Abstract
Thioredoxin-like protein 1 (TXNL1), one of the thioredoxin superfamily known as redox-regulator, plays an essential in maintaining cell survival via various antioxidant and anti-apoptotic mechanisms. It is well known that relationship between ischemia and oxidative stress, however, the role of TXNL1 protein in ischemic damage has not been fully investigated. In the present study, we aimed to determine the protective role of TXNL1 against on ischemic injury in vitro and in vivo using cell permeable Tat-TXNL1 fusion protein. Transduced Tat-TXNL1 inhibited ROS production and cell death in H2O2-exposed hippocampal neuronal (HT-22) cells and modulated MAPKs and Akt activation, and pro-apoptotic protein expression levels in the cells. In an ischemia animal model, Tat-TXNL1 markedly decreased hippocampal neuronal cell death and the activation of astrocytes and microglia. These findings indicate that cell permeable Tat-TXNL1 protects against oxidative stress in vitro and in vivo ischemic animal model. Therefore, we suggest Tat-TXNL1 can be a potential therapeutic protein for ischemic injury.
Keywords: Apoptosis, Ischemic injury, MAPK, Protein therapy, Tat-TXNL1
INTRODUCTION
Thioredoxin-like protein 1 (TXNL1), known as thioredoxin-related protein of 32 kDa (TRP32), is a member of the thioredoxin family and is localized in the nucleus and cytoplasm (1, 2). TXNL1 processes two domains, the N-terminal Trx domain and a C-terminal domain which possess the unknown function 1000 (DUF1000) and this domain interacts with the 26S proteasome (3, 4). It is well known that this protein alters the pathogenesis of a number of diseases through their anti-oxidative mechanism. It is reported that TXNL1 protects against glucose deprivation-induced cytotoxicity in human embryonic kidney 293 cells (HEK-293 cells) (5) and overexpression of TXNL1 inhibits mammalian cell proliferation and acts as a transcriptional repressor through direct binding to the transcription factor B-Myb in SNU-1 cells (6). Furthermore overexpression of TXNL1 prevents cell death and cancer progression through the inactivation of oxidative stress-induced phosphatase of regenerating liver (PRL) (7). Xu et al. also reported that reduced expression of base excision repair protein XRCC1 (X-ray repair cross complementing group1) in gastric cancer tissues correlates with a significant survival benefit from chemotherapy. Upregulation of TXNL1 promoted the degradation of XRCC1 expression, in contrast, TXNL1 downregulated whereas XRCC1 was upregulated in BGC823/DDP cells. Thus, authors suggested that expression of TXNL1 and XRCC1 may have an important role in gastric cancer cells responsive to oxidative stress, contribute to chemotherapy resistance and potential drug targets for adjuvant chemotherapy in gastric cancer (8).
Reactive oxygen species (ROS) like H2O2 cause oxidative damage that is highly associated with a wide range of diseases including neuronal diseases and cancers (9, 10). Increased production of ROS induced toxic effects on DNA damage in neuronal cells and high levels of ROS are associated with brain ischemic injury (11). It is well known that Trx1 protein plays important role in reducing oxidative stress in the brain, therefore, it was hypothesized that inhibition or regulation of ROS production through TXNL1 may have a protective effect against brain ischemic injury (12, 13). However, the precise protective mechanism of this protein against ischemic insult is not well studied yet.
Protein transduction domains (PTDs) including trans-acting activator of transcription (Tat) PTD are well described tools for protein delivery into cells. Notably, Tat PTD fusion proteins are not be limited by the protein size of the protein and various cell permeable fusion proteins have been showed protective effects in vitro and in vivo (14-24). Thus, in the present study, we determine the protective effects of Tat-TXNL1 in HT-22 cells and in an ischemic animal model.
RESULTS
Purification and transduction of Tat-TXNL1
We showed a diagram of Tat-TXNL1 and TXNL1 protein. After the construction of both proteins, we confirmed the purified proteins. Purified Tat-TXNL1 and TXNL1 protein displayed the expected molecular weights (Supplementary Fig. 1).
The HT-22 cells were exposed to Tat-TXNL1 (0.5-7 μM) for 1 h or Tat-TXNL1 (7 μM) for 5-60 min, confirm the transduced Tat-TXNL1. Tat-TXNL1 transduced into HT-22 cells in a concentration and a time-dependent manner. Also, Transduced Tat-TXNL1 showed for to 12 h and distributed in the cells cytoplasm and nucleus. However, TXNL1 did not transduce into the cells (Supplementary Fig. 2).
Effects of Tat-TXNL1 on cell death
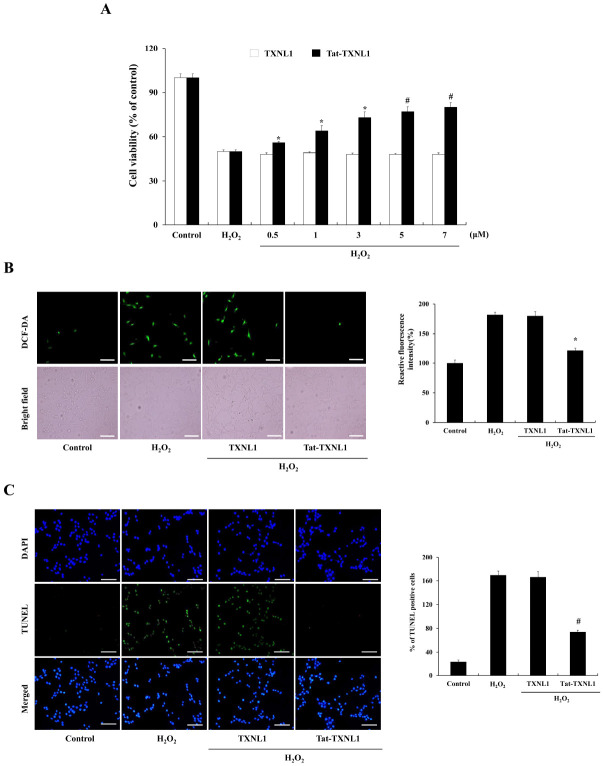
To examine the effects of Tat-TXNL1 against oxidative stress-induced HT-22 cell damage; the cells were treated with hydrogen peroxide (H2O2) and assessed for viability, ROS generation, and DNA fragmentation. Tat-TXNL1 transduced HT-22 cells showed markedly increased cell survival compared with only H2O2 treated HT-22 cells (Fig. 1A). Also, transduced Tat-TXNL1 markedly reduced ROS generation and DNA damage (Fig. 1B, C). On the other hand, there was no significant difference between H2O2 alone and TXNL1 treated cells.
Fig. 1.
Effects of Tat-TXNL1 protein against oxidative stress-induced HT-22 cell damage. (A) Effect of transduced Tat-TXNL1 on cell viability. HT-22 cells were pretreated with Tat-TXNL1 (0.5-7 μM) for 1 h and exposed to H2O2 (1 mM). Cell viabilities were estimated using a colorimetric assay using MTT. Effects of Tat-TXNL1 against H2O2-induced ROS production and DNA fragmentation. HT-22 cells were treated with Tat-TXNL1 (7 μM) or TXNL1 for 1 h and exposed to H2O2 (1 mM). Then, (B) ROS production levels were determined by DCF-DA staining. Fluorescence intensity was quantified using an ELISA plate reader. (C) DNA fragmentation levels were determined by TUNEL staining and quantitative evaluation of TUNEL positive cells confirmed by cell counting under a phase-contrast microscopy (×200 magnification). Scale bar = 50 μm. *P < 0.05 and #P < 0.01 compared with H2O2 treated cells. The bars in the figure represent the mean ± SEM obtained from 3 independent experiments.
Effects of Tat-TXNL1 on MAPK and apoptotic signaling pathways
Mitogen activated protein kinases (MAPKs) and Akt signaling pathways play crucial roles in cell death regulation and survival on oxidative stress (25-28). We examined whether Tat-TXNL1 inhibited the activation of MAPK and Akt signaling (Fig. 2). In the H2O2 treated cells, phosphorylated MAPKs and Akt levels were increased compared to the control cells; the phosphorylated MAPKs and Akt levels were not changed in the TXNL1 treated cells. However, Tat-TXNL1 treated cells showed markedly reduced the phosphorylated MAPKs and Akt levels.
Fig. 2.
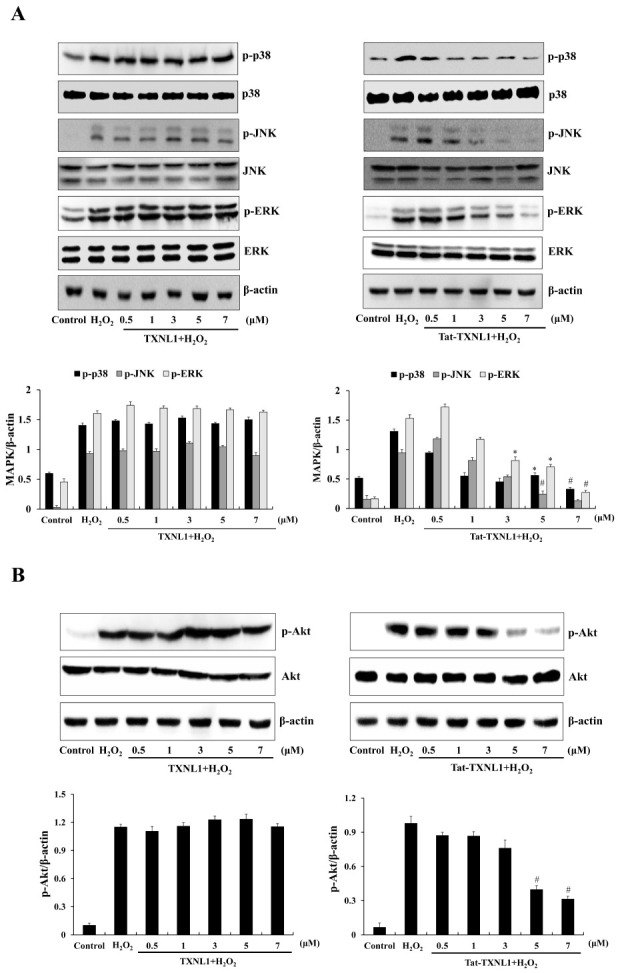
Effects of Tat-TXNL1 protein on H2O2-induced MAPKs and Akt activation in HT-22 cells. The cells were treated with Tat-TXNL1 (0.5-7 μM) or TXNL1 for 1 h and exposed to H2O2 (1 mM). (A) MAPK and (B) Akt activation was analyzed by Western blotting. Band intensity was measured by densitometry. *P < 0.05 and #P < 0.01 compared with H2O2 treated cells. The bars in the figure represent the mean ± SEM obtained from 3 independent experiments.
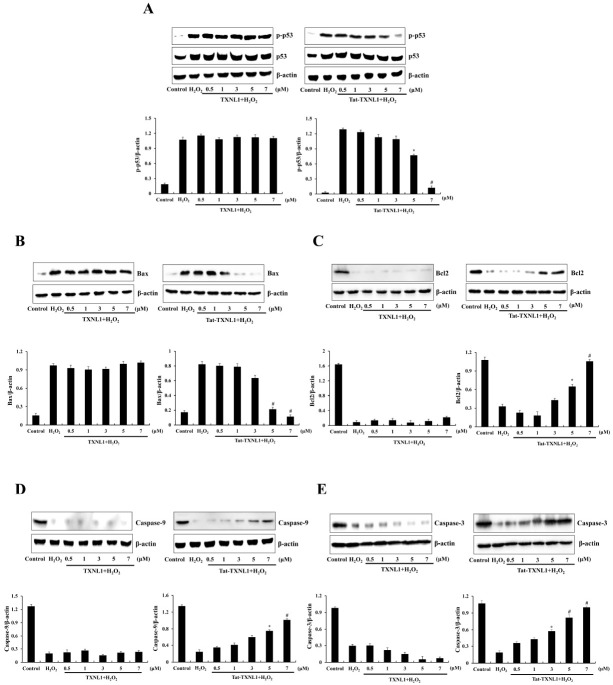
As shown in Fig. 3, p53 and Bax levels were markedly increased in the H2O2 treatment HT-22 cells while Tat-TXNL1 significantly reduced the phosphorylated p53 and Bax levels. On the other hand, Tat-TXNL1 increased levels of Bcl-2, pro-caspase-3, and pro-caspase-9 levels. There was no significant difference between H2O2 alone and TXNL1 treated cells.
Fig. 3.
Effect of Tat-TXNL1 protein against H2O2-induced apoptotic protein expression in HT-22 cells. The cells were treated with Tat-TXNL1 (0.5-7 μM) or TXNL1 for 1 h and exposed to H2O2 (1 mM). The expression of (A) p53, (B) Bax, (C) Bcl-2, (D) Pro-caspase-9 and (E) Pro-caspase-3 were analyzed by Western blotting. The band intensity was measured by densitometry. *P < 0.05 and #P < 0.01 compared with H2O2 treated cells. The bars in the figure represent the mean ± SEM obtained from 3 independent experiments.
Effects of Tat-TXNL1 on ischemic brain injury
We performed an immunohistochemistry test to determine the effects of Tat-TXNL1 on neuronal damage after ischemic brain injury (Fig. 4). Cresyl violet (CV) staining was used to examine changes in the cellular distribution and morphology in the brain. In the vehicle- and TXNL-ischemia group, a significant loss of CV-positive neuronal cells was observed at 7 days after ischemic brain injury. However, a large number of CV-positive neuronal cells were observed in the Tat-TXNL1-treated ischemia group. Also, Fluoro-Jade B (F-JB) specifically binds to degenerating neurons and is known as a marker for degenerating or dead cells. In this study, after F-JB staining, F-JB positive cells were markedly increased in the vehicle- and TXNL-ischemia group. However, F-JB positive cells were markedly reduced in the Tat-TXNL1-treated ischemia group.
Fig. 4.
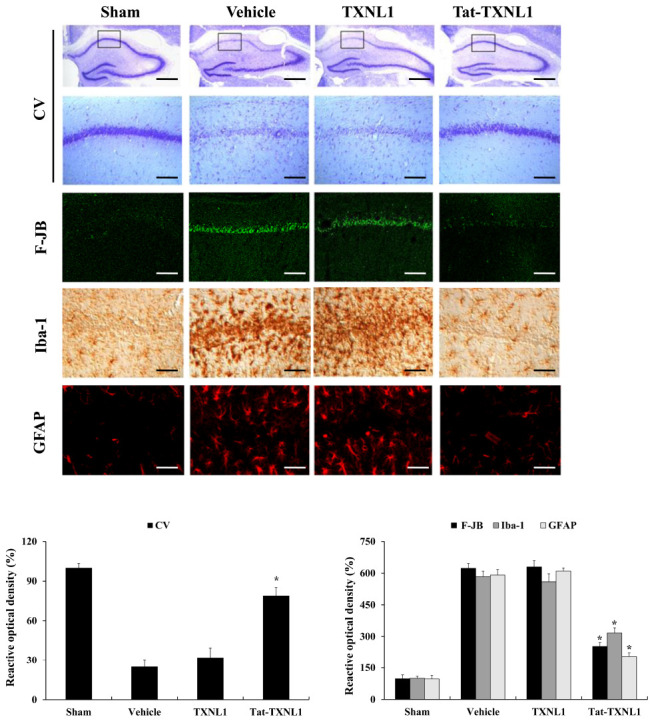
Protective effects of Tat-TXNL1 protein on ischemic injury. Gerbils were treated with single injections Tat-TXNL1 (2 mg/kg) and killed after 7 days. Then, the effects of Tat-TXNL1 on neuronal cell viability determined using immunostaining. The hippocampus was stained with CV, F-JB, Iba-1, and GFAP in sham-, vehicle-, TXNL1-, and Tat-TXNL1-treated animals 7 days after ischemia-reperfusion. Relative numeric analysis of CV-, F-JB-, Iba-1-, and GFAP-positive neurons in CA1 region. Scale bar = 400 and 50 μm. *P < 0.01 significantly different from the vehicle group.
Further, we examined the changes in ionized calcium-binding adaptor molecule 1 (Iba-1; a marker for microglia) and glial fibrillary acidic protein (GFAP; a marker for astrocyte)-positive cells after ischemic brain injury (Fig. 4). In the vehicle- and TXNL-ischemia group, Iba-1, and GFAP-positive cells were significantly increased and hypertrophied compared to that in the sham control group. In the Tat-TXNL1-treated ischemia group, there was no significant change in the morphology of Iba-1 and GFAP-positive cells compared to the sham control group.
DISCUSSION
Thioredoxin plays a critical role in several biological processes including the maintenance of cellular homeostatic and redox balance (29-31) and TXNL1, a member of the thioredoxin family, is present in the cytoplasm, and the nucleus of the cells (1, 2). TXNL1 is involved in the function of the regulatory particle non-ATPase 11, a subunit of the 26S proteasome, translation elongation factor 1A, and in the transfer of misfolded protein degradation (32-34). Other studies have shown that overexpression of TXNL1 protected cell death by reducing cellular cytotoxicity (5, 7). Recent study reported that recombinant seahorse TXNL1 has antioxidant and free radical scavenging activity (35) and several studies have reported that excessive ROS enhances the modification of macromolecules and cell death (36) and involved in a wide range of diseases including neural diseases like brain ischemic injury (9-11). Although Sugawara and Chan reported that inhibition of excessive ROS production can protect against ischemic brain injury (13), they didn’t show the precise protective mechanism. Therefore, in the present study, we investigated protective mechanism of TXNL1 protein against hippocampal neuronal cell damage induced by oxidative stress by using the cell permeable Tat-TXNL1 fusion protein.
It has been reported that excessive ROS increases phosphorylation of MAPKs leading to neuronal cell death (26, 37, 38) and overexpression of TXNL1 shows reduction of neuronal cell damage by regulation of MAPKs signaling pathways and plays an anti-apoptotic effect in vitro. Therefore, they conjectured that TXNL1 might be a potential therapeutic target for ischemic injury (39-41). In the present study, we demonstrated that Tat-TXNL1 significantly inhibits the levels of phosphorylated MAPKs in oxidative stress-induced HT-22 cells, suggesting that Tat-TXNL1 protects HT-22 cell death through the regulation MAPKs signaling pathways.
Since it is well known that apoptosis plays a pivotal role in ischemic brain injury, it is necessary to investigate the proteins such as Bax and Bcl-2 involved in the apoptotic signaling pathways. Bax and Bcl-2 play a key role in the apoptosis because overexpression of Bax is primarily executed apoptosis and down-regulation of Bcl-2 initiates caspase-dependent cell death and it was reported that the regulation of Bax and Bcl-2 expressions has been suggested as a therapeutic strategy in apoptosis-related disorders (42-44). Other studies have reported that treatment with coenzyme Q10 (CoQ10), a strong anti-oxidant agent, markedly increased Bcl-2 levels in cerebral ischemic injury, suggesting that CoQ10 protects ischemic injury by inhibiting apoptosis (45).
We have shown that Tat-TXNL1 increased Bcl-2, pro-caspase-3, and pro-caspase-9 levels, whereas Bax levels were reduced in H2O2-exposed HT-22 cells. These findings indicate that transduced Tat-TXNL1 suppressed ROS-induced apoptosis through the regulation of pro-apoptotic proteins and anti-apoptotic proteins. We confirmed the Yoo et al. report that they showed knockdown of Trx1 markedly increased apoptosis and cell death in EMT6 cells (46). Other studies have shown that ROS is necessary for the induction of apoptosis via pro-caspase-9 and pro-caspase-3 signaling (47) and excessive ROS leads to the disruption of mitochondrial membrane and the release of cytochrome c into the cytoplasm which activates pro-caspase-9 and pro-caspase-3 to initiate apoptosis (48).
At high levels of ROS, p53 protein plays an important role in the progression of apoptosis through interaction with the mitochondrial apoptotic proteins Bax and Bcl-2 which are known as signaling proteins that regulate the direct activation of pro-caspase-9 and pro-caspase-3 (49-51). A potent ROS inducer H2O2 reduces pro-caspase-9 and pro-caspase-3 in PC12 cells in a time-dependent manner and promotes phosphorylation of p53 through activation of pro-caspase-9 and pro-caspase-3 signaling by Bax and Bcl-2 (52). Recently, there are several reports involved in the relationship between ischemic neuronal cell damage and apoptotic signaling. Tan et al. (2021) reported that mitochonic acid 5 (MA-5) reduced the lipopolysaccharide (LPS)-induced cleaved-caspase-3 expression in BV-2 cells, suggesting that MA-5 may promote the survival of microglial cells in response to LPS-induced inflammation (53). Wang et al. (2021) showed that Senkyunolide I (SEI) have been used in traditional Chinese medicine for the treatment of stroke by significant inhibition of the increasing the protein level of cleaved caspase-3 in glutamate-induced Neuro2a cells, suggesting that SEI has beneficial effects against focal cerebral ischemia-reperfusion in rats (54). In addition, Kang et al. (2021) reported that retinoic acid (RA) markedly reduced the expression of cleaved caspase-3 and -9 in the cerebral cortex of middle cerebral artery occlusion (MCAO) animals and they suggested that RA exhibits a neuroprotective effect against ischemic damage by modulating apoptosis signaling pathway (55). As mentioned above, we have shown that Tat-TXNL1 drastically increased the level of Bcl-2 expression and significantly reduced Bax in the H2O2 treatment HT-22 cells. These results indicate that Tat-TXNL1 exert a protective effect against oxidative stress-induced HT-22 cell death by regulating apoptosis signaling pathways. However, further studies of precise protective mechanism of Tat-TXNL1 are remains to be elucidated.
Since the oxidative stress is significantly involved in ischemia, inhibition of oxidative stress is a potential target for neuroprotection in ischemic injury (11-13, 56). Activation of astrocytes and microglia in the brain plays crucial roles in the pathogenesis of cerebral ischemic injury and some studies suggested that the inhibition of astrocytes and microglia activations is a potential target for neuroprotective strategies in ischemic injury (57-61). In a previous study, we have reported that Tat fused glyoxalase and CIAPIN1 (Tat-glyoxalase and Tat-CIAPIN1) protein reduced astrocytes and microglia activation in an ischemic animal model (19, 22). We showed that Tat-TXNL1 markedly increased CV-positive neuronal cells whereas F-JB-positive neuronal cells were reduced in the ischemic animal model. We further have shown that Iba-1 and GFAP-positive cells were significantly increased in the ischemic injury animal model. However, Tat-TXNL1 reduced the Iba-1 and GFAP-positive cells and no significant change in the morphology compared to the normal animal. These results indicate that Tat-TXNL1 reduced neuronal cell death and activation of astrocytes and microglia in the ischemia animal model.
In summary, we demonstrated that Tat-TXNL1 transduced into HT-22 cells and showed protective effects against H2O2-induced cytotoxicity by inhibiting excessive ROS production through MAPK and apoptotic signaling pathways. Increasing intracellular ROS can lead to MAPK activation and apoptotic cell signaling and transduced Tat-TXNL1 prevented hippocampal neuronal cell death and this fusion protein plays a protective role in ischemic brain injury. Those results indicate that TXNL1 protein can be a putative therapeutic agent for brain ischemic injury.
MATERIALS AND METHODS
See supplementary information for this section.
Funding Statement
ACKNOWLEDGEMENTS This research was supported by Basic Science Research Program (2018R1D1A3B07049265 & 2019R1A6A1A11036849) through the National Research Foundation of Korea (NRF) funded by the Ministry of Education.
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
REFERENCES
- 1.Lee KK, Murakawa M, Takahashi S, et al. Purification, molecular cloning, and characterization of TRP32, a novel thioredoxin-related mammalian protein of 32 kDa. J Biol Chem. 1998;273:19160–19166. doi: 10.1074/jbc.273.30.19160. [DOI] [PubMed] [Google Scholar]
- 2.Miranda-Vizuete A, Gustafsson JA, Spyrou G. Molecular cloning and expression of a cDNA encoding a human thioredoxin-like protein. Biochem Biophys Res Commun. 1998;243:284–288. doi: 10.1006/bbrc.1997.8003. [DOI] [PubMed] [Google Scholar]
- 3.Goroncy AK, Koshiba S, Tochio N, et al. Solution structure of the C-terminal DUF1000 domain of the human thioredoxin-like 1 protein. Proteins. 2010;78:2176–2180. doi: 10.1002/prot.22719. [DOI] [PubMed] [Google Scholar]
- 4.Jin J, Chen X, Zhou Y, et al. Crystal structure of the catalytic domain of a human thioredoxin-like protein. Eur J Biochem. 2002;269:2060–2068. doi: 10.1046/j.1432-1033.2002.02844.x. [DOI] [PubMed] [Google Scholar]
- 5.Jimenez A, Pelto-Huikko M, Gustafsson JA, Miranda-Vizuete A. Characterization of human thioredoxin-like-1: potential involvement in the cellular response against glucose deprivation. FEBS Lett. 2006;580:960–967. doi: 10.1016/j.febslet.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 6.Kim KY, Lee JW, Park MS, Jung MH, Jeon GA, Nam MJ. Expression of a thioredoxin-related protein-1 is induced by prostaglandin E(2) Int J Cancer. 2006;118:1670–1679. doi: 10.1002/ijc.21572. [DOI] [PubMed] [Google Scholar]
- 7.Ishii T, Funato Y, Miki H. Thioredoxin-related protein 32 (TRP32) specifically reduces oxidized phosphatase of regenerating liver (PRL) J Biol Chem. 2013;288:7263–7270. doi: 10.1074/jbc.M112.418004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu W, Wang S, Chen Q, et al. TXNL1-XRCC1 pathway regulates cisplatin-induced cell death and contributes to resistance in human gastric cancer. Cell Death Dis. 2014;5:e1055. doi: 10.1038/cddis.2014.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown JD, Day AM, Taylor SR, Tomalin LE, Morgan BA, Veal EA. A peroxiredoxin promotes H2O2 signaling and oxidative stress resistance by oxidizing a thioredoxin family protein. Cell Rep. 2013;5:1425–1435. doi: 10.1016/j.celrep.2013.10.036.9cc8c00a0d4343e0af668d7ca601d652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li PA, He QP, Nakamura L, Csiszar K. Free radical spin trap alpha-phenyl-N-tert-butyl-nitron inhibits caspase-3 activation and reduces brain damage following a severe forebrain ischemic injury. Free Radic Biol Med. 2001;31:1191–1197. doi: 10.1016/S0891-5849(01)00700-6. [DOI] [PubMed] [Google Scholar]
- 11.Floyd RA. Role of oxygen free radicals in carcinogenesis and brain ischemia. FASEB J. 1990;4:2587–2597. doi: 10.1096/fasebj.4.9.2189775. [DOI] [PubMed] [Google Scholar]
- 12.Ginsberg MD, Becker DA, Busto R, et al. Stilbazulenyl nitrone, a novel antioxidant, is highly neuroprotective in focal ischemia. Ann Neurol. 2003;54:330–342. doi: 10.1002/ana.10659. [DOI] [PubMed] [Google Scholar]
- 13.Sugawara T, Chan PH. Reactive oxygen radicals and pathogenesis of neuronal death after cerebral ischemia. Antioxid Redox Signal. 2003;5:597–607. doi: 10.1089/152308603770310266. [DOI] [PubMed] [Google Scholar]
- 14.El-Andaloussi S, Holm T, Langel U. Cell-penetrating peptides: mechanisms and applications. Curr Pharm Des. 2005;11:3597–3611. doi: 10.2174/138161205774580796. [DOI] [PubMed] [Google Scholar]
- 15.Kim MJ, Park M, Kim DW, et al. Transduced PEP-1-PON1 proteins regulate microglial activation and dopaminergic neuronal death in a Parkinson's disease model. Biomaterials. 2015;64:45–56. doi: 10.1016/j.biomaterials.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 16.Kubo E, Fatma N, Akagi Y, Beier DR, Singh SP, Singh DP. TAT-mediated PRDX6 protein transduction protects against eye lens epithelial cell death and delays lens opacity. Am J Physiol Cell Physiol. 2008;294:C842–C855. doi: 10.1152/ajpcell.00540.2007. [DOI] [PubMed] [Google Scholar]
- 17.Moon JI, Han MJ, Yu SH, et al. Enhanced delivery of protein fused to cell penetrating peptides to mammalian cells. BMB Rep. 2019;52:324–329. doi: 10.5483/BMBRep.2019.52.5.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakurazawa M, Katsura KI, Saito M, Asoh S, Ohta S, Katayama Y. Mild hypothermia enhanced the protective effect of protein therapy with transductive anti-death FNK protein using a rat focal transient cerebral ischemia model. Brain Res. 2012;1430:86–92. doi: 10.1016/j.brainres.2011.10.041. [DOI] [PubMed] [Google Scholar]
- 19.Shin MJ, Kim DW, Lee YP, et al. Tat-glyoxalase protein inhibits against ischemic neuronal cell damage and ameliorates ischemic injury. Free Radic Biol Med. 2014;67:195–210. doi: 10.1016/j.freeradbiomed.2013.10.815. [DOI] [PubMed] [Google Scholar]
- 20.van den Berg A, Dowdy SF. Protein transduction domain delivery of therapeutic macromolecules. Curr Opin Biotechnol. 2011;22:888–893. doi: 10.1016/j.copbio.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 21.Wadia JS, Dowdy SF. Protein transduction technology. Curr Opin Biotechnol. 2002;13:52–56. doi: 10.1016/S0958-1669(02)00284-7. [DOI] [PubMed] [Google Scholar]
- 22.Yeo HJ, Shin MJ, Yeo EJ, et al. Tat-CIAPIN1 inhibits hippocampal neuronal cell damage through the MAPK and apoptotic signaling pathways. Free Radic Biol Med. 2019;135:68–78. doi: 10.1016/j.freeradbiomed.2019.02.028. [DOI] [PubMed] [Google Scholar]
- 23.Yeo HJ, Shin MJ, Kim DW, Kwon HY, Eum WS, Choi SY. Tat-CIAPIN1 protein prevents against cytokine-induced cytotoxicity in pancreatic RINm5F β-cells. BMB Rep. 2021;54:458–446. doi: 10.5483/BMBRep.2021.54.9.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang X, Li Y, Cheng Y, et al. Tat PTD-endostatin: A novel anti-angiogenesis protein with ocular barrier permeability via eye-drops. Biochim Biophys Acta. 2015;1850:1140–1149. doi: 10.1016/j.bbagen.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 25.Ahn KH, Kim YS, Kim SY, Huh Y, Park C, Jeong JW. Okadaic acid protects human neuroblastoma SH-SY5Y cells from 1-methyl-4-phenylpyridinium ion-induced apoptosis. Neurosci Lett. 2009;449:93–97. doi: 10.1016/j.neulet.2008.10.103. [DOI] [PubMed] [Google Scholar]
- 26.Kim EK, Choi EJ. Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta. 2010;1802:396–405. doi: 10.1016/j.bbadis.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 27.Uranga RM, Katz S, Salvador GA. Enhanced phosphatidylinositol 3-kinase (PI3K)/Akt signaling has pleiotropic targets in hippocampal neurons exposed to iron-induced oxidative stress. J Biol Chem. 2013;288:19773–19784. doi: 10.1074/jbc.M113.457622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu H, Shi L, Qi G, Zhao S, Gao Y, Li Y. Gypenoside protects cardiomyocytes against ischemia-reperfusion injury via the inhibition of mitogen-activated protein kinase mediated nuclear factor kappa B pathway in vitro and in vivo. Front Pharmacol. 2016;7:148. doi: 10.3389/fphar.2016.00148.f3524f0505c443d1be6c5641214a14ff [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev. 2002;82:373–428. doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- 30.Sarin R, Sharma YD. Thioredoxin system in obligate anaerobe Desulfovibrio desulfuricans: identification and characterization of a novel thioredoxin 2. Gene. 2006;376:107–115. doi: 10.1016/j.gene.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 31.Zhang H, Cheng D, Liu H, Zheng H. Differential responses of a thioredoxin-like protein gene to Vibrio parahaemolyticus challenge in the noble scallop Chlamys nobilis with different total carotenoids content. Fish Shellfish Immunol. 2018;72:377–382. doi: 10.1016/j.fsi.2017.11.020. [DOI] [PubMed] [Google Scholar]
- 32.Andersen KM, Madsen L, Prag SO, et al. Thioredoxin Txnl1/TRP32 is a redox-active cofactor of the 26 S proteasome. J Biol Chem. 2009;284:15246–15254. doi: 10.1074/jbc.M900016200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chuang SM, Chen L, Lambertson D, Anand M, Kinzy TG, Madura K. Proteasome-mediated degradation of cotranslationally damaged proteins involves translation elongation factor 1A. Mol Cell Biol. 2005;25:403–413. doi: 10.1128/MCB.25.1.403-413.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gonen H, Smith CE, Siegel NR, et al. Protein synthesis elongation factor EF-1 alpha is essential for ubiquitin-dependent degradation of certain N alpha-acetylated proteins and may be substituted for by the bacterial elongation factor EF-Tu. Proc Natl Acad Sci USA. 1994;91:7648–7652. doi: 10.1073/pnas.91.16.7648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liyanage DS, Omeka WKM, Godahewa GI, Lee J. Molecular characterization of thioredoxin-like protein 1 (TXNL1) from big-belly seahorse hippocampus abdominalis in response to immune stimulation. Fish Shellfish Immunol. 2018;75:181–189. doi: 10.1016/j.fsi.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 36.Circu ML, Aw TY. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med. 2010;48:749–762. doi: 10.1016/j.freeradbiomed.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jia L, Chen Y, Tian YH, Zhang G. MAPK pathway mediates the anti-oxidative effect of chicoric acid against cerebral ischemia-reperfusion injury in vivo. Exp Ther Med. 2018;15:1640–1646. doi: 10.3892/etm.2017.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kwon SH, Hong SI, Kim JA, et al. The neuroprotective effects of Lonicera japonica THUNB. against hydrogen peroxide-induced apoptosis via phosphorylation of MAPKs and PI3K/Akt in SH-SY5Y cells. Food Chem Toxicol. 2011;49:1011–1019. doi: 10.1016/j.fct.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 39.Ang YLE, Yong WP, Tan P. Translating gastric cancer genomics into targeted therapies. Crit Rev Oncol Hematol. 2016;100:141–146. doi: 10.1016/j.critrevonc.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 40.Saitoh M, Nishitoh H, Fujii M, et al. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou F, Gomi M, Fujimoto M, et al. Attenuation of neuronal degeneration in thioredoxin-1 overexpressing mice after mild focal ischemia. Brain Res. 2009;1272:62–70. doi: 10.1016/j.brainres.2009.03.023. [DOI] [PubMed] [Google Scholar]
- 42.Atalay S, Soylu B, Aykac A, et al. Protective effects of St. John's wort in the hepatic ischemia/reperfusion injury in rats. Turk J Surg. 2018;34:198–204. doi: 10.5152/turkjsurg.2018.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ge Y, Zhang Q, Li H, Bai G, Jiao Z, Wang H. Adipose-derived stem cells alleviate liver apoptosis induced by ischemia-reperfusion and laparoscopic hepatectomy in swine. Sci Rep. 2018;8:16878. doi: 10.1038/s41598-018-34939-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 45.Hassanzadeh S, Jameie SB, Soleimani M, Farhadi M, Kerdari M, Danaei N. Coenzyme Q10 influences on the levels of TNF-α and IL-10 and the ratio of Bax/Bcl2 in a menopausal rat model following lumbar spinal cord injury. J Mol Neurosci. 2018;65:255–264. doi: 10.1007/s12031-018-1090-6. [DOI] [PubMed] [Google Scholar]
- 46.Yoo MH, Carlson BA, Gladyshev VN, Hatfield DL. Abrogated thioredoxin system causes increased sensitivity to TNF-α-induced apoptosis via enrichment of p-ERK 1/2 in the nucleus. PLoS One. 2013;8:e71427. doi: 10.1371/journal.pone.0071427.ac07033f4db5454db16a16a260a08da7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brentnall M, Rodriguez-Menocal L, de Guevara RL, Cepero E, Boise LH. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013;14:32. doi: 10.1186/1471-2121-14-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jahan N, Chowdhury A, Li T, Xu K, Wei F, Wang S. Neferine improves oxidative stress and apoptosis in benign prostate hyperplasia via Nrf2-ARE pathway. Redox Rep. 2021;26:1–9. doi: 10.1080/13510002.2021.1871814.33d68478ecb942df85faf2cd897491f0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pallepati P, Averill-Bates D. Mild thermotolerance induced at 40 degrees C increases antioxidants and protects HeLa cells against mitochondrial apoptosis induced by hydrogen peroxide: role of p53. Arch Biochem Biophys. 2010;495:97–111. doi: 10.1016/j.abb.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 50.Redza-Dutordoir M, Averill-Bates DA. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim Biophys Acta. 2016;1863:2977–2992. doi: 10.1016/j.bbamcr.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 51.Fulda S, Gorman AM, Hori O, Samali A. Cellular stress responses: cell survival and cell death. Int J Cell Biol. 2010;2010:214074. doi: 10.1155/2010/214074.77e5fa1c812e47c08de13962e79513d5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamakawa H, Ito Y, Naganawa T, et al. Activation of caspase-9 and -3 during H2O2-induced apoptosis of PC12 cells independent of ceramide formation. Neurol Res. 2000;22:556–564. doi: 10.1080/01616412.2000.11740718. [DOI] [PubMed] [Google Scholar]
- 53.Tan J, Chen SX, Lei QY, et al. Mitochonic acid 5 regulates mitofusin 2 to protect microglia. Neural Regen Res. 2021;16:1813–1820. doi: 10.4103/1673-5374.306094.80b2ec926e3448ba9478bc873b1c8dfb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang M, Hayashi H, Horinokita I, et al. Neuroprotective effects of Senkyunolide I against glutamate-induced cells death by attenuating JNK/caspase-3 activation and apoptosis. Biomed Pharmacother. 2021;140:111696. doi: 10.1016/j.biopha.2021.111696. [DOI] [PubMed] [Google Scholar]
- 55.Kang JB, Park DJ, Shah MA, Koh PO. Retinoic acid exerts neuroprotective effects against focal cerebral ischemia by preventing apoptotic cell death. Neurosci Lett. 2021;757:135979. doi: 10.1016/j.neulet.2021.135979. [DOI] [PubMed] [Google Scholar]
- 56.Leak RK, Li P, Zhang F, et al. Apurinic/apyrimidinic endonuclease 1 upregulation reduces oxidative DNA damage and protects hippocampal neurons from ischemic injury. Antioxid Redox Signal. 2015;22:135–148. doi: 10.1089/ars.2013.5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Endoh M, Maiese K, Wagner J. Expression of the inducible form of nitric oxide synthase by reactive astrocytes after transient global ischemia. Brain Res. 1994;651:92–100. doi: 10.1016/0006-8993(94)90683-1. [DOI] [PubMed] [Google Scholar]
- 58.Hur J, Lee P, Kim MJ, Kim Y, Cho YW. Ischemia-activated microglia induces neuronal injury via activation of gp91phox NADPH oxidase. Biochem Biophys Res Commun. 2010;391:1526–1530. doi: 10.1016/j.bbrc.2009.12.114. [DOI] [PubMed] [Google Scholar]
- 59.Chu K, Yin B, Wang J, et al. Inhibition of P2X7 receptor ameliorates transient global cerebral ischemia/reperfusion injury via modulating inflammatory responses in the rat hippocampus. J Neuroinflammation. 2012;9:69. doi: 10.1186/1742-2094-9-69.080da2c640724c7d81f58d9b7baefe90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ha SK, Moon E, Ju MS, et al. 6-Shogaol, a ginger product, modulates neuroinflammation: a new approach to neuroprotection. Neuropharmacology. 2012;63:211–223. doi: 10.1016/j.neuropharm.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 61.Park JH, Park CW, Ahn JH, et al. Neuroprotection and reduced gliosis by pre- and post-treatments of hydroquinone in a gerbil model of transient cerebral ischemia. Chem Biol Interact. 2017;278:230–238. doi: 10.1016/j.cbi.2017.01.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.