment, in the 12 months prior to study entry and be at least 12 years of age, with a weight of at least 43.5 kg to allow for the frequent blood collections. The daily e‐diary captured a patient‐reported VOC pain crisis as a VOC day, if the patient responded yes to whether they experienced a pain crisis in the last 24 h. The pain crisis module triggered at‐home blood collections (within 24 h of the initial event, with follow‐up collections at 48 h and 72 h after VOC event resolution) using mobile phlebotomy to collect blood samples in the subjects’ home. Alternatively, when VOC involved an emergency room visit with or without hospital stay, the event was captured through patient medical records. Baseline non‐VOC blood collections were conducted at three‐week intervals in the subject’s home during the six‐month period.
Flow adhesion biomarkers
Standardized microfluidic flow adhesion assays were conducted as described previously. 24 Briefly, blood for FA‐WB‐VCAM, FA‐WB‐Psel, FA‐WBC‐VCAM and FA‐WBC‐Psel assays was drawn in sodium citrate tubes and stored at 4°C for up to 48 h, which in our control experiments did not result in statistically significant changes in VCAM‐1 or P‐selectin flow adhesion. Isolated WBC suspension was prepared according to a standard procedure. 35 A commercial well plate microfluidic flow adhesion system, BioFlux 1000Z (Fluxion Bio, San Francisco, CA, USA) was utilized. Whole blood samples (1:1 diluted with Hank's Balanced Salt Solution buffer) or isolated WBC suspension (at 5 million cells/ml) were perfused through VCAM‐1 (20 µg/ml) ‐ or Psel (5 µg/ml)‐coated microfluidic channels (350 µm wide x 70 µm tall) using pulsatile (1·67 Hz) shear stress (1 dyne/cm2) and washed with the buffer at the same flow rate to eliminate non‐adhering cells. Images were acquired with a high‐resolution CCD camera and analyzed with Montage imaging software (Molecular Devices, Downingtown, PA, USA). An adhesion index (AI) was established for each sample by quantifying adherent cells within a standard viewing area (cells/mm2).
Statistical analysis
Baseline characteristics were summarized for the entire cohort using standard descriptive statistics. Box and whiskers plots were utilized to depict the range of baseline values for each flow adhesion biomarker for individual subjects during this six‐month investigation. Geometric means were used, unless otherwise specified, to account for the effect of data outliers that are particularly pronounced in flow adhesion results. As geometric mean is a monotonic function of the mean of the logarithms, significance of the difference between the means of the logarithms implies significance of the difference between geometric means themselves. Comparison between the geometric means of each biomarker was performed using mixed‐model accounting for the repeated measurements.
An extended time‐dependent Cox regression model [Anderson–Gill Model (AG‐CP)] was adopted in this data analysis. 36 The data structure for fitting AG‐CP models was based on the VOC‐free time, and defined the time of enrolment as day zero and the day of the end of the study. The interval between the VOC events was calculated as the difference between the start and the end of VOC‐free time (only the VOC events confirmed during the survey and associated with blood samples were used in this analysis). The model did not account for VOC durations, which were small compared to in‐between VOC times. The start of each interval was the first day of the previous event or day zero, whichever comes later. The end of each interval was the first day of the following VOC event or the day the study ends, whichever comes later. Thus, subjects with no VOC events had one VOC‐free interval; subjects with one VOC event – two intervals, subjects with two events – three intervals, and so on. The covariates were used as categorical variables and included FA‐WB‐VCAM, FA‐WB‐Psel, FA‐WBC‐VCAM and FA‐WBC‐Psel.
Nine quantiles (at 2·5% intervals starting with 60%) were selected as candidate cut‐off values for each flow biomarker. Univariable Cox regression analysis was used with each of the four adhesion biomarkers to assess their relationship to VOC risk. The optimal cut‐off value for each biomarker was determined based on statistical significance (defined as P‐value < 0·05) of the likelihood ratio test (LRT), and on the minimum of the Akaike information criterion (AIC).
The multivariable Cox regression model utilized biomarkers from the univariable Cox regression analysis to provide a combination of biomarkers with significance on a LRT and with the lowest AIC value. Using the identified combination of biomarkers, a flow adhesion multimarker score was defined as the number of flow adhesion biomarkers present at high levels in a given patient. This multibiomarker score was used as a continuous variable to assess the collective effect of biomarker level on VOC risk in subsequent analysis. The proportional hazards assumption was evaluated and confirmed using the Schoenfeld residual test for all Cox proportional hazard regression models.
The concordance index (C‐index) was used to compare the predictive accuracy and discriminative ability of the multimarker score and individual biomarkers to classify subjects with regard to VOC at the six‐month period. Data analysis was performed using the statistics software R, version 4·0. (R Foundation for Statistical Computing, Vienna, Austria). All statistical tests were two‐tailed and P‐values < 0·05 were used to determine statistical significance.
Results
Clinical characteristics of the subjects
Thirty‐five SCD subjects had been followed for six months with the basic patient information given in Table I. Twenty‐nine percent of the subjects did not experience VOCs during the study while 71% of the subjects experienced one or more VOCs, totalling 48 VOC days (two continuous VOC days were counted as 1). Intersubject longitudinal variability for all four flow adhesion biomarkers at both at steady state and during VOCs is shown in Fig 1.
Table I.
Study population data and vaso‐occlusion crisis (VOC) incidence.
| Characteristics | Values |
|---|---|
| Total subjects | 35 |
| Study duration | 179 ± 9 days |
| Male | 13 (37%) |
| Female | 22 (63%) |
| Age | 25 ± 9 years |
| Less than 18 years old | 12 (34%) |
| No VOC | 10 (29%) |
| 1 VOC | 5 (14%) |
| More than 1 VOC | 20 (57%) |
| Average time between VOC* | 18 ± 31 days(range: 1–133) |
For subjects experiencing more than 1 VOC during the study.
Fig 1.
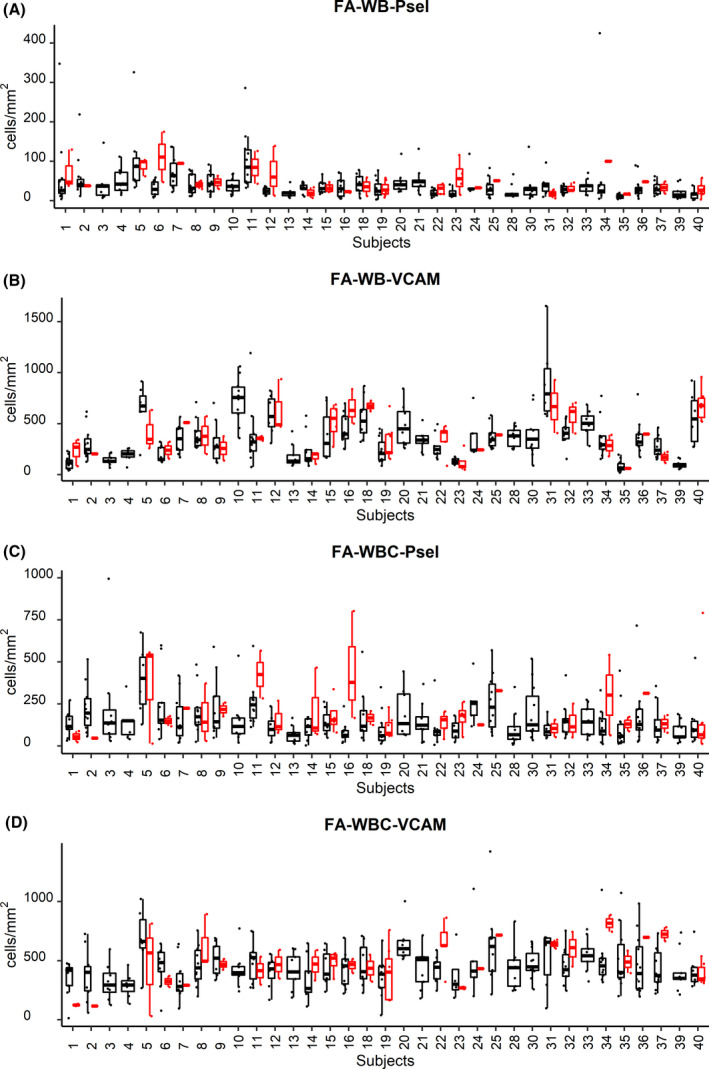
Interpatient variation of flow adhesion biomarkers. Interpatient variation of biomarkers at steady state in whole blood (WB; A and B) and isolated white blood cell (WBC; C and D) for flow adhesion on P‐selectin (A, C) and VCAM‐1 (B, D) substrates presented as box and whiskers plots. Bottom and top of the box correspond to the first and third quantiles; band inside is the median. Whiskers show 1·5 interquartile range. (black points are steady‐state samples, red points are vaso‐occlusion crisis samples).
Group variance between steady state and VOC
The average values of the four flow adhesion biomarkers from 68 VOC samples received from the 25 subjects who had at least one VOC event during the study were elevated as compared to the 314 VOC‐negative samples (from 35 subjects; Table II). The comparison between the baseline and VOC‐positive biomarker values (see Methods) indicates that the increase, accounting for repeated measurements, was statistically significant for FA‐WB‐Psel (P = 0·015) and was approaching significance for FA‐WBC‐Psel (P = 0·056). The difference between the baseline and VOC‐positive values was not significant for FA‐WB‐VCAM or FA‐WBC‐VCAM (Table II).
Table II.
Average Flow Adhesion biomarker values at baseline and during vaso‐occlusion crisis (VOC).
| Biomarker | Steady State* | VOC* | Significance † | Fold difference(95% CI) ‡ |
|---|---|---|---|---|
| FA‐WB‐VCAM | 282 (260–305) | 320 (268–382) | 0·16 | 12% (−1 to 26%) |
| FA‐WBC‐VCAM | 404 (382–429) | 417 (362–408) | 0·56 | 7% (−4 to 18%) |
| FA‐WB‐Psel | 29 (26–31) | 34 (28–41) | 0·015 | 36% (8–64%) |
| FA‐WBC‐Psel | 110 (99–122) | 134 (108–167) | 0·056 | 41% (4–78%) |
Steady State and VOC data are presented as geometric mean with 95% confidence interval.
Significance of the difference in biomarker values between steady‐state and VOC conditions was calculated with a mixed model accounting for the repeated measurements (see Methods) accounting for both inter‐ and intrasubject variability through inclusion of both fixed and random effects.
Fold difference, given in percent, was calculated as the ratio between adhesion values (VOC minus steady state)/(steady state), accounting for the repeat measurements and thus taking into consideration intersubject variability.
Determination of optimal cut‐off values
Statistical models were compared using LRT and AIC. 37 While both LRT and AIC use the value of the likelihood function for assessing model fit, the latter also includes a penalty for using more parameter‐rich models. In analysis, we preferentially used AIC for model selection for forecasting and LRT for significance testing. Based on fluctuation of each flow adhesion biomarker at steady state, nine quantiles, ranging from 60% to 80%, at 2·5% intervals, were calculated for each biomarker and used as candidate cut‐off values.
Calculated AIC and LRT values for univariable Cox regression analysis models based on cut‐off values for FA‐WB‐VCAM and FA‐WB‐Psel are given in Fig 2. The difference in the calculated values of AIC for the FA‐WB‐Psel model shows its sensitivity to cut‐off values, thus allowing for selection of the best fit model (cut‐off = 46 cells/mm2, AIC = 335). Models with cut‐off values of less than 42 cells/mm2 and over 48 cells/mm2 have higher AIC values with the difference being more than 2 AIC units, often taken as the level of significance in model comparison. 37 The implication is that the range for cut‐off values showing statistical significance for the FA‐WB‐Psel model is 46 ± 4 cells/mm2 (Fig 2A). For FA‐WB‐VCAM, the difference in AIC values for models with the cut‐off values in the range of 360 to 460 cells/mm2 was less than 2, indicating no preference in the quality of the fit between the models (Fig 2C). LRT values (Figs 2B and 2D) showed a weak preference for the cut‐off values suggested by the AIC analysis. Lack of variability in the calculated AIC values allowed for no justifiable fit model selection for either FA‐WBC‐VCAM or FA‐WBC‐Psel (data not shown).
Fig 2.
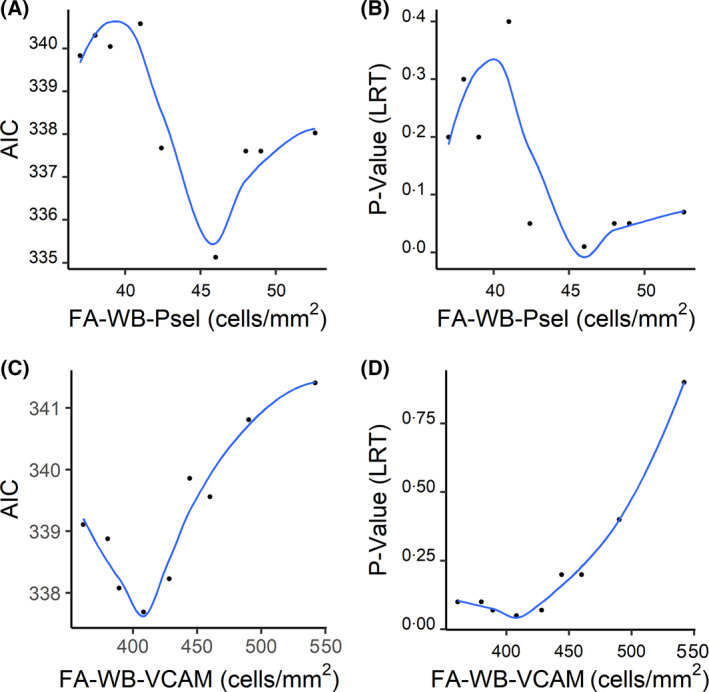
Determination of the optimal cut‐off values. A LOESS (Locally Estimated Scatterplot Smoothing) curve was fitted to Akaike information criterion (AIC) or likelihood ratio test (LRT) values plotted as a function of cell adhesion cut‐off values. The optimal cut‐off values for FA‐WB‐Psel (A and B) and FA‐WB‐VCAM (C and D) were determined using lowest AICs.
Univariable Cox regression analysis indicates that SCD subjects with the FA‐WB‐Psel values over the optimized cut‐off were more than twice as likely (HR 2·33) to experience a VOC event than the subjects with the biomarker values below the cut‐off (Fig 3A). Similar analysis for the FA‐WB‐VCAM provided a lower HR of about 1·8, consistent with the weaker statistical significance compared to that of FA‐WB‐Psel (Fig 3B). The final optimal multivariable Cox regression model included both flow adhesion biomarkers FA‐WB‐VCAM (P = 0·031) and FA‐WB‐Psel (P < 0·001).
Fig 3.
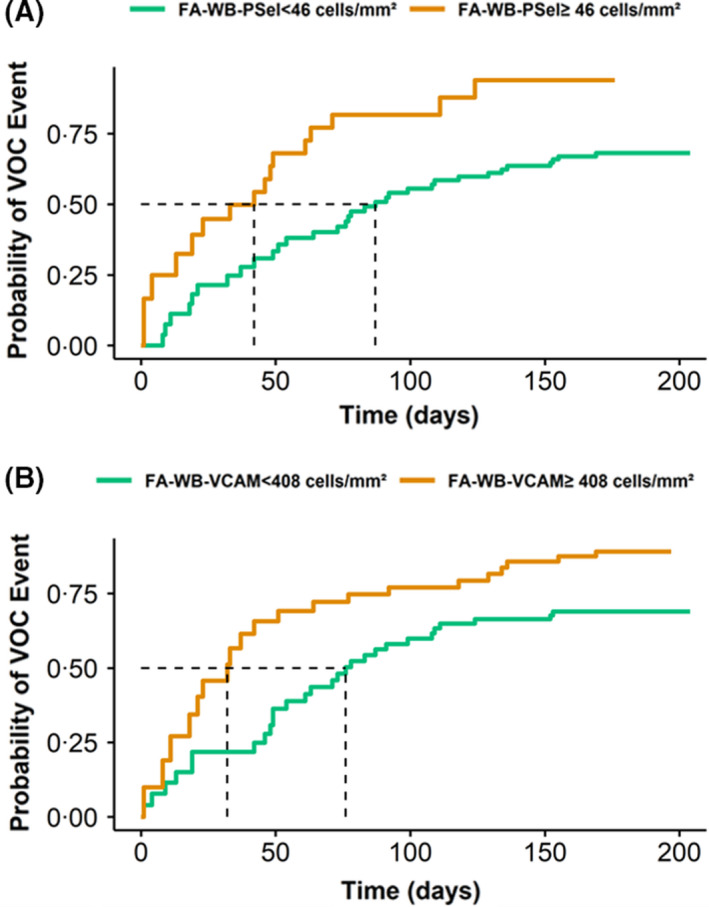
Kaplan–Meier (KM) curves of the cumulative probability of vaso‐occlusion crisis (VOC) risk. Kaplan–Meier curves of the cumulative probability of VOC risk for sickle cell disease (SCD) subjects stratified based on the adhesion indexes being above or below the cut‐off values. (A) FA‐WB‐Psel: If SCD patient’s adhesion index is above the cut‐off of 46 cells/mm2, the patient has 50% chance to experience a VOC at approximately 40 days compared to about 85 days if the adhesion index is below the cut‐off point; (B) FA‐WB‐VCAM: If SCD patient’s adhesion index is above the cut‐off of 408 cells/mm2, the patient has 50% chance to experience a VOC at approximately 30 days compared to about 75 days if the adhesion index is below the cut‐off point.
Flow adhesion multimarkers risk‐stratify individuals with SCD according to VOC risk
Using the biomarkers included in the optimal Cox regression model, we defined a new composite variable, the multimarker score, as the number of markers with levels exceeding the respective cut‐off values. The cut‐off values of 46 cells/mm² for FA‐WB‐Psel and 408 cells/mm² for FA‐WB‐VCAM established through the optimization process described above were selected, which corresponded to the model with minimum AIC value. Therefore, the multimarker score ranged from 0 to 2. That calculated score was found to have statistically significant correlation with the risk of VOC event. The AIC for the the multimarker score model was 329·66 compared to 335·13 (P < 0·001) for FA‐WB‐Psel model and 337·69 (P < 0·001) for the FA‐WB‐VCAM model. The size of the difference in calculated AIC values indicates the potential of the multimarker score model to be a better predictor of VOC events than each of the two component biomarkers.
Figure 4 shows the Kaplan–Meier curves depicting the cumulative probability of VOC events for SCD subjects with low, intermediate, and high multimarker scores. Multimarker HRs for VOC events for SCD subjects with low, intermediate, and high multimarker scores were established and are shown in Fig 5. Analysis of the models for these groups indicated that for SCD subjects with intermediate score the risk of VOC is more than twice that same risk for SCD subjects with the low score (reference). SCD subjects with a high multimarker score are nearly six times as likely to have VOC as SCD subjects with a low score.
Fig 4.
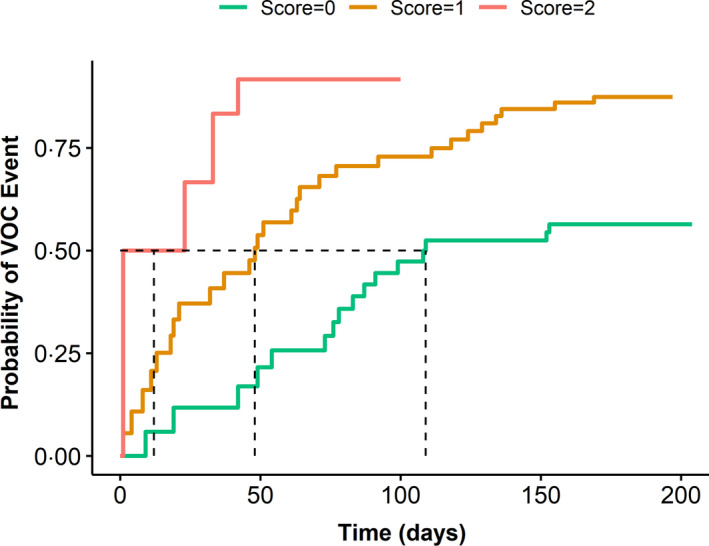
Cumulative probability of vaso‐occlusion crisis (VOC) risk according to multimarker score. Kaplan–Meier curves of the cumulative probability of VOC risk for sickle cell disease (SCD) subjects stratified based on the multimarker score. Probability of VOC event increases with the increase of multimarker adhesion score. Fifty percent probability of the time‐to‐VOC event constituting about 110 days (score = 0), 50 days (score = 1), and about 10 days (score = 2).
Fig 5.
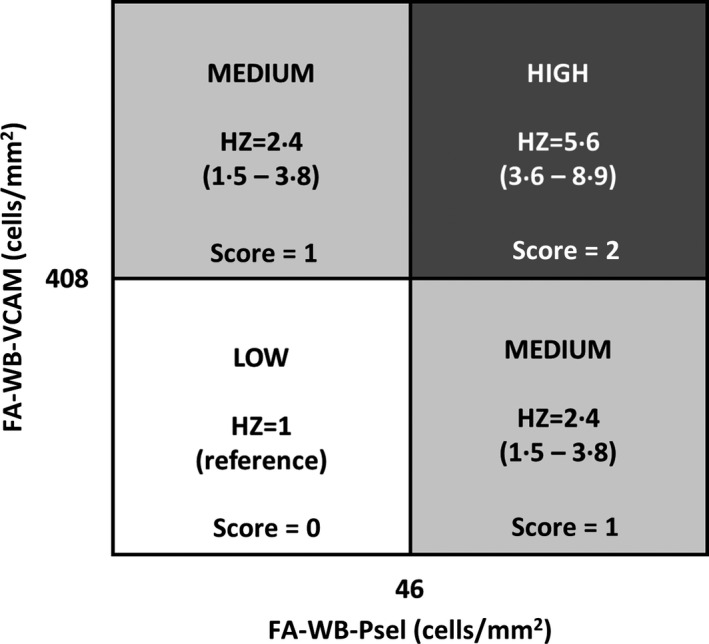
Vaso‐occlusion crisis (VOC) risk stratification. Hazard ratio (HR) and HR range for patient groups stratified as high (score = 2), medium (score =1) and low (score = 0) risk of VOC events. Stratification was based on the cut‐off values described above (408 cells/mm2 for FA‐WB‐VCAM and 46 cells/mm2 for FA‐WB‐Psel).
The C‐index for the multimarker score was 0·63 in the six‐month period (95% CI: 0·56–0·70), indicating a better ability to distinguish individual risk of VOC, compared to the individual biometrics FA‐WB‐VCAM (C‐index: 0·57; 95% CI: 0·49–0·65) or FA‐WB‐Psel (C‐index: 0·58; 95% CI: 0·53–0·62).
Discussion
This study evaluated data from a six‐month longitudinal study (ELIPSIS) to determine if standardized flow adhesion biomarkers could stratify SCD subjects based on prospective risk of self‐reported VOCs. Flow adhesion of whole blood to P‐selectin (FA‐WB‐Psel) was the best prognostic biomarker in risk‐stratifying in individuals with SCD according to their prospective risk of self‐reported VOCs. Given the heterogeneous and complex nature of VOC pathophysiology, it is unlikely that any single biomarker would be sufficient to reflect and fully predict the overall pathogenesis of the event. Instead, combining multiple biomarkers into a scoring system is likely to offer a more promising strategy. The multivariable Cox regression analysis presented here demonstrated that prognostic capability of the FA‐WB‐Psel biomarker alone can be further improved when it is supplemented with another adhesion biomarker (FA‐WB‐VCAM).
In this report, we described the application of a standardized, microfluidic flow‐based adhesion bioassay measuring flow adhesion at both steady state and VOC for its potential to assess VOC risk for SCD subjects. In clinical practice, biomarker data, which are usually collected as a continuous measurement, are often divided into categories based on an optimal cut‐off value. This kind of categorization makes it easier to interpret the effects of a biomarker on the outcome via an odds ratio or risk ratio analysis. It also offers clinicians an objective criterium to determine the risk of an individual patient for future adverse events. An outcome‐oriented approach was used for the selection of cut‐off values, which has the advantage of accounting for the association between the measured biomarker and the observed clinical outcome. Such an outcome‐oriented approach was enabled by dichotomization of the continuous biomarkers measured by our flow adhesion assays.
Among the limitations of this study, the most significant is that it was a single‐centre study with a relatively low number of enrolled subjects. Additionally, the described multimarker score model was built based on the same data set used for determination of the optimal cut‐off values; thus its reliability and performance should be re‐assessed through independent external validation. A greater degree of confidence in the discriminatory results between low risk and high risk of VOC in SCD subjects could be achieved with a larger sample size, ideally with subjects recruited at multiple centres for higher diversity. Additional limitations arise from the fact that the steady state samples were collected at a three‐week interval, and thus the scale and the rates of elevation of flow adhesion values before the VOC cannot be precisely evaluated.
To the best of our knowledge, this is the first study to utilize a composite flow adhesion biomarker score for the stratification of risk of self‐reported VOC both at home and in the acute‐care environment. The results of this study indicate a consistent tendency towards higher VOC risk in SCD subjects with higher flow adhesion indices. The multimarker score defined in this study is intuitive and easy for clinicians to implement into clinical practice. It is worth noting that whole blood flow adhesion to P‐selectin (FA‐WB‐Psel) was a better predictor of VOC than isolated WBC adhesion to P‐selectin. RBCs present in whole blood, but not in isolated WBC, can also bind to P‐selectin and thus contribute to adhesion in the FA‐WB‐Psel assay. Although the impact of possible RBC–WBC interactions may contribute to the predictive value of the FA‐WB‐Psel assay, we feel it is more plausible that the presence of sickle donor plasma in the FA‐WB‐Psel (whole blood) assay but not in the FA‐WBC‐Psel (isolated WBCs) assay is a greater contributor to the difference in performance. While the interaction of leukocyte L‐selectin glycoprotein ligand‐1 (LSGL‐1) ligand with P‐selectin (CD62P) expressed on endothelial cells does not require mediators, patient‐specific adhesion may be affected by plasma‐containing promoters and/or inhibitors, both endogenously generated as well as present due to medical treatment. In general, clinical blood function assays should reflect critical aspects of microvascular physiology in order to minimize the influence of in vitro variables and sample processing (washing, centrifugation, etc.). In addition to removing patient plasma, isolation of WBC subjects the cells to a number of wash steps and centrifugation‐induced shear that may alter the clinical predictive utility of adhesion acquired under these less physiologic conditions. Further experiments would be required to determine if any of these are indeed the culprit. However, in terms of assays’ potential clinical utility, ability to use whole blood without the need for WBC isolation is a clear advantage as it makes the assay faster and easier to use. The ability to intervene in patients at steady state who are at increased risk of future VOCs will allow providers to focus more on health maintenance and less on crisis management, which could substantially reduce healthcare costs and improve quality of life for individuals living with SCD. Studies are under way to assess the ability of assays such as FA‐WB‐Psel to monitor response to SCD‐modifying therapies (e.g., crizanlizumab, L‐glutamine, voxelotor).
Funding information
This study was sponsored by Pfizer Inc. Pfizer Inc. has no input in the interpretation of the data.
Conflicts of interest
PCH, XG, KL, JW and MT are employees and shareholders of Functional Fluidics. MUC has received grants and personal fees from Bayer, Biomarin, Global Blood Therapeutics, Hema Biologics, Kedrion, Octapharma, Pfizer, Roche/Genentech, Sanofi/Bioverativ, Spark Therapeutics, and Takeda.
Author contributions
PCH contributed to study design, data analysis, and manuscript preparation. MUC contributed to study design and data analysis. AUZ contributed to study design and data analysis. XG performed all flow adhesion assays and contributed to analysis of biomarker data, and manuscript preparation. KL contributed to the flow adhesion data analysis. JW participated in flow experiments and data analysis. MT contributed to the data analysis and manuscript preparation. All authors contributed to interpretation of the data, participated in the critical review and revision of the manuscript, and provided approval of the final manuscript. All authors had access to the data and assume responsibility for the completeness and accuracy of the data and data analyses.
Acknowledgements
The authors acknowledge all subjects and individuals who contributed to the study. We thank David Beidler and Debra D. Pittman for their role in the design and initiation of the study. We also thank Sanguine Biosciences, Clinical Ink, and CRF Health for their expertise and efforts to make this study possible.
References
- 1. NHIBI . Sickle Cell Disease. https://wwwnhlbinihgov/health‐topics/sickle‐cell‐disease. Accessed 26 October 2020.
- 2. Shah N, Bhor M, Xie L, Paulose J, Yuce H. Sickle cell disease complications: prevalence and resource utilization. PLoS One. 2019;14(7): e0214355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ballas SK, Bauserman RL, McCarthy WF, Castro OL, Smith WR, Waclawiw MA. Hydroxyurea and acute painful crises in sickle cell anemia: effects on hospital length of stay and opioid utilization during hospitalization, outpatient acute care contacts, and at home. J Pain Symptom Manage. 2010;40(6):870–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Charache S, Terrin ML, Moore RD, Dover GJ, Barton FB, Eckert SV, et al. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. N Engl J Med. 1995;332(20):1317–22. [DOI] [PubMed] [Google Scholar]
- 5. Papageorgiou DP, Abidi SZ, Chang H‐Y, Li X, Kato GJ, Karniadakis GE, et al. Simultaneous polymerization and adhesion under hypoxia in sickle cell disease. Proc Natl Acad Sci. 2018;115(38):9473–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ilboudo Y, Garrett ME, Bartolucci P, Brugnara C, Clish CB, Hirschhorn JN, et al. Potential causal role of l‐glutamine in sickle cell disease painful crises: a Mendelian randomization analysis. Blood Cells Mol Dis. 2021;86; 102504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Oksenberg D, Dufu K, Patel MP, Chuang C, Li Z, Xu Q, et al. GBT 440 increases haemoglobin oxygen affinity, reduces sickling and prolongs RBC half‐life in a murine model of sickle cell disease. Br J Haematol. 2016;175(1):141–53. [DOI] [PubMed] [Google Scholar]
- 8. Ali MA, Ahmad A, Chaudry H, Aiman W, Aamir S, Anwar MY, et al. Efficacy and safety of recently approved drugs for sickle cell disease: a review of clinical trials. Exp Hematol. 2020;92:11–18.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thom H, Jansen J, Shafrin J, Zhao L, Joseph G, Cheng H‐Y, et al. Crizanlizumab and comparators for adults with sickle cell disease: a systematic review and network meta‐analysis. BMJ open. 2020;10(9):e034147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vichinsky EP, Telfer P, Inati A, Tonda M, Tong B, Agodoa I, et al. Incidence of vaso‐occlusive crisis does not increase with achieving higher hemoglobin levels on voxelotor treatment or after discontinuation: analyses of the HOPE study. DC: American Society of Hematology Washington. 2019. [Google Scholar]
- 11. Porter M. Rapid fire: sickle cell disease. Emerg Med Clin. 2018;36(3):567–76. [DOI] [PubMed] [Google Scholar]
- 12. Kato GJ, Piel FB, Reid CD, Gaston MH, Ohene‐Frempong K, Krishnamurti L, et al. Sickle cell disease. Nat Rev Disease Primers. 2018;4(1):1–22. [DOI] [PubMed] [Google Scholar]
- 13. Ellory J, Robinson H, Browning J, Stewart G, Gehl K, Gibson J. Abnormal permeability pathways in human red blood cells. Blood Cells Mol. Dis. 2007;39(1):1–6. [DOI] [PubMed] [Google Scholar]
- 14. Wood KC, Hebbel RP, Lefer DJ, Granger DN. Critical role of endothelial cell‐derived nitric oxide synthase in sickle cell disease‐induced microvascular dysfunction. Free Radic Biol Med. 2006;40(8):1443–53. [DOI] [PubMed] [Google Scholar]
- 15. Space SL, Lane PA, Pickett CK, Weil JV. Nitric oxide attenuates normal and sickle red blood cell adherence to pulmonary endothelium. Am J Hematol. 2000;63(4):200–4. [DOI] [PubMed] [Google Scholar]
- 16. Loscalzo J. Nitric oxide insufficiency, platelet activation, and arterial thrombosis. Circ Res. 2001;88(8):756–62. [DOI] [PubMed] [Google Scholar]
- 17. Hebbel RP, Eaton J, Balasingam M, Steinberg MH. Spontaneous oxygen radical generation by sickle erythrocytes. J Clin Investig. 1982;70(6):1253–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. George A, Pushkaran S, Konstantinidis DG, Koochaki S, Malik P, Mohandas N, et al. Erythrocyte NADPH oxidase activity modulated by Rac GTPases, PKC, and plasma cytokines contributes to oxidative stress in sickle cell disease. Blood J Am Soc Hematol. 2013;121(11):2099–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mohanty J, Nagababu E, Rifkind JM. Red blood cell oxidative stress impairs oxygen delivery and induces red blood cell aging. Front Physiol. 2014;5:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hebbel RP. Perspectives series: cell adhesion in vascular biology. Adhesive interactions of sickle erythrocytes with endothelium. J Clin Investig. 1997;99(11):2561–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Takano M, Meneshian A, Sheikh E, Yamakawa Y, Wilkins KB, Hopkins EA, et al. Rapid upregulation of endothelial P‐selectin expression via reactive oxygen species generation. Am J Physiol‐Heart Circulat Physiol. 2002;283(5):H2054–61. [DOI] [PubMed] [Google Scholar]
- 22. Antwi‐Boasiako C, Donkor ES, Sey F, Dzudzor B, Dankwah GB, Otu KH, et al. Levels of soluble endothelium adhesion molecules and complications among sickle cell disease patients in Ghana. Diseases. 2018;6(2):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Manwani D, Frenette PS. Vaso‐occlusion in sickle cell disease: pathophysiology and novel targeted therapies. Blood J Am Soc Hematol. 2013;122(24):3892–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Embury SH, Matsui NM, Ramanujam S, et al. The contribution of endothelial cell P‐selectin to the microvascular flow of mouse sickle erythrocytes in vivo. Blood. 2004;104(10):3378–85. [DOI] [PubMed] [Google Scholar]
- 25. Turhan A, Weiss LA, Mohandas N, Coller BS, Frenette PS. Primary role for adherent leukocytes in sickle cell vascular occlusion: a new paradigm. Proc Natl Acad Sci. 2002;99(5):3047–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gutsaeva DR, Parkerson JB, Yerigenahally SD, Kurz JC, Schaub RG, Ikuta T, et al. Inhibition of cell adhesion by anti–P‐selectin aptamer: a new potential therapeutic agent for sickle cell disease. Blood J Am Soc Hematol. 2011;117(2):727–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Matsui NM, Borsig L, Rosen SD, Yaghmai M, Varki A, Embury SH. P‐selectin mediates the adhesion of sickle erythrocytes to the endothelium. Blood. 2001;98(6):1955–62. [DOI] [PubMed] [Google Scholar]
- 28. Matsui NM, Varki A, Embury SH. Heparin inhibits the flow adhesion of sickle red blood cells to P‐selectin. Blood. 2002;100(10):3790–6. [DOI] [PubMed] [Google Scholar]
- 29. Man Y, Goreke U, Kucukal E, Hill A, An R, Liu S, et al. Leukocyte adhesion to P‐selectin and the inhibitory role of Crizanlizumab in sickle cell disease: a standardized microfluidic assessment. Blood Cells Mol Dis. 2020;83.102424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ataga KI, Kutlar A, Kanter J, Liles D, Cancado R, Friedrisch J, et al. Crizanlizumab for the prevention of pain crises in sickle cell disease. N Engl J Med. 2017;376(5):429–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kutlar A, Kanter J, Liles DK, Alvarez OA, Cançado RD, Friedrisch JR, et al. Effect of crizanlizumab on pain crises in subgroups of patients with sickle cell disease: a SUSTAIN study analysis. Am J Hematol. 2019;94(1):55–61. [DOI] [PubMed] [Google Scholar]
- 32. Styles LA, Lubin B, Vichinsky E, Lawrence S, Hua M, Test S, et al. Decrease of very late activation antigen‐4 and CD36 on reticulocytes in sickle cell patients treated with hydroxyurea. Blood J Am Soc Hematol. 1997;89(7):2554–9. [PubMed] [Google Scholar]
- 33. Perkins LA, Nyiranshuti L, Little‐Ihrig L, Latoche JD, Day KE, Zhu Q, et al. Integrin VLA‐4 as a PET imaging biomarker of hyper‐adhesion in transgenic sickle mice. Blood Advan. 2020;4(17):4102–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. White J, Lancelot M, Sarnaik S, Hines P. Increased erythrocyte adhesion to VCAM‐1 during pulsatile flow: application of a microfluidic flow adhesion bioassay. Clin Hemorheol Micro. 2015;60(2):201–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. SIGMA‐ALDRICH . Histopaque‐1077. https://wwwsigmaaldrichcom/content/dam/sigma‐aldrich/docs/Sigma/Product_Information_Sheet/1/10771pispdf. Accessed 20 January 2016.
- 36. Amorim LD, Cai J. Modelling recurrent events: a tutorial for analysis in epidemiology. Int J Epidemiol. 2015;44(1):324–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Beier P. Model selection and inference: a practical information‐theoretic approach. JSTOR. 2001;65(3):606. [Google Scholar]


