Abstract
The application of nucleic acid amplification methods to the detection of food-borne pathogens could be facilitated by concentrating the organisms from the food matrix before detection. This study evaluated the utility of metal hydroxide immobilization for the concentration of bacterial cells from dairy foods prior to detection by cultural and molecular methods. Using reconstituted nonfat dry milk (NFDM) as a model, two food-borne pathogens (Listeria monocytogenes and Salmonella enterica serovar Enteritidis) were concentrated from 25-ml samples by the sequential steps of clarification and high-speed centrifugation (designated primary concentration) and immobilization with zirconium hydroxide and low-speed centrifugation (designated secondary concentration). Sample volume reduction after immobilization with zirconium hydroxide was 50-fold, with total bacterial recoveries ranging from 78 to 96% of input for serovar Enteritidis and 65 to 96% of input for L. monocytogenes. Immobilized bacteria remained viable and could be enumerated by standard cultural procedures. When followed by RNA extraction and subsequent detection by reverse transcription (RT)-PCR, detection limits of 101 to 102 CFU/25 ml of reconstituted NFDM were achieved for both organisms. The bacterial-immobilization step was relatively nonspecific, resulting in recovery of >50% of the input cells when evaluated on a panel of representative bacterial strains of significance to foods. The method could be adapted to more complex dairy products, such as whole milk and ice cream, for which bacterial recoveries after immobilization ranged from 64 to >100%, with subsequent RT-PCR detection limits of ≥102 CFU/ml for whole milk and ≥101 CFU for ice cream for both serovar Enteritidis and L. monocytogenes. The bacterial-immobilization method is easy, rapid, and inexpensive and may have applications for the concentration of a wide variety of food-borne bacteria prior to detection by both conventional and alternative methods.
Food-borne pathogens are a major cause of disease in the United States, resulting in substantial costs to individuals, food processors, and the national economy. Since contamination levels are generally low and there is a zero tolerance for many bacterial pathogens in foods, detection methods require lengthy culture enrichment steps to increase target bacterial numbers before isolation and identification using standard cultural procedures. These conventional food microbiological techniques often require several days or weeks to complete and may fail to detect important bacterial pathogens present in foods at low levels. Although the alternative immunological (enzyme-linked immunosorbent assay) and nucleic acid-based (DNA or RNA hybridization) detection protocols are faster, they remain limited by relatively high detection limits, and hence, culture enrichment has not been eliminated.
The introduction of nucleic acid amplification techniques, such as PCR, has stimulated a flurry of activity by food microbiologists seeking to adapt this methodology to the detection of food-borne pathogens, with hopes of increased detection sensitivity and significantly reduced testing time (1, 27, 33). Disappointingly, detection limits have remained higher than desired and most PCR applications for the detection of food-borne pathogens still require enrichment steps. Specifically, the widespread application of PCR in food microbiology has been limited by (i) high sample volumes compared to amplification volumes, (ii) residual food components which inhibit PCR enzymatic reactions, (iii) low levels of contaminating pathogens, and (iv) the inability to discriminate between live and dead pathogens (1).
It has been suggested that the application of many rapid molecular methods could be improved if the bacteria were separated, concentrated, and purified from the food matrix before detection (20, 27, 33). Although methods such as centrifugation (2), filtration (4), cationic- and anionic-exchange resins (29), aqueous two-phase partitioning (11, 21), immobilized lectins (19), and immunomagnetic separation (16, 25) have been reported for bacterial concentration in food systems, none of these methods is ideal, and in many cases, a technique optimized for one food system or microorganism is not readily adaptable to others.
Immobilization of bacterial cells with metal hydroxides was first reported by Kennedy et al. (10) and was later applied in the concentration of cells from culture media, clinical samples, and foods prior to detection by solid-phase immunoassay (8, 9). The method was rapid, efficient, and inexpensive, resulting in the removal of compounds that caused nonspecific binding in immunoassays and increasing assay detection limits greater than 100-fold. More recently, Berry and Siragusa (3) have reported the use of hydroxyapatite to concentrate indigenous bacteria from meat slurries and environmental samples. The purpose of this study was to evaluate the utility of bacterial immobilization using metal hydroxides for the concentration and purification of bacterial cells from dairy foods in preparation for detection by nucleic acid amplification. Thus, we report data supporting the feasibility of metal hydroxide immobilization for the concentration of representative food-borne microorganisms, the use of the method in conjunction with detection by reverse transcription (RT)-PCR, and the potential adaptability of the method to multiple dairy commodities.
MATERIALS AND METHODS
Bacterial cultures.
Stock cultures of Salmonella enterica serovar Enteritidis, Listeria monocytogenes Scott A, Escherichia coli O157:H7 (HC 122), and E. coli ATCC 25922 were obtained courtesy of Brian Sheldon, Department of Food Science, North Carolina State University. Cultures were grown overnight at 35°C in brain heart infusion (BHI) broth (Difco, Detroit, Mich.) before their use in recovery experiments. Lactobacillus sp. strain ATCC 4356, Lactococcus lactis subsp. lactis NCK 203, and Bacillus cereus NCK 143 were provided by Todd Klaenhammer, Department of Food Science, North Carolina State University. These overnight cultures were grown in MRS broth (Difco) at 37°C, Elliker broth (Difco) at 30°C, and BHI broth at 30°C, respectively. Pseudomonas aeruginosa (ATCC 10145) was obtained from the American Type Culture Collection (Manassas, Va.) and grown overnight in BHI broth at 37°C. In recovery experiments, serial 10-fold dilutions were done in 0.9% NaCl (sterile saline), and plating for recovery was performed by the spread plate technique on the agar-solidified broth medium designated for each organism.
Preparation of metal hydroxides.
Metal hydroxide solutions were prepared as previously reported with minor modifications (8, 9). For zirconium hydroxide and hafnium hydroxide, a 40-ml volume of distilled water was added to 2.0 g of zirconium(IV) chloride or hafnium chloride 98% (Aldrich Chemical Co., Milwaukee, Wis.). For titanous hydroxide, a 1.3 mM solution was prepared by the addition of 200 ml of distilled water to 356 μl of titanium(III) chloride (Aldrich Chemical Co.). The solutions were adjusted to pH 7.0 ± 0.2 by the dropwise addition of ammonium hydroxide (5 M) and continuous agitation. Each metal hydroxide solution was then washed three times with 200 ml of sterile saline solution to remove excess ammonium ions (10). In the washing procedure, the hydroxide was mixed gently with the sterile saline solution and allowed to settle over a 10-min period, and then approximately 40% of the top phase (consisting of saline solution and debris) was decanted. The final volume of each hydroxide was between 200 and 300 ml, and the hydroxide solutions were stored in the dark at room temperature for up to 6 months.
Immobilization Studies. (i) Feasibility studies with serovar Enteritidis and L. monocytogenes.
In the initial immobilization studies, 200 μl of each metal hydroxide was mixed with 100 μl of an overnight culture of serovar Enteritidis or L. monocytogenes serially diluted in sterile saline solution to approximately 107, 105, and 103 CFU/100 μl. This represented a 1:2 volume ratio of sample to metal hydroxide. The suspensions were gently agitated at room temperature for 10 min to keep the metal hydroxides in suspension, followed by a brief vortex and centrifugation at 500 × g for 5 min at 7°C using an Eppendort microfuge (Brinkmann Instrument Co., Westbury, N.Y.). The supernatants were poured off and retained, and the bacterium-containing pellets were reconstituted in 100 μl of sterile saline solution. Bacterial loss to the supernatant was determined after the serial dilution of supernatants and subsequent plating. Percent recovery was calculated as previously reported (8): [percent immobilization = (total population in sample before immobilization − total population in supernatant after immobilization) × 100/(total population in sample before immobilization)]. Plating was also performed on dilutions that were treated identically except without the addition of the metal hydroxide (control). All experiments were done in triplicate.
(ii) Bacterial immobilization applied to dairy products.
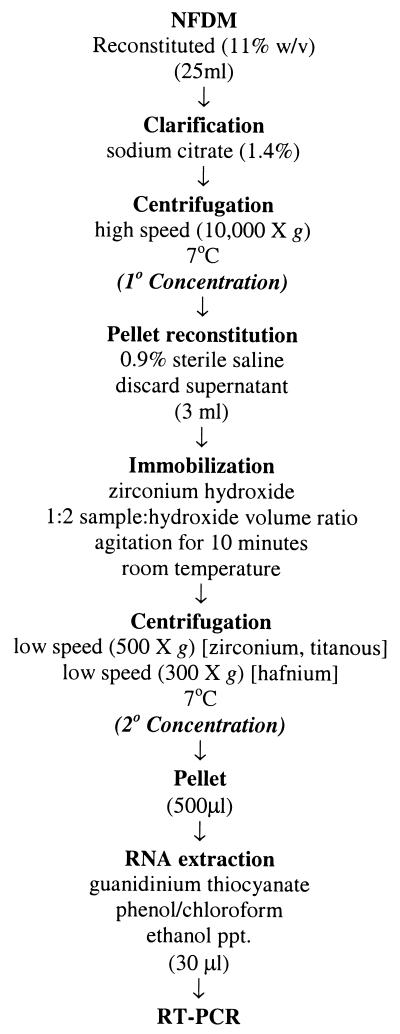
The efficacy of bacterial concentration with metal hydroxides was initially investigated using a nonfat dry milk (NFDM) model. Twenty-five-milliliter samples of NFDM reconstituted in sterile water (11% [wt/vol]) were seeded with a 1-ml volume of diluted overnight cultures of serovar Enteritidis or L. monocytogenes to achieve final inoculum concentrations of 104, 103, 102, or 101 CFU/25 ml of NFDM. Serial dilutions of the NFDM samples were plated on BHI agar both before and after inoculation to evaluate the level of the indigenous microflora and to confirm the pathogen levels, respectively. Sample clarification was achieved by the addition of 1.5 ml of 25% (wt/vol) sodium citrate (Fisher Chemical Co., Fair Lawn, N.J.) (22) with 5 min of shaking by hand at room temperature. An initial separation step (designated primary concentration) was performed by centrifugation at 10,000 × g for 10 min at 7°C in a Sorvall RC-2 refrigerated centrifuge (DuPont, Wilmington, Del.). The resulting pellet was resuspended in 3.0 ml of sterile saline by gentle mixing with a pipette. Zirconium hydroxide solution was added to the reconstituted sample in a 1:2 sample-to-hydroxide volume ratio. The suspension was agitated by horizontal shaking on a vortexer (speed setting, 2.5) for 10 min to promote bacterial immobilization. A second separation step, to pellet the hydroxide (designated secondary concentration), was performed by centrifugation (on a Sorvall RC-2 unit) at 500 × g at 7°C for 10 min. The supernatant was carefully removed by pipette, and the bacterium-containing pellet was reconstituted to 1 ml in sterile saline and vortexed gently to thoroughly mix it. The two supernatants (from the primary and secondary concentration steps) and the final reconstituted pellet were plated in duplicate for recovery on BHI agar after serial dilution. Total bacterial recoveries after immobilization were calculated based on loss to the supernatants (see the formula above). In instances where recovery was based on direct plating of the pellet obtained from bacterial immobilization, it was calculated using the following formula: [percent immobilization = (total population in pellet after immobilization) × 100/(total population in sample before immobilization)]. Control samples consisted of inoculated NFDM samples treated for bacterial concentration using the above scheme but without the addition of metal hydroxide. Duplicate and parallel samples were extracted for RNA isolation and subsequent nucleic acid amplification by RT-PCR. All experiments were done in triplicate. The entire concentration scheme is outlined in Fig. 1.
FIG. 1.
Flow chart of bacterial-immobilization method. For NFDM, whole milk, and ice cream, the immobilization efficiencies of L. monocytogenes and serovar Enteritidis were evaluated with zirconium hydroxide. For specificity studies, zirconium, titanous, and hafnium hydroxides were evaluated for the concentrations of E. coli, E. coli O157:H7, serovar Enteritidis, P. aeruginosa, L. monocytogenes, Lactobacillus spp., B. cereus, and L. lactis.
To demonstrate the applicability of the method to other dairy commodities, 900-μl samples of pasteurized whole milk or melted vanilla ice cream were seeded with 100 μl of serial dilutions of an overnight culture of serovar Enteritidis or L. monocytogenes to achieve final concentrations of 104, 103, 102, or 101 CFU/ml of milk or ice cream. These samples were subjected to the bacterial-immobilization scheme outlined in Fig. 1 except that the volumes were adjusted to account for the reduced sample size. As with the NFDM model, the two supernatants (from the primary and secondary concentration steps) and the final reconstituted pellet were plated for recovery and parallel samples were extracted for RNA isolation and subsequent nucleic acid amplification by RT-PCR.
(iii) Immobilization of other food-borne bacteria.
In order to evaluate the specificity of metal hydroxide adsorption for the recovery of various gram-positive and gram-negative bacteria of significance to foods, overnight cultures of representative food-borne bacteria (serovar Enteritidis, L. monocytogenes Scott A, E. coli O157:H7, E. coli ATCC 25922, Lactobacillus sp. strain ATCC 4356, L. lactis subsp. lactis NCK 203, B. cereus NCK 143 and P. aeruginosa ATCC 25922) were serially diluted in sterile growth media to approximately 106 CFU/ml. One-milliliter volumes were mixed gently by vortexing and subjected to bacterial immobilization according to the scheme outlined in Fig. 1 except that volumes were adjusted to account for the reduced sample sizes. Zirconium hydroxide, titanous hydroxide, and the heavier hafnium hydroxide were evaluated in these studies. Due to the large size of the hafnium hydroxide complex, centrifugation speeds were reduced to 300 × g for the bacterial-immobilization experiments using that agent. Bacterial loss to the supernatant and recovery in precipitates were calculated as described above. Tubes containing bacterial suspensions but no metal hydroxide (control) were treated in the same manner to evaluate the efficacy of centrifugation alone.
(iv) Viability of immobilized cells.
To confirm that bacterial cells remained viable after exposure to metal hydroxide solutions, 1-ml volumes of overnight cultures of serovar Enteritidis and L. monocytogenes, serially diluted 100-fold in sterile saline solution, were seeded with 2 ml of zirconium hydroxide and plated on BHI agar at 0, 2, 6, 12, 24, and 48 h. Control tubes consisted of the diluted overnight cultures without the addition of zirconium hydroxide. Plate counts for treated and untreated samples were compared at each time point to evaluate cell survival in the presence of zirconium hydroxide.
(v) Statistical analyses.
When statistical comparisons were necessary, analysis of variance and the Tukey-Kramer multiple comparisons test, or Student's t test, were done on the log-transformed bacterial population or percent recovery data using the InStat 2 statistical analysis package (GraphPad Software, San Diego, Calif.).
Molecular biological methods. (i) RNA extraction.
Total RNA in the reconstituted metal hydroxide pellets was extracted directly with TRI reagent BD (Molecular Research Center, Inc., Cincinnati, Ohio) using the method described by the manufacturer for extraction of RNA from whole blood. Briefly, cells in the metal hydroxide pellet were lysed by the addition of 0.75 ml of TRI reagent BD supplemented with 20 μl of 5 N acetic acid. The lysed samples were extracted with 0.2 ml of chloroform with vigorous vortexing for 15 s and spun at 10,000 × g for 15 min at 4°C. The aqueous phase was supplemented with 5 μl of a 10-mg/ml solution of yeast tRNA (Sigma Chemical Co., St. Louis, Mo.) to act as a carrier, and the RNA was precipitated in 0.5 ml of isopropanol for 5 to 10 min at room temperature followed by centrifugation at 10,000 × g for 8 min at 4°C. The RNA pellet was washed in 75% ethanol and centrifuged again at 7,500 × g for 5 min at 4°C. The precipitated RNA was air dried for 5 min to remove residual alcohol, resuspended in 30 μl of sterile diethyl pyrocarbonate-treated water, and used directly in RT-PCR amplifications.
(ii) RT-PCR primers and oligoprobes.
The oligonucleotide primer and probe sequences for L. monocytogenes and serovar Enteritidis have been previously described (12, 30, 31) and corresponded to unique 16S rRNA sequences for each organism. All primers and probes for PCR were synthesized by GenoSys (The Woodlands, Tex.). Primers for Salmonella were based upon two regions of sequence variability between the V3 and V5 regions of the 16S rRNA specific to Salmonella (12) (5′ primer, 5′-TGTTGTGGTTAATAACCGCA-3′; 3′ primer, 5′-CACAAATCCATCTCTGGA-3′) and generated a 575-bp amplicon. L. monocytogenes primers were based on region 1228 to 1297 of the 16S rRNA (30, 31) (primer L1, 5′-CACGTGCTACAATGGATAG-3′; primer L2, 5′-AGAATAGTTTTATGGGATTAG-3′) and selectively amplified a 70-bp region specific for L. monocytogenes to the exclusion of other Listeria species. The internal oligoprobe (RLM3; 5′-GTCGCGAAGCCGCGAGGT-3′) for the confirmation of L. monocytogenes amplicons by Southern hybridization was also previously reported (30, 31).
(iii) RT-PCR.
RT-PCRs were done with the Gene-Amp kit (Roche Molecular Systems, Branchburg, N.J.) according to the manufacturer's instructions except that the volumes for RT were increased from 20 to 30 μl to accommodate a 10-μl sample size and Ampli-Taq Gold (Applied Biosystems, Foster City, Calif.) was used as the source of thermostable DNA polymerase. RT was done at 42°C for 1 h with random hexamer primers, and then the tubes were heated to 99°C for 5 min to inactivate the enzyme. After being chilled on ice, the tubes were supplemented with 2.5 U of AmpliTaq Gold and 100 ng of each primer as appropriate. PCR amplification was done in a DNA thermal cycler (Perkin-Elmer, Norwalk, Conn.). For L. monocytogenes, amplification was performed as previously reported (30) and consisted of one cycle at 90°C for 3 min followed by 40 cycles of 95°C for 20 s, 48°C for 20 s, 73°C for 40 s, and a final extension at 73°C for 3 min. PCR amplification for serovar Enteritidis was also performed as previously reported (12) and consisted of one cycle of 90°C for 10 min followed by 40 cycles of 95°C for 1.5 min, 54°C for 1.5 min, and 72°C for 1.5 min and a final extension cycle of 73°C for 3 min. A 10- to 15-μl portion of RT-PCR product, which represented 10 to 15% of the total reaction volume, was analyzed by gel electrophoresis on 2% (serovar Enteritidis) or 4% (L. monocytogenes) agarose, stained with ethidium bromide, and visualized by UV light. In some experiments, the inhibitory effect of food components was evaluated by serial dilution of sample concentrates (i.e., pellets after primary and secondary concentration steps) and the addition of a constant amount of bacterial RNA immediately followed by nucleic acid amplification by RT-PCR.
(iv) Oligoprobe hybridization.
RT-PCR products from L. monocytogenes amplification were transferred to nylon membranes using the method of Southern (26). The DNA was bound by cross-linking with shortwave UV light (Ultraviolet Products, Inc., San Gabriel, Calif.) for 3 to 5 min at a distance of 15 cm. The oligoprobes for target amplicons were 3′-end labeled with digoxigenin-dUTP using terminal transferase, purified by ethanol precipitation, and used in hybridization reactions according to the instructions in the Genius nonradioactive end-labeling kit (Boehringer Mannheim Biochemicals, Indianapolis, Ind.). Briefly, membranes were prehybridized for 4 h and hybridized overnight at 55°C with the recommended hybridization solutions (5× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate], 0.1% [wt/vol] N-laurylsarcosine, 0.02% [wt/vol] sodium dodecyl sulfate, and 1% [wt/vol] Boehringer Mannheim Biochemical's proprietary blocking reagent) containing labeled probe at a concentration of 5 to 10 ng per ml of hybridization solution. The blots were washed five times in 6× SSC–0.05% pyrophosphate at 55°C. Immunological detection of RT-PCR product–oligoprobe hybrids was performed using an anti-digoxigenin–alkaline phosphatase antibody conjugate and enzyme-catalyzed colorimetric reaction with 5-bromo-4-chloro-3-indolylphosphate and nitroblue tetrazolium salt as substrates.
(v) LCR.
The identities of Salmonella RT-PCR amplicons were confirmed by the ligase chain reaction (LCR) (23). A set of four primers (SEA1, SEA2, SEA3, and SEA4) were designed to specifically detect a single-base-pair difference within the Salmonella PCR amplicon. Primers with the following sequences were synthesized by GenoSys: SEA1, 5′-TAGTCCACGCCGTAAACGATGTCT-3′; SEA2, 5′-ACTTGGAGGTTGTGCCCTTGAG-3′; SEA3, 5′-GACATCGTTTACGGCGTGGACTA-3′; SEA4, 5′-CTCAAGGGCACAACCTCCAAGTA-3′.
SEA2 and SEA3 were 5′ phosphorylated during manufacture; SEA1 was synthesized with a universal primer sequence (5′-TGGCACTGGCCGTCGTTTTAC-3′), designated UP, at the 5′ end, and SEA4 was biotinylated during manufacture. LCR was performed with the LCR kit (Stratagene, La Jolla, Calif.) according to the manufacturer's instructions. Briefly, 1 μl of RT-PCR product was added to 20 μl of LCR mixture (2 μl of 10× LCR buffer, 20 ng of LCR primers, 4 U of Pfu ligase, and 15 μl of sterile, diethyl pyrocarbonate-treated water) and incubated at 94°C for 5 min (denaturation) and 60°C for 4 min (annealing). The reaction mixtures were then cycled 25 times at 95°C for 1 min and 60°C for 2 min. LCR products were detected with the AmpliTek Detection Module (Bio-Rad, Hercules, Calif.) according to the manufacturer's instructions. In this method, LCR products diluted 1:10 or 1:50 in 1× SSC were incubated for 1 h at 37°C in streptavidin-coated microtiter plate wells. The wells were washed five times with 1× proprietary wash buffer and then incubated for 1 h at 37°C with alkaline phosphatase conjugated to an oligonucleotide sequence complimentary to the UP sequence of the SEA1 primer. The wells were again washed five times to remove unbound enzyme and then incubated for 1 h at 37°C with the NADPH substrate. Addition of a second substrate activated a secondary enzyme system, creating a redox cycle which produced a molecule of colored formazan. The reaction products were detected colorimetrically by endpoint determination at 490 nm.
RESULTS
Initial immobilization studies.
Initial bacterial-immobilization studies were done using diluted overnight cultures of L. monocytogenes and serovar Enteritidis. Small sample volumes of 200 μl were concentrated fourfold to 50 μl. While immobilization efficiency was partially dependent upon the initial concentration of bacterial cells and the concentration of metal hydroxide (data not shown), once these parameters were optimized, consistently high recoveries could be achieved. Percent recovery data for L. monocytogenes and serovar Enteritidis immobilized by titanous or zirconium hydroxide were based on loss to the supernatant (Table 1) (8). Immobilization efficiencies ranged from 95 to 99%, and there were no statistically significant differences between the metal hydroxides, the organisms, or the initial inoculum levels when evaluated by the Tukey-Kramer pairwise statistical comparison (P > 0.05). The immobilized cells could be enumerated by direct plating of the reconstituted pellet on BHI agar (data not shown).
TABLE 1.
Immobilization of L. monocytogenes and serovar Enteritidis in pure culture using titanous hydroxide and zirconium hydroxide
| Organism | Inoculum (CFU/ml) | Avg % immobilizationa
|
|
|---|---|---|---|
| Titanous hydroxide | Zirconium hydroxide | ||
| L. monocytogenes | 107 | 98 ± 2 | 98 ± 2 |
| 105 | 98 ± 3 | 97 ± 2 | |
| 103 | 95 ± 3 | 98 ± 2 | |
| Serovar Enteritidis | 107 | 98 ± 1 | 97 ± 2 |
| 105 | 98 ± 1 | 98 ± 1 | |
| 103 | 98 ± 1 | 99 ± 1 | |
Average percent immobilization (mean ± standard deviation) of three replicate samples; calculated as previously reported (8): [percent immobilization = (total population in sample before immobilization − total population in supernatant after immobilization) × 100/(total population in sample before immobilization)].
Bacterial recovery and detection in the NFDM model.
Reconstituted NFDM was used as a model to investigate the efficiency of bacterial immobilization in foods. The entire sample concentration scheme was applied to 25-ml samples and included the sequential steps of clarification and high-speed centrifugation (designated primary concentration) followed by immobilization with metal hydroxides and low-speed centrifugation (designated secondary concentration) (Fig. 1). The combined steps of primary and secondary concentration resulted in an overall 50-fold sample volume reduction from 25 ml to 500 μl. By following the bacterial concentration with an RNA extraction step, a final sample concentrate of 20 to 30 μl could be obtained, representing an additional 20-fold concentration factor.
To evaluate the effect of food-related RT-PCR inhibition, reconstituted NFDM samples at various points in the concentration process were serially diluted and seeded with RNA corresponding to approximately 107 CFU of L. monocytogenes just prior to RT reactions. The ability to obtain detectable amplicons at various dilutions by RT-PCR was indicative of the degree of sample inhibition. These RT-PCR compatibility studies indicated minimal PCR inhibition (10-fold dilution of the sample was required for positive amplification) from untreated NFDM and NFDM treated by primary concentration alone. There was little to no inhibition after bacterial immobilization with titanous or zirconium hydroxide, as evidenced by the ability to amplify target rRNA sequences without prior sample dilution (data not shown).
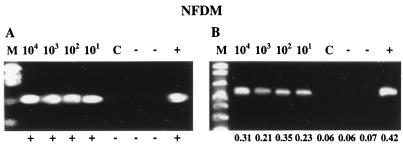
To evaluate both bacterial recovery and RT-PCR detection in this model system, 25-ml samples of reconstituted NFDM were seeded with a 1-ml aliquot of 104, 103, 102, or 101 CFU of an overnight culture of L. monocytogenes or serovar Enteritidis and processed by the combined clarification, centrifugation, and bacterial-immobilization procedure. Recoveries were assessed in parallel by both bacteriological plating (supernatants after primary and secondary concentration and the final immobilized pellet) and RT-PCR amplification. Plating of the uninoculated NFDM samples revealed background microflora levels of <101 CFU/ml. Total bacterial recoveries after the primary concentration steps were 85 to 98 and 78 to 98% for serovar Enteritidis and L. monocytogenes, respectively (Table 2). The secondary concentration step, which employed bacterial immobilization, resulted in efficient recovery of 51 to 96% of inoculated serovar Enteritidis and 95 to 98% of inoculated L. monocytogenes. The immobilized cells were apparently viable and could be enumerated by direct plating of the reconstituted pellet. These recoveries ranged from 65 to 95% for serovar Enteritidis and 78 to 96% for L. monocytogenes (Table 2). Low-speed centrifugation without prior immobilization was extremely inefficient and variable, resulting in recoveries of <36% (data not shown). Total RNA from the immobilization sample concentrates (500 μl) was extracted to a final volume of 25 μl, representing an overall 1,000-fold sample volume reduction. When subjected to nucleic acid amplification by RT-PCR, direct detection of both serovar Enteritidis and L. monocytogenes was consistently possible at initial bacterial input levels of 102 CFU/25 ml and higher cell concentrations in reconstituted NFDM (Fig. 2). At low input levels of 101 CFU/25 ml of NFDM, RT-PCR detection was possible for serovar Enteritidis in one out of three samples and in two out of three samples for L. monocytogenes. The identities of RT-PCR amplicons were confirmed by internal oligoprobe hybridization (L. monocytogenes) or LCR (serovar Enteritidis).
TABLE 2.
Recovery and detection of serovar Enteritidis and L. monocytogenes in 25-ml reconstituted NFDM samples after bacterial immobilization with zirconium hydroxide and subsequent RT-PCR
| Inoculuma (CFU/25 ml) | Serovar Enteritidis
|
L. monocytogenes
|
||||||
|---|---|---|---|---|---|---|---|---|
| Avg % recoveryb
|
Detection (PCR/LCR)c | Avg % recoveryb
|
Detection (PCR/Hyb)c | |||||
| 1o concn | 2o concn | Ppt | 1o concn | 2o concn | Ppt | |||
| 104 | 97 ± 2x | 96 ± 3xy | 69 ± 4z | 3/3 | 98 ± 4x | 98 ± 1x | 96 ± 1x | 3/3 |
| 103 | 85 ± 3y | 95 ± 3yz | 85 ± 3y | 3/3 | 96 ± 1x | 96 ± 1x | 92 ± 1y | 3/3 |
| 102 | 98 ± 2x | 96 ± 1xy | 95 ± 1x | 3/3 | 89 ± 2y | 97 ± 2x | 89 ± 9xy | 3/3 |
| 101 | 94 ± 6xy | 51 ± 41z | 65 ± 16yz | 1/3 | 78 ± 10y | 95 ± 5x | 78 ± 12y | 2/3 |
Total CFU at input is taken as 100%; input level confirmed by plating samples before bacterial concentration.
Average percent recovery (mean ± standard deviation) of three replicate samples; for primary concentration (1o concn) and secondary concentration (2o concn), percent recovery was calculated as previously reported (8): [percent recovery = (total population in sample before concentration − total population in supernatant after 1o concentration or after 2o concentration {i.e., immobilization}) × 100/(total population in sample before concentration)]. For the immobilized pellet (Ppt), percent recovery was calculated as follows: [percent recovery = (total population in pellet after immobilization {i.e., after 1o and 2o concentrations} × 100/(total population in sample before concentration)]. Different superscript letters (x, y, and z) identify statistically significant differences (P ≤ 0.05) in percent recovery at different input levels of each organism. Boldface data identify statistically significant differences (P ≤ 0.05) between percent recovery values when calculations based on loss to the supernatant versus direct plating of the immobilized pellet were compared.
Detection was done by agarose gel electrophoresis of PCR products and subsequent confirmation by DNA hybridization (PCR/Hyb for L. monocytogenes) or LCR (PCR/LCR for serovar Enteritidis).
FIG. 2.
Detection of L. monocytogenes and serovar Enteritidis in artificially contaminated reconstituted NFDM after bacterial immobilization with zirconium hydroxide, RNA extraction, and RT-PCR. Reconstituted NFDM (25-ml) samples were inoculated with 104, 103, 102, or 101 CFU of L. monocytogenes (A) or serovar Enteritidis (B) and processed for bacterial concentration followed by RNA isolation and RT-PCR amplification. The corresponding initial inoculum level (CFU/25 ml of reconstituted NFDM) is given above each gel lane. Southern hybridization results (L. monocytogenes) and LCR absorbance readings (serovar Enteritidis) for confirmation of RT-PCR amplicons are displayed below each amplicon. Lanes: M, marker; C, uninoculated 25-ml reconstituted-NFDM sample processed for bacterial concentration; −, complete RT-PCR cocktail without sample (i.e., water); +, positive control reaction for amplification (i.e., RNA extracted from approximately 106 CFU of L. monocytogenes or serovar Enteritidis in pure culture).
Specificity of bacterial concentration.
The specificity of metal hydroxide adsorption for the concentration of various gram-positive and gram-negative bacteria of significance to foods was evaluated using eight representative bacterial strains in pure culture. Percent recovery was based on both loss to the supernatant and recovery from direct plating of the precipitate. Bacterial recoveries based on loss to the supernatant were 88 to 100% for titanous hydroxide, 89 to 98% for zirconium hydroxide, and 70 to 97% for hafnium hydroxide (data not shown). Standard deviations for the loss-to-supernatant recoveries were generally small, and statistically significant differences (P ≤ 0.05) in recoveries were seen with only one organism (E. coli) for the hafnium hydroxide treatment. In all cases, the efficacy of centrifugation alone was highly erratic.
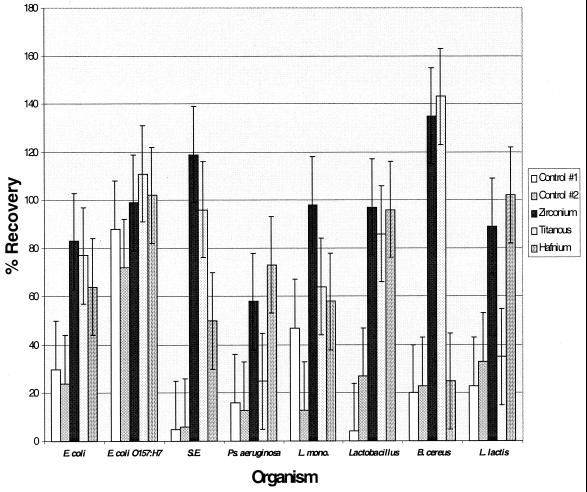
When calculations were based on direct plating of the precipitate, recoveries ranged from 25 to 143% for titanous hydroxide, 58 to 135% for zirconium hydroxide, and 25 to 102% for hafnium hydroxide (Fig. 3). Standard deviations for these recoveries were much higher than when recovery was calculated by loss to the supernatant. Statistically significant differences (P ≤ 0.05) between organisms were apparent for titanous and hafnium hydroxides, whereas zirconium hydroxide recoveries were reasonably consistent (P > 0.05) regardless of the organism tested. As with the loss-to-supernatant data, the bacterial recoveries from pellets obtained after centrifugation alone were inconsistent and highly organism dependent. Statistical comparison among metal hydroxides revealed that, while all metal hydroxides were capable of removing all of the organisms to varying degrees, in almost all cases, zirconium hydroxide performed better than titanous and hafnium hydroxides in providing equal or better recoveries by direct plating of the precipitate. Furthermore, subsequent experiments indicated that hafnium hydroxide was inhibitory to RT-PCRs (data not shown). Because of its consistent performance in bacterial immobilization, as well as its compatibility with enzymatic nucleic acid amplification, zirconium hydroxide was the only immobilization agent chosen for subsequent studies.
FIG. 3.
Percent recovery of selected food-borne bacteria by metal hydroxide immobilization as calculated by direct plating of the immobilized pellet. The error bars represent standard deviations. Control 1 corresponds to centrifugation alone at 500 × g, while control 2 corresponds to centrifugation alone at 300 × g. Specific food-borne microorganisms tested include the following: E. coli, E. coli O157:H7, serovar Enteritidis (S.E.), P. (Ps.) aeruginosa, L. monocytogenes (L. mono), Lactobacillus, B. cereus, and L. lactis.
Viability of immobilized cells.
To confirm that bacterial cells remained viable after exposure to metal hydroxide solutions, serially diluted overnight cultures of serovar Enteritidis and L. monocytogenes were seeded with zirconium hydroxide and plated for recovery at 0, 2, 6, 12, 24, and 48 h. Control tubes consisted of the diluted overnight cultures without the addition of zirconium hydroxide. Subsequent analysis indicated no statistically significant increase or reduction (P > 0.05) in total bacterial population over time for either the control or treatment samples for both serovar Enteritidis and L. monocytogenes. Furthermore, there was no significant difference (P > 0.05) between log-transformed counts for the control and hydroxide treatments at any one time point for either organism.
Bacterial recovery and detection in whole milk and ice cream.
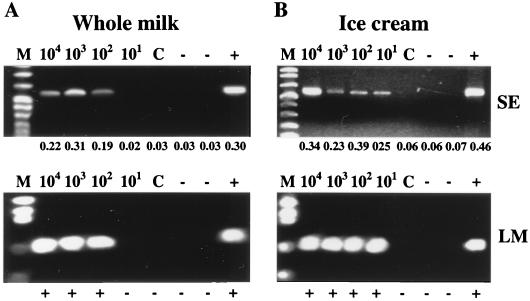
To evaluate the bacterial-concentration scheme in more complex dairy products, 900-μl samples of milk and ice cream were seeded with serial dilutions of L. monocytogenes or serovar Enteritidis to achieve a final concentration of 104, 103, 102, or 101 CFU/ml of milk or ice cream. These samples were then processed by the combined clarification, centrifugation, and bacterial immobilization procedure (Fig. 1). Recoveries were evaluated based on loss to the supernatant (after secondary concentration) and direct plating of the immobilized cells using standard cultural procedures. Plating of the uninoculated milk and ice cream samples revealed background microflora levels of <101 CFU/ml (ice cream) and <102 CFU/ml (whole milk). Total bacterial recoveries after immobilization in milk, as calculated by loss to the supernatant, were 96 to 97 and 92 to 96% for serovar Enteritidis and L. monocytogenes, respectively (Table 3). Recoveries from ice cream were 71 to 95 and 61 to 91% for serovar Enteritidis and L. monocytogenes, respectively (Table 3). Recoveries based on loss to the supernatant were extremely consistent both among organisms and among different inoculum levels for whole milk. In the case of ice cream, these recoveries were less consistent, yet there was no notable reduction in recovery efficiency at lower inoculum levels. Recovery efficiencies were essentially equal for L. monocytogenes and serovar Enteritidis. The immobilized cells could be enumerated by direct plating of the reconstituted pellet, and recoveries in milk ranged from 77 to 107% for serovar Enteritidis and 93 to 122% for L. monocytogenes (Table 3). Recoveries for ice cream were 64 to 79 and 71 to 161% for serovar Enteritidis and L. monocytogenes, respectively (Table 3). Low-speed centrifugation without prior immobilization was extremely inefficient, resulting in >50% recovery of the input bacteria (data not shown). Total RNA from the immobilization sample precipitates was extracted to a final volume of 10 μl, representing an overall 100-fold sample volume reduction. When subjected to nucleic acid amplification using RT-PCR, direct detection (without sample dilution) of both serovar Enteritidis and L. monocytogenes was possible at initial bacterial input levels of ≥102 CFU/ml for whole milk and at input levels of ≥101 CFU/ml for ice cream (Fig. 4).
TABLE 3.
Recovery and detection of serovar Enteritidis and L. monocytogenes in 1-ml samples of whole milk or ice cream after bacterial immobilization with zirconium hydroxide and subsequent RT-PCR
| Organism | Whole milk
|
Ice cream
|
||||||
|---|---|---|---|---|---|---|---|---|
| Inoculuma (CFU/ml) | Avg % recoveryb
|
Detection (PCR/confirm)c | Inoculuma (CFU/ml) | Avg % recoveryb
|
Detection (PCR/confirm)c | |||
| 2o concn | Ppt | 2o concn | Ppt | |||||
| L. monocytogenes | 104 | 96 ± 1x | 114 ± 5x | 3/3 | 104 | 91 ± 1x | 104 ± 23y | 3/3 |
| 103 | 92 ± 1y | 122 ± 12x | 3/3 | 103 | 67 ± 1y | 78 ± 7y | 3/3 | |
| 102 | 92 ± 1y | 100 ± 8x | 3/3 | 102 | 61 ± 3y | 71 ± 7y | 3/3 | |
| 101 | 95 ± 4x | 93 ± 20x | 0/3 | 101 | 84 ± 13x | 161 ± 11x | 2/3 | |
| Serovar Enteritidis | 104 | 97 ± 2x | 83 ± 7x | 3/3 | 104 | 95 ± 1x | 69 ± 8x | 3/3 |
| 103 | 96 ± 1x | 90 ± 19xy | 3/3 | 103 | 89 ± 1y | 65 ± 2x | 3/3 | |
| 102 | 97 ± 2x | 77 ± 11x | 3/3 | 102 | 87 ± 1z | 64 ± 4x | 1/3 | |
| 101 | 96 ± 5x | 107 ± 1y | 0/3 | 101 | 71 ± 14w | 79 ± 14x | 2/3 | |
Total CFU at input is taken as 100%; input level confirmed by plating samples before bacterial concentration.
Average percent recovery (mean ± standard deviation) of three replicate samples; for secondary concentration (2o concn), percent recovery was calculated as previously reported (8): [percent recovery = (total population in sample before concentration − total population in supernatant after immobilization {i.e., after 1o and 2o concentration} × 100/(total population in sample before concentration)]. For the immobilized pellet (Ppt), percent recovery was calculated as follows: [percent recovery = (total population in pellet after immobilization {i.e., after 1o and 2o concentrations} × 100/(total population in sample before concentration)]. Different superscript letters (w, x, y, and z) identify statistically significant differences (P ≤ 0.05) in percent recovery at different input levels of each organism. Boldface data identify statistically significant differences (P ≤ 0.05) between percent recovery values when calculations based on loss to supernatant versus direct plating of the immobilized pellet were compared.
Detection was done by agarose gel electrophoresis of PCR products and subsequent confirmation (PCR/confirm) by DNA hybridization (L. monocytogenes) or LCR (serovar Enteritidis).
FIG. 4.
Detection of L. monocytogenes and serovar Enteritidis in artificially contaminated whole milk or ice cream after bacterial immobilization with zirconium hydroxide, RNA extraction, and RT-PCR. One-milliliter samples of whole milk (A) or ice cream (B) were inoculated with 104, 103, 102, or 101 CFU of L. monocytogenes (LM; bottom) or serovar Enteritidis (SE; top) and processed for bacterial concentration followed by RNA isolation and subsequent RT-PCR amplification. The corresponding initial inoculum level (CFU/1 ml of whole milk or ice cream) is given above each gel lane. Southern hybridization results (L. monocytogenes) and LCR absorbance readings (serovar Enteritidis) for confirmation of RT-PCR amplicons are displayed below each amplicon. Lanes: M, marker; C, 1-ml samples of uninoculated whole milk or ice cream processed for bacterial concentration; −, complete RT-PCR cocktail without sample (i.e., water); +, positive control reaction for amplification (i.e., RNA extracted from approximately 106 CFU of L. monocytogenes or serovar Enteritidis in pure culture).
DISCUSSION
Despite their apparent promise as effective bacterial concentration agents in clinical, environmental, and food samples (8, 9), little is known about the exact chemical mechanism(s) whereby metal hydroxides are able to effectively immobilize bacterial cells. Kennedy et al. (10) reported that the dissolution of zirconium(IV) chloride in water results in a cation that polymerizes into a tetrameric complex, [Zr4(OH)8(H2O)16]8+. With increasing pH, the complex is able to polymerize further, resulting in multiple tetrameric complexes in which the zirconium ions are connected by hydroxide bridges. The resulting gelatinous precipitate (zirconium hydroxide) has been used to immobilize enzymes, most likely due to the formation of partial covalent bonds between the hydroxyl groups of the metal hydroxide and suitable amino acid side chains. Since these same ligands and many others are plentiful on the surface of the bacterial cell, it is likely that metal hydroxide complexes immobilize bacterial cells by the formation of partial covalent bonds with ligands on the surface of the bacterial cell wall (10).
Previous investigators have demonstrated that bacterial cells immobilized on metal hydroxides remain viable. For instance, Kennedy et al. (10) demonstrated that both prokaryotic (E. coli) and eukaryotic (Saccharomyces cerevisiae) cells continued to respire when immobilized by titanous or zirconium hydroxide. Using a related compound, Berry and Siragusa (3) reported that E. coli cells immobilized by hydroxyapatite remained viable as evaluated by fluorescent-staining techniques. Our results are consistent with these reports, demonstrating that representative gram-negative and gram-positive bacterial species remained viable after exposure to zirconium hydroxide for up to 48 h.
The observation that bacterial immobilization with metal hydroxides is nonspecific is also consistent with the reports of other investigators (3, 8, 9, 10). As with our data, these investigators failed to observe a significant effect on immobilization efficiency associated with the Gram reaction or cell morphology, frequently reporting bacterial recoveries exceeding 80 to 90% for a variety of gram-positive and gram-negative bacteria (3, 8, 9). All of these previous studies have assessed recoveries based on loss to the supernatant, and from this perspective, the recoveries reported in our study are equal to or better than those reported in previous papers. However, our study also demonstrates that viable cells can be recovered and enumerated directly from precipitates collected after metal hydroxide immobilization and low-speed centrifugation. In cases where recoveries based on direct plating of the immobilized pellet were on the low side, particularly when compared to calculations based on loss to the supernatant, we can hypothesize that bacterial adherence to tubes or bacterial clumping during centrifugation may be at least partially responsible. In instances where the bacterial recovery obtained by direct plating of the precipitated pellet was in excess of 100%, we suspect that the simultaneous immobilization of the indigenous microflora may be responsible. Efforts are under way to ascertain the cause of these variations in recovery and to further optimize metal hydroxide adsorption to effectively reduce sample volumes yet still maintain cellular viability, and hence detectability by cultural, immunological, and nucleic acid-based methodologies.
This study demonstrates that metal hydroxide immobilization may be a highly feasible approach to bacterial concentration in foods and one that offers significant advantages over other approaches currently under investigation. Although the most widely used bacterial-concentration method is centrifugation (5, 15, 17, 28), high centrifugal forces are generally necessary to effectively sediment cells. The use of metal hydroxides prior to centrifugation, as demonstrated in this work, enabled a reduction in centrifugal force, presumably due to an increase in the total mass of the bacterium-hydroxide complex as opposed to the uncomplexed bacterial cells. This reduction in centrifugation speed most likely reduces the tendency to coprecipitate PCR inhibitors and may also aid in maintaining cellular viability, facilitating direct detection of the bacteria in the precipitated floc by standard cultural procedures. Furthermore, the adsorption of bacteria to the surfaces of the metal hydroxides allows effective removal without the need for subsequent elution steps, since the immobilized cells remain viable and can be enumerated by culturing. The metal hydroxide method also circumvents problems that may be associated with filtration, such as clogged filters and simultaneous concentration of inhibitory compounds (18) or immunomagnetic separation, which is organism specific and expensive and requires small sample sizes (16, 25).
We are cautiously optimistic that the metal hydroxide immobilization procedure will also be adaptable to more complex dairy products. Recognizing that the reconstituted NFDM system is “ideal” with respect to minimizing PCR inhibitors and control of product composition, we are encouraged by the reasonably good recoveries and detection limits demonstrated. The method clearly worked for whole milk and ice cream, having achieved final RT-PCR detection levels similar to those for NFDM. It is important to note that the present study did not address the volume reductions necessary for practical application of the method to larger food sample sizes (≥25 ml or g) of these more complex dairy products. As we continue our efforts in this area, it is becoming increasingly clear that sample pretreatments to simultaneously remove PCR inhibitors and maintain bacterial cell viability will remain necessary (24, 33).
The detection limits reported in this work are equal to if not better than those reported by other investigators. When surveying the literature on PCR-based detection of food-borne pathogens, we found that detection limits rarely exceed 102 to 103 CFU/ml or g in samples that have not previously undergone culture enrichment (1, 27, 33). Detection limits of ≤1 CFU/ml or g have been reported after 8 to 48 h of culture enrichment (1, 27, 33). Only a few investigators have examined more than one form of bacterial concentration, applications to larger sample volumes, or elimination of enrichment steps (6, 7, 30). Most recently, Herman et al. (6, 7) reported a chemical extraction method that enabled the detection of as little as 1 CFU of L. monocytogenes cells or Clostridium tyrobutyricum spores from 25 ml of raw milk using a nested PCR approach. While our detection limits are not quite as good as those of Herman et al. (6, 7), we have purposely avoided nested PCR due to the potential for cross contamination, which increases the probability of false-positive results. Alternatively, we used an rRNA target for the nucleic acid amplification because of the high copy number of this molecule in viable cells (32). It was also chosen because of its relative instability, compared to DNA, when used as the target for nucleic acid amplification in food systems, thus reducing the likelihood of detecting dead cells (13, 14). Further work to refine the rRNA-based RT-PCR assay, specifically with respect to the detection of viable cells (14) should improve the overall detection limits of the assay, allowing sensitive detection of bacterial contamination without the need for prior culture enrichment. Such developmental efforts are under way in our laboratory.
Three major issues remain when considering the successful adaptation of PCR to the detection of food-borne pathogens, i.e., reduction of sample size, concentration of viable bacteria, and removal of PCR inhibitors. Immobilization of food-borne bacteria by metal hydroxides effectively addresses all of these issues. The method, when applied to 25-ml samples of reconstituted NFDM, resulted in a 50-fold sample concentration factor and recovery of 65 to 96% of the input bacteria. When coupled with an RNA extraction step, the final sample volume was reduced 1,000-fold, and the resulting RT-PCR detection limits for both L. monocytogenes and serovar Enteritidis were approximately 102 CFU/25 ml without prior culture enrichment. The immobilization step is rapid (<1 h), inexpensive ($0.50/sample), and simple, requiring no sophisticated equipment or personnel training. Since it is nonspecific and apparently results in the recovery of viable cells, it may also be applicable to sample preparation prior to the use of other rapid-detection methodologies, such as enzyme-linked immunosorbent assay. Furthermore, the use of bacterial concentration prior to conventional cultural procedures, such as enrichment or plating, may facilitate improved detection limits and reduced medium costs. We hope to explore some of these issues in future studies.
ACKNOWLEDGMENTS
This work was funded by the Southeast Dairy Foods Research Center, Dairy Management, Inc.; the North Carolina Dairy Foundation; the North Carolina Agricultural Foundation; and the North Carolina Agricultural Research Service.
We gratefully acknowledge Danielle Robins Datz for her help in the preparation of the manuscript.
Footnotes
This paper is number FSR-99-38 in the journal series of the Department of Food Science, North Carolina State University, Raleigh, NC 27695-7624.
REFERENCES
- 1.Bej A K, Mahbubani M H. Detection of food borne microbial pathogens. In: Griffin H G, Griffin A M, editors. PCR technology: current innovations. Boca Raton, Fla: CRC Press; 1994. pp. 341–365. [Google Scholar]
- 2.Bernhardt M, Pennell D R, Almer L S, Schell R F. Detection of bacteria in blood by centrifugation and filtration. J Clin Microbiol. 1991;29:422–425. doi: 10.1128/jcm.29.3.422-425.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berry E D, Siragusa G R. Hydroxyapatite adherence as a means to concentrate bacteria. Appl Environ Microbiol. 1997;63:4069–4074. doi: 10.1128/aem.63.10.4069-4074.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernandez-Astorga A, Hijarrubia M J, Lazaro B, Barcina I. Effect of the pre-treatments for milk samples filtration on direct viable cell counts. J Appl Bacteriol. 1996;80:511–516. doi: 10.1111/j.1365-2672.1996.tb03250.x. [DOI] [PubMed] [Google Scholar]
- 5.Fliss I, Emond E, Simard R E, Pandian S. A rapid and efficient method of lysis of Listeria and other gram-positive bacteria using mutanolysin. BioTechniques. 1991;11:453–457. [PubMed] [Google Scholar]
- 6.Herman L M, De Block J H G E, Waes G M S B J. A direct PCR detection method for Clostridium tyrobutyricum spores in up to 100 milliliters of raw milk. Appl Environ Microbiol. 1995;61:4141–4146. doi: 10.1128/aem.61.12.4141-4146.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herman L M F, DeBlock J H G E, Moermans R J B. Direct detection of Listeria monocytogenes in 25 milliliters of raw milk by a two-step PCR with nested primers. Appl Environ Microbiol. 1995;61:817–819. doi: 10.1128/aem.61.2.817-819.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ibrahim F, Lyons M J, Walker R A, Fleet G H. Immobilization of microorganisms for detection by solid-phase immunoassay. J Clin Microbiol. 1985;22:361–365. doi: 10.1128/jcm.22.3.361-365.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ibrahim G F, Lyons M J, Walker R A, Fleet G H. Rapid detection of Salmonellae by immunoassays with titanous hydroxide as the solid phase. Appl Environ Microbiol. 1985;50:670–675. doi: 10.1128/aem.50.3.670-675.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kennedy J F, Barker S A, Humphreys J D. Microbial cells living immobilized on metal hydroxides. Nature. 1976;261:242–244. doi: 10.1038/261242a0. [DOI] [PubMed] [Google Scholar]
- 11.Lantz P G, Tjerneld F, Borch E, Han-Hagerdal B, Radstrom P. Enhanced sensitivity of PCR detection of Listeria monocytogenes in soft cheese through use of an aqueous two-phase system as a sample preparation method. Appl Environ Microbiol. 1994;60:3416–3418. doi: 10.1128/aem.60.9.3416-3418.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin C K, Tsen H Y. Use of two 16S DNA targeted oligonucleotides as PCR primers for the specific detection of Salmonella in foods. J Appl Bacteriol. 1996;80:659–666. doi: 10.1111/j.1365-2672.1996.tb03271.x. [DOI] [PubMed] [Google Scholar]
- 13.McKillip J L, Jaykus L, Drake M. Nucleic acid persistence in heat-killed Escherichia coli O157:H7 from contaminated skim milk. J Food Prot. 1999;62:839–844. doi: 10.4315/0362-028x-62.8.839. [DOI] [PubMed] [Google Scholar]
- 14.McKillip J L, Jaykus L, Drake M. Ribosomal RNA stability in heat-killed and UV-irradiated enterotoxigenic Staphylococcus aureus and Escherichia coli O157:H7. Appl Environ Microbiol. 1998;64:4264–4268. doi: 10.1128/aem.64.11.4264-4268.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyer R, Luthy J, Candrian U. Direct detection by polymerase chain reaction (PCR) of Escherichia coli in water and soft cheese and identification of enterotoxigenic strains. Lett Appl Microbiol. 1991;13:268–271. [Google Scholar]
- 16.Muramatsu Y, Yanase T, Okabayashi T, Ueno H, Morita C. Detection of Coxiella burnetti in cow's milk by PCR-enzyme-linked immunosorbent assay combined with a novel sample preparation method. Appl Environ Microbiol. 1997;63:2142–2146. doi: 10.1128/aem.63.6.2142-2146.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neiderhauser C, Candrian U, Hofelein C, Jermini M, Buhler H P, Luthy J. Use of polymerase chain reaction for detection of Listeria monocytogenes in food. Appl Environ Microbiol. 1992;58:1564–1568. doi: 10.1128/aem.58.5.1564-1568.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oyofo B A, Rollins D M. Efficacy of filter types for detecting Campylobacter jejuni and Campylobacter coli in environmental water samples by polymerase chain reaction. Appl Environ Microbiol. 1993;59:4090–4095. doi: 10.1128/aem.59.12.4090-4095.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Payne M J, Campbell S, Patchett R A, Kroll R G. The use of immobilized lectins in the separation of Staphylococcus aureus, Escherichia coli, Listeria and Salmonella spp. from pure cultures and foods. J Appl Bacteriol. 1992;73:41–52. doi: 10.1111/j.1365-2672.1992.tb04967.x. [DOI] [PubMed] [Google Scholar]
- 20.Payne M J, Kroll R G. Methods for the separation and concentration of bacteria from foods. Trends Food Sci Technol. 1991;1991(Dec.):315–319. [Google Scholar]
- 21.Pedersen L H, Skouboe P, Rossen L, Rasmussen O F. Separation of Listeria monocytogenes and Salmonella berta from a complex food matrix by aqueous polymer two-phase partitioning. Lett Appl Microbiol. 1998;26:47–50. doi: 10.1046/j.1472-765x.1998.00275.x. [DOI] [PubMed] [Google Scholar]
- 22.Reid J R, Ng K H, Moore C H, Coolbear T, Pritchard G G. Comparison of bovine β-casein hydrolysis by PI and PIII-type proteinases from Lactobacillus lactis subsp. cremoris. Appl Microbiol Biotechnol. 1991;36:344–351. doi: 10.1007/BF00208154. [DOI] [PubMed] [Google Scholar]
- 23.Rosenfield S I. Development of reverse transcription polymerase chain reaction (RT-PCR) approaches to the detection of bacterial and viral foodborne disease agents. M.S. thesis. Raleigh: North Carolina State University; 1998. [Google Scholar]
- 24.Rossen L, Norskov P, Holmstrom K, Rasmussen O F. Inhibition of PCR by components of food samples, microbial diagnostic assays and DNA-extraction solutions. Int J Food Microbiol. 1992;17:37–45. doi: 10.1016/0168-1605(92)90017-w. [DOI] [PubMed] [Google Scholar]
- 25.Safarik I, Safarikova M. Use of magnetic techniques for the isolation of cells. J Chromatogr B. 1999;722:33–53. [PubMed] [Google Scholar]
- 26.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 27.Swaminathan B, Feng P. Rapid detection of food-borne pathogenic bacteria. Annu Rev Microbiol. 1994;48:401–426. doi: 10.1146/annurev.mi.48.100194.002153. [DOI] [PubMed] [Google Scholar]
- 28.Tjhie J H T, van Kuppeveld F J M, Roosendaal R, Melchers W J G, Gordijn R, MacLaren D M, Walboomers J M M, Meijer C J L M, van der Brule A J C. Direct PCR enables detection of Mycoplasma pneumoniae in patients with respiratory tract infections. J Clin Microbiol. 1994;32:11–16. doi: 10.1128/jcm.32.1.11-16.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turpin P E, Maycroft K A, Rowlands C L, Wellington E M H. An ion-exchange based extraction method for the detection of salmonellas in soil. J Appl Bacteriol. 1993;74:181–190. doi: 10.1111/j.1365-2672.1993.tb03013.x. [DOI] [PubMed] [Google Scholar]
- 30.Wang R F, Cao W W, Johnson M G. 16S rRNA based probes and polymerase chain reaction method to detect Listeria monocytogenes cells added to foods. Appl Environ Microbiol. 1992;58:2827–2831. doi: 10.1128/aem.58.9.2827-2831.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang R F, Cao W W, Johnson M G. Development of a 16S rRNA-based oligomer probe specific for Listeria monocytogenes. Appl Environ Microbiol. 1991;57:3666–3670. doi: 10.1128/aem.57.12.3666-3670.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waters A P, McCatchan T F. Ribosomal RNA: nature's own polymerase-amplified target for diagnosis. Parasitol Today. 1990;6:56–59. doi: 10.1016/0169-4758(90)90071-b. [DOI] [PubMed] [Google Scholar]
- 33.Wilson I G. Minireview: inhibition and facilitation of nucleic acid amplification. Appl Environ Microbiol. 1997;63:3741–3751. doi: 10.1128/aem.63.10.3741-3751.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]