Abstract
Purpose
To investigate the association between alcohol consumption and kidney stones in American adults.
Materials and methods
National Health and Nutrition Examination Survey (NHANES) datasets from 2007 to 2016 were utilized. Participants with a history of kidney stones and alcohol consumption aged 20 or older were included. Weighted proportions and regression analysis were used to assess the association between alcohol consumption and kidney stones by adjusting age, gender, race, marital status, education, recreational activities, smoking, and several comorbidities.
Results
Eleven population samples (Q1-Q11) were included from the NHANES dataset based on 11 questions compiled from the Alcohol Use Questionnaire (ALQ). In the fully adjusted regression model, none of these 11 samples demonstrated a significant association with urolithiasis, that is, alcohol consumption was not significantly associated with the incidence of kidney stones, even among heavy drinkers.
Conclusion
Alcohol consumption is not significantly associated with the prevalence of kidney stones. This finding requires a more adequate sample size and a more detailed review of the history of kidney stones to be further verified.
Keywords: kidney stones, alcohol consumption, NHANES, association, cross-sectional analysis
Introduction
Kidney stones have a high incidence rate worldwide that has been increasing every year (1–4). Alcohol is a common addictive substance, with about a third of the world’s population currently consuming it for different reasons (5). Previous studies have shown that fluid intake may be associated with kidney stone formation (6, 7), and alcohol is one of the important liquids. Some researchers believe that alcohol intake is negatively associated with the formation of kidney stones (8–12), while others believe that alcohol consumption is a risk factor for kidney stone formation (13, 14). We believe that research on the relationship between alcohol consumption and kidney stones is necessary to generate new ideas for the prevention and treatment of kidney stones, given the differing opinions.
The purpose of this study was to investigate the relationship between different drinking profiles and history of kidney stones by analyzing National Health and Nutrition Examination Survey (NHANES) cross-sectional data.
Materials and methods
Data resource and participants
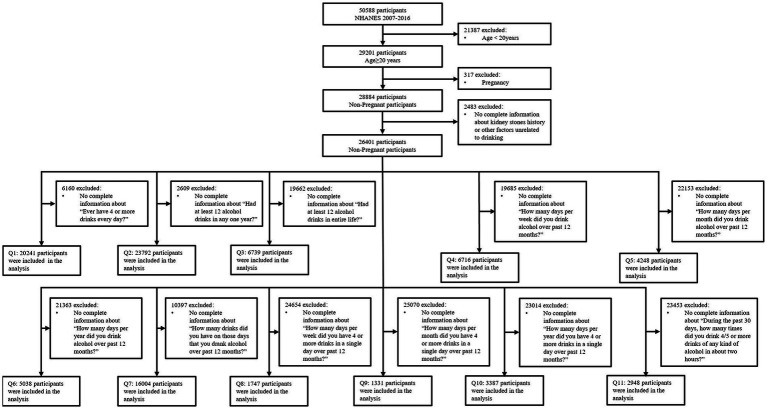
NHANES, a major program of the National Center for Health Statistics (NCHS), is a program of studies designed to estimate the health and nutritional status of participants in the United States. The survey combines interviews and physical examinations. We utilized 5 continuous cycles of NHANES data from year 2007 to 2016. This cross-sectional study included 29,201 adult American participants aged 20 years or older. 317 subsequently pregnant participants were excluded. Furthermore, 2,483 participants with incomplete kidney stones history information or other factors unrelated to drinking were excluded. Finally, we compiled 11 questions from the Alcohol Use Questionnaire (ALQ), and excluded participants with incomplete information for each question separately to obtain 11 different samples, named Q1 to Q11. Detailed inclusion and exclusion standards are shown in Figure 1. Additionally, there were minor variations observed in the ALQs of certain cycles in NHANES. Therefore, the questions used to collect specific samples (Q1, Q4, Q5, Q6, Q8, Q9, Q10) underwent processing, as described in Supplementary Figure S1.
Figure 1.
Flow chart of the study population.
Results and exposure factors
Results of the questionnaire determines whether the participant has a history of nephrolithiasis. If the answer to the question “Have you ever had kidney stones?” is “yes,” we assume that the participant has a history of kidney stones. The veracity of self-reported was confirmed in a previous study (15).
The main exposure factor was the lifetime and current (during the past 12 months) alcohol consumption of the participants, as determined by the ALQ in NHANES 2007–2016. The type of alcohol consumed was not specified.
Covariates
In order to increase accuracy and credibility, we included the following covariates: age, gender, race, marital status, education, recreational activities, smoking, asthma, overweight, gout, congestive heart failure, coronary heart disease, angina, stroke, cancer, diabetes, hypertension, and BMI. According to the recommendations of NHANES, we devided the age into three groups: 20–39, 40–59, and ≥ 60 years. Races included Mexican American, other Hispanic, non-Hispanic white, non-Hispanic black, and other races. Marital status was categorized as married, widowed, divorced, separated, never married, and living with partner. Education level included less than 9th grade, 9-11th grade (includes 12th grade with no diploma), high school graduate/GED or equivalent, some college or AA degree, and college graduate or above. BMI was divided into <25, 25–30, and > 30 kg/m2. The comorbidities included asthma, overweight, gout, congestive heart failure, coronary heart disease, angina, stroke, cancer, diabetes, and hypertension.
Statistical methods
We used the NHANES recommended weighting data and the merging method. In the baseline characteristics table, categorical variables are expressed as proportions, and all continuous variables are treated as categorical variables, also expressed as proportions. The difference between those with and without kidney stones history was tested by survey-weighted Chi-square test.
To investigate the relationship between alcohol consumption and kidney stones prevalence, we applied three logistic regression models, including unadjusted, slightly adjusted, and fully adjusted covariates. Crude model was unadjusted. Model I was adjusted for age, gender and race. Model II was adjusted for age, gender, race, marital status, education, recreational activities, smoking, asthma, overweight, gout, congestive heart failure, coronary heart disease, angina, stroke, cancer, diabetes, hypertension, and BMI. The same method was used to examine 11 different population samples separately to explore the association between alcohol consumption and kidney stones prevalence. To better evaluate the relationship, we treated the continuous variables in the 11 samples as categorical variables and analyzed them to estimate trends. To examine the association between heavy drinking and kidney stones, we classified Q11 as <5 and ≥ 5, based on the Substance Abuse and Mental Health Services Administration (SAMHSA) definitions of heavy drinking and binge drinking, respectively. Additionally, we conducted a separate analysis to explore the relationship between the number of binge drinking days and kidney stones, using Q11 as a continuous variable. Finally, we performed a univariate analysis of the association of all confounders listed in the baseline tables with kidney stones.
We merged the sample weights of 5 continuous cycles according to the recommended method on the NHANES website.1 According to the suggestions, we used a weight that was appropriate for the variable of interest that was collected on the smallest number of respondents. All analyses were performed using R packages (http://www.R-project.org; The R Foundation) and EmpowerStats (www.empowerstats.com, X&Y solutions Inc., Boston, MA.). A 2-tailed p < 0.05 was considered statistically significant.
Ethics statement
The studies involving human participants were reviewed and approved by National Center for Health Statistics (NCHS) research ethics review board. The patients/participants provided their written informed consent to participate in this study
Results
Participants characteristics
Figure 1 shows the study design, inclusion and exclusion criteria. Eleven groups of American adults were included in the corresponding sample (Q1-Q11), and the number of people in each group is also shown. Supplementary Figure S1 shows the process of some questions in the ALQs of certain cycles in NHANES for better accessibility to the study sample (Q1, Q4, Q5, Q6, Q8, Q9, Q10). Table 1 shows the baseline demographic characteristics of the participants based on kidney stones history, using the population prior to classification by the ALQ. Patients with a history of kidney stones may be more likely to have other comorbidities than those without a history of kidney stones. Different histories of kidney stones may not be related to the level of education.
Table 1.
Baseline characteristics of participants with and without kidney stone history.
| Without history | With history | p valueb | |||
|---|---|---|---|---|---|
| Non-weighted NO. | % (95% CI)a | Non-weighted NO. | % (95% CI)a | ||
| Total number of participants | 23,986 | 2,415 | |||
| Age | <0.0001 | ||||
| 20–39 | 8,532 | 38.46 (36.99,39.94) | 467 | 21.36 (19.32,23.56) | |
| 40–59 | 8,025 | 37.37 (36.37,38.38) | 834 | 42.49 (39.41,45.63) | |
| ≥60(%) | 7,429 | 24.18 (23.12,25.26) | 1,114 | 36.14 (33.56,38.81) | |
| Gender | <0.0001 | ||||
| Male | 11,559 | 47.85 (47.18,48.52) | 1,338 | 54.78 (52.20,57.34) | |
| Famale | 12,427 | 52.15 (51.48,52.82) | 1,077 | 45.22 (42.66,47.80) | |
| Race | <0.0001 | ||||
| Mexican American | 3,684 | 8.72 (7.18,10.55) | 320 | 6.08 (4.53,8.10) | |
| Other Hispanic | 2,543 | 5.78 (4.76,7.00) | 286 | 5.19 (3.82,7.01) | |
| Non-Hispanic White | 9,719 | 65.71 (62.46,68.83) | 1,306 | 77.33 (73.79,80.52) | |
| Non-Hispanic Black | 5,290 | 11.90 (10.33,13.68) | 321 | 5.92 (4.84,7.22) | |
| Other Race - Including Multi-Racial | 2,750 | 7.89 (6.96,8.92) | 182 | 5.48 (4.28,7.00) | |
| Marital status | <0.0001 | ||||
| Married | 12,134 | 54.28 (52.77,55.78) | 1,406 | 63.45 (60.56,66.24) | |
| Widowed | 1825 | 5.61 (5.23,6.02) | 243 | 6.54 (5.67,7.53) | |
| Divorced | 2,544 | 10.00 (9.43,10.60) | 315 | 12.35 (10.77,14.13) | |
| Separated | 806 | 2.39 (2.13,2.67) | 85 | 2.23 (1.63,3.04) | |
| Never married | 4,733 | 19.64 (18.24,21.12) | 223 | 9.58 (8.14,11.25) | |
| Living with partner | 1944 | 8.09 (7.52,8.70) | 143 | 5.85 (4.75,7.19) | |
| Education level | 0.262 | ||||
| Less than 9th grade | 2,600 | 5.80 (5.19,6.47) | 277 | 5.48 (4.54,6.60) | |
| 9-11th grade (Includes 12th grade with no diploma) | 3,492 | 11.05 (10.08,12.11) | 354 | 11.29 (9.82,12.94) | |
| High school graduate/GED or equivalent | 5,426 | 22.03 (20.99,23.11) | 549 | 23.23 (20.61,26.07) | |
| Some college or AA degree | 6,895 | 31.26 (30.21,32.34) | 733 | 32.65 (30.25,35.14) | |
| College graduate or above | 5,573 | 29.86 (27.91,31.87) | 502 | 27.35 (23.81,31.21) | |
| Moderate or vigorous recreational activities at least 10 min continuously | <0.0001 | ||||
| NO | 12,423 | 45.43 (43.64,47.24) | 1,453 | 53.62 (50.74,56.48) | |
| YES | 11,563 | 54.57 (52.76,56.36) | 962 | 46.38 (43.52,49.26) | |
| Smoked at least 100 cigarettes in life | 0.0001 | ||||
| NO | 13,523 | 56.14 (54.89,57.38) | 1,194 | 50.20 (47.05,53.35) | |
| YES | 10,463 | 43.86 (42.62,45.11) | 1,221 | 49.80 (46.65,52.95) | |
| Asthma | 0.0004 | ||||
| NO | 20,654 | 85.55 (84.85,86.22) | 2,000 | 82.45 (80.45,84.29) | |
| YES | 3,332 | 14.45 (13.78,15.15) | 415 | 17.55 (15.71,19.55) | |
| Overweight | <0.0001 | ||||
| NO | 16,137 | 67.00 (66.05,67.94) | 1,324 | 52.84 (49.93,55.73) | |
| YES | 7,849 | 33.00 (32.06,33.95) | 1,091 | 47.16 (44.27,50.07) | |
| Gout | <0.0001 | ||||
| NO | 23,026 | 96.66 (96.27,97.01) | 2,207 | 91.84 (90.33,93.13) | |
| YES | 960 | 3.34 (2.99,3.73) | 208 | 8.16 (6.87,9.67) | |
| Congestive heart failure | <0.0001 | ||||
| NO | 23,341 | 97.98 (97.71,98.23) | 2,261 | 95.29 (94.26,96.15) | |
| YES | 645 | 2.02 (1.77,2.29) | 154 | 4.71 (3.85,5.74) | |
| Coronary heart disease | <0.0001 | ||||
| NO | 23,178 | 97.23 (96.89,97.54) | 2,221 | 93.30 (91.98,94.42) | |
| YES | 808 | 2.77 (2.46,3.11) | 194 | 6.70 (5.58,8.02) | |
| Angina | <0.0001 | ||||
| NO | 23,503 | 98.33 (98.08,98.54) | 2,287 | 95.48 (94.53,96.28) | |
| YES | 483 | 1.67 (1.46,1.92) | 128 | 4.52 (3.72,5.47) | |
| Stroke | <0.0001 | ||||
| NO | 23,191 | 97.51 (97.23,97.76) | 2,279 | 95.58 (94.80,96.24) | |
| YES | 795 | 2.49 (2.24,2.77) | 136 | 4.42 (3.76,5.20) | |
| Cancer | <0.0001 | ||||
| NO | 21,873 | 90.56 (90.02,91.07) | 2,050 | 84.02 (82.22,85.67) | |
| YES | 2,113 | 9.44 (8.93,9.98) | 365 | 15.98 (14.33,17.78) | |
| Diabetes | <0.0001 | ||||
| NO | 21,124 | 91.34 (90.72,91.92) | 1,865 | 81.71 (80.01,83.30) | |
| YES | 2,862 | 8.66 (8.08,9.28) | 550 | 18.29 (16.70,19.99) | |
| Hypertension | <0.0001 | ||||
| NO | 15,878 | 70.29 (69.21,71.35) | 1,209 | 54.51 (51.91,57.08) | |
| YES | 8,108 | 29.71 (28.65,30.79) | 1,206 | 45.49 (42.92,48.09) | |
| BMI | <0.0001 | ||||
| <25 | 7,345 | 31.73 (30.53,32.96) | 496 | 20.58 (18.39,22.96) | |
| 25–30 | 7,977 | 33.61 (32.70,34.54) | 837 | 33.41 (31.12,35.78) | |
| >30 | 8,664 | 34.65 (33.54,35.78) | 1,082 | 46.01 (43.69,48.36) | |
% (95% CI), survey-weighted percentage (95% CI).
value of p was by survey-weighted Chi-square test.
Regression analysis
We put 11 samples (Q1-Q11) related to alcohol consumption into three logical regression models for analysis. Although Q1 was negatively correlated with the risk of kidney stones in crude model (OR = 0.70; 95%CI 0.60 to 0.82) and model I (OR = 0.76; 95%CI 0.64 to 0.90), it was not associated with kidney stones in model II (OR = 0.86; 95%CI 0.72 to 1.03). Q4 was positively correlated with the risk of kidney stones in crude model (OR = 1.34; 95%CI 1.02 to 1.76), but not in model I (OR = 1.01; 95%CI 0.76 to 1.33) and model II (OR = 1.06; 95%CI 0.79 to 1.40). The remaining samples were not associated with kidney stones in all three logical regression models (Table 2). In addition, we individually analyzed the effect of each covariate on the risk of kidney stones. When confounders were not considered, women appeared to be less likely to have kidney stones than men (OR = 0.76; 95% CI 0.68 to 0.85), and the risk of kidney stones seemed to be higher in those who smoked, were obese, and had other medical conditions. Interestingly, if covariates were not considered, those who were never married appeared to have a lower risk of kidney stones than those who were married (OR = 0.42; 95% CI 0.34 to 0.51). Furthermore, there was no association between education level and the risk of kidney stones (Table 3).
Table 2.
Association between alcohol consumption and kidney stone.
| Exposure | Crude Modela | Model Ib | Model IIc | |||
|---|---|---|---|---|---|---|
| OR (95%CI) | value of p | OR (95%CI) | p-value | OR(95%CI) | p-value | |
| Q1 | ||||||
| NO | Ref. | Ref. | Ref. | |||
| YES | 0.70 (0.60, 0.82) | <0.0001 | 0.76 (0.64, 0.90) | 0.0023 | 0.86 (0.72, 1.03) | 0.1128 |
| Q2 | ||||||
| NO | Ref. | Ref. | Ref. | |||
| YES | 1.07 (0.94, 1.22) | 0.2862 | 1.13 (0.98, 1.30) | 0.089 | 1.14 (0.99, 1.32) | 0.0716 |
| Q3 | ||||||
| NO | Ref. | Ref. | Ref. | |||
| YES | 0.91 (0.71, 1.16) | 0.4441 | 0.94 (0.73, 1.22) | 0.6616 | 1.02 (0.79, 1.31) | 0.8703 |
| Q4 | ||||||
| 1–3 | Ref. | Ref. | Ref. | |||
| 4–7 | 1.34 (1.02, 1.76) | 0.039 | 1.01 (0.76, 1.33) | 0.9633 | 1.06 (0.79, 1.40) | 0.7115 |
| Q5 | ||||||
| 1–10 | Ref. | Ref. | Ref. | |||
| 11–20 | 1.48 (0.62, 3.52) | 0.3803 | 1.32 (0.55, 3.16) | 0.5388 | 1.47 (0.63, 3.45) | 0.3766 |
| 21–30 | 0.49 (0.12, 1.99) | 0.3229 | 0.32 (0.08, 1.32) | 0.1194 | 0.43 (0.10, 1.79) | 0.2512 |
| P-value for trend | 0.9079 | 0.5987 | 0.9721 | |||
| Q6 | ||||||
| 1–120 | Ref. | Ref. | Ref. | |||
| 121–240 | 1.95 (0.61, 6.22) | 0.2607 | 1.51 (0.48, 4.73) | 0.4811 | 1.59 (0.51, 4.95) | 0.4263 |
| 241–365 | 0.11 (0.02, 0.53) | 0.0072 | 0.08 (0.02, 0.38) | 0.0023 | 0.13 (0.03, 0.60) | 0.0126 |
| P-value for trend | 0.5342 | 0.2073 | 0.4758 | |||
| Q7 | ||||||
| 1–15 | Ref. | Ref. | Ref. | |||
| ≥16 | 0.48 (0.15, 1.54) | 0.2232 | 0.64 (0.20, 2.03) | 0.4507 | 0.63 (0.19, 2.09) | 0.4565 |
| Q8 | ||||||
| 1–3 | Ref. | Ref. | Ref. | |||
| 4–7 | 1.18 (0.72, 1.95) | 0.5113 | 0.89 (0.49, 1.62) | 0.703 | 0.80 (0.43, 1.49) | 0.4875 |
| Q9 | ||||||
| 1–10 | Ref. | Ref. | Ref. | |||
| 11–20 | 0.55 (0.07, 4.25) | 0.5677 | 0.57 (0.07, 4.64) | 0.6001 | 0.64 (0.08, 5.44) | 0.6858 |
| 21–30 | 0.00 (0.00, 0.00) | <0.0001 | 0.00 (0.00, 0.00) | <0.0001 | 0.00 (0.00, 0.00) | <0.0001 |
| p-value for trend | 0.3672 | 0.4079 | 0.5611 | |||
| Q10 | ||||||
| 1–120 | Ref. | Ref. | Ref. | |||
| 121–240 | 4.98 (1.06, 23.39) | 0.0452 | 5.09 (1.07, 24.11) | 0.0442 | 5.23 (1.24, 22.15) | 0.0292 |
| 241–365 | 0.00 (0.00, 0.00) | <0.0001 | 0.00 (0.00, 0.00) | <0.0001 | 0.00 (0.00, 0.00) | <0.0001 |
| p-value for trend | 0.4208 | 0.5468 | 0.5108 | |||
| Q11(Continuous) | 0.95 (0.85, 1.06) | 0.3381 | 0.97 (0.89, 1.07) | 0.5559 | 0.98 (0.90, 1.07) | 0.7434 |
| <5 | Ref. | Ref. | Ref. | |||
| ≥5 | 0.72 (0.25, 2.02) | 0.5338 | 0.85 (0.30 2.39) | 0.7588 | 0.88 (0.31, 2.53) | 0.8497 |
Crude Model: adjusted for none.
Model I: adjusted for age, gender and race.
Model II: adjusted for age, gender, race, marital status, education, recreational activities, smoking, asthma, overweight, gout, congestive heart failure, coronary heart disease, angina, stroke, cancer, diabetes, hypertension, and BMI.
Table 3.
Univariate analysis for kidney stone.
| Covariate | (Na) % (95%CI)b | OR (95%CI) | p-valuec |
|---|---|---|---|
| Age(years) | (26401) 9.50 (8.98,10.01) | 1.02 (1.02, 1.03) | <0.0001 |
| 20–39 | (8999) 5.51 (4.89,6.13) | Ref. | |
| 40–59 | (8859) 10.66 (9.67,11.65) | 2.05 (1.75, 2.40) | <0.0001 |
| ≥60 | (8543) 13.56 (12.57,14.55) | 2.69 (2.35, 3.08) | <0.0001 |
| Gender | |||
| Male | (12897) 10.72 (9.99,11.46) | Ref. | |
| Famale | (13504) 8.34 (7.66,9.02) | 0.76 (0.68, 0.85) | <0.0001 |
| Race | |||
| Mexican American | (4004) 6.82 (5.90,7.73) | Ref. | |
| Other Hispanic | (2829) 8.61 (7.26,9.97) | 1.29 (1.02, 1.63) | 0.0356 |
| Non-Hispanic White | (11025) 10.99 (10.29,11.69) | 1.69 (1.44, 1.98) | <0.0001 |
| Non-Hispanic Black | (5611) 4.96 (4.40,5.51) | 0.71 (0.59, 0.86) | 0.0009 |
| Other Race - Including Multi-Racial | (2932) 6.80 (5.43,8.17) | 1.00 (0.76, 1.32) | 0.9857 |
| Marital status | |||
| Married | (13540) 10.93 (10.13,11.72) | Ref. | |
| Widowed | (2068) 10.89 (9.59,12.19) | 1.00 (0.84, 1.18) | 0.9652 |
| Divorced | (2859) 11.48 (9.85,13.10) | 1.06 (0.89, 1.26) | 0.532 |
| Separated | (891) 8.92 (6.38,11.47) | 0.80 (0.59, 1.09) | 0.1569 |
| Never married | (4956) 4.87 (4.11,5.63) | 0.42 (0.34, 0.51) | <0.0001 |
| Living with partner | (2087) 7.06 (5.69,8.42) | 0.62 (0.49, 0.78) | 0.0001 |
| Education level | |||
| Less than 9th grade | (2877) 9.03 (7.68,10.37) | Ref. | |
| 9-11th grade (Includes 12th grade with no diploma) | (3846) 9.68 (8.55,10.81) | 1.08 (0.87, 1.33) | 0.4767 |
| High school graduate/GED or equivalent | (5975) 9.96 (8.80,11.13) | 1.12 (0.92, 1.36) | 0.2774 |
| Some college or AA degree | (7628) 9.88 (9.06,10.69) | 1.10 (0.93, 1.31) | 0.2622 |
| College graduate or above | (6075) 8.77 (7.80,9.74) | 0.97 (0.78, 1.21) | 0.7791 |
| Moderate or vigorous recreational activities at least 10 min continuously | |||
| NO | (13876) 11.02 (10.34,11.70) | Ref. | |
| YES | (12525) 8.19 (7.55,8.82) | 0.72 (0.65, 0.80) | <0.0001 |
| Smoked at least 100 cigarettes in life | |||
| NO | (14717) 8.58 (8.02,9.14) | Ref. | |
| YES | (11684) 10.65 (9.74,11.55) | 1.27 (1.13, 1.43) | 0.0002 |
| Asthma | |||
| NO | (22654) 9.19 (8.66,9.71) | Ref. | |
| YES | (3747) 11.30 (10.06,12.54) | 1.26 (1.11, 1.43) | 0.0008 |
| Overweight | |||
| NO | (17461) 7.64 (7.07,8.21) | Ref. | |
| YES | (8940) 13.04 (12.06,14.02) | 1.81 (1.61, 2.04) | <0.0001 |
| Gout | |||
| NO | (25233) 9.07 (8.55,9.58) | Ref. | |
| YES | (1168) 20.40 (17.03,23.76) | 2.57 (2.06, 3.20) | <0.0001 |
| Congestive heart failure | |||
| NO | (25602) 9.26 (8.75,9.77) | Ref. | |
| YES | (799) 19.66 (15.64,23.69) | 2.40 (1.84, 3.12) | <0.0001 |
| Coronary heart disease | |||
| NO | (25399) 9.15 (8.62,9.68) | Ref. | |
| YES | (1002) 20.26 (16.89,23.63) | 2.52 (2.01, 3.17) | <0.0001 |
| Angina | |||
| NO | (25790) 9.25 (8.73,9.77) | Ref. | |
| YES | (611) 22.05 (18.12,25.99) | 2.78 (2.18, 3.54) | <0.0001 |
| Stroke | |||
| NO | (25470) 9.33 (8.80,9.85) | Ref. | |
| YES | (931) 15.69 (13.01,18.37) | 1.81 (1.46, 2.24) | <0.0001 |
| Cancer | |||
| NO | (23923) 8.87 (8.33,9.41) | Ref. | |
| YES | (2478) 15.08 (13.53,16.64) | 1.82 (1.59, 2.10) | <0.0001 |
| Diabetes | |||
| NO | (22989) 8.58 (8.06,9.11) | Ref. | |
| YES | (3412) 18.14 (16.43,19.85) | 2.36 (2.07, 2.69) | <0.0001 |
| Hypertension | |||
| NO | (17087) 7.53 (6.98,8.07) | Ref. | |
| YES | (9314) 13.84 (12.86,14.83) | 1.97 (1.76, 2.21) | <0.0001 |
| BMI(kg/m2) | (26401) 9.50 (8.98,10.01) | 1.04 (1.03, 1.04) | <0.0001 |
| <25 | (7841) 6.37 (5.63,7.11) | Ref. | |
| 25–30 | (8814) 9.44 (8.66,10.23) | 1.53 (1.31, 1.80) | <0.0001 |
| >30 | (9746) 12.23 (11.33,13.13) | 2.05 (1.78, 2.36) | <0.0001 |
CI, confidence interval. OR, odds ratio.
N: Number of observed.
% (95%CI): survey-weighted percentage (95% CI).
For Kidney stone: survey-weighted OR (95%CI) p-value.
Discussion
This cross-sectional study investigated the association between alcohol consumption and history of kidney stones by analyzing 5 cycles of the NHANES dataset. The results displayed that there was no significant correlation between both lifetime and current (past 12 months) alcohol consumption and a history of kidney stones, that is, there was no significant association between the amount and frequency of alcohol consumption and the prevalence of kidney stones, even among heavy drinkers.
Consuming alcohol is a mean of socializing in many cultures. Unfortunately, of the population that do consume alcohol, many is addicted and drink it without restraint. Alcohol consumption is associated with a series of health problems, such as diabetes, obesity (16, 17) etc. Diabetes and obesity have previously been reported to be risk factors for kidney stone formation (18, 19). Furthermore, alcohol consumption can cause kidney injury, inflammation and fibrosis, all of which are associated with the formation of kidney stones (20–22). Nevertheless, few studies have elucidated the direct correlation between alcohol consumption and kidney stones. In this study, although the direct association between alcohol consumption and kidney stones does not appear to be significant, other diseases associated with kidney stone formation due to alcohol consumption should be considered.
This study poses some limitations. First, we noticed that some questions in the ALQ explored alcohol consumption of participants in the last 12 months, however, the specific times of kidney stone occurrence for the corresponding participants were not specified in NHANES. As a result, it is unclear how data of participants with kidney stones that occurred before the last 12 months affect the results of this study. In addition, the ALQ questionnaire did not specify the type of alcohol consumed, which limits our ability to assess whether there is an association between different types of alcohol or alcoholic beverages and the formation of kidney stones. Furthermore, detailed data on the composition, size, and treatment history of kidney stones were not available in NHANES, therefore limiting our ability to investigate the correlation between alcohol consumption and different types of kidney stones.
Conclusion
By conducting a cross-sectional study of the available data in the NHANES dataset, we found that alcohol consumption is not significantly associated with the prevalence of kidney stones. This finding requires a more adequate sample size and a more detailed review of the history of kidney stones to be further verified.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: NHANES (https://www.cdc.gov/nchs/nhanes/index.htm).
Ethics statement
The studies involving human participants were reviewed and approved by National Center for Health Statistics (NCHS) research ethics review board. The patients/participants provided their written informed consent to participate in this study.
Author contributions
ZZ and WZ contributed to conception and design of the study. ZZ organized the database. ZZ, ZH, GA, and GZ participated in acquisition of data. ZZ performed the statistical analysis. ZZ wrote the first draft of the manuscript. XG contributed to manuscript revision. All authors contributed to the article and approved the submitted version.
Funding
This work was granted and financed by the Young Talent Support Project of Guangzhou Association for Science and Technology, and the Guangzhou Science Technology and Innovation Commission (no. 202102010214).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
This paper has not been published elsewhere and are only being considered by this journal, although an earlier version of this manuscript has been presented as a preprint in Research square according to the following link: https://www.researchsquare.com/article/rs-2438471/v1. The authors thank the participants and staff of NHANES.
Footnotes
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2023.1156097/full#supplementary-material
References
- 1.Soucie JM, Thun MJ, Coates RJ, McClellan W, Austin H. Demographic and geographic variability of kidney stones in the United States. Kidney Int. (1994) 46:893–9. doi: 10.1038/ki.1994.347, PMID: [DOI] [PubMed] [Google Scholar]
- 2.Pozdzik A, Maalouf N, Letavernier E, Brocheriou I, Body JJ, Vervaet B, et al. Meeting report of the "symposium on kidney stones and mineral metabolism: calcium kidney stones in 2017". J Nephrol. (2019) 32:681–98. doi: 10.1007/s40620-019-00587-1, PMID: [DOI] [PubMed] [Google Scholar]
- 3.Scales CJ, Smith AC, Hanley JM, Saigal CS. Prevalence of kidney stones in the United States. Eur Urol. (2012) 62:160–5. doi: 10.1016/j.eururo.2012.03.052, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Croppi E, Ferraro PM, Taddei L, Gambaro G. Prevalence of renal stones in an Italian urban population: a general practice-based study. Urol Res. (2012) 40:517–22. doi: 10.1007/s00240-012-0477-z [DOI] [PubMed] [Google Scholar]
- 5.Ilhan MN, Yapar D. Alcohol consumption and alcohol policy. Turk J Med Sci. (2020) 50:1197–202. doi: 10.3906/sag-2002-237, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gamage KN, Jamnadass E, Sulaiman SK, Pietropaolo A, Aboumarzouk O, Somani BK. The role of fluid intake in the prevention of kidney stone disease: a systematic review over the last two decades. Turk J Urol. (2020) 46:S92–S103. doi: 10.5152/tud.2020.20155, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheungpasitporn W, Rossetti S, Friend K, Erickson SB, Lieske JC. Treatment effect, adherence, and safety of high fluid intake for the prevention of incident and recurrent kidney stones: a systematic review and meta-analysis. J Nephrol. (2016) 29:211–9. doi: 10.1007/s40620-015-0210-4, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turney BW, Appleby PN, Reynard JM, Noble JG, Key TJ, Allen NE. Diet and risk of kidney stones in the Oxford cohort of the European prospective investigation into cancer and nutrition (EPIC). Eur J Epidemiol. (2014) 29:363–9. doi: 10.1007/s10654-014-9904-5, PMID: [DOI] [PubMed] [Google Scholar]
- 9.Littlejohns TJ, Neal NL, Bradbury KE, Heers H, Allen NE, Turney BW. Fluid intake and dietary factors and the risk of incident kidney stones in UK biobank: a population-based prospective cohort study. Eur Urol Focus. (2020) 6:752–61. doi: 10.1016/j.euf.2019.05.002, PMID: [DOI] [PubMed] [Google Scholar]
- 10.Hirvonen T, Pietinen P, Virtanen M, Albanes D, Virtamo J. Nutrient intake and use of beverages and the risk of kidney stones among male smokers. Am J Epidemiol. (1999) 150:187–94. doi: 10.1093/oxfordjournals.aje.a009979, PMID: [DOI] [PubMed] [Google Scholar]
- 11.de Lorimier AA. Alcohol, wine, and health. Am J Surg. (2000) 180:357–61. doi: 10.1016/S0002-9610(00)00486-4 [DOI] [PubMed] [Google Scholar]
- 12.Wang H, Fan J, Yu C, Guo Y, Pei P, Yang L, et al. Consumption of tea, alcohol, and fruits and risk of kidney stones: a prospective cohort study in 0.5 million Chinese adults. Nutrients. (2021) 13:1119. doi: 10.3390/nu13041119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee YH, Huang WC, Tsai JY, Lu CM, Chen WC, Lee MH, et al. Epidemiological studies on the prevalence of upper urinary calculi in Taiwan. Urol Int. (2002) 68:172–7. doi: 10.1159/000048445, PMID: [DOI] [PubMed] [Google Scholar]
- 14.Khalili P, Jamali Z, Sadeghi T, Esmaeili-Nadimi A, Mohamadi M, Moghadam-Ahmadi A, et al. Risk factors of kidney stone disease: a cross-sectional study in the southeast of Iran. BMC Urol. (2021) 21:141. doi: 10.1186/s12894-021-00905-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor EN, Stampfer MJ, Curhan GC. Obesity, weight gain, and the risk of kidney stones. JAMA. (2005) 293:455–62. doi: 10.1001/jama.293.4.455, PMID: [DOI] [PubMed] [Google Scholar]
- 16.Liu M, Park S. A causal relationship between alcohol intake and type 2 diabetes mellitus: a two-sample Mendelian randomization study. Nutr Metab Cardiovasc Dis. (2022) 32:2865–76. doi: 10.1016/j.numecd.2022.08.013, PMID: [DOI] [PubMed] [Google Scholar]
- 17.Traversy G, Chaput JP. Alcohol consumption and obesity: an update. Curr Obes Rep. (2015) 4:122–30. doi: 10.1007/s13679-014-0129-4, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song L, Maalouf NM. Nephrolithiasis. South Dartmouth (MA): MDText.com, Inc; (2020). 2000 p. [Google Scholar]
- 19.Carbone A, Al SY, Tasca A, Palleschi G, Fuschi A, Nunzio CD, et al. Obesity and kidney stone disease: a systematic review. Minerva Urol Nefrol. (2018) 70:393–400. doi: 10.23736/S0393-2249.18.03113-2. [DOI] [PubMed] [Google Scholar]
- 20.Yang Q, Chen HY, Wang JN, Han HQ, Jiang L, Wu WF, et al. Alcohol promotes renal fibrosis by activating Nox2/4-mediated DNA methylation of Smad7. Clin Sci (Lond). (2020) 134:103–22. doi: 10.1042/CS20191047, PMID: [DOI] [PubMed] [Google Scholar]
- 21.Varga ZV, Matyas C, Paloczi J, Pacher P. Alcohol misuse and kidney injury: epidemiological evidence and potential mechanisms. Alcohol Res. (2017) 38:283–8. [PMC free article] [PubMed] [Google Scholar]
- 22.Yu JT, Hu XW, Chen HY, Yang Q, Li HD, Dong YH, et al. DNA methylation of FTO promotes renal inflammation by enhancing m(6)a of PPAR-alpha in alcohol-induced kidney injury. Pharmacol Res. (2021) 163:105286. doi: 10.1016/j.phrs.2020.105286, PMID: [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: NHANES (https://www.cdc.gov/nchs/nhanes/index.htm).