Abstract
Background
The development of DNA amplification for the direct detection of M. tuberculosis from clinical samples has been a major goal of clinical microbiology during the last ten years. However, the limited sensitivity of most DNA amplification techniques restricts their use to smear positive samples. On the other hand, the development of automated liquid culture has increased the speed and sensitivity of cultivation of mycobacteria. We have opted to combine automated culture with rapid genotypic identification (ARDRA: amplified rDNA restriction analysis) for the detection resp. identification of all mycobacterial species at once, instead of attempting direct PCR based detection from clinical samples of M. tuberculosis only.
Results
During 1998–2000 a total of approx. 3500 clinical samples was screened for the presence of M. tuberculosis. Of the 151 culture positive samples, 61 were M. tuberculosis culture positive. Of the 30 smear positive samples, 26 were M. tuberculosis positive. All but three of these 151 mycobacterial isolates could be identified with ARDRA within on average 36 hours. The three isolates that could not be identified belonged to rare species not yet included in our ARDRA fingerprint library or were isolates with an aberrant pattern.
Conclusions
In our hands, automated culture in combination with ARDRA provides with accurate, practically applicable, wide range identification of mycobacterial species. The existing identification library covers most species, and can be easily updated when new species are studied or described. The drawback is that ARDRA is culture-dependent, since automated culture of M. tuberculosis takes on average 16.7 days (range 6 to 29 days). However, culture is needed after all to assess the antibiotic susceptibility of the strains.
Background
Rapid and accurate detection, identification and susceptibility testing of mycobacteria remains important i) because the overall incidence of tuberculosis is increasing, also due to the HIV pandemic [1], ii) because of the increasing resistance to antituberculous agents [2] and iii) because an increasing number of mycobacterial species are being recognized as potentially pathogenic [3]. The importance of M. avium infection has increased in HIV patients [4], and clinical infections have been described with species like M. heidelbergense[5], M. conspicuum[6], M. branderi [7] and M. interjectum[8,9], which have been recognized only recently.
Current DNA amplification based diagnostic tests are expensive, have limited senstivity, are usually restricted to the detection of M. tuberculosis only and provide no or limited information on susceptibility (e.g. rifampicin only: RifTB LiPA, Innogenetics, Zwijnaarde, Belgium). Therefore, the need for culture has not been circumvented. The CDC decided to restrict the use of genotypic tests to confirmation of smear positive samples, so that they cannot be used to test the large number of specimens processed for mycobacterial detection every year in an average laboratory [10]. Only recently, the enhanced AMTD (Gen-Probe, San Diego, CA) gained FDA approval for direct detection of M. tuberculosis from smear-negative samples, but several problems are reported [e.g. [11], and the high costs keep restricting its use.
Here, we present our findings with the use of ARDRA [12] for the identification of Mycobacterium species. The method consists of amplification of the 16S rRNA gene (rDNA) and subsequent restriction digestion of the amplicon. The restriction patterns obtained with different restriction enzymes and combination of these patterns into a restriction profile was shown to enable identification of most clinically important mycobacteria by comparison of the obtained profiles with a library of ARDRA profiles obtained for reference strains of different species [12]. This PCR-RFLP analysis of the 16S rRNA gene, was published almost simultaneously with the more widely used technique (known as PRA), which is based on the amplification of the hsp65 gene [13-15].
Results
The initial study describing the applicability of ARDRA for the identification of mycobacteria [12] used universal bacterial primers. However, this sometimes resulted in the false positive amplification from decontaminated samples of organisms other than mycobacteria. Therefore primers were developed, aimed at more specific amplification of mycobacteria. During the three year evaluation period, of which the results are reported here, amplification of nonmycobacterial organisms occurred in two cases. These organisms, namely Corynebacterium glutamicum and Actinomyces odontolyticum, stained acid fast on direct smear and are relatives of the mycobacteria.
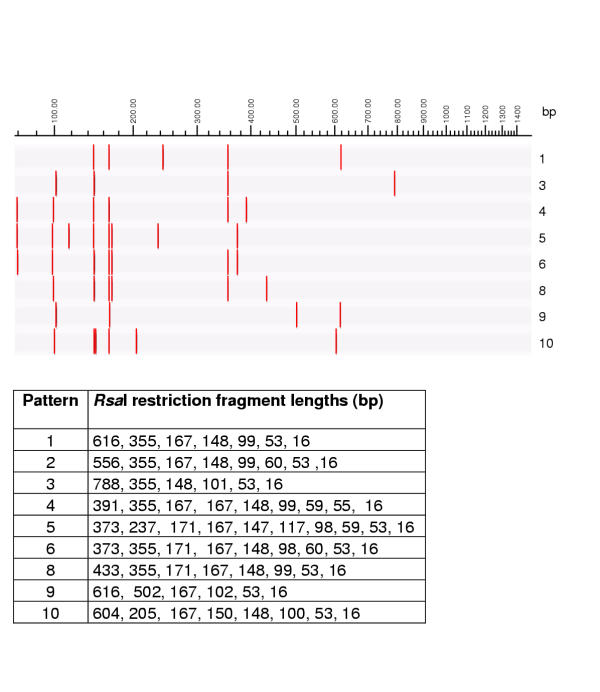
The restriction patterns obtained with the enzymes HhaI (isoschizomer of CfoI), MboI and RsaI for the different species are numbered arbitrarily and are presented in Figures 1, 2 and 3 respectively. Figures 4,5,6 represent the restriction patterns obtained when digital restriction is carried out with the same enzymes on published GenBank sequences. The combination of these patterns is designed as ARDRA profiles and these are listed in Table 1. For example, strains of the M. tuberculosis complex can be recognized by an ARDRA profile 1-1-1, while M. grodonae strains have ARDRA profile 8-4-2. For some species the ARDRA pattern obtained with enzyme is already characteristic, e.g. HhaI 1 is observed only for species of the M. tuberculosis complex.
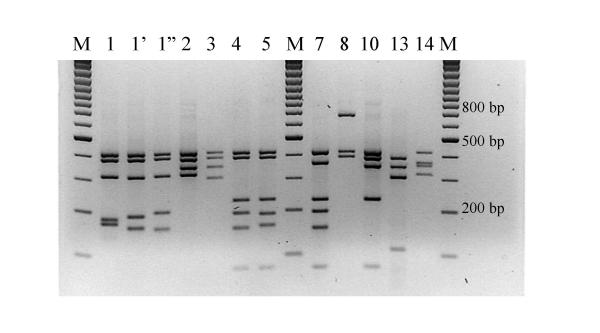
Figure 1.
HhaI (CfoI) restriction patterns of amplified mycobacterial 16S rRNA genes. Legend: M: marker (100 base pair ladder, Fermentas, Vilnius, Lithuania)
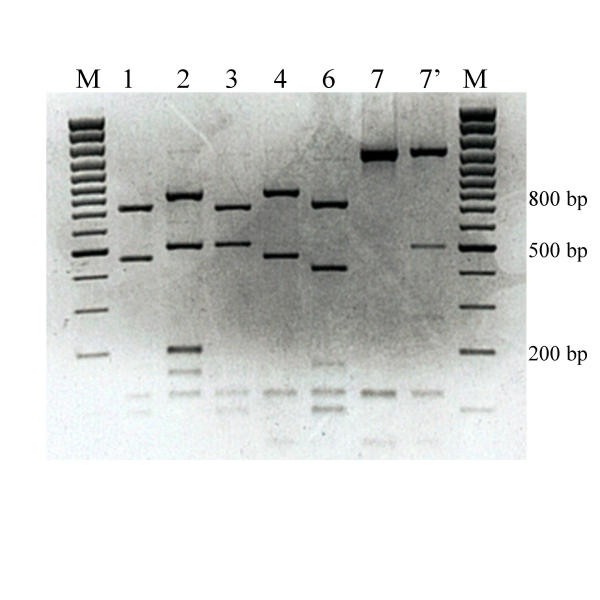
Figure 2.
MboI restriction patterns of amplified mycobacterial 16S rRNA genes. Legend: M: marker (100 base pair ladder, Fermentas, Vilnius, Lithuania)
Figure 3.
RsaI restriction patterns of amplified mycobacterial 16S rRNA genes. Legend: M: marker (100 base pair ladder, Fermentas, Vilnius, Lithuania)
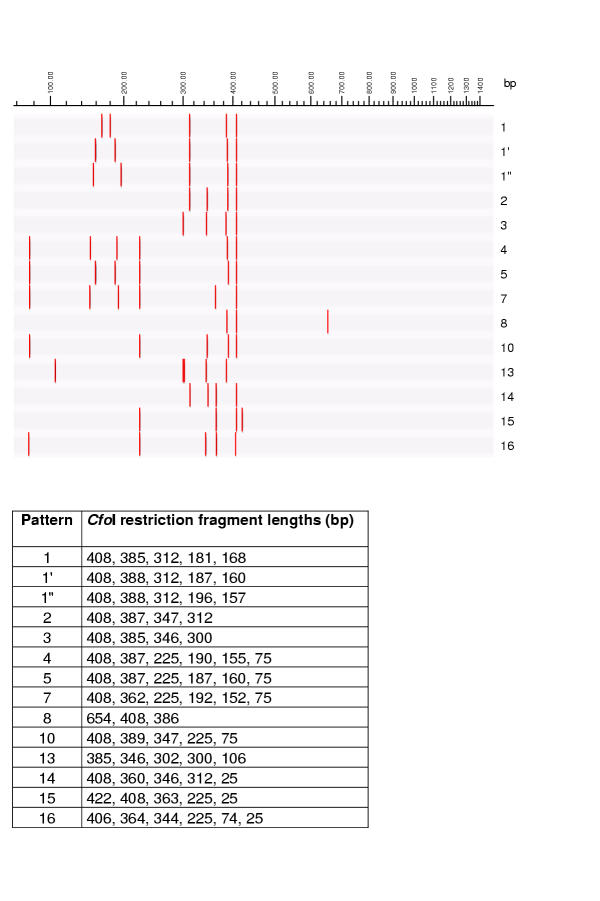
Figure 4.
HhaI (CfoI) restriction patterns of mycobacterial 16S rRNA genes, theoretically calculated using RFLP (Applied Maths) and published GenBank sequences. Graphical representation and table of restriction fragment lengths for each of the possible patterns.
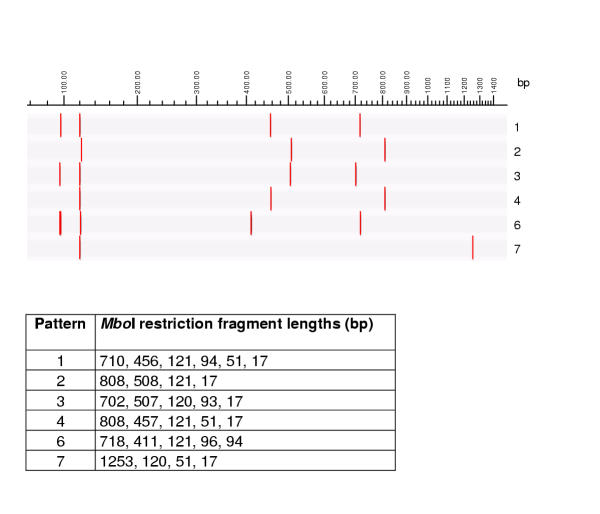
Figure 5.
MboI restriction patterns of mycobacterial 16S rRNA genes, theoretically calculated using RFLP (Applied Maths) and published GenBank sequences. Graphical representation and table of restriction fragment lengths for each of the possible patterns.
Figure 6.
RsaI restriction patterns of mycobacterial 16S rRNA genes, theoretically calculated using RFLP (Applied Maths) and published GenBank sequences. Graphical representation and table of restriction fragment lengths for each of the possible patterns.
Table 1.
Library of ARDRA profiles (combination of restriction patterns) obtained for mycobacterial species.
| Species | Genbank Number | HhaI | MboI | RsaI | BstUI | Reference strains useda |
| M. tuberculosis complex | X52917 | 1 | 1 | 1 | 1 | ITG 8021, IPB 92/0805 |
| M. conspicuum | X88922 | 1' | 1 | 2 | 2 | |
| M. intracellulare | X52927 | 1' | 2 | 2 | 3 | ITG 5913, ITG 5917 |
| M. gastri | X52919 | 1' | 4 | 1 | 1 | |
| M. kansasii | M29575 | 1' | 4 | 1 | 1 | ITG 8201 |
| M. bohemicum | AJ277283 | 1' | 4 | 1 | 3 | |
| M. haemophilum | U06638 | 1' | 4 | 1 | 3 | ITG 3065 |
| M. malmoense | AF152560 | 1' | 4 | 1 | 3 | ITG 940611 |
| M. szulgai | X52926 | 1' | 4 | 2 | 1 | ITG 4981 |
| M. scrofulaceum | X52924 | 1' | 4 | 2 | 3 | ITG 4988 |
| M. xenopi | X52929 | 1' | 4 | 3 | 2 | ITG 4986 |
| M. heckeshornense | AF174290 | 1' | 4 | 3 | 4 | |
| M. marinum | AF251565 | 2 | 1 | 1 | 1 | ITG 1728 |
| M. asiaticum | M29556 | 2 | 1 | 2 | 5 | ITG 8182 |
| M. terrae | X52925 | 2 | 1 | 4 | 2 | ITG 4922 |
| M. ulcerans | Z13990 | 2 | 1 | 9 | 1 | ITG 724, ITG 1837 |
| M. avium | AF306455 | 2 | 2 | 2 | 3 | ITG 4991, ITG 2666 |
| M. botniense | AJ012756 | 2 | 4 | 3 | 2 | |
| M. terrae-like MCRO6b | X93032 | 2 | 6 | 4 | 7 | |
| M. nonchromogenicum | X52928 | 2 | 6 | 4 | 7 | ITG 4980 |
| M. phlei | M29566 | 3 | 1 | 6 | 2' | |
| M. elephantis | AJ010747 | 3 | 1 | 8 | 3' | |
| M. chelonae group IVc | AJ416940 | 3 | 3 | 5 | 2' | ITG 7701 |
| M. abscessus group IIIc | AJ419970 | 3 | 3 | 6 | 2' | ITG 98-1296 |
| M. chelonae group 1c | AJ419969 | 3 | 3 | 6 | 2' | ITG 95-0026 |
| M. immunogenum | AJ011771 | 3 | 3 | 6 | 2' | ITG 98-1289 |
| M. farcinogenes | AF055333 | 4 | 1 | 6 | 7 | |
| M. fortuitum | X52933 | 4 | 1 | 6 | 7 | SLZ A046 |
| M. senegalense | M29567 | 4 | 1 | 6 | 7 | |
| M. septicum | AF111809 | 4 | 1 | 6 | 7 | ITG 4166 |
| M. intermedium | X67847 | 5 | 4 | 6 | 1' | |
| M. interjectum | AJ272088 | 5 | 4 | 6 | 2' | ITG 96-116 |
| M. heidelbergense | X70960 | 5 | 4 | 8 | 2' | |
| M. simiae | X52931 | 5 | 7 | 6 | 2' | ITG 4485 |
| M. lentiflavum | X80769 | 5 | 7 | 6 | 2' | |
| M. peregrinumd | X52921 | 7 | 1 | 6 | 9 | ITG 99-2069, ATCC 14467 |
| M. gordonae | M29563 | 8 | 4 | 2 | 5 | ITG 7838 |
| M. wolinskyi | Y12871 | 10 | 1 | 1 | 2' | |
| M. speciesa | AF028712 | 10 | 1 | 1 | 2' | |
| M. goodii | Y12872 | 10 | 1 | 2 | 2' | |
| M. smegmatis | AJ131761 | 10 | 1 | 2 | 2' | ITG 4995 |
| M. kubicae | AF133902 | 10 | 1 | 6 | 1' | |
| M. tusciae | AF058299 | 10 | 1 | 6 | 2' | |
| M. flavescens | X52932 | 10 | 1 | 6 | 2' | VUB A016 |
| M. genavense | X60070 | 10 | 7 | 6 | 2' | ITG 97-102 |
| M. triplex | U57632 | 10 | 7 | 6 | 2' | |
| M. chelonae group IIc | AJ419968 | 13 | 3 | 6 | 6 | ITG 96-0295 |
| M. branderi | X82234 | 14 | 1 | 1 | 9 | |
| M. celatume | L08169 | 14 | 1 | 1 | 10 | |
| M. triviale | X88924 | 15 | 1 | 10 | 10' | |
| M. aurum | X55595 | 16 | 1 | 6 | 8 |
a. ITG: Institute for Tropical Medicine, Antwerp, Belgium; IPB: Institute Pasteur du Brabant, Brussels, Belgium; SLZ: Streeklaboratorium Zeeland, Goes, the Netherlands; VUB: Free University of Brussels, Brussels, Belgium. b. Strains designated MCRO6 have been studied by Turenne et al.[22] and Torkko et al.[33]. c. Sequences determined in this study. Roman numbering according to Portaels et al.[16], who distinguished four groups within the M. abscessus/M. chelonae complex, based on the 16S–23S rRNA spacer region. d. The GenBank sequence (AF058712) of strain ATCC 14467, submitted to GenBank as M. peregrinum, did not cluster within the M. fortuitum complex (Figure 8) and was highly similar to sequence Y12871 (M. wolinskyi). Moreover, the ARDRA profile calculated from sequence AF058712 (10-1-1-2') did not correspond with the profile we obtained for strain ATCC 14467 (4-1-6-7). The sequence obtained in this study from strain ATCC 14467 (submitted as AJ422046) was identical to the M. fortuitum sequence X52933. Sequence AF058712 is indicated as M. species in the table. e. Received as M. xenopi.
Most mycobacterial species could be readily identified by comparison of the obtained ARDRA profile with the profiles from Table 1. The species of the M. tuberculosis complex can not be differentiated on the basis of the 16S rDNA sequence, and therefore restriction digestion of this gene could not either. Most of the clinically relevant and the most abundant species were readily differentiated from each other. The following species could not be differentiated from each other after digestion with HhaI, MboI, RsaI and BstUI: M. gastri and M. kansasii (1'-4-1-1), M. bohemicum, M. haemophilum and M. malmoense (1'-4-1-3), MCRO6 and M. nonchromogenicum (2-6-4-7), M. chelonae group I, M. abscessus and M. immunogenicum (3-3-6-2'), M. farcinogenes, M. fortuitum, M. senegalense and M. septicum (4-1-6-7), M. simiae and M. lentiflavum (5-7-6-2'), M. goodii and M. smegmatis (10-1-2-2'), M. tusciae and M. flavescens (10-1-6-2'), and finally M. genavense and M. triplex (10-7-6-2').
During 1998–2000, approximately 3500 samples were sent to the laboratory for direct smear examination and mycobacterial culture. Of these, 151 specimens, from 149 patients, were culture positive, and 20% of these were also smear positive. Table 2 summarizes the obtained identifications. For 148 of the 151 isolates, identification by ARDRA was straightforward and was obtained after an average of 36 hours after receipt of the cultured strain. Only three difficulties were encountered. One isolate presented with the ARDRA profile 5-4-6, not present in the ARDRA library at that time. Sequencing lead to an identification as M. interjectum[8], a species that was not yet covered by the library. A second isolate was first misidentified as M. xenopi (profile 1'-4-3), but later on it was observed that the HhaI fingerprint differed clearly from the M. xenopi HhaI 1' pattern by its low molecular size fragments (Figure 1). This HhaI pattern was designated HhaI 1", resulting in the unique ARDRA profile 1"-4-3. The isolate was identified as M. heckeshornense by sequencing of the 16S rRNA gene, and can now also be identified by ARDRA. The third problematic isolate had ARDRA profile 1-1-3, again a profile that had never been observed for any mycobacterial strain studied thus far. Sequencing of the 16S rRNA gave a 99.8 % similarity to the 16S rRNA sequence of the type strain of M. tuberculosis. The sequence revealed a mutation at E. coli position 646 from A to G, abolishing the RsaI restriction site GTAC at that place. This mutation shifts the RsaI pattern 1 to RsaI pattern 3 because the two fragments of resp. 620 and 180 bp are replaced by a single fragment of 800 bp. Further morphological and biochemical tests revealed an identification as probably M. africanum, one of the species of the M. tuberculosis complex. It should be mentioned that M. africanum reference strains used in a previous study [12], were found to have the regular M. tuberculosis complex ARDRA profile 1-1-1.
Table 2.
Distribution of the Mycobacterium species during 1998–2000 in the Ghent University Hospital, number of smear positives and time to positivity of culture.
| Mycobacterium species | Number of isolates (%) | Number of samples positive on direct smear a (% of culture positive) | Average time until positive culture in days (smear positives; negatives) |
| M. tuberculosis complex | 61 (40) | 26 (46b) | 16.7 (12.9; 20) |
| M. avium | 12 | 2 | 6.5 |
| M. heckeshornense | 1 | 1 | 32.5 |
| M. chelonae | 4 | 0 | 18 |
| M. fortuitum | 2 | 0 | 35.5 |
| M. gordonae | 41 (27) | 1 | 22.3 |
| M. interjectum | 1 | 0 | 30.5 |
| M. intracellulare | 1 | 0 | 11.6 |
| M. kansasii/M. gastri | 2 | 0 | No data |
| M. malmoense | 1 | 0 | No data |
| M. szulgai | 3 | 0 | 20.3 |
| M. xenopi | 22 (15) | 0 | 38.1 |
| Total | 151 | 40 (26) |
a: No data for 6 of the 61 samples. All six of these samples were M. tuberculosis culture positive. b. Calculated as 26 positives on a total of those 56 culture positives for which data on smear positivity were available.
To construct artificial ARDRA patterns for the recently described species, we applied the programme RFLP on the published Genbank sequences. The resulting, theoretically to be expected, ARDRA profiles are presented in Table 1.
M. tuberculosis was found to be the most prevalent species, with 40% of the isolates, followed by M. gordonae and M. xenopi, species mostly isolated form non-pulmonar samples with low clinical relevance.
Similarity calculation of published sequences and of sequences obtained in this study (Figure 8) indicates that the M. abscessus/M. chelonae complex (M. chehnae, M. abscessus and M. immunogenum) clusters separately from all other mycobacteria. Portaels et al.[16] described the presence of four groups within the M. abscessus/M. chelonae complex, based on the 16S–23S spacer sequences, as used in the INNO-LiPA Mycobacteria kit (Innogenetics, Zwijnaarde, Belgium). Groups II and IV consisted of genuine M. chelonae strains, which were usually from environmental sources. These two groups have characteristic ARDRA profiles, namely 13-3-6 and 3-3-5. Group III, which was found to represent genuine M. abscessus and M. chelonae group I, which most probably corresponds with M. immunogenum, a M. abscessus-like species that was recently described as being frequently involved in bronchoscope related pseudo-outbreaks [17], can not be distinguished from each other by ARDRA (profile 3-3-6). Strains of groups I and III are usually isolated from clinical samples, with only group III strains (genuine M. abscessus) being pathogenic [16].
Figure 8.
Neighbour-joining similarity tree for 16S rRNA gene sequences of most mycobacterial species. Legend: N. asteroides ATCC 49872 (Genbank Z82229) was used as the outgroup. Table 1 lists the GenBank accession numbers of the sequences used to construct this tree. ARDRA patterns for HhaI, MboI, RsaI and BstUI are listed after the species name. a: GenBank AF028712. Erroneously listed in GenBank as M. peregrinum (see also legend of Table 1). b: M. gastri clusters below 100% with M. kansasii, although it is generally agreed that the 16S rRNA gene sequences for M. kansasii and M. gastri are identical. This can be explained by the fact that the only available GenBank M. gastri sequence (X52919) contained several ambiguities. c. M. lentiflavum, initially not included in the manuscript is not presented in this tree. It clusters close to the branch including M. heidelbergense, M. simiae, M. triplex and M. genavense.
Figure 7.
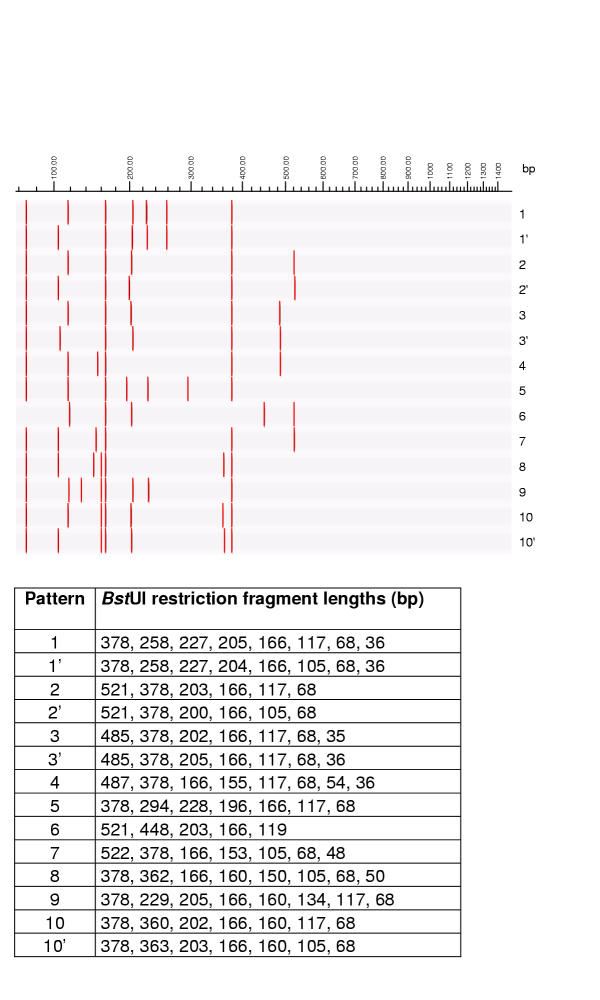
BstUI restriction patterns of mycobacterial 16S rRNA genes, theoretically calculated using RFLP (Applied Maths) and published GenBank sequences. Graphical representation and table of restriction fragment lengths for each of the possible patterns.
M. tuberculosis was the most frequently cultured species (40% of all culture positives), followed by M. gordonae (27%) and M. xenopi (15%) The latter two species were mostly isolated from non-pulmonar samples. 46% of the samples culture positive for M. tuberculosis, were auramine staining positive, and the average time of incubation until a positive liquid culture of M. tuberculosis was 12.9 days for the auramine positive samples and 20 days for auramine negative samples. The range of time to positivity for all M. tuberculosis positive samples was between 6 and 29 days. The average culture times for species other than M. tuberculosis, as far as data were available, are presented in Table 2.
Discussion
Restriction analysis of the amplified 16S rRNA gene, or amplified rDNA restriction analysis (ARDRA) was introduced into the Laboratory for Bacteriology of the Ghent University Hospital for the identification of cultured mycobacteria in 1993 [12]. Since then, several comparable approaches, based on restriction digestion of the amplified rRNA genes and spacer regions have been described [18-21]. During that period, this approach has been updated and refined. This was possible due to some technical changes, like increased quality control of gel electrophoresis and pattern interpretation and the use of primers specific for species of the order of the Actinomycetales instead of universal bacterial primers. Refinement was also possible because of the improvement of mycobacterial taxonomy and the possibility offered by PCR-RFLP techniques, like ARDRA, to easily adapt to this new information. Indeed, when new species are described, there is no need to develop new probes or primers. Instead, new ARDRA profiles can be easily added to the existing library. Also, ARDRA profiles for newly described species can be predicted by applying computer aided digestion of the available GenBank sequences, given the availability of sequences of sufficient quality [22].
ARDRA was found to be a useful tool for identification of mycobacterial isolates in a clinical routine laboratory, because of its speed – compared to phenotypic identification, its reliability, practical applicability, flexibility and the possibility to identify most nontuberculous mycobacteria together with and at the same cost as M. tuberculosis, at an affordable price. Of the 151 isolates during the last three years, 148 could be identified without problems. The other three isolates, respectively M. interjectum, M. heckeshornense and an M. africanum-like strain, should be identifiable when met again in the future, since they presented with specific ARDRA profiles.
Practical applicability of ARDRA
The theoretical turnaround time of ARDRA is 6 hours, and the average identification time in practice during this study was 36 hours. It should be emphasized that the technique was not fully implemented in the routine laboratory, but was carried out by the research laboratory technicians, which means that the practical turnaround time should be far less than 36 hours in a routine diagnostic laboratory. Technically, ARDRA is nondemanding, comprising only basic molecular biology techniques like simple DNA extraction, PCR, restriction digestion and submarine agarose gel electrophoresis.
General considerations
We have addressed previously the several limitations of molecular biology based detection in diagnostic bacteriology [23]. Others agree that the expectations that DNA amplification technologies would supplant microscopy, accurately predict culture results and provide an immediate definitive diagnosis were premature and that these claims have to be replaced with a more realistic view of the limitations and of the practical value of molecular diagnostics of tuberculosis [10,24,25]. Also the expectation that susceptibility would be carried out solely by means of DNA technology, had to be moderated [26]. Despite the fact that during the last ten years a tremendous effort, both in academic and commercial research, has been put into the applicability of nucleotide amplification techniques for the detection of mycobacteria directly from clinical samples, the CDC approved application of these techniques only for smear positive samples. This implicates that for 52% of the culture positive samples with M. tuberculosis encountered in this study, DNA technology would not have accelerated detection, since microscopy was negative. Recently, AMTD2 (GenProbe) gained FDA approval for testing smear negative sputa, but the cost of the technique keeps limiting its use to only those smear negative samples with strong clinical suspicion of tuberculosis. Moreover, since this kind of direct detection amplification technology is technically demanding or requires specialized equipment and kits, many laboratories carry out these tests only at well-set time intervals [e.g. [11]], delaying diagnosis with several days on average, and as such loosing some of the time gain offered by these direct detection methods. As a final remark, one should keep in mind that direct detection without direct antimicrobial susceptibility testing does not obviate the need for culture after all.
ARDRA compared to other culture based genotypic identification techniques
ARDRA and other gene restriction techniques [14,15,21,27,28] have been developed as a practical short cut to full sequence determination. From this study and others it is clear that the discriminatory power of these RFLP approaches for identification of mycobacteria is almost as high as that of sequencing. The discriminatory power and reliability of a commercially available rRNA-spacer based hybridisation assay (INNO-LiPA Mycobacteria) is high, but again this approach is somewhat more laborious and more expensive than in house PCR-RFLP-based techniques. Restriction digestion of a 439 bp stretch of the hsp65 gene for identification of mycobacteria was described almost simultaneously with ARDRA and designated PCR-RFLP Analysis (PRA) [15]. Several laboratories have published their experience using this technique [e.g. [14,27,28]]. Possible drawbacks of hsp65 gene restriction analysis are the smaller sized restriction fragments and the higher intraspecific variability, which may make interpretation more difficult. The small size differences have led to the use of polyacrylamide gel electrophoresis [27], which is less practical than agarose gel electrophoresis and the interpretation difficulties in general have led to reconsideration of the hsp65 gene restriction profiles used thus far [28]. Comparable remarks can be made for PCR-RFLP analysis of the rRNA spacer region [21].
Future developments
At present, in an effort to have the best of both worlds, we are performing a double PCR, directly on smear positive, decontaminated samples, extracting DNA with the commerially available QiaAmp Tissue kit (Qiagen, Hilden, Germany). One PCR attempts to amplify the full length 16S rRNA gene (1500 bp), which can be used for ARDRA, at an annealing at 55°C, and one PCR amplifies a 123 bp region of the IS6110 at an optimal annealing of 68.6°C [29]. Both PCRs are carried out simultaneously in a T-gradient thermocycler (Biometra, Göttingen, Germany), programmed to have both annealing temperatures in the 96 well block. In case of the presence of the 123 bp fragment on an agarose gel, the identification of M. tuberculosis is completed, and can optionally be confirmed with ARDRA. Due to the higher sensitivity of the IS6110 PCR compared to the rDNA PCR, we could amplify the IS6110 fragment of M. tuberculosis directly from all smear positive, M. tuberculosis culture positive samples thus far. In case only the 16S rDNA fragment of 1500 bp is present, the absence of M. tuberculosis can be confirmed by ARDRA which also immediately provides with the identification of the Mycobacterium species other than tuberculosis. Thus far, this yielded in all cases an identification as a nontuberculous strain, confirming the absence of M. tuberculosis. Also in case both amplifications of the smear positive sample remain negative, this can be interpreted as the absence of M. tuberculosis. A positive culture is then awaited to identify the nontuberculous organism with ARDRA.
Taxonomical considerations
This study also confirmed the robustness of ARDRA based identifications. For example, for the first study [12], we received strains identified by reference laboratories as M. avium and M. xenopi, using phenotypic methods. During the first study already, some strains that had been sent as M. avium could be shown by ARDRA (and subsequent confirmation by another laboratory) to be M. scrofulaceum. For M. xenopi, ARDRA indicated the presence of several groups. In the meantime mycobacterial taxonomy has been refined and the groups we indicated as M. xenopi A and M. xenopi B [12] appear to correspond to genuine M. xenopi, resp. M. celatum (ITG 6147)[30], as becomes apparent when applying the programme RFLP on the 16S rRNA sequence data we obtained. Similarly, strains that we classified as M. fortuitum A and B [12], appear to correspond to M. fortuitum subsp. fortuitum, resp. M. peregrinum.
Materials and Methods
Strains
A collection of well characterized reference strains belonging to different mycobacterial species was used to create an ARDRA profile library. The strains used are listed in Table 1. The 151 clinical strains used in this evaluation were collected during the period between January 1998 and December 2000 in the routine clinical laboratory of the Ghent University Hospital.
Processing and culturing of the samples
Decontamination of the samples was done by mixing 1 ml of sample with 1 ml of decontamination buffer (3% NaOH/N-acetyl L-cysteine (NALC)). After 15 min of incubation at room temperature the mixture was neutralized by adding 40 ml of 0.067 M phosphate buffer (pH 6.8), followed by centrifugation at 11600 g during 15 min. The supernatant was removed and part of the pellet was used for auramine staining and microscopy. The remaining pellet was suspended in 1 ml of phosphate buffer and used for inoculation of culture media. 100 μl was used for inoculation of a solid medium (Ogawa, Sanofi-Pasteur, Marnes la Coquette, France) and 500 μl for the inoculation of a liquid medium. During this study the automated liquid culture system was changed from the Bactec system (Becton Dickinson, Cockeysville, Md.) to the 3D BacT/Alert system (Organon Teknika, Boxtel, The Netherlands).
DNA extraction
Starting from liquid culture, 500 μl of a positive culture was transferred to a 1.5 ml screw cap Eppendorf tube. After centrifugation at 13.000 rpm for 15 minutes, the supernatant was removed and the resulting pellet was resuspended in 50 μl TE buffer (100 mM Tris-HCl – 10 mM EDTA, pH 8.0). The mixture was heated for 30 minutes at 95°C, followed by a freezing step at -20°C for at least 30 minutes. Starting from solid culture, a loopful of a bacterial colony was suspended in 500 μl of TE buffer. The mixture was heated at 95°C for 30 minutes, followed by a freezing step at -20°C for at least 30 minutes. Prior to PCR, DNA extracts were thawed at 4°C and centrifuged shortly to pellet the debris.
ARDRA for mycobacteria consists of the amplification of the 16S rRNA gene, followed by separate restriction digestion with HhaI, MboI and RsaI The combination of the three obtained fingerprints is designated an ARDRA profile which can be compared with a library of ARDRA profiles, obtained from well-identified mycobacterial strains. In some cases, more discriminatory identification is possible by additional restriction with BstUI.
Amplification of the 16S rRNA gene
The primers used to amplify the full length 16S rRNA gene (approximately 1500 bp) were MBUZ1 (GAC GAA CGC TGG CGG CGT GCT TAA C) and MBUZ2 (CGT CCC AAT CGC CGA TC). These primers are designated to amplify only the 16S rRNA gene for species of the order Actinomycetales. The PCR mixture consisted of 25 μl Qiagen Mastermix (Qiagen, Hilden, Germany), 0.2 μM of each primer, 5 μl of DNA extract, and was adjusted to 50 μl with distilled water. Thermal cycling consisted of an initial denaturation of 5 min at 94°C, followed by three cycles of 1 min at 94°C, 2 min at 55°C and 1 min at 72°C, followed by 30 cycles of 20 sec at 94°C, 1 min at 55°C and 1 min 72°C, with a final extension of 7 min at 72°C, and cooling at 10°C.
Amplification of 123 bp of the IS6110 region was carried out as described [29] after DNA extraction from decontaminated sputum samples using the QiaAmp Tissue kit (QiaGen, Hilden, Germany).
Restriction digestion
The restriction enzymes used were HhaI (isoschizomer of CfoI)(Amersham Pharmacia Biotech Benelux, Roosendaal, the Netherlands), MboI (Fermentas, Vilnius, Lithuania), RsaI (Amersham Pharmacia). When necessary for further discrimination, digestion with BstUI (New England Biolabs, Beverly, Ma.) was carried out. Each 16S rDNA amplicon was divided in three separate tubes in aliquots of 10 μl, to which 10 U of the respective restriction enzymes were added, with 2 μl of the corresponding enzyme buffer (10× concentrated, final concentration 2×) and each restriction digestion mixture was adjusted to 20 μl with distilled water and incubated during 2 hours at 37°C in a heater.
Electrophoresis
The DNA restriction fragments were electrophoresed in a 2.5% agarose electrophoresis gel, containing 2% Methaphor (FMC Bioproducts, Rockland, Me.) and 0.5% MP agarose (Roche) in the presence of ethidium bromide (50 ng/ml). The gels were photographed and the fingerprints were compared visually with the overview gels (Figures 1, 2 and 3).
16S rDNA sequencing and comparative analysis
A fragment of the 16S rRNA gene (corresponding to positions 10-1507 in the Escherichia coli numbering system) was sequenced as described previously [31]. Sequencing primers were MB UZ1 (GACGAACGCTGGCGGCGTGCT TAAC, E. coli position 27-50), MB UZ2 (CGTCCCAATCGCCGATC, 1493 – 1476), MBP1 (CCGGCCAACTACGTGCCAGC, 502 – 522), MBP2 (CTGGAATTCCTGGTGTAGCGG, 673 – 693), MBP3R (GCATGTCAAACCCAGGTAAGG, 1006 – 986) and MBP4R (CCACCTTCCTCCGAGTTGACC, 1185 – 1165)
The 16S rDNA sequences obtained in this study are indicated in Table 1. All steps of the comparative sequence analysis were performed by using the GeneBase software package (Applied Maths, St. Martens Latem, Belgium), as described [32]. First, pairwise alignment using UPGMA was carried out with a gap penalty of 100 %, a unit gap cost of 20 % and an ambiguity cost of 50 % of the mismatch cost. Subsequently, global alignment – with N. asteroides ATCC 49872 (Genbank Z82229) used as the outgroup – was carried out on the region corresponding to positions 67 through 1444 of the 16S rRNA gene of E. coli, with costs as above. Finally, a similarity matrix of the aligned sequences was constructed by global alignment homology calculation and a gap penalty of 20 %. The neighbour-joining method was used to construct the dendrogram based on this similarity matrix. Bootstrap values were calculated.
Theoretical calculation of restriction patterns was done by means of RFLP (Applied Maths), which makes it possible to obtain restriction patterns using sequences in EMBL format, for every restriction enzyme. The programme GelCompar (Applied Maths) was then used to display the obtained fingerprints.
Table 3.
Overview of the theoretical ARDRA profiles of the species that were newly described since the start of the evaluation period (1998), as obtained after computer aided digestion of the published sequences (see also Figures 4,5,6,7).
| Species | Source of isolation | Reference | GenBank Accession Numbers | Hhal | Mbol | Rsal | BstUI |
| M. bohemicum | [34] | AJ277284, U84502 | 1' | 4 | 1 | 3 | |
| M. botniense | Water | [35] | AF012756 | 2 | 4 | 3 | 2 |
| M. elephantis | Elephant | [36] | AF010747 | 3 | 1 | 8 | 3' |
| M. goodii | Human wounds | [37] | Y12872 | 10 | 1 | (1) | 2' |
| Y12872a | 10 | 1 | 2 | 2' | |||
| M. heckeshornense | Lung disease | [38] | AF1174290 | 1 | 4 | 3 | 4 |
| M. kubicae | [39] | AF133902 | 10 | 1 | 6 | 1' | |
| M. murale | Walls of childrens daycare centre | [40] | Unpublished | ||||
| M. septicum | Catheter related bacteraemia | [41] | AF111809 | 4 | 1 | 6 | 7 |
| M. tuberculosis subsp. caprae | Goats in Spain | [42] | AJ131120 | 1 | 1 | 1 | 1 |
| M. tusciae | [43] | AF058299 | 10 | 1 | 6 | 2' | |
| M. wolinskyi | Human wounds | [37] | Y12871, Y12873 | 10 | 1 | 1 | 2' |
a. After resolving the ambiguity (N) present at E. coli position 95 in the Genbank sequence Y12872. An identical base (G) is found at this position in all mycobacteria.
Acknowledgments
Acknowledgements
We thank Leen Rigouts for biochemical identification of some of the strains.
Contributor Information
Thierry De Baere, Email: Thierry.DeBaere@rug.ac.be.
Ricardo de Mendonça, Email: Ricardo.de.Mendonca@ulb.ac.be.
Geert Claeys, Email: Geert.Claeys@rug.ac.be.
Gerda Verschraegen, Email: Gerda.Verschraegen@rug.ac.be.
Wouter Mijs, Email: Wouter_Mijs@Innogenetics.com.
Rita Verhelst, Email: Rita.Verhelst@rug.ac.be.
Sylvianne Rottiers, Email: Sylvianne.Rottiers@ulb.ac.be.
Leen Van Simaey, Email: Leen.VanSimaey@rug.ac.be.
Catharine De Ganck, Email: Catharine_De_Ganck@yahoo.com.
Mario Vaneechoutte, Email: Mario.Vaneechoutte@rug.ac.be.
References
- Wolinsky E. Mycobacterial diseases other than tuberculosis. Clin Infect Dis. 1992;15:1–12. doi: 10.1093/clinids/15.1.1. [DOI] [PubMed] [Google Scholar]
- Espinal MA, Laserson K, Camacho M, Fusheng Z, Kim SJ, Tlali RE, Smith I, Suarez P, Antunes ML, George AG, et al. Determinants of drug-resistant tuberculosis: analysis of 11 countries. Int J Tubere Lung Dis. 2001;5:887–93. [PubMed] [Google Scholar]
- Wayne LG, Sramek HA. Agents of newly recognized or infrequent encountered mycobacterial disease. Clin Microbiol Rev. 1992;5:1–25. doi: 10.1128/cmr.5.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsburg C, Metchok B, McGowen J, Thompson S. Clinical implications of recovery of M. avium complex from stool or respiratory tract of HIV infected individuals. AIDS. 1992;6:512–514. [PubMed] [Google Scholar]
- Haas WH, Butler WR, Kirschner P, Plikaytis BB, Coyle MB, Amthor B, Steigerwalt AG, Brenner DJ, Salfinger M, Crawford JT, et al. A new agent of mycobacterial lymphadenitis in children: Mycobacterium heidelbergense sp. nov. J Clin Microbiol. 1997;35:3203–3209. doi: 10.1128/jcm.35.12.3203-3209.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer B, Tortoli E, Richter I, Grünewald R, Rüsch-Gerdes S, Uschmann K, Suter F, Collins MD, Kroppenstedt RM, Böttger ED. Mycobacterium conspicuum sp. nov., a new species isolated from patients with disseminated infections. J Clin Microbiol. 1995;33:2805–2811. doi: 10.1128/jcm.33.11.2805-2811.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koukila-Kähkölä P, Springer B, Böttger EC, Paulin I, Jantzen E, Katila ML. Mycobacterium branderi sp. nov., a new potential human pathogen. Int J Syst Bacteriol. 1995;45:549–553. doi: 10.1099/00207713-45-3-549. [DOI] [PubMed] [Google Scholar]
- De Baere T, Moerman M, Rigouts L, Dhooge C, Vermeersch H, Verschraegen G, Vaneechoutte M. Mycobacterium interjectum as causative agent of cervical lymphadenitis. J Clin Microbiol. 2001;39:725–727. doi: 10.1128/JCM.39.2.725-727.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortoli E, Bartoloni A, Burrini C, Colombrita D, Mantella A, Pinsi G, Simonetti MT, Swierczynski G, Bottger EC. Characterization of an isolate of the newly described species Mycobacterium interjectum. Zentralbl Bakteriol. 1996;283:286–294. doi: 10.1016/s0934-8840(96)80062-4. [DOI] [PubMed] [Google Scholar]
- Doern GV. Diagnostic mycobacteriology: where are we today? J Clin Microbiol. 1996;34:1873–1876. doi: 10.1128/jcm.34.8.1873-1876.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artiles F, Jose Pena M, Isolina Campos-Herrero M, Lafarga B. Clinical evaluation of the Amplified Mycobacterium Tuberculosis Direct 2 test. Enferm Infec Microbiol Clin. 2001;19:53–56. doi: 10.1016/s0213-005x(01)72560-8. [DOI] [PubMed] [Google Scholar]
- Vaneechoutte M, De Beenhouwer H, Claeys G, Verschraegen G, De Rouck A, Paepe N, Elaichouni A, Portaels F. Identification of Mycobacterium species by using amplified ribosomal DNA restriction analysis. J Clin Microbiol. 1993;31:2061–2065. doi: 10.1128/jcm.31.8.2061-2065.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plikaytis BB, Plikaytis BD, Yakrus MA, Butler WR, Woodley CL, Silcox VA, Shinnick TM. Differentiation of slowly growing Mycobacterium species, including Mycobacterium tuberculosis by gene amplification and restriction fragment length polymorphism analysis. J Clin Microbiol. 1992;30:1815–1822. doi: 10.1128/jcm.30.7.1815-1822.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steingrube VA, Gibson JL, Brown BA, Zhang Y, Wilson RW, Rajagopalan M, Wallace RJ., Jr PCR amplification and restriction endonuclease analysis of a 65-Kilodalton heat schock protein gene sequence for taxonomic separation of rapidly growing mycobacteria. J Clin Microbiol. 1995;33:149–153. doi: 10.1128/jcm.33.1.149-153.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telenti A, Marchesi F, Balz M, Bally F, Bottger EC, Bodmer T. Rapid identification of mycobacteria to the species level by polymerase chain reaction and restriction enzyme analysis. J Clin Microbiol. 1993;31:175–178. doi: 10.1128/jcm.31.2.175-178.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portaels F, De Rijk P, Jannes G, Lemans R, Mijs W, Rigouts L, Rossau R. The 16S–23S rRNA spacer, a useful tool for taxonomical and epidemiological studies of the M. chelonae complex. Tuberc Lung Dis. 1996;77:17–18. [Google Scholar]
- Wilson RW, Steingrube VA, Bottger EC, Springer B, Brown-Elliott BA, Vincent V, Jost KC, Jr, Zhang Y, Garcia MJ, Chiu SH, et al. Mycobacterium immunogenum sp. nov., a novel species related to Mycobacterium abscessus and associated with clinical disease, pseudo-outbreaks and contaminated metalworking fluids: an international cooperative study on mycobacterial taxonomy. Int J Syst Evol Microbiol. 2001;51:1751–1764. doi: 10.1099/00207713-51-5-1751. [DOI] [PubMed] [Google Scholar]
- Hughes MS, Skuce RA, Beck L-A, Neill SD. Identification of mycobacteria from animals by restriction enzyme analysis and direct DNA cycle sequencing of polymerase chain reaction-amplified 16S rRNA gene sequences. J Clin Microbiol. 1993;31:3216–3222. doi: 10.1128/jcm.31.12.3216-3222.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobner P, Feldmann K, Rifai M, Loscher T, Rinder H. Rapid identification of mycobacterial species by PCR amplification of hypervariable 16S rRNA gene promoter region. J Clin Microbiol. 1996;34:866–869. doi: 10.1128/jcm.34.4.866-869.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tötsch M, Brömmelkamp E, Stücker A, Fille M, Gross R, Wiesner P, Werner Schmid K, Bocker W, Dockhorn-Dworniczak B. Identification of mycobacteria to the species level by automated restriction enzyme fragment length polymorphism analysis. Virchows Archives. 1996;298:1–5. doi: 10.1007/BF00203742. [DOI] [PubMed] [Google Scholar]
- Roth A, Reisehl U, Streubel A, Naumann L, Kroppenstedt RM, Habicht M, Fischer M, Mauch H. Novel diagnostic algorithm for identification of mycobacteria using genus-specific amplification of the 16S–23S rRNA gene spacer and restriction endonucleases. J Clin Microbiol. 2000;38:1094–1104. doi: 10.1128/jcm.38.3.1094-1104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turenne CY, Tschetter L, Wolfe J, Kabani A. Necessity of quality-controlled 16S rRNA gene sequence databases: identifying nontuberculous Mycobacterium species. J Clin Microbiol. 2001;39:3637–3648. doi: 10.1128/JCM.39.10.3638-3648.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaneechoutte M, Van Eldere J. The possibilities and limitations of nucleic acid amplification technology in diagnostic microbiology. J Med Microbiol. 1997;46:188–194. doi: 10.1099/00222615-46-3-188. [DOI] [PubMed] [Google Scholar]
- Roth A, Schaberg T, Mauch H. Molecular diagnosis of tuberculosis: current clinical validity and future perspectives. Eur Respir J. 1997;10:1877–1891. doi: 10.1183/09031936.97.10081877. [DOI] [PubMed] [Google Scholar]
- Gallina M, Troupioti P, Rocco G, Sensalari G, Libanori E. Predicting culture results for Mycobacterium tuberculosis complex. Chest. 1999;118:28–32. doi: 10.1378/chest.118.1.28. [DOI] [PubMed] [Google Scholar]
- Fluit AC, Visser MR, Schmitz FJ. Molecular detection of antimicrobial resistance. Clin Microbiol Rev. 2001;14:836–871. doi: 10.1128/CMR.14.4.836-871.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunello G, Ligozzi M, Cristelli E, Bonora S, Tortoli E, Fontana R. Identification of 54 mycobacterial species by PCR-restriction fragment length polymorphism analysis of the hsp65 gene. J Clin Microbiol. 2001;39:2799–2806. doi: 10.1128/JCM.39.8.2799-2806.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong DA, Yip PC, Cheung DT, Kam KM. Simple and rational approach to the identification of Mycobacterium tuberculosis, Mycobacterium avium complex species, and other commonly isolated mycobacteria. J Clin Microbiol. 2001;39:3768–3771. doi: 10.1128/JCM.39.10.3768-3771.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellyer TJ, DesJardin LE, Assaf MK, Bates JH, Cave MD, Eisenach KD. Specificity of IS6110-base amplification assays for Mycobacterium tuberculosis complex. J Clin Microbiol. 1996;34:2843–2846. doi: 10.1128/jcm.34.11.2843-2846.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler WR, O'Connor SP, Yakrus MA, Smithwick RW, Plikaytis BB, Moss CW, Floyd MM, Woodley CL, Kilburn JO, Vadney FS. Mycobacterium celatum sp. nov. Int J Syst Bacteriol. 1993;43:539–548. doi: 10.1099/00207713-43-3-539. [Published erratum in Int J Syst Bacteriol 1993, 43:868.] [DOI] [PubMed] [Google Scholar]
- Vaneechoutte M, Claeys G, Steyaert S, De Baere T, Peleman R, Verschraegen G. Isolation of Moraxella canis from an ulcerated metastatic lymph node. J Clin Microbiol. 2000;38:3870–3871. doi: 10.1128/jcm.38.10.3870-3871.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemec A, De Baere T, Tjernberg I, Vaneechoutte M, van der Reijden TJK, Dijkshoorn L. Acinetobacter ursingii sp. nov. and Acinetobacter schindleri sp. nov., isolated from human clinical specimens. Int J Syst Evol Microbiol. 2001;51:1891–1899. doi: 10.1099/00207713-51-5-1891. [DOI] [PubMed] [Google Scholar]
- Torkko P, Suutari M, Suomalainen S, Paulin L, Larsson L, Katila ML. Separation among species of Mycobacterium terrae complex by lipid analyses: comparison with biochemical tests and 16S rRNA sequencing. J Clin Microbiol. 1998;36:499–505. doi: 10.1128/jcm.36.2.499-505.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisehl U, Emler S, Horak Z, Kaustova J, Kroppenstedt RM, Lehn N. Mycobacterium bohemicum sp. nov., a slow-growing scotochromogenic mycobacterium. Int J Syst Bacteriol. 1998;48:1349–1355. doi: 10.1099/00207713-48-4-1349. [DOI] [PubMed] [Google Scholar]
- Torkko P, Suomalainen S, livanainen E, Suutari M, Tortoli E, Paulin L, Katila ML. Mycobacterium xenopi and related organisms isolated from stream waters in Finland and description of Mycobacterium botniense sp. nov. Int J Syst Bacteriol. 2000;50:283–289. doi: 10.1099/00207713-50-1-283. [DOI] [PubMed] [Google Scholar]
- Shojaei H, Magee JG, Freeman R, Yates M, Horadogoda NU, Goodfellow M. Mycobacterium elephantis sp. nov., a rapidly growing non-chromogenic Mycobacterium isolated from an elephant. Int J Syst Evol Microbiol. 2000;50:1817–1819. doi: 10.1099/00207713-50-5-1817. [DOI] [PubMed] [Google Scholar]
- Brown BA, Springer B, Steingrube VA, Wilson RW, Pfyffer GE, Garcia MJ, Menedez MC, Rodriguez-Salgada B, Jost KC, Jr, Chiu SH, et al. Mycobacterium wolinskyi sp. nov. and Mycobacterium goodii sp. nov., two new rapidly growing species related to Mycobacterium smegmatis and associated with human wound infections: a cooperative study from the International Working Group on Mycobacterial Taxonomy. Int J Syst Bacteriol. 1999;49:1493–1511. doi: 10.1099/00207713-49-4-1493. [DOI] [PubMed] [Google Scholar]
- Roth A, Reisehl U, Schonfeld N, Naumann L, Elmer S, Fischer M, Mauch H, Loddenkemper R, Kroppenstedt RM. Mycobacterium heckeshornense sp. nov., a new pathogenic slowly growing Mycobacterium sp. causing cavitary lung disease in an immunocompetent patient. J Clin Microbiol. 2000;38:4102–4107. doi: 10.1128/jcm.38.11.4102-4107.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd MM, Gross WM, Bonato DA, Silcox VA, Smithwick RW, Metchock B, Crawford JT, Butler WR. Mycobacterium kubicae sp. nov., a slowly growing, scotochromogenic Mycobacterium. Int J Syst Evol Microbiol. 2000;50:1811–1816. doi: 10.1099/00207713-50-5-1811. [DOI] [PubMed] [Google Scholar]
- Vuorio R, Andersson MA, Rainey FA, Kroppenstedt RM, Kämpfer P, Busse HJ, Viljanen M, Salkinoja-Salonen M. A new rapidly growing mycobacterial species, Mycobacterium murale sp. nov., isolated from the indoor walls of a children's day care centre. Int J Syst Bacteriol. 1999;49:25–35. doi: 10.1099/00207713-49-1-25. [DOI] [PubMed] [Google Scholar]
- Schinsky MMF, McNeil MM, Whitney AM, Steigerwalt AG, Lasker BA, Floyd MM, Hogg GG, Brenner DJ, Brown JM. Mycobacterium septicum sp. nov., a new rapidly growing species associated with catheter-related bacteraemia. Int J Syst Evol Microbiol. 2000;50:575–581. doi: 10.1099/00207713-50-2-575. [DOI] [PubMed] [Google Scholar]
- Aranaz A, Liébana E, Gomez-Mampaso E, Galan JC, Cousins D, Ortega A, Blazquez J, Baquero F, Mateos A, Suarez G, Dominguez L. Mycobacterium tuberculosis subsp. caprae subsp. nov.: a taxonomic study of a new member of the Mycobacterium tuberculosis complex isolated from goats in Spain. Int J Syst Bacteriol. 1999;49:1263–1273. doi: 10.1099/00207713-49-3-1263. [DOI] [PubMed] [Google Scholar]
- Tortoli E, Kroppenstedt RM, Bartoloni A, Caroli G, Jan I, Pawlowski J, Emler S. Mycobacterium tusciae sp. nov. Int J Syst Bacteriol. 1999;48:1839–1844. doi: 10.1099/00207713-49-4-1839. [DOI] [PubMed] [Google Scholar]