Abstract
Background
Innovative surgical procedures and devices are often modified throughout their development and introduction into clinical practice. A systematic approach to reporting modifications may support shared learning and foster safe and transparent innovation. Definitions of ‘modifications’, and how they are conceptualized and classified so they can be reported and shared effectively, however, are lacking. This study aimed to explore and summarize existing definitions, perceptions, classifications and views on modification reporting to develop a conceptual framework for understanding and reporting modifications.
Methods
A scoping review was conducted in accordance with PRISMA Extension for Scoping Reviews (PRISMA-ScR) guidelines. Targeted searches and two database searches were performed to identify relevant opinion pieces and review articles. Included were articles relating to modifications to surgical procedures/devices. Data regarding definitions, perceptions and classifications of modifications, and views on modification reporting were extracted verbatim. Thematic analysis was undertaken to identify themes, which informed development of the conceptual framework.
Results
Forty-nine articles were included. Eight articles included systems for classifying modifications, but no articles reported an explicit definition of modifications. Some 13 themes relating to perception of modifications were identified. The derived conceptual framework comprises three overarching components: baseline data about modifications, details about modifications and impact/consequences of modifications.
Conclusion
A conceptual framework for understanding and reporting modifications that occur during surgical innovation has been developed. This is a first necessary step to support consistent and transparent reporting of modifications, to facilitate shared learning and incremental innovation of surgical procedures/devices. Testing and operationalization is now needed to realize the value of this framework.
This paper describes the conduct of a scoping review and the development of a conceptual framework for reporting modifications in surgical innovation.
Introduction
Surgical innovation has the potential to improve outcomes for patients and advance standards of surgical care1. Innovation in surgical practice can include new surgical techniques or devices (for example minimally invasive surgery) or can involve incremental changes to existing methods and devices (for example technological improvements)2,3. In the early stages of surgical innovation, new techniques or devices are modified or refined as innovations are optimized4. Whilst modifications may be made with the intention to improve or optimize innovative procedures or devices, this can be associated with uncertainty, unknown risks, and sometimes adverse events and harm. Understanding and sharing how and why modifications are made is therefore important for safe and efficient innovation.
Frameworks to improve transparency of surgical innovation and facilitate standardized approaches to evaluating surgical innovation have been introduced. The Idea, Development, Exploration, Assessment and Long-Term follow-up (IDEAL) framework, for example, outlines a pathway of five stages in surgical innovation (from first in human to long-term study) with recommendations on how new surgical procedures and devices should be developed and evaluated at each stage4,5. A core outcome set for standardized evaluation of innovative surgical procedures and devices (COHESIVE COS) has recently been developed to define outcomes to be measured and reported in early phase studies of surgical innovation6,7. Reporting of modifications is a key component of both stages 2a/2b within the IDEAL framework. Reporting of modification is also recommended as part of the recently developed COHESIVE COS, which was identified as a core outcome domain to report all early phase studies of surgical procedures/devices6. This included modifications to technical components, patient selection criteria and co-interventions (for example administration of intravenous analgesia). There are currently, however, no standardized frameworks for reporting modifications in studies of surgical innovation. Indeed, vague terminology is sometimes seen in literature/guidance without further definition or explanation, for example referring to modifications as ‘major’ or ‘minor’8,9. Current approaches to reporting modifications in surgical innovation, and the incremental learning arising from them, are informal and inconsistent2. Deficient reporting and a lack of opportunity for sharing incremental learning may result in surgeons unknowingly repeating ineffective or detrimental modifications and may increase the risk of avoidable patient harm. In contrast, sharing experiences of effective modifications may also be compromised, meaning efficient uptake of promising innovations is hindered. A systematic and transparent approach to reporting modifications when introducing new procedures or devices into clinical practice is recommended to support shared learning, promote efficiency and protect patient safety10.
This study aimed to explore and summarize existing literature about modifications related to: definitions, perception, classifications and views on modification reporting, to inform a conceptual framework for understanding and reporting modifications in surgical innovation.
Methods
A scoping review was conducted and reported in accordance with relevant PRISMA guidelines (PRISMA-ScR, see Appendix S1)11. The multidisciplinary study team consisted of surgeons, methodologists and trialists with extensive experience in health services research. A study protocol has previously been published12. Ethical approval was not required for this review as no empirical patient data or sensitive information was collected.
Search strategy
Multiple consecutive searches were required to identify relevant articles due to a lack of index terms for the specific subject areas of surgical innovation and modifications5,13,14. Targeted internet searches using keywords for ‘modifications’, ‘definition’, ‘classification’ and ‘invasive procedures’ were initially performed to identify relevant key papers. The terms used in electronic medical databases for indexing the key papers were identified, including additional keywords, and were used to inform the search terms for the current review. Two separate database searches were conducted in MEDLINE (Ovid version) to identify review articles and opinion pieces. Snowball searching (that is backward and forward searching of reference lists) was completed for all articles included in the review, to identify further relevant articles15. Searches were conducted between July and October 2020. The search strategy is detailed in Tables S1, S2.
Study eligibility
Included were any review articles or opinion pieces published in a peer-reviewed journal and relevant to surgical innovation (that is topics related to the development or use of new surgical procedures and devices), that provided one or more of the following: a scientific or descriptive definition of modifications; a description of how modifications may be perceived or understood; a classification, typology and/or taxonomy for categorizing modifications; and views and/or opinions on how modifications could be reported.
Excluded were non-English language publications, conference abstracts and studies reporting empirical examples of modifications. Review articles published before 2001 were excluded to allow for an anticipated manageable number of records to be screened whilst maintaining a broad time interval. Publications within the last 20 years were included because this time frame was identified as an interval of increasing interest in methodology relating to evaluation of surgical innovation2,16. No publication date limit was set for opinion pieces because fewer records were retrieved.
Study selection
A two-stage screening process was performed independently by two reviewers (S.H., C.H.). First, titles, abstracts and keywords were examined to identify potentially eligible articles. This was followed by an in-depth full-text review. All articles where inclusion was uncertain were discussed between the two reviewers and assessed independently by a third reviewer (R.M.) to reach a final consensus on their inclusion. Input from the wider study team was sought wherever necessary.
Data extraction and analysis
Data extraction was performed independently by two reviewers (C.H., S.H.) using a predesigned standardized proforma, directly into an electronic database (see Table S3). Data related to the definition, perception, classification or views on reporting of modifications were extracted verbatim. Publication characteristics, author affiliation, conflict of interest, funding statements and institution characteristics were recorded to examine any patterns in potential influences on the available evidence. Initially, each reviewer independently extracted data from the same five articles. Extracted data were then compared and discussed to ensure that subsequent data extraction was consistent and sufficient quality, that is extracted data met one of the four study eligibility criteria specified above. Data extraction of remaining articles was performed independently by the two reviewers, with regular meetings for further quality assurance.
Article types were grouped into categories (for example commentaries, letters and perspectives were grouped as ‘opinion pieces’). Publication characteristics were summarized using descriptive statistics. Other verbatim extracted data relating to the review objectives were analysed using a narrative synthesis to identify and iteratively derive common themes using principles of thematic analysis, consisting of data familiarization, systematic coding of textual data, and iterative refinement of themes and subthemes17,18. Where a narrative synthesis was not possible due to a paucity of data for a specific review objective, content analysis (quantifying the presence of certain words) of extracted data was performed to explore the terminology used in the included articles. Narrative synthesis was performed separately for articles relating to surgical procedures and devices. Analyses were assisted using qualitative analysis software (NVivo; version 12)19.
Development of a conceptual framework
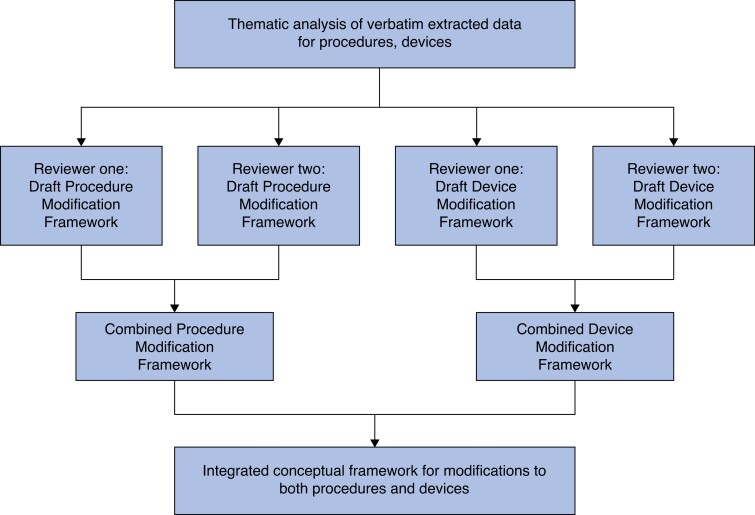
A conceptual framework for modifications was developed following the principles of framework analysis in three steps20,21. First, the inductively derived themes were reviewed to qualitatively compare their content, with a view to highlighting similarities and differences (for example whilst several articles described the broader concept of underlying drivers for modifications, they differed in the specific justifications described, such as improving patient outcomes, resolving technical problems or expanding patient selection)22–25. Second, themes considered to be similar were grouped, and subsequently organized according to relationships between them. Two reviewers (C.H., S.H.) independently developed draft conceptual frameworks informed by these themes, separately for surgical procedures and devices to ensure maximum validity. Third, the reviewers’ independent draft frameworks were compared and discussed between the reviewers (C.H., S.H.) and combined to produce agreed frameworks for surgical procedures and devices. Finally, through further rounds of discussion with the wider study team, the two frameworks were integrated to produce a single conceptual framework for modifications to procedures and devices, focusing on applicability of the framework to clinical practice. This process is summarized in Fig. 1. Existing reviews of empirical studies of surgical innovation known to the study team5,7,13,14 were examined to aid organization of the framework throughout this process.
Fig. 1.
Overview of steps in the development of an integrated conceptual framework for modifications to procedures and devices
Results
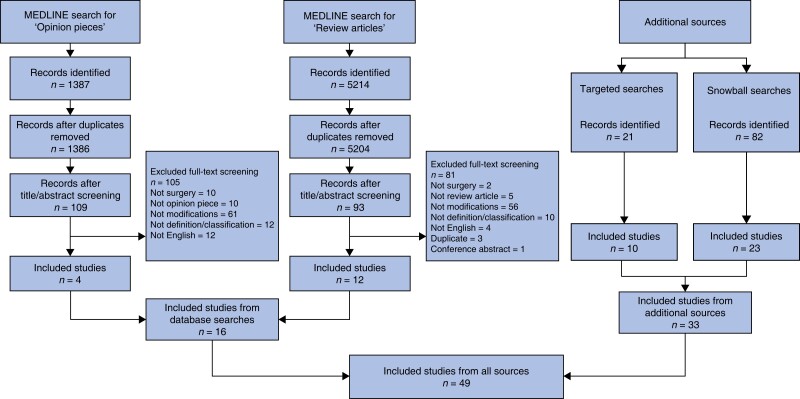
The database search yielded a total of 6601 records (Fig. 2). Of these, 1387 and 5214 records were identified through searches for opinion pieces and review articles respectively. Additional targeted and snowball searches identified a further 103 potentially relevant articles. A total of 49 articles were eligible for analysis.
Fig. 2.
PRISMA flow diagram
Study characteristics
Characteristics of included articles are summarized in Table 1 and Table S4. Article types were predominately narrative reviews (N = 22, 45 per cent) and opinion pieces (N = 14, 29 per cent). Most articles were relevant to surgical procedures (N = 37, 76 per cent) and discussed procedures generically (N = 31, 63 per cent) rather than referring to a specific operative technique (N = 6, 12 per cent). Most articles discussed surgical devices in general (N = 15, 31 per cent), whilst others discussed a specific device (N = 6, 12 per cent). Most articles were published by author(s) affiliated to higher education institutions such as universities and teaching hospitals (N = 41, 84 per cent) and conducted in the USA (N = 28, 57 per cent), followed by Europe (N = 23, 47 per cent). Included articles were mostly published in the last decade (N = 30, 32 per cent).
Table 1.
Summary of characteristics of included articles
| n (%) | |
|---|---|
| Type of article | |
| Narrative review | 22 (45) |
| Opinion pieces (comments, debates, editorials) | 14 (29) |
| Guideline | 7 (14) |
| Database review | 4 (8) |
| Systematic review | 2 (4) |
| General topics discussed | |
| Surgical procedures* | 37 (76) |
| Surgical devices* | 20 (41) |
| Both | 8 (16) |
| Details about content of the article | |
| Surgical procedures in general | 31 (63) |
| Surgical devices in general | 15 (31) |
| Specific surgical technique | 6 (12) |
| Specific surgical specialty | 6 (12) |
| Conflict of interest | |
| Conflict of interest statement present | 38 (78) |
| Conflict of interest statement not present | 11 (22) |
| Details about presented statements | |
| Authors declared no conflict of interest | 27 (55) |
| Statement is unclear | 9 (18) |
| Authors declared a conflict of interest | 2 (4) |
| Funding | |
| Funding or sponsorship statement present | 36 (73) |
| Funding or sponsorship statement not present | 13 (27) |
| Details about presented statements | |
| Funding or sponsorship was received | 15 (31) |
| No funding or sponsorship was received | 13 (26) |
| Statement is unclear | 8 (16) |
| Year of publication | |
| 2020–2016 | 15 (31) |
| 2015–2011 | 15 (31) |
| 2010–2006 | 12 (24) |
| 2005–1999 | 7 (14) |
| Authors' affiliation | |
| Higher education affiliation | 41 (84) |
| Research collaboration | 3 (6) |
| Industry affiliation | 2 (4) |
| Country* | |
| USA | 28 (57) |
| Europe | 23 (47) |
| Canada | 7 (14) |
| Asia | 4 (8) |
| Single country | 41 (84) |
| Multinational | 8 (16) |
Categories are not mutually exclusive.
Definitions, perception, classifications and views on how to report modifications
Definitions
No scientific or descriptive definition of modifications was identified in the included articles.
Classifications and views on modification reporting
The included articles referenced a total of eight different classifications (see Table S5). The most frequently cited were the IDEAL framework, which proposes reporting of iterative modifications in the early stages of surgical innovation4,7,26–29, and the US Food and Drug Administration (FDA) Premarket Approval (PMA) pathway0–4, which describes the FDA regulatory framework through which modifications to devices are approved.
Perceptions
A total of 13 themes were identified from verbatim extracted descriptions of modifications (see Table S6). Of these, six themes were common to both procedures and devices and are described in more detail below. A summary of these six themes with example extracts from the included articles can be found in Table S7.
Three further themes were unique to procedures: phase of surgical innovation (that is modifications are a necessary stage in the development of procedures with an aim to reach a point of stability); failure and mistakes (that is are inevitable elements of modifications which should be (prospectively) defined and responded to); increased complexity (that is modifications can lead to technically more complex procedures to achieve better outcomes, which requires managing potentially conflicting outcomes). The remaining four themes were unique to devices which are characterized by: transparency issues (that is lack of comprehensive reporting of information primarily related to minor device modifications, which require improvements in regulation, oversight, registries and/or greater risk management); collaboration with industry (that is the need for joint working between industry, regulatory bodies and clinicians); access (that is the perceived right for patients to access innovation which often justifies rapid introduction of modified devices) and the relationship between surgeon learning and patient selection (that is changes in patient selection criteria in response to the surgeon’s learning curve).
Perceptions of modifications common to both procedures and devices
Modifications as a process
Modifications were often described as a process in relation to both procedures and devices. Modifications to procedures were characterized as a stepwise and goal-directed process23,24,35. Further descriptions revealed goals may be changeable and evolving with experience, fixed (for example clinical outcomes, measures of failure and success) or independent of clinical outcomes (for example practical concerns)23,24. In contrast, modifications to devices were viewed as a dynamic process during which changes occur frequently and continuously36. This process was described as an open-ended cycle (without an end goal) of postmarket product development, where incremental minor improvements to devices can lead to an entirely new product emerging over time. Reference was made to existing US FDA regulations facilitating this process and labelled ‘predicate creep’ or ‘design drift’37,38.
Types/understandings of modifications
Articles reported different types/understandings of modifications for both procedures and devices. Changes to technical components of the surgery, physical changes to the device, changes to patient selection and changes to co-interventions were all described. Two distinct types of modification were evident from descriptions of surgical procedures: a change to a single aspect (for example a technical modification) of an existing procedure which may lead to an entirely new procedure or multiple, cumulative changes to different aspects of a procedure (including new indication, combining of established techniques)39,40. In contrast, device modifications were broadly described as either: manufacturer-led modifications that were primarily design-focused and clinician-led modifications that focused on addressing a clinical need31,41.
Magnitude of modifications
Articles referred to the magnitude of modifications for both procedures and devices, broadly described as ‘major’ and ‘minor’. ‘Major’ procedure modifications were considered to represent changes conferring greater levels of risk or uncertainty, necessitating greater surgeon training needs and performance assessment, or requiring external scrutiny42. ‘Minor’ procedure modifications were consistent with smaller technical modifications within an accepted procedure, requiring less extensive surgeon training. For surgical devices, articles referred to ‘minor’ device modifications such as smaller design changes, in contrast to ‘major’ device modifications that might include new indications for use32.
Drivers for modifications
In some articles, modifications were described as being motivated by underlying rationale that could be considered to represent ‘drivers’ for modifications (that is the reasons that influence why modifications are made). Examples included the desire to advance existing treatments/foster further innovation, improve existing techniques, improve outcomes, respond to technical problems/failures, improve efficiency or broaden patient selection/indication24,43.
Enablers of modifications
Modifications were described in some articles as being influenced by ‘enablers’ (that is factors that allow or make modifications easier to happen) or facilitated the stepwise process of development43,44. For example iterative developments may be influenced by the dissemination of existing innovations or technological advancements, or by shared learning.
Relationship to formal research
Some articles referred to the limitations of current research or governance structures in identifying the need for oversight of modified procedures. Other articles considered how evolving procedures or devices that were continually being modified, presented researchers with challenges when interpreting short- versus long-term safety and effectiveness data from clinical studies44,45.
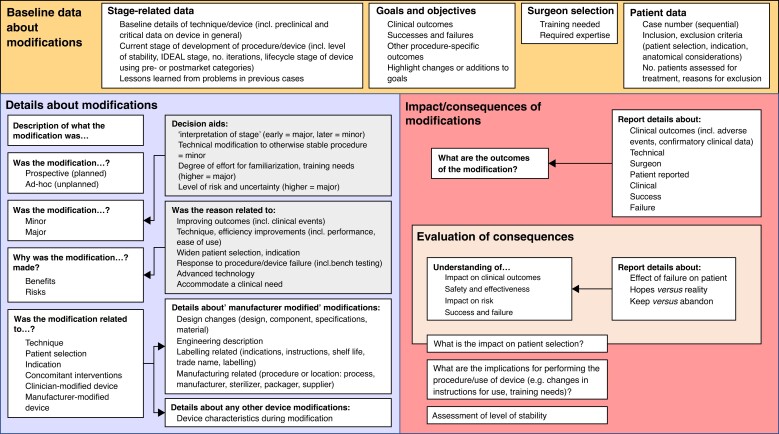
Conceptual framework for understanding and reporting modifications
The themes listed above were organized to produce draft conceptual frameworks for reporting modifications, separately for procedures and devices (Figs S1, S2). Figure 3 presents the single integrated conceptual framework relevant to modifications to procedures and devices. The framework comprises three overarching components to represent the organized themes derived from the data: baseline data about modifications, details about modifications and impact/consequences of modifications.
Fig. 3.
Integrated conceptual framework for modifications to procedures and devices
The ‘baseline data about modifications’ component of the integrated conceptual framework broadly includes background contextual features that may be relevant to understanding modifications to a procedure or device. Within this component, the stage of development, goals and objectives, patient data (for example patient selection criteria/indication and reasons for exclusion) and lessons learned from previous cases may contextualize modifications to procedures or devices. The ‘details about modifications’ component broadly outlines the modification itself and includes other identified themes relevant to understanding the modification, for example what aspect of the procedure/device the modification related to, why the modification was made and the perceived magnitude of the modification. The ‘impact/consequences of modifications’ component includes an assessment of actual or perceived outcomes of a modification, and considers the subsequent implications on future cases, including an assessment of whether the modification might be kept, abandoned or modified further.
Discussion
This study systematically analysed existing literature to identify definitions, perception, classifications, and views on modifications to surgical procedures and devices, with the aim of developing a conceptual framework for understanding and reporting modifications undertaken during early phase studies. Syntheses of verbatim data, extracted from 49 review articles and opinion pieces, identified 13 relevant themes. Six themes were common to modifications to procedures and devices, whilst three themes were unique to procedures and four unique to devices. All themes were organized to develop an integrated conceptual reporting framework for modifications to procedures and devices, comprising three overarching components: baseline data about modifications, details about modifications and impact/consequences of modifications. Use of the framework is recommended to increase transparency in surgical innovation and enhance the safe and efficient introduction of new procedures and devices into clinical practice.
This work completes an initial step towards operationalizing a core outcome domain of the COHESIVE COS, which recommends reporting modifications in all studies of early phase surgical procedures and devices6. Prior studies have highlighted considerable heterogeneity when reporting innovation-specific outcomes including modifications13. The proposed framework provides a summary of the conceptual areas that may be considered relevant to informing a future reporting framework for modifications. The framework can be used in context alongside existing guidance for reporting surgical innovation. For example the IDEAL checklist, developed to facilitate reporting for surgical studies throughout their lifecycle specifies a minimum list of reporting items, including broad information about modifications in stages 2a and 2b (for example number, time point, magnitude and reason for modifications made)7. Findings from the current study complement these recommendations to facilitate how modifications can be conceptualized, described and reported in detail. The framework may, for example help surgeons to determine a modification’s magnitude and describe their rationale for making the modification, ultimately facilitating shared learning. The eight classifications identified in this review may also provide helpful systems in deciding how to adopt processes for recording information in specific settings.
Findings from this review emphasize the important role of modifications during the development phase of innovative surgical devices and procedures. Understanding when and why modifications occur in practice is crucial for shared learning of surgical innovation. Importantly, improved understanding and real-time sharing of modifications may accelerate efficient evaluation of innovative procedures and devices. Modifications can serve as an indicator of the stability of innovative procedures and devices, crucial to establishing the point at which definite evaluation through an RCT is recommended46,47. It is anticipated that the framework will be useful to those reporting innovation in real-time, to indicate whether a procedure/device has sufficiently stabilized and is ready for evaluation in an RCT. It may also be useful, for example to establish the stage of innovation (that is level of stabilization of a new procedure/device) retrospectively48.
There is no agreement in the current literature about how to define modifications, and no definitions were identified in this review. Further work and stakeholder consensus is therefore needed to achieve a commonly accepted definition to progress standardized reporting. However, challenges in this regard have been noted during attempts to define ‘surgical innovation’ which was found to be underpinned by conceptual areas rather than a single, practical definition44,49. The conceptual framework proposed in this study may be used to inform work to define modifications in surgical innovation.
This study had several strengths. Robust scoping review methodology was used to examine existing literature on a topic area with little prior exploration11. The search strategy was comprehensive and designed to identify potentially relevant literature in a broad range of article types, which were considered to be specifically relevant to achieving the review objectives. A deliberately broad approach was applied during the conduct of this study to include as many potentially relevant articles as possible and to ensure no important evidence would be excluded. This means that there was no a priori definition of a reporting or classification system, however, synthesis included important aspects of modifications that may have been overlooked in other studies and therefore allowed development of a comprehensive conceptual framework. Specifically, the study adopted a broad view of modifications as suggested in the COHESIVE COS (for example modifications to patient selection criteria and co-interventions) and included evidence for modifications to procedures and devices by undertaking multiple searches (two databases, targeted and snowball searches)6. The approach to data analysis consisted of established qualitative methods, drawing on both thematic analysis and on principles of framework analysis to inductively generate an understanding of important concepts and organize themes17,18. Scoping review methodology has been recognized as a valid and insightful means to synthesize evidence in healthcare and various other areas of research with little prior evidence50–52. Framework analyses is an established method for organizing data20,21 and has been previously applied to develop conceptual frameworks in healthcare53 or reporting frameworks in other fields54,55. Synthesis was conducted by two independent experienced reviewers with input from a multidisciplinary team consisting of methodologists, health services researchers and surgeons to ensure rigour.
Study limitations should also be noted. It is acknowledged that some relevant publications may have been missed, as identification of all potentially relevant articles remains dependent on indexing. Included articles were limited to those published in the English language and from peer-reviewed journals from mostly higher education institutions and academic surgeons. This could potentially have limited the ‘real-world’ understanding of modifications in other types of clinical settings or institutions. Likewise, it is unknown whether the conceptual framework is appropriate in wider settings (for example modifications to an entire technique versus a small component of an existing procedure). Refinements of the conceptual framework to accommodate such variability may be needed in future work to accelerate standardized reporting of modifications and evaluation of surgical innovation.
This work has completed a necessary first step and developed a conceptual framework to understand modifications to innovative procedures and devices. Testing and operationalizing the proposed conceptual framework into a validated and clinically applicable tool for identifying, describing and reporting modifications are now needed, alongside identification of optimal ways to integrate findings into practice. A validated reporting framework for modifications will complement the Template for Intervention Description and Replication (TIDieR) checklist and CONSORT statement for Non-Pharmacological Treatments (NPT), which both refer to modifications and tailoring but lack detail in several areas relevant to effective reporting56,57. This is being explored within the National Institute for Health and Care Research (NIHR) funded Bristol Biomedical Research Centre (BRC) in the UK, which aims to improve the introduction and evaluation of innovative surgical procedures and devices.
Routine use of a framework to describe and report modifications will support a more systematic and standardized approach to reporting modifications and ultimately increase transparency in surgical innovation, facilitate shared learning amongst surgeon innovators and minimize research waste and patient harm.
Supplementary Material
Acknowledgements
We would like to thank Sarah Herring (Subject Information Specialist) for help in developing the search strategy. A data table summarizing the included articles is provided in Table S4; a complete data set including verbatim extracted data is available upon request. S.H. and C.H. are joint first authors. K.A., S.P. and R.M. are joint senior authors.
Contributor Information
Sina Hossaini, Department of Population Health Sciences, National Institute for Health and Care Research Bristol Biomedical Research Centre, Bristol Centre for Surgical Research, Bristol Medical School, University of Bristol, Bristol, UK.
Christin Hoffmann, Department of Population Health Sciences, National Institute for Health and Care Research Bristol Biomedical Research Centre, Bristol Centre for Surgical Research, Bristol Medical School, University of Bristol, Bristol, UK.
Sian Cousins, Department of Population Health Sciences, National Institute for Health and Care Research Bristol Biomedical Research Centre, Bristol Centre for Surgical Research, Bristol Medical School, University of Bristol, Bristol, UK.
Natalie Blencowe, Department of Population Health Sciences, National Institute for Health and Care Research Bristol Biomedical Research Centre, Bristol Centre for Surgical Research, Bristol Medical School, University of Bristol, Bristol, UK; Department of Gastrointestinal Surgery, North Bristol NHS Trust, Bristol, UK.
Angus G K McNair, Department of Population Health Sciences, National Institute for Health and Care Research Bristol Biomedical Research Centre, Bristol Centre for Surgical Research, Bristol Medical School, University of Bristol, Bristol, UK; Department of Gastrointestinal Surgery, North Bristol NHS Trust, Bristol, UK.
Jane M Blazeby, Department of Population Health Sciences, National Institute for Health and Care Research Bristol Biomedical Research Centre, Bristol Centre for Surgical Research, Bristol Medical School, University of Bristol, Bristol, UK; Division of Surgery, Bristol Royal Infirmary, University Hospitals Bristol and Weston NHS Foundation Trust, Bristol, UK.
Kerry N L Avery, Department of Population Health Sciences, National Institute for Health and Care Research Bristol Biomedical Research Centre, Bristol Centre for Surgical Research, Bristol Medical School, University of Bristol, Bristol, UK.
Shelley Potter, Department of Population Health Sciences, National Institute for Health and Care Research Bristol Biomedical Research Centre, Bristol Centre for Surgical Research, Bristol Medical School, University of Bristol, Bristol, UK; Bristol Breast Care Centre, North Bristol NHS Trust, Bristol, UK.
Rhiannon Macefield, Department of Population Health Sciences, National Institute for Health and Care Research Bristol Biomedical Research Centre, Bristol Centre for Surgical Research, Bristol Medical School, University of Bristol, Bristol, UK.
Funding
This work was supported by the National Institute for Health and Care Research (NIHR) Bristol Biomedical Research Centre (BRC) at University Hospitals Bristol and Weston NHS Foundation Trust and the University of Bristol (BRC-1215-20011). The views expressed in this publication are those of the authors and not necessarily those of the NHS or NIHR. N.B. is a Medical Research Council (MRC) Clinician Scientist. S.P. and A.G.K.M. are NIHR Clinician Scientists (NIHR CS-2016-16-019, NIHR CS-2017-17-010). J.B. is a NIHR Senior Investigator.
Contributions
R.M., K.A., S.P. and J.B. conceived and initiated the study. R.M., K.A., S.P., S.C., S.H. and C.H. designed the study protocol. J.B. developed the ideas for the NIHR Bristol BRC Surgical Innovation theme. R.M., K.A. and S.P. had joint senior oversight and co-directed the study. All named co-authors contributed to oversight of the study, aspects of study design and/or acquisition of data and/or interpretation of data. S.H. and C.H. wrote the first draft of this manuscript. All co-authors critically revised the manuscript and gave final approval of the version to be published.
Disclosure
All authors declare no conflict of interest.
Supplementary material
Supplementary material is available at BJS Open online.
Data availability
No new data were collected or generated from this study. All study materials have been made available in the supplementary file.
References
- 1. Surgeons RCo . Surgical Innovation, New Techniques and Technologies. In: England RCoSo, editor. RCSEng Website. RCS Professional Standards, 2019. https://www.rcseng.ac.uk/-/media/files/rcs/standards-and-research/standards-and-policy/good-practice-guides/old-documents/surgical-innovation-new-techniques-and-technologies.pdf(accessed 1 July 2022)
- 2. Barkun JS, Aronson JK, Feldman LS, Maddern GJ, Strasberg SM, Altman DGet al. Evaluation and stages of surgical innovations. Lancet 2009;374:1089–1096 [DOI] [PubMed] [Google Scholar]
- 3. Riskin DJ, Longaker MT, Gertner M, Krummel TM. Innovation in surgery: a historical perspective. Ann Surg 2006;244:686–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McCulloch P, Altman DG, Campbell WB, Flum DR, Glasziou P, Marshall JCet al. No surgical innovation without evaluation: the IDEAL recommendations. Lancet 2009;374:1105–1112 [DOI] [PubMed] [Google Scholar]
- 5. Avery K, Blazeby J, Wilson N, Macefield R, Cousins S, Main Bet al. Development of reporting guidance and core outcome sets for seamless, standardised evaluation of innovative surgical procedures and devices: a study protocol for content generation and a delphi consensus process (COHESIVE study). BMJ Open 2019;9:e029574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Avery KNL, Wilson N, Macefield R, McNair A, Hoffmann C, Blazeby JMet al. A core outcome set for seamless, standardized evaluation of innovative surgical procedures and devices (COHESIVE): a patient and professional stakeholder consensus study. Ann Surg 2023;277:238–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bilbro NA, Hirst A, Paez A, Vasey B, Pufulete M, Sedrakyan Aet al. The IDEAL reporting guidelines: a delphi consensus statement stage specific recommendations for reporting the evaluation of surgical innovation. Ann Surg 2021;273:82–85 [DOI] [PubMed] [Google Scholar]
- 8. NICE . Interventional Procedures Programme. In: Excellence TNIfHaC, editor. London: National Institute for Health and Care Excellence (NICE), 2022
- 9. Cousins S, Richards HS, Zahra J, Robertson H, Mathews JA, Avery KNLet al. Healthcare organization policy recommendations for the governance of surgical innovation: review of NHS policies. Br J Surg 2022;109;1004–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yu J, Shan F, Hirst A, McCulloch P, Li Y, Sun X. Identifying research waste from surgical research: a protocol for assessing compliance with the IDEAL framework and recommendations. BMJ Surg Interv Health Technol 2021;3:e000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac Det al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 2018;169:467–473 [DOI] [PubMed] [Google Scholar]
- 12. Hoffmann C, Hossaini S, Cousins S, Blencowe N, McNair AGK, Blazeby JMet al. Reporting modifications in surgical innovation: a systematic scoping review protocol. Int J Surg Protoc 2021;25:250–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Macefield RC, Wilson N, Hoffmann C, Blazeby JM, McNair AGK, Avery KNLet al. Outcome selection, measurement and reporting for new surgical procedures and devices: a systematic review of IDEAL/IDEAL-D studies to inform development of a core outcome set. BJS Open 2020;4:1072–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hoffmann C, Macefield RC, Wilson N, Blazeby JM, Avery KNL, Potter Set al. A systematic review and in-depth analysis of outcome reporting in early phase studies of colorectal cancer surgical innovation. Colorectal Dis 2020;22:1862–1873 [DOI] [PubMed] [Google Scholar]
- 15. Choong MK, Galgani F, Dunn AG, Tsafnat G. Automatic evidence retrieval for systematic reviews. J Med Internet Res 2014;16:e223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pennell CP, Hirst AD, Campbell WB, Sood A, Agha RA, Barkun JSet al. Practical guide to the idea, development and exploration stages of the IDEAL framework and recommendations. Br J Surg 2016;103:607–615 [DOI] [PubMed] [Google Scholar]
- 17. Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77–101 [Google Scholar]
- 18. Thomas J, Harden A. Methods for the thematic synthesis of qualitative research in systematic reviews. BMC Med Res Methodol 2008;8:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.https://www.qsrinternational.com/nvivo-qualitative-data-analysis-software/home Ltd. QIP. NVivo. (Version 12) ed.
- 20.https://methods.sagepub.com/book/the-qualitative-researchers-companion Huberman AM, Miles MB. The Qualitative Researcher’s Companion. Thousand Oaks, California: SAGE Publications, Inc., 2022.
- 21. Srivastava A, Thomson S. Framework analysis: a qualitative methodology for applied policy research. J Administration Governance 2009;4:72–79 [Google Scholar]
- 22. Coobs BR, Xiong A, Clohisy JC. Contemporary concepts in the young adult hip patient: periacetabular osteotomy for hip dysplasia. J Arthroplasty 2015;30:1105–1108 [DOI] [PubMed] [Google Scholar]
- 23. Agich GJ. Ethics and innovation in medicine. J Med Ethics 2001;27:295–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Das S, McKneally M. The surgeon-in-chief should oversee innovative surgical practice. Am J Bioeth 2019;19:34–36 [DOI] [PubMed] [Google Scholar]
- 25. Lau WY, Lai EC. Modifications of ALPPS—from complex to more complex or from complex to less complex operations. Hepatobiliary Pancreat Dis Int 2017;16:346–352 [DOI] [PubMed] [Google Scholar]
- 26. Hirst A, Agha RA, Rosin D, McCulloch P. How can we improve surgical research and innovation?: the IDEAL framework for action. Int J Surg 2013;11:1038–1042 [DOI] [PubMed] [Google Scholar]
- 27. Hirst A, Philippou Y, Blazeby J, Campbell B, Campbell M, Feinberg Jet al. No surgical innovation without evaluation: evolution and further development of the IDEAL framework and recommendations. Ann Surg 2019;269:211–220 [DOI] [PubMed] [Google Scholar]
- 28. McCulloch P, Feinberg J, Philippou Y, Kolias A, Kehoe S, Lancaster Get al. Progress in clinical research in surgery and IDEAL. Lancet 2018;392:88–94 [DOI] [PubMed] [Google Scholar]
- 29. Diener MK, Simon T, Büchler MW, Seiler CM. Surgical evaluation and knowledge transfer–methods of clinical research in surgery. Langenbecks Arch Surg 2012;397:1193–1199 [DOI] [PubMed] [Google Scholar]
- 30. Garber AM. Modernizing device regulation. N Engl J Med 2010;362:1161–1163 [DOI] [PubMed] [Google Scholar]
- 31. Rathi VK, Ross JS, Samuel AM, Mehra S. Postmarket modifications of high-risk therapeutic devices in otolaryngology cleared by the US Food and Drug Administration. Otolaryngol Head Neck Surg 2015;153:400–408 [DOI] [PubMed] [Google Scholar]
- 32. Ezaldein HH, Scott JF, Yin ES, Ventura A, DeRuyter NP, Leffell DJ. Transparency and dermatologic device approval by the US Food and Drug Administration. JAMA Dermatol 2018;154:273–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rome BN, Kramer DB, Kesselheim AS. FDA approval of cardiac implantable electronic devices via original and supplement premarket approval pathways, 1979–2012. JAMA 2014;311:385–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Administration USFaD . PMA Supplements and Amendments. In: Devices M, editor. 2019. https://www.fda.gov/medical-devices/premarket-approval-pma/pma-supplements-and-amendments#references(accessed 1 July 2022)
- 35. Currie A, Brigic A, Blencowe NS, Potter S, Faiz OD, Kennedy RHet al. Systematic review of surgical innovation reporting in laparoendoscopic colonic polyp resection. Br J Surg 2015;102:e108–e116 [DOI] [PubMed] [Google Scholar]
- 36. Zheng SY, Redberg RF. Premarket approval supplement pathway: do we know what we are getting? Ann Intern Med 2014;160:798–799 [DOI] [PubMed] [Google Scholar]
- 37. Mangir N, Roman S, MacNeil S. The changing regulatory landscape for biomedical implants and its relationship to withdrawal of some vaginal mesh products. Curr Opin Urol 2019;29:414–418 [DOI] [PubMed] [Google Scholar]
- 38. Olaiya OR, Oyesile D, Stone N, Mbuagbaw L, McRae MH. Postmarket modifications of high-risk plastic surgery devices. Plast Reconstr Surg Glob Open 2020;8:e2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Biffl WL, Spain DA, Reitsma AM, Minter RM, Upperman J, Wilson Met al. Responsible development and application of surgical innovations: a position statement of the society of university surgeons. J Am Coll Surg 2008;206:1204–1209 [DOI] [PubMed] [Google Scholar]
- 40. McCulloch P, Taylor I, Sasako M, Lovett B, Griffin D. Randomised trials in surgery: problems and possible solutions. BMJ 2002;324:1448–1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Campbell B. Home-made, adapted and modified devices in surgical practice. Ann R Coll Surg Engl 2008;90:251–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stefanidis D, Fanelli RD, Price R, Richardson W, Committee SG. SAGES guidelines for the introduction of new technology and techniques. Surg Endosc 2014;28:2257–2271 [DOI] [PubMed] [Google Scholar]
- 43. Waninger MS, Whirley RG, Smith LJ, Wolf BS. Manufacturer evaluations of endograft modifications. J Vasc Surg 2013;57:826–828 [DOI] [PubMed] [Google Scholar]
- 44. Birchley G, Ives J, Huxtable R, Blazeby J. Conceptualising surgical innovation: an eliminativist proposal. Health Care Anal 2020;28:73–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sedrakyan A, Marinac-Dabic D, Normand SL, Mushlin A, Gross T. A framework for evidence evaluation and methodological issues in implantable device studies. Med Care 2010;48:S121–S128 [DOI] [PubMed] [Google Scholar]
- 46. Cook JA. The challenges faced in the design, conduct and analysis of surgical randomised controlled trials. Trials 2009;10:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lilford R, Braunholtz D, Harris J, Gill T. Trials in surgery. Br J Surg 2003;91:6–16 [DOI] [PubMed] [Google Scholar]
- 48. Kamarajah SK, Chapman SJ, Glasbey J, Morton D, Smart N, Pinkney Tet al. Systematic review of the stage of innovation of biological mesh for complex or contaminated abdominal wall closure. BJS Open 2018;2:371–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hutchison K, Rogers W, Eyers A, Lotz M. Getting clearer about surgical innovation: a new definition and a new tool to support responsible practice. Ann Surg 2015;262:949–954 [DOI] [PubMed] [Google Scholar]
- 50. Peterson J, Pearce PF, Ferguson LA, Langford CA. Understanding scoping reviews: definition, purpose, and process. J Am Assoc Nurse Pract 2017;29:12–16 [DOI] [PubMed] [Google Scholar]
- 51. Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol 2018;18:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pham MT, Rajić A, Greig JD, Sargeant JM, Papadopoulos A, McEwen SA. A scoping review of scoping reviews: advancing the approach and enhancing the consistency. Res Synth Methods 2014;5:371–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Oliver SR, Rees RW, Clarke-Jones L, Milne R, Oakley AR, Gabbay Jet al. A multidimensional conceptual framework for analysing public involvement in health services research. Health Expect 2008;11:72–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Trankle SA, Reath J. Partners in recovery: an early phase evaluation of an Australian mental health initiative using program logic and thematic analysis. BMC Health Serv Res 2019;19:524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Olesson E, Albert E, Coroneos R, Leeson R, Wyatt R. Options for a Local Government Framework for Measuring Liveability. Sydney: Australian Centre of Excellence for Local Government, 2012
- 56. Boutron I, Altman DG, Moher D, Schulz KF, Ravaud P, Group CN. CONSORT statement for randomized trials of nonpharmacologic treatments: a 2017 update and a CONSORT extension for nonpharmacologic trial abstracts. Ann Intern Med 2017;167:40–47 [DOI] [PubMed] [Google Scholar]
- 57. Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher Det al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:g1687. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No new data were collected or generated from this study. All study materials have been made available in the supplementary file.