Abstract
Background and Aim: This study aimed to compare the efficacy and safety of endoscopic ultrasound-guided gallbladder drainage and percutaneous transhepatic gallbladder drainage as a bridge to surgery in patients with acute cholecystitis unfit for urgent cholecystectomy. Methods: This retrospective study included 46 patients who underwent cholecystectomy following endoscopic ultrasound-guided gallbladder drainage (EUS-GBD) or percutaneous transhepatic gallbladder drainage (PTGBD) for acute cholecystitis in NTT Tokyo Medical Center. We surveyed 35 patients as the EUS-GBD group and 11 patients as the PTGBD group, and compared the rate of technical success of the cholecystectomy and periprocedural adverse events. A 7-F, 10-cm double pigtail plastic stent was used for ultrasound-guided gallbladder drainage. Results: The rate of technical success of cholecystectomy was 100% in both groups. Regarding postsurgical adverse events, no significant difference was noted between the two groups (EUS-GBD group, 11.4%, vs. PTGBD group, 9.0%; p = 0.472). Conclusions: EUS-GBD as a BTS seems to be an alternative for patients with AC because it can ensure lower adverse events. On the other hand, there are two major limitations in this study––the sample size is small and there is a risk of selection bias.
Keywords: EUS drainage, acute cholecystitis, bridge to surgery
1. Introduction
Cholecystectomy is the curative treatment for acute cholecystitis (AC). Early cholecystectomy is mandatory for AC; however, emergency cholecystectomy for AC is associated with high morbidity (20–30%) and mortality (6–30%) rates in patients with significant comorbidities [1,2,3]. As a result, some surgeons prefer non-surgical procedures as makeshift treatments, such as antibiotic administration with/without percutaneous/endoscopic drainage, as an alternative to emergent cholecystectomy. However, elective surgery may lead to several complications, including empyema, gangrene, perforation, pericholecystitis with abscess formation, peritonitis, and sepsis [4,5]. Emergent surgery may not be safe and practical in patients with high surgical risk [6]. Percutaneous transhepatic gallbladder drainage (PTGBD) has been performed as a bridge for delayed surgical treatment in vulnerable patients with high surgical risk. The presence of a drainage tube may increase the risk of an adverse event during surgery by 16.2% to 25% [7,8,9]. Recently, endoscopic ultrasound-guided gallbladder drainage (EUS-GBD) has gained attention as a treatment for internal drainage of the gallbladder in high-risk patients [10,11,12,13,14,15,16,17,18]. Although PTGBD followed by late laparoscopic cholecystectomy for high-risk patients has been accepted as the standard procedure [19,20,21], there are limitations of PTGBD, such as inconvenience for patients and risk of its dislocation. However, there are no reports on alternatives to PTGBD focusing on the bridge to surgery (BTS). Thus, the present study aimed to validate the efficacy and safety of EUS-GBD as a BTS in patients with AC who are considered unfit for urgent cholecystectomy.
2. Materials and Methods
2.1. Study Design
This was a retrospective study conducted between April 2016 and July 2021. This study protocol was approved by the Institutional Review Board (ID18-313) of our institute. The study was investigator-initiated and conducted according to the ethical principles of the Declaration of Helsinki. Written informed consent was obtained from all patients.
2.2. Patients
Patients with a diagnosis of AC admitted to our institute between April 2016 and July 2021 were retrospectively identified. The diagnosis of AC was made using a combination of patient history, physical examination, laboratory analysis, and imaging (abdominal ultrasonography, computed tomography, and magnetic resonance imaging), and based on the Tokyo Guideline 2018 [22]. Patients with common bile duct stones were excluded because they had concurrent cholangitis. Patients were divided into two groups: one group who underwent cholecystectomy following EUS-GBD during the period from April 2019 to June 2021, and another group who underwent cholecystectomy following PTGBD during the period from April 2016 to June 2018.
2.3. Procedures
2.3.1. EUS-GBD
EUS-GBD was performed by endoscopists who had performed over 500 interventional-EUS procedures and over 500 therapeutic ERCP procedures. Endoscopists used an oblique-viewing, curved-linear array echoendoscope (GF-UCT260 or GF-UCT240; Olympus Medical Systems, Tokyo, Japan) and a dedicated processor (ME-1/2; Olympus Medical Systems). The gallbladder was depicted by ultrasound imaging from the duodenal bulb or gastric antrum and punctured using a 19-gauge fine aspiration needle (EZ Shot 3 Plus; Olympus Medical Systems). Thereafter, a 0.025-inch guidewire (VisiGlide2; Olympus Medical Systems) was inserted into the gallbladder lumen, and the tract was dilated using a 4-mm balloon catheter with a tapered tip (REN; Kaneka Corporation, Tokyo, Japan). Finally, a 7-Fr 10-cm double-pigtail plastic stent (DPPS) (Through & Pass DP; Gadelius Medical K.K, Tokyo, Japan) was placed in the gallbladder through the duodenal bulb or gastric antrum (Figure 1). The inclusion criteria were: obvious cholecystitis identified by the presence of gallstones, no gallbladder perforation, and the provision of written informed consent.
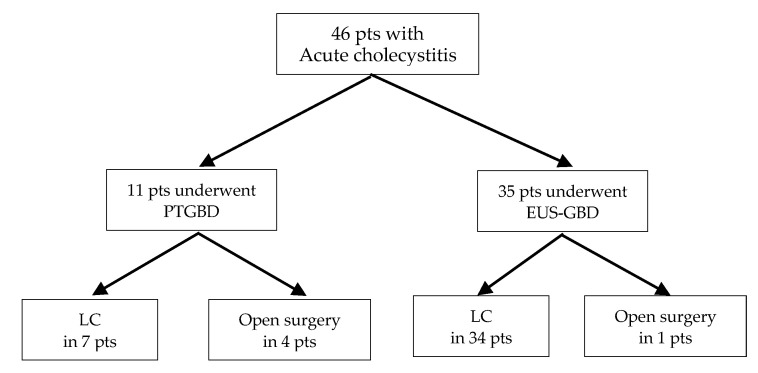
Figure 1.
Result of analyzed patients (pts) with acute cholecystitis.
2.3.2. PTGBD
PTGBD was performed under local anesthesia by trained interventional radiologists in the interventional suite. A transhepatic route was used in all patients, and a 7-Fr pigtail drainage catheter (Hanako Medical Co., Ltd., Saitama, Japan) was placed between the seventh or eighth intercostal space under combined sonographic and fluoroscopic guidance.
2.4. Follow-Up
All patients underwent plain abdominal radiography and laboratory tests the day after the procedure and leading up to the surgery. Oral diet was started when clinical symptoms improved without any severe adverse events. DPPS was kept in place without periodical exchange until the surgery.
2.5. Laparoscopic Cholecystectomy
Laparoscopic cholecystectomy was performed for eligible patients at least 1 month after EUS-GBD. The previous day before the surgery, the DPPS was endoscopically removed. The surgery was performed under general anesthesia using a standard four-trocar technique. Surgeons identified the enterocholecysto fistula, which was then immediately cut using a stapler. If the laparoscopic surgery was difficult to complete, conversion to open cholecystectomy was performed at the operator’s discretion. All laparoscopic cholecystectomy procedures were performed by one hepatobiliary pancreatic surgeon who had previously performed more than 500 laparoscopic cholecystectomies.
Difficult laparoscopic cholecystectomy (DLC) was defined as a procedure with an operative time ≥ 3 h, bleeding volume ≥ 300 mL common bile duct injury, partial cholecystectomy, the need for a second surgeon, and/or conversion to open surgery [22].
2.6. Outcomes
The primary outcome was technical success of the cholecystectomy after EUS-GBD. Technical success was defined as successful gallbladder removal during cholecystectomy without complications. Clinical success was defined as clinical improvement (resolution of fever, decrease in white blood cell count, and resolution of pain and tenderness) within 72 h after the procedure. The secondary outcome was periprocedural adverse events including prolonged surgical time after cholecystectomy.
2.7. Statistical Analysis
Data are summarized as mean ± standard deviation for continuous data and as frequency and percentages for categorical data. For continuous data, characteristics and outcomes of the two groups were compared using the student’s t-test or Mann–Whitney U test based on the viability of the normality assumption. The Chi-squared or Fisher’s exact test was used to compare the two groups with regard to categorical characteristics and outcomes. The level of significance was set at a two-sided p-value < 0.05. Statistical analysis was performed using BellCurve for Excel (Social Survey Research Information Co., Ltd., Tokyo, Japan).
3. Results
3.1. Patient Characteristics
In this period, 46 patients were included in this study (Figure 1): 35 patients underwent EUS-GBD (62.9% male; average age, 69.2 ± 13.4 years) and 11 patients underwent PTGBD (90.9% male; average age, 72.4 ± 12.2 years), followed by cholecystectomy. No statistical differences were found in age, sex, or body mass index between the two groups (Table 1). The etiology of cholecystitis was gallstone disease (n = 35, 100%) in the EUS-GBD group and gallstone disease (n = 10, 81.8%), acalculous disease (n = 1, 9.1%), and gallbladder cancer (n = 1, 9.1%) in the PTGBD group (p = 0.005). No significant differences were noted regarding baseline diseases, advanced cancers (p = 0.721), cerebrovascular disorder (p = 0.912), or cardiopulmonary disease (p = 0.886) between the two groups. The severities for cholecystitis were moderate (n = 33, 94.3%) and severe (n = 2, 5.7%) in the EUS-GBD group and moderate (n = 11, 100%) in the PTGBD group. Cholecystectomy was proposed for all patients at the initial diagnosis for AC; however, if the surgeons, endoscopists, and radiologists regarded these patients as unsuitable surgical candidates, either EUS-GBD or PTGBD was performed.
Table 1.
Patient characteristics.
| Variable | EUS-GBD (n = 35) | PTGBD (n = 11) | p Value |
|---|---|---|---|
| Age (years) | 69.2 ± 13.4 (34–88) | 72.4 ± 12.2 (44–82) | 0.900 |
| Sex (male/female) | 22/13 | 10/1 | 0.052 |
| BMI | 24.2 ± 3.8 (15–32.8) | 22.9 ± 2.6 (18.9–25.8) | 0.286 |
| Etiology of cholecystitis | |||
| Gallstone | 35 (100) | 9 (81.8) | 0.005 |
| Acalculous | 0 | 1 (9.0) | |
| Gallbladder cancer | 0 | 1 (9.0) | |
| Underlying conditions | |||
| Baseline disease | |||
| Advanced cancer | 6 (17.1) | 3 (27.3) | 0.721 |
| Cerebrovascular disorder | 2 (5.7) | 1 (9.1) | 0.912 |
| Cardiopulmonary disease | 8 (22.9) | 2 (18.2) | 0.886 |
| ASA-PS I | 5 (14.3) | 1 (9.0) | 0.445 |
| ASA-PS II | 28 (80.0) | 6 (54.5) | 0.094 |
| ASA-PS III | 2 (57.1) | 3 (27.3) | 0.272 |
| ASA-PS IV | 0 | 1 (9.0) | |
| Severity of cholecystitis (based on Tokyo guideline 2018) | |||
| Moderate | 33 (94.3) | 11 (100) | 0.201 |
| Severe | 2 (5.7) | 0 |
Numbers are shown in number (%) or average ± SD (range); EUS-GBD, endoscopic ultrasound-guided gallbladder drainage; PTGBD, percutaneous transhepatic gallbladder drainage; BMI, body mass index; ASA-PS, American Society of Anesthesiologists physical status; SD, standard deviation.
3.2. Primary Outcome
Clinical success of gallbladder drainage was achieved in 100% of patients in the EUS-GBD group and 81.8% of patients in the PTGBD group; two patients in the PTGBD group exhibited catheter dislodgement. No significant difference was observed regarding the duration from drainage to cholecystectomy between the two groups (p = 0.512).
Technical success of cholecystectomy was achieved in 100% of patients in both groups (Table 2). All patients in the EUS-GBD group underwent laparoscopic cholecystectomy, and only one (2.9%) patient required conversion to open surgery. In the PTGBD group, eight patients (72.7%) underwent laparoscopic cholecystectomy, three patients (27.3%) underwent open cholecystectomy, and one patient (12.5%) required conversion to open cholecystectomy. The number of patients who required conversion was not statistically different between the two groups (p = 0.400). No significant differences were noted regarding operation time (p = 0.707), estimated blood loss (p = 0.493), or duration from operation to discharge (p = 0.541) between the two groups.
Table 2.
Comparison of drainage procedure outcomes.
| Variable | EUS-GBD (n = 35) | PTGBD (n = 11) | p Value |
|---|---|---|---|
| Technical success of gallbladder drainage | 35 (100) | 11 (100) | |
| Clinical success of gallbladder drainage | 35 (100) | 9 (81.8) | 0.005 |
| Procedure time (min) | 25.1 ± 9.2 (13–52) | No record | |
| Time from drainage to cholecystectomy (days) | 86.7 ± 113.7 (29–632) | 62.0 ± 87.8 (7–308) | 0.512 |
| Technical success of cholecystectomy | 35 (100) | 11 (100) | |
| Type of cholecystectomy | |||
| Laparoscopic | 35 (100) | 8 (72.7) | 0.002 |
| Open | 0 | 3 (27.3) | |
| Laparoscopic converted to open | 1 (2.9) | 1 (12.5) | 0.4 |
| Operating time (min) | 171.9 ± 71.7 (46–368) | 182.0 ± 53.5 (110–302) | 0.707 |
| Estimated blood loss (ml) | 75.5 ± 99.5 (5–400) | 103.2 ± 130.8 (10–440) | 0.493 |
| Time from operation to discharge (days) | 5.4 ± 2.5 (3–14) | 6.5 ± 2.8 (3–13) | 0.541 |
Numbers are shown as number (%) or average ± SD (range); EUS-GBD, endoscopic ultrasound-guided gallbladder drainage; PTGBD, percutaneous transhepatic gallbladder drainage; SD, standard deviation.
3.3. Secondary Outcome
Postsurgical adverse events were observed in four patients (11.4%) in the EUS-GBD group and in one patient (9.0%) in the PTGBD group; no significant differences were found between the two groups (p = 0.472) (Table 3). In the EUS-GBD group, four patients suffered from abscesses that were managed by adjusting the position of the drain placed at the time of cholecystectomy. In the PTGBD group, the single adverse event was postoperative heart failure, managed with medication.
Table 3.
Comparison of adverse events.
| Variable | EUS-GBD (n = 35) | PTGBD (n = 11) | p Value |
|---|---|---|---|
| Post procedural adverse events | 6 (17.1) | 3 (27.2) | 0.361 |
| Types of adverse events | |||
| Recurrent cholecystitis | 0 | 1 (9.0) | 0.035 |
| Drain dislodging | 0 | 2 (18.2) | 0.005 |
| Peritonitis | 6 (17.1) | 0 | 0.071 |
| Patients requiring repeat procedure | 0 | 0 | |
| Postsurgical adverse events | 4 (11.4) | 1 (9.0) | 0.472 |
| Recurrent biliary events | 0 | 0 | |
| Abscess | 4 (11.4) | 0 | 0.418 |
Numbers are shown as number (%) or average ± SD (range); EUS-GBD, endoscopic ultrasound-guided gallbladder drainage; PTGBD, percutaneous transhepatic gallbladder drainage; SD, standard deviation.
4. Discussion
This paper indicated that EUS-GBD could be an alternative to PTGBD as a BTS. Ryu’s meta-analysis and systematic review reported EUS-GBD was comparable with PTGBD regarding clinical success, with less reintervention and readmission, for acute cholecystitis with high surgical risk [23]. However, postprocedural adverse events, which could be conservatively managed, occurred in 6 of 35 patients (17.1%) in the EUS-GBD group; controllable peritonitis occurred in all patients. As bile leak reportedly occurs in one in eight (12.5%) patients with DPPS [24], the rate of bile leak in this study (17.1%) was relatively high. Although a 4-mm balloon catheter was used in all patients in our study, a high rate of bile leak may have occurred due to the use of this catheter, and leakage after the dilation procedure was convertibly countered. On the other hand, postprocedural adverse events occurred in 3 of 11 patients (27.2%) in the PTGBD group, and 2 of these patients exhibited drain dislodging. Bile leak peritonitis can be treated conservatively with antibiotics, but drain dislodging is a serious adverse event. This suggests that EUS-GBD is an acceptable method for BTS in terms of adverse events. In the PTGBD group, one patient had gallbladder cancer as the etiology of acute cholecystitis. The length of time from operating to discharge for this patient was 5 days. In addition, this patient did not require conversion to open. Therefore, in our report, gallbladder cancer was not affected by length of time from operating to discharge and conversion to open.
Moreover, concerning difficult LC (DLC), the rate of DLCs was relatively high compared to a previous paper [25] (45.7% vs. 26.3%). In other reports, 3 of 12 (25%) patients and 2 of 23 (9%) patients required conversion to open cholecystectomy [26,27]; in our study, only 1 of 35 (2.9%) patients required conversion. Thus, LC led EUS-GBD could be endured when it comes to patients with DLC.
Jang et al. [27] reported rates of conversion to open cholecystectomy after EUS-GBD had an adverse effect on laparoscopic cholecystectomy and showed that EUS-GBD did not cause severe inflammation or adhesion to surrounding gallbladder tissue; however, this study only included surgical candidates, and cholecystectomy was performed after a median of 5 days after EUS-GBD. In our study, cholecystectomy was performed as elective surgery based on the results of Altieri et al. [28], who revealed that a duration of ≤8 weeks (n = 1211) was associated with a higher overall rate of complications.
A well-timed LC 8 weeks after EUS-GBD would be preferable, since the inflammation would be ameliorated, ensuring better surgical outcomes [28]. In our study, the duration from drainage to cholecystectomy in the EUS-GBD group was 86.7 days; this was >8 weeks and longer than the duration in the PTGBD group. However, in the report by Altieri et al., the average time to cholecystectomy was 203 days in the >8 weeks group [28]. Therefore, the rate of DLC in our study could be lower if the waiting period for the surgery was lowered. All patients in our study demonstrated moderate or mild adhesions and fibrosis during surgery; nevertheless, surgery was performed safely, and despite the presence of adhesions and fibrosis, only one patient required conversion to open cholecystectomy. This also indicated that the inflammation due to EUS-GBD can be a surmountable event for experienced laparoscopic surgeons. The EUS-GBD group showed moderate and mild adhesions and fibrosis in all of the patients, yet despite these adhesions and fibrosis, as far as we can observe, there are no long-term postoperative complications such as upper gastrointestinal obstruction in the two groups.
Adverse events (AEs) due to drainage present an independent risk for postsurgical adverse events. In our study, peritonitis and drain dislodging were the most common postprocedural AEs, with bile leak closely related to these events. Bile leak may make cholecystectomy difficult due to the severe adhesion around the gallbladder and enterocholecysto fistula; thus, to minimize the risk of bile leak in EUS-GBD, lumen-apposing metal stent (LAMS) is used. EUS-GBD using LAMS is becoming a widely accepted therapeutic approach for gallbladder drainage with high clinical and technical success rates and low rates of adverse events, as shown by several studies [29]; however, it is only covered by insurance for pancreatic pseudocyst and walled-off necrosis in Japan. Therefore, although plastic stents were used in the EUS-GBD group in our study, it may be that LAMS provides more safety during the procedure [29].
In one previous report, AC had clinical particularities in aged patients with an increased rate of postoperative complications [30]. We obtained the same result in our study. In an aging society, PTGBD is a routine procedure; however, dislocation would be critical for patients with AC. Indeed, drain migration is reported in 0.3–12% of patients [1,31,32,33,34]; besides, EUS-GBD in our study resulted in few cases of drain migration. Therefore, EUS-GBD will be safer and more reliable in the future. EUS-GBD would be more patient-friendly than the PTGBD without dislocation and inconvenience.
A review conducted by Lee et al. [35] revealed that nine patients demonstrated rapid clinical improvement within 72 h after EUS-GBD. Elective laparoscopic cholecystectomy was eventually performed in seven patients and was successful in six patients, and transduodenal cholecystostomy was converted to open cholecystectomy in one patient (14.3%) without complication. The rate of technical success of cholecystectomy was 100% in the report of both Lee et al. and our own report, whereas the rates of conversion to open cholecystectomy were 14.3% and 2.9%; thus, both studies demonstrate that LC following EUS-GBD was safe.
This study had some limitations. First, this was a retrospective study. Doctors’ treatment preferences may have resulted in a bias. The decision to PTGBD or EUS drainage was made at the discretion of the surgeons, endoscopists, and radiologists, and it may have led to a selection bias. Furthermore, due to the characteristics of our hospital in this study, the proportion of patients with underlying medical conditions was high, so the population may be slightly different from the usual acute cholecystitis patients. This may limit the generalizability of the study.
Second, the sample size of PTGBD patients included was small. In Ryu’s meta-analysis and systematic review, reported EUS-GBD was associated with fewer adverse events than PTGBD [23]. However, in our study, post procedural adverse events were observed in six patients (17.1%) in the EUS-GBD group and in three patients (27.2%) in the PTGBD group; no significant differences were found between the two groups (p = 0.472). However, no significant difference is seen, although that does not mean there are no differences between EUS-GBD and PTGBD. Hence, randomized controlled trials or non-inferiority trials with more patients should be planned to prove the present results.
Third, our study was conducted by only one expert hepatobiliary pancreatic surgeon; therefore, it may not be valid to generalize our results across other centers, as the surgeons may have varying levels of clinical experience and familiarity with cholecystectomy for high-risk patients with acute cholecystitis. Hence, larger prospective studies are required to confirm our results. Third, since LAMS cannot be used for EUS-GBD in Japan, we hope that a global study using LAMS will be conducted in the future.
In conclusion, this paper indicated that EUS-GBD could be an alternative to PTGBD as a BTS. However, further studies are needed to confirm this.
Author Contributions
Conceptualization, Y.F.; methodology, Y.K.; software, E.S.; validation, S.T.; formal analysis, Y.K.; investigation, Y.F.; resources, Y.F.; data curation, Y.K.; writing—original draft preparation, K.I.; writing—review and editing, K.I.; visualization, A.N. (Atsuki Nagao); supervision, K.H.; project administration, T.T.; funding acquisition, A.N. (Atsushi Nakajima) and K.K. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This was a retrospective study conducted between April 2016 and July 2021. This study protocol was approved by the Institutional Review Board (ID18-313 in January 2016) of our institute. The study was investigator-initiated and conducted according to the ethical principles of the Declaration of Helsinki.
Informed Consent Statement
Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Glenn F. Cholecystostomy in the high risk patient with biliary tract disease. Ann. Surg. 1977;185:185–191. doi: 10.1097/00000658-197702000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Margiotta S.J., Jr., Willis I.H., Wallack M.K. Cholecystectomy in the elderly. Am. Surg. 1988;54:34–39. doi: 10.1016/S0002-9610(88)80541-5. [DOI] [PubMed] [Google Scholar]
- 3.Edlund G., Ljungdahl M. Acute cholecystitis in the elderly. Am. J. Surg. 1990;159:414–416. doi: 10.1016/S0002-9610(05)81285-1. [DOI] [PubMed] [Google Scholar]
- 4.Lai P.B.S., Kwong K.H., Leung K.L., Kwok S.P.Y., Chan A.C.W., Chung S.C.S., Lau W.Y. Randomized trial of early versus delayed laparoscopic cholecystectomy for acute cholecystitis. Br. J. Surg. 1998;85:764–767. doi: 10.1046/j.1365-2168.1998.00708.x. [DOI] [PubMed] [Google Scholar]
- 5.Weigelt J.A., Norcross J.F., Aurbakken C.M. Cholecystectomy after tube cholecystostomy. Am. J. Surg. 1983;146:723–726. doi: 10.1016/0002-9610(83)90327-6. [DOI] [PubMed] [Google Scholar]
- 6.De Geus T., Moriarty H.K., Waters P.S., O’Reilly M.K., Lawler L., Geoghegan T., Conneely J., McEntee G., Farrelly C. Outcomes of patients treated with upfront cholecystostomy for severe acute cholecystitis. Surg. Laparosc. Endosc. Percutan. Tech. 2020;30:79–84. doi: 10.1097/SLE.0000000000000747. [DOI] [PubMed] [Google Scholar]
- 7.Kortram K., de Vries Reilingh T.S., Wiezer M.J., van Ramshorst B., Boerma D. Percutaneous drainage for acute calculous cholecystitis. Surg. Endosc. 2011;25:3642–3646. doi: 10.1007/s00464-011-1771-5. [DOI] [PubMed] [Google Scholar]
- 8.Winbladh A., Gullstrand P., Svanvik J., Sandström P. Systematic review of cholecystostomy as a treatment option in acute cholecystitis. HPB. 2009;11:183–193. doi: 10.1111/j.1477-2574.2009.00052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGillicuddy E.A., Schuster K.M., Barre K., Suarez L., Hall M.R., Kaml G.J., Davis K.A., Longo W.E. Non-operative management of acute cholecystitis in the elderly. Br. J. Surg. 2012;99:1254–1261. doi: 10.1002/bjs.8836. [DOI] [PubMed] [Google Scholar]
- 10.Tyberg A., Saumoy M., Sequeiros E.V., Giovannini M., Artifon E., Teoh A., Nieto J., Desai A., Kumta N.A., Gaidhane M., et al. EUS-guided versus percutaneous gallbladder drainage: Isn’t it time to convert? J. Clin. Gastroenterol. 2018;52:79–84. doi: 10.1097/MCG.0000000000000786. [DOI] [PubMed] [Google Scholar]
- 11.Irani S., Ngamruengphong S., Teoh A., Will U., Nieto J., Abu Dayyeh B.K., Gan S.I., Larsen M., Yip H.C., Topazian M.D., et al. Similar efficacies of endoscopic ultrasound gallbladder drainage with a lumen-apposing metal stent versus percutaneous transhepatic gallbladder drainage for acute cholecystitis. Clin. Gastroenterol. Hepatol. 2017;15:738–745. doi: 10.1016/j.cgh.2016.12.021. [DOI] [PubMed] [Google Scholar]
- 12.Teoh A.Y.B., Serna C., Penas I., Chong C.C.N., Perez-Miranda M., Ng E.K.W., Lau J.Y.W. Endoscopic ultrasound-guided gallbladder drainage reduces adverse events compared with percutaneous cholecystostomy in patients who are unfit for cholecystectomy. Endoscopy. 2017;49:130–138. doi: 10.1055/s-0042-119036. [DOI] [PubMed] [Google Scholar]
- 13.Choi J.H., Kim H.W., Lee J.-C., Paik K.-H., Seong N.J., Yoon C.J., Hwang J.-H., Kim J. Percutaneous transhepatic versus EUS-guided gallbladder drainage for malignant cystic duct obstruction. Gastrointest. Endosc. 2017;85:357–364. doi: 10.1016/j.gie.2016.07.067. [DOI] [PubMed] [Google Scholar]
- 14.Dollhopf M., Larghi A., Will U., Rimbaş M., Anderloni A., Sanchez-Yague A., Teoh A.Y.B., Kunda R. EUS-guided gallbladder drainage in patients with acute cholecystitis and high surgical risk using an electrocautery-enhanced lumen-apposing metal stent device. Gastrointest. Endosc. 2017;86:636–643. doi: 10.1016/j.gie.2017.02.027. [DOI] [PubMed] [Google Scholar]
- 15.Choi J.H., Lee S.S., Choi J.H., Park D.H., Seo D.W., Lee S.K., Kim M.H. Long-term outcomes after endoscopic ultrasonography-guided gallbladder drainage for acute cholecystitis. Endoscopy. 2014;46:656–661. doi: 10.1055/s-0034-1365720. [DOI] [PubMed] [Google Scholar]
- 16.de la Serna-Higuera C., Pérez-Miranda M., Gil-Simón P., Ruiz-Zorrilla R., Diez-Redondo P., Alcaide N., Val L.S.-D., Nuñez-Rodriguez H. EUS-guided transenteric gallbladder drainage with a new fistula-forming, lumen-apposing metal stent. Gastrointest. Endosc. 2013;77:303–308. doi: 10.1016/j.gie.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 17.Kamata K., Takenaka M., Kitano M., Omoto S., Miyata T., Minaga K., Yamao K., Imai H., Sakurai T., Watanabe T., et al. Endoscopic ultrasound-guided gallbladder drainage for acute cholecystitis: Long-term outcomes after removal of a self-expandable metal stent. World J. Gastroenterol. 2017;23:661–667. doi: 10.3748/wjg.v23.i4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan S.M., Teoh A.Y.B., Yip H.C., Wong V.W.Y., Chiu P.W.Y., Ng E.K.W. Feasibility of per-oral cholecystoscopy and advanced gallbladder interventions after EUS-guided gallbladder stenting (with video) Gastrointest. Endosc. 2017;85:1225–1232. doi: 10.1016/j.gie.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 19.McKay A., Abulfaraj M., Lipschitz J. Short- and long-term outcomes following percutaneous cholecystostomy for acute cholecystitis in high-risk patients. Surg. Endosc. 2012;26:1343–1351. doi: 10.1007/s00464-011-2035-0. [DOI] [PubMed] [Google Scholar]
- 20.Akyürek N., Salman B., Yüksel O., Tezcaner T., İrkörücü O., Yücel C., Oktar S., Tatlicioğlu E. Management of acute calculous cholecystitis in high-risk patients: Percutaneous cholecystotomy followed by early laparoscopic cholecystectomy. Surg. Laparosc. Endosc. Percutan. Tech. 2005;15:315–320. doi: 10.1097/01.sle.0000191619.02145.c0. [DOI] [PubMed] [Google Scholar]
- 21.Patterson E.J., McLoughlin R.F., Mathieson J.R., Cooperberg P.L., MacFarlane J.K. An alternative approach to acute cholecystitis. Percutaneous cholecystostomy and interval laparoscopic cholecystectomy. Surg. Endosc. 1996;10:1185–1188. doi: 10.1007/s004649900275. [DOI] [PubMed] [Google Scholar]
- 22.Kiriyama S., Kozaka K., Takada T., Strasberg S.M., Pitt H.A., Gabata T., Hata J., Liau K.-H., Miura F., Horiguchi A., et al. Tokyo Guidelines 2018, diagnostic criteria and severity grading of acute cholangitis (with videos) J. Hepatobiliary Pancreat. Sci. 2018;25:17–30. doi: 10.1002/jhbp.512. [DOI] [PubMed] [Google Scholar]
- 23.Lyu Y., Li T., Wang B., Cheng Y., Chen L., Zhao S. Ultrasound-Guided Gallbladder Drainage Versus Percutaneous Transhepatic Gallbladder Drainage for Acute Cholecystitis with High Surgical Risk: An Up-to-Date Meta-Analysis and Systematic Review. J. Laparoendosc. Adv. Surg. Tech. A. 2021;11:1232–1240. doi: 10.1089/lap.2020.0786. [DOI] [PubMed] [Google Scholar]
- 24.Song T.J., Park D.H., Eum J.B., Moon S.-H., Lee S.S., Seo D.W., Lee S.K., Kim M.-H. EUS-guided cholecystoenterostomy with single-step placement of a 7F double-pigtail plastic stent in patients who are unsuitable for cholecystectomy: A pilot study (with video) Gastrointest. Endosc. 2010;71:634–640. doi: 10.1016/j.gie.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 25.Maehira H., Kawasaki M., Itoh A., Ogawa M., Mizumura N., Toyoda S., Okumura S., Kameyama M. Prediction of difficult laparoscopic cholecystectomy for acute cholecystitis. J. Surg. Res. 2017;216:143–148. doi: 10.1016/j.jss.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 26.Matsubara S., Isayama H., Nakai Y., Kawakubo K., Yamamoto N., Saito K., Saito T., Takahara N., Mizuno S., Kogure H., et al. Endoscopic ultrasound-guided gallbladder drainage with a combined internal and external drainage tubes for acute cholecystitis. J. Gastroenterol. Hepatol. 2020;35:1821–1827. doi: 10.1111/jgh.15065. [DOI] [PubMed] [Google Scholar]
- 27.Jang J.W., Lee S.S., Song T.J., Hyun Y.S., Park D.H., Seo D., Lee S., Kim M., Yun S. Endoscopic ultrasound-guided transmural and percutaneous transhepatic gallbladder drainage are comparable for acute cholecystitis. Gastroenterology. 2012;142:805–811. doi: 10.1053/j.gastro.2011.12.051. [DOI] [PubMed] [Google Scholar]
- 28.Altieri M.S., Yang J., Yin D., Brunt L.M., Talamini M.A., Pryor A.D. Early cholecystectomy (≤ 8 weeks) following percutaneous cholecystostomy tube placement is associated with higher morbidity. Surg. Endosc. 2020;34:3057–3063. doi: 10.1007/s00464-019-07050-z. [DOI] [PubMed] [Google Scholar]
- 29.Zain A., Christina L., Tarun R. Endoscopic Ultrasound-Guided Gallbladdar Drainage. Dig. Dis. Sci. 2021;66:2154–2161. doi: 10.1007/s10620-020-06520-y. [DOI] [PubMed] [Google Scholar]
- 30.Serban D., Socea B., Balasescu S., Badiu C., Tudor C., Dascalu A., Vancea G., Spataru R., Sabau A., Sabau D., et al. Safety of laparoscopic cholecystectomy for acute cholecystitis in the elderly: A multivariate analysis of risk factors for intra and postoperative complications. Medicina. 2021;57:230. doi: 10.3390/medicina57030230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kiviniemi H., Mäkelä J.T., Autio R., Tikkakoski T., Leinonen S., Siniluoto T., Perälä J., Päivänsalo M., Merikanto J. Percutaneous cholecystostomy in acute cholecystitis in high-risk patients: An analysis of 69 patients. Int. Surg. 1998;83:299–302. [PubMed] [Google Scholar]
- 32.Sugiyama M., Tokuhara M., Atomi Y. Is percutaneous cholecystostomy the optimal treatment for acute cholecystitis in the very elderly? World J. Surg. 1998;22:459–463. doi: 10.1007/s002689900416. [DOI] [PubMed] [Google Scholar]
- 33.Ito K., Fujita N., Noda Y., Kobayashi G., Kimura K., Sugawara T., Horaguchi J. Percutaneous cholecystostomy versus gallbladder aspiration for acute cholecystitis: A prospective randomized controlled trial. AJR Am. J. Roentgenol. 2004;183:193–196. doi: 10.2214/ajr.183.1.1830193. [DOI] [PubMed] [Google Scholar]
- 34.Hatzidakis A.A., Prassopoulos P., Petinarakis I., Sanidas E., Chrysos E., Chalkiadakis G., Tsiftsis D., Gourtsoyiannis N.C. Acute cholecystitis in high-risk patients: Percutaneous cholecystostomy vs. conservative treatment. Eur. Radiol. 2002;12:1778–1784. doi: 10.1007/s00330-001-1247-4. [DOI] [PubMed] [Google Scholar]
- 35.Lee S.S., Park D.H., Hwang C.Y., Ahn C.-S., Lee T.Y., Seo D.-W., Lee S.K., Kim M.-W. EUS-guided transmural cholecystostomy as rescue management for acute cholecystitis in elderly or high-risk patients: A prospective feasibility study. Gastrointest. Endosc. 2007;66:1008–1012. doi: 10.1016/j.gie.2007.03.1080. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.