Abstract
DNA methylation is important for establishing and maintaining cell identity and for genomic stability. This is achieved by regulating the accessibility of regulatory and transcriptional elements and the compaction of subtelomeric, centromeric, and other inactive genomic regions. Carcinogenesis is accompanied by a global loss in DNA methylation, which facilitates the transformation of cells. Cancer hypomethylation may also cause genomic instability, for example through interference with the protective function of telomeres and centromeres. However, understanding the role(s) of hypomethylation in tumor evolution is incomplete because the precise mutational consequences of global hypomethylation have thus far not been systematically assessed. Here we made genome-wide inventories of all possible genetic variation that accumulates in single cells upon the long-term global hypomethylation by CRISPR interference-mediated conditional knockdown of DNMT1. Depletion of DNMT1 resulted in a genomewide reduction in DNA methylation. The degree of DNA methylation loss was similar to that observed in many cancer types. Hypomethylated cells showed reduced proliferation rates, increased transcription of genes, reactivation of the inactive X-chromosome and abnormal nuclear morphologies. Prolonged hypomethylation was accompanied by increased chromosomal instability. However, there was no increase in mutational burden, enrichment for certain mutational signatures or accumulation of structural variation to the genome. In conclusion, the primary consequence of hypomethylation is genomic instability, which in cancer leads to increased tumor heterogeneity and thereby fuels cancer evolution.
Subject terms: Genomic instability, DNA methylation, Cancer genetics, Cancer genomics
Introduction
Epigenetic mechanisms confer cell identity by regulating gene activity, allowing cells to have different phenotypes while sharing the same genotype1. To switch phenotypes cells need to remodel their epigenetic landscapes, for example during differentiation2, reprogramming3, or the transformation of healthy cells into tumor cells4. A key epigenetic modification in mammalian cells is DNA methylation, the covalent attachment of a methyl group to the 5th carbon atom of cytosine. In healthy human cells, around 85% of the cytosines that are flanked by guanines (CpG sites) are methylated. Notable exceptions are CpG islands (CGIs), regions with high GC content that are mostly unmethylated (< 10%) and are associated with active promoter regions of housekeeping genes and tumor suppressor genes (TSGs)5.
The DNA methylation landscape is sculpted by the joint activity of DNA methyltransferases (DNMT) and ten-eleven translocation (TET) enzymes. DNMT3A and DNMT3B are responsible for the de novo establishment of DNA methylation. DNMT1 maintains DNA methylation in dividing cells and is supported by UHRF1 that recognizes hemi-methylated substrates at replication forks6. Active demethylation is accomplished by TET1, TET2, and TET3, through the hydroxylation of methylcytosine, followed by oxidation of hydroxymethylcytosine to 5-formylcytosine (5fC) and then to 5-carboxycytosine (5caC). These bases are subsequently recognized by base excision repair and replaced by cytosine. DNMT and TET enzymes are frequently amplified or mutated in cancer and can have a causal role in carcinogenesis7–14.
Many studies have provided evidence that genetically or chemically induced loss of DNA methylation results in genomic instability15–20. DNA methylation supports genomic stability by promoting DNA condensation and the inhibition of transcription of constitutive heterochromatic genomic elements including centromeric and pericentric heterochromatin, LINE elements and subtelomeric regions21,22. Tumorigenesis is generally accompanied by a global decrease in DNA methylation23,24, which leads to the concomitant deprotection of these heterochromatic regions. Pericentromeric heterochromatin, LINE elements and subtelomeric regions are frequently affected in cancer25–28, indicating that the cancer-associated changes to the DNA methylation landscape coincide with genome instability. Thus, the epigenetic instability of transforming cells may interfere with the protective role of DNA methylation in maintaining genomic integrity, leading to genomic instability. However, the genetic—epigenetic relationship is complex and our knowledge on the genomic consequences of the epigenetic reprogramming that coincide with cancer development is far from complete.
To improve our view on cancer evolution and tumor heterogeneity we need a better understanding of how reprogramming of the epigenetic software influences the genetic hardware. In recent years, whole genome sequencing of cancer genomes has increasingly been used for the identification of mutational signatures, distinct patterns of mutation accumulation that provide insight into past mutational processes, such as DNA repair deficiencies, endogenous mutational processes, and exposure to exogenous mutagens such as tobacco-smoking, UV-light or anti-cancer treatments29. Over 100 mutational signatures have been described that involve single, double and clustered base substitution signatures and indel signatures30,31. In addition, sixteen signatures have been identified that are based on patterns of structural variation such as deletions, tandem duplications, and inversions32. The etiology for many mutational signatures is still unknown.
In spite of the myriad genomic consequences that may follow DNA methylation loss, the genomic consequences of long-term DNA hypomethylation have thus far not been systematically characterized and no mutational signatures have been linked to DNA methylation loss. In this study, we aimed to fully characterize all genomic consequences of global methylation loss; from the whole chromosome level down to single base resolution. We performed CRISPR/CAS9 mediated conditional knockdown of DNMT1 and examined the genetic consequences after prolonged culture by whole genome sequencing at 30X coverage of expanded clones to capture events at high resolution affecting few or single bases and by single cell DNA sequencing to capture chromosome scale events.
Results
For these experiments we made use of a human female TERT immortalized retinal pigment epithelial cell line (RPE-1)33,34, which lacks a transformed phenotype and is near diploid. This is a valuable and powerful cell line for the study of DNA damage, genomic instability, and mutational signature analysis, because RPE-1 cells can be cultured long-term, while maintaining a stable karyotype with a modal chromosome number of 4635–37. Because of its key role in maintenance of methylation, loss of DNMT1 activity should lead to a progressive loss of DNA methylation in cycling cells. In most cell lines, DNMT1 is an essential gene38,39. In line with these observations, our attempts to create a DNMT1 knockout in RPE-1 cells failed, while knockouts for non-essential genes were successful (data not shown). Therefore, we decided to use CRISPR interference (CRISPRi) to knock down DNMT1 gene expression40. In CRISPRi, a nuclease dead version of Cas9 (dCAS9) fused to the transcriptional inhibitor KRAB41 (dCAS9-KRAB) is guided to the transcriptional start site by a sgRNA, thereby blocking the transcription machinery and resulting in decreased expression of the gene of interest. In combination with a doxycycline inducible sgRNA, CRISPRi allows temporal downregulation in full isogenic cell lines.
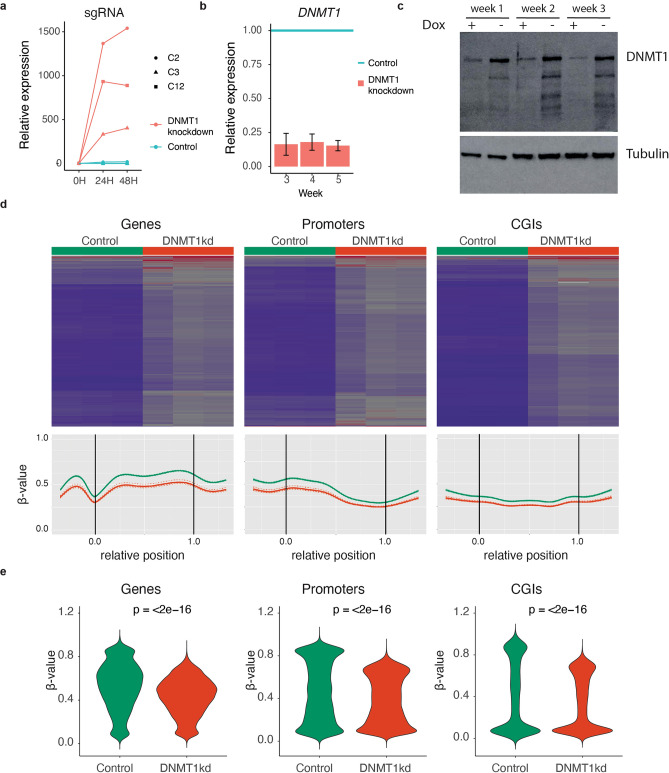
We established clonal hTERT RPE-1 dCAS9-KRAB doxycycline inducible sgRNADNMT1 cell lines (hereafter referred to as DNMT1 knockdown cells). The lines were P53-/- knock-out (Supplementary Fig. 1a,b) to increase the permissiveness of cells for all types of mutations and avoid loss of such events due to P53-induced senescence or apoptosis. Three independent clonal DNMT1 knockdown lines showed clear upregulation of the sgRNA upon addition of doxycycline (Fig. 1a). All next experiments were performed with clonal line 2, which showed robust and long-term downregulation of DNMT1 at the mRNA and protein levels upon sgRNA induction (Fig. 1a-c, Supplementary Fig. 2). Methylation arrays covering over 850,000 sites with extensive coverage of CpG islands, genes, and enhancers revealed a genome wide reduction in methylation levels in DNMT1 knockdown cells in three independent experiments (Fig. 1d).Genome-wide, the mean degree of methylation was reduced by 10% upon DNMT1 inhibition, while regions with β-values > 0.5 showed a reduction of 16% in methylation levels. These reductions in DNA methylation levels are similar to or even larger than has been previously described in a wide variety of solid tumors, including colorectal, lung, and breast cancer that mostly show reductions of around 10% 24,42. Therefore, our DNMT1 knockdown cell line is a highly suitable model to study if the loss in methylation as observed in cancer can induce mutations or genomic instability. Methylation loss was independent of genomic location and observed in genes, promoters and CpG islands (Fig. 1d,e).
Figure 1.
Characterization of DNMT1 knockdown cells (a) Relative increase in doxycycline-inducible sgRNA expression upon 24 h and 48 h hours of treatment with doxycycline. (b) Relative DNMT1 expression levels upon doxycycline treatment of DNMT1 knockdown cells. n = 4 per time point. (c) Western blot images of DNMT1 protein expression levels upon prolonged doxycycline treatment of DNMT1 knockdown cells for the indicated time points. (d) EPIC DNA methylation array analysis of DNMT1 knockdown cells cultured with or without doxycycline for 6 weeks. Top panels: heatmaps with methylation values for control (n = 3) and DNMT1 knockdown (n = 3) cells. Lower panels: Regional methylation profiles (composite plots) according to sample groups. DNMT1 knockdown cells consistently show reduced methylation density represented by lower β-values. For each region in the corresponding region type, relative coordinates of 0 and 1 correspond to the start and end coordinates of that region respectively. Coordinates smaller than 0 and greater than 1 denote flanking regions normalized by region length. Horizontal lines indicate region boundaries. (e) Violin plots of the β-values for genes, promoters and CGIs. Statistical test: paired Wilcoxon signed-rank test.
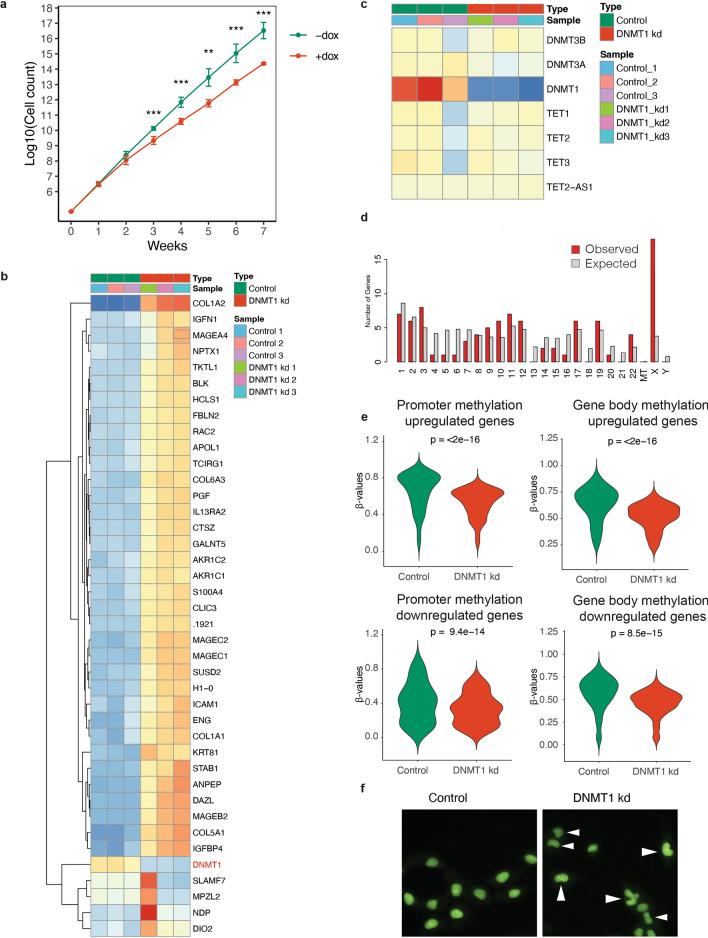
Prolonged downregulation of DNMT1 delayed growth by approximately 50%, without leading to a full cell cycle arrest (Fig. 2a), which is in line with previous observations19,20. Further characterization of DNMT1 knockdown cells by RNA seq revealed that global hypomethylation resulted in a relative increase in gene activity. In total, 501 genes were up- and 73 genes were downregulated (p adj < 0.05) in DNMT1 knockdown cells. Reassuringly, DNMT1 was among the down regulated genes, while other writers and erasers of DNA methylation were not differentially expressed between both conditions (Fig. 2b,c). Strikingly, more genes on the X-chromosome were upregulated than expected, indicating partial reactivation of the inactive X-chromosome (Fig. 2d).
Figure 2.
Phenotype of DNMT1 knockdown cells (a) Growth curves of doxycycline induced DNMT1 knockdown cells and control cells (n = 4 per condition per time-point). (b) Unsupervised hierarchical clustering heatmap of the 40 most differentially expressed genes between doxycycline induced DNMT1 knockdown cells and control cells. (c) heatmap of the expression of the writers (DNMT genes) and erasers (TET enzymes) of DNA methylation in doxycycline induced DNMT1 knockdown cells and control cells. (d) Distribution of genes on chromosomes as determined by ShinyGO 0.7681. Chi-square test p = 5.8E-08. (e) Violin plots of the β-values for genes, promoters and CGIs for the differentially expressed genes (p adj < 0.05). Statistical test: paired Wilcoxon signed-rank test.(f) Morphology of control cells and cells with hypomethylated genomes. Arrowheads denote cells with abnormal nuclear morphology.
To further examine the correlation between DNA methylation and gene expression, we examine the DNA methylation status at promoter regions and gene bodies of genes that were differentially expressed. Promoter regions of upregulated genes were hypermethylated in control cells and showed a significant reduction of 13% in methylation status upon DNMT1 knockdown (Fig. 2e), which is in line with the repressive nature of promoter methylation43. In contrast, promoter regions of downregulated genes showed much lower methylation levels in control cells when compared to upregulated genes. Also the reduction in DNA methylation was less pronounced (7.5%). In gene bodies, DNA methylation was reduced in both the upregulated gene set as well as the downregulated geneset, with respectively 12% and 11% (Fig. 2e).
Doxycycline treated cells acquired an abnormal kidney-shaped nuclear morphology, indicating that DNA methylation loss disturbs global genome organization (Fig. 2f).
CRISPRi mediated knockdown of DNMT1 in RPE-1 cells is a relatively clean method to reduce DNA methylation compared to alternative methods such as short hairpin mediated knockdown or chemical inhibition that have more risk of adverse side-effects unrelated to the loss of DNA methylation and are generally restricted to short-term consequences44–46. Together, this conditional DNMT1 knockdown cell line is a powerful tool to study the long-term consequences of DNA methylation loss.
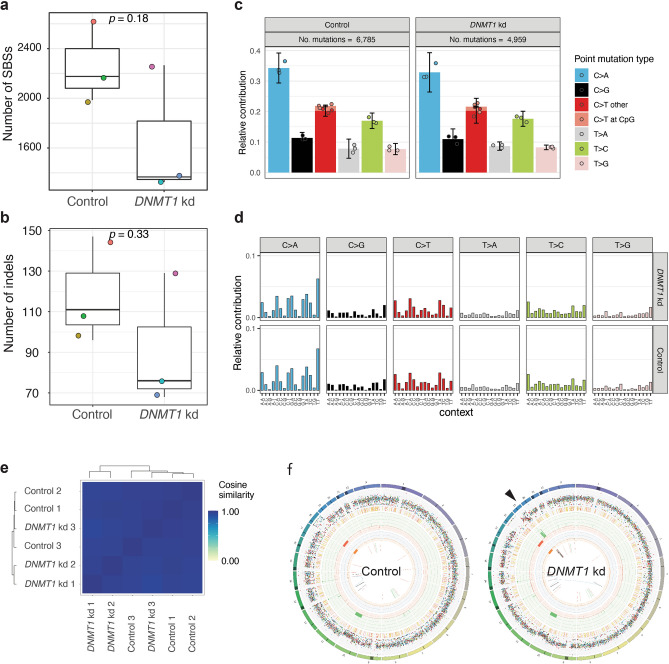
To examine the genomic consequences of global DNA methylation loss in single cells at nucleotide resolution we performed prolonged downregulation of DNMT1 (6 weeks) to allow putative genetic changes to occur, followed by single clone expansion. Whole genome sequencing at 30X coverage of expanded clones followed by computational analysis enables the identification of all types of genetic variation, including single base substitutions (SBSs) and indels that are private to each clone. We previously applied this highly sensitive method to identify and characterize the mutational signatures in individual cells in healthy, diseased, and perturbed conditions47–53. Loss of DNA methylation has previously been associated with mismatch repair (MMR) deficiency54 and therefore we anticipated to find an elevated mutational burden as well as an increase in the contribution of MMR-related mutational signatures. In contrast to our expectations we did not observe an increase in SBS burden (Student’s t-test, p value = 0.18; Fig. 3a) or in the number of indels (Student’s t-test, p value = 0.33; Fig. 3b), although numbers tended to be lower in the hypomethylated clones when compared to the control condition, possibly reflecting the difference in proliferation rates (Fig. 2a). The mutational spectrum (Fig. 3c) and 96 mutational profiles (Fig. 3d-e) were highly similar between hypomethylated cells and control cells, indicating that reduced methylation did not lead to a shift in the activity of mutational processes. Notably, in this study we relied on a sample size set (n = 3 per condition) similar to previous studies that also used manipulated tissue culture systems and which has been adequate for the detection of differences in mutation rates and to identify induced mutational patterns47,50,55. Structural variant (SVs) analysis revealed that most SVs were shared between clones of both conditions indicating these SVs were acquired prior to DNMT1 knockdown (Fig. 3f). Notable exception was a chromothripsis event that affected the q-arm of Chromosome 19, which was only present in two of the three clones of the hypomethylated cells (Fig. 3f).
Figure 3.
Mutational impact of global DNA methylation loss (a) Box-plot with the individual data points of the number of single base substitutions per clone. Each clone is represented by a unique color. (b) Box-plot with the individual data points of the number of indels per clone. Each clone is represented by a unique color. Same color scheme as in a. (c) Average mutational spectra of (left) control clones and (right) hypomethylated DNMT1 knockdown clones. (d) Average mutational profiles of (top) hypomethylated DNMT1 knockdown clones and (bottom) control clones. (e) Cosine similarity between the mutational profiles of hypomethylated DNMT1 knockdown clones and control cells. (f) Circos plots from a clone with (left) normal DNA methylation levels and (right) a hypomethylated DNMT1 knockdown clone. Arrowhead denotes a chromothripsis event at the q-arm of chromosome 19, which was observed in 2 out of 3 clones.
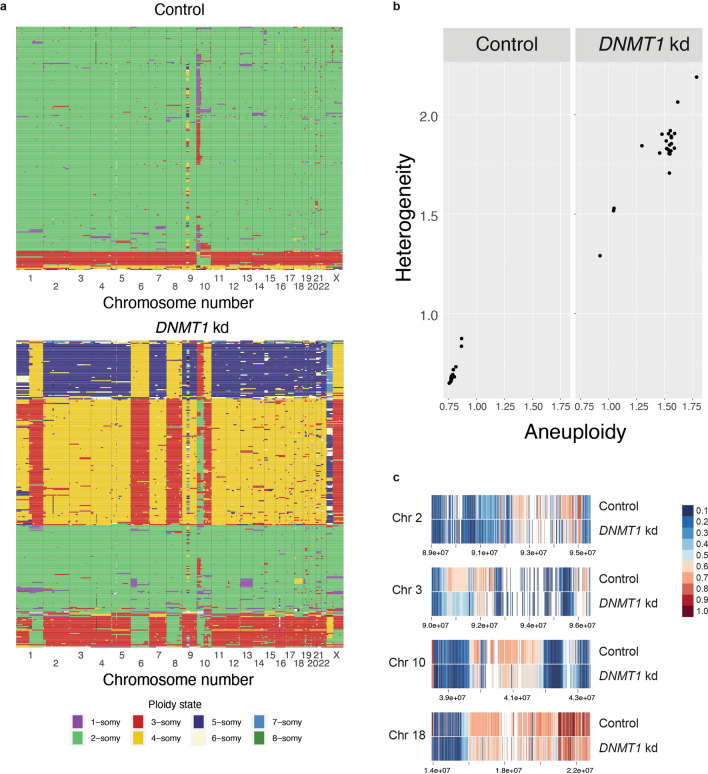
Chromothripsis has mechanistically been linked to the missegregation of chromosomes or chromosome arms followed by damage and non-homologous repair in micronuclei56. The observed chromothripsis event may therefore be the result of increased chromosomal instability (CIN), as has been described previously for hypomethylated cells57. To further investigate CIN, we performed single cell DNA sequencing (Fig. 4a). Doxycycline treatment for 6 weeks of DNMT1 knockdown cells resulted in increased aneuploidy and higher cellular heterogeneity (Fig. 4b). We reasoned that the increased chromosomal instability was the result of the loss of DNA methylation in peri-centromeric regions. However, the repetitive nature of these regions precludes assessment of DNA methylation by DNA methylation arrays. Therefore, we decided to perform long-read Nanopore sequencing of peri-centromeres, which allows both the direct measurement of methylated cytosines as well as unique mapping to the telomere-to-telomere reference genome58. Similar to the genomic regions that were assessed by methylation arrays, pericentromeric regions of doxycycline-treated cells showed a consistent reduction in DNA methylation levels (Fig. 4c).
Figure 4.
Impact of global DNA methylation loss on chromosomal instability (a) Single cell DNA sequencing results showing the copy number state per chromosome per cell for (top) control clones and (bottom) hypomethylated DNMT1 knockdown clones. Each row represents a single cell and each column represents a chromosome. For each condition 384 cells were sorted. Shown are the cells with good quality libraries. (b) Aneuploidy and heterogeneity scores for (left) control clones and (right) hypomethylated DNMT1 knockdown clones calculated with Aneufinder. Each dot represents a chromosome. (c) Reduced DNA methylation levels in pericentromeric regions of chromosomes 2, 3, 10, and 18 upon DNMT1 knockdown as determined by Nanopore DNA sequencing. Coordinate values are indicated.
Discussion and Conclusion.
Ample evidence has established that DNA methylation is required for genomic integrity15–20. However, DNA methylation is associated with many different genomic elements providing different routes towards genomic instability upon methylation loss59. Reduced methylation can destabilize pericentromeric heterochromatin inducing rearrangements of chromosome arms, as has been described in for example Wilms tumors and hepatocellular carcinoma60,61, and upon chemical interference of cell lines with the DNA hypomethylating drug 5-aza-2’-deoxycytidine20. Additionally, loss of methylation at repetitive elements may lead to genomic instability through secondary DNA structures that cause replication stress, DNA breaks and recombination between repeats59. Methylation loss can also lead to derepression of transposable elements that in turn can promote genomic rearrangements26,62,63. A decrease in methylation could also lead to more transcription and formation of R-loops, which are a source of replication stress, DNA breaks and genome instability64. Furthermore, reduced methylation at subtelomeric regions could lead to telomere instability65. Finally, DNA methylation has been implicated in DNA mismatch-repair54 and the absence of DNA methylation may lead to mild MMR-deficiency. Because of these possible effects of DNA methylation loss, we hypothesized that experimentally induced DNA demethylation would lead to a broad spectrum of genetic variation, ranging from SBSs, to indels, and from SVs to chromosomal aberrations. With a systematic approach, using highly sensitive methods to measure all types of genetic variation in individual cells47,49,66, we identified chromosomal instability as the foremost mutational consequence of DNA methylation loss.
There are several possible explanations for why we did not observe other types of genetic variation. In our model, DNA methylation loss was incomplete, so it is possible that more genetic events beyond chromosomal instability will occur if DNA methylation is further reduced, although this would likely severely impact on viability of the cells and may not be physiologically relevant in the context of cancer. Additionally, the hypomethylation effects may be cell-type specific. For example, the RPE-1 cell line that we used was immortalized by overexpression of hTERT, which may counteract any destabilization of telomeres caused by DNA methylation loss. Other cell types may be more sensitive to DNA hypomethylation in peri-telomeric regions. The effects of DNA methylation loss may also be context dependent and require additional circumstances. For example, global DNA demethylation may derepress transposons, but these may only become active when accompanied by the loss of repressive histone modifications and when post-transcriptional and post-translation defense mechanisms that suppress their mobility also fail. The mutational patterns induced by DNA methylation loss may also be too subtle to be detected above the background of more common mutational processes, e.g. those induced by culturing conditions47. Taken together, while our observations do not support an important role for DNA methylation in mutational processes other than CIN, subtle effects may be detected by capturing more variants, for example by extending the culture period of hypomethylated cells or by the analysis of vast amounts of clones expanded from single cells, which would be a very costly endeavor.
The exact mechanism through which hypomethylation causes CIN is as yet unknown. Proposed non-exclusive mechanisms include increased recombination of centromeric repeats, increased DNA breaks in centromeres, dysregulation of the centromeric protein network, increased transcription of α-satellite transcripts, defective assembly of centromeres/kinetochores and premature cohesion loss22,32. Future work may uncover the mechanistic link between pericentromeric hypomethylation and chromosomal instability. In conclusion, we observed that the degree of hypomethylation that can be observed in many cancer types primarily promotes CIN, without inducing other mutational processes. Hypomethylation induced CIN may promote tumor heterogeneity and cancer evolution.
Materials and methods
Molecular cloning and virus production
We replaced the shRNA scaffold of pLV.FUTG.Tet.Inducible.shRNA with the sgRNA scaffold from lenticrispr v267, by InFusion cloning (Takara). Next, we annealed oligonucleotides for the guideRNA of DNMT1 (Top: CACCGGGTACGCGCCGGCATCTCGG; Bottom: AAACCCGAGATGCCGGCGCGTACCC) and subsequently ligated the product into BsmbI digested pLV.TETi.sgRNA to obtain pLV.TETi.sgDNMT1. Selection of this sgRNA sequence was predicted to be highly effective for CRISPR interference based on chromatin, position, and sequence features40. To make RPE-1 cells more permissive to the accumulation of genetic variation, TP53 knock-outs were generated with sgRNAs targeting the gene sequence 5′-GGGCAGCTACGGTTTCCGTC-3′. The annealed DNA oligonucleotides were cloned in px458 that also contains Cas9 followed by a 2A-EGFP sequence68. For virus production, lentiviral plasmids carrying the transgenes of interest were co-transfected with pRSV-REV, pMD2g and pMDLG-pRRE in HEK293T cells. Virus was harvested 3 days post-transfection and collected for immediate use or concentrated using Lenti-X Concentrator solution and stored at -80. Concentrated or undiluted virus was added to RPE-1 cells in a dilution series together with 4 µg/ml polybrene. Successfully transduced cells were subsequently selected with the appropriate mammalian selection marker.
Cell culture
hTERT RPE1 cells (ATCC, CRL-4000) and HEK239T cells were cultured in DMEM, 10% fetal bovine serum, 1% penicillin, 1% streptomycin at 37 °C, and 5% CO2.
RPE1 TP53-/- cells were transduced with lentivirus carrying lenti.EF1a.dCas9.KRAB.Puro41 followed by transduction with pLV.TETi.sgDNMT1 lentivirus. RPE1-p53ko-dCas9-KRAB-Teti-DNMT1 cells were cultured with 10 ug/ml puromycin for selection of the dCas9-KRAB plasmid, 10 µg/ml blasticidin for selection of guide RNA plasmid. Clonal cell lines were established by limiting dilution series. The expression of the guideRNA was induced by stimulation with doxycyclin 2 µg/ml, which was refreshed every 2–3 days. Cell counts were performed every week and 5.3 × 10E + 3 cells/cm2 were seeded. Cell counts were multiplied by split ratio to correct for passage. To establish hTERT-RPE1 CRISPR knock-out cell lines, cells were seeded in a 10 cm dish and transfected with vectors encoding both Cas9 and sgRNA target sequence using an Amaxa Nucleofector II instrument (Lonza). Forty-eight hours after transfection single GFP + cells were sorted into 96-wells plates on FACS ARIA II/III Flow Cytometer (BD Biosciences) and expanded. Clones were tested for genome editing with PCR and Sanger sequencing and analyzed with the ICE CRISPR tool69.
RT-qPCR
RNA was isolated using the RNeasy Mini kit (Qiagen) and further purified using 3 M NaAc and isopropanol if required. RT-qPCR was performed using Luna Universal One-Step RT-qPCR kit (NEB) using the following primers: DNMT1 forward: 5′-AGCGGAGGTGTCCCAATATG-3′, DNMT1 reverse: 5′-GAGACACAGTCCCCCACTTC-3′, sgRNA forward: TTTAGAGCTAGAAATAGC, sgRNA reverse: CGACTCGGTGCCACTTTTTC, GAPDH forward: 5′-AAATCCCATCACCATCTTCCAGGAGC-3′, GAPDH reverse: 5′-CATGGTTCACACCCATGACGAACA-3′. GAPDH was used as a reference gene and relative gene expression levels were calculated by ΔΔCt analysis70, comparing cells cultured with doxycycline to cells cultured without doxycycline.
Western Blot
Samples were collected in Laemmli buffer and incubated at 100 °C for 10 min. Pageruler plus prestained protein ladder (ThermoFisher) and 20 ug of each sample were loaded on a 6% or 10% SDS-PAGE gel and transferred to a nitrocellulose membrane using Trans-Blot Turbo Transfer System (Bio-Rad). Western Blot was blocked using 5% ELK and incubated overnight with primary antibody mouse monoclonal anti-UHRF1 (Santa Cruz, SC-373750, 1:250) or rabbit polyclonal anti-DNMT1 (Invitrogen, PA3-16,556, 1:1000) or monoclonal mouse anti-tubulin (Sigma-Aaldrich, T5168, 1:4000) and secondary HRP-conjugated antibody goat anti-mouse (1:2500) and goat anti-rabbit (1:2500). Blot was imaged using Amersham ECL (GE Healthcare) and Amersham Imager 600.
Staining and microscopy
Cell culture medium was removed and 1 ml of staining solution, consisting of 2.5 uM SYTO 11 Green Fluorescent Nucleic Acid Stain (Invitrogen) in cell culture medium was added to each well of a 6-well plate and cells were incubated at 37 °C for 60 min. Cells were imaged using an EVOS M5000 Imaging System at 10X magnification.
Single cell DNA sequencing
Single cell sequencing was performed by the Single Cell Sequencing Core facility at the Hubrecht Institute. In short, cells were cultured with doxycycline for 6 weeks were resuspended and incubated with 2 ml of staining solution, consisting of 5 ug/ml Hoechst 34,580 in cell culture medium. Cell pellet was resuspended in PBS. Cells were FACS sorted in 384-well plates and lysis of individual cells was performed for 2 h at 55 °C using Proteinase K (Ambion) in 1 × Cutsmart (New England Biolabs) followed by heat inactivation at 80 °C for 10 min. The genomic DNA was subsequently fragmented with 100 nl NLAIII (R0125L, New England Biolabs) in 1 × Cutsmart (New England Biolabs) for 2 h at 37 °C followed by heat inactivation at 65 °C for 20 min. Then, 50 nl of 50 mM barcoded double-stranded NLAIII adapters and 400 nl of 40 U T4 DNA ligase (New England Biolabs) in 1 × T4 DNA ligase buffer (New England Biolabs) supplemented with 10 mM ATP (Invitrogen) was added to each well and ligated overnight at 16 °C. Libraries were sequenced on an Illumina Nextseq 2000 with 1 × 50 bp double-end sequencing. The fastq files were mapped to GRCH38 using the Burrows–Wheeler aligner. The mapped data were further analyzed using custom scripts in Python, which parsed for library barcodes, removed reads without a NlaIII sequence and removed PCR-duplicated reads. Copy number analysis was performed as described previously66.
RNA sequencing
For RNA sequencing, cells were cultured for 6 weeks with doxycycline. RNA was isolated using RNeasy Mini kit (Qiagen) and samples with low purity were further purified by isopropanol precipitation. RNA-seq libraries were prepared with the TruSeq Stranded Total RNA Library Prep Kit (Illumina) according to the manufacturer’s instructions. RNA-seq libraries were pooled and sequenced on a NextSeq2000 (Illumina) as 1 × 50 bp single end reads. RNA sequencing reads were aligned against human reference genome 37. The Bioconductor package DESeq2 was used to normalize raw read counts and to perform differential gene expression analysis71.
DNA methylation analysis
DNMT1 knockdown cells were cultured with doxycycline for 6 weeks. DNA was isolated using the DNeasy Blood & Tissue kit (Qiagen) followed by bisulfite conversion with the EZ DNA Methylation™ Kit (Zymo) according to the manufacturer’s instructions. The samples were subsequently run on an Infinium EPIC DNA methylation array. Data was analyzed with RNBeads72.
Nanopore sequencing of centromeres
For ONT sequencing of centromeres, pericentromeric regions were isolated by AlphaHOR-RES (alpha higher-order repeat restriction and enrichment by size)73. In short, genomic DNA was extracted from ~ 25 million cells using an NEB High Molecular Weight DNA extraction kit followed by elution. Fully solubilized DNA was digested with MscI, and AseI. Digested DNA was loaded onto a 0.3% TAE agarose gel and run at 2 V/cm for 1 h. Fragments larger than 20 kb were purified using a Zymoclean Large Fragment DNA Recovery Kit. DNA was subsequently prepared for DNA sequencing using an ONT native library prep kit (LSK-109) and sequenced on a MinION (r9) flow cell. Nanopore raw data was basecalled using Guppy v5.0.17, and subsequently mapped against the CHM13 draft version 1.1 using minimap2 (map-ont settings). Reads were filtered for a mapping quality > = 10. Methylation was then called using nanopolish 0.11.1 using the nanopolish call-methylation command. For binarized methylation calls, a cutoff of 2 was used so that scores > 2 are interpreted as methylated and scores < -2 are interpreted as unmethylated.
Sequencing and data analysis.
For whole genome sequencing, DNA was isolated from cell pellets with the Qiasymphony (Qiagen) DNA isolation method and the Illumina TruSeq Nano DNA Library Prep Kit was used for library preparation. Samples were sequenced on HiSeq Xten or NovaSeq6000 platforms (Illumina) with 30 × coverage. All samples were analysed with the HMF pipeline V4.8 (https://github.com/hartwigmedical/pipeline) which was locally deployed using GNU Guix with the recipe from https://github.com/UMCUGenetics/guix-additions. Full pipeline description is explained in74, and details and settings of all the tools can be found at their Github page. Briefly, sequence reads were mapped against the human reference genome GRCh37 using Burrows-Wheeler Alignment (BWA-MEM) v0.7.5a75. Subsequently, somatic single base substitutions (SBSs), double base substitutions (DBSs) and small insertions and deletions (INDELS) were determined by Strelka v1.0.1476 that are further annotated by PURPLE. PURPLE (v2.53) combines B-allele frequency (BAF) from AMBER (v3.3), read depth ratios from COBALT (v1.7), and structural variants from GRIDSS77 to estimate copy number profiles, variant allele frequency (VAF), variant clonality and microhomology context at the breakpoints. To obtain high-quality somatic mutations that can be attributed to in vivo mutagenesis in the ASC clones, we only considered somatic mutations with a PURPLE derived variant allele frequency higher than 30% as mutations that fall outside this range were potentially induced in vitro after the clonal passage. Analysis of the SVs was based on the LINX (v1.26)78 output which interprets and annotates simple and complex SV events from PURPLE and GRIDSS output. LINX chains individual SVs into SV clusters and classifies these clusters into various event types. Clusters can have one SV (for simple events such as deletions and duplications which all have 1 clusterId), or multiple SVs, with ClusterId > 1 and here considered as complex SV. We defined SV load as the total number of simple SV events. We quantified deletions and duplications (ResolvedType is 'DEL’ or ‘DUP’) stratified by length (1–10 kb, 10–100 kb, 100 kb–1 Mb, 1–10 Mb, > 10 Mb). For complex SVs, we included “Complex_SV”, |”Complex_DEL “, “RECIP_INV” and “RECIP_TRANS” under resolved_Type annotation feature.
Mutation burden analysis
The SBS, DBS and indel mutations were parsed from PURPLE vcfs by our developed R package Mutational Patterns79 that was recently updated with DBS and indel functionality as well as COSMIC compatibility80. For each mutation type, we defined mutation burden as the total number of mutations of the autosomal genome.
Supplementary Information
Acknowledgements
We are grateful for the support of Useq for facilitating the sequencing that was performed in this study and the Single Cell Sequencing Core facility at the Hubrecht Institute for their help with the single cell DNA sequencing. We would also like to thank Livio Kleij for his support with the microscopy and our colleagues from the genome diagnostics department for their support with the methylation arrays.
Author contributions
N.B. and J.K. performed wet-lab experiments. C.V., A.v.H., and E.K. performed bioinformatic analyses. S.B. supported data management. E.C. and E.K. were involved in the conceptual design of the study. E.C. and J.d.R. provided financial and lab support. E.K. wrote the paper. All authors proofread, made comments, and approved the paper.
Data availability
The datasets generated and analysed during the current study are available in the EGA repository, EGAS00001006845.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Nicolle Besselink and Janneke Keijer
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-33932-3.
References
- 1.Goldberg AD, Allis CD, Bernstein E. Epigenetics: A landscape takes shape. Cell. 2007;128:635–638. doi: 10.1016/j.cell.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 2.Mohn F, Schübeler D. Genetics and epigenetics: Stability and plasticity during cellular differentiation. Trends Genet. 2009;25:129–136. doi: 10.1016/j.tig.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 4.Suvà ML, Riggi N, Bernstein BE. Epigenetic reprogramming in cancer. Science. 2013;339:1567–1570. doi: 10.1126/science.1230184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergman Y, Cedar H. DNA methylation dynamics in health and disease. Nat. Struct. Mol. Biol. 2013;20:274–281. doi: 10.1038/nsmb.2518. [DOI] [PubMed] [Google Scholar]
- 6.Liu X, et al. UHRF1 targets DNMT1 for DNA methylation through cooperative binding of hemi-methylated DNA and methylated H3K9. Nat. Commun. 2013;4:1563. doi: 10.1038/ncomms2562. [DOI] [PubMed] [Google Scholar]
- 7.Brunetti L, Gundry MC, Goodell MA. DNMT3A in leukemia. Cold Spring Harb. Perspect. Med. 2017;7:a030320. doi: 10.1101/cshperspect.a030320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao T, Pan W, Sun X, Shen H. Increased expression of TET3 predicts unfavorable prognosis in patients with ovarian cancer-a bioinformatics integrative analysis. J. Ovarian Res. 2019;12:101. doi: 10.1186/s13048-019-0575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fang J-Y, et al. Expression of Dnmt1, demethylase, MeCP2 and methylation of tumor-related genes in human gastric cancer. World J. Gastroenterol. 2004;10:3394–3398. doi: 10.3748/wjg.v10.i23.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simó-Riudalbas L, Melo SA, Esteller M. DNMT3B gene amplification predicts resistance to DNA demethylating drugs. Genes Chromosomes Cancer. 2011;50:527–534. doi: 10.1002/gcc.20877. [DOI] [PubMed] [Google Scholar]
- 11.Langemeijer SMC, et al. Acquired mutations in TET2 are common in myelodysplastic syndromes. Nat. Genet. 2009;41:838–842. doi: 10.1038/ng.391. [DOI] [PubMed] [Google Scholar]
- 12.Xiong Y, et al. Opposite alterations of DNA methyltransferase gene expression in endometrioid and serous endometrial cancers. Gynecol. Oncol. 2005;96:601–609. doi: 10.1016/j.ygyno.2004.11.047. [DOI] [PubMed] [Google Scholar]
- 13.Zhu Y-M, et al. Expression of human DNA methyltransferase 1 in colorectal cancer tissues and their corresponding distant normal tissues. Int. J. Colorectal Dis. 2007;22:661–666. doi: 10.1007/s00384-006-0224-4. [DOI] [PubMed] [Google Scholar]
- 14.Gaudet F, et al. Induction of tumors in mice by genomic hypomethylation. Science. 2003;300:489–492. doi: 10.1126/science.1083558. [DOI] [PubMed] [Google Scholar]
- 15.Chen RZ, Pettersson U, Beard C, Jackson-Grusby L, Jaenisch R. DNA hypomethylation leads to elevated mutation rates. Nature. 1998;395:89–93. doi: 10.1038/25779. [DOI] [PubMed] [Google Scholar]
- 16.Karpf AR, Matsui S-I. Genetic disruption of cytosine DNA methyltransferase enzymes induces chromosomal instability in human cancer cells. Cancer Res. 2005;65:8635–8639. doi: 10.1158/0008-5472.CAN-05-1961. [DOI] [PubMed] [Google Scholar]
- 17.Sheaffer KL, Elliott EN, Kaestner KH. DNA hypomethylation contributes to genomic instability and intestinal cancer initiation. Cancer Prev. Res. 2016;9:534–546. doi: 10.1158/1940-6207.CAPR-15-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen T, et al. Complete inactivation of DNMT1 leads to mitotic catastrophe in human cancer cells. Nat. Genet. 2007;39:391–396. doi: 10.1038/ng1982. [DOI] [PubMed] [Google Scholar]
- 19.Barra V, Schillaci T, Lentini L, Costa G, Di Leonardo A. Bypass of cell cycle arrest induced by transient DNMT1 post-transcriptional silencing triggers aneuploidy in human cells. Cell Div. 2012;7:2. doi: 10.1186/1747-1028-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Costa G, Barra V, Lentini L, Cilluffo D, Di Leonardo A. DNA demethylation caused by 5-Aza-2′-deoxycytidine induces mitotic alterations and aneuploidy. Oncotarget. 2016;7:3726–3739. doi: 10.18632/oncotarget.6897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saksouk N, Simboeck E, Déjardin J. Constitutive heterochromatin formation and transcription in mammals. Epigenetics Chromatin. 2015 doi: 10.1186/1756-8935-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scelfo A, Fachinetti D. Keeping the centromere under control: A promising role for DNA methylation. Cells. 2019;8:912. doi: 10.3390/cells8080912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berman BP, et al. Regions of focal DNA hypermethylation and long-range hypomethylation in colorectal cancer coincide with nuclear lamina-associated domains. Nat. Genet. 2011;44:40–46. doi: 10.1038/ng.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Timp W, et al. Large hypomethylated blocks as a universal defining epigenetic alteration in human solid tumors. Genome Med. 2014;6:61. doi: 10.1186/s13073-014-0061-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jafri MA, Ansari SA, Alqahtani MH, Shay JW. Roles of telomeres and telomerase in cancer, and advances in telomerase-targeted therapies. Genome Med. 2016;8:69. doi: 10.1186/s13073-016-0324-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodriguez-Martin B, et al. Pan-cancer analysis of whole genomes identifies driver rearrangements promoted by LINE-1 retrotransposition. Nat. Genet. 2020;52:306–319. doi: 10.1038/s41588-019-0562-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saha AK, et al. The genomic landscape of centromeres in cancers. Sci. Rep. 2019;9:11259. doi: 10.1038/s41598-019-47757-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Slee RB, et al. Cancer-associated alteration of pericentromeric heterochromatin may contribute to chromosome instability. Oncogene. 2012;31:3244–3253. doi: 10.1038/onc.2011.502. [DOI] [PubMed] [Google Scholar]
- 29.Tate JG, et al. COSMIC: The catalogue of somatic mutations in cancer. Nucleic Acids Res. 2019;47:D941–D947. doi: 10.1093/nar/gky1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Degasperi A, et al. Substitution mutational signatures in whole-genome-sequenced cancers in the UK population. Science. 2022;376:abl9283. doi: 10.1126/science.abl9283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alexandrov LB, et al. The repertoire of mutational signatures in human cancer. Nature. 2020;578:94–101. doi: 10.1038/s41586-020-1943-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Y, et al. Patterns of somatic structural variation in human cancer genomes. Nature. 2020;578:112–121. doi: 10.1038/s41586-019-1913-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bodnar AG, et al. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 34.Jiang XR, et al. Telomerase expression in human somatic cells does not induce changes associated with a transformed phenotype. Nat. Genet. 1999;21:111–114. doi: 10.1038/5056. [DOI] [PubMed] [Google Scholar]
- 35.Janssen A, van der Burg M, Szuhai K, Kops GJPL, Medema RH. Chromosome segregation errors as a cause of DNA damage and structural chromosome aberrations. Science. 2011;333:1895–1898. doi: 10.1126/science.1210214. [DOI] [PubMed] [Google Scholar]
- 36.Umbreit NT, et al. Mechanisms generating cancer genome complexity from a single cell division error. Science. 2020;368:eaba0712. doi: 10.1126/science.aba0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reijns MAM, et al. Signatures of TOP1 transcription-associated mutagenesis in cancer and germline. Nature. 2022;602:623–631. doi: 10.1038/s41586-022-04403-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen W-H, Lu G, Chen X, Zhao X-M, Bork P. OGEE v2: An update of the online gene essentiality database with special focus on differentially essential genes in human cancer cell lines. Nucleic Acids Res. 2017;45:D940–D944. doi: 10.1093/nar/gkw1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hart T, et al. High-resolution CRISPR screens reveal fitness genes and genotype-specific cancer liabilities. Cell. 2015;163:1515–1526. doi: 10.1016/j.cell.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 40.Horlbeck MA, et al. Compact and highly active next-generation libraries for CRISPR-mediated gene repression and activation. Elife. 2016;5:e19760. doi: 10.7554/eLife.19760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ho S-M, et al. Evaluating synthetic activation and repression of neuropsychiatric-related genes in hiPSC-derived NPCs, neurons, and astrocytes. Stem Cell Rep. 2017;9:615–628. doi: 10.1016/j.stemcr.2017.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Visone R, et al. DNA methylation of shelf, shore and open sea CpG positions distinguish high microsatellite instability from low or stable microsatellite status colon cancer stem cells. Epigenomics. 2019;11:587–604. doi: 10.2217/epi-2018-0153. [DOI] [PubMed] [Google Scholar]
- 43.Bird JBA. DNA methylation inhibits transcription indirectly via a methyl-CpG binding protein. Cell. 1991;64:1123–1134. doi: 10.1016/0092-8674(91)90267-3. [DOI] [PubMed] [Google Scholar]
- 44.Kaelin WG., Jr Molecular biology. Use and abuse of RNAi to study mammalian gene function. Science. 2012;337:421–422. doi: 10.1126/science.1225787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maslov AY, et al. 5-aza-2′-deoxycytidine-induced genome rearrangements are mediated by DNMT1. Oncogene. 2012;31:5172–5179. doi: 10.1038/onc.2012.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Santi DV, Norment A, Garrett CE. Covalent bond formation between a DNA-cytosine methyltransferase and DNA containing 5-azacytosine. Proc. Natl. Acad. Sci. U. S. A. 1984;81:6993–6997. doi: 10.1073/pnas.81.22.6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kuijk E, et al. The mutational impact of culturing human pluripotent and adult stem cells. Nat. Commun. 2020;11:2493. doi: 10.1038/s41467-020-16323-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuijk E, et al. Early divergence of mutational processes in human fetal tissues. Sci. Adv. 2019;5:eaaw1271. doi: 10.1126/sciadv.aaw1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jager M, et al. Deficiency of nucleotide excision repair is associated with mutational signature observed in cancer. Genome Res. 2019;29:1067–1077. doi: 10.1101/gr.246223.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Christensen S, et al. 5-Fluorouracil treatment induces characteristic T>G mutations in human cancer. Nat. Commun. 2019;10:4571. doi: 10.1038/s41467-019-12594-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blokzijl F, et al. Tissue-specific mutation accumulation in human adult stem cells during life. Nature. 2016;538:260–264. doi: 10.1038/nature19768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nguyen L, et al. Precancerous liver diseases do not cause increased mutagenesis in liver stem cells. Commun. Biol. 2021;4:1301. doi: 10.1038/s42003-021-02839-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kuijk E, Kranenburg O, Cuppen E, Van Hoeck A. Common anti-cancer therapies induce somatic mutations in stem cells of healthy tissue. Nat. Commun. 2022;13:5915. doi: 10.1038/s41467-022-33663-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang K-Y, James Shen C-K. DNA methyltransferase Dnmt1 and mismatch repair. Oncogene. 2004;23:7898–7902. doi: 10.1038/sj.onc.1208111. [DOI] [PubMed] [Google Scholar]
- 55.Drost J, et al. Use of CRISPR-modified human stem cell organoids to study the origin of mutational signatures in cancer. Science. 2017;358:234–238. doi: 10.1126/science.aao3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang C-Z, et al. Chromothripsis from DNA damage in micronuclei. Nature. 2015;522:179–184. doi: 10.1038/nature14493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eden A, Gaudet F, Waghmare A, Jaenisch R. Chromosomal instability and tumors promoted by DNA hypomethylation. Science. 2003;300:455. doi: 10.1126/science.1083557. [DOI] [PubMed] [Google Scholar]
- 58.Nurk S, et al. The complete sequence of a human genome. Science. 2022;376:44–53. doi: 10.1126/science.abj6987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pappalardo XG, Barra V. Losing DNA methylation at repetitive elements and breaking bad. Epigenetics Chromatin. 2021;14:25. doi: 10.1186/s13072-021-00400-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wong N, et al. Hypomethylation of chromosome 1 heterochromatin DNA correlates with q-arm copy gain in human hepatocellular carcinoma. Am. J. Pathol. 2001;159:465–471. doi: 10.1016/S0002-9440(10)61718-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Qu GZ, Grundy PE, Narayan A, Ehrlich M. Frequent hypomethylation in Wilms tumors of pericentromeric DNA in chromosomes 1 and 16. Cancer Genet. Cytogenet. 1999;109:34–39. doi: 10.1016/S0165-4608(98)00143-5. [DOI] [PubMed] [Google Scholar]
- 62.Feng S, Jacobsen SE, Reik W. Epigenetic reprogramming in plant and animal development. Science. 2010;330:622–627. doi: 10.1126/science.1190614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jönsson ME, et al. Activation of neuronal genes via LINE-1 elements upon global DNA demethylation in human neural progenitors. Nat. Commun. 2019;10:3182. doi: 10.1038/s41467-019-11150-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.García-Muse T, Aguilera A. R loops: From physiological to pathological roles. Cell. 2019;179:604–618. doi: 10.1016/j.cell.2019.08.055. [DOI] [PubMed] [Google Scholar]
- 65.Gonzalo S, et al. DNA methyltransferases control telomere length and telomere recombination in mammalian cells. Nat. Cell Biol. 2006;8:416–424. doi: 10.1038/ncb1386. [DOI] [PubMed] [Google Scholar]
- 66.Bakker B, et al. Single-cell sequencing reveals karyotype heterogeneity in murine and human malignancies. Genome Biol. 2016;17:115. doi: 10.1186/s13059-016-0971-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sanjana NE, Shalem O, Zhang F. Improved vectors and genome-wide libraries for CRISPR screening. Nat. Methods. 2014;11:783–784. doi: 10.1038/nmeth.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ran FA, et al. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 2013;8:2281–2308. doi: 10.1038/nprot.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Conant D, et al. Inference of CRISPR edits from sanger trace data. CRISPR J. 2022 doi: 10.1089/crispr.2021.0113. [DOI] [PubMed] [Google Scholar]
- 70.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 71.Eccles, D. A. Creating differential transcript expression results with DESeq2 v2. 10.17504/protocols.io.8epv51686l1b/v2.
- 72.Müller F, et al. RnBeads 2.0: Comprehensive analysis of DNA methylation data. Genome Biol. 2019 doi: 10.1186/s13059-019-1664-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Altemose, N. et al. AlphaHOR-RES: A method for enriching centromeric DNA v1. protocols.io (2021) 10.17504/protocols.io.bv9vn966
- 74.Priestley, P. et al. Pan-cancer whole genome analyses of metastatic solid tumors. 10.1101/415133 (2018).
- 75.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Saunders CT, et al. Strelka: Accurate somatic small-variant calling from sequenced tumor-normal sample pairs. Bioinformatics. 2012;28:1811–1817. doi: 10.1093/bioinformatics/bts271. [DOI] [PubMed] [Google Scholar]
- 77.Cameron DL, et al. GRIDSS2: Comprehensive characterisation of somatic structural variation using single breakend variants and structural variant phasing. bioRxiv. 2020;22:1–25. doi: 10.1101/2020.07.09.196527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cameron DL, et al. GRIDSS, PURPLE, LINX: Unscrambling the tumor genome via integrated analysis of structural variation and copy number. BioRxiv. 2019 doi: 10.1101/781013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Blokzijl F, Janssen R, van Boxtel R, Cuppen E. Mutational patterns: Comprehensive genome-wide analysis of mutational processes. Genome Med. 2018;10:33. doi: 10.1186/s13073-018-0539-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Manders F, et al. Mutational patterns: The one stop shop for the analysis of mutational processes. bioRxiv. 2021 doi: 10.1101/2021.11.01.466730. [DOI] [Google Scholar]
- 81.Ge SX, Jung D, Yao R. ShinyGO: A graphical gene-set enrichment tool for animals and plants. Bioinformatics. 2019;36:2628–2629. doi: 10.1093/bioinformatics/btz931. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analysed during the current study are available in the EGA repository, EGAS00001006845.