Abstract
Background: Pancreatic surgery has been associated with important postoperative morbidity, mortality and prolonged length of hospital stay. In pancreatic surgery, the effect of poor preoperative nutritional status and muscle wasting on postsurgery clinical outcomes still remains unclear and controversial. Materials and Methods: A total of 103 consecutive patients with histologically proven carcinoma undergoing elective pancreatic surgery from June 2015 through to July 2020 were included and retrospectively studied. A multidimensional nutritional assessment was performed before elective surgery as required by the local clinical pathway. Clinical and nutritional data were collected in a medical database at diagnosis and after surgery. Results: In the multivariable analysis, body mass index (OR 1.25, 95% CI 1.04–1.59, p = 0.039) and weight loss (OR 1.16, 95% CI 1.06–1.29, p = 0.004) were associated with Clavien score I–II; weight loss (OR 1.13, 95% CI 1.02–1.27, p = 0.027) affected postsurgery morbidity/mortality, and reduced muscle mass was identified as an independent, prognostic factor for postsurgery digestive hemorrhages (OR 0.10, 95% CI 0.01 0.72, p = 0.03) and Clavien score I–II (OR 7.43, 95% CI 1.53–44.88, p = 0.018). No association was identified between nutritional status parameters before surgery and length of hospital stay, 30 days reintervention, 30 days readmission, pancreatic fistula, biliary fistula, Clavien score III–IV, Clavien score V and delayed gastric emptying. Conclusions: An impaired nutritional status before pancreatic surgery affects many postoperative outcomes. Assessment of nutritional status should be part of routine preoperative procedures in order to achieve early and appropriate nutritional support in pancreatic cancer patients. Further studies are needed to better understand the effect of preoperative nutritional therapy on short-term clinical outcomes in patients undergoing pancreatic elective surgery.
Keywords: pancreatic surgery, cancer, nutritional status, reduced muscle mass, sarcopenia, BMI, weight loss, malnutrition
1. Introduction
Despite the large number of efforts to enhance the efficacy of varied therapeutic opportunities over the past decade, pancreatic tumors are still one of the most deadly cancers, and five-year survival rates are currently within the range of 6% to 10% [1,2]. Worldwide, exocrine pancreatic cancer is the seventh leading cause of cancer death in both sexes [3].
Among pancreatic cancer patients, survival rates are much better in those who have undergone surgery than those who are unresectable [4]. Regrettably, less than 20% of pancreatic cancer patients will be eligible for resectable surgery [5]. This low resection rate is strongly linked to an advanced cancer stage, the location of the tumor, patients’ comorbidities and an impaired performance status [4]. Indeed, in pancreatic cancer patients, poor oral nutritional intake, catabolism due to malignancy and reduced intestinal absorption because of obstruction or exocrine insufficiency can synergically affect nutritional status and lead to malnutrition and loss of muscle mass [6]. These in turn worsen the patients’ nutritional and performance status and their suitability for surgery.
Pancreatic surgery has been associated with important postoperative morbidity, mortality and prolonged length of hospital stay [7,8,9,10], primarily linked to pancreatic anastomotic leak [11]. Even though technological advances in surgical techniques and perioperative management have greatly improved the mortality rate after pancreatic resection, postoperative morbidity continues to be a significant critical issue [12,13,14].
Pancreatic resection has been identified as one of the most complex surgical procedures as a result of the extended resection, the resulting metabolic stress and the comparatively high rate of complications. This specific kind of surgery strongly modifies metabolic activities and nutritional conditions by triggering inflammation, stress hormones and cytokines. In this specific clinical setting, nutritional status before surgery can also affect postsurgery clinical outcomes. Indeed, in cancer patients, impaired muscle mass before pancreatic surgery is associated with worse long-term survival [15,16,17,18]. However, the effect of preoperative poor nutritional status and muscle wasting on postoperative complications, in-hospital mortality and length of stay still remains unclear and controversial; furthermore, studies on heterogeneity and risk of bias limit the strength of this conclusion [16,17,18,19]. Further research in this area is needed to obtain a definitive answer.
Our retrospective observational study aims to investigate the association between nutritional status before pancreatic elective surgery and short-term clinical outcomes in cancer patients.
2. Materials and Methods
This single-center, retrospective study was approved by the local Ethics Committee (n◦: 67/2022/OSS/AOUMO), and all living patients provided written informed consent.
Patients with histologically proven carcinoma undergoing elective pancreatic surgery in University Hospital of Modena from June 2015 through July 2020 were consecutively included and retrospectively studied. No neoadjuvant chemotherapy was administered to enrolled patients. Nutritional assessment was performed before elective surgery as required by local clinical pathway. Oral food intake was assessed by 24 h recall in order to define energy and protein intake. A 24 h dietary recall (24HR) is a structured interview that aims to collect detailed informations and knowledges about all foods, beverages and oral nutritional supplements consumed by patients in the last 24 h. Food models, images and other visual aids were used to support patients in judging and describing volume of portions. Energy requirement was defined in line with European Society for Clinical Nutrition and Metabolism (ESPEN) guidelines on nutrition in cancer patients [20]. Before surgery, computed tomography (CT) scan was performed in order to stage cancer disease and define muscle mass. Diagnosis of cancer-related malnutrition (CRM) was detected in line with Global Leadership Initiative on Malnutrition (GLIM) criteria [21], which include three phenotypic criteria (unintentional weight loss, reduced body mass index and loss of muscle mass) and two etiologic criteria (inflammation and reduced energy intake or absorption). To diagnose malnutrition, at least one phenotypic and one etiologic criterion must be identified [21]. Phenotypic metrics for staging severity of malnutrition as Stage 1 (moderate) and Stage 2 (severe) were available [21].
Clinical and nutritional data were collected in medical records and the hospital electronic medical database at diagnosis and after surgery, including the following variables: age, gender, height, weight, body mass index (BMI), unintentional weight loss %, American Society of Anesthesiology (ASA) score, kind of surgery, operation time and vascular resection.
Postsurgery clinical outcomes (length of hospital stay, morbidity, in-hospital mortality, 30 days reintervention, 30 days readmission, pancreatic fistula, biliary fistula, delayed gastric emptying and digestive hemorrhage) were recorded for all patients. The Clavien score was collected in order to grade adverse events that occur as a result of surgical procedures.
2.1. Muscle Mass Measurement
The CT scans performed by the patients for pancreatic disease staging were also used for the evaluation of the body composition, and in particular for the muscle mass analysis. CT investigations were performed with two pieces of equipment: General Electric VCT 64 slice CT scanner (Milwaukee) and General Electric Optima 64 slice CT scanner (Milwaukee). For the reconstructions and the acquisition of the anthropometric parameters, a GE Healthcare AW Volume Share 7 workstation was used, with software that allows one to selectively visualize certain tissues, such as that of muscle, by setting threshold values of density typical of the tissue, and in this case between −29 and +150 Hounsfield units (HU).
Thanks to selective visualization, it was possible to perform a more precise segmentation of the skeletal muscle tissue at the level of the third lumbar vertebra (L3), in which both transverse processes were clearly visible. Areas of interest (ROI) were then drawn using a software tool corresponding to the compartments to be analyzed, within which the area expressed in cm2 and the average density value were calculated automatically.
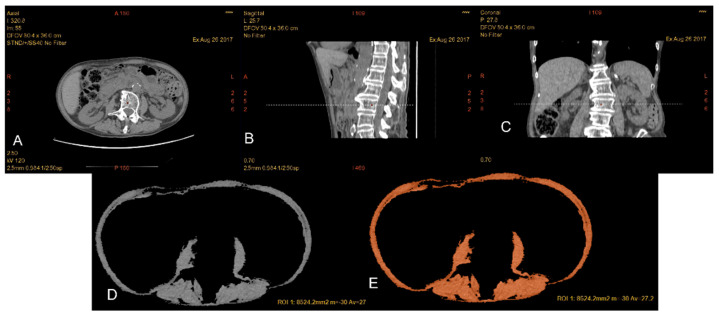
Total lumbar muscle area (TLA) (cm2), including paraspinal and abdominal wall muscles at the L3 level, was calculated by the software after manually tracing an ROI including the psoas muscles, paraspinal muscles (erector spine, quadratus lumborum, multifidus) and wall muscles (transversus, internal and external oblique, rectus abdominis). The skeletal muscle index (SMI) is the parameter obtained from the ratio between the total area of the lumbar muscles (TLA) and the square of the height (cm2/m2)Figure 1. It is an index of normalization of skeletal muscle mass with respect to the patient’s height. Reduced muscle mass was defined using default sex-specific SMI cutoff values: 52.4 cm2/m2 for men and 38.5 cm2/m2 for women [22].
Figure 1.
Patient with reduced muscle mass (A–E): in (A), axial CT image at the level of L3 as confirmed by the corresponding reference line in the sagittal (B) and coronal (C) planes. By applying the threshold −29/+ 150 HU, it is possible to selectively choose the muscle component and draw a ROI including all the musculature at the level of L3 (TLA). Automatically, it is possible to read the overall value of the traced area in cm2 and the average value of the density.
2.2. Statistical Analysis
Continuous variables were expressed using mean and standard deviation or median and interquartile range; binary and categorical data were reported as frequencies and percentages.
The associations between length of hospital stay and the patients’ characteristics were assessed using linear regression models, whereas logistic regression models were adopted to investigate the associations with respect to the other outcome measures. In the first place, for each outcome, we performed univariable analyses, and then, when appropriate, a multivariable model was estimated, considering all subjects with nonmissing data. The covariates included in the multivariable models were selected based on the results obtained from the univariable analysis and their clinical importance. In particular, for each outcome, all variables that were statistically associated with that outcome (nominal p-value less than 0.05) were selected; furthermore, the main clinical variables of this study, such as the reduced muscle mass indicator, were included. Subsequently, covariates with high association with respect to other covariates were excluded from the models to avoid issues of multicollinearity. Regarding propensity scores, they were used to estimate the probability that a subject has reduced muscle mass, holding other covariates constant. The selection of covariates for the propensity scores was carried out using the same methods described above. A multivariable logistic regression model was then estimated by including in the propensity score model that the variables potentially associated with reduced muscle mass.
Results were reported as the mean difference (MD) or odds ratio (OR) with 95% confidence intervals (CI). Analyses were carried out using R 4.2.1 statistical software.
3. Results
3.1. Patients’ Characteristics
A total of 103 consecutive patients with a confirmed diagnosis of carcinoma and treated with elective pancreatic surgery in the University Hospital of Modena from June 2015 to July 2020 were retrospectively selected and included in the study. The main characteristics of the enrolled patients are summarized in Table 1. The mean age was 68.7 (±11.2)years, and 59.2% were male. The ASA score was 2 in 61.6% of patients. Pancreatic adenocarcinoma was the most prevalent (76.6%) cancer diagnosis and Vater papilla adenocarcinoma was the second most frequent (10.5%) histological diagnosis. Concerning surgery, pancreaticoduodenectomy was the most common (61.2%) procedure. A vascular resection was performed in 21.2% of surgery. Mean operation time was about 430 min.
Table 1.
General characteristics.
| Number | Percentage | Mean | ±SD | ||
|---|---|---|---|---|---|
| Gender | Male | 61 | 59.2 | ||
| Female | 42 | 40.8 | |||
| Age | Years | 68.7 | 11.2 | ||
| ASA Score | 1 | 2 | 2 | ||
| 2 | 59 | 59.6 | |||
| 3 | 38 | 38.4 | |||
| Site of Cancer | Pancreatic adenocarcinoma |
79 | 76.6 | ||
| NET | 6 | 5.9 | |||
| Vater papilla carcinoma |
10 | 10.5 | |||
| Biliary carcinoma | 8 | 8 | |||
| Type of Surgery | Pancreaticoduodenectomy | 62 | 61.2 | ||
| Distal pancreasectomy | 6 | 5.8 | |||
| Total pancreasectomy | 35 | 34 | |||
| Operation Time | Minutes | 430 | 107.1 | ||
| Vascular Resection Performing | 21 | 21.2 | |||
Missing values were excluded from calculations. Abbreviations: ASA: American Society of Anesthesiologists; NET: neuroendocrine tumor; SD: standard deviation.
Regarding nutritional status before surgery, the mean BMI was 24.6 kg/m2 (DS ± 4.6), and unintentional weight loss was detected in 70.5% of the population. An unintentional weight loss higher than 5% was detected in 56.8% of our population. The mean SMI was 43.24 cm2/m2 (DS ± 13.3), the mean SMI in females was 39.89 cm2/m2 (DS ± 10.7) and the mean SMI in males was 48.24 cm2/m2 (DS ± 9.96). A condition of reduced muscle mass was observed in 56.3% of patients. A total of 48% of patients showed an energy oral intake of less than 75% of the daily energy nutritional requirement; differently52% of patients showed an energy oral intake of more than 75% of the daily energy nutritional requirement. Cancer-related malnutrition (CRM) was recognized in 75.7% of patients, in line with GLIM criteria (one phenotypic and one etiologic criterion). Nutritional parameters are summarized in Table 2.
Table 2.
Nutritional parameters before surgery.
| Number | Percentage | Mean | ±SD | ||
|---|---|---|---|---|---|
| BMI | kg/m2 | 24.6 | 4.6 | ||
| Unintentional Weight Loss % | No weight Loss | 28 | 29.5 | ||
| <5% | 13 | 13.7 | |||
| 5–10% | 22 | 23.2 | |||
| ≥10% | 32 | 33.6 | |||
| Oral Intake | >75% | 51 | 52 | ||
| 75–50% | 23 | 23.5 | |||
| <50% | 24 | 24.5 | |||
| SMI | Total—cm2/m2 | 43.24 | 13.34 | ||
| Female—cm2/m2 | 39.89 | 10.76 | |||
| Male—cm2/m2 | 48.24 | 9.96 | |||
| Reduced Muscle Mass | Yes | 58 | 56.3 | ||
| Diagnosis of Malnutrition (GLIM) | Yes | 78 | 75.7 | ||
Missing values were excluded from calculations. Abbreviations: BMI: body mass index; SMI: skeletal muscle index; GLIM: Global Leadership Initiative on Malnutrition.
Regarding postsurgery outcomes, the mean length of hospital stay was 16.2 (±11.8) days. We observed a prevalence of 73% (number 73) for postsurgery morbidity and 1.9% (number 2 events) for in-hospital mortality. A Clavien score of I–II was recognized in 53.5% (number 54 events) of patients, and the prevalence of digestive hemorrhage was 10% (number 10 events).
3.2. Role of Nutritional Status before Surgery
Our analysis was aimed at searching for clinical and nutritional prognostic parameters. Following adjustment for significantly prognostic covariates at the univariate analysis, a multivariable analysis was performed, which confirmed weight loss before surgery (OR 1.13, 95% CI 1.02–1.27, p = 0.027) and total pancreasectomy (OR 8.91, 95% CI 1.79–70.71, p = 0.016) as independent prognostic factors in terms of postsurgery morbidity/mortality (Table 3).
Table 3.
Multivariable analysis for the risk of adverse clinical outcomes.
| Length of Hospital Stay | Morbidity–Mortality | Clavien I–II | |||||
|---|---|---|---|---|---|---|---|
| Category | MD (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Age | 0.27 (−0.03; 0.56) |
0.083 | 1.01 (0.95; 1.08) |
0.694 | 1.02 (0.96; 1.08) |
0.558 | |
| Pancreaticoduodenectomy | 1 | Reference | Reference | Reference | |||
| Distal pancreasectomy | 2 | −7.88 (−22.16; 6.41) |
0.284 | 0.83 (0.06; 12.54) |
0.891 | 1.33 (0.09; 21.98) |
0.830 |
| Total pancreasectomy | 3 | 7.08 (0.83; 13.34) |
0.030 | 8.91 (1.79; 70.71) |
0.016 | 1.07 (0.30; 3.83) |
0.913 |
| BMI (kg/m2) | −0.14 (−0.84; 0.57) |
0.702 | 1.13 (0.99; 1.35) |
0.103 | 1.25 (1.04; 1.59) |
0.039 | |
| Unintentional weight loss % | −0.08 (−0.48; 0.32) |
0.705 | 1.13 (1.02; 1.27) |
0.027 | 1.16 (1.06; 1.29) |
0.004 | |
| ASA | 1–2 | Reference | Reference | Reference | |||
| ASA | 3 | 1.13 (−5.36; 7.62) |
0.734 | 0.35 (0.07; 1.57) |
0.177 | 0.70 (0.20; 2.43) |
0.575 |
| Operation time (min) | 0.01 (−0.02; 0.04) |
0.682 | 1.00 (0.99; 1.01) |
0.709 | 0.99 (0.99; 1.00) |
0.155 | |
| Vascular resection | No | Reference | Reference | Reference | |||
| Yes | 2.61 (−4.43; 9.65) |
0.470 | 0.74 (0.14; 4.24) |
0.720 | 1.35 (0.32; 6.02) |
0.685 | |
| Reduced muscle mass | No | Reference | Reference | Reference | |||
| Yes | −2.39 (−10.45; 5.68) |
0.564 | 2.28 (0.37; 18.03) |
0.395 | 7.43 (1.53; 44.88) |
0.018 | |
Abbreviations: MD: mean difference; CI: confidence interval; OR: odds ratio; BMI: body mass index; ASA: American Society of Anesthesiologists; min: minutes.
We also evaluated the prognostic impact of anthropometric measures before surgery. Following adjustment for significantly prognostic covariates at univariate analysis, a multivariable analysis was performed, which confirmed a significant and independent interaction between BMI (OR 1.25, 95% CI 1.04–1.59, p = 0.039), weight loss (OR 1.16, 95% CI 1.06–1.29, p = 0.004), reduced muscle mass (OR 7.43, 95% CI 1.53–44.88, p = 0.018) and Clavien score I–II. Table 3. Overall, no correlation was highlighted between nutritional status parameters before surgery and length of hospital stay, 30 days reintervention, 30 days readmission and pancreatic fistula (Table 3).
To define the association between reduced muscle mass and each outcome when the outcome’s characteristics did not meet the requirements to estimate the desirable multivariable model, propensity scores were performed to adjust possible differences in covariates. Loss of muscle mass before surgery was associated with postsurgery digestive hemorrhage (OR 0.10, 95%CI 0.01 0.72, p = 0.03) (Table 4). No association was identified between reduced muscle mass before surgery, pancreatic fistula, biliary fistula, delayed gastric emptying, Clavien Score III–V, 30 days reintervention and 30 days readmission (Table 4).
Table 4.
Multivariable analysis with propensity scores for the risk of adverse clinical outcomes.
| OR | 95% CI | p-Value | ||
|---|---|---|---|---|
| Pancreatic Fistula | ||||
| Reduced muscle mass | 1.79 | 0.35 | 10.88 | 0.50 |
| Propensity score | 0.28 | 0.02 | 2.92 | 0.30 |
| Biliary Fistula | ||||
| Reduced muscle mass | 0.41 | 0.01 | 18.69 | 0.62 |
| Propensity score | 4.51 | 0.01 | 5207.77 | 0.62 |
| Delayed Gastric Emptying | ||||
| Reduced muscle mass | 1.78 | 0.42 | 8.49 | 0.45 |
| Propensity score | 1.08 | 0.10 | 11.21 | 0.95 |
| Digestive Hemorrhage | ||||
| Reduced muscle mass | 0.10 | 0.01 | 0.72 | 0.03 |
| Propensity score | 101.86 | 3.07 | 10,309.36 | 0.02 |
| Clavien Score III IV | ||||
| Reduced muscle mass | 0.27 | 0.06 | 1.08 | 0.07 |
| Propensity score | 10.24 | 1.11 | 119.37 | 0.05 |
| Clavien Score V | ||||
| Reduced muscle mass | 0.55 | 0.01 | 27.12 | 0.74 |
| Propensity score | 2.13 | 0.01 | 1119.19 | 0.79 |
| 30 days Reintervention | ||||
| Reduced muscle mass | 2.14 | 0.43 | 13.45 | 0.38 |
| Propensity score | 2.14 | 0.16 | 31.86 | 0.57 |
| 30 days Readmission | ||||
| Reduced muscle mass | 0.40 | 0.06 | 2.75 | 0.35 |
| Propensity score | 1.03 | 0.05 | 19.74 | 0.98 |
Abbreviations: OR: odds ratio; CI: confidence interval.
4. Discussion
Pancreatic resection has been identified as one of the most complex surgical procedures as a result of the extended resection, the resulting metabolic stress and the comparatively high rate of complications. This specific kind of surgery strongly modifies metabolism and nutritional status by triggering inflammation, stress hormones and cytokines [23].
In order to support proper tissue healing and recovery or maintenance of organ functions after surgery, an effective anabolic response and adequate qualitative and quantitative nutritional substrates are required. Malnourished patients deplete their nutritional reserves quickly, which thereby affects their recovery and healing [23]. The development and progression of CRM can be associated with reduced oral nutritional intake and/or increased catabolism [24,25]. Recently, malnutrition has been defined through (one phenotypic and one etiologic criterion) weight loss, low body mass index, muscle wasting, poor energy intake and increased catabolism, in line with GLIM criteria [21].
In our study, the prevalence of CRM before surgery was very high (75.7%); as supposed by some preliminary publications [26,27,28], unintentional weight loss, low BMI, loss of muscle mass and Onodera’s prognostic nutrition index (PNI) have been identified as possible independent prognostic factors for several adverse clinical outcomes after pancreatic surgery.
In particular, BMI (OR 1.25, 95% CI 1.04–1.59, p = 0.039) and weight loss (OR 1.16, 95% CI 1.06–1.29, p = 0.004) were associated with Clavien score I–II, while weight loss before surgery (OR 1.13, 95% CI 1.02–1.27, p = 0.027) affected postsurgery morbidity/mortality.
Our results also highlight the effect of reduced muscle mass before pancreatic surgery on postoperative clinical outcomes, since muscle mass before surgery has been identified as an independent, negative prognostic factor for postsurgery digestive hemorrhages (OR 0.10, 95% CI 0.01 0.72, p = 0.03) and Clavien score I–II (OR 7.43, 95% CI 1.53–44.88, p = 0.018).
Many publications improperly define sarcopenia only as a condition of reduced muscle mass without performing muscle function measurements as required [29,30,31,32,33,34]. In addition, different tools are available for the assessment of sarcopenia, and the interpretation of results across studies is particularly difficult [16,34]. In pancreatic cancer patients, the impact of preoperative loss of muscle mass on the surgical outcome is still unclear and controversial [16,17,18,19], and available publications show several limitations. In particular, studies included patients receiving pancreatic surgery for both benign and malignant diseases, and not all studies used comparable parameters (different methods, tools and/or cutoffs) to define reduced muscle mass; moreover, a comprehensive nutritional assessment was not performed [16,17,18,19] as required [29,34]. Otherwise, in order to reduce the bias described above, in our study, all included patients had a histologically proven carcinoma, and a global assessment of nutritional status was achieved for each patient before elective pancreatic surgery to diagnose CRM. Furthermore, a quantitative CT analysis of muscle mass was performed by applying the most widely used cutoff for reduced muscle mass as parameter to define malnutrition as recommended [17,19,20,21,22,34]. Indeed, our study was carried out in a high-volume institution for pancreatic surgery, and all the main adverse clinical outcomes after pancreatic surgery were taken into account.
Unfortunately, the retrospective design of this study limits the strength of its conclusions, and for this reason, these findings definitely need to be confirmed in a larger prospective study. Our findings strongly support the relationship between poor nutritional status before pancreatic surgery and short-term adverse clinical outcomes, since not only CT-detected reduced muscle mass but also unintentional weight loss and BMI could negatively affect several short-term clinical outcomes in pancreatic surgery. Further research is needed to better evaluate the effect of severity of malnutrition before surgery on short-term clinical outcomes.
Although they overlap, sarcopenia, reduced muscle mass and CRM are different conditions, the term sarcopenia is unfortunately extensively used to define two different clinical situations: muscle wasting alone and reduced muscle mass associated with an impaired muscle function [29]. This is a significant source of doubts, confusion and mistakes in the research field and in many clinical settings.
Some recent studies have investigated the single effect of the depletion of skeletal muscle mass on short-term clinical outcomes after pancreatic surgery, achieving unclear and controversial results [15,16,17,18,19]. Our findings greatly highlight the need to take not only muscle wasting into account, but all the diagnostic parameters for malnutrition as required by GLIM criteria [21], as part of a nutritional assessment before elective pancreatic surgery.
It should also be remembered that a large number of nutritional assessment tools and scores are available to properly identify cancer patients with malnutrition in surgical settings. Nevertheless, it is still unclear which of these tools are the most appropriate and careful in predicting postoperative adverse outcomes in pancreatic cancer patients [35]. For a long time, hematological biomarkers of status of visceral proteins and liver function have also been used as indicators of impaired nutritional status. Nevertheless, the real predictive efficacy of these biomarkers still remains unclear [36]. Additional research is needed in this area.
Notably, it is strictly recommended that all cancer patients undergoing pancreatic surgery should receive an early, comprehensive and multidimensional evaluation of their nutritional status before elective surgery [23]. Our research supports the advice to assess nutritional status before and after major pancreatic surgery using a validated tool. A multidimensional and comprehensive nutritional assessment is required in order to detect early muscle wasting and/or malnutrition, in line with GLIM criteria, which include three phenotypic criteria (unintentional weight loss, reduced body mass index and loss of muscle mass) and two etiologic criteria (inflammation and reduced energy intake or absorption) [21].
In malnourished patients and in patients at risk of malnutrition, nutritional therapy should be started prior to major cancer surgery, even if operations must be delayed. A period from 7 to 14 days can be suitable [37].
In addition, after elective pancreatic surgery, adjuvant chemotherapy is often indicated to reduce the risk of cancer recurrence. A large number of available publications in this specific clinical setting have reported an impaired response, a reduced tolerance and worse survival rates in pancreatic cancer patients with reduced muscle mass [16]. In light of this, during pancreatic surgery, the early prevention of malnutrition and/or proper perioperative nutritional therapy for CRM is required in order to improve tolerance to antineoplastic therapy and clinical outcome.
In this situation, the introduction of a Nutritional Oncology Board (NOB) in daily practice, aimed at a multidisciplinary assessment of patients and at implementing an early nutritional therapy from oncological diagnosis onward seems to be the right path to take [6]. The NOB, sharing common experiences, goals, obstacles and unmet needs, can be an optimal fertile ground for the birth of collaborative research activities. Indeed, the NOB aims to enhance a shared pathway of care from both a clinical and an organizational point of view, and ideally to also improve awareness towards clinical nutrition [6].
5. Conclusions
Our findings highlight that an impaired nutritional status before pancreatic surgery can strongly affect many short-term postoperative outcomes. CRM is a well-known risk factor for surgery-related complications. In cancer patients, before and after pancreatic surgery, proper and appropriate recognition and management of CRM are central clinical concerns and warrant a specific and multidisciplinary (clinical nutrition, oncology, surgery) approach to improve clinical outcomes. The measurement of nutritional status supported by CT analysis of body composition parameters, especially the muscle component, should be a gold standard for preoperative assessment in order to achieve early and appropriate nutritional support.
Further studies are needed to better understand the effect of preoperative nutritional therapy on short-term clinical outcomes in patients undergoing elective pancreatic surgery.
Author Contributions
Conception and design: R.M., F.V., A.P. and R.C.C. Analysis and interpretation of data: all authors. Statistical analysis: R.D. and R.C.C. Supervision: R.M., A.P. and P.T. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The Ethical Review Board of each Institutional Hospital approved the present study. This study was performed in line with the principles of the Declaration of Helsinki.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are available on request from the authors.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Mizrahi J.D., Surana R., Valle J.W., Shroff R.T. Pancreatic cancer. Lancet. 2020;395:2008–2020. doi: 10.1016/S0140-6736(20)30974-0. [DOI] [PubMed] [Google Scholar]
- 2.Arnold M., Rutherford M.J., Bardot A., Ferlay J., Andersson T.M.L., Myklebust T.Å. Progress in cancer survival, mortality, and incidence in seven high-income countries 1995–2014 (ICBP SURVMARK-2): A population-based study. Lancet Oncol. 2019;20:1493–1505. doi: 10.1016/S1470-2045(19)30456-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 4.Ducreux M., Cuhna A.S., Caramella C., Hollebecque A., Burtin P., Goéré D., Seufferlein T., Haustermans K., Van Laethem J.L., Conroy T., et al. ESMO Guidelines Committee. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015;26((Suppl. S5)):v56–v68. doi: 10.1093/annonc/mdv295. [DOI] [PubMed] [Google Scholar]
- 5.Wolfgang C.L., Herman J.M., Laheru D.A., Klein A.P., Erdek M.A., Fishman E.K., Hruban R.H. Recent progress in pancreatic cancer. CA Cancer J. Clin. 2013;63:318–348. doi: 10.3322/caac.21190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rovesti G., Valoriani F., Rimini M., Bardasi C., Ballarin R., Di Benedetto F., Menozzi R., Dominici M., Spallanzani A. Clinical Implications of Malnutrition in the Management of Patients with Pancreatic Cancer: Introducing the Concept of the Nutritional Oncology Board. Nutrients. 2021;13:3522. doi: 10.3390/nu13103522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmidt C.M., Choi J., Powell E.S., Yiannoutsos C.T., Zyromski N.J., Nakeeb A., Pitt H.A., Wiebke E.A., Madura J.A., Lillemoe K.D. Pancreatic fistula following pancreaticoduodenectomy: Clinical predictors and patient outcomes. HPB Surg. 2009;2009:404520. doi: 10.1155/2009/404520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Addeo P., Delpero J.R., Paye F., Oussoultzoglou E., Fuchshuber P.R., Sauvanet A., Cunha A.S., Le Treut Y.P., Adham M., Mabrut J.Y., et al. Pancreatic fistula after a pancreaticoduodenectomy for ductal adenocarcinoma and its association with morbidity: A multicentre study of the French Surgical Association. HPB. 2014;16:46e55. doi: 10.1111/hpb.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Heek N.T., Kuhlmann K.F., Scholten R.J., de Castro S.M., Busch O.R., van Gulik T.M., Obertop H., Gouma D.J. Hospital volume and mortality after pancreatic resection—A systematic review and an evaluation of intervention in The Netherlands. Ann. Surg. 2005;242:781e90. doi: 10.1097/01.sla.0000188462.00249.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nimptsch U., Krautz C., Weber G., Mansky T., Grützmann R. Nationwide inhospital mortality following pancreatic surgery in Germany is higher than anticipated. Ann. Surg. 2016;264:1082e90. doi: 10.1097/SLA.0000000000001693. [DOI] [PubMed] [Google Scholar]
- 11.Uzunoglu F.G., Reeh M., Vettorazzi E., Ruschke T., Hannah P., Nentwich M.F., Vashist Y.K., Bogoevski D., König A., Janot M., et al. Preoperative Pancreatic Resection (PREPARE) score: A prospective multicenter-based morbidity risk score. Ann. Surg. 2014;260:857e64. doi: 10.1097/SLA.0000000000000946. [DOI] [PubMed] [Google Scholar]
- 12.Greenblatt D.Y., Kelly K.J., Rajamanickam V., Wan Y., Hanson T., Rettammel R., Winslow E.R., Cho C.S., Weber S.M. Preoperative factors predict perioperative morbidity and mortality after pancreaticoduodenectomy. Ann. Surg. Oncol. 2011;18:2126e35. doi: 10.1245/s10434-011-1594-6. [DOI] [PubMed] [Google Scholar]
- 13.DeOliveira M.L., Winter J.M., Schafer M., Cunningham S.C., Cameron J.L., Yeo C.J., Clavien P.A. Assessment of complications after pancreatic surgery: A novel grading system applied to 633 patients undergoing pancreaticoduodenectomy. Ann. Surg. 2006;244:931e9. doi: 10.1097/01.sla.0000246856.03918.9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Büchler M.W., Wagner M., Schmied B.M., Uhl W., Friess H., Z’graggen K. Changes in morbidity after pancreatic resection: Toward the end of completion pancreatectomy. Arch. Surg. 2003;138:1310e4. doi: 10.1001/archsurg.138.12.1310. [DOI] [PubMed] [Google Scholar]
- 15.Miligkos M., Wächter S., Manoharan J., Maurer E., Bartsch D.K. Sarcopenia and sarcopenic obesity are significantly associated with poorer overall survival in patients with pancreatic cancer: Systematic review and meta-analysis. Int. J. Surg. 2018;59:19–26. doi: 10.1016/j.ijsu.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 16.Chan M.Y., Chok K.S.H. Sarcopenia in pancreatic cancer—Effects on surgical outcomes and chemotherapy. World J. Gastrointest Oncol. 2019;11:527–537. doi: 10.4251/wjgo.v11.i7.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pierobon E.S., Moletta L., Zampieri S., Sartori R., Brazzale A.R., Zanchettin G., Serafini S., Capovilla G., Valmasoni M., Merigliano S., et al. The Prognostic Value of Low Muscle Mass in Pancreatic Cancer Patients: A Systematic Review and Meta-Analysis. J. Clin. Med. 2021;10:3033. doi: 10.3390/jcm10143033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bundred J., Kamarajah S.K., Roberts K.J. Body composition assessment and sarcopenia in patients with pancreatic cancer: A systematic review and meta-analysis. HPB. 2019;21:1603–1612. doi: 10.1016/j.hpb.2019.05.018. [DOI] [PubMed] [Google Scholar]
- 19.Ratnayake C.B., Loveday B.P., Shrikhande S.V., Windsor J.A., Pandanaboyana S. Impact of preoperative sarcopenia on postoperative outcomes following pancreatic resection: A systematic review and meta-analysis. Pancreatology. 2018;18:996–1004. doi: 10.1016/j.pan.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 20.Arends J., Bachmann P., Baracos V., Barthelemy N., Bertz H., Bozzetti F., Fearon K., Hütterer E., Isenring E., Kaasa S., et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017;36:11–48. doi: 10.1016/j.clnu.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 21.Cederholm T., Jensen G.L., Correia M.I.T.D., Gonzalez M.C., Fukushima R., Higashiguchi T., Baptista G., Barazzoni R. GLIM Core Leadership Committee; GLIM Working Group. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2019;38:1–9. doi: 10.1016/j.clnu.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Prado C.M., Lieffers J.R., McCargar L.J., Reiman T., Sawyer M.B., Martin L., Baracos V.E. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol. 2008;9:629–635. doi: 10.1016/S1470-2045(08)70153-0. [DOI] [PubMed] [Google Scholar]
- 23.Gianotti L., Besselink M.G., Sandini M., Hackert T., Conlon K., Gerritsen A., Griffin O., Fingerhut A., Probst P., Abu Hilal M., et al. Nutritional support and therapy in pancreatic surgery: A position paper of the International Study Group on Pancreatic Surgery (ISGPS) Surgery. 2018;164:1035–1048. doi: 10.1016/j.surg.2018.05.040. [DOI] [PubMed] [Google Scholar]
- 24.Fearon K., Strasser F., Anker S.D., Bosaeus I., Bruera E., Fainsinger R.L., Jatoi A., Loprinzi C., MacDonald N., Mantovani G., et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011;12:489–495. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 25.Cooper C., Burden S.T., Molassiotis A. An explorative study of the views and experiences of food and weight loss in patients with operable pancreatic cancer perioperatively and following surgical intervention. Support Care Cancer. 2015;23:1025–1033. doi: 10.1007/s00520-014-2455-1. [DOI] [PubMed] [Google Scholar]
- 26.Bozzetti F., Mariani L. Perioperative nutritional support of patients undergoing pancreatic surgery in the age of ERAS. Nutrition. 2014;30:1267–1271. doi: 10.1016/j.nut.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 27.Pausch T., Hartwig W., Hinz U., Swolana T., Bundy B.D., Hackert T., Grenacher L., Büchler M.W., Werner J. Cachexia but not obesity worsens the postoperative outcome after pancreatoduodenectomy in pancreatic cancer. Surgery. 2012;152:S81. doi: 10.1016/j.surg.2012.05.028. [DOI] [PubMed] [Google Scholar]
- 28.Kanda M., Fujii T., Kodera Y., Nagai S., Takeda S., Nakao A. Nutritional predictors of postoperative outcome in pancreatic cancer. Br. J. Surg. 2011;98:268–274. doi: 10.1002/bjs.7305. [DOI] [PubMed] [Google Scholar]
- 29.Cruz-Jentoft A.J., Bahat G., Bauer J., Boirie Y., Bruyère O., Cederholm T. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fielding R.A., Vellas B., Evans W.J., Bhasin S., Morley J.E., Newman A.B., Abellan van Kan G., Andrieu S., Bauer J., Breuille D., et al. Sarcopenia: An undiagnosed condition in older adults. Current consensus definition: Prevalence, etiology, and consequences. International working group on sarcopenia. J. Am. Med. Dir. Assoc. 2011;12:249–256. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muscaritoli M., Anker S.D., Argilés J., Aversa Z., Bauer J.M., Biolo G., Boirie Y., Bosaeus I., Cederholm T., Costelli P., et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: Joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin. Nutr. 2010;29:154–159. doi: 10.1016/j.clnu.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 32.Morley J.E., Abbatecola A.M., Argiles J.M., Baracos V., Bauer J., Bhasin S., Cederholm T., Coats A.J., Cummings S.R., Evans W.J., et al. Society on Sarcopenia, Cachexia and Wasting Disorders Trialist Workshop. Sarcopenia with limited mobility: An international consensus. J. Am. Med. Dir. Assoc. 2011;12:403–409. doi: 10.1016/j.jamda.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen L.K., Woo J., Assantachai P., Auyeung T.W., Chou M.Y., Iijima K., Jang H.C., Kang L., Kim M., Kim S., et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020;21:300–307.e2. doi: 10.1016/j.jamda.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 34.Cruz-Jentoft A.J., Gonzalez M.C., Prado C.M. Sarcopenia ≠ low muscle mass. Eur. Geriatr. Med. 2023;14:225–228. doi: 10.1007/s41999-023-00760-7. [DOI] [PubMed] [Google Scholar]
- 35.Probst P., Haller S., Bruckner T., Ulrich A., Strobel O., Hackert T., Diener M.K., Büchler M.W., Knebel P. Prospective trial to evaluate the prognostic value of different nutritional assessment scores in pancreatic surgery (NURIMAS Pancreas) Br. J. Surg. 2017;104:1053–1062. doi: 10.1002/bjs.10525. [DOI] [PubMed] [Google Scholar]
- 36.Lee J.L., Oh E.S., Lee R.W., Finucane T.E. Serum albumin and prealbumin in calorically restricted, nondiseased individuals: A systematic review. Am. J. Med. 2015;128:1023.e1–1023.e22. doi: 10.1016/j.amjmed.2015.03.032. [DOI] [PubMed] [Google Scholar]
- 37.Weimann A., Braga M., Carli F., Higashiguchi T., Hübner M., Klek S., Laviano A., Ljungqvist O., Lobo D.N., Martindale R.G., et al. ESPEN practical guideline: Clinical nutrition in surgery. Clin. Nutr. 2021;40:4745–4761. doi: 10.1016/j.clnu.2021.03.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on request from the authors.