Abstract
Plasmodium vivax (P. vivax) is one of the human’s most common malaria parasites. P. vivax is exceedingly difficult to control and eliminate due to the existence of extravascular reservoirs and recurring infections from latent liver stages. Traditionally, licorice compounds have been widely investigated against viral and infectious diseases and exhibit some promising results to combat these diseases. In the present study, computational approaches are utilized to study the effect of licorice compounds against P. vivax Duffy binding protein (DBP) to inhibit the malarial invasion to human red blood cells (RBCs). The main focus is to block the DBP binding site to Duffy antigen receptor chemokines (DARC) of RBC to restrict the formation of the DBP–DARC complex. A molecular docking study was performed to analyze the interaction of licorice compounds with the DARC binding site of DBP. Furthermore, the triplicates of molecular dynamic simulation studies for 100 ns were carried out to study the stability of representative docked complexes. The leading compounds such as licochalcone A, echinatin, and licochalcone B manifest competitive results against DBP. The blockage of the active region of DBP resulting from these compounds was maintained throughout the triplicates of 100 ns molecular dynamic (MD) simulation, maintaining stable hydrogen bond formation with the active site residues of DBP. Therefore, the present study suggests that licorice compounds might be good candidates for novel agents against DBP-mediated RBC invasion of P. vivax.
Keywords: Plasmodium vivax, molecular docking, molecular dynamic simulation, DBP inhibition
1. Introduction
Malaria, an infectious disease, has posed a significant threat to human health. [1]. There are nine unique species of the unicellular eukaryotic parasite Plasmodium that infect humans, which comprise P. falciparum, P. vivax, P. malariae, P. ovale curtisi, P. ovale wallikeri [2], P. knowlesi, P. cynomolgi [3], P. simium [4,5], and P. brasilianum [6]. Only two of the nine Plasmodium species, P. falciparum and P. vivax, appear to constitute a significant threat to the global malaria burden. Around 80% of the global P. vivax burden is carried by Asia and the Asia-Pacific area. In these areas, P. vivax is by far the most prevalent cause of human malaria [7]. The proportion of malaria caused by P. vivax has increased in regions where the P. falciparum load has been lowered by vigorous malaria control strategies [8]. To treat the disease, quinine-like drugs (such as the cinchona alkaloid quinine, 4-aminoquinoline chloroquine (CQ), hydroxychloroquine, primaquine, and pyronaridine) and artemisinin-based combination therapy are employed. However, drug resistance has evolved, presumably as a result of mutations in the active regions of drug targets or biochemical alterations in drug receptors, necessitating the development of innovative techniques [9].
Plasmodium sporozoite remains in the liver and matures into merozoite to invade human RBCs in the blood stage. P. vivax infiltrates human RBCs by engaging a protein known as Duffy binding protein (DBP). P. vivax DBP interacts with the Duffy antigen receptor for chemokines on the surface of red blood cells (DARC). DARC is a transmembrane, glycosylated protein of approximately 35–40 kDa that is found on chromosome 1 (1.q22-1.q23). It has an intracellular C-terminal domain and an extracellular N-terminal region that includes chemokine binding receptors [10]. Plasmodium releases micronemes and rhoptries, two specialized apical organelles, to enter an RBC. DBP binds DARC with great affinity via Duffy binding-like domains and localizes to micronemes. DBP is a promising vaccine and therapeutic option for P. vivax malaria [11].
Herbs have long been utilized in traditional medicine and include a wide range of phytochemical components including terpenoids, phenols, lignins, stilbenes, tannins, flavonoids, quinones, coumarins, alkaloids, amines, betalains, and other metabolites [12]. The success of two plant-based antimalarial medications, quinine and artemisinin, prompted researchers to look for antimalarial compounds from a variety of botanic sources. Without a doubt, licorice is one of the most popular medicinal herbs [13]. Glycyrrhiza glabra is 1 of roughly 30 species in the genus Glycyrrhiza L. Glycyrrhiza glabra L. is a traditional medicinal herb that grows all over the world. [14]. Ancient historical literature from China, India, and Greece references its application to treat hepatic, viral, and respiratory tract diseases [15]. Glycyrrhiza glabra has been discovered to have a potential therapeutic effect on P. falciparum [16]. Given the rising resistance of Plasmodium strains to well-known anti-malarial treatments [17], the development of novel anti-malarial therapies is in high demand.
Computer-aided drug design (CADD) has become an increasingly important tool in the field of drug discovery and development. The use of computational methods and software allows for the rapid and efficient screening of large numbers of compounds, reducing the time and cost associated with traditional experimental methods [18]. CADD allows for the creation of detailed models of proteins, enzymes, and other biological molecules, providing a deeper understanding of the underlying biology [19]. This can be used to identify new drug targets and optimize the properties of existing drugs. In this work, computational approaches are used to investigate the therapeutic potential of licorice compounds against the DARC binding domain of P. vivax DBP. Therefore, the literature survey was performed to screen the best and most recently reported biologically active licorice compounds. Molecular docking studies predict five compounds to be potent against DBP. Furthermore, molecular dynamic modeling studies were carried out to predict the best compounds and examine the sustainability of the docking complexes based on the efficacy of DBP binding site blockage.
2. Results
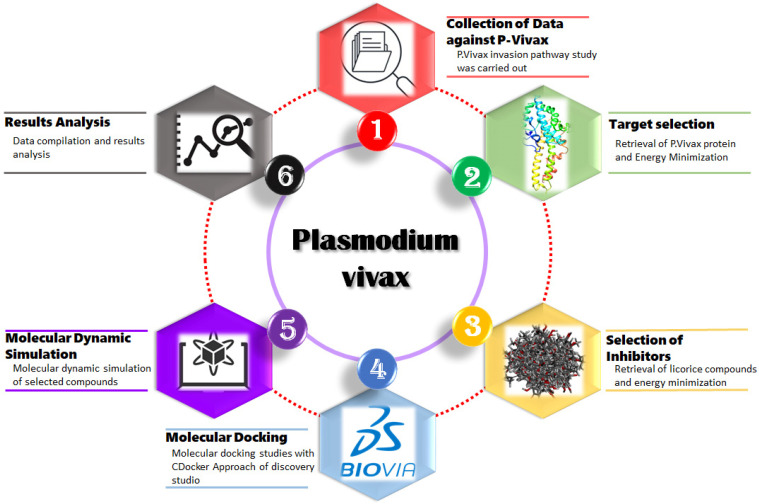
Figure 1 depicts the suggested research workflow. To anticipate and observe the interactions of licorice compounds with DBP protein, six stages were taken, including protein retrieval, retrieval of best licorice compounds, molecular docking, MD simulation experiments, and data analysis.
Figure 1.
Workflow diagram for the screening of licorice inhibitors against DBP.
2.1. Structural Assessment of P. vivax DBP
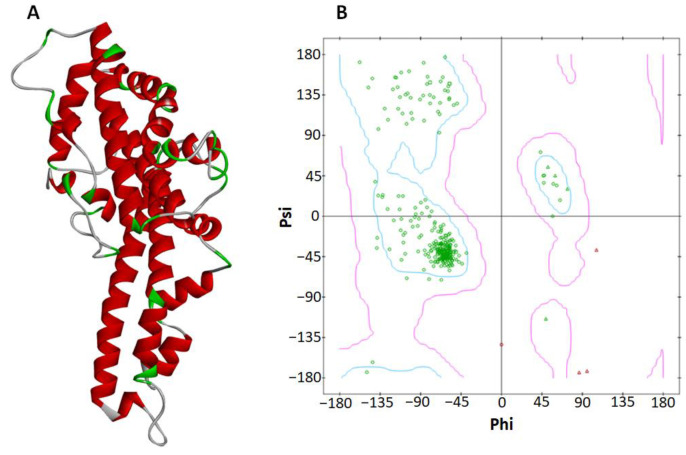
P. vivax DBP has 317 amino acids, 62% of which are helices, 1% are sheets, 36% are coils, and 22% are turns. P. vivax DBP Ramachandran plots show that 95.8% of all residues were in favorable areas. In total, 100% of all residues were in permitted areas. There were no outliers in the dataset. Figure 2A,B depicts the 3D structure and Ramachandran graph values of DBP.
Figure 2.
The 3D structure of P. vivax DBP after energy minimization is shown on the right (A). The (B) Ramachandran graph exhibiting that all of the amino acid residues are in the permitted zone and there are no outliers.
2.2. Binding Pocket Analysis
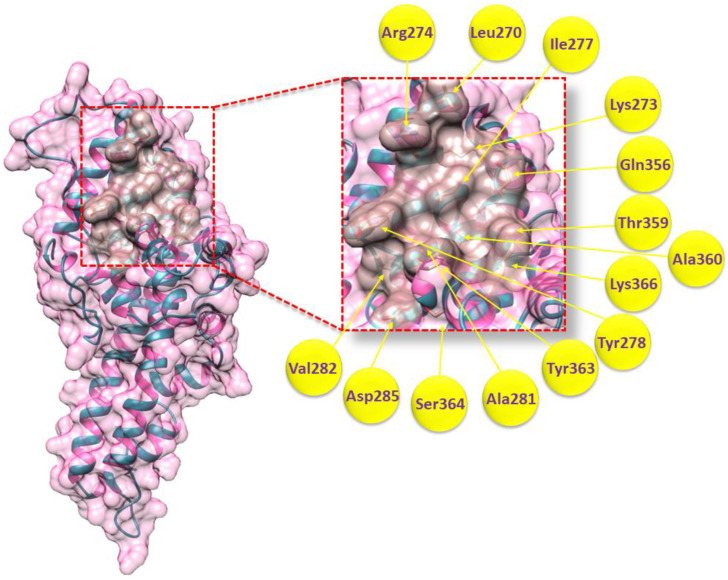
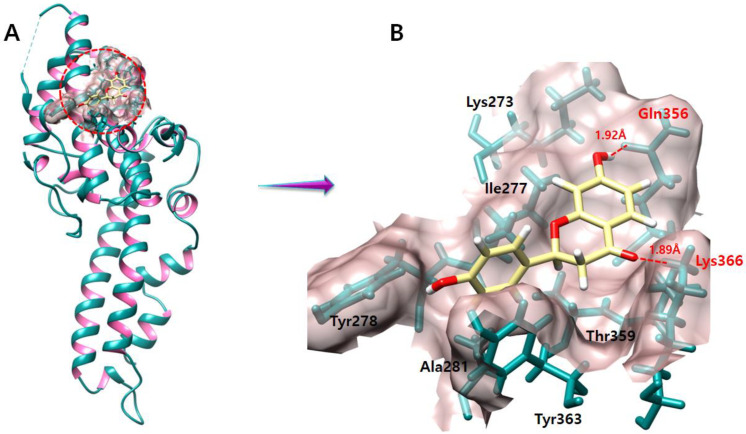
The DBP interacts with DARC on the surface of RBCs for P. vivax to invade RBCs. A significant opportunity for parasite control is provided by inhibiting this important interaction (Osii, 2022). Leu270, Lys273, Arg274, Ile277, Tyr278, Ala281, Val282, Asp285, Gln356, Thr359, Ala360, Tyr363, Ser364, and Lys366, which were chosen as the binding residues of P. vivax DBP (Figure 3), are previously published in a research paper [20]. These residues are crucial in the development of the DBP–DARC complex. Therefore, such residues are being studied further in molecular docking.
Figure 3.
DBP’s binding pocket surface is emphasized in rosy brown color, whereas the rest of the protein surface appears pink. The ribbon color is dark cyan, while the interiors of the ribbon helix are identified with a hot pink tint. Furthermore, binding site residues that participate in the DBP–DARC complex are labeled with their amino acid number based on their position in the active binding site of the protein.
2.3. Licorice (Ligands) Preparation
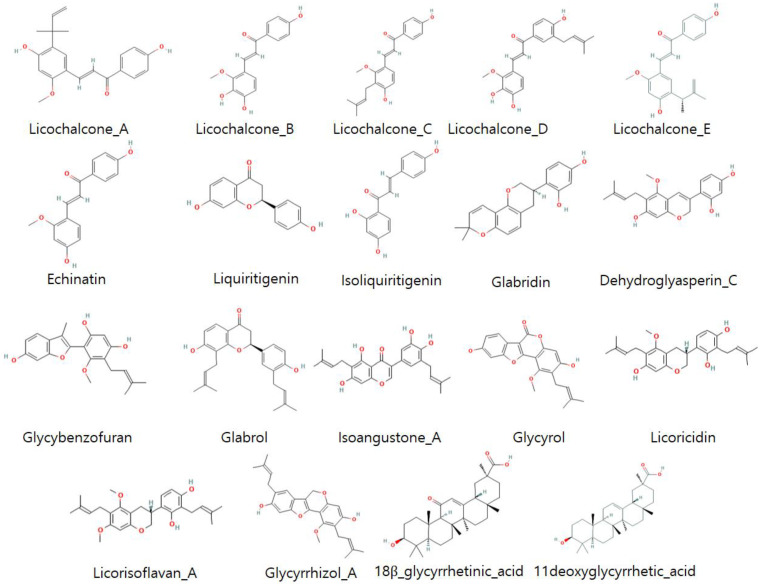
Licorice compounds are most prevalently used to combat many types of diseases such as cancer, viral infections, and parasitic infection. Their 3D models are downloaded and displayed in PyMOL and Discovery Studio for extensive 2D and 3D evaluation. Therefore, 19 licorice compounds (licochalcone_A, licochalcone_B, licochalcone_C, licochalcone_D, licochalcone_E, echinatin, liquiritigenin, isoliquiritigenin, glabridin, dehydroglyasperin_C, glycybenzofuran, glabrol, isoangustone_A, glycyrol, licoricidin, licorisoflavan_A, glycyrrhizol_A, 18β_glycyrrhetinic_acid, and 11deoxyglycyrrhetic_acid) were selected on the basis of their best structural conformation (Figure 4) and biological activity against various diseases (see Table 1) for our molecular docking studies.
Figure 4.
The 2D structure analysis of licorice compounds.
Table 1.
Recent biological activities of selected compounds.
| Sr No | Ligand Name | Activity | Reference |
|---|---|---|---|
| 1 | Licochalcone B | Antitumor, anti-inflammatory, antiviral | [21,22,23] |
| 2 | Echinatin | Antiviral | [22,24] |
| 3 | Licochalcone A | Antiviral, antimicrobial, immunoregulatory |
[21,25] |
| 4 | Licochalcone E | Antiviral, antimicrobial | [25] |
| 5 | Liquiritigenin | Antiviral, antimicrobial, immunoregulatory |
[21,25] |
| 6 | Licochalcone C | Anticancer | [26] |
| 7 | Isoliquiritigenin | Antiviral | [27] |
| 8 | Glabridin | Antiviral | [28,29] |
| 9 | Dehydroglyasperin C | Hepatoprotective | [30] |
| 10 | Licochalcone D | Antiviral, antioxidant | [31] |
| 11 | Glycygenzofuran | Diabetes mellitus, obesity | [32] |
| 12 | Glabrol | Hepatoprotective | [25] |
| 13 | Isoangustone A | Adenocarcinoma | [33] |
| 14 | Glycyrol | Antiviral | [29] |
| 15 | Licoricidin | Antiviral | [34] |
| 16 | Licorisoflavan A | Antimicrobial | [35] |
| 17 | Glycyrrhizol A | Anticaries, antimicrobial | [36,37] |
| 18 | 18β Glycyrrhetinic acid | Antiviral, antimicrobial | [25] |
| 19 | 11Deoxyglycyrrhetic acid | Antiviral | [38] |
2.4. Molecular Docking Analysis
All docked complexes against DBP were examined independently and evaluated based on minimum energy values and ligand interaction patterns (Table 2). The top five licorice compounds (Licochalcone B, echinatin, licochalcone A, licochalcone E, and liquiritigenin) were ranked and chosen based on their lowest docking energies and ligand interaction patterns for further MD simulation analysis. The five ligands demonstrated the lowest docking energy and bound in the active region of the target protein.
Table 2.
Docking energy table of the screened flavonoids calculated by CDocker.
| No | Ligand Name | CDocker Energy | CDocker Interaction Energy |
|---|---|---|---|
| 1 | Licochalcone B | −40.646 | −47.6473 |
| 2 | Echinatin | −36.4715 | −44.7142 |
| 3 | Licochalcone A | −33.3302 | −52.3068 |
| 4 | Licochalcone E | −30.6573 | −54.8755 |
| 5 | Liquiritigenin | −22.072 | −27.4566 |
| 6 | Licochalcone C | −20.0702 | −52.0653 |
| 7 | Isoliquiritigenin | −14.967 | −26.2369 |
| 8 | Glabridin | −11.7479 | −31.5265 |
| 9 | Dehydroglyasperin C | −5.66293 | −48.4455 |
| 10 | Licochalcone D | −1.45512 | −33.7997 |
| 11 | Glycygenzofuran | 6.02888 | −34.8505 |
| 12 | Glabrol | 12.2899 | −37.0889 |
| 13 | Isoangustone A | 12.5396 | −35.0905 |
| 14 | Glycerol | 18.4799 | −32.3047 |
| 15 | Licoricidin | 20.9019 | −36.0512 |
| 16 | Licorisoflavan A | 25.2122 | −35.4716 |
| 17 | Glycyrrhizol A | 35.3237 | −33.7042 |
| 18 | 18β Glycyrrhetinic acid | 43.0477 | −38.4474 |
| 19 | 11Deoxyglycyrrhetic acid | 51.5132 | −38.6611 |
2.5. Interaction Analyses of the Top Five Ligands against DBP
The determination of the top five compounds that manifest the lowest molecular docking energy was carried out through binding mode analysis to see the exact interactions and blockage of the targeted protein’s active site.
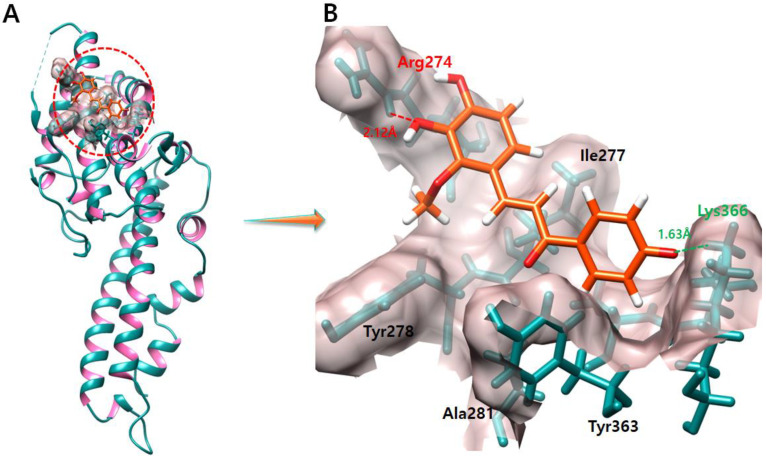
2.6. Licochalcone B
The docking result of the licochalcone B–DBP depicts one hydrogen bond and one salt bridge at Arg274 and Lys366 residues. The oxygen atom of licochalcone B forms a hydrogen bond with Arg274 with a bond length of 2.12 Å, and the other oxygen atom of licochalcone B forms a salt bridge with Lys366 with a bonding distance of 1.63 Å (Figure 5).
Figure 5.
The figure (A) shows the licochalcone B–DBP complex. In figure (B) salt bridges and hydrogen bonds formed during the docking experiment are depicted in green and red color, respectively. One oxygen atom of daidzein forms a salt bridge and a hydrogen bond with Arg274 with a bond length of 2.12 Å. On the other hand, another oxygen atom forms a salt bridge with Lys366, and the length of the salt bridge is 1.63 Å.
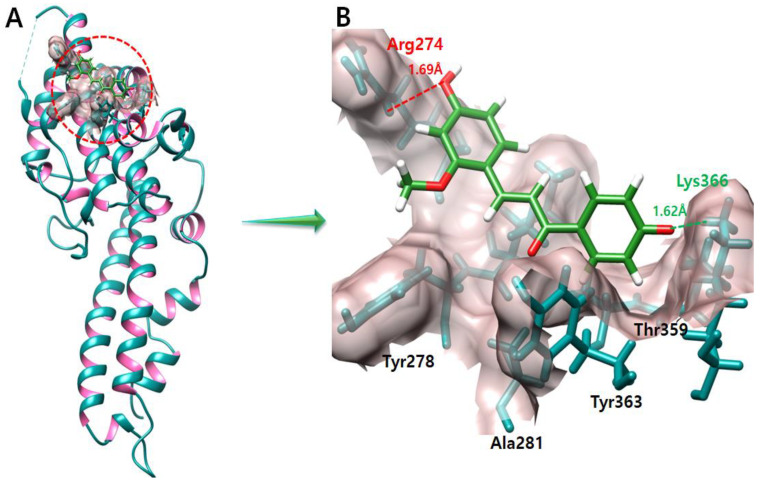
2.7. Echinatin
The docking result of the echinatin–DBP docked complex showed that one hydrogen bond and one salt bridge were formed by echinatin and Arg274 and Lys366 residues, respectively (Figure 6). The oxygen atom of echinatin forms a hydrogen bond with Arg274 with a bond length of 1.69 Å, and the other oxygen atom of echinatin forms a salt bridge with Lys366 with a bond length of 1.62 Å.
Figure 6.
The figure (A) shows the echinatin and DBP complex. In figure (B) salt bridges and hydrogen bonds produced during the docking experiment are depicted in green and red color, respectively. One oxygen atom of echinatin forms a salt bridge with Lys366 with a length of 1.62 Å. On the other hand, another oxygen atom of echinatin forms a hydrogen bond with Arg274 with a length of 1.69 Å.
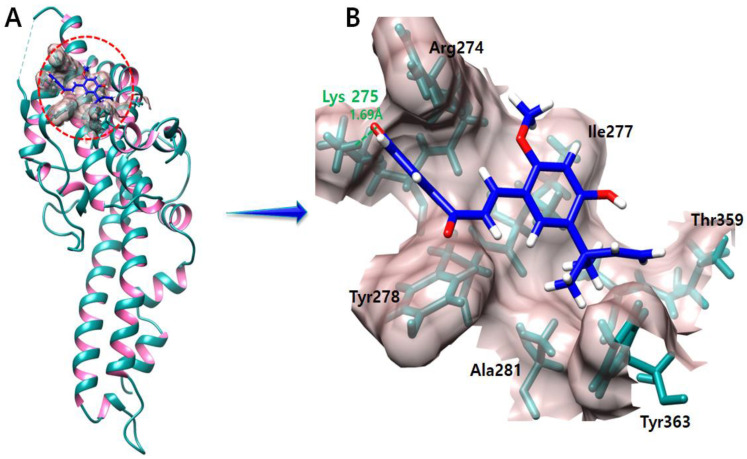
2.8. Licochalcone A
The binding interaction results of the licochalcone A–DBP docked complex manifest that the oxygen atom of licochalcone A forms a salt bridge with Lys275 with a bond length of 1.69 Å (Figure 7). Furthermore, the other interacting amino acids were also depicted in the graphical representation.
Figure 7.
The licochalcone A–DBP complex is depicted in this figure (A). In figure (B) salt bridge produced during the docking experiment is shown in green color. An oxygen atom forms a salt bridge with the Lys275 with a length of 1.69 Å.
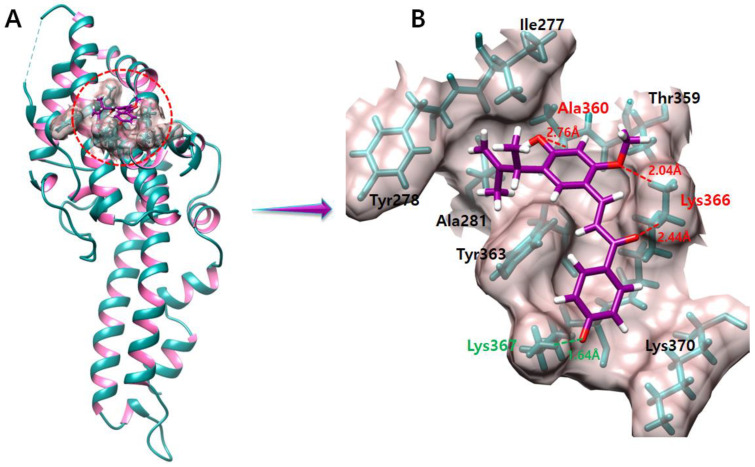
2.9. Licochalcone E
The complex of licochalcone E–DBP demonstrates one hydrogen bond and one salt bridge with Ala360, lys366, and Lys367 residues (Figure 8). The oxygen atom of licochalcone E forms a hydrogen bond with Ala360 with a bond length of 2.76 Å. Another two oxygen atoms of licochalcone E produce two hydrogen bonds with the same Lys366 with bond lengths of 2.04 Å and 2.44 Å. Furthermore, another oxygen atom of licochalcone E makes a salt bridge with Lys366 with a length of 1.64 Å.
Figure 8.
The figure (A) shows the licochalcone E and DBP complex. The salt bridges and hydrogen bonds produced during our docking experiment are shown in green and red color, respectively in figure (B).
2.10. Liquiritigenin
The liquiritigenin molecule forms two hydrogen bonds with Lys366 and Gln356 residues. The oxygen atom of liquiritigenin forms a hydrogen bond with Lys366 with a length of 1.89 Å, while another oxygen atom of liquiritigenin forms a hydrogen bond with Gln356 with a length of 1.92 Å (Figure 9) in the active region of the targeted protein.
Figure 9.
The figure (A) shows the liquiritigenin and DBP complex. In figure (B) the hydrogen bonds are represented in red color and the bonding residues are also labeled in red color. An oxygen atom of pulchellidine forms a salt bridge with Lys366 with a length of 1.68 Å.
2.11. Molecular Dynamic Simulation
To test the stability of the best-docked complexes with the lowest binding energy conformation, the MD simulation approach was used. GROMACS, a Linux-based program, was utilized to conduct triplicates of 100 ns MD simulations [39]. The stability of licorice compounds in the binding region of P. vivax DBP were determined by RMSD analysis, binding mode analysis, and interaction energy analysis.
2.12. RMSD Analysis
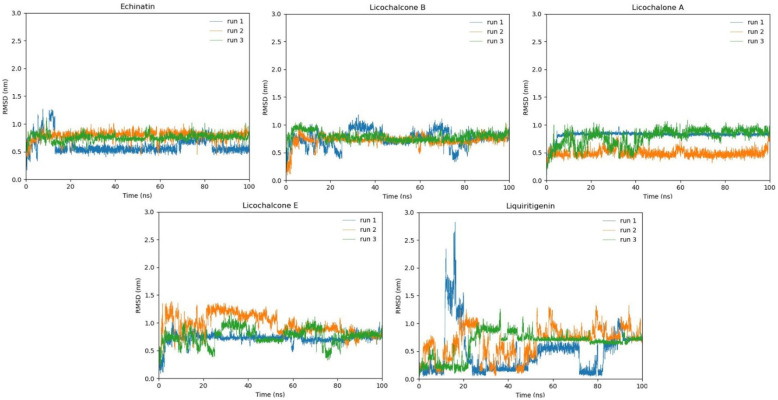
To evaluate the flexibility and overall stability of the docked complexes, 100 ns long MD simulations using GROMACS were conducted. The fluctuations of ligands inside the active site of the DBP protein were determined by root-mean-square-deviation (RMSD) from MD trajectories. Figure 10 shows the RMSD plots of licorice compounds against DBP protein during the 100 ns simulation.
Figure 10.
The graph exhibits the root mean square values of five selected compounds throughout a 100 ns MD simulation period. Three runs are indicated by different colors: run1 is indicated in blue color, run2 is exhibited in orange color, and run3 is determined by light green color. All three runs manifest RMSD values for 100 ns carried individually.
The licochalcone B molecule, which depicted the lowest molecular docking score and salt bridge formation in molecular docking studies, manifests approximately the same conformation in run2 and run3, while a little fluctuation in the graph can be seen in run1. The echinatin compound depicts the most stable RMSD values in run2 and run3, while, in run1, the graph shows that the RMS deviation point changes to σ = 0.7 from 70 ns to 83 ns in 100 ns MD simulation. The compound licochalcone A depicts the most stable RMSD in run1, while run2 also predicts a stable conformation. Meanwhile, run3 is a little fluctuating. The RMSD values of licochalcone E manifest stable RMSD in run1. Therefore, run2 and 3 are fluctuating at the start, but they are merging at the same point at 90 ns and become stable from 90 ns to 100 ns. The liquiritigenin compound, which exhibits high energy values in molecular docking, depicts the most fluctuating bar graph. In all three runs, the bar graph keeps fluctuating until the end, where the RMSD values remain stabilized to σ = 0.9 (Figure 10).
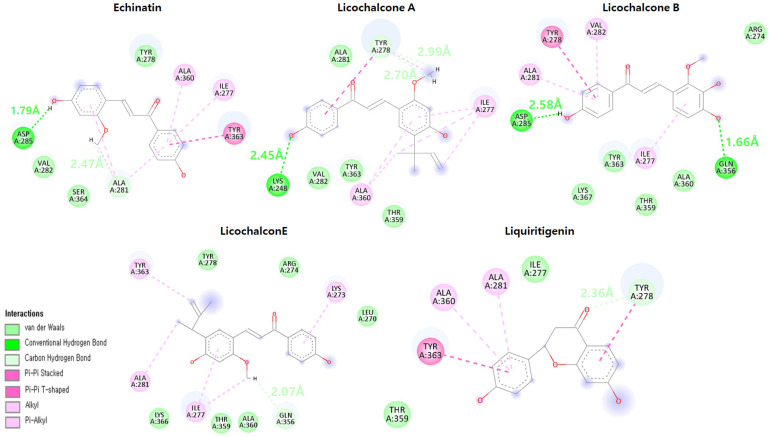
2.13. Binding Modes Analysis after the MD Simulation
The snapshots of all five complexes were obtained from 100 ns MD simulations, and the interactions were visualized using Discovery Studio and UCSF Chimera tools [40,41]. Licochalcone A, which showed only one hydrogen bond in molecular docking with Lys275, maintained one conventional hydrogen bond with Gln356 with a bond length of 2.11 Å and one carbon hydrogen bond with Ala360 with a bond length of 2.41 Å, which shows that the bounded ligand remained intact to the binding pocket throughout the 100 ns MD simulation experiment (Figure 11). Echinatin also maintained one conventional hydrogen bond with Asp285 with a bond length of 1.85 Å and the second carbon hydrogen bond with Val282 with a bond length of 2.61 Å. Echinatin also predicts a very stable RMS deviation graph and remains intact in the active region of the protein (Figure 11).
Figure 11.
The figure depicts the interaction of licorice compounds in the active region of DBP. The conventional hydrogen bonds are predicted in green color, while carbon hydrogen bonds are depicted in light green color, and the hydrogen bonding distances are mentioned with respective colors.
Licochalcone B, which showed the lowest binding energy in molecular docking and the most stable RMSD values both in run1 and run2, maintained one conventional hydrogen bond with Gln356 with a bond length of 2.52 Å and a secure active binding site of the target protein (Figure 11), while licochalcone E and liquiritigenin, which predict high fluctuations in RMSD and higher energy values as compared to licochalcone A, licochalcone B, and echinatin in molecular docking, also exhibit good interaction with the target protein. Licochalcone E carries one conventional hydrogen bond and two carbon hydrogen bonds with DBP active region amino acids, while liquiritigenin has two conventional hydrogen bonds with the target protein with bond lengths of 1.96 Å and 3.02 Å.
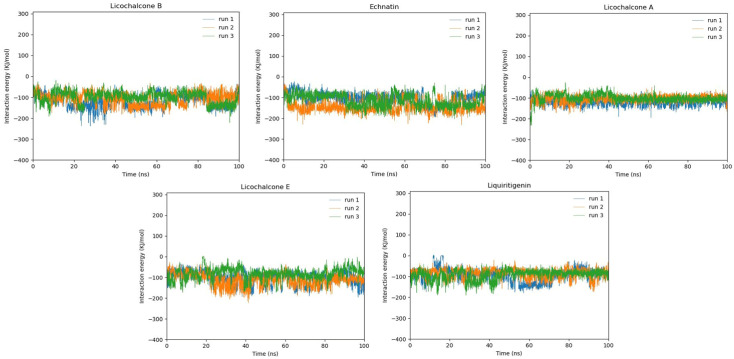
2.14. MD Simulation Interaction Energy
Along with RMSD and binding mode analysis, interaction energy calculations for all those five compounds against DBP were also carried out during 100 ns MD simulation of all three runs to examine the interaction energy score values. The interaction energy was analyzed in two forms, Coulombic interaction energy and Lennard-Jones interaction energy, and the sum of both energies was denoted by total interaction energy. The echinatin molecule showed the lowest interaction energy, at –119.7481. Meanwhile, licolchalcone B, which showed the lowest energy values in molecular docking studies, acquired a little more interaction energy than echinatin in MD simulation. Furthermore, licochalcone A, which showed the most stable RMSD value in run1, exhibited an average energy of –105.9473 for the 3 runs. Moreover, the licochalcone E and liquiritigenin molecules, which manifest high energy values in molecular docking and had higher RMS deviation rates than others, manifest the highest interaction energy in MD simulation interaction energy analysis (Table 3). Moreover, the total interaction energy of all these five compounds was also depicted in the bar chart, which predicts the most stable and similar plotting pattern for all five compounds in the respective three runs (Figure 12).
Table 3.
The MD simulation interaction energy of the top five compounds was predicted in the table.
| Ligand Name | Total Energy | Average Energy | ||
|---|---|---|---|---|
| R1 | R2 | R3 | ||
| Licochalcone B | –105.8469 | –116.8744 | –99.7185 | –107.4799 |
| Echinatin | –102.3061 | –143.673 | –113.2652 | –119.7481 |
| Licochalcone A | –112.8598 | –104.1913 | –100.7909 | –105.9473 |
| Liquiritigenin | –84.437 | –92.9508 | –93.1907 | –90.1928 |
| Licochalcone E | –110.7497 | –101.6259 | –81.5792 | –97.9849 |
Figure 12.
Bar graph depicting the stability of total interaction energy for licochalcone B, echinatin, licochalcone A, licochalcone E, and liquiritigenin for all three runs of 100 ns. Every run is indicated by a different color (run1—blue, run2—orange, run3—light green). The triplicates of every compound were predicted separately.
3. Discussion
Glycyrrhiza glabra L., also known as licorice, has shown potential therapeutic effects in the treatment of gastric ulcers, malaria, and hepatic disorders [16]. The results in this study demonstrate that licochalcone B, echinatin, and licochalcone A exhibit a good profile against P. vivax DBP. Discovery Studio was employed to dock 19 recently biologically active compounds against DBP. The molecular docking studies demonstrate that the ligands bind in the active region of DBP and block its active site by hindering the active site amino acid residues with the lowest molecular docking energies. The prediction of best compounds based on the docking score was recently found to be a non-promising approach [42]. Therefore, MD confirmations are required to predict the best fit against the receptor. The top five docked complexes based on their lowest docking energy were indulged in MD simulation analysis. The triplicate runs were carried out to check the stability of the compound with different starting valencies. All five compounds were keenly observed through RMSD, binding mode, and interaction energy analysis. The RMSD analysis manifests that licochalcone B, echinatin, and licochalcone A exhibit the most stable RMS deviation as compared to licochalcone E and liquiritigenin. Furthermore, the binding mode analysis was carried out to obtain the interacting amino acids and to confirm if the ligands bind with the active amino acids throughout 100 ns. The snapshots were taken at 100 ns, and interactions were analyzed using Discovery Studio. The binding mode analysis reveals that the ligands bind with the active amino acid residues until 100 ns, and conventional hydrogen bonds, carbon hydrogen bonds, and electrostatic forces were observed. Moreover, the MD interaction energy calculation was carried out using Coulomb and Lennard-Jones contributions, and the sum of both was mentioned as total interaction energy, while the average of three runs was calculated and exhibited in tabular form. The average MD interaction energy was also manifested in a graphical representation that depicts the almost similar energy patterns for all five compounds.
The current study employed the particle mesh Ewald (PME) technique, a frequently used approach for computing long-range electrostatic interactions between charged particles in molecular dynamic simulations. Electrostatic interaction calculations are computationally costly, particularly in systems with a number of charged particles. The PME method provides an efficient way to calculate these long-range electrostatic interactions while maintaining high accuracy [43]. Although there are several other algorithms to calculate electrostatic interactions, currently, Reaction Field (RF) has been found to be less computational costly as compared to PME. In the RF approach, the electrostatic interaction energy between two charged particles is determined by combining the direct interaction energy and the reaction field energy. Using a cutoff distance beyond which the interaction is presumed to be negligible, the direct interaction energy is determined [44].
Computer-aided drug discovery (CADD) has shown to be an incredible resource in accelerating the development of epigenetic inhibitors by assisting in the selection, screening, designing, and optimizing of existing drugs, predicting their efficacy against new targets, identifying potential side effects, and improving pharmacokinetic properties, which leads to the discovery of novel compounds that can also be used to predict the properties of drug candidates and evaluate their effectiveness in silico before experimental testing [45,46]. Although in the least understood, novel, and complex cases, CADD is found to be struggling in many cases, the computationally predicted drugs have shown promising in vitro results, and they are being used against many diseases commercially. Captopril, Saquinavir, Zanamivir, Boceprevir, Nolatrexed, Rupintrivir, Aliskiren, Dorzolamide, and Oseltamivir are the drugs that were developed using CADD initially, and they have shown promising results against heart failure, human immunodeficiency virus (HIV), swine flu, hepatitis C virus (HCV), liver cancer, human rhinovirus (HRV), human renin, ocular hypertension, and influenza in vitro, with some of them being in clinical phase 3 trials [47]. Therefore, biological studies are necessary to confirm computational results. The examination of the binding inhibition efficacy of licorice compounds using ELISA assay and cell culture with purified DBP and DARC proteins is needed to validate the results. Furthermore, the biological activity of licorice compounds using human blood samples or animal models needs to be examined.
4. Methodology
4.1. Repossession of 4NUV from PDB
P. vivax DBP heterotetramer structure was obtained from the protein data bank (PDB) with PDBID 4NUV (www.rcsb.org (accessed on 15 February 2023)). 4NUV’s heterotetrameric structure was reduced into a single chain monomeric form and designated as the target protein (DBP). The target protein’s energy minimization was performed by utilizing Discovery Studio [40]. Furthermore, the DBP Ramachandran graph was evaluated using Discovery Studio [48]. The online web server VADAR 1.8 (VADAR) was used to obtain the protein architecture and statistical percentage values of helices, beta sheets, coils, and turns.
4.2. Binding Site Assessment of DBP
The position of a ligand in the protein’s holo-structure most likely determines the binding pocket of the targeted protein and channels [49]. It is composed of certain amino acid residues that catalyze a reaction with that substrate (catalytic site) and residues that temporarily interact with the ligand (binding site) [50]. The active binding site residues are chosen from the data that have already been published [20] and acknowledged by Discovery Studio and the UCSF Chimera tool. Therefore, the binding site is specified by the current selection approach and the docking sphere constricted in the discovery studio to be constrained to our selected amino acid residues.
4.3. Licorice (Ligands) Preparation
Licorice compounds change the erythrocyte membrane at the concentration area where anti-plasmodial action is exhibited [51]. The ligand molecules are selected based on their recently reported biological activity (Table 1). The 3D structure of these 19 representative ligand molecules (licorice) were retrieved from PubChem and further minimized by visualizing in Discovery Studio and PyMOL. The representative ligands were also evaluated on the basis of their structures (2D, 3D) for molecular docking studies.
4.4. Molecular Docking Analysis Using Discovery Studio
Molecular docking is the most widely used method for evaluating the interactions and conformations of ligands with target proteins [52]. By using scoring functions, it is feasible to anticipate the connection strength or binding interaction between two molecules based on preferred orientation [53]. To perform molecular docking of licorice compounds against DBP, Discovery Studio’s “CDocker” approach was used. The binding pocket sphere values were adjusted as (X = _−12.1487, Y = _ 51.0354, and Z = _ 53.4578) and the radius value was adjusted as 7.4148 for improved conformational location in the active region of the target protein [20]. All of the compounds were docked against DBP individually using the default orientation and conformation 10, 10 correspondingly. Meanwhile, the top hits were chosen as 05. The lowest binding energy (Kcal/mol) values were used to assess the anticipated docked complexes. Discovery Studio (4.1) and UCSF Chimera 1.10.1 [41] were used to create a three-dimensional (3D) graphical depiction of the top five docked complexes.
4.5. Molecular Dynamic Simulation
The simulation methodology and parameters were obtained from previously published data [39] and extended to run the triplicates of 100 ns MD simulation experiments. The best complexes based on their molecular docking score and binding patron to the active region of the targeted protein were subjected to an MD simulation experiment. The GROMACS tool (version 2019.3) was used under the Linux operating system to examine the structural behavior of protein and ligand complexes [54]. The CHARMM-GUI server’s solution builder protocol (www.charm-gui.org (accessed on 20 March 2023)) was used to generate the CHARMM36 force field, and the same interface was used to construct input files for MD simulations in GROMACS [55]. TIP3P solutions were used to solvate the existing model into a periodic, cubic box that was expanded by 10 Å beyond each protein atom. Counter ions were added until the system was neutralized. The Verlet cutoff technique with 10 Å was employed for electrostatic and van der Waals interactions, while the LINCS algorithm was applied to restrict bonds. Furthermore, the particle mesh Ewald (PME) technique was used to calculate the electrostatic interactions. The solvated systems were subjected to the steepest descent energy minimization. Following that, the systems proceeded through two rounds of equilibration. Systems were first brought into equilibrium under the NVT condition. During the NVT equilibration phase, the number of particles, volume, and temperature are kept constant; then, under the NPT condition, during the NPT equilibration phase, the number of particles, pressure, and temperature are kept constant. Thus, the system can exchange energy and particles with the thermostat and barostat to maintain a constant temperature and pressure, respectively. Therefore, the CHARMM-GUI includes a Python script for converting GROMACS topology (top) and parameter (itp) files for MD simulations in GROMACS. To execute production dynamics in GROMACS, a 2 fs time step was used, and coordinates were written to a file every picosecond for analysis.
5. Conclusions
In the blood stage, P. vivax DBP invades red blood cells through specific types of receptors present on their surface (DARC). To block the DBP–DARC complex formation, DARC binding residues of DBP are subjected to blockage with the screened library of licorice compounds. Therefore, licorice compounds showed significant compatibility against P. vivax DBP. Licochalcone B, echinatin, licochalcone A, licochalcone E, and liquiritigenin exhibited the lowest docking energy values in molecular docking analysis and bind to the active region of the protein in binding analysis. Furthermore, these five ligands were subjected to MD simulation for the analysis of the stability of docked complexes, and the triplicates of MD simulation were carried out for each compound. Licochalcone B, echinatin, and licochalcone A blocked the active site of DPB protein with the lowest interaction energy both in molecular docking and MD simulation and higher stability in RMSD analysis, followed by licochalcone E and liquiritigenin. Therefore, licochalcone B, echinatin, and licochalcone A compounds appear to be promising against P. vivax DBP, which binds to the active region of DBP and remains intact throughout the entire study. In conclusion, licorice compounds should be investigated as a promising possibility for inhibiting P. vivax invasion into human RBCs via DBP–DARC interaction. Furthermore, modifying licorice compounds might be a promising approach for future prospects to obtain more efficient ligands against P. vivax DBP.
Author Contributions
M.Y. and J.P. were involved in the experimental operation and data analysis. E.-T.H., W.S.P. and J.-H.H. were involved in data curation and the methodology. Y.-S.K. was involved in the project administration. W.C. was involved in the conceptualization, writing, reviewing, and editing of the manuscript. H.-J.L. and W.C. confirmed the authenticity of all the raw data. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data are available in the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not applicable.
Funding Statement
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government [2021-R1A4A1031574].
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Tizifa T.A., Kabaghe A.N., McCann R.S., Berg H.V.D., Van Vugt M., Phiri K.S. Prevention Efforts for Malaria. Curr. Trop. Med. Rep. 2018;5:41–50. doi: 10.1007/s40475-018-0133-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sutherland C.J., Tanomsing N., Nolder D., Oguike M., Jennison C., Pukrittayakamee S., Dolecek C., Hien T.T., Rosário V.E.D., Arez A.P., et al. Two Nonrecombining Sympatric Forms of the Human Malaria Parasite Plasmodium ovale Occur Globally. J. Infect. Dis. 2010;201:1544–1550. doi: 10.1086/652240. [DOI] [PubMed] [Google Scholar]
- 3.Ta T.H., Hisam S., Lanza M., Jiram A.I., Ismail N., Rubio J.M. First case of a naturally acquired human infection with Plasmodium cynomolgi. Malar. J. 2014;13:68. doi: 10.1186/1475-2875-13-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deane L.M. Simian malaria in Brazil. Mem. Inst. Oswaldo Cruz. 1992;87:1–20. doi: 10.1590/S0074-02761992000700001. [DOI] [PubMed] [Google Scholar]
- 5.Brasil P., Zalis M.G., de Pina-Costa A., Siqueira A.M., Júnior C.B., Silva S., Areas A.L.L., Pelajo-Machado M., de Alvarenga D.A.M., da Silva Santelli A.C.F., et al. Outbreak of human malaria caused by Plasmodium simium in the Atlantic Forest in Rio de Janeiro: A molecular epidemiological investigation. Lancet Glob. Health. 2017;5:e1038–e1046. doi: 10.1016/S2214-109X(17)30333-9. [DOI] [PubMed] [Google Scholar]
- 6.Lalremruata A., Magris M., Vivas-Martínez S., Koehler M., Esen M., Kempaiah P., Jeyaraj S., Perkins D.J., Mordmüller B., Metzger W.G. Natural infection of Plasmodium brasilianum in humans: Man and monkey share quartan malaria parasites in the Venezuelan Amazon. Ebiomedicine. 2015;2:1186–1192. doi: 10.1016/j.ebiom.2015.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howes R.E., Battle K.E., Mendis K.N., Smith D.L., Cibulskis R.E., Baird J.K., Hay S.I. Global Epidemiology of Plasmodium vivax. Am. J. Trop. Med. Hyg. 2016;95:15–34. doi: 10.4269/ajtmh.16-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kar S., Sinha A. Plasmodium vivax Duffy Binding Protein-Based Vaccine: A Distant Dream. Front. Cell. Infect. Microbiol. 2022;12:990. doi: 10.3389/fcimb.2022.916702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soeiro M.D.N.C., Vergoten G., Bailly C. Mechanism of action of glycyrrhizin against Plasmodium falciparum. Mem. Inst. Oswaldo Cruz. 2021;116:e210084. doi: 10.1590/0074-02760210084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adams J.H., Ntumngia F., Thomson-Luque R., Pires C.V. The role of the human Duffy antigen receptor for chemokines in malaria susceptibility: Current opinions and future treatment prospects. J. Recept. Ligand Channel Res. 2016;9:1–11. doi: 10.2147/JRLCR.S99725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen E., Salinas N.D., Ntumngia F.B., Adams J.H., Tolia N.H. Structural Analysis of the Synthetic Duffy Binding Protein (DBP) Antigen DEKnull Relevant for Plasmodium vivax Malaria Vaccine Design. PLoS Negl. Trop. Dis. 2015;9:e0003644. doi: 10.1371/journal.pntd.0003644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karimi A., Majlesi M., Rafieian-Kopaei M. Herbal versus synthetic drugs; beliefs and facts. J. Nephropharmacol. 2015;4:27–30. [PMC free article] [PubMed] [Google Scholar]
- 13.Siracusa L., Saija A., Cristani M., Cimino F., D’Arrigo M., Trombetta D., Rao F., Ruberto G.J.F. Phytocomplexes from liquorice (Glycyrrhiza glabra L.) leaves—Chemical characterization and evaluation of their antioxidant, anti-genotoxic and anti-inflammatory activity. Fitoterapia. 2011;82:546–556. doi: 10.1016/j.fitote.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 14.Mostafa D.M., Abd El-Alim S.H., Kassem A.A. Chapter 6-Nanoemulsions: A New Approach for Enhancing Phytonutrient Efficacy. In: Oprea A.E., Grumezescu A.M., editors. Nanotechnology Applications in Food. Academic Press; Cambridge, MA, USA: 2017. pp. 107–127. [Google Scholar]
- 15.Akbar S. Handbook of 200 Medicinal Plants. Springer; Cham, Switzerland: 2020. Glycyrrhiza glabra L. (Fabaceae/Leguminosae): (Syns.: G. glandulifera Waldst. & Kit.; G. hirsuta Pall.; G. pallida Boiss. & Noe; G. violacea Boiss. & Noe) pp. 963–980. [DOI] [Google Scholar]
- 16.Rashidzadeh H., Mosavi F.S., Shafiee T., Adyani S.M., Eghlima G., Sanikhani M., Kheiry A., Amiri M., Tavakolizadeh M., Ramazani A. Anti-Plasmodial Effects of Different Ecotypes of Glycyrrhiza glabra Traditionally Used for Malaria in Iran. Rev. Bras. Farm. 2023;33:310–315. doi: 10.1007/s43450-022-00353-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nasri H. Cisplatin therapy and the problem of gender-related nephrotoxicity. J. Nephropharmacol. 2013;2:13–14. [PMC free article] [PubMed] [Google Scholar]
- 18.Talevi A. Computer-Aided Drug Design: An Overview. Comput. Drug Discov. Des. 2018;1762:1–19. doi: 10.1007/978-1-4939-7756-7_1. [DOI] [PubMed] [Google Scholar]
- 19.Huang H.-J., Yu H.W., Chen C.-Y., Hsu C.-H., Chen H.-Y., Lee K.-J., Tsai F.-J., Chen C.Y.-C. Current developments of computer-aided drug design. J. Taiwan Inst. Chem. Eng. 2010;41:623–635. doi: 10.1016/j.jtice.2010.03.017. [DOI] [Google Scholar]
- 20.Batchelor J.D., Malpede B.M., Omattage N.S., DeKoster G.T., Henzler-Wildman K., Tolia N.H. Red Blood Cell Invasion by Plasmodium vivax: Structural Basis for DBP Engagement of DARC. PLoS Pathog. 2014;10:e1003869. doi: 10.1371/journal.ppat.1003869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang R., Wang L.-Q., Yuan B.-C., Liu Y. The Pharmacological Activities of Licorice. Planta Med. 2015;81:1654–1669. doi: 10.1055/s-0035-1557893. [DOI] [PubMed] [Google Scholar]
- 22.Hu Y., Liu M., Qin H., Lin H., An X., Shi Z., Song L., Yang X., Fan H., Tong Y. Artemether, Artesunate, Arteannuin B, Echinatin, Licochalcone B and andrographolide effectively inhibit SARS-CoV-2 and related viruses in vitro. Front. Cell. Infect. Microbiol. 2021;11:680127. doi: 10.3389/fcimb.2021.680127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao Y., Lei E., Wang X., Qi X., Li L., Ren J., Yang J., Wang S. Licochalcone A inhibits enterovirus A71 replication in vitro and in vivo. Antivir. Res. 2021;195:105091. doi: 10.1016/j.antiviral.2021.105091. [DOI] [PubMed] [Google Scholar]
- 24.Mittal A., Kakkar R. Synthetic methods and biological applications of retrochalcones isolated from the root of Glycyrrhiza species: A review. Results Chem. 2021;3:100216. doi: 10.1016/j.rechem.2021.100216. [DOI] [Google Scholar]
- 25.Wang L., Yang R., Yuan B., Liu Y., Liu C. The antiviral and antimicrobial activities of licorice, a widely-used Chinese herb. Acta Pharm. Sin. B. 2015;5:310–315. doi: 10.1016/j.apsb.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oh H.N., Seo J.H., Lee M.H., Kim C., Kim E., Yoon G., Cho S.S., Cho Y.S., Choi H.W., Shim J.H. Licochalcone C induced apoptosis in human oral squamous cell carcinoma cells by regulation of the JAK2/STAT3 signaling pathway. J. Cell. Biochem. 2018;119:10118–10130. doi: 10.1002/jcb.27349. [DOI] [PubMed] [Google Scholar]
- 27.Traboulsi H., Cloutier A., Boyapelly K., Bonin M.-A., Marsault É., Cantin A.M., Richter M.V. The Flavonoid Isoliquiritigenin Reduces Lung Inflammation and Mouse Morbidity during Influenza Virus Infection. Antimicrob. Agents Chemother. 2015;59:6317–6327. doi: 10.1128/AAC.01098-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pastorino G., Cornara L., Soares S., Rodrigues F., Oliveira M.B.P.P. Liquorice (Glycyrrhiza glabra): A phytochemical and pharmacological review. Phytother. Res. 2018;32:2323–2339. doi: 10.1002/ptr.6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adianti M., Aoki C., Komoto M., Deng L., Shoji I., Wahyuni T.S., Lusida M.I., Fuchino H., Kawahara N., Hotta H. Anti-hepatitis C virus compounds obtained from Glycyrrhiza uralensis and other Glycyrrhiza species. Microbiol. Immunol. 2014;58:180–187. doi: 10.1111/1348-0421.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim H.J., Lim S.S., Park I.S., Lim J.S., Seo J.Y., Kim J.-S. Neuroprotective effects of dehydroglyasperin C through activation of heme oxygenase-1 in mouse hippocampal cells. J. Agric. Food Chem. 2012;60:5583–5589. doi: 10.1021/jf300548b. [DOI] [PubMed] [Google Scholar]
- 31.Seo J.-H., Choi H.W., Oh H.-N., Lee M.-H., Kim E., Yoon G., Cho S.-S., Park S.-M., Cho Y.S., Chae J., et al. Licochalcone D directly targets JAK2 to induced apoptosis in human oral squamous cell carcinoma. J. Cell. Physiol. 2018;234:1780–1793. doi: 10.1002/jcp.27050. [DOI] [PubMed] [Google Scholar]
- 32.Hosseinzadeh H., Nassiri-Asl M. Pharmacological effects of Glycyrrhiza spp. and its bioactive constituents: Update and review. Phytother. Res. 2015;29:1868–1886. doi: 10.1002/ptr.5487. [DOI] [PubMed] [Google Scholar]
- 33.Huang W., Tang S., Qiao X., Ma W., Ji S., Wang K., Ye M., Yu S. Isoangustone A induces apoptosis in SW480 human colorectal adenocarcinoma cells by disrupting mitochondrial functions. Fitoterapia. 2014;94:36–47. doi: 10.1016/j.fitote.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 34.Shah A., Patel V., Parmar B. Discovery of some antiviral natural products to fight against novel coronavirus (SARS-CoV-2) using an in silico approach. Comb. Chem. High Throughput Screen. 2021;24:1271–1280. doi: 10.2174/1386207323666200902135928. [DOI] [PubMed] [Google Scholar]
- 35.Kırmızıbekmez H., Uysal G.B., Masullo M., Demirci F., Bağcı Y., Kan Y., Piacente S. Prenylated polyphenolic compounds from Glycyrrhiza iconica and their antimicrobial and antioxidant activities. Fitoterapia. 2015;103:289–293. doi: 10.1016/j.fitote.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 36.Gazzani G., Daglia M., Papetti A. Food components with anticaries activity. Curr. Opin. Biotechnol. 2012;23:153–159. doi: 10.1016/j.copbio.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 37.He J., Chen L., Heber D., Shi W., Lu Q.-Y. Antibacterial Compounds from Glycyrrhiza u ralensis. J. Nat. Prod. 2006;69:121–124. doi: 10.1021/np058069d. [DOI] [PubMed] [Google Scholar]
- 38.Baltina L.A., Kondratenko R.M., Plyasunova O.A., Pokrovskii A.G., Khalilov L.M., Galin F.Z., Tolstikov G.A. Synthesis and anti-HIV activity of triterpene conjugates of α-d-glucosamine. Pharm. Chem. J. 2008;42:64–67. doi: 10.1007/s11094-008-0061-6. [DOI] [Google Scholar]
- 39.Park J.-Y., Lee Y., Lee H.J., Kwon Y.-S., Chun W. In silico screening of GABA aminotransferase inhibitors from the constituents of Valeriana officinalis by molecular docking and molecular dynamics simulation study. J. Mol. Model. 2020;26:1–13. doi: 10.1007/s00894-020-04495-1. [DOI] [PubMed] [Google Scholar]
- 40.DJA Studio . Discovery Studio. Dassault Systemes BIOVIA; San Diego, CA, USA: 2008. [Google Scholar]
- 41.Pettersen E.F., Goddard T.D., Huang C.C., Couch G.S., Greenblatt D.M., Meng E.C., Ferrin T.E. UCSF Chimera? A visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 42.Breznik M., Ge Y., Bluck J.P., Briem H., Hahn D.F., Christ C.D., Mortier J., Mobley D.L., Meier K. Prioritizing Small Sets of Molecules for Synthesis through in-silico Tools: A Comparison of Common Ranking Methods. Chemmedchem. 2022;18:e202200425. doi: 10.1002/cmdc.202200425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abraham M.J., Gready J.E. Optimization of parameters for molecular dynamics simulation using smooth particle-mesh Ewald in GROMACS 4.5. J. Comput. Chem. 2011;32:2031–2040. doi: 10.1002/jcc.21773. [DOI] [PubMed] [Google Scholar]
- 44.Ge Y., Hahn D.F., Mobley D.L. A Benchmark of Electrostatic Method Performance in Relative Binding Free Energy Calculations. J. Chem. Inf. Model. 2021;61:1048–1052. doi: 10.1021/acs.jcim.0c01424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu W., Zhang R., Jiang H., Zhang H., Luo C. Computer-Aided Drug Design in Epigenetics. Front. Chem. 2018;6:57. doi: 10.3389/fchem.2018.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang G., Bai Y., Cui J., Zong Z., Gao Y., Zheng Z. Computer-Aided Drug Design Boosts RAS Inhibitor Discovery. Molecules. 2022;27:5710. doi: 10.3390/molecules27175710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Talele T.T., Khedkar S.A., Rigby A.C. Successful Applications of Computer Aided Drug Discovery: Moving Drugs from Concept to the Clinic. Curr. Top. Med. Chem. 2010;10:127–141. doi: 10.2174/156802610790232251. [DOI] [PubMed] [Google Scholar]
- 48.Lovell S.C., Davis I.W., Arendall W.B., III, De Bakker P.I., Word J.M., Prisant M.G., Richardson J.S., Richardson D.C. Structure validation by Cα geometry: ϕ, ψ and Cβ deviation. Proteins Struct. Funct. Bioinform. 2003;50:437–450. doi: 10.1002/prot.10286. [DOI] [PubMed] [Google Scholar]
- 49.Hassan M., Yasir M., Shahzadi S., Kloczkowski A. Exploration of Potential Ewing Sarcoma Drugs from FDA-Approved Pharmaceuticals through Computational Drug Repositioning, Pharmacogenomics, Molecular Docking, and MD Simulation Studies. ACS Omega. 2022;7:19243–19260. doi: 10.1021/acsomega.2c00518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yasir M., Park J., Han E.-T., Park W.S., Han J.-H., Kwon Y.-S., Lee H.-J., Hassan M., Kloczkowski A., Chun W. Exploration of Flavonoids as Lead Compounds against Ewing Sarcoma through Molecular Docking, Pharmacogenomics Analysis, and Molecular Dynamics Simulations. Molecules. 2023;28:414. doi: 10.3390/molecules28010414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ziegler H.L., Hansen H.S., Stærk D., Christensen S.B., Haägerstrand H., Jaroszewski J.W. The Antiparasitic Compound Licochalcone A Is a Potent Echinocytogenic Agent That Modifies the Erythrocyte Membrane in the Concentration Range Where Antiplasmodial Activity Is Observed. Antimicrob. Agents Chemother. 2004;48:4067–4071. doi: 10.1128/AAC.48.10.4067-4071.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hassan M., Abbasi M.A., Aziz ur R., Siddiqui S.Z., Hussain G., Shah S.A.A., Shahid M., Seo S.-Y. Exploration of synthetic multifunctional amides as new therapeutic agents for Alzheimer’s disease through enzyme inhibition, chemoinformatic properties, molecular docking and dynamic simulation insights. J. Theor. Biol. 2018;458:169–183. doi: 10.1016/j.jtbi.2018.09.018. [DOI] [PubMed] [Google Scholar]
- 53.Sharma M., Kohli D., Chaturvedi S., Sharma S. Molecular modelling studies of some substitued 2-butylbenzimidazoles angiotensin ii receptor a ntagonists as antihypertensive agents. Dig. J. Nanomater. Biostruct. 2009;4:843–856. [Google Scholar]
- 54.Berendsen H.J.C., Van Der Spoel D., Van Drunen R. GROMACS: A message-passing parallel molecular dynamics implementation. Comput. Phys. Commun. 1995;91:43–56. doi: 10.1016/0010-4655(95)00042-E. [DOI] [Google Scholar]
- 55.Jo S., Kim T., Iyer V.G., Im W. CHARMM-GUI: A web-based graphical user interface for CHARMM. J. Comput. Chem. 2008;29:1859–1865. doi: 10.1002/jcc.20945. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data are available in the manuscript.