Abstract
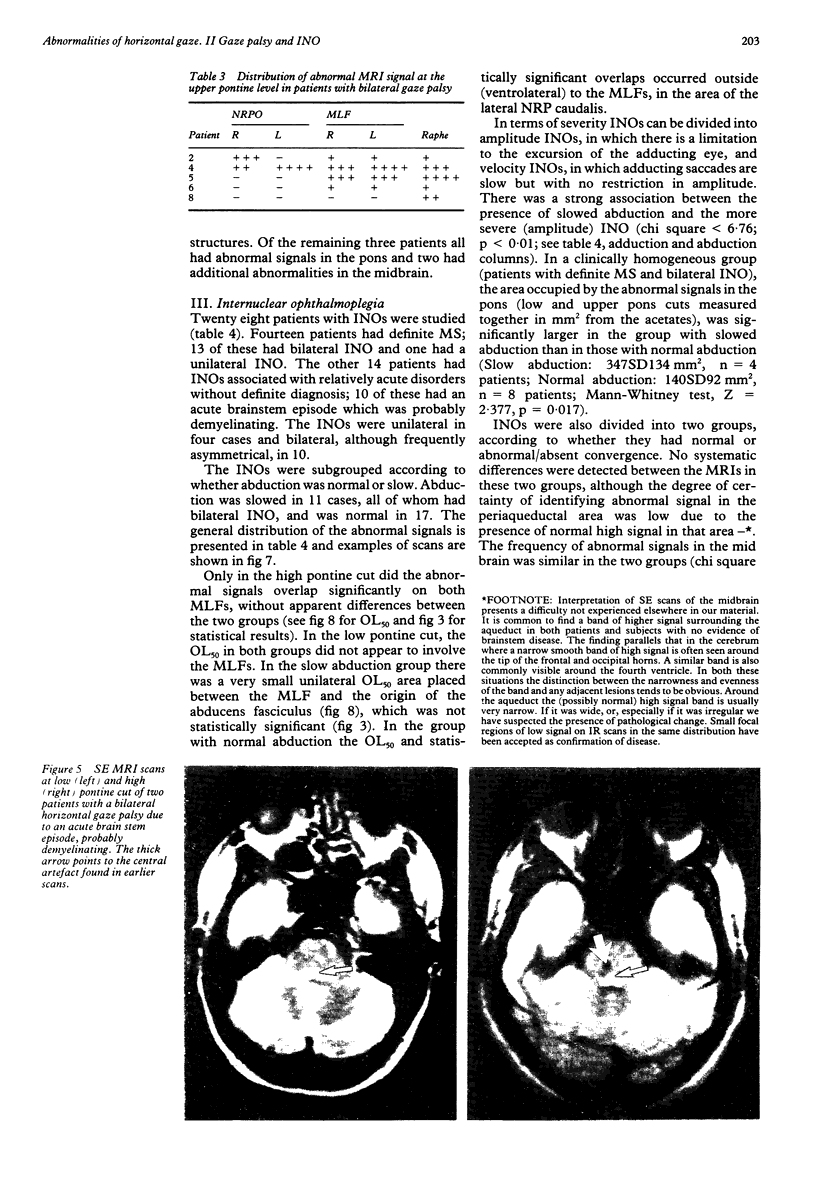
The site of lesions responsible for horizontal gaze palsy and various types of internuclear ophthalmoplegia (INO) was established by identifying the common areas where the abnormal MRI signals from patients with a given ocular-motor disorder overlapped. Patients with unilateral gaze palsy had lesions in the paramedian area of the pons, including the abducens nucleus, the lateral part of the nucleus reticularis pontis caudalis and the nucleus reticularis pontis oralis. Patients with abducens nucleus lesions showed additional clinical signs of lateral rectus weakness. Lesions responsible for bilateral gaze palsy involved the pontine tegmental raphe. Since this region contains the saccadic omnipause neurons, this finding suggests that damage to omnipause cells produces slowing of saccades rather than opsoclonus, as previously proposed. All INOs, regardless of the presence of impaired abduction or convergence, had similar MRI appearances. Frequently the lesions in patients with INO, were not confined to the medial longitudinal fasciculus (MLF) but also involved neighbouring structures at the pontine and mid-brain levels. There was a statistically significant association between the clinical severity of the INO and the presence of abnormal abduction or convergence. The findings suggest that the lesions outside the MLF, which may affect abducens, gaze or convergence pathways, are responsible for the presence of features additional to INO, depending on the magnitude of functional disruption they produce.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atlas S. W., Grossman R. I., Savino P. J., Schatz N. J., Sergott R. C., Bosley T. M., Hackney D. B., Goldberg H. I., Bilaniuk L. T., Zimmerman R. A. Internuclear ophthalmoplegia: MR-anatomic correlation. AJNR Am J Neuroradiol. 1987 Mar-Apr;8(2):243–247. [PMC free article] [PubMed] [Google Scholar]
- Bird A. C., Leech J. Internuclear ophthalmoplegia. An electro-oculographic study of peak angular saccadic velocities. Br J Ophthalmol. 1976 Sep;60(9):645–651. doi: 10.1136/bjo.60.9.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronstein A. M., Morris J., Du Boulay G., Gresty M. A., Rudge P. Abnormalities of horizontal gaze. Clinical, oculographic and magnetic resonance imaging findings. I. Abducens palsy. J Neurol Neurosurg Psychiatry. 1990 Mar;53(3):194–199. doi: 10.1136/jnnp.53.3.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büttner-Ennever J. A., Cohen B., Pause M., Fries W. Raphe nucleus of the pons containing omnipause neurons of the oculomotor system in the monkey, and its homologue in man. J Comp Neurol. 1988 Jan 15;267(3):307–321. doi: 10.1002/cne.902670302. [DOI] [PubMed] [Google Scholar]
- Büttner-Ennever J. A., Henn V. An autoradiographic study of the pathways from the pontine reticular formation involved in horizontal eye movements. Brain Res. 1976 May 21;108(1):155–164. doi: 10.1016/0006-8993(76)90171-2. [DOI] [PubMed] [Google Scholar]
- CARPENTER M. B., McMASTERS R. E., HANNA G. R. Disturbances of conjugate horizontal eye movements in the monkey. I. Physiological effects and anatomical degeneration resulting from lesions of the abducens nucleus and nerve. Arch Neurol. 1963 Mar;8:231–247. doi: 10.1001/archneur.1963.00460030015001. [DOI] [PubMed] [Google Scholar]
- Cheney P. D., Preston J. B. Classification and response characteristics of muscle spindle afferents in the primate. J Neurophysiol. 1976 Jan;39(1):1–8. doi: 10.1152/jn.1976.39.1.1. [DOI] [PubMed] [Google Scholar]
- Cogan D. G. Internuclear ophthalmoplegia, typical and atypical. Arch Ophthalmol. 1970 Nov;84(5):583–589. doi: 10.1001/archopht.1970.00990040585005. [DOI] [PubMed] [Google Scholar]
- Cohen B., Henn V. Unit activity in the pontine reticular formation associated with eye movements. Brain Res. 1972 Nov 13;46:403–410. doi: 10.1016/0006-8993(72)90030-3. [DOI] [PubMed] [Google Scholar]
- Feldon S. E., Hoyt W. F., Stark L. Disordered inhibition in internuclear ophthalmoplegia: analysis of eye movement recordings with computer simulations. Brain. 1980 Mar;103(1):113–137. doi: 10.1093/brain/103.1.113. [DOI] [PubMed] [Google Scholar]
- Fisher C. M. Some neuro-ophthalmological observations. J Neurol Neurosurg Psychiatry. 1967 Oct;30(5):383–392. doi: 10.1136/jnnp.30.5.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs A. F., Kaneko C. R., Scudder C. A. Brainstem control of saccadic eye movements. Annu Rev Neurosci. 1985;8:307–337. doi: 10.1146/annurev.ne.08.030185.001515. [DOI] [PubMed] [Google Scholar]
- Hanson M. R., Hamid M. A., Tomsak R. L., Chou S. S., Leigh R. J. Selective saccadic palsy caused by pontine lesions: clinical, physiological, and pathological correlations. Ann Neurol. 1986 Aug;20(2):209–217. doi: 10.1002/ana.410200206. [DOI] [PubMed] [Google Scholar]
- Henn V., Lang W., Hepp K., Reisine H. Experimental gaze palsies in monkeys and their relation to human pathology. Brain. 1984 Jun;107(Pt 2):619–636. doi: 10.1093/brain/107.2.619. [DOI] [PubMed] [Google Scholar]
- Highstein S. M., Maekawa K., Steinacker A., Cohen B. Synaptic input from the pontine reticular nuclei to absucens motoneurons and internuclear neurons in the cat. Brain Res. 1976 Aug 6;112(1):162–167. doi: 10.1016/0006-8993(76)90344-9. [DOI] [PubMed] [Google Scholar]
- Keller E. L. Participation of medial pontine reticular formation in eye movement generation in monkey. J Neurophysiol. 1974 Mar;37(2):316–332. doi: 10.1152/jn.1974.37.2.316. [DOI] [PubMed] [Google Scholar]
- Loeffler J. D., Hoyt W. F., Slatt B. Motor excitation and inhibition in internuclear palsy. An electromyographic study. Arch Neurol. 1966 Dec;15(6):664–671. doi: 10.1001/archneur.1966.00470180104012. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny C., Chain F., Serdaru M., Gray F., Lhermitte F. The 'one-and-a-half' syndrome. Electro-oculographic analyses of five cases with deductions about the Physiological mechanisms of lateral gaze. Brain. 1981 Dec;104(Pt 4):665–699. doi: 10.1093/brain/104.4.665. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny C., Goasguen J. Isolated abducens nucleus damage due to histiocytosis X. Electro-oculographic analysis and physiological deductions. Brain. 1984 Dec;107(Pt 4):1019–1032. doi: 10.1093/brain/107.4.1019. [DOI] [PubMed] [Google Scholar]
- Pola J., Robinson D. A. An explanation of eye movements seen in internuclear ophthalmoplegia. Arch Neurol. 1976 Jun;33(6):447–452. doi: 10.1001/archneur.1976.00500060053011. [DOI] [PubMed] [Google Scholar]
- Pola J., Robinson D. A. Oculomotor signals in medial longitudinal fasciculus of the monkey. J Neurophysiol. 1978 Mar;41(2):245–259. doi: 10.1152/jn.1978.41.2.245. [DOI] [PubMed] [Google Scholar]
- Ridley A., Kennard C., Scholtz C. L., Büttner-Ennever J. A., Summers B., Turnbull A. Omnipause neurons in two cases of opsoclonus associated with oat cell carcinoma of the lung. Brain. 1987 Dec;110(Pt 6):1699–1709. doi: 10.1093/brain/110.6.1699. [DOI] [PubMed] [Google Scholar]
- Scudder C. A. A new local feedback model of the saccadic burst generator. J Neurophysiol. 1988 May;59(5):1455–1475. doi: 10.1152/jn.1988.59.5.1455. [DOI] [PubMed] [Google Scholar]
- Strassman A., Evinger C., McCrea R. A., Baker R. G., Highstein S. M. Anatomy and physiology of intracellularly labelled omnipause neurons in the cat and squirrel monkey. Exp Brain Res. 1987;67(2):436–440. doi: 10.1007/BF00248565. [DOI] [PubMed] [Google Scholar]
- Strassman A., Highstein S. M., McCrea R. A. Anatomy and physiology of saccadic burst neurons in the alert squirrel monkey. I. Excitatory burst neurons. J Comp Neurol. 1986 Jul 15;249(3):337–357. doi: 10.1002/cne.902490303. [DOI] [PubMed] [Google Scholar]
- Zee D. S., Robinson D. A. A hypothetical explanation of saccadic oscillations. Ann Neurol. 1979 May;5(5):405–414. doi: 10.1002/ana.410050502. [DOI] [PubMed] [Google Scholar]