Abstract
Maintaining internal homeostasis and regulating innate behaviors are essential for animal survival. In various animal species, a highly conserved neuroendocrine system integrates sensory inputs and regulates physiological responses to environmental and internal changes. Diuretic hormones 44 and 31, which are homologs of mammalian corticotropin-releasing factor (CRF) and calcitonin gene-related peptide (CGRP), respectively, control body fluid secretion in Drosophila. These neuropeptides and their re-ceptors have multiple physiological roles, including the regulation of body-fluid secretion, sleep:wake cycle, internal nutrient-sensing, and CO2-dependent response. This review discusses the physiological and behavioral roles of DH44 and DH31 signaling pathways, consisting of neuroendocrine cells that secrete DH44 or DH31 peptides and their receptor-expressing organs. Further research is needed to understand the regulatory mechanisms of the behavioral processes mediated by these neuroendocrine systems.
Keywords: DH31, DH31 receptor, DH44, DH44 receptors, Drosophila, Feeding, Fluid secretion, Nutrient sensing, Sleep:wake cycle
INTRODUCTION
The neuroendocrine system maintains metabolic and physiological homeostasis and regulates internal ion balance and stress response. Multiple synaptic inputs from the sensory organs produced by the environmental and internal changes are integrated into various neuroendocrine systems and stimulate the secretion of hormones to regulate the activity of peripheral organs and behaviors, including feeding, mating, fighting, and sleep (1). The output of those neuroendocrine systems leads to motor activity regulation of effector organs to regulate the metabolic processes and control specific behaviors. These overall processes are tightly tuned and highly conserved among various animal species from nematodes to mammals (1-3).
Drosophila melanogaster, the fruit fly, is a powerful genetic model system that has enabled the identification of numerous novel functions of neuropeptide signaling. Neuropeptides and their signaling cascades play critical roles in controlling various behaviors, such as olfaction, feeding, sleep:wake cycle, mating, and aggression, as well as regulating development, growth, and lifespan (4). Notably, many neuropeptides and their signaling pathways have highly conserved mammalian homologs. For instance, the feeding-regulating neuropeptide F in Drosophila has a mammalian homolog, neuropeptide Y, which is a well-known feeding-controlling neuropeptide in mammals. There are numerous examples of highly functionally conserved neuropeptides between Drosophila and mammals (4, 5).
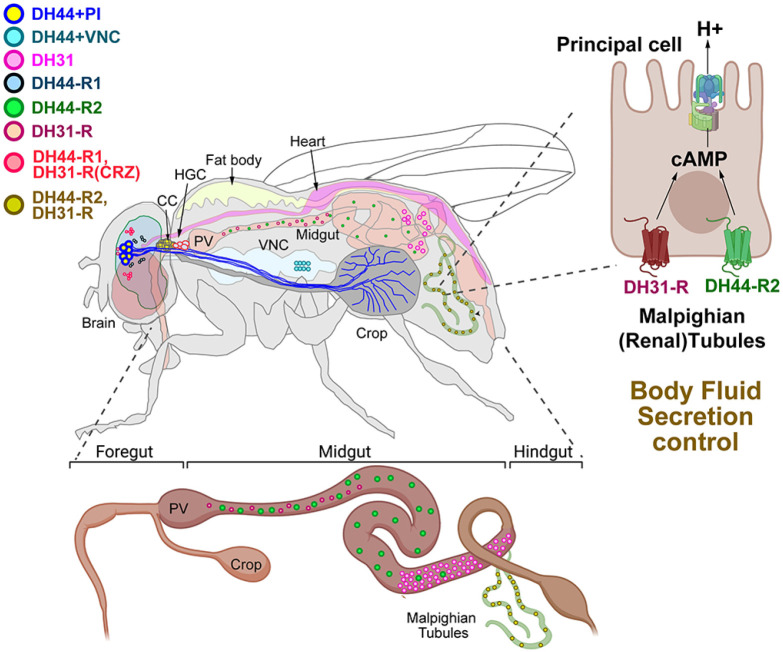
Among various neuroendocrine systems, we focus on the biological roles of diuretic hormones (DHs), DH44 and DH31, in Drosophila. Two distinct diuretic hormone (DH) genes, DH44 and DH31, encode Drosophila DH44 and DH31 peptides (6). DH44 and DH31 peptides also called Drome-DH44 and -DH31, indicate that the Drosophila diuretic hormones consist of 44 or 31 amino acid residues, respectively. DH44-expressing neurons have six cell bodies in the pars intercerebralis (PI) region of the brain, a neuroendocrine center in flies (Fig. 1) (7). The DH44 + PI neurons project axons and dendrites to the crop and project dendrites to the subesophagus zone (SEZ) in the adult fly brain (7, 8). The DH44 receptor 1 (DH44-R1) is expressed in the brain and the DH44 receptor 2 (DH44-R2) is mainly expressed in the midgut and the Malpighian tubules (Fig. 1) (9). The DH31 is primarily expressed and secreted in a subset of enteroendocrine cells, which mainly secrete intestinal hormones in Drosophila, located in the posterior midgut (10). The DH31 receptor (DH31-R) is expressed in the brain, midgut, and the Malpighian tubules (Fig. 1) (9, 11, 12). The brain expression of DH44-R1 is overlapped with those of DH31-R in the corazonin (CRZ)-expressing neurons in the lateral area of the brain (12). The Drosophila DH44 peptide has significant sequence similarities to mammalian CRF expressed in the hypothalamus, a well-known regulator of the hypothalamic-pituitary-adrenal (HPA) axis in mammals, and other diuretic hormones expressed in various insect species (13-15). The Drosophila DH31 peptide is related to mammalian calcitonin and CGRP which are essential in regulating calcium metabolism and maintaining calcium homeostasis in mammals (14).
Fig. 1.
The expression and projection patterns and body fluid secretion function of DH44 and DH31-expressing cells and their receptors in adult Drosophila. DH44 + PI neurons project along the esophagus to innervate the crop. DH44-R1 and DH31 receptors are expressed in CRZ neurons located in the lateral part of the brain. DH31-expressing enteroendocrine (DH31 + EE) cells are located around the posterior midgut. DH44-R2 and DH31-R are expressed in the midgut and the principal cells of the Malpighian tubules. DH44-R2 and DH31-R are expressed on the principal cells of the Malpighian tubules, where they work together to increase the concentration of cAMP, activating V-type ATPase and playing a role in body fluid secretion. DH44 + PI, diuretic hormone 44 expressing neurons located in the pars intercerebralis of the brain; DH44 + VNC, diuretic hormone 44 expressing neurons located in the ventral nerve cord; DH31, diuretic hormone 31 expressing cells; DH44-R1, diuretic hormone 44 receptor 1 expressing neurons; DH44-R2, diuretic hormone 44 receptor 2 expressing cells; DH31, diuretic hormone 31 expressing cells; DH31-R, diuretic hormone 31 receptor-expressing cells; CRZ, corazonin; PV, proventriculus; CC, corpora cardiaca; HCG, hypocerebral ganglion; VNC, ventral nerve cord; cAMP, cyclic adenosine monophosphate.
In this mini-review, we discuss the biological functions of DH44 and DH31-mediated neuroendocrine systems that integrate sensory inputs and regulate various physiological processes and behaviors in Drosophila. We also discuss further research direction to understand how those systems tightly balance internal metabolic needs and produce output circuits to control peripheral organs.
BODY FLUID SECRETION
Fluid secretion from Malpighian tubules
In Drosophila, body fluid secretion is mainly controlled by the Malpighian (renal) tubules and the hindgut. Malpighian tubules are branched from the midgut, connected with the hindgut, and bathed in the hemolymph of the main body cavity (16). They have a similar role to that of the glomerulus of the nephron of mammals. They filtrate the hemolymph using osmotic filtration and produce the primary urine using diffusion and active transportation (2, 16). There are two distinct neuropeptide families, including the mammalian corticotropin-releasing factor (CRF) related diuretic peptides and the kinin family of neuropeptides, which have been shown to stimulate the fluid secretion of isolated Malpighian tubules (17). DH44 and DH31 both activate the Malpighian tubules to stimulate body fluid secretion through a cAMP-dependent signaling pathway (6, 18). The principal cells of Malpighian tubules express DH44-R2 and DH31-R, which are crucial for regulating the body fluid secretion by accepting DH44 and DH31 signals (9, 12). Both pathways stimulate cAMP increases in the principal cells of Malpighian tubules, yet their mechanisms to increase cAMP levels and activate a vacuolar-type ATPase (V-ATPase) in the principal cells are not overlapped because DH44 and DH31 are working synergically when they are treated to the Malpighian tubules at the same time (12, 18).
Stress tolerance and immune response
Suppressing body fluid secretion is essential to overcome metabolic and physiological stress induced by desiccation and starvation. In Drosophila, the suppression of DH44-R2 signaling in principal cells in Malpighian tubules increases desiccation and starvation tolerance (19, 20). This occurs because the DH44-R2 pathway in Malpighian tubules mediates the suppression of body fluid secretion, which minimizes the loss of body fluid. This suppression is beneficial in overcoming desiccation, starvation, and ionic stress (9, 19, 20). A subset of DH44-expressed neurons in the ventral nerve cord (DH44 + VNC) expresses both DH44 and leucokinin (LK). Both of these molecules induce body fluid secretion through the Malpighian tubules and regulate stress physiology by controlling the fluid secretory function of the tubules (20).
Because DH44 and DH31 are working on the digestive tract and Malpighian tubules which are mainly affected by pathogens and bacteria, those two peptides have an essential role in the elimination of bacteria and toxic reactive oxygen species (ROS) made by the immune responses (21, 22). DH31 is secreted from enteroendocrine cells and promotes strong visceral contractions through its DH31-R to secrete ROS and toxic molecules produced by pathogens (21). The expression of DH44 is induced upon immune challenge and is essential for activating the excretion during the infection or injury (22, 23). The lipid-binding protein Materazzi induces the removal of hemolymphatic lipids under ROS-enriched conditions and prevents lipid peroxidation and tubule dysfunction derived from the infection or severe injury (22). Further investigation needs to be conducted to understand the role of DH44 and DH31 in regulating the pathogen secretion and the other innate immune responses in Drosophila.
CIRCADIAN AND SLEEP REGULATION
The essential regulator in the circadian clock output circuit
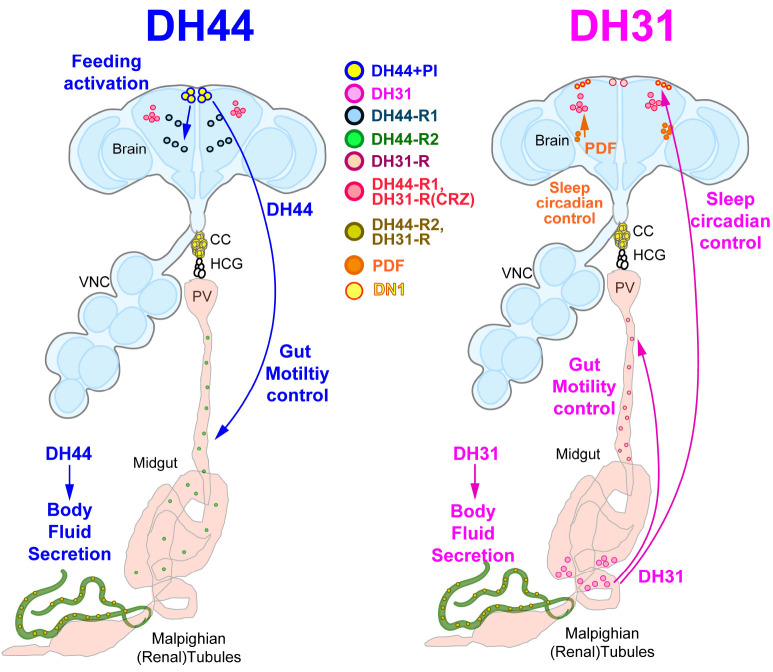
The regulation of circadian locomotion rhythm is essential for the survival of animals who live in environments with day and night changes. In Drosophila, a subset of 150 central clock neurons expressing core clock genes is required to maintain the locomotor rhythms with approximately 24 hours period for a day (24). After a historical finding of the first clock gene period, the detailed molecular mechanism to make daily rhythms on several pacemaker neurons in the brain has been identified (25-28). The next question after the investigations of the core clock mechanism is to identify the circadian clock output circuit that sends the daily rhythm produced by the pacemaker neurons in the brain to the peripheral organs regulating the body movement, metabolic rate, immune responses, and timing of food intake. Recent studies showed that DHs have an impor-tant role in regulating the circadian output circuit and temperature preference rhythm in flies (29-31). The DH44-expressing PI neurons (DH44 + PI) receive a circadian output signal produced from the master pacemaker small lateral ventral neurons (s-LNvs) through dorsal neurons (DNs) and send the signal to Hugin-expressing neurons in the SEZ area of the brain and ventral nerve cord (VNC) to direct the peripheral movement according to the circadian control (30, 32). This discovery was a breakthrough in the field because finding the circadian output circuit that receives the signal from DNs and relays it to the peripheral body was one of the main issues of the field.
DH31 peptide is also essential in maintaining normal circadian locomotor activity (33). Even though the loss of function mutants of DH31 showed normal circadian rhythm, DH31 and pigment-dispersing factor (PDF) double mutant flies exhibited more severe disruption of rhythmicity than those of PDF single mutant flies (Fig. 2) (33). DH31 peptide regulates free-running rhythmicity through PDF receptor (PDFR) in posterior dorsal neuron 1 (DN1ps), not DH31-R (33) (Fig. 2). DH31 also has a vital role in regulating night onset temperature preference rhythm through PDFR in dorsal neurons 2 (DN2s) (31). The daily body temperature rhythm is regulated by DH31 peptide through DH31-R expressing clock cells (34). Collectively, both DH44 and DH31 have a significant function in maintaining circadian rhythm, yet they have distinct mechanisms to regulate the daily rhythm in flies.
Fig. 2.
The distribution and physiological functions of DH44 and DH31 neurons and their receptors in adult Drosophila. A schematic summary is presented to depict the anatomical distribution and diverse biological functions of DH44 and DH31 signaling in adult Drosophila. DH44 + PI neurons secrete the DH44 peptide to neurons expressing DH44-R1, which increases food intake. They also secrete DH44 peptide to the midgut to activate gut motility and to the Malpighian tubules to stimulate body fluid secretion. DH31-producing enteroendocrine cells located in the posterior midgut secrete the DH31 peptide to DN1 neurons in the brain, which play a role in regulating sleep/circadian behavior. These cells also regulate gut contraction via the DH31-R expressed on the midgut. In addition, a sleep and circadian rhythm-regulating peptide PDF activate DH31-expressing neurons, further regulating sleep and circadian behavior in the adult brain. DH44 + PI, diuretic hormone 44 expressing neurons located in the pars intercerebralis of the brain; DH31, diuretic hormone 31 expressing cells; DH44-R1, diuretic hormone 44 receptor 1 expressing neurons; DH44-R2, diuretic hormone 44 receptor 2 expressing cells; DH31, diuretic hormone 31 expressing cell; DH31-R, diuretic hormone 31 receptor-expressing cells; CRZ, corazonin; PDF, pigment dispersing factor; DN1, dorsal neuron 1; PV, proventriculus; CC, corpora cardiaca; HCG, hypocerebral ganglion; VNC, ventral nerve cord.
Controlling sleep:wake cycle
Sleep is an essential physiological process in diverse animals and is regulated by circadian output signals and sleep homeostasis mediated by the accumulation of sleep needs (35, 36). Diuretic hormones not only maintain circadian rhythms but also regulate the sleep:wake patterns in flies. DH31-expressing DN1s neurons are the circadian clock output circuit that mediates nighttime sleep regulation (29). PDF secreted from the lateral ventral neurons (LNvs) stimulates DH31-expressing DN1s neurons through PDFR and stimulates the secretion of DH31 peptide during the late night (29). The secreted DH31 activates DH31-R-dependent downstream pathways and suppresses sleep late at night (29). Additionally, male-specific P1 neurons activate sleep-controlling DN1s neurons to secrete DH31 to suppress sleep when the male flies receive enough courtship-inducing sensory inputs (37). These findings illustrate that DH31-secreted DN1s neurons suppress sleep during the night and integrate courtship sensory inputs and internal sleep needs to balance mating and sleep based on their physiological condition in male flies (Fig. 2).
Additionally, DH44 + PI neurons promote wakefulness when the internal thermosensory anterior cells (ACs) detect ambient temperature shifting and secrete acetylcholine to evoke CNMa secretion from the subset of DN1p neurons (38). CNMa peptide secreted from DN1ps neurons after receiving acetylcholine signal from ACs affects DH44 neurons to promote wakefulness in response to a drastic temperature shift (38). This AC-DN1p-DH44 + PI neural circuit integrates thermosensory inputs and promotes wakefulness to increase survival rate within a rapid temperature-shifting environment (38). We suppose that the DH44 circuit would have an essential role in controlling the sleep:wake cycle because it is one of the primary regulators of circadian output circuits in flies.
INTERNAL CHEMICAL SENSING AND METABOLIC REGULATION
Taste-independent sugar and amino acid sensor
Animals mainly detect sugars and proteins using their taste receptors (39), yet they can also monitor the nutritive sugars and proteins using internal nutrient sensors in the brain (7, 40-42). The six-cell bodies of DH44 + PI neurons evoke calcium oscillation when circulating sugar levels are increased (7). The increased inter-cellular calcium level promotes the secretion of DH44 peptide that activates proboscis extension response (PER) via the DH44-R1 downstream pathway and stimulates gut motility using the DH44-R2 downstream path to maximize the consumption of nutritive sugars only in a starved condition (Fig. 2) (7). Interestingly, DH44 + PI neurons are not activated by nutritive sugar in the sated flies indicating that the upstream inhibitory signal specifically works on the sated conditions (7). The following research showed that the two independent upstream signals from the periphery, circulating glucose levels and gut stretch, suppress the glucose response of DH44 + PI neurons only in a fed condition (8).
DH44 + PI neurons are activated by circulating sugars and stimulated by several amino acids, including L-Glu, L-Ala, and L-Asp, which are supplied from food sources (40, 43). DH44 + PI neurons are essential to increase the feeding amount which contains nutritive sugars and those three amino acids, L-Glu, L-Ala, and L-Asp (40, 43). However, it is unclear whether detecting those three amino acids by DH44 + PI neurons would be necessary for shifting the food preference between carbohydrates and proteins in a protein-deprived condition. The other previous study showed that the essential amino acid sensing by the dopaminergic neurons through general control nonderepressible 2 (GCN2)/activating transcription factor 4 (ATF4)-dependent mechanism is necessary for inducing the rejection of a protein-deficient diet during the larval stage (44). Further investigations need to answer why DH44 + PI neurons are activated by the three non-essential amino acids rather than the other essential amino acids and regulate the feeding behavior. Additionally, a subpopulation of DH31- and tachykinin-expressing enteroendocrine cells are activated by the proteins and amino acid-containing food intake (45). These results indicate that not only DH44 + PI neurons but also DH31-expressing cells in the posterior midgut detect the protein or amino acids and secrete neuropeptides to maintain metabolic homeostasis.
Regulation of food intake, gut motility, and defecation
Environmental cues and internal energy status influence feeding behavior. The regulation of food intake is crucial for maintaining metabolic homeostasis in animals. Feeding behaviors are mainly controlled by the mechanisms regulated by brain homeostasis and hedonic systems (46). Because DH44 and DH31 respond to internal nutrient level changes, those neuropeptides may be involved in regulating feeding-related behaviors. They also possibly mediate gut motility and defecation because they are important factors controlling body fluid secretion. When the nutritive sugar sensory function of DH44 + PI neurons was investigated, it was also reported that manipulating DH44 + PI neurons does not affect normal food intake which contains carbohydrates and proteins (7). However, the following study showed that the suppression or activation of DH44 + PI neurons decreases or increases sucrose food intake in adult flies (8). To prevent carbohydrate food overconsumption driven by the activation of DH44 + PI neurons, those neurons are suppressed by the mechanical and chemical information from the peripheral organs when the flies are sated (8).
Additionally, the DH44 peptide stimulates gut motility and increases defecation via the DH44-R2 expressed in the midgut (Fig. 2) (7). The enhancement of PER through the DH44-R1 circuit and activation of gut motility and defecation via DH44-R2 maximize nutritive sugar consumption, especially in starved flies (7). This is why the DH44 + PI neurons must be suppressed in a sated condition. Additionally, the axons of DH44 + PI neurons project to the crop, homolog of the mammalian stomach, indicating that DH44 signaling may regulate the crop size control based on the internal energy status (8). Further study must investigate whether crop movement control is mediated by DH44 signaling. DH31 peptide also triggers intestinal contractions and fosters bowel emptying gut movement, especially in an infected status, to eliminate the harmful bacteria and ROS (21). The drastic stimulation of gut movement is a common function of the DH44 and DH31 circuits (Fig. 2). Additionally, DH31 expressed in enteroendocrine cells also controls intestinal stem cell (ISC) proliferation and midgut senescence which impacts the longevity of adult flies as a counter partner of allatostatin A (AstA) (47).
CO2-dependent response in a larval stage
Detecting CO2 mainly produces aversive behavior in adult flies via a single population of olfactory sensory neurons through Gr21a and Gr63a receptor (48, 49). The other study showed that the flies detect carbohydrate water, and CO2 dissolved water, through their gustatory system and evoke the feeding acceptance behavior (50). Based on these previous studies, the higher CO2 level in the air is detected by an odorant receptor and stimulates the aversive behavior of the flies to avoid an overcrowded environment. In contrast, the CO2 dissolved water is sensed by the gustatory receptor and induces acceptance behavior because carbonated water implies the rotten fruits nearby. An independent study recently showed that a subset of tracheal dendritic (TD) neurons respond to CO2 levels. Those signals are integrated via several interneurons and activate DH44 + PI and CRZ neurons (51). Although they did not report whether the activation of DH44 + PI and CRZ neurons evokes acceptance or aversive behavior, they showed that mediating CO2-dependent response is a novel function of DH44 signaling in a larval stage.
REGULATION OF SEXUAL BEHAVIOR
Regulation of the female sperm ejection
Female flies eject a large portion of injected sperm after mating, and less than 20% of the sperm can be stored in their uterus (52). To avoid post-copulatory sexual selection and increase sperm acceptance, male flies need to prepare their sperm to reduce the female’s sperm ejection behavior (53). On the female side, they evaluate the quality of the sperm and decide whether to retain or eject it from the uterus. Female flies assess the nutritive value of the sperm using DH44 + PI neurons as nutritive sensors and determine whether to eject the sperm based on its nutritional value (54). By using these neurons as a sensor for evaluating sperm quality, female flies can increase the fertility rate and health quality of their offspring. The inactivation of DH44 + PI neurons in female flies resulted in an inability to store nutritive sperm. The DH44-R1 expressing neurons that also express doublesex, a gene involved in sex determination and regulation of sexual behaviors in flies, modulate sperm ejection behavior (54). These findings demonstrate that flies utilize the nutritional sensory function of DH44 + PI neurons to regulate female sperm ejection behavior.
Balancing courtship and feeding behavior
The animal must prioritize their behaviors, including sleep, feeding, and mating, based on the environmental situation and internal nutritive status (3). In this process, a specific neuronal circuit must detect internal nutritive status and send this information to the mating or sleep regulatory center. For example, starving flies sense their energy reduction and reduce sleep and increase locomotion activity to increase the chance of finding nutritive food (55). This starvation-induced hyperactivity results from resetting the priority among behaviors and reducing sleep to find nutritive food. In a recent publication, DH31 secreted from the midgut after consuming amino acids suppresses feeding and promotes male courtship behavior (56). This finding is another example in which male flies change the priority of their behaviors and promote courtship behavior after they fulfilled their nutritive needs (56). Before this study, another previous study showed that DH31-expressing enteroendocrine cells in the posterior midgut are activated explicitly by the protein and amino acid intake in the larval stage (45). The gut-secreted DH31 peptide induced by the intestinal proteins is transferred into the brain and activates the secretion of CRZ peptide, promoting male courtship behavior through DH31-R (56). Further research needs to follow to determine the biological role of DH31 in balancing feeding and courtship behavior.
SUMMARY
Diuretic hormones were first identified as stimulators of fluid secretion in the Malpighian tubules and hindgut of various insect species (14, 15). Over the past two decades of research, we have learned that diuretic hormones control various physiological processes and behaviors, including stress responses, gut movement, feeding behaviors, circadian rhythm, sleep behavior, and internal nutrition sensing (as depicted in Fig. 3 and 4). Despite the important roles that DH44 and DH31 play in regulating these behaviors, further research is needed to investigate how these neuroendocrine systems interact to maintain complex physiological and behavioral balances in Drosophila. Overall, the neuroendocrine systems mediated by DH44 and DH31 integrate internal status and environmental cues to control a variety of behaviors and maintain metabolic and physiologic homeostasis (as shown in Fig. 3 and 4).
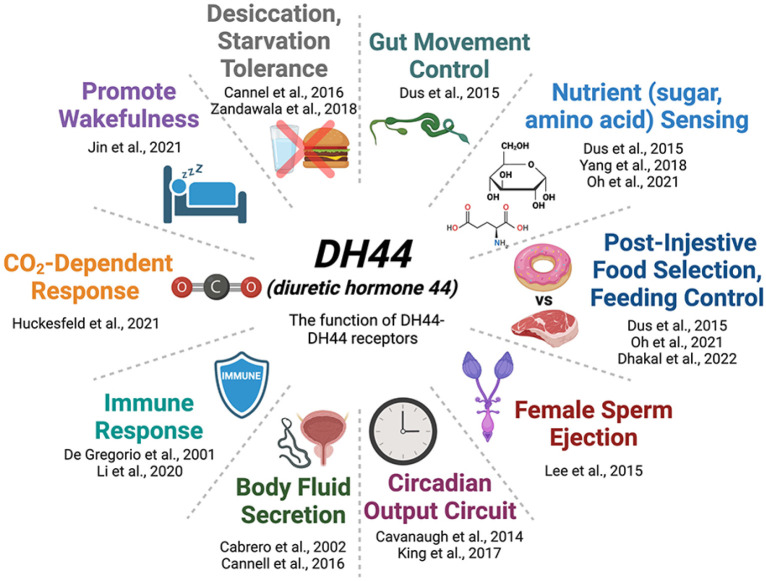
Fig. 3.
The biological function of DH44 and its receptors in Drosophila. The illustration shows the physiological and behavioral roles of the DH44 peptide and its receptors with representative references.
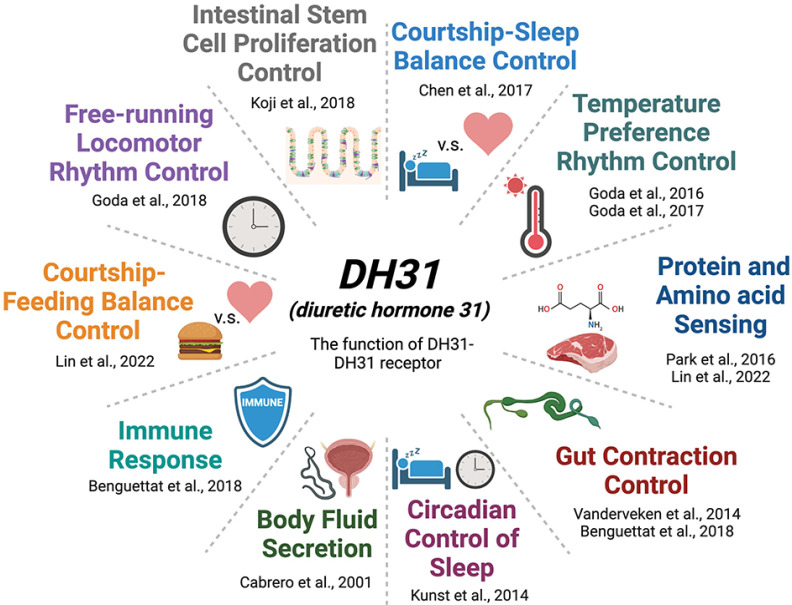
Fig. 4.
The biological function of DH31 and its receptor in Drosophila. The illustration shows the physiological and behavioral roles of the DH31 peptide and its receptor with representative references.
In mammals, the HPA axis regulates stress response and this axis depends on the CRF secretion from the hypothalamus of the brain (57). The sequence homology of the Drosophila DH44 peptide is more than 40% with mammalian CRF (6), indicating that those conserved neuropeptides may have similar biological functions in each species. Based on the twenty years of investigation, we found that the sequences and biological functions of those two peptides are identical, but their functions do not perfectly match each other. Both DH44 and CRF regulate the stress response, yet the DH44 circuit only regulates body fluid secretion and gut motility in flies, not the CRF-dependent circuits in mammals. DH31 peptide is also conserved with the mammalian CGRP, whereas it is still elusive whether the function of the DH31 circuit is related to those of calcitonin or CGRP in mammals. Future investigation is essential to understand the detailed mechanisms of how DH44 and DH31 signals regulate various behaviors in flies. Additionally, future studies will determine whether the mammalian CRF and CGRP signaling have similar functions with the DH44 and DH31 signaling in flies.
Funding Statement
ACKNOWLEDGEMENTS This work was supported by the Ewha Womans University Research Grant of 2022 (1-2022-0352-001-1), the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. RS-2023-00212599) to Y.O.
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
REFERENCES
- 1.Pribram KH. A review of theory in physiological psychology. Annu Rev Psychol. 1960;11:1–40. doi: 10.1146/annurev.ps.11.020160.000245. [DOI] [Google Scholar]
- 2.Phillips J. Comparative physiology of insect renal function. Am J Physiol Regul Integr Comp Physiol. 1981;241:241–257. doi: 10.1152/ajpregu.1981.241.5.R241. [DOI] [PubMed] [Google Scholar]
- 3.McFarland DJ. Decision making in animals. Nature. 1977;269:15–21. doi: 10.1038/269015a0. [DOI] [Google Scholar]
- 4.Nässel DR, Zandawala M. Recent advances in neuropeptide signaling in Drosophila, from genes to physiology and behavior. Prog Neurobiol. 2019;179:101607. doi: 10.1016/j.pneurobio.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Chung BY, Ro J, Hutter SA, et al. Drosophila neuropeptide F signaling independently regulates feeding and sleep-wake behavior. Cell Rep. 2017;19:2441–2450. doi: 10.1016/j.celrep.2017.05.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cabrero P, Radford JC, Broderick KE, et al. The Dh gene of Drosophila melanogaster encodes a diuretic peptide that acts through cyclic AMP. J Exp Biol. 2002;205:3799–3807. doi: 10.1242/jeb.205.24.3799. [DOI] [PubMed] [Google Scholar]
- 7.Dus M, Lai Jason SY, Gunapala Keith M, et al. Nutrient sensor in the brain directs the action of the brain-gut axis in Drosophila. Neuron. 2015;87:139–151. doi: 10.1016/j.neuron.2015.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oh Y, Lai JSY, Min S, et al. Periphery signals generated by Piezo-mediated stomach stretch and Neuromedin-mediated glucose load regulate the Drosophila brain nutrient sensor. Neuron. 2021;109:1979–1995. doi: 10.1016/j.neuron.2021.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hector CE, Bretz CA, Zhao Y, Johnson EC. Functional differences between two CRF-related diuretic hormone receptors in Drosophila. J Exp Biol. 2009;212:3142–3147. doi: 10.1242/jeb.033175. [DOI] [PubMed] [Google Scholar]
- 10.Veenstra JA, Agricola H-J, Sellami A. Regulatory peptides in fruit fly midgut. Cell Tissue Res. 2008;334:499–516. doi: 10.1007/s00441-008-0708-3. [DOI] [PubMed] [Google Scholar]
- 11.Johnson EC, Bohn LM, Taghert PH. Drosophila CG8422 encodes a functional diuretic hormone receptor. J Exp Biol. 2004;207:743–748. doi: 10.1242/jeb.00818. [DOI] [PubMed] [Google Scholar]
- 12.Johnson EC, Shafer OT, Trigg JS, et al. A novel diuretic hormone receptor in Drosophila: evidence for conservation of CGRP signaling. J Exp Biol. 2005;208:1239–1246. doi: 10.1242/jeb.01529. [DOI] [PubMed] [Google Scholar]
- 13.Audsley N, Kay I, Hayes TK, Coast GM. Cross reactivity studies of CRF-related peptides on insect Malpighian tubules. Comp Biochem Physiol A. 1995;110:87–93. doi: 10.1016/0300-9629(94)00132-D. [DOI] [PubMed] [Google Scholar]
- 14.Furuya K, Harper MA, Schegg KM, Schooley DA. Isolation and characterization of CRF-related diuretic hormones from the whitelined sphinx moth Hyles lineata. Insect Biochem Mol Biol. 2000;30:127–133. doi: 10.1016/S0965-1748(99)00106-X. [DOI] [PubMed] [Google Scholar]
- 15.Baldwin DC, Schegg KM, Furuya K, Lehmberg E, Schooley DA. Isolation and identification of a diuretic hormone from Zootermopsis nevadensis. Peptides. 2001;22:147–152. doi: 10.1016/S0196-9781(00)00371-5. [DOI] [PubMed] [Google Scholar]
- 16.O'Donnell MJ, Dow JA, Huesmann GR, Tublitz NJ, Maddrell SH. Separate control of anion and cation transport in malpighian tubules of Drosophila melanogaster. J Exp Biol. 1996;199:1163–1175. doi: 10.1242/jeb.199.5.1163. [DOI] [PubMed] [Google Scholar]
- 17.Coast GM. Neuropeptides implicated in the control of diuresis in insects. Peptides. 1996;17:327–336. doi: 10.1016/0196-9781(95)02096-9. [DOI] [PubMed] [Google Scholar]
- 18.Coast GM, Webster SG, Schegg KM, Tobe SS, Schooley DA. The Drosophila melanogaster homologue of an insect calcitonin-like diuretic peptide stimulates V-ATPase activity in fruit fly Malpighian tubules. J Exp Biol. 2001;204:1795–1804. doi: 10.1242/jeb.204.10.1795. [DOI] [PubMed] [Google Scholar]
- 19.Cannell E, Dornan AJ, Halberg KA, Terhzaz S, Dow JAT, Davies S-A. The corticotropin-releasing factor-like diuretic hormone 44 (DH44) and kinin neuropeptides modulate desiccation and starvation tolerance in Drosophila melanogaster. Peptides. 2016;80:96–107. doi: 10.1016/j.peptides.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zandawala M, Marley R, Davies SA, Nässel DR. Characterization of a set of abdominal neuroendocrine cells that regulate stress physiology using colocalized diuretic peptides in Drosophila. Cell Mol Life Sci. 2018;75:1099–1115. doi: 10.1007/s00018-017-2682-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benguettat O, Jneid R, Soltys J, et al. The DH31/CGRP enteroendocrine peptide triggers intestinal contractions favoring the elimination of opportunistic bacteria. PLoS Pathog. 2018;14:e1007279. doi: 10.1371/journal.ppat.1007279.154cda8f811f491fa900b34507d8318a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X, Rommelaere S, Kondo S, Lemaitre B. Renal purge of hemolymphatic lipids prevents the accumulation of ROS-induced inflammatory oxidized lipids and protects Drosophila from tissue damage. Immunity. 2020;52:374–387. doi: 10.1016/j.immuni.2020.01.008. [DOI] [PubMed] [Google Scholar]
- 23.De Gregorio E, Spellman PT, Rubin GM, Lemaitre B. Genome-wide analysis of the Drosophila immune response by using oligonucleotide microarrays. Proc Natl Acad Sci U S A. 2001;98:12590–12595. doi: 10.1073/pnas.221458698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taghert PH, Shafer OT. Mechanisms of clock output in the Drosophila circadian pacemaker system. J Biol Rhythms. 2006;21:445–457. doi: 10.1177/0748730406293910. [DOI] [PubMed] [Google Scholar]
- 25.Konopka RJ, Benzer S. Clock mutants of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1971;68:2112–2116. doi: 10.1073/pnas.68.9.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reddy P, Zehring WA, Wheeler DA, et al. Molecular analysis of the period locus in Drosophila melanogaster and identification of a transcript involved in biological rhythms. Cell. 1984;38:701–710. doi: 10.1016/0092-8674(84)90265-4. [DOI] [PubMed] [Google Scholar]
- 27.Shin H-S, Bargiello TA, Clark BT, Jackson FR, Young MW. An unusual coding sequence from a Drosophila clock gene is conserved in vertebrates. Nature. 1985;317:445–448. doi: 10.1038/317445a0. [DOI] [PubMed] [Google Scholar]
- 28.Vosshall LB, Price JL, Sehgal A, Saez L, Young MW. Block in nuclear localization of period protein by a second clock mutation, timeless. Science. 1994;263:1606–1609. doi: 10.1126/science.8128247. [DOI] [PubMed] [Google Scholar]
- 29.Kunst M, Hughes Michael E, Raccuglia D, et al. Calcitonin gene-related peptide neurons mediate sleep-specific circadian output in Drosophila. Curr Biol. 2014;24:2652–2664. doi: 10.1016/j.cub.2014.09.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cavanaugh Daniel J, Geratowski Jill D, Wooltorton Julian RA, et al. Identification of a circadian output circuit for rest: activity rhythms in Drosophila. Cell. 2014;157:689–701. doi: 10.1016/j.cell.2014.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goda T, Tang X, Umezaki Y, et al. Drosophila DH31 neuropeptide and PDF receptor regulate night-onset temperature preference. J Neurosci. 2016;36:11739. doi: 10.1523/JNEUROSCI.0964-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.King AN, Barber AF, Smith AE, et al. A peptidergic circuit links the circadian clock to locomotor activity. Curr Biol. 2017;27:1915–1927. doi: 10.1016/j.cub.2017.05.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goda T, Umezaki Y, Alwattari F, Seo HW, Hamada FN. Neuropeptides PDF and DH31 hierarchically regulate free-running rhythmicity in Drosophila circadian locomotor activity. Sci Rep. 2019;9:838. doi: 10.1038/s41598-018-37107-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goda T, Doi M, Umezaki Y, et al. Calcitonin receptors are ancient modulators for rhythms of preferential temperature in insects and body temperature in mammals. Genes Dev. 2018;32:140–155. doi: 10.1101/gad.307884.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Allada R, Siegel JM. Unearthing the phylogenetic roots of sleep. Curr Biol. 2008;18:670–679. doi: 10.1016/j.cub.2008.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borbély AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 37.Chen D, Sitaraman D, Chen N, et al. Genetic and neuronal mechanisms governing the sex-specific interaction between sleep and sexual behaviors in Drosophila. Nat Commun. 2017;8:154. doi: 10.1038/s41467-017-00087-5.b2bf3637c1d941c1986c1030c17eb3a7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jin X, Tian Y, Zhang ZC, Gu P, Liu C, Han J. A subset of DN1p neurons integrates thermosensory inputs to promote wakefulness via CNMa signaling. Curr Biol. 2021;31:2075–2087. doi: 10.1016/j.cub.2021.02.048. [DOI] [PubMed] [Google Scholar]
- 39.Yarmolinsky DA, Zuker CS, Ryba NJP. Common sense about taste: from mammals to insects. Cell. 2009;139:234–244. doi: 10.1016/j.cell.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang Z, Huang R, Fu X, et al. A post-ingestive amino acid sensor promotes food consumption in Drosophila. Cell Res. 2018;28:1013–1025. doi: 10.1038/s41422-018-0084-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miyamoto T, Slone J, Song X, Amrein H. A fructose receptor functions as a nutrient sensor in the Drosophila brain. Cell. 2012;151:1113–1125. doi: 10.1016/j.cell.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dus M, Min S, Keene AC, Lee GY, Suh GSB. Taste-independent detection of the caloric content of sugar in Drosophila. Proc Natl Acad Sci U S A. 2011;108:11644–11649. doi: 10.1073/pnas.1017096108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen YCD, Dahanukar A. DH44 neurons: gut-brain amino acid sensors. Cell Res. 2018;28:1048–1049. doi: 10.1038/s41422-018-0101-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bjordal M, Arquier N, Kniazeff J, Pin Jean P, Léopold P. Sensing of amino acids in a dopaminergic circuitry promotes rejection of an incomplete diet in Drosophila. Cell. 2014;156:510–521. doi: 10.1016/j.cell.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 45.Park JH, Chen J, Jang S, et al. A subset of enteroendocrine cells is activated by amino acids in the Drosophila midgut. FEBS Lett. 2016;590:493–500. doi: 10.1002/1873-3468.12073. [DOI] [PubMed] [Google Scholar]
- 46.Saper CB, Chou TC, Elmquist JK. The need to feed: homeostatic and hedonic control of eating. Neuron. 2002;36:199–211. doi: 10.1016/S0896-6273(02)00969-8. [DOI] [PubMed] [Google Scholar]
- 47.Koji T, Takashi O, Mayu T, Mio Y, Kiichiro T, Takashi AY. Drosophila peptide hormones allatostatin A and diuretic hormone 31 exhibiting complementary gradient distribution in posterior midgut antagonistically regulate midgut senescence and adult lifespan. Zool Sci. 2018;35:75–85. doi: 10.2108/zs160210. [DOI] [PubMed] [Google Scholar]
- 48.Jones WD, Cayirlioglu P, Grunwald Kadow I, Vosshall LB. Two chemosensory receptors together mediate carbon dioxide detection in Drosophila. Nature. 2007;445:86–90. doi: 10.1038/nature05466. [DOI] [PubMed] [Google Scholar]
- 49.Suh GSB, Wong AM, Hergarden AC, et al. A single population of olfactory sensory neurons mediates an innate avoidance behaviour in Drosophila. Nature. 2004;431:854–859. doi: 10.1038/nature02980. [DOI] [PubMed] [Google Scholar]
- 50.Fischler W, Kong P, Marella S, Scott K. The detection of carbonation by the Drosophila gustatory system. Nature. 2007;448:1054–1057. doi: 10.1038/nature06101. [DOI] [PubMed] [Google Scholar]
- 51.Hückesfeld S, Schlegel P, Miroschnikow A, et al. Unveiling the sensory and interneuronal pathways of the neuroendocrine connectome in Drosophila. eLife. 2021;10:e65745. doi: 10.7554/eLife.65745.9c88b8a123134ed3babb3375a0264da2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Birkhead TR, Pizzari T. Postcopulatory sexual selection. Nat Rev Genet. 2002;3:262–273. doi: 10.1038/nrg774. [DOI] [PubMed] [Google Scholar]
- 53.Manier MK, Belote JM, Berben KS, Novikov D, Stuart WT, Pitnick S. Resolving mechanisms of competitive fertilization success in Drosophila melanogaster. Science. 2010;328:354–357. doi: 10.1126/science.1187096. [DOI] [PubMed] [Google Scholar]
- 54.Lee K-M, Daubnerová I, Isaac RE, et al. A neuronal pathway that controls sperm ejection and storage in female Drosophila. Curr Biol. 2015;25:790–797. doi: 10.1016/j.cub.2015.01.050. [DOI] [PubMed] [Google Scholar]
- 55.Keene AC, Duboué ER, McDonald DM, et al. Clock and cycle limit starvation-induced sleep loss in Drosophila. Curr Biol. 2010;20:1209–1215. doi: 10.1016/j.cub.2010.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin H-H, Kuang MC, Hossain I, et al. A nutrient-specific gut hormone arbitrates between courtship and feeding. Nature. 2022;602:632–638. doi: 10.1038/s41586-022-04408-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Charmandari E, Tsigos C, Chrousos G. Endocrinology of the stress response. Annu Rev Physiol. 2004;67:259–284. doi: 10.1146/annurev.physiol.67.040403.120816. [DOI] [PubMed] [Google Scholar]