Abstract
The human body is vastly colonised by microorganisms, whose impact on health is increasingly recognised. The human genital tract hosts a diverse microbiota, and an increasing number of studies on the male genital tract microbiota suggest that bacteria have a role in male infertility and pathological conditions, such as prostate cancer. Nevertheless, this research field remains understudied. The study of bacterial colonisation of the male genital tract is highly impacted by the invasive nature of sampling and the low abundance of the microbiota. Therefore, most studies relied on the analysis of semen microbiota to describe the colonisation of the male genital tract (MGT), which was thought to be sterile. The aim of this narrative review is to present the results of studies that used next-generation sequencing (NGS) to profile the bacterial colonisation patterns of different male genital tract anatomical compartments and critically highlight their findings and their weaknesses. Moreover, we identified potential research axes that may be crucial for our understanding of the male genital tract microbiota and its impact on male infertility and pathophysiology.
Keywords: male genital tract, microbiota, bacteria, infertility, sperm, prostate
1. Genital Microbiota
Mammal colonising microbes share a long history of coevolution with their hosts. This is the case for bacteria colonising the gut of modern primates, including humans, which arose from ancient bacteria that coevolved with the common ancestors of the lineage [1]. Gut microbiota remains the most studied bacterial community in the human body [2,3]. Despite important advances in the field, we are only starting to appreciate the impact of bacteria in the digestive tract on physiology, ranging from immunological and metabolic roles to unexpected neurobehavioral implications [4]. Today we know that most parts of the human body are colonised by a plethora of bacteria, which may greatly influence the homeostasis of these particular and very diverse ecological niches [4,5,6].
The genital tract is not an exception, and an increasing number of studies exploring the role of bacteria on pregnancy, infertility, and infection are being carried out [7,8,9]. Nevertheless, the majority of studies focus on the female tract, with the vaginal microbiota being the most studied environment. In a healthy state, vaginal microbiota is dominated by members of the Lactobacillus genus, which greatly influence this environment [10]. Recently, the existence of a specific microbiota is also being recognised in the female upper genital tract, including the uterus, fallopian tubes, and ovaries [11], which were previously considered “sterile”. In contrast, MGT microbiota has been completely neglected except in recent years, with a limited number of studies addressing the composition of bacterial communities of this particular niche [12,13,14,15].
The human male reproductive system sustains the production of spermatozoa and their transfer into the female genital tract for reproductive purposes. Due to its anatomical conformation, sampling of the MGT is highly invasive. Availability of samples is therefore restricted to pathological conditions such as prostate cancer, a requirement for orchiectomy or testicular biopsies in the case of infertility. On the other side, semen does not involve major restrictions for sampling and may be used as a proxy for studying the bacterial colonisation of the entire MGT.
2. Initial Studies on Bacteria Colonising the MGT
The presence of bacteria in the MGT has been initially associated with an infective state. The most common outcomes of bacterial infections of the MGT comprise orchitis, epididymitis, prostatitis, and urethritis. The majority of these infections are caused by sexually transmitted pathogens and ascending uropathogens. Chlamydia trachomatis, the most common sexually transmitted disease, and Neisseria gonorrhoeae, are predominant in epididymo-orchitis and urethritis [16]. On the other hand, acute and chronic prostatitis are mainly caused by Escherichia coli, along with other Enterobacteriaceae (Klebsiella spp., Proteus spp., and Pseudomonas aeruginosa), Enterococcus spp., and Staphylococcus aureus [17,18].
Bacteriospermia (presence of bacteria in the semen) was therefore linked to infections of the MGT. Bacteriological analysis of semen using conventional microbiology techniques and growth media magnified the influence and importance of known pathogenic bacteria to the detriment of nonculturable bacteria. The first studies on bacteria present in semen relied mainly on classical microbiology methods. Clinical samples were inoculated on different solid media under aerobic or anaerobic conditions to isolate bacteria. Members of Staphylococcus, Enterococcus, Escherichia, and Ureaplasma genera were the most isolated bacteria from human semen [19]. In general, these studies led to the notion that the presence of bacteria in the semen was associated with a pathological condition and that the semen of normospermic men was sterile. More recently, however, the application of polymerase chain reaction (PCR)-based methods has highlighted that bacterial DNA is present in almost all semen samples, even when microbiological investigation using conventional methods revealed the absence of bacteria [20,21]. Further characterisation of bacteria colonising human semen has been performed recently using mass spectrometry-related methods (matrix-assisted laser desorption ionisation time of flight mass spectrometry, MALDI-TOF MS) [22]. Although this technique provides a quick way to identify microorganisms, the potential presence of unidentified bacterial species could be a limitation. It is, therefore, only with the advent of NGS techniques that we started to appreciate the extent of bacterial colonisation of the MGT fully.
3. Microbiota of the MGT
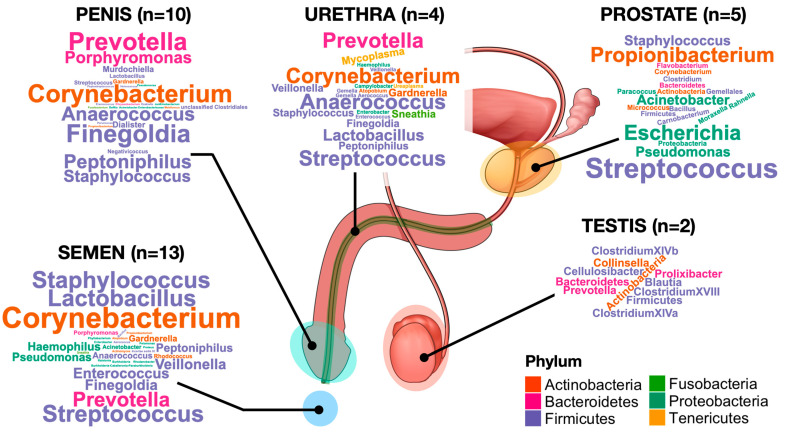
Compared to the female counterpart, metagenomic characterisation of the MGT is still in its initial phases. Except for semen and penis, collection of samples of the MGT is highly invasive and therefore difficult to perform, also from the ethical perspective. Most of the studies performed so far rely on patients with pathologies such as prostate cancer or infertility. While paramount questions remain to be answered, such as the stability of the MGT microbiota over time or possible differences due to geographical or genetic background or hormonal imbalance, the increasing number of studies suggest that it is typically a low bacterial abundance ecosystem with relatively diverse bacterial communities. Figure 1 depicts the major genera identified with NGS in the different parts of the MGT. Further efforts should be made to understand the effect of the bacterial colonisation of the MGT for several reasons, which include a possible link with male infertility issues, its role in sexually transmitted diseases and its impact on the bacterial colonisation of the female genital tract, whose role in gynaecological and obstetrical outcomes has been established.
Figure 1.
Word cloud representation of the major bacterial taxa identified in the MGT. The size of each taxon is proportional to its occurrence in all the NGS studies used to characterise the microbiota of specific parts of the MGT. The number of studies used to generate the graphs is indicated in brackets.
3.1. Testes, Epididymis and Vas Deferens
Testes ensure the maintenance of the self-renewing stem cell reserve and host spermatogenesis. Replication of spermatogonia and differentiation into spermatozoa occurs in the seminiferous tubules, which constitute most of the testis’s content. The maturation of spermatozoa takes place in the epididymis, where motility and fertilising ability are acquired and where spermatozoa are stored prior to ejaculation [23].
The presence of bacteria in the upper genital tract has been associated with active infections, viral or bacterial, with subsequent acute or chronic inflammation [24]. The main bacterial agents involved may be sexually transmitted (Chlamydia trachomatis, Neisseria gonorrhoeae, and Mycoplasma genitalium) or associated with urinary tract infections (Escherichia coli, Klebsiella pneumoniae as Staphylococcus aureus, among others) and may lead to orchitis, epididymitis, or epididymo-orchitis [25]. Nevertheless, recent studies have suggested that testes without apparent signs of infection and inflammation harbour a low abundant microbiota. In a first assessment of testicular microbiota, Alfano et al. compared bacterial colonisation of men with idiopathic nonobstructive azoospermia and normal germline maturation undergoing unilateral orchiectomy for nonmetastatic seminoma [15]. As a negative control, the authors included the PC3 cell line that was grown in the presence of antibiotics, while the buccal mucosa samples were used as a positive control. Characterisation of microbiota was carried out at the phylum and class levels and revealed that Firmicutes and Actinobacteria had the highest relative abundance. At the phylum level, men with azoospermia showed a significant increase in Actinobacteria abundance, while a decrease was observed for Proteobacteria and Bacteroidetes. A more recent study questioned the existence of a testicular microbiota, with rigorous approaches concerning possible contaminations [26]. A series of negative controls and stringent in silico elimination of possible contaminants allowed the identification of specific bacterial genera specific to the testicular milieu. More in detail, these genera include Blautia, Cellulosibacter, Clostridium XIVa, Clostridium XIVb, Clostridium XVIII, Collinsella, Prevotella, Prolixibacter, Robinsoniella, and Wandonia. Interestingly, the Prevotella genus was described as one of the major components of seminal microbiota [13,27]. The putative impact of testicular bacteria on seminal microbiota composition may be evaluated by studying semen samples from men undergoing vasectomy. This is a common procedure aiming at definitive male contraception and eliminates the participation of testis and epididymis in the composition of semen. A pilot study in this context compared the seminal microbiota of men undergoing vasectomy prior to and after the surgery [28]. The authors concluded that in both paired and unpaired semen samples, vasectomy resulted in a decrease in α-diversity. Nevertheless, the bacterial composition of the samples (β-diversity) was not significantly different between the two groups.
3.2. Accessory Glands
Most of the seminal fluid is composed of by secretions of accessory glands, which comprise the prostate, the seminal vesicles, and the bulbourethral glands. There is increasing interest in the prostate microbiota due to its potential relationship with prostate cancer [29]. Therefore, despite the invasive procedure, the availability of clinical samples is possible due to the high number of prostatectomies that are performed since prostate cancer is among the most frequently diagnosed cancers among men [30]. The prostate is one of the major accessory glands of the male reproductive tract [31]. Approximately a quarter of the ejaculate is composed of secretions produced by the prostatic epithelium [31]. While previous studies suggested the existence of a prostate microbiota [32,33], only studies relying on NGS could reveal an unbiased composition of the bacterial taxa (Table 1).
Table 1.
Characterisation of prostatic tissue microbial communities using NGS.
| Study | Sequencing Method | Sample Size | Negative Controls | Bacterial Count | Main Findings |
|---|---|---|---|---|---|
| Cavarretta et al., 2017 [34] | Pyrosequencing (V3–V5) | 16 | No | No |
|
| Yow et al., 2017 [35] | Illumina (V2–V3 and V4) | 10 | Yes | No |
|
| Feng et al., 2019 [36] | Illumina (shotgun) | 65 | No | No |
|
| Jain et al., 2020 [37] | Illumina (V3) | 20 | No | No |
|
| Wu et al., 2020 [38] | Illumina (V4) | 63 | No | No |
|
An initial analysis of the prostate microbiota of patients with an aggressive form of prostate cancer that had undergone radical prostatectomy showed that an unidentified member of the Enterobacteriaceae family was highly prevalent (37.2 to 81.2% of the total reads). The dominance of the Proteobacteria phylum was further denoted by the presence of Escherichia spp. in all samples (20.9% of the total reads). Other genera identified consistently in most of the samples comprised Actinobacteria, Pseudomonas, and Streptococcus, although at very low abundance.
Cavarretta et al. analysed the bacterial colonisation patterns of prostatic biopsies from patients undergoing radical prostatectomy [34]. They divided the samples into three categories, which included tumoral tissue, peritumoral tissue, and nontumoral tissue. Of note, DNA was extracted from samples embedded in paraffin, previously fixed in formalin. Among all samples, Actinobacteria was the dominating phylum, followed by Firmicutes and Proteobacteria. Major genera included Propionibacterium, Corynebacterium, and Staphylococcus, which comprised between 60% and 82% of all bacteria, depending on the sample group. Interestingly, Prevotella spp. and, more generally, members of the Bacteroidetes phylum were not detected, while this taxon was highly prevalent in other studies.
Colonisation by members of the Proteobacteria phylum was predominant (~50%) in samples from patients with benign prostate hyperplasia [37]. Viable bacteria could be isolated from the prostatic tissue, although the colonisation patterns did not match those determined by NGS due to the limitations of laboratory cultivation of nonconventional bacterial isolates. An analogous study revealed that Firmicutes dominate in samples of patients with benign prostate hyperplasia [39]. Evidence of bacterial colonisation of the prostate was demonstrated by performing fluorescent in situ hybridisation in accordance with a low bacterial biomass microbiota. Concomitant analysis of urine samples showed that prostate microbiota was significantly different, further suggesting the existence of a local microbiota.
While several hits suggest the presence of the prostate microbiota, additional studies are required to characterise it fully. Unfortunately, the two studies about benign prostate hyperplasia [37,39] did not present the resolution of the bacterial colonisation patterns up to the genus level, making their comparison difficult. On the other side, the major differences observed at the phylum level may reflect differences due to sample and NGS processing.
Little is known about the bacterial colonisation of the seminal vesicles and the bulbourethral glands, whose samplings remain very challenging and performed only in patients with pathologies. In a pioneer study, Lei et al. analysed bacterial colonisation profiles of samples obtained through transurethral seminal vesiculoscopy in patients with seminal vesicle complications [40]. At the phylum level, Firmicutes were the predominant taxon (52.08%), followed by Bacteroidetes (21.69%), Proteobacteria (12.72%), Actinobacteria (9.64%), and Fusobacteria (1.62%). Bacterial genera with over 5% relative abundance were Bacteroides (9.13%), Lactobacillus (5.38%), Bifidobacterium (5.35%), and Faecalibacterium (5.10%). Colonisation patterns were not significantly different between samples from patients with and without signs of infection. Nevertheless, colonisation profiles of the main bacterial genera of samples obtained from seminal vesicles did not differ significantly from urine samples obtained prior to vesiculoscopy, thus highlighting the serious risk of sample contamination during the procedure.
3.3. Colonisation of the Urethra
The urethra is the common duct for the evacuation of urine and the transition of semen throughout the penis. Several studies have analysed the bacterial content of the urethra by analysing first void urine samples [41,42,43]. Nevertheless, it is still not clear whether the observed taxa were present specifically in the urethra or are part of the bladder microbiota, which has not been fully elucidated yet [44]. Microbiota of first-catch urine and urethral swabs have been compared by Dong et al. [41]. Swabbing of the urethra is routinely performed in testing for sexually transmitted infections but creates discomfort for the patients. First-catch urine showed a similar microbiota compared to the swab samples. The most recurrent genera were Lactobacillus, Streptococcus, Sneathia, Veillonella, Corynebacterium, and Prevotella. In addition, the highest abundant genus in subjects with a confirmed sexually transmitted infection was Neisseria. Hrbacek et al. sampled first-catch urine, mid-stream urine, and aseptically catheterised urine and showed that the microbial community structure of the latter was significantly different compared to the first two [42]. This finding indirectly implies the existence of a specific urethral microbiota since the alpha diversity of first-catch and mid-stream urine samples was significantly higher. Relative abundance analyses showed that Prevotella_1, Streptococcus, and Campylobacter genera were specifically enriched in the first-catch urine samples and may represent specific taxa present in the urethra. Evidence of a specific urethral microbiota was also suggested by Nelson et al. by comparing voided urine specimens with swabs of the coronal sulcus [45].
Bacterial colonisation of the urethra has also been studied in the context of idiopathic urethritis [43]. The results suggested that microbiota is significantly different between controls and men with urethritis and that the sex of the partner also influenced the composition of the microbiota. H. influenzae was significantly increased in men with male partners, while Corynebacterium spp. Was significantly increased in men with female partners.
3.4. Penis Glans and Coronary Sulcus
The penis acts as an erectile penetrating tool during sexual intercourse and allows the introduction of semen into the vagina. It is composed of several distinct parts with different physical and immunological properties. Sampling is relatively easy to perform and does not represent particular issues for the patients. Penile skin microbiota has been analysed on several occasions [46,47,48,49] (Table 2). The results showed similar colonisation patterns, dominated by Corynebacterium and Staphylococcus genera, which are typical commensals of the skin microbiota [50,51]. Different microbiota studies have focused on the foreskin mucosa and penis glans, which are the entry sites of sexually transmitted viral diseases such as human immunodeficiency virus or human papillomavirus [12]. Liu et al. identified that dysbiosis, driven by an increase in anaerobic bacteria, augmented the risk of HIV seroconversion [52]. Additional studies have suggested that microbiota dominated by anaerobic bacteria influence the local production of inflammatory chemokines that modulate the human immune system, which is the target of HIV infection [53]. More specifically, species belonging to Peptostreptococcus, Prevotella, and Dialister genera increased cytokine production, which resulted in the attraction of HIV-susceptible CD4+ T cells to the inner foreskin and was linked to an increased risk of HIV acquisition [54].
Table 2.
Characterisation of penile microbial communities using NGS.
| Study | Sequencing Method | Sample Size | Negative Controls | Bacterial Count | Main Findings |
|---|---|---|---|---|---|
| Price et al., 2010 [55] | Pyrosequencing (V3–V4) | 12 | No | No |
|
| Nelson et al., 2012 [45] | Pyrosequencing (V1–V3, V3–V5 and V6–V9) | 18 | No |
|
|
| Liu et al., 2013 [56] | Pyrosequencing (V3–V6) | 156 | No | Yes |
|
| Liu et al., 2015 [47] | Pyrosequencing (V3–V6) | 165 | No | Yes |
|
| Liu et al., 2017 [53] | Illumina (V3–V4) | 182 | No | Yes |
|
| Mehta et al., 2020 [57] | Illumina (V3–V4) | 231 | No | No |
|
| Onywera et al., 2020 [48] | Illumina (V3–V4) | 238 | Yes | No |
|
| Plummer et al., 2021 [49] | Illumina (V3–V4) | 34 | Yes | No |
|
| Watchorn et al., 2021 [58] | Illumina (V3–V4) | 40 | No | Yes |
|
| Prodger et al., 2021 [54] | Pyrosequencing (V3–V6) | 188 | No | Yes |
|
| Mehta et al., 2020 [59] | Illumina (V3–V4) | 168 | Yes | No |
|
Penile microbiota may be drastically impacted by the circumcision procedure [55]. Increased exposition to aerobic conditions significantly reduced the abundance of putative anaerobic genera, such as Prevotella, Anaerococcus, Finegoldia, and Peptoniphilus. This was accompanied by a reduction in bacterial load and diversity, resulting in an increase in Corynebacterium spp. and Staphylococcus spp., which, as previously stated, are members of the skin microbiota. Interestingly, circumcision was also linked to a decrease in bacterial vaginosis in female partners [60]. Therefore, it has been suggested that the penile microbiota, mainly anaerobic bacteria, may trigger the activation of the immune system leading to a surge of susceptibility to sexually transmitted viral infections [61].
3.5. Semen Microbiota
Semen is a complex biological fluid that may be used as a proxy to study the colonisation of the MGT. Sampling is not invasive and it is routinely performed for the assessment of fertility status. Most of the studies are seminal microbiota focused, therefore, on male partners of infertile couples; the principal study question is the putative association of seminal bacteria with semen parameters (total spermatozoa count and concentration, total and progressive motility and morphology). Since the infertility factor may involve the female partner, such cohorts comprise fertile male partners with normal spermiogram parameters that can be used as internal controls for men with abnormal spermiogram parameters. In some cases, healthy sperm donors may be used as the control group, although this procedure may be limited due to ethical concerns. Alternatively, seminal microbiota was correlated to a pathological state, such as viral infections (human immunodeficiency virus or human papillomavirus) [52,62] or prostatitis [63]. Table 3 depicts all the NGS studies performed on human semen and summarises the major findings.
Table 3.
Characterisation of seminal microbiota using NGS.
| Study | Sequencing Method | Sample Size | Negative Controls | Bacterial Count | Main Findings |
|---|---|---|---|---|---|
| Hou et al., 2013 [64] | Pyrosequencing (V1–V2) | 77 | No | No |
|
| Weng et al., 2014 [27] | Illumina (V4) | 96 | No | No |
|
| Liu et al., 2014 [52] | Pyrosequencing (V3–V6) | 49 | No | No |
|
| Mändar et al., 2015 [65] | Illumina (V6) | 23 | No | Yes |
|
| Mändar et al., 2017 [63] | Illumina (V6) | 67 | No | No |
|
| Chen et al., 2018 [66] | Illumina (V4) | 17 | No | No |
|
| Monteiro et al., 2018 [67] | Ion Torrent (V3–V6) | 118 | No | No |
|
| Baud et al., 2019 [13] | Illumina (V1–V2) | 94 | Yes | Yes |
|
| Yang et al., 2020 [68] | Illumina (V1–V2) | 159 | No | No |
|
| Amato et al., 2020 [69] | Illumina (V3–V4) | 23 | No | Yes |
|
| Štšepetova et al., 2020 [70] | Pyrosequencing (NA) | 50 | Yes | Yes |
|
| Lundy et al., 2021 [71] | Illumina (V3–V4) | 37 | No | NA |
|
| Tuominen et al., 2021 [62] | Illumina (V3–V4) | 31 | No | No |
|
| Yao et al., 2021 [72] | Illumina (V3–V4) | 87 | No | No |
|
| Bukharin et al., 2022 [73] | Illumina (NA) | 72 | No | NA |
|
Almost one decade ago, Hou and colleagues performed the first metagenomic study on semen microbiota [64]. They concluded that bacteria could be identified in both fertile and infertile men and corroborated their NGS results with microscopy observation of semen samples processed with Gram staining. The most abundant genera included Ralstonia, Lactobacillus, Corynebacterium, Streptococcus, and Staphylococcus. Anaerococcus spp., the eighth most abundant genus, could be linked with negative sperm quality.
Subsequent studies have further characterised seminal microbiota and identified specific colonisation patterns. Weng et al. identified three seminal bacterial community types that they termed G1 (Pseudomonas-predominant group), G2 (Lactobacillus-predominant group), and G3 (Prevotella-predominant group) in a cohort of 96 patients. Lactobacillus dominance was positively associated with sperm quality, while the opposite was seen with samples in which Prevotella spp. was dominant. Moreover, the presence of G. vaginalis, previously associated with bacterial vaginosis [74], was positively correlated with sperm quality. Similarly, we have previously reported three main bacterial colonisation profiles in a similar cohort of infertile couples, in two of which Prevotella spp. and Lactobacillus spp. were the predominant taxa [13]. Again, a higher proportion of semen samples of patients with abnormal spermiogram and low motility was significantly enriched with Prevotella spp. On the other hand, an increased abundance of Lactobacillus spp. positively correlated with normal semen morphology, while the presence of Staphylococcus spp. was linked to a normal spermiogram and high total motility. This was also the first study to quantify the bacterial load in semen using a panbacterial quantitative PCR assay, which showed that most samples carried between 104 and 106 copies of 16S rRNA genes per ml of semen, thus confirming the low bacterial abundance nature of this sample. Additional studies revealed the presence of Lactobacillus, Prevotella, Staphylococcus, and Corynebacterium genera in semen [68,70,72].
Nevertheless, other studies did not show the same agreement. Monteiro and colleagues analysed a seminal microbiota of 118 samples from two fertility centres in Portugal (89 patients and 29 controls). The authors found that pathogenic bacteria (Neisseria spp., Klebsiella spp., and Pseudomonas spp.) were associated with seminal hyperviscosity and oligoasthenoteratozoospermia. They observed a high prevalence of Enterococcus spp., which represented approximately 25% of total sequences. Other relatively abundant genera included Staphylococcus, Anaerococcus, Corynebacterium, Peptoniphilus, and Propionibacterium. The overall absence of Lactobacillus spp. and Prevotella spp. in semen samples was also observed by Lundy et al., which found Gardnerella, Veilonella, Enterococcus, Streptococcus, and Anaerococcus to be the most represented genera [71]. In their analysis, increased relative abundance of Aerococcus, Rhodocytophaga, and Gemella genera was increased in infertile patients, while Colinsella spp. was associated with normospermic men.
In addition to differences in the composition of the microbiota, positive or negative associations of semen parameters with relative abundances of specific bacterial genera also showed several discordances. This was, for example, the case for Lactobacillus spp. [13,27,68] and Staphylococcus spp. [13,73], suggesting that the resolution of taxa at the genus level may not be sufficient to fully appreciate the possible impact of seminal bacteria on sperm parameters.
The different outcomes observed between studies of bacterial colonisation of semen and, more generally, of the male genital tract may be explained by multiple factors. Microbiota itself may not be stable over time, leading to different colonisation patterns being observed. Therefore, longitudinal studies should be performed to understand the dynamics of this microbiota better. Geographical variability may also be a factor, as studies have been conducted on patients from different continents. This could potentially affect the composition of the microbiota, leading to different results.
Processing of samples is another important factor that can impact the results. Sample collection methods, DNA extraction techniques, and the presence of contaminants can all affect the composition of the microbiota that is observed. Furthermore, analysis of different variable regions of the 16S rRNA genes can also impact the final results. Finally, the bioinformatic pipeline used to analyse the data can also affect the results. Different approaches to data analysis can lead to different conclusions about the composition and function of the microbiota. Ideally, there should be standardisation of the techniques used to process samples and analyse data in order to minimise variability and ensure comparability between studies. This would improve the overall quality of research in this field and increase the reliability of findings.
4. How May Genital Microbiota Impair Male Fertility?
Spermatogenesis is a constant process that generates millions of spermatozoa each day in the testes [75]. It takes approximately 30–40 days for spermatogonia to undergo mitosis, meiosis, and morphological changes that will result in highly specialised cells whose goal is to fertilise the oocyte [75]. Nevertheless, spermatozoa are not functional and require a maturation stage that takes place in the epididymis and involves progressive motility [23,76]. This is also the place where motile spermatozoa are stored until ejaculation or reabsorbed in the absence of ejaculation. It is, therefore, in testes and epididymis that spermatozoa may have a higher chance of being impacted by resident microbiota. However, as seen above, studies on testicular and epididymal microbiota are scarce and warrant further analysis. On the other hand, microbiota may indirectly impact spermatozoa physiology by changing the properties of the seminal fluid. Therefore, a potential dysbiosis in the accessory glands may change the composition of prostatic or seminal vesicle fluids, thus providing a hostile environment which would not support the function of spermatozoa.
For several decades, the presence of bacteria in semen was linked to infection status and, therefore, to a reduction in spermatozoa count and an increase in leukocytes detected in sperm [77]. The adverse effect of several pathogenic bacteria on sperm physiology has been shown in the past, including Mycoplasma genitalium, Mycoplasma hominis [78], Ureaplasma urealyticum [79], Chlamydia trachomatis [80,81], and Chlamydia-like bacteria [82]. Direct exposure to these pathogens leads to decreased spermatozoa motility [79,83] or increased apoptosis [80,84]. Moreover, direct interaction with spermatozoa could be observed and was negatively linked with spermatozoa physiology [79,82,85,86,87].
More generally, bacteria may release soluble factors, such as lipopolysaccharides (LPS) [88,89], hemolysins [90,91], and other soluble spermatotoxic factors that can affect sperm physiology [92,93]. The outcomes of bacterial infection may be multiple, comprising reduction in motility, induction of teratozoospermia (abnormal sperm morphology), apoptosis, DNA fragmentation, sperm agglutination, and exposure to oxidative stress through the formation of reactive oxygen species (ROS) [94,95]. Bacteria in seminal fluid seem to trigger a local immune reaction, usually inducing leukocytospermia and cytokine secretion and leading to inflammation [96,97,98].
Other than producing cytotoxic effectors, bacteria may significantly influence their ecological niches through their metabolic activity [99,100]. A striking example is the human vagina, whose pH is acidic due to the activity of lactobacilli in a healthy state and protects against vaginal infections [101,102]. The effect of MGT microbiota on metabolites present in semen is still unknown. Metabolomic analysis of semen samples in a 660-men Chinese cohort revealed several metabolites that may represent biomarkers for the discrimination of high-quality and low-quality semen samples [103]. For example, increased levels of carnitine and its derivatives were negatively associated with semen quality. It is well established that bacteria may interfere with this metabolite and have an impact on the homeostasis of the host [104,105]. Further analyses combining metagenomics and metabolomics may unravel the potential effects of microbiota on the composition of seminal fluid and therefore explain the impact on fertility.
5. Future Directions and Missing Gaps
The increasing number of studies of the bacterial colonisation of the MGT, along with continuous improvements in sequencing techniques, will facilitate our understanding of MGT microbiota and its potential impact on male fertility, sexual dysfunction, and MGT pathologies, such as prostate cancer. This may also bring novel insights into the field of sexually transmitted diseases from both viral and bacterial origin. Actual discrepancies between studies may be explained by several variables, including geographical and genetic differences between cohorts, but also technical issues due to different protocols used for DNA extraction and performing of NGS. Although challenging from a technical standpoint, more research should be devoted to investigating the role of the urinary tract in the bacterial colonisation of the MGT. While there is some overlap in the bacterial genera found in both the urinary and genital tracts [106], it is crucial to determine the specific niches in which different genital bacteria reside.
One of the most important conclusions from the characterisation of MGT microbiota is that bacterial abundance is highly variable. Bacterial abundance in semen, for example, may vary from 108 16S rRNA copies per ml of semen to a virtually sterile condition [13]. Nevertheless, most semen samples contain a relatively low number of bacteria. Other body sites with these characteristics include the lower respiratory tract or the female upper genital tract [11,107]. Clinical samples with a low biomass microbiota are, therefore, very prone to contaminations, which may occur at several steps: (1) during the sampling procedure, (2) during the DNA extraction procedure, and (3) during the amplification steps required for the NGS process [108,109]. In order to accurately profile microbial communities, the inclusion of negative controls is crucial. Negative controls serve as a baseline for detecting and correcting for potential sources of contamination throughout sampling, sample processing, and data analysis. During DNA extraction, ultrapure water should be processed to ensure that the extraction process does not introduce any unwanted contaminants. Furthermore, negative controls should be included during the amplification of target DNA by PCR to account for any potential amplification of contaminations that may have occurred during this step. In this context, most of the studies on semen microbiota (Table 1) did not include negative controls in the NGS analysis, for example.
In addition to these procedures, it is also important to include in silico decontamination approaches that will use negative controls as input and filter data postanalysis, which may influence the analysis and therefore make wrong conclusions [110,111]. Moreover, an ideal pipeline used for microbial analysis should also include commercially available artificial bacterial communities, which can be used to compare the analysis outcome with their theoretical composition. Therefore, studies such as the one from Molina et al. should be taken as an example for the analysis of possible contaminations [26]. In addition, the determination of the bacterial load should be assessed using quantitative PCR.
Human ejaculate consists of a heterogeneous pool of sperm, varying in features such as shape, size, and motility, that affect the process of fertilisation. Most of the mechanisms involved in the production of this heterogenous pool are only partially known, as well as conditions affecting the dynamic changes of sperm features. Moreover, selecting the optimal population of spermatozoa is a crucial step in the ART process, and there is general agreement that the quality of sperm selection must be improved in order to optimise oocyte fertilisation. MGT microbiota analysis represents a new tool to disentangle the complexity of human sperm as well as a potential target to improve male fertility.
Future studies should evaluate the stability of the bacterial populations of the MGT, by performing longitudinal surveys of the microbiota. The presence of a dynamic and diverse microbiota may further complicate our understanding of its impact on the host. Analysis of normospermic men will be important to assess the physiological seminal microbiota. Additional strategies may be used to confirm the presence of specific bacteria in the MGT, such as FISH or electron microscopy. Furthermore, the analysis of the metabolomic impact of bacterial colonisation will be paramount to understanding the broad impact of the microbiota on the MGT and also on the female genital tract. Despite all the possible limitations, the impact of specific bacterial taxa on spermatozoa physiology should be assessed using in vitro infection models. With this regard, it will be of great importance to establish collections of genital bacteria, and more specifically, MGT bacteria, and make them available to the scientific community for further studies.
Author Contributions
Conceptualisation, M.S.; Writing—Original Draft Preparation, M.S., A.Z. and A.P.; Writing—Review and Editing, M.S., A.Z., A.P., N.P. and D.B. All the authors approved the final version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the Department Women-Mother-Child, Service of Obstetrics, Lausanne University Hospital, Switzerland and by the SNSF grant numbers 310030-156169/1, 320030-169853/1, and 320030-169853/2 obtained by D.B. D.B. is also supported by the ‘Fondation Leenaards’ through the ‘Bourse pour la relève académique’, by the ‘Fondation Divesa’, and by the ‘Loterie Romande’.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Moeller A.H., Caro-Quintero A., Mjungu D., Georgiev A.V., Lonsdorf E.V., Muller M.N., Pusey A.E., Peeters M., Hahn B.H., Ochman H. Cospeciation of Gut Microbiota with Hominids. Science. 2016;353:380–382. doi: 10.1126/science.aaf3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van de Guchte M., Blottière H.M., Doré J. Humans as Holobionts: Implications for Prevention and Therapy. Microbiome. 2018;6:81. doi: 10.1186/s40168-018-0466-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lloyd-Price J., Arze C., Ananthakrishnan A.N., Schirmer M., Avila-Pacheco J., Poon T.W., Andrews E., Ajami N.J., Bonham K.S., Brislawn C.J., et al. Multi-Omics of the Gut Microbial Ecosystem in Inflammatory Bowel Diseases. Nature. 2019;569:655–662. doi: 10.1038/s41586-019-1237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valdes A.M., Walter J., Segal E., Spector T.D. Role of the Gut Microbiota in Nutrition and Health. BMJ. 2018;361:2179. doi: 10.1136/bmj.k2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilbert J.A., Blaser M.J., Caporaso J.G., Jansson J.K., Lynch S.V., Knight R. Current Understanding of the Human Microbiome. Nat. Med. 2018;24:392–400. doi: 10.1038/nm.4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dominguez-Bello M.G., Godoy-Vitorino F., Knight R., Blaser M.J. Role of the Microbiome in Human Development. Gut. 2019;68:1108–1114. doi: 10.1136/gutjnl-2018-317503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta P., Singh M.P., Goyal K. Diversity of Vaginal Microbiome in Pregnancy: Deciphering the Obscurity. Front. Public. Health. 2020;8:326. doi: 10.3389/fpubh.2020.00326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Callaghan J.L., Turner R., Dekker Nitert M., Barrett H.L., Clifton V., Pelzer E.S. Re-Assessing Microbiomes in the Low-Biomass Reproductive Niche. BJOG. 2020;127:147–158. doi: 10.1111/1471-0528.15974. [DOI] [PubMed] [Google Scholar]
- 9.Heil B.A., Paccamonti D.L., Sones J.L. Role for the Mammalian Female Reproductive Tract Microbiome in Pregnancy Outcomes. Physiol. Genom. 2019;51:390–399. doi: 10.1152/physiolgenomics.00045.2019. [DOI] [PubMed] [Google Scholar]
- 10.Amabebe E., Anumba D.O.C. The Vaginal Microenvironment: The Physiologic Role of Lactobacilli. Front. Med. 2018;5:181. doi: 10.3389/fmed.2018.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peric A., Weiss J., Vulliemoz N., Baud D., Stojanov M. Bacterial Colonization of the Female Upper Genital Tract. Int. J. Mol. Sci. 2019;20:3405. doi: 10.3390/ijms20143405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Onywera H., Williamson A.-L., Ponomarenko J., Meiring T.L. The Penile Microbiota in Uncircumcised and Circumcised Men: Relationships With HIV and Human Papillomavirus Infections and Cervicovaginal Microbiota. Front. Med. (Lausanne) 2020;7:383. doi: 10.3389/fmed.2020.00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baud D., Pattaroni C., Vulliemoz N., Castella V., Marsland B.J., Stojanov M. Sperm Microbiota and Its Impact on Semen Parameters. Front. Microbiol. 2019;10:234. doi: 10.3389/fmicb.2019.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ding N., Zhang X., Zhang X.D., Jing J., Liu S.S., Mu Y.P., Peng L.L., Yan Y.J., Xiao G.M., Bi X.Y., et al. Impairment of Spermatogenesis and Sperm Motility by the High-Fat Diet-Induced Dysbiosis of Gut Microbes. Gut. 2020;69:1608–1619. doi: 10.1136/gutjnl-2019-319127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alfano M., Ferrarese R., Locatelli I., Ventimiglia E., Ippolito S., Gallina P., Cesana D., Canducci F., Pagliardini L., Viganò P., et al. Testicular Microbiome in Azoospermic Men-First Evidence of the Impact of an Altered Microenvironment. Hum. Reprod. 2018;33:1212–1217. doi: 10.1093/humrep/dey116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Putman S.B., Chanmugam A.S. Urethritis, Prostatitis, and Epididymitis. In: Chanmugam A.S., Rothman R., Desai S., Putman S., editors. Infectious Diseases Emergencies. Oxford University Press; Oxford, UK: 2016. [Google Scholar]
- 17.Lam J.C., Lang R., Stokes W. How I Manage Bacterial Prostatitis. Clin. Microbiol. Infect. 2023;29:32–37. doi: 10.1016/j.cmi.2022.05.035. [DOI] [PubMed] [Google Scholar]
- 18.Bacterial Prostatitis: Current Opinion in Infectious Diseases. [(accessed on 12 February 2023)]. Available online: https://journals.lww.com/co-infectiousdiseases/Fulltext/2016/02000/Bacterial_prostatitis.15.aspx.
- 19.Farahani L., Tharakan T., Yap T., Ramsay J.W., Jayasena C.N., Minhas S. The Semen Microbiome and Its Impact on Sperm Function and Male Fertility: A Systematic Review and Meta-Analysis. Andrology. 2021;9:115–144. doi: 10.1111/andr.12886. [DOI] [PubMed] [Google Scholar]
- 20.Jarvi K., Lacroix J.M., Jain A., Dumitru I., Heritz D., Mittelman M.W. Polymerase Chain Reaction-Based Detection of Bacteria in Semen. Fertil. Steril. 1996;66:463–467. doi: 10.1016/S0015-0282(16)58520-3. [DOI] [PubMed] [Google Scholar]
- 21.Lacroix J.-M., Jarvi K., Batra S.D., Heritz D.M., Mittelman M.W. PCR-Based Technique for the Detection of Bacteria in Semen and Urine. J. Microbiol. Methods. 1996;26:61–71. doi: 10.1016/0167-7012(96)00844-5. [DOI] [Google Scholar]
- 22.Theiler T., Olaru I.D., Kilzer C., Schuler F., Schaumburg F. Ejaculate for Microbiological Culture: To Wash or Not To Wash? Microbiol. Spectr. 2022;10:e0326922. doi: 10.1128/spectrum.03269-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.James E.R., Carrell D.T., Aston K.I., Jenkins T.G., Yeste M., Salas-Huetos A. The Role of the Epididymis and the Contribution of Epididymosomes to Mammalian Reproduction. Int. J. Mol. Sci. 2020;21:5377. doi: 10.3390/ijms21155377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang S., Zhang K., Yao Y., Li J., Deng S. Bacterial Infections Affect Male Fertility: A Focus on the Oxidative Stress-Autophagy Axis. Front. Cell. Dev. Biol. 2021;9:727812. doi: 10.3389/fcell.2021.727812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nistal M., González-Peramato P. Infectious Disease of the Testis. In: van Krieken J.H.J.M., editor. Encyclopedia of Pathology. Encyclopedia of Pathology; Springer International Publishing; Cham, Switzerland: 2019. pp. 1–5. [Google Scholar]
- 26.Molina N.M., Plaza-Díaz J., Vilchez-Vargas R., Sola-Leyva A., Vargas E., Mendoza-Tesarik R., Galán-Lázaro M., Mendoza-Ladrón de Guevara N., Tesarik J., Altmäe S. Assessing the Testicular Sperm Microbiome: A Low-Biomass Site with Abundant Contamination. Reprod. BioMed. Online. 2021;43:523–531. doi: 10.1016/j.rbmo.2021.06.021. [DOI] [PubMed] [Google Scholar]
- 27.Weng S.-L., Chiu C.-M., Lin F.-M., Huang W.-C., Liang C., Yang T., Yang T.-L., Liu C.-Y., Wu W.-Y., Chang Y.-A., et al. Bacterial Communities in Semen from Men of Infertile Couples: Metagenomic Sequencing Reveals Relationships of Seminal Microbiota to Semen Quality. PLoS ONE. 2014;9:e110152. doi: 10.1371/journal.pone.0110152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suarez Arbelaez M.C., Israeli J.M., Tipton C.D., Loloi J., Deebel N., Leong J.Y., Ramasamy R. Pilot Study: Next-Generation Sequencing of the Semen Microbiome in Vasectomized Versus Nonvasectomized Men. Eur. Urol. Focus. 2022;11:10. doi: 10.1016/j.euf.2022.11.010. [DOI] [PubMed] [Google Scholar]
- 29.Rizzo A., Santoni M., Mollica V., Fiorentino M., Brandi G., Massari F. Microbiota and Prostate Cancer. Semin. Cancer Biol. 2022;86:1058–1065. doi: 10.1016/j.semcancer.2021.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 31.Verze P., Cai T., Lorenzetti S. The Role of the Prostate in Male Fertility, Health and Disease. Nat. Rev. Urol. 2016;13:379–386. doi: 10.1038/nrurol.2016.89. [DOI] [PubMed] [Google Scholar]
- 32.Lee J.C. Microbiology of the Prostate. Curr. Urol. Rep. 2000;1:159–163. doi: 10.1007/s11934-000-0052-y. [DOI] [PubMed] [Google Scholar]
- 33.Berger R.E., Krieger J.N., Rothman I., Muller C.H., Hillier S.L. Bacteria in the Prostate Tissue of Men With Idiopathic Prostatic Inflammation. J. Urol. 1997;157:863–865. doi: 10.1016/S0022-5347(01)65066-3. [DOI] [PubMed] [Google Scholar]
- 34.Cavarretta I., Ferrarese R., Cazzaniga W., Saita D., Lucianò R., Ceresola E.R., Locatelli I., Visconti L., Lavorgna G., Briganti A., et al. The Microbiome of the Prostate Tumor Microenvironment. Eur. Urol. 2017;72:625–631. doi: 10.1016/j.eururo.2017.03.029. [DOI] [PubMed] [Google Scholar]
- 35.Yow M.A., Tabrizi S.N., Severi G., Bolton D.M., Pedersen J., Giles G.G., Southey M.C. Australian Prostate Cancer BioResource Characterisation of Microbial Communities within Aggressive Prostate Cancer Tissues. Infect. Agents Cancer. 2017;12:4. doi: 10.1186/s13027-016-0112-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feng Y., Ramnarine V.R., Bell R., Volik S., Davicioni E., Hayes V.M., Ren S., Collins C.C. Metagenomic and Metatranscriptomic Analysis of Human Prostate Microbiota from Patients with Prostate Cancer. BMC Genom. 2019;20:146. doi: 10.1186/s12864-019-5457-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jain S., Samal A.G., Das B., Pradhan B., Sahu N., Mohapatra D., Behera P.K., Satpathi P.S., Mohanty A.K., Satpathi S., et al. Escherichia Coli, a Common Constituent of Benign Prostate Hyperplasia-Associated Microbiota Induces Inflammation and DNA Damage in Prostate Epithelial Cells. Prostate. 2020;80:1341–1352. doi: 10.1002/pros.24063. [DOI] [PubMed] [Google Scholar]
- 38.Wu Y., Jiang H., Tan M., Lu X. Screening for Chronic Prostatitis Pathogens Using High-Throughput next-Generation Sequencing. Prostate. 2020;80:577–587. doi: 10.1002/pros.23971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okada K., Takezawa K., Tsujimura G., Imanaka T., Kuribayashi S., Ueda N., Hatano K., Fukuhara S., Kiuchi H., Fujita K., et al. Localization and Potential Role of Prostate Microbiota. Front. Cell. Infect. Microbiol. 2022;12:1776. doi: 10.3389/fcimb.2022.1048319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lei H., Han H., Feng Y., Zhang X., Xin Z., Tian L. Altered Microbiota Profile in Seminal Vesicles of Men Presenting with Refractory Hematospermia. Mol. Biol. Rep. 2022;50:2381–2389. doi: 10.1007/s11033-022-08139-w. [DOI] [PubMed] [Google Scholar]
- 41.Dong Q., Nelson D.E., Toh E., Diao L., Gao X., Fortenberry J.D., Van Der Pol B. The Microbial Communities in Male First Catch Urine Are Highly Similar to Those in Paired Urethral Swab Specimens. PLoS ONE. 2011;6:e19709. doi: 10.1371/journal.pone.0019709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hrbacek J., Morais D., Cermak P., Hanacek V., Zachoval R. Alpha-Diversity and Microbial Community Structure of the Male Urinary Microbiota Depend on Urine Sampling Method. Sci. Rep. 2021;11:23758. doi: 10.1038/s41598-021-03292-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Plummer E.L., Ratten L.K., Vodstrcil L.A., Murray G.L., Danielewski J.A., Fairley C.K., Garland S.M., Chow E.P.F., Bradshaw C.S. The Urethral Microbiota of Men with and without Idiopathic Urethritis. mBio. 2022;13:e0221322. doi: 10.1128/mbio.02213-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roth R.S., Liden M., Huttner A. The Urobiome in Men and Women: A Clinical Review. Clin. Microbiol. Infect. 2022 doi: 10.1016/j.cmi.2022.08.010. [DOI] [PubMed] [Google Scholar]
- 45.Nelson D.E., Dong Q., Pol B.V.D., Toh E., Fan B., Katz B.P., Mi D., Rong R., Weinstock G.M., Sodergren E., et al. Bacterial Communities of the Coronal Sulcus and Distal Urethra of Adolescent Males. PLoS ONE. 2012;7:e36298. doi: 10.1371/journal.pone.0036298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zozaya M., Ferris M.J., Siren J.D., Lillis R., Myers L., Nsuami M.J., Eren A.M., Brown J., Taylor C.M., Martin D.H. Bacterial Communities in Penile Skin, Male Urethra, and Vaginas of Heterosexual Couples with and without Bacterial Vaginosis. Microbiome. 2016;4:16. doi: 10.1186/s40168-016-0161-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu C.M., Hungate B.A., Tobian A.A.R., Ravel J., Prodger J.L., Serwadda D., Kigozi G., Galiwango R.M., Nalugoda F., Keim P., et al. Penile Microbiota and Female Partner Bacterial Vaginosis in Rakai, Uganda. mBio. 2015;6:e00589-15. doi: 10.1128/mBio.00589-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Onywera H., Williamson A.-L., Cozzuto L., Bonnin S., Mbulawa Z.Z.A., Coetzee D., Ponomarenko J., Meiring T.L. The Penile Microbiota of Black South African Men: Relationship with Human Papillomavirus and HIV Infection. BMC Microbiol. 2020;20:78. doi: 10.1186/s12866-020-01759-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Plummer E.L., Vodstrcil L.A., Doyle M., Danielewski J.A., Murray G.L., Fehler G., Fairley C.K., Bulach D.M., Garland S.M., Chow E.P.F., et al. A Prospective, Open-Label Pilot Study of Concurrent Male Partner Treatment for Bacterial Vaginosis. mBio. 2021;12:e02323-21. doi: 10.1128/mBio.02323-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Byrd A.L., Belkaid Y., Segre J.A. The Human Skin Microbiome. Nat. Rev. Microbiol. 2018;16:143–155. doi: 10.1038/nrmicro.2017.157. [DOI] [PubMed] [Google Scholar]
- 51.Gonçalves M.F.M., Fernandes Â.R., Rodrigues A.G., Lisboa C. Microbiome in Male Genital Mucosa (Prepuce, Glans, and Coronal Sulcus): A Systematic Review. Microorganisms. 2022;10:2312. doi: 10.3390/microorganisms10122312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu C.M., Osborne B.J.W., Hungate B.A., Shahabi K., Huibner S., Lester R., Dwan M.G., Kovacs C., Contente-Cuomo T.L., Benko E., et al. The Semen Microbiome and Its Relationship with Local Immunology and Viral Load in HIV Infection. PLoS Pathog. 2014;10:e1004262. doi: 10.1371/journal.ppat.1004262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu C.M., Prodger J.L., Tobian A.A.R., Abraham A.G., Kigozi G., Hungate B.A., Aziz M., Nalugoda F., Sariya S., Serwadda D., et al. Penile Anaerobic Dysbiosis as a Risk Factor for HIV Infection. mBio. 2017;8:e00996-17. doi: 10.1128/mBio.00996-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Prodger J.L., Abraham A.G., Tobian A.A.R., Park D.E., Aziz M., Roach K., Gray R.H., Buchanan L., Kigozi G., Galiwango R.M., et al. Penile Bacteria Associated with HIV Seroconversion, Inflammation, and Immune Cells. JCI Insight. 2021;6:e147363. doi: 10.1172/jci.insight.147363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Price L.B., Liu C.M., Johnson K.E., Aziz M., Lau M.K., Bowers J., Ravel J., Keim P.S., Serwadda D., Wawer M.J., et al. The Effects of Circumcision on the Penis Microbiome. PLoS ONE. 2010;5:e8422. doi: 10.1371/journal.pone.0008422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu C.M., Hungate B.A., Tobian A.A.R., Serwadda D., Ravel J., Lester R., Kigozi G., Aziz M., Galiwango R.M., Nalugoda F., et al. Male Circumcision Significantly Reduces Prevalence and Load of Genital Anaerobic Bacteria. mBio. 2013;4:e00076. doi: 10.1128/mBio.00076-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mehta S.D., Nandi D., Agingu W., Green S.J., Bhaumik D.K., Bailey R.C., Otieno F. Vaginal and Penile Microbiome Associations With Herpes Simplex Virus Type 2 in Women and Their Male Sex Partners. J. Infect. Dis. 2022;226:644–654. doi: 10.1093/infdis/jiaa529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Watchorn R.E., van den Munckhof E.H.A., Quint K.D., Eliahoo J., de Koning M.N.C., Quint W.G.V., Bunker C.B. Balanopreputial Sac and Urine Microbiota in Patients with Male Genital Lichen Sclerosus. Int. J. Dermatol. 2021;60:201–207. doi: 10.1111/ijd.15252. [DOI] [PubMed] [Google Scholar]
- 59.Mehta S.D., Zhao D., Green S.J., Agingu W., Otieno F., Bhaumik R., Bhaumik D., Bailey R.C. The Microbiome Composition of a Man’s Penis Predicts Incident Bacterial Vaginosis in His Female Sex Partner With High Accuracy. Front. Cell. Infect. Microbiol. 2020;10:433. doi: 10.3389/fcimb.2020.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gray R.H., Kigozi G., Serwadda D., Makumbi F., Nalugoda F., Watya S., Moulton L., Chen M.Z., Sewankambo N.K., Kiwanuka N., et al. The Effects of Male Circumcision on Female Partners’ Genital Tract Symptoms and Vaginal Infections in a Randomized Trial in Rakai, Uganda. Am. J. Obs. Gynecol. 2009;200:42.e1-7. doi: 10.1016/j.ajog.2008.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Prodger J.L., Kaul R. The Biology of How Circumcision Reduces HIV Susceptibility: Broader Implications for the Prevention Field. AIDS Res. Ther. 2017;14:49. doi: 10.1186/s12981-017-0167-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tuominen H., Rautava J., Kero K., Syrjänen S., Collado M.C., Rautava S. HPV Infection and Bacterial Microbiota in the Semen from Healthy Men. BMC Infect. Dis. 2021;21:373. doi: 10.1186/s12879-021-06029-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mändar R., Punab M., Korrovits P., Türk S., Ausmees K., Lapp E., Preem J.-K., Oopkaup K., Salumets A., Truu J. Seminal Microbiome in Men with and without Prostatitis. Int. J. Urol. 2017;24:211–216. doi: 10.1111/iju.13286. [DOI] [PubMed] [Google Scholar]
- 64.Hou D., Zhou X., Zhong X., Settles M.L., Herring J., Wang L., Abdo Z., Forney L.J., Xu C. Microbiota of the Seminal Fluid from Healthy and Infertile Men. Fertil. Steril. 2013;100:1261–1269. doi: 10.1016/j.fertnstert.2013.07.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mändar R., Punab M., Borovkova N., Lapp E., Kiiker R., Korrovits P., Metspalu A., Krjutškov K., Nõlvak H., Preem J.-K., et al. Complementary Seminovaginal Microbiome in Couples. Res. Microbiol. 2015;166:440–447. doi: 10.1016/j.resmic.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 66.Chen H., Luo T., Chen T., Wang G. Seminal Bacterial Composition in Patients with Obstructive and Non-obstructive Azoospermia. Exp. Ther. Med. 2018;15:2884–2890. doi: 10.3892/etm.2018.5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Monteiro C., Marques P.I., Cavadas B., Damião I., Almeida V., Barros N., Barros A., Carvalho F., Gomes S., Seixas S. Characterization of Microbiota in Male Infertility Cases Uncovers Differences in Seminal Hyperviscosity and Oligoasthenoteratozoospermia Possibly Correlated with Increased Prevalence of Infectious Bacteria. Am. J. Reprod. Immunol. 2018;79:e12838. doi: 10.1111/aji.12838. [DOI] [PubMed] [Google Scholar]
- 68.Yang H., Zhang J., Xue Z., Zhao C., Lei L., Wen Y., Dong Y., Yang J., Zhang L. Potential Pathogenic Bacteria in Seminal Microbiota of Patients with Different Types of Dysspermatism. Sci. Rep. 2020;10:6876. doi: 10.1038/s41598-020-63787-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Amato V., Papaleo E., Pasciuta R., Viganò P., Ferrarese R., Clementi N., Sanchez A.M., Quaranta L., Burioni R., Ambrosi A., et al. Differential Composition of Vaginal Microbiome, but Not of Seminal Microbiome, Is Associated With Successful Intrauterine Insemination in Couples With Idiopathic Infertility: A Prospective Observational Study. Open. Forum Infect. Dis. 2020;7:ofz525. doi: 10.1093/ofid/ofz525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Štšepetova J., Baranova J., Simm J., Parm Ü., Rööp T., Sokmann S., Korrovits P., Jaagura M., Rosenstein K., Salumets A., et al. The Complex Microbiome from Native Semen to Embryo Culture Environment in Human in Vitro Fertilization Procedure. Reprod. Biol. Endocrinol. 2020;18:3. doi: 10.1186/s12958-019-0562-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lundy S.D., Sangwan N., Parekh N.V., Selvam M.K.P., Gupta S., McCaffrey P., Bessoff K., Vala A., Agarwal A., Sabanegh E.S., et al. Functional and Taxonomic Dysbiosis of the Gut, Urine, and Semen Microbiomes in Male Infertility. Eur. Urol. 2021 doi: 10.1016/j.eururo.2021.01.014. [DOI] [PubMed] [Google Scholar]
- 72.Yao Y., Qiu X.-J., Wang D.-S., Luo J.-K., Tang T., Li Y.-H., Zhang C.-H., Liu H., Zhou L., Zhao L.-L. Semen Microbiota in Normal and Leukocytospermic Males. Asian J. 2021;24:398–405. doi: 10.4103/aja202172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bukharin O., Perunova N., Ivanova E., Chaynikova I., Bekpergenova A., Bondarenko T., Kuzmin M. Semen Microbiota and Cytokines of Healthy and Infertile Men. Asian J. 2022;24:353. doi: 10.4103/aja202169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Onderdonk A.B., Delaney M.L., Fichorova R.N. The Human Microbiome during Bacterial Vaginosis. Clin. Microbiol. Rev. 2016;29:223–238. doi: 10.1128/CMR.00075-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Griswold M.D. Spermatogenesis: The Commitment to Meiosis. Physiol. Rev. 2016;96:1–17. doi: 10.1152/physrev.00013.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sullivan R., Mieusset R. The Human Epididymis: Its Function in Sperm Maturation. Hum. Reprod. Update. 2016;22:574–587. doi: 10.1093/humupd/dmw015. [DOI] [PubMed] [Google Scholar]
- 77.Hosseinzadeh S., Eley A., Pacey A.A. Semen Quality of Men with Asymptomatic Chlamydial Infection. J. Androl. 2004;25:104–109. doi: 10.1002/j.1939-4640.2004.tb02764.x. [DOI] [PubMed] [Google Scholar]
- 78.Ahmadi M.H., Mirsalehian A., Sadighi Gilani M.A., Bahador A., Talebi M. Asymptomatic Infection With Mycoplasma Hominis Negatively Affects Semen Parameters and Leads to Male Infertility as Confirmed by Improved Semen Parameters After Antibiotic Treatment. Urology. 2017;100:97–102. doi: 10.1016/j.urology.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 79.Nunez-Calonge R., Caballero P., Redondo C., Baquero F., Martinez-Ferrer M., Meseguer M.A. Ureaplasma Urealyticum Reduces Motility and Induces Membrane Alterations in Human Spermatozoa. Hum. Reprod. 1998;13:2756–2761. doi: 10.1093/humrep/13.10.2756. [DOI] [PubMed] [Google Scholar]
- 80.Satta A., Stivala A., Garozzo A., Morello A., Perdichizzi A., Vicari E., Salmeri M., Calogero A.E. Experimental Chlamydia Trachomatis Infection Causes Apoptosis in Human Sperm. Hum. Reprod. 2006;21:134–137. doi: 10.1093/humrep/dei269. [DOI] [PubMed] [Google Scholar]
- 81.Hosseinzadeh S., Brewis I.A., Eley A., Pacey A.A. Co-Incubation of Human Spermatozoa with Chlamydia Trachomatis Serovar E Causes Premature Sperm Death. Hum. Reprod. 2001;16:293–299. doi: 10.1093/humrep/16.2.293. [DOI] [PubMed] [Google Scholar]
- 82.Baud D., Vulliemoz N., Ammerdorffer A., Gyger J., Greub G., Castella V., Stojanov M. Waddlia Chondrophila, a Chlamydia-Related Bacterium, Has a Negative Impact on Human Spermatozoa. Hum. Reprod. 2017;33:3–10. doi: 10.1093/humrep/dex342. [DOI] [PubMed] [Google Scholar]
- 83.Huang C., Long X., Jing S., Fan L., Xu K., Wang S., Zhu W. Ureaplasma Urealyticum and Mycoplasma Hominis Infections and Semen Quality in 19,098 Infertile Men in China. World J. Urol. 2016;34:1039–1044. doi: 10.1007/s00345-015-1724-z. [DOI] [PubMed] [Google Scholar]
- 84.Eley A., Pacey A.A., Galdiero M., Galdiero M., Galdiero F. Can Chlamydia Trachomatis Directly Damage Your Sperm? Lancet Infect. Dis. 2005;5:53–57. doi: 10.1016/S1473-3099(04)01254-X. [DOI] [PubMed] [Google Scholar]
- 85.Svenstrup H.F., Fedder J., Abraham-Peskir J., Birkelund S., Christiansen G. Mycoplasma Genitalium Attaches to Human Spermatozoa. Hum. Reprod. 2003;18:2103–2109. doi: 10.1093/humrep/deg392. [DOI] [PubMed] [Google Scholar]
- 86.Wølner-Hanssen P., Mårdh P.-A. In Vitro Tests of the Adherence of Chlamydia Trachomatis to Human Spermatozoa. Fertil. Steril. 1984;42:102–107. doi: 10.1016/S0015-0282(16)47966-5. [DOI] [PubMed] [Google Scholar]
- 87.Erbengi T. Ultrastructural Observations on the Entry of Chlamydia Trachomatis into Human Spermatozoa. Hum. Reprod. 1993;8:416–421. doi: 10.1093/oxfordjournals.humrep.a138063. [DOI] [PubMed] [Google Scholar]
- 88.Gorga F., Galdiero M., Buommino E., Galdiero E. Porins and Lipopolysaccharide Induce Apoptosis in Human Spermatozoa. Clin. Diagn. Lab. Immunol. 2001;8:206–208. doi: 10.1128/CDLI.8.1.206-208.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li Z., Zhang D., He Y., Ding Z., Mao F., Luo T., Zhang X. Lipopolysaccharide Compromises Human Sperm Function by Reducing Intracellular CAMP. Tohoku J. Exp. Med. 2016;238:105–112. doi: 10.1620/tjem.238.105. [DOI] [PubMed] [Google Scholar]
- 90.Boguen R., Treulen F., Uribe P., Villegas J.V. Ability of Escherichia Coli to Produce Hemolysis Leads to a Greater Pathogenic Effect on Human Sperm. Fertil. Steril. 2015;103:1155–1161. doi: 10.1016/j.fertnstert.2015.01.044. [DOI] [PubMed] [Google Scholar]
- 91.Qiang H., Jiang M.-S., Lin J.-Y., He W.-M. Influence of Enterococci on Human Sperm Membrane in Vitro. Asian J. 2007;9:77–81. doi: 10.1111/j.1745-7262.2007.00219.x. [DOI] [PubMed] [Google Scholar]
- 92.Prabha V., Gupta T., Kaur S., Kaur N., Kala S., Singh A. Isolation of a Spermatozoal Immobilization Factor from Staphylococcus Aureus Filtrates. Can. J. Microbiol. 2009;55:874–878. doi: 10.1139/W09-032. [DOI] [PubMed] [Google Scholar]
- 93.Answal M., Prabha V. Escherichia Coli Recombinant Sperm Immobilizing Factor RecX as a Potential Vaginal Contraceptive. Reprod. Biol. Endocrinol. 2018;16:88. doi: 10.1186/s12958-018-0407-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Oghbaei H., Rastgar Rezaei Y., Nikanfar S., Zarezadeh R., Sadegi M., Latifi Z., Nouri M., Fattahi A., Ahmadi Y., Bleisinger N. Effects of Bacteria on Male Fertility: Spermatogenesis and Sperm Function. Life Sci. 2020;256:117891. doi: 10.1016/j.lfs.2020.117891. [DOI] [PubMed] [Google Scholar]
- 95.Tvrdá E., Ďuračka M., Benko F., Lukáč N. Bacteriospermia—A Formidable Player in Male Subfertility. Open. Life Sci. 2022;17:1001–1029. doi: 10.1515/biol-2022-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Eini F., Kutenaei M.A., Zareei F., Dastjerdi Z.S., Shirzeyli M.H., Salehi E. Effect of Bacterial Infection on Sperm Quality and DNA Fragmentation in Subfertile Men with Leukocytospermia. BMC Mol. Cell. Biol. 2021;22:42. doi: 10.1186/s12860-021-00380-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fraczek M., Hryhorowicz M., Gill K., Zarzycka M., Gaczarzewicz D., Jedrzejczak P., Bilinska B., Piasecka M., Kurpisz M. The Effect of Bacteriospermia and Leukocytospermia on Conventional and Nonconventional Semen Parameters in Healthy Young Normozoospermic Males. J. Reprod. Immunol. 2016;118:18–27. doi: 10.1016/j.jri.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 98.Eldamnhoury E.M., Elatrash G.A., Rashwan H.M., El-Sakka A.I. Association between Leukocytospermia and Semen Interleukin-6 and Tumor Necrosis Factor-Alpha in Infertile Men. Andrology. 2018;6:775–780. doi: 10.1111/andr.12513. [DOI] [PubMed] [Google Scholar]
- 99.Visconti A., Le Roy C.I., Rosa F., Rossi N., Martin T.C., Mohney R.P., Li W., de Rinaldis E., Bell J.T., Venter J.C., et al. Interplay between the Human Gut Microbiome and Host Metabolism. Nat. Commun. 2019;10:4505. doi: 10.1038/s41467-019-12476-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Franzosa E.A., Sirota-Madi A., Avila-Pacheco J., Fornelos N., Haiser H.J., Reinker S., Vatanen T., Hall A.B., Mallick H., McIver L.J., et al. Gut Microbiome Structure and Metabolic Activity in Inflammatory Bowel Disease. Nat. Microbiol. 2019;4:293–305. doi: 10.1038/s41564-018-0306-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.O’Hanlon D.E., Come R.A., Moench T.R. Vaginal PH Measured in Vivo: Lactobacilli Determine PH and Lactic Acid Concentration. BMC Microbiol. 2019;19:13. doi: 10.1186/s12866-019-1388-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.O’Hanlon Vaginal PH and Microbicidal Lactic Acid When Lactobacilli Dominate the Microbiota. [(accessed on 16 September 2020)]; doi: 10.1371/journal.pone.0080074. Available online: https://pubmed.ncbi.nlm.nih.gov/24223212/ [DOI] [PMC free article] [PubMed]
- 103.Wang Y.-X., Wu Y., Chen H.-G., Duan P., Wang L., Shen H.-Q., Lu W.-Q., Sun B., Wang Q., Zhang B., et al. Seminal Plasma Metabolome in Relation to Semen Quality and Urinary Phthalate Metabolites among Chinese Adult Men. Environ. Int. 2019;129:354–363. doi: 10.1016/j.envint.2019.05.043. [DOI] [PubMed] [Google Scholar]
- 104.Koeth R.A., Wang Z., Levison B.S., Buffa J.A., Org E., Sheehy B.T., Britt E.B., Fu X., Wu Y., Li L., et al. Intestinal Microbiota Metabolism of L-Carnitine, a Nutrient in Red Meat, Promotes Atherosclerosis. Nat. Med. 2013;19:576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hulme H., Meikle L.M., Strittmatter N., van der Hooft J.J.J., Swales J., Bragg R.A., Villar V.H., Ormsby M.J., Barnes S., Brown S.L., et al. Microbiome-Derived Carnitine Mimics as Previously Unknown Mediators of Gut-Brain Axis Communication. Sci. Adv. 2020;6:eaax6328. doi: 10.1126/sciadv.aax6328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Perez-Carrasco V., Soriano-Lerma A., Soriano M., Gutiérrez-Fernández J., Garcia-Salcedo J.A. Urinary Microbiome: Yin and Yang of the Urinary Tract. Front. Cell. Infect. Microbiol. 2021;11:617002. doi: 10.3389/fcimb.2021.617002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Man W.H., de Steenhuijsen Piters W.A.A., Bogaert D. The Microbiota of the Respiratory Tract: Gatekeeper to Respiratory Health. Nat. Rev. Microbiol. 2017;15:259–270. doi: 10.1038/nrmicro.2017.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Eisenhofer R., Minich J.J., Marotz C., Cooper A., Knight R., Weyrich L.S. Contamination in Low Microbial Biomass Microbiome Studies: Issues and Recommendations. Trends Microbiol. 2019;27:105–117. doi: 10.1016/j.tim.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 109.Karstens L., Asquith M., Davin S., Fair D., Gregory W.T., Wolfe A.J., Braun J., McWeeney S. Controlling for Contaminants in Low-Biomass 16S RRNA Gene Sequencing Experiments. mSystems. 2019;4:e00290-19. doi: 10.1128/mSystems.00290-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.McKnight D.T., Huerlimann R., Bower D.S., Schwarzkopf L., Alford R.A., Zenger K.R. MicroDecon: A Highly Accurate Read-Subtraction Tool for the Post-Sequencing Removal of Contamination in Metabarcoding Studies. Environ. DNA. 2019;1:14–25. doi: 10.1002/edn3.11. [DOI] [Google Scholar]
- 111.Davis N.M., Proctor D.M., Holmes S.P., Relman D.A., Callahan B.J. Simple Statistical Identification and Removal of Contaminant Sequences in Marker-Gene and Metagenomics Data. Microbiome. 2018;6:226. doi: 10.1186/s40168-018-0605-2. [DOI] [PMC free article] [PubMed] [Google Scholar]