Learning objectives.
By reading this article, you should be able to:
-
•
Describe the burden of mitral valve regurgitation.
-
•
Discuss the haemodynamic goals for managing patients with severe mitral regurgitation.
-
•
Explain the key procedural steps for transcatheter edge-to-edge repair of the mitral valve.
-
•
Detail the anaesthetist's interventions that affect the ease of the procedure.
-
•
Identify patients eligible for fast-track care and those who required prolonged monitoring.
Key points.
-
•
Mitral regurgitation is common and the use of transcatheter techniques for mitral repair is increasing.
-
•
Anaesthetists are key members of structural heart teams.
-
•
Manipulation of cardiac physiology to decrease mitral annular size and increase leaflet coaptation can improve procedural ease during transcatheter mitral repair.
-
•
Anaesthetic techniques for transcatheter mitral valve repair should be chosen to facilitate rapid recovery.
Mitral regurgitation (MR) is common, with a prevalence of moderate or severe MR reaching 10% at age 75 yrs.1 Mitral regurgitation may relate to structural abnormalities of the valve itself (primary MR) or result from changes in the size and geometry of the left ventricle (secondary MR). Patients with severe MR are limited by dyspnoea and fatigue, and can develop overt heart failure. Severe MR results in higher-than-expected healthcare use, with up to 50% more heart failure admissions per year and excess mortality of 6.3% per year.2,3
Traditionally, patients with severe, symptomatic MR have been offered surgical valve replacement or repair. However, increasingly patients are being treated with transcatheter valve repair. In this article we discuss the management of patients undergoing transcatheter repair of the mitral valve (MV).
Indications and contraindications
A transcatheter approach to repair may be chosen in patients whose valvular anatomy is favourable for transcatheter repair and for patients at high surgical risk.4,5 Patients with severe primary MR and high operative risk can generally proceed directly to transcatheter repair. Patients with severe secondary MR should first receive optimal medical therapy for heart failure and management of concomitant coronary artery disease. Then, if symptoms of heart failure persist and MR remains severe, patients with secondary MR can be offered transcatheter MV repair. With increasing clinical experience, the anatomical features for transcatheter repair eligibility have expanded. However, certain anatomical features are relative contraindications to transcatheter repair (Table 1).
Table 1.
Relative contraindications for transcatheter edge-to-edge repair of the mitral valve. Expanded eligibility criteria for transcatheter edge-to-edge repair is far more permissive than suggested in initial MitraClip trials.
| Valve features | Valve area <3 cm Leaflet length <6 mm Mean pressure gradient >5 mmHg Calcification at grasping area Thrombus or mass on valve or annulus Valve perforation Leaflet cleft with significant regurgitation Regurgitation at commissure |
| Atrial access | Inability to achieve >35 mm above mitral valve on intra-atrial septum Very small left atrium |
Devices and evidence for transcatheter MV repair
The success of transcatheter aortic valve implantation has stimulated the development of transcatheter approaches to the MV. Although several devices are available for transcatheter MV repair (Table 2), the dominant approach is edge-to-edge repair, whereby clips are placed on the free edge of the mitral leaflets.6 In this review, we focus on the management of patients undergoing transcatheter edge-to-edge repair.
Table 2.
Transcatheter mitral valve repair devices that have received the Conformité Européenne mark. The MitraClip and PASCAL are the only two devices with approval from the US Food and Drug Administration.
| Repair strategy | Device | Manufacturer | Delivery to mitral valve |
|---|---|---|---|
| Edge-to-edge repair | MitraClip | Abbott | Femoral vein, transseptal |
| PASCAL | Edwards Lifesciences | Femoral vein, transseptal | |
| Annuloplasty | Cardioband | Edwards Lifesciences | Femoral vein, coronary sinus, transseptal |
| Carillon Mitral Contour | Cardiac Dimensions | Jugular vein, coronary sinus | |
| Chordae | NeoChord | NeoChord | Transapical |
| HARPOON | Edwards Lifesciences | Transapical |
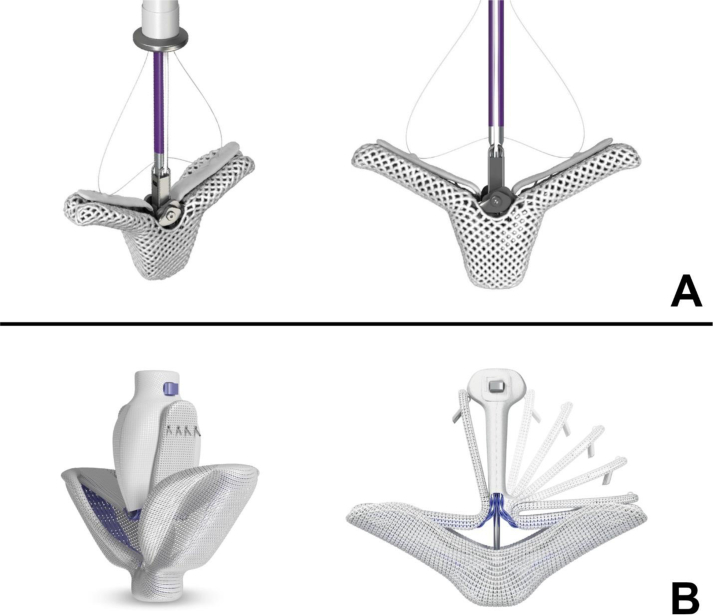
Two devices for transcatheter edge-to-edge repair have been approved by the US Food and Drug Administration, the MitraClip (Abbott Laboratories, Abbott Park, IL, USA) and PASCAL (Edwards Lifesciences, Irvine, CA, USA). The MitraClip device is the most used and best studied, whereas experience with the PASCAL device is rapidly increasing (Fig 1). The Endovascular Valve Edge-to-Edge Repair Study (EVEREST) II trial compared surgical and transcatheter MV repair and demonstrated transcatheter repair was associated with fewer adverse events but a smaller reduction in MR compared with surgery.7 Subgroup analysis of the EVEREST II trial suggested transcatheter MV repair was as effective as surgery in older patients with secondary MR, which led to two subsequent randomised trials comparing transcatheter repair with medical therapy, Mitra-FR and Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients With Functional Mitral Regurgitation (COAPT).8,9 Mitra-FR showed no difference in hospitalisation or mortality at 1 yr year whereas COAPT showed a 42.1% per year decrease in hospitalisations and a 17% decrease in death at 2 yrs with transcatheter repair.8,9 The discrepant results from the two trials may reflect differences in the patients studied and the more aggressive treatment of MR in the COAPT trial.10
Fig 1.
Currently approved devices for transcatheter edge-to-edge mitral valve repair. (A) MitraClip G4 device by Abbot. (B) PASCAL device by Edwards Lifesciences. Images used with permission.
Initially, transcatheter MV repair was limited to older and high-risk patients with secondary MR. However, it has subsequently been shown that high-risk patients with primary MR can also be treated successfully and obtain durable repairs.11 Moderate risk patients are being enrolled in the Percutaneous MitraClip Device or Surgical Mitral Valve REpair in PAtients with PrImaRy Mitral Regurgitation who are Candidates for Surgery (REPAIR MR) trial (ClinicalTrials.gov Identifier NCT04198870).
Preparation for procedure
An expert heart team consisting of a structural interventional cardiologist, a heart surgeon skilled in mitral repair, an echocardiographer and an anaesthetist with expertise in anaesthesia for structural heart procedures should review all patients being considered for transcatheter MV repair. In our institution, the roles of anaesthetist and echocardiographer are combined.
Patients presenting for transcatheter MV repair are typically frail, older, and have coexisting conditions. Therefore, careful preoperative assessment is essential. In addition to the usual considerations, the anaesthetist should focus on left ventricular (LV) size and function. Patients with low LV ejection fraction (LVEF <40%) are at risk of afterload mismatch after the procedure, whereby correction of MR – with consequent increase in LV afterload – leads to a further decrease in LVEF. The risk of afterload mismatch in patients with low LVEF is less after transcatheter repair compared with surgical repair, as the goal with transcatheter repair is to reduce – not eliminate – MR.
Patients with severe MR frequently have coexisting pulmonary hypertension and tricuspid regurgitation. Neither condition, including severe pulmonary hypertension (systolic pressure >50 mmHg), appear to be associated with adverse short-term outcome after transcatheter repair.12,13 Therefore, preprocedural interventions targeting these conditions are unlikely to improve the outcome from transcatheter repair.
Our practice is to continue β-blockers, antiarrhythmics, antiplatelets and diuretics. We stop warfarin for 5 days and direct-acting anticoagulants (e.g. dabigatran, rivaroxaban) for 2 days before the procedure. We leave pacemakers and internal cardioverter–defibrillators in their usual settings but have the devices checked by a pacemaker technician to verify proper functioning after the procedure.
Unlike transcatheter aortic valve replacement, discussion regarding emergency cardiac surgery is rarely undertaken before the procedure, as the need for emergency cardiac surgery is exceedingly rare.
Management
Transcatheter MV repair is performed either in a cardiac catheterisation laboratory or – more commonly nowadays – a hybrid operating room. Transcatheter MV repair requires the simultaneous use of both fluoroscopy and transoesophageal echocardiography (TOE). To facilitate TOE, patients typically require general anaesthesia with tracheal intubation. Although there have been cases of MitraClip implantation performed using either a combination of transthoracic and intracardiac echocardiography or with TOE, using only topical anaesthesia of the oropharynx, these approaches are not routine.14
Patients are positioned supine on the fluoroscopy table with their arms by their side. Equipment, drapes and additional personnel (e.g. an echocardiographer) limit access to the patient during the procedure. Therefore, it is essential that monitoring devices and vascular access lines are carefully secured. When TOE imaging is difficult, wedging the patient in the left or right partial decubitus position may improve image quality.
Principles of anaesthesia management
Owing to frailty and advanced age, patients undergoing transcatheter MV repair are at high risk of developing post-procedural delirium. To help mitigate this risk, our practice for inducing anaesthesia is to use a combination of propofol (0.5–1.5 mg kg−1), remifentanil (0.5–1 μg kg−1) and rocuronium (0.6 mg kg−1). We maintain anaesthesia with an infusion of remifentanil (0.1–0.2 μg kg−1 min−1) in combination with an inhaled volatile anaesthetic agent or a target-controlled infusion of propofol. To facilitate rapid emergence and minimise the risk of delirium, we avoid using benzodiazepines.
Haemodynamic goals during the procedure include maintaining cardiac output while avoiding exacerbating MR. Decreased LV afterload owing to general anaesthesia helps maintain cardiac output and reduces the severity of MR. Hypotension may be treated with an i.v. β-agonist (e.g. ephedrine 5–10 mg). Vasopressors (e.g. phenylephrine) have the potential to reduce cardiac output and exacerbate MR. Similarly, i.v. fluid loading can exacerbate MR. Bradycardia (HR <60 min−1) decreases cardiac output and should be avoided. Remifentanil-induced bradycardia can be treated by stopping the infusion or by giving an anticholinergic drug (e.g. glycopyrrolate 200–300 μg as an i.v. bolus). Chronic atrial fibrillation is common in patients with severe MR, and fast ventricular rates should be treated (e.g. amiodarone 150–300 mg i.v.) to optimise cardiac output and to facilitate leaflet grasping during the procedure. After TOE examination of the left atrial appendage to exclude thrombus, cardioversion can be considered, as sinus rhythm increases cardiac output and facilitates leaflet grasping by the device.
In addition to routine monitors, an intra-arterial catheter is appropriate, as it provides continuous blood pressure monitoring and allows blood sampling for checking the activated clotting time (ACT). A central venous catheter is rarely necessary but may be appropriate for patients with limited peripheral venous access or in those with impaired LV function (LVEF <40%). Occasionally, the intracardiac wires and catheters used during the procedure can cause malignant arrythmias. Therefore, external defibrillator pads should be placed in all patients. A urinary catheter is not routinely indicated. Measurement of left atrial (LA) pressure directly with the valve repair delivery system provides additional information on the patient's haemodynamic state. Changes in the v-wave on the LA pressure trace may provide useful information on dynamic changes in MR severity, which can then be further assessed with TOE.
Haemodynamic and ventilatory interventions that affect procedural ease
Haemodynamic interventions can affect the ease with which the proceduralist can perform the procedure. When the LV is dilated, the gap between the leaflets at end-systole (coaptation gap) increases, particularly in patients with secondary MR. Interventions that reduce LV size make it easier to grasp the leaflets and place clips in an optimal position. Administering diuretics or commencing inotropes at the time of anaesthetic induction provides sufficient time for these agents to decrease LA and LV end-diastolic pressure, thereby decreasing LV size and helping to reduce the coaptation gap.
Furosemide (20–40 mg i.v.) results in a rapid, brisk diuresis, and is particularly helpful in patients with heart failure who have intravascular fluid overload and high LV filling pressures. Furosemide also helps mitigate the effects of the i.v. fluid patients receive from the catheter flushing system, which may exceed 2 L. Infusions of dobutamine (2–5 μg kg−1 min−1) or milrinone (0.05–0.5 μg kg−1 min−1) are suitable inotropic drugs and have the advantage of being able to be given safely via a peripheral i.v. catheter. In addition to reducing LV size (and therefore the coaptation gap), inotropic drugs also help mitigate afterload mismatch in patients with impaired LV systolic function.15 Although these agents (diuretics and inotropes) are useful in selected patients – particularly those with impaired LV systolic function (LVEF <40%) and elevated LA pressure (<20 mmHg) – their use not been studied sufficiently to show an effect on the success of the procedure.
Moderate PEEP (5–10 cmH2O) reduces systemic venous return, which may decrease the coaptation gap, increase available coaptation length and help with leaflet grasping with the device. However, high PEEP can reduce cardiac output, particularly in patients with impaired ventricular function. Changes to the patient's tidal volume and PEEP should not be made when the delivery system is only slightly across the atrial septum, as it can cause the device to retract into the right atrium. Ventilatory changes should also be avoided when clips are applied to the MV but remain attached to the delivery system, as this can increase tension and damage the mitral leaflets. Mechanical ventilation with low tidal volumes (3–6 ml kg−1) reduces breath-to-breath movement of the heart and makes it easier to grasp the leaflets with the device. Conversely, large tidal volumes make harder to grasp the leaflets.
The procedure
The anaesthetist must be familiar with the steps of the procedure so as to be able to target anaesthetic and haemodynamic management accordingly. The procedure begins with a transfemoral venous puncture with a small-bore needle and wire insertion. Confirmation of venous puncture can be done with fluoroscopy, surface ultrasound or TOE – with the wire visualised in the inferior vena cava or right atrium. Using a Seldinger technique, the femoral venous access site is dilated to allow the atrial puncture and clip delivery systems to be advanced over the wire.
Heparin must be given to prevent thrombus forming on the wires and catheters, particularly after atrial septal puncture, as embolisation of LA thrombus can cause stroke. We give heparin immediately after femoral venous access is obtained. The target ACT is 250–300 s, which is usually achieved with an i.v. bolus of 125–150 IU kg−1 of unfractionated heparin. Additional heparin doses (25–50 IU kg−1) may be required during the procedure to maintain the ACT in the target range.
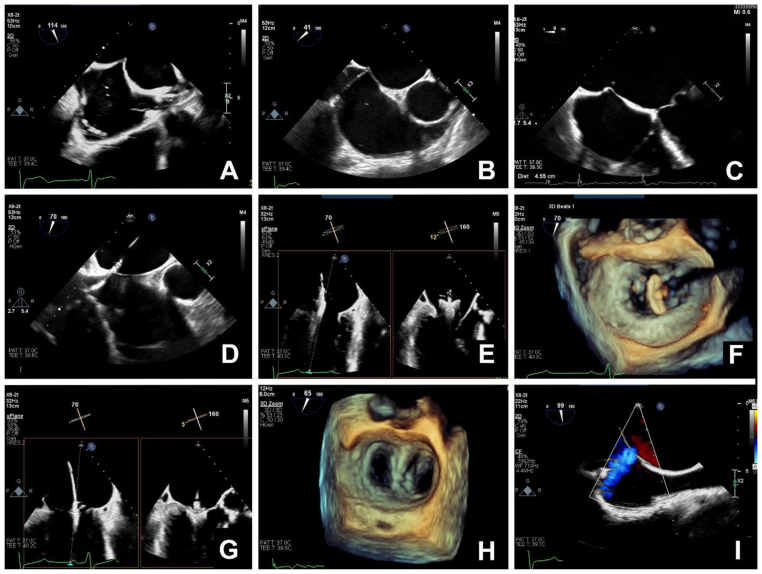
Performing the transseptal puncture in the correct location is critical to the success and safety of the procedure. With current devices, transseptal puncture should be in mid-position when the atrial septum is visualised in a mid-oesophageal bicaval view (Fig 2A) and posteriorly when visualised in mid-oesophageal aortic valve short-axis view (Fig 2B). In a mid-oesophageal four-chamber view (Fig 2C), the puncture site should be 4–4.5 cm above the mitral annulus. The target of 4–4.5 cm can be reduced when there is leaflet tethering, such as occurs with secondary MR. However, it is better to have excess height, as inadequate height may result in difficulty retracting the clip, if required.
Fig 2.
Transoesophageal echocardiographic imaging during transcatheter mitral valve repair. (A) Ideal position for puncture of the atrial septum in the mid-oesophageal bicaval view (Video 1). (B) Ideal position for puncture of the atrial septum in the on mid-oesophageal aortic valve short axis view (Video 2). (C) In the mid-oesophageal four-chamber view, the puncture site should be 4–4.5 cm above the mitral annulus. (D) Insertion of the delivery system and dilator into the left atrium. It is important to carefully monitor the position of the distal tip of the device to prevent perforation of the left atrial wall (Video 3). (E) Mid-oesophageal commissural and long axis views with X-plane imaging showing the clip and delivery system positioned above the mitral valve. Orthogonal imaging helps confirm correct positioning of the device (Video 4). (F) Three-dimensional (3D) imaging of the mitral valve from the left atrial aspect showing the clip positioned above the valve and oriented perpendicular to the line of coaptation (Video 5). (G) Mid-oesophageal commissural and long-axis views with X-plane imaging showing the clip attached to the leaflets in a closed position (Videos 6 and 7). (H) Three-dimensional imaging of the mitral valve from the left atrial aspect of a repaired mitral valve (Video 8). (I) Mid-oesophageal bicaval view with colour Doppler imaging showing a small iatrogenic atrial septal defect with left-to-right flow (Video 9). If reading the pdf online, please click on the respective panels to view the videos.
After atrial septal puncture, a wire is inserted into the left atrium and the puncture site dilated with a 6.0–8.0 mm balloon. A supportive wire is then placed in the left upper pulmonary vein or the left atrium, and the delivery system advanced under continuous TOE imaging to ensure proper placement and to avoid injury to the left atrium and the MV (Fig 2D).
Once the delivery system is in the left atrium, a clip is passed through the delivery system and sequentially steered towards the MV (Fig 2E). The clip is opened, positioned and aligned above the target application site on the MV. Three-dimensional echocardiography is helpful to assist with positioning and aligning the clip (Fig 2F). The MV is then crossed, the clip is opened and leaflets are grasped (Fig 2G).
Once the clip has been attached to the valve, the MV is carefully assessed for adequate leaflet insertion within the clip, residual MR or new mitral stenosis (Fig 2H). In one study, elevated MV gradient (mean diastolic gradient 7.2 mmHg) resulted in no difference in symptoms or echocardiographic findings at 2 yrs but moderate or more residual MR resulted in worse heart failure symptoms.16 Our goal is to apply further clips if there is an appropriate anatomical target to reduce MR severity while avoiding mitral stenosis by targeting a mean diastolic gradient ≤6 mmHg. We also stop applying clips when there is no further reduction in the magnitude of the v-wave on the LA trace or there is increased LA pressure, as measured through the delivery system. Typically, one to three clips are required but we have used up to five. The goal is to reduce MR severity to mild or less (≤2+), which can be achieved in most patients.
When assessing for mitral residual MR or new mitral stenosis, it is essential to adjust the patient's haemodynamics to match the awake state (i.e. when MR was judged to be severe) to ensure any change in MR can be attributed to the clips alone. Hypotension and hypovolaemia tend to reduce MR severity and hypertension and hypervolaemia tend to increase MV severity. Manoeuvres described previously to facilitate easier clip application will reduce the severity of the MR. Changes in ventilation should be avoided while the clip is being deployed. Unlike valve deployment during transcatheter aortic valve insertion, placing clips on the MV does not interfere with LV filling and does not cause hypotension. Therefore, clip deployment can be done in a careful and unhurried manner.
Once of the results of the procedure have been assessed and it is decided that additional clips are not required, the delivery system is retracted into the right atrium. A wire can be left across the atrial septum to assess for direction and size of the shunt (Fig 2I). The importance of closing the atrial septal defect is debated and remains an active area of investigation. Our approach is to close defects with severe left-to-right shunting, continuous right-to-left shunting or intermittent right-to-left shunting in the presence of severe tricuspid regurgitation. Once assessment of the atrial septum is complete, the transseptal wire is withdrawn and heparin may be reversed with protamine, although this is not done in all centres. The femoral venous access site is repaired with vascular closure device.
After the procedure, a complete TOE examination is performed to confirm procedural success and exclude complications (see below), particularly pericardial tamponade.
Postoperative care and complications
Routine postoperative care
The great majority of patients can be woken at the completion of the procedure and recover in the PACU. When transcatheter MV repair was first introduced, postprocedural monitoring in the ICU was routine; however, this is no longer the case.17,18 Intermediate care units or coronary care units are suitable for most patients.
Venous access sites should be monitored for bleeding and haematoma formation. With the use of vascular closure devices, there is no need for routine compression of the femoral venous access site. Our approach is for patients to have at least 1 h of bed rest after the procedure.
Postoperative pain is uncommon, as local anaesthesia is used at the vascular access site. Discomfort related to the TOE probe can be reduced with topicalisation of the oropharynx before insertion. If oropharyngeal topicalisation is performed, patients should avoid oral intake for 1–2 h after the procedure to allow time for the local anaesthetic to wear off.
Patients should have a chest radiograph to verify that the position of the clips is unchanged compared with the final fluoroscopy imaging and to rule out complications such as pulmonary oedema or pneumothorax. All patients should undergo a transthoracic echocardiogram within the first 24 h to confirm procedural success and to exclude complications. Residual MR and the presence of new mitral stenosis should be carefully assessed and documented. If the atrial septal defect was not closed during the procedure, the volume and direction of flow across the atrial septum should be assessed and the need for closure reconsidered for large defects.
Anticoagulation is not required after MitraClip procedures. Patients should receive an antiplatelet agent (aspirin, clopidogrel) as per the manufacturer's recommendations and recognised practice. Patients taking oral anticoagulants before the procedure (e.g. for atrial fibrillation) can recommence these agents the following day.
Hospital discharge is typically within 24–48 h of the procedure. Selected patients can be considered for discharge on the day of the procedure, provided they have the necessary support and appropriate follow-up is arranged.18 Patients with an elevated risk score (EuroScore, Society of Thoracic Surgeons) are more likely to require a longer hospital stay, particularly those with baseline kidney dysfunction.19 Decreased LVEF and New York Heart Association (NYHA) class III or IV heart failure symptoms are predictive of the need for rehospitalisation and are associated with an increased 1-yr mortality.20
Complications
Major bleeding occurs in about 3% of cases after transcatheter MV repair.21,22 Bleeding from the femoral access site is rare and usually obvious. However, access-site bleeding can be missed. During the procedure, the operator may be task-distracted. After the procedure, bleeding may be obscured by gowns and bedclothes. Bleeding into the retroperitoneum is less common with femoral venous access for transcatheter MV repair than with femoral arterial access for transcatheter aortic valve implantation but, when it does occur, it is easily missed. Unexplained hypotension, hypovolemia or anaemia are all suggestive of retroperitoneal bleeding.
Oesophageal and gastric injury caused by the TOE probe occurs in more than 5% of patients undergoing transcatheter MV repair.23 Acute kidney injury is relatively common, occurring in up to 18% of patients, despite the procedure not requiring the use of iodinated contrast agents.24
Delayed pericardial tamponade can occur and is one of the most feared complications of transcatheter cardiac interventions. Urgent transthoracic echocardiography is mandatory in the event of post-procedural haemodynamic instability. New arrhythmias are uncommon after transcatheter repair. In one study, atrial fibrillation was the most common new arrhythmia, occurring in 2.4% of patients.25
Hypoxaemia resulting from pulmonary oedema is possible, arising as a consequence of new mitral stenosis or excessive fluids given. In rare cases, hypoxaemia can result from shunt reversal (right-to-left) across the atrial septal defect. If there is uncertainty as to the cause, hypoxaemia should be investigated with a transthoracic echocardiogram.
Device embolisation is rare compared with thrombotic embolisation, but both complications should be considered in patients with new organ malperfusion, particularly new neurological dysfunction.
Patients undergoing urgent transcatheter MV repair are at increased risk of complications compared with patients undergoing elective repair. In a non-randomised cohort, complications such as atrial fibrillation, pericardial collection, acute kidney injury and in-hospital mortality were higher in those who had an urgent procedure.26 In our own experience of transcatheter MV repair in patients in cardiogenic shock, 30-day mortality was 26%. All deaths were secondary to progression of shock; none were anaesthesia or procedural related.27 These findings were confirmed in a subsequent study involving a larger cohort of patients with cardiogenic shock.28 Patients undergoing urgent procedures or those in a critical state before the intervention should be cared for in a high dependency unit (HDU) or ICU environment after the procedure.
Conclusions
The scope of transcatheter mitral repair is expanding because of the increasing burden of MR in an ageing population and the excellent outcomes with current devices, particularly in high-risk patients. Technology is developing rapidly and new transcatheter approaches to the MV continue to be developed and tested. In this review, we have highlighted three key principles of anaesthetic management: (i) choosing a technique for general anaesthesia that facilitates rapid and safe recovery; (ii) TOE guidance for successful puncture of the atrial septum and device deployment; and (iii) haemodynamic strategies that facilitate the procedure. The principles of anaesthesia developed for current technologies are likely to form the basis of anaesthetic techniques for new devices as they become available.
Declaration of interests
The authors declare that they have no conflicts of interest.
Biographies
Benjamin Hibbert MD PhD FRCPC is a consultant interventional cardiologist and the director of the Vascular Biology and Experimental Medicine Laboratory at the University of Ottawa Heart Institute. He is involved in clinical trials on percutaneous valve repair technologies and has published on the use of these technologies in patients experiencing cardiogenic shock.
Mark Hynes MD FRCPC is a consultant anaesthesiologist and echocardiographer at the University of Ottawa Heart Institute. He is an assistant professor at the University of Ottawa and a founding member of the Percutaneous Valve Repair Review Committee.
Adam Dryden MD FRCPC is a consultant anaesthesiologist and the director of perioperative echocardiography at the University of Ottawa Heart Institute. He lectures extensively on the use of echocardiography for surgical and percutaneous valve repair.
Matrix codes: 1A02; 2A03; 3G00
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bjae.2023.01.004.
MCQs
The associated MCQs (to support CME/CPD activity) will be accessible at www.bjaed.org/cme/home by subscribers to BJA Education.
Appendix A. Supplementary data
The following are the Supplementary data to this article: The videos associated with this article can all be viewed from the article in BJA Education online.
References
- 1.Nkomo V.T., Gardin J.M., Skelton T.N., Gottdiener J.S., Scott C.G., Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368:1005–1011. doi: 10.1016/S0140-6736(06)69208-8. [DOI] [PubMed] [Google Scholar]
- 2.Nishimura R.A., Vahanian A., Eleid M.F., Mack M.J. Mitral valve disease—current management and future challenges. Lancet. 2016;387:1324–1334. doi: 10.1016/S0140-6736(16)00558-4. [DOI] [PubMed] [Google Scholar]
- 3.McCullough P.A., Mehta H.S., Barker C.M., et al. The economic impact of mitral regurgitation on patients with medically managed heart failure. Am J Cardiol. 2019;124:1226–1231. doi: 10.1016/j.amjcard.2019.07.033. [DOI] [PubMed] [Google Scholar]
- 4.Otto C.M., Nishimura R.A., Bonow R.O., et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. Circulation. 2021;143:e72–e227. doi: 10.1161/CIR.0000000000000923. [DOI] [PubMed] [Google Scholar]
- 5.Vahanian A., Beyersdorf F., Praz F., et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease: developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2022;43:561–632. [Google Scholar]
- 6.McInerney A., Marroquin-Donday L., Tirado-Conte G., et al. Transcatheter treatment of mitral regurgitation. J Clin Med. 2022;11:2921. doi: 10.3390/jcm11102921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feldman T., Foster E., Glower D.D., et al. Percutaneous repair or surgery for mitral regurgitation. N Engl J Med. 2011;364:1395–1406. doi: 10.1056/NEJMoa1009355. [DOI] [PubMed] [Google Scholar]
- 8.Obadia J.F., Messika-Zeitoun D., Leurent G., et al. Percutaneous repair or medical treatment for secondary mitral regurgitation. N Engl J Med. 2018;379:2297–2306. doi: 10.1056/NEJMoa1805374. [DOI] [PubMed] [Google Scholar]
- 9.Stone G.W., Lindenfeld J., Abraham W.T., et al. Transcatheter mitral-valve repair in patients with heart failure. N Engl J Med. 2018;379:2307–2318. doi: 10.1056/NEJMoa1806640. [DOI] [PubMed] [Google Scholar]
- 10.Pibarot P., Delgado V., Bax J.J. MITRA-FR vs. COAPT: lessons from two trials with diametrically opposed results. Eur Heart J Cardiovasc Imaging. 2019;20:620–624. doi: 10.1093/ehjci/jez073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiarito M., Pagnesi M., Martino E.A., et al. Outcome after percutaneous edge-to-edge mitral repair for functional and degenerative mitral regurgitation: a systematic review and meta-analysis. Heart. 2018;104:306–312. doi: 10.1136/heartjnl-2017-311412. [DOI] [PubMed] [Google Scholar]
- 12.Tigges E., Blankenberg S., von Bardeleben R.S., et al. Implication of pulmonary hypertension in patients undergoing MitraClip therapy: results from the German transcatheter mitral valve interventions (TRAMI) registry. Eur J Heart Fail. 2018;20:585–594. doi: 10.1002/ejhf.864. [DOI] [PubMed] [Google Scholar]
- 13.Ben-Yehuda O., Shahim B., Chen S., et al. Pulmonary hypertension in transcatheter mitral valve repair for secondary mitral regurgitation: the COAPT trial. J Am Coll Cardiol. 2020;76:2595–2606. doi: 10.1016/j.jacc.2020.09.609. [DOI] [PubMed] [Google Scholar]
- 14.Horn P., Hellhammer K., Minier M., et al. Deep sedation vs. general anesthesia in 232 patients undergoing percutaneous mitral valve repair using the MitraClip((R)) system. Catheter Cardiovasc Interv. 2017;90:1212–1219. doi: 10.1002/ccd.26884. [DOI] [PubMed] [Google Scholar]
- 15.Essandoh M.K. Afterload mismatch after MitraClip implantation: the potential impact of pharmacologic support. J Cardiothorac Vasc Anesth. 2017;31:702–706. doi: 10.1053/j.jvca.2016.05.047. [DOI] [PubMed] [Google Scholar]
- 16.Halaby R., Herrmann H.C., Gertz Z.M., et al. Effect of mitral valve gradient after MitraClip on outcomes in secondary mitral regurgitation: results from the COAPT Trial. JACC Cardiovasc Interv. 2021;14:879–889. doi: 10.1016/j.jcin.2021.01.049. [DOI] [PubMed] [Google Scholar]
- 17.Di Prima A.L., Covello D.R., Franco A., et al. Do patients undergoing MitraClip implantation require routine ICU admission? J Cardiothorac Vasc Anesth. 2014;28:1479–1483. doi: 10.1053/j.jvca.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Chowdhury M., Buttar R., Rai D., et al. Same-day discharge after transcatheter mitral valve repair using MitraClip in a tertiary community hospital: a case series. Eur Heart J Case Rep. 2021;5 doi: 10.1093/ehjcr/ytab397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ledwoch J., Bertog S., Wunderlich N., et al. Predictors for prolonged hospital stay after transcatheter mitral valve repair with the MitraClip. Catheter Cardiovasc Interv. 2014;84:599–605. doi: 10.1002/ccd.25460. [DOI] [PubMed] [Google Scholar]
- 20.Kessler M., Seeger J., Muche R., Wohrle J., Rottbauer W., Markovic S. Predictors of rehospitalization after percutaneous edge-to-edge mitral valve repair by MitraClip implantation. Eur J Heart Fail. 2019;21:182–192. doi: 10.1002/ejhf.1289. [DOI] [PubMed] [Google Scholar]
- 21.Eggebrecht H., Schelle S., Puls M., et al. Risk and outcomes of complications during and after MitraClip implantation: experience in 828 patients from the German TRAnscatheter mitral valve interventions (TRAMI) registry. Catheter Cardiovasc Interv. 2015;86:728–735. doi: 10.1002/ccd.25838. [DOI] [PubMed] [Google Scholar]
- 22.Korber M.I., Silwedel J., Friedrichs K., et al. Bleeding complications after percutaneous mitral valve repair with the MitraClip. Am J Cardiol. 2018;121:94–99. doi: 10.1016/j.amjcard.2017.09.027. [DOI] [PubMed] [Google Scholar]
- 23.Freitas-Ferraz A.B., Rodes-Cabau J., Junquera Vega L., et al. Transesophageal echocardiography complications associated with interventional cardiology procedures. Am Heart J. 2020;221:19–28. doi: 10.1016/j.ahj.2019.11.018. [DOI] [PubMed] [Google Scholar]
- 24.Spieker M., Hellhammer K., Katsianos S., et al. Effect of acute kidney injury after percutaneous mitral valve repair on outcome. Am J Cardiol. 2018;122:316–322. doi: 10.1016/j.amjcard.2018.03.358. [DOI] [PubMed] [Google Scholar]
- 25.Schnitzler K., Hell M., Geyer M., Kreidel F., Munzel T., von Bardeleben R.S. Complications following MitraClip implantation. Curr Cardiol Rep. 2021;23:131. doi: 10.1007/s11886-021-01553-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Musuku S.R., Mustafa M., Pulavarthi M., et al. Procedural, short-term, and intermediate-term outcomes in propensity-matched patients with severe mitral valve regurgitation undergoing urgent versus elective MitraClip percutaneous mitral valve repair. J Cardiothorac Vasc Anesth. 2022;36:1268–1275. doi: 10.1053/j.jvca.2021.12.008. [DOI] [PubMed] [Google Scholar]
- 27.Chan V., Messika-Zeitoun D., Labinaz M., et al. Percutaneous mitral repair as salvage therapy in patients with mitral regurgitation and refractory cardiogenic shock. Circ Cardiovasc Interv. 2019;12 doi: 10.1161/CIRCINTERVENTIONS.119.008435. [DOI] [PubMed] [Google Scholar]
- 28.Jung R.G., Simard T., Kovach C., et al. Transcatheter mitral valve repair in cardiogenic shock and mitral regurgitation: a patient-level, multicenter analysis. JACC Cardiovasc Interv. 2021;14:1–11. doi: 10.1016/j.jcin.2020.08.037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.