Abstract
The present systematic review investigated the concentration of chromium (Cr) and cobalt (Co) in serum in patients who have undergone total hip arthroplasty (THA). The first outcome of interest was to investigate the mean concentration in serum of Cr and Co using different material combinations and to verify whether their concentrations change significantly using different patterns of head and liner in THA. The second outcome of interest was to investigate whether the time elapsed from the index surgery to the follow-up, BMI, sex, and side exert an influence on the mean concentration of Cr and Co in serum in patients who have undergone THA. The following material combinations were investigated (head-liner): Ceramic-Co Cr (CoCr), CoCr-CoCr, CoCr-Polyethylene, CoCr high carbide-CoCr high carbide. Data from 2756 procedures were retrieved. The mean length of follow-up was 69.3 ± 47.7 months. The ANOVA test evidenced good comparability in age, length of follow-up, BMI, and sex (P > 0.1). In patients who have undergone THA, the mean concentration in the serum of Co ranged between 0.5 µg/L and 3.5 µg/L, and the mean concentration of Cr from 0.6 to 2.6 µg/L. The difference in the concentration of Co and Cr in serum is strictly related to the implant configuration, with the coupling CoCr-CoCr showing the highest and CoCr-Polyethylene showing the lowest concentration. Patient characteristics, BMI, sex, side and the time elapsed from the index surgery to the last follow-up did not exert a significant influence on the concentration of Co and Cr in serum in patients who have undergone total hip arthroplasty (THA).
Subject terms: Medical research, Signs and symptoms, Materials science
Introduction
Total hip arthroplasty (THA) is a common procedure for patients with hip osteoarthritis. THA is associated with a significant improvement in patient reported outcome measures (PROMs)1–3. The weight bearing on the mobile components (head and liner) of THA produce friction, wear, tear, and deformation, and consequently the release of metal elements4. Particles release in implants with metallic mobile components, especially chromium (Cr) and cobalt (Co), is a concern1,5. These particles might remain into the joint capsule or migrate to the periarticular tissues or to other body sites though the blood and lymphatic circulation. The concentrations of Co and Cr in patients who have undergone THA with Co-Cr components are detectable in their serum. Several studies have been conducted to assess the serum concentration of Co and Cr in patients with such mobile components4,6–10. However, variability in implant components may impair a proper estimation of the serum concentration. Whether different mobile component configurations in THA (Ceramic-CoCr, CoCr-CoCr, CoCr-Polyethylene) is associated with differences in serum concentrations of Co and Cr is unclear and evidence is missing. Moreover, whether patient demographic may influence the serum concentration of Co and Cr has not been systematically evaluated. Recently, Co-Cr alloys have been enhanced with high carbide alloy (Co-CrHC) additives to increase the stability of the metals, and therefore, reduce wear, tear, and deformation over the time11–13. However, whether Co-CrHC is associated with a lower concentration of Co and Cr is also unclear.
The present systematic review investigated the concentration of Co and Cr in the serum of patients who had undergone THA. The first outcome of interest was to investigate the mean serum concentration of Cr and Co in patients who have undergone THA using different material combinations, and to verify whether their concentrations change significantly using different head and liner coupling. The second outcome of interest was to investigate whether the time elapsed from the index surgery to the follow-up, BMI, sex, and side exert an influence in the mean concentration in serum of Cr and Co. The following material combinations were investigated (head- liner): Ceramic-CoCr, CoCr-CoCr, CoCr-Polyethylene, CoCrHC-CoCrHC. It was hypothesised that patient characteristics and the time elapsed from the index surgery to the last follow-up did not exert a significant influence on the concentration of Co and Cr in serum.
Methods
Eligibility criteria
All the clinical trials investigating the concentration (µg/L) of Cr and/ or Co in serum in patients who have undergone THA were considered. Only studies which clearly stated the composition of head and/ or liner components were eligible. Reviews, opinions, letters, editorials were not considered. In vitro, computational, biomechanics, and animal studies were not eligible. Prospective studies level I to II of evidence, according to Oxford Centre of Evidence-Based Medicine14, were considered. Given the authors language abilities, articles in English, German, Italian, French and Spanish were eligible. Missing data on the mean serum concentration (µg/L) of Cr and Co warranted the exclusion from the present study.
Search strategy
This study compiles with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the 2020 PRISMA checklist15. The PICOTD algorithm was preliminary pointed out:
P (Problem): end-stage OA;
I (Intervention): THA;
C (Comparison): Ceramic-CoCr, CoCr-CoCr, CoCr-Polyethylene, CoCrHC-CoCrHC;
(Outcomes): concentration in serum;
T (Time): minimum 24 months follow-up;
D (Design): clinical trial.
In December 2022, the following databases were accessed: PubMed, Web of Science, Google Scholar, Embase. No time constrain was set for the search. The following matrix of keywords were used in each database to accomplish the search using the Boolean operator AND/OR: THA AND (OR hip OR arthroplasty OR replacement OR prosthesis) AND (serum OR blood OR plasma) AND (CoCr OR Cr Co OR Cr OR Co OR metal OR steel OR high carbide). No additional filters were used in the databases search.
Selection and data collection
Two authors (F. M. and R.M.) separately performed selection and data collection. The full-text of the studies which matched the topic of interest were accessed. If the full-text was not, the article was excluded. The references of the full-text articles were screened by hand by the reviewers for inclusion. In case of disagreements, a third author (N.M.) took the final decision.
Data extraction
Two authors (F.M. and R.M.) independently performed data extraction in a Microsoft Office Excel spreadsheet (version 16, Microsoft Corporation, Redmond, USA). The following generalities were retrieved: first author, year, length of the follow-up, and journal of publication. The following data at baseline were collected: number of patients, women, side, mean age, and mean BMI (Kg/m2). Data concerning the mean serum concentration (µg/L) of Cr and Co were extracted at last follow-up.
Assessment of the risk of bias
The risk of bias was evaluated in accordance with the guidelines in the Cochrane Handbook for Systematic Reviews of Interventions16. Two reviewers (R.G. and A.B.) evaluated the risk of bias of the extracted studies independently using the risk of bias of the software Review Manager 5.3 (The Nordic Cochrane Collaboration, Copenhagen). The following endpoints were evaluated: selection, detection, performance, attrition, reporting, and other bias. Disagreements were solved by a third author (N.M.).
Synthesis methods
The statistical analyses were performed by the main author (F.M.) following the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions17. For descriptive statistics, mean and standard deviation were used. To evaluate baseline comparability of patient demographic, the SPSS software was used. The analysis of variance (ANOVA) was performed assuming that values of P > 0.05 indicated comparability. The STATA/MP software (Stata Corporation, College Station, Texas, USA) was used for the network meta-analysis. The analyses were performed through the STATA routine for Bayesian hierarchical random-effects model. Continuous variables were analysed through the inverse variance method with standardized mean difference (SMD) effect measure. The confidence interval was set at 0.95. Heterogeneity was assessed using χ2 and Higgins-I2 tests. If χ2 > 0.05, no statistically significant heterogeneity was found. A fixed model effect was used. If χ2 < 0.05 and Higgins-I2 > 60% high heterogeneity was found and a random model effect was used for analysis. A multiple linear model regression analysis through the Pearson Product-Moment Correlation Coefficient (r) was used. The Cauchy–Schwarz formula was used for inequality: + 1 is considered as positive linear correlation, while and − 1 a negative one. Values of 0.1 <| r |< 0.3, 0.3 <| r |< 0.5, and | r |> 0.5 were considered to have weak, moderate, and strong correlation, respectively. The overall significance was assessed through the χ2 test, with values of P < 0.05 considered statistically significant.
Ethical approval
This study complies with ethical standards.
Results
Study selection
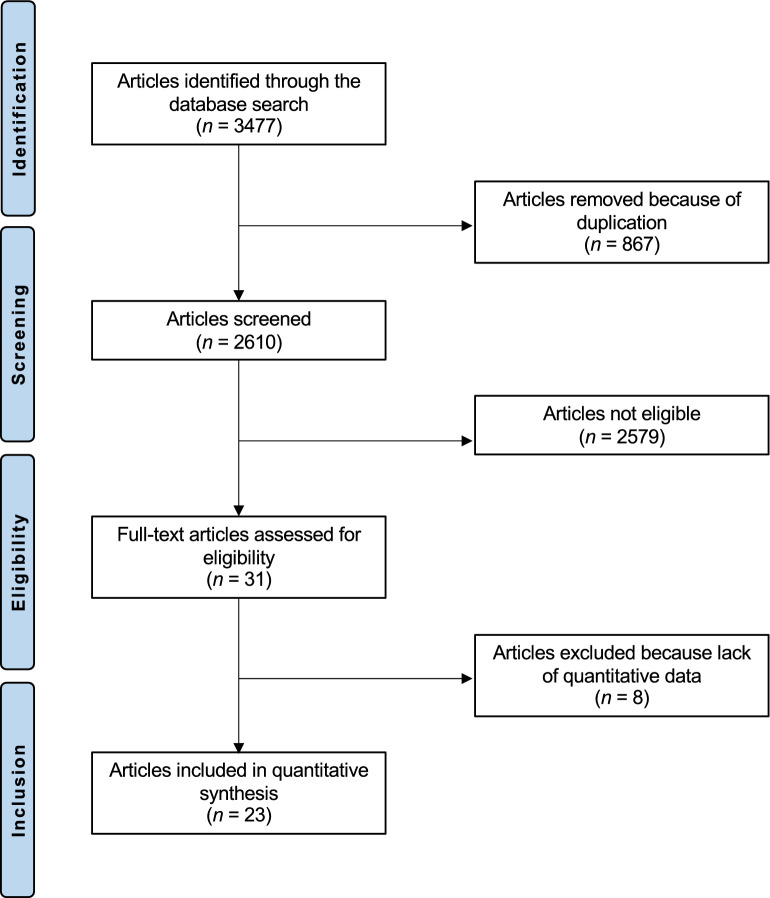
The initial databases research resulted in 3477 articles. Of them 867 were excluded as they were duplicates. A further 2579 articles were excluded as they did not match the eligibility criteria: not reporting data on the concentration in Co and/ or Cr in serum (N = 1733), study design (N = 385), not focusing on THA (N = 329), poor level of evidence (N = 84), not clearly reported the composition of head and/ or liner (N = 45), language limitations (N = 3). A further eight studies were excluded as they did not report quantitative data under the outcomes of interests. Finally, 23 studies were included: 15 nonRCTs and 8 RCTs. The results of the literature search are shown in Fig. 1.
Figure 1.
PRISMA flow chart of the literature search.
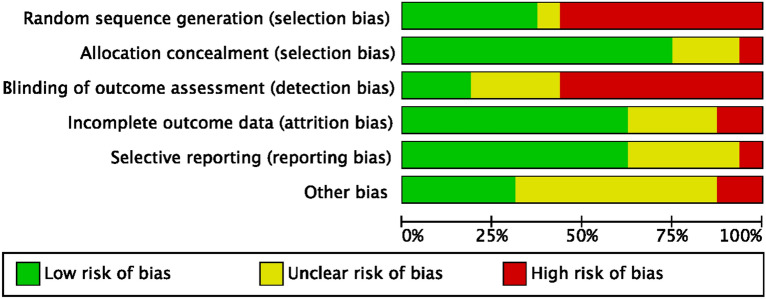
Risk of bias assessment
The risk of bias tool of the Cochrane Collaboration was used to evaluate the risk of bias. Given the prospective nature of the included studies, the overall risk of selection bias was low to moderate. Most studies did not perform assessor blinging or gave no information on it. Therefore, the risk of detection bias was moderate to high. The overall risk of attrition and reporting biases were both low to moderate, and the risk of other bias was moderate. Concluding, the overall quality of the methodological assessment was low to moderate (Fig. 2).
Figure 2.
Cochrane risk of bias tool.
Study characteristics and results of individual studies
Data from 2756 THAs were retrieved. Of them, 53% (1461 of 2756) were performed on women. The mean length of the follow-up was 69.3 ± 47.7 months. The mean age was 59.9 ± 8.6 years and the mean BMI was 28.2 ± 1.9 kg/m2. The ANOVA test evidenced good comparability in age, follow-up, BMI and sex of the patient demographic (P > 0.1). The generalities of the included studies are shown in Table 1, and the patient demographic of each group is shown in Table 2.
Table 1.
Generalities and patient baseline of the included studies.
| Author and year | Design | Head material | Liner material | Procedures | Mean age | Mean BMI | Women (%) |
|---|---|---|---|---|---|---|---|
| Briggs et al. 20159 | NonRCT | CoCr | Polyethylene | 22 | 73 | 28.7 | 77% |
| Cocr | CoCr | 23 | 67 | 28.1 | 74% | ||
| Cadossi et al. 201618 | NonRCT | Ceramic | CoCr | 20 | 65.9 | 28.5 | 70% |
| CoCr | CoCr | 29 | 61.8 | 26 | 41% | ||
| Chen et al. 20168 | NonRCT | CoCrHC | CoCrHC | 25 | 36 | ||
| CoCr | Polyethylene | 25 | 35.4 | ||||
| Dahlstrand et al. 201710 | RCT | CoCr | CoCr | 41 | 65 | 27 | 51% |
| CoCr | Polyethylene | 44 | 67 | 27 | 54% | ||
| Darrith et al. 202019 | NonRCT | CoCr | CoCr | 49 | 57.59 | 33.58 | 51% |
| Al2O3, CoCr | CoCr, ceramic, polyethylene | 26 | 58.65 | 33.72 | 50% | ||
| Engh et al. 201420 | RCT | CoCr | Polyethylene | 33 | 61.6 | 29.9 | 30% |
| CoCr | CoCr | 22 | 62.2 | 28.7 | 60% | ||
| CoCr | CoCr | 30 | 63.4 | 29.1 | 29% | ||
| CoCr | CoCr | 30 | 63.4 | 29.1 | 29% | ||
| Grübl et al. 200621 | RCT | Al2O3 | Al2O3 | 15 | 58.2 | 28.2 | 53% |
| CoCr | CoCr | 13 | 66.8 | 28.2 | 77% | ||
| Al2O3 | Al2O3 | 15 | 58.2 | 28.2 | 53% | ||
| CoCr | CoCr | 13 | 66.8 | 28.2 | 77% | ||
| Gustafson et al. 201422 | RCT | CoCr | CoCr | 19 | 64 | 26 | 53% |
| Al2O3, CoCr | Polyethylene | 25 | 64 | 27 | 72% | ||
| CoCr | CoCr | 19 | 64 | 26 | 53% | ||
| Al2O3, CoCr | Polyethylene | 25 | 64 | 27 | 72% | ||
| Higgins et al. 202023 | RCT | CoCr | CoCr | 87 | 65.2 | 37% | |
| AMC/ZTA | CoCr | 92 | 65.2 | 37% | |||
| Malviya et al. 201124 | RCT | CoCr | CoCr | 50 | 63.9 | 28.6 | 62% |
| CoCr | Polyethylene | 50 | 64.9 | 29.4 | 54% | ||
| CoCr | CoCr | 50 | 63.9 | 28.6 | 62% | ||
| CoCr | Polyethylene | 50 | 64.9 | 29.4 | 54% | ||
| Martin et al. 201825 | NonRCT | AMC/ZTA | AMZ/ZTA | 42 | 60 | 26.4 | 14% |
| CoCr | CoCr | 40 | 54 | 30.6 | 55% | ||
| Moroni et al. 201226 | NonRCT | CoCr | PCU | 15 | 67 | 27.7 | 60% |
| CoCr | CoCr | 15 | 61 | 25.5 | 60% | ||
| CoCr | PCU | 15 | 67 | 27.7 | 60% | ||
| CoCr | CoCr | 15 | 61 | 25.5 | 60% | ||
| Nam et al. 201527 | NonRCT | CoCr | Polyethylene | 10 | 54.2 | 27.3 | 50% |
| Ceramic | Polyethylene | 15 | 45.1 | 26 | 80% | ||
| OxZr | Polyethylene | 11 | 43.5 | 30.3 | 36% | ||
| Pozzuoli et al. 202028 | NonRCT | CoCr | CoCr | 34 | 66.1 | 24.3 | 68% |
| Ceramic | AMZ/ZTA | 34 | 68.6 | 25.5 | 62% | ||
| Savarino et al. 200829 | NonRCT | CoCr | CoCr | 32 | 72 | 75% | |
| Al2O3 | Al2O3 | 16 | 54 | 56% | |||
| Control group | Control group | 47 | 43 | 21% | |||
| Savarino et al. 200230 | NonRCT | CoCr | CoCr | 26 | 48 | 54% | |
| CoCr | Polyethylene | 15 | 64 | 80% | |||
| Control group | Control group | 22 | 56 | 59% | |||
| Control group | Control group | 22 | 43 | 36% | |||
| Savarino et al. 200829 | NonRCT | CoCr | CoCr | 32 | 72 | 75% | |
| Al2O3 | Al2O3 | 16 | 54 | 56% | |||
| Control group | Control group | 47 | 43 | 21% | |||
| Savarino et al. 200230 | NonRCT | CoCr | CoCr | 26 | 48 | 54% | |
| CoCr | Polyethylene | 15 | 64 | 80% | |||
| Control group | Control group | 22 | 56 | 59% | |||
| Control group | Control group | 22 | 43 | 36% | |||
| Schouten et al. 201731 | NonRCT | AMC/ZTA | CoCr | 36 | 62 | 30 | 50% |
| CoCr | CoCr | 31 | 64 | 30 | 32% | ||
| Schouten et al. 201232 | RCT | AMC/ZTA | CoCr | 41 | 61.5 | 29 | 45% |
| CoCr | CoCr | 36 | 63.8 | 29 | 36% | ||
| AMC/ZTA | CoCr | 41 | 61.5 | 29 | 45% | ||
| CoCr | CoCr | 36 | 63.8 | 29 | 36% | ||
| Tiusanen et al. 201333 | NonRCT | CoCrHC | CoCrHC | 46 | 62 | 50% | |
| CoCrHC | Polyethylene | 46 | 60 | 48% | |||
| CoCrHC | CoCrHC | 46 | 62 | 50% | |||
| CoCrHC | Polyethylene | 46 | 60 | 48% | |||
| White et al. 201634 | NonRCT | AMC/ZTA | Polyethylene | 370 | 60.6 | 27.5 | 43% |
| CoCr | Polyethylene | 313 | 74.2 | 27.2 | 60% | ||
| Zijlstra et al. 201435 | RCT | CoCr | Polyethylene | 32 | |||
| CoCr | CoCr | 28 | |||||
| CoCr | Polyethylene | 32 | |||||
| CoCr | CoCr | 28 |
RCT randomised controlled trial, Al2O3 Alumina oxide ceramic, AMC/ZTA Alumina matrix composite/Zirconia toughed alumina, OxZR Oxidized zirconium, PCU Polycarbidate Urethan, CoCR Co Cr, CoCRHC CoCr—high carbid.
Table 2.
Demographic of the patients of each group (CoCR: Co Cr; CoCRHC: CoCr—high carbid).
| Materials (head-liner) | THAs | Mean age | Mean BMI | Women |
|---|---|---|---|---|
| Ceramic-CoCr | 230 | 63.2 ± 2.2 | 29.1 ± 0.6 | 49% |
| CoCr-CoCr | 981 | 62.3 ± 5.5 | 28.1 ± 2.1 | 52% |
| CoCr-polyethylene | 811 | 63.0 ± 9.8 | 27.7 ± 2.4 | 57% |
| CoCrHC-CoCrHC | 258 | 59.6 ± 11.0 | 28.0 ± 1.4 | 45% |
| Control group | 232 | 50.4 ± 26.4 | 26.4 ± 3.8 | 35% |
Mean concentration of Co and Cr in serum
The mean concentration of Co in serum ranged between 0.5 µg/L and 3.5 µg/L. The mean concentration of Cr in serum ranged between 0.6 and 2.6 µg/L. The concentration of both materials according to the different head- liner compositions is shown in Table 3.
Table 3.
Mean concentration in serum of Co and Cr using different materials combination.
| Materials | Co (µg/L) | Cr (µg/L) |
|---|---|---|
| Ceramic-CoCr | 1.7 ± 1.0 | 1.3 ± 0.6 |
| CoCr-CoCr | 3.5 ± 5.1 | 2.6 ± 4.4 |
| CoCr-Polyethylene | 0.5 ± 0.5 | 0.6 ± 0.4 |
| CoCrHC-CoCrHC | 0.7 ± 1.1 | 1.1 ± 1.7 |
| Control Group | 0.3 ± 0.1 | 0.3 ± 0.2 |
Chromium
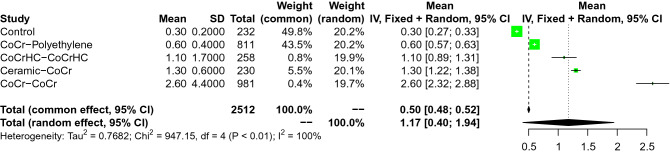
The coupling CoCr-Polyethylene demonstrated the lowest concentration of Cr in serum, followed by CoChHC-CoChHC, and Ceramic-CoCr. The coupling CoCr-CoCr demonstrated the highest concentration of Cr in serum. The overall effect was significant (95% CI: 0.0781 to 0.1225, Fig. 3). All network comparisons are showed in Appendix A.
Figure 3.
Forest plot of the comparison on Cr.
Cobalt
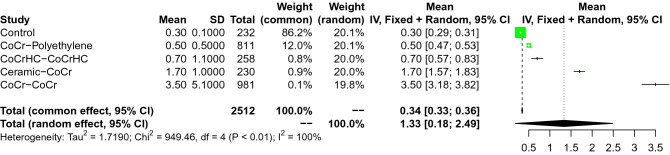
As expected, the control group and the coupling CoCr-CoCr demonstrated the lowest and the highest concentration of Cr in serum, respectively. After the control group, the coupling CoCr-Polyethylene demonstrated the lowest concentration of Cr in serum, followed by the coupling CoChHC-CoChHC, Ceramic-CoCr. The overall effect was significant (95% CI 0. 0.1345–0.1871, Fig. 4). All network comparisons are showed in Appendix B.
Figure 4.
Forest plot of the comparison on Co.
Multiple linear regressions
There was evidence of a weak association between BMI and the concentration of Co in serum (r = 0.3; P = 0.03). The time elapsed from the index surgery to the last follow-up, sex, and side did not evidence any statistically significant association with the concentration of Co and Cr in serum (Table 4).
Table 4.
Multiple linear regressions.
| Endpoint | Co | Cr | ||
|---|---|---|---|---|
| r | P | r | P | |
| Follow-up | 0.1 | 0.9 | 0.1 | 0.9 |
| BMI | 0.3 | 0.03 | 0.2 | 0.2 |
| Male/female | 0.6 | 0.6 | 0.1 | 0.9 |
| Right/left | 0.3 | 0.3 | 0.2 | 0.2 |
Discussion
According to the main findings of the present study, the mean concentration of Co in the serum of patients who have undergone THA ranged between 0.5 µg/L and 3.5 µg/L, and the mean concentration of Cr from 0.6 to 2.6 µg/L. The difference in the concentration of Co and Cr in serum is strictly related to the implant configuration, with the coupling CoCr-CoCr showing the highest and the coupling CoCr-Polyethylene showing the lowest concentration. These results confirm our hypothesis that patient characteristics and the time elapsed from the index surgery to the last follow-up did not exert any significant influence on the concentration of Co and Cr in serum in patients who have undergone THA.
Co exists in two forms: Co2+ and Co3+ and the absorption is mediated by the same receptor of Fe2+36,37. Co has an important role as a constituent of vitamin B 12 (hydroxocobalamin)38. Occupational exposure to Co typically happens in hard metal industry, with the inhalation of dust; in the construction industry, through skin contact with cement; in the e-waste recycling industry, from the release of Co from several electronic devices36,39,40. Co can be toxic for different organs due to the accumulation and the oxidative stress36. Co can cause a rapid and reversible decline of cardiac systolic function41. Co can cross the blood–brain barrier and cause peripheral and central nervous system deficit42. Hearing loss, optic nerve atrophy, cognitive decline, motor axonopathy, and sensitive symptoms have been documented43–45. Co inhalation is associated with the ‘hard metal lung disease’46. Skin contact provokes contact dermatitis and it is considered an occupational disease 47. The hematologic effect of Co is uncertain: some studies show an association between red blood cell count and haemoglobin levels and Co concentration48,49. Co decreases the iodine uptake by the thyroid resulting in gout and the development of hypothyroidism50. Exposure to Co, associated with tungsten carbide (WC–CO) can augment the risk of developing lung cancer51,52. The WC–CO nanoparticles generate ROS and promote cells proliferation and inflammation53.
Cr exists in different oxidation states from − 2 to + 654. Cr enters the cells through specific transporters, and it is reduced by glutathione reductase55. During this process, several reactive oxygen species can be formed, including ion superoxide and hydrogen peroxide55. Cr is excreted by the kidneys and through bile and hair in lower proportion56. Cr hazard has spread given its industrial usage57. Because of the heavy water contamination, urban areas are more at risk than rural areas 58. The established threshold of Cr in drinking water is 0.1 mg/l59. Inhalation of Cr can cause parenchymal pneumonia, asthma, wheezing and mucosal lung damage60,61. Gastrointestinal symptoms of Cr ingestion are bloody diarrhoea, abdominal pain, vomiting and ulceration62. High concentration of Cr can provoke hepatotoxicity, causing necrosis of liver cells and lymphocytes infiltration, leading to liver dysfunction55,63. Cr has toxic effects on the reproductive system64,65. Cr induces an increase in IGF-1 receptors, FOXO1 and an elevation in p53 expression level in kidney cells66. Chronic exposure can cause tubular necrosis and renal failure66. Contact dermatitis is common among workers in leather factories, and it is classified as an occupational disease67. Cr is an extremely sensitizing agent, both through inhalation and skin contact67,68. Cr is a genotoxic agent and is carcinogen69. Professional exposure can cause lung and sinonasal cancer70. It can also be related with gastrointestinal tract cancer71.
Adverse reaction to metal debris (ARMD) was described after metal-on-metal (MOM) THA, caused by the corrosion of the head and neck component72. Metal particles induce a local inflammatory reaction that can provokes fibrosis and osteolysis36. ARMD includes different histological findings73. In metallosis, the activation of innate response induces the formation of a granuloma surrounding metal debris74. Aseptic lymphocytic vasculitis associated lesion is characterized by perivascular lymphocytic infiltration and lymphoid aggregates of B and T cells, similar to a type IV reaction75. Type I reaction is mediated by immunoglobulin76. Radiography is the first line investigation for the diagnosis although it is not sensitive (62–64%)73. Periprosthetic osteolysis or a radiodense joint effusion can be identified77–79. MRI is the most sensitive imaging to diagnose ARMD77. It can detect indirect signs such as wear-induced synovitis, and direct signs generated by magnetic field variation, produced by metal fragments80–82.
Our systematic review includes the most updated articles in the present literature. 8 RCT studies were included in this review. The other studies had an overall low-moderate risk of bias. This makes our conclusion very reliable. Our study did not examine only one type of implant, but it compared ions concentrations using different materials patterns. It allows the surgeon to have a comprehensive understanding of the risks of ions related diseases when a specific type of implant is chosen. To our knowledge, this is the first systematic review that examined the association between ions concentration and the patients’ characteristics. This is another step ahead for the personalised surgery.
The present study has limitations. Firstly, the retrospective nature of some studies included in our review. Patient selection was different among the included studies. Patients suffering from renal failure were not excluded in studies8,10,20,23–26,29,30. The predominant mechanism of Cr and Co excretion is glomerular filtration without reabsorption54,83. Renal failure can lead to an accumulation of the two ions and an increase in their toxicity, but no association was found between GFR and ion levels84,85. It is not clear whether renal failure is a contraindication for metal-on-metal implants, but in these patients, a strict follow-up is advised21,86,87. It could influence the ion concentration values. It was not used a standardised method for blood sample collection. Pre-operative data were not available in two studies19,28. Country, region, city closeness to the factory, pollution of the ground and even the season can influence ion levels in the blood serum88. The diameter of the femoral head implant was not well clarified among the included studies. It is shown that a femoral head diameter greater than 36 mm is correlated with ARMD33,89,90.
Conclusion
The mean concentration of Co in the serum of patients who have undergone THA ranged between 0.5 µg/L and 3.5 µg/L, and the mean concentration of Cr from 0.6 to 2.6 µg/L. The difference in the concentration of Co and Cr in serum is strictly related to the implant configuration, with the coupling CoCr-CoCr showing the highest and the coupling CoCr-Polyethylene showing the lowest concentration. Patient characteristics and the time elapsed from the index surgery to the last follow-up did not exert any significant influence on the concentration of Co and Cr in serum.
Supplementary Information
Author contributions
F.M.: literature search, data extraction, conception and design, statistical analysis, writing; N.M.: supervision, revision; M.P.: writing; R.M.: literature search, data extraction. AB: risk of bias assessment; R.G.: risk of bias assessment. All authors have agreed to the final version to be published and agree to be accountable for all aspects of the work.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data availability
The datasets generated during and/or analysed during the current study are available throughout the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-34177-w.
References
- 1.Ferguson RJ, Palmer AJ, Taylor A, Porter ML, Malchau H, Glyn-Jones S. Hip replacement. Lancet. 2018;392(10158):1662–1671. doi: 10.1016/S0140-6736(18)31777-X. [DOI] [PubMed] [Google Scholar]
- 2.Learmonth ID, Young C, Rorabeck C. The operation of the century: Total hip replacement. Lancet. 2007;370(9597):1508–1519. doi: 10.1016/S0140-6736(07)60457-7. [DOI] [PubMed] [Google Scholar]
- 3.Lau RL, Gandhi R, Mahomed S, Mahomed N. Patient satisfaction after total knee and hip arthroplasty. Clin. Geriatr. Med. 2012;28(3):349–365. doi: 10.1016/j.cger.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Hartmann A, Hannemann F, Lutzner J, Seidler A, Drexler H, Gunther KP, Schmitt J. Metal ion concentrations in body fluids after implantation of hip replacements with metal-on-metal bearing–systematic review of clinical and epidemiological studies. PLoS One. 2013;8(8):e70359. doi: 10.1371/journal.pone.0070359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Varnum C (2017) Outcomes of different bearings in total hip arthroplasty - implant survival, revision causes, and patient-reported outcome. Dan Med J 64 (3) [PubMed]
- 6.Gkiatas I, Sharma AK, Greenberg A, Duncan ST, Chalmers BP, Sculco PK. Serum metal ion levels in modular dual mobility acetabular components: A systematic review. J. Orthop. 2020;21:432–437. doi: 10.1016/j.jor.2020.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheung AC, Banerjee S, Cherian JJ, Wong F, Butany J, Gilbert C, Overgaard C, Syed K, Zywiel MG, Jacobs JJ, Mont MA. Systemic cobalt toxicity from total hip arthroplasties: Review of a rare condition Part 1—History, mechanism, measurements, and pathophysiology. Bone Jt. J. 2016;98(1):6–13. doi: 10.1302/0301-620X.98B1.36374. [DOI] [PubMed] [Google Scholar]
- 8.Chen SY, Chang CH, Hu CC, Chen CC, Chang YH, Hsieh PH. Metal ion concentrations and semen quality in patients undergoing hip arthroplasty: A prospective comparison between metal-on-metal and metal-on-polyethylene implants. J. Orthop. Res. 2016;34(3):544–551. doi: 10.1002/jor.23037. [DOI] [PubMed] [Google Scholar]
- 9.Briggs TW, Hanna SA, Kayani B, Tai S, Pollock RC, Cannon SR, Blunn GW, Carrington RW. Metal-on-polyethylene versus metal-on-metal bearing surfaces in total hip arthroplasty: A prospective randomised study investigating metal ion levels and chromosomal aberrations in peripheral lymphocytes. Bone Jt. J. 2015;97-B(9):1183–1191. doi: 10.1302/0301-620X.97B9.34824. [DOI] [PubMed] [Google Scholar]
- 10.Dahlstrand H, Stark A, Wick MC, Anissian L, Hailer NP, Weiss RJ. Comparison of metal ion concentrations and implant survival after total hip arthroplasty with metal-on-metal versus metal-on-polyethylene articulations. Acta Orthop. 2017;88(5):490–495. doi: 10.1080/17453674.2017.1350370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aroukatos P, Repanti M, Repantis T, Bravou V, Korovessis P. Immunologic adverse reaction associated with low-carbide metal-on-metal bearings in total hip arthroplasty. Clin. Orthop. Relat. Res. 2010;468(8):2135–2142. doi: 10.1007/s11999-009-1187-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Repantis T, Vitsas V, Korovessis P. Poor mid-term survival of the low-carbide metal-on-metal Zweymuller-plus total hip arthroplasty system: A concise follow-up, at a minimum of ten years, of a previous report. J. Bone Jt. Surg. Am. 2013;95(6):e331–334. doi: 10.2106/JBJS.L.00031. [DOI] [PubMed] [Google Scholar]
- 13.Affatato S, Traina F, Ruggeri O, Toni A. Wear of metal-on-metal hip bearings: Metallurgical considerations after hip simulator studies. Int. J. Artif. Organs. 2011;34(12):1155–1164. doi: 10.5301/ijao.5000065. [DOI] [PubMed] [Google Scholar]
- 14.Howick JCI, Glasziou P, Greenhalgh T, Carl H, Liberati A, Moschetti I, Phillips B, Thornton H, Goddard O, Hodgkinson M. The 2011 Oxford CEBM Levels of Evidence. Oxford Centre for Evidence-Based Medicine; 2011. [Google Scholar]
- 15.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hrobjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, Thomas J. Updated guidance for trusted systematic reviews: A new edition of the cochrane handbook for systematic reviews of interventions. Cochrane Database Syst. Rev. 2019;10:ED000142. doi: 10.1002/14651858.ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins JPT TJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA . Cochrane Handbook for Systematic Reviews of Interventions version 6.2. Cochrane 2021. www.training.cochrane.org/handbook. Accessed February 2022.
- 18.Cadossi M, Mazzotti A, Baldini N, Giannini S, Savarino L. New couplings, old problems: Is there a role for ceramic-on-metal hip arthroplasty? J. Biomed. Mater. Res. B Appl. Biomater. 2016;104(1):204–209. doi: 10.1002/jbm.b.33383. [DOI] [PubMed] [Google Scholar]
- 19.Darrith B, Rahman TM, Ananthasubramaniam K, Culvern C, Jacobs JJ, Silverton CD. Echocardiographic changes in the context of metal-on-metal versus nonmetal-on-metal total hip arthroplasty. J. Arthroplasty. 2020;35(11):3230–3236 e3233. doi: 10.1016/j.arth.2020.06.020. [DOI] [PubMed] [Google Scholar]
- 20.Engh CA, MacDonald SJ, Sritulanondha S, Korczak A, Naudie D, Engh C. Metal ion levels after metal-on-metal total hip arthroplasty: A five-year, prospective randomized trial. J. Bone Jt. Surg. Am. 2014;96(6):448–455. doi: 10.2106/JBJS.M.00164. [DOI] [PubMed] [Google Scholar]
- 21.Grubl A, Weissinger M, Brodner W, Gleiss A, Giurea A, Gruber M, Poll G, Meisinger V, Gottsauner-Wolf F, Kotz R. Serum aluminium and cobalt levels after ceramic-on-ceramic and metal-on-metal total hip replacement. J. Bone Jt. Surg. Br. 2006;88(8):1003–1005. doi: 10.1302/0301-620X.88B8.17870. [DOI] [PubMed] [Google Scholar]
- 22.Gustafson K, Jakobsen SS, Lorenzen ND, Thyssen JP, Johansen JD, Bonefeld CM, Stilling M, Baad-Hansen T, Soballe K. Metal release and metal allergy after total hip replacement with resurfacing versus conventional hybrid prosthesis. Acta Orthop. 2014;85(4):348–354. doi: 10.3109/17453674.2014.922730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higgins JE, Conn KS, Britton JM, Pesola M, Manninen M, Stranks GJ. Early results of our international, multicenter, multisurgeon, double-blinded, prospective, randomized, controlled trial comparing metal-on-metal with ceramic-on-metal in total hip arthroplasty. J. Arthroplasty. 2020;35(1):193–197 e192. doi: 10.1016/j.arth.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Malviya A, Ramaskandhan JR, Bowman R, Hashmi M, Holland JP, Kometa S, Lingard E. What advantage is there to be gained using large modular metal-on-metal bearings in routine primary hip replacement? A preliminary report of a prospective randomised controlled trial. J. Bone Jt. Surg. Br. 2011;93(12):1602–1609. doi: 10.1302/0301-620X.93B12.27533. [DOI] [PubMed] [Google Scholar]
- 25.Martin JR, Jennings JM, Watters TS, Levy DL, Miner TM, Dennis DA. Midterm prospective comparative analysis of 2 hard-on-hard bearing total hip arthroplasty designs. J. Arthroplasty. 2018;33(6):1820–1825. doi: 10.1016/j.arth.2018.01.019. [DOI] [PubMed] [Google Scholar]
- 26.Moroni A, Nocco E, Hoque M, Diremigio E, Buffoli D, Cantu F, Catalani S, Apostoli P. Cushion bearings versus large diameter head metal-on-metal bearings in total hip arthroplasty: A short-term metal ion study. Arch. Orthop. Trauma Surg. 2012;132(1):123–129. doi: 10.1007/s00402-011-1364-8. [DOI] [PubMed] [Google Scholar]
- 27.Nam D, Keeney JA, Nunley RM, Johnson SR, Clohisy JC, Barrack RL. Metal ion concentrations in young, active patients following total hip arthroplasty with the use of modern bearing couples. J. Arthroplasty. 2015;30(12):2227–2232. doi: 10.1016/j.arth.2015.06.025. [DOI] [PubMed] [Google Scholar]
- 28.Pozzuoli A, Berizzi A, Crimi A, Belluzzi E, Frigo AC, Conti G, Nicolli A, Trevisan A, Biz C, Ruggieri P. Metal ion release, clinical and radiological outcomes in large diameter metal-on-metal total hip arthroplasty at long-term follow-up. Diagnostics (Basel) 2020;10(11):941. doi: 10.3390/diagnostics10110941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Savarino L, Padovani G, Ferretti M, Greco M, Cenni E, Perrone G, Greco F, Baldini N, Giunti A. Serum ion levels after ceramic-on-ceramic and metal-on-metal total hip arthroplasty: 8-year minimum follow-up. J. Orthop. Res. 2008;26(12):1569–1576. doi: 10.1002/jor.20701. [DOI] [PubMed] [Google Scholar]
- 30.Savarino L, Granchi D, Ciapetti G, Cenni E, Nardi Pantoli A, Rotini R, Veronesi CA, Baldini N, Giunti A. Ion release in patients with metal-on-metal hip bearings in total joint replacement: A comparison with metal-on-polyethylene bearings. J. Biomed. Mater. Res. 2002;63(5):467–474. doi: 10.1002/jbm.10299. [DOI] [PubMed] [Google Scholar]
- 31.Schouten R, Malone AA, Frampton CM, Tiffen C, Hooper G. Five-year follow-up of a prospective randomised trial comparing ceramic-on-metal and metal-on-metal bearing surfaces in total hip arthroplasty. Bone Jt. J. 2017;99-B(10):1298–1303. doi: 10.1302/0301-620X.99B10.BJJ-2016-0905.R1. [DOI] [PubMed] [Google Scholar]
- 32.Schouten R, Malone AA, Tiffen C, Frampton CM, Hooper G. A prospective, randomised controlled trial comparing ceramic-on-metal and metal-on-metal bearing surfaces in total hip replacement. J. Bone Jt. Surg. Br. 2012;94(11):1462–1467. doi: 10.1302/0301-620X.94B11.29343. [DOI] [PubMed] [Google Scholar]
- 33.Tiusanen H, Makela K, Kiilunen M, Sarantsin P, Sipola E, Pesola M. The effect of different bearing surfaces on metal ion levels in urine following 28 mm metal-on-metal and 28 mm metal-on-polyethylene total hip arthroplasty. Scand. J. Surg. 2013;102(3):197–203. doi: 10.1177/1457496913491874. [DOI] [PubMed] [Google Scholar]
- 34.White PB, Meftah M, Ranawat AS, Ranawat CS. A comparison of blood metal ions in total hip arthroplasty using metal and ceramic heads. J. Arthroplasty. 2016;31(10):2215–2220. doi: 10.1016/j.arth.2016.03.024. [DOI] [PubMed] [Google Scholar]
- 35.Zijlstra WP, van der Veen HC, van den Akker-Scheek I, Zee MJ, Bulstra SK, van Raay JJ. Acetabular bone density and metal ions after metal-on-metal versus metal-on-polyethylene total hip arthroplasty; short-term results. Hip. Int. 2014;24(2):136–143. doi: 10.5301/hipint.5000087. [DOI] [PubMed] [Google Scholar]
- 36.Leyssens L, Vinck B, Van Der Straeten C, Wuyts F, Maes L. Cobalt toxicity in humans-A review of the potential sources and systemic health effects. Toxicology. 2017;387:43–56. doi: 10.1016/j.tox.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 37.Valberg LS, Ludwig J, Olatunbosun D. Alteration in cobalt absorption in patients with disorders of iron metabolism. Gastroenterology. 1969;56(2):241–251. doi: 10.1016/S0016-5085(69)80123-X. [DOI] [PubMed] [Google Scholar]
- 38.Taylor A, Marks V. Cobalt: A review. J. Hum. Nutr. 1978;32(3):165–177. doi: 10.3109/09637487809144525. [DOI] [PubMed] [Google Scholar]
- 39.Wang BJ, Wu JD, Sheu SC, Shih TS, Chang HY, Guo YL, Wang YJ, Chou TC. Occupational hand dermatitis among cement workers in Taiwan. J. Formos. Med. Assoc. 2011;110(12):775–779. doi: 10.1016/j.jfma.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 40.Kusaka Y, Yokoyama K, Sera Y, Yamamoto S, Sone S, Kyono H, Shirakawa T, Goto S. Respiratory diseases in hard metal workers: An occupational hygiene study in a factory. Br. J. Ind. Med. 1986;43(7):474–485. doi: 10.1136/oem.43.7.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Packer M. Cobalt cardiomyopathy: A critical reappraisal in light of a recent resurgence. Circ. Heart Fail. 2016 doi: 10.1161/CIRCHEARTFAILURE.116.003604. [DOI] [PubMed] [Google Scholar]
- 42.Hock A, Demmel U, Schicha H, Kasperek K, Feinendegen LE. Trace element concentration in human brain. Activation analysis of cobalt, iron, rubidium, selenium, zinc, chromium, silver, cesium, antimony and scandium. Brain. 1975;98(1):49–64. doi: 10.1093/brain/98.1.49. [DOI] [PubMed] [Google Scholar]
- 43.Oldenburg M, Wegner R, Baur X. Severe cobalt intoxication due to prosthesis wear in repeated total hip arthroplasty. J. Arthroplasty. 2009;24(5):825.e15–825.e20. doi: 10.1016/j.arth.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 44.Apel W, Stark D, Stark A, O'Hagan S, Ling J. Cobalt-chromium toxic retinopathy case study. Doc. Ophthalmol. 2013;126(1):69–78. doi: 10.1007/s10633-012-9356-8. [DOI] [PubMed] [Google Scholar]
- 45.Steens W, von Foerster G, Katzer A. Severe cobalt poisoning with loss of sight after ceramic-metal pairing in a hip–A case report. Acta Orthop. 2006;77(5):830–832. doi: 10.1080/17453670610013079. [DOI] [PubMed] [Google Scholar]
- 46.Lison D, Lauwerys R, Demedts M, Nemery B. Experimental research into the pathogenesis of cobalt/hard metal lung disease. Eur. Respir. J. 1996;9(5):1024–1028. doi: 10.1183/09031936.96.09051024. [DOI] [PubMed] [Google Scholar]
- 47.Schmidt M, Goebeler M. Immunology of metal allergies. J. Dtsch. Dermatol. Ges. 2015;13(7):653–660. doi: 10.1111/ddg.12673. [DOI] [PubMed] [Google Scholar]
- 48.Davis JE, Fields JP. Experimental production of polycythemia in humans by administration of cobalt chloride. Proc. Soc. Exp. Biol. Med. 1958;99(2):493–495. doi: 10.3181/00379727-99-24395. [DOI] [PubMed] [Google Scholar]
- 49.Bowie EA, Hurley PJ. Cobalt chloride in the treatment of refractory anaemia in patients undergoing long-term haemodialysis. Aust. N. Z. J. Med. 1975;5(4):306–314. doi: 10.1111/j.1445-5994.1975.tb03263.x. [DOI] [PubMed] [Google Scholar]
- 50.Paustenbach DJ, Tvermoes BE, Unice KM, Finley BL, Kerger BD. A review of the health hazards posed by cobalt. Crit. Rev. Toxicol. 2013;43(4):316–362. doi: 10.3109/10408444.2013.779633. [DOI] [PubMed] [Google Scholar]
- 51.Leonard A, Lauwerys R. Mutagenicity, carcinogenicity and teratogenicity of cobalt metal and cobalt compounds. Mutat. Res. 1990;239(1):17–27. doi: 10.1016/0165-1110(90)90029-b. [DOI] [PubMed] [Google Scholar]
- 52.Armstead AL, Li B. Nanotoxicity: emerging concerns regarding nanomaterial safety and occupational hard metal (WC-Co) nanoparticle exposure. Int. J. Nanomed. 2016;11:6421–6433. doi: 10.2147/IJN.S121238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lison D, van den Brule S, Van Maele-Fabry G. Cobalt and its compounds: Update on genotoxic and carcinogenic activities. Crit. Rev. Toxicol. 2018;48(7):522–539. doi: 10.1080/10408444.2018.1491023. [DOI] [PubMed] [Google Scholar]
- 54.Ducros V. Chromium metabolism. A literature review. Biol. Trace Elem. Res. 1992;32:65–77. doi: 10.1007/BF02784589. [DOI] [PubMed] [Google Scholar]
- 55.Hossini H, Shafie B, Niri AD, Nazari M, Esfahlan AJ, Ahmadpour M, Nazmara Z, Ahmadimanesh M, Makhdoumi P, Mirzaei N, Hoseinzadeh E. A comprehensive review on human health effects of chromium: Insights on induced toxicity. Environ. Sci. Pollut. Res. Int. 2022;29(47):70686–70705. doi: 10.1007/s11356-022-22705-6. [DOI] [PubMed] [Google Scholar]
- 56.Senft AW, Philpott DE, Pelofsky AH. Electron microscope observations of the integument, flame cells, and gut of Schistosoma mansoni. J. Parasitol. 1961;47:217–229. doi: 10.2307/3275292. [DOI] [PubMed] [Google Scholar]
- 57.Ayele A, Suresh A, Benor S, Konwarh R. Optimization of chromium(VI) removal by indigenous microalga (Chlamydomonas sp.)-based biosorbent using response surface methodology. Water Environ. Res. 2021;93(8):1276–1288. doi: 10.1002/wer.1510. [DOI] [PubMed] [Google Scholar]
- 58.Wilbur S, Abadin H, Fay M, Yu D, Tencza B, Ingerman L, Klotzbach J, James S (2012). In: Toxicological Profile for Chromium. Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profiles. Atlanta (GA), [PubMed]
- 59.Murthy MK, Khandayataray P, Padhiary S, Samal D. A review on chromium health hazards and molecular mechanism of chromium bioremediation. Rev. Environ. Health. 2022 doi: 10.1515/reveh-2021-0139. [DOI] [PubMed] [Google Scholar]
- 60.Sanz P, Nogue S, Munne P, Torra R, Marques F. Acute potassium dichromate poisoning. Hum. Exp. Toxicol. 1991;10(3):228–229. doi: 10.1177/096032719101000315. [DOI] [PubMed] [Google Scholar]
- 61.Derelanko MJ, Rinehart WE, Hilaski RJ, Thompson RB, Loser E. Thirteen-week subchronic rat inhalation toxicity study with a recovery phase of trivalent chromium compounds, chromic oxide, and basic chromium sulfate. Toxicol. Sci. 1999;52(2):278–288. doi: 10.1093/toxsci/52.2.278. [DOI] [PubMed] [Google Scholar]
- 62.Suh M, Wikoff D, Lipworth L, Goodman M, Fitch S, Mittal L, Ring C, Proctor D. Hexavalent chromium and stomach cancer: A systematic review and meta-analysis. Crit. Rev. Toxicol. 2019;49(2):140–159. doi: 10.1080/10408444.2019.1578730. [DOI] [PubMed] [Google Scholar]
- 63.Pascale LR, Waldstein SS, Engbring G, Dubin A, Szanto PB. Chromium intoxication, with special reference to hepatic injury. J. Am. Med. Assoc. 1952;149(15):1385–1389. doi: 10.1001/jama.1952.02930320025008. [DOI] [PubMed] [Google Scholar]
- 64.Elbetieha A, Al-Hamood MH. Long-term exposure of male and female mice to trivalent and hexavalent chromium compounds: Effect on fertility. Toxicology. 1997;116(1–3):39–47. doi: 10.1016/s0300-483x(96)03516-0. [DOI] [PubMed] [Google Scholar]
- 65.Banu SK, Stanley JA, Sivakumar KK, Arosh JA, Taylor RJ, Burghardt RC. Chromium VI—Induced developmental toxicity of placenta is mediated through spatiotemporal dysregulation of cell survival and apoptotic proteins. Reprod. Toxicol. 2017;68:171–190. doi: 10.1016/j.reprotox.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu YH, Lin JC, Wang TY, Lin TJ, Yen MC, Liu YH, Wu PL, Chen FW, Shih YL, Yeh IJ. Hexavalent chromium intoxication induces intrinsic and extrinsic apoptosis in human renal cells. Mol. Med. Rep. 2020;21(2):851–857. doi: 10.3892/mmr.2019.10885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bregnbak D, Johansen JD, Jellesen MS, Zachariae C, Menne T, Thyssen JP. Chromium allergy and dermatitis: Prevalence and main findings. Contact Dermat. 2015;73(5):261–280. doi: 10.1111/cod.12436. [DOI] [PubMed] [Google Scholar]
- 68.Lockman LE. Case report: Allergic contact dermatitis and new-onset asthma. Chromium exposure during leather tanning. Can. Fam. Physician. 2002;48:1907–1909. [PMC free article] [PubMed] [Google Scholar]
- 69.O'Brien TJ, Ceryak S, Patierno SR. Complexities of chromium carcinogenesis: Role of cellular response, repair and recovery mechanisms. Mutat. Res. 2003;533(1–2):3–36. doi: 10.1016/j.mrfmmm.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 70.Wang Y, Su H, Gu Y, Song X, Zhao J. Carcinogenicity of chromium and chemoprevention: A brief update. Onco Targets Ther. 2017;10:4065–4079. doi: 10.2147/OTT.S139262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cullen JM, Ward JM, Thompson CM. Reevaluation and classification of duodenal lesions in B6C3F1 mice and F344 rats from 4 studies of hexavalent chromium in drinking water. Toxicol. Pathol. 2016;44(2):279–289. doi: 10.1177/0192623315611501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Waterson HB, Whitehouse MR, Greidanus NV, Garbuz DS, Masri BA, Duncan CP. Revision for adverse local tissue reaction following metal-on-polyethylene total hip arthroplasty is associated with a high risk of early major complications. Bone Jt. J. 2018;100-B(6):720–724. doi: 10.1302/0301-620X.100B6.BJJ-2017-1466.R1. [DOI] [PubMed] [Google Scholar]
- 73.Shon WY, Gupta S, Biswal S, Han SH, Hong SJ, Moon JG. Pelvic osteolysis relationship to radiographs and polyethylene wear. J. Arthroplasty. 2009;24(5):743–750. doi: 10.1016/j.arth.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 74.Natu S, Sidaginamale RP, Gandhi J, Langton DJ, Nargol AV. Adverse reactions to metal debris: Histopathological features of periprosthetic soft tissue reactions seen in association with failed metal on metal hip arthroplasties. J. Clin. Pathol. 2012;65(5):409–418. doi: 10.1136/jclinpath-2011-200398. [DOI] [PubMed] [Google Scholar]
- 75.Ng VY, Lombardi AV, Jr, Berend KR, Skeels MD, Adams JB. Perivascular lymphocytic infiltration is not limited to metal-on-metal bearings. Clin. Orthop. Relat. Res. 2011;469(2):523–529. doi: 10.1007/s11999-010-1570-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thyssen JP, Jakobsen SS, Engkilde K, Johansen JD, Soballe K, Menne T. The association between metal allergy, total hip arthroplasty, and revision. Acta Orthop. 2009;80(6):646–652. doi: 10.3109/17453670903487008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Maloney E, Ha AS, Miller TT. Imaging of adverse reactions to metal debris. Semin. Musculoskelet. Radiol. 2015;19(1):21–30. doi: 10.1055/s-0034-1396764. [DOI] [PubMed] [Google Scholar]
- 78.Salem KH, Lindner N, Tingart M, Elmoghazy AD. Severe metallosis-related osteolysis as a cause of failure after total knee replacement. J. Clin. Orthop. Trauma. 2020;11(1):165–170. doi: 10.1016/j.jcot.2019.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Giustra F, Bistolfi A, Bosco F, Fresia N, Sabatini L, Berchialla P, Sciannameo V, Masse A. Highly cross-linked polyethylene versus conventional polyethylene in primary total knee arthroplasty: Comparable clinical and radiological results at a 10-year follow-up. Knee Surg. Sports Traumatol. Arthrosc. 2023;31(3):1082–1088. doi: 10.1007/s00167-022-07226-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nawabi DH, Gold S, Lyman S, Fields K, Padgett DE, Potter HG. MRI predicts ALVAL and tissue damage in metal-on-metal hip arthroplasty. Clin. Orthop. Relat. Res. 2014;472(2):471–481. doi: 10.1007/s11999-013-2788-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hargreaves BA, Worters PW, Pauly KB, Pauly JM, Koch KM, Gold GE. Metal-induced artifacts in MRI. AJR Am. J. Roentgenol. 2011;197(3):547–555. doi: 10.2214/AJR.11.7364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee MJ, Kim S, Lee SA, Song HT, Huh YM, Kim DH, Han SH, Suh JS. Overcoming artifacts from metallic orthopedic implants at high-field-strength MR imaging and multi-detector CT. Radiographics. 2007;27(3):791–803. doi: 10.1148/rg.273065087. [DOI] [PubMed] [Google Scholar]
- 83.Simonsen LO, Harbak H, Bennekou P. Cobalt metabolism and toxicology–A brief update. Sci. Total Environ. 2012;432:210–215. doi: 10.1016/j.scitotenv.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 84.Brodner W, Grohs JG, Bitzan P, Meisinger V, Kovarik J, Kotz R. Serum cobalt and serum chromium level in 2 patients with chronic renal failure after total hip prosthesis implantation with metal-metal gliding contact. Z. Orthop. Ihre Grenzgeb. 2000;138(5):425–429. doi: 10.1055/s-2000-10172. [DOI] [PubMed] [Google Scholar]
- 85.Lainiala O, Reito A, Jamsa P, Eskelinen A. Mild or moderate renal insufficiency does not increase circulating levels of cobalt and chromium in patients with metal-on-metal hip arthroplasty. Bone Jt. J. 2017;99-B(9):1147–1152. doi: 10.1302/0301-620X.99B9.BJJ-2016-0773.R2. [DOI] [PubMed] [Google Scholar]
- 86.Manninen E, Lainiala O, Karsikas M, Reito A, Jamsa P, Eskelinen A. Do cobalt or chromium accumulate in metal-on-metal hip arthroplasty patients who have mild, moderate, or severe renal insufficiency? Bone Jt. J. 2021;103-B(7):1231–1237. doi: 10.1302/0301-620X.103B7.BJJ-2020-0836.R2. [DOI] [PubMed] [Google Scholar]
- 87.Vigni GE, Bosco F, Cioffi A, Camarda L. Mortality risk assessment at the admission in patient with proximal femur fractures: Electrolytes and renal function. Geriatr. Orthop. Surg. Rehabil. 2021;12:2151459321991503. doi: 10.1177/2151459321991503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xiong Q, Zhao W, Zhao J, Zhao W, Jiang L. Concentration levels, pollution characteristics and potential ecological risk of dust heavy metals in the metropolitan area of Beijing, China. Int. J. Environ. Res. Public Health. 2017;14(10):1159. doi: 10.3390/ijerph14101159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pandit H, Glyn-Jones S, McLardy-Smith P, Gundle R, Whitwell D, Gibbons CL, Ostlere S, Athanasou N, Gill HS, Murray DW. Pseudotumours associated with metal-on-metal hip resurfacings. J. Bone Jt. Surg. Br. 2008;90(7):847–851. doi: 10.1302/0301-620X.90B7.20213. [DOI] [PubMed] [Google Scholar]
- 90.Ollivere B, Darrah C, Barker T, Nolan J, Porteous MJ. Early clinical failure of the Birmingham metal-on-metal hip resurfacing is associated with metallosis and soft-tissue necrosis. J. Bone Jt. Surg. Br. 2009;91(8):1025–1030. doi: 10.1302/0301-620X.91B8.21701. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available throughout the manuscript.