Abstract
Pleuroparenchymal fibroelastosis (PPFE) is a rare form of interstitial pneumonitis. Although most cases of PPFE are idiopathic, some cases of PPFE occur secondary to stem cell transplantation. We report a 41-year-old woman developed pneumonia after autologous peripheral blood system cell transplantation (PBSCT). Eleven years after PBSCT, she presented with dyspnea. A computed tomographic scan showed pleuroparenchymal thickening and predominantly in the upper lobes. She was diagnosed with PPFE secondary to PBSCT. She was started nintedanib and administered oxygen therapy. Most cases of PPFE secondary to stem cell transplantation have been reported. However, we experienced the case of PPFE post-autologous PBSCT.
Keywords: Pleuroparenchymal fibroelastosis (PPFE), Idiopathic interstitial pneumonia (IIP), Hematopoietic stem cell transplantation (HSCT), Peripheral blood stem cell transplantation (PBSCT), Graft-versus-host disease (GVHD)
1. Background
The number of patients receiving hematopoietic stem cell transplantation (HSCT) is increasing. Therefore, the number of patients with pulmonary complications after HSCT has increased. The majority of these cases are associated with bronchiolitis obliterans [1]. Pleuroparenchymal fibroelastosis (PPFE) has also been reported as a pulmonary complication after HSCT, occurring in approximately 0.3% of these patients [2]. The characteristics of this disease have not been well described, which may lead to a misdiagnosis. PPFE is a rare interstitial pneumonia and is a fibrosis of the upper lobes. PPFE is recognized as a novel clinicopathologic entity [3] and is included in the updated classification of idiopathic interstitial pneumonia [4]. We report a patient with PPFE secondary to autologous peripheral blood system cell transplantation (PBSCT). She developed chronic respiratory failure with pleural and subpleural elastic fibrosis predominantly in the upper bilateral lobes.
2. Case presentation
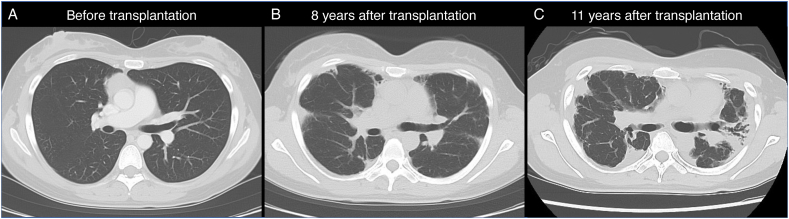
A 41-year-old Japanese woman was admitted to Kyushu University Hospital with dyspnea for several months. She was diagnosed with diffuse large B-cell lymphoma 11 years previously and then received chemotherapy followed by autologous PBSCT, which is a type of HSCT. A chest computed tomographic (CT) scan before transplantation was normal (Fig. 1A). Eight years after the transplantation, a chest CT scan showed bilateral irregular pleuroparenchymal thickening (Fig. 1B). Pulmonary function tests showed restrictive ventilatory dysfunction and decreased diffusion capacity (Table 1). Bronchoscopy showed non-specific findings. Predominant neutrophils were observed in bronchoalveolar lavage fluid, but all cultures were negative. Malignant cells were not detected. At this admission 11 years after the transplantation, a chest CT showed progression of bilateral pleuroparenchymal thickening with traction bronchiectasis and loss of the lung volume, which was most marked in the upper lobes (Fig. 1C). Pulmonary function tests also showed progression of restrictive ventilatory dysfunction and decreased diffusion capacity. The forced vital capacity was 1.27 L (49.4% of predicted), and the ratio of the forced expiratory volume in 1 s to the forced vital capacity was 98.43%. The diffusion capacity of carbon monoxide was 6.61 mmol/min/kPa (35.0% of predicted) (Table 1). An arterial blood gas analysis showed type 1 respiratory failure, with a PaO2 of 78.0 Torr and PaCO2 of 38.1 Torr. Bronchoscopy and a lung biopsy were not performed without consent. The patient had no other relevant comorbidities, no history of smoking, no known exposure, and was not prescribed regular medication. On the basis of her medical history, clinical manifestations, and serial CT findings, she was diagnosed with PPFE secondary to autologous PBSCT. The patient was administered nintedanib, which is an antifibrotic drug, but it was discontinued because of pneumothorax 1 week later. She was administered long-term oxygen therapy and is still waiting for a lung transplant.
Fig. 1.
Chest computed tomographic imaging.
A computed tomographic (CT) scan shows normal lungs before chemotherapy followed by autologous hematopoietic stem cell transplantation (PBSCT) for the treatment of diffuse large B-cell lymphoma (A). A CT scan shows bilateral irregular pleuroparenchymal thickening 8 years after the transplantation (B). A CT scan shows progression of bilateral pleuroparenchymal thickening and subpleural elastotic fibrosis accompanied by traction bronchiectasis, and a loss of lung volume predominantly in the upper lobes at admission to the hospital 11 years after the transplantation (C).
Table 1.
Pulmonary function test results.
| Before transplantation |
8 years after transplantation |
11 years after transplantation |
|||||
|---|---|---|---|---|---|---|---|
| Value | % Predicted | Value | % Predicted | Value | % Predicted | ||
| VC | (L) | 3.08 | 104.1 | 1.77 | 56.2 | 1.30 | 42.5 |
| FVC | (L) | 3.13 | 105.7 | 1.74 | 57.4 | 1.27 | 49.4 |
| FEV1 | (L) | 2.74 | 95.5 | 1.68 | 64.1 | 1.25 | 49.4 |
| FEV1/FVC | % | 87.53 | 103.5 | 96.55 | 110.0 | 98.43 | 113.4 |
| DLCO | (mL/min/mmHg) | 17.96 | 90.5 | 11.52 | 59.1 | 6.61 | 35.0 |
VC: vital capacity, FVC: forced vital capacity, FEV1: forced expiratory volume in 1 s, DLCO: diffusing capacity of the lungs for carbon monoxide.
3. Discussion and conclusions
We describe a case of PPFE that appeared after chemotherapy followed by autologous PBSCT for diffuse large B-cell lymphoma. Several cases of PPFE secondary to allogenic HSCT have been reported, but we experienced a rare case of development of PPFE secondary to autologous HSCT. Our findings suggest that autologous HSCT is possible in the development of PPFE.
PPFE is an unusual pulmonary disease and was first described by Amitani et al. [5]. Five cases were diagnosed as PPFE, and shared a pattern of chronic interstitial and pleural fibrosis that did not fit within other categories of idiopathic interstitial pneumonia [3]. PPFE is characterized by intense elastic fibrosis involving the pleura and subpleural lung parenchyma predominantly in the upper lobes [3,6], and was designated a rare idiopathic interstitial pneumonia in 2013 [4].
Little is known regarding the etiology of PPFE, and most cases are considered idiopathic. However, a few cases of PPFE have been reported as a late-onset pulmonary complication that occurs after HSCT, representing a rare post-transplantation complication. A study by Mariani et al. who retrospectively reviewed high-resolution CT scans from 700 HSCT recipients showed that the prevalence of PPFE was 0.28% among these recipients [2]. Although the mechanism by which transplantation leads to PPFE remains to be fully determined, possible causes may include reactions to graft-versus-host disease (GVHD) because the majority of the reported cases occurred after allogenic HSCT [4,7]. However, in the present case, PPEF occurred after autologous PBSCT, which does not usually cause GVHD. Some autologous cases have also been reported in the literature. One patient developed PPFE after autologous bone marrow transplant in the treatment of acute myeloid leukemia, and another patient developed PPFE after chemotherapy and radiotherapy followed by autologous HSCT for Hodgkin's lymphoma [8,9]. PPFE was observed in both cases in allogeneic and autologous HSCT. Therefore, PPFE after HSCT is a heterogeneous condition, with possible involvement of GVHD or other conditions associated with HSCT, such as chemotherapy or radiotherapy.
The prognosis for PPFE remains poor. The use of large doses of corticosteroids or the use of immunosuppressive agents is usually avoided because of the increased risk of infection in these patients. Pirfenidone, which is an antifibrotic agent, has been used for PPFE with insufficient results [10,11]. In our case, another antifibrotic drug, nintedanib, was started but discontinued because of pneumothorax. Therefore, the effect of nintedanib is unclear. Nintedanib suppresses the progression of a decline in lung capacity in patients with fibrosing interstitial lung diseases [12]. Therefore, nintedanib may be beneficial if started early in this disease. Lung transplantation has been reported as a treatment for end-stage PPFE, but long-term outcome data are unclear [13,14].
To the best of our knowledge, the present case is the first case of PPFE secondary to autologous PBSCT. PPFE is a rare disease, but this disease as a complication of autologous HSCT including PBSCT needs to be better understood.
Declarations
Ethics approval and consent to participate: Clinical data were used with written informed consent of the patient.
Consent for publication
We reports this case with written informed consent of the patient.
Availability of data and materials
The datasets used during the current study available from the corresponding author on reasonable request.
Funding
Not applicable.
Authors’ contributions
A. Egashira: methodology, data curation, investigation, and writing–original draft. Y. Yoneshima: conceptualization, methodology, formal analysis, data curation, investigation, writing–original draft, and writing–review and editing. S. Mizusaki: investigation and writing–review and editing. Y. Tsuneoka: investigation and writing–review and editing. K. Tsubouchi: investigation and writing–review and editing. I. Okamoto: supervision and writing–review and editing.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgements
Not applicable.
Handling Editor: DR AC Amit Chopra
List of abbreviations
- Partial pressure of arterial oxygen
(PaO2)
- Partial pressure of arterial carbon dioxide
(PaCO2)
References
- 1.Williams K.M. How I treat bronchiolitis obliterans syndrome after hematopoietic stem cell transplantation. Blood. 2017;129(4):448–455. doi: 10.1182/blood-2016-08-693507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mariani F., Gatti B., Rocca A., Bonifazi F., Cavazza A., Fanti S., Tomassetti S., Piciucchi S., Poletti V., Zompatori M. Pleuroparenchymal fibroelastosis: the prevalence of secondary forms in hematopoietic stem cell and lung transplantation recipients. Diagn. Interv. Radiol. 2016;22(5):400–406. doi: 10.5152/dir.2016.15516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frankel S.K., Cool C.D., Lynch D.A., Brown K.K. Idiopathic pleuroparenchymal fibroelastosis: description of a novel clinicopathologic entity. Chest. 2004;126(6):2007–2013. doi: 10.1378/chest.126.6.2007. [DOI] [PubMed] [Google Scholar]
- 4.Travis W.D., Costabel U., Hansell D.M., King T.E., Jr., Lynch D.A., Nicholson A.G., Ryerson C.J., Ryu J.H., Selman M., Wells A.U., Behr J., Bouros D., Brown K.K., Colby T.V., Collard H.R., Cordeiro C.R., Cottin V., Crestani B., Drent M., Dudden R.F., Egan J., Flaherty K., Hogaboam C., Inoue Y., Johkoh T., Kim D.S., Kitaichi M., Loyd J., Martinez F.J., Myers J., Protzko S., Raghu G., Richeldi L., Sverzellati N., Swigris J., Valeyre D., A.E.C.o.I.I. Pneumonias An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am. J. Respir. Crit. Care Med. 2013;188(6):733–748. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amitani R N.A., Kuse F. Idiopathic pulmonary upper lobe fibrosis (IPUF) Kokyu. 1992;11 693–639. [Google Scholar]
- 6.Chua F., Desai S.R., Nicholson A.G., Devaraj A., Renzoni E., Rice A., Wells A.U. Pleuroparenchymal fibroelastosis. A review of clinical, radiological, and pathological characteristics. Ann Am Thorac Soc. 2019;16(11):1351–1359. doi: 10.1513/AnnalsATS.201902-181CME. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takeuchi Y., Miyagawa-Hayashino A., Chen F., Kubo T., Handa T., Date H., Haga H. Pleuroparenchymal fibroelastosis and non-specific interstitial pneumonia: frequent pulmonary sequelae of haematopoietic stem cell transplantation. Histopathology. 2015;66(4):536–544. doi: 10.1111/his.12553. [DOI] [PubMed] [Google Scholar]
- 8.von der Thusen J.H., Hansell D.M., Tominaga M., Veys P.A., Ashworth M.T., Owens C.M., Nicholson A.G. Pleuroparenchymal fibroelastosis in patients with pulmonary disease secondary to bone marrow transplantation. Mod. Pathol. 2011;24(12):1633–1639. doi: 10.1038/modpathol.2011.114. [DOI] [PubMed] [Google Scholar]
- 9.Zhang S., Xie W., Wang Z., Tian Y., Da J., Zhai Z. Pleuroparenchymal fibroelastosis secondary to autologous hematopoietic stem cell transplantation: a case report. Exp. Ther. Med. 2019;17(4):2557–2560. doi: 10.3892/etm.2019.7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonifazi M., Montero M.A., Renzoni E.A. Idiopathic pleuroparenchymal fibroelastosis. Curr. Pulmonol. Rep. 2017;6(1):9–15. doi: 10.1007/s13665-017-0160-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shioya M., Otsuka M., Yamada G., Umeda Y., Ikeda K., Nishikiori H., Kuronuma K., Chiba H., Takahashi H. Poorer prognosis of idiopathic pleuroparenchymal fibroelastosis compared with idiopathic pulmonary fibrosis in advanced stage. Cancer Res. J. 2018;2018 doi: 10.1155/2018/6043053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flaherty K.R., Wells A.U., Cottin V., Devaraj A., Walsh S.L.F., Inoue Y., Richeldi L., Kolb M., Tetzlaff K., Stowasser S., Coeck C., Clerisme-Beaty E., Rosenstock B., Quaresma M., Haeufel T., Goeldner R.G., Schlenker-Herceg R., Brown K.K. Nintedanib in progressive fibrosing interstitial lung diseases. N. Engl. J. Med. 2019;381(18):1718–1727. doi: 10.1056/NEJMoa1908681. [DOI] [PubMed] [Google Scholar]
- 13.Chen F., Matsubara K., Miyagawa-Hayashino A., Tada K., Handa T., Yamada T., Sato M., Aoyama A., Date H. Lung transplantation for pleuroparenchymal fibroelastosis after chemotherapy. Ann. Thorac. Surg. 2014;98(5):e115–e117. doi: 10.1016/j.athoracsur.2014.07.045. [DOI] [PubMed] [Google Scholar]
- 14.Hata A., Nakajima T., Yoshida S., Kinoshita T., Terada J., Tatsumi K., Matsumiya G., Date H., Yoshino I. Living donor lung transplantation for pleuroparenchymal fibroelastosis. Ann. Thorac. Surg. 2016;101(5):1970–1972. doi: 10.1016/j.athoracsur.2015.07.056. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used during the current study available from the corresponding author on reasonable request.