Abstract
Introduction
This study aims to describe the demographic, clinical, laboratory, and ultrasonic characteristics of patients with psoriatic arthritis (PsA) in the Psoriatic Arthritis cohort of West China Hospital.
Methods
In this cross-sectional study, we included patients diagnosed with PsA according to the Classification Criteria for Psoriatic Arthritis, collected their demographic information, medical histories, and treatments, evaluated all domains (skin and nail lesions, tenderness, swelling, enthesitis, dactylitis, and axial arthritis) related to PsA, and then performed descriptive statistical analyses of all data.
Results
A total of 275 patients with PsA were included in this study. The ratio of male to female patients was 2.16:1. Skin lesions preceded arthritis in 86.5% of these patients with PsA with a mean interval of 10.1 years. The metacarpophalangeal (MCP) joints, proximal interphalangeal (PIP) joints of fingers, and sacroiliac joints are the most commonly involved sites of tenderness, swelling, and the spine, respectively. Among all comorbidities, fatty liver has the highest incidence with 33.1%. Finally, we noted that the mean disease duration of PsA was 4.2 years, suggesting a delay in the diagnosis of PsA.
Conclusion
Our study proposes that the prevalent population of PsA are male patients with psoriasis over 40 years of age who have a long disease course. For patients with PsA, MCP, PIP joints of fingers, and sacroiliac joints are the most frequently affected anatomical sites. With respect to comorbidities, the association between PsA and fatty liver and the underlying molecular mechanisms are worthy of further exploration.
Keywords: Psoriasis, Psoriatic arthritis, Clinical study, China
Key Summary Points
| Why carry out this study? | |
| A thorough grasp of the clinical characteristics of PsA is urgently required to direct physicians in accurately appreciating the assessment strategies of PsA and further promoting early diagnosis. | |
| This study aims to describe the demographic, clinical, laboratory, and ultrasonic characteristics of patients with PsA in a Chinese center. | |
| What was learned from the study? | |
| Our study proposes that the prevalent population of PsA are male patients with psoriasis over 40 years of age who have a long disease course. | |
| The metacarpophalangeal joints, proximal interphalangeal joints of fingers, and sacroiliac joints are the most frequently affected anatomical sites of patients with PsA. | |
| The association between PsA and fatty liver and the underlying molecular mechanisms are worthy of further exploration. |
Introduction
Psoriatic arthritis (PsA) is a chronic inflammatory musculoskeletal disorder with various prevalence rates in different regions, affecting approximately 6–41% of individuals in the psoriatic population [1]. The clinical manifestations of PsA are complex and involve both peripheral and axial joints characterized by synovitis, enthesitis, dactylitis, and sacroiliitis [2]. Along with arthritis, patients frequently experience extra-articular manifestations such as psoriatic skin lesions and nail psoriasis [2]. Moreover, some patients may also have comorbidities such as cardiovascular disease, metabolic diseases, fatty liver, anxiety, and depression, which significantly burden them [3].
Patients with PsA exhibit highly heterogeneous clinical manifestations that span a wide range of domains, so understanding the severity of PsA requires a thorough evaluation of pain, swelling, function, and disease activities of the peripheral and axial joints, assessment of psoriatic lesions and nails, and laboratory and imaging examinations, which are highly challenging [4]. However, a complete assessment of the patient with PsA is recommended before choosing treatment measures, which may be beneficial in helping the patient to reap better outcomes [5]. Therefore, there is an urgent for a comprehensive understanding of the clinical features of PsA to guide clinicians in precisely comprehending the assessment strategies of PsA and further facilitate early diagnosis and individualized treatment approaches. Although the clinical characteristics of PsA have been summarized in several cohorts [6–10] from various countries since the 1970s, the clinical variables included in different studies are heterogeneous and limited, necessitating further information from other cohorts. Therefore, this study aims to describe the demographic, clinical, laboratory, and ultrasonic characteristics of patients with PsA in a Chinese center, further completing this field.
Methods
Patient Enrollment and Study Design
From April 2020 to October 2022, all patients who came from the Department of Dermatology, West China Hospital, Sichuan University, and met the Classification Criteria for Psoriatic Arthritis (CASPAR) [11] with confirmed PsA diagnosis were enrolled in our PsA cohort, namely the Psoriatic Arthritis cohort of West China Hospital (PARWCH). We conducted this cross-sectional study using baseline data of these patients in the cohort.
The study was approved by the ethics committee of West China Hospital, Sichuan University (approval number 2022(1842)) and performed in accordance with the Helsinki Declaration of 1964, and its later amendments. Each participating patient signed an informed consent form. No identifying information of participants was included in the manuscript.
Data Collection and Clinical Evaluation
All patients in the cohort were interviewed and examined in detail, and a variety of data were collected, including demographics, medical histories, laboratory indexes, imaging findings, personal and family histories, comorbidities, extra-articular manifestations, and treatments.
Regarding the medical histories, we focused on the age at onset of skin lesions and arthritis and the disease duration of psoriasis and PsA. The disease duration of PsA means the interval between the onset of musculoskeletal disease and the time of the diagnosis. We calculated the percentage of patients with various onset orders. Co-occurrence of psoriatic lesions and arthritis means that the interval between the two is less than 1 year. Additionally, we divided the skin lesions of patients into plaque, erythrodermic, and pustular subtypes and used the Psoriasis Area and Severity Index (PASI) and Body Surface Area (BSA) to determine the severity of plaque psoriasis. For patients with nail involvement, we divided the nail damage into two groups: nail matrix lesions (pitting, leukonychia red spots in the lunula, and crumbling) and nail bed lesions (onycholysis, splinter hemorrhages, subungual hyperkeratosis, and oil drop) [12].
All patients with PsA in PARWCH were classified into three subtypes, namely peripheral type, axial type, and mixed type. We defined subtypes based on clinical symptoms, physical examination, and imaging manifestations. Patients with PsA who have only axial involvement present with inflammatory neck/back pain as well as limited mobility with radiographic sacroiliitis and typical structural changes in the spine (nonmarginal syndesmophytes, fusion of facet joints, bone marrow, soft tissue edema, etc.) which could be detected by CT and MRI [13]. Patients with PsA who have only peripheral involvement frequently exhibit swelling, pain, and stiffness in the peripheral joints, and some patients may show sausage digit (dactylitis), with abnormalities such as synovitis, enthesitis, and bone erosion detected by ultrasound [2]. Patients with mixed subtype have both peripheral and axial involvement. For patients with peripheral arthritis, we performed detailed physical examinations and recorded 66/68-swollen and tender joint counts (SJC66/TJC68). Furthermore, we also recorded the visual analog scale (VAS) scores of pain, patient’s and physician’s global assessment of disease activity, disability index of the health assessment questionnaire (HAQ), and acute phase reactant, including high-sensitivity C-reactive protein (hs-CRP) and erythrocyte sedimentation rate (ESR), which are necessary for the American College of Rheumatology (ACR) scoring system [14]. The Leeds enthesitis index (LEI) and Leeds dactylitis index (LDI) were used to evaluate the conditions of enthesitis and dactylitis in these patients, respectively. Moreover, to characterize the types of peripheral joint lesions, we performed ultrasound examinations for each patient in our cohort. The operations were conducted by three sonographers with more than 5 years of experience in musculoskeletal ultrasound imaging according to the guidelines for musculoskeletal ultrasound examination jointly developed by the American Society of Ultrasound in Medicine (AIUM) [15]. The sonographers examined all target joints, tendons, and bursae in grayscale mode and examined blood flow signals in PD mode, focusing on the changes in synovitis, osteophytes, joint effusion, enthesitis, tenosynovitis, bone erosions, bursitis, and dactylitis in these patients with PsA [16]. For patients with axial arthritis, variables associated with axial activity, including Bath Ankylosing Spondylitis Functional Index (BASFI), Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), and VAS scores of back pains, were assessed and recorded.
Regarding personal and family histories, we gathered information on previous smoking, drinking, surgeries, trauma, infections, and family history of psoriasis and PsA. Moreover, comorbidities and extra-articular manifestations, including hypertension, type 2 diabetes, cardiovascular disease, inflammatory bowel disease, uveitis, fatty liver disease, anxiety, and depression, were also recorded. With respect to treatment, we collected the patients’ previous treatment histories as well as current treatment options, focusing on methotrexate, nonsteroidal anti-inflammatory drugs (NSAIDs), traditional Chinese medicine (TCM), biologicals (interleukin (IL)-17 inhibitors; tumor necrosis factor alpha (TNFα) inhibitors, IL-23 inhibitors), and Janus kinase inhibitors (Jaki).
Statistical Analysis
We performed descriptive statistical analyses of all data. Count (%) was used to express categorical data, and the mean (SD) was used to represent continuous variables. The analyses were performed using R (version 4.0.2), and the ggplot2 package in R was used for plotting.
Results
Demographic and Disease-Related Clinical Information
In this cohort of 275 patients with confirmed PsA, the demographic and disease-related clinical information were well documented and detailed in Table 1. The male/female ratio was 2.16:1, with a mean age of 44.6 (SD 11.9). The median body mass index (BMI) was 24.0 (SD 3.8), and the stratified statistics showed that over half of the patients (164, 59.6%) were considered overweight and generally obese. Given that patients with PsA in this cohort were consulted at the Department of Dermatology, all of them were troubled with psoriatic lesions, and most of them (269, 97.8%) were diagnosed with plaque psoriasis with a mean PASI of 5.8 (SD 7.6). The mean age at onset of the skin lesions was 31.0 (SD 12.5) years, which is younger than that of arthritis (40.4 ± 12.1). Likewise, skin lesions preceded arthritis in 86.5% of these patients with PsA, with a mean interval of 10.1 (SD 8.9) years. Nail lesions including nail matrix and nail bed involvement were reported in 171 patients (62.2%). With respect to comorbid conditions, fatty liver disease, hypertension, anxiety, and type 2 diabetes presented relatively high incidences of 33.1%, 15.3%, 9.1%, and 7.3%, respectively.
Table 2.
Previous and current treatments of patients in PARWCH
| Treatment | N = 275 |
|---|---|
| Previousa | |
| Conventional treatment | |
| Methotrexate, n (%) | 132 (48.0) |
| Acitretin, n (%) | 73 (26.6) |
| Ciclosporin, n (%) | 10 (3.6) |
| Phototherapy, n (%) | 61 (22.2) |
| NSAIDs, n (%) | 45 (16.4) |
| TCM, n (%) | 117 (42.6) |
| Without any conventional treatment, n (%) | 10 (3.6) |
| Biologicals, n (%) | 63 (22.9) |
| IL-17i, n (%) | 23 (8.4) |
| TNFαi, n (%) | 59 (21.5) |
| IL-12/23i & IL-23i, n (%) | 0 (0.0) |
| Currentb | |
| Conventional treatment | |
| Methotrexate, n (%) | 83 (30.2) |
| NSAIDs, n (%) | 30 (10.9) |
| TCM, n (%) | 38 (13.8) |
| Biologicals and small molecule inhibitors, n (%) | 141 (51.3) |
| IL-17i, n (%) | 59 (21.5) |
| TNFαi, n (%) | 77 (28.0) |
| IL-12/23i & IL-23i, count (%) | 3 (1.1) |
| Jaki, n (%) | 2 (0.7) |
PARWCH Psoriatic Arthritis cohort of West China Hospital, NSAIDs nonsteroidal anti-inflammatory drugs, TCM traditional Chinese medicine, including Tripterygium wilfordii, total glucosides of Paeonia and glycyrrhizin, IL-17i interleukin-17 inhibitors, TNFαi tumor necrosis factor-alpha inhibitors, IL-12/23i interleukin-12/23 inhibitors, Jaki Janus kinase inhibitors
aSome patients received multiple therapies in the past
bSome people take several different medication combinations
Table 1.
Demographic and clinical features of patients in PARWCH
| Variable | N = 275 |
|---|---|
| Demographic information | |
| Sex, male to female ratio | 2.16:1 (188:87) |
| Age, years, mean (SD) | 44.6 (11.9) |
| BMI, kg/m2, mean (SD) | 24.0 (3.8) |
| Overweight, 23 ≤ BMI < 27.5, n (%) | 121 (44.0) |
| Grade 1 obesity, 27.5 ≤ BMI < 32.5, n (%) | 39 (14.2) |
| Grade 2 obesity, 32.5 ≤ BMI < 37.5, n (%) | 2 (0.7) |
| Grade 3 obesity, BMI ≥ 37.5, n (%) | 2 (0.7) |
| Disease-related clinical information | |
| Disease onset | |
| Onset of skin lesions, years, mean (SD) | 31.0 (12.5) |
| Disease duration of psoriasis, years, mean (SD) | 13.7 (10.0) |
| Onset of PsA, years, mean (SD) | 40.4 (12.1) |
| Disease duration of PsA, years, mean (SD) | 4.2 (6.6) |
| Skin lesions precede arthritis, n (%) | 238 (86.5) |
| Interval between skin lesions and arthritis, years, mean (SD) | 10.1 (8.9) |
| Skin lesions later than arthritis, n (%) | 20 (7.3) |
| Skin lesions and arthritis occurring simultaneously, n (%) | 17 (6.2) |
| Subtypes of psoriasis | |
| Plaque psoriasis, n (%) | 269 (97.8) |
| PASI, mean (SD) | 5.8 (7.6) |
| BSA, mean (SD) | 8.9 (16.5) |
| Erythrodermic psoriasis, n (%) | 3 (1.1) |
| Pustular psoriasis, n (%) | 3 (1.1) |
| Nail lesions | 171 (62.2) |
| Nail matrix involvement, n (%) | 110 (40.0) |
| Nail bed involvement, n (%) | 156 (56.7) |
| Peripheral joint involvement (n = 252a) | |
| TJCs, mean (SD) | 5.7 (9.2) |
| SJCs, mean (SD) | 2.4 (4.6) |
| Patient’s pain VAS, mean (SD) | 3.8 (2.9) |
| Patient’s disease activity VAS, mean (SD) | 4.5 (2.8) |
| Physician’s disease activity VAS, mean (SD) | 4.0 (2.6) |
| LEI, mean (SD) | 0.4 (1.0) |
| LDI, mean (SD) | 0.3 (1.0) |
| Axial joint involvement (n = 140b) | |
| BASFI, mean (SD) | 0.9 (1.8) |
| BASDAI, mean (SD) | 1.1 (2.1) |
| Patient’s global VAS, mean (SD) | 3.4 (3.3) |
| Back pain VAS, mean (SD) | 1.8 (2.8) |
| Functional and laboratory evaluation | |
| HAQ, mean (SD) | 0.2 (0.5) |
| hs-CRP, mg/L, mean (SD) | 11.1 (21.6) |
| ESR, mean (SD) | 20.9 (23.0) |
| Personal and family history | |
| Smoking history, n (%) | 120 (43.6) |
| Drinking history, n (%) | 103 (37.5) |
| Surgery history, n (%) | 115 (41.8) |
| Trauma history, n (%) | 54 (19.6) |
| Infection history, n (%) | 19 (6.9) |
| Family history of psoriasis, n (%) | 67 (24.4) |
| Family history of PsA, n (%) | 14 (5.1) |
| Comorbid conditions | |
| Hypertension, n (%) | 42 (15.3) |
| Type 2 diabetes, n (%) | 20 (7.3) |
| Cardiovascular disease, n (%) | 7 (2.6) |
| Fatty liver disease, n (%) | 91 (33.1) |
| Anxiety, n (%) | 25 (9.1) |
| Depression, n (%) | 11 (4.0) |
| Extra-articular manifestations | |
| Uveitis, n (%) | 5 (1.8) |
| Inflammatory bowel disease, n (%) | 2 (0.7) |
PARWCH Psoriatic Arthritis cohort of West China Hospital, BMI body mass index, cutoff value of BMI was followed by the BMI typing for Asian populations [23], PsA psoriatic arthritis, PASI psoriasis area and severity index, BSA body surface area, TJC tender joint counts, SJC swollen joint counts, VAS visual analog scale, LEI Leeds enthesitis index, LDI Leeds dactylitis index, BASFI Bath ankylosing spondylitis functional index, BASDAI Bath ankylosing spondylitis disease activity index, HAQ health assessment questionnaire, hs-CRP high-sensitivity C-reactive protein, ESR erythrocyte sedimentation rate
a252 patients include those with peripheral and mixed PsA
b140 patients include those with axial and mixed PsA
Characteristics of Joint Involvement
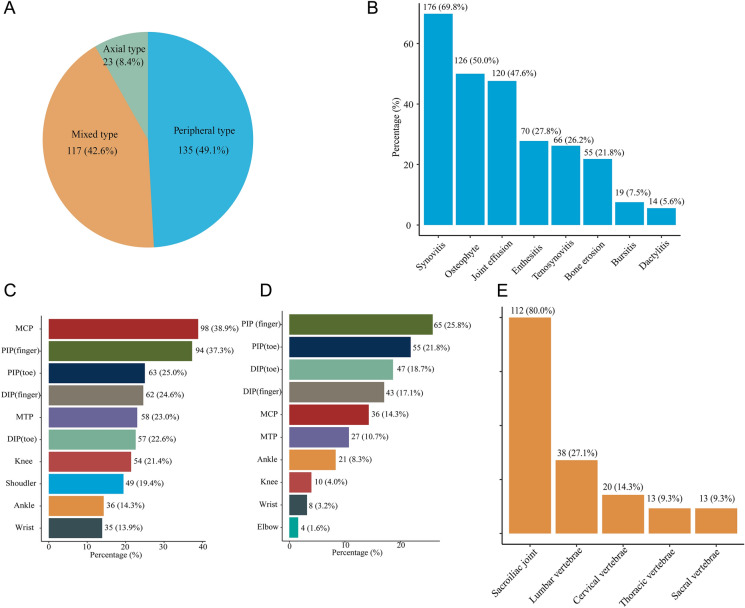
The three subtypes of patients with PsA are shown in the pie diagram (Fig. 1a). All patients with peripheral joint involvement completed the ultrasound assessment, and typical manifestations such as synovitis, enthesitis, bone erosions, and dactylitis were found in 176 (69.8%), 70 (27.8%), 55 (21.8%), and 14 (5.6%) patients, respectively (Fig. 1b). Through physical examination, the mean TJC was 5.7, and the mean SJC was 2.4. The ten most frequently anatomical sites with tenderness and swelling are shown as bar charts in Fig. 1c and d, wherein metacarpophalangeal (MCP) joints and proximal interphalangeal (PIP) joints of fingers rank first, respectively. Subsequently, we also examined enthesitis and dactylitis; the mean LEI was 0.4 (SD 1.0), and the mean LDI was 0.3 (SD 1.0). The mean pain VAS was 3.8 (SD 2.9), and the patients reported higher disease activity VAS than physician (4.5 ± 2.8 vs. 4.0 ± 2.6) (Table 1).
Fig. 1.
Characteristics of joint involvement of patients in PARWCH. a Classification of subtypes in patients with PsA; b ultrasonic changes of peripheral joints; c ten most frequent anatomical sites with tenderness in patients with peripheral arthritis (n = 252, 91.6%); d ten most frequent anatomical sites with swelling in patients with peripheral arthritis (n = 252, 91.6%); e proportion of sites involved in axial arthritis (n = 140, 50.9%); PARWCH the Psoriatic Arthritis cohort of West China Hospital, MCP metacarpophalangeal joints, PIP proximal interphalangeal joints, DIP distal interphalangeal joints, MTP metatarsophalangeal joints
Among patients with axial involvement, the sacroiliac joint (112, 80.0%) was the most frequently detected site, followed by the lumbar vertebrae (38, 27.1%) and cervical vertebrae (20, 14.3%) (Fig. 1e). The mean back pain VAS was 1.8 (SD 2.8). The functional assessment of axial joints was based on BASFI (0.9 ± 1.8). The disease activity assessment, BASDAI, scored 1.1 ± 2.1. Of the 275 patients with PsA, the mean HAQ was 0.2 (SD 0.5), indicating that arthritis had little impact on the lives of the patients in this cohort. Finally, we found that the values of hs-CRP and ESR both increased in patients with PsA.
Previous and Current Therapy
In this cohort, almost all patients with PsA received conventional systematic medication before, and only 10 patients (3.6%) denied ever being treated with this class of medicine. There were 212 biological-naïve patients (77.1%), the remaining 63 patients (22.9%) were biological-experienced, 59 patients (21.5%) had been treated with TNFαi, and 23 patients (8.4%) had been treated with IL-17i. After the diagnosis of PsA was clear, more than half of the patients received biologicals. Seventy-seven patients (28.0%) were treated with TNFαi and 59 patients (21.5%) with IL-17i.
Discussion
In this study, we comprehensively reported the clinical characteristics of patients with PsA and summarized their prevalent subtypes, areas of involvement, and comorbidities.
Our findings demonstrated that men are more likely than women to develop PsA, with a mean peak age of approximately 44 years. The majority of patients initially appear with psoriatic lesions and psoriasis precedes arthritis by an average of 10 years, supporting physicians with a window of opportunity for early screening and therapeutic interventions. These results are consistent with several earlier studies [8, 9] showing that the prevalent population of PsA are male patients with psoriasis over 40 years of age with a long disease duration.
Furthermore, we discovered that peripheral PsA was the most common subtype in this cohort; MCP and PIP joints of fingers were the most frequently affected anatomical sites presenting with tenderness and swelling, respectively. It is noteworthy that this finding can be supported by another Chinese PsA cohort [9]. However, the past view was that distal interphalangeal joint involvement was more specific for PsA and can be utilized as a marker to differentiate PsA from rheumatoid arthritis [17]. Therefore, our findings suggest that it might be more challenging to distinguish between PsA and seronegative rheumatoid arthritis in clinical practice. Moreover, we presented that the most frequently involved axial site was the sacroiliac joint, paralleling the opinion that the sacroiliac joint is one of the most common manifestations of early PsA [13]. Previously, Williamson et al. [18] observed MRI features of sacroiliitis in 38% of unselected patients with PsA. In 2017, Maldonado-Ficco and colleagues [19] evaluated 135 MRI spine scans, and they reported sacroiliac joint involvement in only 24.4% of participants. By comparison, we showed a high proportion of sacroiliac affection because this percentage was based on patients with axial involvement. We ordered radiographic spine examination only for patients with PsA who presented with inflammatory back pain or other suspected symptoms. Moreover, spine joint involvement was defined by combining spine CT and MRI, which are highly complementary in detecting structural changes and inflammatory features, and might enhance the sensitivity and accuracy.
Among all comorbidities included in this study, patients with PsA in our cohort exhibited a high prevalence of fatty liver disease. Notably, it has been demonstrated in both our prior work and the study carried out by Candia and colleagues that non-alcoholic fatty liver disease is quite common in patients with PsA and can be a risk factor for PsA transition [20, 21]. This study further supported this finding, indicating that the relationship between PsA and fatty liver and the underlying molecular mechanisms merit further investigation.
Additionally, we noted that the interval between the onset of musculoskeletal disease and the time of the PsA diagnosis averaged 4.2 years, suggesting that patients’ arthritic symptoms have often been ignored or misdiagnosed over the past several years. As a result of the delayed diagnosis, these patients have not obtained adequate and standardized treatment in the past, as seen by the low usage of NSAIDs, methotrexate, oral small molecule inhibitors, and biologicals. After diagnosis of PsA was clear, the vast majority of patients received the recommended treatments, with the proportion of biologic therapies rising to 51.3%. A study in Ireland [22] found that even a 6-month diagnostic delay contributes to poor radiographic and functional outcomes in PsA, suggesting the importance of accurate and prompt diagnosis for treatment response in patients with PsA.
Our study comprised a thorough analysis of every domain relevant to patients with PsA and laboratory and imaging results, making the data complete and sufficient. However, our study has some limitations. On the one hand, we did not set up controls with other joint diseases or normal people, so our conclusion is descriptive. Given that many of our results are also supported in other cohorts, we believe that these conclusions remain plausible. On the other hand, since this is a single-center study, the conclusions need to be further confirmed by more studies.
Conclusion
The prevalent population of PsA are male patients with psoriasis over 40 years of age who have a long disease course. The three anatomical areas most typically impacted by PsA are the MCP joints, PIP joints of fingers, and sacroiliac joints. Regarding comorbidities, it would be worthwhile to explore the relationship between PsA and fatty liver as well as the underlying molecular pathways.
Acknowledgements
Funding
This study including the Rapid Service Fee was funded by the 135 Project for Disciplines of Excellence, West China Hospital, Sichuan University (ZYJC18003) and Sichuan Provincial Science and Technology Project (2021YFG0306).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Yiyi Wang, Yue Xiao and Furong Li. All authors contributed to drafting and critical revision of the manuscript. All authors reviewed and approved the final version of the manuscript.
Medical Writing, Editorial, and Other Assistance
Ultrasound examinations were provided by Dr. Yuanjiao Tang, Dr. Ling Zhong and Dr. Lingyan Zhang who are from the Department of Ultrasound in West China Hospital of Sichuan University.
Disclosures
Yiyi Wang, Yue Xiao, Furong Li, Yuanxia Gu, Min Yang, Lingyan Zhang, Jing Tang, and Wei Li have nothing to disclose.
Compliance with Ethics Guidelines
The study was approved by the ethics committee of West China Hospital, Sichuan University (approval number 2022(1842)) and performed in accordance with the Helsinki Declaration of 1964, and its later amendments. Each participating patient signed an informed consent form. No identifying information of participants was included in the manuscript.
Data Availability
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Footnotes
Yiyi Wang, Yue Xiao, and Furong Li contributed equally to this work.
References
- 1.Ogdie A, Weiss P. The epidemiology of psoriatic arthritis. Rheum Dis Clin North Am. 2015;41(4):545–568. doi: 10.1016/j.rdc.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ritchlin CT, Colbert RA, Gladman DD. Psoriatic arthritis. N Engl J Med. 2017;376(10):957–970. doi: 10.1056/NEJMra1505557. [DOI] [PubMed] [Google Scholar]
- 3.Perez-Chada LM, Merola JF. Comorbidities associated with psoriatic arthritis: review and update. Clin Immunol. 2020;214:108397. doi: 10.1016/j.clim.2020.108397. [DOI] [PubMed] [Google Scholar]
- 4.Mease PJ. Measures of psoriatic arthritis: Tender and Swollen Joint Assessment, Psoriasis Area and Severity Index (PASI), Nail Psoriasis Severity Index (NAPSI), Modified Nail Psoriasis Severity Index (mNAPSI), Mander/Newcastle Enthesitis Index (MEI), Leeds Enthesitis Index (LEI), Spondyloarthritis Research Consortium of Canada (SPARCC), Maastricht Ankylosing Spondylitis Enthesis Score (MASES), Leeds Dactylitis Index (LDI), Patient Global for Psoriatic Arthritis, Dermatology Life Quality Index (DLQI), Psoriatic Arthritis Quality of Life (PsAQOL), Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F), Psoriatic Arthritis Response Criteria (PsARC), Psoriatic Arthritis Joint Activity Index (PsAJAI), Disease Activity in Psoriatic Arthritis (DAPSA), and Composite Psoriatic Disease Activity Index (CPDAI) Arthritis Care Res (Hoboken) 2011;63(Suppl 11):S64–85. doi: 10.1002/acr.20577. [DOI] [PubMed] [Google Scholar]
- 5.Coates LC, Soriano ER, Corp N, et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA): updated treatment recommendations for psoriatic arthritis 2021. Nat Rev Rheumatol. 2022;18(8):465–479. doi: 10.1038/s41584-022-00798-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gladman DD, Shuckett R, Russell ML, Thorne JC, Schachter RK. Psoriatic arthritis (PSA)–an analysis of 220 patients. QJ Med. 1987;62(1987):127–41. [PubMed] [Google Scholar]
- 7.Kammer GM, Soter NA, Gibson DJ, Schur PH. Psoriatic arthritis: a clinical, immunologic and HLA study of 100 patients. Semin Arthritis Rheum. 1979;9(2):23. doi: 10.1016/S0049-0172(79)80001-3. [DOI] [PubMed] [Google Scholar]
- 8.Torre Alonso JC, Rodriguez Perez A, Arribas Castrillo JM, Ballina Garcia J, Riestra Noriega JL, Lopez Larrea C. Psoriatic arthritis (PA): a clinical, immunological and radiological study of 180 patients. Br J Rheumatol. 1991;30(4):6. doi: 10.1093/rheumatology/30.4.245. [DOI] [PubMed] [Google Scholar]
- 9.Song Z, Deng X, Xie W, Li B, Zhang Z. Clinical characteristics of psoriatic arthritis in Chinese patients: a cross-sectional study. Rheumatol Ther. 2021;8(4):1845–1857. doi: 10.1007/s40744-021-00384-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohara Y, Kishimoto M, Takizawa N, et al. Prevalence and clinical characteristics of psoriatic arthritis in Japan. J Rheumatol. 2015;42(8):1439–1442. doi: 10.3899/jrheum.141598. [DOI] [PubMed] [Google Scholar]
- 11.Taylor W, Gladman D, Helliwell P, et al. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum. 2006;54(8):2665–2673. doi: 10.1002/art.21972. [DOI] [PubMed] [Google Scholar]
- 12.Rich P, Scher RK. Nail psoriasis severity index: a useful tool for evaluation of nail psoriasis. J Am Acad Dermatol. 2003;49(2):206–212. doi: 10.1067/S0190-9622(03)00910-1. [DOI] [PubMed] [Google Scholar]
- 13.Poddubnyy D, Jadon DR, Van den Bosch F, Mease PJ, Gladman DD. Axial involvement in psoriatic arthritis: an update for rheumatologists. Semin Arthritis Rheum. 2021;51(4):880–887. doi: 10.1016/j.semarthrit.2021.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Felson DT, Anderson JJ, Boers M, et al. American College of Rheumatology preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheumat. 1995;38(6):727–735. doi: 10.1002/art.1780380602. [DOI] [PubMed] [Google Scholar]
- 15.American College of Radiology (ACR), Society for Pediatric Radiology (SPR), Society of Radiologists in Ultrasound (SRU). AIUM practice guideline for the performance of a musculoskeletal ultrasound examination. J Ultrasound Med. 2012;31(9):16. [DOI] [PubMed]
- 16.Wang Y, Zhang L, Yang M, et al. Development of a predictive model for screening patients with psoriasis at increased risk of psoriatic arthritis. Dermatol Ther (Heidelb) 2022;12(2):419–433. doi: 10.1007/s13555-021-00663-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merola JF, Espinoza LR, Fleischmann R. Distinguishing rheumatoid arthritis from psoriatic arthritis. RMD Open. 2018;4(2):e000656. doi: 10.1136/rmdopen-2018-000656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williamson L, Dockerty JL, Dalbeth N, McNally E, Ostlere S, Wordsworth BP. Clinical assessment of sacroiliitis and HLA-B27 are poor predictors of sacroiliitis diagnosed by magnetic resonance imaging in psoriatic arthritis. Rheumatol (Oxf) 2004;43(1):85–88. doi: 10.1093/rheumatology/keg475. [DOI] [PubMed] [Google Scholar]
- 19.Maldonado-Ficco H, Sheane BJ, Thavaneswaran A, Chandran V, Gladman DD. Magnetic resonance imaging in psoriatic arthritis: a descriptive study of indications, features and effect on treatment change. J Clin Rheumatol. 2017;23(5):243–245. doi: 10.1097/RHU.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Ding L, Chen J, et al. Risk factors for progression from subclinical to clinical phase of psoriatic arthritis: a case-control study. Rheumatol Ther. 2021;8(1):585–597. doi: 10.1007/s40744-021-00295-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Candia R, Ruiz A, Torres-Robles R, Chavez-Tapia N, Mendez-Sanchez N, Arrese M. Risk of non-alcoholic fatty liver disease in patients with psoriasis: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2015;29(4):656–662. doi: 10.1111/jdv.12847. [DOI] [PubMed] [Google Scholar]
- 22.Haroon M, Gallagher P, FitzGerald O. Diagnostic delay of more than 6 months contributes to poor radiographic and functional outcome in psoriatic arthritis. Ann Rheum Dis. 2015;74(6):1045–1050. doi: 10.1136/annrheumdis-2013-204858. [DOI] [PubMed] [Google Scholar]
- 23.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–63. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.