Abstract
Repairing the wound is a multistep process that includes the spatial and temporal synchronization of a different range of cell types to increase the speed of wound contraction, the proliferation of epithelial cells, and collagen formation. The need for proper management of acute wounds to be cured and not turned into chronic wounds is a significant clinical challenge. The traditional practice of medicinal plants in many regions of the world has been used in wound healing since ancient times. Recent scientific research introduced evidence of the efficacy of medicinal plants, their phyto-components, and the mechanisms underlying their wound-repairing activity. This review aims to briefly highlight the wound-curing effect of different plant extracts and purely natural substances in excision, incision, and burn experimental animal models with or without infection of mice, rats (diabetic and nondiabetic), and rabbits in the last 5 years. The in vivo studies represented reliable evidence of how powerful natural products are in healing wounds properly. They have good scavenging activity against Reactive oxygen species (ROS) and anti-inflammatory and antimicrobial effects that help in the process of wound healing. It is evident that incorporating bioactive natural products into wound dressings of bio- or synthetic polymers in nanofiber, hydrogel, film, scaffold, and sponge forms showed promising results in different phases of the wound-curing process of haemostasis, inflammation, growth, re-epithelialization, and remodelling.
Keywords: Animal models, Burns, Inflammation, Natural products, Wound healing, Wound dressings
Introduction
The human body includes different organs. One of them is the skin which occupies a large body area. It represents the outermost defensive covering of the body and an immunological barrier that regularly faces different external factors. It fortifies against mechanical pressure, microbial contagion, and septicity and maintains normal body temperature. It is responsible for the sensation of touch, heat, and cold (Richmond and Harris 2014; Kwiecien et al. 2019; Kumar P and Kothari 2021).
The antimicrobial protective role of different skin layers was evidenced through different previous studies. An external layer displays the composition of human skin outside the epidermis called microbiota, epidermis, dermis, adipose tissue, glands (sweat and sebaceous), and hair follicles (Kwiecien et al. 2019).
Epidermis is composed of keratinocytes, melanocytes, Langerhans’ cells, and Merkel cells. Keratinocytes are a significant type of cells that has a role in vitamin D formation and produce keratin and lipids to form a water barrier. Keratinocytes could act against chemical and biochemical toxins by creating pro-inflammatory cytokines, e.g., interleukins: IL-1α, IL-1β, IL-3, and IL-6, interferons-alpha and beta, transforming growth factors, tumour necrosis factors, and others (Blume-Peytavi et al. 2016). Melanocytes are responsible for skin pigmentation. The first line of protectors of the skin is represented by Langerhans cells. They transport antigens in the skin to the lymph node. The membranes of Merkel cells interact with free nerve endings in the skin, so they have a sensory function. The dermis layer includes the sweat glands, blood vessels, muscles, and sensory neurons (Yousef et al. 2017). Symbiotic microorganisms of bacteria and fungi are recognized as skin colonies with harmless and vital effects in protecting the skin. They are inside hair follicles, sweat and sebaceous glands to protect the skin against invasive and microbial pathogens. Among them, species of Staphylococcus, Malassezia, Demodex folliculorum, and Demodex brevis were the most important (Grice and Segre 2011; Ibrahim et al. 2020).
Wounds have happened due to the loss of histological composition of the skin tissue due to internal or external factors or sequential loss of function in any layer of the skin, which leads to tissue disturbance (Herman and Bordoni 2020). The existence of wounds permits the entrance of different microbial agents as bacteria and viruses or any foreign elements, into the body. Inflammation of skin wounds is happened because of local microbial infections. Also, a generalized systemic infection (septicemia) could be found, a life-threatening condition (Percival 2002). Consequently, more research should be done to find out simple and effective ways of taking care of skin wounds to heal properly. The main goals are to stop bleeding, get rid of microbial infection of wounds, and help wounds to heal effectively without any complications or deformities (Sarabahi et al. 2012; Jones 2015).
Once any damage has occurred to the skin tissue, multiple cellular and extracellular pathways act in a harmonized way, and their functions must be performed in the appropriate order at a suitable time to achieve repair, growth, and tissue regeneration (Richmond and Harris 2014).
Bleeding due to damaged blood vessels must be stopped, which is considered the initial reaction in the process of wound repair, besides platelet stimulation to compose a fibrin clot. Immediately after that, the disturbed tissues discharge growth factors and pro-inflammatory cytokines. Upon controlling the bleeding, many inflammatory cells such as monocytes, macrophages, and neutrophils are gathered at the wound site to provoke the inflammatory response (inflammatory phase). Moreover, the different self and exogenous antigens trigger the immune system to fight against them (Rodrigues et al. 2019; Alotaibi et al. 2021).
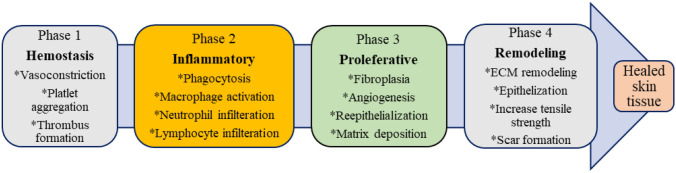
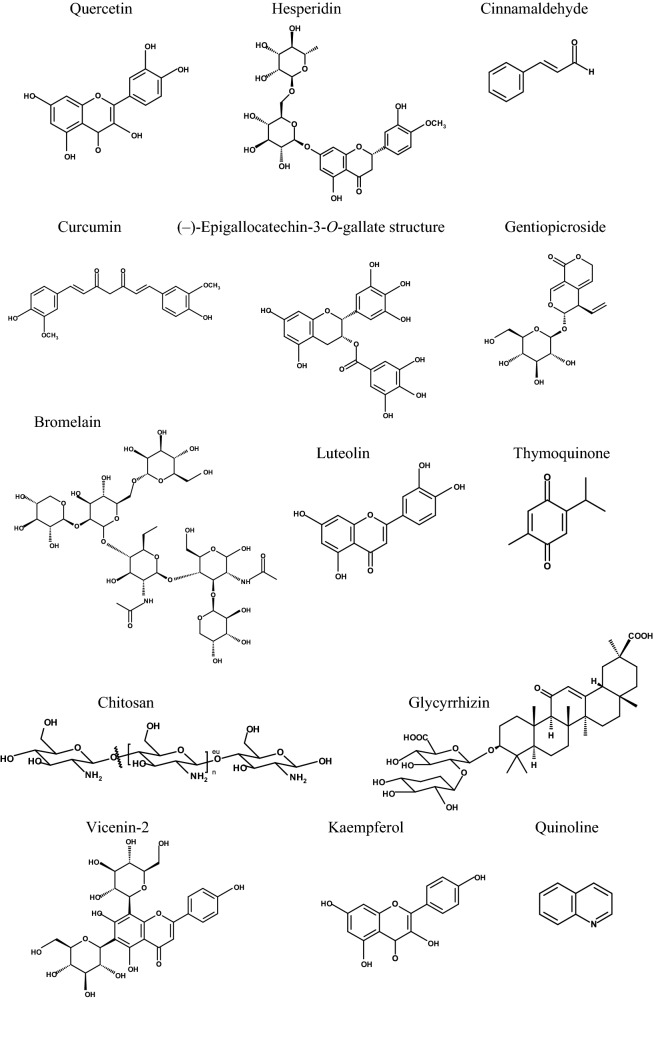
Angiogenesis is the following phase, which is parallel to the inflammation phase. The formation of a new blood vessel characterizes this phase. It is then followed by the growth and proliferative phases, which are predominate by fibroblast relocation and propagation, production of the matrix proteins, keratinocyte proliferation, differentiation, and restoration of hair follicles, etc. lastly, the wound healing process is finished with the remodelling of the extracellular matrix (ECM), besides the reordering of granulation tissue to scar tissue. Collagen synthesis and cross-linking afford stability to the healing tissue (Rodrigues et al. 2019). Figure 1 demonstrates the different phases of wound healing, while Table 1 summarizes herbal extracts studied using in vivo wound healing models. Structures of purely natural substances that were investigated using wound healing in vivo models showed in Fig. 2 and Table 2.
Fig. 1.
Different phases of skin wound healing
Table 1.
Botanical extracts investigated by wound healing in vivo models (animal models)
| No. | Plant name and part used | Family | Wound model and treatment | Animal | Outcome | References |
|---|---|---|---|---|---|---|
| 1 | Aloe megalacantha Baker (Leaf latex) | Xanthorrhoeaceae | Leaf latex was loaded to an ointment base (5% and 10% w/w) | Using for incision Swiss albino mice and excision Sprague Dawley rats' models | Both wound models showed a significant increase in the speed of wound contraction, epithelial cell proliferation, and increased tensile strength | Gebremeskel et al. 2018 |
| 2 | Phyllanthus muellerianus (water extract of aerial part and pure compound geraniin) | Euphorbiaceae | Aqueous creams of the plant (0.25, 0.5, and 1% w/w) and geraniin (0.1, 0.2, and 0.4% w/w) | Male Sprague–Dawley rats were used with induced excision and incision wounds | A Remarkable elevation in fibroblasts, cross-linking, and collagen content in P. muellerianus and geraniin-treated wound tissues were shown. Also, notable levels of TGF-β1 were recorded | Boakye et al. 2018 |
| 3 | The biofunctionalized silver nanoparticle was produced from cloves extract | Myrtaceae | AgNP was loaded into a cream with concentrations of 3% and 5% | Excision and incision male and female albino rats wound models | The wound-repairing impact was notable in animals treated with 5% silver nanoparticles. Collagen | Parveen et al. 2018 |
| 4 | Euphorbia characias subsp. Wulfenii | Euphorbiaceae | 1% ointment of methanol n-hexane, and ethyl acetate extracts E. characias subsp. | It was investigated in the linear incision and circular excision wounds in male Sprague Dawley rats | E. characias subsp. wulfenii displayed significant wound-curing activity | Özbilgin et al. 2018 |
| 5 | Lafoensia pacari A. St.-Hil | Lythraceae | The hydroethanolic leaves extract was tested at 10, 30, or 100 mg/g of gel | Excision and incision-rat (Rattus norvegicus, Wistar strain model) | Increased rates of wound contraction, moderate re-epithelialization, neovascularization, proliferation, and acceleration of the remodeling phase | Pereira et al. 2018 |
| 6 | Alkanna strigose | Boraginaceae | (Hexane extract of roots) Rattus norvgecis model | Excision and incision Albino Wistar rats | The beneficial effect of A. strigosa extract was confirmed | Aburjai et al. 2019 |
| 7 | Vitis labrusca (Hydroalcoholic extract of leaves) | Vitaceae | Oral administration of the extract at 100, 200, and 300 mg/kg | Excision wounds male Wistar rats | Histological evidence showed that the extract could be a potential oral medicine for healing purposes | Santos et al. 2021 |
| 8 | Coccinia grandis (Polyphenol- rich fraction was obtained from the methanol extract of leaves) | Cucurbitaceae | The hydrogel of Coccinia grandis (1.5 mg/ g) was tested | Excision wounds in male albino rats which was infected by B. cereus | Polyphenolic compounds of Coccinia grandis could be utilized as a natural wound-repairing drug | Al-Madhagy et al. 2019 |
| 9 | Jacaranda decurrens (Hydroalcoholic extract of leaves.) | Bignoniaceae | 15 mg/g of extract in the ointment base was tested | Mice-excision wound model | The extract increases rate of wound curing by modulating the action of pro-inflammatory cytokines | Serra et al. 2020 |
| 10 | Phlomis russeliana (n-hexane fraction of methanol extract of the aerial part.) | Lamiaceae | 5% of the extract in carbopol- hydroxypropyl gel | excisional wound model in Swiss mice (Mus musculus) | Phlomis russeliana has a wound-healing effect following the ethnobotanical application | Okur et al. 2020 |
| 11 | Plumeria obtusa (Ethanolic extract of leaves) | Apocynaceae | 2.5, 5, and 10% spray of the plant extract | Excision wound Swiss albino Wistar rats model | The formula with 10% P. rutic extract spray showed the best wound healing effect | Bihani and Mhaske 2020 |
| 12 | Boerhavia diffusa (Methanol and chloroform extracts of the leaves) | Nyctaginaceae | Ointment (10% w/v) of methanol or chloroform extracts | Excision wound assays in a Albino Wistar rat model | The methanol extract of B. diffusa have a significant wound-healing effect | Juneja et al. 2020 |
| 13 | Ephedra ciliata (methanol extract and quercetin) | Ephedraceae | 5, 10, 20% cream of Ephedra ciliata methanol extract and 20% quercetin | Albino male and female rat model with excision and burn wounds was used | The extract rich with quercetin (methanol extract) of Ephedra ciliata promoted natural wound healing. The healing effects of the 20% methanol extract were comparable to the 20% quercetin | (Yaseen et al. 2020) |
| 14 | Moringa oliefera (Hexane extract of seeds | Moringaceae | 5% and 10% hydrogel of n-hexane extracts of Moringa oleifera seeds | Excision and incision Male Swiss albino mice wound healing model | The hydrogel containing n-hexane extract of Moringa oleifera seeds could act as a wound-healing agent | Ali et al. 2021 |
| 15 | Moringa oleifera leaves | Moringaceae | Moringa leaves extract gel | Incision wound male Wister rat (Rattus norvegicus) model | Moringa oleifera leaves extract gel exerted wound healing effect by speeding epithelialization | Ayu et al. 2020 |
| 16 | Curatella americana Linn. (Hydroethanolic extract of leaves) (HECA) | Dilleniaceae | lyophilized extract of C. americana 0.5 and 1% loaded to a gel | Excision Adult Swiss albino mice wound model | treatment with 1% of the extract displayed the highest wound-repairing effect | Fujishima et al. 2020 |
| 17 | Nigella sativa oil | Ranunculaceae | Mats of polyurethane electrospun nanofibrous loaded with Nigella sativa oil were tested as wound healing dressing | The full-thickness excisional wound in female Sprague Dawley rats | The mat of N. sativa-loaded Polyurethane nanofibrous, significantly provoked the wound-healing process | Aras et al. 2021 Nordin et al. 2019 |
| 18 | Dodonaea viscosa (Leaves methanol and chloroform extracts) | Sapindaceae | 10% w/w herbal. Ointment of the extracts | Incision and excision Sprague Dawley rats wound models | Methanolic extract significantly accelerated the epithelization of the excision wound. The extracts exerted a notable elevation in the tensile strength regarding the incision model | Nayeem et al. 2021 |
| 19 | Roylea elegans (Aqueous leaves extract) | Lamiaceae | The cream contained 5 or 10% of the aqueous extract of leaves | Burn Wistar albino rats model | Roylea elegans caused wound-healing acceleration | Upadhyay et al. 2021 |
| 20 | Cupressus macrocarpa (Diethyl ether extract of leaves) (DEEL) | Cupressaceae | DEEL in 20% DMSO in normal saline was applied to wounded and infected rats by methicillin-resistant Staphylococcus aureus clinical isolates | Full-thickness excision wounds male albino rats | DEEL showed epidermis regeneration, granulation tissue maturation, and a decrease in inflammatory cell infiltration | Attallah NGM et al. 2021 |
| 21 | Zehneria scabra (80% Methanol Leaf Extract) | Cucurbitaceae | 5% and 10% (w/w) of 80% methanol extract in an ointment base | Incision and excision wounds in adult albino mice | Z. scabra exerted significant wound-repairing activity | Tekleyes et al. 2021 |
| 22 | Bersama abyssinica (Hydro-methanol, chloroform, hexane, and water fractions of leaves) | Francoaceae | 5% and 10% w/w ointment of the hydro-methanolic extract was investigated | Excision, incision, and burn wounds in adult Swiss albino mice | Both 5% and 10% w/w of hydro-methanolic extract and solvent fractions of the plant have wound-curing effects | Taddese et al. 2021 |
| 23 | Semecarpus anacardium L., Argemone mexicana L., Cocculus hirsutus L., and Woodfordia fruticose K | Anacardiaceae Papaveraceaei Menispermaceae Lythraceae | The polyherbalBhallatakadi Ghrita (BG) formulation is composed of this mixture | Incision and excision Wistar rats model | Quercetin, gallic acid, and fatty acids increased the healing rate by the ghrita formulation | Wayal and Gurav 2021 |
| 24 | Elaeis guineensis Jacq (Leaves) | Arecaceae | Leave extracts | Sprague Dawley rats were used for making excision wounds with microbial infection | E. guineensis promote the healing of wounds even though they were infected, confirming its traditional use in wound curing | Rajoo et al. 2021 |
| 25 | Vernonia auriculifera Hiern (methanol extract of leaves and its fractions | Asteraceae | Ointment preparations of 5% and 10% w/w of methanol and other fractions | Excision, incision, and burn wound models in Swiss albino mice and female Wistar rats | The plant’s different extracts (methanol, aqueous, and ethyl acetate) showed | Lambebo et al. 2021 |
| 26 | Brucea antidysentrica Rhamnus prinoides Dodonaea angustifolia | Simaroubaceae | Brucea antidysentrica (extract of roots bark), Rhamnus prinoides (leaves), and Dodonaea angustifolia (80% methanol extract) | Types of induced wounds in Swiss albino mice were excision and incision wounds | The traditional use of these plants in repairing wounds was confirmed. This plants increase wound contraction rate and tensile strength and decrease the time needed for efficient epithelialization | Tessema and Molla 2021 |
| 27 | Jatropha Neopauciflora Pax Latex | Euphorbiaceae | latex (50%, 75%, and 100%) | Incisions were made in normal and diabetic male mice (Mus musculus) mice | neopauciflora could be beneficial for wound management in diabetes mellitus and speeds up and stimulates the wound-healing process | Hernandez-Hernandez et al. 2021 |
| 28 | Sanguisorba officinalis Roots (the isolated Rhoifolin-Rich Fraction RRF) | Rosoideae | 2% carbopol and hydroxypropyl cellulose gel of RRF | Full-thickness excision wound white albino rat model | RRF enhanced re-epithelization, angiogenesis, and shoed anti-bacterial, immunomodulatory, and anti-inflammatory activities | Negm et al. 2022 |
| 29 | Platycodon grandifloras (Water extract of the dried tuberous roots) | Campanulaceae | The concentrated water extract was mixed with medical vaseline to make an ointment. 10% P. grandiflorus mixed emulsifiable paste was tested | Scald model males specific-pathogen-free (SPF) Sprague–Dawley rat | P. grandiflorus showed a significant healing effect on cutaneous scald lesions. A well-repaired epidermis was observed in rats treated with P. grandifloras | Wang et al. 2022 |
| 30 | Pistacia vera (Italian and Algerian oleoresins) | Anacardiaceae | Oleoresins mixed with vaseline (5% w/w) | Circular wound excision ew Zealand albino rabbits model | Both oleoresins had very high wound-healing activity agents | Boudjelal et al. 2022 |
| 31 | Moringa oleifera (Hydroethanolic extract of seeds) | Moringaceae | 5% and 10% of the extract of Moringa oleifera seeds is added to the hydrogel | Excision and incision wound Male Swiss albino mice models | Hydr-ethanolic extract of M. oleifera could be utilized in wound management as an alternative plan | Ali et al. 2022 |
| 32 | Calendula officinalis L. (Flower extract) | Asteraceae | The wound dressing of collagen film containing flower extract | Excision wound male Sprague–Dawley rat model | The tested dressing for wound repair contained the calendula extract. It was loaded with collagen film and showed safe, stable, and effective effects | Rathod et al. 2022 |
| 33 | Curcuma longa (Aqueous, 70% methanolic, and ethanolic extracts) | Zingiberaceae | Different extracts of C. longa encapsulated in Ethosome were tested to heal wounds.(0.25, 0.5, and 1 g/cm2) | Full-thickness skin wounds in adult Wistar rats | Encapsulation of C. longa led to a better shape of wound, and maturation of granulation tissue, with an accelerated rate of healing, compared to crude extract | Kumar S et al. 2022 |
| 34 | Globularia arabica (Leaf methanol extract) | Plantaginaceae | The study used variable concentrations of G. arabica extract (1%, 5%, and 10%) in ointment base | Excision diabetic and nondiabetic male Wistar rat model | G. arabica could be useful in healing wounds by provoking collagen and hydroxyproline formation when added externally on the wounded skin | Alsarayreh et al. 2022 |
| 35 | Premna integrifolia (Standardized extract) | Lamiaceae | 5% (w/w) ointment of the standardized extract | Excision wound model in male and female Wistar albino rats | Premna integrifolia had a wound-healing impact and could contribute to curing the wounds as a source of bioactive constituents with wound-healing characteristics | Alsareii et al. 2022 |
| 36 | Zizyphus mauritiana | Rhamnaceae | (Fruit extract) | Full-thickness excisional wounds in adult male New Zealand Dutch strain albino rabbits | ZFE might act as a potential alternative drug to speed wound repair due to its antioxidant and anti-inflammatory effects | Shady et al. 2022 |
| 37 | Parkia clappertoniana Keay (Fruit husk extract) | Fabaceae | Ointment of fruit extract (0.3, 1, and 3%) | Excision wound model in male Sprague–Dawley rats | P.clappertoniana exerted wound-healing and antimicrobial effects | Kuma et al. 2022 |
Fig. 2.
Structures of natural pure substances were investigated using wound healing in vivo models (animal models)
Table 2.
Natural pure substances investigated using wound healing in vivo models (animal models)
| No. | Natural products derived substances | Wound model and treatment | Animal | Outcome | References |
|---|---|---|---|---|---|
| 1 | Quercetin | This combination is prepared by taking 15% carbopol and varying the gelatin ratio | Excision albino rat wound model.Multiple phases hydrogel system combined with quercetin loaded to liposomes | The rate of wound repair is raised, with a prominent decrease in wound closure time compared to the drug's dosage form | Jangde et al. 2018 |
| 2 | Quercetin | Two ischemia–reperfusion (I/R) cycles were utilized in each animal to induce ulcer formation. Topical treatment was performed with 1 μmol/L quercetin in DMSO | The animal pressure ulcer mice model was established with two cycles | The treatment by quercetin caused a significant acceleration of wound closure, a reduction in immune cell infiltration, and pro-inflammatory cytokines formation | Yin et al. 2018 |
| 3 | Quercetin | Different treatments of quercetin (0.3%) and quercetin-loaded chitosan nanoparticles (0.03%, 0.1%, 0.3%) in pluronic F-127 gel (20% w/v) | Excision male Wistar rat wound model | Quercetin nanoparticles at 0.03% showed significant hastening wound healing by affecting cytokines and growth factors in inflammatory and proliferative phases | Choudhary et al. 2020 |
| 4 | Quercetin | Quercetin of 0.03, 0.1, and 0.3% in DMSO was tested | Excision wound adult male Wistar rat model | Modulation of growth factors, antioxidant parameters, and different cells of the wound healing process was confirmed by 0.3% quercetin | Kant et al. 2020a, b |
| 5 | Quercetin | quercetin at 10, 20, and 40 mg/mL concentrations in 10% DMSO | Diabetic Sprague Dawley rats, excision wounds, | Conversion from M1 to M2 phenotype by modulation of macrophage polarization led to inhibition of inflammation process by quercetin | Fu et al. 2020 |
| 6 | Quercetin | Quercetin as well as the photo-stimulatory impact of low energy 632.8 nm laser irradiation, were tested. Quercetin was taken by oral gavage at 25 mg/kg b. w. in 5 ml of 1% carboxymethylcellulose (CMC) with or without low-level laser treatment | The wound type was an excisional wound used in nondiabetic and diabetic male albino rats | The quercetin combined with low-level laser treatment improves the wound-curing process more than the utilization of only one of them | Ahmed et al. 2018 |
| 7 | Quercetin | Three different solvents were used to contain 0.3% of quercetin: corn oil, 10% DMSO, and ointment base | Excisional wounded adult male Wistar rats | The most efficient wound healing impact with an accelerated healing rate | Kant, Kumar, et al. 2020 |
| 8 | Quercetin incorporated in a new scaffold:Polyethylene glycol (PEG)ylated graphene oxide/quercetin (GO-PEG/Que) and artificial acellular dermal matrix/quercetin (ADM-GO-PEG/Que) | 1-Polyethylene glycol (PEG)ylated graphene oxide (GO-PEG) /quercetin. 2-Artificial acellular dermal matrix -GO-PEG/quercetin (0.1 mg/mL) | Excision wound model in diabetic male albino mice | Helps in collagen deposition and angiogenesis. ADM-GO-PEG/Que represents a new material for tissue engineering scaffold | Chu et al. 2018 |
| 9 | Quercetin | Secondary intention wound healing model in Wistar rats. 0.2 ml of gel contained 5% quercetin, 5% benzocaine, and glycerin | Palatal wounds of 5 mm diameter in Wistar rats | A reduction in inflammatory cells and an elevation in fibroblast cells were observed | Taskan et al. 2019 |
| 10 | Quercetin (QCN)- and oxygen-carrying 1-bromoperfluorooctane (PFOB)-loaded nano emulsions (QCN-NE and OXY-PFOBNE) | The hydrogel is containing LMWP-GFs/QCN-NE/OXY-PFOB-NE. Low-molecular-weight protamine (LMWP) /skin-permeable growth factors (GFs) | The type of wound was excisional in diabetic C57BL/6 mice (females) | The hydrogel elevated the wound repairing rate in the diabetic mice and downregulated wound size relative to the vehicle and LMWP-GFs. Nano-emulsion was produced to ameliorate the external delivery of quercetin and oxygen | Jee et al. 2019 |
| 11 | Quercetin and ciprofloxacin | PCL-bases nanofiber loaded with ciprofloxacin hydrochloride and quercetin | A full-thickness excisional wound in male Wistar rats | The topical delivery of ciprofloxacin hydrochloride and quercetin functionalized nanofiber. Both drugs could act as a bioactive wound dressing substance | Ajmal et al. 2019a, b |
| 12 | Quercitrin and myricitrin were isolated from Pistacia lentiscus leaves | Pistacia lentiscus leaves methanol extract 5, 20 mg/mL. Quercitrin and myricitrin 1 mg/mL | Excisional wounds in male Wistar rats | PDL, quercitrin, and myricitrin efficiently impact the healing of skin wounds | Elloumi et al. 2022 |
| 13 | Hesperidin | Alginate/chitosan containing different concentrations of hesperidin | Full-thickness excision in male Wistar rats | Hesperidin loaded to alginate/chitosan hydrogels can be utilized to treat skin wounds in humans | Bagher et al. 2020 |
| 14 | Quercetin in nanofiber scaffold | Four treatments were tested:1-gauze, 2-Poly ε-caprolactone-gelatin, 3-Poly ε-caprolactone-gelatin-ciprofloxacin hydrochloride, and 4-Poly ε-caprolactone-gelatin-ciprofloxacin hydrochloride-quercetin nanofibers | Excision wounds in male Wistar rats | A new scaffold showed full repair of wounds, and it could be used as a dressing material for healing wounds | Ajmal et al. 2019a, b |
| 15 | Quercetin | 20% quercetin | Excision wound albino rat model | Animal treated with quercetin and heparin sodium exhibited significant healing effects in comparison to the control group | Yaseen et al. 2020 |
| 16 | Quercetin | Quercetin was loaded to polycaprolactone/gelatin electrospun nanofiber | Excision wound male Wistar rat model | Quercetin nanofibers treated wounds exhibited a significant wound contraction with upregulation of angiogenesis and collagen formation. These nanofibers provided good integrity and hydrophilicity for wound dressing applications | Karuppannan et al. 2022 |
| 17 | A homogeneous polysaccharide (ZWP) from Curcuma zedoaria | Chitosan/silk hydrogel sponge loaded with platelet-rich plasma exosomes (PRP-Exos), ZWP, or PRP-Exos/ZWP | Excision wounds in diabetic emale Sprague Dawley rats | Wound contraction was recognized in the separate or combined treatments, as represented by a reduction in ulcer and an elevation in the thickness of epidermis. PRP-Exos/ZWP combined treatment gave better results in wound closure | Xu et al. 2018 |
| 18 | Curcumin conjugated with hyaluronic acid HA | Wounds of mice treated with 20 ml of 210 mg/ml of hyaluronic (HA) or 20 ml of the 25 mM of curcumin or hyaluronic-curcumin (HA–cur) | Diabetic Swiss male albino mice Excision wounds | Curcumin topical effect enhanced wound healing compared to treatment with HA-free curcumin and HA alone | Sharma et al. 2018 |
| 19 | Cinnamaldehyde | Male diabetic (BSK.Cg-m + / + Leprdb; db/db) and WT mice (C57BL/6 J), and male Kunming mice. Doses of intraperitoneal injection of cinnamaldehyde (25, 50, and 100 mg/kg) | Mice were injured with excisional skin wounds. Normal and diabetic mice were used in the study | Cinnamaldehyde-induced angiogenesis and led to an increased rate of wound repair | Yuan et al. 2018 |
| 20 | Bromelain pineapple (Ananas comosus) | Bromelain was given intraperitoneally in doses of 25 mg/kg or 45 mg/kg | Full thickness incision and diabetic Male Wistar rats wound model | Bromelain significantly enhanced wound contraction and strength, reduced granulation tissue formation, and increased angiogenesis | Fathi et al. 2020 |
| 21 | Bromelain pineapple (Ananas comosus) | Chitosan nanofibers loaded with bromelain were investigated in burn wound repair | Induced burn wounds in rats | The safety significantly improved, good impact on re-epithelialization, reduction of necrosis, and good wound closure were observed | Kalalinia et al. 2021 |
| 22 | Luteolin | Intraperitoneal administration of luteolin 100 mg/kg body weight | Excision wounds diabetic male Wistar rat model | Wounded and diabetic rats experienced wound restoration via improving inflammatory and oxidative stress through the administration of luteolin | Chen et al. 2021 |
| 23 | Luteolin | An ointment of luteolin of different concentrations (0.5% and 1% w/w) was applied topically on wounds | Excision and incision diabetic and nondiabetic male Wistar rate models | Luteolin ointments ameliorated wounds and enhanced skin tissue's healing process in both nondiabetic and diabetic wounds | Özay et al. 2018 |
| 24 | Luteolin | Medical vaseline ointment of 10% luteolin | Skin wound of scald model males specific-pathogen-free Sprague Dawley rats | Inflammation of scalded rats was efficiently reduced with the promotion of proper wounds in luteolin treated group | Wang et al. 2022 |
| 25 | Thymoquinone | 0.5% w/w of thymoquinone nano-emulgel incorporated with Carbopol 940 (TMQ-NEG) | Excision wounds Wistar rats | The examined nano emulgel showed a faster and better wound-healing effect compared to the ordinary hydrogel form of thymoquinone | Algahtani et al. 2021 |
| 26 | Thymoquinone Thymoquinone loaded chitosan-lecithin micelles | An investigation was done using 20 mg/mL of thymoquinone loaded to micelles formulation and with the 2% w/ w thymoquinone loaded to polymeric micelle-hydrogel | Excision wound model of old Balb/c mice | The hydrogel showed a remarkable wound-curing impact on the original thymoquinone and silver sulphadiazine | Negi et al. 2020 |
| 27 | Thymoquinone | The polyvinyl pyrrolidone (PVP) matrix-type films containing 20% w/w of TQ were tested (hydrogel formulation) | Full-thickness excisional wound infection model in male mice (BALB/c) | TQ-containing films exhibited significant activity against Staphylococcus aureus infection | Haq et al. 2020 |
| 28 | Gentiopicroside and Thymoquinone | Mats of co-blended polyvinyl pyrrolidine (PVP) and methyl ether Polyethylene glycol (m-PEG) were loaded with gentiopicroside and thymoquinone | White albino male rats were used | The polymeric mats are loaded with gentiopicroside and thymoquinone, so it could be considered suitable wound dressing | Almukainzi M. et al. 2022 |
| 29 | Vicenin-2 (VCN-2) | VCN-2 in the form of hydrocolloid film | Wounds were inflicted in diabetic male adult Sprague Dawley rats | VCN-2 may have a wound-healing impact as wound treatment with VCN-2 hydrocolloid films could efficiently enhance wound repair in hyperglycemic cases | Tan et al. 2019 |
| 30 | Kaempferol (KM) | The KM ointments 1% w/w were used | Diabetic excisional and nondiabetic incisional male Wistar rats' models | Kaempferol was an efficient wound-healing drug in treating both nondiabetic and diabetic wounds | Özay et al. 2019 |
| 31 | Glycyrrhizin micelle as a genistein nanocarrier | Dipotassium glycyrrhizinate-based micelle ophthalmic solution encapsulating genistein (DG-Gen) 1:15 | Diabetic corneal and nerve-wounded C57BL/6 J male mice | Application of the DG-Gen significantly prompted corneal re-epithelialization and nerve regeneration in wounded diabetic mice | Hou et al. 2021 |
| 32 | Quinoline | A hydrogel loaded with Cu (II) Schiff base 8-hydroxy quinoline complex (CuSQ) solid lipid nanoparticles (SLN) | excision wound healing model in male Wistar albino rats | CuSQ would have a good impact as a drug for cutaneous wound curing through the control of growth factors and different cytokines | El-ezz et al. 2022 |
| 33 | Micro-channeled alkylated chitosan sponge (MACS) | Liver perforation in male Wistar rats and Bama miniature male pigs was performed in this study | Pigs | The Micro-channeled alkylated chitosan sponge introduces higher pro-coagulant and hemostatic effects in lethal conditions of either normal or heparinized animal models. Generally, the MACS displayed promising clinical translational ability in managing fatal noncompressible hemorrhage and improving wound healing | Du et al. 2021 |
| 34 | Green tea catechin (–)-Epigallocatechin-3-O-gallate (EGCG) | EGCG-grafted water-soluble silk fibroin hydrogels (SFEGCG). SFEGCG conjugate was co-crosslinked with tyramine-substituted SF (SF-T) via horseradish peroxidase (HRP)/H2O2 mediated enzymatic reaction to form SF-T/SF-EGCG hydrogels | Male Sprague Dawley rat model of full-thickness skin defect | SF-T70/SF-EGCG30 hydrogels exerted a remarkable wound-healing effect over SF-T hydrogels and a commercial DuoDERM® gel dressing | Lee et al. 2022 |
Botanical extracts have been extensively utilized in managing wounds in traditional medicine. Therefore, in vitro and in vivo studies have assessed different extracts for their wound-curing characteristics. Their phytochemical content is the purpose of their remedial features in wound repair. Other phytochemicals and plant-derived substances were investigated for their wound-healing activity as flavonols, flavanones, isoflavones, flavanols, flavonolignans, proanthocyanidins (Carvalho et al. 2021), β-glucans (Majtan and Jesenak 2018), bromelain (Fathi et al. 2020), curcumin (Akbik et al. 2014). It was disclosed that different botanicals and medicinal plants are widely used as a topical treatment for wound repairing, such as aloe vera, banana leaves (Sivamani et al. 2012), turmeric, Centella asiatica, Rosmarinus officinalis, Calendula officinalis (Artem Ataide et al. 2018).
Natural products such as plant extracts and other plant-derived products and their phytochemicals assist in managing inflammatory diseases, exert antimicrobial effects, and might aid skin tissue regeneration (Alherz et al. 2022; Attallah NG et al. 2022). They could remove oxidative stress and lower inflammation (Shah and Amini-Nik 2017). The wound-repairing ability of different plant extracts and their actives was confirmed in wound-curing animal models. Such plants improved collagen deposition, the proliferation of epithelial cells, and angiogenesis in diabetic and nondiabetic animal models (Binsuwaidan et al. 2022). Different types of plants are widely used in managing wounds and injuries from previous scientific research (Chingwaru et al. 2019).
The current review demonstrates and focuses on the latest findings in the last 5 years (2018–2022) regarding the in vivo studies of wound repairing effect of different plant extracts, the derived substances from plants, and pure natural substances as a new frontier in treating wounds.
Methods of collecting data
Data collected in the frame of this work were generated by common research engines such as ScienceDirect, Web of Science, PubMed, SciFinder-n, and Scopus, using the references “natural products”, “wound healing” and refining with keywords “animal models”, “burns”, “biological”, “plants” “wound dressings” and “inflammation”. A total of 2194 research items were examined out of which 190 fall into the scope of the review, thus, constituting the baseline of the current survey.
Botanicals and pure natural substances in the preclinical studies
The present review provided the research work, which included the preclinical studies (in vivo) of plant extracts and pure natural substances on wound healing in the last 5 years. The preclinical investigation by using animal models is important for acute and chronic wounds, in vitro studies could be used, but they do not assess the complexity of the wound healing process (Dunn et al. 2013; Zindle et al. 2021). Acute wounds occur through known sequential steps (Zindle et al. 2021). but chronic wounds exhibited impaired or delayed healing. The acute wound heals within 2–3 weeks, followed by the remodelling phase in normal healthy people. The normal healing sequence could be interrupted by other diseases such as diabetes, wound infection, foreign bodies, chronic inflammation, and ischemia. Microbial infection is the famous reason for wound-related morbidity (Said et al. 2009; Rajendran et al. 2018). This led to a physiological imbalance in the mechanism of healing. It might get stuck in one of the phases, and the wound then falls into the non-healing chronic type (FrykbergRobert 2015; Rajendran et al. 2018). It was reported that a wound is not healed in more than 6–8 weeks defined as a chronic/ non-healing wound (Rajendran et al. 2018). The universal goal of all studies about wound healing is to treat acute wounds perfectly in due time, so we avoid conversion into chronic ones and discover the appropriate therapy if the patient suffers from chronic wounds. Patients with chronic wounds suffer from pain, depression due to isolation from the community, and risk of amputation (Ivanková and Belovičová 2020).
Wound healing potentials of various plant extracts
Different studies of the wound-repairing effect of various plant extracts revealed the diversity of actives responsible for this activity. It was suggested that D-pinitol and caffeic acid, the major constituents of Boerhavia diffusa leaf methanol extract, contributed to the wound-healing effect (Juneja et al. 2020). In another study, the fraction contained a high level of polyphenolic compounds, separated from leaves methanol extract of Coccinia grandis showed a remarkable wound repair effect. This effect was due to (rutin), quercetin-3-O-neohesperidin, nicotiflorin, kaempferol-3-O-glucorhamnoside, and astragalin as well as seco-iridoids of oleuropein and ligstroside (Al-Madhagy et al. 2019). HPLC metabolic profiling of the methanol extract of Ephedra ciliata recognized quercetin as a major compound. The antioxidant and antimicrobial activities of quercetin were related to the wound-closure effect of the extract (Yaseen et al. 2020). Biological guided study of E. characias subsp. wulfenii extracts (methanol, n-hexane, and ethyl acetate) of the aerial parts were tested. It was explored that the methanol extract displayed significant wound-repairing activity in circular excision and linear incision wound models, as well as anti-inflammatory effects. This study explored whether quercetin derivatives (quercitrin, hyperoside, and guaijaverin) were responsible for the wound-repairing effect (Özbilgin et al. 2018). Regarding Jacaranda decurrens Cham., metabolic profiling was done to find out ten compounds in the extract of flavonoidal and triterpenoidal nature. It was concluded that these compounds improved the healing of wounds in this study (Serra et al. 2020). Hydroethanolic extract of leaves of Lafoensia pacari A. St.-Hil. was evaluated in accelerating the contraction of wounds. The plant contained punicalagin, ellagic acid, punicalin, kaempferol, quercetin-3-O-xylopyranoside, and quercitrin, which could be related to re-epithelialization, improved cell proliferation, and enhanced remodeling phase of the wounds (Pereira et al. 2018). The mats composed of polyurethane loaded with Nigella sativa oil were studied to assess the in vivo wound-repairing effect (Aras et al. 2021). The essential oil of Nigella sativa seeds contains thymoquinone, which was reported to have wound-healing activity (Haq et al. 2020). Different studies were performed to obtain an effective wound healing process e. g. loaded thymoquinone chitosan- lecithin micelles which keep thymoquinone at the site of wounds with controlled release of the drug (Negi et al. 2020). Hydro-ethanol extract from Vitis labrusca leaves was found to advance the healing of wounds due to the total phenolic and flavonoid content (Santos et al. 2021). Aqueous ethanol extract of Leaves of Curatella americana Linn. exerted remarkable wound healing properties due to its active constituents. Leaves contain compounds known as wound-healing agents, mainly quercetin, kaempferol, glucosides, catechin, and epicatechin (Fujishima et al. 2020). A homogenous polysaccharide was separated from the rhizomes of Curcuma zedoaria and tested in the process of healing wounds in diabetic rats. It was added with platelet-rich plasma exosomes and loaded to a hydrogel sponge of chitosan and silk. It was found that the previous combination was effective and safe to speed the curing of wounds in the case of diabetes (Xu et al. 2018). Methanol extract of Dodonaea viscosa leaves caused accelerated epithelization of excision wounds and increased tensile strength of incision wounds of rats. HPTLC chromatogram showed 10 constituents of flavonoids, tannins, and saponins, including rutin and kaempferol, with reported healing effects (Nayeem et al. 2021).
bio- and synthetic polymers of bioactive substances from natural products
Wound dressings can be created from a combination of bio- and synthetic polymers. Loading them with bioactive substances from natural products increased the good features of this combination. The combined bio- and synthetic polymers may have little or no anti-bacterial, anti-inflammatory, and antioxidant effects (Alven et al. 2020). Loading the bioactive natural product to either the combined polymers or to only one of them eliminates this problem. Bioactive materials such as curcumin (Lüer et al. 2012; Tejada et al. 2016), quercetin (Choudhary et al. 2020; Karuppannan et al. 2022), rutin (Zhou et al. 2021), bromelain (Kalalinia et al. 2021), thymoquinone, gentiopicroside (Almukainzi M. et al. 2022; Almukainzi May et al. 2022), hesperidin (Carvalho et al. 2021), and others were reported to enhance wound healing by adding them to bio- or synthetic polymers or both.
Different types of wound dressings have existed as traditional or passive, e.g., plasters and wool dressing which are not favorable nowadays because of the pain and possible re-skin damage. The interactive wound dressing of synthetic or bio-polymers could be represented as hydrogel, foams, sprays, films, and nanofibers, which introduced a moist environment for wound healing and facilitated water vapor transmission but with a limited anti-bacterial effect. Bioactive wound dressings could be represented by the previously mentioned types of interactive wound dressings, which may be composed of synthetic polymers of polyethylene glycol, polyvinyl pyrrolidone polyurethanes, poly-hydroxyethyl methacrylate, polyglycolic acid, polylactide, poly-ε-caprolactone, as well as biopolymers of pectin, chitosan, cellulose, dextran, and alginate, collagen, which are loaded with antibiotics or growth factors or vitamins, and/or bioactive natural products (Zahedi et al. 2010; Aderibigbe and Buyana 2018; Alven et al. 2020).
The merits of combining synthetic and bio-polymer with bioactive natural products in wound dressings for better wound healing were confirmed in many studies e.g., curcumin (Sharma et al. 2018), quercetin, and rutin (Zhou et al. 2021). Curcumin is the active substance of the roots of turmeric or Curcuma longa. It exerts strong antioxidant and anti-inflammatory, anti-bacterial effects but with low water solubility and oral bioavailability. Curcumin was loaded into bio- and synthetic polymers to overcome this problem (Alven et al. 2020). The combination between bio- and synthetic polymers could overcome the problem of poor mechanical support of bio-polymers (Aycan et al. 2019), besides overcoming the problem of lacking biocompatibility, biodegradability, and bad patient compliance of synthetic polymers (Mir et al. 2018). Effective wound dressing for skin burns represents a challenge to the healthcare system due to the probability of skin structure damage leading to an increased risk of infection. Quercetin and rutin are flavonoids with strong antioxidant, antimicrobial and anti-inflammatory effects but have limited water solubility. It was revealed that incorporating quercetin and rutin into polycaprolactone and chitosan oligosaccharides to form a new bioactive electrospun nanofiber membrane, exhibited superior efficacy among all nanofiber membranes for burn injuries (Zhou et al. 2021).
Regarding diabetic wounds, new scaffolds formed of polyethylene glycolylated graphene oxide collagen hybrid for nanoscale drug delivery of quercetin were tested. It was found that it provided a new scaffold with the advantages of being superior, stable, the controllable release of quercetin, biodegradable nanomaterial, and biocompatible, which permitted collagen formation and angiogenesis. Besides, the mesenchymal stem cells' proliferation and differentiation potential were promoted via adhesion to this scaffold. These new scaffolds could help in solving issues of deficient collagen hyperplasia and insufficient blood supply in the case of diabetic wounds (Chu et al. 2018).
Conclusions and future direction
The current review clarifies that nature introduces medicinal plants with remarkable wound-healing effects. Scientific evidence obtained in the last 5 years has allowed us to expand our knowledge about herbal medicines on wound healing and the underlying molecular mechanisms. Plants, with their natural actives, have the ability to cure wounds and to be utilized in skin wound care. Mainly due to their anti-inflammatory, antimicrobial, and antioxidant activities (Pazyar et al. 2014).
Recent literature has proved that different natural substances, such as flavonoids, saponins, phenolic compounds, and polysaccharides, can operate at various phases of the process through diverse mechanisms and are primarily responsible for the activity of herbal remedies active in wound healing. Polyphenolic compounds have been confirmed therapeutical agents in wound healing by regulating and modulating inflammatory responses. Numerous phytochemicals in medicinal plants have been revealed to be important regulators of homeostasis, re-epithelialization, and regeneration by encouraging fibroblast proliferation and/or collagen formation. Scientific research confirmed the powerful impact of medicinal plants and their phytochemicals in wound management through multiple connected mechanisms (Maver et al. 2015; Artem Ataide et al. 2018).
The development of novel wound care techniques that integrate herbal healing agents with modern products and procedures is in line with current trends in wound healing. Nanostructures and nanoformulations have recently shown promise in overcoming the limitations of conventional medications. They control the release of medicines, lower the dosages needed for healing, and enhance the solubility and effectiveness of water-insoluble herbal components in healing wounds. The optimal dressing for wound treatment is made of nanofibers due to their well-controlled porosity and resemblance to skin tissue. The incorporation of natural materials into nanofibrous architectures for wound dressing has been studied. A biocompatible formulation made of natural herbal extracts would give the consumer a “green” option, and almost fewer side effects once put on the skin.
Based on these findings, it is recommended that many therapeutic approaches be employed concurrently in managing wounds, especially chronic wound injuries, to speed up the healing process and prevent complications. Moreover, various problems need to be resolved to improve the efficacy and utilization of natural substances in wound healing. Multidisciplinary efforts are required to confirm the products’ safety, look at their adverse effects, and do double-blind controlled clinical trials. Good production standards and regulatory regulations are equally essential to increase practitioners’ use of phytotherapy and encourage its incorporation into national health systems.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This review received no external funding.
Data availability
The authors confirm that the data supporting this study are available within the article.
Declarations
Conflicts of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
S. A. El-Sherbeni, Email: suzy.elsherbini@pharm.tanta.edu.eg
W. A. Negm, Email: walaa.negm@pharm.tanta.edu.eg
References
- Aburjai T, Al-Janabi R, Al-Mamoori F, Azzam H. In vivo wound healing and antimicrobial activity of Alkanna strigose. Wound Med. 2019;25(1):100152. doi: 10.1016/j.wndm.2019.100152. [DOI] [Google Scholar]
- Aderibigbe BA, Buyana B. Alginate in wound dressings. Pharmaceutics. 2018;10(2):42. doi: 10.3390/pharmaceutics10020042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed OM, Mohamed T, Moustafa H, Hamdy H, Ahmed RR, Aboud E. Quercetin and low level laser therapy promote wound healing process in diabetic rats via structural reorganization and modulatory effects on inflammation and oxidative stress. Biomed Pharmacother. 2018;101:58–73. doi: 10.1016/j.biopha.2018.02.040. [DOI] [PubMed] [Google Scholar]
- Ajmal G, Bonde GV, Mittal P, Khan G, Pandey VK, Bakade BV, Mishra B. Biomimetic PCL-gelatin based nanofibers loaded with ciprofloxacin hydrochloride and quercetin: a potential anti-bacterial and antioxidant dressing material for accelerated healing of a full thickness wound. Int J Pharm. 2019;567:118480. doi: 10.1016/j.ijpharm.2019.118480. [DOI] [PubMed] [Google Scholar]
- Ajmal G, Bonde GV, Thokala S, Mittal P, Khan G, Singh J, Pandey VK, Mishra B. Ciprofloxacin HCl and quercetin functionalized electrospun nanofiber membrane: fabrication and its evaluation in full thickness wound healing. Artif Cells Nanomed Biotechnol. 2019;47(1):228–240. doi: 10.1080/21691401.2018.1548475. [DOI] [PubMed] [Google Scholar]
- Akbik D, Ghadiri M, Chrzanowski W, Rohanizadeh R. Curcumin as a wound healing agent. Life Sci. 2014;116(1):1–7. doi: 10.1016/j.lfs.2014.08.016. [DOI] [PubMed] [Google Scholar]
- Al-Madhagy SA, Mostafa NM, Youssef FS, Awad GE, Eldahshan OA, Singab ANB. Metabolic profiling of a polyphenolic-rich fraction of Coccinia grandis leaves using LC-ESI-MS/MS and in vivo validation of its antimicrobial and wound healing activities. Food Funct. 2019;10(10):6267–6275. doi: 10.1039/C9FO01532A. [DOI] [PubMed] [Google Scholar]
- Algahtani MS, Ahmad MZ, Shaikh IA, Abdel-Wahab BA, Nourein IH, Ahmad J. Thymoquinone loaded topical nanoemulgel for wound healing: formulation design and in-vivo evaluation. Molecules. 2021;26(13):3863. doi: 10.3390/molecules26133863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alherz FA, Negm WA, Elekhnawy E, El-Masry TA, Haggag EM, Alqahtani MJ, Hussein IA. Silver nanoparticles prepared using encephalartos laurentianus de wild leaf extract have inhibitory activity against candida albicans clinical isolates. J Fungi. 2022;8(10):1005. doi: 10.3390/jof8101005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali A, Garg P, Goyal R, Kaur G, Li X, Negi P, Valis M, Kuca K, Kulshrestha S. A novel herbal hydrogel formulation of moringa oleifera for wound healing. Plants. 2021;10(1):25. doi: 10.3390/plants10010025. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ali A, Garg P, Goyal R, Khan A, Negi P, Li X, Kulshrestha S. An efficient wound healing hydrogel based on a hydroalcoholic extract of Moringa oleifera seeds. S Afr J Bot. 2022;145:192–198. doi: 10.1016/j.sajb.2021.05.003. [DOI] [Google Scholar]
- Almukainzi M, El-Masry TA, Negm WA, Elekhnawy E, Saleh A, Sayed AE, Ahmed HM, Abdelkader DH. Co-delivery of gentiopicroside and thymoquinone using electrospun m-PEG/PVP nanofibers: in-vitro and in vivo studies for antibacterial wound dressing in diabetic rats. Int J Pharm. 2022;625:122106. doi: 10.1016/j.ijpharm.2022.122106. [DOI] [PubMed] [Google Scholar]
- Almukainzi M, El-Masry TA, Negm WA, Elekhnawy E, Saleh A, Sayed AE, Khattab MA, Abdelkader DH. Gentiopicroside PLGA nanospheres: fabrication, in vitro characterization, antimicrobial action, and in vivo effect for enhancing wound healing in diabetic rats. Int J Nanomed. 2022;17:1203. doi: 10.2147/IJN.S358606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alotaibi B, Negm WA, Elekhnawy E, El-Masry TA, Elseady WS, Saleh A, Alotaibi KN, El-Sherbeni SA. Antibacterial, immunomodulatory, and lung protective effects of boswellia dalzielii oleoresin ethanol extract in pulmonary diseases in vitro and in vivo studies. Antibiotics. 2021;10(12):1444. doi: 10.3390/antibiotics10121444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aaa A, Attalah Oran S, Shakhanbeh JM. In vitro and in vivo wound healing activities of Globularia arabica leaf methanolic extract in diabetic rats. J Cosmet Dermatol. 2022;2(10):4888–4900. doi: 10.1111/jocd.14882. [DOI] [PubMed] [Google Scholar]
- Alsareii SA, Alzerwi NA, AlAsmari MY, Alamri AM, Mahnashi MH, Shaikh IA. Topical application of premna integrifolia linn on skin wound injury in rats accelerates the wound healing process: evidence from in vitro and in vivo experimental models. Evid-Based Complement Altern Med. 2022;2022:1–14. doi: 10.1155/2022/6449550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alven S, Nqoro X, Aderibigbe BA. Polymer-based materials loaded with curcumin for wound healing applications. Polymers. 2020;12(10):2286. doi: 10.3390/polym12102286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aras C, Tümay Özer E, Göktalay G, Saat G, Karaca E. Evaluation of Nigella sativa oil loaded electrospun polyurethane nanofibrous mat as wound dressing. J Biomater Sci Polym Ed. 2021;32(13):1718–1735. doi: 10.1080/09205063.2021.1937463. [DOI] [PubMed] [Google Scholar]
- Artem Ataide J, Caramori Cefali L, Machado Croisfelt F, Arruda Martins Shimojo A, Oliveira-Nascimento L, Gava MP. Natural actives for wound healing: a review. Phytother Res. 2018;32(9):1664–1674. doi: 10.1002/ptr.6102. [DOI] [PubMed] [Google Scholar]
- Attallah NG, Elekhnawy E, Negm WA, Hussein IA, Mokhtar FA, Al-Fakhrany OM. In vivo and in vitro antimicrobial activity of biogenic silver nanoparticles against Staphylococcus aureus clinical isolates. Pharmaceuticals. 2022;15(2):194. doi: 10.3390/ph15020194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attallah NGM, Negm WA, Elekhnawy E, Elmongy EI, Altwaijry N, El-Haroun H, El-Masry TA, El-Sherbeni SA. Elucidation of phytochemical content of cupressus macrocarpa leaves in vitro and in vivo anti-bacterial effect against methicillin-resistant staphylococcus aureus clinical isolates. Antibiotics (basel). 2021;10(8):890. doi: 10.3390/antibiotics10080890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aycan D, Selmi B, Kelel E, Yildirim T, Alemdar N. Conductive polymeric film loaded with ibuprofen as a wound dressing material. Eur Polymer J. 2019;121:109308. doi: 10.1016/j.eurpolymj.2019.109308. [DOI] [Google Scholar]
- Ayu CA, Weta IW, Aman IGM. Moringa (Moringa oleifera) leaves extract gel improved wound healing by increasing fibroblasts, neovascularization and in male Wistar rats. IJAAM (indones J Anti-Aging Med). 2020;4(1):28–32. [Google Scholar]
- Bagher Z, Ehterami A, Safdel MH, Khastar H, Semiari H, Asefnejad A, Davachi SM, Mirzaii M, Salehi M. Wound healing with alginate/chitosan hydrogel containing hesperidin in rat model. J Drug Deliv Sci Technol. 2020;55:101379. doi: 10.1016/j.jddst.2019.101379. [DOI] [Google Scholar]
- Bihani T, Mhaske N. Evaluation of in vivo wound healing activity of Plumeria obtusa L. (Champa) spray in rats. Wound Med. 2020;28:100176. doi: 10.1016/j.wndm.2019.100176. [DOI] [Google Scholar]
- Binsuwaidan R, Elekhnawy E, Elseady WS, Keshk WA, Shoeib NA, Attallah NG, Mokhtar FA, Abd El Hadi SR, Ahmed E, Magdeldin S. Anti-bacterial activity and wound healing potential of Cycas thouarsii R. Br n-butanol fraction in diabetic rats supported with phytochemical profiling. Biomed Pharmacother. 2022;155:113763. doi: 10.1016/j.biopha.2022.113763. [DOI] [PubMed] [Google Scholar]
- Blume-Peytavi U, Tan J, Tennstedt D. Fragility of epidermis in newborns, children and adolescents (vol 30, pg 3, 2016) J Europ Acade Dermatol Venereol. 2016;30(9):1634–1634. doi: 10.1111/jdv.13636. [DOI] [PubMed] [Google Scholar]
- Boakye YD, Agyare C, Ayande GP, Titiloye N, Asiamah EA, Danquah KO. Assessment of wound-healing properties of medicinal plants: the case of Phyllanthus muellerianus. Front Pharmacol. 2018;9:945. doi: 10.3389/fphar.2018.00945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudjelal A, Napoli E, Benkhaled A, Benazi L, Bey R, Gentile D, Ruberto G. In vivo wound healing effect of Italian and Algerian Pistacia vera L. resins. Fitoterapia. 2022;159:105197. doi: 10.1016/j.fitote.2022.105197. [DOI] [PubMed] [Google Scholar]
- Carvalho MTB, Araújo-Filho HG, Barreto AS, Quintans-Júnior LJ, Quintans JSS, Barreto RSS. Wound healing properties of flavonoids: A systematic review highlighting the mechanisms of action. Phytomedicine. 2021;90:153636. doi: 10.1016/j.phymed.2021.153636. [DOI] [PubMed] [Google Scholar]
- Chen L-Y, Cheng H-L, Kuan Y-H, Liang T-J, Chao Y-Y, Lin H-C. Therapeutic potential of luteolin on impaired wound healing in streptozotocin-induced rats. Biomedicines. 2021;9(7):761. doi: 10.3390/biomedicines9070761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chingwaru C, Bagar T, Maroyi A, Kapewangolo PT, Chingwaru W. Wound healing potential of selected Southern African medicinal plants: a review. J Herb Med. 2019;17–18:100263. doi: 10.1016/j.hermed.2019.100263. [DOI] [Google Scholar]
- Choudhary A, Kant V, Jangir BL, Joshi VG. Quercetin loaded chitosan tripolyphosphate nanoparticles accelerated cutaneous wound healing in Wistar rats. Eur J Pharmacol. 2020;880:173172. doi: 10.1016/j.ejphar.2020.173172. [DOI] [PubMed] [Google Scholar]
- Chu J, Shi P, Yan W, Fu J, Yang Z, He C, Deng X, Liu H. PEGylated graphene oxide-mediated quercetin-modified collagen hybrid scaffold for enhancement of MSCs differentiation potential and diabetic wound healing. Nanoscale. 2018;10(20):9547–9560. doi: 10.1039/C8NR02538J. [DOI] [PubMed] [Google Scholar]
- Du X, Wu L, Yan H, Jiang Z, Li S, Li W, Bai Y, Wang H, Cheng Z, Kong D, et al. Microchannelled alkylated chitosan sponge to treat noncompressible hemorrhages and facilitate wound healing. Nat Commun. 2021;12(1):4733. doi: 10.1038/s41467-021-24972-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn L, Prosser HC, Tan JT, Vanags LZ, Ng MK, Bursill CA. Murine model of wound healing. JoVE (j Visual Exp) 2013;75:e50265. doi: 10.3791/50265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-ezz A, Abdel-Rahman LH, Al-Farhan BS, Mostafa DA, Ayad EG, Basha MT, Abdelaziz M, Abdalla EM. Enhanced in vivo wound healing efficacy of a novel hydrogel loaded with copper (II) schiff base quinoline complex (CuSQ) solid lipid nanoparticles. Pharmaceuticals. 2022;15(8):978. doi: 10.3390/ph15080978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elloumi W, Mahmoudi A, Ortiz S, Boutefnouchet S, Chamkha M, Sayadi S. Wound healing potential of quercetin-3-O-rhamnoside and myricetin-3-O-rhamnoside isolated from Pistacia lentiscus distilled leaves in rats model. Biomed Pharmacother. 2022;146:112574. doi: 10.1016/j.biopha.2021.112574. [DOI] [PubMed] [Google Scholar]
- Fathi AN, Sakhaie MH, Babaei S, Babaei S, Slimabad F, Babaei S. Use of bromelain in cutaneous wound healing in streptozocin-induced diabetic rats: an experimental model. J Wound Care. 2020;29(9):488–495. doi: 10.12968/jowc.2020.29.9.488. [DOI] [PubMed] [Google Scholar]
- Frykberg Robert G. Challenges in the treatment of chronic wounds. Advan Wound Care. 2015;4(9):560–582. doi: 10.1089/wound.2015.0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Huang J, Lin M, Xie T, You T. Quercetin promotes diabetic wound healing via switching macrophages from M1 to M2 polarization. J Surg Res. 2020;246:213–223. doi: 10.1016/j.jss.2019.09.011. [DOI] [PubMed] [Google Scholar]
- Fujishima MAT, Sa DMC, Lima CMDS, Bittencourt JAH, Pereira WLA, Muribeca AdJB, Silva ECYY, de Silva MN, de Sousa FFO, Dos Santos CB. Chemical profiling of Curatella americana Linn leaves by UPLC-HRMS and its wound healing activity in mice. PLoS ONE. 2020;15(1):0225514. doi: 10.1371/journal.pone.0225514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebremeskel L, Bhoumik D, Sibhat GG, Tuem KB. In vivo wound healing and anti-inflammatory activities of leaf latex of aloe megalacantha baker (Xanthorrhoeaceae) Evid-Based Complement Altern Med. 2018;2018:1–7. doi: 10.1155/2018/5037912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grice EA, Segre JA. The Skin Microbiome. Nat Rev Microbiol. 2011;9(4):244–253. doi: 10.1038/nrmicro2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haq A, Kumar S, Mao Y, Berthiaume F, Michniak-Kohn B. Thymoquinone-loaded polymeric films and hydrogels for bacterial disinfection and wound healing. Biomedicines. 2020;8(10):386. doi: 10.3390/biomedicines8100386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman TF, Bordoni B. 2020. Wound classification. [PubMed]
- Hernandez-Hernandez AB, Alarcon-Aguilar FJ, Garcia-Lorenzana M, Rodriguez-Monroy MA, Canales-Martinez MM. Jatropha neopauciflora Pax latex exhibits wound-healing effect in normal and diabetic mice. J Evid-Based Integrat Med. 2021;26:2515690X20986762. doi: 10.1177/2515690X20986762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y, Xin M, Li Q, Wu X. Glycyrrhizin micelle as a genistein nanocarrier: Synergistically promoting corneal epithelial wound healing through blockage of the HMGB1 signaling pathway in diabetic mice. Exp Eye Res. 2021;204:108454. doi: 10.1016/j.exer.2021.108454. [DOI] [PubMed] [Google Scholar]
- Ibrahim AAE, Bagherani N, Smoller BR, Reyes-Baron C, Bagherani N. Functions of the Skin. In: Smoller B, Bagherani N, editors. Atlas of Dermatology, Dermatopathology and Venereology. Cham: Springer International Publishing; 2020. pp. 1–11. [Google Scholar]
- Ivanková V, Belovičová M 2020 Consequences of chronic wounds on patient’s lif. Укpaїнa Здopoв’я нaцiї. 2(3).
- Jangde R, Srivastava S, Singh MR, Singh D. In vitro and In vivo characterization of quercetin loaded multiphase hydrogel for wound healing application. Int J Biol Macromol. 2018;115:1211–1217. doi: 10.1016/j.ijbiomac.2018.05.010. [DOI] [PubMed] [Google Scholar]
- Jee J-P, Pangeni R, Jha SK, Byun Y, Park JW. Preparation and in vivo evaluation of a topical hydrogel system incorporating highly skin-permeable growth factors, quercetin, and oxygen carriers for enhanced diabetic wound-healing therapy. Int J Nanomed. 2019;14:5449. doi: 10.2147/IJN.S213883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones ML. A short history of the development of wound care dressings. Brit J Healthcare Assist. 2015;9(10):482–485. doi: 10.12968/bjha.2015.9.10.482. [DOI] [Google Scholar]
- Juneja K, Mishra R, Chauhan S, Gupta S, Roy P, Sircar D. Metabolite profiling and wound-healing activity of Boerhavia diffusa leaf extracts using in vitro and in vivo models. J Tradit Complement Med. 2020;10(1):52–59. doi: 10.1016/j.jtcme.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalalinia F, Aamiri N, Bayat S, Movaffagh J, Hahsemi M. 671 burn wound healing effect of bromelain-loaded chitosan nanofibers. J Burn Care Res. 2021;42(1):S192–S192. doi: 10.1093/jbcr/irab032.317. [DOI] [PubMed] [Google Scholar]
- Kant V, Jangir BL, Kumar V, Nigam A, Sharma V. Quercetin accelerated cutaneous wound healing in rats by modulation of different cytokines and growth factors. Growth Fact. 2020;38(2):105–119. doi: 10.1080/08977194.2020.1822830. [DOI] [PubMed] [Google Scholar]
- Kant V, Kumar M, Jangir BL, Kumar V 2020b Temporal effects of different vehicles on wound healing potentials of quercetin: biochemical, molecular, and histopathological approaches. The International Journal of Lower Extremity Wounds:1534734620977582. [DOI] [PubMed]
- Karuppannan SK, Dowlath MJH, Ramalingam R, Musthafa SA, Ganesh MR, Chithra V, Ravindran B, Arunachalam KD. Quercetin functionalized hybrid electrospun nanofibers for wound dressing application. Mater Sci Eng, B. 2022;285:115933. doi: 10.1016/j.mseb.2022.115933. [DOI] [Google Scholar]
- Kuma DN, Boye A, Kwakye-Nuako G, Boakye YD, Addo JK, Asiamah EA, Aboagye EA, Martey O, Essuman MA, Atsu Barku VY. Wound healing properties and antimicrobial effects of parkia clappertoniana keay fruit husk extract in a rat excisional wound model. BioMed Res Intern. 2022 doi: 10.1155/2022/9709365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Kothari V. Wound healing research: current trends and future directions. Singapore: Springer Singapore; 2021. [Google Scholar]
- Kumar S, Kumar A, Kumar N, Singh P, Singh TU, Singh BR, Gupta PK, Thakur VK. In vivo therapeutic efficacy of Curcuma longa extract loaded ethosomes on wound healing. Vet Res Commun. 2022;46(4):1033–1049. doi: 10.1007/s11259-022-09952-1. [DOI] [PubMed] [Google Scholar]
- Kwiecien K, Zegar A, Jung J, Brzoza P, Kwitniewski M, Godlewska U, Grygier B, Kwiecinska P, Morytko A, Cichy J. Architecture of antimicrobial skin defense. Cytokine Growth Factor Rev. 2019;49:70–84. doi: 10.1016/j.cytogfr.2019.08.001. [DOI] [PubMed] [Google Scholar]
- Lambebo MK, Kifle ZD, Gurji TB, Yesuf JS. Evaluation of wound healing activity of methanolic crude extract and solvent fractions of the leaves of vernonia auriculifera hiern (asteraceae) in mice. J Exp Pharmacol. 2021;13:677. doi: 10.2147/JEP.S308303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G, Ko Y-G, Bae KH, Kurisawa M, Kwon OK, Kwon OH. Green tea catechin-grafted silk fibroin hydrogels with reactive oxygen species scavenging activity for wound healing applications. Biomat Res. 2022;26(1):1–6. doi: 10.1186/s40824-022-00304-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüer S, Troller R, Aebi C. Anti-bacterial and antiinflammatory kinetics of curcumin as a potential antimucositis agent in cancer patients. Nutr Cancer. 2012;64(7):975–981. doi: 10.1080/01635581.2012.713161. [DOI] [PubMed] [Google Scholar]
- Majtan J, Jesenak M. β-Glucans: multi-functional modulator of wound healing. Molecules. 2018;23(4):806. doi: 10.3390/molecules23040806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Msarabahi S, Tiwari V, Bhattacharya S. Principles and practice of wound care. Thieme Med Sci Pub Private Ltd. 2012;45(1):167–169. [Google Scholar]
- Maver T, Maver U, Stana Kleinschek K, Smrke DM, Kreft S. A review of herbal medicines in wound healing. Int J Dermatol. 2015;54(7):740–751. doi: 10.1111/ijd.12766. [DOI] [PubMed] [Google Scholar]
- Mir M, Ali MN, Barakullah A, Gulzar A, Arshad M, Fatima S, Asad M. Synthetic polymeric biomaterials for wound healing: a review. Prog Biomater. 2018;7(1):1–21. doi: 10.1007/s40204-018-0083-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayeem N, Asdaq SMB, Alamri AS, Alsanie WF, Alhomrani M, Mohzari Y, Alrashed AA, Alotaibi N, Alharbi MA, Aldhawyan NN. Wound healing potential of Dodonaea viscosa extract formulation in experimental animals. J King Saud Univ-Sci. 2021;33(5):101476. doi: 10.1016/j.jksus.2021.101476. [DOI] [Google Scholar]
- Negi P, Sharma G, Verma C, Garg P, Rathore C, Kulshrestha S, Lal UR, Gupta B, Pathania D. Novel thymoquinone loaded chitosan-lecithin micelles for effective wound healing: development, characterization, and preclinical evaluation. Carbohyd Polym. 2020;230:115659. doi: 10.1016/j.carbpol.2019.115659. [DOI] [PubMed] [Google Scholar]
- Negm WA, El-Kadem AH, Elekhnawy E, Attallah NGM, Al-Hamoud GA, El-Masry TA, Zayed A. Wound-healing potential of rhoifolin-rich fraction isolated from sanguisorba officinalis roots supported by enhancing re-epithelization, angiogenesis, anti-inflammatory, and antimicrobial effects. Pharmaceuticals (basel) 2022;15(2):178. doi: 10.3390/ph15020178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordin A, Kamal H, Yazid MD, Saim A, Idrus R. Effect of Nigella sativa and its bioactive compound on type 2 epithelial to mesenchymal transition: a systematic review. BMC Complement Altern Med. 2019;19(1):290. doi: 10.1186/s12906-019-2706-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okur ME, Karadağ AE, Üstündağ Okur N, Özhan Y, Sipahi H, Ayla Ş, Daylan B, Demirci B, Demirci F. In vivo wound healing and in vitro anti-inflammatory activity evaluation of Phlomis russeliana extract gel formulations. Molecules. 2020;25(11):2695. doi: 10.3390/molecules25112695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özay Y, Güzel S, Erdoğdu İH, Pehlivanoğlu B, Aydın Türk B, Darcan S. Evaluation of the wound healing properties of luteolin ointments on excision and incision wound models in diabetic and nondiabetic rats. Rec Nat Prod. 2018;12(4):350–366. doi: 10.25135/rnp.38.17.08.135. [DOI] [Google Scholar]
- Özay Y, Güzel S, Yumrutaş Ö, Pehlivanoğlu B, Erdoğdu İH, Yildirim Z, Türk BA, Darcan S. Wound healing effect of kaempferol in diabetic and nondiabetic rats. J Surg Res. 2019;233:284–296. doi: 10.1016/j.jss.2018.08.009. [DOI] [PubMed] [Google Scholar]
- Özbilgin S, Acıkara ÖB, Akkol EK, Süntar I, Keleş H, İşcan GS. In vivo wound-healing activity of Euphorbia characias subsp. wulfenii: Isolation and quantification of quercetin glycosides as bioactive compounds. J Ethnopharmacol. 2018;224:400–408. doi: 10.1016/j.jep.2018.06.015. [DOI] [PubMed] [Google Scholar]
- Parveen A, Kulkarni N, Yalagatti M, Abbaraju V, Deshpande R. In vivo efficacy of biocompatible silver nanoparticles cream for empirical wound healing. J Tissue Viability. 2018;27(4):257–261. doi: 10.1016/j.jtv.2018.08.007. [DOI] [PubMed] [Google Scholar]
- Pazyar N, Yaghoobi R, Rafiee E, Mehrabian A, Feily A. Skin wound healing and phytomedicine: a review. Skin Pharmacol Physiol. 2014;27(6):303–310. doi: 10.1159/000357477. [DOI] [PubMed] [Google Scholar]
- Percival NJ. Classification of wounds and their management. Surg Infect (larchmt) 2002;20(5):114–117. [Google Scholar]
- Pereira LOM, Vilegas W, Tangerina MMP, Arunachalam K, Balogun SO, Orlandi-Mattos PE, Colodel EM, Martins DTdO. Lafoensia pacari A. St.-Hil.: wound healing activity and mechanism of action of standardized hydroethanolic leaves extract. J Ethnopharmacol. 2018;219:337–350. doi: 10.1016/j.jep.2018.02.038. [DOI] [PubMed] [Google Scholar]
- Rajendran NK, Kumar SSD, Houreld NN, Abrahamse H. A review on nanoparticle based treatment for wound healing. J Drug Deliv Sci Technol. 2018;44:421–430. doi: 10.1016/j.jddst.2018.01.009. [DOI] [Google Scholar]
- Rajoo A, Ramanathan S, Mansor SM, Sasidharan S. Formulation and evaluation of wound healing activity of Elaeis guineensis Jacq leaves in a Staphylococcus aureus infected Sprague Dawley rat model. J Ethnopharmacol. 2021;266:113414. doi: 10.1016/j.jep.2020.113414. [DOI] [PubMed] [Google Scholar]
- Rathod L, Bhowmick S, Patel P, Sawant K. Calendula flower extract loaded collagen film exhibits superior wound healing potential: Preparation, evaluation, in-vitro & in-vivo wound healing study. J Drug Deliv Sci Technol. 2022;72:103363. doi: 10.1016/j.jddst.2022.103363. [DOI] [Google Scholar]
- Richmond JM, Harris JE. Immunology and skin in health and disease. Cold Spring Harb Perspect Med. 2014;4(12):a015339. doi: 10.1101/cshperspect.a015339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues M, Kosaric N, Bonham CA, Gurtner GC. Wound healing: a cellular perspective. Physiol Rev. 2019;99(1):665–706. doi: 10.1152/physrev.00067.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said HK, Roy NK, Gurjala AN, Mustoe TA. Quantifying tissue level ischemia: Hypoxia response element-luciferase transfection in a rabbit ear model. Wound Rep Regenerat. 2009;17(4):473–479. doi: 10.1111/j.1524-475X.2009.00498.x. [DOI] [PubMed] [Google Scholar]
- Santos TS, Santos IDDd, Pereira-Filho RN, Gomes SVF, Lima-Verde IB, Marques MN, Cardoso JC, Severino P, Souto EB, Albuquerque-Júnior RLCd. Histological evidence of wound healing improvement in rats treated with oral administration of hydroalcoholic extract of vitis labrusca. Curr Issues Mol Biol. 2021;43(1):335–352. doi: 10.3390/cimb43010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra MB, Barroso WA, Rocha C, Furtado PGR, Borges ACR, Silva SN, Tangerina MMP, Nascimento JRd, Vilegas W, Alves AC, et al. Chemical characterization and wound healing property of jacaranda decurrens cham. (Bignoniaceae): an experimental study based on molecular mechanisms. Evid-Based Complement Altern Med. 2020;2020:4749712. doi: 10.1155/2020/4749712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shady NH, Soltane R, Maher SA, Saber EA, Elrehany MA, Mostafa YA, Sayed AM, Abdelmohsen UR. Wound healing and antioxidant capabilities of zizyphus mauritiana fruits: in-vitro, in-vivo, and molecular modeling study. Plants. 2022;11(11):1392. doi: 10.3390/plants11111392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah A, Amini-Nik S. The role of phytochemicals in the inflammatory phase of wound healing. Int J Mol Sci. 2017;18(5):1068. doi: 10.3390/ijms18051068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M, Sahu K, Singh SP, Jain B. Wound healing activity of curcumin conjugated to hyaluronic acid: in vitro and in vivo evaluation. Artif Cell Nanomed Biotechnol. 2018;46(5):1009–1017. doi: 10.1080/21691401.2017.1358731. [DOI] [PubMed] [Google Scholar]
- Sivamani RK, Ma BR, Wehrli LN, Maverakis E. Phytochemicals and naturally derived substances for wound healing. Adv Wound Care. 2012;1(5):213–217. doi: 10.1089/wound.2011.0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddese SM, Gurji TB, Abdulwuhab M, Aragaw TJ. Wound healing activities of hydromethanolic crude extract and solvent fractions of bersama abyssinica leaves in mice. Evid-Based Complement Altern Med. 2021;2021:1–20. doi: 10.1155/2021/9991146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan WS, Arulselvan P, Ng S-F, Mat Taib CN, Sarian MN, Fakurazi S. Improvement of diabetic wound healing by topical application of Vicenin-2 hydrocolloid film on Sprague Dawley rats. BMC Complement Altern Med. 2019;19(1):20. doi: 10.1186/s12906-018-2427-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taskan MM, Yuce HB, Karatas O, Gevrek F. Topical quercetin gel application improved wound healing in Wistar rats. Annal Med Res. 2019;26(10):2397. doi: 10.5455/annalsmedres.2019.05.289. [DOI] [Google Scholar]
- Tejada S, Manayi A, Daglia M, Nabavi FS, Sureda A, Hajheydari Z, Gortzi O, Pazoki-Toroudi H, Nabavi MS. Wound healing effects of curcumin: a short review. Curr Pharmaceut Biotechnol. 2016;17(11):1002–1007. doi: 10.2174/1389201017666160721123109. [DOI] [PubMed] [Google Scholar]
- Tekleyes B, Huluka SA, Wondu K, Wondmkun YT. Wound healing activity of 80% methanol leaf extract of zehneria scabra (lf) sond (cucurbitaceae) in mice. J Exp Pharmacol. 2021;13:537. doi: 10.2147/JEP.S303808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessema Z, Molla Y. Evaluation of the wound healing activity of the crude extract of root bark of Brucea antidysentrica, the leaves of Dodonaea angustifolia and Rhamnus prinoides in mice. Heliyon. 2021;7(1):e05901. doi: 10.1016/j.heliyon.2021.e05901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay G, Tiwari N, Maurya H, Upadhyay J, Joshi R, Ansari MN. In vivo wound-healing and antioxidant activity of aqueous extract of Roylea elegans leaves against physically induced burn model in Wistar albino rats. Biotech. 2021;11(10):442. doi: 10.1007/s13205-021-02993-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Hu L, Peng Z, Cao H, Cao D, Long Y, Zou Z. Luteolin is an effective component of platycodon grandiflorus in promoting wound healing in rats with cutaneous scald injury. Clin Cosmet Investig Dermatol. 2022;15:1715–1727. doi: 10.2147/CCID.S372229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayal SR, Gurav SS. Evaluation of wound healing potential of Bhallatakadi Ghrita–cow ghee based polyherbal formulation: in-vivo excision and incision wound model. J Complement Integrat Med. 2021;18(3):507–515. doi: 10.1515/jcim-2020-0179. [DOI] [PubMed] [Google Scholar]
- Xu N, Wang L, Guan J, Tang C, He N, Zhang W, Fu S. Wound healing effects of a Curcuma zedoaria polysaccharide with platelet-rich plasma exosomes assembled on chitosan/silk hydrogel sponge in a diabetic rat model. Int J Biol Macromol. 2018;117:102–107. doi: 10.1016/j.ijbiomac.2018.05.066. [DOI] [PubMed] [Google Scholar]
- Yaseen HS, Asif M, Saadullah M, Asghar S, Shams MU, Bazmi RR, Saleem M, Yousaf HM, Yaseen M. Methanolic extract of Ephedra ciliata promotes wound healing and arrests inflammatory cascade in vivo through downregulation of TNF-α. Inflammopharmacology. 2020;28(6):1691–1704. doi: 10.1007/s10787-020-00713-7. [DOI] [PubMed] [Google Scholar]
- Yin G, Wang Z, Wang Z, Wang X. Topical application of quercetin improves wound healing in pressure ulcer lesions. Exp Dermatol. 2018;27(7):779–786. doi: 10.1111/exd.13679. [DOI] [PubMed] [Google Scholar]
- Yousef H, Alhajj M, Sharma S. 2017. Anatomy, skin (integument), epidermis. [PubMed]
- Yuan X, Han L, Fu P, Zeng H, Lv C, Chang W, Runyon RS, Ishii M, Han L, Liu K, et al. Cinnamaldehyde accelerates wound healing by promoting angiogenesis via up-regulation of PI3K and MAPK signaling pathways. Lab Invest. 2018;98(6):783–798. doi: 10.1038/s41374-018-0025-8. [DOI] [PubMed] [Google Scholar]
- Zahedi P, Rezaeian I, Ranaei-Siadat SO, Jafari SH, Supaphol P. A review on wound dressings with an emphasis on electrospun nanofibrous polymeric bandages. Polym Adv Technol. 2010;21(2):77–95. doi: 10.1002/pat.1625. [DOI] [Google Scholar]
- Zhou L, Cai L, Ruan H, Zhang L, Wang J, Jiang H, Wu Y, Feng S, Chen J. Electrospun chitosan oligosaccharide/polycaprolactone nanofibers loaded with wound-healing compounds of Rutin and Quercetin as anti-bacterial dressings. Int J Biol Macromol. 2021;183:1145–1154. doi: 10.1016/j.ijbiomac.2021.05.031. [DOI] [PubMed] [Google Scholar]
- Zindle JK, Wolinsky E, Bogie KM. A review of animal models from 2015 to 2020 for preclinical chronic wounds relevant to human health. J Tissue Viability. 2021;30(3):291–300. doi: 10.1016/j.jtv.2021.05.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting this study are available within the article.