Abstract
Glioblastoma (GBM) is the most common malignant primary brain tumor and confers a dismal prognosis. With only two FDA-approved therapeutics showing modest survival gains since 2005, there is a great need for the development of other disease-targeted therapies. Due, in part, to the profound immunosuppressive microenvironment seen in GBMs, there has been a broad interest in immunotherapy. In both GBMs and other cancers, therapeutic vaccines have generally yielded limited efficacy, despite their theoretical basis. However, recent results from the DCVax-L trial provide some promise for vaccine therapy in GBMs. There is also the potential that future combination therapies with vaccines and adjuvant immunomodulating agents may greatly enhance antitumor immune responses. Clinicians must remain open to novel therapeutic strategies, such as vaccinations, and carefully await the results of ongoing and future trials. In this review of GBM management, the promise and challenges of immunotherapy with a focus on therapeutic vaccinations are discussed. Additionally, adjuvant therapies, logistical considerations, and future directions are discussed.
Keywords: glioblastoma, GBM, vaccine, dendritic cell, adjuvant
1. Background
Glioblastoma (GBM) is the most common malignant primary brain tumor in adults, representing 14.2% of all central nervous system (CNS) tumors and 50.1% of all malignant tumors [1]. GBM is associated with a high symptom burden and a wide range of neurological symptoms, including cognitive deficits, focal weakness, headaches, and seizures, depending on the tumor’s location [2]. The median overall survival (mOS) is poor at 15 months, despite maximal standard of care therapies [3,4,5]. Only 6.9% of patients survive five years post-diagnosis [1]. This has been unchanged at the population level since 2011 [6]. Despite extensive research, since 2005, only the following two U.S. Food and Drug Administration (FDA)-approved therapeutics have been shown to confer a survival benefit for GBM [7]: the oral chemotherapy agent temozolomide [8] and the tumor-treating fields (TTF) device Optune [9].
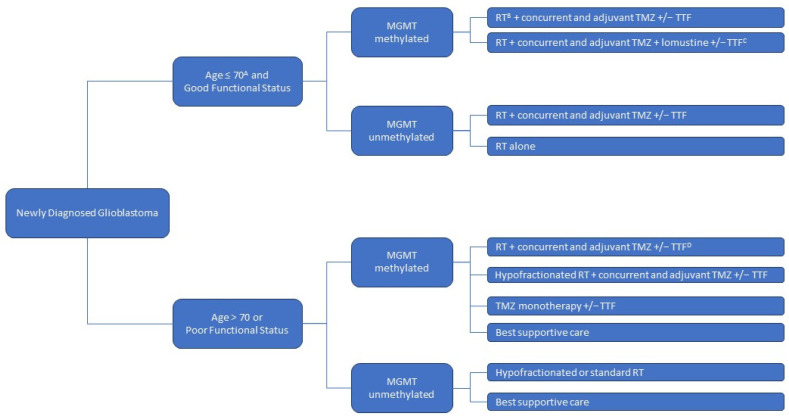
The standard of care management for newly diagnosed GBMs begins with a maximal safe surgical resection, followed by radiation therapy with concurrent and adjuvant temozolomide, with or without TTF [7,10,11]. The treatment is tailored according to the patient’s age, performance status, and tumor molecular profile [7]. The standard course of radiation treatment is administered over six weeks [10]. Temozolomide is typically administered concurrently with radiation and in six adjuvant cycles [10]. For patients with a good performance status and O6-methylguanine-DNA methyltransferase (MGMT)-methylated tumors, the addition of lomustine to their treatment with radiation and temozolomide may confer additional benefits [12]. In elderly patients or patients with a poor functional status, abbreviated courses of radiation therapy can be considered [13]. In similar patients with MGMT-methylated tumors, temozolomide monotherapy is an accepted option [14]. The approach to treatment for newly diagnosed GBMs is summarized in Figure 1. Since none of these treatments is curative, clinical trial enrollment should be considered for all patients [7]. Unfortunately, recurrence is almost inevitable for all patients [1]. The median time to the first recurrence after the diagnosis of GBM is 7 months [10]. To date, there is no proven therapy for improving survival in this setting, despite hundreds of clinical trials since the early 2000s [6,7,15]. The therapeutic options for recurrent diseases include the following: clinical trials, further alkylating chemotherapy (i.e., lomustine/CCNU or temozolomide rechallenge), bevacizumab, and regorafenib, with some selected cases of off-label use with immune checkpoint inhibitors, small-molecule-targeted therapy (i.e., EGFR inhibitors), or re-irradiation, with variable practice patterns across institutions [7].
Figure 1.
Approach to newly diagnosed glioblastoma treatments, following maximal safe surgical resection. MGMT = O6-methylguanine-DNA methyltransferase; RT = radiation therapy; TMZ = temozolomide; TTF = tumor-treating fields. A: Elderly status is defined as ≥65 in certain studies. B: A standard radiation therapy course is administered over 6 weeks. C: For MGMT-methylated patients with a good performance status, the addition of lomustine to the standard treatment with radiation therapy and temozolomide, with or without tumor-treating fields, may provide additional benefits (see text). D: In selected elderly patients with a good performance status, standard 6-week radiation therapy with concurrent and adjuvant temozolomide can be considered (see text).
The reasons for this poor therapeutic response are multifaceted [16]. GBM has well-documented intertumoral and intratumoral heterogeneity that serves as a foundation for resistance to therapy [17,18,19,20]. Glioblastoma was the first systematically studied cancer type as part of The Cancer Genome Atlas Research Network (TCGA), initially leading to the identification of proneural, neural, mesenchymal, and classical molecular tumor subtypes [21,22]. Further studies led to the characterization of three subtypes (proneural, mesenchymal, and classical), with a proneural to mesenchymal transition also described for recurrent tumors [23]. Proneural tumors were initially characterized by platelet-derived growth factor receptor alpha (PDGFRA) and isocitrate dehydrogenase (IDH) mutations [24]. IDH mutant tumors were later grouped separately under a more recent World Health Organization (WHO) tumor classification [24,25]. The mesenchymal and classical subtypes were characterized by neurofibromatosis 1 (NF1) mutations and epidermal growth factor receptor (EGFR) amplifications, respectively [24]. The TCGA studies provided tremendous insight into the intertumoral GBM heterogeneity, demonstrating the presence of different driver mutations for different tumors and raising the possibility of rational molecularly based and highly individualized treatments [23]. Unfortunately, to date, subtype classification has not translated into effective treatment stratifications or improved outcomes, in part due to intratumoral heterogeneity and phenotype switching [26,27]. Traditional chemotherapy and targeted molecular therapeutic options may only work on a subpopulation of the tumor and, ultimately, negatively select for resistant clonal subpopulations [26] or cause phenotype switching, a phenomenon whereby tumor cells transition their invasiveness and/or differentiation [27]. The normal brain architecture itself also serves as a challenge. Even if a promising systemic therapy option is identified, its ability to cross the blood–brain barrier [28] and penetrate within the brain and tumor itself remains a challenge [29,30], such that there has been recent focus on novel strategies (i.e., ultrasound) to open the blood–brain barrier for drug delivery [31]. Among the most important factors in GBM’s resistance to treatment in an immunotherapeutic era is a profound local and systemic immunosuppressive state [32,33,34].
2. Immunosuppressive State and Immune-Targeted Therapies
Several mechanisms contribute to GBM immune evasion, including increased PD-L1 expression [35], release of cytokines (IL-10, IL-6, and TGF-beta1), and, potentially, downregulation of MHC expression [36,37]. This is compounded by aspects related to the tumor microenvironment, including tumor-associated microglia/macrophages, secretion of protumorigenic/survival factors (IL-10 and IDO), vascular-related factors (VEGF, FGF, and pericyte proliferation), and release of immunosuppressive extracellular vesicles [36,38,39]. The GBM microenvironment is increasingly recognized as an important factor in the development of promising therapeutics for GBM. Out of the scope of the present review, several excellent reviews discuss the implications of the GBM microenvironment on the lack of therapeutic success, especially with immunotherapy [40,41,42]. Despite the fact that GBM tumors rarely metastasize outside the central nervous system, patients with GBM exhibit profound systemic immunosuppression, characterized by decreased overall T-cell numbers and function [43] combined with increased circulating immunosuppressive leukocytes, including regulatory T cells and myeloid-derived suppressor cells [44,45,46]. Given the role of immune dysfunction in GBM and the paucity of therapies improving longevity, there has been great interest in developing immune-targeted therapies for GBM [36,38]. Potential therapies fall into the following key classes: (1) immune checkpoint inhibitors (i.e., pembrolizumab, nivolumab, and ipilimumab), (2) T-cell-targeted therapy (i.e., CAR-T), (3) oncolytic viral therapies (i.e., adenovirus leading to cell lysis/death, specifically targeting GBM cell populations), (4) therapeutic vaccines, and (5) cytokine-based therapies [38,47,48,49,50]. These approaches are thoroughly reviewed in a recent manuscript from our group [47]. The focus of the present manuscript will be on therapeutic vaccines for GBM.
There has been great interest in harnessing active tumor-specific immune responses as a disease-targeting therapy for cancers [51], in addition to the non-specific reduction in tumor-mediated immunosuppression. For example, therapeutic cancer vaccines expose antigen-presenting cells to cancer-specific antigens, thereby inducing a cytotoxic T-cell response and ultimately cancer cell death [52,53,54]. However, there are only a few FDA-approved cancer therapeutic vaccines, despite extensive preclinical development and numerous clinical trials [53]. Bacillus Calmette-Guérin (BCG) is approved for patients with early-stage bladder cancer [55,56]. Sipuleucel-T is a dendritic cell (DC)-based vaccine approved in 2010 for use in men with metastatic castration-resistant prostate cancer [57]. Talimogene laherparepvec T-VEC (Imlygic®) was approved for the treatment of metastatic melanomas [58]. Given the promise of immune therapy in targeting a broad spectrum of malignancies, there has been great interest in the development of a therapeutic vaccine for GBM [59,60].
3. Therapeutic Vaccines
Therapeutic cancer vaccines may be categorized based on the target antigen and vehicle or mechanism of delivery. Peptide vaccines are by far the most studied antigen platform in GBM [52,53,61]. Peptides consist of amino acid chains, which are ultimately presented to T cells in lymphoid tissues by DC, priming an immune response. The peptide antigen targets vary in size and number (single or multipeptide) [53,62]. There is also interest in nucleic acid vaccines based on bacterial DNA plasmids and mRNA [63,64]. Once delivered, DNA plasmids enter the nucleus, whereby the target antigen is transcribed and expressed on the target cell through MHC class I/II presentation, generating innate and adaptive immune responses [63]. mRNA vaccines consist of single-stranded mRNA transcripts that encode antigen(s) of interest, which are incorporated into antigen-presenting cells (APCs) that are subsequently activated to generate an immune response, similar to DNA plasmid vaccines [64].
GBM vaccine delivery is often accomplished through DCs [61]. DCs are APCs with an important role in normal immune function [65]. For vaccine development, DCs are exposed to an antigen of interest ex vivo and matured prior to injection. The DCs then migrate to lymphoid tissues, leading to the activation of T cells and other immune mediators. This prompts the immune system to target the antigen of interest, in this case, one common to the primary tumor [66]. Heat-shock proteins (HSPs) [67] are less commonly used vaccine vehicles in GBMs. HSPs are produced in response to various stressors to mitigate the downstream effects of misfolded protein production by assisting protein refolding. If the functional proteins cannot be refolded, HSPs chaperone these misfolded proteins to proteasomes for destruction [67,68]. In vaccine development, HSPs are combined with the peptide of interest, which leads to a T-cell response [69].
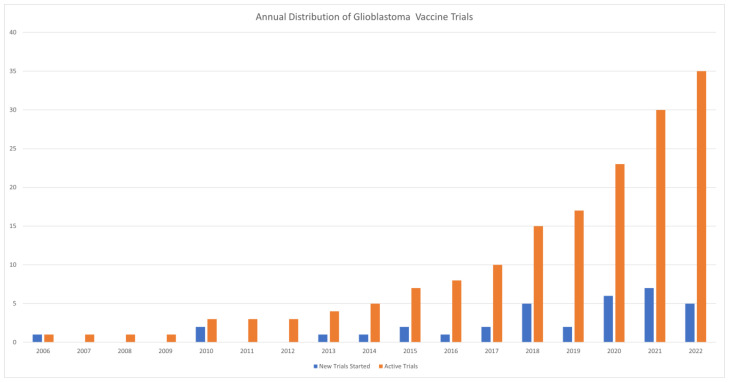
In this review, a selection of the most promising therapeutic vaccines in GBM are discussed, with a complete list of ongoing and future clinical trials shown in Table 1 (as of January 2023; listed on clinicaltrials.gov). The annual distribution of GBM vaccine trials is presented in Figure 2.
Table 1.
Active, recruiting, or soon-to-be-recruiting clinical trials investigating therapeutic vaccinations for glioblastomas.
| ID | Phase | Design | Patients Enrolled | Diagnosis | Vaccine | Delivery | Adjuvant | Control | Primary Endpoint | Age | Status | Start | Completion |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NCT03149003 | Phase 3 | Rand | 236 | rGBM | DSP-7888 | ID | Bevacizumab | Bevacizumab + SOC | DLT, OS | 18+ | Active, not recruiting | 8 December 2017 | 1 November 2023 |
| NCT05100641 | Phase 3 | Rand | 726 | nGBM | AV-GBM-1 | Not listed | GM-CSF | Autologous monocytes | OS | 18+ | Not yet recruiting | 1 March 2022 | 1 March 2027 |
| NCT04277221 | Phase 3 | Rand | 118 | rGBM | ADCTA | SC | Bevacizumab | Bevacizumab + SOC | OS | 18–70 | Unknown status | 19 September 2019 | 31 December 2022 |
| NCT02455557 | Phase 2 | Single Arm | 66 | nGBM | SurVaxM | SC | Montanide ISA 51 and Sargramostim | - | PFS6 | 18+ | Active, not recruiting | 4 May 2015 | 30 December 2023 |
| NCT01204684 | Phase 2 | Rand | 60 | n/rGBM | ATL-DC | ID | Resiquimod or Poly-ICLC | - | Most effective combination | 18–70 | Active, not recruiting | 8 October 2010 | 31 January 2025 |
| NCT03400917 | Phase 2 | Single Arm | 55 | nGBM | AV-GBM-1 | Not listed | GM-CSF | - | OS | 18–70 | Active, not recruiting | 20 June 2018 | 1 February 2023 |
| NCT04523688 | Phase 2 | Single Arm | 28 | GBM | ATL-DC | ID | - | - | PFS, AEs | 18+ | Not yet recruiting | 1 March 2021 | 1 December 2025 |
| NCT04888611 | Phase 2 | Rand | 40 | rGBM | GSC-DCV | Not listed | Camrelizumab | Camrelizumab alone | OS, PFS | 18–70 | Recruiting | 26 October 2021 | 1 May 2024 |
| NCT02465268 | Phase 2 | Rand | 175 | nGBM | CMV-DC | SC | GM-CSF | Autologous monocytes | OS | 18+ | Recruiting | 1 August 2016 | 1 June 2024 |
| NCT04280848 | Phase 2 | Non-Rand | 56 | nGBM | UCPVax | SC | - | - | Immunogenicity | 18–75 | Recruiting | 26 May 2020 | 1 May 2023 |
| NCT03395587 | Phase 2 | Rand | 136 | nGBM | ATL-DC | ID | - | SOC | OS | 18+ | Recruiting | 6 March 2018 | 6 June 2025 |
| NCT05163080 | Phase 2 | Rand | 265 | nGBM | SurVaxM | SC | Montanide and Sargramostim | Montanide and Sargramostim | OS | 18+ | Recruiting | 18 November 2021 | 18 April 2024 |
| NCT03382977 | Phase 1/2 | Non-Rand | 98 | rGBM | VBI-1901 | ID | GM-CSF | SOC | DLT, AEs | 18+ | Active, not recruiting | 6 December 2017 | 1 August 2025 |
| NCT03665545 | Phase 1/2 | Rand | 18 | rGBM | IMA950 | SC | Poly-ICLC +/− Pembrolizumab | - | AEs | 18+ | Active, not recruiting | 25 October 2018 | 31 December 2023 |
| NCT03750071 | Phase 1/2 | Single Arm | 30 | rGBM | VXM01 | Not listed | Avelumab | - | AEs | 18+ | Active, not recruiting | 21 November 2018 | 31 December 2022 |
| NCT04116658 | Phase 1/2 | Non-Rand | 52 | rGBM | EO2401 | Not listed | Nivolumab +/− Bevacizumab | - | AEs | 18+ | Recruiting | 13 July 2020 | 1 August 2023 |
| NCT02649582 | Phase 1/2 | Single Arm | 20 | nGBM | auto-WT1-DC | ID | - | - | OS | 18+ | Recruiting | 1 December 2015 | 1 December 2024 |
| NCT04801147 | Phase 1/2 | Single Arm | 76 | nGBM | ATL-DC | ID | - | - | PFS12 | 18–70 | Recruiting | 1 June 2010 | 1 December 2023 |
| NCT04388033 | Phase 1/2 | Single Arm | 10 | nGBM | ATL-DC | ID | IL-12 | - | AEs, PFS6 | 18–75 | Recruiting | 1 December 2020 | 1 December 2023 |
| NCT04015700 | Phase 1 | Single Arm | 9 | nGBM | GNOS-PV01 | Not listed | INO-9012 (IL-12) | - | DLT, Feasibility | 18+ | Active, not recruiting | 14 July 2020 | 13 April 2023 |
| NCT03223103 | Phase 1 | Single Arm | 13 | nGBM | MTA-based Personalized Vaccine | Not listed | Poly-ICLC | - | DLT | 18+ | Active, not recruiting | 1 March 2018 | 1 May 2023 |
| NCT04642937 | Phase 1 | Sequential | 24 | rGBM | GBM6-AD (Allogeneic TL) | Not listed | hP1A8 + Imiquimod | - | MTD | 18+ | Active, not recruiting | 1 December 2020 | 1 November 2023 |
| NCT00639639 | Phase 1 | Single Arm | 42 | nGBM | CMV-DC (auto) | ID | tetanus toxoid | - | Feasibility | 18+ | Active, not recruiting | 1 January 2006 | 1 December 2022 |
| NCT04741984 | Phase 1 | Sequential | 27 | nGBM | MT-201-GBM | IV | - | - | MTD, Immunogenicity | 18+ | Not yet recruiting | 1 October 2022 | 1 August 2025 |
| NCT05283109 | Phase 1 | Single Arm | 36 | nGBM | P30-EPS (P30-linked EphA2, CMV pp65, survivin) | Not listed | Hiltonol | - | DLT | 18+ | Not yet recruiting | 1 November 2022 | 1 February 2028 |
| NCT04968366 | Phase 1 | Single Arm | 10 | nGBM | ATL-DC (with multiple tumor neoantigen peptides) | ID | - | - | AEs | 18–75 | Recruiting | 30 July 2021 | 1 August 2024 |
| NCT04573140 | Phase 1 | Single Arm | 28 | nGBM | RNA-LP | IV | - | - | Feasibility, DLT, MTD | 21+ | Recruiting | 26 October 2021 | 1 July 2027 |
| NCT04963413 | Phase 1 | Single Arm | 10 | nGBM | CMV-DC (auto) | Not listed | GM-CSF | - | Feasibility | 18–90 | Recruiting | 13 January 2022 | 1 May 2025 |
| NCT02287428 | Phase 1 | Rand | 56 | nGBM | NeoVax | Not listed | Pembrolizumab | - | AEs, Feasibility | 18+ | Recruiting | 1 November 2014 | 1 January 2026 |
| NCT04201873 | Phase 1 | Rand | 40 | rGBM | ATL-DC | ID | Pembrolizumab, Poly-ICLC | IV Placebo | Immunogenicity, AEs | 18+ | Recruiting | 8 January 2020 | 1 August 2025 |
| NCT04552886 | Phase 1 | Non-Rand | 24 | nGBM | ATL-DC (Th-1 specific) | Not listed | - | - | AEs | 18+ | Recruiting | 11 October 2021 | 31 December 2025 |
| NCT04842513 | Phase 1 | Single Arm | 15 | nGBM | Multipeptide vaccine | SC | XS15 | - | AEs, Immunogenicity | 18+ | Recruiting | 3 May 2021 | 2 May 2024 |
| NCT05557240 | Phase 1 | Single Arm | 10 | nGBM | NeoPep Vaccine1/2 (NPVAC1/2) | Not listed | Poly-ICLC | - | AEs | 18–70 | Recruiting | 13 September 2022 | 12 August 2025 |
| NCT03360708 | Early Phase 1 | Single Arm | 20 | rGBM | ATL-DC + Allo GBM lysate | ID | - | - | AEs, Feasibility | 18+ | Active, not recruiting | 3 June 2019 | 1 June 2023 |
| NCT01957956 | Early Phase 1 | Single Arm | 21 | nGBM | auto-DC + Allo GBM lysate | ID | - | - | AEs | 18+ | Active, not recruiting | 11 November 2013 | 15 November 2023 |
Data are taken from clinicaltrials.gov as of January 2023. nGBM = newly diagnosed glioblastoma; rGBM = recurrent glioblastoma; Rand = randomized; Non-Rand = non-randomized; ATL-DC = autologous tumor lysate dendritic cell vaccine; DC = dendritic cell; CMV-DC = CMV-based dendritic cell vaccine; Allo = allogeneic vaccine component; AEs = adverse events; OS = overall survival; PFS = progression-free survival; DLT = dose-limiting toxicity; SOC = standard of care; MTD = maximum tolerated dose; GM-CSF = granulocyte macrophage colony-stimulating factor; ADCTA = autologous dendritic cell/tumor antigen; poly-ICLC = polyinosinic-polycytidylic acid stabilized with polylysine and carboxymethylcellulose; WT1 = Wilms tumor 1; IL-12 = interleukin-12; MTA = mutation-derived tumor antigen; GSC-DC = glioma stem cell dendritic cell; UCPVAx = universal cancer peptide-based vaccination; hP1A8 = human P1A8; P30-EPS = P30-linked Ephrin receptor A2; RNA-LP = ribonucleic acid lipid particle; SC = subcutaneous; ID = intradermal; IV = intravenous.
Figure 2.
Annual Distribution of Glioblastoma Vaccine Clinical Trials.
Multiple DC vaccines have been developed for use in GBMs [70,71,72]. Of these, DCVax-L utilizes an autologous tumor lysate, developed by Northwest Biotherapeutics, Inc., which has been under development as part of a larger DCVax platform. The results from a Phase 3 trial (NCT00045968) have recently been published, which assessed the impact of DCVax-L on the survival rate in patients with newly diagnosed and recurrent GBM who otherwise received standard of care [73] and updated interim analyses [74]. The nonrandomized, externally controlled trial took place from August 2007 to November 2015 at 94 sites in four countries (the USA, Canada, the UK, and Germany). A total of 331 patients with newly diagnosed GBM were enrolled (with a median age of 56 years; 61% were male; 89% were white). They had an mOS (n = 232) of 19.3 months (95% CI, 17.5–21.3) from the time of randomization (22.4 months from surgery) in the DCVax-L cohort relative to 16.5 months (95% CI, 16.0–17.5) from randomization in external controls, with a hazard ratio (HR) of 0.8 (98% CI, 0–0.94, p = 0.002). The mOS in patients with recurrent GBM (n = 64) was 13.2 months (95% CI, 9.7–16.8) from relapse versus 7.8 months (95% CI, 7.2–8.2) in the external controls, with an HR of 0.58 (98% CI, 0–0.76, p < 0.001). In addition to this observed survival benefit in both the newly diagnosed and recurrent GBMs, the authors noted a greater proportion of long-term survivors (36–60 months) in the DCVax-L group. Predefined subgroup analyses showed a potential survival benefit, particularly in patients that were 65 years of age or older, those with subtotal resection at the time of surgery, and those with MGMT promoter methylation. Importantly, DCVax-L showed negligible toxicity, with only five serious adverse events out of 2151 total administered doses. Although the results of this Phase 3 trial are promising, they must be interpreted in light of several limitations. External controls without individual patient-level data were used as a comparison for efficacy. The primary endpoint was changed from the initial design due to prominent radiographic changes after therapy (i.e., radiographic or pseudoprogression). Temozolomide, which may dampen the immune response, was given to most patients [75,76]. Lastly, the DCVax-L used for the recurrent GBM was derived from a tumor at initial diagnosis. While there are likely to be some similarities in the tumor at recurrence, recurrent GBM evolves with changes in the most prominent clonal subpopulations and phenotype switching [26,27].
Survivin (or BIRC5) is an anti-apoptotic protein that inhibits caspase activation and is highly expressed in most cancers, including GBM [77]. BIRC5 expression in GBM is associated with a worse prognosis; however, it is not present in normal glial tissues, making it an ideal vaccine target [78,79]. SurVaxM is a synthetic survivin vaccine, recently studied in early-phase clinical trials. An early clinical study of 9 patients (NCT01250470) demonstrated safety (mostly Grade 1 and no serious adverse events) [80], and the results from a recent Phase 2a trial (NCT02455557) of 64 patients with newly diagnosed GBM confirmed the previous safety data [81]. The survival data demonstrated a median progression-free survival (PFS) of 11.4 months (95.2% of the patients remained progression-free after 6 months), and the mOS was 25.9 months from the time of the first SurVaxM dose [81]. These results have since led to a larger ongoing Phase 2 trial (SURVIVE; NCT05163080), with an estimated completion date of April 2024.
The epidermal growth factor receptor (EGFR) is amplified in approximately 40% of GBMs, with an estimated 20% of GBMs harboring the mutant EGFRvIII, leading to the activation of the signaling pathways and contributing to malignant potential [82,83]. A Phase 3, randomized, double-blind, placebo-controlled clinical trial (Act IV; NCT01480479) of rindopepimut, an EGFRvIII-targeting peptide vaccine, in patients with newly diagnosed GBM (total n = 745; vaccine n = 371; placebo n = 374) was terminated for futility after a preplanned interim analysis [84]. There was no improvement in the mOS between the rindopepimut (20.1 months; 95% CI 18.5–22.1) versus the placebo (20.0 months; 95% CI 18.1–21.9), with an HR of 1.01 (95% CI 0.79–1.30, p = 0.93) [84]. The loss of EGFRvIII expression was described in about 57–59% of the tumors in both the treatment and control arms of the study [85], highlighting the frequency of phenotype switching and its importance when developing molecularly targeted therapeutics for GBMs.
IMA950 is a multipeptide vaccine that contains the following nine MHC class I-restricted peptides: brevican (BCAN), chondroitin sulfate proteoglycan 4 (CSPG4), fatty-acid-binding protein 7 (FABP7), insulin-like growth factor 2 mRNA-binding protein 3 (IGF2BP3), neuronal cell adhesion molecules (NRCAMs), neuroligin 4 X-linked (NLGN4X), protein tyrosine phosphatase, receptor type Z1 (PTPRZ1), and tenascin C (TNC). These antigens are typically overexpressed on the surface of GBM tumor samples and absent in normal glial tissues [86]. It is important to note that with a multipeptide approach, assessing the response may be more challenging than with more targeted peptide approaches. Immunogenicity of individual peptides in the form of a sustained T-cell adaptive response, as well as autocrine effects mediated by the secretion of cytokines and lymphokines by antigen-presenting cells, requires further preclinical and clinical characterization. IMA950 also contains two MHC class II-restricted peptides, c-Met and survivin, which are overexpressed in GBM but not expressed on the cell surface [87]. A recent Phase 1/2 clinical trial assessed IMA950 in combination with adjuvant poly-ICLC in 16 patients with newly diagnosed GBM. This trial (NCT01920191) demonstrated safety (although four patients had short-term cerebral edema with quick recovery) and immunogenicity, with an mOS of 19 months [87], supporting the results from a previous trial (NCT01222221) [88]. An ongoing Phase 1/2 trial is assessing IMA950 and poly-ICLC in combination with the immune checkpoint inhibitor (ICI) pembrolizumab (NCT03665545).
Cytomegalovirus (CMV) proteins have been found in the majority of GBMs [89], with the CMV phosphoprotein 65 (pp65) commonly expressed in tumors but not in normal glial tissues [90]. There has been great interest in CMV pp65-based vaccines, with some promising results in early-phase trials. A single-arm Phase 1 trial assessing a CMV pp65 vaccine in combination with dose-dense temozolomide in 11 patients with newly diagnosed GBM showed a greatly improved PFS (mPFS of 25.3 months; 95% CI 11–∞) and OS (mOS of 41.1 months; 95% CI 21.6–∞) from historical controls. There were also four long-term survivors (36%), who remained progression-free at 59+ months (NCT00639639) [91]. Given these impressive results, several trials assessing CMV pp65-based vaccines alone or in combination with therapy are ongoing and/or soon to be reported as of January 2023 (NCT02465268, NCT04963413, NCT04741984, NCT04573140, NCT00639639, NCT05283109, NCT03382977, and NCT03299309).
Wilm’s tumor 1 (WT1) is a transcription factor found in various malignancies, including GBM [92]. A Phase 1 dose-escalation trial of DSP-7888 (NCT02498665), a peptide vaccine including two synthetic peptides derived from WT1, was completed in patients with multiple malignancies (pancreatic cancers, sarcomas, non-small-cell lung cancers, ovarian cancers, and melanomas), including GBM (n = 7). The results from this trial demonstrated safety (the most common adverse events were low-grade injection site reactions without dose-limiting toxicity) [93], supporting further study in an ongoing Phase 3 trial (NCT03149003) in patients with recurrent or progressive GBMs.
EO2401 is an off-the-shelf, microbiome-derived, multipeptide vaccine that combines peptides that mimic cancer-driver antigens (IL13Ra2, BIRC5, and FOXM1) and the helper peptide UCP2, which is currently being assessed in a Phase 1B/2A trial of patients with progressive GBM +/− ICI/bevacizumab therapy (ROSALIE, NCT04116658) [94,95]. The interim results suggest that the treatment leads to strong immunogenicity with an mPFS of 1.8 months and a 6-month OS of 85% in 40 patients [95], with a further improved PFS (5.5 months) and tumor response with the addition of bevacizumab to EO2401 + ICI (nivolumab) [94].
VXM01 is a DNA plasmid vaccine that contains an attenuated strain of Salmonella typhimurium, which encodes the murine vascular endothelial growth factor receptor 2 (VEGFR-2). VEGFR-2 activation upregulates angiogenesis and cell proliferation, both necessary for tumor growth, and is commonly expressed within tumor microenvironments. Immunologically targeting and inhibiting this receptor likewise impairs tumor progression [96]. A clinical study (n = 14; NCT02718443) of VXM01 in progressive GBMs showed a favorable response in five patients, and the prolonged survivors had lower intratumoral PD-L1 expression, suggesting that combination with ICI therapy may boost the response to VXM01 therapy [97]. An ongoing Phase 1/2 trial (NCT03750071) is assessing VXM01 in combination with the ICI avelumab (a PD-L1 inhibitor) in recurrent GBMs.
There is increasing interest in personalized approaches to vaccination [98]. A Phase 1 trial (GAPVAC-101; NCT02149225) demonstrated overall safety (three serious adverse events attributable to the study drug—one cerebral edema and two anaphylaxis) and immunogenicity (sustained responses of CD8+ and CD4+ T cells) in 15 patients with newly diagnosed GBM [99]. These patients were administered APVAC1, derived from premanufactured unmutated tumor antigens, followed by APVAC2, derived from targeted tumor neoepitopes personalized from mutations in individual tumors (APVAC1: CD8+; APVAC2: CD4+). This trial resulted in an mPFS of 14.2 months and an mOS of 29.0 months [99]. Another ongoing Phase 1 trial is assessing a personalized vaccine (NeoVax) with an ICI (pembrolizumab) in GBM (NCT02287428).
4. Delivery
The method of vaccine delivery is also a consideration in the development of cancer therapeutic vaccines [100,101,102]. The vast majority of vaccination trials in glioblastomas utilize subcutaneous, intradermal, or intravenous delivery methods (Table 1). This is reasonable in that antigen exposure leads to a systemic immune response that ultimately targets the tumor [66]. However, there is increasing interest in novel drug delivery methods that may improve efficacy. Some examples of local delivery include the following: polymeric wafers, nanofibrous scaffolds, and hydrogels [100,101,102]. While out of the scope of the present review, recent articles extensively review advances in drug delivery that may be incorporated into vaccine development for GBM [100,101,102].
5. Challenges
Several factors likely contribute to the failure of most therapeutic vaccines to impact clinically meaningful endpoints [36,52,53]. A candidate vaccine may not lead to robust and sustained immunogenicity, which may be due to the antigen selection or the tumor microenvironment. There is an increasing understanding that GBM is “immunologically cold”, meaning that it is poorly infiltrated by effector cell populations and contains highly suppressive microenvironments. This, in turn, may cause GBM to be less responsive to immunotherapy, particularly monotherapy with ICIs [53,61,103]. GBM leads to a systemic immunosuppressive state [32,34], which should be considered in the design of immunotherapy. This may be further compounded by immunosuppressive therapies, such as temozolomide, which reduce the potential beneficial effects of vaccines. Indeed, alkylating chemotherapy leads to a meaningful and lasting impairment of T- and B-cell responses and proliferative potentials [75,76]. Adjuvant immunotherapies have been increasingly utilized to potentiate a more robust immune response [104,105,106]. Another important factor contributing to the challenges of immunotherapy and glioblastomas is iatrogenic immunosuppression with the use of corticosteroids (i.e., dexamethasone). Corticosteroids are often used in clinical practice to improve symptoms secondary to tumor mass effects, edema, and/or treatment effects (i.e., radiation) [107,108]. As the immunosuppressive effect of corticosteroids is well-established, it has been proposed that it has contributed to the failure of specific immunotherapies (immune checkpoint inhibitors) in GBMs [109]. The use of corticosteroids must be considered in the trial design and interpretation of results [110,111].
6. Adjuvant Therapies
With the lack of success for nearly all cancer therapeutic vaccines, there has been increasing attention on the use of adjuvant therapies, which may potentiate vaccine efficacy by heightening the immune response to the delivered antigen [53,61,104,105]. Multiple classes of adjuvant therapy are increasingly combined. For a more thorough review of adjuvant therapies utilized in peptide-based vaccines, see several excellent references in [104,105,106]. The key adjuvants studied in GBM trials are discussed below.
Bevacizumab is a monoclonal antibody used against the vascular endothelial growth factor (VEGF) and has been FDA-approved for use in GBM [112]. Its primary mechanism is as an antiangiogenic and reducer of cerebral edema. Bevacizumab may also reduce immunosuppression by improving VEGF-associated dysfunction in antigen presentation, lymphocyte trafficking, and DC maturation [36,113,114].
Montanide is a water-in-oil emulsion that works by prolonging the release of vaccine antigens. Two primary formulations are used in humans, primarily Montanide ISA-51 and Montanide ISA-720, which differ based on the included oil [115]. A Phase 2 trial in 64 patients with newly diagnosed GBM assessed SurVaxM in Montanide ISA-51 (plus sargramostim and concurrent/adjuvant temozolomide) and showed no serious adverse events related to the vaccine/Montanide (NCT02455557) [81]. Montanide ISA-51 was also used as adjunctive immunotherapy in an early-phase trial (NOA16; n = 32; NCT02454634) assessing the safety of an IDH1(R132H)-specific peptide vaccine in patients with another primary central nervous system tumor (IDH1 mutant glioma). This trial demonstrated safety (one serious adverse event; no regime-limiting toxicities) and vaccine-induced immune responses in 93% of the participants [116].
Tetanus-diphtheria toxoid (Td) is used as an antigen to precondition vaccination with the goal of promoting a more robust immune response [117,118]. Preclinical and clinical (NCT00639639) data show that a Td treatment prior to a DC vaccine (cytomegalovirus phosphoprotein 65, or CMV pp65) leads to improved DC migration, suppressed tumor growth, and prolonged survival (n = 12), which appeared dependent on the increased levels of the chemokine CCL3 [119].
Toll-like receptors (TLRs) are critical for the activation of the innate immune system and can be stimulated by imiquimod, poly-ICLC, and CpG oligonucleotides [106]. Imiquimod is an imidazoquinoline that activates TLR7/8, thereby leading to the production of inflammatory cytokines and enhanced DC migration. Poly-ICLC acts through TLR3, leading to the production of inflammatory cytokines and interferons and the upregulation of costimulatory molecules [106]. CpG oligonucleotides activate TLR9, leading to the release of inflammatory cytokines. There are three classes of CpG oligonucleotides (CpG A, B, and C) with varying properties.
While inflammatory cytokines are produced through TLR activation, various cytokines may also be administered directly as an adjunctive approach to stimulate the innate and/or adaptive immune system [120]. Interferon alpha has been approved for melanomas, and high-dose interleukin-2 (HDIL-2) has been approved for renal cell carcinomas and melanomas [121,122]. The granulocyte-macrophage colony-stimulating factor (GM-CSF) leads to DC recruitment and maturation with the activation of other immune cells (NK cells, macrophages, and neutrophils) [106,123]. While cytokines can promote immune activation, this class of therapies is most often used as an adjunct rather than a single agent [120]. Of the cytokines, GM-CSF and IL-12 are currently the most studied in GBM.
ICIs are FDA-approved [124] for use in many solid tumors. The primary mechanism of reversing the T-cell inhibitory effects of either the cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) or the programmed cell death protein 1/programmed cell death ligand 1 (PD-1/PD-L1) has been extensively reviewed [124,125,126,127]. While they have been ineffective in newly diagnosed/recurrent GBM trials to date [47], they may be used to augment the immune response when combined with other immune-modulating agents [128,129].
Indoleamine 2,3-dioxygenase 1 (IDO1) has been shown to promote cancer immune escape by catalyzing the breakdown of tryptophan to kynurenine. This is the initial step of the kynurenine pathway that has immunoregulatory functions [130,131]. High expressions of IDO1 have been associated with a worse prognosis in several malignancies [130], including GBM [132,133]. The use of a novel IDO1 inhibitor, BGB-5777, showed survival benefits in a mouse GBM model when combined with ICI and RT [134], forming the basis for ongoing/future trials (combined with ICI and RT +/− other therapies), including BMS-986205 (NCT04047706) and epacadostat (NCT03532295).
7. Logistical Considerations
It is important to consider the inevitable logistical challenges that arise from the more widespread use of vaccine therapies. DCVax-L, for example, is a personalized vaccine that uses an autologous tumor homogenate and, therefore, represents a highly individualized therapy [135,136]. Several steps are needed for broad implementation. Each institution performing resection would need to provide enough tissue to the vaccine manufacturer for antigen loading and the ultimate maturation of the DC for delivery. Patients would need to undergo leukapheresis prior to the start of standard therapy, potentially delaying treatment initiation. Multiple resections may be needed to ensure that the individualized vaccine is designed against antigens relevant to the recurrent rather than the primary tumor [26,27]. The feasibility of such an approach is also likely to hamper widespread adoption unless there is a robust survival advantage or improvement in the quality of life/functional status. The logistical challenges with an autologous antigen DC vaccine are lessened with the use of a standardized vaccine with target antigens commonly found in GBM [135,136,137] or the use of allogeneic tumor lysates [138]. Although there have been limited meaningful clinical data to support these approaches to date [139], several trials are ongoing.
8. Conclusions
GBM is the most common malignant primary brain tumor and confers a dismal prognosis. With only two FDA-approved therapeutics showing modest survival gains since 2005, there is a great need for the development of other disease-targeted therapies. Due, in part, to the profound immunosuppressive microenvironment seen in GBM, there has been a broad interest in immunotherapy. In both GBMs and other cancers, therapeutic vaccines have generally yielded limited efficacy, despite their theoretical basis [53]. However, recent results from the DCVax-L trial [73] provide some promise for vaccine therapy in GBMs. There is also potential that future combination therapies with vaccines and adjuvant immunomodulating agents [105,106] may greatly enhance antitumor immune responses [53]. The neuro-oncology community must remain open to novel therapeutic strategies, such as vaccinations, and carefully await the results of ongoing and future trials.
Author Contributions
Writing—original draft preparation, B.J.N.; data curation, M.J.W.; supervision, I.F.P. and U.T.S.; conceptualization, U.T.S.; writing—review and editing, B.J.N., M.J.W., I.F.P. and U.T.S. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This publication was supported by Grant Number UL1 TR002377 from the National Center for Advancing Translational Sciences (NCATS). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Ostrom Q.T., Price M., Neff C., Cioffi G., Waite A.K., Kruchko C., Barnholtz-Sloan J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2015–2019. Neuro-Oncology. 2022;24:v1–v95. doi: 10.1093/neuonc/noac202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roelcke U., Schwyzer L., Zeitlberger A.M., Hundsberger T. Symptom burden in glioblastoma-a prospective pilot study from diagnosis to first progression. Oncology. 2022;101:145–152. doi: 10.1159/000525651. [DOI] [PubMed] [Google Scholar]
- 3.Lacroix M., Abi-Said D., Fourney D.R., Gokaslan Z.L., Shi W., DeMonte F., Lang F.F., McCutcheon I.E., Hassenbusch S.J., Holland E., et al. A multivariate analysis of 416 patients with glioblastoma multiforme: Prognosis, extent of resection, and survival. J. Neurosurg. 2001;95:190–198. doi: 10.3171/jns.2001.95.2.0190. [DOI] [PubMed] [Google Scholar]
- 4.Tykocki T., Eltayeb M. Ten-year survival in glioblastoma. A systematic review. J. Clin. Neurosci. 2018;54:7–13. doi: 10.1016/j.jocn.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Delgado-López P.D., Corrales-García E.M. Survival in glioblastoma: A review on the impact of treatment modalities. Clin. Transl. Oncol. 2016;18:1062–1071. doi: 10.1007/s12094-016-1497-x. [DOI] [PubMed] [Google Scholar]
- 6.Neth B.J., Carabenciov I.D., Ruff M.W., Johnson D.R. Temporal Trends in Glioblastoma Survival: Progress then Plateau. Neurologist. 2021;27:119–124. doi: 10.1097/NRL.0000000000000393. [DOI] [PubMed] [Google Scholar]
- 7.Wen P.Y., Weller M., Lee E.Q., Alexander B.M., Barnholtz-Sloan J.S., Barthel F.P., Batchelor T.T., Bindra R.S., Chang S.M., Chiocca E.A., et al. Glioblastoma in adults: A Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro Oncol. 2020;22:1073–1113. doi: 10.1093/neuonc/noaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mutter N., Stupp R. Temozolomide: A milestone in neuro-oncology and beyond? Expert Rev. Anticancer Ther. 2006;6:1187–1204. doi: 10.1586/14737140.6.8.1187. [DOI] [PubMed] [Google Scholar]
- 9.Fabian D., Eibl M.D.P.G.P., Alnahhas I., Sebastian N., Giglio P., Puduvalli V., Gonzalez J., Palmer J.D. Treatment of Glioblastoma (GBM) with the Addition of Tumor-Treating Fields (TTF): A Review. Cancers. 2019;11:174. doi: 10.3390/cancers11020174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stupp R., Mason W.P., van den Bent M.J., Weller M., Fisher B., Taphoorn M.J.B., Belanger K., Brandes A.A., Marosi C., Bogdahn U., et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 11.Stupp R., Taillibert S., Kanner A.A., Kesari S., Steinberg D.M., Toms S.A., Taylor L.P., Lieberman F., Silvani A., Fink K.L., et al. Maintenance Therapy with Tumor-Treating Fields Plus Temozolomide vs Temozolomide Alone for Glioblastoma. JAMA. 2015;314:2535–2543. doi: 10.1001/jama.2015.16669. [DOI] [PubMed] [Google Scholar]
- 12.Herrlinger U., Tzaridis T., Mack F., Steinbach J.P., Schlegel U., Sabel M., Hau P., Kortmann R.-D., Krex D., Grauer O., et al. Lomustine-temozolomide combination therapy versus standard temozolomide therapy in patients with newly diagnosed glioblastoma with methylated MGMT promoter (CeTeG/NOA–09): A randomised, open-label, phase 3 trial. Lancet. 2019;393:678–688. doi: 10.1016/S0140-6736(18)31791-4. [DOI] [PubMed] [Google Scholar]
- 13.Perry J.R., Laperriere N., O’Callaghan C.J., Brandes A.A., Menten J., Phillips C., Fay M., Nishikawa R., Cairncross J.G., Roa W., et al. Short-Course Radiation plus Temozolomide in Elderly Patients with Glioblastoma. N. Engl. J. Med. 2017;376:1027–1037. doi: 10.1056/NEJMoa1611977. [DOI] [PubMed] [Google Scholar]
- 14.Malmström A., Grønberg B.H., Marosi C., Stupp R., Frappaz D., Schultz H., Abacioglu U., Tavelin B., Lhermitte B., Hegi E.M., et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: The Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13:916–926. doi: 10.1016/S1470-2045(12)70265-6. [DOI] [PubMed] [Google Scholar]
- 15.Shergalis A., Bankhead A., 3rd, Luesakul U., Muangsin N., Neamati N. Current Challenges and Opportunities in Treating Glioblastoma. Pharmacol. Rev. 2018;70:412–445. doi: 10.1124/pr.117.014944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goenka A., Tiek D., Song X., Huang T., Hu B., Cheng S.-Y. The Many Facets of Therapy Resistance and Tumor Recurrence in Glioblastoma. Cells. 2021;10:484. doi: 10.3390/cells10030484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dirkse A., Golebiewska A., Buder T., Nazarov P.V., Muller A., Poovathingal S., Brons N.H.C., Leite S., Sauvageot N., Sarkisjan D., et al. Stem cell-associated heterogeneity in Glioblastoma results from intrinsic tumor plasticity shaped by the microenvironment. Nat. Commun. 2019;10:1787. doi: 10.1038/s41467-019-09853-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perrin S.L., Samuel M.S., Koszyca B., Brown M.P., Ebert L.M., Oksdath M., Gomez G.A. Glioblastoma heterogeneity and the tumour microenvironment: Implications for preclinical research and development of new treatments. Biochem. Soc. Trans. 2019;47:625–638. doi: 10.1042/BST20180444. [DOI] [PubMed] [Google Scholar]
- 19.Qazi M.A., Vora P., Venugopal C., Sidhu S.S., Moffat J., Swanton C., Singh S.K. Intratumoral heterogeneity: Pathways to treatment resistance and relapse in human glioblastoma. Ann. Oncol. 2017;28:1448–1456. doi: 10.1093/annonc/mdx169. [DOI] [PubMed] [Google Scholar]
- 20.Soeda A., Hara A., Kunisada T., Yoshimura S.I., Iwama T., Park D.M. The evidence of glioblastoma heterogeneity. Sci. Rep. 2015;5:7979. doi: 10.1038/srep07979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The Cancer Genome Atlas Research Network Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brennan C.W., Verhaak R.G.W., McKenna A., Campos B., Noushmehr H., Salama S.R., Zheng S., Chakravarty D., Sanborn J.Z., Berman S.H., et al. The Somatic Genomic Landscape of Glioblastoma. Cell. 2013;155:462–477. doi: 10.1016/j.cell.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Behnan J., Finocchiaro G., Hanna G. The landscape of the mesenchymal signature in brain tumours. Brain. 2019;142:847–866. doi: 10.1093/brain/awz044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verhaak R.G.W., Hoadley K.A., Purdom E., Wang V., Wilkerson M.D., Miller C.R., Ding L., Golub T., Jill P., Alexe G., et al. Integrated Genomic Analysis Identifies Clinically Relevant Subtypes of Glioblastoma Characterized by Abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Louis D.N., Perry A., Wesseling P., Brat D.J., Cree I.A., Figarella-Branger D., Hawkins C., Ng H.K., Pfister S.M., Reifenberger G., et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology. 2021;23:1231–1251. doi: 10.1093/neuonc/noab106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang J., Cazzato E., Ladewig E., Frattini V., Rosenbloom D.I.S., Zairis S., Abate F., Liu Z., Elliott O., Shin Y.-J., et al. Clonal evolution of glioblastoma under therapy. Nat. Genet. 2016;48:768–776. doi: 10.1038/ng.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang L., Jung J., Babikir H., Shamardani K., Jain S., Feng X., Gupta N., Rosi S., Chang S., Raleigh D., et al. A single-cell atlas of glioblastoma evolution under therapy reveals cell-intrinsic and cell-extrinsic therapeutic targets. Nat. Cancer. 2022;3:1534–1552. doi: 10.1038/s43018-022-00475-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daneman R., Prat A. The blood-brain barrier. Cold Spring Harb. Perspect. Biol. 2015;7:a020412. doi: 10.1101/cshperspect.a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ou A., Yung W.K.A., Majd N. Molecular Mechanisms of Treatment Resistance in Glioblastoma. Int. J. Mol. Sci. 2020;22:351. doi: 10.3390/ijms22010351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sarkaria J.N., Hu L.S., Parney I.F., Pafundi D.H., Brinkmann D.H., Laack N.N., Giannini C., Burns T.C., Kizilbash S., Laramy J.K., et al. Is the blood–brain barrier really disrupted in all glioblastomas? A critical assessment of existing clinical data. Neuro-Oncology. 2017;20:184–191. doi: 10.1093/neuonc/nox175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu H.-L., Hua M.-Y., Chen P.-Y., Chu P.-C., Pan C.-H., Yang H.-W., Huang C.-Y., Wang J.-J., Yen T.-C., Wei K.-C. Blood-Brain Barrier Disruption with Focused Ultrasound Enhances Delivery of Chemotherapeutic Drugs for Glioblastoma Treatment. Radiology. 2010;255:415–425. doi: 10.1148/radiol.10090699. [DOI] [PubMed] [Google Scholar]
- 32.Nduom E.K., Weller M., Heimberger A.B. Immunosuppressive mechanisms in glioblastoma: Figure 1. Neuro-Oncology. 2015;17:vii9–vii14. doi: 10.1093/neuonc/nov151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jackson C.M., Choi J., Lim M. Mechanisms of immunotherapy resistance: Lessons from glioblastoma. Nat. Immunol. 2019;20:1100–1109. doi: 10.1038/s41590-019-0433-y. [DOI] [PubMed] [Google Scholar]
- 34.Waziri A. Glioblastoma-Derived Mechanisms of Systemic Immunosuppression. Neurosurg. Clin. North Am. 2010;21:31–42. doi: 10.1016/j.nec.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 35.Parsa A.T., Waldron J.S., Panner A., Crane C.A., Parney I.F., Barry J.J., Cachola K.E., Murray J.C., Tihan T., Jensen M.C., et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat. Med. 2007;13:84–88. doi: 10.1038/nm1517. [DOI] [PubMed] [Google Scholar]
- 36.Brown N.F., Carter T.J., Ottaviani D., Mulholland P. Harnessing the immune system in glioblastoma. Br. J. Cancer. 2018;119:1171–1181. doi: 10.1038/s41416-018-0258-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zagzag D., Salnikow K., Chiriboga L., Yee H., Lan L., Ali M.A., Garcia R., Demaria S., Newcomb E.W. Downregulation of major histocompatibility complex antigens in invading glioma cells: Stealth invasion of the brain. Lab. Investig. 2005;85:328–341. doi: 10.1038/labinvest.3700233. [DOI] [PubMed] [Google Scholar]
- 38.Lim M., Xia Y., Bettegowda C., Weller M. Current state of immunotherapy for glioblastoma. Nat. Rev. Clin. Oncol. 2018;15:422–442. doi: 10.1038/s41571-018-0003-5. [DOI] [PubMed] [Google Scholar]
- 39.Himes B.T., Peterson E.T., de Mooij T., Garcia L.M.C., Jung M.-Y., Uhm S., Yan D., Tyson J., Jin-Lee H.J., Parney D., et al. The role of extracellular vesicles and PD-L1 in glioblastoma-mediated immunosuppressive monocyte induction. Neuro-Oncology. 2020;22:967–978. doi: 10.1093/neuonc/noaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Himes B.T., Geiger P.A., Ayasoufi K., Bhargav A.G., Brown D.A., Parney I.F. Immunosuppression in Glioblastoma: Current Understanding and Therapeutic Implications. Front. Oncol. 2021;11:770561. doi: 10.3389/fonc.2021.770561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Desland F.A., Hormigo A. The CNS and the Brain Tumor Microenvironment: Implications for Glioblastoma Immunotherapy. Int. J. Mol. Sci. 2020;21:7358. doi: 10.3390/ijms21197358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jackson C., Ruzevick J., Phallen J., Belcaid Z., Lim M. Challenges in Immunotherapy Presented by the Glioblastoma Multiforme Microenvironment. J. Immunol. Res. 2011;2011:732413. doi: 10.1155/2011/732413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bornschlegl S., Gustafson M.P., Delivanis A.D., Ryder M., Liu M.C., Vasmatzis G., Hallemeier C.L., Park S.S., Roberts L.R., Parney I.F., et al. Categorisation of patients based on immune profiles: A new approach to identifying candidates for response to checkpoint inhibitors. Clin. Transl. Immunol. 2021;10:e1267. doi: 10.1002/cti2.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chongsathidkiet P., Jackson C., Koyama S., Loebel F., Cui X., Farber S.H., Woroniecka K., Elsamadicy A.A., Dechant C.A., Kemeny H.R., et al. Sequestration of T cells in bone marrow in the setting of glioblastoma and other intracranial tumors. Nat. Med. 2018;24:1459–1468. doi: 10.1038/s41591-018-0135-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gustafson M.P., Lin Y., New K.C., Bulur P.A., O’Neill B.P., Gastineau D.A., Dietz A.B. Systemic immune suppression in glioblastoma: The interplay between CD14+HLA-DRlo/neg monocytes, tumor factors, and dexamethasone. Neuro-Oncology. 2010;12:631–644. doi: 10.1093/neuonc/noq001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rodrigues J.C., Gonzalez G.C., Zhang L., Ibrahim G., Kelly J.J., Gustafson M.P., Lin Y., Dietz A., Forsyth P.A., Yong V.W., et al. Normal human monocytes exposed to glioma cells acquire myeloid-derived suppressor cell-like properties. Neuro-Oncology. 2009;12:351–365. doi: 10.1093/neuonc/nop023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sener U., Ruff M.W., Campian J.L. Immunotherapy in Glioblastoma: Current Approaches and Future Perspectives. Int. J. Mol. Sci. 2022;23:7046. doi: 10.3390/ijms23137046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilcox J.A., Ramakrishna R., Magge R. Immunotherapy in glioblastoma. World Neurosurg. 2018;116:518–528. doi: 10.1016/j.wneu.2018.04.020. [DOI] [PubMed] [Google Scholar]
- 49.Xu S., Tang L., Li X., Fan F., Liu Z. Immunotherapy for glioma: Current management and future application. Cancer Lett. 2020;476:1–12. doi: 10.1016/j.canlet.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 50.van Putten E.H.P., Kleijn A., Van Beusechem V.W., Noske D., Lamers C.H., De Goede A.L., Idema S., Hoefnagel D., Kloezeman J.J., Fueyo J., et al. Convection Enhanced Delivery of the Oncolytic Adenovirus Delta24-RGD in Patients with Recurrent GBM: A Phase I Clinical Trial Including Correlative Studies. Clin. Cancer Res. 2022;28:1572–1585. doi: 10.1158/1078-0432.CCR-21-3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Esfahani K., Roudaia L., Buhlaiga N., Del Rincon S.V., Papneja N., Miller W.H., Jr. A Review of Cancer Immunotherapy: From the Past, to the Present, to the Future. Curr. Oncol. 2020;27((Suppl. S2)):S87–S97. doi: 10.3747/co.27.5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weller M., Roth P., Preusser M., Wick W., Reardon D.A., Platten M., Sampson J.H. Vaccine-based immunotherapeutic approaches to gliomas and beyond. Nat. Rev. Neurol. 2017;13:363–374. doi: 10.1038/nrneurol.2017.64. [DOI] [PubMed] [Google Scholar]
- 53.Saxena M., van der Burg S.H., Melief C.J., Bhardwaj N. Therapeutic cancer vaccines. Nat. Rev. Cancer. 2021;21:360–378. doi: 10.1038/s41568-021-00346-0. [DOI] [PubMed] [Google Scholar]
- 54.Guo C., Manjili M.H., Subjeck J.R., Sarkar D., Fisher P.B., Wang X.Y. Therapeutic cancer vaccines: Past, present, and future. Adv. Cancer Res. 2013;119:421–475. doi: 10.1016/B978-0-12-407190-2.00007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.De Jager R., Guinan E., Lamm D., Khanna O., Brosman S., De Kernion J., Williams R., Richardson C., Muenz L., Reitsma D., et al. Long-term complete remission in bladder carcinoma in situ with intravesical TICE bacillus Calmette Guerin. Overview analysis of six phase II clinical trials. Urology. 1991;38:507–513. doi: 10.1016/0090-4295(91)80166-5. [DOI] [PubMed] [Google Scholar]
- 56.Sfakianos J.P., Salome B., Daza J., Farkas A., Bhardwaj N., Horowitz A. Bacillus Calmette-Guerin (BCG): Its fight against pathogens and cancer. Urol. Oncol. Semin. Orig. Investig. 2020;39:121–129. doi: 10.1016/j.urolonc.2020.09.031. [DOI] [PubMed] [Google Scholar]
- 57.Kantoff P.W., Higano C.S., Shore N.D., Berger E.R., Small E.J., Penson D.F., Redfern C.H., Ferrari A.C., Dreicer R., Sims R.B., et al. Sipuleucel-T Immunotherapy for Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 58.Chesney J.A., Ribas A., Long G.V., Kirkwood J.M., Dummer R., Puzanov I., Hoeller C., Gajewski T.F., Gutzmer R., Rutkowski P., et al. Randomized, Double-Blind, Placebo-Controlled, Global Phase III Trial of Talimogene Laherparepvec Combined with Pembrolizumab for Advanced Melanoma. J. Clin. Oncol. 2023;41:528–540. doi: 10.1200/JCO.22.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schijns V.E.J.C., Pretto C., Strik A.M., Gloudemans-Rijkers R., Devillers L., Pierre D., Chung J., Dandekar M., Carrillo J.A., Kong X.-T., et al. Therapeutic Immunization against Glioblastoma. Int. J. Mol. Sci. 2018;19:2540. doi: 10.3390/ijms19092540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bausart M., Préat V., Malfanti A. Immunotherapy for glioblastoma: The promise of combination strategies. J. Exp. Clin. Cancer Res. 2022;41:1–22. doi: 10.1186/s13046-022-02251-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Frederico S.C., Hancock J.C., Brettschneider E.E.S., Ratnam N.M., Gilbert M.R., Terabe M. Making a Cold Tumor Hot: The Role of Vaccines in the Treatment of Glioblastoma. Front. Oncol. 2021;11:672508. doi: 10.3389/fonc.2021.672508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu W., Tang H., Li L., Wang X., Yu Z., Li J. Peptide-based therapeutic cancer vaccine: Current trends in clinical application. Cell Prolif. 2021;54:e13025. doi: 10.1111/cpr.13025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang B., Jeang J., Yang A., Wu T.C., Hung C.-F. DNA vaccine for cancer immunotherapy. Hum. Vaccines Immunother. 2014;10:3153–3164. doi: 10.4161/21645515.2014.980686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mitchell A.D., Nair S.K. RNA transfected dendritic cells as cancer vaccines. Curr. Opin. Mol. Ther. 2000;2:76–181. [PubMed] [Google Scholar]
- 65.Banchereau J., Briere F., Caux C., Davoust J., Lebecque S., Liu Y.-J., Pulendran B., Palucka K. Immunobiology of Dendritic Cells. Annu. Rev. Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 66.Reardon D.A., Mitchell D.A. The development of dendritic cell vaccine-based immunotherapies for glioblastoma. Semin. Immunopathol. 2017;39:225–239. doi: 10.1007/s00281-016-0616-7. [DOI] [PubMed] [Google Scholar]
- 67.Schlesinger M.J. Heat shock proteins. J. Biol. Chem. 1990;265:12111–12114. doi: 10.1016/S0021-9258(19)38314-0. [DOI] [PubMed] [Google Scholar]
- 68.Stetler R.A., Gan Y., Zhang W., Liou A.K., Gao Y., Cao G., Chen J. Heat shock proteins: Cellular and molecular mechanisms in the central nervous system. Prog. Neurobiol. 2010;92:184–211. doi: 10.1016/j.pneurobio.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Blachere N.E., Li Z., Chandawarkar R.Y., Suto R., Jaikaria N.S., Basu S., Udono H., Srivastava P.K. Heat Shock Protein–Peptide Complexes, Reconstituted In Vitro, Elicit Peptide-specific Cytotoxic T Lymphocyte Response and Tumor Immunity. J. Exp. Med. 1997;186:1315–1322. doi: 10.1084/jem.186.8.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Buchroithner J., Erhart F., Pichler J., Widhalm G., Preusser M., Stockhammer G., Nowosielski M., Iglseder S., Freyschlag C.F., Oberndorfer S., et al. Audencel Immunotherapy Based on Dendritic Cells Has No Effect on Overall and Progression-Free Survival in Newly Diagnosed Glioblastoma: A Phase II Randomized Trial. Cancers. 2018;10:372. doi: 10.3390/cancers10100372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cho D.-Y., Yang W.-K., Lee H.-C., Hsu D.-M., Lin H.-L., Lin S.-Z., Chen C.-C., Harn H.-J., Liu C.-L., Lee W.-Y., et al. Adjuvant Immunotherapy with Whole-Cell Lysate Dendritic Cells Vaccine for Glioblastoma Multiforme: A Phase II Clinical Trial. World Neurosurg. 2012;77:736–744. doi: 10.1016/j.wneu.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 72.Tan L., Peng J., Liu P., Wu Q. The Efficacy of Dendritic Cell Vaccine for Newly Diagnosed Glioblastoma: A Meta-analysis of Randomized Controlled Studies. Clin. Neuropharmacol. 2021;44:216–221. doi: 10.1097/WNF.0000000000000452. [DOI] [PubMed] [Google Scholar]
- 73.Liau L.M., Ashkan K., Brem S., Campian J.L., Trusheim J.E., Iwamoto F.M., Tran D.D., Ansstas G., Cobbs C.S., Heth J.A., et al. Association of Autologous Tumor Lysate-Loaded Den.dritic Cell Vaccination with Extension of Survival Among Patients with Newly Diagnosed and Recurrent Glioblastoma: A Phase 3 Prospective Externally Controlled Cohort Trial. JAMA Oncol. 2022;9:112–121. doi: 10.1001/jamaoncol.2022.5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liau L.M., Ashkan K., Tran D.D., Campian J.L., Trusheim J.E., Cobbs C.S., Heth J.A., Salacz M., Taylor S., D’Andre S.D., et al. First results on survival from a large Phase 3 clinical trial of an autologous dendritic cell vaccine in newly diagnosed glioblastoma. J. Transl. Med. 2018;16:142. doi: 10.1186/s12967-018-1507-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Litterman A.J., Dudek A.Z., Largaespada A.D. Alkylating chemotherapy may exert a uniquely deleterious effect upon neo-antigen-targeting anticancer vaccination. Oncoimmunology. 2013;2:e26294. doi: 10.4161/onci.26294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Litterman A.J., Zellmer D.M., Grinnen K.L., Hunt M.A., Dudek A.Z., Salazar A.M., Ohlfest J.R. Profound Impairment of Adaptive Immune Responses by Alkylating Chemotherapy. J. Immunol. 2013;190:6259–6268. doi: 10.4049/jimmunol.1203539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ambrosini G., Adida C., Altieri D.C. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat. Med. 1997;3:917–921. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- 78.Chakravarti A., Noll E., Black P.M., Finkelstein D.F., Finkelstein D.M., Dyson N.J., Loeffler J.S. Quantitatively determined survivin expression levels are of prognostic value in human gliomas. J. Clin. Oncol. 2002;20:1063–1068. doi: 10.1200/JCO.2002.20.4.1063. [DOI] [PubMed] [Google Scholar]
- 79.Kajiwara Y., Yamasaki F., Hama S., Yahara K., Yoshioka H., Sugiyama K., Arita K., Kurisu K. Expression of survivin in astrocytic tumors: Correlation with malignant grade and prognosis. Cancer Interdiscipl. Int. J. Am. Cancer Soc. 2003;97:1077–1083. doi: 10.1002/cncr.11122. [DOI] [PubMed] [Google Scholar]
- 80.Fenstermaker R.A., Ciesielski M.J., Qiu J., Yang N., Frank C.L., Lee K.P., Mechtler L.R., Belal A., Ahluwalia M.S., Hutson A.D. Clinical study of a survivin long peptide vaccine (SurVaxM) in patients with recurrent malignant glioma. Cancer Immunol. Immunother. 2016;65:1339–1352. doi: 10.1007/s00262-016-1890-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ahluwalia M.S., Reardon D.A., Abad A.P., Curry W.T., Wong E.T., Figel S.A., Mechtler L.L., Peereboom D.M., Hutson A.D., Withers H.G., et al. Phase IIa Study of SurVaxM Plus Adjuvant Temozolomide for Newly Diagnosed Glioblastoma. J. Clin. Oncol. 2023;41:1453–1465. doi: 10.1200/JCO.22.00996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.An Z., Aksoy O., Zheng T., Fan Q.-W., Weiss W.A. Epidermal growth factor receptor and EGFRvIII in glioblastoma: Signaling pathways and targeted therapies. Oncogene. 2018;37:1561–1575. doi: 10.1038/s41388-017-0045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gan H.K., Kaye A.H., Luwor R.B. The EGFRvIII variant in glioblastoma multiforme. J. Clin. Neurosci. 2009;16:748–754. doi: 10.1016/j.jocn.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 84.Weller M., Butowski N., Tran D.D., Recht L.D., Lim M., Hirte H., Ashby L., Mechtler L., Goldlust S.A., Iwamoto F., et al. Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): A randomised, double-blind, international phase 3 trial. Lancet Oncol. 2017;18:1373–1385. doi: 10.1016/S1470-2045(17)30517-X. [DOI] [PubMed] [Google Scholar]
- 85.Binder D.C., Ladomersky E., Lenzen A., Zhai L., Lauing K.L., Otto-Meyer S.D., Lukas R.V., Wainwright D.A. Lessons learned from rindopepimut treatment in patients with EGFRvIII-expressing glioblastoma. Transl. Cancer Res. 2018;7((Suppl. S4)):S510–S513. doi: 10.21037/tcr.2018.03.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dutoit V., Herold-Mende C., Hilf N., Schoor O., Beckhove P., Bucher J., Dorsch K., Flohr S., Fritsche J., Lewandrowski P., et al. Exploiting the glioblastoma peptidome to discover novel tumour-associated antigens for immunotherapy. Brain. 2012;135:1042–1054. doi: 10.1093/brain/aws042. [DOI] [PubMed] [Google Scholar]
- 87.Migliorini D., Dutoit V., Allard M., Grandjean Hallez N., Marinari E., Widmer V., Philippin G., Corlazzoli F., Gustave R., Kreutzfeldt M., et al. Phase I/II trial testing safety and immunogenicity of the multipeptide IMA950/poly-ICLC vaccine in newly diagnosed adult malignant astrocytoma patients. Neuro-Oncology. 2019;21:923–933. doi: 10.1093/neuonc/noz040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rampling R., Peoples S., Mulholland P.J., James A., Al-Salihi O., Twelves C.J., McBain C., Jefferies S., Jackson A., Stewart W., et al. A Cancer Research UK First Time in Human Phase I Trial of IMA950 (Novel Multipeptide Therapeutic Vaccine) in Patients with Newly Diagnosed Glioblastoma. Clin. Cancer Res. 2016;22:4776–4785. doi: 10.1158/1078-0432.CCR-16-0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cobbs C.S., Harkins L., Samanta M., Gillespie G.Y., Bharara S., King P.H., Nabors L.B., Cobbs C.G., Britt W.J. Human cytomegalovirus infection and expression in human malignant glioma. Cancer Res. 2002;62:3347–3350. [PubMed] [Google Scholar]
- 90.Mitchell D.A., Xie W., Schmittling R., Learn C., Friedman A., McLendon R.E., Sampson J.H. Sensitive detection of human cytomegalovirus in tumors and peripheral blood of patients diagnosed with glioblastoma. Neuro-Oncology. 2008;10:10–18. doi: 10.1215/15228517-2007-035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Batich K.A., Reap E.A., Archer G.E., Sanchez-Perez L., Nair S.K., Schmittling R.J., Norberg P., Xie W., Herndon J.E., II, Healy P., et al. Long-term Survival in Glioblastoma with Cytomegalovirus pp65-Targeted Vaccination. Clin. Cancer Res. 2017;23:1898–1909. doi: 10.1158/1078-0432.CCR-16-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nakahara Y., Okamoto H., Mineta T., Tabuchi K. Expression of the Wilms’ tumor gene product WT1 in glioblastomas and medulloblastomas. Brain Tumor Pathol. 2004;21:113–116. doi: 10.1007/BF02482185. [DOI] [PubMed] [Google Scholar]
- 93.Spira A., Hansen A.R., Harb W.A., Curtis K.K., Koga-Yamakawa E., Origuchi M., Li Z., Ertik B., Shaib W.L. Multicenter, Open-Label, Phase I Study of DSP-7888 Dosing Emulsion in Patients with Advanced Malignancies. Target. Oncol. 2021;16:461–469. doi: 10.1007/s11523-021-00813-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Reardon D.A., Idbaih A., Vieito M., Tabatabai G., Stradella A., Ghiringhelli F., Burger M.C., Mildenberger I., Gonzalez M., Hervieu A., et al. Ctim-17. Eo2401 Therapeutic Vaccine for Patients with Recurrent Glioblastoma: Phase 1/2 Rosalie Study ( NCT04116658) Neuro-Oncology. 2022;24((Suppl. S7)):vii63. doi: 10.1093/neuonc/noac209.249. [DOI] [Google Scholar]
- 95.Baudin E., Jimenez C., Fassnacht M., Grisanti S., Menke C.W., Haak H., Subbiah V., Capdevila J., De La Fouchardiere C., Granberg D., et al. EO2401, A Novel Microbiome-Derived Therapeutic Vaccine for Patients with Recurrent Glioblastoma: ROSALIE Study. American Society of Clinical Oncology; Alexandria, VA, USA: 2022. [Google Scholar]
- 96.Schmitz-Winnenthal F.H., Hohmann N., Niethammer A.G., Friedrich T., Lubenau H., Springer M., Breiner K.M., Mikus G., Weitz J., Ulrich A., et al. Anti-angiogenic activity of VXM01, an oral T-cell vaccine against VEGF receptor 2, in patients with advanced pancreatic cancer: A randomized, placebo-controlled, phase 1 trial. Oncoimmunology. 2015;4:e1001217. doi: 10.1080/2162402X.2014.1001217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wick W., Wick A., Sahm F., Riehl D., Von Deimling A., Bendszus M., Kickingereder P., Beckhove P., Schmitz-Winnenthal F.H., Jungk C., et al. VXM01 phase I study in patients with progressive glioblastoma: Final results. J. Clin. Oncol. 2018;36:2017. doi: 10.1200/JCO.2018.36.15_suppl.2017. [DOI] [Google Scholar]
- 98.Shemesh C.S., Hsu J.C., Hosseini I., Shen B.-Q., Rotte A., Twomey P., Girish S., Wu B. Personalized Cancer Vaccines: Clinical Landscape, Challenges, and Opportunities. Mol. Ther. 2020;29:555–570. doi: 10.1016/j.ymthe.2020.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hilf N., Kuttruff-Coqui S., Frenzel K., Bukur V., Stevanović S., Gouttefangeas C., Platten M., Tabatabai G., Dutoit V., Van Der Burg S.H., et al. Actively personalized vaccination trial for newly diagnosed glioblastoma. Nature. 2019;565:240–245. doi: 10.1038/s41586-018-0810-y. [DOI] [PubMed] [Google Scholar]
- 100.Bu L.-L., Yan J., Wang Z., Ruan H., Chen Q., Gunadhi V., Bell R.B., Gu Z. Advances in drug delivery for post-surgical cancer treatment. Biomaterials. 2019;219:119182. doi: 10.1016/j.biomaterials.2019.04.027. [DOI] [PubMed] [Google Scholar]
- 101.Cha G.D., Kang T., Baik S., Kim D., Choi S.H., Hyeon T., Kim D.-H. Advances in drug delivery technology for the treatment of glioblastoma multiforme. J. Control. Release. 2020;328:350–367. doi: 10.1016/j.jconrel.2020.09.002. [DOI] [PubMed] [Google Scholar]
- 102.Woodring R.N., Gurysh E.G., Bachelder E.M., Ainslie K.M. Drug Delivery Systems for Localized Cancer Combination Therapy. ACS Appl. Bio Mater. 2023;6:934–950. doi: 10.1021/acsabm.2c00973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen D.S., Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541:321–330. doi: 10.1038/nature21349. [DOI] [PubMed] [Google Scholar]
- 104.Bowen W.S., Svrivastava A.K., Batra L., Barsoumian H., Shirwan H. Current challenges for cancer vaccine adjuvant development. Expert Rev. Vaccines. 2018;17:207–215. doi: 10.1080/14760584.2018.1434000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dubensky T.W., Jr., Reed S.G. Seminars in Immunology. Elsevier; Amsterdam, The Netherlands: 2010. Adjuvants for cancer vaccines. [DOI] [PubMed] [Google Scholar]
- 106.Khong H., Overwijk W.W. Adjuvants for peptide-based cancer vaccines. J. Immunother. Cancer. 2016;4:56. doi: 10.1186/s40425-016-0160-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cenciarini M., Valentino M., Belia S., Sforna L., Rosa P., Ronchetti S., D’Adamo M.C., Pessia M. Dexamethasone in Glioblastoma Multiforme Therapy: Mechanisms and Controversies. Front. Mol. Neurosci. 2019;12:65. doi: 10.3389/fnmol.2019.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pitter K.L., Tamagno I., Alikhanyan K., Hosni-Ahmed A., Pattwell S.S., Donnola S., Dai C., Ozawa T., Chang M., Chan T.A., et al. Corticosteroids compromise survival in glioblastoma. Brain. 2016;139:1458–1471. doi: 10.1093/brain/aww046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Swildens K.X., Smitt P.A.E.S., Bent M.J.V.D., French P.J., Geurts M. The effect of dexamethasone on the microenvironment and efficacy of checkpoint inhibitors in glioblastoma: A systematic review. Neuro-Oncology Adv. 2022;4:vdac087. doi: 10.1093/noajnl/vdac087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Giles A.J., Hutchinson M.-K., Sonnemann H.M., Jung J., Fecci P.E., Ratnam N.M., Zhang W., Song H., Bailey R., Davis D., et al. Dexamethasone-induced immunosuppression: Mechanisms and implications for immunotherapy. J. Immunother. Cancer. 2018;6:51. doi: 10.1186/s40425-018-0371-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Iorgulescu J.B., Gokhale P.C., Speranza M.C., Eschle B.K., Poitras M.J., Wilkens M.K., Soroko K.M., Chhoeu C., and Knott A., Gao Y., et al. Concurrent Dexamethasone Limits the Clinical Benefit of Immune Checkpoint Blockade in GlioblastomaDexamethasone Limits Anti–PD-1 Benefit for GBM. Clin. Cancer Res. 2021;27:276–287. doi: 10.1158/1078-0432.CCR-20-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cohen M.H., Shen Y.L., Keegan P., Pazdur R. FDA Drug Approval Summary: Bevacizumab (Avastin®) as Treatment of Recurrent Glioblastoma Multiforme. Oncologist. 2009;14:1131–1138. doi: 10.1634/theoncologist.2009-0121. [DOI] [PubMed] [Google Scholar]
- 113.Ohm J.E., Carbone D.P. VEGF as a Mediator of Tumor-Associated Immunodeficiency. Immunol. Res. 2001;23:263–272. doi: 10.1385/IR:23:2-3:263. [DOI] [PubMed] [Google Scholar]
- 114.Oyama T., Ran S., Ishida T., Nadaf S., Kerr L., Carbone D.P., Gabrilovich D.I. Vascular endothelial growth factor affects dendritic cell maturation through the inhibition of nuclear factor-kappa B activation in hemopoietic progenitor cells. J. Immunol. 1998;160:1224–1232. doi: 10.4049/jimmunol.160.3.1224. [DOI] [PubMed] [Google Scholar]
- 115.Aucouturier J., Dupuis L., Deville S., Ascarateil S., Ganne V. Montanide ISA 720 and 51: A new generation of water in oil emulsions as adjuvants for human vaccines. Expert Rev. Vaccines. 2002;1:111–118. doi: 10.1586/14760584.1.1.111. [DOI] [PubMed] [Google Scholar]
- 116.Platten M., Bunse L., Wick A., Bunse T., Le Cornet L., Harting I., Sahm F., Sanghvi K., Tan C.L., Poschke I., et al. A vaccine targeting mutant IDH1 in newly diagnosed glioma. Nature. 2021;592:463–468. doi: 10.1038/s41586-021-03363-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wishart F.O., Jackson L.K. Tetanus Toxoid—The Recall Dose. Canad. J. Public Health/Rev. Can. Sante’e Publique. 1948;39:181–186. [PubMed] [Google Scholar]
- 118.Alson D., Schuyler S.C., Yan B.-X., Samimuthu K., Qiu J.T. Combination Vaccination with Tetanus Toxoid and Enhanced Tumor-Cell Based Vaccine Against Cervical Cancer in a Mouse Model. Front. Immunol. 2020;11:927. doi: 10.3389/fimmu.2020.00927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mitchell D.A., Batich K.A., Gunn M.D., Huang M.-N., Sanchez-Perez L., Nair S.K., Congdon K.L., Reap E.A., Archer G.E., Desjardins A., et al. Tetanus toxoid and CCL3 improve dendritic cell vaccines in mice and glioblastoma patients. Nature. 2015;519:366–369. doi: 10.1038/nature14320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Conlon K.C., Miljkovic M.D., Waldmann T.A. Cytokines in the Treatment of Cancer. J. Interf. Cytokine Res. 2019;39:6–21. doi: 10.1089/jir.2018.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rosenberg S.A. The Development of New Immunotherapies for the Treatment of Cancer Using Interleukin-2. Ann. Surg. 1988;208:121–135. doi: 10.1097/00000658-198808000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Majidpoor J., Mortezaee K. Interleukin-2 therapy of cancer-clinical perspectives. Int. Immunopharmacol. 2021;98:107836. doi: 10.1016/j.intimp.2021.107836. [DOI] [PubMed] [Google Scholar]
- 123.Gillessen S., Naumov Y.N., Nieuwenhuis E.E.S., Exley M.A., Lee F.S., Mach N., Luster A.D., Blumberg R.S., Taniguchi M., Balk S.P., et al. CD1d-restricted T cells regulate dendritic cell function and antitumor immunity in a granulocyte–macrophage colony-stimulating factor-dependent fashion. Proc. Natl. Acad. Sci. USA. 2003;100:8874–8879. doi: 10.1073/pnas.1033098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hargadon K.M., Johnson C.E., Williams C.J. Immune checkpoint blockade therapy for cancer: An overview of FDA-approved immune checkpoint inhibitors. Int. Immunopharmacol. 2018;62:29–39. doi: 10.1016/j.intimp.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 125.Twomey J.D., Zhang B. Cancer Immunotherapy Update: FDA-Approved Checkpoint Inhibitors and Companion Diagnostics. AAPS J. 2021;23:39. doi: 10.1208/s12248-021-00574-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Robert C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat. Commun. 2020;11:3801. doi: 10.1038/s41467-020-17670-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Postow M.A., Callahan M.K., Wolchok J.D. Immune Checkpoint Blockade in Cancer Therapy. J. Clin. Oncol. 2015;33:1974–1982. doi: 10.1200/JCO.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jenkins R.W., Barbie D.A., Flaherty K.T. Mechanisms of resistance to immune checkpoint inhibitors. Br. J. Cancer. 2018;118:9–16. doi: 10.1038/bjc.2017.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zappasodi R., Merghoub T., Wolchok J.D. Emerging Concepts for Immune Checkpoint Blockade-Based Combination Therapies. Cancer Cell. 2018;33:581–598. doi: 10.1016/j.ccell.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tang K., Wu Y.-H., Song Y., Bin Yu B. Indoleamine 2,3-dioxygenase 1 (IDO1) inhibitors in clinical trials for cancer immunotherapy. J. Hematol. Oncol. 2021;14:68. doi: 10.1186/s13045-021-01080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Savitz J. The kynurenine pathway: A finger in every pie. Mol. Psychiatry. 2019;25:131–147. doi: 10.1038/s41380-019-0414-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhai L., Ladomersky E., Lauing K.L., Wu M., Genet M., Gritsina G., Győrffy B., Brastianos P.K., Binder D.C., Sosman J.A., et al. Infiltrating T Cells Increase IDO1 Expression in Glioblastoma and Contribute to Decreased Patient Survival. Clin. Cancer Res. 2017;23:6650–6660. doi: 10.1158/1078-0432.CCR-17-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhai L., Ladomersky E., Lenzen A., Nguyen B., Patel R., Lauing K.L., Wu M., A Wainwright D. IDO1 in cancer: A Gemini of immune checkpoints. Cell. Mol. Immunol. 2018;15:447–457. doi: 10.1038/cmi.2017.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ladomersky E., Zhai L., Lenzen A., Lauing K.L., Qian J., Scholtens D.M., Gritsina G., Sun X., Liu Y., Yu F., et al. IDO1 Inhibition Synergizes with Radiation and PD-1 Blockade to Durably Increase Survival Against Advanced Glioblastoma. Clin. Cancer Res. 2018;24:2559–2573. doi: 10.1158/1078-0432.CCR-17-3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Türeci Ö., Löwer M., Schrörs B., Lang M., Tadmor A., Sahin U. Challenges towards the realization of individualized cancer vaccines. Nat. Biomed. Eng. 2018;2:566–569. doi: 10.1038/s41551-018-0266-2. [DOI] [PubMed] [Google Scholar]
- 136.Türeci Ö., Vormehr M., Diken M., Kreiter S., Huber C., Sahin U. Targeting the Heterogeneity of Cancer with Individualized Neoepitope Vaccines. Clin. Cancer Res. 2016;22:1885–1896. doi: 10.1158/1078-0432.CCR-15-1509. [DOI] [PubMed] [Google Scholar]
- 137.Bastin D.J., Quizi J., Kennedy M.A., Kekre N., Auer R.C. Current challenges in the manufacture of clinical-grade autologous whole cell vaccines for hematological malignancies. Cytotherapy. 2022;24:979–989. doi: 10.1016/j.jcyt.2022.03.010. [DOI] [PubMed] [Google Scholar]
- 138.Parney I.F., Gustafson M., Solseth M., Bulur P., Peterson E.T., Smadbeck J.B., Johnson S.H., Murphy S.J., Vasmatzis G., Dietz A.B. Novel strategy for manufacturing autologous dendritic cell/allogeneic tumor lysate vaccines for glioblastoma. Neuro-Oncol. Adv. 2020;2:vdaa105. doi: 10.1093/noajnl/vdaa105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Parney I.F., Anderson S.K., Gustafson M.P., Steinmetz S., Peterson E.T., Kroneman T.N., Raghunathan A., O’Neill B.P., Buckner J.C., Solseth M., et al. Phase I trial of adjuvant mature autologous dendritic cell/allogeneic tumor lysate vaccines in combination with temozolomide in newly diagnosed glioblastoma. Neuro-Oncol. Adv. 2022;4:vdac089. doi: 10.1093/noajnl/vdac089. [DOI] [PMC free article] [PubMed] [Google Scholar]