Abstract
This study was conducted to determine the effects of dietary yeast cell wall (YCW) on growth performance, intestinal health, and immune responses of broiler chickens. In a randomized completely block design (block: initial body weight), a total of 800 broilers (Ross 308; 45.18 ± 3.13 g of initial body weight) were assigned to 2 dietary treatments (40 birds/pen; 10 replicates/treatment) and fed for 5 wk: 1) a basal broiler diet based on corn-soybean meal (CON) and 2) CON + 0.05% dietary YCW. Growth performance was measured at intervals in 3 phase feed program. On the final day of the study, one bird per pen was randomly selected and euthanized for sample collection. Broilers fed YCW had decreased (P < 0.05) feed conversion ratio during the grower phase compared with those fed CON. The YCW increased (P < 0.05) villus height to crypt depth ratio in the duodenum, jejunum, and ileum compared with the CON. In addition, the YCW tended to higher (P < 0.10) number of goblet cells in the duodenum than in the CON. Broilers fed YCW had increased (P < 0.05) serum TGF- β1, ileal gene expression of the claudin family, and relative abundance of Lactobacillus, Prevotella, and Enterococcus compared with the CON, but decreased serum TNF-α (P < 0.05), IL-1β (P < 0.05), and IL-6 (P < 0.10), ileal gene expression of IL-6 (P < 0.05), and relative abundance of Clostridium (P < 0.05). The present study demonstrated that the addition of dietary YCW in broiler diets enhanced the intestinal health of broiler chickens and may be associated with modulated intestinal morphology and integrity by upregulating tight junction-related protein gene expression and modifying the ileal microbiota. In addition, dietary YCW modulated immune responses and inflammatory cytokine gene expression in the ileum.
Key words: broiler chicken, immune response, intestinal health, ileal microbiota, yeast cell wall
INTRODUCTION
Provision of adequate nutrients to broiler chickens is important to maintain growth and health in broiler production systems. Gut health is interdependent with nutrition, and is a comprehensive domain that includes physiology, microbiology, and immunology (Jha et al., 2019). It is also important to improve the growth performance of broilers by converting optimal nutrients through their feed into absorbable forms to increase utilization efficiency. Post-hatching is a critical event in broiler production, and the gut system at this time is still immature (Maiorka et al., 2006). In the past, the use of antibiotics along with feed played an important role in preventing diseases as well as improving growth efficiency and gut health. However, antibiotic resistance and public health concerns regarding safe and healthy meat have led to the banning of in-feed antibiotics as growth promoters (Kang et al., 2021; Kim et al., 2021a).
Yeast- or yeast-derived feed additives have been used in livestock production as potential alternatives of in-feed antibiotics (Liu et al., 2018; Pascual et al., 2020; Lee et al., 2021; Kim et al., 2022). Yeast- containing feed additives are commercially available in various forms such as live yeast, yeast culture, yeast cell wall, and their components (Gao et al., 2008; M'sadeq et al., 2015; Xue et al., 2017; Bonato et al., 2020; He et al., 2021). Particularly, supplementation of yeast cell wall (YCW) to broiler chickens has shown its potential to replace in-feed antibiotics by preventing external infection and promoting growth (Liu et al., 2021). YCW contains β-glucans and mannan oligosaccharides (MOS) that act as bioactive compounds in birds. MOS can prevent the proliferation of harmful bacteria by prohibiting pathogens to bind the gut epithelium (Spring et al., 2000), thereby enhancing mucus defense (Gao et al., 2008). On the other hand, there is evidence that birds fed a diet containing β-glucans presented altered anti-inflammatory immune responses (Cox et al., 2010; Liu et al., 2021). However, the impacts of dietary YCW on intestinal integrity and microbiota are not yet thoroughly explored in broilers. The present study hypothesized that supplementation of dietary YCW in broiler diets would alter immune responses and microbial communities and improve intestinal integrity, thereby improving growth performance. Therefore, the objectives of the present study were to evaluate the effects of dietary YCW on growth performance, immune responses, and intestinal health of broiler chickens.
MATERIALS AND METHODS
Experimental Design, Animals, and Diets
The experimental design and procedures of the present study were reviewed and approved by the Institutional Animal Care and Use Committee of Chungnam National University, Daejeon, Republic of Korea (approval: #201909A-CNU-165).
In a randomized completely block design [block: initial body weight (BW)], a total of 800 broiler chickens (Ross 308 strain; 45.18 ± 3.13 g of initial BW; 1 d of age) were obtained from a commercial hatchery (Maniker Co. Ltd., Yesan, Republic of Korea) and were assigned to 2 dietary treatments. Dietary treatments were 1) a basal broiler diet based on corn-soybean meal (CON) and 2) CON supplemented with 0.05% dietary YCW. Each dietary treatment included 10 replicates with 40 broilers per pen. As shown in Table 1, the basal diet was formulated according to Ross 308 nutrient recommendations (Aviagen, 2016). The present study was conducted in 3 phase feeding program, with the starter phase from d 1 to 7, the grower phase from d 8 to 21, and the finisher phase from d 22 to 35. The YCW product contained 29.58% crude protein, 3.69% crude fiber, 5.88% crude ash, and 28.20% β-glucan (Pathway Intermediates, Seoul, Republic of Korea). The diet was provided in mash form, and birds had free access to the diet and water and were in same sized floor pens (190 × 120 × 91.7 cm) with rice hulls as litter material (depth = 5 cm). All birds were housed in temperature, relative humidity and lighting program-controlled pens according to Ross 308 broiler management guideline (Aviagen, 2018).
Table 1.
Composition of basal diet for broiler chickens (as-fed basis).
| Item | Starter1 | Grower1 | Finisher1 |
|---|---|---|---|
| Ingredient, % | |||
| Corn | 58.35 | 62.10 | 67.60 |
| Soybean meal | 35.00 | 26.80 | 22.00 |
| Corn distiller's dried grains with soluble | – | 5.00 | 22.00 |
| Soy oil | 2.90 | 2.50 | 2.20 |
| L-Lysine | 0.05 | 0.50 | 0.50 |
| L-Methionine | 0.40 | 0.30 | 0.20 |
| Threonine | 0.15 | 0.15 | 0.15 |
| Choline chloride | 0.10 | 0.10 | 0.10 |
| Monocalcium phosphate | 0.70 | 0.20 | 0.10 |
| Limestone | 1.60 | 1.60 | 1.40 |
| Salt | 0.25 | 0.25 | 0.25 |
| Vitamin premix2 | 0.20 | 0.20 | 0.20 |
| Mineral premix3 | 0.20 | 0.20 | 0.20 |
| Phytase4 | 0.10 | 0.10 | 0.10 |
| Calculated composition | |||
| Metabolizable energy, kcal/kg | 3,075 | 3,075 | 3,100 |
| Crude protein, % | 21.8 | 20.3 | 18.8 |
| Lysine, % | 1.25 | 1.18 | 1.12 |
| Methionine + cysteine, % | 1.00 | 0.95 | 0.90 |
Starter phase was from d 1 to 7, grower phase was from d 8 to 21, and finisher phase was from d 22 to 35 of post-hatching.
Vitamin-mineral premix provided the following nutrients per kg of diet: vitamin A, 24,000 IU; vitamin D3, 6,000 IU; vitamin E, 30 IU; vitamin K, 4 mg; thiamin, 4 mg; riboflavin, 12 mg; pyridoxine, 4 mg; folacine, 2 mg; biotin, 0.03 mg; vitamin B8 0.06 mg; niacin, 90 mg; pantothenic acid, 30 mg.
Mineral premix provided the following nutrients per kg of diet: Fe, 80 mg (as FeSO4·H2O); Zn, 80 mg (as ZnSO4·H2O); Mn, 80 mg (as MnSO4·H2O); Co, 0.5 mg (as CoSO4·H2O); Cu, 10 mg (as CuSO4·H2O); Se, 0.2 mg (as Na2SeO3); I, 0.9 mg (as Ca (IO3)·2H2O)
Phytase was added in diets to all diets to supply 750 U/kg of final feed.
Data and Sample Collection
BW and remaining feed were weighed and recorded on a pen basis at d 7, 21, and 35, and average daily gain (ADG), average daily feed intake (ADFI), and feed conversion ratio (FCR) were calculated. FCR was corrected for mortality. On d 35, 1 bird per pen was selected for sample collection. Blood samples were randomly selected from 6 birds per treatment and collected from the jugular vein of broiler chickens using 10 mL serum tubes (BD Vacutainer Systems, Franklin Lakes, NJ). Serum samples were separated by centrifugation (1580R; LaboGene, Lynge, Denmark) at 3,000 × g for 15 min at 4°C and stored at −80°C further immune response analysis (Kim et al., 2021b). After blood collection, selected sample birds were euthanized by cervical dislocation for biopsy. The gizzard, caeca, and whole breast and leg were harvested and weighed. The gizzard and caeca contents were removed before weighing. Relative organ weights were expressed as a percentage to bird final live BW. For small intestinal morphology, middle duodenal, jejunal, and ileal tissues (3 cm each) were gently flushed with distilled water and fixed in 10% neutral buffered formaldehyde. Ileal mucosa samples were scraped from the ileal sample using a microslide and stabilized in a microtube including RNA later reagent (QIAGEN GmbH, Hilden, Germany) for 24 h at room temperature and then were stored at −80°C for further gene expression analysis. Distal ileal digesta samples were collected and stored at −80°C for microbiota measurement.
Intestinal Morphology Analysis
Intestinal morphology was measured according to a previously reported procedure (Song et al., 2022). The fixed small intestinal samples were stained with hematoxylin and eosin (H&E) and imprinted on slide glasses. The H&E stained slides were imaged using a fluorescence microscope (TE2000; Nikon, Tokyo, Japan) equipped with a charge-coupled device camera (DS-Fi1; Nikon, Tokyo, Japan) and NIS-Elements software (Version, 3.00; NIS Elements, Nikon, Tokyo, Japan). The intestinal images were scanned to analyze villus height, villus width, villus area, crypt depth, villus height to crypt depth ratio (VH:CD), and number of goblet cells were determined by selecting 15 straight and integrated villi and their associated crypts and the number of goblet cells (Mun et al., 2021).
Immune Responses Analysis
Serum samples were analyzed the immune responses such as cortisol, tumor necrosis factor-alpha (TNF-α), transforming growth factor-beta1 (TGF-β1), interleukin-1beta (IL-1β), and interleukin-6 (IL-6) using ELISA kits (MyBioSource, San Diego, CA: cortisol, TNF-α, IL-1β, and IL-6; Cusabio Biotech, Wuhan, China: TGF-β1) according to the manufacturer's instructions. Each concentration was determined using a microplate reader at 450nm (Epoch microplate spectrophotometer, BioTek Instruments Inc., Winooski, VT). Intra-assay coefficients of variation for cortisol, TNF-α, TGF-β1, IL-1β, and IL-6 were ≤8%, <8%, <8%, <10%, and ≤6.1%, respectively; the interassay coefficients were ≤12%, <12%, <10%, <12%, and ≤8.6%, respectively.
Ileal Gene Expression Analysis
Total RNA was extracted from ileal mucosa samples using the HiGene Total RNA Prep kit (BIOFACT, Daejeon, Republic of Korea). The RNA concentration and quantities of samples were determined using NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies Inc., Wilmington, DE). Reverse transcription into cDNA was performed using the QuantiTect Reverse Transcription kit (Qiagen, GmbH, Hilden, Germany). The qRT-PCR analysis was carried out by StepOnePlus RT-PCR system (Applied Biosystems, Foster City, CA) and SFCgreen I (BIOFACT, Daejeon, Republic of Korea), and gene-specific designed primers (Bioneer, Daejeon, Republic of Korea). Gene-specific primer sequences including target genes [tight junction-related proteins and inflammatory cytokines: claudin-1 (CLND1), claudin-2 (CLDN2), claudin-3 (CLND3), claudin-4 (CLDN4), occludin (OCLN), tight junction protein-1 (TJP1), mucin-1 (MUC1), TNF-α, IL-1β, IL-6, and C–C motif chemokine ligand-5 (CCL5)] and reference gene as β-actin (ACTB) was designed using Primer Express software (Applied Biosystems) and is shown in Table 2. Relative gene expression levels were calculated using the cycle threshold (Ct) value by the 2−ΔΔCt method (Livak and Schmittgen, 2001) with ACTB as a housekeeping gene against target genes.
Table 2.
Gene-specific primer sequences for gene expression of tight junction proteins and inflammatory cytokines in ileal tissues.
| Item1 | Forward (5′–3′) | Reverse (5′–3′) |
|---|---|---|
| CLDN1 | GAAGATGCGGATGGCTGTCT | GGCCCGAGCCACTCTGTT |
| CLDN2 | CCCTGACAGCACCAAATTTGA | GCAGGAGGCACAGAGGATGA |
| CLDN3 | CGCGCTGCCCATGTG | GCTCTGCACCACGCAGTTC |
| CLDN4 | GGAGGACGAGACAGCCAAAG | GTTGGCCGACCAGCAGAT |
| OCLN | CATCGCCTCCATCGTCTACA | GTAGGCCTGGCTGCACATG |
| TJP1 | ACAAAAACAGAGCTGAACAACTAGCTA | GCTACGCAAACCTCGGAATC |
| MUC1 | CCTACCTGCCAGATACCATTGC | GAGAAGGGCTGGACTTGAGATG |
| TNF-α | TATGTGCAGCAACCCGTAGTG | CTGACTCATAGCAGAGACGTGTCA |
| IL-1β | ACCAACCCGACCAGGTCAA | ACATACGAGATGGAAACCAGCAA |
| IL-6 | CGGCCTGTTCGCCTTTC | CAGGTGCTTTGTGCTGTAGCA |
| CCL5 | CTGATACAACCGTGTGCTGCTT | GCTGCCTGTGGGCATTTG |
| ACTB | AACACCCACACCCCTGTGAT | TGAGTCAAGCGCCAAAAGAA |
CLDN1, claudin-1; CLDN2, claudin-2; CLDN3, claudin-3; CLDN4, claudin-4; OCLN, occludin; TJP1, tight junction protein-1; MUC1, mucin-1; TNF-α, tumor necrosis factor-α; IL-1β, interleukin-1β; IL-6, interleukin-6; CCL5, C–C motif chemokine ligand-5; ACTB, β-actin.
Ileal Microbiota Analysis
Total DNA from the ileal digesta was isolated using the QIAmp DNA Stool Mini kit (QIAGEN, Hilden, Germany). The V3 to V4 regions of the 16s rRNA gene were amplified with primers Bakt 341F and Bakt 805R using the Illumina MiSeq platform (Illumina, San Diego, CA) at Macrogen Inc. (Seoul, Republic of Korea). Paired-end reads were merged using the Fast Length Adjustment of Short reads software (FLASH; v 1.2.11) (Magoč and Salzberg, 2011). Microbial diversity and composition were analyzed using Quantitative Insights Into Microbial Ecology (QIIME) software (Caporaso et al., 2010). The acquired high-quality sequence data were clustered to identify Operational Taxonomic Units (OTUs) with ≥97% similarity using the QIIME-UCLUST (Edgar, 2010). Alpha diversity was expressed within ileal microbial samples using richness as Chao1 and observed OTUs and diversity as Shannon and Inverse Simpson, with an estimated over 0.99 Good's coverage. Beta diversity between the microbial communities of dietary treatments was presented by Principal Coordinate Analysis (PCoA) based on the Bray-Curtis index using the MicrobiomeAnalyst webtool (https://www.microbiomeanalyst.ca/). Taxonomic abundance of ileal microbiota was presented as a ratio based on relative abundance.
Statistical Analyses
Data were analyzed using the General Linear Model procedure of SAS (SAS Inst. Inc., Cary, NC) in a randomized completely block design (block: initial BW). Experimental unit was the pen. Statistical models for growth performance, carcass part yields, intestinal morphology, and immune responses included dietary treatments as a main effect and initial BW as a covariate. The t test was used to compare ileal gene expression between dietary treatments. Microbial alpha and beta diversity results between samples from dietary treatments were checked for statistical differences by Kruskal-Wallis test and permutational multivariate analysis of variance (PERMANOVA), respectively. Results were presented as the mean ± SEM, excluding the ileal microbial diversity, which is presented as the mean ± SD. Statistically differences were considered significant and tendency between dietary treatments at P < 0.05 and 0.05 ≤ P < 0.10, respectively.
RESULTS
Growth Performance and Carcass Part Yields
The mortality throughout the study was <5% without disease clinical lesions and/or sign, and this was not related to the dietary treatments. As shown in Table 3, the effects of YCW on the growth performance of broiler chickens throughout the study. Broilers fed YCW had decreased (P < 0.05) FCR on grower phase compared with those fed CON. However, there were no differences on growth performance over the entire period. There were no differences on carcass meat and organ weights and their relative yields between CON and YCW (Table 4).
Table 3.
Effects of dietary yeast cell wall on growth performance of broiler chickens.1
| Item2 | CON | YCW | SEM | P value |
|---|---|---|---|---|
| BW, g | ||||
| D 1 | 45.18 | 45.17 | 1.02 | 0.996 |
| D 7 | 170.00 | 171.10 | 2.81 | 0.785 |
| D 21 | 937.08 | 951.28 | 9.11 | 0.285 |
| D 35 | 1886.69 | 1912.47 | 15.32 | 0.250 |
| ADG, g/d | ||||
| Starter (d 1–7) | 17.83 | 17.99 | 0.31 | 0.722 |
| Grower (d 8–21) | 54.79 | 55.73 | 0.48 | 0.186 |
| Finisher (d 22–35) | 67.83 | 68.66 | 0.61 | 0.346 |
| Overall (d 1–35) | 52.62 | 53.35 | 0.43 | 0.241 |
| ADFI, g/d | ||||
| Starter (d 1–7) | 19.01 | 19.22 | 0.32 | 0.647 |
| Grower (d 8–21) | 74.64 | 74.80 | 0.56 | 0.839 |
| Finisher (d 22–35) | 118.14 | 119.43 | 1.14 | 0.437 |
| Overall (d 1–35) | 80.91 | 81.54 | 0.67 | 0.519 |
| FCR, g/g | ||||
| Starter (d 1–7) | 1.066 | 1.068 | 0.005 | 0.766 |
| Grower (d 8–21) | 1.363 | 1.342 | 0.006 | 0.031 |
| Finisher (d 22–35) | 1.742 | 1.740 | 0.011 | 0.890 |
| Overall (d 1–35) | 1.538 | 1.528 | 0.006 | 0.258 |
Each value is the mean value of 10 replicates (40 broilers/pen).
CON, a basal diet based on corn-soybean meal; YCW, CON + 0.05% dietary yeast cell wall; BW, body weight; ADG, average daily gain; ADFI, average daily feed intake; FCR, feed conversion ratio.
Table 4.
Effects of dietary yeast cell wall on carcass parts organ weight and yield of broiler chickens.1
| Item2 | CON | YCW | SEM | P value |
|---|---|---|---|---|
| Whole breast | ||||
| Weight, g | 488.21 | 504.38 | 10.09 | 0.272 |
| Yield, % | 25.90 | 26.40 | 0.61 | 0.563 |
| Whole leg | ||||
| Weight, g | 149.54 | 150.27 | 3.29 | 0.878 |
| Yield, % | 7.92 | 7.86 | 0.15 | 0.759 |
| Drumstick | ||||
| Weight, g | 78.61 | 80.40 | 2.18 | 0.568 |
| Yield, % | 4.17 | 4.21 | 0.11 | 0.810 |
| Cecum | ||||
| Weight, g | 4.87 | 4.61 | 0.23 | 0.412 |
| Yield, % | 0.26 | 0.24 | 0.01 | 0.339 |
| Gizzard | ||||
| Weight, g | 30.25 | 29.68 | 1.00 | 0.691 |
| Yield, % | 1.60 | 1.55 | 0.05 | 0.492 |
Each value is the mean value of 10 replicates (1 broiler/pen).
CON, a basal diet based on corn-soybean meal; YCW, CON + 0.05% dietary yeast cell wall.
Intestinal Morphology
The results presented in Table 5 show that dietary YCW influenced the intestinal morphology of broiler chicks. Broilers fed YCW had increased villus height (P < 0.10), VH:CD (P < 0.05), villus width (P < 0.05), and the number of goblet cells (P < 0.10) in the duodenum compared with those fed CON, but decreased (P < 0.05) crypt depth. In addition, the YCW group had increased villus height (P < 0.10) and VH:CD (P < 0.05) in the jejunum, and villus height (P < 0.05) and VH:CD (P < 0.05) in the ileum compared with the CON group.
Table 5.
Effects of dietary yeast cell wall on intestinal morphology of broiler chickens.1
| Item2 | CON | YCW | SEM | P value |
|---|---|---|---|---|
| Duodenum | ||||
| Villus height, μm | 839.64 | 943.11 | 40.24 | 0.099 |
| Crypt depth, μm | 159.82 | 122.15 | 7.97 | 0.008 |
| VH:CD, μm/μm | 5.29 | 7.80 | 0.36 | 0.001 |
| Villus width, μm | 146.10 | 186.86 | 9.69 | 0.014 |
| Villus area, μm2 | 69512.45 | 74835.31 | 8051.39 | 0.650 |
| Goblet cell, n | 90.87 | 125.93 | 13.65 | 0.099 |
| Jejunum | ||||
| Villus height, μm | 616.85 | 754.07 | 53.32 | 0.099 |
| Crypt depth, μm | 71.86 | 64.03 | 5.78 | 0.360 |
| VH:CD, μm/μm | 8.54 | 12.40 | 1.06 | 0.028 |
| Villus width, μm | 150.79 | 151.10 | 8.31 | 0.979 |
| Villus area, μm2 | 70250.02 | 77317.98 | 5124.17 | 0.352 |
| Goblet cell, n | 82.15 | 93.39 | 10.16 | 0.452 |
| Ileum | ||||
| Villus height, μm | 456.56 | 600.35 | 42.51 | 0.038 |
| Crypt depth, μm | 62.99 | 59.35 | 4.32 | 0.564 |
| VH:CD, μm/μm | 7.21 | 10.25 | 0.66 | 0.009 |
| Villus width, μm | 153.10 | 158.93 | 11.91 | 0.736 |
| Villus area, μm2 | 58705.85 | 58397.03 | 7191.29 | 0.976 |
| Goblet cell, n | 63.73 | 78.18 | 6.18 | 0.130 |
Each value is the mean value of 6 replicates (1 broiler/pen).
CON, a basal diet based on corn-soybean meal; YCW, CON + 0.05% dietary yeast cell wall; VH:CD, villus height to crypt depth ratio.
Immune Responses
The effects of supplementation of YCW in broiler diet on immune responses are presented in Table 6. The YCW group had lower serum concentrations of TNF-α (P < 0.05), IL-1β (P < 0.05), and IL-6 (P < 0.10) than CON group. In contrast, broilers fed YCW had a higher (P < 0.05) serum concentration of TGF-β1 than those fed CON. However, there was no difference in serum concentration of cortisol between the dietary treatments.
Table 6.
Effects of dietary yeast cell wall on immune responses of broiler chickens.1
| Item2 | CON | YCW | SEM | P value |
|---|---|---|---|---|
| Cortisol, ng/mL | 35.71 | 32.89 | 4.11 | 0.638 |
| TNF-α, pg/mL | 129.64 | 112.96 | 3.44 | 0.006 |
| TGF-β1, pg/mL | 28.21 | 41.35 | 2.78 | 0.007 |
| IL-1β, pg/mL | 59.61 | 36.02 | 4.77 | 0.006 |
| IL-6, pg/mL | 134.78 | 128.38 | 2.09 | 0.056 |
Each value is the mean value of 6 replicates (1 broiler/pen).
CON, a basal diet based on corn-soybean meal; YCW, CON + 0.05% dietary yeast cell wall; TNF-α, tumor necrosis factor-α; TGF-β1, transforming growth factor-β1; IL-1β, interleukin-1β; IL-6, interleukin-6.
Ileal Gene Expression of Tight Junction-Related Proteins and Inflammatory Cytokines
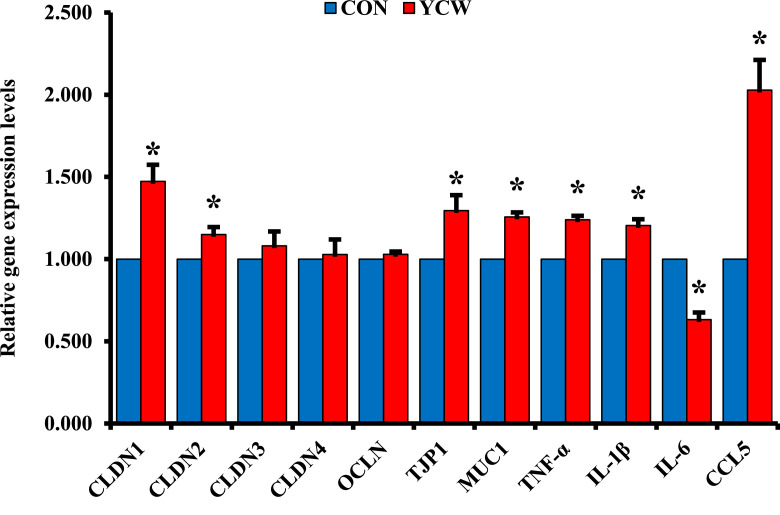
The effects of YCW on gene expression of tight junction-related proteins and cytokines in the ileal mucosa are shown in Figure 1. Supplementation of YCW in broiler diet had upregulated (P < 0.05) the relative expressions of CLDN1, CLDN2, TJP1, and MUC1 in the ileum of broilers compared with the CON diet. However, no differences were found in the relative expressions of ileal CLDN3, CLDN4, and OCLN between the dietary treatments. In the inflammatory cytokine gene expression, the YCW group had upregulated (P < 0.05) relative expressions of TNFα, IL1β, and CCL5 in the ileum of broilers compared with the CON group. In contrast, the birds fed YCW had downregulated (P < 0.05) in the ileal relative expression of IL6 compared with those fed CON.
Figure 1.
Gene expression of tight junction-related proteins and inflammatory cytokines. Each value is the mean of 6 replicates (1 broiler/pen). CON, a basal diet based on corn-soybean meal; YCW, CON + 0.05% dietary yeast cell wall; CLND1, claudin-1; CLDN2, claudin-2; CLDN3, claudin-3; CLDN4, claudin-4; OCLN, occludin; TJP1, tight junction protein-1; MUC1, mucin-1; TNF-α, tumor necrosis factor-α; IL-1β, interleukin-1β; IL-6, interleukin-6; CCL5, C–C motif chemokine ligand-5. *Means with different letters within each variable differ (P < 0.05).
Diversity and Community of Ileal Microbiota
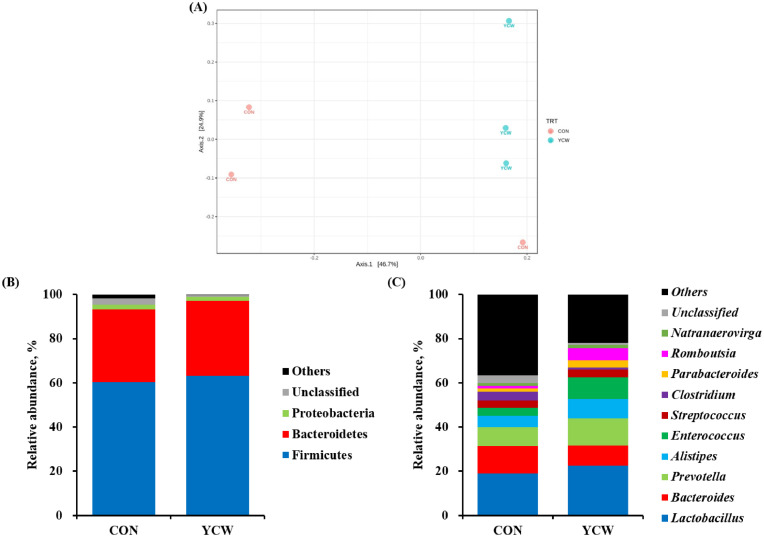
A total of 899,881 read counts were obtained from ileal digesta, with mean read counts of 149,980 ± 22,993 per sample after sequencing assembled using FLASH. After quality filtering using QIIME, the mean read counts were 40,332 ± 8,832 in the CON group and 66,932 ± 3,700 in the YCW the group. The ileal microbial community diversity results obtained by calculating the observed OTUs, Chao1, Shannon, and Inverse Simpson, are presented in Table 7. Our results showed that bacterial alpha diversity did not differ between dietary treatments. Beta diversity analysis using Bray-Curtis index by PCoA plots between dietary treatments was illustrated in Figure 2A. Distinct separation of the microbial population was not observed visually between dietary treatments.
Table 7.
Effects of dietary yeast cell wall on alpha diversity of ileal microbiota of broiler chickens.1
| Item2 | CON | YCW | P value |
|---|---|---|---|
| Observed OTUs | 322.30 ± 85.42 | 306.00 ± 75.23 | 0.816 |
| Chao1 | 333.80 ± 76.17 | 333.30 ± 98.74 | 0.995 |
| Shannon | 5.74 ± 1.60 | 4.53 ± 1.09 | 0.340 |
| Inverse Simpson | 0.92 ± 0.09 | 0.89 ± 0.07 | 0.704 |
Each value in the mean value of 3 replicates (1 broiler/pen) and presented as mean ± standard deviation.
CON, a basal diet based on corn-soybean meal; YCW, CON + 0.05% dietary yeast cell wall; OTUs, operational taxonomic units.
Figure 2.
Summary of ileal microbiota on broiler chickens between dietary treatments (3 replicates/treatment). (A) Beta diversity using PCoA on Bray-Curtis index and assessed using PREMANOVA (R2 = 0.303; P > 0.10). Relative abundance of ileal bacterial community (B) at the phylum level and (C) genus level. The proportion of ileal microbiota of broiler chickens less than 1% was included in others. CON, a basal diet based on corn-soybean meal; YCW, CON + 0.05% dietary yeast cell wall.
Taxonomic composition of the ileal bacterial community at the phylum and genus levels were presented in Figure 2B and C, respectively. At the phylum level, Firmicutes were dominant in both treatments (CON = 60.27%; YCW = 63.22%), followed by Bacteroidetes (CON = 32.98%; YCW = 33.89%) and Proteobacteria (CON = 2.12%; YCW = 1.88%) in the ileal digesta samples. At the genus level, broilers fed YCW had greater (P < 0.05) relative abundance of Lactobacillus (22.51%), Prevotella (12.33%), and Enterococcus (9.75%) than those fed CON (18.94, 8.50, and 3.61%, respectively). In contrast, the YCW group had lower (P < 0.05) relative abundance of Clostridium (1.03%) than the CON group (3.89%).
DISCUSSION
In the current study, YCW supplementation in broiler diets improved FCR in the grower phase compared with CON, which was associated with numerically increased body weight upon dietary YCW. However, this improved grower feed efficiency did not lead to the improvement in growth performance and carcass yield on the final day of the study. Based on these results, it can be suggested that an adaptation period was required before YCW could evoke a positive effect on growth performance, although there were no differences in contrast to numerical advantage in the finisher phase. Similar results on the effect of YCW addition in the growth phase have been reported (Gao et al., 2008; Muthusamy et al., 2011). In addition, the effects of YCW on growth performance are diverse, which may be related to their types and concentrations (Fowler et al., 2015; Li et al., 2016; Alizadeh et al., 2016; Xue et al., 2017; Liu et al., 2018). Furthermore, the beneficial impacts of YCW are primarily associated with immunostimulatory effects due to the presence of polysaccharides components in yeast products (Gao et al., 2008; Reisinger et al., 2012). The beneficial impacts of YCW are primarily associated with activated immune functions due to the presence of β-glucan and MOS in yeast products.
Development of intestinal morphology can reflect intestinal integrity. VH:CD can be used as an indicator of gut development and functional capacity. A higher VH:CD indicates an increased surface area for nutrient digestion and absorption (Gao et al., 2008). The beneficial impacts of yeast-derived products on intestinal morphology have been consistent in broilers, improving and/or maintaining a healthy gut architecture (Gao et al., 2008; Muthusamy et al., 2011; Ghosh et al., 2012; He et al., 2021). Correspondingly, our results clearly showed the notable alterations in intestinal morphology upon YCW supplementation, in which supplemental YCW heightened villus length and VH:CD compared with birds fed CON throughout the small intestine. These positive results are likely due to the components of YCW (e.g., β-glucan and MOS), which can protect the mucosa via preventing pathogens and/or pathogenic bacteria with type I fimbriae from binding to the villi and allowing fewer antigens to come into contact with the villi (Spring et al., 2000; Gao et al., 2008; Muthusamy et al., 2011). Hence, YCW supplementation numerically increased the number of goblet cells in all small intestinal segments, indicating the stimulation of epithelial cell renewal and increased mucin secretion (Muthusamy et al., 2011). However, the fast turnover rate of intestinal cells might have increased the energy requirement for maintenance, thereby reducing energy for growth. This likely explains the negligible impact of supplemental YCW on growth performance in the present study. Nevertheless, this clear positive effect of YCW on gut functionality is expected to exert positive impacts on growth performance when birds are challenged with subclinical intestinal diseases. In this regard, further research is warranted to investigate if YCW supplementation can mitigate the negative symptoms of any infection in the gastrointestinal tract of birds.
The observed enhanced gut function is again supported by the upregulated tight junction-related protein expressions in the present study. The formation of tight junctions between epithelial cells with multiprotein complexes acts as intestinal barrier functionality by regulating gut permeability (Chen et al., 2015; Awad et al., 2017). In the present study, CLDN1 and CLDN2 genes were upregulated by YCW supplementation. Claudin family comprise major transmembrane proteins and contribute to pericellular sealing (Yu and Turner, 2008). Furthermore, TJP1 and MUC1 genes were upregulated by YCW addition. MUC1 genes are responsible for the secretion of mucin that acts as the first defense line against foreign invaders through the mucosa (Dhar and McAuley, 2019). Our results suggest that YCW supplementation may have the potential to modulate gut integrity in broiler chickens.
The immune system protects the host from external pathogens, through a process of recognition and response. In the immune system, cytokines regulate immune responses, including infection, injury, and inflammation, through the cell to cell communications (Parkin and Cohen, 2001). In our study, YCW supplementation suppressed the concentrations of serum TNF-α, IL-1β, and IL-6 compared with the CON, but promoted the concentration of serum TGF-β1. These results are consistent with previous results showing the anti-inflammatory effects of yeast-derived products (Xue et al., 2017; Lee et al., 2021; Kim et al., 2022). The small intestine is one of the most important organs of the immune system and plays an important role in the nutritional system. Particularly, the balance between T helper type 1 (Th1) and Th2 cytokines is important in the regulation of the body's immune system. For instance, Th1 cytokines stimulate the cell-mediated immune responses, secreting IFN-γ, TNF-α, and IL-1. On the other hand, Th2 cytokines activate the humoral immune responses, producing IL-4, IL-6, and IL-10. In the present study, ileal gene expression results showed that TNF-α and IL-1β as Th1 indicators were upregulated, but IL-6 as Th2 indicator was downregulated following addition of YCW compared with CON. Changes in inflammatory cytokine gene expression triggered by YCW addition seem to result from cross-regulatory effects between Th1 and Th2, and recover from relative immaturity of the intestinal immune system due to post-hatching. Our results suggest that YCW addition is beneficial for systemic/local immune regulation due to the anti-inflammatory effects of its constituent β-glucan through stimulating immune cells (Cox et al., 2010; Liu et al., 2021).
Gut microbial communities in broilers play an important role in promoting health status through digestion and absorption of nutrients, development of the immune system, and inhibition of pathogen colonization (Wei et al., 2013; Stanley et al., 2014; Shang et al., 2018). Therefore, the early development of the gut microbiota is the key to maintaining and improving host health and resistance against microbial infection (Kers et al., 2018). In general, high alpha-diversity of the gut micro-ecosystem was considered beneficial for host health. However, diversity is not as simple as more is better because not all microbes are beneficial (Reese and Dunn, 2018). The presence of highly competitive microbial communities in commensal microbes can affect microbial stability (Bauer et al., 2018). Furthermore, it has been reported that there is a negative correlation between microbial stability and diversity (Coyte et al., 2015). Thus, the stability of the core microbial community in relation to the microbial competition can be considered as a healthy microbial community. Although the addition of YCW did not affect alpha diversity in ileal microbiota, it was confirmed that each dietary treatment clustered as a result of beta diversity in ileal microbiota. In the present study, alterations in the relative taxonomic abundance of ileal microbiota were found for increased genera Lactobacillus, Prevotella, and Enterococcus, and decreased genera Clostridium following YCW addition compared with CON. This may be related to no differences in ileal microbial diversity following colonization of YCW-altered microbiota. Yeast-derived products have been reported to modulate the gut microbial composition in broilers (Muthusamy et al., 2011; Ghosh et al., 2012; Wang et al., 2016; Bonato et al., 2020; Kim et al., 2022). The genus Lactobacillus is well known for its gene encoding functional abilities related to transport and utilization in relation to carbohydrate metabolism (Cai et al., 2009; Schwab and Gänzle, 2011; Gänzle and Follador, 2012). Prevotella is associated with improved glucose metabolism (Kovatcheva-Datchary et al., 2015; Ley, 2016), nonstarch carbohydrate degradation (Xia et al., 2021), and short-chain fatty acid production (Megahed et al., 2019). Among Enterococcus strains derived from chickens (i.e., E. faecium) produce bacteriocins as potential antibacterial factors (Strompfová and Lauková, 2007; Levkut et al., 2012; Royan, 2018). In contrast, although Clostridium strains are often found in the poultry intestinal tract, the relatively high abundance of Clostridium perfringens can be pathogenic for birds, producing necrotic enteritis toxins (Fasina and Lillehoj, 2019; Sandvang et al., 2021). MOS, a component of YCW, is known for its ability to bind pathogenic bacteria with type-1 fimbriae (Spring et al., 2000), thereby exerting prebiotic properties. Therefore, this may explain positive alterations in ileal microbiota composition in the present study.
CONCLUSIONS
In conclusion, our findings suggested that dietary YCW enhanced the intestinal health of broiler chickens by modulating intestinal morphology and integrity through upregulating tight junction-related protein gene expression and modifying the microbial community. Furthermore, dietary YCW modulates serum immune responses and inflammatory cytokine gene expression in the ileum. Therefore, the addition of dietary YCW in broiler diets can enhance the intestinal health of broiler chickens.
ACKNOWLEDGMENTS
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea Government (MSIT) (No. NRF-2021R1I1A3046876) and Pathway Intermediates, Seoul, Republic of Korea.
DISCLOSURES
The authors declare that they have no competing interests.
REFERENCES
- Alizadeh M., Rodriguez-Lecompte J.C., Yitbarek A., Sharif S., Crow G., Slominski B.A. Effect of yeast-derived products on systemic innate immune response of broiler chickens following a lipopolysaccharide challenge. Poult. Sci. 2016;95:2266–2273. doi: 10.3382/ps/pew154. [DOI] [PubMed] [Google Scholar]
- Aviagen . Ross Broiler Management Handbook. Aviagen Inc.; Huntsville, AL: 2018. [Google Scholar]
- Aviagen . Ross 308 Nutrition Specifications. Aviagen Inc; Huntsville, AL: 2016. [Google Scholar]
- Awad W.A., Hess C., Hess M. Enteric pathogens and their toxin-induced disruption of the intestinal barrier through alteration of tight junctions in chickens. Toxins. 2017;9:60. doi: 10.3390/toxins9020060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer M.A., Kainz K., Carmona-Gutierrez D., Madeo F. Microbial wars: competition in ecological niches and within the microbiome. Microb. Cell. 2018;5:215–219. doi: 10.15698/mic2018.05.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonato M., Borges L.L., Ingberman M., Fávaro C., Mesa D., Caron L.F., Beirão B.C.B. Effects of yeast cell wall on immunity, microbiota, and intestinal integrity of Salmonella-infected broilers. J. Appl. Poult. Res. 2020;29:545–558. [Google Scholar]
- Cai H., Thompson R., Budinich M.F., Broadbent J.R., Steele J.L. Genome sequence and comparative genome analysis of Lactobacillus casei: insights into their niche-associated evolution. Genome Biol. Evol. 2009;1:239–257. doi: 10.1093/gbe/evp019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso J.G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F.D., Costello E.K., Fierer N., Peña A.G., Goodrich J.K., Gordon J.I., Huttley G.A., Kelley S.T., Knights D., Koenig J.E., Ley R.E., Lozupone C.A., McDonald D., Muegge B.D., Pirrung M., Reeder J., Sevinsky J.R., Turnbaugh P.J., Walters W.A., Widmann J., Yatsunenko T., Zaneveld J., Knight R. QIIME allows anlaysis of high-throughput community sequencing data. Nat. Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Tellez G., Richards J.D., Escobar J. Identification of potential biomarkers for gut barrier failure in broiler chickens. Front. Vet. Sci. 2015;2:14. doi: 10.3389/fvets.2015.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox C.M., Sumners L.H., Kim S., Mcelroy A.P., Bedford M.R., Dalloul R.A. Immune responses to dietary β-glucan in broiler chicks during an Eimeria challenge. Poult. Sci. 2010;89:2597–2607. doi: 10.3382/ps.2010-00987. [DOI] [PubMed] [Google Scholar]
- Coyte K.Z., Schluter J., Foster K.R. The ecology of the microbiome: networks, competition, and stability. Science. 2015;350:663–666. doi: 10.1126/science.aad2602. [DOI] [PubMed] [Google Scholar]
- Dhar P., McAuley J. The role of the cell surface mucin MUC1 as a barrier to infection and regulator of inflammation. Front. Cell. Infect. Microbiol. 2019;9:117. doi: 10.3389/fcimb.2019.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- Fasina Y.O., Lillehoj H.S. Characterization of intestinal immune response to Clostridium perfringens infection in broiler chickens. Poult. Sci. 2019;98:188–198. doi: 10.3382/ps/pey390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler J., Kakani R., Haq A., Byrd J.A., Bailey C.A. Growth promoting effects of prebiotic yeast cell wall products in starter broilers under an immune stress and Clostridium perfringens challenge. J. Appl. Poult. Res. 2015;24:66–72. [Google Scholar]
- Gänzle M.G., Follador R. Metabolism of oligosaccharides and starch in lactobacilli: a review. Front. Microbiol. 2012;3:340. doi: 10.3389/fmicb.2012.00340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J., Zhang H.J., Yu S.H., Wu S.G., Yoon I., Quigley J., Gao Y.P., Qi G.H. Effects of yeast culture in broiler diets on performance and immunomodulatory functions. Poult. Sci. 2008;87:1377–1384. doi: 10.3382/ps.2007-00418. [DOI] [PubMed] [Google Scholar]
- Ghosh T.K., Haldar S., Bedford M.R., Muthusami N., Samanta I. Assessment of yeast cell wall as replacements for antibiotic growth promoters in broiler diets: effects on performance, intestinal histo-morphology and humoral immune responses. J. Anim. Physiol. Anim. Nutr. (Berl.) 2012;96:275–284. doi: 10.1111/j.1439-0396.2011.01155.x. [DOI] [PubMed] [Google Scholar]
- He T., Mahfuz S., Piao X., Wu D., Wang W., Yan H., Ouyang T., Liu Y. Effects of live yeast (Saccharomyces cerevisiae) as a substitute to antibiotic on growth performance, immune function, serum biochemical parameters and intestinal morphology of broilers. J. Appl. Anim. Res. 2021;49:15–22. [Google Scholar]
- Jha R., Singh A.K., Yadav S., Berrocoso J.F.D., Mishra B. Early nutrition programming (in ovo and post-hatch feeding) as a strategy to modulate gut health of poultry. Front. Vet. Sci. 2019;6:82. doi: 10.3389/fvets.2019.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J., Lee J.J., Cho J.H., Choe J., Kyoung H., Kim S.H., Kim H.B., Song M. Effects of dietary inactivated probiotics on growth performance and immune responses of weaned pigs. J. Anim. Sci. Technol. 2021;63:520–530. doi: 10.5187/jast.2021.e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kers J.G., Velkers F.C., Fischer E.A.J., Hermes G.D.A., Stegeman J.A., Smidt H. Host and environmental factors affecting the intestinal microbiota in chickens. Front. Microbiol. 2018;9:235. doi: 10.3389/fmicb.2018.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Cho J.H., Kim H.B., Song M. Evaluation of brown rice to replace corn in weanling pig diet. J. Anim. Sci. Technol. 2021;63:1344–1354. doi: 10.5187/jast.2021.e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K., Kim B., Kyoung H., Liu Y., Campbell J., Song M., Ji P. Dietary spray-dried plasma supplementation in late-gestation and lactation enhanced productive performance and immune responses of lactating sows and their litters. J. Anim. Sci. Technol. 2021;63:1076–1085. doi: 10.5187/jast.2021.e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E., Kyoung H., Hyung Koh N., Lee H., Lee S., Kim Y., il Park K., Min Heo J., Song M. Supplementation of live yeast culture modulates intestinal health, immune responses, and microbiota diversity in broiler chickens. J. Anim. Sci. 2022;100:skac122. doi: 10.1093/jas/skac122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovatcheva-Datchary P., Nilsson A., Akrami R., Lee Y.S., de Vadder F., Arora T., Hallen A., Martens E., Björck I., Bäckhed F. Dietary fiber-induced improvement in glucose metabolism is associated with increased abundance of Prevotella. Cell. Metab. 2015;22:971–982. doi: 10.1016/j.cmet.2015.10.001. [DOI] [PubMed] [Google Scholar]
- Lee J.J., Kyoung H., Cho J.H., Choe J., Kim Y., Liu Y., Kang J., Lee H., Kim H.B., Song M. Dietary yeast cell wall improves growth performance and prevents of diarrhea of weaned pigs by enhancing gut health and anti-inflammatory immune responses. Animals. 2021;11:2269. doi: 10.3390/ani11082269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levkut M., Revajová V., Lauková A., Ševčíková Z., Spišáková V., Faixová Z., Levkutová M., Strompfová V., Pistl J., Levkut M. Leukocytic responses and intestinal mucin dynamics of broilers protected with Enterococcus faecium EF55 and challenged with Salmonella Enteritidis. Res. Vet. Sci. 2012;93:195–201. doi: 10.1016/j.rvsc.2011.06.021. [DOI] [PubMed] [Google Scholar]
- Ley R.E. Gut microbiota in 2015: Prevotella in the gut: choose carefully. Nat. Rev. Gastroenterol. Hepatol. 2016;13:69–70. doi: 10.1038/nrgastro.2016.4. [DOI] [PubMed] [Google Scholar]
- Li X.H., Chen Y.P., Cheng Y.F., Yang W.L., Wen C., Zhou Y.M. Effect of yeast cell wall powder with different particle sizes on the growth performance, serum metabolites, immunity and oxidative status of broilers. Anim. Feed Sci. Technol. 2016;212:81–89. [Google Scholar]
- Liu N., Wang J.Q., Jia S.C., Chen Y.K., Wang J.P. Effect of yeast cell wall on the growth performance and gut health of broilers challenged with aflatoxin B1 and necrotic enteritis. Poult. Sci. 2018;97:477–484. doi: 10.3382/ps/pex342. [DOI] [PubMed] [Google Scholar]
- Liu Y., Wu Q., Wu X., Algharib S.A., Gong F., Hu J., Luo W., Zhou M., Pan Y., Yan Y.Y., Wang Y. Structure, preparation, modification, and bioactivities of β-glucan and mannan from yeast cell wall: a review. Int. J. Biol. Macromol. 2021;173:445–456. doi: 10.1016/j.ijbiomac.2021.01.125. [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Magoč T., Salzberg S.L. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011;27:2957–2963. doi: 10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiorka A., Fabiano D., de Azevedo Morgulis M.S.F. Broiler adaptation to post-hatching period. Ciência Rural. 2006;36:701–708. [Google Scholar]
- Megahed A., Zeineldin M., Evans K., Maradiaga N., Blair B., Aldridge B., Lowe J. Impacts of environmental complexity on respiratory and gut microbiome community structure and diversity in growing pigs. Sci. Rep. 2019;9:13773. doi: 10.1038/s41598-019-50187-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- M'sadeq S.A., Wu S.B., Choct M., Forder R., Swick R.A. Use of yeast cell wall extract as a tool to reduce the impact of necrotic enteritis in broilers. Poult. Sci. 2015;94:898–905. doi: 10.3382/ps/pev035. [DOI] [PubMed] [Google Scholar]
- Mun D., Kyoung H., Kong M., Ryu S., Jang K.B., Baek J., il Park K., Song M., Kim Y. Effects of Bacillus-based probiotics on growth performance, nutrient digestibility, and intestinal health of weaned pigs. J. Anim. Sci. Technol. 2021;63:1314–1327. doi: 10.5187/jast.2021.e109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthusamy N., Haldar S., Ghosh T.K., Bedford M.R. Effects of hydrolysed Saccharomyces cerevisiae yeast and yeast cell wall components on live performance, intestinal histo-morphology and humoral immune response of broilers. Br. Poult. Sci. 2011;52:694–703. doi: 10.1080/00071668.2011.633072. [DOI] [PubMed] [Google Scholar]
- Parkin J., Cohen B. An overview of the immune system. Lancet. 2001;357:1777–1789. doi: 10.1016/S0140-6736(00)04904-7. [DOI] [PubMed] [Google Scholar]
- Pascual A., Pauletto M., Giantin M., Radaelli G., Ballarin C., Birolo M., Zomeño C., Dacasto M., Bortoletti M., Vascellari M., Xiccato G., Trocino A. Effect of dietary supplementation with yeast cell wall extracts on performance and gut response in broiler chickens. J. Anim. Sci. Biotechnol. 2020;11:1–11. doi: 10.1186/s40104-020-00448-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese A.T., Dunn R.R. Drivers of microbiome biodiversity: a review of general rules, feces, and ignorance. mBio. 2018;9 doi: 10.1128/mBio.01294-18. e01294-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisinger N., Ganner A., Masching S., Schatzmayr G., Applegate T.J. Efficacy of a yeast derivative on broiler performance, intestinal morphology and blood profile. Livest. Sci. 2012;143:195–200. [Google Scholar]
- Royan M. The use of enterococci as probiotics in poultry. Iran J. Appl. Anim. Sci. 2018;8:559–565. [Google Scholar]
- Sandvang D., Skjoet-Rasmussen L., Cantor M.D., Mathis G.F., Lumpkins B.S., Blanch A. Effects of feed supplementation with 3 different probiotic Bacillus strains and their combination on the performance of broiler chickens challenged with Clostridium perfringens. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab C., Gänzle M. Lactic acid bacteria fermentation of human milk oligosaccharide components, human milk oligosaccharides and galactooligosaccharides. FEMS Microbiol. Lett. 2011;315:141–148. doi: 10.1111/j.1574-6968.2010.02185.x. [DOI] [PubMed] [Google Scholar]
- Shang Y., Kumar S., Oakley B., Kim W.K. Chicken gut microbiota: importance and detection technology. Front. Vet. Sci. 2018;5:254. doi: 10.3389/fvets.2018.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M., Kim B., Cho J.H., Kyoung H., Park S., Cho J.Y., il Park K., Kim H.B., Lee J.J. Effects of dietary protease supplementation on growth rate, nutrient digestibility, and intestinal morphology of weaned pigs. J. Anim. Sci. Technol. 2022;64:462–470. doi: 10.5187/jast.2022.e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spring P., Wenk C., Dawson K.A., Newman K.E. The effects of dietary mannanoligosaccharides on cecal parameters and the concentrations of enteric bacteria in the ceca of Salmonella-challenged broiler chicks. Poult. Sci. 2000;79:205–211. doi: 10.1093/ps/79.2.205. [DOI] [PubMed] [Google Scholar]
- Stanley D., Hughes R.J., Moore R.J. Microbiota of the chicken gastrointestinal tract: influence on health, productivity and disease. Appl. Microbiol. Biotechnol. 2014;98:4301–4310. doi: 10.1007/s00253-014-5646-2. [DOI] [PubMed] [Google Scholar]
- Strompfová V., Lauková A. In vitro study on bacteriocin production of Enterococci associated with chickens. Anaerobe. 2007;13:228–237. doi: 10.1016/j.anaerobe.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Wang X., Farnell Y.Z., Peebles E.D., Kiess A.S., Wamsley K.G.S., Zhai W. Effects of prebiotics, probiotics, and their combination on growth performance, small intestine morphology, and resident Lactobacillus of male broilers. Poult. Sci. 2016;95:1332–1340. doi: 10.3382/ps/pew030. [DOI] [PubMed] [Google Scholar]
- Wei S., Morrison M., Yu Z. Bacterial census of poultry intestinal microbiome. Poult. Sci. 2013;92:671–683. doi: 10.3382/ps.2012-02822. [DOI] [PubMed] [Google Scholar]
- Xia Y., Miao J., Zhang Y., Zhang H., Kong L., Seviour R., Kong Y. Dietary inulin supplementation modulates the composition and activities of carbohydratemetabolizing organisms in the cecal microbiota of broiler chickens. PLoS One. 2021;16 doi: 10.1371/journal.pone.0258663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue G.d.a., Wu S.B., Choct M., Swick R.A. Effects of yeast cell wall on growth performance, immune responses and intestinal short chain fatty acid concentrations of broilers in an experimental necrotic enteritis model. Anim. Nutr. 2017;3:399–405. doi: 10.1016/j.aninu.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D., Turner J.R. Stimulus-induced reorganization of tight junction structure: the role of membrane traffic. Biochim. Biophys. Acta Biomembr. 2008;1778:709–716. doi: 10.1016/j.bbamem.2007.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]