Abstract
The congenital disorder of glycosylation type IIs (ATP6AP1-CDG; OMIM# 300972) is a rare X-linked recessive complex syndrome characterized by liver dysfunction, recurrent bacterial infections, hypogammaglobulinemia, and defective glycosylation of serum proteins. Here, we examine the case of a 1-year-old male patient of Buryat origin, who presented with liver dysfunction. At the age of 3 months, he was hospitalized with jaundice and hepatosplenomegaly. Whole-exome sequencing identified the ATP6AP1 gene missense variant NM_001183.6:c.938A>G (p.Tyr313Cys) in the hemizygous state, which was previously reported in a patient with immunodeficiency type 47. At the age of 10 months, the patient successfully underwent orthotopic liver transplantation. After the transplantation, the use of Tacrolimus entailed severe adverse effect (colitis with perforation). Replacing Tacrolimus with Everolimus led to improvement. Previously reported patients demonstrated abnormal N- and O-glycosylation, but these data were collected without any specific treatment. In contrast, in our patient, isoelectric focusing (IEF) of serum transferrin was performed only after the liver transplant and showed a normal IEF pattern. Thus, liver transplantation could be a curative option for patients with ATP6AP1-CDG.
Keywords: ATP6AP1, liver transplantation, cholestasis, X-linked primary immunodeficiency
1. Introduction
Congenital disorders of glycosylation (CDG) are a clinically and genetically heterogeneous group of disorders caused by errors in different steps along glycan modification pathways [1]. To date, more than 130 types of CDG have been identified, and more than 140 genes are associated with different types of CDG, which are characterized by a broad spectrum of clinical manifestations and severity [2]. CDG type IIs is caused by pathogenic variants of the ATP6AP1 gene and characterized by a defect in N-glycosylation and, in some cases, a defect in O-glycosylation. The ATP6AP1 gene encodes for the ATPase H+ transporting protein. This is an accessory subunit of the vacuolar V-ATPase proton pump complex that regulates pH homeostasis in cells [3]. Defects in certain subunits and accessory proteins of V-ATPase can cause congenital disorders of glycosylation [4]. In 2016, Jansen et al. described a novel ATP6AP1-linked immunodeficiency and identified disease-causing pathogenic variants in ATP6AP1 in 11 male patients with abnormal protein glycosylation [5]. The common clinical symptoms displayed by this cohort of patients included immune abnormalities and hepatopathy. Recurrent bacterial infections were associated with hypogammaglobinemia. Hepatopathy varied from mild hypertransaminasemia to cirrhosis and end-stage liver failure. In addition, common laboratory abnormalities included leukopenia, slightly elevated serum transaminases, low serum copper and ceruloplasmin, and high alkaline phosphatase.
Here, we present the case of a 1-year-old male patient of Buryat origin with liver dysfunction that eventually led to liver transplantation.
2. Case Presentation
2.1. Clinical Data
The family of the affected male were clinically examined at the National Medical Research Center for Obstetrics, Gynecology, and Perinatology named after the academician V.I. Kulakov, as well as at the Federal Research Center of Nutrition and Biotechnology and the Research Centre for Medical Genetics, Moscow, Russia.
2.2. Genetic Testing
Blood samples from the proband and his unaffected parents were collected, and genomic DNA was extracted using the Wizard® Genomic DNA Purification Kit (Promega, Madison, WI, USA) according to the manufacturer’s recommendations. Clinical exome sequencing was performed for the proband. Target enrichment with a SeqCap EZ HyperCap Workflow solution capture array (Roche Sequencing Solutions, Santa Clara, CA, USA), including the coding regions of 6640 genes currently described as clinically significant in the OMIM and the Human Gene Mutation Database (HGMD), and sequencing were carried out using Illumina NextSeq 500. The sequencing data were processed using a standard computer-based algorithm from Illumina and BaseSpace software (Enrichment 3.1.0). The sequenced fragments were visualized with Integrative Genomics Viewer (IGV) software (© 2023–2018 Broad Institute and the Regents of the University of California, Berkeley, CA, USA). Filtering of the variants was based on their frequency of less than 1% in gnomAD and coding region sequence effects such as missense, nonsense, coding indels, and splice sites. The variants’ clinical significance was evaluated according to the guidelines for massive parallel sequencing (MPS) data interpretation [6]. Automatic Sanger sequencing was carried out on ABIPrism 3500 (Applied Biosystems, Waltham, MA, USA) according to the manufacturer’s protocol. The primer sequences (ATP6AP1F CTCTAAGATGCCAAAGGCCCTC ATP6AP1R CTTCGTCTCTCAACCACTAGCC) were chosen according to the GeneBank database (NM_001183.6). The size of the PCR fragment was 491 base pairs.
2.3. Ethical Consideration
The study was approved by the local ethics committee of the Research Centre for Medical Genetics (the approval number 2018-1/3).
2.4. Clinical Evaluation
The proband was an affected 1-year-old boy born to non-consanguineous Buryat parents. The pregnancy was complicated by proteinuria in the 3rd trimester. The boy was born at 39 weeks of gestation. The birth weight was 3670 g (Z-score 1.65 SD), the birth length was 53 cm (Z-score 0.64 SD), and the Apgar score was 8/9 (Table 1). The perinatal period was normal. Myotonic syndrome was diagnosed at the age of one month. At the age of three months, the patient was admitted to the local hospital with jaundice and hepatosplenomegaly. Laboratory evaluation revealed thrombocytopenia, hypoglycemia, elevated transaminases, as well as a significant increase in alkaline phosphatase (more than tenfold) and in alpha-fetoprotein (Table 1). Based on the clinical picture and laboratory findings, the patient was diagnosed with idiopathic hepatitis (probably due to an inherited metabolic disease). Tandem mass spectrometry (MS/MS analysis) of acylcarnitines and amino acids in plasma detected elevated tyrosine levels, with a normal level of succinylacetone. Oncologists excluded hepatic tumors, using ultrasound and MRI (magnetic resonance imaging) methods of visualization. During hospitalization, the patient was treated with ursodeoxycholic acid at a dose of 25 mg/kg/day with clinical improvement.
Table 1.
Anthropometric parameters and laboratory data (from birth to one year and four months).
| 0 m | 3 m | 5 m | 7 m | 10 m | 1 y 1 m | 1 y 4 m | ||
|---|---|---|---|---|---|---|---|---|
| Weight, g/SD | 3670/0.64 | 7100/0.26 | 7800/1.44 | 7900/−0.06 | 8300/−1.58 | 8600/−1.1 | ||
| Length, cm/SD | 53/1.65 | 62/0.25 | 69/1.44 | 72/2.01 | 74.5/−1.0 | 76.5/−0.77 | ||
| Blood tests | ||||||||
| No data | 3 m | 5 m | 7 m | Liver transplantation | 1 y 1 m | 1 y 4 m | Normal ranges | |
| hemoglobin | 105 | 102 | 90 | 84 | 114 | 110–140 g/L | ||
| red blood cells | - | 3.28 | 3.24 | 2.85 | 4.04 | 3.5–4.5 × 1012/L | ||
| white blood cells | - | 6.6 | 5.8 | 6.9 | 5.28 | 6–17.5 × 109/L | ||
| platelets | - | 100 | - | 96 | 174 | 160–390 × 109/L | ||
| total bilirubin | 80 | 98.1 | 54.5 | 5.8 | 8.2 | 5–21 mkM/ | ||
| direct bilirubin | 23.8 | 75.6 | 24.1 | 2.9 | 4.5 | <3.4 mkM/L | ||
| ALT | 69 | 51 | 50.6 | 17.3 | 42 | 0–40 U/L | ||
| AST | 190 | 183 | 133.5 | 33.9 | 34 | 0–40 U/L | ||
| ALP | 4164 | 2523 | 1754 | 387 | 612 | 82–383 U/L | ||
| GGT | - | - | 29.4 | 45.4 | 187 | 0–6 m: <204; 6–12 m: <34; 1–3 y:< 18 U/L |
||
| glucose | 1.9 | 2.81 | 5.01 | 5.03 | 5.12 | 3.3–5.5 mM/L | ||
| urea | - | - | 3.1 | 4.2 | 2.4 | 2.8–7.2 mM/L | ||
| cholesterol | - | 5.1 | 2.26 | 1.86 | 1.86 | 3.2–5.2 mM/L | ||
| total protein | - | - | 61.5 | 54.8 | 65.6 | 64–83 g/L | ||
| albumin | - | - | 39.8 | 35.3 | 38.9 | 35–52 g/L | ||
| AFP | 47,241 | 85,602 | 6306 | - | - | 0.5–50,000 IU/mL | ||
| fibrinogen | - | 1.1 | - | 3.18 | 2–4 g/L | |||
| prothrombin index | 58.1 | 32 | 38 | 85 | - | 81–138% | ||
| aPTT | 75.6 | 100.2 | 51 | 27 | 44.3 | 25–35 s | ||
| INR | 1.36 | - | 3.2 | 1.13 | 1.1 | 0.88–1.1 | ||
Note: m: month; y: years; ALT: alanine aminotransferase; AST: aspartate aminotransferase; ALP: alkaline phosphatase; GGT: gamma-glutamyltranspeptidase; AFP: alpha-fetoprotein; aPTT: activated partial thromboplastin time, INR: international normalized ratio.
One month later, the child was hospitalized due to a progression of hepatosplenomegaly and abnormal liver function tests. Laboratory investigations revealed mild anemia, secondary thrombocytopenia (probably due to hypersplenism and lack of thrombopoietin), cholestasis, and impaired coagulation.
At the age of 7 months, the child was first examined in the National Medical Research Center for Obstetrics, Gynecology, and Perinatology. On examination, jaundice was noted, and the liver was slightly increased, but it was dense. The spleen was significantly enlarged (+7 cm felt below the left costal margin). Diffuse muscle hypertonia was noted. The examination revealed cholestasis, elevated transaminases and alkaline phosphatase, as well as hypocoagulation (see Table 1). Ultrasonography demonstrated a mild hepatomegaly (the liver length in midclavicular line was 86 mm, +1.5 SD) and a significant enlargement of the spleen (the spleen length was 84 mm, +2.5 SD) without signs of portal hypertension. There were no esophageal varicose veins. Differential diagnosis was carried out between hereditary metabolic diseases, including lysosomal storage diseases, peroxisomal disorders, and others. A gas chromatography–mass spectrometry blood analysis showed normal concentrations of very-long-chain fatty acids and phytanic acid. An NGS-based panel analysis including 52 genes associated with cholestasis was also performed. There were no causative variants in the genes included in the panel.
At the age of 10 months, due to the development of liver failure, the child successfully underwent orthotopic living-donor liver transplantation. His father was the donor. The patient received Tacrolimus with monitoring of the whole-blood concentrations of the drug and dosage adjustments. On the 10th postoperative day, he developed an intestinal perforation of the terminal ileum, lying within 15 cm of the ileocecal valve. A right hemicolectomy was performed. It was regarded as an infectious complication due to immunosuppressive therapy and immunodeficiency.
Few days after the surgery, loose stools, abdominal distension, and bloating appeared. Over the next two months, the patient’s condition deteriorated rapidly, with a developing fever and cough. The patient was admitted to the hospital with acute respiratory distress syndrome, acute kidney injury, dyspeptic syndrome, and pancytopenia. On admission, his blood test results were as shown in Table 1. Due to drug intolerance and adverse side effects, the treatment with Tacrolimus was discontinued. The patient received a transfusion of fresh frozen plasma, as well as a correction of electrolyte disorders and hypoalbuminemia. Therapy with antibiotics and immunoglobulin was started, leading to clinical improvement.
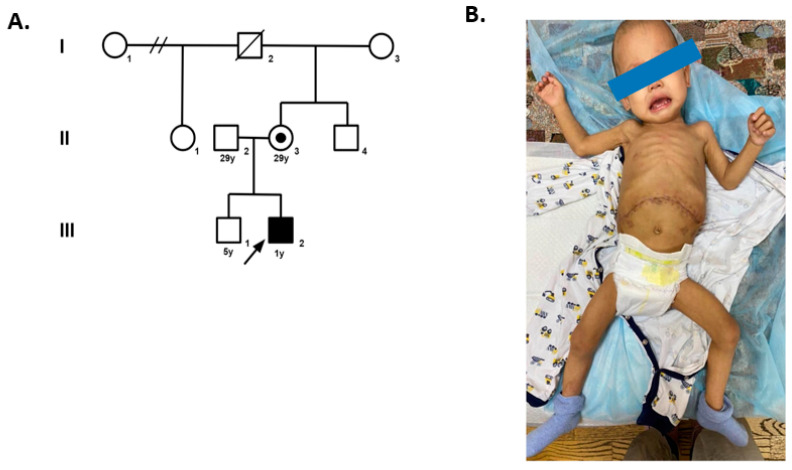
One month later, at the age of 1 year 4 months, the patient’s weight was 8.6 kg (with a Z-score of 1.1 SD), and his length was 76.5 cm (with a Z-score of 0.77 SD) (Figure 1); the main symptoms were frequent stool and poor weight gain. A colonoscopy showed diffuse active colitis. A colonic biopsy revealed focal surface erosions, increased infiltration of lymphocytes, and eosinophils in the lamina propria. A gastroscopy demonstrated gastritis. Given the patient’s stable condition, restarting the treatment with Tacrolimus with monitoring of the whole-blood concentrations of the drug and dosage adjustments was recommended. Eleven days after the start of the treatment with Tacrolimus, the patient developed fever, dyspeptic syndrome, and face swelling. Laboratory studies revealed anemia (hemoglobin of 79 g/L), thrombocytopenia (platelet cell count of 57 × 103/μL), neutropenia, hypoalbuminemia, hypokalemia, and an elevated level of CRP (150 mg/L). Bone marrow aspiration showed no signs of hemophagocytosis. The decision was made to switch from Tacrolimus to Everolimus at a starting dose of 0.25 mg twice a day. The patient received antibiotic therapy and intravenous fluids to correct electrolyte disturbances and hypoalbuminemia, with improvement.
Figure 1.
Pedigree chart of the patient (A) and patient’s general appearance at the age of 1 year (B).
2.5. Genetic Analysis
Whole-exome sequencing was performed to find the causative gene defect. As a result, the missense variant NM_001183.6:c.938A>G (p.Tyr313Cys) in the hemizygous state was identified in the ATP6AP1 gene. The described variant was previously reported in a patient with immunodeficiency type 47, characterized by liver dysfunction, recurrent bacterial infections, hypogammaglobulinemia, and defective glycosylation of serum proteins. The variant was not registered in the gnomAD database or the control group of Russian patients’ exomes. The p.Tyr313Cys variant is predicted by SIFT, PolyPhen2, Provean, and MutationTaster to have a deleterious effect on protein function. According to the ACMG-AMP criteria, the mentioned variant is of uncertain significance (PM2, PP3, PP5). Sanger sequencing validated this variant as hemizygous in the proband (III.2) and heterozygous in his unaffected mother (II.3). No mutation was identified in the patient’s healthy brother and other members of the family from the mother’s side, excluding the grandfather. We could not test him because he died many years ago.
3. Discussion
The ATP6AP1 gene, localized on the X chromosome, encodes a subunit of the proton-transporting V-ATPase enzyme, which plays an important role in numerous physiological processes in the human body. Therefore, mutations in genes encoding subunits of the V-ATPase enzyme are associated with numerous pathological conditions, including renal tubular acidosis, deafness, osteoporosis, and cancer [7,8,9]. According to currently existing research literature data, pathogenic variants in the ATP6AP1 gene lead to X-linked recessive complex primary immunodeficiency syndrome type 47 (OMIM#300972). The core symptoms of this condition reported by Jansen et al. (2016) in a cohort of 11 males with hemizygous missense variants in the ATP6AP1 gene include defective glycosylation of serum proteins and liver dysfunction with neonatal jaundice and hepatosplenomegaly [5]. Six patients from three families also presented with neurological symptoms, including seizures, mild intellectual disability, and behavioral abnormalities. Dimitrov et al. (2018) and Witters et al. (2018) reported two patients with IMD47, both of whom had hepatopathy (elevated levels of transaminases and total bilirubin with a normal level of GGT; hyperechogenic liver parenchyma, normal liver size but enlarged size spleen (+3.7 SD) by ultrasonography), immune abnormalities, glycosylation defects, and cutis laxa [10,11]. Tvina et al. (2020) described a prenatal phenotype of an X-linked ATP6AP1 gene mutation and the association of this gene mutation with increased nuchal translucency (NT), elevated alpha-fetoprotein (AF-AFP), positive acetylcholinesterase (AchE), and Aplasia Cutis Congenita [12]. In our case, in contrast, there were no pathological signs during the prenatal period.
In this case report, we described a 1-year-old male patient of Buryat ancestry with primary immunodeficiency and liver dysfunction that led to liver transplantation. Whole-exome sequencing identified the ATP6AP1 gene missense variant NM_001183.6:c.938A>G (p.Tyr313Cys) in the hemizygous state, as previously reported by Jansen et al. in a patient with immunodeficiency type 47. Our patient had clinical symptoms and laboratory findings similar to the 4-year-old of Irish descent described by Jansen et al., including mild hepatomegaly and severe splenomegaly, as well as increased levels of transaminases. Interestingly, our patient did not have recurrent infections before the liver transplantation and immunosuppressive treatment. For the above-mentioned reasons, screening for N-glycosylation by isoelectric focusing of serum transferrin should be performed in patients with cholestasis, hepatomegaly, and severe splenomegaly without portal hypertension. The patient described by Jansen et al. demonstrated abnormal N- and O-glycosylation, while for our patient, isoelectric focusing of serum transferrin was performed only after the liver transplant and showed a normal IEF pattern. We suggest that a possible explanation for the normal glycosylation of transferrin in our patient might be a normalization of the glycosylation profile due to the liver transplant, as Mirian et al. reported when describing the first successful liver transplantation in a patient with a congenital disorder of glycosylation, after which, normal N-glycosylation of transferrin was found [13].
4. Conclusions
We discussed the case of a 1-year-old male patient of Buryat origin, who presented with liver dysfunction, caused by the ATP6AP1 gene missense variant NM_001183.6:c.938A>G (p.Tyr313Cys) in the hemizygous state. At the age of 10 months, the child successfully underwent orthotopic liver transplantation. After the transplantation, the use of Tacrolimus entailed a severe side effect (colitis with perforation). A change in therapy from Tacrolimus to Everolimus led to improvement. This case report highlights the importance of performing biochemical screening and genetic tests in infants with hepatic dysfunction and cholestasis for differential diagnosis and successful therapy.
Acknowledgments
We thank Natasha Grigorian for her help with proofreading the manuscript.
Abbreviations
ALT: alanine aminotransferase; AST: aspartate aminotransferase; CDG: congenital disorder of glycosylation; GGT: gamma-glutamyltranspeptidase; LDG: lactate dehydrogenase; OMIM: Online Mendelian Inheritance in Man; MS/MS analysis: tandem mass spectrometry; MRI: magnetic resonance imaging; NT: nuchal translucency; AF-AFP: alpha-fetoprotein; AchE: acetylcholinesterase.
Author Contributions
Conceptualization, N.S. and T.S.; methodology, N.S., A.M. and T.S.; investigation, N.S., O.S. (Olga Shatokhina), O.S. (Olga Shchagina), E.K. and N.T.; resources, O.S. (Olga Shatokhina), O.S. (Olga Shchagina), E.K., A.D., N.T., and T.S.; writing—original draft preparation, N.S.; writing—review and editing, O.S. (Olga Shchagina), A.M., A.D. and T.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The clinical and molecular genetic study was performed in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of the Research Centre for Medical Genetics, Moscow, Russia (approval number 2018-1/3), with written informed consent obtained from each participant and/or their legal representative as appropriate.
Informed Consent Statement
Written informed consent was obtained from the patient’s legal guardians for the publication of this case report, any accompanying images, and population studies. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Data Availability Statement
The datasets used and/or analyzed during this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare that they have no competing interests.
Funding Statement
The work was funded by a state assignment of the Ministry of Science and Higher Education of the Russian Federation. The funder had no role in the design of the study; in the collection, analysis, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Chang I.J., He M., Lam C.T. Congenital disorders of glycosylation. Ann. Transl. Med. 2018;6:477. doi: 10.21037/atm.2018.10.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson M.P., Matthijs G. The evolving genetic landscape of congenital disorders of glycosylation. Biochim. Biophys. Acta Gen. Subj. 2021;1865:129976. doi: 10.1016/j.bbagen.2021.129976. [DOI] [PubMed] [Google Scholar]
- 3.Miles A.L., Burr S.P., Grice G.L., Nathan J.A. The vacuolar-ATPase complex and assembly factors, TMEM199 and CCDC115, control HIF1α prolyl hydroxylation by regulating cellular iron levels. Elife. 2017;6:e22693. doi: 10.7554/eLife.22693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rujano M.A., Cannata Serio M., Panasyuk G., Péanne R., Reunert J., Rymen D., Hauser V., Park J.H., Freisinger P., Souche E., et al. Mutations in the X-linked. J. Exp. Med. 2017;214:3707–3729. doi: 10.1084/jem.20170453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jansen E.J., Timal S., Ryan M., Ashikov A., van Scherpenzeel M., Graham L.A., Mandel H., Hoischen A., Iancu T.C., Raymond K., et al. ATP6AP1 deficiency causes an immunodeficiency with hepatopathy, cognitive impairment and abnormal protein glycosylation. Nat. Commun. 2016;7:11600. doi: 10.1038/ncomms11600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richards S., Aziz N., Bale S., Bick D., Das S., Gastier-Foster J., Grody W.W., Hegde M., Lyon E., Spector E., et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pamarthy S., Kulshrestha A., Katara G.K., Beaman K.D. The curious case of vacuolar ATPase: Regulation of signaling pathways. Mol. Cancer. 2018;17:41. doi: 10.1186/s12943-018-0811-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith A.N., Skaug J., Choate K.A., Nayir A., Bakkaloglu A., Ozen S., Hulton S.A., Sanjad S.A., Al-Sabban E.A., Lifton R.P., et al. Mutations in ATP6N1B, encoding a new kidney vacuolar proton pump 116-kD subunit, cause recessive distal renal tubular acidosis with preserved hearing. Nat. Genet. 2000;26:71–75. doi: 10.1038/79208. [DOI] [PubMed] [Google Scholar]
- 9.Kartner N., Manolson M.F. Novel techniques in the development of osteoporosis drug therapy: The osteoclast ruffled-border vacuolar H(+)-ATPase as an emerging target. Expert. Opin. Drug Discov. 2014;9:505–522. doi: 10.1517/17460441.2014.902155. [DOI] [PubMed] [Google Scholar]
- 10.Witters P., Breckpot J., Foulquier F., Preston G., Jaeken J., Morava E. Expanding the phenotype of metabolic cutis laxa with an additional disorder of N-linked protein glycosylation. Eur. J. Hum. Genet. 2018;26:618–621. doi: 10.1038/s41431-017-0044-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dimitrov B., Himmelreich N., Hipgrave Ederveen A.L., Lüchtenborg C., Okun J.G., Breuer M., Hutter A.M., Carl M., Guglielmi L., Hellwig A., et al. Cutis laxa, exocrine pancreatic insufficiency and altered cellular metabolomics as additional symptoms in a new patient with ATP6AP1-CDG. Mol. Genet. Metab. 2018;123:364–374. doi: 10.1016/j.ymgme.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 12.Tvina A., Thomsen A., Palatnik A. Prenatal and postnatal phenotype of a pathologic variant in the ATP6AP1 gene. Eur. J. Med. Genet. 2020;63:103881. doi: 10.1016/j.ejmg.2020.103881. [DOI] [PubMed] [Google Scholar]
- 13.Janssen M.C., de Kleine R.H., van den Berg A.P., Heijdra Y., van Scherpenzeel M., Lefeber D.J., Morava E. Successful liver transplantation and long-term follow-up in a patient with MPI-CDG. Pediatrics. 2014;134:e279–e283. doi: 10.1542/peds.2013-2732. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during this study are available from the corresponding author upon reasonable request.