Abstract
Background
Deficits of physical function are associated with poor quality of life and adverse health outcomes, but data informing the association of these assessments among Black and Hispanic outpatients with heart failure (HF) are limited.
Methods
The multicentre, prospective Screening for Cardiac Amyloidosis With Nuclear Imaging for Minority Populations (SCAN-MP) study identified Black and Hispanic subjects with stable HF, collected baseline characteristics, and took measures using the short physical performance battery. Subjects completed a Kansas City Cardiomyopathy Questionnaire (KCCQ), and the clinical outcomes of HF hospitalization and death were ascertained by telephone and review of the electronic health record.
Results
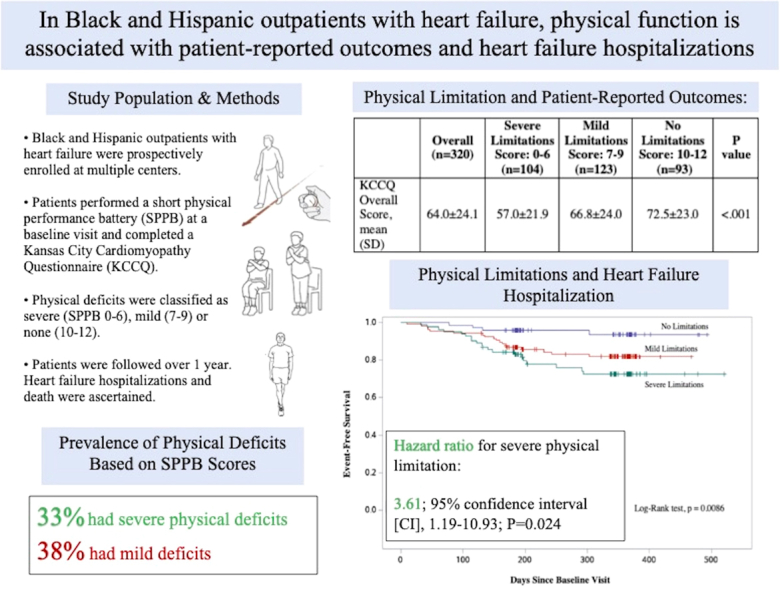
Of 320 participants, 227 (70.9%) had physical deficits, defined by a battery score of ≤ 9. Patients with severe physical deficits reported overall lower KCCQ scores compared to those with no deficits (KCCQ score of 57.0 vs 72.4, P < 0.001). Physical limitation was significantly associated with risk of HF hospitalization, after adjustments for age, sex, and New York Heart Association class (severe physical deficit hazard ratio, 3.61; 95% confidence interval [CI], 1.19-10.93; P = 0.024; mild physical deficit hazard ratio, 2.59; 95% CI, 0.86-7.75; P = 0.090).
Conclusions
Reduced physical performance is highly prevalent among Black and Hispanic outpatients with HF, and it is associated with overall KCCQ score, as well as an increased risk for HF hospitalization.
Graphical abstract
Résumé
Contexte
La limitation physique est associée à une détérioration de la qualité de vie et à une aggravation de l’état de santé, mais il y a peu de données sur la corrélation entre ces paramètres chez les patients externes noirs et hispaniques atteints d’insuffisance cardiaque.
Méthodologie
Dans l’étude multicentrique et prospective de dépistage de l’amylose cardiaque chez les populations minoritaires (SCAN-MP), on a ciblé des sujets noirs et hispaniques atteints d’insuffisance cardiaque stable, recueilli les caractéristiques initiales et mesuré les capacités au moyen du court test d’évaluation de la performance physique (short physical performance battery [SPPB]). Les sujets ont répondu au questionnaire de cardiomyopathie de Kansas City (KCCQ), et les critères cliniques des hospitalisations et des décès liés à l’insuffisance cardiaque ont été évalués par téléphone et par examen des dossiers de santé électroniques.
Résultats
Des 320 participants, 227 (70,9 %) avaient des déficits physiques, définis par un score au SPPB de 9 ou moins. Les patients ayant des déficits physiques graves ont obtenu des scores globaux au KCCQ inférieurs aux patients sans déficit (score KCCQ de 57,0 contre 72,4; p < 0,001). La limitation physique est fortement associée au risque d’hospitalisation liée à l’insuffisance cardiaque, après ajustement pour tenir compte de l’âge, du sexe et de la classe d’insuffisance cardiaque de la New York Heart Association (rapport des risques instantanés [RRI] du déficit physique grave : 3,61; intervalle de confiance [IC] à 95 % de 1,19 à 10,93; p = 0,024; RRI du déficit physique léger : 2,59; IC à 95 % de 0,86 à 7,75; p = 0,090).
Conclusions
La diminution de la performance physique est très fréquente chez les patients externes noirs et hispaniques atteints d’insuffisance cardiaque, et elle est corrélée au score global au KCCQ ainsi qu’à une augmentation du risque d’hospitalisation liée à l’insuffisance cardiaque.
Poor physical performance is associated with adverse outcomes, in both the general population1, 2, 3, 4 and those with heart failure (HF),5, 6, 7, 8 independent of other comorbidities. The short physical performance battery (SPPB), developed by Guralnik et al. in 1994, is commonly used as an objective surrogate for physical function, given its reliability, its reproducibility, and the relative ease with which it can be used in a clinic or inpatient setting.9,10 The exam tests an individual’s balance, gait speed, and the ability to repeatedly stand from a seated position, and it is scored on a scale from 0 to 12, with higher scores reflecting better lower-extremity function. Since its development, numerous studies have demonstrated its association with a variety of health outcomes. In the general population, lower SPPB scores have been predictive of disability, poor quality of life, incident hospitalization among outpatients, rehospitalization among inpatients, and overall mortality.1,11, 12, 13 More recently, these associations have been studied in the HF population. In patients hospitalized with decompensated HF, lower SPPB scores are associated with length of hospital stay, quality of life, risk of rehospitalization, and overall mortality.14, 15, 16, 17 However, data exploring the association of SPPB with health outcomes in outpatients with established HF are limited.18 Furthermore, data exploring these associations in individuals who identify as Black or Hispanic are particularly sparse. Accordingly, we sought to evaluate the extent of physical disability in this specific outpatient population with HF, and to explore the association of SPPB scores with patient-reported outcomes, HF-related hospitalization, and death.
Materials and Methods
Study design
This study was a substudy of the multicentre, prospective Screening for Cardiac Amyloidosis With Nuclear Imaging for Minority Populations (SCAN-MP) study. The SCAN-MP study is an ongoing actively enrolling cohort study of older self-identified Black or Caribbean Hispanic community–dwelling outpatients with HF. All participants who were enrolled in the SCAN-MP study from May 14, 2019 until March 9, 2022 and completed an SPPB were included in this substudy.
Study setting and eligibility criteria
As part of the SCAN-MP study, participants were recruited at 2 study sites in New York (Columbia University Irving Medical Centre and Harlem Hospital Centre) and 1 site in Boston (Boston Medical Centre). This study was approved by the Western Institutional Review Board (WIRB) and respective IRBs of Boston University Medical Campus and Columbia University Irving Medical Centre. As a primary goal of the SCAN-MP study is to identify previously undiagnosed transthyretin cardiac amyloidosis (ATTR-CA), men and women were eligible if they self-identified as Black or Hispanic of Caribbean origin, were aged ≥ 60 years, had a diagnosis of HF, and had an interventricular septum or posterior wall ≥ 12 millimeters on echocardiography. Age ≥ 60 years and left ventricular wall diameters were added to the inclusion criteria, as ATTR-CA most commonly affects older individuals and causes increased wall thickness. Participants were considered to have HF if they fulfilled at least 1 of 2 definitions described by Rich et al.19 or the National Health and Nutrition Examination Survey (NHANES).20 The former requires a history of acute pulmonary edema or the occurrence of at least 2 of the following that improved with diuretic therapy without another identifiable cause: dyspnea on exertion, paroxysmal nocturnal dyspnea, orthopnea, bilateral lower-extremity edema, or exertional fatigue. The latter tallies the presence of clinical variables, which include dyspnea, tachycardia, rales, jugulovenous distention, edema, hepatomegaly, and typical chest radiograph findings. As the primary goal of the SCAN-MP study is to identify previously undiagnosed ATTR-CA, participants were excluded if their HF was the result of another clearly evident cause, as determined by the study physicians. Thus, subjects with HF primarily due to ischemic or valvular heart disease were not included in the SCAN-MP study, and therefore were not included in this substudy. Individuals were also excluded if they had any of the following: life expectancy < 1 year due to noncardiac disease; left ventricular ejection fraction (LVEF) < 30%; chronic kidney disease (CKD) with estimated glomerular filtration rate (eGFR) < 15 mL/min per 1.73 m2; end-stage renal disease (ESRD), anticipated need for ventricular assist device or inotropic support in the next 6 months; dementia; debilitating stroke; or another medical disorder that precluded participation in the study. All recruited participants provided written consent, which they could provide in English, Spanish, French, or Haitian–Creole.
Data collection
At the initial study visit, research staff completed a demographic and medical history survey based on electronic chart review. If data were missing after electronic chart review, participants were asked directly for this information in their native language. The demographic survey included sex, age, racial identity (Black, non-Black, decline to specify), Hispanic origin (yes, no, decline to specify), marital status, and highest level of education (Table 1). Medical history collected by research staff included atrial fibrillation or flutter, coronary artery disease (CAD) or prior myocardial infarction, hypertension, hyperlipidemia, peripheral vascular disease (PVD), stroke, CKD, hyper- or hypothyroidism, liver disease (chronic hepatitis or hepatic cirrhosis), anemia, chronic obstructive pulmonary disease (COPD), asthma, osteoarthritis, carpal tunnel syndrome, spinal stenosis, polyneuropathy, benign prostatic hypertrophy or enlarged prostate, gastric or duodenal ulcers, cancer, and diabetes. Subjects were also asked about their smoking history and alcohol intake. All demographic and medical history surveys were completed in paper format and were subsequently transferred by research staff to a Research Electronic Data Capture (REDCap) database.
Table 1.
Baseline characteristics by short physical performance battery category
|
Characteristic |
Overall (n = 320) | Severe limitations score: 0–6 (n = 104) | Mild limitations score: 7–9 (n = 123) | No limitations score: 10–12 (n = 93) | P | Tukey's Studentized range (HSD) test comparisons significant at the 0.05 level are indicated by ∗∗∗ |
||
|---|---|---|---|---|---|---|---|---|
| Severe limitations – Mild limitations |
Severe limitations – No limitations |
Mild limitations – No limitations |
||||||
| Age, y | 72.8 ± 8.9 | 75.3 ± 8.8 | 71.8 ± 8.6 | 69.5 ± 7.2 | < 0.001 | ∗∗∗ | ∗∗∗ | |
| Gender | < 0.001 | |||||||
| Male | 173 (54.1) | 39 (37.5) | 70 (56.9) | 64 (68.8) | ||||
| Female | 147 (45.9) | 65 (62.5) | 53 (43.1) | 29 (31.2) | ||||
| Racial identity | 0.108 | |||||||
| Black | 256 (80.0) | 77 (74.0) | 104 (84.6) | 75 (80.7) | ||||
| Non-Black | 7 (2.2) | 1 (1.0) | 4 (3.3) | 2 (2.2) | ||||
| Not reported/ declined to specify | 57 (17.8) | 25 (25.0) | 15 (12.2) | 16 (17.2) | ||||
| Hispanic origin | 0.026 | |||||||
| Yes | 104 (32.5) | 40 (38.5) | 29 (23.6) | 35 (37.6) | ||||
| No | 216 (67.5) | 64 (61.5) | 94 (76.4) | 58 (62.4) | ||||
| Unknown or not reported | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||||
| BMI | 32.9 ± 7.2 | 34.7 ± 7.9 | 32.5 ± 7.0 | 32.3 ± 6.3 | 0.025 | ∗∗∗ | ||
| Marital status | 0.033 | |||||||
| Married | 92 (28.9) | 26 (25.2) | 32 (26.2) | 34 (36.6) | ||||
| Divorced/separated | 57 (17.9) | 15 (14.6) | 28 (23.0) | 14 (15.1) | ||||
| Widowed | 60 (18.9) | 21 (20.4) | 24 (19.7) | 15 (16.1) | ||||
| Living as married/living with partner | 8 (2.5) | 0 (0) | 2 (1.6) | 6 (6.5) | ||||
| Single (never married) | 99 (31.1) | 41 (39.8) | 35 (28.7) | 23 (24.7) | ||||
| Did not answer | 2 (0.6) | 0 (0) | 1 (0.8) | 1 (1.1) | ||||
| Highest level of education | 0.001 | |||||||
| Less than high school | 110 (34.7) | 54 (52.4) | 33 (27.1) | 23 (25.0) | ||||
| High school/GED | 122 (38.5) | 35 (34.0) | 49 (40.2) | 38 (41.3) | ||||
| 2-year or 4-year college degree | 66 (20.8) | 11 (10.7) | 30 (24.6) | 25 (27.2) | ||||
| Graduate or postgraduate degree | 16 (5.1) | 3 (2.9) | 8 (6.6) | 5 (5.4) | ||||
| Did not answer | 3 (1) | 0 (0) | 2 (1.6) | 1 (1.1) | ||||
| Comorbidities | ||||||||
| Coronary artery disease | 77 (24.1) | 36 (34.6) | 25 (20.3) | 16 (17.2) | 0.008 | |||
| Atrial fibrillation or flutter | 93 (29.1) | 40 (38.5) | 32 (26.0) | 21 (22.6) | 0.032 | |||
| Hypertension | 308 (96.3) | 100 (96.2) | 120 (97.6) | 88 (94.6) | 0.534 | |||
| Hyperlipidemia | 235 (73.4) | 79 (76.0) | 84 (68.3) | 72 (77.4) | 0.251 | |||
| Stroke | 40 (12.5) | 13 (12.5) | 13 (10.6) | 14 (15.1) | 0.615 | |||
| Chronic kidney disease | 110 (34.4) | 43 (41.4) | 48 (39.0) | 19 (20.4) | 0.003 | |||
| Diabetes mellitus | 181 (56.6) | 64 (61.5) | 67 (54.5) | 50 (53.8) | 0.458 | |||
| Number of comorbidities | 5.7 ± 2.0 | 6.4 ± 1.9 | 5.7 ± 1.9 | 4.8 ± 1.7 | < 0.001 | ∗∗∗ | ∗∗∗ | ∗∗∗ |
| Smoking history | 0.684 | |||||||
| Yes | 47 (21.7) | 17 (21.3) | 20 (24.4) | 10 (18.2) | ||||
| No | 170 (78.3) | 63 (78.8) | 62 (75.6) | 45 (81.8) | ||||
| Current alcohol intake | 0.069 | |||||||
| Yes | 110 (34.4) | 28 (26.9) | 51 (41.5) | 31 (33.3) | ||||
| No | 210 (65.6) | 76 (73.1) | 72 (58.5) | 62 (66.7) | ||||
| Echo LVEF | 59.1 ± 11.5 | 58.2 ± 12.2 | 59.6 ± 10.8 | 58.1 ± 12.0 | 0.583 | |||
| NYHA class | < 0.001 | |||||||
| I | 69 (21.6) | 9 (8.7) | 25 (20.3) | 35 (37.6) | ||||
| II | 172 (53.9) | 51 (49.5) | 71 (57.7) | 50 (53.8) | ||||
| III | 77 (24.1) | 42 (40.8) | 27 (22.0) | 8 (8.6) | ||||
| IV | 1 (0.3) | 1 (1.0) | 0 (0) | 0 (0) | ||||
| 6MWT | 261.5 ± 122.9 | 162.1 ± 85.7 | 277.0 ± 102.8 | 341.7 ± 110.7 | < 0.001 | ∗∗∗ | ∗∗∗ | ∗∗∗ |
| ATTR-CA cases | 17 (5.3) | 9 (8.7) | 2 (1.6) | 6 (6.5) | 0.055 | |||
Values are n (%), or mean (± standard deviation), unless otherwise indicated.
ATTR-CA, cardiac transthyretin amyloidosis; BMI, body mass index; GED, general equivalency diploma; HSD, honestly significant difference; LVEF, left venctricular ejection fraction; NYHA, New York Heart Association; SPPB, short physical performance battery; 6MWT, 6-minute walk test.
Weight and height were measured by trained research staff at the initial visit, and body mass index (BMI) was calculated from these measurements. New York Heart Association (NYHA) classification was determined by study physicians. As part of the larger SCAN-MP study, participants also had basic lab tests, an electrocardiogram, an echocardiogram, and a 99m-technetium pyrophosphate scan. Genetic testing was performed to determine if mutant or wild-type ATTR-CA was present.
Physical performance evaluation
During the initial study visit, participants completed an SPPB assessment. The SPPB tests an individual’s balance, gait speed, and ability to repeatedly sit and stand from a chair. To test balance, individuals are asked to stand for 10 seconds with their feet at first side-by-side, then second in semi-tandem, and third in tandem. If the individual is unable to perform any of the stances, the balance assessment is terminated without attempting the next stance. One point is awarded for each 10-second stance completed, with an additional point added for completing all 3 stances. To evaluate gait speed, individuals complete a timed 4-meter walk, with faster speeds corresponding to higher scores, ranging from 0 to 4. Finally, individuals are asked to sit and stand from a chair 5 times. Again, faster times correspond to higher scores, ranging from 0 to 4. Overall, the SPPB is scored from 0 to 12, with higher scores reflecting better physical function. SPPB scores were stratified into severe physical deficit (0-6), mild physical deficit (7-9) and no physical deficit (10-12), similar to groupings in previous reports.18 Participants also performed a 6-minute walk test (6MWT) per the guidelines of the American Thoracic Society.21 A trained research staff member was present throughout the physical performance evaluations and provided instructions in each participant’s preferred language.
Patient-reported outcomes
At the initial visit, participants completed the 23-item Kansas City Cardiomyopathy Questionnaire (KCCQ).22 Paper copies of the KCCQ were administered in English, Spanish, French, or Haitian–Creole. Subjects completed the survey independently when possible, and research staff was available if questions arose. If a participant was illiterate or had vision problems, research staff read the survey out loud in the individual’s native language. Since its initial development as a measurement tool for disease-specific patient-reported outcomes in individuals with HF, the KCCQ has been validated for both patients with reduced and those with preserved LVEF.23,24 The survey items are specific to individuals with HF and can be stratified into the following multiple domains: physical limitations, symptom frequency, symptom severity, symptom stability, self-efficacy, quality of life, and social limitation. The KCCQ is scored from 0 to 100, with higher scores reflecting better patient-reported health status.
Primary outcome ascertainment
All-cause survival and HF-related hospitalization were determined by review of the electronic health record (EHR) with verification of discharge diagnosis, as well as follow-up phone calls at 6 and 12 months. If HF hospitalizations were documented in the EHR, this was confirmed with participants over the phone. If no outcomes were recorded in the EHR, individuals were contacted by phone and asked if they had been hospitalized since their initial visit. If the participant had been hospitalized at another medical facility that was not within the EHR system, research staff obtained primary documentation from the outside hospital to discern the cause for hospitalization. HF-related hospitalization was determined by diagnosis related group (DRG) coding in the medical record. Hospitalization data were missing in 4 subjects who died, in 3 subjects before the 6-month follow-up, and in 1 subject before the 12-month follow-up.
Statistical analysis
Differences in baseline characteristics between the SPPB groups were determined using analysis of variance (ANOVA) and Pearson’s χ2 tests. When more than 20% of the expected counts were less than 5, Fisher's exact test was performed, or Monte Carlo estimates were determined in rare circumstances. For continuous variables, if the omnibus 1-way ANOVA was significant at the 5% level, post hoc testing was determined using Tukey’s multiple-comparison test. A univariate linear regression analysis of each predictor was also performed using SPPB score as the dependent variable. Significant variables in the univariate linear regression analysis (P < 0.05) were then included in a multiple linear regression model. The association of SPPB score with event-free survival was analyzed using Kaplan-Meier survival estimates, with events being the combined outcome of HF-related hospitalization or death. Subjects were censored if, at the time of the 6- or 12-month follow-up, no HF hospitalization or death was reported; or, for individuals unable to be contacted, if they had not had an event according to electronic chart review. Time to event was calculated from initial visit to the day of hospitalization for HF or day of death. Subjects with no follow-up data in the REDCap database were not included in the survival analysis. For individuals who died and did not have hospitalization data, follow-up time was calculated up to their date of death, regardless of whether HF hospitalization occurred. Comparison of the survival distributions of the SPPB stratifications was evaluated with log-rank testing. Multivariable analysis, adjusted for age, sex, and NYHA class, was performed by Cox proportional hazards modeling, with hazard ratios and 95% confidence intervals reported. All tests were 2-sided and were considered statistically significant at P < 0.05. Statistical analysis was performed using SAS Studio 3.8 (Enterprise Edition; SAS. Cary, NC).
Results
Study participants and covariates
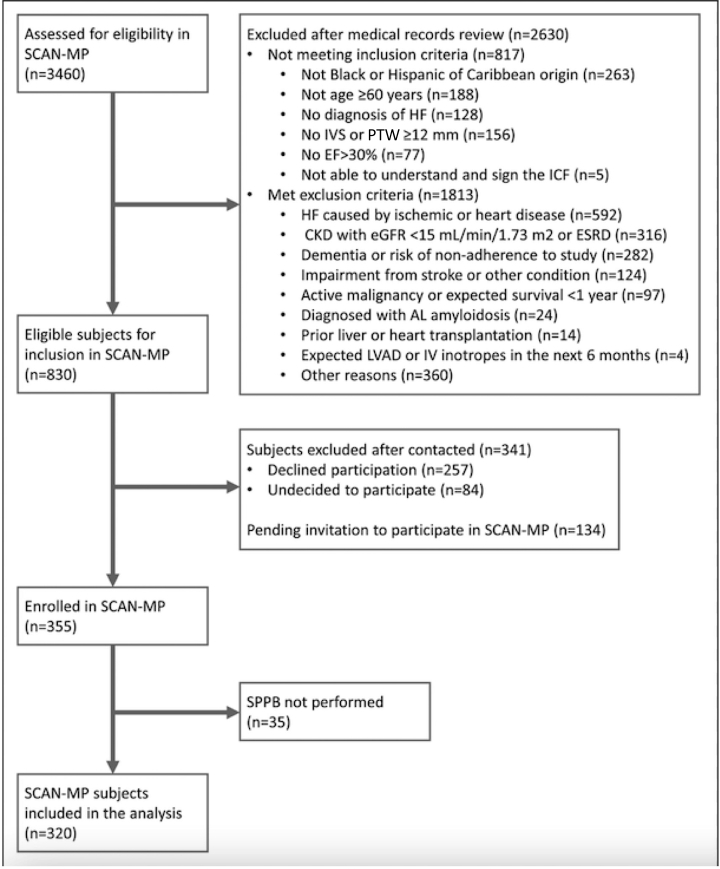
Between May 14, 2019 and March 9, 2022, a total of 3460 individuals were assessed for eligibility in the SCAN-MP study, of whom 830 were determined to be eligible for inclusion (Fig. 1). Of the 830 eligible subjects, 355 agreed to enroll in the study, and 320 completed an SPPB. Of these individuals, 104 (32.5%) had an SPPB score ≤ 6; 123 (38.4%) had an SPPB score of 7-9; and 93 (29.1%) had an SPPB score ≥ 10. The baseline demographics are shown in Table 1. Of the 147 women enrolled in the substudy, 65 (44.2%) had severe physical limitations (SPPB score ≤ 6), and 39 of the 173 men (22.5%) had severe deficits. Two hundred fifty six (80.0%) identified as Black, and 104 (32.5%) identified as Hispanic. A total of 57 participants did not report their race, and 7 reported their race as “non-Black”; all of these 64 subjects were Hispanic. The mean age was 72.8 ± 8.9 years, and the mean LVEF was 59.1% ± 11.5%. A total of 260 subjects (81.3%) had an LVEF ≥ 50%, and 60 (18.8%) had an LVEF between 30% and 49%. Compared to patients with an SPPB score ≥ 10, individuals with an SPPB score ≤ 6 were more likely to be older (mean age, 75.3 vs 69.5 years, P < 0.001), be female (χ2 < 0.001), have a higher BMI (34.7 vs 32.3 kg/m2, P = 0.025), have more comorbidities (6.4 vs 4.8, P < 0.001), and have a higher NYHA class (χ2 < 0.001). Severe physical deficits were also associated with shorter walking distances in the 6MWT (162.1 vs 341.7 m, P < 0.001). No significant difference was present in number of ATTR-CA cases among SPPB categories. In the univariate linear regression using SPPB score as the dependent variable, the covariates age, sex, Black race, BMI, marital status, education, CAD, atrial fibrillation, CKD, number of comorbidities, and 6MWT distance were all significantly associated. When these variables were included in a multiple linear analysis, sex (P = 0.004), marital status (P = 0.042), education (P = 0.001), and 6MWT distance (P < 0.001) remained significant.
Figure 1.
Screening, inclusion, and exclusion. AL, light-chain amyloidosis; CKD, chronic kidney disease; EF, left ventricular ejection fraction; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; HF, heart failure; ICF, informed consent form; IV, intravenous; IVS, interventricular septum; LVAD, left ventricular assist device; PTW, posterior wall thickness; SCAN-MP, Screening for Cardiac Amyloidosis With Nuclear Imaging for Minority Populations; SPPB, short physical performance battery.
Patient-reported outcomes
Severe physical deficits were also associated with lower overall KCCQ scores, compared to the scores for those without physical deficits (KCCQ overall score, respectively, 57.0 vs 72.4, P < 0.001; Table 2). This association remained significant for several subdomains of the KCCQ that extended beyond the physical limitation subdomain. Lower SPPB scores were associated with worse scores in the subdomains of symptom frequency, symptom burden, quality of life, and social limitations as well (Table 2). The significant association between SPPB score and KCCQ score remained true in both the univariate and the multivariate linear regression models after adjusting for age, sex, BMI, marital status, education, history of CAD, history of CKD, NYHA class, and 6MWT distance (P = 0.04).
Table 2.
KCCQ subdomain scores by short physical performance battery category
|
Subdomain |
Overall | Severe limitations score: 0–6 (n = 104) | Mild limitations score: 7–9 (n = 123) | No limitations score: 10–12 (n = 93) | P | Tukey's Studentized range (HSD) test comparisons significant at the 0.05 level are indicated by ∗∗∗ |
||
|---|---|---|---|---|---|---|---|---|
| Severe limitations – Mild limitations |
Severe limitations – No limitations |
Mild limitations – No limitations |
||||||
| KCCQ overall score | 64.0 ± 24.1 | 57.0 ± 21.9 | 66.8 ± 24.0 | 72.5 ± 23.0 | < 0.001 | ∗∗∗ | ∗∗∗ | |
| Physical limitation | 60.7 ± 28.9 | 51.2 ± 27.2 | 65.4 ± 27.3 | 71.8 ± 26.7 | < 0.001 | ∗∗∗ | ∗∗∗ | |
| Symptom stability | 52.8 ± 22.5 | 52.4 ± 21.2 | 52.6 ± 22.8 | 55.2 ± 22.7 | 0.635 | |||
| Symptom frequency | 66.7 ± 26.6 | 58.8 ± 25.1 | 70.0 ± 26.3 | 75.3 ± 24.7 | < 0.001 | ∗∗∗ | ∗∗∗ | |
| Symptom burden | 70.0 ± 26.7 | 63.4 ± 26.4 | 72.6 ± 25.8 | 77.4 ± 25.3 | 0.001 | ∗∗∗ | ∗∗∗ | |
| Self-efficacy | 75.6 ± 25.7 | 73.0 ± 26.7 | 76.2 ± 25.1 | 76.4 ± 26.1 | 0.582 | |||
| Quality of life | 62.1 ± 26.4 | 56.5 ± 25.8 | 63.8 ± 27.1 | 67.8 ± 24.2 | 0.009 | ∗∗∗ | ||
| Social limitations | 64.0 ± 31.5 | 58.0 ± 29.7 | 66.4 ± 31.5 | 72.4 ± 29.8 | 0.007 | ∗∗∗ | ||
Values are mean ± standard deviation, unless otherwise indicated.
HSD, honestly significant difference; KCCQ, Kansas City Cardiomyopathy Questionnaire.
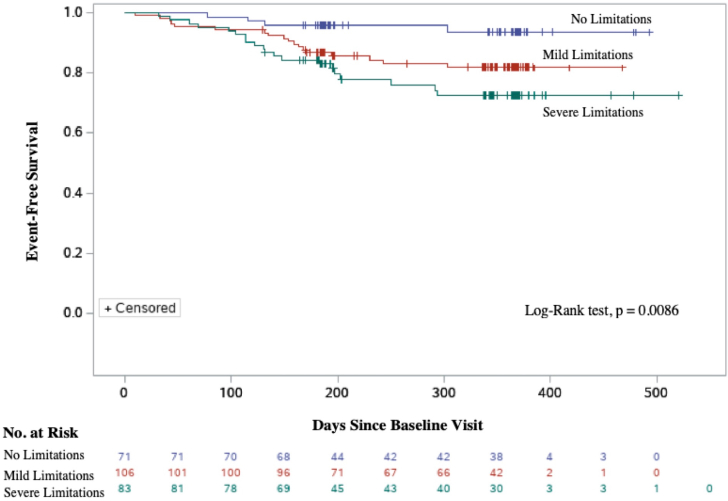
Clinical outcomes
Kaplan-Meier event-free survival estimates according to SPPB stratification are shown in Figure 2. Over a median follow-up period of 338 days, 44 of the 320 participants (13.8%) had been hospitalized for HF, and 15 (4.7%) had died. Female patients accounted for 21 of the 44 hospitalizations (47.7%) and 4 of the 15 deaths (26.7%). In the multivariate Cox regression model, adjusted for age and sex and NYHA class, the risk of HF hospitalization was significantly associated with SPPB strata (Table 3). In the group with no physical limitations, 4 HF hospitalizations occurred during the follow-up period. Comparatively, 23 HF hospitalizations occurred in the severe deficit group (hazard ratio 3.61; 95% confidence interval [CI], 1.19-10.93; P = 0.024), and 17 occurred in the group with mild physical deficits (hazard ratio 2.59; 95% CI, 0.86-7.75; P = 0.090). No statistical differences were present in number of deaths among the groups. In the subgroup analysis for sex, SPPB score ≤ 6 was associated with HF hospitalization in women, after adjustments for age (hazard ratio 8.07; 95% CI, 1.05-62.13, P = 0.045). For men, mild physical limitations (SPPB score 7-9) were associated with HF hospitalization (hazard ratio 4.45; 95% CI, 1.28-15.51; P = 0.019).
Figure 2.
Event-free survival for the composite outcome of heart failure–related hospitalizations or death, stratified by short physical performance battery (SPPB) score.
Table 3.
Outcomes by short physical performance battery (SPPB) score
| Outcome (SPPB score), n | n (%) | Hazard ratio (95% CI) | P |
|---|---|---|---|
| Overall | |||
| HF hospitalization | |||
| Severe (0–6), 104 | 23 (22.1) | 3.61 (1.19–10.93) | 0.024 |
| Mild (7–9), 123 | 17 (13.8) | 2.59 (0.86–7.75) | 0.090 |
| No (10–12), 93 | 4 (4.3) | Reference | |
| Death | |||
| Severe (0–6), 104 | 4 (3.9) | 1.01 (0.22–4.59) | 0.989 |
| Mild (7–9), 123 | 7 (5.7) | 1.32 (0.38–4.63) | 0.668 |
| No (10–12), 93 | 4 (4.3) | Reference | |
| HF hospitalization or death | |||
| Severe (0–6), 104 | 26 (25) | 2.58 (1.06–6.30) | 0.038 |
| Mild (7–9), 123 | 21 (17.1) | 1.97 (0.83–4.68) | 0.126 |
| No (10–12), 93 | 7 (7.5) | Reference | |
| Female participants | |||
| HF hospitalization | |||
| Severe (0–6), 65 | 17 (26.2) | 8.07 (1.05-62.13) | 0.045 |
| Mild limitations (7–9), 53 | 3 (5.7) | 1.54 (0.16–14.81) | 0.710 |
| No limitations (10–12), 29 | 1 (3.5) | Reference | |
| Death | |||
| Severe (0–6), 65 | 2 (3.1) | 0.65 (0.05–8.35) | 0.737 |
| Mild (7–9), 53 | 1 (1.9) | 0.45 (0.03–7.45) | 0.580 |
| No (10–12), 29 | 1 (3.5) | Reference | |
| HF hospitalization or death | |||
| Severe (0–6), 65 | 19 (29.2) | 4.39 (0.99–19.43) | 0.052 |
| Mild (7–9), 53 | 3 (5.7) | 0.76 (0.13–4.57) | 0.765 |
| No (10–12), 29 | 2 (6.9) | Reference | |
| Male participants | |||
| HF hospitalization | |||
| Severe (0–6), 39 | 6 (15.4) | 3.4 (0.84–13.8) | 0.087 |
| Mild (7–9), 70 | 14 (20.0) | 4.45 (1.28–15.51) | 0.019 |
| No (10–12), 64 | 3 (4.7) | Reference | |
| Death | |||
| Severe (0–6), 39 | 2 (5.1) | 1.03 (0.17–6.28) | 0.977 |
| Mild (7–9), 70 | 6 (8.6) | 1.73 (0.43–6.93) | 0.443 |
| No (10–12), 64 | 3 (4.7) | Reference | |
| HF hospitalization or death | |||
| Severe (0–6), 39 | 7 (18.0) | 2.32 (0.73–7.41) | 0.157 |
| Mild (7–9), 70 | 18 (25.7) | 3.41 (1.26–9.19) | 0.016 |
| No (10–12), 64 | 5 (7.8) | Reference |
Overall hazard ratios were adjusted by age, sex, and New York Heart Association class. Hazard ratios for female and male participants were adjusted by age. “Severe,” “Mild,” and “No” indicate level of limitations.
CI, confidence interval; HF, heart failure.
Discussion
This study examined a large cohort of Black and Hispanic outpatients with stable HF and showed that physical deficits, as defined by an SPPB score ≤ 9, are highly prevalent among this population. The principal finding of the study is that in Black and Hispanic outpatients with HF, the SPPB score is independently associated with HF hospitalizations, after adjustments for age, sex, and NYHA class. These results support the utility of the SPPB as an easy and reliable tool to evaluate physical function in outpatients with HF, and they suggest that using SPPB in the clinic setting could be beneficial in predicting health-related outcomes.
Physical limitation prevalence and covariates
Our study adds to the growing literature highlighting the prevalence of physical limitation in outpatients with HF.7,14,18,25, 26, 27, 28 However, many of the past studies have utilized the Fried Frailty Phenotype to characterize physical limitation, rather than the SPPB.7,25, 26, 27, 28 Meanwhile, the SPPB has been studied primarily in hospitalized patients with HF, or in outpatients without cardiac disease.1,9,11, 12, 13,29 To our knowledge, this study is one of the first utilizing the SPPB to evaluate outpatients with HF, and the first to do so in an exclusively Black and Hispanic population. Results from this study showed that 227 of 320 participants (70.9%) had physical limitations, as defined by an SPPB score ≤ 9. More specifically, 32.5% had severe physical limitations (SPPB score ≤ 6), whereas 38.4% had mild physical limitations (SPPB score 7-9), similar to the findings of a prior study examining predominantly White outpatients with HF.18 A point worth noting is that although patients with LVEF ≥ 30% were eligible for enrollment, the mean and standard deviation of LVEF in our results rendered our cohort to be overwhelmingly one of patients with preserved LVEF (59.1% ± 11.5%). Notably, the LVEF was not associated with SPPB scores in either the ANOVA or the linear regression model. When results were stratified by sex, women were found to be more likely to have poor physical performance. Of the 147 women enrolled in the study, 65 (44.2%) had severe physical limitation, compared to 39 of the 173 men (22.5%); these differences were significant in the ANOVA, as well as in the univariate and multivariate linear regressions. Unsurprisingly, the SPPB score, which incorporates an assessment of walking speed, was also significantly associated with 6MWT distances,
Association with KCCQ
Since its development in the early 2000s, the KCCQ has grown in popularity and become a valuable tool in the disease-specific evaluation of HF. The survey, which has 7 subdomains, has been utilized to quantify physical limitations, symptom burden, symptom stability, symptom frequency, self-efficacy, quality of life, and social limitations. As the emphasis placed on quality of life is growing, the KCCQ has become a common endpoint in recent literature. Our study showed that multiple subdomains of the KCCQ were associated with physical function. Predictably, the association included the physical limitation subdomain, but also symptom frequency, symptom burden, quality of life, and social limitations. The overall KCCQ score was also associated with SPPB scores in the ANOVA, and both the univariate and multivariate linear regression analysis. These findings suggest that if lower-extremity strength were improved and SPPB scores increased, patients might have improvements in their quality of life, social interactions, and perceived symptom burden and frequency.
Association with hospitalizations
The most important finding of the study was that SPPB score, when adjusted for age, sex, and NYHA class, was significantly associated with HF hospitalization. During the follow-up period of the substudy (median, 338 days), 59 clinical events occurred, including 44 HF hospitalizations and 15 deaths. Kaplan-Meier event-free survival estimates according to SPPB stratification revealed a significant association in the combined outcome of HF hospitalization or death. This result was driven primarily by HF hospitalizations, with severe physical deficits (SPPB ≤ 6) portending a hazard ratio of 3.61 (95% CI, 1.19-10.93). Women, though they were more likely to have severe physical deficits, had similar rates of hospitalization (23 of 147; 14.3%) compared to those of men (23 of 173; 13.3%). These findings add to a growing body of literature showing the associations of lower-extremity function and frailty with clinical outcomes in HF.5,18
Future direction
This study suggests that the SPPB, which measures an individual’s physical function with balance, gait speed, and leg strength, could help clinicians prognosticate outcomes for Black and Hispanic outpatients in the HF clinic. The results also suggest that by improving this population’s SPPB score via lower-extremity strengthening, clinicians could enact a multifaceted improvement in their HF-related health status, and may reduce the risk of HF-related hospitalization or death. Past work has suggested that physical function is a dynamic process and that SPPB scores can be improved with directed interventions.30,31 Multidisciplinary rehabilitation programs have shown promising effects for patients with various cardiovascular diseases.32, 33, 34, 35, 36 Moreover, a meta-analysis examining exercise-based cardiac rehabilitations for patients with HF showed that structured physical activity was associated with improvements in both exercise capacity and quality of life.37 Recently, the Rehabilitation Therapy in Older Acute Heart Failure Patients (REHAB-HF) trial showed that in patients recently admitted for decompensated HF, a tailored rehabilitation intervention program led to improved SPPB scores at 3 months.38 These findings highlight the importance of interventions aimed at improving physical status for patients with HF.
Strengths and limitations
A primary strength of this study is its characterization of physical function in an exclusively Black and Hispanic outpatient population with HF. In addition, older adults, who are traditionally excluded from research, were included in this study. Finally, the cohort had thorough phenotyping, with demographic information, clinical history, functional status, and objective measurements. The study had several limitations. Patients with ischemic and valvular heart disease were excluded from this study, and therefore results cannot be generalized to those populations. In addition, the study results cannot be generalized to patients with HF who have left ventricular wall thickness < 12 mm. However, previous results show that patients with HF with preserved ejection fraction due to hypertension have similar degrees of left ventricular hypertrophy and therefore, our study results are likely applicable to a large proportion of patients with HF with preserved ejection fraction.39 Finally, the results of this substudy were mainly cross-sectional, with follow-up data mostly limited to ascertainment of death and hospitalization.
Conclusion
Among Black and Hispanic outpatients with HF, physical limitations as measured by the SPPB are highly prevalent. SPPB scores are associated with HF-related hospitalizations, as well as overall KCCQ score, and scores across a variety of KCCQ subdomains. Our data suggest that targeted interventions to improve physical function could reduce the risk of hospitalization and improve quality of life in this patient population.
Acknowledgments
Funding Sources
This study was supported by a grant from the National Institutes of Health (NIH) (R01HL139671).
Disclosures
F.L.R. is a consultant for Alexion and Attralus and has research support from NIH R01HL139671, Pfizer, Akcea, and Alnylam. M.S.M. has grants support from NIH R01HL139671, R21AG058348, and K24AG036778, consulting income from Eidos, Prothena, Ionis, Alnylam and Intellia, and institutional support in the form of clinical trial funding from Pfizer, Ionis, Eidos, and Alnylam. The other authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: This study was approved by the Western Institutional Review Board and the respective institutional review boards of Boston University Medical Centre and Columbia University Irving Medical Centre. All recruited participants provided written consent, which could be provided in English, Spanish, French, or Haitian–Creole.
See page 301 for disclosure information.
References
- 1.Penninx B.W., Ferrucci L., Leveille S.G., et al. Lower extremity performance in nondisabled older persons as a predictor of subsequent hospitalization. J Gerontol A Biol Sci Med Sci. 2000;55:M691–M697. doi: 10.1093/gerona/55.11.m691. [DOI] [PubMed] [Google Scholar]
- 2.Khan H., Kalogeropoulos A.P., Georgiopoulou V.V., et al. Frailty and risk for heart failure in older adults: the health, aging, and body composition study. Am Heart J. 2013;166:887–894. doi: 10.1016/j.ahj.2013.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Studenski S., Perera S., Wallace D., et al. Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003;51:314–322. doi: 10.1046/j.1532-5415.2003.51104.x. [DOI] [PubMed] [Google Scholar]
- 4.Pavasini R., Guralnik J., Brown J.C., et al. Short physical performance battery and all-cause mortality: systematic review and meta-analysis. BMC Med. 2016;14:215. doi: 10.1186/s12916-016-0763-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pandey A., Kitzman D., Whellan D.J., et al. Frailty among older decompensated heart failure patients: prevalence, association with patient-centered outcomes, and efficient detection methods. JACC Heart Fail. 2019;7:1079–1088. doi: 10.1016/j.jchf.2019.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boxer R., Kleppinger A., Ahmad A., et al. The 6-minute walk is associated with frailty and predicts mortality in older adults with heart failure. Congest Heart Fail. 2010;16:208–213. doi: 10.1111/j.1751-7133.2010.00151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Madan S.A., Fida N., Barman P., et al. Frailty assessment in advanced heart failure. J Card Fail. 2016;22:840–844. doi: 10.1016/j.cardfail.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Newman A.B., Gottdiener J.S., Mcburnie M.A., et al. Cardiovascular Health Study Research Group. Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci. 2001;56:M158–M166. doi: 10.1093/gerona/56.3.m158. [DOI] [PubMed] [Google Scholar]
- 9.Guralnik J.M., Simonsick E.M., Ferrucci L., et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 10.Guralnik J.M., Ferrucci L., Simonsick E.M., Salive M.E., Wallace R.B. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–562. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campo G., Maietti E., Tonet E., et al. The assessment of scales of frailty and physical performance improves prediction of major adverse cardiac events in older adults with acute coronary syndrome. J Gerontol A Biol Sci Med Sci. 2020;75:1113–1119. doi: 10.1093/gerona/glz123. [DOI] [PubMed] [Google Scholar]
- 12.Ashikaga K., Saji M., Takanashi S., et al. Physical performance as a predictor of midterm outcome after mitral valve surgery. Heart Vessels. 2019;34:1665–1673. doi: 10.1007/s00380-019-01397-y. [DOI] [PubMed] [Google Scholar]
- 13.Saji M., Higuchi R., Tobaru T., et al. Impact of frailty markers for unplanned hospital readmission following transcatheter aortic valve implantation. Circ J. 2018;82:2191–2198. doi: 10.1253/circj.CJ-17-0816. [DOI] [PubMed] [Google Scholar]
- 14.Chiarantini D., Volpato S., Sioulis F., et al. Lower extremity performance measures predict long-term prognosis in older patients hospitalized for heart failure. J Card Fail. 2010;16:390–395. doi: 10.1016/j.cardfail.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Lilamand M., Saintout M., Vigan M., et al. Quality of life, physical performance and nutritional status in older patients hospitalized in a cardiology department. J Geriatr Cardiol. 2020;17:410–416. doi: 10.11909/j.issn.1671-5411.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Volpato S., Cavalieri M., Guerra G., et al. Performance-based functional assessment in older hospitalized patients: feasibility and clinical correlates. J Gerontol A Biol Sci Med Sci. 2008;63:1393–1398. doi: 10.1093/gerona/63.12.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Volpato S., Cavalieri M., Sioulis F., et al. Predictive value of the short physical performance battery following hospitalization in older patients. J Gerontol A Biol Sci Med Sci. 2011;66:89–96. doi: 10.1093/gerona/glq167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hornsby W.E., Sareini M.A., Golbus J.R., et al. Lower extremity function is independently associated with hospitalization burden in heart failure with preserved ejection fraction. J Card Fail. 2019;25:2–9. doi: 10.1016/j.cardfail.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rich M.W., Beckham V., Wittenberg C., et al. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. N Engl J Med. 1995;333:1190–1195. doi: 10.1056/NEJM199511023331806. [DOI] [PubMed] [Google Scholar]
- 20.Schocken D.D., Arrieta M.I., Leaverton P.E., Ross E.A. Prevalence and mortality rate of congestive heart failure in the United States. J Am Coll Cardiol. 1992;20:301–306. doi: 10.1016/0735-1097(92)90094-4. [DOI] [PubMed] [Google Scholar]
- 21.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 22.Dolgin M., New York Heart Association. Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels. 9th ed. Little Brown; Boston: 1994. [Google Scholar]
- 23.Green C.P., Porter C.B., Bresnahan D.R., Spertus J.A. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35:1245–1255. doi: 10.1016/s0735-1097(00)00531-3. [DOI] [PubMed] [Google Scholar]
- 24.Spertus J., Peterson E., Conard M.W., et al. Monitoring clinical changes in patients with heart failure: a comparison of methods. Am Heart J. 2005;150:707–715. doi: 10.1016/j.ahj.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 25.Chaudhry S.I., McAvay G., Chen S., et al. Risk factors for hospital admission among older persons with newly diagnosed heart failure: findings from the Cardiovascular Health Study. J Am Coll Cardiol. 2013;61:635–642. doi: 10.1016/j.jacc.2012.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McNallan S.M., Singh M., Chamberlain A.M., et al. Frailty and healthcare utilization among patients with heart failure in the community. JACC Heart Fail. 2013;1:135–141. doi: 10.1016/j.jchf.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McNallan S.M., Chamberlain A.M., Gerber Y., et al. Measuring frailty in heart failure: a community perspective. Am Heart J. 2013;166:768–774. doi: 10.1016/j.ahj.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reeves G.R., Whellan D.J., Patel M.J., et al. Comparison of frequency of frailty and severely impaired physical function in patients ≥ 60 years hospitalized with acute decompensated heart failure versus chronic stable heart failure with reduced and preserved left ventricular ejection fraction. Am J Cardiol. 2016;117:1953–1958. doi: 10.1016/j.amjcard.2016.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bellettiere J., Lamonte M.J., Unkart J., et al. Short physical performance battery and incident cardiovascular events among older women. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.016845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gill T.M., Gahbauer E.A., Allore H.G., Han L. Transitions between frailty states among community-living older persons. Arch Intern Med. 2006;166:418–423. doi: 10.1001/archinte.166.4.418. [DOI] [PubMed] [Google Scholar]
- 31.Xue Q.L., Bandeen-Roche K., Tian J., et al. Progression of physical frailty and the risk of all-cause mortality: Is there a point of no return? J Am Geriatr Soc. 2021;69:908–915. doi: 10.1111/jgs.16976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lutz A.H., Delligatti A., Allsup K., et al. Cardiac rehabilitation is associated with improved physical function in frail older adults with cardiovascular disease. J Cardiopulm Rehabil Prev. 2020;40:310–318. doi: 10.1097/HCR.0000000000000537. [DOI] [PubMed] [Google Scholar]
- 33.Goldfarb M., Afilalo J., Chan A., et al. Early mobility in frail and non-frail older adults admitted to the cardiovascular intensive care unit. J Crit Care. 2018;47:9–14. doi: 10.1016/j.jcrc.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 34.Molino-Lova R., Pasquini G., Vannetti F., et al. Effects of a structured physical activity intervention on measures of physical performance in frail elderly patients after cardiac rehabilitation: a pilot study with 1-year follow-up. Intern Emerg Med. 2013;8:581–589. doi: 10.1007/s11739-011-0654-z. [DOI] [PubMed] [Google Scholar]
- 35.Rogers P., Al-Aidrous S., Banya W., et al. Cardiac rehabilitation to improve health-related quality of life following trans-catheter aortic valve implantation: a randomised controlled feasibility study: RECOVER-TAVI pilot, ORCA 4, for the Optimal Restoration of Cardiac Activity group. Pilot Feasibility Stud. 2018;4:185. doi: 10.1186/s40814-018-0363-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Voller H., Salzwedel A., Nitardy A., et al. Effect of cardiac rehabilitation on functional and emotional status in patients after transcatheter aortic-valve implantation. Eur J Prev Cardiol. 2015;22:568–574. doi: 10.1177/2047487314526072. [DOI] [PubMed] [Google Scholar]
- 37.Taylor R.S., Walker S., Ciani O., et al. Exercise-based cardiac rehabilitation for chronic heart failure: the EXTRAMATCH II individual participant data meta-analysis. Health Technol Assess. 2019;23:1–98. doi: 10.3310/hta23250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kitzman D.W., Whellan D.J., Duncan P., et al. Physical rehabilitation for older patients hospitalized for heart failure. N Engl J Med. 2021;385:203–216. doi: 10.1056/NEJMoa2026141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shah A.M., Shah S.J., Anand I.S., et al. TOPCAT Investigators. Cardiac structure and function in heart failure with preserved ejection fraction: baseline findings from the echocardiographic study of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist trial. Circ Heart Fail. 2014;7:104–115. doi: 10.1161/CIRCHEARTFAILURE.113.000887. [DOI] [PMC free article] [PubMed] [Google Scholar]