Abstract
Purpose
Cancer antigen 15-3 (CA15-3) is a serum tumor marker for breast cancer (BC) extensively used in clinical practice. CA15-3 is non-invasive, easily available, and a cost-effective tumor marker for immediate diagnosis, monitoring and prediction of BC recurrence. We hypothesized that an elevation of CA15-3 may have prognostic impact in patients with early BC with normal serum CA15-3 level.
Methods
This was a retrospective cohort study, which included patients with BC who received curative surgery at a comprehensive single institution between 2000 and 2016. CA15-3 levels from 0 to 30 U/mL were considered normal, and patients who had CA15-3 > 30 U/mL, were excluded from the study.
Results
The mean age of study participants (n = 11,452) was 49.3 years. The proportion of participants with elevated CA15-3 ≥ 1 standard deviation (SD) compared with the previous examination during follow-up was 23.3% (n = 2,666). During the follow-up (median follow-up 5.8 years), 790 patients experienced recurrence. The fully-adjusted hazard ratio (HR) for recurrence comparing participants with stable CA15-3 level to subjects with elevated CA15-3 level was 1.76 (95% confidence interval [CI], 1.52–2.03). In addition, if the CA15-3 was elevated ≥ 1 SD, the risk was much higher (HR, 6.87; 95% CI, 5.81–8.11) than in patients without elevated CA15-3 ≥ 1 SD. In sensitivity analysis, the recurrence risk was consistently higher in participants with elevated CA15-3 levels than in participants without elevated CA15-3 levels. The association between elevated CA15-3 levels and incidence of recurrence was observed in all subtypes and the association was stronger in patients with N+ than in patients with N0 stage (p-value for interaction < 0.01).
Conclusion
The results of the present study demonstrated that elevation of CA15-3 in patients with early BC and initial normal serum CA15-3 levels has a prognostic impact.
Keywords: Breast Neoplasms, Prognosis, Survival
INTRODUCTION
Breast cancer (BC) is the most common cancer in the world as well as the most common malignancy in Korean women, and the incidence continues to increase [1,2]. Due to increased early detection with cancer screening programs and advances in systemic treatment such as chemotherapy, anti-hormone therapy, and human epidermal growth factor 2 (HER2)-targeted therapy, more patients are surviving after treatment for BC [3].
Current surveillance guidelines for follow-up after a diagnosis of BC recommend regular mammography (MMG) and physical examinations as well as further symptom-related laboratory tests and imaging tests, such as computed tomography (CT) or positron emission tomography-CT scans [4,5]. These guidelines are based on data from clinical trials performed in the early 1990’s, which did not show any survival benefit with the early detection of distant metastasis [6,7]. However, those clinical trials were mainly conducted using imaging modalities with poor sensitivity (e.g., chest X-ray), physical examinations with examiner-dependent variation of sensitivity (e.g., abdominal sonography), or procedures with limited specificity (e.g., bone scan), and did not include tumor markers (e.g., cancer antigen 15-3 [CA15-3]).
CA15-3 is a serum tumor marker for BC extensively used in clinical practice. CA15-3 is non-invasive, easily available, and a cost-effective tumor marker for immediate diagnosis, monitoring, and prediction of BC in early, advanced, and metastatic BC [8,9,10]. However, to the best of our knowledge, its clinical value within normal range has not been assessed. We hypothesized that an elevation of CA15-3 levels which were initially within normal ranges in patients with early BC could affect recurrence of BC; thus, the association between elevated CA15-3 levels and BC recurrence was analyzed in the present study.
METHODS
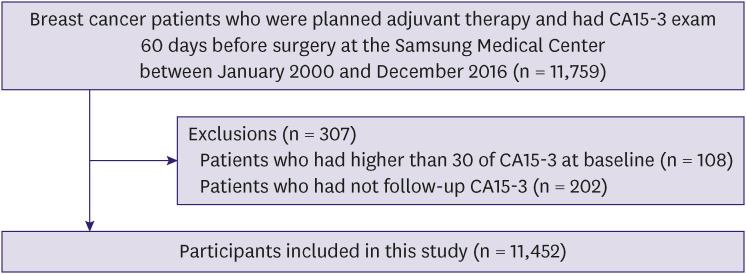
This was a retrospective cohort study conducted using a Samsung Medical Center Breast Cancer Surgery Treatment Registry (SMC_BCSTR) [11]. The registry included patients with BC who received curative surgery followed by adjuvant treatment at Samsung Medical Center (SMC) between January 2000 and December 2016. Patients who underwent neoadjuvant chemotherapy, had distant metastasis, had experienced BC and/or other cancer were excluded. We measured CA15-3 levels at baseline when BC was diagnosed, and followed up regularly for monitoring because CA15-3 is non-invasive, easily available, and cost-effective. Since the objective was to evaluate the prospective association between changes in CA15-3 levels and recurrence among patients whose initial CA15-3 levels were within normal range, the analysis was restricted to subjects who underwent CA15-3 examination within 60 days before surgery (n = 11,759). Participants who had CA15-3 > 30 U/mL (n = 108) and who did not have a follow-up CA15-3 (n = 202) were excluded. The final sample size was 11,452 (Figure 1). De-identified data for the events of recurrence were used and visits extracted from the Clinical Data Warehouse of SMC (DARWIN-C), which contains data from 1994 onward. Recurrence was defined as any BC recurrence including locoregional recurrence, distant metastasis, and contralateral BC diagnosed by imaging and/or biopsy. We followed up every six months with physical examination, breast ultrasonography and/or breast MMG and tumor markers, and every year with physical examination, CT scan and tumor marker estimations. The Institutional Review Board of SMC approved this study (IRB No. 2018-06-137) and waived the requirement for informed consent because only de-identified data routinely collected during health screening visits were used.
Figure 1. Schematic diagram for participants inclusion.
CA15-3 = cancer antigen 15-3.
Measurements
The measurement of serum CA15-3 was performed using immunoradiometric assay. CA15-3 levels from 0 to 30 U/mL were considered normal. The measurement of serum CA15-3 was performed using a commercially available immunoradiometric kit (ELSA-CA15-3; CIS Diagnostici, Vercelli, Italy). There was no modification in the methods of immunoradiometric assay in our institute between January 2000 and December 2016. CA15-3 levels from 0 to 30 U/mL were considered normal, in accordance with the manufacturer’s protocol. In this study, recurrence was defined as the first detected event of local or/and distant BC recurrence. The pathologic stage was based on the criteria of the American Joint Committee on Cancer, 7th edition. Two experienced pathologists reviewed and determined the primary tumor characteristics based on size, axillary nodal status, and receptor status (estrogen receptor [ER], progesterone receptor (PR), and anti-HER2) using immunohistochemical (IHC) staining. ER positivity (ER+) and PR positivity (PR+) were defined as an Allred score of 3–8 based on IHC staining with antibodies against ER (Immunotech, Marseille, France) and PR (Novocastra, Newcastle upon Tyne, UK), respectively. HER2 status was evaluated using the appropriate antibody (Dako, Carpinteria, USA) and/or silver in situ hybridization (SISH). HER2 grades 0 and 1 indicated a negative result and grade 11 indicated a positive result. HER2 amplification was confirmed using SISH for results of 2+. Triple negative BC was defined as BC with negative ER (ER−) and PR (PR−) expression and lack of HER2 overexpression.
Statistical analysis
Participants were included in the study on the date of surgery (baseline) and were followed up until study endpoint, death, or the last available visit. The study endpoints were the development of recurrence. In a sensitivity analysis, we excluded patients who had CA15-3 levels above 30 U/mL during follow-up, as this could have led to more intensive surveillance.
The study exposure was change in the CA15-3 levels as a time-varying variable [12]. In time-dependent exposure design, all changes at each visit contributed until the next examination (e.g., visit 2). The changes were continuedly updated by the new examination results. Then the weighted average risk of recurrence of all the visits were calculated using log rank test and a cox proportional hazards regression model [13]. In statistics, the standard deviation (SD) is a measure of the amount of variation or dispersion of a set of values. Thus, we performed analyses when the CA15-3 changed more than the acceptable amount of variation. In addition to categorical analysis, we modeled changes in the CA15-3 level compared with previous examination as continuous variables using restricted cubic splines with knots at the 5th, 35th, 65th, and 95th percentiles of the sample distribution to provide a flexible estimate of the dose-response relationship between change in the CA15-3 level and recurrence. To account for additional potential confounding factors, we adjusted for age, pathological T stage, pathological N stage, hormone receptor, HER2, chemotherapy, radiation therapy, hormone therapy, and targeted therapy.
We also conducted an additional sensitivity analysis to investigate the association between elevated CA15-3 levels compared to the baseline and recurrence. We defined the elevated CA15-3 group as those who experienced a 1 SD or greater increase in CA15-3 levels compared to their baseline measurement. The patients were considered as an elevated CA15-3 group from the initial elevated exam date to the end of the follow-up.
All reported p-values were two-sided and the statistical significance was set at 0.05. All analyses were performed using STATA version 14 (StataCorp LP, College Station, USA).
RESULTS
The mean ± SD age of study participants (n = 11,452) was 49.3 ± 9.8 years and 57.5% were premenopausal. The proportion of participants with elevated CA15-3 ≥ 1 SD compared with previous examinations during follow-up was 23% (n = 2,666). The median time interval from the baseline to the time when CA15-3 increased was 293 days (9.7 months). Compared with patients with stable CA15-3 level, those with elevated CA15-3 ≥ 1 SD were less likely to have N0 stage (66.3% vs. 54.9%, p < 0.01), ER+ or PR+ (77.8% vs. 73.9%, p < 0.01), were more likely to receive chemotherapy (61.0% vs. 75.5%, p < 0.01), radiation therapy (73.4% vs. 77.2%, p < 0.01), and to have higher CA15-3 levels at surgery (9.6 vs. 11.4, p < 0.01; Table 1).
Table 1. Characteristics of study participants.
| Characteristics | Overall (n = 11,452) | Stable (n = 8,786) | 1 SD elevated* (n = 2,666) | p-value | ||
|---|---|---|---|---|---|---|
| Age (yr) | 49.3 ± 9.8 | 49.3 ± 9.7 | 49.5 ± 10.1 | 0.59 | ||
| Pathologic T stage | ||||||
| In situ | 28 (0.2) | 21 (0.2) | 7 (0.3) | |||
| T1 | 6,997 (61.1) | 5,472 (62.3) | 1,525 (57.2) | |||
| T2 | 3,945 (34.5) | 2,954 (33.6) | 991 (37.2) | |||
| T3 | 445 (3.9) | 310 (3.5) | 135 (5.1) | |||
| T4 | 13 (0.1) | 6 (0.1) | 7 (0.3) | |||
| Unknown | 24 (0.2) | 23 (0.3) | 1 (0.0) | |||
| Pathologic N stage | < 0.01 | |||||
| N0 | 7,286 (63.6) | 5,823 (66.3) | 1,463 (54.9) | |||
| N1 | 2,980 (26) | 2,155 (24.5) | 825 (30.9) | |||
| N2 | 747 (6.5) | 514 (5.9) | 233 (8.7) | |||
| N3 | 410 (3.6) | 271 (3.1) | 139 (5.2) | |||
| Unknown | 29 (0.3) | 23 (0.3) | 6 (0.2) | |||
| Subtype | < 0.01 | |||||
| Luminal A (ER or PR+/HER2−) | 7,504 (65.5) | 5,815 (66.2) | 1,689 (63.4) | |||
| Luminal B (ER or PR+/HER2+) | 1,209 (10.6) | 929 (10.6) | 280 (10.5) | |||
| HER2+ (ER and PR−/HER2+) | 1,070 (9.3) | 815 (9.3) | 255 (9.6) | |||
| TNBC (ER and PR−/HER2−) | 1,432 (12.5) | 1,040 (11.8) | 392 (14.7) | |||
| Unknown | 237 (2.1) | 187 (2.1) | 50 (1.9) | |||
| Treatment | ||||||
| Chemotherapy | < 0.01 | |||||
| No | 4,020 (35.1) | 3,373 (38.4) | 647 (24.3) | |||
| Yes | 7,373 (64.4) | 5,359 (61) | 2,014 (75.5) | |||
| Unknown | 59 (0.5) | 54 (0.6) | 5 (0.2) | |||
| Radiation therapy | < 0.01 | |||||
| No | 2,858 (25.0) | 2,259 (25.7) | 599 (22.5) | |||
| Yes | 8,505 (74.3) | 6,447 (73.4) | 2,058 (77.2) | |||
| Unknown | 89 (0.8) | 80 (0.9) | 9 (0.3) | |||
| Hormone therapy | < 0.01 | |||||
| No | 2,518 (22.0) | 1,879 (21.4) | 639 (24.0) | |||
| Yes | 8,831 (77.1) | 6,815 (77.6) | 2,016 (75.6) | |||
| Unknown | 103 (0.9) | 92 (1.0) | 11 (0.4) | |||
| Baseline CA15-3 (U/mL) | 10.0 (4.5) | 9.6 (4.3) | 11.4 (5.0) | < 0.01 | ||
Values are presented as mean ± standard deviation or number (%).
SD = standard deviation; ER = estrogen receptor; PR = progesterone receptor; HER2 = human epidermal growth factor 2; CA15-3 = cancer antigen 15-3; TNBC = triple negative breast cancer.
*Elevated ≥ 1 SD of baseline CA15-3 (4.5).
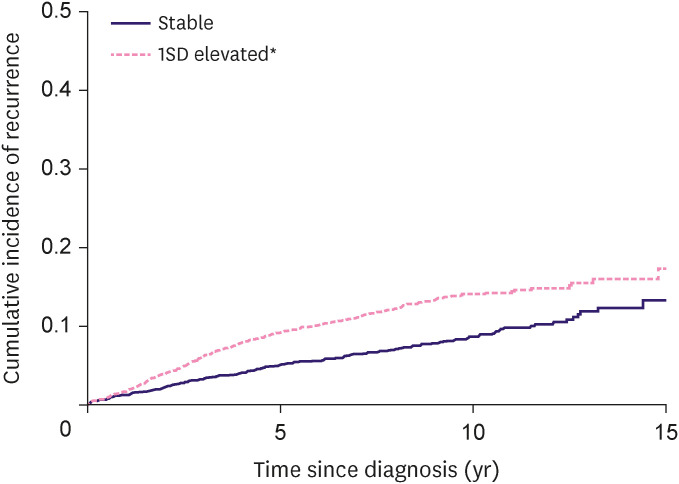
During the follow-up (median follow-up of 5.8 years), 790 patients experienced recurrence. The incidence rate per 100 person-years in participants with stable and elevated CA15-3 levels was 1.0 and 1.7, respectively. Patients with elevated CA15-3 levels were likely to have a higher cumulative incidence of recurrence than patients with stable CA15-3 levels (log rank test p-value < 0.01; Figure 2). Compared with patients with stable CA15-3 level, patients with elevated CA15-3 level had a higher risk of recurrence (hazard ratio [HR], 1.76; 95% confidence interval [CI], 1.52–2.03; Table 2). In addition, if the CA15-3 levels were elevated ≥ 1 SD, the risk was significantly higher (HR, 6.87; 95% CI, 5.81–8.11) than in patients without CA15-3 levels elevated ≥ 1 SD (Table 2). The median time of increase of CA15-3 and the time at which the recurrence was actually diagnosed were 293 days (9.7 months) and 835 days (27.6 months). In sensitivity analysis, even when CA15-3 was < 30 U/mL, the recurrence risk was consistently higher in participants who had elevated CA15-3 level than in subjects who did not (Table 2).
Figure 2. Kaplan-Meier curve for recurrence by elevated cancer antigen 15-3 level.
SD = standard deviation.
Table 2. Hazard ratio for recurrence based on change in cancer antigen 15-3.
| Change between previous examination and current examination | No. of recurrence (incidence rate per 100 person-years) | HR (95% CI) | ||
|---|---|---|---|---|
| Crude | Adjusted* | |||
| Change of CA15-3 | ||||
| Stable | 298 (1.0) | Reference | Reference | |
| Elevated | 492 (1.7) | 1.72 (1.49–1.99) | 1.75 (1.52–2.02) | |
| More than 1 SD change of CA15-3 | ||||
| Stable | 844 (1.3) | Reference | Reference | |
| Elevated 1 SDa | 166 (8.8) | 7.05 (5.97–8.33) | 6.86 (5.80–8.11) | |
Adjusted for age, pathological N stage, hormone receptor, human epidermal growth factor 2, chemotherapy, radiation therapy, and hormone therapy.
HR = hazard ratio; CI = confidence interval; CA15-3 = cancer antigen 15-3; SD = standard deviation.
*Elevated ≥ 1 SD of baseline CA15-3 (4.5).
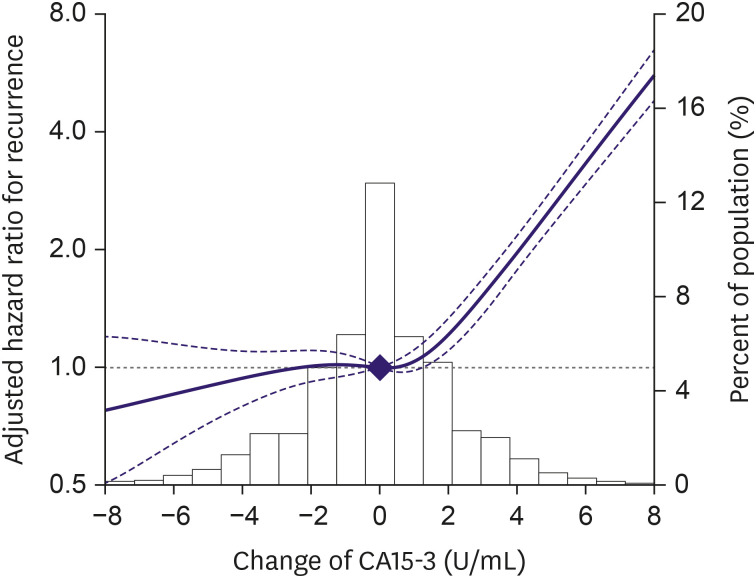
The association between elevated CA15-3 levels and the incidence of recurrence was observed in all subtypes (Table 3), although the association was stronger in patients with N+ than in those with N0 stage (p-value for interaction < 0.01). In spline regression models, the association between changes in CA15-3 levels and the incidence of recurrence was nonlinear, with a stronger association at elevated CA15-3 levels than at decreased levels (p-value for nonlinear spline terms < 0.01; Figure 3).
Table 3. Subgroup analysis for hazard ratio for recurrence based on change in cancer antigen 15-3.
| Change between previous examination and current examination | Adjusted HR (95% CI) | ||
|---|---|---|---|
| Elevated | Elevated 1 SD* | ||
| Pathologic N stage | |||
| N0 | 1.43 (1.17–1.75) | 5.31 (4.05–6.95) | |
| N+ | 2.12 (1.73–2.60) | 8.82 (6.72–10.31) | |
| p-value for interaction | < 0.01 | 0.01 | |
| Subtype | |||
| Luminal A (ER or PR+/HER2−) | 2.07 (1.69–2.53) | 8.00 (6.38–10.02) | |
| Luminal B (ER or PR+/HER2+) | 1.58 (1.05–2.38) | 6.53 (3.95–10.79) | |
| HER2+ (ER and PR−/HER2+) | 1.32 (0.85–2.07) | 4.52 (2.57–7.94) | |
| TNBC (ER and PR−/HER2−) | 1.47 (1.09–1.98) | 6.00 (4.21–8.55) | |
| p-value for interaction | 0.22 | 0.34 | |
Adjusted for age, pathological N stage, hormone receptor, HER2, chemotherapy, radiation therapy, and hormone therapy.
HR = hazard ratio; CI = confidence interval; SD = standard deviation; ER = estrogen receptor; PR = progesterone receptor; HER2 = human epidermal growth factor 2; TNBC = triple negative breast cancer.
*Elevated ≥ 1 SD of baseline CA15-3 (4.5).
Figure 3. Adjusted hazard ratio for recurrence based on changes in cancer antigen 15-3 level.
CA15-3 = cancer antigen 15-3.
In the sensitivity analysis, patients who had elevated CA15-3 from baseline were more likely to have higher risk of recurrence (HR, 2.61; 95% CI, 2.27–3.00) than patients without CA15-3 elevated (Supplementary Table 1).
DISCUSSION
In the present study, changes in CA15-3 levels were analyzed in more than 11,000 patients with BC and a significant association was found between elevated CA15-3 levels within normal range and recurrence. The association between elevated CA15-3 levels and recurrence was observed in all molecular subtypes.
In numerous studies, elevated CA15-3 level was associated with prognosis of BC with various ranges and situations. In patients with metastatic BC, elevated CA15-3 level showed poor overall survival (OS) with cut-off values ranging from 20.1 to 40 U/mL [14,15,16,17]. Patients with BC with bone metastasis, which shows better prognosis among metastatic BCs, also had elevated CA15-3 levels and a significantly poor progression-free survival with cut-off values ranging from 30 to 35 U/mL [18,19]. Furthermore, in many studies, patients with BC with stage I–III with elevated CA15-3 levels had poor disease-free survival (DFS) with cut-off values ranging from 20.11 to 40 U/mL [20,21,22,23]. In previous studies, fewer than 1,000 patients were reported and cases with CA15-3 below the cut-off value were ignored. In the present study, a large number of operable patients with CA15-3 below the cut-off value (mean CA15-3 10.0 U/mL) were included and the change below the cut-off value analyzed.
Although current guidelines do not recommend routine imaging such as abdominal CT, chest CT, or bone scan to detect distant metastasis in patients with asymptomatic BC, many clinicians routinely use intensive imaging and tumor markers (CA15-3, carcinoembryonic antigen [CEA]) to detect distant metastasis [24,25,26]. Because clinical trials conducted decades ago showed no benefit for routine intensive imaging and do not reflect recent modern imaging and target therapies, routine intensive imaging is currently used to detect distant metastasis [7,27]. Furthermore, the survival of patients with metastatic BC has significantly improved over the last several decades, and some patients with metastatic BC, especially oligometastases, experience durable clinical remission if they are treated with intensive treatment [28,29,30]. Similarly, the European Society for Medical Oncology [31] and American Society of Clinical Oncology do not recommend serial measurement of CA15-3 during the follow-up of early BC due to the lack of data that it increases survival benefit [26]. However, in contrast to CT or bone scan, which can potentially harm patients, CA15-3 is very non-invasive, easily available, and a cost-effective tumor marker. Many clinicians use a serial assessment of tumor markers such as CA15-3 as part of routine follow-up in patients with asymptomatic early BC.
Notably, in the current study, elevated CA15-3 level within normal range in the early BC was associated with worse DFS. Recently, liquid biopsy based on circulating tumor DNA (ctDNA) or cell-free DNA (cfDNA) is an emerging new technique used to diagnose and monitor BC [32,33]. Clatot et al. [34] analyzed the relation of circulating ESR1 mutation, CA15-3, and circulating cfDNA in 103 patients with hormone receptor positive metastatic BC. They demonstrated that CA15-3 elevation was correlated with progression, while as cfDNA was not correlated to progression. Tang et al. [35] directly compared CA15-3, CEA, and cfDNA levels between patients with BC and normal healthy carriers. They showed that combined diagnosis with tumor marker and cfNDA significantly improved the diagnostic accuracy of BC, with sensitivity of 97.5% and negative predictive value of 96.4%. However, there were limited data of cfDNA or ctDNA compared to CA15-3; a future study comparing ctDNA or cfDNA with CA15-3 is warranted. The present study had several limitations. First, this was a retrospective study conducted at a single institution which limits generalization. Second, other tumor markers such as CEA were not analyzed; thus, the risk of recurrence could not be compared with other tumor markers. External validation and comparison with other tumor markers are necessary. Third, correlation between CA15-3 level and a detailed recurrence pattern such as distant metastasis, locoregional recurrence, contralateral BC, and OS was not demonstrated because of de-identified dataset for the research purpose. Fourth, since the patients had multiple examinations and the CA15-3 level was changed in the examination, it might be associated with false-positive results. However, when we performed a sensitivity analysis that a patient who had elevated CA15-3 from baseline did not change the group across the follow-up, the results were consistent. Further studies are necessary for correlation between CA15-3 levels and detailed recurrence or survival outcomes. Lastly, we could not determine if benign diseases, such as liver disease, ovarian cysts, or benign breast disease, could affect CA15-13 levels. However, we used time-dependent exposure design; the same individual could contribute person-time to all change level categories in each examination. This study was conducted with a large homogenous BC patient cohort and was the first in which elevated CA15-3 levels within normal range were shown to affect BC recurrence.
In conclusion, the results of the present study demonstrated that elevation of CA15-3 levels in patients with early BC and normal serum CA15-3 level has a prognostic impact. Whether early detection of elevation of CA15-3 level within normal range can improve DFS should be investigated in future studies.
Footnotes
Funding: This study was supported by Future Medicine 20*30 Project of the Samsung Medical Center (SMC1230451). This work was supported by National IT Industry Promotion Agency grant funded by the Ministry of Science and ICT.
Conflict of Interest: The authors declared that have no competing interests.
- Conceptualization: Yu JH, Kang M.
- Data curation: Lee JE, Kim SW, Nam SJ, Lee SK, Im YH, Ahn JS, Park YH, Kim JY.
- Investigation: Kang D.
- Methodology: Cho J.
- Supervision: Yu JH, Lee H.
- Visualization: Kang D.
- Writing - original draft: Ryu JM, Kang D.
- Writing - review & editing: Kim YJ, Ryu JM.
SUPPLEMENTARY MATERIAL
Sensitivity analysis of hazard ratio for recurrence according to change of cancer antigen 15-3 censoring after cancer antigen 15-3 > 30
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Kang SY, Lee SB, Kim YS, Kim Z, Kim HY, Kim HJ, et al. Breast cancer statistics in Korea, 2018. J Breast Cancer. 2021;24:123–137. doi: 10.4048/jbc.2021.24.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suh M, Choi KS, Park B, Lee YY, Jun JK, Lee DH, et al. Trends in cancer screening rates among Korean men and women: results of the Korean National Cancer Screening Survey, 2004-2013. Cancer Res Treat. 2016;48:1–10. doi: 10.4143/crt.2014.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith TJ. Breast cancer surveillance guidelines. J Oncol Pract. 2013;9:65–67. doi: 10.1200/JOP.2012.000787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flowers CI, Mooney BP, Drukteinis JS. Clinical and imaging surveillance following breast cancer diagnosis. Am Soc Clin Oncol Educ Book. 2012:59–64. doi: 10.14694/EdBook_AM.2012.32.220. [DOI] [PubMed] [Google Scholar]
- 6.Ghezzi P, Magnanini S, Rinaldini M, Berardi F, Di Biagio G, Testare F, et al. Impact of follow-up testing on survival and health-related quality of life in breast cancer patients. A multicenter randomized controlled trial. JAMA. 1994;271:1587–1592. doi: 10.1001/jama.1994.03510440047031. [DOI] [PubMed] [Google Scholar]
- 7.Rosselli Del Turco M, Palli D, Cariddi A, Ciatto S, Pacini P, Distante V. Intensive diagnostic follow-up after treatment of primary breast cancer. A randomized trial. National Research Council Project on Breast Cancer follow-up. JAMA. 1994;271:1593–1597. doi: 10.1001/jama.271.20.1593. [DOI] [PubMed] [Google Scholar]
- 8.Li X, Dai D, Chen B, Tang H, Xie X, Wei W. Clinicopathological and prognostic significance of cancer antigen 15-3 and carcinoembryonic antigen in breast cancer: a meta-analysis including 12,993 patients. Dis Markers. 2018;2018:9863092. doi: 10.1155/2018/9863092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Svobodova S, Kucera R, Fiala O, Karlikova M, Narsanska A, Zedníková I, et al. CEA, CA 15-3, and TPS as prognostic factors in the follow-up monitoring of patients after radical surgery for breast cancer. Anticancer Res. 2018;38:465–469. doi: 10.21873/anticanres.12245. [DOI] [PubMed] [Google Scholar]
- 10.Di Gioia D, Stieber P, Schmidt GP, Nagel D, Heinemann V, Baur-Melnyk A. Early detection of metastatic disease in asymptomatic breast cancer patients with whole-body imaging and defined tumour marker increase. Br J Cancer. 2015;112:809–818. doi: 10.1038/bjc.2015.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim JY, Lee YS, Yu J, Park Y, Lee SK, Lee M, et al. Deep learning-based prediction model for breast cancer recurrence using adjuvant breast cancer cohort in tertiary cancer center registry. Front Oncol. 2021;11:596364. doi: 10.3389/fonc.2021.596364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cho IS, Chae YR, Kim JH, Yoo HR, Jang SY, Kim GR, et al. Statistical methods for elimination of guarantee-time bias in cohort studies: a simulation study. BMC Med Res Methodol. 2017;17:126. doi: 10.1186/s12874-017-0405-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dekker FW, de Mutsert R, van Dijk PC, Zoccali C, Jager KJ. Survival analysis: time-dependent effects and time-varying risk factors. Kidney Int. 2008;74:994–997. doi: 10.1038/ki.2008.328. [DOI] [PubMed] [Google Scholar]
- 14.Berruti A, Tampellini M, Torta M, Buniva T, Gorzegno G, Dogliotti L. Prognostic value in predicting overall survival of two mucinous markers: CA 15-3 and CA 125 in breast cancer patients at first relapse of disease. Eur J Cancer. 1994;30A:2082–2084. doi: 10.1016/0959-8049(94)00356-a. [DOI] [PubMed] [Google Scholar]
- 15.Albuquerque KV, Price MR, Badley RA, Jonrup I, Pearson D, Blamey RW, et al. Pre-treatment serum levels of tumour markers in metastatic breast cancer: a prospective assessment of their role in predicting response to therapy and survival. Eur J Surg Oncol. 1995;21:504–509. doi: 10.1016/s0748-7983(95)96935-7. [DOI] [PubMed] [Google Scholar]
- 16.Darlix A, Lamy PJ, Lopez-Crapez E, Braccini AL, Firmin N, Romieu G, et al. Serum HER2 extra-cellular domain, S100ß and CA 15-3 levels are independent prognostic factors in metastatic breast cancer patients. BMC Cancer. 2016;16:428. doi: 10.1186/s12885-016-2448-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JS, Park S, Park JM, Cho JH, Kim SI, Park BW. Elevated levels of preoperative CA 15-3 and CEA serum levels have independently poor prognostic significance in breast cancer. Ann Oncol. 2013;24:1225–1231. doi: 10.1093/annonc/mds604. [DOI] [PubMed] [Google Scholar]
- 18.James JJ, Evans AJ, Pinder SE, Gutteridge E, Cheung KL, Chan S, et al. Bone metastases from breast carcinoma: histopathological - radiological correlations and prognostic features. Br J Cancer. 2003;89:660–665. doi: 10.1038/sj.bjc.6601198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turanli S, Cetin A. Prognostic role of serum cancer antigen 15-3 in breast cancer patients with isolated bone metastases. Biomarkers. 2010;15:418–423. doi: 10.3109/1354750X.2010.482672. [DOI] [PubMed] [Google Scholar]
- 20.Park BW, Oh JW, Kim JH, Park SH, Kim KS, Kim JH, et al. Preoperative CA 15-3 and CEA serum levels as predictor for breast cancer outcomes. Ann Oncol. 2008;19:675–681. doi: 10.1093/annonc/mdm538. [DOI] [PubMed] [Google Scholar]
- 21.Cañizares F, Sola J, Pérez M, Tovar I, De Las Heras M, Salinas J, et al. Preoperative values of CA 15-3 and CEA as prognostic factors in breast cancer: a multivariate analysis. Tumour Biol. 2001;22:273–281. doi: 10.1159/000050627. [DOI] [PubMed] [Google Scholar]
- 22.Di Gioia D, Dresse M, Mayr D, Nagel D, Heinemann V, Stieber P. Serum HER2 in combination with CA 15-3 as a parameter for prognosis in patients with early breast cancer. Clin Chim Acta. 2015;440:16–22. doi: 10.1016/j.cca.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Martín A, Corte MD, Alvarez AM, Rodriguez JC, Andicoechea A, Bongera M, et al. Prognostic value of pre-operative serum CA 15.3 levels in breast cancer. Anticancer Res. 2006;26:3965–3971. [PubMed] [Google Scholar]
- 24.Cardoso F, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rubio IT, et al. Early breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30:1674. doi: 10.1093/annonc/mdz189. [DOI] [PubMed] [Google Scholar]
- 25.Runowicz CD, Leach CR, Henry NL, Henry KS, Mackey HT, Cowens-Alvarado RL, et al. American Cancer Society/American Society of Clinical Oncology breast cancer survivorship care guideline. J Clin Oncol. 2016;34:611–635. doi: 10.1200/JCO.2015.64.3809. [DOI] [PubMed] [Google Scholar]
- 26.De Cock L, Heylen J, Wildiers A, Punie K, Smeets A, Weltens C, et al. Detection of secondary metastatic breast cancer by measurement of plasma CA 15.3. ESMO Open. 2021;6:100203. doi: 10.1016/j.esmoop.2021.100203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kokko R, Hakama M, Holli K. Role of chest X-ray in diagnosis of the first breast cancer relapse: a randomized trial. Breast Cancer Res Treat. 2003;81:33–39. doi: 10.1023/A:1025419114857. [DOI] [PubMed] [Google Scholar]
- 28.Cardoso F, Spence D, Mertz S, Corneliussen-James D, Sabelko K, Gralow J, et al. Global analysis of advanced/metastatic breast cancer: Decade report (2005-2015) Breast. 2018;39:131–138. doi: 10.1016/j.breast.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 29.Lee ES, Jung SY, Kim JY, Kim JJ, Yoo TK, Kim YG, et al. Identifying the potential long-term survivors among breast cancer patients with distant metastasis. Ann Oncol. 2016;27:828–833. doi: 10.1093/annonc/mdw036. [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi T, Ichiba T, Sakuyama T, Arakawa Y, Nagasaki E, Aiba K, et al. Possible clinical cure of metastatic breast cancer: lessons from our 30-year experience with oligometastatic breast cancer patients and literature review. Breast Cancer. 2012;19:218–237. doi: 10.1007/s12282-012-0347-0. [DOI] [PubMed] [Google Scholar]
- 31.Cottu P, D’Hondt V, Dureau S, Lerebours F, Desmoulins I, Heudel PE, et al. Letrozole and palbociclib versus chemotherapy as neoadjuvant therapy of high-risk luminal breast cancer. Ann Oncol. 2018;29:2334–2340. doi: 10.1093/annonc/mdy448. [DOI] [PubMed] [Google Scholar]
- 32.Duque G, Manterola C, Otzen T, Arias C, Galindo B, Mora M, et al. Clinical utility of liquid biopsy in breast cancer: a systematic review. Clin Genet. 2022;101:285–295. doi: 10.1111/cge.14077. [DOI] [PubMed] [Google Scholar]
- 33.Cullinane C, Fleming C, O’Leary DP, Hassan F, Kelly L, O’Sullivan MJ, et al. Association of circulating tumor DNA with disease-free survival in breast cancer: a systematic review and meta-analysis. JAMA Netw Open. 2020;3:e2026921. doi: 10.1001/jamanetworkopen.2020.26921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clatot F, Perdrix A, Beaussire L, Lequesne J, Lévy C, Emile G, et al. Risk of early progression according to circulating ESR1 mutation, CA-15.3 and cfDNA increases under first-line anti-aromatase treatment in metastatic breast cancer. Breast Cancer Res. 2020;22:56. doi: 10.1186/s13058-020-01290-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang Z, Li L, Shen L, Shen X, Ju S, Cong H. Diagnostic value of serum concentration and integrity of circulating cell-free DNA in breast cancer: a comparative study with CEA and CA15-3. Lab Med. 2018;49:323–328. doi: 10.1093/labmed/lmy019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sensitivity analysis of hazard ratio for recurrence according to change of cancer antigen 15-3 censoring after cancer antigen 15-3 > 30