Abstract
Background
COVID-19 has been shown to differently affect various demographic and clinical population subgroups. We aimed to describe trends in absolute and relative COVID-19-related mortality risks across clinical and demographic population subgroups during successive SARS-CoV-2 pandemic waves.
Methods
We did a retrospective cohort study in England using the OpenSAFELY platform with the approval of National Health Service England, covering the first five SARS-CoV-2 pandemic waves (wave one [wild-type] from March 23 to May 30, 2020; wave two [alpha (B.1.1.7)] from Sept 7, 2020, to April 24, 2021; wave three [delta (B.1.617.2)] from May 28 to Dec 14, 2021; wave four [omicron (B.1.1.529)] from Dec 15, 2021, to April 29, 2022; and wave five [omicron] from June 24 to Aug 3, 2022). In each wave, we included people aged 18–110 years who were registered with a general practice on the first day of the wave and who had at least 3 months of continuous general practice registration up to this date. We estimated crude and sex-standardised and age-standardised wave-specific COVID-19-related death rates and relative risks of COVID-19-related death in population subgroups.
Findings
18 895 870 adults were included in wave one, 19 014 720 in wave two, 18 932 050 in wave three, 19 097 970 in wave four, and 19 226 475 in wave five. Crude COVID-19-related death rates per 1000 person-years decreased from 4·48 deaths (95% CI 4·41–4·55) in wave one to 2·69 (2·66–2·72) in wave two, 0·64 (0·63–0·66) in wave three, 1·01 (0·99–1·03) in wave four, and 0·67 (0·64–0·71) in wave five. In wave one, the standardised COVID-19-related death rates were highest in people aged 80 years or older, people with chronic kidney disease stage 5 or 4, people receiving dialysis, people with dementia or learning disability, and people who had received a kidney transplant (ranging from 19·85 deaths per 1000 person-years to 44·41 deaths per 1000 person-years, compared with from 0·05 deaths per 1000 person-years to 15·93 deaths per 1000 person-years in other subgroups). In wave two compared with wave one, in a largely unvaccinated population, the decrease in COVID-19-related mortality was evenly distributed across population subgroups. In wave three compared with wave one, larger decreases in COVID-19-related death rates were seen in groups prioritised for primary SARS-CoV-2 vaccination, including people aged 80 years or older and people with neurological disease, learning disability, or severe mental illness (90–91% decrease). Conversely, smaller decreases in COVID-19-related death rates were observed in younger age groups, people who had received organ transplants, and people with chronic kidney disease, haematological malignancies, or immunosuppressive conditions (0–25% decrease). In wave four compared with wave one, the decrease in COVID-19-related death rates was smaller in groups with lower vaccination coverage (including younger age groups) and conditions associated with impaired vaccine response, including people who had received organ transplants and people with immunosuppressive conditions (26–61% decrease).
Interpretation
There was a substantial decrease in absolute COVID-19-related death rates over time in the overall population, but demographic and clinical relative risk profiles persisted and worsened for people with lower vaccination coverage or impaired immune response. Our findings provide an evidence base to inform UK public health policy for protecting these vulnerable population subgroups.
Funding
UK Research and Innovation, Wellcome Trust, UK Medical Research Council, National Institute for Health and Care Research, and Health Data Research UK.
Research in context.
Evidence before this study
We searched MEDLINE on Dec 8, 2022, for studies investigating risk factors for COVID-19 mortality in the UK, published from Jan 1, 2020, to Dec 6, 2022, using the terms “((coronavir* OR covid* OR sars*) AND (mortality OR death) AND risk AND (UK OR United Kingdom))”, with no language restrictions. Multiple studies showed that various population subgroups were disproportionately affected by COVID-19 depending on their demographic and clinical profile. Previous studies in the UK have reported higher COVID-19-related mortality in people with older age, male sex, minority ethnicities, public-facing occupations (eg, social care workers), households of multigenerational living, social deprivation, learning disability, and clinical comorbidities, such as obesity, kidney disease, and immunodeficiency. The studies were predominantly done in the first year of the COVID-19 pandemic (2020) and focused on a specific demographic or clinical factor.
Added value of this study
Much of what is known regarding population subgroups at greater risk of COVID-19-related death comes from the early stages of the pandemic. This study describes changes in the distributions of absolute and relative COVID-19-related mortality risks across population subgroups over the course of five waves in England from 2020 to 2022. This study shows that, although absolute COVID-19-related death rates decreased over time, demographic and clinical relative risk profiles persisted and worsened for people with low vaccination coverage or impaired immune response. Each of the COVID-19 waves after the first wave showed a different pattern in the distribution of the risk reduction across population subgroups. Before the roll-out of the national vaccination programme in wave two (alpha [B.1.1.7]-dominant), the risk reductions were broadly evenly distributed across population subgroups. In wave three, larger decreases in COVID-19-related mortality risk were seen in the subgroups prioritised for vaccination, except for in the prioritised subgroups with a lower immune response. In wave four, in a population who had mostly been vaccinated, risk reductions were again broadly evenly distributed across most population subgroups, but were smaller in subgroups with lower vaccination coverage or an impaired immune response.
Implications of all the available evidence
Improvements in the management of COVID-19 led to a reduction in population-level COVID-19-related mortality risks over subsequent waves of the pandemic. These improvements, however, have not resolved the social and clinical inequalities in the COVID-19 mortality burden, and inequalities have worsened for groups of people with low vaccination coverage or with conditions associated with a low immune response. Our findings provide an evidence base to inform UK public health policy to protect these vulnerable population subgroups.
Introduction
COVID-19 has been shown to disproportionately affect various subgroups of the population depending on their demographic and clinical profile.1, 2, 3, 4, 5 Previous studies in the UK have reported higher COVID-19-related death rates in people with older age,6, 7 male sex,8 minority ethnicities,9, 10 public-facing occupations (eg, social care workers),11, 12 households of multigenerational living,13 social deprivation,14, 15 learning disabilities,16, 17 and clinical comorbidities, such as obesity,18 kidney disease,19, 20, 21 immunodeficiency,22, 23 chronic obstructive pulmonary disease,24 and cancer.25
Much of what is known regarding population subgroups at greater risk of COVID-19-related death is based on the early stages of the pandemic. During the third pandemic wave in the UK, which was dominated by the delta (B.1.617.2) variant and lasted until mid-December, 2021, overall monthly COVID-19-related death rates were considerably lower than in the first and second pandemic waves.26 This reduction might be attributed in part to the widespread implementation of COVID-19 vaccines among groups at high risk of severe outcomes,27 as well as improvements in clinical management (eg, timely administration of antivirals28 or corticosteroids29 and non-pharmacological interventions,30 such as physical distancing and shielding). Subsequently, a reduction in the risk of COVID-19-related death after infection was seen during the omicron (B.1.1.529)-dominant period (omicron became the dominant variant in the UK from mid-December, 2021) compared with during the delta-dominant period.31, 32
Despite these improvements, inequalities in COVID-19-related mortality burden persist between clinical and demographic population subgroups. For example, in a study of post-immunisation infection among people who had completed a two-dose COVID-19 vaccination series, population subgroups who had higher rates of COVID-19-related death—including people with kidney disease and malignancies—had similar death rates to those seen at the start of the pandemic.33
We aimed to describe the burden of COVID-19-related mortality in population subgroups and how this has changed over successive waves of the pandemic, and report trends in absolute and relative COVID-19-related mortality risks across clinical and demographic population subgroups from 2020 to 2022.
Methods
Study design and participants
We did a retrospective cohort study in England using the OpenSAFELY platform to access primary care records managed by the general practice software provider TPP.
In March, 2020, the Secretary of State for Health and Social Care used powers under the UK Health Service (Control of Patient Information) Regulations 2002 to require organisations to process confidential patient information for the purposes of protecting public health, providing health-care services to the public, and monitoring and managing the COVID-19 outbreak and incidents of exposure; therefore, the requirement for informed consent was waived.34 This was extended in November, 2022, for the National Health Service (NHS) England OpenSAFELY COVID-19 research platform.35
From March 23, 2020, to Aug 3, 2022, five cohorts were extracted covering the first five SARS-CoV-2 pandemic waves in England (wave one [wild-type] from March 23 to May 30, 2020; wave two [alpha (B.1.1.7)] from Sept 7, 2020, to April 24, 2021; wave three [delta] from May 28 to Dec 14, 2021; wave four [omicron] from Dec 15, 2021, to April 29, 2022; and wave five [omicron] from June 24 to Aug 3, 2022).36 Details on the determination of these dates are provided in the appendix (p 2). In each of the five cohorts, we included people aged 18–110 years who were registered with a TPP practice on the first day of the wave and who had at least 3 months of continuous general practice registration up to this date, to ensure that baseline data could be adequately captured. We excluded people who had missing data on sex or demographics (including the Sustainability and Transformation Partnership region [an NHS administrative region] or index of multiple deprivation).
This study was approved by the Health Research Authority (20/LO/0651) and by the London School of Hygiene & Tropical Medicine Ethics Board (21863).
Procedures
The outcome of interest was COVID-19-related deaths, based on linked death registry records from the Office for National Statistics. COVID-19-related deaths were defined as having an underlying or contributory cause of death listed as COVID-19 (International Classification of Diseases 10th Revision codes U07.1 or U07.2).
Participants were categorised into subgroups for analysis, which included by demographics, health conditions listed in UK guidance on groups at higher risk of severe COVID-19,37 other common conditions that might cause immunodeficiency (inherently or through medication), and other postulated risk factors for severe outcomes. A detailed description of the subgroups for analysis is provided in the appendix (p 2).
The number of COVID-19 vaccine doses received from wave two onwards was also reported in the full population and within subgroups. Because the number of doses changed over time within each wave, we reported the total person-time spent with each number of doses. Vaccination dose status was categorised as none to three doses in wave two; none to four doses in wave three; and none to five doses in wave four and wave five. People who received more than the maximum number of vaccination doses in a specific wave were categorised as having received the maximum number. A 2-week lag was used before updating vaccination dose status, to reflect delayed-onset immunity after vaccination.
Information on all covariates was obtained from primary care records by searching TPP SystmOne records for specific coded data. Missing data were expected for BMI, smoking, and ethnicity. No missing data were expected in comorbidities because these were coded as present or absent. In our analysis, people with missing ethnicity data were coded as unknown; those with missing BMI records were coded as no obesity; and those with missing smoking information were coded as non-smokers, on the assumption that both obesity and smoking would be likely to be recorded if present. People with no serum creatinine measurement and not coded as having renal replacement therapy were included in the group with no chronic kidney disease or renal replacement therapy. People with diabetes but no glycated haemoglobin (HbA1c) measurement were included in a separate category of diabetes without recent HbA1c measurement. These assumptions have previously been tested in sensitivity analyses.1
Statistical analysis
Crude COVID-19-related death rates were calculated for the overall population and then stratified by subgroup for the five pandemic waves. To account for age and sex differences between subgroups, death rates were directly standardised for age and sex to the European Standard Population using 5-year age bands, except for rates of death by age group (not standardised) and rates of death by sex (standardised for age only). 95% CIs were obtained taking the normal approximation to the binomial distribution.38 To capture changes in absolute COVID-19-related death rates over time, fold-changes in death rates (defined as the ratio between two death rates) were calculated for waves two, three, four, and five compared with wave one.
For each of the five waves and each of the clinical and demographic covariates of interest, a Cox proportional hazards model was fitted. Models were adjusted for age using restricted cubic splines with four knots, except for the estimation of relative hazard of death by age group; adjusted for sex, except for the estimation of relative hazard of death by sex; and stratified by Sustainability and Transformation Partnership region to account for regional differences in infection rates. Follow-up began on the first date of the wave and ended at the earliest occurrence of COVID-19-related death, death by other causes, or the end date of the wave. For clinical conditions, people without the clinical condition were the reference group. For the variables with more than two categories, we used reference values of 50–59 years for age, 5 (least deprived) for index of multiple deprivation, and never or unknown for smoking. Proportional hazards assumptions were assessed by testing for a zero slope in the scaled Schoenfeld residuals and graphical methods. To capture changes in the relative risk of COVID-19-related death over time, fold-changes in the relative hazard (defined as the ratio between relative hazards) were calculated for waves two, three, four, and five compared with wave one.
Data management was performed using Python (version 3.8), and analyses were done using R (version 4.0).
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
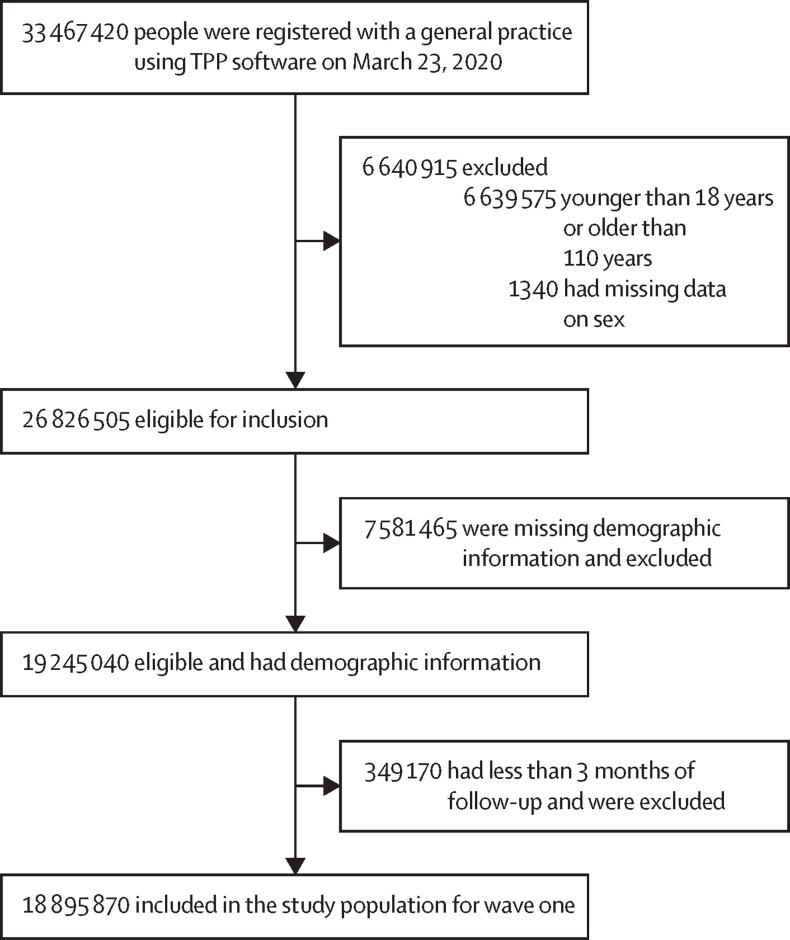
18 895 870 adults were included in wave one, 19 014 720 in wave two, 18 932 050 in wave three, 19 097 970 in wave four, and 19 226 475 in wave five (figure 1 , table ; appendix pp 3–4). In the final included study population, 5 096 200 (27·0%) people in wave one, 5 435 225 (28·6%) in wave two, 5 925 225 (31·3%) in wave three, 6 426 430 (33·6%) in wave four, and 6 922 705 (36·0%) in wave five had missing data on BMI; 805 205 (4·3%), 867 175 (4·6%), 918 670 (4·9%), 989 355 (5·2%), and 1 054 240 (5·5%) had missing data on smoking status; and 1 510 040 (8·0%), 1 533 890 (8·1%), 1 536 035 (8·1%), 1 573 330 (8·2%), and 1 611 940 (8·4%) had missing data on ethnicity. COVID-19-related death was recorded in linked registration data for 15 725 people in the study population in wave one, 31 975 in wave two, 6635 in wave three, 7110 in wave four, and 1420 in wave five. Follow-up time in the overall population was 3 512 400 person-years in wave one, 11 875 400 in wave two, 10 337 200 in wave three, 7 043 600 in wave four, and 2 101 400 in wave five. The percentage of people in the various population subgroups stayed approximately stable over time (table), except for some minor differences.
Figure 1.
Participant selection for wave one of the SARS-CoV-2 pandemic
All counts were rounded to the nearest five.
Table.
Participant characteristics in the first five SARS-CoV-2 pandemic waves in England
| Wave one (n=18 895 870) | Wave two (n=19 014 720) | Wave three (n=18 932 050) | Wave four (n=19 097 970) | Wave five (n=19 226 475) | |
|---|---|---|---|---|---|
| Age, years | |||||
| 18–39 | 6 725 965 (35·6%) | 6 742 875 (35·5%) | 6 665 485 (35·2%) | 6 711 790 (35·1%) | 6 793 445 (35·3%) |
| 40–49 | 3 076 590 (16·3%) | 3 076 590 (16·3%) | 3 076 590 (16·3%) | 3 087 230 (16·2%) | 3 087 230 (16·2%) |
| 50–59 | 3 270 775 (17·3%) | 3 270 775 (17·3%) | 3 297 225 (17·4%) | 3 320 805 (17·4%) | 3 316 370 (17·2%) |
| 60–69 | 2 555 985 (13·5%) | 2 585 615 (13·6%) | 2 606 015 (13·8%) | 2 642 395 (13·8%) | 2 674 170 (13·9%) |
| 70–79 | 2 064 500 (10·9%) | 2 091 885 (11%) | 2 110 905 (11·1%) | 2 131 140 (11·2%) | 2 144 025 (11·2%) |
| ≥80 | 1 202 055 (6·4%) | 1 208 840 (6·4%) | 1 192 170 (6·3%) | 1 204 610 (6·3%) | 1 211 820 (6·3%) |
| Sex | |||||
| Female | 9 496 635 (50·3%) | 9 548 245 (50·2%) | 9 515 160 (50·3%) | 9 563 885 (50·1%) | 9 623 880 (50·1%) |
| Male | 9 399 235 (49·7%) | 9 466 475 (49·8%) | 9 416 890 (49·7%) | 9 534 085 (49·9%) | 9 602 595 (49·9%) |
| Ethnicity | |||||
| White | 15 058 520 (79·7%) | 15 108 180 (79·5%) | 14 989 530 (79·2%) | 15 047 885 (78·8%) | 15 046 150 (78·3%) |
| Mixed | 227 745 (1·2%) | 232 780 (1·2%) | 236 810 (1·3%) | 243 975 (1·3%) | 251 780 (1·3%) |
| South Asian | 1 244 045 (6·6%) | 1 269 475 (6·7%) | 1 287 085 (6·8%) | 1 321 685 (6·9%) | 1 365 515 (7·1%) |
| Black | 422 975 (2·2%) | 431 055 (2·3%) | 437 195 (2·3%) | 447 510 (2·3%) | 463 075 (2·4%) |
| Other | 432 545 (2·3%) | 439 345 (2·3%) | 445 390 (2·4%) | 463 590 (2·4%) | 488 015 (2·5%) |
| Unknown | 1 510 040 (8·0%) | 1 533 890 (8·1%) | 1 536 035 (8·1%) | 1 573 330 (8·2%) | 1 611 940 (8·4%) |
| Region | |||||
| North East | 899 915 (4·8%) | 903 135 (4·7%) | 897 645 (4·7%) | 901 265 (4·7%) | 906 215 (4·7%) |
| North West | 1 648 900 (8·7%) | 1 653 580 (8·7%) | 1 647 735 (8·7%) | 1 655 970 (8·7%) | 1 664 080 (8·7%) |
| Yorkshire and the Humber | 2 668 670 (14·1%) | 2 689 445 (14·1%) | 2 681 755 (14·2%) | 2 697 500 (14·1%) | 2 718 855 (14·1%) |
| East Midlands | 3 296 615 (17·4%) | 3 311 015 (17·4%) | 3 290 915 (17·4%) | 3 314 045 (17·4%) | 3 346 630 (17·4%) |
| West Midlands | 763 055 (4·0%) | 767 420 (4·0%) | 759 560 (4·0%) | 761 300 (4·0%) | 762 285 (4·0%) |
| East of England | 4 367 135 (23·1%) | 4 400 625 (23·1%) | 4 376 130 (23·1%) | 4 400 165 (23·0%) | 4 395 375 (22·9%) |
| London | 1 315 950 (7·0%) | 1 335 490 (7·0%) | 1 336 580 (7·1%) | 1 395 025 (7·3%) | 1 431 910 (7·4%) |
| South East | 1 270 625 (6·7%) | 1 275 550 (6·7%) | 1 267 320 (6·7%) | 1 275 820 (6·7%) | 1 285 665 (6·7%) |
| South West | 2 665 005 (14·1%) | 2 678 460 (14·1%) | 2 674 410 (14·1%) | 2 696 880 (14·1%) | 2 715 455 (14·1%) |
| Index of Multiple Deprivation quintile | |||||
| 5 (least deprived) | 3 548 820 (18·8%) | 3 570 255 (18·8%) | 3 555 735 (18·8%) | 3 577 560 (18·7%) | 3 602 985 (18·7%) |
| 4 | 3 908 825 (20·7%) | 3 932 355 (20·7%) | 3 918 315 (20·7%) | 3 957 260 (20·7%) | 3 984 360 (20·7%) |
| 3 | 4 028 240 (21·3%) | 4 049 630 (21·3%) | 4 031 870 (21·3%) | 4 071 765 (21·3%) | 4 099 655 (21·3%) |
| 2 | 3 758 275 (19·9%) | 3 782 190 (19·9%) | 3 764 715 (19·9%) | 3 802 715 (19·9%) | 3 836 800 (20·0%) |
| 1 (most deprived) | 3 651 710 (19·3%) | 3 680 290 (19·4%) | 3 661 410 (19·3%) | 3 688 670 (19·3%) | 3 702 670 (19·3%) |
| BMI | |||||
| No obesity | 14 769 835 (78·2%) | 14 929 790 (78·5%) | 14 971 695 (79·1%) | 15 214 405 (79·7%) | 15 439 985 (80·3%) |
| Obesity class I (30·0–34·9 kg/m2) | 2 480 740 (13·1%) | 2 451 220 (12·9%) | 2 367 555 (12·5%) | 2 316 960 (12·1%) | 2 253 460 (11·7%) |
| Obesity class II (35·0–39·9 kg/m2) | 1 008 565 (5·3%) | 1 000 385 (5·3%) | 973 600 (5·1%) | 956 220 (5·0%) | 934 105 (4·9%) |
| Obesity class III (≥40·0 kg/m2) | 636 730 (3·4%) | 633 325 (3·3%) | 619 200 (3·3%) | 610 390 (3·2%) | 598 925 (3·1%) |
| Smoking status | |||||
| Never or unknown | 9 490 920 (50·2%) | 9 595 080 (50·5%) | 9 587 155 (50·6%) | 9 709 585 (50·8%) | 9 848 195 (51·2%) |
| Previous smoking | 6 184 190 (32·7%) | 6 208 280 (32·6%) | 6 210 865 (32·8%) | 6 263 090 (32·8%) | 6 299 560 (32·8%) |
| Current smoking | 3 220 760 (17%) | 3 211 360 (16·9%) | 3 134 030 (16·6%) | 3 125 295 (16·4%) | 3 078 720 (16%) |
| Asthma | |||||
| No asthma | 15 814 235 (83·7%) | 15 912 575 (83·7%) | 15 846 620 (83·7%) | 16 006 850 (83·8%) | 16 131 825 (83·9%) |
| Asthma with no oral steroid use | 2 757 105 (14·6%) | 2 806 845 (14·8%) | 2 889 425 (15·3%) | 2 840 335 (14·9%) | 2 806 590 (14·6%) |
| Asthma with oral steroid use | 324 530 (1·7%) | 295 300 (1·6%) | 196 000 (1·0%) | 250 785 (1·3%) | 288 060 (1·5%) |
| Diabetes | |||||
| No diabetes | 16 987 330 (89·9%) | 17 091 580 (89·9%) | 16 935 210 (89·5%) | 17 028 460 (89·2%) | 17 074 535 (88·8%) |
| Controlled diabetes | 1 617 210 (8·6%) | 1 536 745 (8·1%) | 1 523 125 (8·0%) | 1 682 005 (8·8%) | 1 797 890 (9·4%) |
| Diabetes not controlled | 80 390 (0·4%) | 76 995 (0·4%) | 75 170 (0·4%) | 72 350 (0·4%) | 70 985 (0·4%) |
| No recent HbA1c measure | 210 940 (1·1%) | 309 400 (1·6%) | 398 540 (2·1%) | 315 155 (1·7%) | 283 065 (1·5%) |
| CKD or RRT | |||||
| None | 17 870 605 (94·6%) | 18 010 570 (94·7%) | 17 925 880 (94·7%) | 18 083 705 (94·7%) | 18 200 150 (94·7%) |
| CKD stage 3a | 680 330 (3·6%) | 665 920 (3·5%) | 667 050 (3·5%) | 670 045 (3·5%) | 680 175 (3·5%) |
| CKD stage 3b | 251 460 (1·3%) | 245 825 (1·3%) | 246 490 (1·3%) | 249 595 (1·3%) | 250 295 (1·3%) |
| CKD stage 4 | 60 595 (0·3%) | 59 490 (0·3%) | 59 510 (0·3%) | 61 195 (0·3%) | 61 990 (0·3%) |
| CKD stage 5 | 6615 (<0·1%) | 6600 (<0·1%) | 6660 (<0·1%) | 6840 (<0·1%) | 7165 (<0·1%) |
| RRT (dialysis) | 13 645 (0·1%) | 13 735 (0·1%) | 13 875 (0·1%) | 14 050 (0·1%) | 14 140 (0·1%) |
| RRT (transplant) | 12 615 (0·1%) | 12 580 (0·1%) | 12 585 (0·1%) | 12 540 (0·1%) | 12 560 (0·1%) |
| Organ transplant | |||||
| No transplant | 18 875 550 (99·9%) | 18 994 325 (99·9%) | 18 911 585 (99·9%) | 19 077 500 (99·9%) | 19 205 935 (99·9%) |
| Kidney transplant | 15 005 (0·1%) | 15 055 (0·1%) | 15 105 (0·1%) | 15 090 (0·1%) | 15 140 (0·1%) |
| Other organ transplant | 5315 (<0·1%) | 5345 (<0·1%) | 5360 (<0·1%) | 5380 (<0·1%) | 5400 (<0·1%) |
| Comorbidities | |||||
| Hypertension | 6 369 885 (33·7%) | 6 347 260 (33·4%) | 6 341 955 (33·5%) | 6 350 220 (33·3%) | 6 377 980 (33·2%) |
| Chronic respiratory disease | 755 655 (4·0%) | 747 180 (3·9%) | 742 030 (3·9%) | 742 950 (3·9%) | 743 560 (3·9%) |
| Chronic cardiac disease | 1 251 760 (6·6%) | 1 246 395 (6·6%) | 1 245 520 (6·6%) | 1 253 705 (6·6%) | 1 260 525 (6·6%) |
| Cancer (non-haematological) | 923 890 (4·9%) | 924 990 (4·9%) | 936 405 (4·9%) | 950 925 (5·0%) | 963 080 (5·0%) |
| Haematological malignancy | 108 355 (0·6%) | 108 935 (0·6%) | 110 125 (0·6%) | 111 510 (0·6%) | 112 435 (0·6%) |
| Chronic liver disease | 110 095 (0·6%) | 111 515 (0·6%) | 114 775 (0·6%) | 118 085 (0·6%) | 120 795 (0·6%) |
| Stroke | 396 830 (2·1%) | 395 445 (2·1%) | 397 485 (2·1%) | 403 730 (2·1%) | 409 110 (2·1%) |
| Dementia | 47 085 (0·2%) | 43 090 (0·2%) | 40 995 (0·2%) | 40 530 (0·2%) | 39 485 (0·2%) |
| Other neurological disease | 186 335 (1·0%) | 184 530 (1·0%) | 183 535 (1·0%) | 184 715 (1·0%) | 185 405 (1·0%) |
| Asplenia | 30 255 (0·2%) | 30 235 (0·2%) | 30 090 (0·2%) | 30 190 (0·2%) | 30 380 (0·2%) |
| Rheumatoid arthritis, lupus, or psoriasis | 949 935 (5·0%) | 952 865 (5·0%) | 953 495 (5·0%) | 958 965 (5·0%) | 964 710 (5·0%) |
| Immunosuppressive condition | 234 890 (1·2%) | 237 140 (1·2%) | 241 915 (1·3%) | 249 740 (1·3%) | 256 495 (1·3%) |
| Learning disability | 106 710 (0·6%) | 108 145 (0·6%) | 111 890 (0·6%) | 113 950 (0·6%) | 115 850 (0·6%) |
| Severe mental illness | 224 545 (1·2%) | 226 300 (1·2%) | 229 040 (1·2%) | 231 275 (1·2%) | 232 270 (1·2%) |
Data are n (%). All counts were rounded to the nearest five and might therefore not sum to the total; percentages were rounded to the first decimal place. CKD=chronic kidney disease. RRT=renal replacement therapy.
The crude COVID-19-related death rates in the overall population per 1000 person-years decreased from 4·48 deaths (95% CI 4·41–4·55) in wave one to 2·69 (2·66–2·72) in wave two, 0·64 (0·63–0·66) in wave three, 1·01 (0·99–1·03) in wave four, and 0·67 (0·64–0·71) in wave five. Similar trends in COVID-19-related death rates over time were seen in the population subgroups (appendix pp 5–9). Crude COVID-19-related death rates decreased in all population subgroups from wave one to wave two and then further decreased in wave three, except in people who had received an organ transplant (eg, in people who had received a kidney transplant, the crude death rate per 1000 person-years was 15·58 deaths [95% CI 13·05–18·12] in wave two and 15·91 [13·17–18·64] in wave three). In most population subgroups, the crude COVID-19-related death rate increased in wave four (omicron-dominant) compared with wave three (delta-dominant) but then decreased again in wave five (a pattern that was pronounced in some subgroups, such as in people aged 80 years or older, where the crude death rate per 1000 person-years of 4·54 deaths [95% CI 4·38–4·71] in wave three increased to 10·01 [9·71–10·30] in wave four and decreased to 6·7 [6·26–7·14] in wave five).
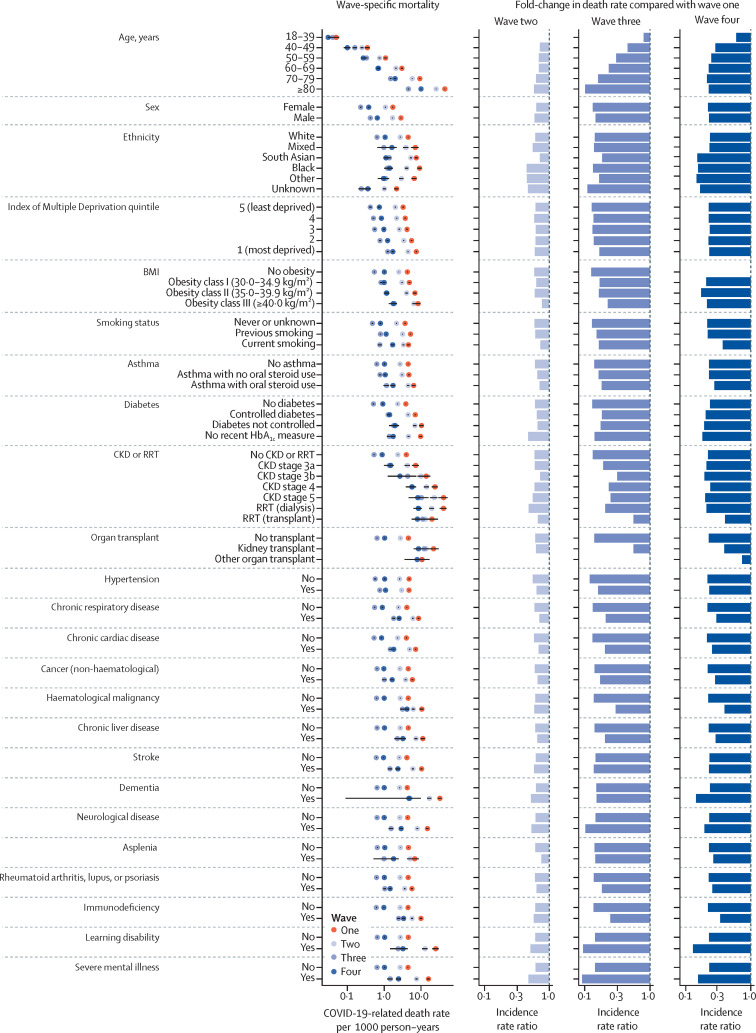
The sex-standardised and age-standardised COVID-19-related death rates in the overall population per 1000 person-years decreased from 4·56 deaths in wave one (95% CI 4·49–4·64) to 2·77 (2·73–2·80) in wave two, 0·65 (0·64–0·67) in wave three, 1·04 (1·01–1·06) in wave four, and 0·69 (0·65–0·72) in wave five, representing an overall fold-change in death rate of 0·61 for wave two versus wave one, 0·14 for wave three versus wave one, 0·23 for wave four versus wave one, and 0·15 for wave five versus wave one. Across population subgroups, the standardised COVID-19-related death rates were consistently lower in waves two, three, four, and five compared with wave one (figure 2 ; appendix pp 4, 10–13).
Figure 2.
Sex-standardised and age-standardised COVID-19-related death rates per 1000 person-years in OpenSAFELY-TPP in the first four pandemic waves by population subgroup
Models were standardised for age and sex using the European standard population, except for the death rates by age group (not standardised) and death rates by sex (standardised by age). The three columns on the right present the fold-changes in death rate in each wave compared with wave one. Equivalent data relating to wave five are shown in the appendix (pp 11–13). CKD=chronic kidney disease. RRT=renal replacement therapy.
In wave one, the sex-standardised and age-standardised COVID-19-related death rates were highest in people aged 80 years or older, people with chronic kidney disease stage 5 or 4, people receiving dialysis, people with dementia or learning disabilities, and people who had received a kidney transplant (ranging from 19·85 to 44·41 deaths per 1000 person-years compared with from 0·05 to 15·93 deaths per 1000 person-years in other subgroups; figure 2). In wave two versus wave one, the decrease in standardised COVID-19-related death rates was broadly evenly distributed across population subgroups (fold-change ranging from 0·45 to 0·77 for 69 of 71 subgroups), except for in people who had received non-kidney organ transplants and people aged 18–39 years, who had a fold-change for wave two versus wave one of 1·00. Consequently, the population subgroups who had the highest COVID-19-related death rates in wave two were consistent with those observed in wave one (with absolute death rates ranging from 12·91 to 25·75 in the mentioned highest risk groups compared with from 0·05 to 10·71 deaths per 1000 person-years in other subgroups).
More variation in the decline in standardised COVID-19-related death rates across population subgroups was seen in wave three than in wave one (fold-changes ranging from 0·09 to 1·00; figure 2). The largest decreases in death rates in wave three compared with wave one were in groups of older age and people with neurological disease, learning disabilities, or severe mental illness (fold-changes ranging from 0·09 to 0·10). Conversely, decreases in COVID-19-related death rates were substantially attenuated in younger age groups, people who had received organ transplants, and people with chronic kidney disease, haematological malignancies, or immunosuppressive conditions (fold-changes ranging from 0·75 to 1·00).
In general, less variation in standardised COVID-19-related death rates between subgroups was seen when comparing wave four with wave one (fold-changes from 0·15 to 0·30 for 61 of 71 clinical and demographic subgroups). The largest decreases in death rates were seen in people with learning disabilities, dementia, or severe mental illness, and Black, south Asian, or other ethnicities (fold-changes ranging from 0·13 to 0·15). The decrease in COVID-19-related death rates was attenuated in people who had received a kidney transplant, people with haematological malignancies or immunosuppressive conditions, and current smokers (fold-changes ranging from 0·34 to 0·40) and highly attenuated in people aged 18–39 years (fold-change 0·60 vs 0·21–0·29 in older age groups) and people who had received a non-kidney organ transplant (fold-change of 0·74). Fold-changes in wave five compared with wave one were similar to those for wave four versus wave one (appendix pp 11–13).
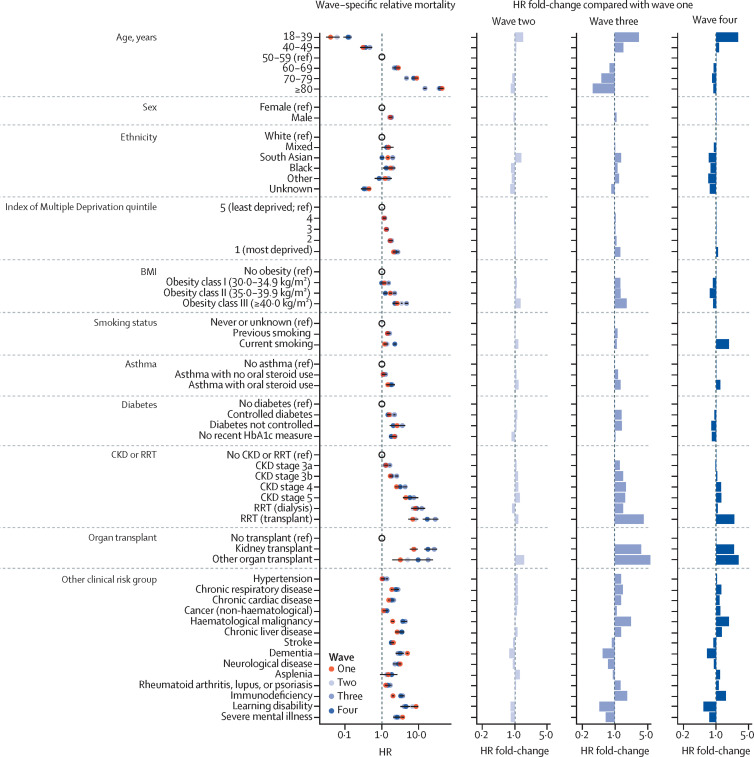
Patterns in subgroup-specific sex and age adjusted relative hazards of COVID-19-related death (stratified by Sustainability and Transformation Partnership region) were consistent throughout the five pandemic waves (figure 3 , appendix pp 14–16). In wave one, the relative hazard of COVID-19-related death was increased in groups of people aged 60 years or older versus people aged 50–59 years, males versus females, people with non-White ethnicity versus people with White ethnicity, people living in deprived areas versus people living in the least deprived areas, and people with various clinical comorbidities versus people without the comorbidity. Although the direction of these associations was consistent across successive waves, the scale differed. We quantified this change by measuring the ratio of hazard ratios (HRs) relative to those observed in wave one.
Figure 3.
Relative hazard of COVID-19-related death in OpenSAFELY-TPP in the first four pandemic waves by population subgroup
Models were adjusted for age using a four-knot cubic spline, except for estimation of relative hazard of death by age group; adjusted for sex, except for estimation of relative hazard of death by sex; and stratified by region. The three columns on the right present the fold-change in HR for COVID-19-related death in each wave compared with wave one. Equivalent data relating to wave five are shown in the appendix (pp 14–16). CKD=chronic kidney disease. HR=hazard ratio. RRT=renal replacement therapy.
Specifically, the fold-changes in HR for COVID-19-related death were relatively small in wave two compared with wave one (HR fold-changes ranging from 0·75 to 1·58; figure 3). In wave three, HRs relative to wave one increased in all subgroups (HR fold-changes ranging from 1·03 to 5·71), except for in people aged 60 years or older and those with unknown ethnicity, dementia, other neurological disease, learning disability, severe mental illness, stroke, or asplenia (HR fold-changes ranging from 0·34 to 0·91). The largest decreases in HR were seen in people aged 80 years or older (HR fold-change relative to wave one of 0·34) and in people with learning disability (HR fold-change relative to wave one of 0·47). By contrast, the largest increases in HR were seen in people aged 18–39 years (HR fold-change relative to wave one of 3·30) and in people who had received an organ transplant (HR fold-change relative to wave one of 3·60 in people who had received a kidney transplant and 5·70 in people who had received other organ transplants). For people with non-White ethnicity, there was a modest increase in HR for wave three compared with wave one (HR fold-changes ranging from 1·03 to 1·23).
In wave four, fold-changes in COVID-19-related death HR relative to wave one for clinical comorbidities were generally consistent with those observed in wave three (HR fold-changes ranging from 0·74 to 2·22 in wave three compared with from 0·53 to 1·89 in wave four), with the largest increases again observed in people who had received organ transplants (HR fold-changes relative to wave one of 2·44–3·05). The fold-change in HR in different age groups in wave four compared with wave one ranged from 0·97 to 1·07, except for in people aged 18–39 years, who retained the increased HR observed in wave three (HR fold-change relative to wave one of 3·00). By contrast to the pattern observed in wave three, for groups with non-White ethnicity, there was a modest decrease in HR for wave four compared with wave one (HR fold-change of 0·68–0·90). Fold-changes in wave five compared with wave one were similar to those seen for wave four compared with wave one (appendix pp 14–16).
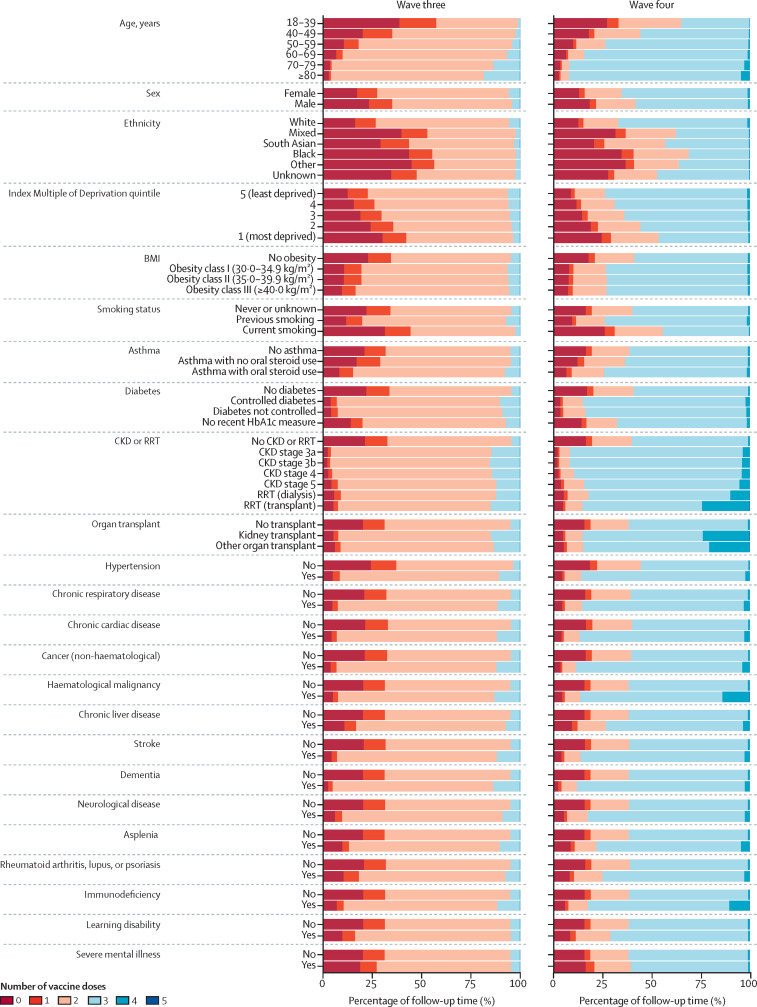
In wave two, the percentage of follow-up time with two or more COVID-19 vaccine doses was 1% in the overall population, but was much higher in people aged 80 years or older, at 8% (appendix pp 17–23). In wave three, two-dose vaccination coverage was higher in people with conditions that were risk factors for severe COVID-19 who were prioritised for vaccination, except for people with severe mental illness. Two-dose vaccination coverage was lower in people with non-White ethnicity, people living in socially deprived areas (based on index of multiple deprivation quintile), and in current smokers (figure 4 , appendix pp 17–23). For example, 96% of follow-up time in people aged 80 years or older was with two or more vaccine doses compared with 42% in people aged 18–39 years, while 77% of follow-up time among people living in the least deprived areas was with two or more vaccine doses compared with 58% in people living in the most deprived areas. Similar patterns were seen in three-dose coverage in wave four and wave five (figure 4, appendix pp 17–23).
Figure 4.
Percentage of follow-up time with number of COVID-19 vaccine doses in waves three and four by population subgroup
Equivalent data for waves two to five are shown in the appendix (p 17). CKD=chronic kidney disease. RRT=renal replacement therapy.
Discussion
In this observational, retrospective cohort study in England that included about 19 million adults per wave in the first five sequential SARS-CoV-2 pandemic waves from 2020 to 2022, we provided a comprehensive description of COVID-19-related mortality in various demographical and clinical subgroups. The relative hazard of COVID-19-related death remained increased through successive pandemic waves in people aged 60 years or older versus people aged 50–59 years, males versus females, people with non-White ethnicity versus people with White ethnicity, people living in deprived areas versus people living in the least deprived areas, and people with various clinical comorbidities versus people without the comorbidity. For ethnic minorities, there was a decrease in relative hazard in waves four and five. For conditions such as kidney disease, people with greater severity disease were at greater risk of COVID-19-related death. Overall, COVID-19-related death rates were consistently lower in waves two, three, four, and five compared with wave one. Comparing wave two with wave one, the decrease in risk of COVID-19-related death was evenly distributed across population subgroups. For wave three compared with wave one, larger decreases in COVID-19-related death rates were seen in groups with older age and people with neurological disease, learning disability, or severe mental illness. Conversely, smaller decreases in COVID-19-related death rates were observed in younger age groups, people who had received organ transplants, and people with chronic kidney disease, haematological malignancies, or immunosuppressive conditions. Consequently, relative hazards of COVID-19-related death in people with these clinical conditions versus people without the clinical condition increased by two to six times in wave three compared with wave one. Omicron was associated with increased transmissibility compared with the delta variant, which was reflected by an increase in population-level COVID-19-related death rates in wave four compared with wave three. Even though a reduction in the risk of COVID-19-related death after infection was seen in the omicron-dominant period compared with the delta-dominant period,31, 32 the incidence of infection was high in wave four, resulting in a higher population-level COVID-19-related mortality than in wave three. For wave four versus wave one, an increase in relative risk of COVID-19-related death was observed in groups with lower vaccination coverage (eg, relative hazard three times higher in people aged 18–39 in wave four compared with wave one) and with conditions associated with immunosuppression (eg, relative hazard three times higher in people who had received non-kidney organ transplants in wave four compared with wave one).
Many of the observed changes in COVID-19-related mortality in population subgroups are likely to reflect the growing vaccination coverage across successive waves. The COVID-19 vaccination programme was introduced in England on Dec 8, 2020, midway through wave two, with the oldest and most vulnerable groups in the population being highest priority. The absolute COVID-19-related mortality rate fell by 90% in wave three compared with wave one among people aged 80 years or older, who were eligible for vaccination at the outset of the vaccination programme, whereas the corresponding decline was 20% in people aged 18–39 years, among whom only the most vulnerable people were eligible for vaccination during the early stages of the vaccine roll-out.39, 40 Notably, people with severe mental illness had a larger reduction in COVID-19-related mortality rate than those without severe mental illness, which was not associated with corresponding discrepancies in vaccine uptake. Although high vaccination uptake was seen in people who had received organ transplants, COVID-19-related death rates in this population subgroup stayed relatively constant over the successive waves, and as larger decreases were seen among people who had not received transplants, the relative risk of COVID-19-related death rose in people who had received transplants across successive waves. Similar patterns in relative COVID-19-related mortality were seen in other subgroups known to have an impaired vaccine response,41, 42 including people with chronic kidney disease (including those receiving dialysis treatment), people with haematological malignancies, and people with immunosuppression. Overall, the relatively low vaccination uptake in young people, people living in socially deprived areas, current smokers, and people with non-White ethnicity was reflected in their COVID-19-related mortality risks. However, decreases in relative risk were seen in wave four compared with wave one among people with non-White ethnicity (when compared with the reference subgroup of people with White ethnicity) despite lower vaccination uptake in these subgroups, possibly explained by higher immunity from previous infection in these groups.
Other mechanisms that are likely to have driven the decrease in COVID-19-related death rates are the improved clinical management of COVID-19 over time in both inpatient and outpatient settings,43, 44 non-pharmaceutical measures, such as lockdowns and physical distancing restrictions,45, 46 immunity from previous infections, and changing severity of disease after infection by the dominant circulating SARS-CoV-2 variant.
This study used large-scale, routinely collected primary care records, linked to death registry data. This allowed us to describe a substantial proportion of the population in England in the first five pandemic waves and to describe changes over time in population-level COVID-19-related mortality.
This study has several important limitations. Our study focused on clinical and demographic subgroups. Other factors, such as occupation and population density, also influence infection risk and therefore COVID-19-related mortality risk. This study only evaluated short-term COVID-19-related mortality, and morbidity associated with SARS-CoV-2 infection was not captured. We used information on death certificates to classify COVID-19-related deaths. Classification inconsistencies or errors in International Classification of Diseases codes used to identify COVID-19-related deaths might have occurred. COVID-19-related death might have been misclassified as being due to other causes, particularly early in the first pandemic wave when mass testing was not available. Conversely, non-COVID-19-related deaths might have been falsely classified as being caused by COVID-19. A study on COVID-19 phenotypes in electronic health records across 57 million patients in England showed that 9·8% of COVID-19-related deaths (as defined by death within 28 days of a COVID-19 diagnosis) had no mention of a COVID-19 diagnosis on death certificates and 6·9% of deaths had only a COVID-19 diagnosis on the death certificate but no evidence of COVID-19 elsewhere in the health records.47 Availability and access to mass testing changed across successive waves and across subgroups and, as a consequence, the likelihood of a COVID-19 diagnosis on a death certificate might have changed over time and across different subgroups and clinical settings. The observed changes in COVID-19-related death rates across successive waves in our study might therefore be influenced by inconsistencies in COVID-19 death classification. Additionally, ascertainment of clinical conditions might have been imperfect, as this relied on appropriate and timely clinical coding in the primary care record. Patients must have presented to the general practitioner, or have had information fed back properly from secondary care settings, to be coded correctly. Under-ascertainment might be more pronounced for less severe illnesses or early-stage conditions. Small declines were observed in the proportion of people with asthma and diabetes over the period of the pandemic, which might reflect a reduction in health-care utilisation as a consequence of the COVID-19 pandemic, rather than a real change in disease incidence.
This study could not disentangle the pathway from infection to disease to death because the ascertainment of infection status was not consistently reliable in routinely collected health data and underlying community infection rates, test availability and access (particularly early in the pandemic and after April 1, 2022, when free mass testing ended in the UK), health-seeking behaviours, and symptoms changed over time. We therefore did not quantify the risk of COVID-19-related death following SARS-CoV-2 infection or the risk of infection separately. The population-level COVID-19-related mortality rates studied here are affected by background infection rates. The risk of infection is influenced by contact patterns of individuals and their local infection incidence. Public health guidance and support for contact-reducing interventions (eg, shielding), as well as public perception of risk, changed across the study period, and therefore the risk of infection in people who were deemed clinically vulnerable might have varied from wave to wave. The risk of infection also changed for circulating SARS-CoV-2 variants, with the delta variant being associated with increased transmissibility compared with the alpha variant,48 and the omicron variant being more infectious than the delta variant. The Cox proportional hazard models were stratified by Sustainability and Transformation Partnership region to account for regional differences in infection rates. Our analysis did not account for differences (eg, in infection rates or patient characteristics) on a more granular level (eg, at the general practice level).
The population included in this study, although extremely large, was geographically clustered because of variation in the electronic health record system used by general practices. For example, only 17% of general practices in London use TPP software, leading to under-representation of this area in our study population. We excluded 20% of our population with missing demographic information and an additional 1% with fewer than 3 months of follow-up to ensure that baseline data were adequately captured, which potentially affected the generalisability of our study. However, the OpenSAFELY platform has been shown to be broadly representative of the English population,49 and there are no a priori reasons to expect that this geographical clustering in our data and the exclusion criteria applied would substantially affect estimates of COVID-19-related mortality over time in population subgroups.
Previous studies of COVID-19-related mortality in demographic and clinical subgroups have shown differences in risks consistent with those presented in this report, including an increased risk in groups of people with older age, male sex, greater social deprivation, minority ethnicities, and clinical conditions such as kidney disease, organ transplant, and learning disability.1, 37 Our findings highlight the extent to which death rates have changed over the course of the pandemic. Notably, during wave three, when primary vaccination using the ChAdOx1-S or BNT162b2 vaccines had been offered to groups at high risk of severe outcomes in England, death rates in older people and some clinical subgroups (eg, people with learning disability) were markedly attenuated compared with waves one and two. This decline is in line with the high effectiveness of primary vaccination against COVID-19-related death.50
An impaired response to primary COVID-19 vaccination has been reported in people with immunosuppression, including people who have received an organ transplant, people receiving dialysis, people with haematological malignancies, and people receiving immunosuppressive therapy.41, 42, 51 Notably, these studies have mostly focused on post-vaccination antibody concentrations or effectiveness against symptomatic or severe disease. In the present study, we highlight the excess COVID-19-related mortality that persists among clinical subgroups that are associated with impaired primary vaccine response. Compared with the overall population, these groups had more modest declines in absolute COVID-19-related death rates in waves three, four, and five (after widespread vaccine implementation) compared with waves one and two.
Some of the demographic inequalities in COVID-19-related mortality burden observed here are mirrored by inequality in COVID-19 vaccine coverage, including reduced uptake among people with minority ethnicities and people living in areas with greater socioeconomic deprivation. Given the efficacy of COVID-19 vaccines in reducing COVID-19-related death,50 better targeting of vaccines to these groups would seem likely to have helped to reduce their mortality burden in waves two to five. Targeted efforts to enhance vaccine uptake in populations with low vaccination coverage during ongoing and future COVID-19 booster vaccination campaigns could help mitigate these inequalities in mortality burden.
Reducing the mortality risk might be more challenging for population subgroups where vaccine efficacy might be lower, such as people with immunosuppressive conditions. Despite such subgroups being prioritised for primary vaccination and multiple booster doses, the relative COVID-19-related mortality burden rose across successive waves. An increased understanding of the mechanisms underlying impaired vaccine response might offer insight into novel approaches to enhance protection in people with immunosuppression (eg, novel vaccine formulations or adjuvants). Our findings provide an evidence base to inform UK public health policy for protecting these vulnerable population subgroups.
Despite population-level reductions in COVID-19-related mortality over successive pandemic waves, we found evidence of persistent inequalities among specific clinical and demographic population subgroups. We showed that some population subgroups remain highly vulnerable, particularly people with conditions associated with impaired immune response and people with low vaccination coverage.
Data sharing
All data were linked, stored, and analysed securely within the OpenSAFELY platform (https://opensafely.org/). The data include pseudonymised data, such as coded diagnoses, medications, and physiological parameters. No free-text data are included. All code is shared openly for review and re-use under Massachusetts Institute of Technology open licence (https://github.com/opensafely/covid_mortality_over_time). Detailed pseudonymised patient data are potentially reidentifiable and therefore not shared. We rapidly delivered the OpenSAFELY data analysis platform without previous funding to deliver timely analyses on urgent research questions in the context of the global COVID-19 health emergency; now that the platform is established, we are developing a formal process for external users to request access in collaboration with NHS England (details of this process are available on the OpenSAFELY website). Detailed information on compilation and sources for every individual code list are available at https://codelists.opensafely.org/ and the lists are available for inspection and re-use by the wider research community.
Declaration of interests
BG has received personal income from speaking and writing for lay audiences on the misuse of science and has been a non-executive director of NHS Digital. EJW has received payment for providing training for AstraZeneca (unrelated to the submitted work). All other authors declare no competing interests.
Acknowledgments
Acknowledgments
We are very grateful for all the support received from the TPP Technical Operations team throughout this work, and for generous assistance from the information governance and database teams at NHS England and NHSX. This research used data assets made available as part of the Data and Connectivity National Core Study, led by Health Data Research UK in partnership with the Office for National Statistics. This research was funded in whole, or in part, by the UK Research Institute (UKRI; MC_PC_20058) and the Wellcome Trust (222097/Z/20/Z). For the purpose of Open Access, the author has applied a CC BY public copyright licence to any author accepted manuscript version arising from this submission. In addition, the OpenSAFELY Platform is supported by grants from Medical Research Council (MR/V015757/1, MC_PC-20059, MR/W016729/1), National Institute for Health and Care Research (NIHR; NIHR135559, COV-LT2-0073), and Health Data Research UK (HDRUK2021.000, 2021.0157). BG has received research funding to the Bennett Institute from the Bennett Foundation (ongoing), Laura and John Arnold Foundation (past), NIHR (ongoing), NIHR School of Primary Care Research (past), NIHR Oxford Biomedical Research Centre (past), Mohn-Westlake Foundation (ongoing), NIHR Applied Research Collaboration Oxford and Thames Valley (ongoing), Wellcome Trust (ongoing), Good Thinking Foundation (ongoing), Health Data Research UK (past), Health Foundation (past), WHO (past), UKRI (ongoing), Asthma UK (past), British Lung Foundation (past), and Longitudinal Health and Wellbeing strand of the National Core Studies programme (ongoing). All Bennett Institute staff are supported by BG's grants on this work. JM is the recipient of a Wellcome Trust Doctoral Studentship. RME is funded by HDRUK and Medical Research Council. EJW holds grants from Medical Research Council. The views expressed are those of the authors and not necessarily those of NIHR, NHS England, UK Health Security Agency, or the Department of Health and Social Care.
Acknowledgments
Contributors
The study was conceptualised by LN, EPKP, SJWE, BG, LAT, and AJW. Data were curated by LN, PI, CEM, SCJB, GH, DE, TW, RMS, SD, ID, SM, BFCB-C, TO, CLS, LB, CB, JC, JP, FH, and SH; and formally analysed by LN, EPKP, CDA, WJH, and LF. Funding was acquired by BG. The investigation was done by LN, EPKP, CDA, WJH, and LF. LN, EPKP, WJH, SJWE, BG, LAT, and AJW contributed to the methodology. Project administration was done by LN, WJH, BM, and AJW. Resources were provided by JM, AM, BM, BG, and AJW. Software was developed by LN, EPKP, PI, CEM, SCJB, GH, DE, TW, RMS, SD, ID, SM, BFCB-C, TO, CLS, and LB. The project was supervised by WJH, BM, BG, LAT, and AJW. The study was validated by EPKP, CDA, WJH, and LF. The results were visualised by EPKP and LN. The original draft of the manuscript was written by LN. All authors were involved in draft revisions and approving the final draft for submission. All authors had full access to the aggregated data in the OpenSAFELY platform and accept responsibility for the decision to submit for publication. Bennett Institute for Applied Data Science developers and principal investigators (BG, CEM, SCJB, AJW, WJH, DE, PI, SD, GH, BBC, RMS, ID, TW, TO, SM, CLS, LB, and EJW) holding contracts with NHS England have access to the underlying OpenSAFELY pseudonymised data tables as needed to develop the OpenSAFELY tools; these tools in turn enable researchers with OpenSAFELY Data Access Agreements to write and execute code for data management and data analysis (LN, EPKP, CDA, WJH, LF, and AJW) without direct access to the underlying raw pseudonymised patient data, and to review the outputs of this code. CB, RC, JP, FH, and SH had full unrestricted access to all data underlying the study. Further details on data access and verification are given in the appendix (pp 26–27).
Supplementary Material
References
- 1.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raleigh V. The King's Fund; 2022. Deaths from Covid-19 (coronavirus)https://www.kingsfund.org.uk/publications/deaths-covid-19 [Google Scholar]
- 3.Wan TK, Huang RX, Tulu TW, et al. Identifying predictors of COVID-19 mortality using machine learning. Life (Basel) 2022;12:547. doi: 10.3390/life12040547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cummins L, Ebyarimpa I, Cheetham N, Tzortziou Brown V, Brennan K, Panovska-Griffiths J. Factors associated with COVID-19 related hospitalisation, critical care admission and mortality using linked primary and secondary care data. Influenza Other Respir Viruses. 2021;15:577–588. doi: 10.1111/irv.12864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Public Health England CHIME—COVID-19 health inequalities. https://analytics.phe.gov.uk/apps/chime/
- 6.Bonanad C, García-Blas S, Tarazona-Santabalbina F, et al. The effect of age on mortality in patients with COVID-19: a meta-analysis with 611,583 subjects. J Am Med Dir Assoc. 2020;21:915–918. doi: 10.1016/j.jamda.2020.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ho FK, Petermann-Rocha F, Gray SR, et al. Is older age associated with COVID-19 mortality in the absence of other risk factors? General population cohort study of 470,034 participants. PLoS One. 2020;15 doi: 10.1371/journal.pone.0241824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katikireddi SV, Hainey KJ, Beale S. The impact of COVID-19 on different population subgroups: ethnic, gender and age-related disadvantage. J R Coll Physicians Edinb. 2021;51:S40–S46. doi: 10.4997/JRCPE.2021.240. [DOI] [PubMed] [Google Scholar]
- 9.Siddiq S, Ahmed S, Akram I. Clinical outcomes following COVID-19 infection in ethnic minority groups in the UK: a systematic review and meta-analysis. Public Health. 2022 doi: 10.1016/j.puhe.2022.05.019. published online June 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathur R, Rentsch CT, Morton CE, et al. Ethnic differences in SARS-CoV-2 infection and COVID-19-related hospitalisation, intensive care unit admission, and death in 17 million adults in England: an observational cohort study using the OpenSAFELY platform. Lancet. 2021;397:1711–1724. doi: 10.1016/S0140-6736(21)00634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matz M, Allemani C, van Tongeren M, et al. Excess mortality among essential workers in England and Wales during the COVID-19 pandemic. J Epidemiol Community Health. 2022;76:660–666. doi: 10.1136/jech-2022-218786. [DOI] [PubMed] [Google Scholar]
- 12.Nafilyan V, Pawelek P, Ayoubkhani D, et al. Occupation and COVID-19 mortality in England: a national linked data study of 14.3 million adults. Occup Environ Med. 2022;79:433–441. doi: 10.1136/oemed-2021-107818. [DOI] [PubMed] [Google Scholar]
- 13.Wing K, Grint DJ, Mathur R, et al. Association between household composition and severe COVID-19 outcomes in older people by ethnicity: an observational cohort study using the OpenSAFELY platform. Int J Epidemiol. 2022;51:1745–1760. doi: 10.1093/ije/dyac158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foster HME, Ho FK, Mair FS, et al. The association between a lifestyle score, socioeconomic status, and COVID-19 outcomes within the UK Biobank cohort. BMC Infect Dis. 2022;22:273. doi: 10.1186/s12879-022-07132-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woodward M, Peters SAE, Harris K. Social deprivation as a risk factor for COVID-19 mortality among women and men in the UK Biobank: nature of risk and context suggests that social interventions are essential to mitigate the effects of future pandemics. J Epidemiol Community Health. 2021;75:1050–1055. doi: 10.1136/jech-2020-215810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williamson EJ, McDonald HI, Bhaskaran K, et al. Risks of COVID-19 hospital admission and death for people with learning disability: population based cohort study using the OpenSAFELY platform. BMJ. 2021;374 doi: 10.1136/bmj.n1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baksh RA, Pape SE, Smith J, Strydom A. Understanding inequalities in COVID-19 outcomes following hospital admission for people with intellectual disability compared to the general population: a matched cohort study in the UK. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2021-052482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yates T, Zaccardi F, Islam N, et al. Obesity, chronic disease, age, and in-hospital mortality in patients with COVID-19: analysis of ISARIC clinical characterisation protocol UK cohort. BMC Infect Dis. 2021;21:717. doi: 10.1186/s12879-021-06466-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Savino M, Santhakumaran S, Currie CSM, et al. Comparison of outcomes of in-centre haemodialysis patients between the 1st and 2nd COVID-19 outbreak in England, Wales, and Northern Ireland: a UK Renal Registry analysis. Nephron. 2022;146:469–480. doi: 10.1159/000523731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Savino M, Santhakumaran S, Evans KM, et al. Outcomes of patients with COVID-19 on kidney replacement therapy: a comparison among modalities in England. Clin Kidney J. 2021;14:2573–2581. doi: 10.1093/ckj/sfab160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahalingasivam V, Craik A, Tomlinson LA, et al. A systematic review of COVID-19 and kidney transplantation. Kidney Int Rep. 2021;6:24–45. doi: 10.1016/j.ekir.2020.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacKenna B, Kennedy NA, Mehrkar A, et al. Risk of severe COVID-19 outcomes associated with immune-mediated inflammatory diseases and immune-modifying therapies: a nationwide cohort study in the OpenSAFELY platform. Lancet Rheumatol. 2022;4:e490–e506. doi: 10.1016/S2665-9913(22)00098-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shields AM, Burns SO, Savic S, Richter AG. COVID-19 in patients with primary and secondary immunodeficiency: the United Kingdom experience. J Allergy Clin Immunol. 2021;147:870–875. doi: 10.1016/j.jaci.2020.12.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uruma Y, Manabe T, Fujikura Y, Iikura M, Hojo M, Kudo K. Effect of asthma, COPD, and ACO on COVID-19: a systematic review and meta-analysis. PLoS One. 2022;17 doi: 10.1371/journal.pone.0276774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang H, Han H, He T, et al. Clinical characteristics and outcomes of COVID-19-infected cancer patients: a systematic review and meta-analysis. J Natl Cancer Inst. 2021;113:371–380. doi: 10.1093/jnci/djaa168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Office for National Statistics Coronavirus (COVID-19) latest insights. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/articles/coronaviruscovid19latestinsights/deaths#deaths
- 27.Public Health England Impact of COVID-19 vaccines on mortality in England. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/977249/PHE_COVID-19_vaccine_impact_on_mortality_March.pdf
- 28.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of COVID-19—final report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sterne JAC, Murthy S, Diaz JV, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324:1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davies NG, Kucharski AJ, Eggo RM, et al. Effects of non-pharmaceutical interventions on COVID-19 cases, deaths, and demand for hospital services in the UK: a modelling study. Lancet Public Health. 2020;5:e375–e385. doi: 10.1016/S2468-2667(20)30133-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ward IL, Bermingham C, Ayoubkhani D, et al. Risk of COVID-19 related deaths for SARS-CoV-2 omicron (B.1.1.529) compared with delta (B.1.617.2): retrospective cohort study. BMJ. 2022;378 doi: 10.1136/bmj-2022-070695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nyberg T, Ferguson NM, Nash SG, et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet. 2022;399:1303–1312. doi: 10.1016/S0140-6736(22)00462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Green A, Curtis H, Hulme W, et al. Describing the population experiencing COVID-19 vaccine breakthrough following second vaccination in England: a cohort study from OpenSAFELY. BMC Med. 2022;20:243. doi: 10.1186/s12916-022-02422-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.UK Government Coronavirus (COVID-19): notification to organisations to share information. https://web.archive.org/web/20200421171727/https://www.gov.uk/government/publications/coronavirus-covid-19-notification-of-data-controllers-to-share-information
- 35.UK Government Coronavirus (COVID-19): notice under Regulation 3(4) of the Health Service (Control of Patient Information) Regulations 2002 [Withdrawn] 2022. https://www.gov.uk/government/publications/coronavirus-covid-19-notification-to-organisations-to-share-information/coronavirus-covid-19-notice-under-regulation-34-of-the-health-service-control-of-patient-information-regulations-2002
- 36.UK Government The R value and growth rate. https://www.gov.uk/guidance/the-r-value-and-growth-rate
- 37.UK National Health Service Who is at high risk from coronavirus (COVID-19) 2021. https://www.nhs.uk/conditions/coronavirus-covid-19/people-at-higher-risk/who-is-at-high-risk-from-coronavirus/
- 38.van Belle G, Fisher LD, Heagerty PJ, Lumley TS. Biostatistics: a methodology for the health sciences. John Wiley & Sons; Hoboken, NJ: 2004. Rates and proportions; pp. 640–660. [Google Scholar]
- 39.UK Government COVID-19: the green book, chapter 14a. https://www.gov.uk/government/publications/covid-19-the-green-book-chapter-14a
- 40.Curtis HJ, Inglesby P, Morton CE, et al. Trends and clinical characteristics of COVID-19 vaccine recipients: a federated analysis of 57.9 million patients' primary care records in situ using OpenSAFELY. Br J Gen Pract. 2021;72:e51–e62. doi: 10.3399/BJGP.2021.0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee ARYB, Wong SY, Chai LYA, et al. Efficacy of COVID-19 vaccines in immunocompromised patients: systematic review and meta-analysis. BMJ. 2022;376 doi: 10.1136/bmj-2021-068632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parker EPK, Desai S, Marti M, et al. Response to additional COVID-19 vaccine doses in people who are immunocompromised: a rapid review. Lancet Glob Health. 2022;10:e326–e328. doi: 10.1016/S2214-109X(21)00593-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mouffak S, Shubbar Q, Saleh E, El-Awady R. Recent advances in management of COVID-19: a review. Biomed Pharmacother. 2021;143 doi: 10.1016/j.biopha.2021.112107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.National Institutes of Health Hospitalized adults: therapeutic management. https://www.covid19treatmentguidelines.nih.gov/management/clinical-management/hospitalized-adults-therapeutic-management/
- 45.Institute for Government Timeline of UK Government coronavirus lockdowns and restrictions. https://www.instituteforgovernment.org.uk/charts/uk-government-coronavirus-lockdowns
- 46.Talic S, Shah S, Wild H, et al. Effectiveness of public health measures in reducing the incidence of COVID-19, SARS-CoV-2 transmission, and COVID-19 mortality: systematic review and meta-analysis. BMJ. 2021;375 doi: 10.1136/bmj-2021-068302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thygesen JH, Tomlinson C, Hollings S, et al. COVID-19 trajectories among 57 million adults in England: a cohort study using electronic health records. Lancet Digit Health. 2022;4:e542–e557. doi: 10.1016/S2589-7500(22)00091-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hart WS, Miller E, Andrews NJ, et al. Generation time of the alpha and delta SARS-CoV-2 variants: an epidemiological analysis. Lancet Infect Dis. 2022;22:603–610. doi: 10.1016/S1473-3099(22)00001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Andrews C, Schultze A, Curtis H, et al. OpenSAFELY: representativeness of electronic health record platform OpenSAFELY-TPP data compared to the population of England. Wellcome Open Res. 2022;7:191. doi: 10.12688/wellcomeopenres.18010.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hulme WJ, Williamson EJ, Green ACA, et al. Comparative effectiveness of ChAdOx1 versus BNT162b2 COVID-19 vaccines in health and social care workers in England: cohort study using OpenSAFELY. BMJ. 2022;378 doi: 10.1136/bmj-2021-068946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.WHO Interim recommendations for an extended primary series with an additional vaccine dose for COVID-19 vaccination in immunocompromised persons. 2022. https://www.who.int/publications-detail-redirect/WHO-2019-nCoV-vaccines-SAGE_recommendation-immunocompromised-persons
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data were linked, stored, and analysed securely within the OpenSAFELY platform (https://opensafely.org/). The data include pseudonymised data, such as coded diagnoses, medications, and physiological parameters. No free-text data are included. All code is shared openly for review and re-use under Massachusetts Institute of Technology open licence (https://github.com/opensafely/covid_mortality_over_time). Detailed pseudonymised patient data are potentially reidentifiable and therefore not shared. We rapidly delivered the OpenSAFELY data analysis platform without previous funding to deliver timely analyses on urgent research questions in the context of the global COVID-19 health emergency; now that the platform is established, we are developing a formal process for external users to request access in collaboration with NHS England (details of this process are available on the OpenSAFELY website). Detailed information on compilation and sources for every individual code list are available at https://codelists.opensafely.org/ and the lists are available for inspection and re-use by the wider research community.