Abstract
Purpose
Fine-needle aspiration cytology (FNAC) of axillary lymph nodes (AxLNs) is performed to diagnose nodal metastasis in patients with breast cancer. Although the sensitivity of ultrasound-guided FNAC for identifying AxLN metastasis is in the range of 36%–99%, whether sentinel lymph node biopsy (SLNB) should be performed for neoadjuvant chemotherapy (NAC) patients with negative FNAC results is uncertain. This study aimed to determine the role of FNAC before NAC in the evaluation and management of AxLN in early breast cancer patients.
Methods
We retrospectively analyzed 3,810 clinically node-negative (a lymph node with no clinical metastasis without FNAC or radiological suspicion of metastasis with negative FNAC results) patients with breast cancer who underwent SLNB between 2008 and 2019. We compared the positivity rate of sentinel lymph nodes (SLNs) between patients who received and those who did not receive NAC with negative FNAC results or without FNAC and axillary recurrence rate in the neoadjuvant group with negative SLNB results.
Results
In the non-neoadjuvant (primary surgery) group, the positivity rate of SLNs in patients with negative FNAC results was higher than that in patients without FNAC (33.2% vs. 12.9%; p < 0.001). However, the SLN positivity rate of patients with negative FNAC results (false-negative rate for FNAC) in the neoadjuvant group was lower than that in the primary surgery group (3.0% vs. 33.2%; p < 0.001). After a median follow-up of 3 years, one axillary nodal recurrence was observed, which was a case from the neoadjuvant non-FNAC group. None of the patients in the neoadjuvant group with negative FNAC results had axillary recurrence.
Conclusion
The false-negative rate for FNAC in the primary surgery group was high; however, SLNB was the proper axillary staging procedure for NAC patients who have clinically suspicious AxLN metastases on radiologic examination but negative FNAC results.
Keywords: Biopsy, Fine-Needle; Breast Neoplasms; Neoadjuvant Therapy; Sentinel Lymph Node Biopsy
INTRODUCTION
We often perform fine-needle aspiration cytology (FNAC) of radiologically suspicious axillary lymph nodes (AxLNs) in patients with early breast cancer (EBC) to diagnose nodal metastasis. The decision to perform axillary lymph node dissection (ALND) or sentinel lymph node biopsy (SLNB) is based on the FNAC results. Studies report that the sensitivity of ultrasound-guided FNAC for identifying axillary lymph node metastasis in patients with breast cancer is in the range of 36%–99%, and the specificity ranges from 80%–100% [1,2,3,4,5,6,7].
Neoadjuvant chemotherapy (NAC) is administered for EBC before surgery to downstage tumors for breast-conserving surgery. Recently, it has been used for response-guided chemotherapy after surgery [8,9]. However, whether SLNB should be performed for NAC patients, especially with clinically suspicious AxLN metastases on radiologic examination and negative FNAC results, is uncertain, and no studies have reported SLNB results for these NAC patients with negative FNAC.
Hence, we aimed to assess the necessity of FNAC in such cases by analyzing the differences in the positivity rate of sentinel lymph node (SLN) in clinically node-negative (cN0) patients with and without FNAC stratified according to NAC.
METHODS
Study population
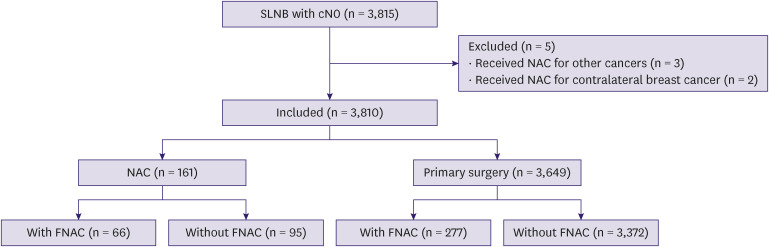
A total of 3,815 patients with cN0 EBC underwent SLNB at our hospital between January 2008 and December 2019. We retrospectively assessed the clinical and pathological data from the clinical records and counted two cases of synchronous or metachronous bilateral breast cancer. We excluded patients who received NAC for other cancers (three cases) and patients with non-invasive ductal carcinoma who received NAC for contralateral invasive breast cancer (two cases) (Figure 1). Thus, we analyzed 3,810 patients with cN0 EBC who underwent SLNB. This study adhered to the principles of the Declaration of Helsinki and was approved by the Ethics Committee of Aichi Cancer Center Hospital (approval number: 2021-1-092). Written and oral informed consent was obtained from each patient before inclusion in the study.
Figure 1. Schema of this analysis.
SLNB = sentinel lymph node biopsy; NAC = neoadjuvant chemotherapy; FNAC = fine-needle aspiration cytology.
Assessment of AxLN metastasis
To evaluate AxLN metastasis, all patients underwent palpation, followed by breast and axillary ultrasonography. Patients underwent additional radiological examinations before primary treatment (computed tomography, positron emission tomography/computed tomography, or magnetic resonance imaging), according to the physician’s preference. Physicians performed FNAC for suspected cases of AxLN metastasis. FNAC was performed under ultrasonography guidance with a 21G needle. Cytology results were categorized in accordance with the Japanese guidelines (General Rules for Clinical and Pathological Recording of Breast Cancer, 18th edition) for quality assurance in breast cancer screening as follows: inadequate, normal or benign, indeterminate, suspicious for malignancy, or malignant [10]. Malignant lesions were defined as node-positive. In this study, cN0 was defined as a lymph node with no clinical metastasis without FNAC or radiological suspicion of metastasis with FNAC and cytology-negative lymph nodes.
Surgical treatment
SLNB was performed using technetium (99mTc) and a blue dye (indigo carmine or indocyanine green). 99mTc was injected into the areola, and lymphoscintigraphy was performed 3 hours after the injection on the day before surgery. Indigo carmine or indocyanine green was injected into the areola immediately before surgery. Resected SLNs were evaluated for metastases by a pathologist intraoperatively and postoperatively with hematoxylin and eosin-stained specimens. Positive or negative lymph node metastasis was defined according to the UICC (Union for International Cancer Control) 8th edition TNM staging system for breast cancer; therefore, isolated tumor cells were defined as negative. In the NAC group, all patients underwent SLNB at the same time as breast surgery after NAC. ALND was performed in all patients with positive SLNB results and omitted in all patients with negative SLNB results.
Pathological assessment and definition of molecular subtypes
Breast cancer subtypes were evaluated from biopsy specimens before NAC, according to the immunohistochemical classification proposed by the 2011 St. Gallen Expert Panel as follows: Luminal: estrogen receptor (ER) and/or progesterone receptor (PgR) positive, and human epidermal growth factor receptor 2 (HER2)-negative; Luminal-HER2: ER and/or PgR positive, and HER2 positive; HER2: ER and PgR negative and HER2 positive; Triple negative: ER, PgR, and HER2 negative [11]. ER positivity was defined as an Allred score ≥ 3. HER2-positive was defined as a Hercep test score of 3+ or fluorescent in situ hybridization positivity following a Hercep test score of 2+. Tumor stage was stratified according to the UICC for International Cancer Control 8th edition TNM staging system for breast cancer.
In this study, we defined pathological complete response (pCR) as pT0/is and pN0.
Systemic and radiation therapies in the NAC group
In the NAC group, perioperative systemic treatment was performed according to the general guidelines. Patients who underwent breast-conserving surgery also underwent whole-breast radiotherapy. Additionally, patients with high-risk T3 or T4 tumors who underwent mastectomy received postmastectomy radiotherapy.
Statistical analysis
We assessed the positivity rates of SLN with subgroups (primary surgery group and NAC groups, each divided into with or without FNAC). We compared patient characteristics, axillary recurrence rate (ARR), and 3-year distant recurrence-free survival (3-y DRFS) in the NAC group with negative SLNB divided into those with or without FNAC. DRFS was defined as the time to distant recurrence or all-cause mortality. Differences in the positivity rates of SLN and patient characteristics were calculated using the χ2 test. The Kaplan–Meier method was used to plot distant recurrence-free survival and cumulative incidence of axillary recurrence events. The log-rank test was used to test the differences in the Kaplan–Meier curves. Survival was calculated from the date of surgery to the date of the event or latest follow-up. A two-tailed p value < 0.05 was considered significant. Statistical analysis was performed using the Stata software (version 15.0; StataCorp, College Station, TX, USA).
RESULTS
We assessed 3,810 patients with cN0, a lymph node with no clinical metastasis without FNAC or radiological suspicion of metastasis with negative FNAC results, EBC who underwent SLNB; the analysis is schematically represented in Figure 1. Of the 3,810 patients, 161 received NAC (NAC group) and 3,649 did not (primary surgery group). In the NAC group, 66 patients underwent FNAC before chemotherapy, and 95 patients did not. In the primary surgery group, 277 patients underwent FNAC before surgery, and 3,372 patients did not. All these patients who underwent FNAC had negative FNAC results.
The patient and pathological tumor characteristics of the NAC group are shown in Table 1. Statistically significant differences were observed in clinical tumor size (p = 0.003), progesterone receptor status (p = 0.021), histological grade (p = 0.009), and radiotherapy (p = 0.0013) between patients with and without FNAC. However, other factors (ER status, HER2 status, subtype, surgery, chemotherapy, number of nodes removed, and pathological response) showed no significant differences between patients with and without FNAC.
Table 1. Patient characteristics in neoadjuvant chemotherapy group.
| Variables | With FNAC | Without FNAC | p-value | |
|---|---|---|---|---|
| All patients | 66 | 95 | ||
| Age | 49 (23–78) | 51 (29–78) | ||
| Clinical tumor size | 0.003 | |||
| cT1 | 3 (4.6) | 17 (17.9) | ||
| cT2 | 49 (74.2) | 73 (76.8) | ||
| cT3 | 9 (13.6) | 3 (3.2) | ||
| cT4 | 5 (7.6) | 2 (2.1) | ||
| Estrogen receptor status | 0.286 | |||
| Positive | 28 (42.4) | 50 (52.6) | ||
| Negative | 38 (57.6) | 44 (46.3) | ||
| Unknown | 0 | 1 (1.1) | ||
| Progesterone receptor status | 0.021 | |||
| Positive | 13 (19.7) | 37 (38.9) | ||
| Negative | 53 (80.3) | 57 (60.0) | ||
| Unknown | 0 | 1 (1.1) | ||
| HER2 status | 0.178 | |||
| Positive | 32 (48.5) | 58 (61.0) | ||
| Negative | 34 (51.5) | 36 (37.9) | ||
| Unknown | 0 | 1 (1.1) | ||
| Histological grade | 0.009 | |||
| 1 | 2 (3.0) | 3 (3.2) | ||
| 2 | 11 (16.7) | 36 (37.9) | ||
| 3 | 53 (80.3) | 53 (55.8) | ||
| Unknown | 0 | 3 (3.2) | ||
| Subtype | 0.243 | |||
| Luminal | 13 (19.7) | 18 (18.9) | ||
| Luminal-HER2 | 16 (24.2) | 33 (34.7) | ||
| HER2 | 16 (24.2) | 26 (27.4) | ||
| TN | 21 (31.8) | 17 (17.9) | ||
| Unknown | 0 | 1 (1.1) | ||
| Surgery | 0.057 | |||
| Mastectomy | 35 (53.0) | 36 (37.9) | ||
| Lumpectomy | 31 (47.0) | 59 (62.1) | ||
| Chemotherapy | 0.585 | |||
| Anthracycline | 8 (12.1) | 13 (13.7) | ||
| Taxane | 3 (4.6) | 8 (8.4) | ||
| Anthracycline-taxane | 55 (83.3) | 74 (77.9) | ||
| Number of nodes removed | 0.185 | |||
| 1 | 25 (37.9) | 42 (44.2) | ||
| 2 | 26 (39.4) | 35 (36.8) | ||
| 3 | 7 (10.6) | 13 (13.7) | ||
| 4 | 7 (10.6) | 2 (2.1) | ||
| 5 | 1 (1.5) | 3 (3.2) | ||
| Radiotherapy | 0.013 | |||
| PMRT | 9 (13.6) | 3 (3.2) | ||
| WBRT | 29 (44.0) | 59 (62.1) | ||
| None | 28 (42.4) | 33 (34.7) | ||
| Pathological response | 0.618 | |||
| pCR (pT0/is pN0) | 29 (43.9) | 38 (40.0) | ||
| Non-pCR | 37 (56.1) | 57 (60.0) | ||
Values are presented as number (%) or median (range).
FNAC = fine-needle aspiration cytology, HER2 = human epidermal growth factor receptor-2, PMRT = postmastectomy radiotherapy, WBRT = whole-breast radiotherapy, pCR = pathological complete response.
The positivity rates of SLN are shown in Table 2. In the primary surgery group, the positivity rate of SLN in patients with negative FNAC results was significantly higher than that in patients without FNAC (33.2% vs. 12.9%; p < 0.001). In the NAC group, there was no difference in the positivity rate of SLN in patients without FNAC (3.0% vs. 7.4%; p = 0.311). Furthermore, in patients with negative FNAC results, the positivity rate of SLN in the NAC group was significantly lower than that in the primary surgery group (3.0% vs. 33.2%; p < 0.001).
Table 2. Positivity rate of sentinel lymph node with/without fine-needle aspiration cytology.
| Variables | NAC | Primary surgery | p-value |
|---|---|---|---|
| With FNAC | 3.0% | 33.2% | < 0.001 |
| Without FNAC | 7.4% | 12.9% | 0.109 |
| p-value | 0.311 | < 0.001 |
NAC = neoadjuvant chemotherapy; FNAC = fine-needle aspiration cytology.
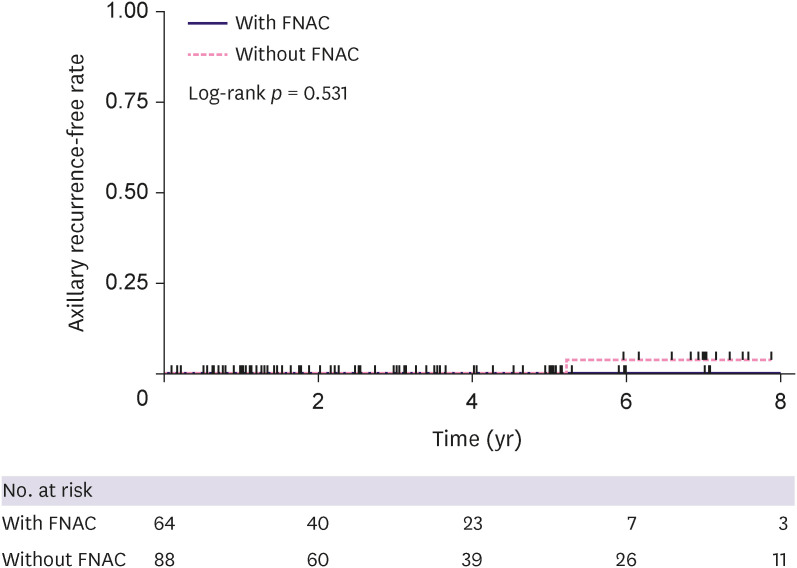
In the 152 patients with negative SLN who underwent NAC, we assessed ARR in each group (with FNAC [n = 64] and without FNAC [n = 88]). One patient (1.1%) without FNAC showed axillary recurrence 5.2 years after surgery, and no patient with negative FNAC results showed axillary recurrence (median follow-up, 3.0 [range 0.1–11.5]). The cumulative incidence of ARR is shown in Figure 2 (log-rank p = 0.531).
Figure 2. Axillary recurrence-free rate in the neoadjuvant chemotherapy group.
FNAC = fine-needle aspiration cytology.
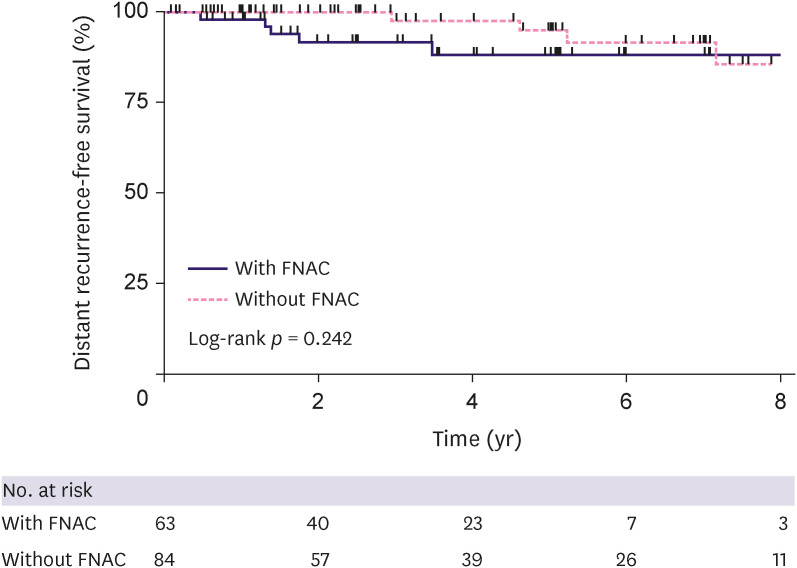
Among 147 patients (excluding patients with bilateral breast cancer), we assessed DRFS in each group (with FNAC [n = 63] and without FNAC [n = 84]); 3-y DRFS was 92.0% (95% confidence interval [CI], 80.0–97.0) and 97.8% (95% CI, 85.3.5–99.7) in patients with negative FNAC results and without FNAC, respectively. There was no statistical difference in the DRFS between the two groups (p = 0.242, Figure 3).
Figure 3. Distant recurrence-free survival in the neoadjuvant chemotherapy group.
FNAC = fine-needle aspiration cytology.
DISCUSSION
In our study, the positivity rate of SLN in the primary surgery group with negative FNAC results was statistically higher than that in the group without FNAC. These results indicate that lymph nodes suspected to be clinically or radiologically metastatic are more likely to be metastasis-positive, even if they are diagnosed as metastasis-negative using FNAC. In this study, the false-negative rate for FNAC and the positivity rate of SLN in patients who underwent FNAC were synonymous, and the false-negative rate for FNAC in the primary surgery group (33.2%) was similar to that reported in a previous study (31%) [7]. However, in patients who underwent FNAC, the SLN positivity rate in the NAC group was significantly lower than that in the primary surgery group. This may indicate that AxLN metastasis resolves after NAC, or that the result of SLNB was a false negative. Previous systematic reviews reported that the false-negative rate of SLNB in patients undergoing NAC was 6% (95% CI, 3–8) [12]. In either case, it is important that the ARR was very low in this study and that ALND could have been avoided in many cN0 patients after NAC. Therefore, SLNB should be performed even in patients with negative FNAC results prior to NAC. On the other hand, SLNB before NAC is one option to accurately evaluate AxLN metastasis; however, SLNB before NAC is considered unnecessary because no patients with negative FNAC results have had axillary recurrence despite the high false-negative rate for FNAC before NAC. Thus, we could perform FNAC instead of SLNB before NAC in the evaluation and management of AxLN from the point of view of minimally invasive procedures.
In contrast, FNAC in some patients with radiologically suspected AxLN metastasis may cause overdiagnosis. ALND after NAC for patients with positive FNAC results before NAC may be overtreated because currently, in majority of Japanese institutions, patients with cN1 who convert to cN0 after NAC still undergo ALND, to account for the false-negative rates in accordance with the Japanese Breast Cancer Society Clinical Practice Guidelines, 2018 edition [13]. However, at the 17th St. Gallen International Breast Cancer Consensus Conference in 2021, expert panelists considered that patients with cN1 who convert to cN0 after NAC are potential candidates for SLNB. If these patients have no residual nodal disease when clipped lymph nodes or at least three SLNs are identified and resected, they do not require ALND [14]. To begin with, the definition of ‘cN0’ used by physicians, countries, and clinical trials is ambiguous; therefore, we need to keep in mind that FNAC may cause overdiagnosis. Thus, it may be unnecessary to perform FNAC in the NAC group. To prove this hypothesis, we compared the ARRs of patients with negative FNAC results and those with positive FNAC results who converted to cN0 after NAC. However, we could not evaluate the latter patients because all patients underwent ALND in our hospital if they converted to cN0 after NAC. We will consider omitting ALND in these patients in the future and investigating this hypothesis.
In a previous study with a design similar to ours, the positivity rate of SLN in the NAC group was 19.1%, which was higher than that in our study (3.0% and 7.4%) [15]. This difference may be because previous studies included more Luminal patients and fewer TN and HER2 patients than in our study. According to another report [16], the pCR rates of TN and HER2 breast cancer were higher than those of Luminal breast cancer; thus, in this study, pre-existing AxLN metastasis could have been resolved with NAC, especially in TN and HER2 patients. Therefore, we should carefully choose patients with TN and HER2 breast cancers to undergo FNAC.
This study had several limitations. This was a single-center retrospective study, and the decision to perform FNAC was based on physician preference. We would recommend defining objective criteria for FNAC, such as diffuse cortical thickening, focal cortical mass, and/or effacement or replacement of the fatty hilum of the lymph node [17]; further prospective studies are required. Another limitation is the short median follow-up time (3.0 years); a long follow-up period could have been more informative. However, our study demonstrated few early AxLN recurrences in cN0 patients who underwent SLNB after NAC. Few early recurrences are important for high-grade tumors, such as TN and HER2, because previous studies have reported that patients with TN and HER2 breast cancer had a high risk of early recurrence [18,19]. Furthermore, we did not show the patient and pathological tumor characteristics in the primary surgery group. More Luminal patients were included in the primary surgery group than in the NAC group. However, Vane et al. [20] reported no significant difference in the sensitivity of axillary ultrasonography between the subtypes.
In conclusion, our findings showed that SLNB was the proper axillary staging procedure for NAC patients, even in those with clinically suspicious AxLN metastases on radiologic examination and negative FNAC results. Thus, we do not need to perform SLNB before NAC, although the false negative rate for FNAC before NAC is high. Conversely, in some ‘cN1’ cases, FNAC before NAC may cause overdiagnosis and ALND may lead to overtreatment. Thus, it may be unnecessary to perform FNAC in the NAC group; however, further studies are needed in the future.
ACKNOWLEDGMENTS
The authors received no grant for this study. We thank Editage (www.editage.com) for editing the manuscript.
Footnotes
Conflicts of Interest: The authors declare that they have no competing interests.
- Conceptualization: Iwata H.
- Investigation: Yoshimura A, Sawaki M, Hattori M, Kotani H, Kataoka A, Horisawa N, Ozaki Y, Endo Y, Nozawa K.
- Supervision: Iwata H.
- Visualization: Takatsuka D. Writing - original draft.
- Writing - review & editing: Takatsuka D.
References
- 1.Krishnamurthy S, Sneige N, Bedi DG, Edieken BS, Fornage BD, Kuerer HM, et al. Role of ultrasound-guided fine-needle aspiration of indeterminate and suspicious axillary lymph nodes in the initial staging of breast carcinoma. Cancer. 2002;95:982–988. doi: 10.1002/cncr.10786. [DOI] [PubMed] [Google Scholar]
- 2.Kuenen-Boumeester V, Menke-Pluymers M, de Kanter AY, Obdeijn IM, Urich D, Van Der Kwast TH. Ultrasound-guided fine needle aspiration cytology of axillary lymph nodes in breast cancer patients. A preoperative staging procedure. Eur J Cancer. 2003;39:170–174. doi: 10.1016/s0959-8049(02)00501-4. [DOI] [PubMed] [Google Scholar]
- 3.Bonnema J, van Geel AN, van Ooijen B, Mali SP, Tjiam SL, Henzen-Logmans SC, et al. Ultrasound-guided aspiration biopsy for detection of nonpalpable axillary node metastases in breast cancer patients: new diagnostic method. World J Surg. 1997;21:270–274. doi: 10.1007/s002689900227. [DOI] [PubMed] [Google Scholar]
- 4.Ciatto S, Brancato B, Risso G, Ambrogetti D, Bulgaresi P, Maddau C, et al. Accuracy of fine needle aspiration cytology (FNAC) of axillary lymph nodes as a triage test in breast cancer staging. Breast Cancer Res Treat. 2007;103:85–91. doi: 10.1007/s10549-006-9355-0. [DOI] [PubMed] [Google Scholar]
- 5.Jung J, Park H, Park J, Kim H. Accuracy of preoperative ultrasound and ultrasound-guided fine needle aspiration cytology for axillary staging in breast cancer. ANZ J Surg. 2010;80:271–275. doi: 10.1111/j.1445-2197.2009.05090.x. [DOI] [PubMed] [Google Scholar]
- 6.Fung AD, Collins JA, Campassi C, Ioffe OB, Staats PN. Performance characteristics of ultrasound-guided fine-needle aspiration of axillary lymph nodes for metastatic breast cancer employing rapid on-site evaluation of adequacy: analysis of 136 cases and review of the literature. Cancer Cytopathol. 2014;122:282–291. doi: 10.1002/cncy.21384. [DOI] [PubMed] [Google Scholar]
- 7.Kane G, Fleming C, Heneghan H, McCartan D, James P, Trueick R, et al. False-negative rate of ultrasound-guided fine-needle aspiration cytology for identifying axillary lymph node metastasis in breast cancer patients. Breast J. 2019;25:848–852. doi: 10.1111/tbj.13402. [DOI] [PubMed] [Google Scholar]
- 8.Masuda N, Lee SJ, Ohtani S, Im YH, Lee ES, Yokota I, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376:2147–2159. doi: 10.1056/NEJMoa1612645. [DOI] [PubMed] [Google Scholar]
- 9.von Minckwitz G, Huang CS, Mano MS, Loibl S, Mamounas EP, Untch M, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380:617–628. doi: 10.1056/NEJMoa1814017. [DOI] [PubMed] [Google Scholar]
- 10.Japanese Breast Cancer Society. General Rules for Clinical and Pathological Recording of Breast Cancer. 18th ed. Tokyo: Kanahara Shuppan; 2018. pp. 77–78. [Google Scholar]
- 11.Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, Senn HJ, et al. Strategies for subtypes--dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22:1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geng C, Chen X, Pan X, Li J. The feasibility and accuracy of sentinel lymph node biopsy in initially clinically node-negative breast cancer after neoadjuvant chemotherapy: a systematic review and meta-analysis. PLoS One. 2016;11:e0162605. doi: 10.1371/journal.pone.0162605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inokuchi M, Kutomi G, Kijima Y, Sakai T, Sawaki M, Shien T, et al. The Japanese Breast Cancer Society clinical practice guidelines for surgical treatment of breast cancer, 2018 edition. Breast Cancer. 2020;27:4–8. doi: 10.1007/s12282-019-01030-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burstein HJ, Curigliano G, Thürlimann B, Weber WP, Poortmans P, Regan MM, et al. Customizing local and systemic therapies for women with early breast cancer: the St. Gallen International Consensus Guidelines for treatment of early breast cancer 2021. Ann Oncol. 2021;32:1216–1235. doi: 10.1016/j.annonc.2021.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nogi H, Uchida K, Mimoto R, Kamio M, Shioya H, Toriumi Y, et al. Long-term follow-up of node-negative breast cancer patients evaluated via sentinel node biopsy after neoadjuvant chemotherapy. Clin Breast Cancer. 2017;17:644–649. doi: 10.1016/j.clbc.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164–172. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 17.Neal CH, Daly CP, Nees AV, Helvie MA. Can preoperative axillary US help exclude N2 and N3 metastatic breast cancer? Radiology. 2010;257:335–341. doi: 10.1148/radiol.10100296. [DOI] [PubMed] [Google Scholar]
- 18.Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 19.Bradley R, Braybrooke J, Gray R, Hills R, Liu Z, Peto R, et al. Trastuzumab for early-stage, HER2-positive breast cancer: a meta-analysis of 13 864 women in seven randomised trials. Lancet Oncol. 2021;22:1139–1150. doi: 10.1016/S1470-2045(21)00288-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vane ML, van Nijnatten TJ, Nelemans PJ, Lobbes MB, van Roozendaal LM, Kooreman LF, et al. Does the subtype of breast cancer affect the diagnostic performance of axillary ultrasound for nodal staging in breast cancer patients? Eur J Surg Oncol. 2019;45:573–577. doi: 10.1016/j.ejso.2019.01.012. [DOI] [PubMed] [Google Scholar]